Abstract
Chemical signals are conveyed to cells through ligand-receptor binding, triggering cascades of biochemical reactions and resulting in pivotal cellular functions. These binding events are important in understanding membrane signaling and drug interactions. To probe ligand-receptor binding, surface enhanced Raman scattering (SERS) tags are a promising tool. SERS tags are plasmonic nanostructures functionalized with a protective coating, a Raman reporter molecule, and a biorecognition element. In biological fluids, native proteins have affinity for bare nanoparticles and form a protein corona. SERS tags have a protective shell which eliminates this complication. It is important to analyze ligand-receptor binding with SERS tags in live cells since cell fixatives alter protein structure, leading to spectral changes and data misinterpretation. In this study, we synthesized a novel SERS tag by creating a mixed monolayer of the small cyclic arginine-glycine-aspartic acid-phenylalanine-cysteine (RGDFC) peptide and 4-mercaptobenzonitrile (MBN) on the surface of spherical gold nanoparticles (Au NP). Au-RGDFC-MBN NP showed resistance to PC formation and were successfully detected in both fixed and living human metastatic colon cancer cells.
Graphical Abstract
Introduction
The use of gold nanoparticles (Au NP) as diagnostic and therapeutic probes in vitro is well established, and their simple synthesis,1 surface functionalization,2 and utility for surface enhanced Raman scattering (SERS)3 led to routine incorporation of Au NP as the plasmonic core of SERS tags. However, the relationship between the physiochemical properties of SERS tags and their interactions with biological fluids is rarely reported in these studies, limiting progression of their use in live cell assays (in vivo).4
When immersed in biological fluids such as blood, human albumin serum, or fetal bovine serum (FBS), bare Au NP are coated with that fluid’s native proteins, forming a protein corona (PC). There are two types of PCs, hard and soft. Proteins with high affinity to the NP bind irreversibly to their surface and form a hard PC. Conversely, a soft PC forms when proteins with lower affinity for the NP’s surface bind reversibly.5 The proteins that comprise the hard PC mediate the interactions between the NP and the biological environment, dictating the NP’s bio-identity. Hard PC formation governs the fate of NP in vitro and in vivo by affecting their efficacy, targeting, toxicity, cellular interactions, and spatial distribution.6 For this paper, PC refers to the hard PC, unless otherwise indicated.
Surface modifications have been investigated to retain effective targeting and long circulation times of NP by avoiding PC formation. SERS tags are plasmonic NP modified to add stability, to provide a characteristic Raman signal, and to bind selectively to a target molecule.7 Typically, SERS tags have four parts: a metal nanosubstrate, a protective shell, a Raman reporter molecule, and a biorecognition element.7 The protective shell of the SERS tag should provide stability and preserve the SERS signal.8 The Raman reporter should produce a strong characteristic Raman signal which is used to indirectly sense a target.7 Finally, the biorecognition molecule should have affinity and specificity for the target molecule. With tag use in biological fluids, the protective coating becomes of primary importance because of its direct role in avoiding PC formation,5 and therefore maintaining the original and intended bio-identity of the NP.9
Different coatings have been utilized as protecting agents to eliminate PC formation. Traditionally, NP are coated with bovine serum albumin (BSA), which advantageously exploits the formation of the PC in a controlled manner.10 Another coating is thiolated polyethylglycol (PEG), which produces steric hindrance of PC formation.11 Polyvinylpyrrolidone can also be used as a protecting agent since it has weak affinity with interference molecules.12 Other coatings are zwitterionic in nature, with both positively and negatively charged groups, which achieves bio-stability of NP.13 Although successful in protecting NP, all of these coatings are typically large, which alters the function of SERS tags. For example, high concentrations or long chains of PEG lead to reduced targeted cell binding caused by steric hindrance between the targeting ligand and the receptor.14
It has recently been demonstrated that the small cyclic arginine-glycine-aspartic acid-phenylalanine-cysteine (RGDFC) peptide can be directly functionalized to the surface of gold nanostars (Au NS), creating a zwitterionic surface, which protects against PC formation.15 Both bare Au NS and RGDFC-functionalized Au NS were incubated with cell media supplemented with FBS and were then characterized. The results show that RGDFC protected against aggregation and unwanted amino acid adsorption, suggesting this cyclic peptide protects Au NS from PC formation. In addition to its protective function, RGDFC also acts as a biorecognition element. Peptides that include the RGD motif have been shown to selectively bind to integrin αvβ3.16 This integrin is highly expressed in new blood vessels and some tumor cells but not in resting endothelial cells, making it a target in anti-angiogenic cancer therapy.17 RGDFC-functionalized Au NS were used to probe the cell membrane receptor αvβ3 integrin on fixed cells. The interesting dual functionality of RGDFC, behaving as both a protective coating and as a biorecognition element, shows exciting promise. It was important to investigate if this property could be applied to other solution-based plasmonic nanostructures, and if it could retain these properties when functionalized along with a Raman reporter in the synthesis of SERS tags.
In addition to the formation of the PC, another factor hampering the use of NP in nanomedicine is the transition from fixed to live cell studies. Traditionally, cell imaging techniques involve fixatives which preserve and prevent degradation of biological samples. The most commonly used fixative is formaldehyde. It functions by cross-linking proteins, which changes their secondary, tertiary, and quaternary structures.18 Spectral changes of cellular proteins therefore occur with fixative use, leading to data misinterpretation.19 It is vital that fixed cell studies can be complemented with live cell studies.
There have been many variations of live cell imaging. In one of the first Raman live cell studies, red blood cells were suspended in phosphate buffered saline (PBS) and were then transferred to a Petri dish for Raman measurements.20 This method is only viable for short-term analysis, however. In order to truly reflect the environment in which cells live and grow, cells need to be supplied with fresh media that maintains a physiological pH and temperature.21 Physiological conditions are dependent on cell type. For instance, cancer cells create an acidic extracellular pH22 unlike healthy cells. To provide the proper conditions, Raman spectrometers have been paired with cell incubators.23
The aim of this study is to demonstrate a new SERS tag that can help characterize and eliminate the negative implications of PC formation on Au NP under cell culture conditions (e.g. cell media supplemented with fetal bovine serum (FBS)). Au NP were functionalized with small peptides and Raman reporter molecules to create SERS tags, protecting against PC formation. The tags were then applied to target αvβ3 integrins on both fixed and living human metastatic colon cancer cells.
Experimental
Chemicals
99.995% tetrachloroauric (III) acid (HAuCl4), ≥99.0% sodium citrate tribasic dihydrate (C6H9Na3O9), 100% sodium phosphate dibasic heptahydrate (Na2HPO4 · 7H2O), ≥99.0% sodium phosphate monobasic monohydrate (H4NaO5P), and 37% hydrochloric acid (HCl) were purchased from Sigma Aldrich (USA). Ultrapure water from a Milli-Q Integral Water Purification System was used. Cyclic(Arg-Gly-Asp-D-Phe-Cys) was purchased from Peptide International. 4-mercaptobenzonitrile (MBN) was purchased from Toronto Research Chemicals Inc. All cell culture reagents were purchased from Thermo Fischer Scientific (USA).
Instrumentation
Raman measurements for characterization of NP were carried out using a Snowy Range IM-52 with 638 nm laser excitation (40 mW, 10 s acquisition times). Size and zeta-potential measurements were carried out using a Malvern Zetasizer ZS. Extinction measurements were carried out using a VWR V-1600 Spectrophotometer. Scanning electron microscope (SEM) images were obtained using a Magellan XHR SEM. Cell mapping Raman measurements were carried out using a Renishaw InVia Raman microscope with 633 nm laser excitation.
Synthesis of Au NP
Citrate-capped spherical Au NP were synthesized by adding HAuCl4 (14 mg) to Milli-Q water (100 mL). The solution was constantly stirred while being heated to 90°C. Sodium citrate (15 mg) was added and the solution was heated and stirred for an additional 15 minutes. The solution changed colors from light yellow, to black, and finally to red. The bare Au NP were characterized using extinction spectroscopy, SEM, DLS, and nanosizer measurements. The extinction spectrum and SEM image are shown in Figure S1 (ESI).
SERS tag synthesis
RGDFC (5 μL of 0.043 mM) and MBN (10 μL of 0.1 mM) were mixed. Au NP (1 mL) were added and the resulting solution was left to shake overnight. The Au-RGDFC-MBN NP were characterized using extinction spectroscopy and Raman spectroscopy with 638 nm laser excitation.
Protein corona formation and characterization
FBS supplemented RPMI cell media (200 μL) was added to Au, Au-MBN, Au-RGDFC and Au-RGDFC-MBN NP (800 μL) and shaken for two hours. The resulting solutions were characterized by using extinction spectroscopy, DLS, and nanosizer measurements.
Cell culture
Human SW620 colon cancer cells derived from commercial cell lines (ATCC, Manassas, VA, USA) were passaged at approximately 80% confluency in RPMI-1640 medium supplemented with 10% FBS. The cells were cultured in a humidified atmosphere containing 5% CO2 with a temperature of 37°C.
NP fixed cell incubation and SERS analysis
Cells and RPMI media were transferred to a 35 mm diameter culture flask (2 mL) with a 22×22 mm square coverslip. The culture flask was left to incubate for two days, allowing the cells time to adhere to the coverslip. Au NP, Au-RGDFC NP, Au-MBN NP, and Au-RGDFC-MBN NP (200 μL each) were added to four different culture flasks and were incubated for 2 hours. The cell media was then removed and the adherent cells on the coverslip were washed with sterile PBS (1 mL) three times. Next, they were fixed by immersing the coverslip in 4% formaldehyde for 15 minutes. The coverslip was then removed from formaldehyde, was washed with PBS and H2O, and was left to dry. Once dry, the cells were imaged by focusing on the cell membrane with a 50x laser objective (NA 0.75). They were Raman mapped using a 1×1 μm step size and 10 second exposure per acquisition. A 633 nm laser excitation with a power of 4 mW was used, scanning between 1069–1584 cm−1.
Phosphate buffer synthesis
H4NaO5P (10 mL of 0.3773 M) and Na2HPO4 · 7H2O (5 mL of 0.6228 M) were mixed and HCl was added until pH 6.5 was achieved.
Live cell viability analysis
Cells and RPMI media were transferred to a 50 mm diameter culture flask (2 mL) with a 40 mm diameter coverslip. The culture flask was left to incubate for two days, allowing the cells time to adhere to the coverslip. Au-RGDFC-MBN NP (400 μL) were added to the culture flask and were incubated for 2 hours. A 0.459 M phosphate buffer (363 μL) was added to media (7.637 mL) to maintain pH 6.5. Additionally, Au-RGDFC-MBN NP (2 mL) and 0.4% trypan blue dye (100 μL) were added to the media. The acidic media, Au-RGDFC-MBN NP, and trypan blue were flowed through the FCS3 Bioptechs System (pictured in Figure S7) and over the surface of the cells using a peristaltic pump. Cells were imaged after 4.5 hours of incubation.
NP live cell incubation and SERS analysis
The Bioptechs FCS3 System with the same conditions as in the live cell viability analysis, excluding the trypan blue dye, was used for SERS analysis. The cells were imaged by focusing on the cell membrane with a 20x laser objective (NA 0.40). They were Raman mapped using a 2×2 μm step size and 3 second exposure per acquisition. A 633 nm laser excitation with a power of 4 mW was used, scanning between between 1069–1584 cm−1 and 848–1384 cm−1.
Results and Discussion
SERS tag synthesis
NP in biological fluids require protective coatings to prevent PC formation, and we recently demonstrated that the small cyclic peptide RGDFC protects against PC formation through its zwitterionic nature. Previously, Au-RGDFC NS were used as label free probes to determine the location and vibrational fingerprint of αvβ3 integrins on colon cancer cells by Raman mapping with 633 nm laser excitation. When compared to Au NS, spherical Au NP have a simpler synthesis that yields homogenous particles. However, spherical Au NP have a much lower SERS enhancement and behave relatively poorly as label free probes.3 To overcome this weakness, Au NP can be used as the core metal nanostructure of SERS tags, having strong bands provided by the Raman reporter molecule which acts as an internal standard. Our SERS tags are spherical Au NP functionalized with MBN, the Raman reporter molecule, and RGDFC to protect against PC formation and to act as the biorecognition molecule for αvβ3 integrin. Due to RGDFC’s dual functionality, these SERS tags have only three elements as opposed to the typical four.7 By Raman mapping cells, the SERS tags provide an indirect detection of αvβ3 integrin location through detecting the strong SERS bands of MBN.
The synthesis and optimization of the proposed SERS tag were carried out by first optimizing the concentration of the protective and selective cyclic RGDFC. The structure of the cyclic peptide is shown in Figure S2a (ESI). Different concentrations of cyclic RGDFC (0, 43, 215, 430, 645, and 860 nM) were functionalized to the surface of 40 nm citrate-capped Au NP. The resulting Au-RGDFC NP were analyzed with extinction spectroscopy and Raman spectroscopy with 638 nm laser excitation, results shown in Figure S2b and c (ESI).
RGDFC easily conjugates to the surface of Au NP via the cysteine residue thiol. Additionally, RGDFC has a distinctive SERS signal used to confirm successful conjugation. The SERS spectra in Figure S2c (ESI) show phenylalanine is observed at 1000 cm −1 and 1205 cm−1, arginine at 1030 cm−1, and cysteine at 836 cm−1. The intensity of the RGDFC signal was dependent on concentration. The maximum intensity was obtained with a concentration of 645 nM, however this concentration was associated with aggregation. When 430 nM of RGDFC was conjugated to Au NP, a strong SERS signal was obtained. However, when analyzing the extinction spectrum corresponding to this concentration, there was the beginning of a 2nd peak forming at roughly 700 nm. This is indicative of aggregation, and it was thought that the addition of MBN to this conjugate would be unstable. Another piece of evidence to back up this claim is the larger error in size measurement associated with the 430 nM conjugates. This indicated the NP are non-homogenous and perhaps already exhibiting signs of aggregation or instability. At 215 nM RGDFC, the NP did not aggregate and they gave characteristic RGDFC SERS signal. The results are shown in Table S1 (ESI). This concentration was used in future experiments.
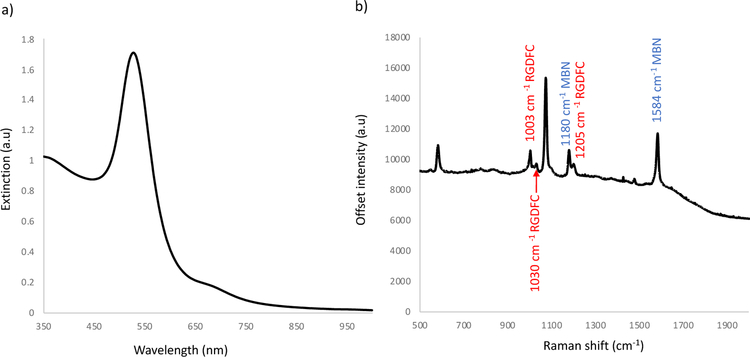
Although RGDFC produces a characteristic SERS spectrum, the intensity is weak on Au NP. Additionally, RGDFC binds to αvβ3 integrins, which could change the molecule’s conformation and ultimately its vibrational fingerprint. For this reason, RGDFC is not the ideal marker molecule for SERS detection of integrin location. To create a tag with a strong SERS signature that will not change once bound to its target, the non-resonant Raman reporter MBN was functionalized to the surface of Au NP, creating a mixed monolayer with the RGDFC molecules. Different concentrations of MBN (0.1, 0.5, 1, 2 and 3 μM), whose structure is shown in Figure S3 (ESI), were added to Au NP at the same time as cyclic-RGDFC (215 nM) and left to functionalize overnight. The resulting SERS tags were characterized using extinction spectroscopy and Raman spectroscopy with 638 nm laser excitation. The optimized result is shown in Fig. 1.
Figure 1.
a) Extinction spectrum and b) SERS spectrum of optimized Au-RGDFC-MBN NP in solution, highlighting the peaks from the RGDFC molecule (1000, 1030, and 1200 cm−1) and from the MBN molecule (1074, 1180, and 1584 cm−1). SERS spectrum is the average of three scans obtained using 638 nm laser excitation.
MBN was selected because it produces a strong SERS signal and because of its thiol group, which allows for easy conjugation to the Au NP. The molecule’s characteristic peaks at 1074, 1180, and 1584 cm−1 are seen in the spectra in Figure 1 and Figure S3c (ESI). As expected, increased MBN concentration with constant 215 nM RGDFC resulted in increased MBN SERS intensity of Au-MBN-RGDFC NP. At 1 μM of MBN, both the strong MBN signal as well as the RGDFC signal existed, demonstrating a mixed monolayer. Little aggregation was observed in the dynamic light scattering (DLS) measurements, results in Table S2 (ESI). The possibility of a slight aggregation was observed in the extinction spectrum but not observed in other experiments, results in Figure S3 (ESI). To create a stable SERS tag with the most desirable properties, the concentration of MBN was five times that of RGDFC. To explain the difference in concentration between MBN and RGDFC, it is possible that MBN’s smaller footprint relative to RGDFC requires a higher concentration to observe it in the SERS spectra. Another potential explanation is that the higher concentration of MBN increases tag stability by better covering the surface of the NP. These two explanations are not necessarily mutually exclusive.
Protein corona characterization
When bare NP are immersed in biological fluids, the PC forms, changing the bio-identity of the NP. For tags to function effectively, they must retain their reporter and biorecognition properties. This retention is provided by a protective coating. PC formation can be characterized by the tags’ ability, or lack thereof, to maintain the same characteristics in biological fluids as in water. In this study, FBS supplemented cell media was used as the model biological fluid. FBS is often added to cell media to enhance the growth and survival of cultured cells, since it has a low abundance of antibodies and a high concentration of growth factors.24 FBS not only has an impact on cell growth and survival, but also has an impact on PC formation. Without the addition of FBS, positively charged amino acids, such as lysine and arginine,25 are attracted to the NP’s negative surface and are electrostatically adsorbed. The resulting SERS spectrum of NP in cell media are shown in Figure S4a (ESI). When FBS is added to cell media the SERS peaks of amino acids are no longer observed. This suggests that in FBS supplemented cell media the small amino acids do not form the PC and it is the larger components of the FBS which adsorb to the surface. Because the large proteins of the FBS have poor scattering properties, they were not observed in the SERS spectrum. The SERS spectrum of the Au NP with FBS supplemented media is shown in Figure S4b (ESI) and indicates that the FBS fluoresces, hiding the Raman signal.
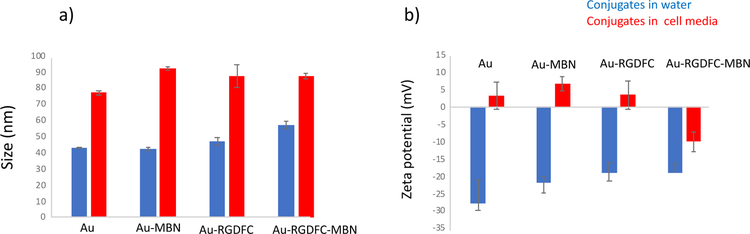
When the FBS supplemented media was added to each sample (Au, Au-MBN, Au-RGDFC, or Au-RGDFC-MBN NP) the extinction spectra red-shifted, as indicated in Figure S5 (ESI). This suggests that the proteins of the FBS supplemented cell media were adsorbed onto the surface of all four samples. The tags had already been functionalized with RGDFC and MBN, making this result unexpected. It is possible that some native proteins were transiently associated with the tags, forming a soft PC. Proteins that comprise a soft PC have greater dissociation rates and are readily desorbed,26 detracting the negative consequences of PC formation. Further evidence of soft PC formation is demonstrated in the size and zeta measurements shown in the bar graphs in Figure 2.
Figure 2.
a) Bar graph depicting size (nm) of Au, Au-MBN, Au-RGDFC, and Au-RGDFC-MBN NP in water (blue) and in FBS supplemented cell media (red) b) Bar graph depicting zeta potential (mV) of Au, Au-MBN, Au-RGDFC, and Au-RGDFC-MBN NP in water (blue) and in FBS supplemented cell media (red)
Au NP almost double in size in FBS supplemented media. While this is usually indicative of aggregation, that is not supported by the extinction spectrum, shown in Figure S5 a) (ESI), and the lack of color change. These data suggest that a large concentration of protein is now adsorbed onto the surface, increasing the size. The PC provides stability, and the Au NP do not aggregate even with the high salt concentration of the media.27, 28 However, PC does ultimately change the bio-identity of the Au NP, which are now positively charged. The same trend is observed for Au-MBN NP, suggesting MBN does little to protect against PC formation. A smaller change in size is experienced with Au-RGDFC NP in FBS supplemented media when compared to Au NP or Au-MBN NP. With the RGDFC molecule, a larger portion of the surface is involved in functionalization, decreasing the surface area exposed to the FBS and media proteins. Recent studies have also suggested that the zwitterionic nature of RGDFC limits protein adsorption.15 Because a relatively low concentration of RGDFC was added to the NP, RGDFC alone was not enough to completely block PC formation. Au-RGDFC NP increased in size and became positively charged in FBS supplemented media, indicating a change in their bio-identity. When both MBN and RGDFC were functionalized onto the surface of the Au NP, Au-RGDFC-MBN NP experienced only a small increase in size and retained their negative charge. Minimal surface area remained exposed to the biological fluid with both RGDFC and MBN functionalized onto the Au NP, preserving the tags’ original and intended bio-identity.
Detection of αvβ3 integrin with SERS tag
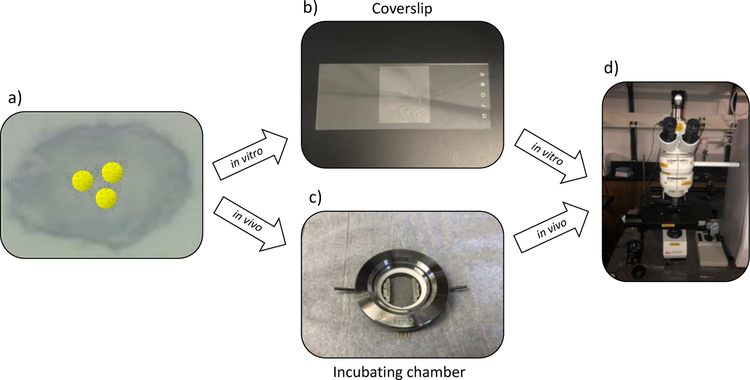
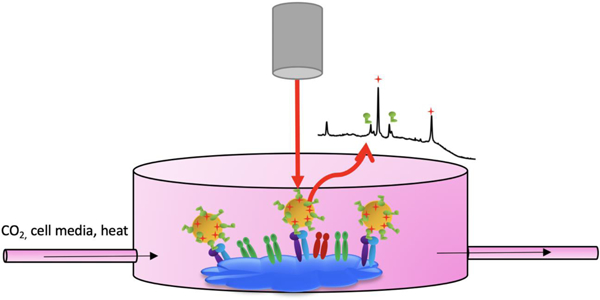
SERS tags are ideal tools for cell studies because they are noninvasive and have great sensitivity.29, 30 To conduct detection of αvβ3 integrin on the surface of fixed and living human metastatic colon cancer cells, Au-RGDFC-MBN NP were utilized. Cells were incubated with tags and the RGDFC tag component selectively bound to the cells’ αvβ3 integrin. After this incubation period, the cells were either fixed or were transferred to the FCS3 incubating chamber for living cell studies (Fig. 3). Then the cells were Raman mapped using confocal Raman microscopy. The tags’ strong characteristic MBN SERS bands allowed for facile detection which indicated location of the tags, and indirectly the location of αvβ3 integrin, on the cell surface. Information about αvβ3 integrin location and behavior on cells is key to creating and optimizing therapeutic agents targeting this transmembrane protein. Probing αvβ3 integrin on living cells is most impactful because it paints a picture of integrin behavior and location in a physiological environment.
Figure 3).
Workflow to detect αvβ3 integrin on the cell surface with SERS tags. a) Cells were incubated with SERS tags. b) Cells were either fixed onto a coverslip for living cell studies or c) were incubated in a chamber for living cell studies. d) Cell samples, either fixed or living, were Raman mapped.
Fixed cell studies
The protective qualities of RGDFC were demonstrated; however, to ensure that RGDFC possesses its biorecognition function when bound to Au NP in a mixed monolayer with MBN, the SERS tags were used as probes to detect αvβ3 integrin on the surface of a human metastatic cell line. Au-RGDFC-MBN NP were added to the FBS supplemented cell media and the cells were incubated in this solution for two hours, which was sufficient time to allow RGDFC to bind to the αvβ3 integrin on the cell surface. The cells were then washed and fixed with formaldehyde and analyzed by Raman mapping with 633 nm laser excitation. The results are shown in Figure 4.
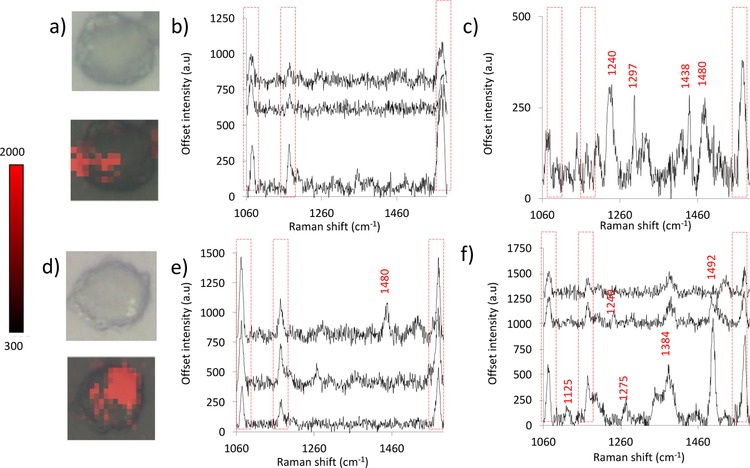
Figure 4.
a and d) White light and false color images of fixed cells incubated with SERS tags. b, c, e, and f) SERS spectra acquired via Raman mapping of cells.
Figure 4 displays the white light and false color images of two cells incubated with Au-RGDFC-MBN NP. The false color images were processed by analyzing the intensity of the 1074 cm−1 peak of MBN for each spectrum of the map. The location of the red pixels highlights the areas were MBN is present, which indirectly shows the location of RGDFC-αvβ3 binding. Clusters of integrin are sensed by the tags, meaning αvβ3 is not evenly dispersed throughout the membrane when bound to its RGDFC ligand, which is consistent with previous findings.15 The SERS spectra in Figures 4b, c, e, and f demonstrate that all three MBN SERS peaks (1074, 1180, and 1584 cm−1) are present on the cell membrane. However, it should be noted that additional peaks are present. We attribute these peaks to the integrin protein and other cell membrane components. The peaks assigned to tryptophan, CH bending and an amide were mapped and the resulting false color images shown in figure S6 (ESI).15 The distribution of these peaks matched that of MBN. However, in some circumstances the MBN signal was weaker. This could be due to the strong scattering by the bound integrin.
Alongside the incubations of Au-RGDFC-MBN NP with cells, control experiments took place. Au, Au-RGDFC, and Au-MBN NP were also incubated with cells, using the same protocol as previously described. Figure S7 (ESI) shows the white light and false color images produced when analyzing the intensity of the 1074 cm−1 peak for cells incubated with Au, Au-RGDFC and Au-MBN NP, respectively. It is clear that the signal from the Au-RGDFC-MBN NP is the strongest.
Live cell studies
Transitioning from fixed to live cell studies is important since fixatives have been shown to alter protein structure, leading to spectral changes and data misinterpretation. In Figure 4, Au-RGDFC-MBN NP were demonstrated to bind to integrin αvβ3 on the surface of fixed cells, but their ability to detect this interaction in living cells under culture conditions remained undetermined and needed to be investigated.
To provide the cells a physiological environment within a confocal Raman setup, a live cell incubator stage was used. A full description of the set-up is shown in figure S8 (ESI). Both the temperature and pH of cell media are important factors in maintaining cell viability. Because it has been reported that cancer cells create an acidic extracellular pH, the media was buffered to pH 6.5, mimicking the cells’ physiological conditions. It was also important to investigate the cytotoxicity of the SERS tags. To do this, Au-RGDFC-MBN NP and trypan blue were added to the buffered media. The trypan blue dye was used in an exclusion test to determine cell viability. Intact cell membranes are impermeable to the dye, whereas disrupted membranes are not.31 After 4.5 hours in the chamber, the cells were imaged. Figure S9 (ESI) shows a picture of the cells, which are all clear in color, evidencing cell viability. Figure S10 (ESI) indicates the change in color expected from cells that have died. They are stained blue as the damaged cell membrane becomes permeable to trypan blue dye.
The cells remained 100% viable in the microscope stage incubator in an acidic environment with SERS tags for at least 4.5 hours, so the tags were used as probes to detect αvβ3 integrin on the surface of a human metastatic cell line. Cells were incubated in FBS supplemented media with Au-RGDFC-MBN NP for two hours. They were then placed in the FCS3 Bioptechs chamber, which maintained physiological temperature at 37 degrees Celsius and provided fresh media buffered to pH 6.5 supplemented with Au-RGDFC-MBN NP. The Raman laser was focused on the cell membrane and then the cell was Raman mapped within a 4.5-hour window of time. In the case of the fixed cells, the scanning center was chosen to include the main peaks of MBN. This was replicated in the live cell experiments and the false colour images and associated spectra shown in figure S11 (ESI). However, this range did not include the RGDFC phenylalanine peak. Hence, to explore any changes that could be seen in this region, live cell maps included these Raman shifts. Results of live Raman cell mapping are shown in Figure 5.
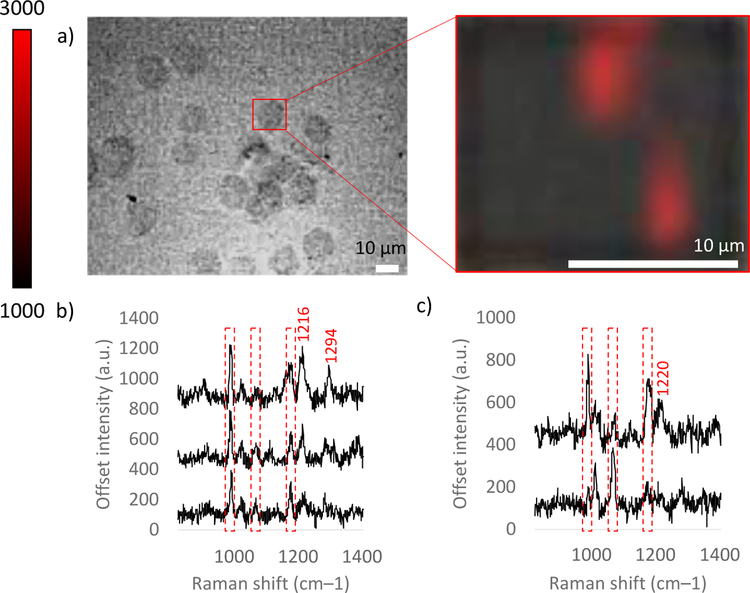
Figure 5.
a) White light and zoomed in SERS false color images of live cell incubated with SERS tags in the FCS3 system. Red pixels indicate SERS detection of MBN on the cell surface. b) Representative SERS spectra from the upper middle and c) the bottom right cluster of red pixels. Repeat live cell experiments are shown in Figures S12.
After Raman mapping, the false color image was created using the intensity of the 1074 cm−1 peak of MBN for each spectrum in the map. The red pixels depict where the 1074 cm−1 peak of MBN is present. These data indicate where the Au-RGDFC-MBN NP bound to the cell. It has been previously shown that Au-RGDFC NP are specific for αvβ3 integrin on the surface of a live SW620 cells, consistent with the fixed cell results for our Au-RGDFC-MBN NP above. The MBN peak was strongest in two regions on the cell, indicating integrin clustering. In addition to the 1074 cm−1 and 1180 cm−1 peaks of MBN, the 1000 cm−1 Phe peak of RGDFC was also present in the spectra. The MBN peak at 1584 cm−1 used for the fixed cells was out of the scanning range in this experiment. In addition, there were peaks not attributed to MBN or RGDFC. Similar to the fixed cell experiments and previous reports,32, 33 these peaks are attributed to integrin and other cell membrane components. Further investigations of these peaks may provide new insights into probe targeting.
The SERS peak at 1295 cm−1 seems to be common to both fixed and living SERS analysis of SW620 cells with Au-RGDFC-MBN NP (Figure 4c). However, most peaks not attributed to MBN or RGDFC within the overlapped wavenumber range differ between fixed and living cell SERS spectra. It is important to note that the scanning range was altered in the live cell experiment to insure the intense 1074 cm−1 band was not lost on the edges of the CCD; however, the overlap is only 315 wavenumbers between fixed and live cell maps. It is possible that outside of this overlapped wavenumber range, more SERS peaks of integrin and cell membrane components align between fixed and living analysis. Alternatively, the differences in peaks observed could be explained by the use of formaldehyde in vitro. Formaldehyde crosslinks proteins, changing their secondary, tertiary, and quaternary structures, which alters their vibrational fingerprints and ultimately their SERS spectra. SERS tags used in live cells circumvent this complication, giving a better picture of the physiological targets and processes probed. In these initial experiments, the use of a reporter molecule provided a reliable signal of the NP; however, it has been shown that higher enhancement structures give rise to SERS signals from the intrinsic molecules. Thus, live cell probing has the potential to take into consideration many additional relevant time-dependent events, such as cell viability; progression through the cell cycle; integrin recycling and integrin localization; and continual tag binding, tag detachment, and flexibility of tag-integrin interactions.
In this study, we probed the αvβ3 integrin with Au-RGDFC-MBN NP on the surface of a living human metastatic colon cancer cell in a physiological environment. SERS cell mapping with the tag provides information about integrin location on the cell surface while ensuring a physiological environment for normal cancer cell activity, which is valuable for developing and optimizing therapeutic agents targeting this integrin. SERS tags may prove to be a viable tool for in vivo probing of membrane proteins.
Conclusion
In conclusion, we created a novel SERS tag using MBN as a Raman reporter molecule and RGDFC, having dual functionality as a biorecognition molecule for human αvβ3 integrin as well as a zwitterionic protecting agent against PC formation. This tag prevents PC formation in FBS supplemented cell media, and thus retains the original and intended bio-identity of the NP as seen in the extinction spectroscopy, DLS, and zeta potential results. Additionally, Au-RGDFC-MBN NP conserved their affinity and selectivity for αvβ3 integrin in fixed cells. We further demonstrated these tags could be detected interacting with live SW620 human metastatic colon cancer cells viability in a flow cell microscope incubator, which maintained cell viability. Au-RGDFC-MBN NP were used as SERS probes for αvβ3 integrin in living cells. PC-free SERS tag use in live cells will be useful in identifying locations of membrane receptors in a truly reflective physiological environment, giving valuable information about cell signaling and potential drug targets.
Supplementary Material
Acknowledgements
The project was funded by National Institutes of Health Award R01 GM109988 to ZDS and the by the Ohio State University and Ohio5 undergraduate research program.
Footnotes
Conflicts of Interest
There are no conflicts of interest to declare.
References
- 1.Yeh YC, Creran B, and Rotello VM. Nanoscale, 2012, 4, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Xianyu Y, and Jiang X. Acc. Chem. Res, 2017, 50, 310–319. [DOI] [PubMed] [Google Scholar]
- 3.Tian F, Bonnier F, Casey A, Shanahan AE, and Byrne HJ. Anal. Methods, 2014, 6, 9116–9123. [Google Scholar]
- 4.Charbgoo F, Nejabat M, Abnous K, Soltani F, Taghdisi SM, Alibolandi M, Thomas Shier W, Steele TWJ, Ramezani M. J. Controlled Release, 2018, 272, 39–53. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen VH and Lee BJ. Int. J. Nanomed, 2017, 12, 3137–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand N, Grenier P, Mahmoudi M, Lima EM, Appel EA, Dormont F, Lim JM, Karnik R, Langer R, and Farokhzad OC. Nat. Commun, 2017, 8, 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Yan B, and Chen L. Chemical Reviews, 2013, 113, 1391–1428. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Qiu Y, Fan C, Cui K, Zhang Y, and Xiao Z. Acta Pharm. Sin. B, 2018, 8, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischer CC and Payne CK. The Journal of Physical Chemistry B, 2014, 118, 14017–14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrini L, Alvarez-Puebla RA, and Pazos-Perez N. Materials, 2018, 11, 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suk JS, Xu Q, Kim N, Hanes J, and Ensign LM. Adv. Drug Delivery Rev, 2016, 99(Pt A), 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durán N, Silveira CP, Durán M, and Martinez DST. J. Nanobiotechnol, 2015,13, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safavi-Sohi R, Maghari S, Raoufi M, Jalali S, Hajipour MJ, Ghassempour A, and Mahmoudi M. ACS Appl. Mater. Interfaces, 2016, 8, 22808–22818. [DOI] [PubMed] [Google Scholar]
- 14.Jokerst JV, Lobovkina T, Zare RN, and Gambhir SS. Nanomedicine, 2011, 6, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloan-Dennison S and Schultz ZD. Chem. Sci, 2019, 10, 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danhier F, Le Breton A, and Préat A. Mol. Pharmaceutics, 2012, 9, 2961–2973. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Wang F, and Chen X. Drug Dev. Res, 2008, 69, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, and Ranganathan K. J. Oral Maxillofac. Pathol, 2012, 16, 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meade AD, Clarke C, Draux F, Sockalingum GD, Manfait M, Lyng FM, and Byrne HJ. Anal. Bioanal. Chem, 2010, 396, 1781–1791. [DOI] [PubMed] [Google Scholar]
- 20.Wood BR, Hammer L, Davis L, and McNaughton D. J. Biomed. Opt, 2005, 10, 14005–14005. [DOI] [PubMed] [Google Scholar]
- 21.Smith R, Wright KL, and Ashton L. Analyst, 2016, 141, 3590–3600. [DOI] [PubMed] [Google Scholar]
- 22.Chiche J, Brahimi-Horn MC, and Pouysségur J. J. Cell. Mol. Med, 2010, 14, 771–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoladek A, Pascut F, Patel P, and Notingher I. Spectroscopy, 2010, 24, 131–136. [Google Scholar]
- 24.Zheng X, Baker H, Hancock WS, Fawaz F, McCaman M, and Pungor E Jr. Biotechnol. Prog, 2006, 22, 1294–300. [DOI] [PubMed] [Google Scholar]
- 25.Salazar A, Keusgen M, and von Hagen J. Amino Acids, 2016, 48, 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safavi-Sohi R, Maghari S, Raoufi M, Jalali SA, Hajipour MJ, Ghassempour A, and Mahmoudi M. ACS Appl. Mater. Interfaces, 2016, 8, 22808–22818. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez-Medina S, Blankenburg J, Olson J, Landes CF, and Link S. ACS Sustainable Chem. Eng, 2013, 1, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells MA, Abid A, Kennedy IM, and Barakat AI. Nanotoxicology, 2012, 6, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zengin A, Tamer U, and Caykara T. J. Mater. Chem. B, 2015, 3, 306–315. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Lorenzo L, Álvarez-Puebla A, Pastoriza-Santos I, Mazzucco S, Stéphan O, Kociak M, Liz-Marzán LM, and García de Abajo J. J. Am. Chem. Soc 2009, 131, 4616–4618. [DOI] [PubMed] [Google Scholar]
- 31.Strober W. Curr. Protoc. Immunol, 2001, 10, 10.1002/0471142735.ima03bs21. [DOI] [PubMed] [Google Scholar]
- 32.Xiao L, Bailey KA, Wang H, Schultz ZD. Anal. Chem, 2017, 89, 9091–9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao L, Sloan-Dennison S, Schultz ZD. J. Soc. Photo-Opt. Instrum. Eng, 2018, 10726, 10.1117/12.2321300. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.