Abstract
Drug uptake transporters are membrane proteins responsible for the trans-membrane transport of endo- and xenobiotics, including numerous drugs. They are important for the uptake of drugs into target tissues or into organs for metabolism and excretion. Many drug uptake transporters have a broad spectrum of structural-independent substrates, which make them vulnerable to drug-drug interactions. Recent studies have shown more and more complex pharmacokinetics involving transporters, and regulatory agencies now require studies to be performed to measure the involvement of transporters in drug development. A better understanding of the factors affecting the expression of transporters is needed. Despite many efforts devoted to the functional characterization of different drug uptake transporters, transporter in vitro to in vivo extrapolations are far from predicting the behavior under physiological conditions. There is an increasing number of uptake transporters demonstrated to form protein-protein interactions or to oligomerize. This raises the possibility that these interactions between or among transporters could help explaining the gap between in vitro and in vivo measurement of drug transporters. In this review, we summarized protein-protein interactions of drug uptake transporters that are important for pharmacokinetics, especially those in the liver and the kidneys.
1. Introduction
Membrane transport proteins are expressed at the cell membrane and facilitate the transport of chemicals and macromolecules in and out of the cells. Since cell membranes consist of a lipid bilayer which only allows certain compounds with distinct physiochemical properties to penetrate freely, transporters are required for cells to take up or efflux the majority of large and hydrophilic endo- and xenobiotics [1]. Some transporters like the Na+/K+ ATPase, which establishes and maintains electro-chemical gradients across the cell membranes, are ubiquitously expressed. In contrast, the expression and function of other transporters are unique to certain cell types. Therefore, this distinct expression pattern of transporters in only certain cell types determines what kinds of chemicals can enter or exit these cells [2].
Transporters can be divided into passive and active transporters based on their energy consumption. Passive transporters move substrates along an electrochemical gradient, while active transporters transfer their substrates against such gradients and consume energy [1]. Based on how the energy is consumed, active transporters are divided into two groups: 1) the primary-active transporters that use energy directly from ATP hydrolysis, and 2) the secondary-active transporters that operate using the electrochemical gradients which were established by primary active transporters. Primary active transporters include ATP-binding cassette (ABC) transporters and numerous ion pumps, while secondary active transporters are found in many Solute Carrier (SLC) families [1]. Depending on their transport mechanisms secondary-active transporters can be further classified into antiporters or exchangers and symporters or co-transporters.
1.1. Significance of Membrane Transporter Proteins
Membrane transport proteins play an essential role not only in physiology but also in pharmacology. They contribute to the absorption, distribution and excretion of nutrients, signaling molecules, metabolic end products and drugs. Under normal physiological conditions, the enterohepatic circulation of bile acids is facilitated by several transporters: NTCP mediates the uptake of bile acids into hepatocytes and the Bile Salt Export Pump (BSEP) secretes them into bile canaliculi. Once they reach the ileum, the Apical Sodium-Dependent Bile Acid Transporter (ASBT) mediates their uptake from the intestine into the enterocytes. The Organic Solute Transporter α/β (OSTα/β) mediate their exit across the basolateral membrane into portal blood which brings them back to the hepatocytes [3]. Another example is the sodium-glucose cotransporter (SGLT1) expressed primarily in the apical membrane of enterocytes in the small intestine [4]. SGLT1 is important for the removal of glucose from the intestine and if non-functional, leads to glucose-galactose malabsorption, a disease where newborns suffer from severe diarrhea that can be life threatening [5].
Transporters are also important in pharmacology because they can affect drug disposition. They are major determinants of the pharmacokinetics for numerous drugs: certain uptake transporters, like the Organic Anion Transporting Polypeptides (OATPs), can affect the bioavailability of drugs. In particular the OATPs that are expressed in hepatocytes can remove orally administered drugs by rapid uptake into hepatocytes, also called first pass effect. In the case of statins this is a desirable effect because the drug target, HMG-CoA reductase, is expressed mainly in hepatocytes. However, a single nucleotide polymorphism in the SLCO1B1 gene coding for an amino acid change from valine to alanine at position 174 results in OATP1B1*5, a transporter that shows reduced uptake of simvastatin into hepatocytes. This results in increased plasma concentrations of simvastatin and can result in simvastatin-induced myopathy [6]. Similarly, efflux transporters like the Multidrug Resistance Protein (MDR1), some Multidrug Resistance associated Proteins (MRPs) and the Breast Cancer Resistance Protein (BCRP) will pump endo- and xenobiotics out of cells and therefore determine whether they can reach the circulation [7].
Transporters can also be drug targets either to deliver drugs to a specific cell or organ, or by using inhibitors. At least 84 SLC transporters have been described to be involved in 100 or more disorders [8]. Two examples of targeting members of the SLC10 family are highlighted in the following. With respect to hepatitis B and D, the Na+/taurocholate cotransporting polypeptide (NTCP, SLC10A1) has been identified as the receptor to which the hepatitis B and D virus bind. Myrcludex B, an NTCP-directed inhibitor peptide with an IC50 of approximately 80 pM, has been developed and is currently in Phase II clinical trials [9, 10]. Another member of the same gene family, the apical sodium-dependent bile acid transporter (ASBT, SLC10A2) is expressed in the ileum, and is responsible for the efficient uptake of bile acids from the intestine. Several pharmaceutical companies are developing ASBT-inhibitors to treat hypercholesterolemia by decreasing the reabsorption of bile acids from the small intestine [11].
Extensive studies have been performed to investigate and characterize the regulation of drug transporters at the transcriptional and post-translational level. However, recent evidence suggests that drug transporters can also be regulated via protein-protein interactions. In this review we will try to summarize the current knowledge regarding protein-protein interactions and how this can affect the expression and function of the involved drug transporters.
1.2. Overview of protein-protein interactions in membrane transport proteins
Although most researchers think of the proteins they study as a monomeric single amino acid polypeptide, in nature most proteins form protein-protein interactions either with very similar homologous proteins (homo-oligomers) or with different proteins (hetero-oligomers) [12]. It is estimated that about 50% of human proteins are involved in protein interactions [13, 14]. However, of the 650,000 predicted interactions [15] so far only ~53,000 high-quality protein-protein interactions have been identified as part of the Human Reference Interactome (http://interactome.baderlab.org; last accessed July 2, 2019). Oligomerization is an important physiological phenomenon and represents an evolutionary advantage. It can yield additional function, functional control, such as allosteric regulation, and post-translational regulation due to the interacting protein network [14, 16]. For example, G protein-coupled receptors (GPCRs) interact with heterotrimeric G proteins to transduce signals from the extracellular space into the cells. Interacting partners include scaffold proteins like β-arrestin and the 5-HT4d(HTR4) receptor, a target for Alzheimer disease [17, 18]. The GPCR interactome includes 299 membrane proteins with many different cellular functions and these interactions can alter the surface expression and function of GPCRs [18]. In addition, oligomerization can also lead to changes in expression levels by affecting the stability of the protein. Protein-protein interactions affecting the function have not only been reported for receptors and enzymes, but also for transporters. Examples are the aquaporins [19] or the sodium-proton exchanger NHE3 which is expressed in the proximal tubule and functionally modulated by carbonic anhydrase II [20]. Protein-protein interactions have also been identified and characterized for drug transporters of the solute carrier (SLC) and the ATP-binding cassette (ABC) families. One of the earliest and best characterized examples is the L-type amino acid transporter-1 (LAT1) that was cloned in 1998 and shown to only transport amino acids in the presence of the 4F2 heavy chain (also known as CD98) [21, 22]. The 12-transmembrane protein LAT1 (SLC7A5) heterodimerizes with a type II membrane glycoprotein, 4F2hc (SLC3A2) to form the functional transport protein [23]. 4F2hc is required for the proper expression of LAT1 in the plasma membrane [23] but there seems to be controversy regarding its effect on function. Reconstitution of either LAT1 or 4F2hc (CD98) alone into proteoliposomes resulted in LAT1- but not 4F2hc-mediated histidine transport [24] while leucine transport was dependent on the coexpression of LAT1 together with 4F2hc [25]. Given that LAT1 is overexpressed in many tumor cell lines and numerous human cancers it became an important drug target in cancer therapy, and LAT1 inhibitors have been developed and are currently in clinical trials as anticancer drugs [26]. In this review, examples of drug transporter-related protein interactions, for transporters that are important for pharmacokinetics, will be provided and their potential consequences are discussed.
2. Protein interactions of transporters in the liver
The liver is the major organ for drug metabolism and together with the kidneys crucial for drug elimination. The focus of drug metabolism studies in the liver was for a long time mainly on the role and significance of the phase I and phase II drug metabolizing enzymes of the CYPP450 and UGT families. However, more recently it has been acknowledged that drug transporters including members of the SLCO, the SLC22 and the SLC10 families, contribute significantly to the first pass effect and are involved in many drug-drug interactions [27]. The uptake of drugs into hepatocytes, also referred to as Phase 0 metabolism, is mainly mediated by OATP1B1 (SLCO1B1), OATP1B3 (SLCO1B3), OCT1 (SLC22A1), OAT2 (SLC22A7), and NTCP. Drugs are effluxed from hepatocytes by members of the ABC family, including MDR1 (ABC1B1), MRP2 (ABCC2), and BCRP (ABCG2), as well as by OSTα/β (SLC51A and SLC51B). Most of these transporters have a broad spectrum of drug substrates. Therefore, the interactions of these drug transporters with other liver transporters and potentially with other proteins expressed in hepatocytes could potential lead to significant effects on their expression. This in turn could affect pharmacokinetics of a number of substrates and modulators. Therefore, it is important to not only consider single nucleotide polymorphisms (SNPs) that could affect the expression levels of the affected genes, but to look at the effects that such SNPs could have on the expression and function of interacting drug transporters.
2.1. OATPs
As mentioned briefly above, OATP1B1 (SLCO1B1) and OATP1B3 (SLCO1B3) are members of the SLCO family and are selectively expressed at the basolateral membrane of human hepatocytes. They are important for the uptake of endogenous compounds like bilirubin, unconjugated bile acids, and conjugated steroid hormones, as well as xenobiotics, including numerous drugs like statins, sartans and antibiotics, into hepatocytes [28]. Their combined absence leads to the Rotor Syndrome, a benign conjugated hyperbilirubinemia [29]. Based on the FDA guidance for industry, OATP1B1 and OATP1B3 are currently the only two liver drug uptake transporters which are required to be tested for drug-drug interactions during drug development [30]. Both, OATP1B1 and OATP1B3, can form homo-oligomers [31, 32] (Figure 1). For OATP1B1 this was demonstrated using differently tagged OATP1B1 constructs and transfection of HEK293 cells. The formation of homo-oligomers occurs through disulfide bonds as shown with experiments using cleavable cross-linkers [32]. In addition, there are three GXXXG motifs present in OATP1B1. These motifs have previously been shown to be involved in the stabilization of transmembrane domains and in the formation of oligomerizations. Mutation of two of the glycine residues, G219A and G393A, reduced OATP1B1 activity. However, only G394A resulted in decreased association of the transporter, suggesting that this position or this GXXXG motif may be involved in oligomerization [32]. Interestingly, both positions are highly conserved in all human OATPs and thus might play a role in general for oligomerization of OATPs [32].
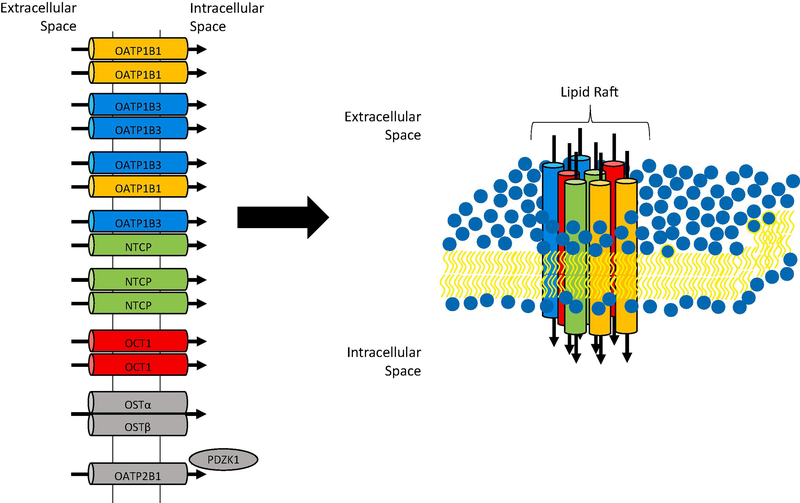
Figure 1: Illustration of the interactions among liver uptake transporters.
Summary of the published protein-protein interactions at the basolateral membrane of human hepatocytes (left). A proposed membrane microdomain containing selected transporters is illustrated at right with the following color code: OATP1B1 (orange), OATP1B3 (blue), NTCP (green) and OCT1(red).
We have recently investigated the homo- and hetero-oligomerization of OATP1B3 [31] (Figure 1). We used co-immunoprecipitation of FLAG- and His-tagged OATP1B3 to demonstrate homo-oligomerization. In addition, the proximity ligation assay visualized the close proximity between the differently tagged OATP1B3 constructs. Using this assay in HEK293 cells, we also demonstrated that OATP1B3 interacts with OATP1B1 and NTCP. We could also confirm the interaction between OATP1B3 and NTCP on frozen human liver sections. Similar to the NTCP where the individual units in the homo-oligomers are the functional units, the individual subunits of OATP1B3 in the oligomers are the functional units [31]. We are currently further investigating the functional consequences of the hetero-oligomerization as well as the underlying mechanism using HEK293 cells and human hepatocytes. One of the hypotheses that we are testing is that these transporters are co-localizing in plasma membrane micro domains, like lipid rafts, and that their expression and function could be affected by the plasma membrane cholesterol content, as has been indicated for rat NTCP [33] (Figure 1).
The expression and function of other OATPs, including the human OATP1A2 and OATP2B1 and several rodent OATPs, are also modulated by their interaction with other proteins, like PDZK1 [34–36]. In a recent study Wang et al. [37] reported that expression of the PDZ domain containing rat OATP1A1 is required for optimal expression of rat OATP1A4 which does not contain a PDZ domain. Using immunofluorescence and co-immunoprecipitation studies the authors could demonstrate that the two rat OATPs interacted with each other. Unlike OATP1B1 and OATP1B3 which are lacking a C-terminal PDZ consensus sequence, both OATP1A2 and OATP2B1 can interact with PDZK1 via their C-terminal PDZ domains. In all studies published so far, PDZK1 is involved in the correct trafficking of these transporters to the plasma membrane and in stabilizing them in the plasma membrane. In general, this will affect the expression level of the functional transporter at the membrane and thus the Vmax rather than the Km values of substrate transport are affected [34].
2.2. OCT1
The organic cation transporter 1 (OCT1; SLC22A1), a member of the SLC22A family, is mainly expressed in the liver at the basolateral membrane of hepatocytes. In addition, it is expressed at the basolateral membrane in human enterocytes and at the brush border membrane in the proximal tubule [38]. The physiological role of OCT1 is to facilitate the uptake of endo- and xenobiotics into hepatocytes for further metabolism and elimination. For example, OCT1 mediates the uptake of organic cations, including choline, several neurotransmitters, the model substrates tetraethylammonium (TEA) and 1-methyl-4-phenylpyridinium (MPP+), and numerous drugs, including metformin, into hepatocytes [38]. Several SNPs result in reduced OCT1 function that can lead to clinically significant increased drug exposure (AUC and Cmax) [39]. The oligomeric state of OCT1 was studied in 2008 using the rat protein [40]. Using a cell free expression system rat OCT1 was synthesized and reconstituted into proteoliposomes. In parallel, rat OCT2 and rat OAT1 were also reconstituted. The formation of OCT1 and OAT1 homo-oligomers was demonstrated by co-immunoprecipitation but no hetero-oligomers between the two transporters were identified [40] (Figure 1). The functional studies performed with the reconstituted OCT1 demonstrated that affinities for transported substrates were similar to values obtained in overexpressing HEK293 cells but non-transported inhibitors showed a 10- to 17-fold lower affinity in the proteoliposomes [40]. A possible explanation was the different lipid composition of the membranes. In another study the same group confirmed the formation of oligomers in X. laevis oocytes that were injected with rat OCT1 cRNA [41]. In addition, cysteine residues in extracellular loop 1 were replaced with serine residues, and as a consequence these mutants did not oligomerize anymore, suggesting that disulfide bonds are crucial for the dimerization of rat OCT1. Changes in substrate affinity were due to the mutations of the cysteine residues rather than the inability to oligomerize and these results were confirmed when substrate affinities were the same before and after treatment with dithiothreitol which breaks disulfide bonds and resulted in monomeric OCT1 [41]. Taken together, these studies demonstrated that rat OCT1 can form homo-oligomers and that oligomerization does not affect the transport function.
2.3. NTCP
NTCP (SLC10A1) belongs to SLC10 family of bile acid transporters. It is almost exclusively expressed at the basolateral membrane of hepatocytes where it plays a crucial role for the uptake of conjugated bile acids [42]. Its major physiological role is to maintain the enterohepatic circulation of bile acids by transporting circulating conjugated bile acids from portal blood into hepatocytes. Besides bile acids, NTCP can also transport several statins, estrone-3-sulfate and conjugate thyroid hormones [43]. Recent studies also demonstrated that human NTCP is the receptor for hepatitis B and D virus infection and as a consequence NTCP inhibitors are being tested as virus entry inhibitors to treat hepatitis B and D infections [44].
NTCP is another liver transporter that can form homo- and hetero-dimers [45, 46] (Figure 1). After identifying NTCP dimers in rat livers using cross-linkers, dimerization was also demonstrated using differently tagged human NTCP constructs and coimmunoprecipitation from transiently transfected U-2 OS cells. Co-expression of wild-type NTCP with a mutant that is retained in the ER resulted in retention of the wild-type protein in the ER, suggesting that dimerization occurs early during protein synthesis and processing in the secretory pathway. In addition, Bijsmans et al. [45] could demonstrate that NTCP also formed hetero-dimers with other members of the SLC10 family, namely with P4 (SLC10A4) and the sodium-dependent organic anion transporter (SOAT; SLC10A6), but not with P3 (SLC10A3), P5 (SLC10A5) and P7 (SLC10A7) [45]. Higher bands on western blots potentially corresponding to dimerized proteins were observed for other members of SLC10 family, including ASBT (SLC10A2), P4 and P5 [47, 48]. Co-expression of wild-type NTCP with a loss-of-function mutant of NTCP did not affect the Km and Vmax values and thus suggests that NTCP dimers function as individual functional units. However, the interaction between NTCP and P4 did reduce NTCP-mediated bile salt uptake [45]. In a more recent and more systematic study all human members of the SLC10 family were investigated for homo- and hetero-oligomerization [46]. Using a modified yeast-two-hybrid system strong homodimerization could be demonstrated for NTCP, ASBT and SLC10A7, while heterodimerization was observed for most of the family members with each other. Co-expression experiments in HEK293 cells using different tagged constructs demonstrated that NTCP-mediated taurocholate uptake was strongly reduced when co-expressed with SLC10A4 or SOAT but less affected by co-expression with SLC10A5 or SLC10A7 [46]. However, it is not clear how important these interactions are physiologically given the liver specific expression of NTCP and the fact that SOAT is not expressed in the liver. Taken together, these studies indicate that hetero- rather than homo-oligomerization has functional consequences, at least in the SLC10 family.
2.4. OSTα/β
In 2005, the heteromeric organic solute transporter OSTα-OSTβ (OSTα/β; SLC51A and SLC51B) was identified from mouse intestine and characterized as the basolateral bile acid transporter [49]. It is expressed in many tissues but most abundant in organs important for bile acid and steroid homeostasis like the liver, the small intestine and the kidneys [50]. OSTα/β plays an important role in the enterohepatic circulation of bile acids by mediating the efflux of bile acids out of enterocytes. In cholestatic liver disease it protects hepatocytes by facilitating the efflux of bile acids across the sinusoidal membrane to blood. OSTα/β is a unique transporter because it requires heterodimerization of two subunits encoded by the SLC51A and SLC51B genes for proper membrane expression and therefore proper function of this transporter [51] (Figure 1). Further characterization revealed that the 50 amino acids at the N-terminus of OSTα are crucial for the maintenance of heterodimerization, membrane expression and normal function of OSTα/β [51, 52]. While this is an example of a transporter that requires absolutely heterodimerization, additional protein interactions have so far not been described.
3. Protein interactions of transporters in the kidney
The kidneys are the other major organ for the elimination of drugs, mainly of smaller and water-soluble compounds. Transporters play also a vital role in the kidneys and are involved in the secretion and reabsorption of endo- and xenobiotics, mainly in the proximal tubule, but also throughout the nephron. Besides drugs that can be filtered in the glomerulus, most of the drugs need to be transported into the cells and then secreted into the lumen through drug transporters, including the organic anion transporters OAT1, OAT2, OAT3, the organic cation transporter 2 (OCT2) and OATP4C1. Similar to the liver, drug-drug interactions also occur at transporters in the kidneys [27]. Because such transporter-based drug-drug interactions can cause significant adverse effects and alter pharmacokinetic parameters, such as renal clearance, these transporters have been carefully characterized regarding their expression and function. Besides the well-known transcriptional and post-translational regulation, protein-protein interactions of renal transporters can also contribute to their physiological role.
3.1. OATs
Organic anion transporters 1 (OAT1; SLC22A6), OAT2 (SLC22A7) and OAT3 (SLC22A8) belong to the SLC22 family of solute carriers and are involved in the secretion of mainly small hydrophilic endo- and xenobiotics in the kidney [38, 53]. OAT1 and OAT3 are mainly expressed in the kidney at the basolateral side of proximal tubule cells. They work as anion-exchangers and transport mainly smaller hydrophilic substances including mono- and dicarboxylates, second messengers, bile salts, hormones and hormone derivatives, and numerous drugs like ACE inhibitors, antibiotics, anticancer drugs, diuretics, NSAIDs, and statins [51]. Unlike OATPs or OCTs where numerous SNPs have been identified, only few SNPs that change the amino acid sequence and thus affect the function have been identified in the SLC22A6 and SLC22A8 genes [38].
Human OAT1 was the first renal transporters identified to form homo-oligomers [54] (Figure 2). Initially detected as a double sized band on western blots and further characterized using gel filtration and immunoprecipitation of differently tagged proteins, homo-oligomerization was also confirmed in rat kidney [54]. In follow-up studies, the same group investigated the effect of co-transfecting OAT1 with short peptides corresponding to the different transmembrane domains (TMDs). They demonstrated that oligomerization of OAT1 was reduced in the presence of a TMD6 peptide, and cell membrane expression was decreased, leading to a reduction in the Vmax of substrate uptake [55]. In another study they demonstrated that the GXXXG motifs in TMD1 and 5 are important for OAT1 expression [56]. Mutation of glycine residues in the TMD2 GXXXG motif to alanine residues resulted in a complete loss of OAT1 expression at the plasma membrane while only mutation to the second glycine in the GXXXG motif in TMD5 reduced transporter expression at the membrane. These results could indicate that the GXXXG motifs may be involved in the oligomerization of OAT1 and thus contribute to correct membrane trafficking [56]. Although no specific studies have been published yet showing the oligomerization status of OAT3, western blots of rat OAT3 showed bands corresponding to double or triple size of the OAT3 monomer and were interpreted as possible multimeric forms of the protein [57]. Interestingly however, OAT3 can interact with cytoskeletal proteins, including β-actin and myosin, that are involved in the formation of lipid rafts and was found in caveolin-1 containing lipid raft domains isolated from rat kidney or from HEK293 cells expressing human OAT3 [58] (Figure 2). Furthermore, disruption of the cytoskeleton or depletion of cholesterol from rat renal cortical slices lead to a reduction of OAT3 transport function while cholesterol depletion did not affect organic cation transport [58]. Thus, these studies suggest that OAT3 expression and function does not only depend on transcriptional and post-translational regulation, but also on the cholesterol content of the plasma membrane which can vary depending on the individual’s health or disease states.
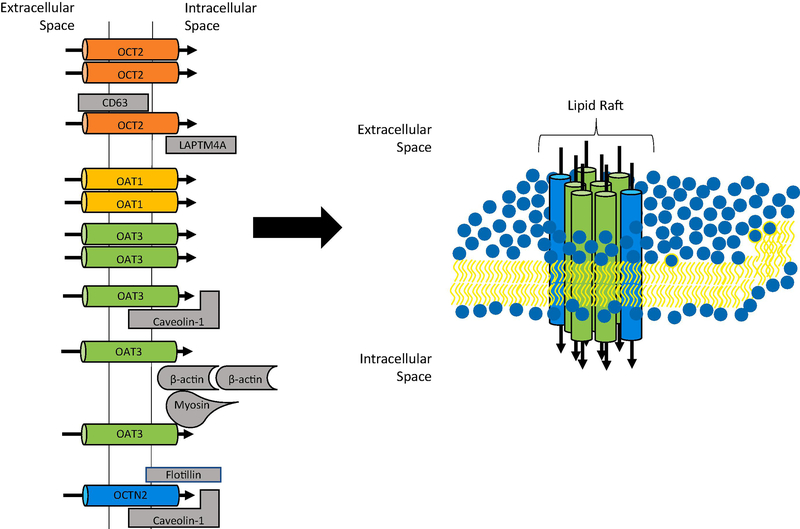
Figure 2: Illustration of the interactions among renal uptake transporters.
Published protein-protein interactions at the basolateral membrane of renal tubular epithelial cells are summarized on the left. A proposed lipid domain with transporters that have been shown to interact with caveolin is illustrated at right: OAT3 (green) and OCTN2 (blue).
3.2. OCT/OCTNs
Human OCT2 (SLC22A2) is mainly expressed at the basolateral membrane in the proximal tubule and represents the major organic cation transporters expressed in the kidney [38]. Besides the kidney, OCT2 expression was also detected in the small intestine, lung, placenta, thymus, brain, and inner ear. It transports numerous endogenous compounds including neurotransmitters and neuromodulators, but also various cationic drugs, such as cisplatin, oxaliplatin and metformin [38]. Similarly to OATs, the cationic OCT2 substrates are smaller and more hydrophilic as compared to compounds that are excreted via bile. Physiologically, OCT2 mediates the first step of organic cation excretion in the kidney by mediating their uptake across the basolateral membrane into the cells in the proximal tubule. MATE1 and MATE2K then excrete these cations across the brush border membranes into the tubular lumen. Pharmacologically, OCT2-mediated uptake of cisplatin can lead to nephrotoxicity and therefore selective OCT2 inhibitors might reduce the renal concentrations of cisplatin and thus reduce its adverse effect.
Regarding its potential oligomerization, two studies indicated that OCT2 forms homodimers [59, 60] (Figure 2). Using a two-hybrid system that works for membrane proteins and Fluorescent Resonance Energy Transfer (FRET) between OCT2-CFP and OCT2-YFP, Brast et al. [59] demonstrated that OCT2 can interact with itself. Truncation experiments narrowed the interaction sites to amino acids between position 50 and 152 but the nearby GXXXG motif does not seem to be involved. This oligomerization also occurs in vivo in the proximal tubule and the large extracellular loop between TMD1 and 2 seems to be involved [59]. An in silico study recently predicted that the above mentioned FRET interactions would only be possible in a dimeric but not a trimeric model, because only in the dimeric model the amino- and carboxy-terminal amino acids would be close enough for energy transfer [60]. In addition to the homo-oligomerization, OCT2 also interacts with other proteins. The Ciarimboli group identified the lysosomal-associated protein transmembrane 4 alpha (LAPTM4A) and CD63 as interacting partners of OCT2 (Figure 2), as well as of OCT1 and OCT3 [61, 62]. These interactions between OCT2 and LAPTM4A or CD63 were first predicted by a modified yeast two-hybrid system, the split-ubiquitin system and then confirmed using co-immunoprecipitation, co-localization and FRET. Interaction with LAPTM4A occurs in lysosomes and late endosomes, and seems to lead to degradation of OCT2 via endocytosis [61]. Interaction with CD63, which is localized in the endosomal system and at the plasma membrane, seems to be important for proper expression of OCT2 at the plasma membrane and mainly affected transporter trafficking [62].
OCTN2 (SLC22A5) is a high affinity sodium-dependent L-carnitine cotransporter. It is expressed in numerous tissues including the liver, the kidney, skeletal muscles, heart, and small intestine. Based on studies with knockout mice, absence of functional OCTN2 could lead to systemic carnitine deficiency [38]. While no oligomeric studies have been reported so far, immunoprecipitation experiments with rat astrocytes demonstrated that OCTN2 interacts with caveolin-1 and flotillin-1, two proteins found in lipid rafts [63] (Figure 2). Thus, like OAT3, OCTN2 seems to reside in cholesterol rich plasma membrane microdomains where it may interact with other proteins (Figure 2). Furthermore, its expression and thus function could also be regulated by the amount of cholesterol in the plasma membrane.
4. Conclusion and future direction
Drug uptake transporters in the liver and the kidneys form an efficient but complex system to eliminate endogenous compounds and xenobiotics. The FDA requires that some of these transporters are tested for drug interactions, based on the recommendations of the International Transporter Consortium [27, 30]. One of the major difficulties is to determine the contribution of each transporter in the pharmacokinetics of a certain drug given the sometimes overlapping substrate specificities. For example, OATP1B3 transports a broad spectrum of structurally independent chemicals, including both conjugated and unconjugated bile acids, hormones and drugs like statins, anti-hypertensives and antibiotics [64]. However, only a small portion of its substrates are transported by OATP1B3 selectively, including CCK-8 and telmisartan [65, 66]. Many of the other substrates are shared with other transporters from different solute carrier families. This makes an in vivo study of individual transporters challenging. Furthermore, transporters are typically characterized using cell-based assays where a single transporter is transiently or stably expressed in a cell line. These cell lines are then used to perform uptake experiments to characterize the function of the expressed transporter and to investigate potential drug-drug interactions [67–69]. This is an efficient method to determine substrates, inhibitors and stimulators of transporters, and it can provide important information for drug development. For example, the drug interactions between grape fruit juice and fexofenadine were discovered using such cell based models and the results eventually affected the recommendations on how to take fexofenadine [70, 71]. However, the pharmacokinetic parameters generated by using an isolated in vitro study may not always predict the situation in vivo well. For example, to build physiologically based pharmacokinetic (PBPK) models for in vitro-in vivo extrapolations (IVIVE) involving transporters, relative activity factors (RAF) need to be used to compensate for Vmax (or Jmax) differences between the in vitro and in vivo environment [72]. Although studies have been performed to try to explain the physiological meaning of RAFs [73], they remain a black box because of the complexity of the in vivo situation. Protein-protein interactions between or among transporters may be a part of the black box of RAFs, and therefore a thorough understanding of these interactions could help improving the IVIVE.
In this review, we summarized recent studies describing oligomerization of drug uptake transporters, which might make the already complex situation even more challenging. As discussed, studies have shown that many of the drug transporters can form homo- or hetero-oligomers. More interestingly, it seems that most of these interactions do not change the affinity of their substrates, but do lead to a significant change of the turnover rate and membrane expression levels. No direct studies have been performed to extrapolate these in vitro observations into animal models or clinical studies. During in vitro cultivation of human hepatocytes, the uptake function of OATP1B3 increased with culture time [66], while the function of OCT1 decreased [74]. It is currently not known how the OCT1 function changes and whether it could affect OATP1B3 function, but one possibility is that the increased OATP1B3 uptake is due to an increased expression level because of the decrease of OCT1 expression. We have preliminary data from studies using transient expression of OATP1B3 together with OCT1 in HEK293 cells which support such a mechanism, but additional experiments using shRNA and primary human hepatocytes are required to demonstrate that this mechanism indeed works in vivo.
Studies of OATP1B1 [32] and OAT1 [56] along with previous reports on other transporters and receptors suggest that the GXXXG motifs could affect the formation of oligomers and the function of transporters. It is well possible that additional drug transporters, including efflux transporters of the ABC family that form homo- or hetero-oligomers use the same mechanisms. For example, the GXXXG motifs in OATP1B1 are highly conserved in other OATPs and similarly, the GXXXG motifs found in OAT1 are also conserved among other OATs. Additional studies of the mechanisms of oligomerization and the functional consequences will provide insight into a different level of transporter regulation and will lead to a better understanding of the interactions within or between transporters.
To conclude, although more and more protein interactions of drug transporters in the liver and the kidney are being discovered, the mechanisms of oligomerization and the consequences for their in vivo function are still not well known. However, based on the current way in vitro transporter studies are performed with cell lines that express a single transporter and thus lack hetero-oligomerization with other transporters, we could well under- or over-estimate certain transport parameters, such as kcat or Km values. Thus, a full characterization of protein-protein interactions and their functional consequences are needed to explain some of the prediction errors when applying the currently used transport parameters to the PBPK models. A better understanding of the oligomerization of transporters will increase the accuracy of in vitro to in vivo extrapolation, and is therefore needed to develop better in vivo transporter models.
Table 1:
Summary of drug uptake transporters involved in protein-protein interaction
| Transporter | Interaction Partner | Functional Change?* | Expression Change?* |
|---|---|---|---|
| NTCP | NTCP | No | N/A |
| SOAT | ↓ | ↓ | |
| OATP1B1 | OATP1B1 | ↓ | ↓ |
| OATP1B3 | OATP1B3 | No | No |
| OATP1B1 | N/A | N/A | |
| NTCP | N/A | N/A | |
| OSTα | OSTβ | Required for function | Required for membrane expression |
| OCT1 | OCT1 | No | N/A |
| OCT2 | OCT2 | N/A | N/A |
| LAPTM4A | N/A | N/A | |
| CD63 | N/A | N/A | |
| OAT1 | OAT1 | ↓ | ↓ |
| OAT3 | OAT3 | N/A | N/A |
| Caveolin-1 | ↑ due to gain of protein-cytoskeletal interaction | Redistribution to non-LRD-rich compartments | |
| β-actin | |||
| Myosin | |||
| OCTN2 | Caveolin-1 | N/A | N/A |
| Flotillin | N/A | N/A |
Functional and expression change means that the potential changes happened to the target transporter by the interactions with the partner transporters. Arrows represent the increase (↑) or decrease (↓) of function or membrane expression.
Acknowledgements
This work was supported by a National Institutes of Health grant (R01GM077336) to B.H.
Footnotes
Conflict of interest
The authors do not have any conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hediger MA, Clemencon B, Burrier RE, Bruford EA, The ABCs of membrane transporters in health and disease (SLC series): introduction, Molecular aspects of medicine 34(2–3) (2013) 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nigam SK, What do drug transporters really do?, Nature reviews. Drug discovery 14(1) (2015) 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kosters A, Karpen SJ, Bile acid transporters in health and disease, Xenobiotica; the fate of foreign compounds in biological systems 38(7–8) (2008) 1043–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wright EM, Glucose transport families SLC5 and SLC50, Molecular aspects of medicine 34(2–3) (2013) 183–96. [DOI] [PubMed] [Google Scholar]
- [5].Wright EM, Turk E, Martin MG, Molecular basis for glucose-galactose malabsorption, Cell Biochem Biophys 36(2–3) (2002) 115–21. [DOI] [PubMed] [Google Scholar]
- [6].Kalliokoski A, Niemi M, Impact of OATP transporters on pharmacokinetics, Br J Pharmacol 158(3) (2009) 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Konig J, Muller F, Fromm MF, Transporters and drug-drug interactions: important determinants of drug disposition and effects, Pharmacological reviews 65(3) (2013) 944–66. [DOI] [PubMed] [Google Scholar]
- [8].Lin L, Yee SW, Kim RB, Giacomini KM, SLC transporters as therapeutic targets: emerging opportunities, Nature reviews. Drug discovery 14(8) (2015) 543–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, Stindt J, Koniger C, Nassal M, Kubitz R, Sultmann H, Urban S, Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes, Gastroenterology 146(4) (2014) 1070–83. [DOI] [PubMed] [Google Scholar]
- [10].Schulze A, Schieck A, Ni Y, Mier W, Urban S, Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction, Journal of virology 84(4) (2010) 1989–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dawson PA, Role of the intestinal bile acid transporters in bile acid and drug disposition, Handb Exp Pharmacol (201) (2011) 169–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hashimoto K, Nishi H, Bryant S, Panchenko AR, Caught in self-interaction: evolutionary and functional mechanisms of protein homooligomerization, Phys Biol 8(3) (2011) 035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kotlyar M, Pastrello C, Malik Z, Jurisica I, IID 2018 update: context-specific physical protein-protein interactions in human, model organisms and domesticated species, Nucleic Acids Res 47(D1) (2019) D581–D589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Garcia-Seisdedos H, Villegas JA, Levy ED, Infinite Assembly of Folded Proteins in Evolution, Disease, and Engineering, Angew Chem Int Ed Engl 58(17) (2019) 5514–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stumpf MP, Thorne T, de Silva E, Stewart R, An HJ, Lappe M, Wiuf C, Estimating the size of the human interactome, Proc Natl Acad Sci U S A 105(19) (2008) 6959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ali MH, Imperiali B, Protein oligomerization: how and why, Bioorganic & medicinal chemistry 13(17) (2005) 5013–20. [DOI] [PubMed] [Google Scholar]
- [17].Hall RA, Lefkowitz RJ, Regulation of G protein-coupled receptor signaling by scaffold proteins, Circ Res 91(8) (2002) 672–80. [DOI] [PubMed] [Google Scholar]
- [18].Sokolina K, Kittanakom S, Snider J, Kotlyar M, Maurice P, Gandia J, Benleulmi-Chaachoua A, Tadagaki K, Oishi A, Wong V, Malty RH, Deineko V, Aoki H, Amin S, Yao Z, Morato X, Otasek D, Kobayashi H, Menendez J, Auerbach D, Angers S, Przulj N, Bouvier M, Babu M, Ciruela F, Jockers R, Jurisica I, Stagljar I, Systematic protein-protein interaction mapping for clinically relevant human GPCRs, Mol Syst Biol 13(3) (2017) 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roche JV, Tornroth-Horsefield S, Aquaporin Protein-Protein Interactions, Int J Mol Sci 18(11) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krishnan D, Liu L, Wiebe SA, Casey JR, Cordat E, Alexander RT, Carbonic anhydrase II binds to and increases the activity of the epithelial sodium-proton exchanger, NHE3, Am J Physiol Renal Physiol 309(4) (2015) F383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H, Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98), The Journal of biological chemistry 273(37) (1998) 23629–32. [DOI] [PubMed] [Google Scholar]
- [22].Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F, Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family, Nature 395(6699) (1998) 288–91. [DOI] [PubMed] [Google Scholar]
- [23].Fotiadis D, Kanai Y, Palacin M, The SLC3 and SLC7 families of amino acid transporters, Molecular aspects of medicine 34(2–3) (2013) 139–58. [DOI] [PubMed] [Google Scholar]
- [24].Napolitano L, Scalise M, Galluccio M, Pochini L, Albanese LM, Indiveri C, LAT1 is the transport competent unit of the LAT1/CD98 heterodimeric amino acid transporter, Int J Biochem Cell Biol 67 (2015) 25–33. [DOI] [PubMed] [Google Scholar]
- [25].Yan R, Zhao X, Lei J, Zhou Q, Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex, Nature 568(7750) (2019) 127–130. [DOI] [PubMed] [Google Scholar]
- [26].Hafliger P, Charles RP, The L-Type Amino Acid Transporter LAT1-An Emerging Target in Cancer, Int J Mol Sci 20(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L, Membrane transporters in drug development, Nature reviews. Drug discovery 9(3) (2010) 215–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hagenbuch B, Stieger B, The SLCO (former SLC21) superfamily of transporters, Molecular aspects of medicine 34(2–3) (2013) 396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van de Steeg E, Stranecky V, Hartmannova H, Noskova L, Hrebicek M, Wagenaar E, van Esch A, de Waart DR, Oude Elferink RP, Kenworthy KE, Sticova E, al-Edreesi M, Knisely AS, Kmoch S, Jirsa M, Schinkel AH, Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver, The Journal of clinical investigation 122(2) (2012) 519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].FDA, In Vitro Metabolism and Transporter-Mediated Drug-Drug Interaction Studies: Guidance for Industry, 2017.
- [31].Zhang Y, Boxberger KH, Hagenbuch B, Organic anion transporting polypeptide 1B3 can form homo- and hetero-oligomers, PLoS One 12(6):e0180257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ni C, Yu X, Fang Z, Huang J, Hong M, Oligomerization Study of Human Organic Anion Transporting Polypeptide 1B1, Molecular pharmaceutics 14(2) (2017) 359–367. [DOI] [PubMed] [Google Scholar]
- [33].Molina H, Azocar L, Ananthanarayanan M, Arrese M, Miquel JF, Localization of the Sodium-Taurocholate cotransporting polypeptide in membrane rafts and modulation of its activity by cholesterol in vitro, Biochimica et biophysica acta 1778(5) (2008) 1283–91. [DOI] [PubMed] [Google Scholar]
- [34].Ferreira C, Hagen P, Stern M, Hussner J, Zimmermann U, Grube M, Meyer Zu Schwabedissen HE, The scaffold protein PDZK1 modulates expression and function of the organic anion transporting polypeptide 2B1, European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 120 (2018) 181–190. [DOI] [PubMed] [Google Scholar]
- [35].Zheng J, Chan T, Cheung FS, Zhu L, Murray M, Zhou F, PDZK1 and NHERF1 regulate the function of human organic anion transporting polypeptide 1A2 (OATP1A2) by modulating its subcellular trafficking and stability, PLoS One 9(4) (2014) e94712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang P, Wang JJ, Xiao Y, Murray JW, Novikoff PM, Angeletti RH, Orr GA, Lan D, Silver DL, Wolkoff AW, Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface, The Journal of biological chemistry 280(34) (2005) 30143–9. [DOI] [PubMed] [Google Scholar]
- [37].Wang P, Wang WJ, Choi-Nurvitadhi J, Lescaille Y, Murray JW, Wolkoff AW, Rat Organic Anion Transport Protein 1A1 Interacts Directly With Organic Anion Transport Protein 1A4 Facilitating Its Maturation and Trafficking to the Hepatocyte Plasma Membrane, Hepatology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Koepsell H, The SLC22 family with transporters of organic cations, anions and zwitterions, Molecular aspects of medicine 34(2–3) (2013) 413–35. [DOI] [PubMed] [Google Scholar]
- [39].Wagner DJ, Hu T, Wang J, Polyspecific organic cation transporters and their impact on drug intracellular levels and pharmacodynamics, Pharmacol Res 111 (2016) 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Keller T, Schwarz D, Bernhard F, Dotsch V, Hunte C, Gorboulev V, Koepsell H, Cell free expression and functional reconstitution of eukaryotic drug transporters, Biochemistry 47(15) (2008) 4552–64. [DOI] [PubMed] [Google Scholar]
- [41].Keller T, Egenberger B, Gorboulev V, Bernhard F, Uzelac Z, Gorbunov D, Wirth C, Koppatz S, Dotsch V, Hunte C, Sitte HH, Koepsell H, The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization, The Journal of biological chemistry 286(43) (2011) 37874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Claro da Silva T, Polli JE, Swaan PW, The solute carrier family 10 (SLC10): beyond bile acid transport, Molecular aspects of medicine 34(2–3) (2013) 252–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Visser WE, Wong WS, van Mullem AA, Friesema EC, Geyer J, Visser TJ, Study of the transport of thyroid hormone by transporters of the SLC10 family, Mol Cell Endocrinol 315(1–2) (2010) 138–45. [DOI] [PubMed] [Google Scholar]
- [44].Eller C, Heydmann L, Colpitts CC, Verrier ER, Schuster C, Baumert TF, The functional role of sodium taurocholate cotransporting polypeptide NTCP in the life cycle of hepatitis B, C and D viruses, Cell Mol Life Sci 75(21) (2018) 3895–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bijsmans IT, Bouwmeester RA, Geyer J, Faber KN, van de Graaf SF, Homo- and hetero-dimeric architecture of the human liver Na(+)-dependent taurocholate co-transporting protein, The Biochemical journal 441(3) (2012) 1007–15. [DOI] [PubMed] [Google Scholar]
- [46].Noppes S, Muller SF, Bennien J, Holtemeyer M, Palatini M, Leidolf R, Alber J, Geyer J, Homo- and heterodimerization is a common feature of the solute carrier family SLC10 members, Biol Chem (2019). [DOI] [PubMed] [Google Scholar]
- [47].Fernandes CF, Godoy JR, Doring B, Cavalcanti MC, Bergmann M, Petzinger E, Geyer J, The novel putative bile acid transporter SLC10A5 is highly expressed in liver and kidney, Biochemical and biophysical research communications 361(1) (2007) 26–32. [DOI] [PubMed] [Google Scholar]
- [48].Geyer J, Fernandes CF, Doring B, Burger S, Godoy JR, Rafalzik S, Hubschle T, Gerstberger R, Petzinger E, Cloning and molecular characterization of the orphan carrier protein Slc10a4: expression in cholinergic neurons of the rat central nervous system, Neuroscience 152(4) (2008) 990–1005. [DOI] [PubMed] [Google Scholar]
- [49].Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N, The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter, The Journal of biological chemistry 280(8) (2005) 6960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ballatori N, Christian WV, Wheeler SG, Hammond CL, The heteromeric organic solute transporter, OSTalpha-OSTbeta/SLC51: a transporter for steroid-derived molecules, Molecular aspects of medicine 34(2–3) (2013) 683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sun AQ, Balasubramaniyan N, Xu K, Liu CJ, Ponamgi VM, Liu H, Suchy FJ, Protein-protein interactions and membrane localization of the human organic solute transporter, American journal of physiology. Gastrointestinal and liver physiology 292(6) (2007) G1586–93. [DOI] [PubMed] [Google Scholar]
- [52].Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL, OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids, Frontiers in bioscience 14 (2009) 2829–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Burckhardt G, Drug transport by Organic Anion Transporters (OATs), Pharmacol Ther 136(1) (2012) 106–30. [DOI] [PubMed] [Google Scholar]
- [54].Hong M, Xu W, Yoshida T, Tanaka K, Wolff DJ, Zhou F, Inouye M, You G, Human organic anion transporter hOAT1 forms homooligomers, The Journal of biological chemistry 280(37) (2005) 32285–90. [DOI] [PubMed] [Google Scholar]
- [55].Duan P, Li S, You G, Transmembrane peptide as potent inhibitor of oligomerization and function of human organic anion transporter 1, Molecular pharmacology 79(3) (2011) 569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Duan P, Wu J, You G, Mutational analysis of the role of GXXXG motif in the function of human organic anion transporter 1 (hOAT1), International journal of biochemistry and molecular biology 2(1) (2011) 1–7. [PMC free article] [PubMed] [Google Scholar]
- [57].Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I, Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition, Am J Physiol Renal Physiol 287(1) (2004) F124–38. [DOI] [PubMed] [Google Scholar]
- [58].Srimaroeng C, Cecile JP, Walden R, Pritchard JB, Regulation of renal organic anion transporter 3 (SLC22A8) expression and function by the integrity of lipid raft domains and their associated cytoskeleton, Cell Physiol Biochem 31(4–5) (2013) 565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Brast S, Grabner A, Sucic S, Sitte HH, Hermann E, Pavenstadt H, Schlatter E, Ciarimboli G, The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 26(3) (2012) 976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sajib AA, Islam T, Paul N, Yeasmin S, Interaction of rs316019 variants of SLC22A2 with metformin and other drugs-an in silico analysis, Journal of Genetic Engineering and Biotechnology %@ 1687–157X (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Grabner A, Brast S, Sucic S, Bierer S, Hirsch B, Pavenstadt H, Sitte HH, Schlatter E, Ciarimboli G, LAPTM4A interacts with hOCT2 and regulates its endocytotic recruitment, Cell Mol Life Sci 68(24) (2011) 4079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schulze U, Brast S, Grabner A, Albiker C, Snieder B, Holle S, Schlatter E, Schroter R, Pavenstadt H, Herrmann E, Lambert C, Spoden GA, Florin L, Saftig P, Ciarimboli G, Tetraspanin CD63 controls basolateral sorting of organic cation transporter 2 in renal proximal tubules, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 31(4) (2017) 1421–1433. [DOI] [PubMed] [Google Scholar]
- [63].Czeredys M, Samluk L, Michalec K, Tulodziecka K, Skowronek K, Nalecz KA, Caveolin-1--a novel interacting partner of organic cation/carnitine transporter (Octn2): effect of protein kinase C on this interaction in rat astrocytes, PLoS One 8(12) (2013) e82105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Stieger B, Hagenbuch B, Organic anion-transporting polypeptides, Curr Top Membr 73 (2014) 205–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Roth M, Obaidat A, Hagenbuch B, OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies, Br J Pharmacol 165(5) (2012) 1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhu Q, Xia H, Xia CQ, Yang Q, Doshi U, Li AP, Liao M, Culture duration-, donor-, and medium-dependent changes in OATP1B3-mediated telmisartan uptake in human hepatocytes, Drug Metab Lett 7(2) (2014) 117–25. [DOI] [PubMed] [Google Scholar]
- [67].Zhao W, Zitzow JD, Ehresman DJ, Chang SC, Butenhoff JL, Forster J, Hagenbuch B, Na+/Taurocholate Cotransporting Polypeptide and Apical Sodium-Dependent Bile Acid Transporter Are Involved in the Disposition of Perfluoroalkyl Sulfonates in Humans and Rats, Toxicol Sci 146(2) (2015) 363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bednarczyk D, Boiselle C, Organic anion transporting polypeptide (OATP)-mediated transport of coproporphyrins I and III, Xenobiotica; the fate of foreign compounds in biological systems 46(5) (2016) 457–66. [DOI] [PubMed] [Google Scholar]
- [69].De Bruyn T, van Westen GJ, Ijzerman AP, Stieger B, de Witte P, Augustijns PF, Annaert PP, Structure-based identification of OATP1B1/3 inhibitors, Molecular pharmacology 83(6) (2013) 1257–67. [DOI] [PubMed] [Google Scholar]
- [70].Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB, Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine, Clinical pharmacology and therapeutics 71(1) (2002) 11–20. [DOI] [PubMed] [Google Scholar]
- [71].Bailey DG, Fruit juice inhibition of uptake transport: a new type of food-drug interaction, British journal of clinical pharmacology 70(5) (2010) 645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Harwood MD, Neuhoff S, Carlson GL, Warhurst G, Rostami-Hodjegan A, Absolute abundance and function of intestinal drug transporters: a prerequisite for fully mechanistic in vitro-in vivo extrapolation of oral drug absorption, Biopharmaceutics & drug disposition 34(1) (2013) 2–28. [DOI] [PubMed] [Google Scholar]
- [73].Kumar V, Yin J, Billington S, Prasad B, Brown CDA, Wang J, Unadkat JD, The Importance of Incorporating OCT2 Plasma Membrane Expression and Membrane Potential in IVIVE of Metformin Renal Secretory Clearance, Drug metabolism and disposition: the biological fate of chemicals 46(10) (2018) 1441–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liao M, Zhu Q, Zhu A, Gemski C, Ma B, Guan E, Li AP, Xiao G, Xia CQ, Comparison of uptake transporter functions in hepatocytes in different species to determine the optimal model for evaluating drug transporter activities in humans, Xenobiotica; the fate of foreign compounds in biological systems 49(7) (2019) 852–862. [DOI] [PubMed] [Google Scholar]