Abstract
Human infection with parasites is still one of the big problems worldwide. Medicinal plants succeeded to overcome a variety of protozoan and helminthic parasites. In this study, Salvadora persica root extracts (SE) were used to treat helminthosis and coccideosis. Three doses were used (200, 100 and 50 mg/ml) to study the anthelmintic activity of S. persica. Allolobophora caliginosa was used as a model worm. Also, Albendazole was used as a reference drug. In order to study the anticoccideal activity of SE, a group of mice were infected with Eimeria papillata sporulated oocysts. Experimental mice were treated with SE (300 mg/Kg) for 5 days. The extract was able to decrease the number of meronts and gamonts of the parasite in jejunum. Also, it regulates the level of glutathione and malondialdehyde and the activity of catalase as well. We conclude that S. persica possesses a powerful Anthelmintic, anticoccidial and antioxidant activity.
Keywords: Salvadora persica, Helminths, Eimeria, Antioxidant, Mice, Jejunum
1. Introduction
Parasitic infections caused by protozoa and helminths induce considerable death and led to economic loss in many countries (Mehlhorn, 2014). Weakness due to malnutrition and anemia is the major complains of the infection with worms (Jones and Berkley, 2014). The currently used Anthelmintic drugs induce some problems in human body especially in liver and kidney (Tripathi, 2008, Hong, 2018). Also, coccidiosis due to Eimeria infection is a major health problem in poultry animals (Mehlhorn, 2014). The oocyst of Eimeria present in the feces of the infected animals could induced upon engulfment of a new host to these oocysts and induce a severe injuries to the target organ. Eimeria papillata infect the mouse jejunum causing a lot of pathological changes (Dkhil and Al-Quraishy, 2012). Anticoocidial drugs (e.g. dindamycin, narasin and decoquinate) against eimeriosis are harmful to the host tissues due to several side effects (Wunderlich et al., 2014). Recently, herbal medications are proven to be effective against complains of eimeriosis (Habibi et al., 2016).
Salvadora persica belongs to family Salvadoraceae. The plant roots are commonly used in Islamic countries due to their excellent biological activities (Eid et al., 1990, Sher et al., 2010). It is considered to be a plant with medicinal value because it contains many active chemical components with antibacterial (Almas and Stakiw, 2000), antifungal (Noumi et al., 2010) and antiparasitic activity (Abdul Majeed, 2011).
In this study, S. persica root extracts are used as anthelmintic, anticoccidial and antioxidant agent.
2. Methods
2.1. Collection of roots
Roots of Salvadora persica were obtainedted from Jazan city, Saudi Arabia. Methanolic extracts from the plant root were prepared based on Amer et al. (2015) method. In brief, S. Persica dry roots were grinded then extracted by methanol (70%). For both the in vivo and the in vitro studies, the extract powder was dissolved in water.
2.2. Extract analysis
Concentration of phenolic and flavonoid compounds in SE were determined as gallic acid equivalents per ml (Kim et al., 2003) and quercetin equivalents per ml (Dewanto et al., 2002), respectively.
2.3. Anthelmintic activity of S. persica.
According to the method of Ajaiyeoba et al. (2001), Allolobophora caliginosa was used as a model worm. Three doses were used (200, 100 and 50 mg/ml to study the anthelmintic activity of S. Persica. We used a reference drug, Albendazole (Saudi Pharmaceutical Industries, Riyadh, Saudi Arabia) with a concentration of 10 mg/ml (Murugamani et al., 2012). Worms in distilled water were used as a control. In this experiment, the time to reach paralysis and death state was expressed in minutes (Dkhil, 2013).
2.4. Mice and coccidial infection
Male mice of the strain C57Bl/6 (9–12 weeks old) were used as experimental animals. We obtained mice from the animal facility of Zoology Department at King Saud University and we followed the ethical rules for animal protection. E. papillata was used as a model coccidial parasite. Oocysts of E. papillata were passaged in laboratory mice. Unsporulated oocysts were collected from mice faeces, sporulated in 2.5% potassium dichromate, and then washed in buffered phosphate solution (Schito et al., 1996).
Eight mice were served as a vehicle control. These animals received only saline. Sixteen mice were orally infected with 1000 sporulated oocysts. After 60 min, eight mice from this group were orally treated with S. Persica extract (300 mg/Kg) (Thagfan et al., 2017). Treatment was daily for 5 days. Faeces were daily collected to count the number of oocysts (Dkhi et al., 2015a). On day 5 postinfection, all mice were killed and part of the jejunum was isolated and stored at −80C for the oxidative stress study while the other part was fixed in 10% formalin to prepare paraffin sections for counting the parasitic stages.
2.5. Parasitic stages
Tissue paraffin sections were prepared according to Adam and Caihak (1964). To differentiate the different parasitic stages in mice jejunum, the sections were stained with hematoxylin and eosin then examined by microscope then we counted meronts, gamonts and developing oocysts in infected and infected-treated groups. Values were expressed in 10 villous crypt units (VCU).
2.6. Oxidative damage in jejunum
Mice jejuna were prepared from the control, infected and infected-treated groups to determine the change in oxidative status in mice jejuna (Dkhi et al., 2015a).
The level of glutathione in jejunal homogenate was estimated by the fluorometric method as reported in Hissin and Hilf (1976). It is expressed in mg/g. Also, catalase activity (U/g) was determined spectrophotometrically by following the Aebi method (Aebi, 1984). In addition, the level of malonaldehyde was assayed by the method of Satoh (1978) and finally evaluated as nmol/g tissue.
In this study, all values were expressed as mean and standard deviation. Statistical significance between groups (P ≤ 0.5) was compared using one-way ANOVA, and Duncan’s test.
3. Results
The methanolic extracts from the root of S. persica were able to exert greater anthelmintic activity against live adult A. caliginosa worms (Table 1). The most efficient dose, 200 mg/kg showed the time to paralysis and death at about 5 and 6 min, respectively. However, the reference drug albendazole (10 mg/ml) showed less effect compared to the 200 mg/kg S. persica root extract.
Table 1.
Anthelminthic action of Salvadora persica root extract.
| Group | Time to paralysis (min) | Time to death (min) |
|---|---|---|
| SPE (50 mg/ml) | 45 ± 6 | 50 ± 3 |
| SPE (100 mg/ml) | 24 ± 3 | 29 ± 4 |
| SPE (200 mg/ml) | 5 ± 2 | 6 ± 1 |
| Vehicle control | – | – |
| Albendazole (10 mg/ml) | 19 ± 2 | 24 ± 1 |
Data are means ± SD. N = 7.
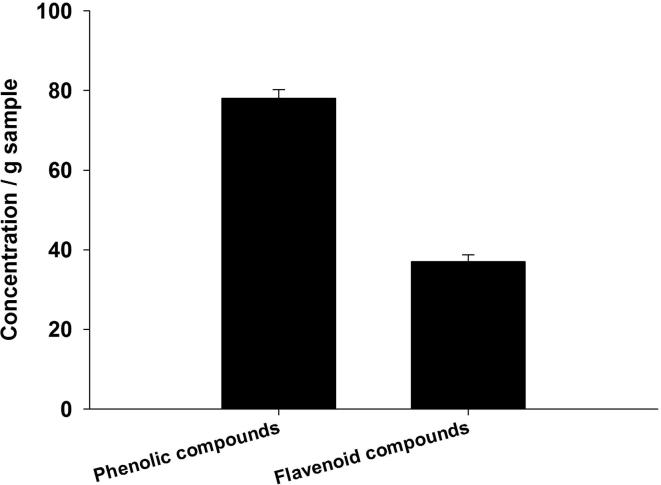
The total concentration of flavonoids and phenolics in the S. persica root methanolic extracts were found to be 37 ± 1.7 as mg quercetin equivalents/ g of the sample and, 78 ± 2.2 mg gallic acid equivalents/ g of the sample, respectively (Fig. 1).
Fig. 1.
Concentration of phenolics (mg) and flavenoids (mg) in root extract of Salvadora persica.
Oocysts output were at its highest level on the fifth day post infection being about 6242.7 ± 731.5 oocysts/g faeces in infected animals. After treatment with S. persica extract, a significantly (p < 0.01) reduced the oocyst output by 2696. 7 ± 441.3 was observed (Thagfan et al., 2017).
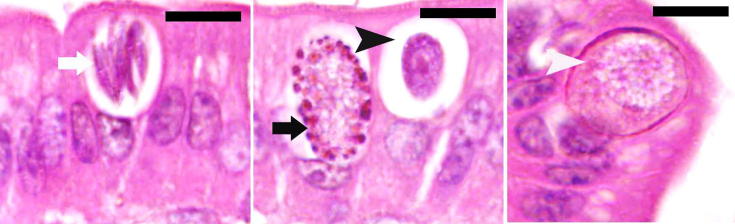
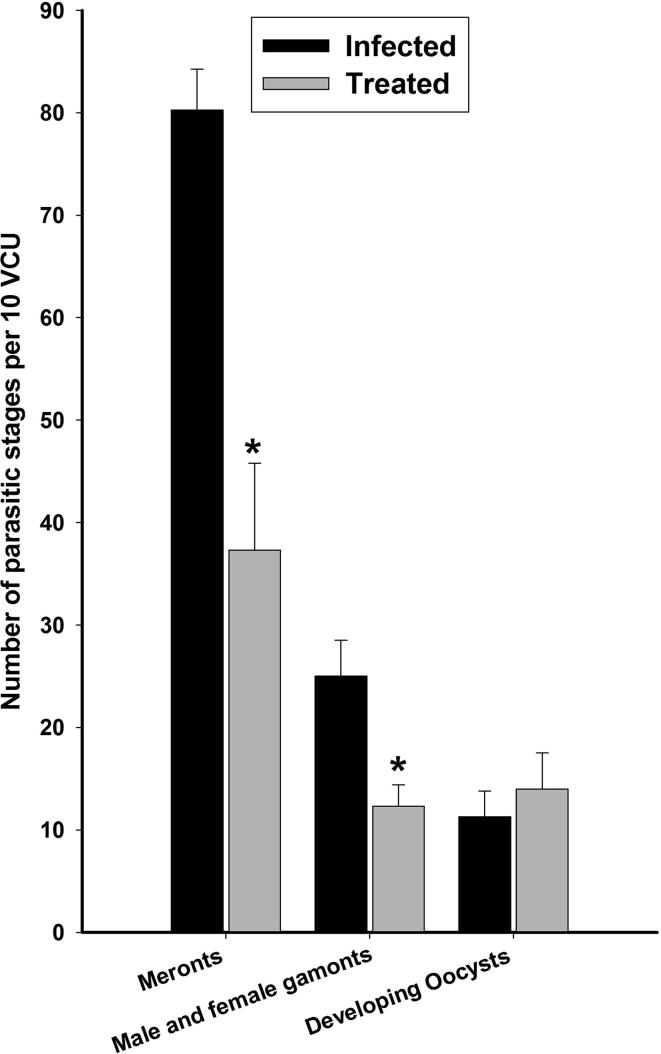
In this study, we counted 80 ± 4 meronts, 25 ± 3 gamonts, and 11 ± 2 developing oocysts in jejunal villi (Fig. 2). Remarkably, the number of meronts and male and female gamonts were significantly (p < 0.01) decreased after treatment by 37 ± 8 and 12 ± 2, respectively (Fig. 3).
Fig. 2.
Parasitic stages of E. papillata in jejunum of mice. Meronts (white arrow), male (black arrow head) and female (black arrow) gamonts and developing oocysts (white arrow head). Sections stained with eosin and hematoxylin. Bar = 10 µm.
Fig. 3.
Number of meronts, gamonts and developing oocysts per 10 villous crypt units (VCU) in mouse jejunum ifected with E. papillata. *, Significance against infected group.
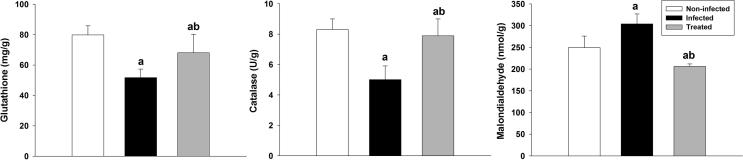
Glutathione level was significantly decreased from 79.8 ± 12 in non-infected group to 51.6 ± 11.2 mg/g in infected group. While, the level of glutathione of mice treated with the extract was increased to 68 ± 24.1 mg/g, (Fig. 4).
Fig. 4.
Effect of S. persica on glutathione level, malondialdehyde levels and catalase activity in mouse jejunum infected with E. papillata. Data expressed as mean and standard deviation. a, significant change against control animals. b, significant change against infected animals.
The activity of catalase enzyme was diminished from 8.3 ± 1.3 to 5 ± 1.9 U/g (Fig. 4). Upon oral administration of infected mice with 300 mg/kg S. persica root, an improvement in the antioxidant system within infected jejunum tissue occurred. Here, the antioxidant activity of catalase was significantly raised in treated mice to 7.9 ± 2.2 U/g. Also, malondialdehyde level was significantly (p < 0.01) increased from 249.3 ± 53.5 in non-infected group to 304 ± 45.8 nmol/g in infected group. While, the level of malondialdehyde of mice treated with S. persica was significantly (p < 0.01) decreased to 206.3 ± 11.2 nmol/g (Fig. 4).
4. Discussion
Several studies have reported the anthelmintic role of certain herbal extracts (Klimpel et al., 2011, Mehlhorn et al., 2011, Yadav, 2012). The earth worms have been chosen as a model for the antihelmintic activity experiment due to the physiological similarity between some intestinal round worms infecting man (Awad, 2004). S. persica could perfectly kill worms in a short time compared to Albendazole, probably owing to the presence of active phytochemical constituents in the root extract.
Coccidiosis in poultry animals caused by Eimeria spp is responsible for economic losses a cross the world (Schito and Barta, 1997, Mehlhorn, 2014, Wunderlich et al., 2014). Previous studies have attempted to determine a solution for this issue.
This study investigated the anthelmintic, anticoccidial and antioxidant activity of S. persica. The used extracts exhibited adequate anticoccidial properties, probably attributed to the extract composition. Khan et al. (2010) reported that of S. persica extracts contain flavonoids, alkaloids, glycosides, steroids, carbohydrates, tannins and saponins.
E. papillata persisting in the intestinal epithelia are associated with infiltration of inflammatory cells as macrophages, neutrophils, mast cells and T-cells (Laurent et al., 2001). This lead to initiation of cytotoxic and oxidative damage within infected mucosal tissue leading to their destruction via reactive oxygen production and nitrogen intermediates, and severe disturbance in the protective antioxidant systems (Allen, 1997, Georgieva et al., 2006).
E. papillata infection is associated with severe local and systemic inflammatory response and oxidative damage to the mice jejunum (Dkhil et al., 2015b).
Treatment of the infected animals with SE showed an excellent modulation of oxidative damage and enhancing antioxidant capability of mice jejunum tissue. The pronounced potential effect of S. persica results from the antioxidant (Mohamed and Khan, 2013) and anti-inflammatory (Ezmirly et al., 1979) activities of the components of the plant extract.
The oxidation of lipid peroxides finally yields numerous carbonyl compounds production, such as malondialdehyde (Shinmoto et al., 1992) which in turn increase after treatment with SE. This reflects the potential role of SE as antioxidant. Previous studies have reported that SE contained active compounds as flavonoids and other derivatives (Abd ELRahman et al., 2003, Dkhil et al., 2015a, Sher et al., 2010). In this study, the presence of flavonoids in SE reflecting its biological role (Duh et al., 2001).
Based on the presented results, we conclude that Salvadora persica possesses a powerful anthelmintic, anticoccidial and antioxidant activity. Future studies are needed to know the mechanism of S. persica action on both of the parasite and the host.
Acknowledgement
The authors extend appreciations to the Deanship of Scientific Research at King Saud University for funding the work through the research group project number RG-198.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd ELRahman H.F., Skaug N., Whyatt A.M., Francis G.W. Volatile compounds in crude Salvadora persica extracts. Pharm. Biol. 2003;41(6):399–404. [Google Scholar]
- Abdul Majeed S. Anthelmintic activity of Salvadora persica root extract against Pheretima posthuma. Int. J. Pharm. Sci. Res. 2011;2(9):2343–2346. [Google Scholar]
- Adam H., Caihak G. Arbeitsmethoden der makroskopischen und mikroskopischen anatomie mit Abbildungen. Gustav Fischer Verlag, Stuttgart; 1964. Grosses zoologisches parktikum tell. [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ajaiyeoba E.O., Onocha P.A., Olarenwaju O.T. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharm. Biol. 2001;39:217–220. [Google Scholar]
- Allen P.C. Production of free radical species during Eimeria maxima infections in chickens. Poult. Sci. 1997;76(6):814–821. doi: 10.1093/ps/76.6.814. [DOI] [PubMed] [Google Scholar]
- Almas K., Stakiw J.E. The effect of miswak extract from Salvadora persica stored for 18 years on microbes in vitro. Egypt. Dent. J. 2000;46:227–230. [Google Scholar]
- Amer O.S., Dkhil M.A., Hikal W.M., Al-Quraishy S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. Biomed. Res. Int. 2015;2015:219670. doi: 10.1155/2015/219670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad N.E. Bioactive brominated diterpenes from the marine red alga Jania Rubens (L.) Lamx. Phytother. Res. 2004;18(4):275–279. doi: 10.1002/ptr.1273. [DOI] [PubMed] [Google Scholar]
- Dewanto V., Wu X., Adom K.K., Liu R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food. Chem. 2002;50(10):3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A., Al-Quraishy S., Wahab R. Anticoccidial and antioxidant activities of zinc oxide nanoparticles on Eimeria papillata-induced infection in the jejunum. Int. J. Nanomed. 2015;10:1961–1968. doi: 10.2147/IJN.S79944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M.A. Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol. Res. 2013;112(7):2639–2646. doi: 10.1007/s00436-013-3430-3. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A., Al-Quraishy S. Metabolic disturbance and hepatic tissue damage induced by Eimeria papillata infection. Afr. Zool. 2012;47:255–260. [Google Scholar]
- Dkhil M.A., Metwaly M.S., Al-Quraishy S., Sherif N.E., Delic D., Al Omar S.Y., Wunderlich F. Anti-Eimeria activity of berberine and identification of associated gene expression changes in the mouse jejunum infected with Eimeria papillata. Parasitol. Res. 2015;114(4):1581–1593. doi: 10.1007/s00436-015-4344-z. [DOI] [PubMed] [Google Scholar]
- Duh P.D., Yen G.C., Yen W.J., Chang L.W. Antioxidant effects of water extracts from barley (Hordeum vulgare L.) prepared under different roasting temperatures. J. Agr. Food Chem. 2001;49(3):1455–1463. doi: 10.1021/jf000882l. [DOI] [PubMed] [Google Scholar]
- Eid M.A., Selim H.A., Al-Shammery A.R. Relationship between chewing sticks (Miswak) and periodontal health. Part 1. Quintessence Int. 1990;21(11):913–917. [PubMed] [Google Scholar]
- Ezmirly S., Cheng J., Wilson S. Saudi Arabian medicinal plants: Salvadora persica. Planta. Med. 1979;35(2):191–192. doi: 10.1055/s-0028-1097205. [DOI] [PubMed] [Google Scholar]
- Georgieva N.V., Koinarski V., Gadjeva V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 2006;172(3):488–492. doi: 10.1016/j.tvjl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Habibi H., Firouzi S., Nili H., Razavi M., Asadi S.L., Daneshi S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: in vitro and in vivo study. J. Parasit. Dis. 2016;40(2):401–407. doi: 10.1007/s12639-014-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissin P.J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Hong S.T. Albendazole and praziquantel: review and safety monitoring in Korea. Infect. Chemother. 2018;50(1):1–10. doi: 10.3947/ic.2018.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.D., Berkley J.A. Severe acute malnutrition and infection. Paediatr. Int. Child. Health. 2014;34(1):S1–S29. doi: 10.1179/2046904714Z.000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W., Mujum A., Shaikh T., Katekar S.M., Tambe R., Rub R.A. Pharmacognostic and preliminary phytochemical investigation of Salvadora persica Linn (Salvadoraceae) Res. J. Pharmacog. Phytochem. 2010;2(4):319–323. [Google Scholar]
- Kim D.O., Chun O.K., Kim Y.J., Moon H.Y., Lee C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003;51(22):6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- Klimpel S., Abdel-Ghaffar F., Al-Rasheid K.A.S., Aksu G., Fischer K., Strassen B., Mehlhorn H. The effects of different plant extracts on nematodes. Parasitol. Res. 2011;108:1047–1054. doi: 10.1007/s00436-010-2168-4. [DOI] [PubMed] [Google Scholar]
- Laurent F., Mancassola R., Lacroix S., Menezes R., Naciri M. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 2001;69(4):2527–2534. doi: 10.1128/IAI.69.4.2527-2534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H. (fourth ed). Springer Press; Berlin: 2014. Encyclopedic Reference of Parasitology. (V. 1, Ed.) [Google Scholar]
- Mehlhorn H., Al-Quraishy S., Al-Rasheid K.A.S., Jatzlau A., Abdel-Ghaffar F. Addition of a combination of onion (Allium cepa) and coconut (Cocos nucifera) to food of sheep stops gastrointestinal helminthic infections. Parasitol Res. 2011;108:1041–1046. doi: 10.1007/s00436-010-2169-3. [DOI] [PubMed] [Google Scholar]
- Mohamed S.A., Khan J.A. Antioxidant capacity of chewing stick miswak Salvadora persica. BMC. Compl. Alternative Med. 2013;13(1):40–45. doi: 10.1186/1472-6882-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugamani V., Raju L., Anand Raj V.B., Sarma Kataki M., Sankar G.G. The new method developed for evaluation of anthelmintic activity by housefly worms and compared with conventional earthworm method. ISRN Pharmacol. 2012;2012:709860. doi: 10.5402/2012/709860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noumi E., Snoussi M., Hajlaoui H., Valentin E., Bakhrouf A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:81–88. doi: 10.1007/s10096-009-0824-3. [DOI] [PubMed] [Google Scholar]
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim Acta; Int. J. Clin. Chem. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Schito M.L., Barta J.R. Nonspecific immune responses and mechanisms of resistance to Eimeria papillata infections in mice. Infect. Immun. 1997;65(8):3165–3170. doi: 10.1128/iai.65.8.3165-3170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schito M.L., Barta J.R., Chobotar B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J. Parasitol. 1996;82:255–262. [PubMed] [Google Scholar]
- Sher H., Al-Yemeni M.N., Masrahi Y.S., Shah A.H. Ethnomedicinal and ethnoecological evaluation of Salvadora persica L.: a threatened medicinal plant in Arabian Peninsula. J. Med. Plants Res. 2010;4(12):1209–1215. [Google Scholar]
- Shinmoto H., Dosako S., Nakajima I. Antioxidant activity of bovine lactoferrin on iron/ascorbate induce lipid peroxidation. Biosci. Biotechnol. Biochem. 1992;56(12):2079–2080. [Google Scholar]
- Thagfan F.A., Dkhil M.A., Al-Quraishy S. In vivo anticoccidial activity of Salvadora persica root extracts. Pakistan J. Zool. 2017;49(1):53–57. doi: 10.1016/j.sjbs.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi K.D. 6th ed. Jaypee Brothers Medical Publishers; New Delhi: 2008. Essentials of Medical Pharmacology; p. 810. [Google Scholar]
- Wunderlich F., Al-Quraishy S., Steinbrenner H., Sies H., Dkhil M.A. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol. Res. 2014;113(10):3547–3556. doi: 10.1007/s00436-014-4101-8. [DOI] [PubMed] [Google Scholar]
- Yadav A.K. In vivo anthelmintic activity of Clerodendrum colebrookianum Walp., a traditionally used taenicidal plant in Northeast India. Parasitol. Res. 2012;111:1841–1846. doi: 10.1007/s00436-012-2908-8. [DOI] [PubMed] [Google Scholar]