Abstract
BACKGROUND:
Health care–associated infections are a common cause of patient morbidity and mortality. We sought to describe the trends in these infections in acute care hospitals, using data from 3 national point-prevalence surveys.
METHODS:
The Canadian Nosocomial Infection Surveillance Program (CNISP) conducted descriptive point-prevalence surveys to assess the burden of health care–associated infections on a single day in February of 2002, 2009 and 2017. Surveyed infections included urinary tract infection, pneumonia, Clostridioides difficile infection, infection at surgical sites and bloodstream infections. We compared the prevalence of infection across the survey years and considered the contribution of antimicrobial-resistant organisms as a cause of these infections.
RESULTS:
We surveyed 28 of 33 (response rate 84.8%) CNISP hospitals (6747 patients) in 2002, 39 of 55 (response rate 71.0%) hospitals (8902 patients) in 2009 and 47 of 66 (response rate 71.2%) hospitals (9929 patients) in 2017. The prevalence of patients with at least 1 health care–associated infection increased from 9.9% in 2002 (95% confidence interval [CI] 8.4%–11.5%) to 11.3% in 2009 (95% CI 9.4%–13.5%), and then declined to 7.9% in 2017 (95% CI 6.8%–9.0%). In 2017, device-associated infections accounted for 35.6% of all health care–associated infections. Methicillin-resistant Staphylococcus aureus (MRSA) accounted for 3.9% of all organisms identified from 2002 to 2017; other antibiotic-resistant organisms were uncommon causes of infection for all survey years.
INTERPRETATION:
In CNISP hospitals, there was a decline in the prevalence of health care–associated infection in 2017 compared with previous surveys. However, strategies to prevent infections associated with medical devices should be developed. Apart from MRSA, few infections were caused by antibiotic-resistant organisms.
Health care–associated infections represent substantial burden on health care systems in highly developed countries, including Canada.1–3 In 2002, health care–associated infection developed in an estimated 5% of patients admitted to hospital in the United States, resulting in 1.7 million infections and 98 000 deaths.1 A study using 2015 data from the European Antimicrobial Resistance Surveillance Network (EARS-Net) from 30 countries estimated 426 277 infections with antibiotic-resistant bacteria were associated with health care, with an attributable mortality of 33 110.2 A point-prevalence study conducted in 2015 estimated that there were 687 200 health care–associated infections in US hospitals.3
Timely data on the occurrence of health care–associated infections and antimicrobial resistant organisms in Canadian hospitals are essential to the response to an evolving epidemiologic situation. Internationally, prevalence surveys are widely used to estimate the incidence and burden of disease from these infections. 3–10 The Canadian Nosocomial Infection Surveillance Program (CNISP) provides data on the incidence of selected health care–associated infections and antimicrobial resistant organisms11–15 and conducted point-prevalence surveys in 2002 and 2009.16,17 In 2017, we replicated a point-prevalence survey in CNISP hospitals, to provide an up-to-date estimate of the burden of health care–associated infections and antimicrobial-resistant organisms causing these infections in Canadian hospitals, and to describe the trends observed over time in the 3 surveys.
Methods
Sources of data and study population
The Canadian Nosocomial Infection Surveillance Program is a collaboration of the Public Health Agency of Canada (PHAC) and sentinel hospitals across Canada that participate as members of the Canadian Hospital Epidemiology Committee, a subcommittee of the Association of Medical Microbiology and Infectious Disease Canada (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190361/-/DC1). Canadian Nosocomial Infection Surveillance Program hospitals from 9 provinces participated in the 2002 and 2009 descriptive point-prevalence surveys, and hospitals from all 10 provinces participated in 2017. Patients of any age who were admitted to a participating CNISP hospital for 48 hours or longer were eligible for inclusion. Patients who had been admitted for less than 48 hours but were admitted within the last month to the survey hospital were also included. We excluded patients admitted to long-term care, maternity, mental health, day surgery or rehabilitation units.
Case definitions
We defined health care–associated infections using the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network standard definitions,18 except for central line–associated bloodstream infections for which we used the CNISP definition.19 We considered an infection to be present if the patient was symptomatic of, or was receiving antimicrobial therapy to treat, a health care–associated infection on the day of the survey. We collected data on the following: pneumonia, urinary tract infection (UTI), primary and secondary bloodstream infection, infection at surgical sites and infection caused by Clostridioides difficile.
Data collection
We identified eligible patients by hospital census on a specified day in February of each survey year. The 2002 survey was conducted in February owing to the timing of budget allocation. To limit the influence of seasonal variation in health care–associated infections and to permit comparison among surveys, the 2009 and 2017 surveys were also conducted in February.
Experienced and trained staff reviewed the medical records of eligible patients for demographic data (age, sex, date of admission and type of ward where the patient was located on the day of the survey) and information on health care–associated infection (infection type, specimen collection date and microbiological etiology when available). In 2017, we collected data on ventilator-associated pneumonia, surgical site infections associated with a prosthetic implant, catheter-associated UTI and central line–associated bloodstream infections. We collected data on methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci and extended-spectrum β-lactamase–producing organisms for all 3 surveys. Carbapenemase-producing organisms emerged as a concern in Canada in 2010 and were surveyed in 2017 only.14 Hospital staff who were experienced in collection of surveillance data, use of National Healthcare Safety Network case definitions and trained in the use of the prevalence survey protocol (infection control professionals) collected data on a standardized form and submitted these forms to the PHAC for data entry, validation and analysis. We performed double-entry verification, and any inconsistencies in the data were compared with the submitted form and verified by the hospital if required. The Canadian Nosocomial Infection Surveillance Program collects hospital-level data (e.g., bed size, specialized services provided and type of hospital) annually using a standardized hospital profile form. We extracted hospital profile data for CNISP hospitals that participated in the 3 surveys and included this data in the analysis.
Statistical analysis
We analyzed the data using SAS software (version 9.3). We compared the characteristics of participating hospitals and patients who were surveyed, the prevalence of health care–associated infections and organisms causing infection using standard differences,20 χ2 tests, Fisher–Freeman–Halton exact tests for categorical variables or Kruskal–Wallis tests for continuous variables. We considered a 2-sided p value of 0.05 or less as significant.
We calculated the prevalence of health care–associated infection as the percentage of the number of patients with at least 1 infection over the total number of patients surveyed. We used Poisson regression with the survey year as the exposure variable to calculate the differences in prevalence of infection. We used generalized estimating equations to account for clustering by hospital, and to calculate p values and robust standard errors.
Ethics approval
These surveys were either considered exempt as quality assurance projects or approved by the research ethics boards at participating hospitals if required by institution-specific policies.
Results
Twenty-eight of 33 CNISP acute care hospitals (6747 patients) participated in the 2002 point-prevalence survey (response rate 84.8%), 39 of 55 hospitals (8902 patients) in 2009 (response rate 71.0%) and 47 of 66 hospitals (9929 patients) in 2017 (71.2% response rate). Table 1 provides the characteristics of the participating hospitals. Over the 3 surveys, the hospitals remained similar with respect to geographic distribution, bed size, hospital type and specialized services provided.
Table 1:
Selected characteristics of participating hospitals for the point-prevalence surveys (2002, 2009 and 2017)
| Variable | No. (%) of hospitals* | p value | ||
|---|---|---|---|---|
| 2002 n = 28 |
2009 n = 39 |
2017 n = 47 |
||
| Participating provinces | BC, AB, SK, MB, ON, QC, NL, NB, NS | BC, AB, SK, MB, ON, QC, NL, NB, NS | BC, AB, SK, MB, ON, QC, NL, NB, NS, PEI | |
| Region | ||||
| Eastern Canada† | 6 (21.4) | 8 (20.5) | 13 (27.7) | |
| Central Canada‡ | 12 (42.9) | 16 (41.0) | 16 (34.0) | 0.9 |
| Western Canada§ | 10 (35.7) | 15 (38.5) | 18 (38.3) | |
| Hospital bed size | ||||
| Median (IQR) | 441 (246–620) | 342 (165–487) | 290 (203–436) | 0.2 |
| Mean ± SD | 445 ± 241 | 354 ± 213 | 343 ± 222 | |
| Hospital type | ||||
| Adult | 13 (46.4) | 20 (51.3) | 23 (48.9) | |
| Mixed | 9 (32.1) | 12 (30.8) | 17 (36.2) | 1.0 |
| Pediatric | 6 (21.4) | 7 (18.0) | 7 (14.9) | |
| Specialized services | ||||
| ICU | 28 (100) | 37 (94.9) | 44 (93.6) | 0.45 |
| Hematology–oncology | 19 (67.9) | 25 (64.1) | 26 (55.3) | 0.5 |
| Dialysis | 18 (64.3) | 28 (71.8) | 31 (66.0) | 0.8 |
| Burn unit | 16 (57.1) | 14 (35.9) | 15 (31.9) | 0.1 |
| Solid organ transplant | 15 (53.6) | 16 (41.0) | 16 (34.0) | 0.3 |
| Teaching hospital | ||||
| Yes | 28 (100.0) | 36 (92.3) | 41 (87.2) | 0.1 |
Note: ICU = intensive care unit, IQR = interquartile range, SD = standard deviation.
Unless specified otherwise.
Eastern Canada includes Nova Scotia (NS), New Brunswick (NB), Prince Edward Island (PEI) and Newfoundland and Labrador (NL).
Central Canada includes Ontario (ON) and Quebec (QC).
Western Canada includes Manitoba (MB), Saskatchewan (SK), Alberta (AB) and British Columbia (BC).
Table 2 provides the characteristics of the patients who were surveyed. Although there were differences in the age distribution and there was an increased proportion of patients located in the intensive care unit (ICU) in 2017, the size of the effect was small (< 0.2) for all characteristics.
Table 2:
Selected characteristics of patients who were surveyed in 2002, 2009 and 2017
| Characteristic | No. (%) of patients* | Standardized difference† | ||
|---|---|---|---|---|
| 2002 n = 6747 |
2009 n = 8902 |
2017 n = 9929 |
||
| Sex, male | 3485 (51.7) | 4569 (51.5) n = 8865 |
5217 (52.8) n = 9881 |
0.03 |
| Age, mean ± SD; yr | 56.1 ± 27.0 | 57.6 ± 27.9 n = 8869 |
58.3 ± 27.9 n = 9896 |
0.08 |
| Age group, yr | n = 8869 | n = 9896 | ||
| Infants (< 1) | 493 (7.3) | 672 (7.6) | 837 (8.5) | 0.04 |
| Children (1–17) | 481 (7.1) | 619 (7.0) | 554 (5.6) | 0.06 |
| Adults (18–64) | 2444 (36.2) | 3052 (34.4) | 3235 (32.7) | 0.07 |
| ≥ 65 | 3329 (49.3) | 4526 (51.0) | 5270 (53.3) | 0.08 |
| Location of patient in hospital on survey day | n = 6736 | n = 8864 | n = 9912 | |
| Medical/surgical | 4882 (72.4) | 5934 (66.7) | 5664 (57.1) | 0.17 |
| ICU | 713 (10.6) | 1027 (11.5) | 1227 (12.4) | 0.06 |
| Adult | 296 (4.4) | 497 (5.6) | 583 (5.9) | 0.07 |
| Neonatal | 355 (5.3) | 475 (5.4) | 534 (5.4) | 0.006 |
| Pediatric | 62 (0.9) | 55 (0.6) | 110 (1.1) | 0.05 |
| Hematology/oncology/bone marrow transplant | 295 (4.4) | 446 (5.0) | 526 (5.3) | 0.04 |
| Pediatrics | 336 (5.0) | 376 (4.2) | 404 (4.1) | 0.04 |
| Critical/coronary care (not ICU) | 169 (2.5) | 209 (2.4) | 348 (3.5) | 0.07 |
| Gynecology/obstetrics | 123 (1.8) | 153 (1.7) | 207 (2.1) | 0.03 |
| Trauma/burn | 104 (1.5) | 92 (1.0) | 115 (1.2) | 0.05 |
| Solid organ transplant | 104 (1.5) | 184 (2.1) | 94 (1.0) | 0.09 |
| Other | 10 (0.2) | 2 (0.02) | 174 (1.8) | 0.30 |
| No. of days patients had been in hospital on survey day, median (IQR) | 10 (5–23) n = 6125 |
11 (5–27) n = 8809 |
10 (5–24) n = 9841 |
0.10 |
Note: ICU = intensive care unit, IQR = interquartile range, SD = standard deviation.
Unless specified otherwise.
Largest absolute standardized difference.
For all 3 surveys combined, a total of 2647 health care–associated infections were reported in 2447 patients with infection (1.08 health care–associated infections per infected patient).
The prevalence of patients with at least 1 health care–associated infection increased from 9.9% in 2002 (95% confidence interval [CI] 8.4%–11.5%) to 11.3% in 2009 (95% CI 9.4%–13.5%) followed by a significant decline to 7.9% in 2017 (95% CI 6.8%–9.0%). For all 3 surveys combined, prevalence of health care–associated infection was higher in patients admitted to ICU, where 16.2% of these patients had at least 1 health care–associated infection compared with 8.7% of patients in all other units combined (p < 0.001). We observed a major decline in the prevalence of infection in patients in the ICU, decreasing from 20.1% in 2002 (95% CI 15.8%–25.5%) to 17.8% in 2009 (95% CI 13.9%–22.8%) to 12.6% in 2017 (95% CI 10.1%–15.7%).
In an analysis restricted to the 18 hospitals that participated in all 3 surveys, we found that the prevalence of patients with a health care–associated infection was 9.8% in 2002 (95% CI 7.8%–12.2%) 10.4% in 2009 (95% CI 7.9%–13.7%) and 8.0% in 2017 (95% CI 6.4%–10.1%). Similarly, the prevalence of health care–associated infections in patients in the ICU in these 18 hospitals also declined from 20.2% in 2002 (95% CI 14.9%–27.4%) to 14.3% in 2009 (95% CI 9.9%–20.5%) to 13.9% in 2017 (95% CI 10.8%–17.8%).
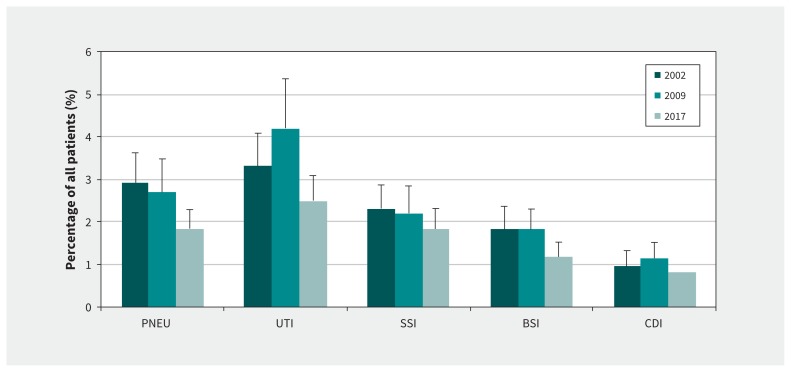
Over the 3 surveys, UTIs (31.9%) were the most common infection type, followed by pneumonia (23.4%), surgical site infection (20.2%), bloodstream infection (15.2%) and C. difficile infection (9.3%). The prevalence of patients with a UTI, surgical site infection and C. difficile infection declined over time, although not significantly. However, the prevalence of patients with pneumonia and bloodstream infection did significantly decrease from 2.9% in 2002 (95% CI 2.4%–3.6%) to 2.7% in 2009 (95% CI 2.1%–3.5%) to 1.8% in 2017 (95% CI 1.5%–2.3%) for pneumonia, and from 1.8% in 2002 (95% CI 1.4%–2.4%) and 2009 (95% CI 1.4%–2.3%) to 1.2% in 2017 (95% CI 0.9%–1.5%) for bloodstream infection (Figure 1).
Figure 1:
Prevalence of health care–associated infection types in Canada in 2002, 2009 and 2017. Note: BSI = bloodstream infection, CDI = Clostridioides difficile infection, PNEU = pneumonia, SSI = surgical site infection, UTI = urinary tract infection. Bars represent 95% confidence intervals.
In 2017, device-associated infections (i.e., ventilator-associated pneumonia, catheter-associated UTI, surgical site infections associated with a prosthetic implant and central line–associated bloodstream infection) accounted for 35.6% of all health care–associated infections (278 of 780 infections). Of the device-associated infections, catheter-associated UTI accounted for 37.1%, ventilator-associated pneumonia for 22.3%, central line–associated bloodstream infection for 21.2% and surgical site infections associated with a prosthetic implant for 19.4%.
Table 3 presents some selected antimicrobial resistant organisms that cause health care–associated infection. Overall, antimicrobial-resistant organisms remained an uncommon cause of health care–associated infection across all survey years. The most common resistant organism was MRSA, which was present in 6.2% of pneumonia infections, 5.6% of bloodstream infections, 5.0% of surgical site infections and 1.1% of UTIs. Of organisms associated with a bloodstream infection, the proportion of MRSA more than doubled from 3.8% in 2009 to 8.5% in 2017 (p = 0.1). Vancomycin-resistant enterococci infrequently caused infection at any site (1.0%, 0.5% and 0.8% of organisms associated with UTIs, surgical site infection and bloodstream infection, respectively). Carbapenemase-producing organisms were identified in only 3 infections (2 Escherichia coli and 1 Enterobacter species) in the 2017 survey. Infections associated with extended-spectrum β-lactamases significantly increased in frequency between 2002 (0.4%) and 2017 (2.8%) (p = 0.01), and were most common in patients with UTIs.
Table 3:
Selected antibiotic-resistant organisms causing health care–associated infection in 2002, 2009 and 2017
| Type of infection | No. (%) of infections* | Total no. (%) of infections* (2002–2017) | p value | ||
|---|---|---|---|---|---|
| 2002 | 2009 | 2017 | |||
| Urinary tract infection | |||||
| Escherichia coli, ESBL | 0 (0.0) | 14 (3.6) | 9 (3.6) | 23 (2.6) | 0.01 |
| Staphylococcus aureus, MRSA | 2 (0.8) | 4 (1.0) | 4 (1.6) | 10 (1.1) | 0.9 |
| Enterococcus species, VRE | 2 (0.8) | 4 (1.0) | 3 (1.2) | 9 (1.0) | 0.9 |
| Klebsiella species, ESBL | 0 (0.0) | 2 (0.5) | 3 (1.2) | 5 (0.6) | 0.2 |
| Escherichia coli, CPE | NA | NA | 2 (0.8) | 2 (0.2) | 0.6 |
| Total no. of UTI organisms | 238 | 392 | 248 | 878 | |
| Pneumonia | |||||
| Staphylococcus aureus, MRSA | 10 (5.4) | 13 (7.0) | 8 (6.1) | 31 (6.2) | 0.1 |
| Escherichia coli, ESBL | 0 (0.0) | 1 (0.5) | 2 (1.5) | 3 (0.6) | 0.2 |
| Klebsiella species, ESBL | 0 (0.0) | 0 (0.0) | 1 (0.8) | 1 (0.2) | 0.6 |
| Total no. of pneumonia organisms | 185 | 187 | 132 | 504 | |
| Surgical site infection | |||||
| Staphylococcus aureus, MRSA | 11 (6.5) | 9 (4.0) | 10 (4.9) | 30 (5.0) | 0.7 |
| Enterococcus species, VRE | 0 (0.0) | 1 (0.4) | 2 (1.0) | 3 (0.5) | 0.2 |
| Escherichia coli, ESBL | 0 (0.0) | 1 (0.4) | 3 (1.5) | 4 (0.7) | 0.2 |
| Enterobacter species, CPE | NA | NA | 1 (0.5) | 1 (0.2) | 0.6 |
| Klebsiella species, ESBL | 2 (1.2) | 0 (0.0) | 1 (0.5) | 3 (0.5) | 0.4 |
| Total no. of surgical site infection organisms | 169 | 224 | 202 | 595 | |
| Bloodstream infection | |||||
| Staphylococcus aureus, MRSA | 5 (4.8) | 6 (3.8) | 11 (8.5) | 22 (5.6) | 0.5 |
| Escherichia coli, ESBL | 1 (1.0) | 2 (1.3) | 0 (0.0) | 3 (0.8) | 0.2 |
| Enterococcus species, VRE | 0 (0.0) | 1 (0.6) | 2 (1.6) | 3 (0.8) | 0.2 |
| Klebsiella species, ESBL | 0 (0.0) | 2 (1.3) | 1 (0.8) | 3 (0.8) | 0.2 |
| Total no. of bloodstream infection organisms | 104 | 157 | 129 | 390 | |
| All infections | |||||
| MRSA | 28 (4.0) | 32 (3.3) | 33 (4.6) | 93 (3.9) | 0.9 |
| ESBL | 3 (0.4) | 22 (2.3) | 20 (2.8) | 45 (1.9) | 0.01 |
| VRE | 2 (0.3) | 6 (0.6) | 7 (1.0) | 15 (0.6) | 0.3 |
| CPE | NA | NA | 3 (0.4) | 3 (0.1) | 0.4 |
| Total no. of organisms | 696 | 960 | 711 | 2367 | |
Note: CPE = carbapenamase-producing Enterobacteriaceae (surveyed only in 2017), ESBL = extended-spectrum β-lactamase–producing gram-negative bacilli, MRSA = methicillin-resistant S. aureus, NA = not available (data not collected), UTI = urinary tract infection; VRE = vancomycin-resistant Enterococcus.
Unless specified otherwise.
Among all health care–associated infections, the percentage of S. aureus isolates that were methicillin resistant remained consistent from 31.4% (2002) to 28.3% (2009) to 31.4% (2017). Conversely, the percentage of Enterococcus species isolates that were vancomycin resistant increased from 1.9% (2002) to 5.0% (2009) to 8.2% (2017) (p = 0.12).
Interpretation
We tracked the burden of health care–associated infections among sentinel Canadian acute care hospitals based on findings from 3 repeated point-prevalence surveys performed in 2002, 2009 and 2017. We found a significant reduction in health care–associated infections, representing a 30.1% decline in prevalence from 2009 to 2017. For patients in the ICU, we found a 29.2% decline in prevalence of infection from 2009 to 2017. Of the different types of infections measured in all 3 surveys, the prevalence of pneumonia and bloodstream infection significantly declined; however, we also observed a decrease for all other types. In addition, prevalence of health care–associated infections among patients in the ICU markedly declined. These results are consistent with other CNISP data,11,20 which suggests improvements in the prevention of health care–associated infections in Canadian acute care hospitals. This trend has occurred despite some changes in hospital patient populations that would be expected to increase infection risk, such as a higher proportion of patients in the ICU.
No single intervention is likely to have produced a decline in all infection types, suggesting that Canadian hospitals have undertaken multiple interventions to address health care–associated infections.21 Examples of interventions that have been adopted include improved hand hygiene compliance, multidisciplinary implementation of bundles (e.g., central catheter insertion and maintenance) and antimicrobial stewardship to prevent C. difficile infection.22–24 In our 2017 survey, device-associated infections accounted for 35.6% of all health care–associated infections. In the future, action to address both the need for and safety of these devices is likely to be the most successful approach to reduce the burden of these infections further.
An important finding of our study is that antimicrobial-resistant organisms other than MRSA remain an uncommon cause of health care–associated infection in the Canadian hospitals that were surveyed; however, their prevalence has increased. Methicillin-resistant S. aureus is now widely prevalent as a cause of infection across types, increasingly as a cause of bloodstream infection, reaching 8.5% in 2017. This is a cause for great concern because MRSA-associated bloodstream infection is associated with a mortality rate of greater than 20% in patients admitted to hospital.25
The prevalence of infection associated with extended-spectrum β-lactamases, while remaining low, was highest in 2017. We collected data on carbapenemase-producing organisms in the 2017 survey and found only 3 infections. The proportion of MRSA (31.4%) and very low frequency of carbapenemase resistance seen in 2017 compares to the prevalence of 45% for MRSA and 5% for carbapenemase-producing organisms in a study of infections in a sample of US hospitals in 2015.3 However, the rising MRSA bacteremia data and emerging signs of resistant gram-negative infections in 2017 indicates a need for vigilance and preventive actions to avoid a worsening antibiotic-resistance problem among infections in CNISP hospitals.
The prevalence of health care–associated infections in our surveys (11.3% in 2009 and 7.9% in 2017) are higher than those reported by the CDC (4.0% in 2011 and 3.2% in 2015).3 This is likely because our surveys represent data from large, tertiary care hospitals that typically serve patient populations at higher risk for infection compared with general hospitals that were included in the CDC surveys. The distribution and trends in infection in our surveys differed from those found by CDC: in their surveys, pneumonia and C. difficile infection were predominant; only surgical site infection and UTI fell in prevalence. The prevalence of health care–associated infections in our 2017 survey (7.9%) was comparable to results reported by a 2016/2017 prevalence survey by the European Centre for Disease Control and Prevention (7.1%) among tertiary care hospitals;5 however, by excluding low-to very low–risk units such as mental health and maternity, our prevalence could be expected to be slightly higher. Differences in frequency and trends in health care–associated infections among jurisdictions highlights the importance of collecting Canadian data to direct prevention strategies.
Limitations
Our surveys have several limitations. First, our findings may not be representative of the general inpatient population in Canada because the populations examined in these surveys were mainly in large, tertiary acute care hospitals. However, our results provide a robust estimate of health care–associated infections in hospitals of this type in Canada. The Public Health Agency of Canada is conducting additional prevalence surveys in hospital settings that were not included or underrepresented in these surveys. Second, results were not disaggregated by province; this was to protect the confidentiality of individual hospitals because some provinces have few reporting hospitals. Third, slight changes to the National Healthcare Safety Network surveillance definitions occurred between the 2009 and 2017 surveys. For example, both the UTI and pneumonia definitions were more specific in 2017 than in 2009. In 2017, a reduction in follow-up period defining surgical site infections occurred, which could reduce the hospital prevalence of these infections.26 Fourth, laboratory practices have changed over time; for example, laboratories now use more sensitive assays to detect C. difficile infection, which could result in an increase in prevalence.27 Nevertheless, by adopting the same methods, timing, similar definitions, hospital type and case mix, we have attempted to minimize the potential for protocol variation. Fifth, there is a risk of inconsistent adjudication considering turnover of hospital staff reviewing the medical charts. However, we provided standardized training to data collectors to reduce inconsistencies in data collection. Sixth, although patients in maternity wards are susceptible to health care–associated infections, they were excluded as most infections among this population present after the patient’s brief hospital stay. For consistency, and to permit comparison among surveys, the decision to exclude maternity patients in the 2002 survey was maintained in 2009 and 2017.
Conclusion
Using 3 sequential point-prevalence studies in a sentinel group of Canadian hospitals between 2002 and 2017, we found a reduction in the prevalence of health care–associated infections overall and that infections caused by antimicrobial-resistant organisms remain uncommon. However, continued efforts in infection prevention and control are required to reduce the burden of health care–associated infections further.
Acknowledgements
The authors gratefully acknowledge the contributions of the epidemiologists, infection control practitioners and staff for their data collection and submission at each participating hospital, as well as the following members of the Canadian Nosocomial Infection Surveillance Program who participated in the 2002, 2009 and 2017 point-prevalence surveys: Bonita E. Lee, Stollery Children’s Hospital, Edmonton, Alta.; Camille Lemieux, University Health Network, Toronto, Ont.; Chelsea Ellis, The Moncton Hospital, Moncton, NB; Deanna Hembroff, University Hospital of Northern BC, Prince George, BC; Elizabeth Bryce, Vancouver General Hospital, Vancouver, BC; Elizabeth Henderson, Alberta Health Services, Calgary, Alta.; Eva Thomas, BC Children’s Hospital, BC Women’s Hospital, Vancouver, BC; Gerald A. Evans, Kingston General Hospital, Kingston, Ont.; Ghada Al-Rawahi, BC Children’s Hospital, BC Women’s Hospital, Vancouver, BC; Gregory German, Queen Elizabeth Hospital, Charlottetown, PEI; Ian Davis, QEII Health Sciences Centre, Halifax, NS; Janice de Heer, Interior Health Authority, Kelowna, BC; Jeannette Comeau, IWK Health Centre, Halifax, NS; Jerome Leis, Sunnybrook Health Sciences Centre, Toronto, Ont.; Jessica Minion, Regina Qu’Appelle Health Region, Regina, Sask.; Joanne Embree, Health Sciences Centre, Winnipeg, Man.; Joanne M. Langley, IWK Health Centre, Halifax, NS; Jocelyn Srigley, BC Children’s Hospital, BC Women’s Hospital, Vancouver, BC; Johan Delport, London Health Sciences Centre, London, Ont.; John Conly, Foothills Medical Centre, Calgary, Alta.; John Embil, Health Sciences Centre, Winnipeg, Man.; Karl Weiss, Maisonneuve-Rosemont Hospital, Montréal, Que.; Kathy Malejczyk, Reginal Qu’Appelle Health Region, Regina, Sask.; Kevin C. Katz, North York General Hospital, Toronto, Ont.; Lynn Johnston, QEII Health Sciences Centre, Halifax, NS; Marie-Astrid Lefebvre, Montreal Children’s Hospital, Montréal, Que.; Mark Loeb, McMaster University and Hamilton Health Sciences, Hamilton, Ont.; Mary Vearncombe, Sunnybrook Health Sciences Centre, Toronto, Ont.; Natalie Bridger, Eastern Health-HSC, St. John’s, Nld.; Nisha Thampi, Children’s Hospital of Eastern Ontario, Ottawa, Ont.; Pamela Kibsey, Royal Jubilee Hospital, Victoria, BC; Paula Stagg, Western Memorial Hospital, Corner Brook, Nld.; Sarah Forgie, Stollery Children’s Hospital, Edmonton, Alta.; Stephanie Smith, University of Alberta Hospital, Edmonton, Alta.; Susan Richardson, Hospital for Sick Children, Toronto, Ont.; Susy Hota, University Health Network, Toronto, Ont.; Titus Wong, Vancouver General Hospital, Vancouver, BC; Valerie Wood, Interior Health Authority, Kelowna, BC; Virginia Roth, The Ottawa Hospital, Ottawa, Ont.; Yves Longtin, SMBD-Jewish General Hospital, Montréal, Que.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.190948
Footnotes
Visual abstract available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190361/-/DC2
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Robyn Mitchell and Wallis Rudnick performed the data analysis. Robyn Mitchell and Geoffrey Taylor interpreted the data and drafted the initial manuscript. All of the authors contributed to conception and design of the work, and data aquisiton; revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The Public Health Agency of Canada provided funding for the Canadian Nosocomial Infection Surveillance Program.
Data sharing: Study protocols are available. Data-sharing requests will be considered and reviewed by the Public Health Agency of Canada and individual site investigators.
References
- 1.Calfee DP. Crisis in hospital-acquired, health care-associated infections. Annu Rev Med 2012;63:359–71. [DOI] [PubMed] [Google Scholar]
- 2.Cassini A, Högberg LD, Plachouras D, et al. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS, O’Leary E, Janelle SJ, et al. Emerging Infections Program Hospital Prevalence Survey Team. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018;379:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Point prevalence survey of health care-associated infections and antimicrobial use in European acute care hospitals: 2011–2012. Stockholm: European Centre for Disease Prevention and Control; 2013. Available: https://ecdc.europa.eu/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf (accessed 2019 Mar. 26). [Google Scholar]
- 5.Suetens C, Latour K, Kärki T, et al. The Health care-Associated Infections Prevalence Study Group. Prevalence of health care-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017 [published erratum in Euro Surveill 2018;23:pii: 181122e1]. Euro Surveill 2018;23:1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksen HM, Iversen BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect 2005;60:40–5. [DOI] [PubMed] [Google Scholar]
- 7.Olona M, Limón E, Barcenilla F, et al. VINCat Program. Prevalence of nosocomial infections in acute care hospitals in Catalonia (VINCat Program). Enferm Infecc Microbiol Clin 2012;30(Suppl 3):7–12. [DOI] [PubMed] [Google Scholar]
- 8.van der Kooi TII, Manniën J, Wille JC, et al. Prevalence of nosocomial infections in The Netherlands, 2007–2008: results of the first four national studies. J Hosp Infect 2010;75:168–72. [DOI] [PubMed] [Google Scholar]
- 9.Antonioli P, Manzalini MC, Stefanati A, et al. Temporal trends of health care associated infections and antimicrobial use in 2011–2013, observed with annual point prevalence surveys in Ferrara University Hospital, Italy. J Prev Med Hyg 2016;57:E135–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Y, Venkatachalam I, Tee NW, et al. Prevalence of health care-associated infections and antimicrobial use among adult inpatients in Singapore acute-care hospitals: results from the First National Point Prevalence Survey. Clin Infect Dis 2017;64(Suppl 2):S61–7. [DOI] [PubMed] [Google Scholar]
- 11.Katz KC, Golding GR, Choi KB, et al. Canadian Nosocomial Infection Surveillance Program. The evolving epidemiology of Clostridium difficile infection in Canadian hospitals during a postepidemic period (2009–2015). CMAJ 2018; 190: E758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd DA, Mataseje LF, Pelude L, et al. Canadian Nosocomial Infection Surveillance Program. Results from the Canadian Nosocomial Infection Surveillance Program for detection of carbapenemase-producing Acinetobacter spp. in Canadian hospitals, 2010–2016. J Antimicrob Chemother 2019;74:315–20. [DOI] [PubMed] [Google Scholar]
- 13.Roth VR, Mitchell R, Vachon J, et al. Canadian Nosocomial Infection Surveillance Program. Periprosthetic infection following primary hip and knee arthroplasty: the impact of limiting the postoperative surveillance period. Infect Control Hosp Epidemiol 2017;38:147–53. [DOI] [PubMed] [Google Scholar]
- 14.Mataseje LF, Abdesselam K, Vachon J, et al. Results from the Canadian Nosocomial Infection Surveillance Program on carbapenemase-producing Enterobacteriaceae, 2010 to 2014. Antimicrob Agents Chemother 2016;60:6787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simor AE, Gilbert NL, Gravel D, et al. Canadian Nosocomial Infection Surveillance Program. Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: national surveillance and changing epidemiology, 1995–2007. Infect Control Hosp Epidemiol 2010;31:348–56. [DOI] [PubMed] [Google Scholar]
- 16.Rutledge-Taylor K, Matlow A, Gravel D, et al. Canadian Nosocomial Infection Surveillance Program. A point prevalence survey of health care-associated infections in Canadian pediatric inpatients. Am J Infect Control 2012;40:491–6. [DOI] [PubMed] [Google Scholar]
- 17.Taylor G, Gravel D, Matlow A, et al. Canadian Nosocomial Infection Surveillance Program. Assessing the magnitude and trends in hospital acquired infections in Canadian hospitals through sequential point prevalence surveys. Antimicrob Resist Infect Control 2016;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- 19.Central venous catheter-associated blood stream infections in intensive care units in Canadian acute-care hospitals: surveillance report January 1, 2006 to December 31, 2006 and January 1, 2009 to December 31, 2011. Ottawa: Centre for Communicable Diseases and Infection Control, Public Health Agency of Canada; 2014. Available: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-90-2013-eng.pdf (accessed 2019 July 12). [Google Scholar]
- 20.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. SAS Global Forum; 2012. Available: https://support.sas.com/resources/papers/proceedings12/335-2012.pdf (accessed 2019 June 12).
- 21.Yokoe DS, Anderson DJ, Berenholtz SM, et al. Introduction to “A compendium of strategies to prevent health care-associated infections in acute care hospitals: 2014 updates” [published erratum in Infect Control Hosp Epidemiol 2014;35:1081]. Infect Control Hosp Epidemiol 2014;35:455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AHS report on performance Q2 2018–19: hand hygiene compliance. Edmonton: Alberta Health Services; 2019. Available: www.albertahealthservices.ca/assets/about/publications/ahs-pub-pr-2018-19-q2-objective-07.pdf (accessed 2019 Mar. 26). [Google Scholar]
- 23.Gonzales M, Rocher I, Fortin E, et al. A survey of preventive measures used and their impact on central line-associated bloodstream infections (CLABSI) in intensive care units (SPIN-BACC). BMC Infect Dis 2013;13:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valiquette L, Cossette B, Garant MP, et al. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis 2007; 45(Suppl 2):S112–21. [DOI] [PubMed] [Google Scholar]
- 25.Simor AE, Pelude L, Golding G, et al. Canadian Nosocomial Infection Surveillance Program. Determinants of outcome in hospitalized patients with methicillin-resistant Staphylococcus aureus bloodstream infection: results from national surveillance in Canada 2008–2012. Infect Control Hosp Epidemiol 2016;37:390–7. [DOI] [PubMed] [Google Scholar]
- 26.Roth VR, Mitchell R, Vachon J, et al. Canadian Nosocomial Infection Surveillance Program. Periprosthetic infection following primary hip and knee arthroplasty: the impact of limiting the postoperative surveillance period. Infect Control Hosp Epidemiol 2017;38:147–53. [DOI] [PubMed] [Google Scholar]
- 27.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015;313:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]