Abstract
Corollary discharge (CD) signals are motor-related signals that exert an influence on sensory processing. They allow mobile organisms to predict the sensory consequences of their imminent actions. Among the many functions of CD is to provide a means by which we can distinguish sensory experiences caused by our own actions from those with external causes. In this way, they contribute to a subjective sense of agency. A disruption in the sense of agency is central to many of the clinical symptoms of schizophrenia, and abnormalities in CD signaling have been theorized to underpin particularly those agency-related psychotic symptoms of the illness. Characterizing abnormal CD associated with eye movements in schizophrenia and their resulting influence on visual processing and subsequent action plans may have advantages over other sensory and motor systems. That is because the most robust psychophysiological and neurophysiological data regarding the dynamics and influence of CD as well as the neural circuitry implicated in CD generation and transmission comes from the study of eye movements in humans and non-human primates. In this paper, we review studies of oculomotor CD signaling in the schizophrenia spectrum and possible neurobiological correlates of CD disturbances. We conclude by speculating upon the ways in which oculomotor CD dysfunction, specifically, may invoke specific experiences, clinical symptoms and cognitive impairments. These speculations lay the groundwork for empirical study, and we conclude by outlining potentially fruitful research directions.
Keywords: Schizophrenia, corollary discharge, efference copy, saccade, eye movements, predictive coding
Corollary discharge (CD) signals are motor-related signals that exert an influence on sensory processing. They allow mobile organisms to predict the sensory consequences of their imminent actions (1). These sensory predictions permit the attenuation of sensory experiences that result from our own actions. They contribute to our perception of a stable world despite the perturbations in our experience of the environment caused by moving about within it. They underpin our abilities to make rapid changes to our action plans without waiting for sensory feedback and to quickly execute movement sequences. And they provide a simple sensorimotor basis for a subjective experience of agency by demarcating sensory experiences caused by oneself from those with external causes: sensations that are correctly predicted are deemed to be self-generated (2).
A disruption in the sense of agency is central to many of the clinical symptoms of schizophrenia, which features a core disturbance in a sense of self (3–5). Given the role of CD in supporting a sense of agency, abnormalities in CD signaling may underpin this blurring of self-other boundaries (6, 7). Passivity delusions are one example. The individual feels as though their thoughts, emotions, or actions are controlled by an outside force—perhaps that one’s limbs are being manipulated like a marionette or that one’s thoughts are being sucked out by a vacuum extractor (8). A loss of agency may also contribute to auditory hallucinations, as they may partly reflect confusion regarding the source of inner speech and thoughts (9, 10).
Thus, CD signaling is a promising pathophysiological treatment target, particularly for those self-related symptoms that have been trickiest to understand at the level of brain and biology. This motivates a deeper understanding of the precise nature of CD signaling abnormalities in psychosis and the ways in which specific alterations in neural circuitry may underpin altered CD signaling. To this end, characterizing abnormal CD associated with eye movements in persons with schizophrenia (PSZ) and their resulting influence on visual processing and subsequent action plans may have advantages over using other sensory and motor systems. Indeed, the most robust psychophysiological and neurophysiological data regarding the dynamics and influence of CD as well as the neural circuitry implicated in CD generation and transmission comes from the study of eye movements in humans and non-human primates.
CD signals associated with eye movements serve several important anticipatory functions in visual perception and oculomotor processing. For example, CD supports stable perception of the world in spite of saccadic eye movements that cause the image of the world to be displaced on our retinas several times per second. Saccade-related CD signals allow the visual system to predict and obviate that imminent change in visual input (11). CD signals may also play a role in preventing visual awareness of what would undoubtedly be a disorienting blur while our eyes are in flight (12). They additionally facilitate rapid, sequential eye movements by providing information about the future location of gaze that can be used to plan saccades in parallel (13). Robust psychophysiological paradigms are available to quantify these influences of CD on action and perception around the time of an eye movement. Furthermore, an elegant body of primate neurophysiology and human lesion data has highlighted the crucial role of the thalamus in relaying these oculomotor CD signals (14, 15).
Here, we review studies of oculomotor CD signaling in the schizophrenia spectrum and possible neurobiological correlates of CD disturbances, and speculate upon the ways in which oculomotor CD dysfunction, specifically, may contribute to specific experiences, clinical symptoms and cognitive impairments. These speculations lay the groundwork for empirical study, and we conclude by outlining potentially fruitful research directions.
Review of oculomotor CD in the schizophrenia spectrum
We begin by describing selected functions of oculomotor CD signals related to perception and action preparation, the psychophysical paradigms developed to study those functions, and findings from individuals with schizophrenia and related conditions, which include differences from healthy individuals and correlations with clinical symptom severity.
Guiding sequential saccadic eye movements
Sequential saccades are prepared in parallel. For example, in a saccadic double-step task where participants make a saccade to two briefly-flashed targets in sequence, the second saccade is planned even before the eyes move to the first target (16, 17). When performed in total darkness and targets are extinguished before the first saccade, no visual reference cues allow participants to localize the second target. Furthermore, proprioceptive signals related to the eyes’ position in their orbit seems to play a negligible role (18, 19). Thus, to accurately hit the second target, a CD signal is required to predict where the second target should be once the first saccade has been executed. PSZ compensate less for the first saccade when executing a saccade to the second target (20). No correlation with symptom severity was observed in this study, but, in a non-clinical sample, the degree of delusional ideation correlated with less compensation for the first saccade (21).
Localizing visual stimuli across and throughout saccadic eye movements
Visual stability and the ability to accurately localize visual stimuli following saccadic eye movements is thought to rely on predictive remapping, the finding that visual neurons begin responding to a visual stimulus before an imminent saccade brings it into its receptive field (22–26, but see 27). These visual neurons update activity in their retinotopic maps, allowing them to prepare for the imminent displacement of the retinal image. CD signals conveying spatial information about the impending saccade allow for this predictive updating (14, 28, 29).
The blanking task provides insights into this updating process (30, 31). In this behavioral paradigm, a saccadic target disappears upon saccade initiation and reappears after a brief delay, somewhat offset from its pre-saccadic location. Participants must indicate the perceived direction of the shift. In healthy individuals, these perceptual judgments are independent of the distance between saccade landing site and pre-saccadic target—the landing site error (31). Thus, participants do not use post-saccadic eye position as a proxy for the pre-saccadic target location. Rather, accurate performance on this task requires an intact CD signal associated with the actual (rather than ideal) saccade vector to correctly relate the pre-saccadic to the postsaccadic target location. Performance in PSZ performing the blanking task is consistent with compromised CD. PSZ were, in general, less accurate at judging the postsaccadic displacement of a visual target (32, 33). These data suggest a greater reliance on retinal error signals, rather than an internal CD signal, which was related to positive symptoms generally (32) and agency-related phenomena in particular (33).
CD signals that serve to adjust visual perception following a saccade may also cause mislocalization of visual stimuli flashed immediately before or during a saccade. Traditionally, peri-saccadic compression—the fact that a visual stimulus that is flashed before a saccade is mislocalized closer to the saccade target than it is—was considered a perceptual consequence of a transient saccadic CD signal (34). Thus, if CD is disturbed in schizophrenia we may expect altered peri-saccadic compression. On the contrary, studies consistently reported no difference in compression between PSZ and healthy controls (35, 36). However, more recent work has revealed that compression is due to interruptions of visual input more generally, either by eye movements or by intermittently masking visual input (37). That compression can be observed in the absence of a saccade, therefore, suggests that compression is due to reduced perisaccadic visual sensitivity rather than a CD signal. Unaltered peri-saccadic compression in schizophrenia does thus not directly challenge other evidence for a disturbed transient oculomotor CD signal. On the other hand, one study (35) reported greater mislocalization of the flashed target in the direction of the saccade in persons with schizophrenia. Based on a formal model of perisaccadic mislocalization, the authors argued that the effect was consistent with a noisy CD signal that was associated with a continuous readout of the predicted eye position during the saccade. Although others (36) did not replicate these results, localization targets in this study were flashed with a delay at which large mislocalizations are not expected (38).
Saccade Adaptation
When a saccade of a particular amplitude and direction consistently falls short or long of the target, the systematic discrepancy between the expected and actual visual input yields an adjustment of the movement’s kinematics (i.e., it will adapt to the systematic target displacement). Saccade adaptation can be probed experimentally by systematically moving a saccade target inward (closer to fixation) or outward (away from fixation) by a consistent distance. Because the displacement occurs while the eyes are in flight, the participant does not perceive the target jump (39); nevertheless, saccade amplitudes gradually adapt to this systematic target shift (40). Indeed, saccades can be corrected midflight to bring gaze closer to the new target position, indicating that a CD-based prediction about the movement’s sensory consequences can alter movement trajectories before any external feedback (41, 42). Compromised saccadic adaptation has been observed in PSZ, but the particular aspect of saccade adaptation that is impaired remains unclear. Some studies found equal strength but slower speed of adaptation in PSZ (43), (36); others reported reduced adaptation strength, but equal speed (44). Neither experimental parameters (e.g. inward versus outward adaptation) nor clinical factors appear to account for these discrepant findings, calling for further studies with larger sample sizes or more sensitive adaptation protocols (45).
Smooth Pursuit
CD also plays an important role in smooth pursuit eye movements. The goals of smooth pursuit are to maintain a moving target on the fovea (where visual acuity is highest) and to reduce motion (and thus blur) of the target on the retina. Both retinal and extra-retinal information guide accurate pursuit. Retinal information includes the comparisons between target and foveal location and velocity. Discrepancies between these two locations (position error) or velocities (retinal slip) trigger catch-up saccades that realign the eye with the target (46). Extra-retinal events play an important role in pursuit maintenance. Pursuit must rely on predictive mechanisms, because once the fovea is stabilized on the moving image, retinal error cannot contribute to pursuit maintenance. One source of this predictive extra-retinal information is a continuous CD signal associated with the pursuit command (47). Thus, disrupted CD may contribute to reduced pursuit accuracy in schizophrenia—one of the most robust and well-replicated findings in the schizophrenia literature (48–50).
Several studies have specifically probed the extraretinal contributions to smooth pursuit by using predictive pursuit paradigms. In these paradigms retinal error is eliminated either by temporarily occluding the target or by stabilizing it on the retina. Using both of these techniques, PSZ as well as their first-degree relatives often have predictive pursuit deficits (51–56). Disturbed CD cannot fully explain the predictive pursuit deficit, however, as predictive impairments are present even at the re-initiation of target movement when eye velocity is starting at zero and there is thus no CD (52). Consistent with predictive pursuit impairments, PSZ have more accurate pursuit than healthy controls following an unexpected change in target motion, suggesting again that they are relying more on retinal rather than predictive, extraretinal sources of information (57). Bipolar patients with a history of psychotic features have similar predictive pursuit failures as PSZ (58, 59), and the degree of disorganized schizophrenia-like traits correlated with poorer predictive pursuit in a non-clinical sample (60). These data suggest that deficits in predictive pursuit may extend trans-diagnostically to individuals prone to psychosis.
Findings of altered visual perception during smooth pursuit eye movements lends additional support for altered oculomotor CD in schizophrenia. CD associated with smooth pursuit can enhance the estimation of the trajectory of moving objects (61) and also suppress or compensate for the perception of background image motion while the eyes are moving. During trajectory estimation, PSZ do not show the same improvement in accuracy as healthy controls when tracking an object with their eyes versus maintaining central fixation (62); CD signals from brain regions controlling smooth pursuit may thus fail to improve motion prediction. Moreover, PSZ can fail to discriminate actual target movement from retinal image motion of a stationary image resulting from smooth pursuit eye movements (63). Although PSZ did not differ from controls in the degree of compensation for pursuit-induced retinal motion, greater delusion severity in PSZ was associated with less compensation for eye movements, corroborating the hypothesized link between positive symptoms and disturbed CD signaling.
Error correction
CD signals are used to rapidly correct motor responses that are inconsistent with higher-level cognitive goals. For example, in the antisaccade task, participants are instructed to inhibit the prepotent response to a salient visual target and instead make a saccade to the mirror location in the opposite hemifield. Substantial evidence from this and other cognitive tasks suggests that error correction can transpire prior to any external feedback about that error to the participant (64–66). CD is a likely mechanism for these internally-driven error corrections. Oculomotor CD disturbances in schizophrenia would then give rise to fewer or slower error corrections in the antisaccade task. Findings regarding such gaze corrections in schizophrenia are mixed.
Equal rates (67–70) and latency (67) of error correction in PSZ and controls have been reported in the antisaccade task. These corrections occurred rapidly, suggesting that they were informed by predictive CD signals rather than proprioceptive or visual feedback. One study (71) observed somewhat fewer and slower corrective saccades in PSZ compared to healthy controls (for a similar finding in first-degree relatives of PSZ see 72). Notably, antisaccade errors were more frequent in this group of participants compared other studies (67–70), possibly explaining the discrepancy in results. Thus far, no empirical data speaks to the relationship between overall error rate and rates of error correction. Another study (73) also described longer antisaccade error correction latency (but equivalent speed of error commission) in PSZ compared to controls, but only in those patients experiencing passivity symptoms, consistent with a link between CD and psychotic symptoms.
A notable aspect of these previous antisaccade studies is that visual feedback was available—participants could see that they made an error. Using a task in which visual information could not guide error correction— a modified double-step paradigm, in which a second target instructed participants to inhibit the response to the first target and instead saccade immediately to the second target—we observed fewer and slower error corrective saccades, despite no difference in the speed of the erroneous saccade (20). We suggest that correction of gaze shifts that are inconsistent with a higher-level task goal are impaired in schizophrenia in the absence of feedback (i.e. when the demand on CD signals is higher), consistent with studies using manual (i.e. keypress or joystick) response tasks (74–76).
Neural circuits implicated in oculomotor CD abnormalities in the schizophrenia spectrum
The thalamus is poised to play a central role in the transmission of CD signals associated with eye movements. Elegant studies in non-human primates (14, 28) identified a neural circuit that conveys CD signals issued from saccade neurons in the superior colliculus (SC) to visual neurons in the frontal eye fields (FEF) via the mediodorsal nucleus of the thalamus (MD). Temporarily inactivating MD resulted in misdirected second saccades in the double-step task and greater use of saccade landing site to inform perception in the blanking task, consistent with compromised use of a CD signal (14, 28, 77). These effects are mirrored in humans with specific MD lesions (15, 78, 79). Additional indirect evidence supports a pathway between the cerebellum and FEF via the ventrolateral thalamus that relays CD signals associated with smooth pursuit velocity (80) and a pathway between the superior colliculus and cortical motion processing areas via the pulvinar (also in the thalamus) that relays a CD signal hypothesized to dampen the perception of retinal image motion during saccades (81, 82).
The role of the thalamus in transmitting CD signals is likely not limited to the visual and oculomotor domains. Rather, Sherman (83) notes that many, and perhaps all, messages sent to the thalamus for relay to cortex are CD signals related to messages sent to lower motor areas. Furthermore, parallel cortico-cortical pathways and trans-thalamic cortico-cortical pathways appear to be a pervasive and general feature of cortical organization (83, 84). Thus, the thalamus may also play a role in relaying CD signals between cortical areas (e.g., between motor cortex and speech production areas to auditory cortex; (85–91).
In schizophrenia, aberrant structure and/or function in a circuit from subcortical and cerebellar motor regions to the cortex via specific thalamic nuclei may underpin compromised transmission of oculomotor CD signals (92). This proposition would be compatible with mechanistic accounts of the illness (93) and an increasing body of evidence from functional and structural neuroimaging studies supporting altered thalamo-cortical and cerebello-thalamo-cortical connectivity in schizophrenia. These neural changes are related to clinical symptoms and cognitive functioning (94–96) and predictive of conversion to the illness in individuals at high risk for developing schizophrenia (97). Altered transmission of CD signals may be a consequence of thalamo-cortical dysconnectivity, and thus the link between alterations in neural circuits and clinical phenomena. Indeed, we recently reported reduced white matter integrity of a pathway between the MD and FEF in individuals with schizophrenia, which was related to oculomotor indices of compromised CD signals and positive symptom severity (98). Finally, case reports from individuals with MD lesions experiencing hallucinations and delusions (99–101) further support a link between thalamic dysfunction and/or thalamo-cortical dysconnectivity and psychosis, although they certainly cannot account for the full clinical picture. One may speculate that compromised function in specific CD pathways may give rise to modality-specific psychotic experiences (e.g. altered SC-MD-FEF function contributing to visual distortions), and that a shared node or nodes within these different CD pathways (e.g. thalamus) can account for the observed co-occurrence of psychotic symptoms across sensory modalities that may have their basis in altered CD.
Potential consequences of disturbed oculomotor CD signaling in schizophrenia
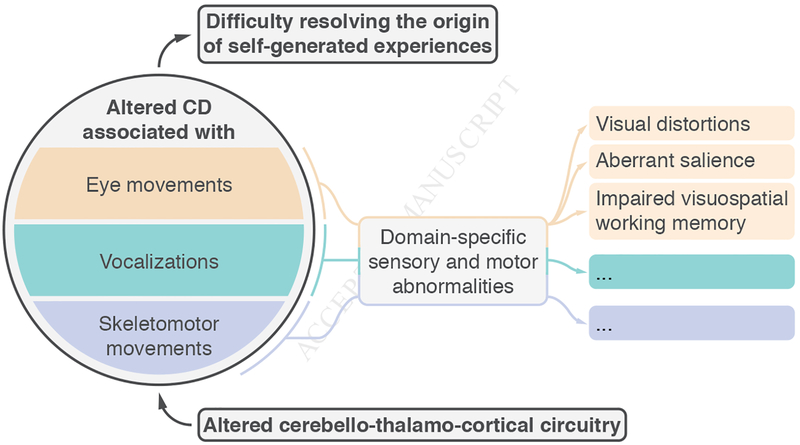
Along with serving as a model system within which to investigate a potential global disruption in CD signaling in schizophrenia, altered CD specifically related to eye movements may also underpin specific clinical and cognitive symptoms of the illness. Although CD dysfunction has broad explanatory power for a subset of clinical symptoms (positive symptoms), the link between oculomotor CD and specific subjective experiences, clinical symptoms, and cognitive functioning has yet to be explored. Given the function of CD within the oculomotor system, we may derive several hypotheses (Figure 1).
Figure 1.
A hypothesized model about causes and correlates of CD dysfunction in schizophrenia. A global disruption in CD signaling may arise due to dysconnectivity in cerebellothalamo-cortical circuits and lead to agency-related symptoms of the illness and a general blurring between self and other. Altered CD associated with specific motor acts, including (but not limited to) eye movements, skeleto-motor movements, and vocalizations, may also be expected to yield their own modality-specific abnormalities that relate to subjective experiences, clinical symptoms, and cognitive disturbances that are relevant to that particular modality. In the eye movement domain, abnormal CD may result in a failure to cancel retinal image motion and displacement, which might contribute to visual distortions such as the pseudomovement of objects. Abnormal CD may also result in the experience of looking without an intention or an expectation regarding the imminent visual input, making sensory input surprising and thus inappropriately imbued with salience and meaning. Finally, a failure in CD-based enhancement of working memory at a future point of gaze may contribute to the robust visuo-spatial working memory deficits in schizophrenia.
First, altered CD in the oculomotor system may contribute to subjective alterations in visual experience in schizophrenia. Visual distortions are common, occurring in more than 60% of PSZ (102). They are associated with important clinical variables, such as suicidality (103), impaired cognition (104, 105), and functional outcome (106, 107), and they are highly predictive of conversion to a psychotic disorder in at-risk individuals (108). As eye-movement-related CD has important effects on visual perception, altered CD may be a contributing factor to these visual distortions. For one, if CD contributes to dampening the perception of motion during eye movements and accounting for the retinal displacement of the world caused by eye movements, a disrupted CD signal may lead to illusory perception of motion. Indeed, one of the so-called basic symptoms of schizophrenia—subjective, subclinical disturbances in thought, emotion, and perception that are common in the prodrome—is pseudomovement of objects or scenes. Given its role in visual stability, could disrupted CD give rise to the perception of a fragmented and unstable environment? If so, could that have downstream consequences for higher-order inferences about the world?
Second, altered CD may also contribute to the aberrant assignment of salience, and thus motivational significance, to external objects in persons with psychosis: unimportant or irrelevant aspects of the environment grab attention and become imbued with meaning, leading to an overwhelming and perplexing experience of the outside world (109–111). This proposition is rooted in the tight coupling between spatial attention and eye movements (112–116). As a saccade is being prepared, the focus of attention shifts to the future location of gaze (117), at which visual sensitivity is predictively enhanced (118). These so-called pre-saccadic shifts in attention result from the impact of oculomotor signals on visual processing (119, 120). Similarly, when preparing a sequence of two saccades, attention selects both target locations ahead of the first movement. Based on CD signals, predictive remapping (see above) updates these locations in an otherwise retinotopic reference frame before the first saccade (11, 121–123). A compelling question arises here: what is the subjective experience that accompanies a failure in CD-mediated predictive attention shifts associated with exploratory eye movements? We may speculate that this unpredicted change in visual input brought about by saccades is surprising in nature, captures attention, and is thus deemed relevant and meaningful.
Finally, there may be a link between oculomotor CD and the robust working memory impairments observed in PSZ (124, 125). Ohl and Rolfs recently reported findings that the accuracy of visual working memory is enhanced at the location of a saccade target—that is, at the future location of gaze—and impaired elsewhere (126, 127). This impact on working memory is presumably supported by a CD signal associated with the planned saccade. Dysfunction in CD, therefore, may contribute to visuo-spatial working memory impairments in schizophrenia, which tend to be more robust than verbal working memory impairments (124). No studies have directly tested this hypothesis, but it is interesting to note that PSZ with more severe deficits in smooth pursuit—a function which relies on CD—have more robust spatial working memory deficits (128).
Conclusions, limitations, and future directions
There is a growing body of evidence that individuals with schizophrenia have abnormal CD signaling associated with saccadic and smooth pursuit eye movements which impair both action plans and visual perception. Based on a rich body of primate neurophysiology work as well neuroimaging studies in clinical populations, we suspect that these CD impairments have their basis in abnormal thalamo-cortical or cerebello-thalamo-cortical connectivity in schizophrenia.
There are several limitations to this body of work, however, that are worth highlighting. First, there is not a clear and consistent relationship between altered CD and clinical symptoms, which is problematic for the argument that disturbed CD is a proximal mechanism for a subset of psychotic symptoms. The failure to observe correlations between clinical and oculomotor measures may be due to the small sample sizes and clinical homogeneity that are typical of the reviewed studies. Alternatively, standard clinical rating scales may not capture the relevant symptom dimensions. However, findings that oculomotor CD is impaired in healthy relatives of PSZ—individuals that are vulnerable but do not manifest full-blown psychotic symptoms—challenge the notion that disrupted CD is directly related to symptoms. On the other hand, schizophrenia-like personality traits, i.e. schizotypy, are much more common in first-degree relatives of PSZ (129, 130). Thus, altered CD in healthy relatives may relate to propensity towards these subclinical, psychotic-like experiences (but see 131). Further research is needed to examine the link, or lack thereof, between CD disruptions and symptomatology. Furthermore, the reviewed studies were nearly all conducted in chronic, medicated samples; thus, neuroleptic use and the psychosocial consequences of having a long-term severe mental illness are potential confounds. Several pieces of evidence suggest that these factors cannot completely explain behavioral evidence for altered oculomotor CD signaling, however: altered CD has been observed in healthy relatives of persons with schizophrenia (53, 72) as well as non-help seeking individuals that are high in schizophrenia-like personality traits (21, 60). Finally, given the central role of CD signals to effective navigation of the world, one may question why schizophrenia does not manifest in profound motor and perceptual impairments if these signals are indeed impaired. In the absence of any signals that could support visual stability, for example, the world would be a dizzying place that was impossible to navigate. In response to this concern, we would first highlight that there cannot be a complete loss of CD signaling. Rather, these impairments can only be partial and thus revealed only in laboratory settings or, perhaps, manifest in mild motor problems (132). Further, individuals with schizophrenia may compensate for a reduction in the integrity of CD signals by relying either on alternate internal (i.e. proprioceptive) and external (i.e. visual) sources of information or on prior beliefs (133) to make inferences about their actions and the sensory consequences thereof. Indeed, the inability to detect displacements of a stimulus across saccades (39) may result from a strong prior that the world is stable (134). In the blanking task (30), stimulus disappearance provides strong evidence for target movement, outweighing that prior. The fact that PSZ are less sensitive to displacement (32, 33) may be indicative of a stronger prior for a stable world in these individuals. Future studies should pit priors against visual information to compare their impact on localization in PSZ and healthy controls.
With these limitations in mind, the reviewed work presents compelling data for compromised oculomotor CD signals in the schizophrenia spectrum and paves the way for future research. The pursuit of relevant clinical questions will likely proceed in tandem with a deeper understanding of CD signals: their nature, function, and related physiology. Behavioral data suggest a reduced influence of oculomotor CD signals on action and perception in schizophrenia, but whether that is because these signals are, occasionally, not generated, appropriately transmitted, or utilized by their target structures is unknown. We may suspect that the problem lies in their transmission, given robust evidence for altered brain connectivity in schizophrenia (135, 136). Our own findings that white matter integrity in a specific thalamo-cortical tract relates to behavioral indices of CD bolsters that notion(137). Furthermore, CD signals can arise from a number of levels along a motor pathway and exert an influence on a number of levels of sensory processing (1). The level at which CD signals are disrupted is likely important. More specifically, these failures of CD would engender aberrant prediction errors, which demand to be reconciled, with phenomenological and clinical consequences (reviewed in 138). Recently, Corlett and colleagues (139) speculated that in a layered inferential hierarchy (exchanging predictions and prediction errors between layers, in service of perception and cognition), aberrant prediction errors will have different consequences depending whether they are more proximal (lower) or distal (higher in the hierarchy) to sensory input. Understanding the interactions between hierarchical levels (and across hierarchies and modalities) may be key to mechanistic conceptualizations of clinical symptoms. The oculomotor and visual systems are particularly appropriate frameworks in which to undertake such work since their circuitry has been clearly delineated in primate studies. Finally, it would be valuable to understand neuromodulatory influences on the transmission or use of CD signals, which would be of particular importance to novel drug discovery and treatment planning.
To conclude, the investigation of CD in the oculomotor system may provide a window into the physiological mechanisms of those symptoms of schizophrenia that have traditionally been most puzzling, and thus may provide insights into novel interventions and may serve as useful clinical assays. Although many questions remain unanswered, these data pave the way for further investigation.
Acknowledgements
This work was supported in part by the National Institute of Health grant R01-MH112644 (KNT, MR), the National Institute of Mental Health grant R21-MH115297 (KNT, MR), a NARSAD Young Investigator Award from the Brain and Behavior Foundation (KNT), and the Deutsche Forschungsgemeinschaft (DFG) grants RO3579/3–1 (MR) and RO3579/8–1 (MR). We would additionally like to thank the four anonymous reviewers for their thoughtful and helpful reactions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Literature cited
- 1.Crapse TB, Sommer MA (2008): Corollary discharge across the animal kingdom. Nat Rev Neurosci. 9:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haggard P (2017): Sense of agency in the human brain. Nature Reviews Neuroscience. 18:197–208. [DOI] [PubMed] [Google Scholar]
- 3.Hur JW, Kwon JS, Lee TY, Park S (2014): The crisis of minimal self-awareness in schizophrenia: a meta-analytic review. Schizophr Res. 152:58–64. [DOI] [PubMed] [Google Scholar]
- 4.Sass LA, Parnas J (2003): Schizophrenia, consciousness, and the self. Schizophr Bull. 29:427–444. [DOI] [PubMed] [Google Scholar]
- 5.van der Weiden A, Prikken M, van Haren NE (2015): Self-other integration and distinction in schizophrenia: A theoretical analysis and a review of the evidence. Neurosci Biobehav Rev. 57:220–237. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg I, Guazzelli M (1999): Schizophrenia--a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J Psychiatry. 174:196–204. [DOI] [PubMed] [Google Scholar]
- 7.Feinberg I (1978): Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 4:636–640. [DOI] [PubMed] [Google Scholar]
- 8.Mellor CS (1970): First rank symptoms of schizophrenia. I. The frequnncy in schizophrenics on admission to hospital. II. Differences between individual first rank symptoms. The British journal of psychiatry : the journal of mental science. 117:15–23. [PubMed] [Google Scholar]
- 9.Ford JM, Mathalon DH (2005): Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int J Psychophysiol. 58:179–189. [DOI] [PubMed] [Google Scholar]
- 10.Green MF, Kinsbourne M (1990): Subvocal activity and auditory hallucinations: clues for behavioral treatments? Schizophr Bull. 16:617–625. [DOI] [PubMed] [Google Scholar]
- 11.Wurtz RH (2018): Corollary Discharge Contributions to Perceptual Continuity Across Saccades. Annu Rev Vis Sci. 4:215–237. [DOI] [PubMed] [Google Scholar]
- 12.Matin E (1974): Saccadic suppression: a review and an analysis. Psychol Bull. 81:899–917. [DOI] [PubMed] [Google Scholar]
- 13.Becker W, Jurgens R (1979): An analysis of the saccadic system by means of double step stimuli. Vision Res. 19:967–983. [DOI] [PubMed] [Google Scholar]
- 14.Sommer MA, Wurtz RH (2002): A pathway in primate brain for internal monitoring of movements. Science. 296:1480–1482. [DOI] [PubMed] [Google Scholar]
- 15.Ostendorf F, Liebermann D, Ploner CJ (2010): Human thalamus contributes to perceptual stability across eye movements. Proc Natl Acad Sci U S A. 107:1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallett PE, Lightstone AD (1976): Saccadic eye movements towards stimuli triggered by prior saccades. Vision Res. 16:99–106. [DOI] [PubMed] [Google Scholar]
- 17.Hallett PE, Lightstone AD (1976): Saccadic eye movements to flashed targets. Vision Res. 16:107–114. [DOI] [PubMed] [Google Scholar]
- 18.Lewis RF, Zee DS, Hayman MR, Tamargo RJ (2001): Oculomotor function in the rhesus monkey after deafferentation of the extraocular muscles. Exp Brain Res. 141:349–358. [DOI] [PubMed] [Google Scholar]
- 19.Steinbach MJ (1987): Proprioceptive knowledge of eye position. Vision Res. 27:1737–1744. [DOI] [PubMed] [Google Scholar]
- 20.Thakkar KN, Schall JD, Heckers S, Park S (2015): Disrupted Saccadic Corollary Discharge in Schizophrenia. J Neurosci. 35:9935–9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malassis R, Del Cul A, Collins T (2015): Corollary Discharge Failure in an Oculomotor Task Is Related to Delusional Ideation in Healthy Individuals. PLoS One. 10:e0134483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba N, Kawano K (2014): Neurons in cortical area MST remap the memory trace of visual motion across saccadic eye movements. Proc Natl Acad Sci U S A. 111:7825–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duhamel JR, Colby CL, Goldberg ME (1992): The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 255:90–92. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Colby CL (2002): Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc Natl Acad Sci U S A. 99:4026–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umeno MM, Goldberg ME (1997): Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 78:1373–1383. [DOI] [PubMed] [Google Scholar]
- 26.Walker MF, Fitzgibbon EJ, Goldberg ME (1995): Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. J Neurophysiol. 73:1988–2003. [DOI] [PubMed] [Google Scholar]
- 27.Zirnsak M, Steinmetz NA, Noudoost B, Xu KZ, Moore T (2014): Visual space is compressed in prefrontal cortex before eye movements. Nature. 507:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer MA, Wurtz RH (2004): What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol. 91:1403–1423. [DOI] [PubMed] [Google Scholar]
- 29.Sommer MA, Wurtz RH (2006): Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 444:374–377. [DOI] [PubMed] [Google Scholar]
- 30.Deubel H, Schneider WX, Bridgeman B (1996): Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Res. 36:985–996. [DOI] [PubMed] [Google Scholar]
- 31.Collins T, Rolfs M, Deubel H, Cavanagh P (2009): Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis. 9:29 21–29. [DOI] [PubMed] [Google Scholar]
- 32.Rosler L, Rolfs M, van der Stigchel S, Neggers SF, Cahn W, Kahn RS, et al. (2015): Failure to use corollary discharge to remap visual target locations is associated with psychotic symptom severity in schizophrenia. J Neurophysiol. 114:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal S, Bray LCJ, Schwartz BL, Joiner WM (2018): Transsaccadic Perception Deficits in Schizophrenia Reflect the Improper Internal Monitoring of Eye Movement Rather Than Abnormal Sensory Processing. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pola J (2011): An explanation of perisaccadic compression of visual space. Vision Res. 51:424–434. [DOI] [PubMed] [Google Scholar]
- 35.Richard A, Churan J, Whitford V, O’Driscoll GA, Titone D, Pack CC (2014): Perisaccadic perception of visual space in people with schizophrenia. J Neurosci. 34:4760–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lencer R, Meermeier A, Silling K, Gremmler S, Lappe M (2016): Instability of visual error processing for sensorimotor adaptation in schizophrenia. Eur Arch Psychiatry Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann E, Born S, Fink GR, Cavanagh P (2014): Masking produces compression of space and time in the absence of eye movements. J Neurophysiol. 112:3066–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Wetter SM, Van Opstal AJ (2008): Experimental test of visuomotor updating models that explain perisaccadic mislocalization. J Vis. 8:8 1–22. [DOI] [PubMed] [Google Scholar]
- 39.Bridgeman B, Hendry D, Stark L (1975): Failure to detect displacement of the visual world during saccadic eye movements. Vision Res. 15:719–722. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin SC (1967): Parametric adjustment in saccadic eye movements. Percept Psychophys. 2:359–362. [Google Scholar]
- 41.Ethier V, Zee DS, Shadmehr R (2008): Changes in control of saccades during gain adaptation. J Neurosci. 28:13929–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R (2008): Adaptive control of saccades via internal feedback. J Neurosci. 28:2804–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coesmans M, Roder CH, Smit AE, Koekkoek SK, De Zeeuw CI, Frens MA, et al. (2014): Cerebellar motor learning deficits in medicated and medication-free men with recent-onset schizophrenia. J Psychiatry Neurosci. 39:E3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Picard HJ, Amado I, Bourdel MC, Landgraf S, Olie JP, Krebs MO (2009): Correlates between neurological soft signs and saccadic parameters in schizophrenia. Prog Neuropsychopharmacol B ol Psychiatry. 33:676–681. [DOI] [PubMed] [Google Scholar]
- 45.Cassanello CR, Ohl S, Rolfs M (2016): Saccadic adaptation to a systematically varying disturbance. J Neurophysiol. 116:336–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lisberger SG, Morris EJ, Tychsen L (1987): Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 10:97–129. [DOI] [PubMed] [Google Scholar]
- 47.Lisberger SG (2010): Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron. 66:477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutton S, Kennard C (1998): Oculomotor abnormalities in schizophrenia: a critical review. Neurology. 50:604–609. [DOI] [PubMed] [Google Scholar]
- 49.Levy DL, Holzman PS, Matthysse S, Mendell NR (1993): Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull. 19:461–536. [DOI] [PubMed] [Google Scholar]
- 50.O’Driscoll GA, Callahan BL (2008): Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain Cogn. 68:359–370. [DOI] [PubMed] [Google Scholar]
- 51.Hong LE, Avila MT, Adami H, Elliot A, Thaker GK (2003): Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophr Res. 63:39–48. [DOI] [PubMed] [Google Scholar]
- 52.Hong LE, Turano KA, O’Neill H, Hao L, Wonodi I, McMahon RP, et al. (2008): Refining the predictive pursuit endophenotype in schizophrenia. Biol Psychiatry. 63:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thaker GK, Avila MT, Hong EL, Medoff DR, Ross DE, Adami HM (2003): A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology. 40:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR (1999): Smooth pursuit eye movements to extra-retinal motion signals: deficits in patients with schizophrenia. Psychiatry Res. 88:209–219. [DOI] [PubMed] [Google Scholar]
- 55.Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, et al. (1998): Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 55:830–836. [DOI] [PubMed] [Google Scholar]
- 56.Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK (2005): Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol Psychiatry. 57:726–732. [DOI] [PubMed] [Google Scholar]
- 57.Hong LE, Avila MT, Thaker GK (2005): Response to unexpected target changes during sustained visual tracking in schizophrenic patients. Exp Brain Res. 165:125–131. [DOI] [PubMed] [Google Scholar]
- 58.Ivleva EI, Moates AF, Hamm JP, Bernstein IH, O’Neill HB, Cole D, et al. (2014): Smooth pursuit eye movement, prepulse inhibition, and auditory paired stimuli processing endophenotypes across the schizophrenia-bipolar disorder psychosis dimension. Schizophr Bull. 40:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moates AF, Ivleva EI, O’Neill HB, Krishna N, Cullum CM, Thaker GK, et al. (2012): Predictive pursuit association with deficits in working memory in psychosis. Biol Psychiatry. 72:752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kattoulas E, Evdokimidis I, Stefanis NC, Avramopoulos D, Stefanis CN, Smyrnis N (2011): Predictive smooth eye pursuit in a population of young men: II. Effects of schizotypy, anxiety and depression. Exp Brain Res. 215:219–226. [DOI] [PubMed] [Google Scholar]
- 61.Spering M, Schutz AC, Braun DI, Gegenfurtner KR (2011): Keep your eyes on the ball: smooth pursuit eye movements enhance prediction of visual motion. J Neurophysiol. 105:1756–1767. [DOI] [PubMed] [Google Scholar]
- 62.Spering M, Dias EC, Sanchez JL, Schutz AC, Javitt DC (2013): Efference copy failure during smooth pursuit eye movements in schizophrenia. J Neurosci. 33:11779–11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT (2005): Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 15:1119–1124. [DOI] [PubMed] [Google Scholar]
- 64.Rabbitt PM (1966): Error correction time without external error signals. Nature. 212:438. [DOI] [PubMed] [Google Scholar]
- 65.Higgins JR, Angel RW (1970): Correction of tracking errors without sensory feedback. J Exp Psychol. 84:412–416. [DOI] [PubMed] [Google Scholar]
- 66.Laming D (1979): Choice reaction performance following an error. Acta Psychol (Amst). 43:199–224. [DOI] [PubMed] [Google Scholar]
- 67.Polli FE, Barton JJ, Vangel M, Goff DC, Iguchi L, Manoach DS (2006): Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophr Res. 82:191–201. [DOI] [PubMed] [Google Scholar]
- 68.Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, et al. (2008): Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 131:971–986. [DOI] [PubMed] [Google Scholar]
- 69.Brownstein J, Krastoshevsky O, McCollum C, Kundamal S, Matthysse S, Holzman PS, et al. (2003): Antisaccade performance is abnormal in schizophrenia patients but not in their biological relatives. Schizophr Res. 63:13–25. [DOI] [PubMed] [Google Scholar]
- 70.Reuter B, Herzog E, Kathmann N (2006): Antisaccade performance of schizophrenia patients: evidence of reduced task-set activation and impaired error detection. J Psychiatr Res. 40:122–130. [DOI] [PubMed] [Google Scholar]
- 71.McDowell JE, Clementz BA (1997): The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res. 115:333–344. [DOI] [PubMed] [Google Scholar]
- 72.Mazhari S, Price G, Dragovic M, Waters FA, Clissa P, Jablensky A (2011): Revisiting the suitability of antisaccade performance as an endophenotype in schizophrenia. Brain Cogn. 77:223–230. [DOI] [PubMed] [Google Scholar]
- 73.Waters F, Price G, Dragovic M, Jablensky A (2009): Electrophysiological brain activity and antisaccade performance in schizophrenia patients with first-rank (passivity) symptoms. Psychiatry Res. 170:140–149. [DOI] [PubMed] [Google Scholar]
- 74.Malenka RC, Angel RW, Thiemann S, Weitz CJ, Berger PA (1986): Central error-correcting behavior in schizophrenia and depression. Biol Psychiatry. 21:263–273. [DOI] [PubMed] [Google Scholar]
- 75.Malenka RC, Angel RW, Hampton B, Berger PA (1982): Impaired central error-correcting behavior in schizophrenia. Arch Gen Psychiatry. 39:101–107. [DOI] [PubMed] [Google Scholar]
- 76.Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM (2003): Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry. 160:1881–1883. [DOI] [PubMed] [Google Scholar]
- 77.Cavanaugh J, Berman RA, Joiner WM, Wurtz RH (2016): Saccadic Corollary Discharge Underlies Stable Visual Perception. J Neurosci. 36:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP (2005): The role of the human thalamus in processing corollary discharge. Brain. 128:1139–1154. [DOI] [PubMed] [Google Scholar]
- 79.Gaymard B, Rivaud S, Pierrot-Deseilligny C (1994): Impairment of extraretinal eye position signals after central thalamic lesions in humans. Exp Brain Res. 102:1–9. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka M (2005): Involvement of the central thalamus in the control of smooth pursuit eye movements. J Neurosci. 25:5866–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berman RA, Wurtz RH (2010): Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci. 30:6342–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berman RA, Wurtz RH (2011): Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J Neurosci. 31:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sherman SM (2016): Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 19:533–541. [DOI] [PubMed] [Google Scholar]
- 84.Mo C, Sherman SM (2019): A Sensorimotor Pathway via Higher-Order Thalamus. J Neurosci. 39:692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schneider DM, Nelson A, Mooney R (2014): A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 513:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eliades SJ, Wang X (2003): Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol. 89:2194–2207. [DOI] [PubMed] [Google Scholar]
- 87.Eliades SJ, Wang X (2008): Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 453:1102–1106. [DOI] [PubMed] [Google Scholar]
- 88.Ford JM, Mathalon DH (2004): Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. Journal of psychiatric research. 38:37–46. [DOI] [PubMed] [Google Scholar]
- 89.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT (2002): Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological psychiatry. 51:485–492. [DOI] [PubMed] [Google Scholar]
- 90.Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT (2001): Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biological psychiatry. 50:540–549. [DOI] [PubMed] [Google Scholar]
- 91.Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT (2001): Cortical responsiveness during inner speech in schizophrenia: an event-related potential study. The American journal of psychiatry. 158:1914–1916. [DOI] [PubMed] [Google Scholar]
- 92.Thakkar KN, Diwadkar VA, Rolfs M (2017): Oculomotor Prediction: A Window into the Psychotic Mind. Trends Cogn Sci. 21:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andreasen NC, Paradiso S, O’Leary DS (1998): “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 24:203–218. [DOI] [PubMed] [Google Scholar]
- 94.Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G (2015): The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 54:57–75. [DOI] [PubMed] [Google Scholar]
- 95.Canu E, Agosta F, Filippi M (2015): A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res. 161:19–28. [DOI] [PubMed] [Google Scholar]
- 96.Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, Voyvodic J, et al. (2018): Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med. 48:2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao H, Chen OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, et al. (2018): Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 9:3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yao B, Neggers SFW, Rolfs M, Rösler L, Thompson IA, Hopman HJ, et al. (in press): Structural thalamo-frontal hypoconnectivity is related to oculomotor corollary discharge dysfunction in schizophrenia. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carrera E, Bogousslavsky J (2006): The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 66:1817–1823. [DOI] [PubMed] [Google Scholar]
- 100.Crail-Melendez D, Atriano-Mendieta C, Carrillo-Meza R, Ramirez-Bermudez J (2012): Schizophrenia-like psychosis associated with right lacunar thalamic infarct. Neurocase. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Y, Fox D, Anand A, Elhaj A, Kapoor A, Najibi F, et al. (2015): Artery of Percheron Infarction as an Unusual Cause of Korsakoff’s Syndrome. Case Rep Neurol Med. 2015:927809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Phillipson OT, Harris JP (1985): Perceptual changes in schizophrenia: a questionnaire survey. Psychol Med. 15:859–866. [DOI] [PubMed] [Google Scholar]
- 103.Grano N, Salmijarvi L, Karjalainen M, Kallionpaa S, Roine M, Taylor P (2015): Early signs of worry: psychosis risk symptom visual distortions are independently associated with suicidal ideation. Psychiatry Res. 225:263–267. [DOI] [PubMed] [Google Scholar]
- 104.Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, et al. (2007): Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 64:1229–1240. [DOI] [PubMed] [Google Scholar]
- 105.Calderone DJ, Hoptman MJ, Martinez A, Nair-Collins S, Mauro CJ, Bar M, et al. (2013): Contributions of low and high spatial frequency processing to impaired object recognition circuitry in schizophrenia. Cereb Cortex. 23:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF (2011): Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 41:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Green MF, Hellemann G, Horan WP, Lee J, Wynn JK (2012): From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F (2001): Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 58:158–164. [DOI] [PubMed] [Google Scholar]
- 109.Gray JA (1998): Integrating schizophrenia. Schizophr Bull. 24:249–266. [DOI] [PubMed] [Google Scholar]
- 110.Kapur S (2003): Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry. 160:13–23. [DOI] [PubMed] [Google Scholar]
- 111.Corlett PR, Frith CD, Fletcher PC (2009): From drugs to deprivation: a Bayesian framework for understanding models of psychosis. Psychopharmacology (Berl). 206:515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sheliga BM, Riggio L, Rizzolatti G (1995): Spatial attention and eye movements. Exp Brain Res. 105:261–275. [DOI] [PubMed] [Google Scholar]
- 113.Moore T, Armstrong KM, Fallah M (2003): Visuomotor origins of covert spatial attention. Neuron. 40:671–683. [DOI] [PubMed] [Google Scholar]
- 114.Bisley JW, Goldberg ME (2003): Neuronal activity in the lateral intraparietal area and spatial attention. Science. 299:81–86. [DOI] [PubMed] [Google Scholar]
- 115.Schall JD (2004): On the role of frontal eye field in guiding attention and saccades. Vision Res. 44:1453–1467. [DOI] [PubMed] [Google Scholar]
- 116.Shepherd M, Findlay JM, Hockey RJ (1986): The relationship between eye movements and spatial attention. Q J Exp Psychol A. 38:475–491. [DOI] [PubMed] [Google Scholar]
- 117.Deubel H, Schneider WX (1996): Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 36:1827–1837. [DOI] [PubMed] [Google Scholar]
- 118.Rolfs M, Carrasco M (2012): Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation. J Neurosci. 32:13744–13752a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rolfs M (2015): Attention in Active Vision: A Perspective on Perceptual Continuity Across Saccades. Perception. 44:900–919. [DOI] [PubMed] [Google Scholar]
- 120.Awh E, Armstrong KM, Moore T (2006): Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 10:124–130. [DOI] [PubMed] [Google Scholar]
- 121.Cavanagh P, Hunt AR, Afraz A, Rolfs M (2010): Visual stability based on remapping of attention pointers. Trends Cogn Sci. 14:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rolfs M, Jonikaitis D, Deubel H, Cavanagh P (2011): Predictive remapping of attention across eye movements. Nat Neurosci. 14:252–256. [DOI] [PubMed] [Google Scholar]
- 123.Rolfs M, Szinte M (2016): Remapping Attention Pointers: Linking Physiology and Behavior. Trends Cogn Sci. 20:399–401. [DOI] [PubMed] [Google Scholar]
- 124.Lee J, Park S (2005): Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 114:599–611. [DOI] [PubMed] [Google Scholar]
- 125.Park S, Holzman PS (1992): Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 49:975–982. [DOI] [PubMed] [Google Scholar]
- 126.Ohl S, Rolfs M (2018): Saccadic selection of stabilized items in visuospatial working memory. Conscious Cogn. 64:32–44. [DOI] [PubMed] [Google Scholar]
- 127.Ohl S, Rolfs M (2016): Saccadic Eye Movements Impose a Natural Bottleneck on Visual Short-Term Memory. J Exp Psychol Learn Mem Cogn. [DOI] [PubMed] [Google Scholar]
- 128.Park S, Holzman PS (1993): Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophr Res. 11:55–61. [DOI] [PubMed] [Google Scholar]
- 129.Kendler KS, Gardner CO (1997): The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychol Med. 27:411–419. [DOI] [PubMed] [Google Scholar]
- 130.Calkins ME, Curtis CE, Grove WM, Iacono WG (2004): Multiple dimensions of schizotypy in first degree biological relatives of schizophrenia patients. Schizophr Bull. 30:317–325. [DOI] [PubMed] [Google Scholar]
- 131.Thaker GK, Cassady S, Adami H, Moran M, Ross DE (1996): Eye movements in spectrum personality disorders: comparison of community subjects and relatives of schizophrenic patients. A J Psychiatry. 153:362–368. [DOI] [PubMed] [Google Scholar]
- 132.Walther S, Mittal VA (2017): Motor System Pathology in Psychosis. Curr Psychiatry Rep. 19:97. [DOI] [PubMed] [Google Scholar]
- 133.Powers AR, Mathys C, Corlett PR (2017): Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 357:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Niemeier M, Crawford JD, Tweed DB (2003): Optimal transsaccadic integration explains distorted spatial perception. Nature. 422:76–80. [DOI] [PubMed] [Google Scholar]
- 135.Narr KL, Leaver AM (2015): Connectome and schizophrenia. Curr Opin Psychiatry. 28:229–235. [DOI] [PubMed] [Google Scholar]
- 136.Uhlhaas PJ (2013): Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol. 23:283–290. [DOI] [PubMed] [Google Scholar]
- 137.Yao B, Neggers SFW, Rolfs M, Rosler L, Thompson IA, Hopman HJ, et al. (2019): Structural thalamo-frontal hypoconnectivity is related to oculomotor corollary discharge dysfunction in schizophrenia. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sterzer P, Adams RA, Fletcher P, Frith C, Lawrie SM, Muckli L, et al. (2018): The Predictive Coding Account of Psychosis. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR 3rd (2018): Hallucinations and Strong Priors. Trends Cogn Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]