Abstract
The strategy of integrating motor signals with sensory information during voluntary behaviour is a general feature of sensory processing. It is required to distinguish externally-applied (exafferent) from self-generated (reafferent) sensory inputs. This distinction, in turn, underlies our ability to achieve both perceptual stability and accurate motor control during everyday activities. In this review, we consider the results of recent experiments that have provided circuit-level insight into how motor-related inputs to sensory areas selectively cancel selfgenerated sensory inputs during active behaviours. These studies have revealed both common strategies as well as important differences across systems. Sensory reafference is suppressed at the earliest stages of central processing in the somatosensory, vestibular and auditory systems, with the cerebellum and cerebellum-like structures playing key roles. Furthermore, motor-related inputs can also suppress reafferent responses at higher levels of processing such as cortex – a strategy preferentially used in visual processing. These recent findings have important implications for understanding how the brain achieves the flexibility required to continuously calibrate relationships between motor signals and the resultant sensory feedback, a computation necessary for our subjective awareness that we control both our actions and their sensory consequences.
Keywords: efference copy, corollary discharge, internal model, cerebellum, active sensing, prediction
Introduction:
Our brain integrates sensory information from multiple modalities to build accurate representations of our environment, which then guide our behaviour. However, during daily activities we are confronted not only with external sensory stimuli (termed exafference) but also with sensory stimulation arising from our own behaviour (termed reafference). In order to ensure accurate perception and motor control, it is absolutely essential that the brain distinguishes these two categories of sensory stimuli. This fundamental ability is important at multiple levels of sensory processing. First, at the level of simple reflexes, it is vital that reafferent sensory signals are interpreted as self-produced rather than externally-generated. For instance, consider a situation where an individual is slipping on ice. This will cause unexpected head motion that drives vestibulo-spinal reflexes to rapidly generate corrective movements to facilitate the maintenance of posture and balance. In contrast, if the same head motion was self-produced and thus expected, the corrective movements produced by vestibulo-spinal reflex pathways would be counterproductive; they would effectively counteract the intended movement. Second, when learning a new motor skill or monitoring ongoing behaviour, it is important to establish whether the sensory consequences of a given motor command are consistent with what is expected from our voluntary behaviour so that, when they are not, the brain can make appropriate changes to adapt our motor planning. Third, the ability to distinguish between sensory exafference and reafference is a hallmark of higher-level perceptual and cognitive processing. Indeed, there is growing interest in the relationship between psychotic illnesses and the ability to distinguish between exafference and reafference as it relates to our subjective awareness of initiating and controlling our own volitional actions in the world (i.e., sense of agency). In this context, a misinterpretation of stimuli as ‘self-generated’ versus ‘externally generated’ has been recently implicated in psychiatric disorders such as schizophrenia (1). In this review, we consider the key role that motor signals play in sensory processing at each of these levels.
Conceptual Framework and Model Systems:
The brain’s ability to distinguish between sensory exafference and reafference has been long appreciated, as was already noted by Helmholtz in the 19th century. Helmholtz made the salient observation that the visual world does not appear to be moving across our retina when we make fast active eye movements. In contrast, tapping on the canthus of our eye to produce movements with comparable velocities creates the illusion that the visual world is moving (2). In order to provide an explanation for why our perception differs in these two conditions, Sperry introduced the concept of “corollary discharge”. Specifically, corollary discharge refers to information that is sent from a motor area to a sensory area to modulate sensory responses (3). Holst and Mittelstaedt concurrently proposed a more specific formulation of this conceptual framework, named the “principle of reafference”. This principle states that, during active movements, a copy of the motor command (i.e., efference copy) is sent to sensory areas to cancel sensory information resulting from active behaviour (4). This idea has become a popular framework for interpreting the result of perceptual studies across many modalities including the visual, somatosensory, vestibular, and auditory systems. For instance, in the example of the visual system discussed above, the principle of reafference has been used to explain why the visual world appears stable during eye movements (5-8).
Over the past several decades, investigations in a number of model systems have provided experimental evidence for the principle of reafference. Example systems include the cricket auditory system, crayfish mechanosensory system, and electrosensory system of the weakly electric momyrid fish. In each of these systems, reafferent sensory stimulation is removed from further processing in order to emphasize and properly interpret externally-generated stimuli (reviewed in 9, 10). Although the strategy of suppressing reafferent information is common across species and sensory systems, the mechanisms underlying cancellation are characterized by important differences. For example, in the cricket auditory system and the crayfish mechanosensory system, reafferent signals are cancelled by motor signals that directly inhibit neurons at the sensory periphery (see Figure 1A red dottedarrow labelled 'efference copy' ,11, 12). In contrast, in the weakly electric mormyrid fish, motor signals do not directly inhibit the periphery but instead inhibit cells in the electrosensory lobe, a cerebellum-like structure that receives afferent sensory information from the periphery as well as efference copy information (13, 14). More recently, a growing number of studies have focused on the processing of self-generated versus externally-applied sensory information in mammalian sensory systems. Below, we review this literature and consider the use of common strategies for the cancellation of sensory reafference, as well as the effects of disruptions of these putative mechanisms/circuits.
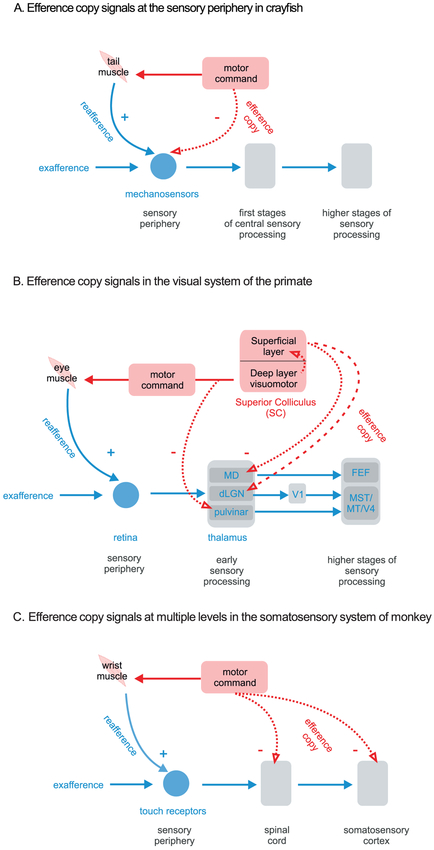
Figure1: Motor signals can influence sensory processing at many levels for a single behavior.
(a) Basic schematic of von Holst and Mittelstaedt's reafference principle illustrated for the crayfish mechanosensory system. In this model, the motor command is sent to both i) the effector muscle (in this case the tail muscle) and ii) to the sensory periphery (i.e. the mechanosensors) (dotted red arrow labeled 'efference copy'). The efference copy of motor command is then subtracted from the incoming sensory signal input, labelled ‘−' and ‘+’, respectively. In situations where sensory inputs and efference copy signals correspond, they will cancel each other out, so that in turn no sensory information relayed to higher levels for further processing. In contrast, in situations where there is a difference between sensory inputs and efference copy signals, this difference – representing externally-generated sensory information (i.e. exafference) - is processed further in higher- order sensory areas. (b) Simplified scheme illustrating sensory-motor convergence in the visual system of the primate during saccadic eye movements. In this scheme, motor signals only weakly modulate responses of neurons early visual processing (i.e., the dLGN of thalamus; (99). However, motor signals strongly influence visual responses at higher levels of processing via the superior colliculus (SC). Specifically, the SC not only sends projections to the premotor pathways to control saccades, but also to the pulvinar (red dashed arrows) where it mediates i) saccadic suppression through the SC-Pulvinar-MT pathway AND ii) shifting of visual receptive fields just before a saccade through the SC-MD thalamus FEF pathway. Together these pathways ensure perceptual stability during eye movements. (c) Simplified scheme illustrating sensory-motor convergence at multiple levels in the somatosensory system of the monkey. In this diagram, a motor signal is sent not only to the muscle controlling the wrist to move the hand but copies are also to both the spinal cord AND cerebral cortex to suppress self-generated somatosensory signals (red dashed arrows). This interaction of motor and sensory signals at multiple levels of processing (e.g. spinal cord and cerebral cortex) likely provides the flexibility required for fine-tuning and updating relationships between motor signals and the resultant sensory feedback during our everyday activities.
Visual System:
During everyday activities, we continually make rapid eye movements (saccades) to scan and explore our visual world. We typically make several saccadic eye movements each second, which can reach impressive speeds (15). This raises a basic question: How does the brain ensure perceptual stability of the visual environment despite such rapid movements of the eyes? Indeed, this topic has been the focus of many well-known scientists of the last century including von Helmholtz, Hering, Mach, and Sherrington (2, 16-18). It is noteworthy that rapid saccadic eye movements will cause both i) a blur on the retina during the saccade and ii) a change in retinal position of objects after as compared to before the saccade. To maintain perceptual stability during the saccade (i.e. avoid image blur on the retina), numerous behavioural studies have shown that our sensitivity to visual stimulation is attenuated during and just before saccadic eye movements. Results of human psychophysical studies suggest that this ‘saccadic suppression’ is the most robust for transient motion-related visual stimuli that preferentially activate the magnocellular visual processing stream (19).
Electrophysiological studies have found little evidence for motor-based suppression of visual input at the first central stages of processing in the dorsal lateral geniculate nucleus (dLGN) of the thalamus and primary visual cortex (area V1) (reviewed in 5). While neurons in the dLGN (20, 21) and V1 (22, 23) demonstrate saccade related modulation, the strength of the observed effects is relatively minor at both these stages, suggesting that the vast majority of suppression occurs at levels beyond the LGN and V1. Indeed, studies have found that the spiking activity of neurons in cortical areas of rhesus monkeys, including V4, medial temporal (MT) and medial superior temporal (MST) cortex, display greater saccade-related suppression than V1 neurons (24, 25). Specifically, the visual responses of neurons in these areas are reduced for image motion during eye movements versus image motion during fixation, consistent with the idea that these neurons discriminate between real and self-generated visual motion (24-29).
The question then arises: what mechanism/pathway leads to saccadic suppression at these higher levels of visual processing? A likely source of this extra-retinal signal is a pathway from the superior colliculus (SC) (Figure 1b; 5, 10). Specifically, the visual responses of neurons in the superficial layers of SC, which receive their primary inputs from the retina and primary visual cortex, are suppressed during saccades (30). This suppression is likely mediated via projections from motor-related neurons in the deeper SC layers, which are known to drive saccadic eye movements (31-35). In turn, the superficial layers of SC project via the pulvinar nucleus of the thalamus to extrastriate visual cortical area including MT (36) and V4 (37). Accordingly, this SC-pulvinar-MT/V4 pathway appears to play a key role in saccadic suppression at these higher levels of visual cortical processing. Interestingly, the saccade-related suppression observed in superficial layer SC neurons is higher for lower spatial frequencies (38), consistent with the view that saccadic suppression is most prominent in the magnocelluar processing stream. Functional Imaging studies (fMRI) in humans have reported markedly similar conclusions, namely that neurons in areas MT and V4, but not V1, discriminate between real and saccade-generated visual motion (39). Furthermore, the use of transcranial stimulation to probe the site of saccadic suppression has provided evidence that a mechanism consistent with the SC-pulvinar-MT pathway described in monkeys provides a motor related cancellation signal to higher cortical areas in humans (40).
A second challenge to maintaining perceptual stability is reconciling retinal object positions before and after saccades. Interestingly, studies have identified a pathway carrying efference copy information to neurons in a visual cortical area that is also involved in the control of saccadic and fixational eye movements. Specifically, this pathway originates in the SC and projects to the frontal eye fields (FEF), through the medial dorsal nucleus of the thalamus (Figure 1B). The saccade-related information (amplitude and direction of saccade) provided by this pathway triggers a shift of the receptive field’s (RF) location just before a saccade from a neurons’ current RF location to that of the future visual field where the RF will rest after the saccade. This enables the neuron to sample the future receptive field location before and after the saccade, which is thought to contribute to visual stability. Consistent with a causal role in reconciling retinal object positions before and after saccades, inactivation of this pathway at the level of the thalamus reduces this presaccadic shift in RF location of FEF neurons (reviewed in 41).
Importantly, visual input to our brain is altered not only by saccades, but also by other types of eye movements we make to scan and interact with our environment. For example, we commonly make voluntary smooth pursuit eye movements to track moving visual targets of interest. The evidence available to date suggests that neurons in higher levels of processing such as ventral intraparietal area (VIP) (42), but not V1 (43, 44), discriminate between real and pursuit-generated visual motion. Thus, similar to the mechanism described above for saccades, pursuit motor signals appear to be primarily integrated with visual information at higher cortical areas to ensure stable vision in the presence of eye movements. Finally, it is also important to consider that eye movements are not the only behaviour that will influence the input to the visual system. For example, locomotion will produce optic flow stimulation as we navigate the world. Recent studies in the mouse visual system, which have controlled the relationship between running speed and optic flow, concluded that visual stimulation alone cannot explain the firing patterns of primary visual cortex neurons. Notably, the visual responses of V1 neurons show strong changes during locomotion (45-49). Specifically, neuronal responses are enhanced when the animal views the same sensory input during walking as compared to when it is stationary. Experiments using intracellular recordings as well as optogenetics have provided insight into cellular mechanisms that underlie this state-dependent enhancement of gain (45-49), however future work will be required to fully understand the underlying circuit.
Taken together studies of the visual system have provide clear evidence that both eye motor signals and signals relating to behavioural state during locomotion are an integral part of visual sensory processing that is essential for ensuring visual stability during our daily activities. These sensory-motor interactions likely underlie our ability to distinguish external and self-generated visual sensory information during daily activities, including both the generation of active eye movements and navigation.
Somatosensory System:
The somatosensory system detects stimuli that provide us with our perception of touch (i.e. pressure on our skin) and proprioception (i.e. position and movement of our muscles). Importantly, this sensory system is also stimulated as we actively move and interact with the world. In fact, our own movements control the nature of the input encountered by the somatosensory system. Gibson observed that the stability of an object determines the constancy of our tactile perception. For instance, if we move the surface of our finger over a corner of an object we usually do not perceive ‘tactile motion’ despite the displacement of the location of pressure relative to the skin (50). Furthermore, self-generated tactile stimulation in human subjects does not result in the same tickling sensation that arises when stimulation is externally produced (51-53). Moreover, experimentally introducing an artificial trajectory perturbation or temporal delay between a movement and the resultant tactile stimulation, will result in self-generated stimulation being perceived as feeling more ticklish (i.e., externally generated) (52). These results have led to the proposal that when sensory sensation does not correspond to what is expected based on the motor command that was generated, the cancellation signal cannot fully cancel the distorted reafference (53). Consistent with this view, self-generated forces are perceived as significantly weaker (i.e., reduced by about half) as compared to externally generated forces of the same magnitude (54).
To understand the interactions between motor and somatosensory signals, experiments have examined the influence of active movements on sensory coding in spinal cord somatosensory neurons and afferents. In alert behaving primates, touch inputs are pre-synaptically inhibited at the level of the spinal cord afferents during active wrist movements (55, 56). This inhibition is strong and precedes electromyographic activity, suggesting that descending motor commands (rather than peripheral feedback from the motion itself) play the dominant role in generating inhibition (55). Somatosensory information is correspondingly suppressed at subsequent stages of processing in the dorsal column-medial lemniscus pathway, the ascending pathway, which conveys touch information from the spinal cord to the somatosensory nuclei of the thalamus (55-60).
Single unit recording experiments in primate have further established that the transmission of self-generated touch information is also attenuated or ‘gated-out’ at the level of somatosensory cortex (61). A recent study (62) examined the mechanisms underlying this sensory gating by electrically stimulating touch afferents while measuring sensory evoked potentials in the spinal cord, as well as across several cortical areas (e.g. primary motor, pre-motor and somatosensory cortex) as monkeys made active limb movements. Suppression preceded movement not only in the spinal cord but also in all cortical areas studied (Figure 1C). Thus, this result is consistent with the finding discussed above that top down mechanisms (e.g., mediated by presynaptic inhibition) play a key role in gating the transmission of self-generated touch information. In addition, bottom up reafference-based mechanisms may also contribute to sensory transmission reduction after movement onset (62).
In addition to sensing touch, the somatosensory system also encodes proprioceptive signals, which provide essential information about the position and movement of limbs. Interestingly, however, proprioceptive inputs are facilitated rather than suppressed at the level of the spinal cord during relevant active movements (63). Such movement-specific facilitation is observed at successive levels of processing along the dorsal column-medial lemniscus pathway to the somatosensory thalamus (59, 63, 64). To examine the cortical coding of proprioceptive signals during active movements, London and Miller recorded in area 2 of primary somatosensory cortex - a region known to have many cells responsive to proprioceptive inputs (65). Neuronal responses were heterogonous with some responding to both active and passive limb movements, others responding only to passive limb movements, and still other responding only during active limb movements. Notably, neuronal responses during active movements began well before the actual onset of voluntary movements, suggesting the integration of proprioceptive and motor-related signals in primary somatosensory cortex (66).
Furthermore, other important pathways also transmit proprioceptive information to higher-order structures, in addition to the dorsal column-medial lemniscus system. In the present context, spinocerebellar pathways are of particular interest since the prevailing view is that the convergence of sensory and motor pathways in the cerebellum underlies the computation of sensory prediction error signals to drive motor learning (67, 68). Recent experiments in mice have shown that spinocerebellar neurons in Clarke’s column of the spinal cord integrate proprioceptive inputs with motor-related inputs via the descending corticospinal tract (69). In addition, spinocerebellar neurons in the ventral spinal cord receive direct excitatory and/or indirect inhibitory signals from corticospinal neurons (70). These two groups of spinocerebellar neurons comprise circuits where motor commands modulate incoming proprioceptive signals. Interestingly, individual spinal interneurons can project both to cervical motor neurons that control forelimb movements and to the lateral reticular nucleus (a pre-cerebellar relay nucleus) (70). Selectively disrupting this cerebellar relay impairs rapid updating of voluntary reaching behaviour, consistent with the role of sensory-motor integration underlying motor learning. Further studies of the specific circuits underlying the integration motor signals and proprioceptive sensory signals at these early stages of somatosensory processing will be fundamental to understanding how the brain ensures accurate motor control. In this context, it is further likely that attention/context -related factors can further modulate sensory processing reviewed in (8, 71), for example facilitating the blind’s ability to read Braille.
In summary, the integration of sensory and motor signals to ensure perceptual stability and accurate motor control is a hallmark of processing in the somatosensory system. Notably, however, there are important differences in the processing of touch versus proprioceptive information during daily activities; touch inputs are suppressed while proprioceptive inputs are facilitated at the level of the spinal cord during active movements. Thus, the integration of motor inputs with somatosensory information is both a behaviour- and modality-specific process.
Vestibular System:
The vestibular system detects our head motion and orientation relative to space. In turn, this information plays a fundamental role in the stabilization of gaze, control of balance and posture and our sense of self-motion and orientation during everyday life. Our ability to distinguish active self-motion from passive self-motion is essential for generating accurate behavioural responses as well as for ensuring perceptual stability. Interestingly, recent studies in the primate vestibular system have revealed that vestibular reafference is cancelled by a sophisticated mechanism at the first central stage of processing.
Vestibular afferents carry information from receptor cells within the semicircular canals and otolith sensory organs of the inner ear to the brain. While afferents encode head motion in the same way regardless of whether it is actively generated or passively applied (72-74), neurons at the next stage of processing in the vestibular nuclei preferentially respond to passive head motion. Specifically, vestibular signals are cancelled in a specific neural population that receives direct afferent input and in turn project both to the spinal cord and thalamus (termed VO neurons) during active head movements (reviewed in 75). Vestibular reafference cancellation occurs in VO neurons for both rotations and translations (76-79) and across species including mice (80), cats (81), and new and old world monkeys (82, 83).
Theoretically, during active motion, there are several extra-vestibular inputs that could contribute to the cancellation of vestibular reafference, including: i) motor efference copy signals, ii) neck proprioceptive feedback, and/or iii) the animal’s explicit knowledge that a movement will occur. To understand the mechanism underlying this cancellation, Roy and Cullen completed a series of experiments to explicitly dissociate the influence of each of these extra-vestibular inputs on neuronal responses during head rotations (77). They found that none of these signals alone directly influence the firing rate of VO neurons. Instead, vestibular reafference is only cancelled in conditions where there is a precise match between the actual proprioceptive feedback and that expected based on the neck motor command that generated the head motion - as is the case during normal active head movements (Fig. 2).
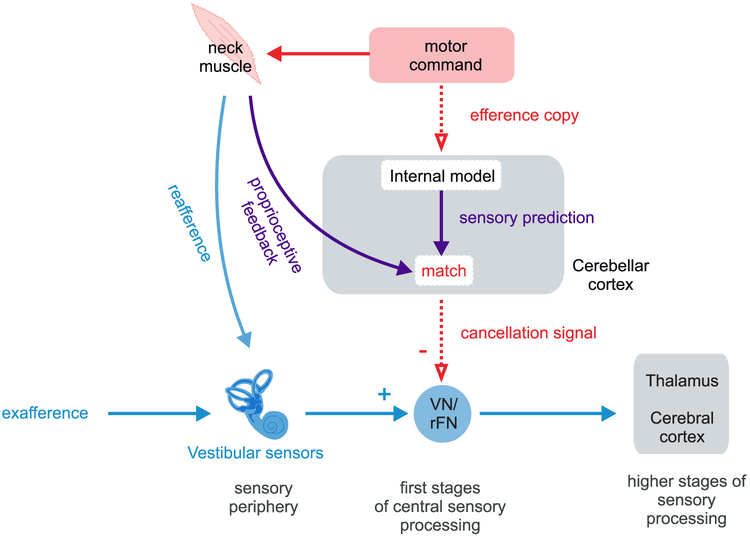
Figure 2: Proposed model for cancellation of sensory reafference in the vestibular system.
In this model, a motor command is sent i) to the neck muscle to move the head, and also ii) to areas that generate internal models of the sensory consequences of active movements, resulting in a prediction of the sensory feedback expected as a result of the head movement command. In situations where there is a match between expected and actual proprioceptive sensory input – as would be the case during normal active head movements - a cancellation signal (negative image of the vestibular afferent signal) is sent to vestibular-only (VO) neurons in the vestibular nuclei (VN) and to the rostral fastigial nuclei (rFN) of the cerebellum to suppress the self-generated vestibular inputs. In situations where actual and predicted sensory inputs do not match, vestibular signals are not suppressed. It is notable that the brain uses a multimodal approach, combining inputs from the vestibular and proprioceptive systems, to both sense self-motion and to suppress the representation of actively generated self-motion.
The first evidence for this model came from an experiment in which monkeys made active head movements, but the monkey was simultaneously rotated with the same velocity profile in the opposite direction to minimize head-in-space velocity (77). In this condition, activation of the vestibular system was negligible but the head moved relative to the body such that there was a match between the actual and expected activation of neck proprioceptors. Because the vestibular system was not stimulated in this condition, a cancellation signal was evident in the form of suppressed VO neuron activity, consistent with the hypothesis schematized in Figure 2. Additional evidence for the model in Fig. 2 came from experiments in which monkeys generated active head movements while external perturbations were simultaneously applied to their heads. The applied perturbation altered the relationship between the motor command and resultant movement, thereby causing a mismatch in sensory feedback (see Figure 2), and, in turn, neurons robustly responded to sensory input even though it was actively generated (84, 85). Experiments focused on the deep cerebellar nuclei have further demonstrated that a cerebellum-based mechanism underlies vestibular reafference cancellation, and that this mechanism is capable of rapid updating. In particular, the vestibular cerebellum can learn to expect modified vestibular inputs when a new relationship is established between motor output and the resultant proprioceptive feedback (84).
Functionally, because VO neurons mediate vestibulo-spinal reflexes, vestibular reafference cancellation underlies behaviour-dependent reflex suppression during active movements. When vestibular inputs are unexpected (i.e., vestibular exafference) the corrective movements generated by vestibulo-spinal reflexes are essential for maintaining balance. In contrast, when similar vestibular input is the result of active movement (i.e., vestibular reafference), vestibulospinal reflexes are gated-out because they are counterproductive; if they remained intact, they would actually counteract the intended movement. In addition, VO neurons project to the vestibular thalamus. Recent studies have further shown that, like their VO neuron inputs, thalamocortical vestibular pathways selectively encode vestibular exafference (86). This selectivity thus also ensures perceptual stability in everyday life by preferentially signalling externally generated (unexpected) self-motion as compared to self-motion that is the consequence of our own actions. Finally, it is noteworthy that vestibular reafference cancellation is not seen in all neuron classes in the vestibular nuclei. In addition to the vestibulo-spinal reflex, the vestibular system generates the vestibulo-ocular reflex (VOR), to stabilize the visual axis of gaze during head movements. Unlike VO neurons, the responses of vestibular nuclei VOR neurons are not suppress during active movements, consistent with their role in maintaining stable gaze during everyday activities.
In summary, active movements influence the processing of vestibular information at multiple levels in order to prevent unnecessary and counterproductive reflex generation, allow for perceptual stability during our daily activities, and allow for accurate and rapidly updated motor plans via sensory-motor interactions within the cerebellum.
Auditory System:
The vertebrate auditory sensory organ, the cochlea, evolved from precursors of the vestibular system (87). Thus, one might predict the auditory system, like the vestibular system, would selectively cancel sensory inputs produced by our own behaviour. Auditory afferents send direct projections from the cochlea to the cochlear nuclei of the brainstem. Notably, afferents project to both the ventral and dorsal cochlear nuclei (VCN and DCN). The DCN also receives strong input from the VCN and also has a cerebellum-like circuitry. A recent study in mice found that while VCN neurons responded in the same way to externally and self-produced licking sounds, DCN neuron responses were strongly suppressed during self-produced licking (88). Interestingly, the source of cancellation signal was identified as a specific class of inhibitory interneurons in the DCN (i.e., cartwheel cells), which share common features with cerebellar Purkinje cells (89). At higher stages of auditory processing, intracellular recording experiments in both mice and marmosets have shown reduced activation of auditory cortex neurons during natural voluntary behaviours including active grooming, head turning, and vocalizations (90). Inputs from inhibitory interneurons in secondary motor cortex likely contribute, at least in part, to this suppression (91). Recent studies using EEG and ECoG in humans have similarly found that responses to self-produced speech are reduced relative to responses to replays of previously recorded speech (92-94). Interestingly, however, no changes in modulation were observed before 100ms suggesting the resolution EEG/ECoG does not allow assessment of the motor-related suppression observed in the early stages of the mouse auditory system pathway.
Common Strategies and Conclusions:
As detailed above, the strategy of integrating motor signals with sensory information during voluntary behaviour is essential for sensory processing. However, across systems and species, there are some important differences. For example, cancellation of reafferent sensory inputs in model systems including the cricket auditory system, crayfish mechanosensory system, and mormyrid fish electrosensory system (reviewed in 9) are largely consistent with the classic view of the reafference principle. In this view, a motor-based signal is sent to cancel self-generated sensory inputs regardless of whether the motor command was successfully executed (e.g., Fig. 1A). Interestingly, more recent studies suggest that a similar mechanism contributes to reafferent cancellation of auditory inputs within the dorsal cochlear nucleus (88), as well as touch-related inputs in the spinal neurons (55). In contrast, reafferent vestibular input is cancelled if and only if the actual sensory feedback matches the brain’s estimate of the sensory consequence of a voluntary movement. This unique, more sophisticated mechanism allows the brain to selectively emphasize unexpected vestibular inputs that occur due to externally-imposed motion (exafference). By computing the difference between expected and actual sensory feedback, this mechanism also contributes to the brain’s ability to rapidly update its motor plan to adapt to new external forces and ensure accurate motor control in changing conditions (84). Interestingly, this vestibular reafference cancellation mechanism depends on a match between the actual and expected proprioceptive feedback – a requirement that is consistent with the fact that proprioceptive inputs from spinal neurons are actually facilitated rather than suppressed during active movements (63).
Finally, recent studies have provided further circuit-level insight into the computations required for reafference suppression. First, as reviewed above, the vestibular cerebellum (84, 95) and cerebellum-like dorsal cochlear nucleus of the auditory system (88) play key roles in reafference cancellation at the earliest stages of central sensory processing. This idea is consistent with previous behavioural and imaging studies in normal subjects and cerebellar patients (53, 96, 97). On the other hand, recent studies in visual (5, 10), touch (62), and auditory (90) systems have emphasized that motor-related inputs can also suppress reafferent sensory responses at the level of cortex. Thus, taken together, results to date suggest sensory-motor convergence all along sensory processing pathways (e.g., Figs. 1B,C & 2). We speculate that this strategy provides the flexibility required for fine-tuning and updating relationships between motor signals and the resultant sensory feedback during our everyday activities. Indeed, continuous dynamic calibration of this computation is vital for lower level functions such as reflex generation as well as accurate perception/cognition. Given that the brain’s ability to distinguish between exafference and reafference is a hallmark of higher-level perceptual and cognitive function it also potentially provides a framework to consider the origin of our sense of agency: the subjective awareness that we control both our actions and their sensory consequences (reviewed in 98).
Acknowledgments:
The authors would like to thank Dr. Lin Wang, Omid Zobeiri, Oliver Stanley, Kantapon Pum Wiboonsaksakul, Graham McAllister and Vanessa Chang and for their helpful comments.
Funding: Canadian Institutes of Health Research (CIHR) and National Institutes of Health (NIH) DC2390.
Footnotes
Financial Disclosures: Drs. Brooks and Cullen reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford JM, Mathalon DH (2005): Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int J Psychophysiol. 58:179–189. [DOI] [PubMed] [Google Scholar]
- 2.Helmholtz HV (1925): Handbuch der Physiologischen Optik [Treatise on PhysiologicalOptics]. Southall: Edition ed.: JPC. [Google Scholar]
- 3.Sperry RW (1950): Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 43:482–489. [DOI] [PubMed] [Google Scholar]
- 4.von Holst E, Mittelstaedt H (1950): Das reafferenzprinzip. Naturwissenschaften. 37:464–476. [Google Scholar]
- 5.Wurtz RH (2008): Neuronal mechanisms of visual stability. Vision Res. 48:2070–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wurtz RH (2018): Corollary Discharge Contributions to Perceptual Continuity Across Saccades. Annu Rev Vis Sci. 4:215–237. [DOI] [PubMed] [Google Scholar]
- 7.Rao HM, Mayo JP, Sommer MA (2016): Circuits for presaccadic visual remapping. J Neurophysiol. 116:2624–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P (2010): Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol. 20:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen KE (2004): Sensory signals during active versus passive movement. Curr Opin Neurobiol. 14:698–706. [DOI] [PubMed] [Google Scholar]
- 10.Crapse TB, Sommer MA (2008): Corollary discharge across the animal kingdom. Nat Rev Neurosci. 9:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards DH, Heitler WJ, Krasne FB (1999): Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 22:153–161. [DOI] [PubMed] [Google Scholar]
- 12.Krasne FB, Bryan JS (1973): Habituation: regulation through presynaptic inhibition. Science. 182:590–592. [DOI] [PubMed] [Google Scholar]
- 13.Meek J, Grant K, Bell C (1999): Structural organization of the mormyrid electrosensory lateral line lobe. J Exp Biol. 202:1291–1300. [DOI] [PubMed] [Google Scholar]
- 14.Mohr C, Roberts PD, Bell CC (2003): The mormyromast region of the mormyrid electrosensory lobe. I. Responses to corollary discharge and electrosensory stimuli. J Neurophysiol. 90:1193–1210. [DOI] [PubMed] [Google Scholar]
- 15.Westheimer G (1954): Eye movement responses to a horizontally moving visual stimulus. AMA Arch Ophthalmol. 52:932–941. [DOI] [PubMed] [Google Scholar]
- 16.Mach E (1875): Grundlinien der Lehre von den Bewegungsempfindungen. (Leipzig: Engelmann; ). [Google Scholar]
- 17.Hering E (1879): “Der Raumsinn und die Bewegungen des Auges”, in Handbuch der Physiologie der Sinnesorgane (Leipzig: Vogel; ) [Google Scholar]
- 18.Sherrington CS (1906): The Integrative Action of the Nervous System. New York: Charles Scribner’s Sons;. [Google Scholar]
- 19.Burr DC, Morrone MC, Ross J (1994): Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature. 371:511–513. [DOI] [PubMed] [Google Scholar]
- 20.Ramcharan EJ, Gnadt JW, Sherman SM (2001): The effects of saccadic eye movements on the activity of geniculate relay neurons in the monkey. Vis Neurosci. 18:253–258. [DOI] [PubMed] [Google Scholar]
- 21.Reppas JB, Usrey WM, Reid RC (2002): Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron. 35:961–974. [DOI] [PubMed] [Google Scholar]
- 22.McFarland JM, Bondy AG, Saunders RC, Cumming BG, Butts DA (2015): Saccadic modulation of stimulus processing in primary visual cortex. Nat Commun. 6:8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troncoso XG, McCamy MB, Jazi AN, Cui J, Otero-Millan J, Macknik SL, et al. (2015): V1 neurons respond differently to object motion versus motion from eye movements. Nat Commun. 6:8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiele A, Henning P, Kubischik M, Hoffmann KP (2002): Neural mechanisms of saccadic suppression. Science. 295:2460–2462. [DOI] [PubMed] [Google Scholar]
- 25.Zanos TP, Mineault PJ, Guitton D, Pack CC (2016): Mechanisms of Saccadic Suppression in Primate Cortical Area V4. J Neurosci. 36:9227–9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson RG, Thier P (1991): A neuronal correlate of spatial stability during periods of self-induced visual motion. Exp Brain Res. 86:608–616. [DOI] [PubMed] [Google Scholar]
- 27.Shenoy KV, Bradley DC, Andersen RA (1999): Influence of gaze rotation on the visual response of primate MSTd neurons. J Neurophysiol. 81:2764–2786. [DOI] [PubMed] [Google Scholar]
- 28.Chukoskie L, Movshon JA (2009): Modulation of visual signals in macaque MT and MST neurons during pursuit eye movement. J Neurophysiol. 102:3225–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman RA, Cavanaugh J, McAlonan K, Wurtz RH (2017): A circuit for saccadic suppression in the primate brain. J Neurophysiol. 117:1720–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson DL, Wurtz RH (1976): Use of an extraretinal signal by monkey superior colliculus neurons to distinguish real from self-induced stimulus movement. J Neurophysiol. 39:852–870. [DOI] [PubMed] [Google Scholar]
- 31.Behan M, Lin CS, Hall WC (1987): The nigrotectal projection in the cat: an electron microscope autoradiographic study. Neuroscience. 21:529–539. [DOI] [PubMed] [Google Scholar]
- 32.Behan M, Appell PP (1992): Intrinsic circuitry in the cat superior colliculus: projections from the superficial layers. J Comp Neurol. 315:230–243. [DOI] [PubMed] [Google Scholar]
- 33.Behan M, Kime NM (1996): Intrinsic circuitry in the deep layers of the cat superior colliculus. Vis Neurosci. 13:1031–1042. [DOI] [PubMed] [Google Scholar]
- 34.Hall WC, Lee P (1997): Interlaminar connections of the superior colliculus in the tree shrew. III: The optic layer. Vis Neurosci. 14:647–661. [DOI] [PubMed] [Google Scholar]
- 35.Doubell TP, Skaliora I, Baron J, King AJ (2003): Functional connectivity between the superficial and deeper layers of the superior colliculus: an anatomical substrate for sensorimotor integration. J Neurosci. 23:6596–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman RA, Wurtz RH (2008): Exploring the pulvinar path to visual cortex. Prog Brain Res. 171:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattass R, Galkin TW, Desimone R, Ungerleider LG (2014): Subcortical connections of area V4 in the macaque. J Comp Neurol. 522:1941–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CY, Hafed ZM (2017): A neural locus for spatial-frequency specific saccadic suppression in visual-motor neurons of the primate superior colliculus. J Neurophysiol. 117:1657–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleiser R, Seitz RJ, Krekelberg B (2004): Neural correlates of saccadic suppression in humans. Curr Biol. 14:386–390. [DOI] [PubMed] [Google Scholar]
- 40.Thilo KV, Santoro L, Walsh V, Blakemore C (2004): The site of saccadic suppression. Nat Neurosci. 7:13–14. [DOI] [PubMed] [Google Scholar]
- 41.Sommer MA, Wurtz RH (2008): Brain circuits for the internal monitoring of movements. Annu Rev Neurosci. 31:317–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T, Heuer HW, Britten KH (2004): Parietal area VIP neuronal responses to heading stimuli are encoded in head-centered coordinates. Neuron. 42:993–1001. [DOI] [PubMed] [Google Scholar]
- 43.Galletti C, Squatrito S, Battaglini PP, Grazia Maioli M (1984): ‘Real-motion’ cells in the primary visual cortex of macaque monkeys. Brain Res. 301:95–110. [DOI] [PubMed] [Google Scholar]
- 44.Ilg UJ, Thier P (1996): Inability of rhesus monkey area V1 to discriminate between self-induced and externally induced retinal image slip. Eur J Neurosci. 8:1156–1166. [DOI] [PubMed] [Google Scholar]
- 45.Keller GB, Bonhoeffer T, Hubener M (2012): Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron. 74:809–815. [DOI] [PubMed] [Google Scholar]
- 46.Niell CM, Stryker MP (2010): Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 65:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polack PO, Friedman J, Golshani P (2013): Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci. 16:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, et al. (2014): A cortical circuit for gain control by behavioral state. Cell. 156:1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M (2013): Integration of visual motion and locomotion in mouse visual cortex. Nat Neurosci. 16:1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibson JJ (1962): Observations on active touch. Psychol Rev. 69:477–491. [DOI] [PubMed] [Google Scholar]
- 51.Blakemore SJ, Wolpert DM, Frith CD (1998): Central cancellation of self-produced tickle sensation. Nat Neurosci. 1:635–640. [DOI] [PubMed] [Google Scholar]
- 52.Blakemore SJ, Frith CD, Wolpert DM (1999): Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci. 11:551–559. [DOI] [PubMed] [Google Scholar]
- 53.Blakemore SJ, Wolpert DM, Frith CD (1999): The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. Neuroimage. 10:448–459. [DOI] [PubMed] [Google Scholar]
- 54.Shergill SS, Bays PM, Frith CD, Wolpert DM (2003): Two eyes for an eye: the neuroscience of force escalation. Science. 301:187. [DOI] [PubMed] [Google Scholar]
- 55.Seki K, Perlmutter SI, Fetz EE (2003): Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci. 6:1309–1316. [DOI] [PubMed] [Google Scholar]
- 56.Fetz EE, Perlmutter SI, Prut Y, Seki K (2002): Functional properties of primate spinal interneurones during voluntary hand movements. Adv Exp Med Biol. 508:265–271. [DOI] [PubMed] [Google Scholar]
- 57.Ghez C, Pisa M (1972): Inhibition of afferent transmission in cuneate nucleus during voluntary movement in the cat. Brain Res. 40:145–155. [DOI] [PubMed] [Google Scholar]
- 58.Coulter JD (1974): Sensory transmission through lemniscal pathway during voluntary movement in the cat. J Neurophysiol. 37:831–845. [DOI] [PubMed] [Google Scholar]
- 59.Tsumoto T, Nakamura S, Iwama K (1975): Pyramidal tract control over cutaneous and kinesthetic sensory transmission in the cat thalamus. Exp Brain Res. 22:281–294. [DOI] [PubMed] [Google Scholar]
- 60.Chapman CE, Jiang W, Lamarre Y (1988): Modulation of lemniscal input during conditioned arm movements in the monkey. Exp Brain Res. 72:316–334. [DOI] [PubMed] [Google Scholar]
- 61.Chapman CE (1994): Active versus passive touch: factors influencing the transmission of somatosensory signals to primary somatosensory cortex. Can J Physiol Pharmacol. 72:558–570. [DOI] [PubMed] [Google Scholar]
- 62.Seki K, Fetz EE (2012): Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci. 32:890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Confais J, Kim G, Tomatsu S, Takei T, Seki K (2017): Nerve-Specific Input Modulation to Spinal Neurons during a Motor Task in the Monkey. J Neurosci. 37:2612–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leiras R, Velo P, Martin-Cora F, Canedo A (2010): Processing afferent proprioceptive information at the main cuneate nucleus of anesthetized cats. J Neurosci. 30:15383–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huffman KJ, Krubitzer L (2001): Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 11:849–867. [DOI] [PubMed] [Google Scholar]
- 66.London BM, Miller LE (2013): Responses of somatosensory area 2 neurons to actively and passively generated limb movements. J Neurophysiol. 109:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebner TJ, Pasalar S (2008): Cerebellum predicts the future motor state. Cerebellum. 7:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ (2007): Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 98:54–62. [DOI] [PubMed] [Google Scholar]
- 69.Hantman AW, Jessell TM (2010): Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci. 13:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azim E, Jiang J, Alstermark B, Jessell TM (2014): Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 508:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stormer VS (2019): Orienting spatial attention to sounds enhances visual processing. Curr Opin Psychol. 29:193–198. [DOI] [PubMed] [Google Scholar]
- 72.Cullen KE, Minor LB (2002): Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci. 22:RC226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadeghi SG, Minor LB, Cullen KE (2007): Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol. 97:1503–1514. [DOI] [PubMed] [Google Scholar]
- 74.Jamali M, Sadeghi SG, Cullen KE (2009): Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. J Neurophysiol. 101:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cullen KE (2012): The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 35:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roy JE, Cullen KE (2001): Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 21:2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roy JE, Cullen KE (2004): Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 24:2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carriot J, Brooks JX, Cullen KE (2013): Multimodal integration of self-motion cues in the vestibular system: active versus passive translations. J Neurosci. 33:19555–19566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carriot J, Jamali M, Brooks JX, Cullen KE (2015): Integration of canal and otolith inputs by central vestibular neurons is subadditive for both active and passive self-motion: implication for perception. J Neurosci. 35:3555–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medrea I, Cullen KE (2013): Multisensory integration in early vestibular processing in mice: the encoding of passive vs. active motion. J Neurophysiol. 110:2704–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCall AA, Miller DM, DeMayo WM, Bourdages GH, Yates BJ (2016): Vestibular nucleus neurons respond to hindlimb movement in the conscious cat. J Neurophysiol. 116:1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gdowski GT, Boyle R, McCrea RA (2000): Sensory processing in the vestibular nuclei during active head movements. Arch Ital Biol. 138:15–28. [PubMed] [Google Scholar]
- 83.Sadeghi SG, Mitchell DE, Cullen KE (2009): Different neural strategies for multimodal integration: comparison of two macaque monkey species. Exp Brain Res. 195:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brooks JX, Carriot J, Cullen KE (2015): Learning to expect the unexpected: rapid updating in primate cerebellum during voluntary self-motion. Nat Neurosci. 18:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brooks JX, Cullen KE (2014): Early vestibular processing does not discriminate active from passive self-motion if there is a discrepancy between predicted and actual proprioceptive feedback. J Neurophysiol. 111:2465–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dale A, Cullen KE (2019): The Ventral Posterior Lateral Thalamus Preferentially Encodes Externally Applied Versus Active Movement: Implications for Self-Motion Perception. Cereb Cortex. 29:305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fritzsch B, Straka H (2014): Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 200:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singla S, Dempsey C, Warren R, Enikolopov AG, Sawtell NB (2017): A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds. Nat Neurosci. 20:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mugnaini E, Berrebi AS, Dahl AL, Morgan JI (1987): The polypeptide PEP-19 is a marker for Purkinje neurons in cerebellar cortex and cartwheel neurons in the dorsal cochlear nucleus. Arch Ital Biol. 126:41–67. [PubMed] [Google Scholar]
- 90.Schneider DM, Nelson A, Mooney R (2014): A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 513:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eliades SJ, Wang X (2008): Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 453:1102–1106. [DOI] [PubMed] [Google Scholar]
- 92.Sitek KR, Mathalon DH, Roach BJ, Houde JF, Niziolek CA, Ford JM (2013): Auditory cortex processes variation in our own speech. PLoS One. 8:e82925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flinker A, Chang EF, Kirsch HE, Barbaro NM, Crone NE, Knight RT (2010): Single-trial speech suppression of auditory cortex activity in humans. J Neurosci. 30:16643–16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Curio G, Neuloh G, Numminen J, Jousmaki V, Hari R (2000): Speaking modifies voice-evoked activity in the human auditory cortex. Hum Brain Mapp. 9:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brooks JX, Cullen KE (2013): The primate cerebellum selectively encodes unexpected self-motion. Curr Biol. 23:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kano M, Kano M, Maekawa K (1991): Binocular interaction and signal components of optokinetic responses of climbing fiber afferents in the cerebellar flocculus and nodulus of the pigmented rabbit. Neurosci Res. 12:151–159. [DOI] [PubMed] [Google Scholar]
- 97.Miall RC, Weir DJ, Wolpert DM, Stein JF (1993): Is the cerebellum a smith predictor? J Mot Behav. 25:203–216. [DOI] [PubMed] [Google Scholar]
- 98.Haggard P (2017): Sense of agency in the human brain. Nat Rev Neurosci. 18:196–207. [DOI] [PubMed] [Google Scholar]
- 99.Ahmadlou M, Zweifel LS, Heimel JA (2018): Functional modulation of primary visual cortex by the superior colliculus in the mouse. Nat Commun. 9:3895. [DOI] [PMC free article] [PubMed] [Google Scholar]