Abstract
Objectives:
Problems with sleep are a common and detrimental occurrence among individuals who receive methadone maintenance for opioid use disorder (OUD).
Methods:
We enrolled ten methadone-maintained persons with insomnia (60% female, mean age 40) in a double-blind trial using actigraphy to confirm daily sleep reports. After a no-medication week to establish baseline sleep patterns, each participant received one week each of mirtazapine (30mg), zolpidem (sustained-release 12.5 mg), mirtazapine (30 mg IR) plus zolpidem (10mg), and placebo with a wash-out week between each medication week. Study medication order was randomized so that the order of each 1-week medication treatment was different for each participant, but all participants received all four regimens.
Results:
We found that mirtazapine alone improved total sleep (mean, 23 minutes), sleep latency (mean, 23 minutes), and sleep efficiency (mean, 3%), surpassing the other regiments.
Conclusions:
This pilot work suggests that mirtazapine is worthy of further testing as a sleep aid for persons with OUD receiving methadone maintenance.
Keywords: opiate use disorder, psychopharmacology, actigraphy
Objectives
Opioid use disorders (OUDs) are a costly and burdensome health concern, affecting approximately 5% of the population each year (Substance Abuse and Mental Heath Services Administration, 2017). Medication-assisted therapy has become part of standard practice in OUD treatment, and includes methadone maintenance therapy (MMT). Despite its effectiveness (Connock, 2007), MMT has been associated with negative effects. For example, more than three quarters of persons receiving MMT report sleep complaints (Oyefeso et al., 1997; Peles, E. et al., 2006; Stein et al., 2004). Sleep disturbance affects quality of life, daytime functioning (Ustinov et al., 2010) and work productivity. Methadone patients with sleep disturbance obtain, on average, less than 6 hours of sleep (Sharkey et al., 2010). These short sleep durations represent sleep restriction that could manifest in daytime impairment (Balkin et al., 2008), lower methadone treatment adherence, and increased relapse risk through behavioral and physiologic mechanisms. Poor sleep efficiency can lead to daytime symptoms such as cognitive difficulties and risk of injury and motor vehicle accidents (National Sleep Foundation, 2009; Solowij et al., 2002). Severity of sleep symptoms in methadone-maintained persons has been associated with comorbid psychiatric disorders, chronic pain, and other drug use (Peles, E. et al., 2006; Stein et al., 2004). More than half of MMT patients report use of both illicit drugs and approved medications to help with sleep (Burke et al., 2008; Peles, E. et al., 2006). Yet those who report using illicit drugs also report more sleep-related problems and greater functional impairment (Burke et al., 2008).
The most common sleep complaints among individuals in methadone treatment include increased sleep latency (difficulty falling asleep) and increased time awake after sleep onset (Peles, E et al., 2006). Subjective sleep complaints have been corroborated by polysomnographic studies by our group and others (Sharkey et al., 2009; Wang and Teichtahl, 2007), demonstrating sleep abnormalities such as decreased REM and slow wave sleep, and shortened total sleep time.
There are several postulated mechanisms to explain insomnia among methadone patients. Opioids decrease acetylcholine release in some brain regions, such as the pontine reticular formation, and therefore may inhibit REM sleep (Lydic and Baghdoyan, 2005). Acute opioid administration suppresses inhibitory GABAergic transmission in the dorsal raphe nucleus, promoting wakefulness. A third potential pathway to sleep disruption in MMT patients is opioid-induced reduction of the nucleoside adenosine in the basal forebrain (Nelson et al., 2009). The notion that reduced adenosine – a neurochemical modulator of the homeostatic drive for sleep – may be responsible for sleep disturbances in MMT patients is further supported by the observation that MMT patients fail to show typical recovery responses after a sleep-deprivation challenge (Trksak et al., 2010).
Methadone patients also have high rates of sleep disordered breathing (both central sleep apnea and obstructive sleep apnea), although neither accounts for complaints of disturbed sleep (Correa et al., 2015; Sharkey et al., 2010; Teichtahl et al., 2001; Van Ryswyk and Antic, 2016; Wang et al., 2005; Webster et al., 2008). Risk factors for sleep-disordered breathing in patients with OUD include smoking, female gender, and increased body weight (Hassamal et al., 2016). Obstructive sleep apnea in this population is likely due to opioid-induced reductions in airway muscle activation. Central sleep apnea may arise from depression of hypoxic and hypercapnic ventilatory drives (Hajiha et al., 2009), which are already reduced during sleep (Van Ryswyk and Antic, 2016).
Clinicians, aware of methadone patients’ sleep problems, prescribe a wide assortment of sleep aids without any evidence basis. For instance, trazodone is the second most commonly prescribed medications for treatment of insomnia (after zolpidem) in the United States (Mendelson et al., 2004) and is the most commonly prescribed soporofic for drug and/or alcohol problems (Friedmann et al., 2008). However, we have demonstrated that trazodone is no better than placebo in its effects on total sleep time or sleep efficiency when tested in methadone patients (Stein et al., 2012).
We considered a variety of FDA-approved medications demonstrated to be safe and effective in persons with sleep disturbance in the search for a potentially efficacious agent to treat insomnia in methadone patients. We wanted to test agents where a broad range of safety parameters have been monitored during prolonged use (De Micco et al., 2006; Richardson, 2005), physical exams and laboratory studies are unnecessary, there is no evidence of rebound insomnia or withdrawal effects (De Micco et al., 2006; Mayer et al., 2009; Roth et al., 2006), there is little potential for drug interaction with methadone, and there is limited addiction potential (Johnson et al., 2006) particularly when used for a short duration. As such, we chose zolpidem because it was the most commonly-prescribed sleep aid and mirtazapine because there was evidence for associated improvements in sleep among individuals in recovery from alcohol use disorder (Alam et al., 2013; Cornelius et al., 2013).
In this randomized, double-blind pilot clinical trial, we used a crossover design to test whether two different active medications and one medication combination (zolpidem CR 12.5 mg, mirtazapine 30 mg, and zolpidem 10 mg plus mirtazapine 30 mg) improved subjective judgment of sleep as compared to placebo among methadone-maintained persons with sleep complaints. Objectively-measured sleep was the primary outcome, and we used wrist actigraphy to objectively corroborate sleep-wake patterns. We hypothesized that compared to baseline and placebo, each of these medication options would be associated with improved sleep quality.
Methods
Participants
Participants were recruited from three methadone clinics in the Providence, Rhode Island metro area. Study researchers posted flyers in the public areas of the clinics, asking “Having trouble sleeping? Call to see if you are eligible for a research study.” Interested individuals were screened in person by study staff at their respective clinics during clinic dosing hours.
Eligibility criteria included: 1) age 21–65; 2) a Pittsburgh Sleep Quality Index (PSQI (Buysse et al., 1989)) score greater than 5, indicating clinically significant insomnia; 3) the ability to sleep, read, and understand English; 4) having been in methadone maintenance treatment for at least the past 6 months and planning to remain for the next 3 months without a methadone taper; 5) access to a phone; 6) Live in a house or apartment (not homeless or transiently housed); and 7) the ability to provide at least two contacts. Individuals who reported current use of any medication known to affect sleep or whose urine toxicology tested positive for illicit substances (opioids, cocaine, amphetamines, benzodiazepines, marijuana) were excluded, as were women who reported current breastfeeding or pregnancy. Any individual with a prior diagnosis of a sleep disorder (e.g., restless leg syndrome or sleep apnea), organic brain disorder, bipolar disorder, psychotic disorder, major depression, a major medical condition that may affect sleep (such as poorly controlled diabetes mellitus or emphysema), and those who worked a swing shift or nighttime hours were also excluded from participation.
Procedure
Interested individuals completed a brief in-person eligibility screen at the MMT site. Persons who were eligible for the study were administered informed consent (approved by the Butler Hospital Institutional Review Board) and scheduled an appointment for a baseline interview (day 0) within one week. Participants were randomized to sequences of medication conditions following a repeated Latin-Square design, which preserves a balance of participants receiving each medication protocol across all medication regimens. At baseline, participants were asked to provide demographic information as well as information regarding sleep history. Participants agreed to take study medications during 4 specified one-week intervals (see below). They were asked to wear a wrist actigraph (see below) and speak with study staff briefly on the phone during weeks 1, 2, 4, 6, and 8. In order to increase study adherence, research staff contacted participants who did not call on a particular day, and there was a financial incentive for making all daily calls. Participants were compensated for phone diaries and in-person interviews, but not for the initial eligibility screen. Participants agreed to an 8-week follow-up period, including daily diaries (recording self-reported bedtime and wake time, plus medication adherence and side effects) and weekly in-person meetings, with follow-up interviews at weeks 2, 4, 6, and 8 at the MMT clinic. During follow-up interviews, actigraphy data was downloaded.
Study Medication and Medication Procedure
This randomized, double blind crossover design study utilized three active medications (zolpidem CR 12.5 mg, mirtazapine 30 mg, and zolpidem 10 mg plus mirtazapine 30 mg) and placebo. Medications were ordered directly from the pharmacy, compounded to look and taste identical and were packaged in identical bottles. The medication combination (zolpidem 10 mg plus mirtazapine 30 mg) was delivered in one pill. Beginning week 2 of study participation, participants took each medication for one week (7 pills were dispensed), then took a week “off,” then were given a week’s supply of a different medication. Study medication order was randomized so that the order of each 1-week medication treatment was different for each participant, but all participants received all four “medications.” Participants were instructed to take one pill immediately before going to bed and were told to expect an immediate effect on sleep. Participants tracked bedtime and wake times, adherence, and side effects with daily diary. Participants were asked not to use sleep aids, alcohol, or illicit substances during the course of the study.
Measures
Participants wore a wrist actigraph (Micro Motionlogger Sleep Watch, AMI, Ardsley, NY) on the non-dominant wrist for each of the study weeks. The device is the size of a large digital wrist watch (1.75” x 1.3” x .38” weighing 2 oz.) and is worn on the non-dominant wrist. The actigraph contains an accelerometer that detects movement and records movement counts in 1-minute bins. The device was initialized by the researcher through a computer interface and required no manipulation by the participant while he or she was wearing it, enabling continuous monitoring for the full week. Participants were instructed to wear the actigraphs 24 hours a day and not to remove them except during bathing or times when the device would be exposed to physical impact. They were asked to note on their daily diaries the times that the actigraph was removed, for how long, and for what purpose. Participants returned the actigraphs to study staff on the day following each recording week to download data and re-calibrate the devices.
Wrist Actigraphy began the day of the baseline interview and continued until day 14 (baseline week and first medication week). From day 14–20, the participant received no study medication (a wash-out period) and did not wear the actigraph or complete sleep diaries. A second randomly assigned study medication was given to participants on day 21 and actigraphy and sleep diaries were re-started. This procedure was repeated with study medications given on days 35 and 49. The data were scored for sleep using the Sadeh algorithm in Action-W software (AMI, Ardsley, NY, USA), an algorithm validated using concomitant polysomnography (Sadeh et al., 1994). First, sleep period time was calculated as minutes between bedtime (as reported by daily diary) and wake-up time. Variables derived from actigraphy were total minutes of sleep during sleep period time, total minutes of being awake during sleep period time, sleep efficiency (total sleep/total wake x100%), sleep latency (time between waking from sleep and new sleep onset), and minutes of wakefulness (total wakefulness during total sleep period).
Analysis Plans
We present descriptive statistics to summarize the characteristics of the participants and describe patterns of medication adherence over time. We used fixed-effects linear regression (Allison, 2009) to estimate intra-person between-medication differences. The within-effects estimator uses only within-subject variation and effectively controls for all time-invariant between subject heterogeneity. To adjust for potential time effects, we included indicator variables for sequential week of observation and for sequential day within the week for each medication protocol. We used difference in likelihood ratio chi-square (LR2) to statistically test for overall differences in medication protocols. We note that given the small sample size, statistical power to reject the null hypothesis of no between-medication differences is low and present between-medication differences in mean sleep outcomes (relative to placebo) and 95% confidence interval estimates for active medication protocols. To facilitate interpretation we present graphs of the predicted means.
Results
Six of the 10 participants were female and 9 were non-Latino Caucasian. Mean age was 40.5 (± 7.2, median = 40, range = 29 – 56). Baseline PSQI scores ranged from 6 to 16 (mean = 12.8, SD = 3.1, median = 14). All but 1 subject had PSQI scores ≥ 10. Six participants reported they had not used any sleep medications in the past month, 3 reported they had used sleep medications less than once a week, one used 3+ times a week. Mean time in methadone maintenance was 146.6 (± 239.8, median = 52, range = 20 to 800) weeks.
Medication adherence data were missing for 1 subject. Among the 9 persons with valid adherence data, 7 reported 100% adherence to all study medication protocols, 1 reported 85.7% adherence, and 1 reported 96.4% adherence. The overall mean adherence rate was 98.0% (± 4.76, median = 100%). Overall adherence rates were 100.0% for mirtazapine, 97.4% for zolpidem, 96.4% for the medication combination, and 98.4% for placebo. Adherence rates by week (not drug) were 98.4%, 96.8%, 100.0%, and 96.8% for medication weeks respectively.
Table 1 gives the estimated adjusted mean difference in sleep outcomes contrasting active medication arms to placebo. Figure 1 graphs the expected means by medication protocol. With the exception of wake after sleep onset (LR2 = 7.97, df = 3, p = .047) between group differences were not statistically significant at the conventional .05 level. Relative to placebo, persons slept an average of 23.1 minutes more per night (95%CI −15.2; 61.4) while receiving mirtazapine, and 16.1 minutes less (95%CI −57.7; 25.6) when receiving zolpidem. While receiving mirtazapine, participants were awake an average of 23.4 (95%CI −58.2; 11.4) minutes less than when receiving placebo. Mean wake minutes was highest when persons were receiving zolpidem. Similar patterns were observed for sleep efficiency, sleep latency, and wake after sleep onset. The highest sleep efficiency, and lowest sleep latency and wake after sleep onset means were observed when persons were receiving mirtazapine; zolpidem was consistently observed to have the poorest sleep outcomes of any of the medication protocols, including placebo.
Table 1.
Estimated Difference in Mean Sleep Outcomes for Active Medication Protocols Minus Placebo. Differences Adjusted for Sequential Week of Administration and Sequential Day within Week of Administration Using Fixed-Effects Regression (n = 10 Persons observed on 254 Days).
| ESTIMATED DIFFERENCES |
|||
|---|---|---|---|
| Sleep Outcome | Mirtazapine-Placebo Mean Difference(95%CI) |
Zolpidem-Placebo Mean Difference(95%CI) |
Combo-Placebo Mean Difference(95%CI) |
| Sleep Minutes | 23.1(−15.2; 61.4) | −16.1(−57.7; 25.6) LR2 = 4.82, df = 3, p = .186 |
17.4(−23.0; 57.8) |
| Wake Minutes | −23.4(−58.2; 11.4) | 20.7(−17.1; 58.6) LR2 = 6.13, df = 3, p = .105 |
−5.8(−42.5; 30.9) |
| Sleep Efficiency | 3.2(−2.0; 8.4) | −3.7(−9.4;1.9) LR2 = 7.02, df = 3, p = .071 |
1.3(−4.2; 6.7) |
| Sleep Latency | −9.7(−27.7; 8.3) | 7.5(−12.0; 27.0) LR2 = 6.82, df = 3, p = .078 |
12.6(−6.3; 31.5) |
| Wake After Sleep Onset | −13.6(−39.3; 12.0) | 20.2(−7.6; 48.0) LR2 = 7.97, df = 3, p = .047 |
−11.3(−38.3; 15.6) |
Note: LR2 = likelihood ratio chi-squared results, df = degrees of freedom
Figure 1.
Predicted Mean (and 95% Confidence Intervals) for Five Sleep Outcomes by Medication.
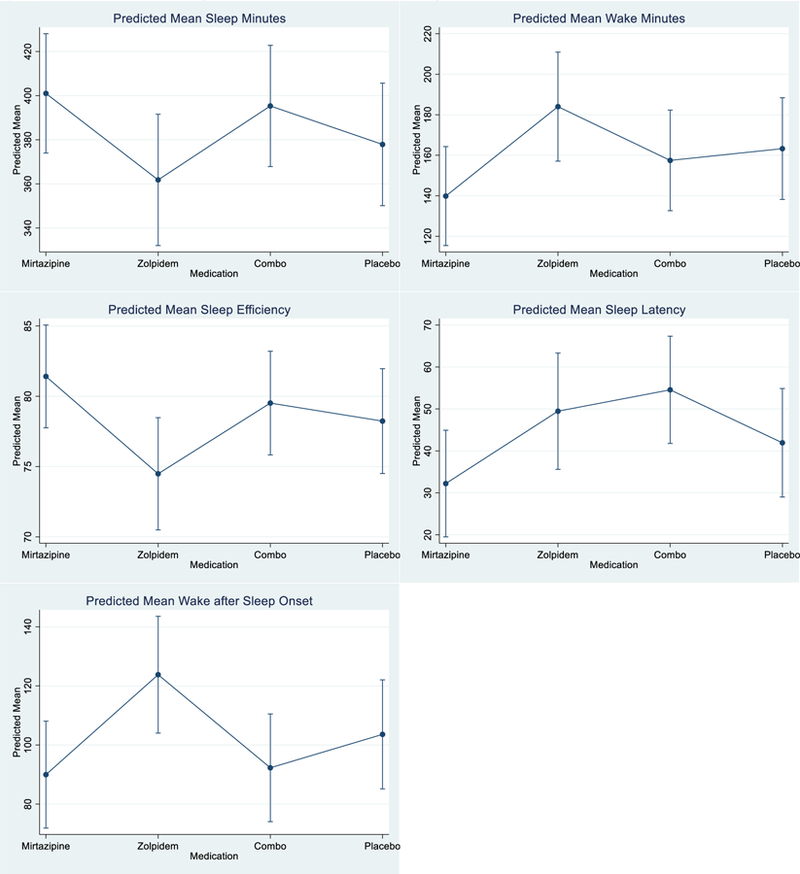
Predicted means were estimated by fixed effects regression. Variables included in the model included dummy indicators for sequential week of observation, sequential day in week of observation, and medication.
Conclusions
This pilot randomized, double-blind clinical trial tested three medication regimens for sleep disturbance among individuals on methadone maintenance. Our findings suggested that mirtazapine was superior in all measures of sleep quality (sleep minutes, wake minutes, sleep efficiency, sleep latency, and wake after sleep onset) compared to zolpidem or combination mirtazapine/zolpidem. Participants were adherent to the medication protocol and reported no negative side effects of medication. As such, mirtazapine appears to be an effective and safe medication for improving sleep quality among individuals on methadone maintenance who have sleep disturbance.
Methadone-maintained persons have many ongoing risks for the chronic insomnia they experience (Chakravorty et al., 2018). Methadone-maintained individuals have high rates of depressive symptoms and chronic pain that may interfere with sleep (Garnaat et al., 2017). Indeed, our sample reported depression symptomatology: eight out of ten reported at least minimal depression. Nine of our ten participants smoked cigarettes and nine out of ten reported using caffeine that interfere with sleep. Ongoing use of illicit drugs that affect sleep is common among persons in methadone maintenance treatment (although excluded here), but again may limit the ability to find an efficacious treatment for insomnia.
While zolpidem likely affects primarily GABAeric neurotransmission (one potential biological mechanism of insomnia), mirtazapine’s biological mechanisms are imprecise. As such, the neurobiological effects of the medications in this population warrant further study. The sleep outcomes of individuals taking zolpidem were surprising, particularly given the efficacy of zolpidem in treating insomnia in other populations. Perhaps mechanisms of sleep disturbance in individuals using opioids chronically differs from more non-drug-using populations. An alternative speculation is that perhaps the high rates of comorbid depression (or other psychiatric disoders) changes GABAergic neurotransmission in this population. The evidence for efficacious insomnia treatment would be welcome. Sleep disturbance reduces physical and emotional well-being (Haack and Mullington, 2005). Sleep disruption lowers pain thresholds (Baghdoyan, 2006; Roehrs et al., 2006) and intensifies chronic pain for many methadone patients (Rosenblum et al., 2003). Additionally, shortened sleep and daytime sleepiness might impair engagement with counseling, leading to continued drug use or relapse.
One potential limitation of this study was our decision to use diary-reported bedtimes and wake times to define the scoring interval for actigraphy data, rather than using event-marked bedtimes and wake times (which frequently were missing) or attempting to visually identify sleep onset and offset times based on inspection of the raw data. We acknowledge that this method may have introduced error into our sleep measures, if participants went to bed or awakened earlier or later than the times they stated during their sleep diary interview the following day. Nevertheless, we believed this method of using self-report data to support actigraphy data was the least likely to introduce bias and avoided the error introduced by defining the scoring interval based on low activity levels when quiet wakefulness could be scored as sleep. Additionally, the study relied on self-report of medication adherence and did not include a weekly pill count or the use of biological verification. Lastly, the study was limited by its small size and our lack of measures regarding the effects of sleep disturbance on daytime function. Given these limitations, the findings of differential effects for these medications should be viewed as preliminary. As such, the finding that zolpidem was associated with the poorest sleep outcomes may warrant more definitive research with a larger sample of persons with opioid use disorder.
This study’s strengths included a randomized, double-blind placebo-controlled design, an excellent rate of follow-up that did not differ between groups in a vulnerable population, and the inclusion of an objective sleep measure. We have previously published a comparison of different approaches to assessing sleep disturbance in this population (Sharkey et al., 2011).
The current study found that mirtazapine was a promising medication for sleep problems among individuals on methadone maintenance. Attention to common co-morbid conditions that may contribute to sleep disturbance in methadone patients, such as mental health disorders, chronic pain, and ongoing substance use, should be emphasized during clinical care. Combined medication and behavioral therapy protocols have been efficacious in other populations (Morin et al., 2009) and warrant continued testing.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr. Blevins is a recipient of an Institutional Development Award (U54GM115677) from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR).
Footnotes
Declarations of interest: none
References
- Alam A, Voronovich Z, Carley JA, 2013. A review of therapeutic uses of mirtazapine in psychiatric and medical conditions. The primary care companion for CNS disorders 15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD, 2009. Fixed Effects Regression Methods for Longitudinal Data Using SAS SAS Institute, Inc., Cary, NC. [Google Scholar]
- Baghdoyan HA, 2006. Hyperalgesia induced by REM sleep loss: a phenomenon in search of a mechanism. Sleep 29(2), 137–139. [PubMed] [Google Scholar]
- Balkin TJ, Rupp T, Picchioni D, Wesensten NJ, 2008. Sleep loss and sleepiness: current issues. Chest 134(3), 653–660. [DOI] [PubMed] [Google Scholar]
- Burke CK, Peirce JM, Kidorf MS, Neubauer D, Punjabi NM, Stoller KB, Hursh S, Brooner RK, 2008. Sleep problems reported by patients entering opioid agonist treatment. J Subst Abuse Treat 35(3), 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Chakravorty S, Vandrey RG, He S, Stein MD, 2018. Sleep Management Among Patients with Substance Use Disorders. The Medical clinics of North America 102(4), 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connock M, 2007. Methadone and buprenorphine for the management of opioid dependence: A systematic review and economic evaluation. Health Technology Assessment 11, 1. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Douaihy AB, Clark DB, Daley DC, Chung TA, Wesesky MA, Wood DS, Salloum I, 2013. Mirtazapine in Comorbid Major Depression and Alcohol Use Disorder: A Long-Term Follow-Up Study. Journal of addictive behaviors, therapy & rehabilitation 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J, 2015. Chronic Opioid Use and Central Sleep Apnea. Anesthesia & Analgesia 120(6), 1273–1285. [DOI] [PubMed] [Google Scholar]
- De Micco M, Wang-Weigand S, Zhang J, 2006. Long-term therapeutic effects of ramelteon treatment in adults with chronic insomnia: A 1-year study. Sleep 29(Abstract Suppl), A234. [Google Scholar]
- Friedmann PD, Rose JS, Swift R, Stout RL, Millman RP, Stein MD, 2008. Trazodone for sleep disturbance after alcohol detoxification: a double-blind, placebo-controlled trial. Alcohol Clin.Exp.Res 32(9), 1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnaat SL, Weisberg RB, Uebelacker LA, Herman DS, Bailey GL, Anderson BJ, Sharkey KM, Stein MD, 2017. The overlap of sleep disturbance and depression in primary care patients treated with buprenorphine. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse 38(4), 450–454. [DOI] [PubMed] [Google Scholar]
- Haack M, Mullington JM, 2005. Sustained sleep restriction reduces emotional and physical well-being. Pain 119(1–3), 56–64. [DOI] [PubMed] [Google Scholar]
- Hajiha M, DuBord MA, Liu H, Horner RL, 2009. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol 587(Pt 11), 2677–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassamal S, Miotto K, Wang T, Saxon AJ, 2016. A narrative review: The effects of opioids on sleep disordered breathing in chronic pain patients and methadone maintained patients. The American Journal on Addictions 25(6), 452–465. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Suess PE, Griffiths RR, 2006. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch.Gen.Psychiatry 63(10), 1149–1157. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA, 2005. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology 103(6), 1268–1295. [DOI] [PubMed] [Google Scholar]
- Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M, 2009. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep 32(3), 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson WB, Roth T, Cassella J, Roehrs T, Walsh JK, Woods JH, Buysse DJ, Meyer RE, 2004. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 New Clinical Drug Evaluation Unit meeting symposium. Sleep Med Rev 8(1), 7–17. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallieres A, Guay B, Ivers H, Savard J, Merette C, Bastien C, Baillargeon L, 2009. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA 301(19), 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation, 2009. 2009 Sleep in America Poll National Sleep Foundation (NSF), Washington, D.C. [Google Scholar]
- Nelson AM, Battersby AS, Baghdoyan HA, Lydic R, 2009. Opioid-induced decreases in rat brain adenosine levels are reversed by inhibiting adenosine deaminase. Anesthesiology 111(6), 1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyefeso A, Sedgwick P, Ghodse H, 1997. Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug Alcohol Depend 48(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M, 2006. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend 82(2), 103–110. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M, 2006. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug and Alcohol Dependence 28(2), 103–110. [DOI] [PubMed] [Google Scholar]
- Richardson GS, 2005. The human circadian system in normal and disordered sleep. J Clin Psychiatry 66 Suppl 9, 3–9; quiz 42–43. [PubMed] [Google Scholar]
- Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T, 2006. Sleep loss and REM sleep loss are hyperalgesic. Sleep 29(2), 145–151. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK, 2003. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 289(18), 2370–2378. [DOI] [PubMed] [Google Scholar]
- Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC, 2006. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry 60(12), 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA, 1994. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 17(3), 201–207. [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD, 2010. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend 108(1–2), 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD, 2011. Assessing sleep in opioid dependence: a comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug Alcohol Depend 113(2–3), 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Corso RP, Brower KJ, Millman RP, Stein MD, 2009. Home polysomnography in methadone maintenance patients with subjective sleep complaints. Am.J.Drug Alcohol Abuse 35(3), 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens R, Roffman R, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J, The Marijuana Treatment Project Research Group, 2002. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287(9), 1123–1131. [DOI] [PubMed] [Google Scholar]
- Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, Anderson BJ, 2004. Sleep disturbances among methadone maintained patients. J.Subst.Abuse Treat 26(3), 175–180. [DOI] [PubMed] [Google Scholar]
- Stein MD, Kurth ME, Sharkey KM, Anderson BJ, Corso RP, Millman RP, 2012. Trazodone for sleep disturbance during methadone maintenance: A double-blind, placebo-controlled trial. Drug and Alcohol Dependence 120(1–3), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Heath Services Administration, 2017. National Survey on Drug Use and Health 2016 Bethesda, MD. [Google Scholar]
- Teichtahl H, Prodromidis A, Miller B, Cherry G, Kronborg I, 2001. Sleep-disordered breathing in stable methadone programme patients: a pilot study. Addiction 96(3), 395–403. [DOI] [PubMed] [Google Scholar]
- Trksak GH, Jensen JE, Plante DT, Penetar DM, Tartarini WL, Maywalt MA, Brendel M, Dorsey CM, Renshaw PF, Lukas SE, 2010. Effects of sleep deprivation on sleep homeostasis and restoration during methadone-maintenance: a [31]P MRS brain imaging study. Drug Alcohol Depend 106(2–3), 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinov Y, Lichstein KL, Wal GS, Taylor DJ, Riedel BW, Bush AJ, 2010. Association between report of insomnia and daytime functioning. Sleep Med 11(1), 65–68. [DOI] [PubMed] [Google Scholar]
- Van Ryswyk E, Antic NA, 2016. Opioids and Sleep-Disordered Breathing. Chest 150(4), 934–944. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H, 2007. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med.Rev 11(1), 35–46. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H, Drummer O, Goodman C, Cherry G, Cunnington D, Kronborg I, 2005. Central sleep apnea in stable methadone maintenance treatment patients. Chest 128(3), 1348–1356. [DOI] [PubMed] [Google Scholar]
- Webster LR, Choi Y, Desai H, Webster L, Grant BJB, 2008. Sleep-Disordered Breathing and Chronic Opioid Therapy. Pain Medicine 9(4), 425–432. [DOI] [PubMed] [Google Scholar]


