Abstract
Patterns of decreased resting cerebral blood flow (CBF) within the inferior temporal gyri, angular gyri, and posterior cingulate are a feature of aging and Alzheimer’s disease (AD) and have shown to be predictive of cognitive decline among older adults. Fitness and physical activity are both associated with many indices of brain health and may positively influence CBF, however, the majority of research to date has examined these measures in isolation, leaving the potential independent associations unknown. The purpose of this study was to determine the unique contributions of fitness and physical activity when predicting CBF in cognitively healthy adults at risk for AD. One hundred participants (63% female) from the Wisconsin Registry for Alzheimer’s Prevention underwent a maximal exercise test, physical activity monitoring, and a 3-D arterial spin labeling magnetic resonance imaging scan. For the entire sample, fitness was significantly associated with CBF while accounting for physical activity, age, gender, APOE ε4, family history of AD, education, and handedness (p = .026). Further, fitness explained significantly more variance than the combined effect of the covariates on CBF (R2 change = .059; p = .047). These results appear to be gender dependent, our data suggest fitness level, independent of physical activity, is associated with greater CBF in regions that are known to decline with age and AD for female (p = .011), but not male participants.
Keywords: Cardiorespiratory fitness, Physical fitness, VO2peak, Arterial spin labeling, Accelerometer
Introduction
Decreased resting cerebral blood flow (CBF) is a feature of aging and Alzheimer’s disease (AD) (Austin et al. 2011; Okonkwo et al. 2014a). Reductions in CBF measured by arterial spin labeling (ASL) have shown to be predictive of cognitive decline and conversion to AD among older adults (Chao et al. 2010; Wang et al. 2013), and can be detected in cognitively normal adults harboring genetic and/or familial risk for AD (Okonkwo et al. 2014a; Thambisetty et al. 2010; Wierenga et al. 2013). However, inconsistencies in the literature are present with some studies reporting increased CBF, typically in anterior brain regions (e.g. anterior cingulate) (Wierenga et al. 2014). Specific brain regions that are susceptible to declining CBF include the inferior temporal gyri, angular gyri, and posterior cingulate (Chen et al. 2011; Wang et al. 2013). For these reasons, ASL-measured CBF is a promising method for investigating early pathophysiological brain changes in preclinical AD and may be a potential target for interventions aimed at delaying the progression of the disease (Wierenga et al. 2014).
Fitness and physical activity are both positively associated with many indices of brain health (Erickson et al. 2014; Okonkwo et al. 2014b) and may favorably influence CBF (Thomas et al. 2013; Zimmerman et al. 2014). However, whether fitness and physical activity differentially affect CBF remains unclear (Chapman et al. 2013; Pereira et al. 2007). Aerobic fitness is defined as the ability to perform moderate-to-high intensity exercise for prolonged periods of time (ACSM 2013; Caspersen et al. 1985) and is objectively measured by a maximal exercise test where the highest rate of oxygen consumption (V̇O2peak) serves as the gold-standard fitness measure (ACSM 2013). Physical activity is any bodily movement that results in energy expenditure (Caspersen et al. 1985) and can be objectively measured through accelerometry, which records the duration and intensity of movement (Lyden et al. 2014). Although fitness and physical activity are related, they are separate physiological and behavioral measures that may explain unique variance when predicting various health outcomes (Lee et al. 2010).
Limitations of previous research investigating relationships between fitness, physical activity, and brain health include unstandardized fitness testing (Dougherty et al. 2018) and the use of self-reported physical activity questionnaires which are subject to recall and social desirability biases (Dyrstad et al. 2014). Importantly, the vast majority of research to date has examined these measures in isolation, leaving the potential independent contributions that fitness and physical activity may have towards mitigating the pathophysiology of AD largely unknown. Further, there is a paucity of studies that have investigated gender differences related to potential AD therapeutics (Dougherty et al. 2017a; Nebel et al. 2018). The purpose of this study was to determine the unique variance explained by fitness and physical activity when predicting CBF in an older adult population at-risk for AD, and ascertain whether this effect is gender dependent.
Methods
Participants
One hundred cognitively healthy adults from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) participated in this study. WRAP is a longitudinal registry composed of more than 1500 late-middle-aged adults who were cognitively normal, and free of major medical conditions (e.g. neurological diseases, psychiatric disorders) at study entry (Sager et al. 2005). For the current study, participants were determined to be cognitively healthy using the mini-mental state examination (MMSE ≥24), and were excluded for any of the following: documented vascular disease, type I or II diabetes mellitus, severe untreated hypertension (>200/100 mmHg), and the inability to safely walk on a treadmill. All 100 participants completed a maximal exercise test, wore a triaxial accelerometer during waking hours for one week, and underwent ASL magnetic resonance imaging (MRI). Similar to the overall WRAP cohort, this study’s sample was enriched with persons who have a family history (FH) of AD (71%) or who were APOE ε4 positive (43%). The University of Wisconsin Institutional Review Board approved all study procedures and informed consent was obtained from all individual participants.
Fitness assessment
Exercise tests were conducted by a certified exercise physiologist along with a trained exercise specialist following a resting twelve-lead electrocardiogram (ECG) assessment. The exercise test was completed on a motor driven treadmill using a modified Balke protocol (Balke and Ware 1959). Comfortable walking speeds were determined during a warm-up period and kept constant for the duration of testing. The majority of participants walked at 3.5 miles per hour. However, if the participant indicated that this speed was uncomfortable, a slower walking speed was chosen. Following a 2-min warm-up at 0% grade, the grade of the treadmill was increased by 2.5% every 2 min until the participant reached volitional exhaustion or indicated they could no longer continue.
During the exercise test, oxygen consumption (V̇O2), carbon dioxide production (V̇CO2), minute ventilation (V̇E), respiratory exchange ratio (RER) and work rate were obtained using a metabolic cart (TrueOne® 2400 Parvomedics, Sandy, UT) and a two-way non-rebreathing mask. Heart rate (HR) was continuously measured through a twelve-channel ECG device (Schiller CS-200 Exercise Stress System, Baar, Switzerland). The metabolic cart was calibrated using standard procedures within 4h of the exercise test to ensure accuracy. Inclusion criteria for a valid exercise test was determined based on American College of Sports Medicine (ACSM) criteria of meeting at least two of the following: (1) RER ≥ 1.1, (2) change in V̇O2 < 200 ml with an increase in work, (3) rating of perceived exertion (RPE) of 17 or greater (Borg 1982), and (4) achieving at least 90% of age predicted maximal heart rate (220-age) (ACSM 2013). These methods were implemented to avoid potential age-driven relationships when volitional effort was not achieved (Dougherty et al. 2018). Aerobic fitness was defined as the highest oxygen consumption (V̇O2peak, ml/kg/min) value recorded during the final stage of the exercise test when criteria were met.
Physical activity assessment
All participants wore a triaxial GT3X+ accelerometer (Actigraph, Pensacola, FL) on their hip for 7 consecutive days following the graded exercise test. Participants were instructed to wear the device during all waking hours, with the exception of when showering, swimming, or bathing. Standard accelerometry inclusion criteria consisted of at least 10 h of valid wear time per day for a minimum of 3 weekdays and 1 weekend day (Troiano et al. 2008). Accelerometer data (in 1-s epochs) were processed using the validated Sojourn-3 Axis method which uses an artificial neural net to identify boundaries between activities of different intensities and to estimate metabolic equivalents (METs) for each bout (Lyden et al. 2014). To calculate minutes spent in different intensity categories of physical activity, estimated METs were determined for each bout interval and then separated into physical activity categories accordingly: <1.5 METs = sedentary, 1.5–2.99 METs = light, 3–6 METs = moderate and > 6 METs = vigorous. Based on current physical activity recommendations (2018 Physical Activity Guidelines Advisory Committee; Piercy et al. 2018), our chosen physical activity measure was time spent engaged in moderate-to-vigorous physical activity which was expressed as a percentage of total accelerometer wear time to account for individual differences.
Neuroimaging protocol
The MRI scans were acquired in the axial plane on a GE X750 Discovery 3.0 T scanner with an eight-channel phased array head coil (General Electric, Waukesha, WI). Three-dimensional (3-D) T1-weighted inversion recovery-prepared spoiled gradient echo scans were collected using the following parameters: inversion time (TI)/echo time (TE)/repetition time (TR) = 450 ms/3.2 ms/8.2 ms, flip angle = 12°, slice thickness = 1 mm no gap, field of view (FOV) = 256, matrix size = 256 × 256. Resting CBF assessments were made using background suppressed pseudocontinuous ASL (pcASL) (Ye et al. 2000; Dai et al. 2008). The protocol featured a 3-D fast spin echo spiral sequence that utilizes a stack of variable-density spiral 4-ms readout and 8 interleaves. Scan parameters included TE/TR = 10.5 ms/4900 ms, slice thickness = 4 mm no gap, FOV = 240, matrix size = 128 × 128, number of excitations = 3, and labeling radiofrequency (RF) amplitude = 0.24 mG. Multislice spin labeling was implemented using a single coil that eliminates off-resonance errors (Garcia et al. 2005) and post-labeling delay was 2025 ms. The three acquisitions that comprise the pcASL sequence (i.e., NEX = 3) were averaged to improve signal-to-noise ratio. The pcASL sequence also included a fluid-suppressed proton density (PD) acquisition, with the same imaging sequence/image slab location as the pcASL but without the RF-labeling preparation, for CBF flow quantitation and image registration. The entire pcASL sequence—all 3 excitations plus PD scan—lasts 4 min and 27 s. To preserve the fidelity of the CBF assessment, scanning was done after a minimum 4-h fast from food, tobacco, caffeine, and medications with vasomodulatory properties. We have previously reported excellent test-retest reliability (r > 0.95) of this pcASL procedure (Xu et al. 2010).
Image processing
The CBF images were processed using SPM12 (www.fil.ion.ucl.ac.uk/spm). The procedure essentially involved registering each participant’s co-localized PD image to their T1 volume, applying the derived transformation matrix to their average quantitative CBF map, then spatially normalizing the T1 volume and associated CBF image to the Montreal Neurological Institute (MNI) template, with resampling to a 2 × 2 × 2 mm3 voxel size. The normalized CBF maps were then smoothed using an 8-mm full-width at half-maximum Gaussian kernel. To focus our analyses on brain regions known to be implicated in AD and reduce the risk of false-positive errors, we used the Alzheimer’s Disease Neuroimaging Initiative (ADNI) FDG Meta-ROI suite to extract CBF values from five AD-relevant regions of interest (ROI) (Landau et al. 2011). The Meta-ROI suite was developed based on a meta-analysis and has shown to discriminate between cognitively healthy, mild cognitive impairment (MCI) and AD participants (Landau et al. 2011). The ROIs identified included the left and right inferior temporal gyri, the left and right angular gyri, and the posterior cingulate. A composite calculated by averaging extractions from all five ROIs served as our CBF measure of interest. Although originally developed for FDG PET data (Landau et al. 2011), the ADNI Meta-ROI suite has shown to be sensitive to age and AD-related CBF changes (Chen et al. 2011; Wang et al. 2013). Additionally, global CBF was extracted to account for individual CBF variability. The composite CBF measure is expressed in both absolute (ml/100 g/min) and relative terms (i.e., scaled to the global CBF measure to account for individual CBF variability). The mean ± SD time between the fitness / physical activity assessment and MRI was 0.32 ± 0.35 years.
Statistical analyses
A model assumption test revealed that physical activity was non-normally distributed (positive skewness). To address the normality violation we subjected the physical activity variable to a log base 10 transformation. Independent-sample t tests (or χ2 tests, as applicable) were used to compare demographics between males and females. Pearson correlations were used to determine the associations between age, fitness, physical activity, and CBF. Hierarchical multiple linear regression was used to assess the independent relationships between CBF and both fitness and physical activity while adjusting for the following covariates: age, gender, APOE-ε4 status, FH status, education and handedness with the significance level set at 0.05. Model 1 included the covariates predicting CBF, while Model 2 added fitness and physical activity to determine if these variables increased the total amount of variance explained (R2 values). To visualize relationships, participants were separated into fitness tertiles. Controlling for the previously described covariates, differences in CBF across the fitness tertiles were tested using pairwise contrasts and Cohen’s d effect sizes (d = M1 – M2 / SDpooled) were used to examine the magnitude of the differences. All analyses were first conducted within the entire sample, and then separated for males and females. Our statistical package was IBM SPSS, version 25.
Results
One hundred cognitively healthy (MMSE = 29.13 ± 1.0) participants (mean age = 63.6 ± 5.9 years, 63% female) completed the study. On average, participants displayed a V̇O2peak (ml/ kg/min) of 26.5 ± 6.3 and engaged in 1.31 ± .64 h of moderate-vigorous physical activity per day. The average absolute and relative composite CBF values were 38.13 ± 8.37 ml/100 g/min, and 1.15 ± .07 respectively. Additional participant characteristics are listed in Table 1. Age was negatively associated with fitness (r = −.41; p < .001), physical activity (r = −.22; p = .027) and CBF (r = −.21; p = .035; Fig. 1). The modest relationship between fitness and physical activity (r = .35; p < .001) suggests that these variables were not completely overlapping constructs and, therefore, may explain unique variance with respect to CBF.
Table 1.
Characteristics of study participants
| Variable | Entire sample, n = 100 | Females, n = 63 | Males, n = 37 | p value |
|---|---|---|---|---|
| Age, years, mean (SD) | 63.64 (5.9) | 63.55 (5.9) | 63.78 | .852 |
| Gender, n | – | 63 | 37 | – |
| APOE ε4 positive, % | 43 | 37 | 54 | .089 |
| Family history of AD, % | 71 | 67 | 78 | .217 |
| Body mass index, kg/m2 | 27.89 (5.42) | 27.55 (6.0) | 28.46 (4.3) | .418 |
| Education, years | 16.4 (2.5) | 16.0 (2.4) | 17.2 (2.6) | .014 |
| Handedness, % Right | 84 | 92 | 70 | .012 |
| Mini-Mental State Examination | 29.13 (1.0) | 29.17 (1.1) | 29.05 (.9) | .564 |
| Fitness, V̇O2peak, ml/kg/min | 26.5 (6.3) | 24.4 (5.0) | 30.0 (6.7) | .000 |
| Total actigraph daily wear time, hours | 15.4 (0.99) | 15.3 (.99) | 15.5 (.98) | .293 |
| Daily moderate-vigorous physical activity, hours | 1.31 (0.64) | 1.28 (.71) | 1.45 (.48) | .089 |
| Daily moderate-vigorous physical activity, % | 8.53 (4.27) | 8.0 (4.7) | 9.4 (3.3) | .107 |
| ROI Composite CBF, ml/100 g/min | 38.13 (8.37) | 40.30 (7.32) | 34.44 (8.85) | .001 |
| Relative ROI Composite CBF, % | 1.15 (.07) | 1.16 (.07) | 1.13 (.08) | .099 |
APOE ε4, apolipoprotein E ε4 allele; AD, Alzheimer’s disease; V̇O2peak, peak oxygen consumption; ROI, region of interest; CBF, resting cerebral blood flow
Values are represented as mean and standard deviation, unless otherwise indicated
Fig. 1. Age and CBF.
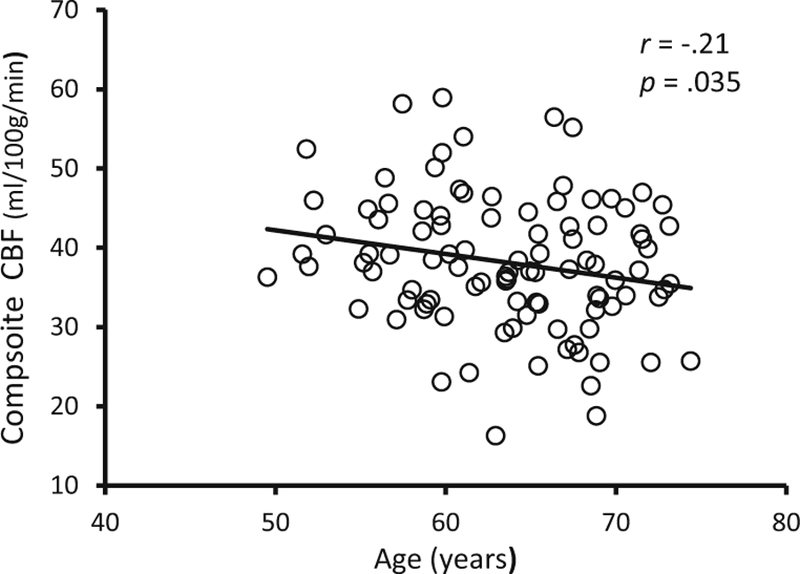
The relationship between age and CBF. CBF = resting cerebral blood flow
For the entire sample (n = 100) Model 1 did not predict CBF (p = .158). In Model 2, adding fitness and physical activity significantly increased the overall variance explained (R2 change = .059; p = .047; Table 2) and resulted in a significant model predicting CBF (p = .050). Model 2 revealed fitness was significantly and positively associated with CBF (p = .026; Table 3) while accounting for variance explained by physical activity, age, gender, APOE ε4 status, FH status, education and handedness. In contrast, physical activity was not (p = .629). For females (n = 63), Model 1 did not predict CBF (p = .155), however, Model 2 increased the overall variance explained (R2 change = .099; p = .036) and resulted in a significant model predicting CBF (p = .039) demonstrating, fitness (p = .011) but not physical activity (p = .574) was positively associated with CBF (Table 4). Neither Model 1 (p = .975) nor Model 2 (p = .874) significantly predicted CBF in the male participants. To visualize this relationship female participants were separated into low (mean = 19.5 ml/ kg/min), moderate (mean = 24.1 ml/kg/min), and high (mean = 29.8 ml/kg/min), fitness tertiles (n = 21, 21, 21). Mean CBF was highest in Tertile 3 (high fitness) and lowest in Tertile 1 (low fitness) (d = 1.4). Participants in Tertile 2 (moderate fitness) displayed greater CBF compared to participants in Tertile 1 (d = .70), and participants in Tertile 3 exhibited higher CBF than participants in Tertile 2 (d = .49; Fig. 2).
Table 2.
Hierarchical multiple linear regression predicting CBF
| Model | R | R2 | Std. error of the estimate | Change statistics |
|||||
|---|---|---|---|---|---|---|---|---|---|
| R2 change | F change | df1 | df2 | Sig. F change | |||||
| All n = 100 | 1 | .305 | .093 | .070 | – | – | 6 | 93 | – |
| 2 | .390 | .152 | .069 | .059 | 3.15 | 2 | 91 | .047 | |
| Femalesa n = 63 | 1 | .358 | .128 | .067 | – | – | 5 | 57 | – |
| 2 | .477 | .227 | .064 | .099 | 3.52 | 2 | 55 | .036 | |
| Malesa n = 37 | 1 | .160 | .025 | .080 | – | – | 5 | 31 | – |
| 2 | .307 | .094 | .080 | .069 | 1.104 | 2 | 29 | .345 | |
Model 1 = age, gender, APOE-ε4 status, FH status, education, handedness
Model 2 = age, gender, APOE-ε4 status, FH status, education, handedness, physical activity, fitness
Model does not include gender
Table 3.
Hierarchical multiple linear regression predicting CBF in the entire sample (n = 100)
| Model | Independent predictors | β (SE) | Standardized β | p value |
|---|---|---|---|---|
| 1 | Age | −.001 (.001) | −.078 | .467 |
| Gender | −.040 (.017) | −.270 | .018 | |
| APOE ε4 | −.008 (.015) | −.052 | .625 | |
| Family History of AD | .016 (.017) | .105 | .335 | |
| Education | .006 (.003) | .220 | .048 | |
| Handedness | .032 (.021) | .165 | .125 | |
| 2 | Age | .001 (.001) | .050 | .668 |
| Gender | −.059 (.018) | −.402 | .001 | |
| APOE ε4 | −.015 (.016) | −.106 | .328 | |
| Family History of AD | .023 (.017) | .148 | .175 | |
| Education | .005 (.003) | .188 | .112 | |
| Handedness | .032 (.020) | .166 | .115 | |
| Physical Activity | .019 (.039) | .056 | .629 | |
| Fitness | .003 (.001) | .295 | .026 |
Fitness, V̇O2peak; Physical activity, moderate-vigorous; APOE ε4, apolipoprotein E ε4 allele; AD, Alzheimer’s disease; CBF, resting cerebral blood flow; β, beta coefficient; SE, standard error
Table 4.
Hierarchical multiple linear regression predicting CBF in Females (n = 63)
| Model | Independent predictors | β (SE) | Standardized β | p value |
|---|---|---|---|---|
| 1 | Age | −.002 (.002) | −.164 | .226 |
| APOE ε4 | −.018 (.020) | −.125 | .369 | |
| Family History of AD | .027 (.019) | .184 | .176 | |
| Education | .007 (.004) | .248 | .055 | |
| Handedness | .039 (.031) | .156 | .215 | |
| 2 | Age | .000 (.002) | .023 | .878 |
| APOE ε4 | −.029 (.019) | −.204 | .141 | |
| Family History of AD | .032 (.019) | .223 | .096 | |
| Education | .004 (.004) | .149 | .267 | |
| Handedness | .035 (.030) | .139 | .248 | |
| Physical Activity | −.024 (.043) | −.080 | .574 | |
| Fitness | .006 (.002) | .426 | .011 |
Fitness, V̇O2peak; Physical activity, moderate-vigorous; APOE ε4, apolipoprotein E ε4 allele; AD, Alzheimer’s disease; CBF, resting cerebral blood flow; β, beta coefficient; SE, standard error
Fig. 2. Fitness and CBF for females.
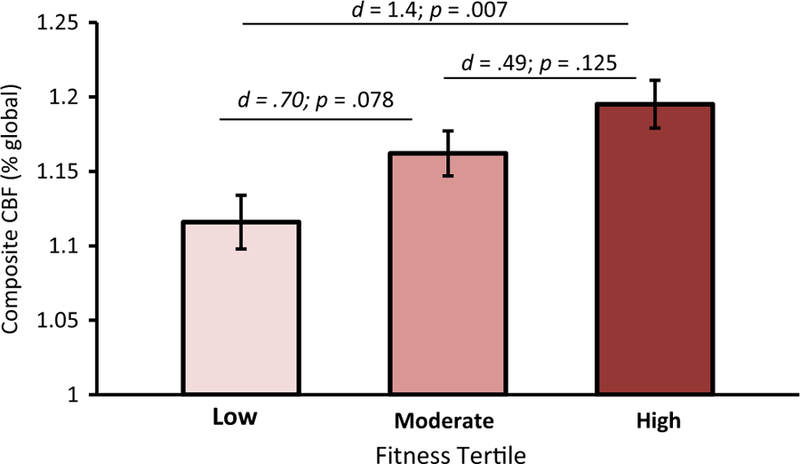
Female participants were separated into equal tertiles (n = 21, 21, 21) based on fitness level (Low = 19.5 ml/ kg/min; Moderate = 24.1 ml/kg/min; High = 29.8 ml/kg/min). Panels show adjusted means and standard error. Effect sizes (d) reflect adjusted means. CBF = resting cerebral blood flow
Discussion
This study concomitantly examined objectively measured fitness and accelerometer-determined physical activity to explore the unique associations with CBF among adults at risk for AD. We found that within the full sample, as well among females, fitness, independent of physical activity was positively associated with CBF in regions known to decline with age and the progression of AD (Chen et al. 2011; Wang et al. 2013). Notably, hierarchical multivariable regression demonstrated that fitness accounted for significantly more variance than the combined effect of the covariates on CBF. This finding was most pronounced in the female participants where fitness level accounted for approximately the same amount of variance (10%) as the covariates. This finding suggests that in our sample, fitness level was a stronger predictor of CBF than the known risk-factors included in Model 1. Interestingly, tertile analyses suggest that fitness might exert a thresholdresponse relationship with CBF. Female participants categorized in the low fitness tertile (19.5 ml/kg/min) showed the lowest CBF compared to the moderate (24.1 ml/kg/min) and high fitness (29.8 ml/kg/min) groups.
There is increasing recognition that CBF is an important preclinical biomarker of AD (Austin et al. 2011; Okonkwo et al. 2014a). In a large-scale study, decreased CBF was found to be the earliest pathophysiological event, preceding traditional AD biomarkers (e.g. amyloid, tau) (Iturria-Medina et al. 2016). Further, patterns of decreased CBF have been shown to predict conversion from MCI to AD (Chao et al. 2010), are associated with an accelerated rate of cognitive decline (Benedictus et al. 2017), and can differentiate cognitively normal adults, MCI and AD patients (Johnson et al. 2006). Notably, studies that used the same ADNI composite measure which includes the inferior temporal gyri, angular gyri, and the posterior cingulate have found CBF in these regions to be negatively associated with age, cognition and AD severity (Chen et al. 2011; Wang et al. 2013). Therefore, treatments that promote CBF and/or delay decline in these regions may mitigate the progression of cognitive decline and AD. That said, it is worth noting that, depending on the brain region, there are data to suggest that an increase in CBF is sometimes observed in AD (Wierenga et al. 2014) and is hypothesized to be the result of compensatory mechanisms (Wierenga et al. 2014; Hays et al. 2016).
Emerging evidence suggests that both fitness and physical activity may be associated with CBF in regions afflicted by aging and AD (Alsop et al. 2010; Wang et al. 2013; Wierenga et al. 2014). Similar to the present findings, estimated fitness mediated the relationship between age and CBF, that is, greater fitness levels negated age-related CBF decline (Zimmerman et al. 2014). Research investigating life-long exercisers (i.e. master athletes) found that these older adults displayed greater fitness and CBF than age-matched sedentary controls (Thomas et al. 2013) and interestingly, cessation of exercise decreased CBF in a similar sample of master athletes (Alfini et al. 2016). Further, 12-weeks of structured physical activity has shown to increase CBF in both young and old adults, however, whether fitness adaptations are necessary to impart this benefit remains unclear (Chapman et al. 2013; Pereira et al. 2007). The extant research has demonstrated a link between fitness, physical activity, and CBF. However, until now, it was uncertain whether fitness and physical activity differentially predict CBF.
In the current study, we observed a modest correlation between fitness and physical activity, replicating what has been observed in similar populations (Burzynska et al. 2014; Voss et al. 2016). Although separate measures, physical activity has been shown to influence fitness levels (Haskell et al. 2007), and therefore fitness has been regarded as a measure of habitual physical activity. Indeed, increasing moderate-vigorous physical activity has been shown to improve fitness (Skinner et al. 2000), and inactivity (i.e. bedrest) has been shown to decrease fitness (Saltin et al. 1968). Further, exercise training performed throughout a lifetime has a strong influence on fitness levels in later life (Carrick-Ranson et al. 2014). Although physical activity is a key factor, other aspects that contribute to fitness include social, environmental, behavioral, physiological, and genetic components (Bouchard and Rankinen 2001; Eaton et al. 1995; Tager et al. 1998). In fact, genetics may account for up to 50% of the individual variability in fitness adaptations (Bouchard et al. 2011, 1999). Because multiple factors contribute to the trait attribute of fitness, delineating the independent contributions of fitness and physical activity is necessary in order to understand the unique health benefits each may confer.
In our sample of older adults at-risk for AD, we found that fitness was significantly associated with CBF, especially among females. These findings demonstrate fitness is a key contributor to CBF, and suggest engagement in activities aimed at improving fitness (e.g. exercise training) may be a promising treatment towards abating age-related CBF decline. Interestingly, there appears to be a gradient where a ~5 ml/kg/ min increase in fitness, which is typically observed following several months of exercise training (Kohrt et al. 1991; Skinner et al. 2000), imparts substantial benefit (Fig. 2). This may be due to exercise training enhancing cardiovascular function (Seals et al. 1984) which promotes peripheral arterial health (Seals et al. 2008) and may lead to improved CBF regulation (Tarumi and Zhang 2017; Barnes and Corkery 2018). Another study of older female adults similarly reported that fitness is positively associated with several metrics of cerebral vascular function (i.e. mean arterial pressure, cerebrovascular conductance) (Brown et al. 2010). Other hypothesized exercise-related mechanisms for improved CBF include increasing neurotrophic factors, angiogenesis, vascular function and neurovascular coupling (Davenport et al. 2012). An alternative interpretation of our findings is that genetic and environmental factors that contribute to fitness levels have a stronger influence on CBF in older adulthood than current physical activity. It could be that participants who have greater fitness due to genetic influences exhibit a healthier CBF profile compared to those with lower fitness levels because of hereditary differences (Bouchard et al. 2011). It is also plausible that a lifetime of physical activity, which has been shown to contribute to higher fitness levels in later life (Carrick-Ranson et al. 2014), may be more influential than present physical activity behavior with respect to CBF.
The gender specific findings observed in the current study may be due to a multitude of factors. There is a large body of literature that describes gender differences in cardiovascular health (see reviews: Miller et al. 2013; Joyner et al. 2015) including blood pressure regulation (Barnes 2017), cerebral metabolic rate of oxygen and cerebral blood flow (Lu et al. 2010). Our gender finding is consistent with our group’s previous work which reported a positive association between fitness and hippocampal volume in female but not male participants (Dougherty et al. 2017a). Further, meta-analyses of exercise trials have found evidence for gender specific effects on cognition (Colcombe and Kramer 2003; Barha et al. 2017), with data suggesting that superior cognitive response in females may be the result of differences in exercise induced hormonal glucometabolic and hypothalamic-pituitaryadrenal axis responses (Baker et al. 2010). This may explain the gender findings reported from the epidemiological Canadian Study of Health and Aging where moderate to high exercise participation resulted in reduced risk of cognitive impairment for females but not males (Middleton et al. 2008). Alternatively, the present study’s results may be explained by gender differences of the current sample. Examining the demographic information (Table 1), males had significantly greater years of education which may translate to increased cognitive reserve (Stern et al. 2012), limiting the potential influence of fitness on cerebral hemodynamics.
Our findings compliment previous research that has investigated both fitness and physical activity simultaneously. Epidemiological studies that examined both measures have routinely found fitness to be a stronger predictor of mortality (Kampert et al. 1996; Park et al. 2009; Wei et al. 2000) even after adjusting for activity level (Lee et al. 2010; Myers et al. 2004). Similar findings have been reported with cardiovascular disease, where fitness appears to be a superior prognostic indicator (DeFina et al. 2015). However, these studies have relied on self-reported physical activity questionnaires, which have shown large variability in comparison to objectively measured physical activity (Dyrstad et al. 2014; Prince et al. 2008).
Research investigating both fitness and physical activity in relation to cognitive health is less clear. The cardiorespiratory fitness hypothesis, first described by Kramer et al. (1999) posits the cognitive benefit to exercise training is due to the physiological adaptations in one’s cardiovascular system (i.e. increased fitness). To date, meta-analytic data do not support this theory (Angevaren et al. 2008; Colcombe and Kramer 2003; Etnier et al. 2006). This may be in part due to variability in fitness testing methods (Dougherty et al. 2018), because there is evidence for the cardiorespiratory fitness hypothesis when applying strict physiological testing criteria (Billinger et al. 2017).
Evidence from our group and others has linked objectively-measured fitness and accelerometer-determined physical activity to many indices of brain health (Dougherty et al. 2017a, b, 2016; Makizako et al. 2015; Hayes et al. 2015), however, research investigating fitness and physical activity concomitantly is limited. In a similar older adult sample, Voss and colleagues reported that fitness was positively associated with functional connectivity in regions afflicted by aging (i.e. default mode network), and this finding remained significant after accounting for accelerometer-determined physical activity (Voss et al. 2016; but see also Burzynska et al. 2014). In agreement with previous research, our findings suggest that fitness has a significant impact on brain health that is not explained by current physical activity behavior.
Future longitudinal studies that assess changes in fitness and physical activity will be needed to better elucidate causality and directionality of the observed relationships. Although our sample’s fitness levels were representative of national normative data (Kaminsky et al. 2015), physical activity was higher than the reported national average (Troiano et al. 2008). Further, we cannot rule out the potential of fluctuations in physical activity behaviors, which would influence our accelerometer measures; however, this does not present a concern with fitness testing. Lastly, due to our sample’s demographic make-up (i.e., predominantly highly-educated non-Hispanic Whites) generalizability of our findings to other ethnic groups may be limited. Studies that include diverse populations are needed to determine whether our findings extend to other ethnic groups.
In summary, the present study provides evidence that fitness, independent of physical activity, is a significant predictor of CBF in regions that decline with age, and disease progression (Chen et al. 2011; Wang et al. 2013). While others have reported that estimated fitness levels (Zimmerman et al. 2014) and habitual physical activity (Thomas et al. 2013) are associated with maintained CBF in older adulthood, our findings suggest fitness is uniquely associated with preserved CBF, particularly among cognitively normal older female adults at risk for AD. This is an important finding as it is now well accepted that the pathophysiology of AD, including reductions in CBF, precedes cognitive impairment (Iturria-Medina et al. 2016; Jack et al. 2010; Wierenga et al. 2014), highlighting the necessity to discover efficacious treatments before cognitive symptoms ensue. This work makes a significant contribution towards research investigating the gender specific mechanisms through which fitness and physical activity reduce the risk of cognitive decline by mitigating AD-related brain changes during the preclinical stage of the disease.
Acknowledgements
The authors gratefully acknowledge Jennifer Oh for her assistance in the data analysis and the support of researchers and staff at the University of Wisconsin-Madison for their assistance in recruitment and data collection. Above all, the authors thank their dedicated volunteers for their participation in this research.
This work was supported by National Institute on Aging grants K23 AG045957 (OCO), R21 AG051858 (OCO), R01 AG027161 (SCJ), R01 AG021155 (SCJ), P50 AG033514 (SA); and by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by the Extendicare Foundation, the Alzheimer’s Association, Wisconsin Alumni Research Foundation, the Helen Bader Foundation, Northwestern Mutual Foundation, and the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI. Ryan J. Dougherty was supported by a National Research Service Award from the National Institute on Aging of the National Institutes of Health under Award Number F31AG062009. Jacob B. Lindheimer was supported by Career Development Award Number IK2 CX001679 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R&D (CSR&D) Service. The contents do not represent the views of the National Institutes of Health, Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of interest Ryan J. Dougherty, Elizabeth A. Boots, Jacob B. Lindheimer, Aaron J. Stegner, Stephanie Van Riper, Dorothy F. Edwards, Catherine L. Gallagher, Cynthia M. Carlsson, Howard A. Rowley, Barbara B. Bendlin, Sanjay Asthana, Bruce P. Hermann, Mark A. Sager, Sterling C. Johnson, Ozioma C. Okonkwo, and Dane B. Cook declare no conflicts of interest.
Ethical approval and informed consent All procedures followed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alfini AJ, Weiss LR, Leitner BP, Smith TJ, Hagberg JM, & Smith JC (2016). Hippocampal and cerebral blood flow after exercise cessation in master athletes. Frontiers in Aging Neuroscience, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Dai W, Grossman M, & Detre JA (2010). Arterial spin labeling blood flow MRI: Its role in the early characterization of Alzheimer’s disease. Journal of Alzheimer’s Disease, 20(3), 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine, ed. (2013). ACSM’s health-related physical fitness assessment manual Lippincott Williams & Wilkins. [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, & Vanhees L (2008). Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews, 3(3). [DOI] [PubMed] [Google Scholar]
- Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, Johnson SC, & Prabhakaran V (2011). Effects of hypoperfusion in Alzheimer’s disease. Journal of Alzheimer’s Disease, 26(s3), 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, … & Duncan GE (2010). Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of neurology, 67(1), 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke B, & Ware RW (1959). An experimental study of physical fitness of air force personnel. United States Armed Forces Medical Journal, 10(6), 675–688. [PubMed] [Google Scholar]
- Barha CK, Davis JC, Falck RS, Nagamatsu LS, & Liu-Ambrose T (2017). Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Frontiers in Neuroendocrinology, 46, 71–85. [DOI] [PubMed] [Google Scholar]
- Barnes JN (2017). Sex-specific factors regulating pressure and flow. Experimental Physiology, 102(11), 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JN, & Corkery AT (2018). Exercise improves vascular function, but does this Translate to the Brain?. Brain Plasticity, (Preprint), 1–15. [DOI] [PMC free article] [PubMed]
- Benedictus MR, Leeuwis AE, Binnewijzend MA, Kuijer JP, Scheltens P, Barkhof F, … & Prins ND (2017). Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. European Radiology, 27(3), 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinger SA, Vidoni ED, Morris JK, Thyfault JP, & Burns JM (2017). Exercise test performance reveals evidence of the cardiorespiratory fitness hypothesis. Journal of Aging and Physical Activity, 25(2), 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg GA (1982). Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise, 14, 377–381. [PubMed] [Google Scholar]
- Bouchard C, & Rankinen T (2001). Individual differences in response to regular physical activity. Medicine and Science in Sports and Exercise, 33.6(Suppl), S446–S451. [DOI] [PubMed] [Google Scholar]
- Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Pérusse L, Leon AS, & Rao DC (1999). Familial aggregation of Vo 2 max response to exercise training: Results from the HERITAGE family study. Journal of Applied Physiology, 87(3), 1003–1008. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, & Rankinen T (2011). Genomic predictors of the maximal O 2 uptake response to standardized exercise training programs. Journal of Applied Physiology, 110(5), 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, & Poulin MJ (2010). Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of Aging, 31(12), 2047–2057. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Chaddock-Heyman L, Voss MW, Wong CN, Gothe NP, Olson EA, et al. (2014). Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One, 9(9), e107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, & Levine BD (2014). The effect of lifelong exercise dose on cardiovascular function during exercise. Journal of Applied Physiology, 116(7), 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, & Christenson GM (1985). Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports, 100(2), 126–131. [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, & Weiner MW (2010). ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Disease and Associated Disorders, 24(1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, DeFina LF, Keebler MW, Didehbani N, & Lu H (2013). Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience, 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wolk DA, Reddin JS, Korczykowski M, Martinez PM, Musiek ES, … & Detre JA (2011). Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology, WNL-0b013e31823a0ef7. [DOI] [PMC free article] [PubMed]
- Colcombe S, & Kramer AF (2003). Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science, 14(2), 125–130. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, De Bazelaire C, & Alsop DC (2008). Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 60(6), 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MH, Hogan DB, Eskes GA, Longman RS, & Poulin MJ (2012). Cerebrovascular reserve: The link between fitness and cognitive function? Exercise and Sport Sciences Reviews, 40(3), 153–158. [DOI] [PubMed] [Google Scholar]
- DeFina LF, Haskell WL, Willis BL, Barlow CE, Finley CE, Levine BD, & Cooper KH (2015). Physical activity versus cardiorespiratory fitness: Two (partly) distinct components of cardiovascular health? Progress in Cardiovascular Diseases, 57(4), 324–329. [DOI] [PubMed] [Google Scholar]
- Dougherty RJ, Ellingson LD, Schultz SA, Boots EA, Meyer JD, Lindheimer JB, et al. (2016). Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 4, 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RJ, Schultz SA, Boots EA, Ellingson LD, Meyer JD, Van Riper S, … & Korcarz CE (2017a). Relationships between cardiorespiratory fitness, hippocampal volume, and episodic memory in a population at risk for Alzheimer’s disease. Brain and Behavior, 7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RJ, Schultz SA, Kirby TK, Boots EA, Oh JM, Edwards D, Gallagher CL, Carlsson CM, Bendlin BB, Asthana S, Sager MA, Hermann BP, Christian BT, Johnson SC, Cook DB, & Okonkwo OC (2017b). Moderate physical activity is associated with cerebral glucose metabolism in adults at risk for Alzheimer’s disease. Journal of Alzheimer’s Disease : JAD, 58(4), 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RJ, Lindheimer JB, Stegner AJ, Van Riper S, Okonkwo OC, & Cook DB (2018). An objective method to accurately measure cardiorespiratory fitness in older adults who cannot satisfy widely used oxygen consumption criteria. Journal of Alzheimer’s Disease, 61(2), 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrstad SM, Hansen BH, Holme IM, & Anderssen SA (2014). Comparison of self-reported versus accelerometermeasured physical activity. Medicine & Science in Sports & Exercise, 46(1), 99–106. [DOI] [PubMed] [Google Scholar]
- Eaton CB, Lapane KL, Garber CE, Assaf AR, Lasater TM, & Carleton RA (1995). Physical activity, physical fitness, and coronary heart disease risk factors. Medicine and Science in Sports and Exercise, 27(3), 340–346. [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, & Weinstein AM (2014). Physical activity, fitness, and gray matter volume. Neurobiology of Aging, 35, S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, & Sibley BA (2006). A metaregression to examine the relationship between aerobic fitness and cognitive performance. Brain Research Reviews, 52(1), 119–130. [DOI] [PubMed] [Google Scholar]
- Garcia DM, Duhamel G, & Alsop DC (2005). Efficiency of inversion pulses for background suppressed arterial spin labeling. Magnetic Resonance in Medicine, 54(2), 366–372. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, … & Bauman A (2007). Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation, 116(9), 1081, 1093. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Alosco ML, Hayes JP, Cadden C, Peterson KM, Allsup K, Forman DE, Sperling RA, & Verfaellie M (2015). Physical activity is positively associated with episodic memory in aging. Journal of the International Neuropsychological Society, 21, 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, & Wierenga CE (2016). The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cellular and Molecular Neurobiology, 36(2), 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC, & Alzheimer’s Disease Neuroimaging Initiative. (2016). Early role of vascular dysregulation on late-onset Alzheimer/’s disease based on multifactorial data-driven analysis. Nature communications, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, … & Trojanowski JQ (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology, 9(1), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, … & Schuff N (2006, June). Pattern of cerebral hypoperfusion in Alzheimer’s disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: Initial experience. In International Congress Series (Vol. 1290, pp. 108–122). Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Barnes JN, Hart EC, Wallin BG,& Charkoudian N (2015). Neural control of the circulation: How sex and age differences interact in humans. Comprehensive Physiology, 5(1), 193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky LA, Arena R, & Myers J (2015, November ). Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: Data from the fitness registry and the importance of exercise national database. In Mayo Clinic Proceedings (Vol. 90, no. 11, pp. 1515–1523). Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampert JB, Blair SN, Barlow CE, & Kohl HW III. (1996). Physical activity, physical fitness, and all-cause and cancer mortality: A prospective study of men and women. Annals of Epidemiology, 6(5), 452–457. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa TAKESHI, Ehsani AA, … & Holloszy JO (1991). Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. Journal of Applied Physiology, 71(5), 2004–2011. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, & Colcombe A (1999). Ageing, fitness and neurocognitive function. Nature, 400(6743), 418–419. [DOI] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. (2011). Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiology of Aging, 32(7), 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Sui X, Ortega FB, Kim YS, Church TS, Winett RA, … & Blair SN (2010). Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. British journal of sports medicine, bjsports66209. [DOI] [PubMed]
- Lyden K, Keadle SK, Staudenmayer J, & Freedson PS (2014). A method to estimate free-living active and sedentary behavior from an accelerometer. Medicine and Science in Sports and Exercise, 46(2), 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, … & Park DC (2010). Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cerebral cortex, 21(6), 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makizako H, Liu-Ambrose T, Shimada H, Doi T, Park H, Tsutsumimoto K, & Suzuki T (2015). Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 70, 480–486. [DOI] [PubMed] [Google Scholar]
- Middleton L, Kirkland S, & Rockwood K (2008). Prevention of CIND by physical activity: different impact on VCI-ND compared with MCI. Journal of the neurological sciences, 269(1–2), 80–84. [DOI] [PubMed] [Google Scholar]
- Miller VM, Garovic VD, Kantarci K, Barnes JN, Jayachandran M, Mielke MM, Joyner MJ, Shuster LT, & Rocca WA (2013). Sex-specific risk of cardiovascular disease and cognitive decline: Pregnancy and menopause. Biology of Sex Differences, 4(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, Yamazaki T, & Froelicher V (2004). Fitness versus physical activity patterns in predicting mortality in men. The American Journal of Medicine, 117(12), 912–918. [DOI] [PubMed] [Google Scholar]
- Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, et al. (2018). Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimer’s & Dementia, 14, 1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, … & LaRue A (2014a). Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cerebral Cortex, 24(4), 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, … & Bendlin BB (2014b). Physical activity attenuates agerelated biomarker alterations in preclinical AD. Neurology, 83(19), 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS, Chung SY, Chang Y, & Kim K (2009). Physical activity and physical fitness as predictors of all-cause mortality in Korean men. Journal of Korean Medical Science, 24(1), 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, & Small SA (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences, 104(13), 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. (2018). Physical activity guidelines advisory committee scientific report Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD (2018). The physical activity guidelines for Americans. JAMA Published online November 12, 2018 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, & Tremblay M (2008). A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 5(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager MA, Hermann B, & La Rue A (2005). Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin registry for Alzheimer’s prevention. Journal of Geriatric Psychiatry and Neurology, 18(4), 245–249. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, et al. (1968). Response to exercise after bed rest and after training: a longitudinal study of adaptive changes in oxygen transport and body composition. Circulation, 37/38(suppl VII), VII-1–VII-78. [PubMed] [Google Scholar]
- Seals DR, Hagberg JM, Hurley BF, Ehsani AA, & Holloszy JO (1984). Endurance training in older men and women. I. Cardiovascular responses to exercise. Journal of applied physiology, 57(4), 1024–1029. [DOI] [PubMed] [Google Scholar]
- Seals DR, DeSouza CA, Donato AJ, & Tanaka H (2008). Habitual exercise and arterial aging. Journal of Applied Physiology, 105(4), 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JS, Wilmore KM, Krasnoff JB, JaskÓlski A, JaskÓlska A, Gagnon J, … & Bouchard C (2000). Adaptation to a standardized training program and changes in fitness in a large, heterogeneous population: The HERITAGE family study. Medicine and Science in Sports and Exercise, 32(1), 157–161. [DOI] [PubMed] [Google Scholar]
- Stern Y (2012). Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology, 11(11), 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager IB, Hollenberg M, & Satariano WA (1998). Association between self-reported leisure-time physical activity and measures of cardiorespiratory fitness in an elderly population. American Journal of Epidemiology, 147(10), 921–931. [DOI] [PubMed] [Google Scholar]
- Tarumi T, & Zhang R (2017). Cerebral blood flow in normal aging adults: Cardiovascular determinants, clinical implications, and aerobic fitness. Journal of Neurochemistry [DOI] [PMC free article] [PubMed]
- Thambisetty M, Beason-Held L, An Y, Kraut MA, & Resnick SM (2010). APOE ε4 genotype and longitudinal changes in cerebral blood flow in normal aging. Archives of Neurology, 67(1), 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, & Lu H (2013). Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. Journal of Magnetic Resonance Imaging, 38(5), 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, & McDowell M (2008). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40(1), 181–188. [DOI] [PubMed] [Google Scholar]
- Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, … & McAuley E (2016). Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage, 131, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Das SR, Xie SX, Arnold SE, Detre JA, Wolk DA, & Alzheimer’s Disease Neuroimaging Initiative. (2013). Arterial spin labeled MRI in prodromal Alzheimer’s disease: A multi-site study. NeuroImage: Clinical, 2, 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, & Blair SN (2000). Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Annals of Internal Medicine, 132(8), 605–611. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Clark LR, Dev SI, Shin DD, Jurick SM, Rissman RA, Liu TT, & Bondi MW (2013). Interaction of age and APOE genotype on cerebral blood flow at rest. Journal of Alzheimer’s Disease, 34(4), 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Hays CC, & Zlatar ZZ (2014). Cerebral blood flow measured by arterial spin labeling MRI as a preclinical mark er of Alzheimer’s disease. Journal of Alzheimer’s Disease, 42(s4), S411–S419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, Christian BT, Oakes TR, & Johnson SC (2010). Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR in Biomedicine, 23(3), 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye FQ, Frank JA, Weinberger DR, McLaughlin AC (2000). Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST). Magn Reson Med 44, 92–100. [DOI] [PubMed] [Google Scholar]
- Zimmerman B, Sutton BP, Low KA, Fletcher MA, Tan CH, Schneider-Garces N, … & Fabiani M (2014). Cardiorespiratory fitness mediates the effects of aging on cerebral blood flow. Frontiers in Aging Neuroscience, 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]


