Abstract
Introduction:
Erectile dysfunction (ED) is frequently encountered in patients with arterial hypertension and there is a recent functional correlation between the expression of thermoreceptor channels TRPM8 (melastatin 8) and alterations in blood pressure in hypertension. The aim of this study was to investigate the function of cold-sensing TRPM8 channel in internal pudendal artery (IPA) in both normotensive and hypertensive rats.
Methods:
We performed experiments integrating physiological, pharmacological, biochemical and cellular techniques.
Results:
TRPM8 channels are expressed in the IPA and in vascular smooth muscle cells from IPA. In addition, TRPM8 activation, by both a cooling compound icilin (82.1 ± 3.0%, n = 6) and cold temperature [thermal stimulus, basal tone (25°C, 41.2 ± 3.4%, n = 5) or pre-contracted tone induced by phenylephrine (25°C, 87.0 ± 3.6%, n = 7)], induced relaxation in IPA. Furthermore, the results showed that the concentration-response curve to icilin was significantly shifted to the right in different conditions, such as: the absence of the vascular endothelium, in the presence of L-NAME (10−4 M), or indomethacin (10−5 M) or by a combination of charybdotoxin (10−7 M) and apamin (5 × 10−6 M), and Y27632 (10−6 M). Interestingly, icilin-induced vasodilation was significantly higher in IPA from spontaneously hypertensive (SHR, E10M−4= 75.3 ± 1.7%) compared to wistar rats (E10M−4= 56.4 ± 2.6%), despite no changes in the TRPM8 expression in IPA between the strains, suggesting that the sensitivity of TRPM8 channels is higher in SHR.
Conclusions:
These data demonstrate for the first time, the expression and function of TRPM8 channels in the IPA involving, at least in part, endothelium-derived relaxing factors and ROCK inhibition. Overall, this channel could potentially be a new target for the treatment of hypertension associated-ED.
Keywords: TRPM8 channels, Spontaneously hypertensive rats, Internal pudendal artery, Vasodilation, Endothelium-derived relaxing factors, ROCK
1. Introduction
Undoubtedly, cardiovascular diseases (CVD) are and will continue to be one of the major health problems of modern society [1]. An equally valuable observation is the fact that sexual dysfunction could indicate asymptomatic CVD. Consistent evidence has accumulated in recent years indicating that sexual dysfunction is associated with CVD, with which it shares major risk factors including hypertension, smoking, and diabetes. In many cases, erectile dysfunction (ED) diagnosis predates symptomatic coronary artery disease or cardiovascular events by years. Additionally, the severity of ED correlates with the severity of CVD, and the presence of ED is an independent marker for increased risk in people with existing CVD. Despite these facts, sexual dysfunction is commonly under-reported, under-recognized and undertreated, yet it could indeed play a role in cardiovascular risk assessment and stratification as well as early treatment [2].
The prevalence of hypertension and ED has grown steadily, and greater than 40% of men with ED share a diagnosis of hypertension [3,4]. To treat patients with both diseases is a clinical challenge due the fact both are correlated and share several risk factors such as diabetes mellitus, obesity, limited or an absence of physical exercise, alcoholism, etc. [3,5,6].
Important advances have occurred in recent years in understanding the pathophysiology of ED resulting in the development of successful oral therapies. The inhibitors of phosphodiesterase type 5 (PDE-5i) are a good example of this. However, the treatment of ED in patients with a high cardiovascular risk profile, diabetes, or vascular endothelial damage, demonstrate low clinical response to PDE-5i [7]. Based on this, additional investigation should be performed to better understand the pathophysiology of ED, with aim to discover new potential targets for treatment of hypertension-associated ED.
Transient Receptor Potential (TRP) channels are critical to vascular function, and dysregulation of these channels is associated with vascular-related pathologies, including hypertension and ischemia-re-perfusion injury (for more details, see review [8–12]). However, despite the strong correlation between ED and hypertension, especially considering their common vascular etiologies, studies investigating the role of TRP channels in the penile vasculature or corpus cavernosum have not been previously reported.
Among TRP channels, TRPM8 (melastatin 8) is a non-selective calcium-permeable channel, mainly localized in the sensory neurons, that to play a critical role in the transduction of moderate cold stimuli [13–15]. TRPM8 behaves as a polymodal receptor activated by membrane depolarization, cold, and chemical compounds such as menthol, icilin, and several inflammatory agents [14,16–18].
In relation to the cardiovascular system, the literature reports a functional correlation between the expression of thermoreceptor channels TRPM8 and alterations in blood pressure in hypertension. Liu et al. [19] demonstrated a decrease in the expression and TRPM8 channel activity in different models of pulmonary hypertension. Interestingly, Xiong et al. [20] demonstrated that the dietary menthol (TRPM8 activator) supplementation reduced the vascular dysfunction and hypertension induced by noxious cold and the treatment with menthol attenuated the vasoconstriction and activation of RhoA/Rho kinase pathway in mice with hypertension induced by cold or Ang II. In line, Sun et al. [21] demonstrated that TRPM8 activation by menthol benefited vascular function and blood pressure by inhibiting RhoA/Rho kinase pathway in the vasculature. In addition, chronic menthol capsule administration improved flow mediated dilatation in prehypertensive individuals, demonstrating that menthol treatment has favorable effects on hypertension.
Although the literature does not demonstrate the role of TRPM8 in the penile vasculature or in erectile function, the use of the menthol (TRPM8 agonist) empirically in different types of preparations for use during sexual intercourse is widespread in the world, but no scientific study has been carried out to evaluate the consequences of TRPM8 activation in tissues of importance for penile erection. Then, the correlation between the thermosensitive TRP channels (TRPM8) and cardiovascular functions in the hypertensive state, together with the importance of restricted thermal regulation of the sexual tissues, ensuring ideal functioning of the germ cells, as well as the erectile tissues, prompted us to investigate the participation of channels TRPs (TRPM8) in hypertension associated-ED. The purpose of this study was to test whether TRPM8 channels are expressed in pudendal artery and if their functions are modified in tissues from hypertensive rats, contributing to vascular dysfunction observed in hypertension associated – ED.
In this study, we demonstrate that TRPM8 channels are expressed in pudendal artery from both normotensive and hypertensive rats and their activation is able to relax these tissues, involving, at least in part, endothelium-derived relaxing factors (EDRFs) and RhoA/ROCK inhibition. Interestingly, higher sensitivity of TRPM8 channels to icilin was observed in the penile vasculature from hypertensive compared to normotensive rats.
2. Methods
2.1. Animals
Male wistar rats, spontaneously hypertensive rats (SHR), both 30 weeks of age, and Sprague Dawley (SD) rats (12–15 weeks old), were used for this investigation (Harlan Laboratories, Indianapolis, IN, USA). All rats were maintained on a 12:12 h light–dark cycle with both rat chow and water ad libitium. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and were reviewed and approved by the Institutional Animal Care and Use Committee of Augusta University. All surgical procedures were undertaken on rats under isoflurane anesthesia, administered via nose cone (5% in 100% O2). Rats were then euthanized by thoracotomy and exsanguination via cardiac puncture.
2.2. Isolation of arteries
Internal pudendal arteries (IPA) were removed from rats and placed in 4 °C physiological salt solution (PSS) containing: NaCl (130 mM), NaHCO3 (14.9 mM), KCl (4.7 mM), KH2PO4 (1.18 mM), MgSO4−7H2O (1.18 mM), CaCl2-2H2O (1.56 mM), EDTA (0.026 mM), and glucose (5.5 mM) (all Sigma-Aldrich, St Louis, MO, USA). Excised arteries were cleaned of perivascular tissue.
2.3. Vascular smooth muscle cell isolation
Primary VSMCs were cultured from IPA using an enzymatic digestion method [22]. Briefly, IPA were cut into small pieces and incubated in enzymatic solution mix, for 60 min at 37 °C under agitation, containing: BSA (2mg/mL), collagenase type 1 (2mg/mL), trypisin inhibitor (0.380 mg/mL) and elastase (0.125 mg/mL) in Hanks’ Balanced Salt solution (HBSS). Cells were passed through a 100-μm nylon mesh filter. The cell suspension was centrifuged at 2500 rpm for 5 min and resuspended in Dulbecco’s modified Eagle’s medium (GE Healthcare, Logan, UT, USA), supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution (Corning, Manassas, VA, USA). VSMCs were identified by determination of α-actin expression and the absence of endothelial cells (EC) was confirmed by assessment of von Willebrand factor, both analyzed by fluorescence microscopy (data not shown). Treatments commenced between passages 3–6, when culture dishes were ≥80% confluent. Twenty-four hours prior to treatments, cells were placed into serum-free media to achieve quiescence.
For some experiments the cell signaling involved in the responses after TRPM8 activation was investigated using Western blotting technique. VSMCs from normotensive rats were treated for 30 min by vehicle (DMSO 0.01%), icilin (10−6 and 10−5 M), a TRPM8 channels activator, or N-(4-tert-butylPhenyl)-4-(3-chloropyridin-2-yl) piperazine-1-carboxamide (BCTC; 2 × 10−6M), a TRPM8 channel antagonist.
2.4. Immunoblotting
IPA were cleaned of perivascular adipose tissue, snapped frozen in liquid nitrogen, and then homogenized in ice-cold tissue protein extraction reagent (T-PER) (Thermo Fisher Scientific, MA, USA), with protease inhibitors [sodium orthovanadate, Phenylmethanesulfonylfluoride (PMSF), protease inhibitor cocktail (Sigma)] and phosphatase inhibitors [sodium fluoride and sodium pyrophosphate] (Sigma), as described in [23]. In some experiments, VSMC from IPA were used for immunoblotting experiments. Protein was extracted and quantified by BCA protein assay (Thermo Fisher Scientific, MA, USA, #23224 and #23228). Equal amounts (30–40 μg) were separated on polyacrylamide gels using a standard SDS-PAGE Western blot protocol. Gels were then transferred to polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Scientific, MA, USA) and probed for protein expression. The membranes were blocked with 5% nonfat dry milk and incubated overnight at 4 °C with primary antibodies raised against anti-TRPM8 (1:1500, abcam, Cambridge, MA, USA, #ab3243); anti-RhoA (1:5000, Cell Signaling Technology, MA, USA, #2117); anti-ROCK II (1:5000; BD Transduction Laboratories, CA, USA, #610623); anti-MYPT (1:1000; BD Transduction Laboratories, #612165); anti-Phosphorylated MYPT1Thr696 (1:5000, Cell Signaling Technology, #5163); anti-GAPDH (1: 20,000, Cell Signaling Technology, #2118); Anti-β-actin antibody (1:2000, abcam, Cambridge, MA, USA; # ab8227);
The membranes were then washed and incubated with horseradish peroxidase-linked secondary antibodies. Immunocomplexes were detected with an enhanced chemiluminescence system on a protein sample imager. Phosphorylated protein expression was normalized to total protein expression; all other proteins were normalized to loading control, GAPDH or β-actin. Expression is presented as arbitrary units (A.U.).
2.5. Immunofluorescence assay
To visualize TRPM8 expression in IPA and VSMCs, confocal microscopy was performed. Arteries were embedded in optimal cutting temperature medium and frozen in liquid nitrogen. Slides containing transverse cross-sections (10 μm) of frozen arteries were immersed into acetone/methanol solution (1:3 proportion) for 10min, washed by physiological buffer solution (PBS, 1X) and blocked with hydrogen peroxide (3%) for 30 min. In the case of the experiments performed with VCMCs, they were fixed in 4% paraformaldehyde (Thermo Fisher Scientific) for 10 min. Cross-sections/cells were blocked in 1X PBS with 0.01% Triton X-100 (Thermo Fisher Scientific) and 5% horse serum. The slides were then incubated with anti-TRPM8 (1:30; Abcam/ab3243) primary antibody overnight. Next, slides were incubated with goat anti-rabbit IgG (H + L) cross-adsorbed secondary antibody Alexa Fluor 594 (1:500) in 1X PBS and 5% BSA for 60 min. Vectashield HardSet Antifade Mounting Medium with DAPI (H-1500) (Vector Laboratories, Inc., Burlingame, CA) was then applied to slides with coverslips. IPA or VSMCs were visualized using a Zeiss LSM 780 Upright confocal microscope (63X objective) (Carl Zeiss MicroImaging, Oberkochen, Germany).
2.6. Vascular function
Internal pudendal arteries were mounted on DMT wire myographs (Danish MyoTech, Aarhus, Denmark). Arteries were bathed in 37 °C physiological salt solution (PSS) with 5% CO2 and 95% O2. Isolated arteries were then normalized to their optimal luminal diameter based on the internal circumference/wall tension ratio of the arteries by setting the internal circumference (L0) to 90% of what the vessels would have if they were exposed to a passive tension equivalent of 100 mmHg (L100) transmural pressure [24,25].
Cumulative concentration-response curves were performed to ACh (10−10−3 × 10−5M), sodium nitroprusside (SNP; 10−10−3 × 10−5M), Phenylephrine (Phe; 10−10−3 × 10−5 M) and icilin (10−8−10−4 M), a TRPM8 channel activator. In some experiments, thirty minutes prior to Phe, arteries were incubated with a TRPM8 channel antagonist [BCTC, (2 × 10−6 M) or M8-B (10−6 M)], cyclooxygenase (COX) inhibitor (indomethacin; 10−5 M), nitric oxide synthase (NOS) inhibitor (L-NAME; 10−4 M), non-selective blocker of the large (BKCa) and intermediate (IKCa) conductance Ca2+-activated K+ channels (charybdotoxin; 10−7M), SK channel blocker (apamin; 5 × 10−6 M), BKCa blocker (iberiotoxin; 10−7 M), non-selective K+ channel blocker (tetraethylammonium – TEA, 3 × 10−3 M), ROCK (Rho-associated protein kinase) inhibitor (Y27632; 10−6M).
In another set of experiments, the effects of cold temperature (35 – 25 °C) activating temperature-sensitive TRPM8 channels were investigated in pudendal arteries under basal or pre-contracted tone. To achieve the desired temperature, the bath solution at the target temperature (using a thermometer) was placed into the chamber, and the heating devices were turned off. The exact temperature (°C) and vascular tone (mN) were informed by the equipment during all the experiment.
For some experiments, the endothelium layer was denuded by rubbing the lumen with a hair shaft to determine whether the relaxant effect of TRPM8 activator (Icilin) was endothelium-dependent. Endothelial integrity was assessed by the degree of relaxation caused by acetylcholine (10−6 M) in vessels pre-contracted with the thromboxane A2 analogue U46619 (10−6 M) (Cayman). Rings were considered to be endothelium-denuded when acetylcholine induced relaxant effects were less than 10% and endothelium-intact when the relaxant effects were more than 90% of the initial contraction. To evaluate contractile integrity, just after the equilibration period and again at the end of the experiments, vessels were exposed to Phenylephrine (10−6 M). Arteries were considered viable if KCl and PE contracted the arteries greater than 10 mN respectively. All compounds and drugs were obtained from Sigma-Aldrich.
2.7. Statistical analysis
Data are presented as the mean ± S.E.M. and n (n = number of animals) represents number of experiments performed from independent rats. The curves were fitted using a variable slope sigmoid fitting routine in GraphPad Prism4 (San Diego, CA, USA), according to the equation: Y = Bottom + (Top-Bottom)/(1 + 10ˆ((LogEC50-X)*HillSlope), where Y is the effect at the concentration test (X), EC50 is the concentration of agonist that gives a response half way between Bottom and Top, HillSlope describes the steepness of the family of curves, and Top and Bottom are plateaus in the units of the Y axis (GraphPad Prism4 guide). Statistical analyses used included one-way analysis of variances (ANOVAs) followed by Bonferroni’s multiple comparisons post-test and unpaired or paired Student’s t-test. Statistical significance was set at p < 0.05.
3. Results
3.1. Identification of TRPM8 channels expression in IPA and VSMCs
Information about the expression of TRPM8 channels in the pudendal artery has not been described in the literature. As a result, protein expression experiments were carried out to determine whether TRPM8 channel proteins are expressed in VSMCs from IPA. In Fig. 1A and B, immunoreactive bands indicating expression of TRPM8 channels in the IPA tissue and VSMC from IPA by using Western blotting technique. Bladder (a nonvascular smooth muscle), mesenteric artery and VSMCs from aorta were used as positive control [26–28]. Bladder and mesenteric artery were isolated and cleaned as described in Teixeira et al. [29] and Silva et al. [30], respectively.
Fig. 1. TRPM8 channels expression in isolated arteries and VSMCs.
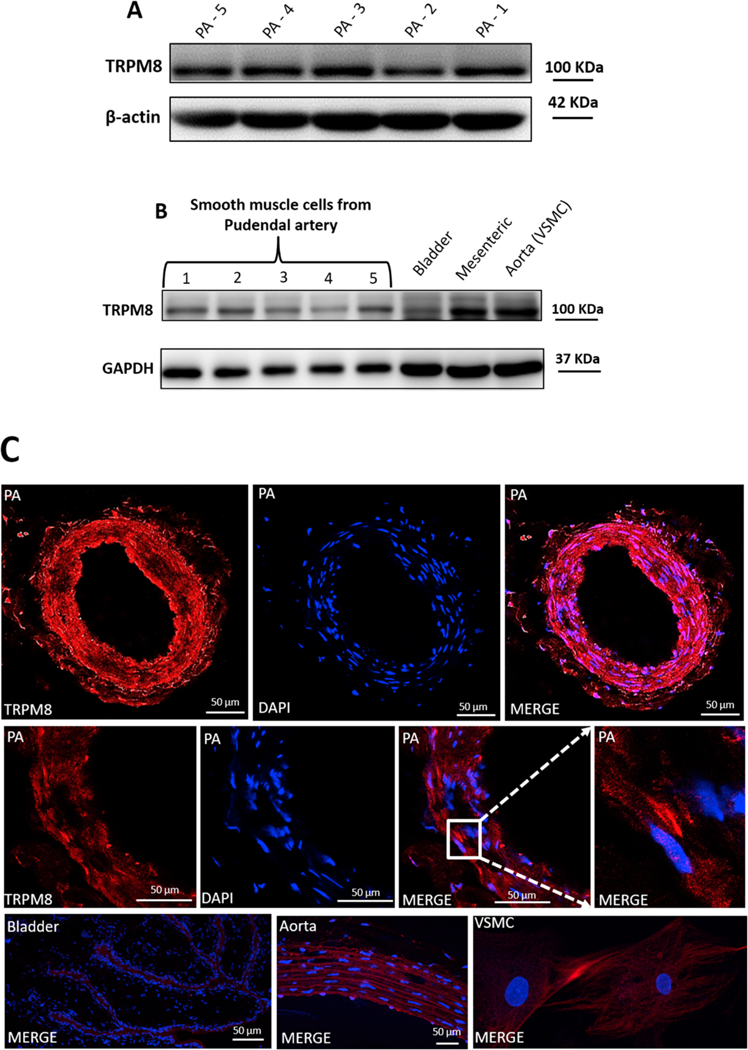
TRPM8 expression analyzed in IPA (A) and VSMCs from IPA (B) using Western blotting technique and immunofluorescence (C). Samples from bladder, mesenteric artery and aorta were used as positive control. VSMC images were taken at 63x magnification. N = 5.
To support these quantitative data, we performed confocal micro-scopy on IPA and VSMCs. First, cultured VSMCs were characterized, using immunofluorescence, by the presence of calponin and by the absence of von Willebrand factor, characteristic of EC (data not shown). IPA characterization is demonstrated in Supplementary material (Fig. 1). In Fig. 1C, immunofluorescence staining showed that TRPM8 was expressed in IPA and VSMCs. Sections from aorta and bladder were used as positive control.
3.2. TRPM8 activation by cold temperature or chemical stimulus induces vasodilation in IPA
In order to investigate the effects induced by TRPM8 channels activation in IPA, experiments were performed using icilin, a TRPM8 activator. Icilin significantly relaxed the sustained contraction induced by Phe (10−6 M, n = 6). The relaxation induced by icilin was reduced in the presence of both, BCTC (2 × 10−6 M, n = 7) or M8-B (10−6 M, n = 6), TRPM8 blockers (Fig. 2A). Furthermore, there was significant change in basal tone after icilin exposure (Fig. 2 in the Supplementary material).
Fig. 2. TRPM8 activation by cold temperature and chemical stimulus induced vasodilation in IPA.
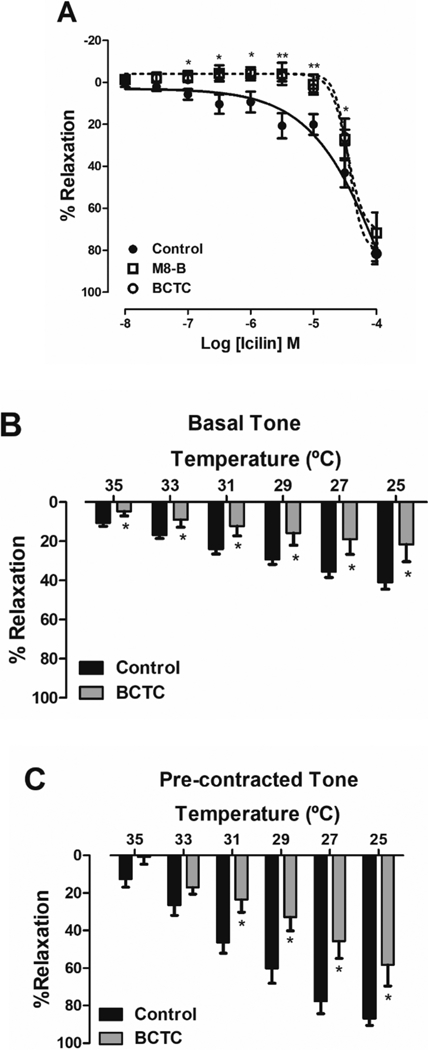
A. Logarithmic concentration-response curves showing the relaxant effect of icilin (TRPM8 activator, 10−8 to 10−4M), in the IPA pre-contracted by Phe (10−6 M), in the absence (●, control, n = 6) or in the presence of BCTC (⚪, 2 × 10−6 M, n = 7) and M8-B (⬜, 10−6 M, n = 6), both TRPM8 blockers. B. Bar graph summary showing that the reduction of temperature of PSS solution induced vasodilation of the basal (A, n = 5) or contracted arterial tone (B, n = 6). Both effects were reduced by BCTC (2 × 10−6 M, A and B, n = 6). Data are the mean ± SEM. The data were examined using one-way ANOVA followed by Bonferroni post-test (Fig. 2A) and Student’s t tests (Fig. 2B and C). *p < 0.05, **p < 0.01 vs control/endothelium intact.
TRPM8 channels are classified as thermosensitive TRP channels because they are activated by cold temperatures between the range of 8 to 27 °C [31–34]. To confirm if TRPM8 activation induces relaxation in IPA, we performed experiments with endothelium-intact rings submitted to cold temperatures (35–25 °C), in two different experimental conditions, basal tone or pre-contracted tone induced by Phe (10−6 M). Fig. 2B and C show that changes in bath temperature, from 37 °C to a temperature that activates TRPM8 channels (25 °C), induced a significant reduction in basal or contracted arterial tone (E25°c = 41.21 ± 3.4, n = 5 and 87.0 ± 3.6%, n = 6, respectively). This relaxing effect induced by cold temperature was significantly decreased by BCTC (2 × 10−6 M) (E25°c = 21.7 ± 8.8 and 58.4 ± 11.3%, respectively, n = 6, p < 0.05).
3.3. Vasorelaxation induced by TRPM8 activation in IPA involves EDRFs
It is well known that the endothelium plays an important role in vascular tone control [35,36] and vascular endothelial cells release several EDRFs that modulate vascular tone. Therefore, we hypothesized that relaxation effect induced by TRPM8 activation would depend on EDRFs. In endothelium-denuded vessels, there was a significant right-ward shift in the concentration-response curve to icilin (p < 0.001) with significant changes on the effect produced by higher concentration of icilin tested (E10−4 = 66.2 ± 4.6%, n = 6) when compared to the endothelium-intact vessels (E10−4 = 82.1 ± 3.0%, n = 6, Fig. 3A).
Fig. 3. Endothelium-dependent and -independent relaxation induced by TRPM8 activation in IPA.
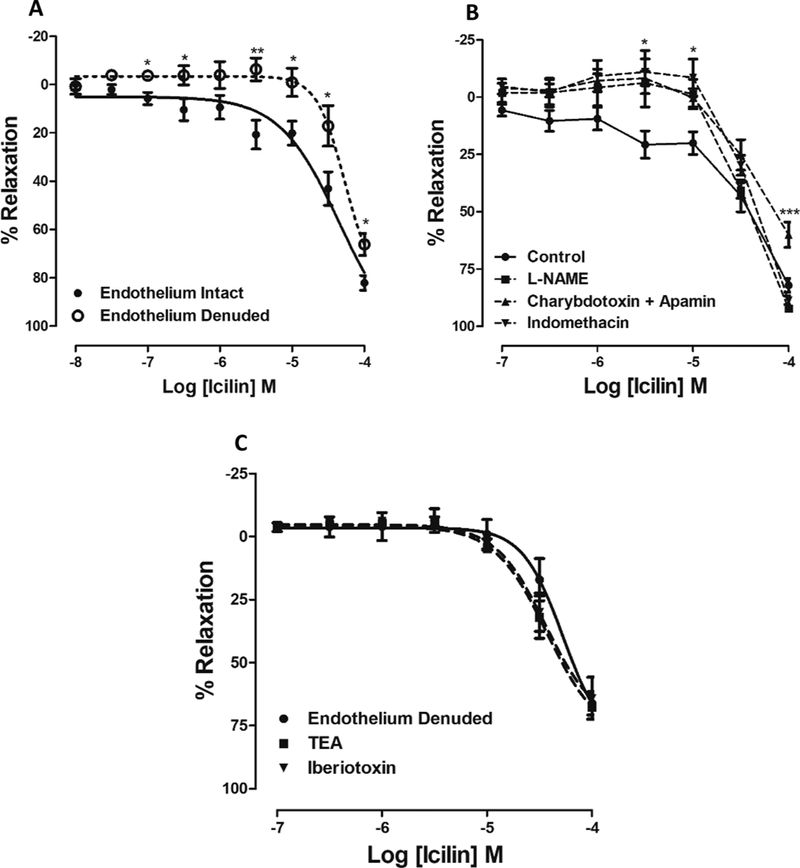
A. The concentration–response curves of icilin in the presence (●, intact endothelium, n = 6) or in the absence of functional endothelium (O, Endothelium denuded, n = 6). The response is expressed as a percentage of relaxation from Phenylephrine-induced contraction. B. Logarithmic concentration response curves to icilin obtained from studies with IPA pre-contracted by Phe (10−6 M), with functional endothelium, in the absence (●, control, n = 6) or in the presence of indomethacin (▼, 10−5 M), n = 6), L-NAME (■, 10−4 M, n = 6) or a combination of charybdotoxin (10−7 M) and apamin (5×10−6 M) (▲; n = 5). C. Logarithmic concentration-response curves showing the relaxant effect of icilin, in the IPA pre-contracted by Phe (10−6 M) without functional endothelium, in the absence (●, endothelium denuded, n = 6) or in the presence of iberiotoxin (▼, 10−7 M, n = 4) or TEA (■, 3 × 10−3 M, n = 6). Values are expressed as means ± S.E.M. The data were examined using unpaired Student’s t tests (Fig. 3A) and one-way ANOVA followed by the Bonferroni post-test (Fig. 3B and 3C). *p < 0.05 and ***p < 0.001 vs control.
To test whether TRPM8-mediated relaxation involves EDRFs, concentration-effect curves to icilin were constructed in the presence of different inhibitors, such as: L-NAME (10−4 M), indomethacin (10−5 M) or a combination of charybdotoxin (10−7 M) and apamin (5 × 10−6 M) to inhibit endothelium-derived hyperpolarizing factor (EDHF). As observed in Fig. 3B, the concentration-response curves to icilin were shifted to the right in arterial groups incubated with L-NAME or in-domethacin, suggesting that after TRPM8 activation, NO production and vasodilators derived from cyclooxygenase pathway (e.g., prostacyclin) seems to be involved in relaxation induced by TRPM8 activator icilin. Furthermore, the pre-incubation of arteries with a combination of charybdotoxin (10−7M) and apamin (5 × 10−6 M) also significantly decreased icilin-mediated relaxation in IPA (E10−4 = 60.1 ± 5.6%, n = 5) compared to the control (E10−4 = 82.1 ± 3.0%).
The subsequent experiments were carried out to explore the endothelium-independent vasorelaxation mechanism. The literature describes very well that TRPM8 is a Ca2+ permeable and we further hypothesized that the activation of BKca channels will occur subsequently to the activation of TRPM8. To determine the possible contribution of BKCa channels to endothelium-independent-icilin-induced relaxation, we examined the influence of their inhibitors, iberiotoxin (10−7 M) and TEA (3 × 10−3 M) in the relaxant response. As shown in Fig. 3C, the vasorelaxant response to icilin was not significantly inhibited by either TEA (E10−4 = 67.6 ± 4.8%, n = 6) or iberiotoxin (E10−4 = 64.2 ± 8.4%, n = 4) compared to control (E10−4 = 66.2 ± 4.6%).
3.4. TRPM8 channel activation inhibits Rho A/ROCK kinase pathway
Regarding the endothelium-independent relaxation, a previous study demonstrated that chronic menthol (TRPM8 agonist) administration reduced vasoconstriction induced by U46619 in mesenteric artery by vascular inhibition of RhoA/ROCK pathway in a TRPM8-dependent manner [21]. Based on this, we hypothesized that TRPM8 activation in IPA could involve RhoA/ROCK pathway inhibition. The Fig. 4A shows that concentration response curve to icilin was desensitized in the presence of Y27632 (ROCK inhibitor, 10−6 M), suggesting that, ROCK, at least in part, is important for relaxation induced by TRPM8 activation. To support these vascular function data, Western blotting was performed for RhoA and ROCK II. However, we observed that in VSMCs isolated from IPA treatment with either Phe (10−6 M) or Phe + icilin (10−5 M) or Phe + icilin + BCTC (2 × 10−6 M) did not change the expression of RhoA/ROCK (Fig. 4B and C).
Fig. 4. TRPM8 channels activation seems inhibit RhoA/ROCK kinase pathway.
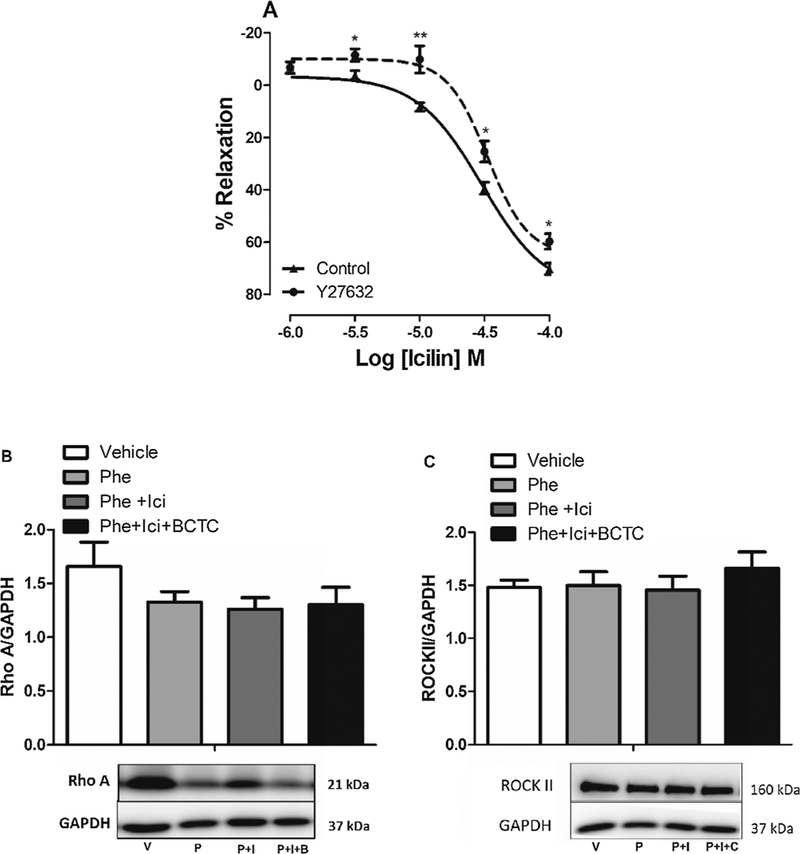
A. Logarithmic concentration response curves to icilin obtained from studies with IPA pre-contracted by Phe (10−6 M), without functional endothelium, in the absence (▲, control, n = 6) or in the presence of Y27632 (●, 10−6 M, n = 6). B and C. Protein expression analysis for RhoA and ROCKII normalized by GAPDH, in VSMCs from IPA, incubated in either vehicle (V, n = 5), Phenylephrine (P, 10−6 M, n = 5), Phenylephrine + icilin (10−5 M, P + I, n = 5) or Phenylephrine + icilin + BCTC (2 × 10−6 M, P + I + B, n = 5). Above, densitometric analysis; below, representative images of immunoblots. The data were examined using unpaired Student’s t tests (Fig. 4A) and one-way ANOVA followed by the Bonferroni post-test (Fig. 4B and 4C).
3.5. Characterization of vascular abnormalities in IPA from hypertensive rats
It has been known for some considerable time that sustained elevation of blood pressure induces structural changes in both heart and blood vessels. However, as the literature is controversial about the vascular abnormalities found in different vascular beds from hypertensive animals, in our set of experiments, we first analyzed the differences on the vascular functions observed in IPA from SHR compared to Wistar rats (30 weeks of age). Fig. 5A showed significant left ventricular hypertrophy in SHR compared to Wistar rats (p < 0.01). However, no differences were observed in proximal or distal internal pudendal arteries diameters from SHR [internal diameter = 406 ± 8 (n = 15) and 398 ± 12 μm (n = 15), respectively] and Wistar rats [internal diameter = 419 ±18 (n = 9) and 382 ± 17 μm (n = 7), respectively] (Fig. 5B). We also analyzed the vascular function changed by hypertensive state in IPA. Data from Fig. 5C shows that IPA with functional endothelium, from SHR, demonstrated higher contractile response to depolarizing KCl solutions (KCl 60 mM; 18.5 ± 0.8 mN, n = 19, p < 0.01) compared to normotensive rats (14.8 ± 0.9 mN, n = 19). On the other hand, no significant changes were observed in contractile response induced by U46619, a thromboxane A2 (TP) receptor agonist, in IPA from both animals (15.6 ± 0.8 and 15.5 ± 0.8 mN, respectively. n = 19). Since the vasoconstriction induced by U46619 was not different between SHR and Wistar rats, subsequent results were presented as % U46619-induced contraction for contraction curves.
Fig. 5. Vascular abnormalities in IPA from hypertensive compared to normotensive rats.
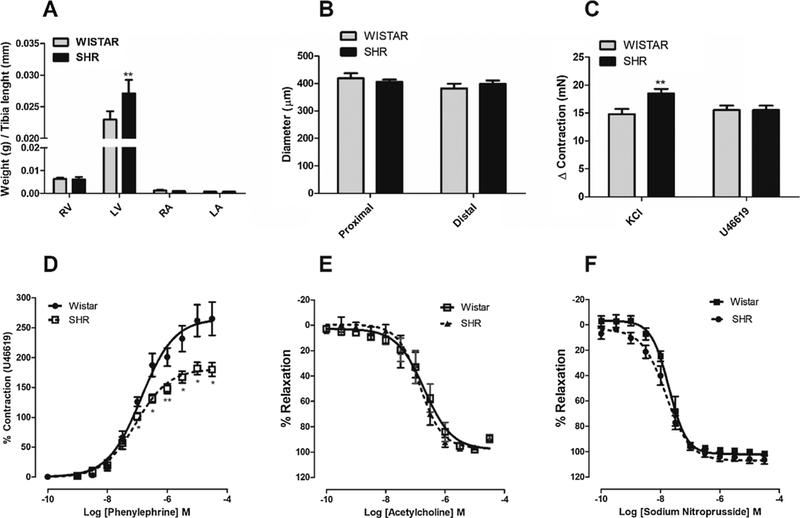
A. Bar graph summary showing weights of the right atrium (RA), left atrium (LA), right ventricle (RV) and left ventricle (LV) from Wistar and SHR (n = 6 each group). Data are mean tissue weight/tibia length (mm). B. Bar graph showing the proximal or distal internal pudendal arteries diameters (μm) predicted from arterial normalization curve. C. Bar graph showing the level of contraction (mN) induced by KCl (60mM) and U46619 (10−6 M) in IPA with functional endothelium, from SHR and Wistar rats (n = 19). Concentration-response curves to Phenylephrine (D), acetylcholine (E) and SNP (F) were performed in the IPA with functional endothelium from SHR and Wistar rats (n = 8 and 9, respectively). Data are the mean ± SEM. The data were examined using unpaired Student’s t tests. *p < 0.05 vs Wistar.
While IPA from SHR demonstrated similar sensitivity to the endothelium-dependent vasoconstrictor Phe (pD2 = 7.04 ± 0.05, n = 8) compared to normotensive rats (pD2 = 6.87 ± 0.07, n = 9), the pharmacological efficacy of Phe was significantly decreased in arteries from SHR [Emax = 180.1 ± 11.6 (n = 8) and 265.1 ± 27.8 (n = 9), respectively; p < 0.05] (Fig. 5D). In contrast, relaxation with the endothelium-independent vasodilator and nitric oxide (NO)-donor SNP or acetylcholine were not different between hypertensive and normotensive animals (Fig. 5E e F).
3.6. The sensitivity but not expression of TRPM8 channels are increased in IPA from hypertensive animals
Hypertension and ED are closely intertwined diseases, which have endothelial dysfunction as a common cause [37]. In our data, we analyzed if TRPM8 expression and activity are changed in IPA from hypertensive rats. Interestingly, in Fig. 6A and B we could not observe significant difference of TRPM8 expression in IPA from SHR and Wistar. However, Fig. 6C shows that icilin-induced vasodilation was significantly higher in IPA from SHR (E10−4 = 75.3 ± 1.7%, n = 12) compared to Wistar (E10−4 = 56.4 ± 2.6%, n = 9), with the concentration-response curve significantly shifted to the left in arteries from hypertensive animals, suggesting that the sensitivity of TRPM8 channels is higher in pudendal arteries from hypertensive compared to normotensive rats (Fig. 6C).
Fig. 6. The sensitivity but not expression of TRPM8 channels are increased in IPA from hypertensive animals.
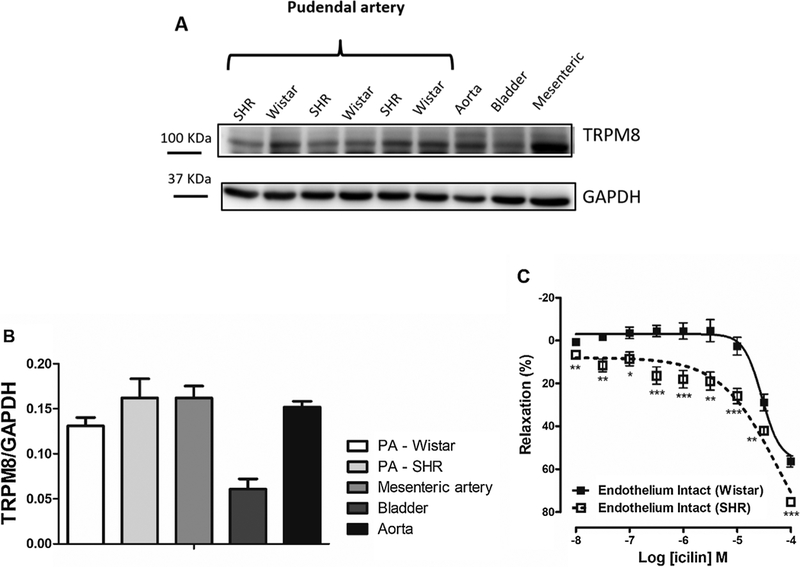
A. Representative images of immunoblots for TRPM8 channels expression obtained of experiments with IPA from SHR and Wistar rats. Aorta, mesenteric artery and bladder were used as positive control. B. Protein expression analysis for TRPM8 channels normalized by GAPDH in IPA from SHR and Wistar rats (n = 5). C. Logarithmic concentration response curves showing the relaxant effect of icilin in IPA from SHR (⬜, n = 12) and Wistar (■, n = 9) rats, pre-contracted by Phe (10−6M) with functional endothelium. Values are expressed as means ± S.E.M. The data were examined using unpaired Student’s t tests. *p < 0.05, **p < 0.01, ***p < 0.001 vs Wistar rats.
3.7. Vasorelaxation induced by TRPM8 activation in IPA from SHR also involves release of EDRFs and RhoA/ROCK pathway inhibition
Since endothelium dysfunction is characterized by alterations in the production and/or bioavailability of EDRFs and endothelium derived constricting factors (EDRFs) in hypertensive rats [38,39], we also analyzed if EDRFs are important for the relaxation induced by TRPM8 activation in IPA from hypertensive animals. Fig. 7A shows that concentration-response curve to icilin was shifted to the right in the presence of both indomethacin or a combination of charybdotoxin and apamin. However, different from that was observed in IPA from normotensive rats, NO production seems to be not important to relaxation induced by TRPM8 activation in SHR.
Fig. 7. Vasorelaxation induced by TRPM8 activation in IPA from SHR also involves release of EDRFs (except NO) and RhoA/ROCK pathway inhibition.
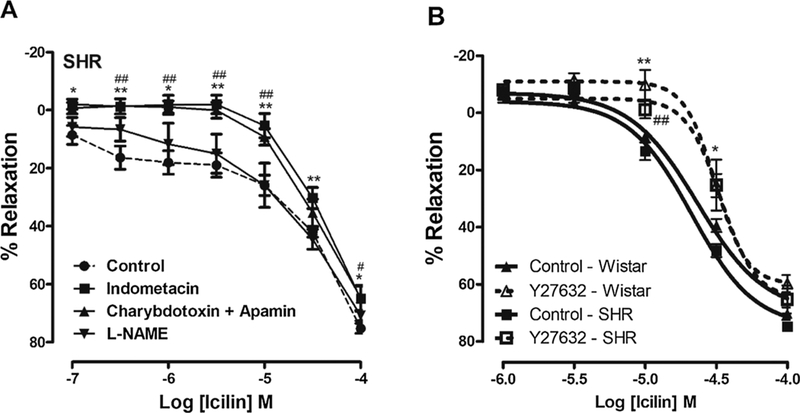
A. Logarithmic concentration response curves showing the relaxant effect induced by icilin in IPA from SHR pre-contracted by Phe (10−6 M), with functional endothelium, in the absence (●, control, n = 12) or in the presence of indomethacin (■, 10−5 M, n = 7), L-NAME (▼, 10−4 M, n = 8) or a combination of charybdotoxin (10−7 M) and apamin (5 × 10−6 M) (▲; n = 6). B. Logarithmic concentration-response curves to icilin in IPA from SHR and Wistar, pre-contracted by Phe (10−6 M), without functional endothelium, in the absence (solid line, control, n = 6) or in the presence of Y27632 (dotted line, 10−6 M, n = 6). The data were examined using one-way ANOVA followed by the Bonferroni post-test (Fig. 7A) and unpaired Student’s t tests (Fig. 7B). *p < 0.05, **p < 0.01, indomethacin vs control or Y27632–wistar vs control wistar. # p < 0.05, ##p < 0.01, charybdotoxin and apamin vs control or Y27632-SHR vs control SHR.
We also analyzed if ROCK inhibition is involved in the TRPM8-mediate relaxation. The Fig. 7B shows that both concentration-response curves to icilin in SHR and Wistar were shifted to the right in the presence of Y27632 (10−6 M), suggesting that, ROCK, at least in part, is important for relaxation induced by TRPM8 activation.
4. Discussion
The present study provides, for the first time, experimental evidence for the expression and function of TRPM8 channels in the pudendal artery, emphasizing the importance of these channels in the penile vasculature, the mechanism of action underlying their activation, increased sensitivity of these channels in IPA from hypertensive rats, and all together, demonstrating the potential of this channel as a new target for the treatment of hypertension associated-ED.
Several TRP channels participate in the function of vasculature and many of these channels are highly expressed in ECs and VSMCs [40,41]. Among the TRP channels family, the subtype TRPM8 is a well-established cold sensor that can be activated by pharmacological agents that mimic the psychophysical sensation of cold, such as menthol [42]. Although the literature does not demonstrate the role of TRPM8 in the penile vasculature or in erectile function, there is an American patent, number US20070060653, investigating the use of menthol (TRPM8 agonist) or any related cooling compound, topically applied to the clitoris, to evoke reflex-induced, vaginal lubrication, initiating the reflex-mediated release of vasoactive polypeptides adjacent to vaginal arterioles. Thus, our results demonstrated, for the first time, the expression of TRPM8 channels in the internal pudendal artery, which controls 70% of blood flow to the erectile tissues. Furthermore, we observed that these channels are also expressed in VSMC from IPA and its activation, by chemical or cold temperature stimulus, signals through vasodilatory pathways.
Once the functional effect induced by the TRPM8 activation was demonstrated in IPA, we aimed to evaluate the mechanism of action involved in the observed responses. The vascular endothelium plays a strategic role in vascular hemodynamics for example by releasing of EDRFs, represented mainly by NO, prostacyclin (PGI2), and EDHF [43,44]. Our results showed that vasorelaxant response was significantly reduced by the absence of the endothelium. Since TRPM8 channels acting as Ca2+ influx channels through plasma membrane and in several vascular beds, a rise in intracellular Ca2+ concentration ([Ca2+]i) in EC activates the enzyme eNOS and cytosolic phospholipase A2 (that activates cyclooxygenase) for the synthesis of NO and prostacyclins, respectively, we hypothesized that EDRFs are important to relaxation induced by TRPM8 activators. In our data, we could observe that the concentration-response curve to icilin was shifted to the right in the presence of L-NAME and indomethacin (Fig. 3). In addition, the endothelium controls vascular tone not only by releasing NO and prostacyclin, but also by release of other factors causing hyperpolarization of the VSMC. The effect induced by these factors can also involve an increase in the [Ca2+]i of the EC, followed by the opening of SKCa and IKCa channels (small and intermediate conductance Ca2+-activated K+ channels respectively) [45,46]. In our studies, the inhibition of SKCa and IKCa channels by combination of charybdotoxin and apamin significantly reduced the relaxation induced by icilin. The literature reports that other TRP channels activated on the vascular endothelium leads to release of EDRFs [47,48].
Since vasodilation induced by TRPM8 activation was reduced, but not abolished by endothelium removal, the subsequent experiments were carried out to explore the endothelium-independent vasorelaxation mechanism. Since TRPM8 is a Ca2 + -permeable cation channel (PCa/PNa ~1–3) [13,14], the activation of TRPM8 present both on the plasma membrane and/or the membrane of the sarcoplasmic reticulum (SR) in the VSMC could increase [Ca2+]i. Initially, we hypothesized that TRPM8 activation on VSMC alters [Ca2+]i and consequently activates BKCa channels. Thus, we performed experiments in the presence of iberiotoxin and TEA, both BKCa channels blockers. No significant changes in the icilin-induced effect were observed, suggesting that, at least in the IPA, this signaling pathway is not involved after subsequent activation of TRPM8. These results demonstrate that activation of TRPM8 channels in vascular tissues may induce endothelium-independent vasodilation by different mechanisms. In IPA TRPM8 activation seems to involve endothelial factors, but not BKca activation. In the mesenteric artery it seems to involve BKca activation [27,30]. On the other hand, in pulmonary artery, TRPM8 activation induces relaxation by inhibition of store-operated calcium entry [49] and RhoA/ROCK pathway inhibition [21]. In addition, TRPM8 activation significantly inhibited the increase in NOX1 and NOX4 in Ang II-treated VSMCs from aorta and suppressed activation of the RhoA-Rock2 and JAK2 signaling pathways [50].
Several works have been published over the years demonstrating that Ca2+ -sensitizing effect of vasoconstrictors is due RhoA/Rho-kinase (ROCK) pathway activation. Since Sun et al. [21] demonstrated that RhoA/ROCK is essential for the effect induced by TRPM8 activation on mesenteric resistance artery, we hypothesized that TRPM8 activation would inhibit RhoA/ROCK in IPA, promoting relaxation. To check this, we performed experiments using Y-27632, a ROCK inhibitor. In our vascular reactivity experiments, the concentration-response curve to icilin was shifted to the right in the presence of Y-27632. Therefore, we can infer that the relaxation of endothelium denuded IPA to icilin involved, at least in part, inhibition of calcium-sensitization and contractility stimulated by RhoA/ROCKII. In addition, we pre-treated VCMCs with TRPM8 activator during 30 min and we analyzed the RhoA and ROCK expression. Interestingly, the treatments did not change the expression of total RhoA and ROCKII, probably due the short duration of treatment. Thus, further studies will be needed to assess the molecular target responsible for ROCK pathway-dependent icilin-induced relaxation.
ED is frequently encountered in patients with arterial hypertension and greatly affects their quality of life of hypertensive patients and their sexual partners [2]. Since TRPM8 channels are downregulated on pulmonary hypertension [19] and renovascular hypertension models (Aorta) [50], and they are expressed and functional in IPA in physiological conditions, we hypothesized that functional changes of these channels may contribute to hypertension-associated erectile dysfunction. To check this, we performed experiments using spontaneously hypertension rats. Behr-Roussel et al. [51] clearly demonstrated the progression of corporal and vascular structural and functional abnormalities associated with development of hypertension and its consequences on erectile function in SHR. They also showed that in SHR, 12 and 24 weeks of age, the magnitude of the erectile responses was drastically reduced compared with WKY rats, besides vascular and penile remodeling.
In our data, we showed the modifications in SHR compared to Wistar rats. Fig. 5 shows that SHR presented marked characteristics of hypertensive disease such as left ventricular hypertrophy and vascular dysfunction. IPA from SHR presented greater contractile capacity for depolarizing agents and less contractility to alpha1-adrenergic activation. Furthermore, as demonstrated in Fig. 6, despite the greater pharmacological efficacy of icilin in activating TRPM8 channels in hypertensive animals compared to normotensive, the expression of TRPM8 channels was not significantly different between the arteries from these animals, suggesting that TRPM8 channels have increased sensitivity in SHR and this would be a compensatory effect in the IPA from hypertensive animals. This highlights that TRPM8 may be a potential therapeutic target for the treatment of erectile dysfunction associated with hypertension. However additional studies will be needed to better evaluate this hypothesis.
Our data also demonstrated that TRPM8 activation involved the EDRFs and ROCK inhibition in IPA from SHR and Wistar rats. However, NO production does not seem to be important for relaxation induced by TRPM8 in these arteries from SHR. In the literature, studies with SHR produced discrepant results regarding the role of NO and endothelial dysfunction in the development of hypertension. These differences depend on many factors and they are vessel-dependent, age-dependent, strain-dependent, and methods-dependent used for determination of vascular function (more details see review written by [52]). In IPA, the exact role of NOS and NO pathway in SHR endothelial dysfunction has not yet been well characterized. Therefore, we could not clarify whether NO-independent relaxation induced by TRPM8 activation, observed in SHR but not in Wistar rats, would be due to a change in NO production/signaling in these arteries from SHR or would be due to a real non-interaction between the signaling of TRPM8 channels and NO in IPA from SHR.
In summary, our data provide strong support that TRPM8 channels are expressed and functional in IPA from both normotensive and hypertensive rats and their activation is able to relax these tissues, involving, at least in part, endothelium derived relaxing factors and RhoA/ROCK inhibition (Fig. 8). In addition, higher sensitivity of TRPM8 channels were observed in penile vasculature from hypertensive compared to normotensive rats suggesting vascular TRPM8 activation can be considered as a potential strategy for the management of hypertension-associated ED.
Fig. 8.
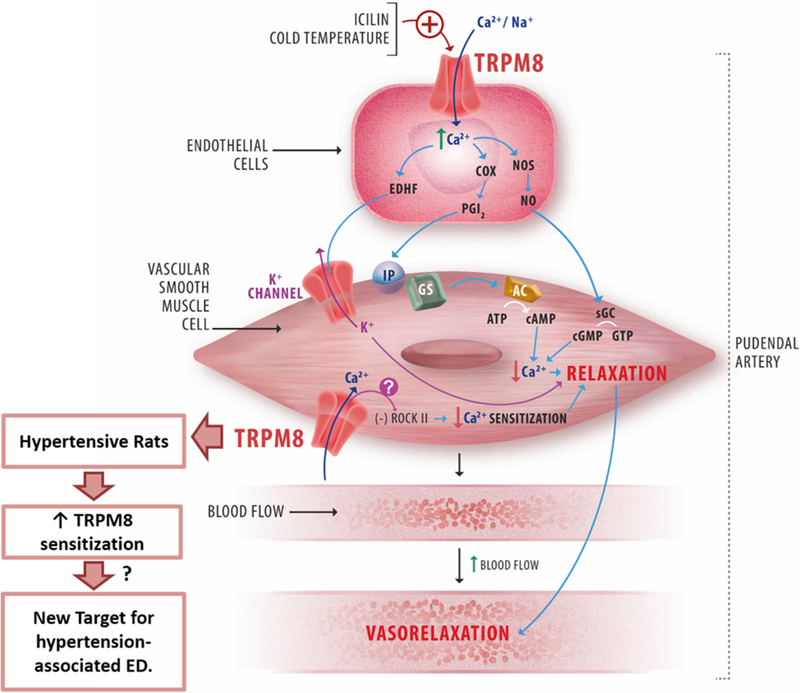
Schematic representation of the mechanism of action underlying the TRPM8 channel activation in IPA. AC, adenylyl cyclase; COX, cyclo-oxygenase; EDHF, endothelium-derived hyperpolarizing factor; sGC, soluble guanylyl cyclase; IP, PGI2 receptor; NOS, NO synthase; PGI2, prostacyclin; ROCK II, Rho-associated protein kinase; transient receptor potential canonical channel; TRPM8, transient receptor potential melastatin 8 channel.
Supplementary Material
Acknowledgments
We would like to thank Ricardo Andrade Garrido for their excellent technical expertise in illustration.
Funding
This study was funded by grants from National Council for Scientific and Technological Development (CNPq), process number 233867/2014–7 and 306106/2017–5; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001; NIDDK Diacomp Pilot & Feasibly Program.
Abbreviations:
- ACC/AHA
American College of Cardiology/American Heart Association
- BKca/ IKca /SKc
large, intermediate and small conductance Ca2+-activated K+ channels, respectively
- CVD
cardiovascular diseases
- EC
Endothelial Cells
- ED
Erectile dysfunction
- EDRF
endothelium-derived relaxing factors
- EDHF
endothelium-derived hyperpolarizing factor
- ICI
Icilin
- IP
Prostacyclin receptor
- IPA
Internal Pudendal Artery
- NOX
NADPH oxidase
- PGI2
Prostacyclin
- ROCK
Rho-associated protein kinase
- SHR
spontaneously hypertensive rats
- TRP
Transient Receptor Potential
- TRPM8
Transient Receptor Potential Melastatin 8
- VSMCs
vascular smooth muscle cells
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2019.104329.
References
- [1].World Health Organization, A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis, WHO, 2013. p. 40. [Google Scholar]
- [2].Viigimaa M, Vlachopoulos C, Lazaridis A, Doumas M, Management of erectile dysfunction in hypertension: tips and tricks, World J. Cardiol. 6 (9) (2014) 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shamloul R, Ghanem H, Erectile dysfunction, Lancet 381 (9861) (2013) 153–165. [DOI] [PubMed] [Google Scholar]
- [4].Patel JP, Lee EH, Mena-Hurtado CI, Walker CN, Evaluation and management of erectile dysfunction in the hypertensive patient, Curr. Cardiol. Rep. 19 (9) (2017) 89 24. [DOI] [PubMed] [Google Scholar]
- [5].Ryan JG, Gajraj J, Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus, J. Diabetes Complications 26 (2012) 141–147. [DOI] [PubMed] [Google Scholar]
- [6].Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, Vlachopoulos C, A systematic review of the association between erectile dysfunction and cardiovascular disease, Eur. Urol. 65 (2014) 968–978. [DOI] [PubMed] [Google Scholar]
- [7].Condorelli RA, Calogero AE, Favilla V, Morgia G, Johnson EO, Castiglione R, et al. , Arterial erectile dysfunction: different severities of endothelial apoptosis between diabetic patients “responders” and “non responders” to sildenafil, Eur. J. Intern. Med. 24 (3) (2013) 234–240. [DOI] [PubMed] [Google Scholar]
- [8].Earley S, Brayden JE, Transient receptor potential channels in the vasculature, Rev. Physiol. Rev. 95 (2) (2015) 645–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhichao Yue, Xie Jia, Yu Albert S., Stock Jonathan, Du Jianyang, Lixia Yue, Role of TRP channels in the cardiovascular system, Am. J. Physiol. Heart Circ. Physiol. 308 (2015) H157–H182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Earley S, Vanilloid and melastatin transient receptor potential channels in vascular smooth muscle, Microcirculation 17 (4) (2010) 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mene P, Punzo G, Pirozzi N, TRP channels as therapeutic targets in kidney disease and hypertension, Curr. Top. Med. Chem. 13 (3) (2013) 386–397. [DOI] [PubMed] [Google Scholar]
- [12].Liu D, Xiong S, Zhu Z, Imbalance and dysfunction of transient receptor potential channels contribute to the pathogenesis of hypertension, Sci. China Life Sci. 57 (8) (2014). [DOI] [PubMed] [Google Scholar]
- [13].McKemy DD, Neuhausser WM, Julius D, Identification of a cold receptor reveals a general role for TRP channels in thermo sensation, Nature 416 (6876) (2002) 52–58. [DOI] [PubMed] [Google Scholar]
- [14].Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A, A TRP channel that senses cold stimuli and menthol, Cell 108 (2002) 705–715. [DOI] [PubMed] [Google Scholar]
- [15].Voets T, Owsianik G, Janssens A, Talavera K, Nilius B, TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli, Nat. Chem. Biol. 3 (2007) 174–182. [DOI] [PubMed] [Google Scholar]
- [16].Latorre R, Brauchi S, Madrid R, Orio P, A cool channel in cold transduction, Physiology 26 (2011) 273–285. [DOI] [PubMed] [Google Scholar]
- [17].Brauchi S, Orio P, Latorre R, Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8, Proc. Natl. Acad. Sci. 101 (2004) 15494–15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B, The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels, Nature 430 (2004) 748–754. [DOI] [PubMed] [Google Scholar]
- [19].Liu XR, Liu Q, Chen GY, Hu Y, Sham JS, Lin MJ, Down-regulation of TRPM8 in pulmonary arteries of pulmonary hypertensive rats, Cell. Physiol. Biochem. 31 (6) (2013) 892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xiong S, Wang B, Lin S, Zhang H, Li Y, Wei X, Cui Y, Wei X, Lu Z, Gao P, Li L, Zhao Z, Liu D, Zhu Z, Activation of transient receptor potential melastatin subtype 8 attenuates cold-induced hypertension through ameliorating vascular mitochondrial dysfunction, J. Am. Heart Assoc. 6 (8) (2017) e005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sun J, Yang T, Wang P, Ma S, Zhu Z, Pu Y, Li L, Zhao Y, Xiong S, Liu S, Zhu Z, Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway, Hypertension 63 (2014) 1354–1363. [DOI] [PubMed] [Google Scholar]
- [22].McGuire PG, Walker-Caprioglio HM, Little SA, McGuffee LJ, Isolation and culture of rat superior mesenteric artery smooth muscle cells, In Vitro Cell. Dev. Biol. 29A (2) (1993) 135–139. [DOI] [PubMed] [Google Scholar]
- [23].Wenceslau CF, McCarthy CG, Szasz T, Webb RC, Lipoxin A4 mediates aortic contraction via RHOA/RHO kinase, endothelial dysfunction and reactive oxygen species, J. Vasc. Res. 51 (6) (2014) 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mulvany MJ, Halpern W, Mechanical properties of vascular smooth muscle cells in situ, Nature 260 (1976) 617–619. [DOI] [PubMed] [Google Scholar]
- [25].McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, Matsumoto T, Webb RC, Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats, Cardiovasc. Res. 107 (1) (2015) 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mistretta FA, Russo A, Castiglione F, Bettiga A, Colciago G, Montorsi F, Brandolini L, Aramini A, Bianchini G, Allegretti M, Bovolenta S, Russo R, Benigni F, Hedlund P, A novel transient receptor potential melastin 8–selective ion channel antagonist, modifies bladder function and reduces bladder overactivity in awake rats, J. Pharmacol. Exp. Ther. 356 (1) (2016) 200–211. [DOI] [PubMed] [Google Scholar]
- [27].Silva DF, de Almeida MM, Chaves CG, Braz AL, Gomes MA, Pinho-da-Silva L, et al. , TRPM8 channel activation induced by monoterpenoid rotundifolone under-lies mesenteric artery relaxation, PLoS One 10 (2015) e0143171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang XR, Lin MJ, McIntosh LS, Sham JS, Functional expression of transient receptor potential melastatin-and vanilloid-related channels in pulmonary arterial and aortic smooth muscle, Am. J. Physiol. Lung Cell Mol. Physiol. 290 (2006) L1267–L1276. [DOI] [PubMed] [Google Scholar]
- [29].Teixeira CE, Jin L, Priviero FB, Ying Z, Webb RC, Comparative pharmacological analysis of Rho-kinase inhibitors and identification of molecular components of Ca2+ sensitization in the rat lower urinary tract, Biochem. Pharmacol. 74 (4) (2007) 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Silva DF, Araujo IG, Albuquerque JG, Porto DL, Dias KL, Cavalcante KV, et al. , Rotundifolone-induced relaxation is mediated by BK(Ca) channel activation and Ca(v) channel inactivation, Basic Clin. Pharmacol. Toxicol. 109 (6) (2011) 465–475. [DOI] [PubMed] [Google Scholar]
- [31].Sokabe T, Tominaga M, A temperature-sensitive TRP ion channel, painless, functions as a noxious heat sensor in fruit flies, Commun. Integr. Biol. 2 (2009) 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, Liu D, Arendshorst WJ, Huang Y, Tepel M, Zhu Z, Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension, Cell Metab. 12 (2) (2010) 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang F, Cui Y, Wang K, Zheng J, Thermosensitive TRP channel pore turret is part of the temperature activation pathway, Proc. Natl. Acad. Sci. 107 (2010) 7083–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zholos A, Pharmacology of transient receptor potential melastatin channels in the vasculature, Br. J. Pharmacol. 159 (2010) 1559–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moncada S, Palmer RMJ, Higgs EA, Biosynthesis of nitric oxide from L-arginine: a pathway for the regulation of cell function and communication, Bioch Pharmac 38 (1989) 1709–1715. [DOI] [PubMed] [Google Scholar]
- [36].Furchgott RF, zawadzki JV, The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine, Nature 288 (1980) 373–376. [DOI] [PubMed] [Google Scholar]
- [37].Nunes KP, Labazi H, Webb RC, New insights into hypertension-associated erectile dysfunction, Curr. Opin. Nephrol. Hypertens. 21 (2) (2012) 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Török J, Participation of nitric oxide in different models of experimental hypertension, Physiol. Res. 57 (2008) 813–825. [DOI] [PubMed] [Google Scholar]
- [39].Vanhoutte PM, Feletou M, Taddei S, Endothelium-dependent contractions in hypertension, Br. J. Pharmacol. 144 (2005) 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yue Z, Xie J, Stock J AS Yu, Du J, Yue L, Role of TRP channels in the cardio-vascular system, Am. J. Physiol. Heart Circ. Physiol. 308 (3) (2015) H157–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alonso-Carbajo L, Kecskes M, Jacobs G, Pironet A, Syam N, Talavera K, Vennekens R, Muscling in on TRP channels in vascular smooth muscle cells and cardiomyocytes, Cell Calcium 66 (2017) 48–61. [DOI] [PubMed] [Google Scholar]
- [42].Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D, The menthol receptor TRPM8 is the principal detector of environmental cold, Nature 448 (2007) 204–208. [DOI] [PubMed] [Google Scholar]
- [43].Lüescher TF, Barton M, Biology of the endothelium, Clin. Cardiol.-Suppl. (1997) 3–10. [PubMed] [Google Scholar]
- [44].Kang KT, Endothelium-derived relaxing factors of small resistance arteries in hypertension, Toxicol. Res. 30 (2014) 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Félétou M, Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br. J. Pharmacol. 156 (4) (2009) 545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Félétou M, SKca and IKca in the eudothelium-dependent hyperpolarization of the guinea-pig isolated carotid artery, Br. J. Pharmacol. 144 (2005) 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sukumaran SV, Singh TU, Parida S, ChE Narasimha Reddy Thangamalai R, Kandasamy K, Singh V, Mishra SK, TRPV4 channel activation leads to endothelium-dependent relaxation mediated by nitric oxide and endothelium-derived hyperpolarizing factor in rat pulmonary artery, Pharmacol. Res. 78 (2013) 18–27. [DOI] [PubMed] [Google Scholar]
- [48].Seki T, Goto K, Kiyohara K, Kansui Y, Murakami N, Haga Y, Ohtsubo T, Matsumura K, Kitazono T, Downregulation of endothelial transient receptor potential vanilloid type 4 channel and small-conductance of Ca2 + -activated K+ channels underpins impaired endothelium-dependent hyperpolarization in hypertension, Hypertension 69 (1) (2017) 143–153. [DOI] [PubMed] [Google Scholar]
- [49].Mu YP, Lin DC, Zheng SY, Jiao HX, Sham JSK, Lin MJ, Transient receptor potential Melastatin-8 activation induces relaxation of pulmonary artery by inhibition of store-operated calcium entry in normoxic and chronic hypoxic pulmonary hypertensive rats, J. Pharmacol. Exp. Ther. 365 (3) (2018) 544–555. [DOI] [PubMed] [Google Scholar]
- [50].Huang F, Ni M, Zhang JM, Li DJ, Shen FM, TRPM8 downregulation by angiotensin II in vascular smooth muscle cells is involved in hypertension, Mol. Med. Rep. 15 (4) (2017) 1900–1908. [DOI] [PubMed] [Google Scholar]
- [51].Behr-Roussel D, Gorny D, Mevel K, Compagnie S, Kern P, Sivan V, Bernabé J, Bedigian MP, Alexandre L, Giuliano F, Erectile dysfunction: an early marker for hypertension? A longitudinal study in spontaneously hypertensive rats, Am. J. Physiol. Regul. Integr. Comp. Physiol. 288 (1) (2004) R276–83. [DOI] [PubMed] [Google Scholar]
- [52].Bernatova I, Endothelial dysfunction in experimental models of arterial hypertension: cause or consequence? Biomed Res. Int. (2014) 598271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


