Abstract
The PI3K/Akt signaling pathway has been implicated in playing an important role in platelet activation during hemostasis and thrombosis involving platelet-matrix interaction and platelet aggregation. Its role in non-physiological shear stress (NPSS)-induced platelet activation relevant to high-shear blood contacting medical devices (BCMDs) is unclear. In the context of blood cells flowing in BCMDs, platelets are subjected to NPSS (>100 Pa) with very short exposure time (<1 sec). In this study, we investigated whether NPSS with short exposure time induces platelet activation through PI3K/Akt signaling pathway. Healthy donor blood treated with or without PI3K inhibitor was subjected to NPSS (150 Pa) with short exposure time (0.5 sec). Platelet activation indicated by the surface P-selectin expression and activated glycoprotein (GP) IIb/IIIa was quantified using flow cytometry. The phosphorylation of Akt, activation of the PI3K signaling, was characterized by western blotting. Changes in adhesion behavior of NPSS-sheared platelets on fibrinogen, collagen and von Willebrand factor (vWF) was quantified with fluorescent microscopy by perfusing the NPSS-sheared and PI3K inhibitor treated blood through fibrinogen, collagen and vWF-coated microcapillary tubes. The results showed that the PI3K/Akt signaling was involved with both NPSS-induced platelet activation and platelet-matrix interaction. NPSS-sheared platelets exhibited exacerbated platelet adhesion on fibrinogen, but had diminished platelet adhesion on collagen and vWF. The inhibition of PI3K signaling reduced P-selectin expression and GPIIb/IIIa activation with suppressed Akt phosphorylation and abolished NPSS-enhanced platelet adhesion on fibrinogen in NPSS-sheared blood. The inhibition of PI3K signaling can attenuate the adhesion of unsheared platelets (baseline) on collagen and vWF, while had no impact on adhesion of NPSS-sheared platelets on collagen and vWF. This study confirmed the important role of PI3K/Akt signaling pathway in NPSS-induced platelet activation. The finding of study suggests that blocking PI3K/Akt signaling pathway could be a potential method to treat thrombosis in patients implanted with BCMDs
Keywords: Non-physiological shear stress, blood contacting medical device, platelet activation, PI3K/Akt signaling pathway, Akt phosphorylation
Introduction
Blood contacting medical devices (BCMDs) are frequently used as treatment or replacement therapies for diseased organs of patients suffering from cardiovascular, cardiopulmonary and renal illnesses. These devices, including ventricular assist devices (VADs), extracorporeal membrane oxygenation (ECMO), heart-lung machines (cardiopulmonary bypass (CPB)), blood oxygenators (artificial lungs), hemodialyzers, and mechanical heart valves (MHVs), improve the survival or extend the lives of many patients and restore quality of their lives [1–3]. While these devices have successfully demonstrated medical benefits, the risks of clinically adverse events, such as thromboembolism, have not been completely eliminated and are associated with increased morbidity and mortality [4, 5]. In certain regions inside these BCMDs, blood cells are exposed to non-physiological shear stress (NPSS) [6, 7]. The typical regions with NPSS are the areas adjacent to the blade surface of a VAD impeller, the rotor surface of blood pumps in ECMO systems and the hinge regions of MHVs. NPSS inside BCMDs is often larger than 100 Pascal (Pa) and sometimes even higher than 600 Pa [8]. The exposure time for related NPSS is short and normally less than 1 sec [6].
Platelets are important blood cells in hemostasis, and are involved not only in physiologic hemostasis but also pathological thrombosis [9]. The activation of platelets can increase the thrombotic risk. Numerous investigations have shown that physical forces, or mechanical shear stresses, affect platelet function [10]. Brown et al. [11] found that the shear stress level of 5 Pa resulted in the liberation of small amounts of ATP, ADP and subsequent platelet aggregation. Holme et al. [12] reported that a pathological shear stress level of 31.5 Pa activated platelets and triggered platelet microparticle generation. Sheriff et al. [13] reported that high shear stresses alone in the absence of von Willebrand factor (vWF) can also induce platelet activation. In our previous study, we found that the NPSS with short exposure time can induce platelet activation [14, 15].
The precise mechanisms of the shear-induced platelet activation are still unknown. Wurzinger et al. suggested that shear stress does not directly cause platelet activation, but rather causes mechanical lysis of platelet and red cells, thereby yielding release of stored agonists, principally ADP, which subsequently activates the intact resting platelets [16]. However, a number of other studies suggested that shear stress has a direct effect on the activation and aggregation of platelets via vWF binding to platelet glycoprotein (GP) Ibα receptor (classical vWF receptor) [17–19]. Kroll et al. [20] stated that it was becoming increasingly clear that the signal transduction mechanisms mediating platelet aggregation by chemical stimuli and shear stress may be distinct. It is believed that the shear-mediated interaction of platelet GPIbα with vWF (A1 domain) initiates a sequence of signaling events in platelets. More specifically, the high shear stress can unfold overall structure of vWF exposing the hidden platelet binding site (A1 domain) in which the platelet receptor GPIbα can bind with vWF [21, 22]. The binding of the GPIbα with vWF mediates platelet tethering and rolling on vWF under shear. This is considered as the first step of the shear-mediated platelet activation and subsequently the mechanical fluid shear force triggers a sequence of signaling events in platelets bound to vWF, resulting in the activation of the ligand binding function of the integrin GPIIb/IIIa (classical fibrinogen receptor) which transmits “outside-in” signals, mediates stable platelet adhesion, aggregation and thrombus formation [23, 24].
It has been indicated that phosphoinositide 3-kinases (PI3K) plays an important role in shear-mediated platelet activation [25, 26]. There are four class I PI3K isoforms (PI3K α, β, γ, δ), which are all expressed in platelets. PI3Kβ is particularly the most abundant isoform in platelets and engaged in the complicated mechanisms of thrombus formation and stabilization at high shear stress condition [27, 28]. Akt (also known as Protein Kinase B) is a serine/threonine-specific protein kinase, which is the major downstream effector of PI3K [29]. Previous studies had shown that the PI3K/Akt signaling pathway played an important role in the shear-mediated vWF-GPIbα interaction and subsequent platelet activation [30–32]. These studies investigated the effect of shear stress on platelets using cone-plate viscometers or parallel-plate flow chamber with generating shear stresses often below 50 Pa and exposure times ranging from several seconds to minutes. This appears to be 3 to 10 orders of magnitude longer than the time that blood actually spends in high shear environment while flowing through VADs, MHVs and arterial stenosis. In contrast to the large surface area to volume ratio and long exposure time in the cone-viscometer or parallel flow chamber, platelets through BCMDs often experience high bulk flow shear for short exposure (< 1 sec). It is unclear whether the platelet activation induced by NPSS with short exposure time is similarly dependent of PI3K/Akt signaling pathway. In the present study, we examined the role of the PI3K/Akt signaling pathways in NPSS-induced platelet activation. Healthy donor blood was treated with or without PI3Kβ inhibitor (TGX221) and then was exposed to NPSS using a special designed blood shearing device.
Materials and methods
Blood collection
Eight healthy adult donors (five men and three women with age from 24 to 31 years) who had not taken any antiplatelet medication 2 weeks prior to blood donation were included in this study. All the participants were informed the aim of the study and gave the written informed consent. The study protocol was reviewed and approved by the Institutional Review Board approval (IRB) of University of Maryland School of Medicine. One unit of blood (450 ml) drawn from an antecubital vein of the volunteers was directly collected into a sterilized collection bag mixed with sodium citrate (ratio: 9 to 1). Complete blood counts were assessed with a blood hematology analyzer (ABX Micros 60 CT, Horiba Medical, Irvine, CA) to ascertain that the collected blood sample was normal.
Blood shearing device
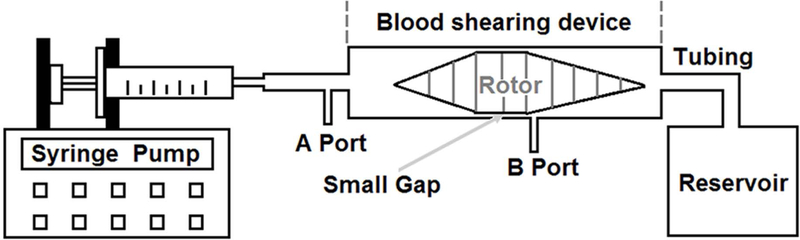
The flow-through Couette-type blood shearing device used for the study was adapted from the adult Jarvik continuous flow VAD (Jarvik Heart, Inc., New York, NY). A high-speed rotating spindle was supported by a pair of tiny pin-bearings. There is a small gap with a uniform width of 100 μm between the middle spindle surface and outer housing wall. The details of the design features and operational principles of this device can be found in our early publication [33]. The whole blood shearing system is shown in Fig. 1. A syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA) was used to pressurize blood in a syringe to flow through the small gap. When passing through the narrow gap, the blood could be exposed to precisely controlled uniform high shear stress generated by the rotating spindle for a short duration. In addition, it should be mentioned that because the shear stress level in other regions of this device was much smaller than that in the narrow gap region [33], the effect of shear-induced blood damage (platelet activation) in the other regions of this device could be negligible.
Fig. 1.
Blood shearing system including a syringe, a syringe pump used to control the flow rate to obtain the desired exposure time, an axial flow-through Couette-type device (blood shearing device), connecting tubes and a waste blood reservoir.
Experimental procedures of blood-shearing experiment
Before experiment, the blood viscosity was measured by using a semi-micro viscometer (Cannon Instrument Company, State College, PA). The desired level of NPSS could be achieved by adjusting the rotational speed of the spindle based on the blood viscosity. The expected exposure time was determined by adjusting the axial flow rate [23]. In this study, the NPSS level of 150 Pa for an exposure time of 0.5 sec was chosen to mimic the typical level in high-shearing BCMDs. To inhibit the PI3K signaling pathway, a commonly used inhibitor, TGX221 (Cayman Chemical, Ann Arbor, MI) was used. Whole blood was incubated with TGX221 (2.2 μM) for 5 minutes at 37°C [34] before being subjected to the high shear stress. Whole blood samples treated with or without TGX221 were loaded into our blood shearing system and exposed to the NPSS of 150 Pa for 0.5 sec. During the experiment, an unsheared blood sample was collected as the baseline sample (taken at the port A in Fig. 1). The sheared blood sample was collected after the blood passed through the narrow gap (taken at B port in Fig. 1). The port B was created close to the narrow gap to ensure that the blood sample was only exposed to the desired NPSS. There were total four experimental conditions in this study, which were (1) untreated + unsheared (baseline) condition, (2) TGX221 treated (2.2 μM) + unsheared condition, (3) untreated + sheared (150 Pa/0.5 sec) condition, and (4) TGX221 treated (2.2 μM) + sheared (150 Pa/0.5 sec) condition. The shearing experiment was repeated for 8 times with blood from 8 different donors.
Flow cytometric analysis of platelet activation
The levels of platelet activation in blood samples of four experimental conditions were quantified by using the marker of GPIIb/IIIa activation and P-selectin expression. Fluorescein isothiocyanate (FITC)-labeled anti-human PAC-1 antibody (PAC-1 FITC) (BD Bioscience, San Jose, CA) and phycoerythrin (PE) conjugated anti-CD62P antibody (CD62P PE) (BioLegend, San Diego, CA) were used to detect platelet GPIIb/IIIa activation and P-selectin expression, respectively. The negative control for PAC-1 and CD62P was FITC-labeled isotype control immunoglobulin IgMK antibody (BioLegend, San Diego, CA) (IgMK FITC) and PE-labeled isotype control IgG1k antibody (IgG1K PE), respectively. V450 labeled Anti-Human CD41a (CD41a-V450) (BD Bioscience, San Jose, CA) was used for platelet identification. For the immunological staining, 5 μl of whole blood was added into a polypropylene tube containing HEPES buffer (10 mM, 25 μl), CD41a-V450 (5 μl), and PAC-1 FITC (20 μl) or IgMK (20 μl), and CD62P (5 μl) or IgG1K antibody (5 μl). After incubation with the antibodies at room temperature in the dark for 30 min, 1 mL of 1% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) was added to fix the samples for 30 min at 4°C in the dark. The samples were analyzed on a flow cytometer (FACSVerse, BD Bioscience, San Jose, CA). The flow cytometry data were analyzed with the FCS Express software (De Novo Software, Los Angeles, CA).
Characterization of platelet adhesion to fibrinogen, collagen, and vWF
Blood samples from four experimental conditions was perfused through protein-coated glass tubes to investigate the effect of PI3K inhibition on platelet adhesion to hemostasis related proteins (fibrinogen, collagen, and vWF). The glass tubes (0.2 mm x 2 mm x 25 mm, VitroCom, Mountain Lakes, NJ) were coated with fibrinogen (1 mg/ml, EMD Millipore, Billerica, MA), collagen (1 mg/ml, Chrono-log, Havertown, PA) and vWF (100 μg/ml, EMD Millipore, Billerica, MA) overnight in a humid environment at 4°C. Then, the glass tubes were blocked with 5% bovine serum albumin in PBS [35] at room temperature for 2 hours. The platelets in blood samples collected from four experimental treatment conditions were labeled with mepacrine (10 μM) (Sigma, St. Louis, MO) and then perfused through the tubes at a shear rate of 500 s−1 for 5 minutes in the dark. This shear rate was within the normal physiological range [36]. Previous studies [37, 38] had shown that platelet function was not affected when a final concentration of mepacrine used for florescence labeling was lower than 20 μM. After the perfusion, PBS was used to wash the tubes gently to remove the nonattached cells. The remaining adherent platelets were fixed with 3.7% PFA and imaged using a fluorescence microscope (IX71, Olympus) equipped with a digital camera (DP80, Olympus). Ten images with 2 mm interval along the center axis of each glass tube were acquired with the Olympus cellSens software (Olympus, Shinjuku-ku, Tokyo, Japan) and used to calculate the average area covered by adhered platelets on each tube using custom-written software in MATLAB (Natick, MA, USA). The details of the platelet adhesion experiment were reported in our previous study [39].
Western blot analysis Akt phosphorylation
Platelet rich plasma (PRP) was obtained from the baseline and sheared blood samples by centrifugation at 250 g for 10 minutes at 20°C. The PRP was then centrifuged at 2000 g for 10 minutes at 4°C. After the second centrifugation, the supernatant was removed. Tyrode’s salts solution including 1.2 mM CaCl2 (Sigma, St. Louis, MO) was used to resuspend and wash platelet pellets for two times. After the second washing, the platelet pellets were obtained by centrifugation of the resuspended platelet solution at 2000 g for 10 minutes at 4°C. The platelet pellets were lysed with radioimmunoprecipitation assay buffer containing protease inhibitor (cOmplete™, Mini, EDTA-free Protease Inhibitor) (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor (PhosSTOP™) (Sigma-Aldrich, St. Louis, MO). Proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using precast protein gel (4–15% Mini-PROTEAN® TGX™ Precast Protein Gels) (Bio-Rad, Hercules, CA), and transferred to a pre-cut 0.2 μm nitrocellulose membrane (Thermo Fisher Scientific, Waltham, MA). The membrane was incubated overnight at 4°C with the primary antibody against phospho-Akt (p-Akt) (Ser473) (1:1000 dilution) (cell signaling Technology, Danvers, MA). Immunoreactivities were detected using enhanced chemiluminescence (ECL) (Amersham Biosciences, UK) and photographic film (Denville Scientific Inc. Metuchen, NJ). Beta actin (1:5000 dilution) (Cell Signaling Technology, Danvers, MA) was used to confirm equal loading conditions for total protein expression. Densitometric quantification of the digitized immunoreactive bands was performed using the UN-SCAN-IT Gel 6.1 software (Silk Scientific, Orem, Utah). The densitometric values were expressed as the ratio of the target protein normalized to loading controls from the same blot individually for each sample.
Statistical Analysis
All data are presented as mean ± SE (standard Error of Mean) (n=8). Statistical differences were determined by using the one-way ANOVA with Tukey multiple comparison post-test (SPSS statistics software package version 18.0, Chicago, IL). *P < 0.05 is considered to be statistically significant.
Results
PI3K inhibition suppresses NPSS-induced platelet activation
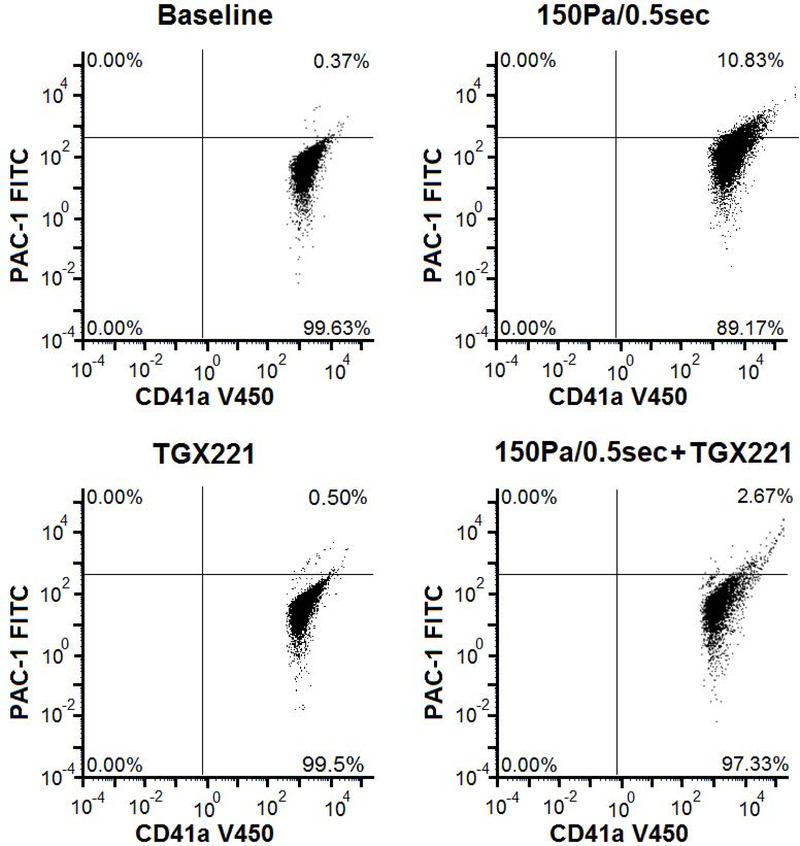
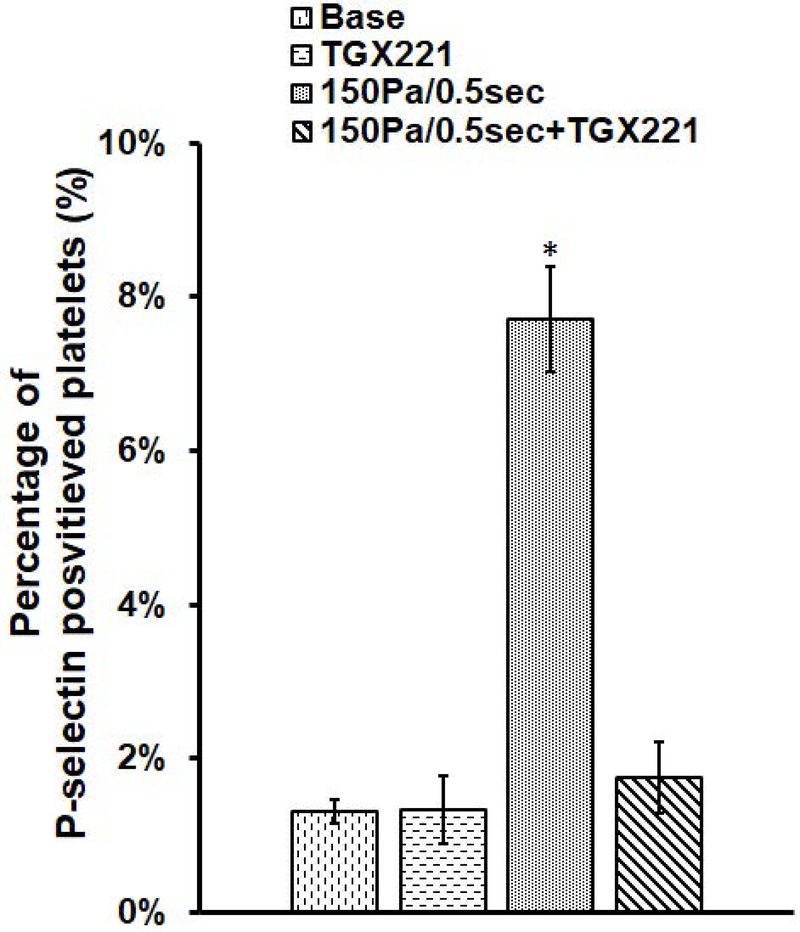
To examine the effect of the PI3K inhibition on the platelet GPIIb/IIIa activation and P-selectin expression, the TGX221 treated blood samples and the normal blood samples were exposed to 150 Pa NPSS for 0.5 sec. The sheared samples were analyzed with their respective unsheared baseline samples by using flow cytometry. Fig. 2A shows typical flow cytometry fluorescence intensity dot plots of platelets in the blood samples collected from the four experimental treatment conditions: (1) Untreated + unsheared (baseline) condition, (2) TGX221 treated (2.2 μM) + unsheared condition, (3) untreated + sheared (150 Pa/0.5 sec) condition, and (4) TGX221 treated (2.2 μM) + sheared (150 Pa/0.5 sec) condition. The upper right quadrant of the plot indicates activated platelets positive for PAC-1 and CD41a markers and the corresponding percentage of activated platelets in the total platelet population. Fig. 2B shows of the average percentages of platelets with GPIIb/IIIa activation over 8 repeated experimental runs in these four experimental conditions, respectively. As indicated in this figure, the percentage of activated platelets with GPIIb/IIIa activation was 9.8% in the NPSS-sheared blood sample, which was significantly higher (*P < 0.001) than 0.12% of the unsheared baseline blood sample. However, when the blood was treated with TGX221 and exposed to the NPSS condition (150 Pa/0.5 sec), the percentage of activated platelets with GPIIb/IIIa activation was 2.1 %, which was significantly lower than that of NPSS-sheared blood sample. Similar to the observation for GPIIb/IIIa activation, the percentage of activated platelets with P-selectin expression significantly (*P < 0.001) increased from 1.32% of baseline blood sample to 7.8% of NPSS-sheared blood sample (Fig. 2C). The percentage of activated platelets with P-selectin expression was 1.76 % when the blood sample was first treated with TGX221 and then exposed to the NPSS condition. The NPSS-induced platelet activation was almost completely suppressed by inhibition of the PI3K signaling pathway. The data indicated that the PI3K inhibition suppressed the NPSS-induced platelet activation.
Fig. 2.
The comparison of platelet activation in (1) baseline (untreated and unsheared) platelets, (2) TGX221 treated (2.2 μM) + unsheared platelets, (3) untreated + NPSS sheared (150 Pa/0.5 sec) platelets, and (4) TGX221 (2.2 μM) treated + NPSS sheared (150 Pa/0.5 sec) platelets. A) Typical images of flow cytometry fluorescence intensity dot plots of platelets; B) Average percentages of platelets with GPIIb/IIIa activation; C) Average percentages of platelets with P-selectin expression; (n=8, *P < 0.05)
PI3K inhibition suppresses NPSS-induced Akt phosphorylation
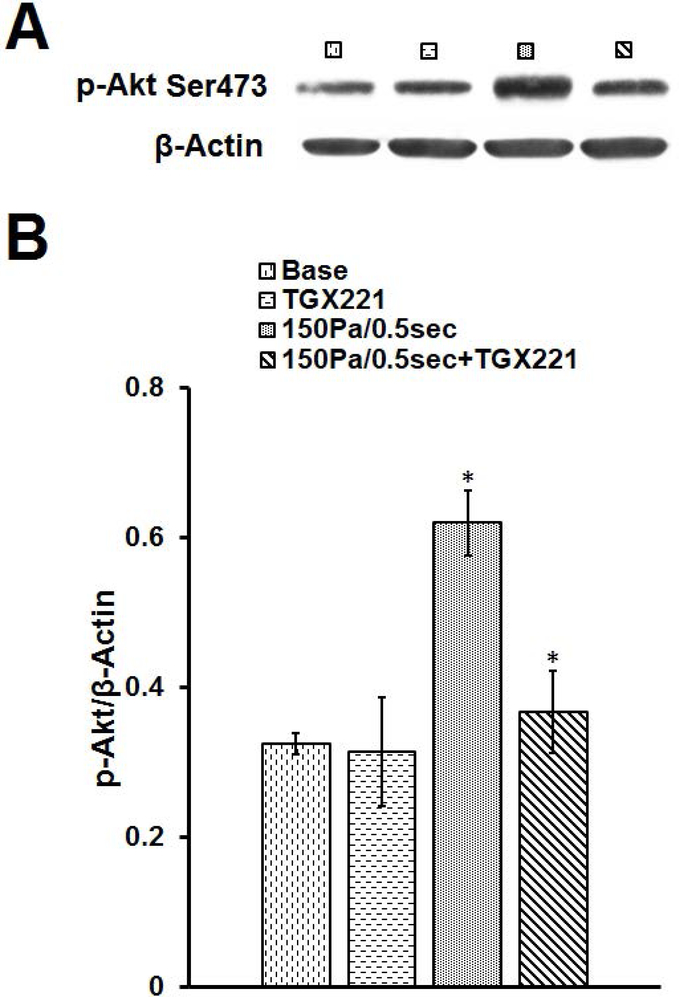
To examine the role of the PI3K signaling pathway in the NPSS-induced platelet activation, we examined the level of Akt phosphorylation with western blotting. Fig. 3A shows typical immunoblots of p-Akt and β-actin in the platelets obtained in the four experimental conditions. Fig. 3B shows the mean values of the normalized immunoblot densities of p-Akt with β-actin in the platelets of the unsheared and sheared blood treated with and without TGX221. As showed in Fig. 3, the level of phosphorylation of Akt significantly increased by 90.8% when the blood was exposed to the NPSS (150 Pa/0.5 sec) compared to the baseline blood (*P < 0.01). However, when the blood was treated with TGX221 and then exposed to the NPSS, the level of phosphorylation of Akt decreased remarkably by 41.1% compared to the untreated blood exposed to the NPSS (*P < 0.05). The data here indicate that the PI3K inhibition through TGX221 suppressed the NPSS-induced Akt phosphorylation.
Fig. 3.
The comparison of Akt phosphorylation in (1) baseline (untreated and unsheared) platelets, (2) TGX221 treated (2.2 μM) + unsheared platelets, (3) untreated + NPSS sheared (150 Pa/0.5 sec) platelets, and (4) TGX221 (2.2 μM) treated + NPSS sheared (150 Pa/0.5 sec) platelets. A) Typical representative immunoblots of p-Akt and β-Actin; B) Mean values of normalized immunoblot densities for p-Akt (normalized to β-Actin) (n=8, *P < 0.05).
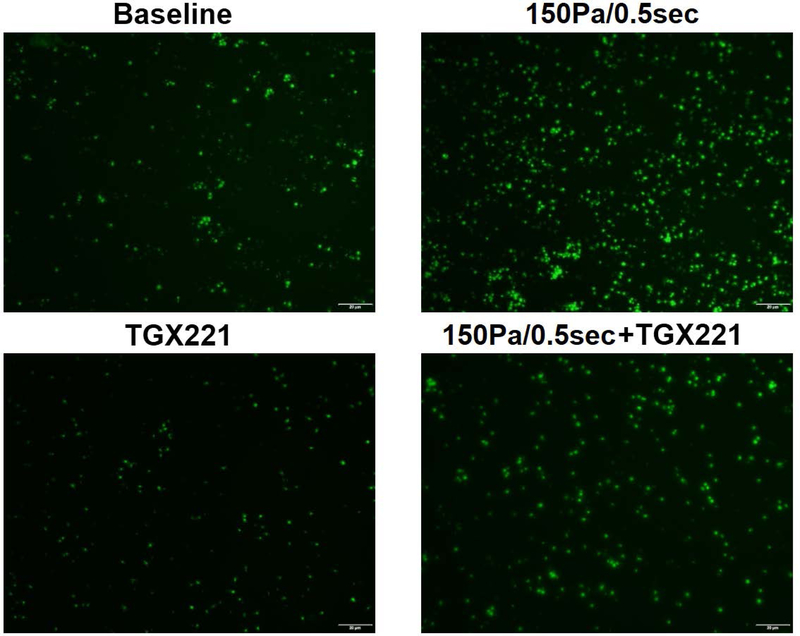
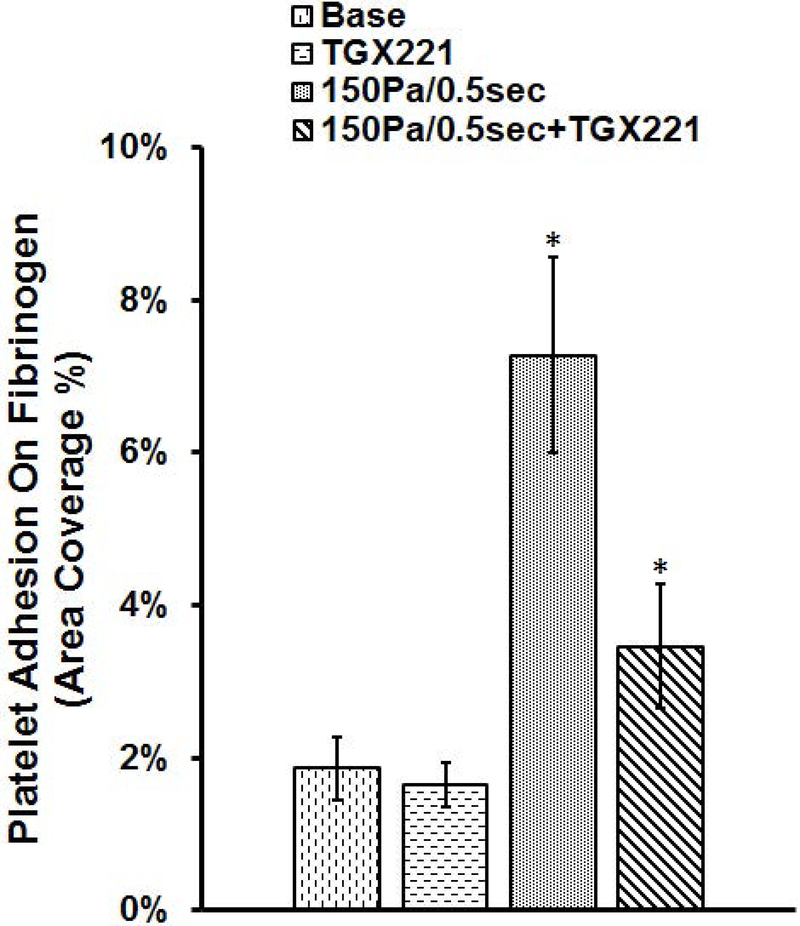
PI3K inhibition abolishes NPSS-induced increase of platelet adhesion to fibrinogen
After being treated with TGX221 and exposed to the NPSS, the blood samples collected from four experimental treatment conditions were perfused through the fibrinogen-coated glass tubes under the physiological shear rate to examine the combined effect of the PI3K signaling inhibition and NPSS exposure on the adhesion of platelets to fibrinogen. Fig. 4A and Fig. 4B show typical images of platelets adhered to fibrinogen after perfusing the blood samples for 5 minutes and the average percentages of areas covered by adhered platelets from the blood samples obtained in the four experimental treatment conditions, respectively. As shown by these figures, the surface coverage on fibrinogen by platelets is 1.8 % in baseline blood sample and increase to 7.3% when the blood sample was exposed to the NPSS. These results indicated that the NPSS could enhance the platelet adhesion to fibrinogen. When the blood was treated with TGX221 and then exposed to the NPSS, the percentage of platelet coverage area was 3.46%, which was significantly lower than that of NPSS-sheared blood sample. The addition of TGX221 to the blood suppressed, but not completely abolished the NPSS-induced increase of platelet adhesion to fibrinogen. The inhibition of the PI3K signaling pathway with TGX221 appeared to have no impact on the platelet adhesion to fibrinogen in the unsheared blood. Both the TGX221 treated and untreated blood samples (baseline) without exposure to the NPSS had a similar level of platelet adhesion to fibrinogen.
Fig. 4.
The blood samples collected from (1) untreated + unsheared (baseline) condition, (2) TGX221 treated (2.2 μM) + unsheared condition, (3) untreated + sheared (150 Pa/0.5 sec) condition, and (4) TGX221 treated (2.2 μM) + sheared (150 Pa/0.5 sec) condition were perfused through the fibrinogen-coated glass tubes under the physiological shear rate (500 s−1). A) Typical fluorescence images of adherent platelets to fibrinogen; B) Average area coverage (ten images of each glass tube) of adherent platelets to fibrinogen (n=8, *P < 0.05).
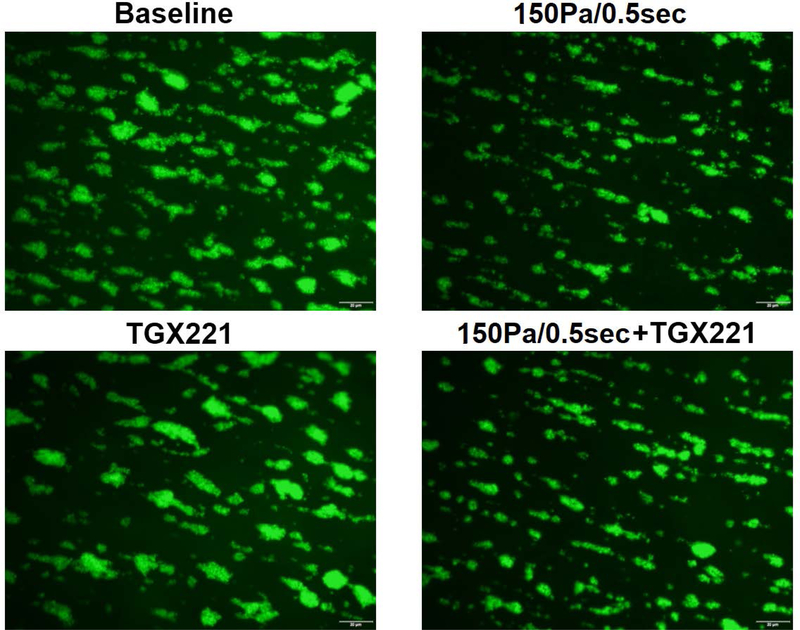
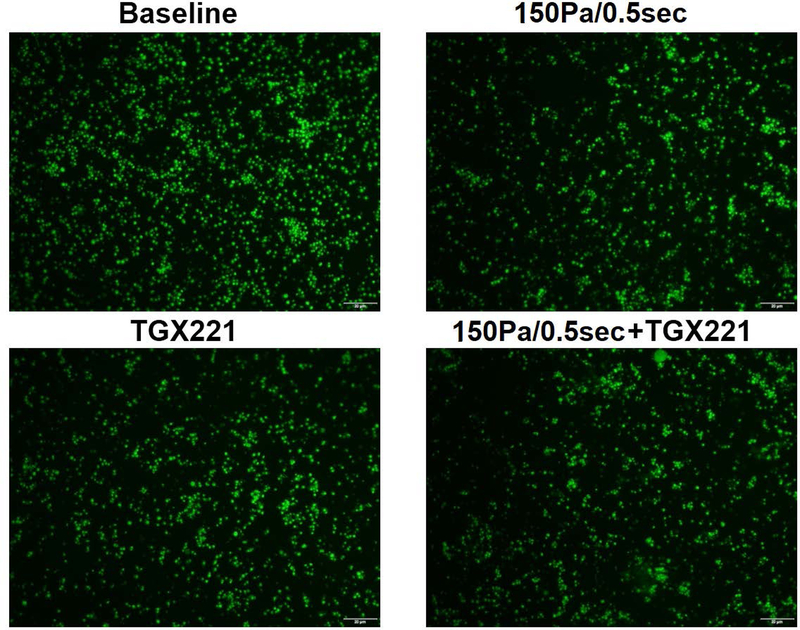
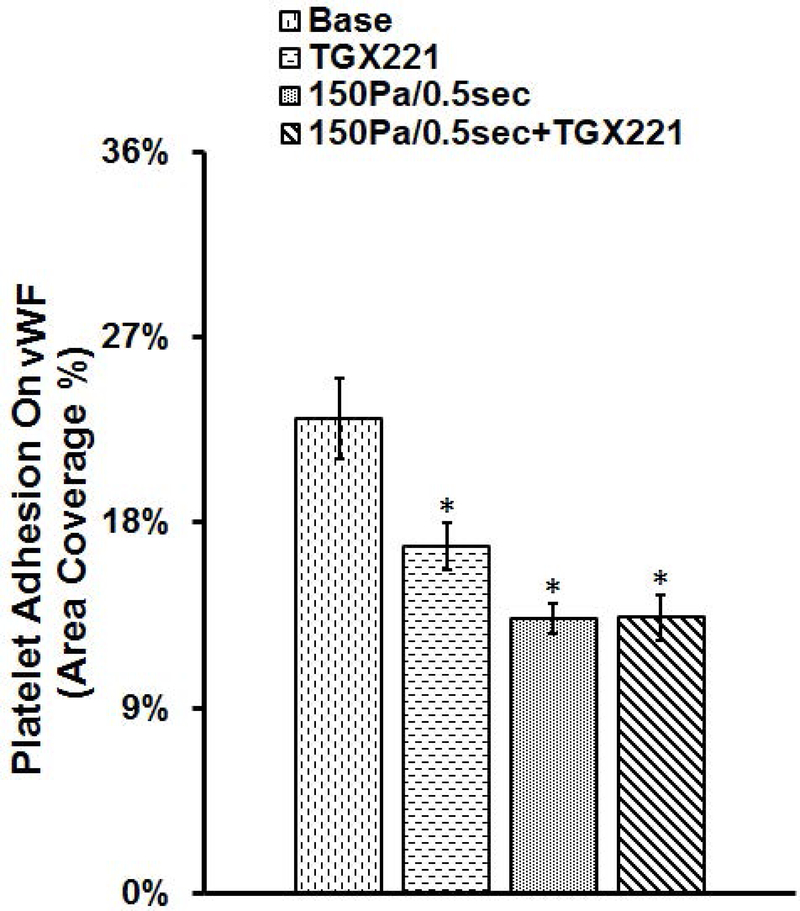
PI3K inhibition has no impact on adhesion of NPSS-sheared platelets to collagen and vWF
The blood samples collected in the four experimental treatment conditions were also perfused through collagen-and vWF-coated glass tubes under the physiological shear rate. Fig. 5 and Fig. 6 show typical images of platelets adhered to collagen and vWF, and the average percentages of areas covered by adhered platelets to collagen and vWF. The area coverage of platelets to collagen and vWF in unsheared baseline samples was 29.1% and 23.1%, respectively. After the blood samples were exposed to NPSS condition, the area coverage of NPSS-damaged platelet adhesion to collagen and vWF was 16.3% and 13.3%, respectively. Compared to the unsheared blood (baseline), both the platelet adhesion to collagen and vWF reduced significantly in the blood sample exposed to the NPSS (*P < 0.001). The area coverage of platelet adhesion to collagen and vWF of only TGX221 treated blood samples was 19.1% and 16.9%. The inhibition of the PI3K signaling pathway in platelets with TGX221 itself did have impact on platelet adhesion to collagen and vWF, which caused a significant reduction (*P < 0.01) compared with the untreated platelets without exposure to the NPSS (baseline). When the blood was treated with TGX221 and then exposed to the NPSS, area coverage of by platelets adhesion on collagen and vWF was 15.8% and 13.4%, which were slightly lower than that of only TGX221 treated blood sample. In addition, it is interesting to note that the reduction in platelet adhesion to collagen and vWF of only NPSS-sheared blood samples was similar with that of TGX221 treated and NPSS-sheared blood samples.
Fig. 5.
The blood samples collected from (1) untreated + unsheared (baseline) condition, (2) TGX221 treated (2.2 μM) + unsheared condition, (3) untreated + sheared (150 Pa/0.5 sec) condition, and (4) TGX221 treated (2.2 μM) + sheared (150 Pa/0.5 sec) condition were perfused through the collagen-coated glass tubes under the physiological shear rate (500 s−1). A) Typical fluorescence images of adherent platelets to collagen; B) Average area coverage (ten images of each glass tube) of adherent platelets to collagen (n=8, *P<0.05).
Fig. 6.
The blood samples collected from (1) untreated + unsheared (baseline) condition, (2) TGX221 treated (2.2 μM) + unsheared condition, (3) untreated + sheared (150 Pa/0.5 sec) condition, and (4) TGX221 treated (2.2 μM) + sheared (150 Pa/0.5 sec) condition were perfused through the vWF-coated glass tubes under the physiological shear rate (500 s−1). A) Typical fluorescence images of adherent platelets to vWF; B) Average area coverage (ten images of each glass tube) of adherent platelets to vWF (n=8, *P<0.05).
Discussion
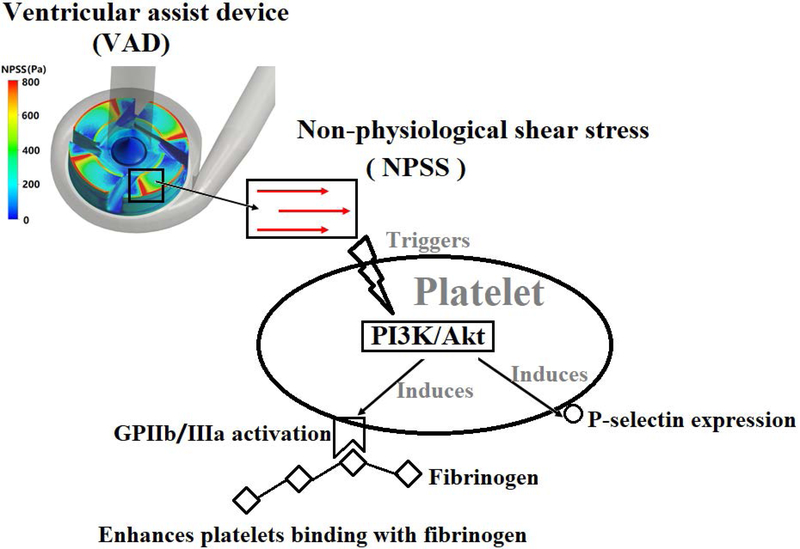
Although it is known that shear stress plays a role in mediating the vWF-GPIbα/PI3K/Akt signaling pathway leading to platelet activation, the impact of NPSS on these signaling events has not been examined. In the present study, the role of the PI3K/Akt signaling pathway in NPSS-induced platelet activation was investigated. As indicated by our study, the PI3K/Akt signaling pathway was involved with both NPSS-induced platelet activation and platelet-matrix interaction (Fig. 7). After being subjected to NPSS, the platelet adhesion to fibrinogen increased compared to the unsheared baseline blood while the platelet adhesion to collagen and vWF decreased. When the blood was treated with TGX221 and then exposed to the NPSS of 150 Pa for 0.5 sec, we found that the inhibition of the PI3K/Akt signaling reduced platelet GPIIb/IIIa activation and P-selectin expression with suppressed Akt phosphorylation and abolished enhanced platelet adhesion to fibrinogen in the NPSS-sheared blood. Additionally, the inhibition of PI3K can attenuate the adhesion of baseline unsheared platelets on collagen and vWF, while, this PI3K/Akt signaling inhibition did not affect NPSS-sheared platelets adhesion to collagen and vWF.
Fig. 7.
The mechanism diagram related with that the PI3K/Akt signaling pathway plays an important role in NPSS-induced platelet activation.
In earlier studies [31, 40, 41], the role of PI3K/Akt signaling pathway in platelet adhesion to fibrinogen, vWF and collagen under shear condition was investigated. The normal platelets were perfused through the protein-coated slide within parallel flow chambers. Cosemans et al.[31] found that continuous outside-in signaling via PI3K isoforms β and γ is required for platelet ADP receptor function in dynamic thrombus stabilization. Yin et al. [40] reported that AKT1 and AKT2 mediate glycoprotein Ib-IX signaling via the cyclic guanosine monophosphate (cGMP)-dependent signaling pathway. Yi et al. [41] showed that using S14161 (Pan-PI3K inhibitor) to block PI3K/Akt signaling pathway can inhibit agonist-induced platelet activation and thrombus formation without significant bleeding tendency. Different with these works, we investigated the role of PI3K/Akt signaling pathway in the adhesion of NPSS-damaged platelets to fibrinogen, vWF and collagen. The platelets were preincubated with/without TGX221 and subjected to NPSS, and then the NPSS-damaged platelets were perfused through the protein-coated glass tubes. This process can mimic the in vivo situation that platelets were damaged by the mechanical shear stress in patients implanted with BCMDs and those damaged platelets continued to circulate in blood vessels. We had found that NPSS could induce activation of the platelet GPIIb/IIIa and shedding of platelet GPVI and GPIbα receptors. This could result in two opposite effects on platelet adhesion [9]. Our data clearly showed that the NPSS-injured (damaged) platelets had increased adhesion on fibrinogen (Fig. 4) and decreased adhesion on collagen and vWF (Fig. 5 and Fig. 6) compared to the unsheared baseline platelets. When the blood was treated with TGX221 and then exposed to the NPSS condition, TGX221 inhibited platelet activation through PI3K/Akt signaling pathway. Less activated platelets in the sheared-blood resulted in less platelet adhesion to fibrinogen compared to the untreated, NPSS-sheared blood (Fig. 4). Additionally, we observed that the platelet adhesion to collagen and vWF was less in the TGX221 treated and unsheared blood compared to the baseline blood, which indicated that the inhibition of PI3K/Akt pathway could attenuate adhesion capacity of platelets to collagen and vWF. Since the TGX221 inhibits platelet activation and NPSS causes platelet receptor shedding (GPVI and GPIbα) leading to weak binding of platelets with substrate proteins (collagen and vWF), the adhesion of the TGX221 treated and NPSS-sheared platelets to collagen and vWF should be less than that of the NPSS-sheared platelets. While, this study showed that the average percentages of areas covered by adherent platelets to collagen and vWF in the TGX221 treated and NPSS-sheared blood were close with that of the NPSS-sheared blood without TGX221 treatment. We postulate that both the TGX221 treated and untreated platelets could make the initial attachment to vWF via GPIbα-vWF interaction or to collagen via GPVI-collagen interaction under shear, but the continuous platelet activation through PI3K/Akt pathway was blocked by TGX221. Without activation of platelet integrins via the PI3K signaling, the TGX221 treated platelets could not form the firm adhesion and aggregation on vWF and collagen [31]. Those platelets that were initially in contact with vWF and collagen could be detached by shear forces because of the weak binding between GPIbα and vWF or GPVI and collagen. Thus there would be less platelets adhered to vWF and collagen (Fig. 5 and Fig. 6). In the case of the TGX221 treated and sheared blood, NPSS induced the shedding of platelet receptors (GPVI and GPIbα), the remaining copies of these receptors on the platelet surface were less. When the NPSS-sheared platelets were perfused over immobilized collagen and vWF, the ability of platelets to bind with these proteins was weak, leading to less platelets tethering and activation. The possibility for triggering PI3K/Akt signaling pathway might be lower due to the TGX221 inhibition. Therefore, the blocking of PI3K/Akt signaling pathway under the TGX221 treated and NPSS-sheared condition could not have extra impact on platelet adhesion to collagen and vWF compared to that of the only NPSS-sheared condition.
Conclusion
In conclusion, the data from the present study provide evidence that both NPSS-induced platelet activation and platelet-matrix interaction involved the PI3K/Akt signaling pathway. The inhibition of PI3K/Akt signaling reduced GPIIb/IIIa activation and P-selectin expression with suppressed Akt phosphorylation and abolished enhanced platelet adhesion to fibrinogen in the NPSS-sheared blood. The inhibition of PI3K can attenuate the adhesion of baseline unsheared platelets on collagen and vWF, while the adhesion of the NPSS-sheared platelets to collagen and vWF was not affected by the inhibition of PI3K/Akt signaling pathway. This study confirmed the important role of PI3K/Akt signaling pathway in NPSS-induced platelet activation. The finding of this study implies that inhibition of PI3K/Akt signaling pathway could be a potential treatment for thrombosis in patients implanted with BCMDs for drug development.
Acknowledgments
This work was funded by the National Institutes of Health (Grant number: R01HL124170).
Footnotes
Declaration of Interest statement
All authors declared no conflicts of interests.
REFERENCES CITED
- 1.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow in patients awaiting heart transplantation. The New England journal of medicine. 2007;357:885–96. [DOI] [PubMed] [Google Scholar]
- 2.Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. Journal of thoracic disease. 2014;6:1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armignacco P, Lorenzin A, Neri M, Nalesso F, Garzotto F, Ronco C. Wearable devices for blood purification: principles, miniaturization, and technical challenges. Seminars in dialysis. 2015;28:125–30. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein D, John R, Salerno C. Increase in left ventricular assist device thrombosis. The New England journal of medicine. 2014;370:1465. [DOI] [PubMed] [Google Scholar]
- 5.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:23–34. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Jena SK, Giridharan GA, Koenig SC, Slaughter MS, Griffith BP, et al. Flow features and device-induced blood trauma in CF-VADs under a pulsatile blood flow condition: A CFD comparative study. International journal for numerical methods in biomedical engineering. 2018;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. Journal of biomechanical engineering. 2012;134:081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Jena SK, Giridharan GA, Sobieski MA, Koenig SC, Slaughter MS, et al. Shear stress and blood trauma under constant and pulse-modulated speed CF-VAD operations: CFD analysis of the HVAD. Medical & biological engineering & computing. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimmino G, Golino P. Platelet biology and receptor pathways. Journal of cardiovascular translational research. 2013;6:299–309. [DOI] [PubMed] [Google Scholar]
- 10.Slepian MJ, Sheriff J, Hutchinson M, Tran P, Bajaj N, Garcia JGN, et al. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. Journal of biomechanics. 2017;50:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown CH 3rd, Leverett LB, Lewis CW, Alfrey CP Jr., Hellums JD. Morphological, biochemical, and functional changes in human platelets subjected to shear stress. The Journal of laboratory and clinical medicine. 1975;86:462–71. [PubMed] [Google Scholar]
- 12.Holme PA, Orvim U, Hamers MJ, Solum NO, Brosstad FR, Barstad RM, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:646–53. [DOI] [PubMed] [Google Scholar]
- 13.Sheriff J, Bluestein D, Girdhar G, Jesty J. High-shear stress sensitizes platelets to subsequent low-shear conditions. Annals of biomedical engineering. 2010;38:1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Griffith BP, et al. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Molecular and cellular biochemistry. 2015;409:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and von Willebrand Factor. Artificial organs. 2016;40:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wurzinger LJ. Histophysiology of the circulating platelet. Advances in anatomy, embryology, and cell biology. 1990;120:1–96. [DOI] [PubMed] [Google Scholar]
- 17.Konstantopoulos K, Wu KK, Udden MM, Banez EI, Shattil SJ, Hellums JD. Flow cytometric studies of platelet responses to shear stress in whole blood. Biorheology. 1995;32:73–93. [DOI] [PubMed] [Google Scholar]
- 18.McCrary JK, Nolasco LH, Hellums JD, Kroll MH, Turner NA, Moake JL. Direct demonstration of radiolabeled von Willebrand factor binding to platelet glycoprotein Ib and IIb-IIIa in the presence of shear stress. Annals of biomedical engineering. 1995;23:787–93. [DOI] [PubMed] [Google Scholar]
- 19.Reininger AJ, Heijnen HF, Schumann H, Specht HM, Schramm W, Ruggeri ZM. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood. 2006;107:3537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525–41. [PubMed] [Google Scholar]
- 21.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–50. [PubMed] [Google Scholar]
- 22.Crawley JT, de Groot R, Xiang Y, Luken BM, Lane DA. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118:3212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–15. [DOI] [PubMed] [Google Scholar]
- 24.Gong H, Shen B, Flevaris P, Chow C, Lam SC, Voyno-Yasenetskaya TA, et al. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 2010;327:340–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson SP, Yap CL, Anderson KE. Phosphoinositide 3-kinases and the regulation of platelet function. Biochemical Society transactions. 2004;32:387–92. [DOI] [PubMed] [Google Scholar]
- 26.Kriplani N, Hermida MA, Brown ER, Leslie NR. Class I PI 3-kinases: Function and evolution. Advances in biological regulation. 2015;59:53–64. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Mangin P, Dangelmaier C, Lillian R, Jackson SP, Daniel JL, et al. Role of phosphoinositide 3-kinase beta in glycoprotein VI-mediated Akt activation in platelets. The Journal of biological chemistry. 2009;284:33763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent PA, Severin S, Hechler B, Vanhaesebroeck B, Payrastre B, Gratacap MP. Platelet PI3Kbeta and GSK3 regulate thrombus stability at a high shear rate. Blood. 2015;125:881–8. [DOI] [PubMed] [Google Scholar]
- 29.Woulfe DS. Akt signaling in platelets and thrombosis. Expert review of hematology. 2010;3:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yap CL, Anderson KE, Hughan SC, Dopheide SM, Salem HH, Jackson SP. Essential role for phosphoinositide 3-kinase in shear-dependent signaling between platelet glycoprotein Ib/V/IX and integrin alpha(IIb)beta(3). Blood. 2002;99:151–8. [DOI] [PubMed] [Google Scholar]
- 31.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108:3045–52. [DOI] [PubMed] [Google Scholar]
- 32.Dayananda KM, Singh I, Mondal N, Neelamegham S. von Willebrand factor self-association on platelet GpIbalpha under hydrodynamic shear: effect on shear-induced platelet activation. Blood. 2010;116:3990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Taskin ME, Fang HB, Pampori A, Jarvik R, Griffith BP, et al. Study of flow-induced hemolysis using novel Couette-type blood-shearing devices. Artificial organs. 2011;35:1180–6. [DOI] [PubMed] [Google Scholar]
- 34.Krajewski S, Kurz J, Geisler T, Peter K, Wendel HP, Straub A. Combined blockade of ADP receptors and PI3-kinase p110beta fully prevents platelet and leukocyte activation during hypothermic extracorporeal circulation. PloS one. 2012;7:e38455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Zhang X, Hao P. The effect of topography and wettability of biomaterials on platelet adhesion. Journal of adhesion science and Technology. 2016;30:878–93. [Google Scholar]
- 36.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282:2035–42. [DOI] [PubMed] [Google Scholar]
- 37.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–97. [DOI] [PubMed] [Google Scholar]
- 38.Jamiolkowski MA, Woolley JR, Kameneva MV, Antaki JF, Wagner WR. Real time visualization and characterization of platelet deposition under flow onto clinically relevant opaque surfaces. Journal of biomedical materials research Part A. 2015;103:1303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Mondal NK, Zheng S, Koenig SC, Slaughter MS, Griffith BP, et al. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets. 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin H, Stojanovic A, Hay N, Du X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood. 2008;111:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi W, Li Q, Shen J, Ren L, Liu X, Wang Q, et al. Modulation of platelet activation and thrombus formation using a pan-PI3K inhibitor S14161. PloS one. 2014;9:e102394. [DOI] [PMC free article] [PubMed] [Google Scholar]