To the Editor:
Penicillin allergy evaluations are important for optimal patient care and public health.1 Practical outpatient strategies to confirm or rule out penicillin allergy are needed to assess as many patients as possible, as safely as possible.2 Although a validated penicillin skin test exists, low-risk penicillin allergy patients can be evaluated by direct amoxicillin challenge without skin testing.2–4
An outpatient pathway for penicillin allergy risk stratification was implemented in January 2017 (Figure 1A). We retrospectively reviewed demographics and allergy history, evaluation detail, and allergy documentation for patients seen for a penicillin allergy procedure from January 2017 through June 2018. We considered the following outcomes: adverse drug reactions (ADRs, any symptoms or signs reported or identified by the treating allergist), hypersensitivity reactions (HSRs, ADRs with objective allergic signs or those precipitating use of anti-allergy treatment), and non-HSRs, side effect reactions or subjective symptoms. HSRs were judged retrospectively by two allergy specialists (KGB, AB) independently and blinded to the penicillin evaluation method used. Descriptive data were presented with chi-squared or Fisher’s exact tests for univariable analyses; logistic regression models were used for multivariable analyses.
Figure 1.
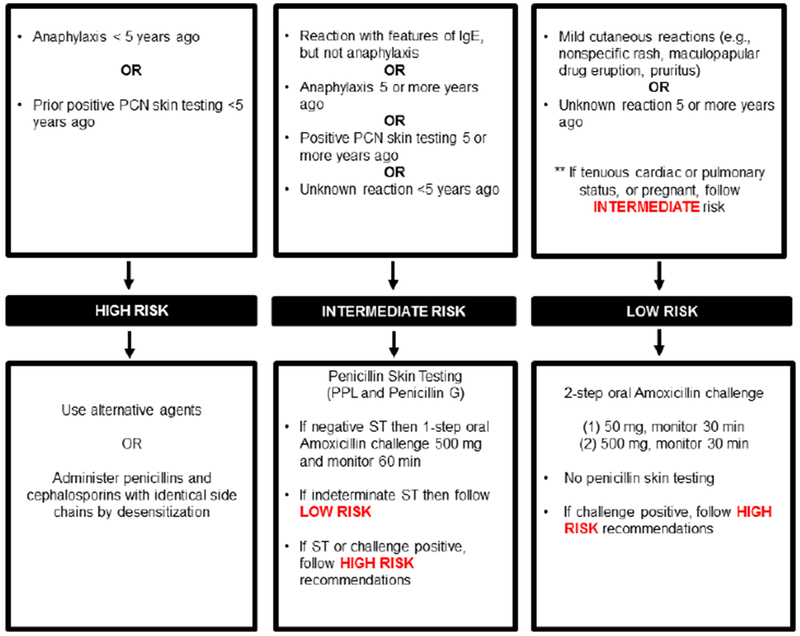
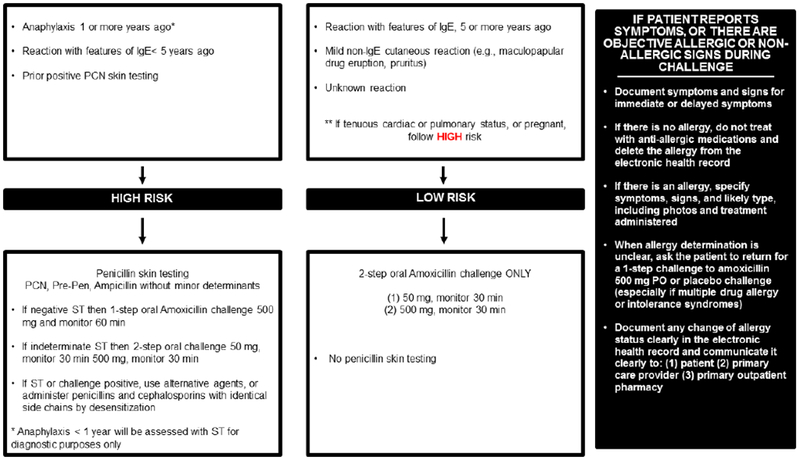
Risk-based pathway for outpatient penicillin allergy evaluations
This penicillin allergy pathway was implemented by allergists, allergy trainees, and an allergy nurse practitioner in 2017 at Massachusetts General Hospital (A). This penicillin allergy pathway was implemented in 2019 at Massachusetts General Hospital (B)
The figures demonstrate the evaluation recommended for all patients presenting for penicillin allergy evaluation whose reaction was possibly IgE-mediated. This clinical pathway does not apply to patients who have a history of a severe cutaneous adverse reaction (such as Stevens-Johnson syndrome, toxic epidermal necrolysis, drug-induced exfoliative dermatitis, drug reaction with eosinophilia and systemic symptoms, or acute generalized exanthematous pustulosis), vasculitis, interstitial nephritis, or hemolytic anemia. Features of IgE included urticaria, angioedema, wheezing/bronchospasm/shortness of breath, and anaphylaxis. Anaphylaxis was defined as reactions that involve two organ systems, or hypotension/arrhythmias.
Abbreviations: PCN, penicillin; IgE, immunoglobulin E; PPL, penicilloyl-polylysine; ST, skin test
Of 509 patients, 426 (83.7%) were penicillin skin tested and 83 (16.3%) received direct amoxicillin challenges. Patients included 486 (95.5%) adults (mean 51.4y [SD 17.2y], range 18-87 y) and 23 (4.5%) children (mean age 8.4y [SD 4.3], range 1.5-17y). Patients were predominantly female (72.7%) and white (86.0%). 184 patients (36.2%) had only a recorded penicillin allergy; 114 patients (22.4%) had one other recorded drug allergy, 73 patients (14.3%) had two other recorded drug allergies, 138 patients (27.1%) had three or more other drug allergies. For 126 patients (24.9%) the culprit was an aminopenicillin. Incident reactions were largely cutaneous (78.8%), such as rash (43.6%) and urticaria (34.2%); angioedema (9.4%) and respiratory symptoms (6.1%) were also reported. 55 patients (10.8%) had an unknown penicillin reaction. Of 408 reactions with known timing, 371 (72.9%) occurred 10 or more years ago and 37 (7.3%) occurred in the year prior to allergy evaluation.
Of 509 patients evaluated, 43 (8.5%; 95%CI 6.2% to 11.2%) had an ADR; 26 patients (5.1%; 95% CI 3.4% to 7.4%) had an HSR and 17 (3.3%; 95%CI 2.0% to 5.3%) had a non-HSR (Table 1). Of 426 penicillin skin tested patients, 62 (14.6%) were pre-cardiac surgery and 30 (7.0%) were pregnant. No patient had a positive skin test. There were 36 ADRs (8.5%, 95%CI 6.0% to 11.5%); 21 were HSRs (4.9%, 95%CI 3.1% to 7.4%) and 15 were non-HSRs (3.5%; 95%CI 2.0% to 5.7%). Of 83 (16.3%) patients who received direct amoxicillin challenges, 7 had ADRs (8.4%, 95%CI 3.5% to 16.6); 5 were HSRs (6.0%; 95%CI 2.0% to 13.5%) and 2 were non-HSRs (2.4%; 95%CI 0.3% to 8.4%). There was no difference in reaction frequency (p=0.59), epinephrine use (p=0.30), or subjective symptom frequency (p=1.0) comparing penicillin allergy evaluation methods.
Table 1.
Reactions resulting from penicillin allergy evaluations
| Number (%) | All patients (n=509) | Penicillin skin tested patients (n=426) | Direct amoxicillin challenge patients (n=83) | P-value* |
|---|---|---|---|---|
| Adverse drug reactions | 43 (8.5) | 36 (8.5) | 7 (8.4) | 1.0 |
| Hypersensitivity reactions | 26 (5.1) | 21 (4.9) | 5 (6.0) | 0.59 |
| Timing | ||||
| Immediate reaction† | 18 (3.5) | 15 (3.5) | 3 (3.6) | 1.0 |
| Delayed reaction | 8 (1.6) | 6 (1.4) | 2 (2.4) | 0.62 |
| Signs and symptoms‡ | 0.64 | |||
| Itching | 13 (2.6) | 10 (2.4) | 3 (3.6) | 0.45 |
| Rash | 10 (2.0) | 9 (2.1) | 1 (1.2) | 1.00 |
| Erythema | 9(1.8) | 7 (1.6) | 2 (2.4) | 0.64 |
| Flushing | 3 (0.6) | 2 (0.5) | 1 (1.2) | 0.41 |
| Difficulty swallowing | 3 (0.6) | 3 (0.7) | 0 (0.0) | 1.0 |
| Gastrointestinal symptoms | 2 (0.4) | 1 (0.2) | 1 (1.2) | 0.30 |
| Hives | 1 (0.2) | 0 (0.0) | 1 (1.2) | 0.16 |
| Other§ | 6 (1.2) | 5 (1.2) | 1 (1.2) | 1.0 |
| Treatment Administered | ||||
| Antihistamines | 16 (3.1) | 12 (2.8) | 4 (4.8) | 0.31 |
| Corticosteroids | 4 (0.8) | 2 (0.5) | 2 (2.4) | 0.13 |
| Epinephrine | 2 (0.4) | 1 (0.2)‖ | 1 (1.2)¶ | 0.30 |
| Non-hypersensitivity reactions (side effect reactions or subjective symptoms) | 17 (3.3) | 15 (3.5) | 2 (2.4) | 1.0 |
| Signs and symptoms‡ | ||||
| Itching | 9 (1.8) | 8 (1.9) | 1 (1.2) | 1.0 |
| Difficulty swallowing | 5 (1.0) | 4 (0.9) | 1 (1.2) | 0.59 |
| Chest tightness | 2 (0.4) | 2 (0.5) | 0 (0.0) | 1.0 |
| Flushing | 1 (0.2) | 1 (0.2) | 0 (0.0) | 1.0 |
| Gastrointestinal symptoms | 1 (0.2) | 0 (0.0) | 1 (1.2) | 0.16 |
| Allergy documentation | ||||
| Allergy label removed, Initial | 482 (94.7) | 405 (95.1) | 77 (92.8) | 0.42 |
| Allergy label removed, 6 months | 461 (90.6) | 385 (90.4) | 76 (91.6) | 0.73 |
| Erroneous reentry, 6 months | 10 (2.0) | 10 (2.4) | 0 (0.0) | 0.31 |
Chi-squared or Fisher’s Exact test (when expected cell number was less than 5).
Within 4 hours.
Patients could have more than one sign or symptom.
Other included chills, dizziness, headache, lightheadedness, hypertension, and pallor.
Patient had a penicillin rash history and developed dizziness, nausea, erythema, facial pallor and hypertension (155/85mmHg) 10 minutes after ingesting amoxicillin 500mg. Treatments included 0.3 mg IM epinephrine, 25mg diphenhydramine PO, and 40mg prednisone PO. A second dose of epinephrine IM was given for rigors and throat tightness, and symptoms improved 90 minutes later.
Patient had a history of idiopathic anaphylaxis and a remote rash to penicillin. Fifteen minutes after the 500mg dose of amoxicillin, facial hives and hypertension (157/93 mmHg) developed and progressed to involve throat itching and abdominal pain despite cetirizine 20mg PO. Treatment with 0.3 mg IM epinephrine resulted in symptom resolution over the next hour.
Abbreviations: IM, intramuscular; PO per orem
Female sex was associated with increased ADR odds (aOR 2.67 [95% CI 1.00 to 7.10], Table E1). Having other recorded drug allergies was associated with increased ADR, HSR, and non-HSR odds.
The penicillin allergy label was removed for 482 (94.7%) patients overall: 405 (95.1%) penicillin skin tested patients and 77 (92.8%) patients who were direct amoxicillin challenged (p=0.42). Penicillin allergy labels were not removed in 11 non-ADR patients due to allergist error (n=9), need for piperacillin testing (n=1), and patient preference (n=1). Six months after penicillin allergy testing, the penicillin allergy remained removed in 461 (90.6%) patients with no difference by evaluation method used (p=0.73). There were 10 patients (2.0%) erroneously relabeled with a penicillin allergy after initial label removal.
We used a penicillin allergy evaluation pathway for outpatient risk stratification at an academic medical center’s large allergy practice that includes high risk referrals from cardiothoracic surgery and obstetrics. HSRs occurred in 5% overall (3% immediate), with other signs and symptoms reported by another 3%. There was no significant difference in HSR frequency, HSR severity, subjective symptom frequency, or documentation accuracy by evaluation method. Overall, more than 90% of penicillin allergy labels were removed and stayed removed 6 months later.
Penicillin skin testing with penicilloyl-polylysine (PPL) and diluted Penicillin G did not identify any allergic patients prior to amoxicillin challenge, although we identified skin test positive patients in our practice outside the study period. We may observe rare positives because patient reactions are often remote and we selected out the highest risk patients (Figure 1A). The 21 patients who reacted to amoxicillin but did not have a positive penicillin skin test may have been sensitized to minor determinants or the amino group, recently appreciated to comprise 65% of skin test positives in a US cohort.5 Although skin testing excluded ampicillin, almost one-quarter of included patients reported aminopenicillin allergy. These data will motivate our expansion of penicillin skin testing to the high-risk group not previously tested (those with recent anaphylaxis and/or prior positive penicillin skin testing), and testing reagents will routinely include ampicillin (Figure 1B).
Our data provide additional evidence for the safety of direct amoxicillin challenges in appropriately selected patients.2 We observed a 6% HSR rate in 83 patients with low-risk penicillin allergy histories, a higher frequency than prior low-risk direct challenge reports (1.3-2.6%).4,6,7 Expanding direct challenges in low-risk patients facilitates more widespread penicillin allergy evaluations, particularly since challenge only evaluations are less resource-intensive and may be more feasibly implemented by non-allergist providers.2,8
Penicillin allergy labels can remain in 28% of patients despite negative testing, and 38% can have erroneous redocumentation.5,9 We identified more modest deficiencies in penicillin allergy label removals and erroneous reentries with no differences by penicillin allergy evaluation method.
While we used multivariable models to investigate risk factors for reactions, the small number of events limited the information gained. Although we present 95%CI for HSR frequencies, we were underpowered because severe beta-lactam allergies are rare events. Because patients were identified by their procedure visit, we were unable to know how many patients had high-risk histories in the study period. HSR determination retrospectively limited the impact of practice heterogeneity, but the allergy outcome may not have been the same as that which was concluded by the treating allergist. Retrospective data collection resulted in limited capture of reaction details.
Direct challenges can be considered for appropriately selected low-risk patients presenting for penicillin allergy evaluation. Penicillin skin testing did not avert a single HSR but is nonetheless advisable for patients with higher risk allergy histories, pregnant patients, and patients with tenuous cardiac or pulmonary status. Penicillin allergy risk stratification tools must include guidance for outcome determination and documentation.
Supplementary Material
Clinical Implications.
Moderate-risk patients received skin testing prior to amoxicillin challenge, and 21 of 426 (4.9%, 95%CI 3.1% to 7.4%) had hypersensitivity reactions (HSRs). Of 83 low-risk patients direct challenged to amoxicillin, there were 5 HSRs (6.0%; 95%CI 2.0% to 13.5%).
Acknowledgements:
The authors thank Lili Xiang.
Funding:
This work was supported by NIH K01AI125631, the American Academy of Allergy Asthma and Immunology Foundation, and the MGH Claflin Distinguished Scholars Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
KGB reports a licensed clinical decision support tool for inpatient beta-lactam allergy evaluation. EMH, XF, YL, GB, ASL, CMM, BRS, AB have nothing to disclose.
References
- 1.Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet 2019;393:183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and Management of Penicillin Allergy: A Review. JAMA 2019;321:188–99. [DOI] [PubMed] [Google Scholar]
- 3.Mill C, Primeau MN, Medoff E, Lejtenyi C, O’Keefe A, Netchiporouk E, et al. Assessing the Diagnostic Properties of a Graded Oral Provocation Challenge for the Diagnosis of Immediate and Nonimmediate Reactions to Amoxicillin in Children. JAMA Pediatr 2016:e160033. [DOI] [PubMed] [Google Scholar]
- 4.Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract 2017;5(3):813–5. [DOI] [PubMed] [Google Scholar]
- 5.Solensky R, Jacobs J, Lester M, Lieberman P, McCafferty F, Nilsson T, et al. Penicillin allergy evaluation: A prospective, multicenter, open label evaluation of a comprehensive penicillin skin testing kit. J Allergy Clin Immunol Pract 2019, in press DOI: 10.1016/j.jaip.2019.02.040 [DOI] [PubMed] [Google Scholar]
- 6.Iammatteo M, Alvarez Arango S, Ferastraoaru D, Akcar N, Lee AY, Cohen HW, et al. Safety and Outcomes of Oral Graded Challenges to Amoxicillin without Prior Skin Testing. J Allergy Clin Immunol Pract 2019;7:236–43. [DOI] [PubMed] [Google Scholar]
- 7.Macy E, Vyles D. Who needs penicillin allergy testing? Ann Allergy Asthma Immunol 2018;121:523–9. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal KG, Li Y, Banerji A, Yun BJ, Long AA, Walensky RP. The Cost of Penicillin Allergy Evaluation. J Allergy Clin Immunol Pract 2018;6:1019–27 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimawi RH, Shah KB, Cook PP. Risk of redocumenting penicillin allergy in a cohort of patients with negative penicillin skin tests. J Hosp Med 2013;8:615–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


