Abstract
Long waiting times for kidney transplant and the high risk of mortality on dialysis have prompted investigation into strategies to utilize Hepatitis C virus (HCV)-infected organs to decrease discard rates of potentially viable kidneys. Due the opioid epidemic, the number of HCV-infected donors has increased significantly. With the development of direct-acting antiviral therapies for HCV infection, now more than 95% of patients who received treatment are cured. Experimental trials have used direct-acting antiviral therapy to treat HCV infection in HCV-uninfected transplant recipients of kidneys from HCV-viremic donors. To date, HCV has been eradicated in all cases. Though these strategies will potentially increase the donor pool of available kidneys, shorten waitlist times, and ultimately decrease mortality in patients waiting for kidney transplant, identifying the ideal candidates and educating them about a protocol to utilize direct-acting antiviral therapy to cure HCV after it is transmitted is essential. We present our approach to patient selection and education for a clinical trial in transplantation of HCV viremic kidneys into uninfected recipients.
Introduction:
According to the Organ Procurement and Transplantation Network, just under 100,000 patients are waiting for a kidney transplant[1] Despite the recent increase in deceased donor transplantation, geographical disparities in access to kidney transplant persist; in some parts of the country waiting times exceed 5–7 years. Because of long wait times combined with the high annual rate of mortality on dialysis, especially for those with longer time since dialysis initiation, it is estimated that more than 25% of patients on the waitlist will die prior to getting a kidney transplant (KT) [2].
Strategies to increase organ allocation are desperately needed. Due to the opioid crisis facing the United States (US), which is leading to a rise in both new Hepatitis C virus (HCV) infections and drug overdose deaths, HCV-viremic deceased donors are increasingly becoming available for transplantation. Accepting a HCV-infected donor kidney substantially shortens waiting time [3]. Additionally, HCV-infected kidneys typically come from younger donors with fewer comorbidities and are higher quality based on kidney donor profile index (KDPI) criteria [4, 5]. Currently, HCV-infected kidneys are under-utilized in the US; Reese et al. estimated that 500 kidneys per year were unnecessarily discarded between 2005 – 2014 due to HCV serostatus of the donor [6], a number that has likely increased since then given the ongoing opioid epidemic. Therefore, it is of paramount importance that we develop strategies to safely utilize these kidneys for transplantation.
Direct acting antiviral therapies (DAAs) have revolutionized the management of HCV. Patients with all genotypes of HCV infection and any stage of chronic kidney disease can now be treated with DAA therapies that are interferon-free [7–10]. Clinical trials and real-world data have demonstrated excellent HCV cure rates in liver and KT recipients and has made it possible to eradicate HCV post KT in >95% who undergo treatment, suggesting that immunosuppression does not decrease the effectiveness of DAAs, nor are there unacceptable interactions with most transplant medications [11–13].
Clinical trials that investigate whether transplantation from HCV-viremic donors followed by immediate post KT treatment can cure HCV in the recipient without leading to symptomatic HCV transmission or adverse events have been performed and are ongoing in academic centers. The Transplanting Hepatitis C Kidneys into Negative Kidney Recipients (THINKER) 1 and 2 trials showed that HCV-viremic organs with genotype 1 infection transplanted into 20 recipients without HCV infection, followed by a 12 week course of grazoprevir plus elbasvir begun shortly after transplant, led to cure of HCV in all participants; recently, the authors reported excellent one-year graft function [14, 15]. The Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients (EXPANDER-1) trial has reported successful cure of 10 HCV-infected KT recipients with preemptive HCV treatment beginning at the time of transplantation from a HCV-infected donor [16].
Other centers, including ours, are investigating similar strategies to transplant HCV-viremic kidneys into HCV-negative recipients. Our investigator-initiated clinical trial sponsored by Merck (NCT02945150) accepts HCV-viremic kidneys from donor with genotype 1 or 4 infection. At the time of transplantation, the patient begins preemptive grazoprevir plus elbasvir “on-call” to the operating room and continues for 12 weeks after transplantation. If resistance associated variants are detected during the analysis of the donor’s virus, this course is extended to 16 weeks and ribavirin is added [17] With this preemptive approach to treating HCV, we have found low levels of viremia developing in the recipient during the first week post-transplant; participants viral loads became undetectable within two weeks (unpublished data). Because strategies that use DAAs after KT are likely to quickly become more commonly deployed in transplant centers across the country, in this report we detail our considerations for patient selection and present our educational materials, informed consent process, and summarize lessons learned and future directions.
Who is the appropriate candidate for an HCV-infected kidney transplant?
Simply put, recipients should be those who are 1) most likely to benefit from shortening their waitlist time and 2) most likely to safely undergo KT from an HCV-viremic donor, which includes not only the ability to strictly comply with DAA therapy, but also excludes those with increased risk of post-operative complications, acute rejection, and early recurrent primary renal disease. Patients must have already met our transplant center’s listing criteria and already be listed for KT alone prior to consent for this protocol. Since the major benefit of this protocol is more rapid access to transplantation, patients who already have substantial accrued waitlist time are not included. The maximum allowed waitlist time is customized by blood group, since waiting time varies by blood group, and is based on average waiting times in our region (See Table 1). In our view, however, the cutoff parameters used to evaluate a patient’s candidacy for receiving a HCV-viremic KT should be individualized and based on the patients age, blood type, prior sensitization, and the transplant center’s expected wait time.
Table 1.
Current and future considerations for choosing recipients of HCV viremic kidney transplantation
| Current Inclusion/Exclusion Criteria | Current Rationale | Recommendations/Future Direction |
|---|---|---|
| Age must be 40–70 years old | Patients < 40 years often have more opportunities for DDKT and have lower waitlist mortality. Participants > 70 years may not be acceptable LT candidates or may have increased perioperative mortality | Remove strict age cutoff, individualized decisions. The need to meet LT criteria may be too strict. |
| No available living kidney donor | Living kidney donor transplantation has survival advantages over DDKT | Continue to restrict participation to those with no available living donor |
| Has ≤ 2 years of accrued transplant waiting time if blood type A and ≤ 3 years of accrued transplant waiting time if blood type B or O. Excluded if blood types AB. | Those with longer accrued transplant waiting time would be less likely to benefit. Blood types B and O typically have significantly longer average waitlist times compared to blood type A. Patients with blood group AB often have shorter waiting times for KT and are less likely to benefit from this protocol. | No strict restrictions on waitlist time, shared decision-making with transplant team and patient, taking into account current practice patterns and likelihood of shortened waitlist time with participation. |
| On chronic hemodialysis or peritoneal dialysis or has eGFR <15mL/min/1.73m2 at the time of screening | Participants with eGFR <15 ml/min/m2 are more likely to be at risk of renal disease-related complications and higher risk of mortality than participant with eGFR 15–20 ml/min/m2. | Per center guidelines on timing of pre-emptive transplantation |
| Must agree to birth control, must not be pregnant or lactating. | Acute HCV infection or the use of study drug might carry risks to a pregnancy that must be avoided. Pregnancy in early post-transplant period should be avoided. | Pregnancy must be avoided |
| Considerations to decrease perioperative risk: - Weigh at least 50kg, BMI < 35, Albumin ≥ 3g/dL, platelet count ≥ 75 × 103/mL |
Exclude patients who are at risk of peri and post-operative complications | Per center guidelines for standard KT |
| Serum ALT within normal limits with no history of liver disease | Patients must have no evidence of liver disease. This is in order to limit the risks of acute HCV infection on the liver. | Patients should be evaluated for liver disease and excluded from participation if it is detected |
| Able to sign informed consent | All patients must have a full understanding of the risk of participation. Surrogate consent is not allowed. | All patients must have a full understanding of the risk of participation. Surrogate consent is not allowed. |
| Sufficient cardiac function: LV ejection fraction > 50% | Patients should have adequate cardiac function to ensure LT candidacy. | Per center guidelines for standard KT. The need to meet LT criteria may be too strict. |
| Exclude patient who may need for non-standard post-transplant immunosuppression: 1) known allergy or intolerance to tacrolimus 2) Positive donor specific antibodies or positive cross-match deemed to be clinically significant 3) Patients with primary focal segmental glomerulosclerosis (FSGS), FSGS recurring after prior transplant, or disease process at increased risk of early graft failure | To minimize participant risks, we exclude participants who may need non-standard post-transplant immunosuppression or desensitization in order to minimize possible drug-drug interactions | Drug-drug interactions will continue to be a challenge |
| HIV-infected recipients | Participants with HIV may need medications with complex drug-drug interactions and are at increased risk of acute rejection. | Working with HIV specialists may allow for conversion of antiretroviral therapy regimen prior to transplant, allowing for safe participation of HIV-infected adults |
| Hepatitis B surface antigen positive | Patients with active Hepatitis B virus infection will be excluded in order to limit the risks of acute HCV infection on the liver. | Recommend excluding patients with active Hepatitis B infection |
Abbreviations: ALT, alanine aminotransferase; BMI, body-mass index; DDKT, deceased donor kidney transplant; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; HCV, hepatitis C virus infection; HIV, human immunodeficiency; KT, kidney transplant; LV, left ventricular; RNA, ribonucleic acid.
Because of the innovative nature of DAA therapy post KT from a HCV-viremic donor, when we designed this study we also ensured all of our candidates would potentially meet candidacy for liver transplantation should a devastating complication of acute HCV occur. It may be argued that this requirement is too strict given the exceedingly small chance that liver transplantation would be needed. Table 1 shows a full list of recipient inclusion and exclusion criteria for NCT02945150 with rationale for each. Additionally, Table 1 includes inclusion considerations for the near future, as HCV viremic transplantation becomes more commonly performed, and more data is available on risk of therapy failure.
Who is the appropriate donor?
Our criteria for accepting a donor for this protocol is that the KDPI be ≤ 0.65 to ensure higher quality kidneys are used while in the experimental stage, justifying the additional risk of HCV infection. Of note, HCV is one of the factors used to calculate the KDPI; HCV infection raises the KDPI by approximately 0.25 [5]. Thus, in the future, higher KDPI cutoffs may be appropriate to maximize organ utilization, and HCV’s influence may be reconsidered in the calculation of KDPI. Because grazoprevir plus elbasvir are being used in this protocol, the donor must have confirmed genotype 1 or 4 infection (rapid genotyping is performed after provisional organ acceptance and prior to final acceptance and transplantation). We exclude donors whose HCV has led to decompensated liver disease or those with known prior receipt of DAA therapy, as these may be associated with a more difficult to treat viral infection in the recipient. Donors with confirmed human immunodeficiency virus (HIV) or Hepatitis B virus (HBV) infection (either surface antigen or HBV DNA positive) are excluded. Beyond that, organ acceptance is at the discretion of the accepting transplant physician and surgeon, and follows common organ acceptance practices.
Patient education and informed consent
Informed consent prior to transplant with an HCV-viremic organ is a critical aspect of any program promoting transplantation of HCV-viremic kidneys into uninfected recipients. While the THINKER and EXPANDER trials have so far shown 100% cure rates with DAAs, it is necessary to inform patients that the numbers who have undergone these protocols are still very small and safety and HCV transmission risk are not fully known. Also, patients selected to participate in these trials went through extensive screening and were a small proportion of the total cohort evaluated for the program. Figure 1 illustrates our process for identifying and selecting patients for this protocol.
Figure 1.
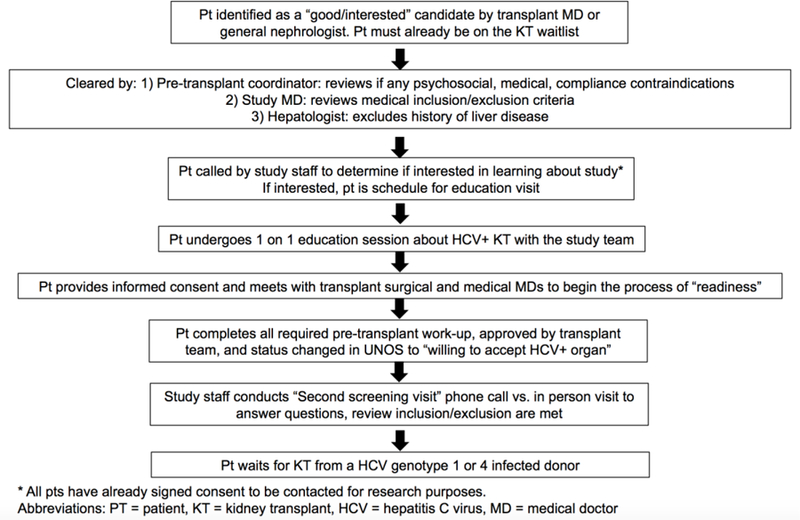
The process of patient identification, education and ensuring readiness for transplantation from a HCV-infected donor
Education session
The patient is invited for an individualized information session with a member of our study team. They are encouraged to bring a family member or close friend, as per routine transplant-related education practice. A twenty-question document is reviewed (Table 2). The full text of the education session is available in the supplemental materials.
Table 2.
Questions discussed in our individual patient education session
| General information about Hepatitis C Virus | What is Hepatitis C infection? How can a person get Hepatitis C infection? What are the different types of Hepatitis C? Can you get Hepatitis C from being in this study? Are there any treatments for Hepatitis C infection? |
| Physical effects of Hepatitis C | What happens when you get Hepatitis C? Are there any other effects of Hepatitis C on other parts of the body? |
| Hepatitis C and kidney donation | Can people with Hepatitis C donate a kidney? How do you know that a kidney donor has Hepatitis C infection? Why are there many donors with Hepatitis C infection? |
| Specific details about the study | Does grazoprevir plus elbasvir treat all types of Hepatitis C? When would the grazoprevir plus elbasvir treatment begin? How long will I take grazoprevir plus elbasvir? How long will I be a part of this study? When will my study visits take place? Will participating in this study effect my transplant medications or my transplant care? Does being a part of this study affect my standing on the kidney transplant list? If I decide not to participate does this affect my standing on the kidney transplant list? What are the side effects of grazoprevir plus elbasvir? Has anyone like me who does not have Hepatitis C ever received a kidney transplant from a patient with Hepatitis C before? What are the benefits to being in this study? |
For full text of education session see Supplemental Materials.
Afterwards, the patient and family member/friend are invited to ask any additional questions. Patients are given a copy of the information session materials and are given as much time as they would like to review these materials prior to signing informed consent. We do allow patients to proceed directly to informed consent on the same day if they choose.
Informed consent: Framing Potential risks
A summary of the risks we discuss are found in Table 3. It is necessary that treatment with DAAs begin early after KT to prevent potential early complications of acute HCV such as fulminant hepatitis or fibrosing cholestatic hepatitis. While we discuss the risks of chronic HCV infection in the post KT setting (Table 3), we explain that preemptive eradication with DAAs beginning at the time of transplant is likely to essentially eliminate these risks since they develop only in the setting of persistent viremia.
Table 3.
Risks discussed in the informed consent process
| Acute HCV infection risk |
| Fulminant hepatitis |
| Fibrosing cholestatic hepatitis |
| Chronic HCV infection |
| Chronic hepatitis leading to cirrhosis |
| End-stage liver disease, liver cancer, need for liver transplant, death |
| Extrahepatic manifestations including mixed cryoglobulinemia |
| Risk of DAA failure |
| Virologic failure, development of resistance |
| Incorrect genotyping test* |
| Risk of HCV transmission to household or sexual partners |
| Side effects of DAAs |
| Risk of undergoing transplantations** |
| Discovery of new medical problems during pre-transplant evaluation |
| Surgical procedure risks |
| Risks of Immunosuppression |
| Risks related to stored blood samples and loss of privacy |
Would not be relevant if a pan-genotypic DAA regimen was used
Additionally, a full comprehensive clinical consent for transplantation takes place outside of this protocol. Abbreviations: HCV = hepatitis C virus, DAAs = direct-acting antivirals
It is important to advise potential participants that a breakthrough infection could occur and that the virus could develop resistance mutations. In our experience, this is one of the most commonly asked questions during the consent process. Reassuringly, recent data show that “salvage regimens” are now available and can successfully treat those patients who have failed first-line DAA therapy with excellent chances of success [18, 19]. Collaboration with hepatologists knowledgeable about first and second-line DAA therapies is an essential part of our team-based approach (Figure 1).
Another commonly asked question by patients considering participation is how to protect household and sexual contacts from getting HCV infection. We recommend avoiding blood exposure (i.e., avoiding sharing razors and toothbrushes) until the patient has been deemed cleared of HCV with a negative viral load test 12 weeks after completing DAA therapy (six months after transplant). Sexual transmission is rare: a study of 500 HIV-negative couples where one partner had HCV infection and the other did not, demonstrated that HCV transmission occurred in approximately one per 190,000 sexual contacts [20]. Therefore, regarding sexual practices, we conservatively recommend barrier protection until sustained virologic response 12 weeks after completing DAA therapy.
Patients may ask about the side effects of DAAs. In the general HCV infected population, side effects of DAAs are relatively mild, with typically no more than 15% experiencing headache, nausea or fatigue. Typically, less than 2 out of 100 patients discontinue therapy due to side effects. Side effects in the post KT setting may be more frequent or severe, although trials and retrospective series in KT recipients do not suggest a dramatic increase in side effects. However, pharmacokinetic interactions between elbasvir and grazoprevir and immunosuppression are important to recognize; both drugs can increase the tacrolimus area under the curve and cyclosporine can dramatically increase grazoprevir [21]. Therefore, careful tacrolimus monitoring may be required after transplant. There is no clinically relevant drug-drug interaction between grazoprevir and elbasvir with mycophenolate mofetil and prednisone.
While separate clinical consents for transplant evaluation and the transplant procedure take place in addition to research consent for our HCV positive to negative protocol, we do remind patients that undergoing transplantation itself involves additional risks. Participants will undergo a thorough pre-transplant “readiness work-up” that may identify contra-indications to, or risks associated with, transplant that will be addressed prior to transplant. Additionally, we remind patients of the surgical risks and the short and long-term risks of immunosuppression.
Controversy: What is the appropriate timing of initiation of DAAs after KT from a HCV viremic donor to an uninfected recipient?
In our trial, DAAs are begun “on-call” to the operating room and continued daily. We have found very low-level viremia detectable at post-operative day 1 which has become undetectable by post-operative day 7. Approaches across the country have differed. In the THINKER trial, DAAs were begun as soon as viremia was detected in the recipient. In the majority of cases this was post-operative day 3. This strategy allows for avoiding unnecessary treatment in a patient who does not develop viremia (although this is the exception, the vast majority of viremic donors will transmit HCV infection). Beginning post-operative day 3 also decreases the chance of interrupting therapy due to periods where patients may be unable to take medications orally. In KT, prolonged periods of nil per os (NPO) are rare, but this is an important consideration in other types of organ transplantation, such as lung transplantation, where patients may have prolonged NPO status. More data is needed on the pharmacokinetics of crushed DAAs to ensure sufficient delivery of DAAs in patients who are NPO. In the EXPANDER trial, grazoprevir plus elbasvir were begun preemptively, “on call” to the operating room, however rapid genotyping did not occur prior to transplantation. If a donor was found to have genotype 2, 3, 5 or 6 infection, sofosbuvir was added to ensure adequate antiviral activity.
Starting preemptively versus within the first few days after KT is unlikely to dramatically change the likelihood of virologic control. However, the safety of longer delays in starting treatment after KT, for example such as those that exceed the first week, are unknown. An extremely concerning report in a recipient of a HCV viremic liver transplant demonstrated HCV-associated membranous nephropathy on post-operative day 18 and subsequent dialysis-requiring renal failure due to a delay in DAA therapy because of reliance on insurance approval [22]. Thus, any program that does not begin DAA therapy within three days after KT (such as any protocol that relies on insurance approval) in our opinion should inform the potential recipient of their risks of HCV infection and suffering its acute complications are higher.
Controversy: Should all HCV infected to uninfected transplants fall under research protocols?
While the THINKER and EXPANDER trials provide tremendous optimism for using HCV-infected donor organs for patients without HCV infection to decrease organ discard rates, more safety data is needed before this approach can be widely adopted as standard of care [23]. As such, we advocate that all clinical protocols proposing utilization of HCV-infected donor organs undergo approval by an Institutional Review Board, and that patients complete written informed consent. Patients need to demonstrate full understanding that they will be infected with HCV via the transplant, and they must be able to comply with post-operative DAA treatment protocols.
At this time, we believe the most important consideration is access to DAA therapy within the first week post KT. Uncertainty remains as to whether DAAs will be approved in a timely manner by insurance companies; thus, any protocol that relies on insurer approval of DAAs must include this risk (i.e. Non-approval) in their informed consent. This is another reason not to consider this as standard of care at this time. Whether longer-term transplant outcomes differ as a function of this experimental protocol is unknown; it is reassuring that one-year outcomes were favorable in THINKER recipients [15].
With more available safety and efficacy data with DAA utilization post KT pan-genotypic regimen adoption, and better identification of donor testing, we foresee such innovative protocols to become the standard of care in the future. As of today, we recommend participation in such protocols to be included in and consented to by an Institutional Review Board-approved research protocol prior to consideration.
Controversy: How should HCV antibody positive, nucleic acid test negative (AB+NAT-) kidneys be allocated and managed?
HCV AB+NAT- donors do not have active infection and are extremely unlikely to transmit HCV infection, except in the case of false negative NAT testing. We recommend that patients be educated on the potential risk of HCV infection from an AB+NAT- donor and sign informed consent. After KT from a, AB+NAT- donor, we do not recommend beginning preemptive DAA therapy, as is recommended for a recipient of a HCV viremic KT. Rather, patients should have close monitoring of HCV viral load after transplant. The optimal frequency and duration of surveillance for HCV after transplantation from a donor who is HCV AB+NAT- is unknown. However, published reports of HCV transmission from increased risk donors with false negative NAT screening to recipients of solid organs were all detected within 12 weeks after transplantation [24]. We propose research protocols that employ monthly screening for HCV RNA for at least six months after transplant from a HCV AB+NAT- donor. If transmission of HCV infection occurs, recipients need immediate access to standard-of-care DAA treatment. Uncontrolled viremia in the context of immunosuppression could have serious medical consequences if there is a delay in starting DAAs due to need for insurance approval.
Lessons Learned and Future Direction
Our patients have generally been extremely eager to participate. Involvement of family and/or close friends in the education process has helped ensure patients are fully supported in their decision. Patients are pre-screened by the coordinators to ensure medical compliance has not been a recent concern. So far, we have not had any lapses in compliance with antiviral therapy post KT. Unfortunately, some patients who consented for the protocol have had serious medical problems identified in their medical and surgical clearance for transplantation. Others have had surgical complications of the transplant procedure itself. Thus, in the patient education process for this program, we have found that it is important to include education about the risks inherent in transplant surgery, per se.
Looking to the future, it is likely that transplantation of HCV viremic donor organs into HCV uninfected recipients may become standard of care. At this point, in our opinion, strict limitations on participation, such as liver transplant candidacy and other restrictions such as age and body mass index limitations, should follow the transplant centers guidelines for general recipients.
We anticipate that in the foreseeable future, the waiting time advantage provided by HCV-viremic kidneys will shrink considerably. This occurs after exhaustion of the so-called “bolus effect” associated with rapid increases in policy-specific kidney transplant rates following new policy implementation. In that case, accepting a HCV viremic organ may only advantage a recipient for a few months less waiting time or allow them to receive a kidney with only a slightly better KDPI than otherwise available. Of course, such results await real-time implementation and outcomes assessment. At this point, decisions will need to be made on a patient-by-patient basis, and shared decision-making discussions between physician and patient regarding risks and benefits
Conclusion
On a patient level, the opportunity to accept an organ from an HCV-infected donor, followed by surveillance for HCV and immediate treatment with DAAs or preemptive treatment with DAAs, will increase access to transplantation and substantially shorten waitlist time. However, appropriate patient selection and thorough education and informed consent are critical to a successful program.
Supplementary Material
Acknowledgement:
Supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp.. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. Additionally, the authors would like to thank the members of the MGH Nephrology Division’s Patient and Family Advisory Council, who reviewed the education session and informed consent, and provided edits and comments to improve comprehensiveness and clarity of the document.
Funding source: MES was supported by NIH K23 DK117014 from the NIDDK. RTC was supported by NIH K24DK078772 from the NIDDK. Merck provided funding for NCT02945150
Disclosure:
MES – Research grants to the institution: Gilead Sciences, Abbvie, Merck.
Scientific advisory board participant: Abbvie and Merck. Consultant to Abbvie.
DW- Research grants to the institution: Novartis, Bristol-Myers Squibb, Oxford Immunotec, Merck, Shire, CSL Behring, Care Dx. Scientific advisory board participant: Merck, Bristol-Myers Squibb.
RTC – Research grants to the institution: Gilead Sciences, Abbvie, Merck, Roche, Bristol-Myers Squibb, Janssen
NE- Research grants to the institution: Novartis, CRICO
WWW – Research grants to the institution: Bristol-Myers Squibb, Vital Therapies Inc. Scientific advisory board member: Veloxis Pharmaceuticals, Vital Therapies, Inc.
REFERENCES
- 1.Hart A, Smith J, Skeans M, Gustafson S, Wilk A, Robinson A, et al. OPTN/SRTR 2016 annual data report: kidney. American Journal of Transplantation 2018;18:18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant 2017;17 Suppl 1:21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scalea JR, Barth RN, Munivenkatappa R, Philosophe B, Cooper M, Whitlow V, et al. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts. Transplantation 2015;99:1192–6. [DOI] [PubMed] [Google Scholar]
- 4.Chute DF, Sise ME. Effect of the Opioid Crisis on the Donor Pool for Kidney Transplantation: An Analysis of National Kidney Deceased Donor Trends from 2010–2016. Am J Nephrol 2018;47:84–93. [DOI] [PubMed] [Google Scholar]
- 5.Amundsen B, Sise M, Lin M, Deirawan H, Heher E, Kimball B, et al. Utilization of HCV-positive Donors’ Kidneys: Potential Benefits in the Era of Direct Acting Antiviral (DAA) Therapy: abstract# P-92. American Journal of Transplantation 2016;16:63. [Google Scholar]
- 6.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting Hepatitis C-Positive Kidneys. N Engl J Med 2015;373:303–5. [DOI] [PubMed] [Google Scholar]
- 7.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr., et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 2015;386:1537–45. [DOI] [PubMed] [Google Scholar]
- 8.Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med 2015;373:2599–607. [DOI] [PubMed] [Google Scholar]
- 9.Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med 2015;373:2608–17. [DOI] [PubMed] [Google Scholar]
- 10.Reau N, Kwo P, Rhee S, Brown R, Agarwal K, Angus P, et al. LBO-03-MAGELLAN-2: safety and efficacy of glecaprevir/pibrentasvir in liver or renal transplant adults with chronic hepatitis C genotype 1–6 infection. Journal of Hepatology 2017;66:S90–S1. [Google Scholar]
- 11.Brown RS Jr., O’Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR Jr., et al. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: Real-world experience from the hepatitis C therapeutic registry and research network. Liver Transpl 2016;22:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo M, Aghemo A, Liu H, Zhang J, Dvory-Sobol H, Hyland R, et al. Treatment With Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients With Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Annals of Internal Medicine 2017;1662:109–17. [DOI] [PubMed] [Google Scholar]
- 13.Chute DF, Chung RT, Sise ME. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney Int 2018;93:560–7. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med 2017;376:2394–5. [DOI] [PubMed] [Google Scholar]
- 15.Reese PP, Abt PL, Blumberg EA, Van Deerlin VM, Bloom RD, Potluri VS, et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann Intern Med 2018. [DOI] [PubMed]
- 16.Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N, et al. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med 2018;168:533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeuzem S, Mizokami M, Pianko S, Mangia A, Han KH, Martin R, et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: Prevalence and effect on treatment outcome. J Hepatol 2017;66:910–8. [DOI] [PubMed] [Google Scholar]
- 18.de Ledinghen Vea. Retreatment with sofosbuvir plus grazoprevir plus elbasvir plus ribavirn of patients with hepatitis c virus genotype 1 or 4 with RASs at failure of a sofosbuvir plus ledipasvir or daclatasvir or simeprevir regimen (REVENGE Study). Hepatology 2016;64.
- 19.Bourliere M, Gordon SC, Ramji A, Ravendhran N, Tran TT, Hyland RH, et al. Sofosbuvir/Velpatasvir/Voxilaprevir for 12 weeks as a Salvage regimen in NS5A inhibitor-experienced patients with genotype 1–6 infection: the phase 3 POLARIS-1 study. Hepatology 2016;64:1–136.27696477 [Google Scholar]
- 20.Terrault NA, Dodge JL, Murphy EL, Tavis JE, Kiss A, Levin TR, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology 2013;57:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng HP, Caro L, Fandozzi CM, Guo Z, Talaty J, Wolford D, et al. Pharmacokinetic Interactions Between Elbasvir/Grazoprevir and Immunosuppressant Drugs in Healthy Volunteers. J Clin Pharmacol 2018;58:666–73. [DOI] [PubMed] [Google Scholar]
- 22.Wadei HM, Pungpapong S, Cortese C, Alexander MP, Keaveny AP, Yang L, et al. Transplantation of HCV‐infected organs into uninfected recipients: Advance with caution. American Journal of Transplantation 2018. [DOI] [PubMed]
- 23.Fishman J, Forns X. Hcv‐positive Donor Organs in Solid Organ Transplantation:“mind the Gap!”. American Journal of Transplantation 2017;17:2755–6. [DOI] [PubMed] [Google Scholar]
- 24.Suryaprasad A, Basavaraju SV, Hocevar SN, Theodoropoulos N, Zuckerman RA, Hayden T, et al. Transmission of Hepatitis C Virus From Organ Donors Despite Nucleic Acid Test Screening. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2015;15:1827–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


