Abstract
Cinnamomum camphora is an excellent tree species for construction of forest construction of Henan Province, China. The diverse bioactive components of nano-catalyzed pyrolyzates form cold-acclimated C. camphora branch (CCB) in North China were explored. The raw powder of CCB treated with nano-catalyst (Ag, NiO, 1/2Ag + 1/2NiO) were pyrolyzed at two temperatures (550 °C and 700 °C), respectively. The main pyrolyzates are bioactive components of bioenergy, biomedicines, food additive, spices, cosmetics and chemical, whose total relative contents at 550 °C pyrolyzates are higher than those at 700 °C pyrolyzates. There are abundant components of spices and biomedicine at 550 °C pyrolyzates, while more spices and food additive at 700 °C pyrolyzates. At 550 °C, the content of biomedicine components reaches the highest by 1/2Ag + 1/2NiO nanocatalysis, while the contents of spices and food additive components reach the highest by NiO nanocatalysis. At 700 °C, the content of bioenergy components reaches the highest by 1/2Ag + 1/2NiO nanocatalysis, and the content of cosmetics components reaches the highest by Ag nanocatalysis. The findings suggested that the branch of the cold-acclimated C. camphora have the potential to develop into valued-added products of bioenergy, biomedicine, cosmetics, spices and food additive by nanocatalysis.
Keywords: Cinnamomum camphora, Nanocatalysis, Pyrolyzates, Bioactive components, Resourcing
1. Introduction
Cinnamomum camphora is one of evergreen trees of Lauraceae, and they are excellent greening trees, street trees and special economic tree species (Li et al., 2018a, Li et al., 2018b). C. camphora is widely cultivated in the south and southwest of China, while is successfully introduced into northern provinces including Henan, a typical province in temperate zone.
The woods, branches, roots and leaves of C. camphora have very high application value (Gao et al., 2017, Li et al., 2018a, Li et al., 2018b). Camphor and camphor oil can be extracted from them. Camphor has the efficacy of killing insects, relieving itching, swelling and pain. It is usually used for medicine, explosives, insecticidal and so on (Zhai et al., 2016). Camphor oil has functions of healing wounds and deodorizing besides insect repellent and mosquito repellent. It can be used as insecticide, soap making, fake paint and essence (Jiang et al., 2016). C. camphora seeds are rich in oil and have special physiological and nutritional functions. They can be made into pillows (Guo et al., 2016). In addition, C. camphora barks and roots, woods, fruit and leaves have medicinal value and can be used as medicine in clinical medicine. The chemical substances such as camphorene, citric hydrocarbon and eugenol emitted from camphor tree have the ability to absorb harmful gases and purify air. Therefore, camphor tree has become the first choice for landscape greening and is widely planted (Guo et al., 2017).
The whole of C. camphora has high economic and applied value. People have paid more attention to the woods, branches, roots and leaves and fruits of C. camphor (Zheng et al., 2016), while the research on C. camphora branch (CCB) is not deep enough, causing environmental pollution and waste of resources. Therefore, using the differential scanning calorimetry (TG) and pyrolysis-gas chromatography-mass spectrometry (Py-GC/MS), the cold-acclimated CCB in north China was selected to analyze the nano-catalyzed pyrolysis characteristics at different high temperature, so that finding some new functional components and providing a new approach for the high-quality utilization of CCB resource.
2. Materials and methods
2.1. Experimental materials
The cold-acclimated CCB were collected in early June, and processed into powder after baking (De et al., 2017). The same batch of solid powder of CCB was sifted through a 200 mesh screen (Ge et al., 2018), and added to the corresponding nano-catalyst in proper order. Samples with different nano-catalysts were represented by A, B, C and D, respectively. A: raw powder of CCB; B: powder of CCB treated by nano-Ag catalyst; C: powder of CCB treated by nano-NiO catalyst; D: powder of CCB treated by nano-1/2Ag + 1/2NiO catalyst. (1/2Ag represents the 1/2 of the quality of nano-Ag catalyst used in B, 1/2NiO represents the 1/2 of the quality of nano- NiO catalyst used in C.) (Fig. 1A).
Fig. 1A.
Experimental process.
2.2. Methods
2.2.1. TG analysis
The cold-acclimated CCB were collected from Henan Agricultural University, Zhengzhou City, Henan Province, China (Fig. 1B). The samples of 8 mg were weighed for detection. The temperature program of TG started at 30 °C, and reached 850 °C at 10 °C/min. The carrier gas is high purity nitrogen, with a flow rate of 40 mL/min (Delaney et al., 2017).
Fig. 1B.
Sampling site.
2.2.2. PY-GC/MS analysis
0.010 g of CCB extracts were placed in the cracking tube, with glass wool in the cracking device sampler. The pyrolysis conditions are as follows: 50 °C, 1 sec standing time, 20 °C/MS flow rate, 700 °C or 550 °C flow rate, hold for 10 s. Interface conditions: 80 °C, flow rate 100 °C/min to 300 °C, keep 2 min (Chen et al., 2018). Valve furnace: 300 °C, transmission line: alpha C, GC–MS./MS conditions: HP-5 capillary column (30 m × 0.25 mm × 0.25 µm); carrier gas, helium, carrier gas flow, 1 mL/min, injection volume, 1 °C, 29 sample injection temperature: 280 °C, split ratio 5:1 (Almeida et al., 2017). Heating procedure: initial temperature is 50 °C, hold for 2 min, then rising to 300 °C at the rate of 10 °C/min, and the residence time is 10 min. Ion source temperature: 230 °C, quadrupole temperature, 150 °C, and detection range 30–700 Da (Gómez et al., 2018).
3. Results
3.1. Behavior of the cold-acclimated CCB during heating
The TG curve decreased continuously, suggesting that the sample was continuously weightless (Fig. 2). The total weight loss rate of the sample is about 90%, between 30 °C and 850 °C. The weightlessness of the sample can be roughly divided into three main stages. The first stage is from the beginning of 30 °C to about 200 °C. A significant peak is found in the corresponding DTG curve. The weight loss rate of the sample is low, and the weight loss of the sample is about 8%. This may be due to the evaporation of water in the sample. The second stage is 200–400 °C. From the TG curve, it can be seen that the mass of the sample decreases rapidly with the increase of temperature. The DTG curve shows obvious weight loss peaks. The weight loss rate of the sample is the highest, about 50%. This may be related to the partial decomposition of the sample. The DTG curves of the samples did not change significantly at 400–850 °C, but the weight of the sample was still declining, and the weight loss rate of the samples was reduced by about 25%. This may be caused by the solid phase transition of the sample.
Fig. 2.
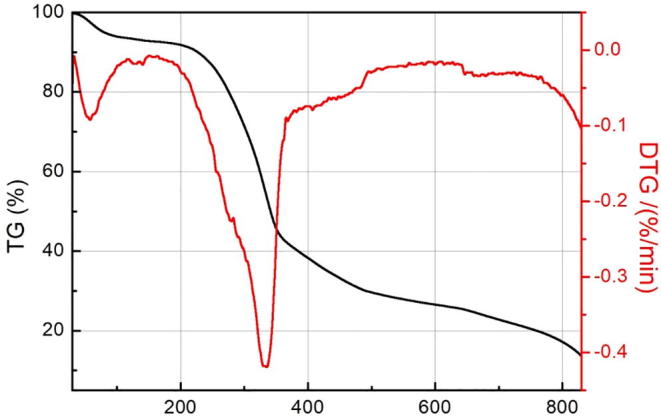
TG-DTG curves were obtained by increasing the temperature of the original powder of the sample at the rate of 10 °C/min.
3.2. Identification of pyrolyzates via nano-catalysts at different temperatures
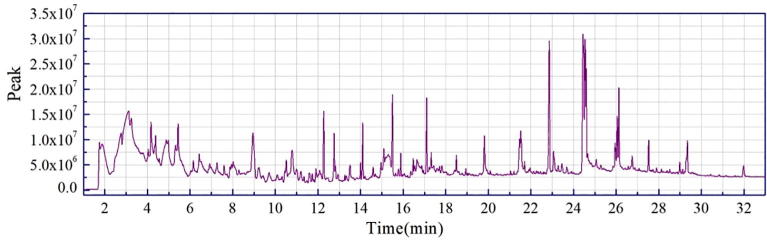
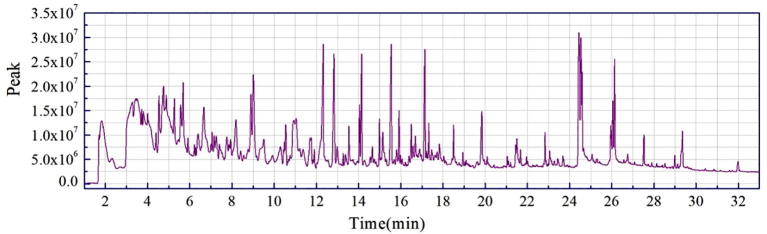
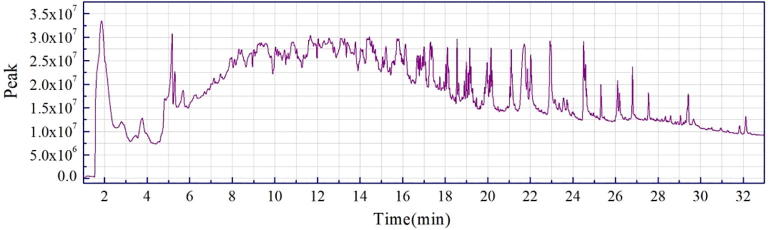
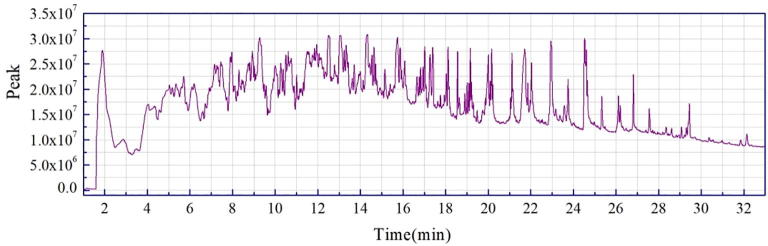
The chromatograms of each peak in the total ion chromatograms of four samples obtained by Py-GC/MS were retrieved using Agilent workstation and Excel (Fig. A1, Fig. A2, Fig. A3, Fig. A4, Fig. A5, Fig. A6, Fig. A7, Fig. A8). The relative content of each component was calculated by peak area normalization method.
Fig. A1.
ion chromatogram of C. camphora branch (CCB) original powder at 550 °C.
Fig. A2.
Ion chromatograms of CCB treatment with the Ag catalyzer at 550 °C.
Fig. A3.
Ion chromatograms of CCB treatment with the NiO catalyzer at 550 °C.
Fig. A4.
Ion chromatograms of CCB treatment with the Ag and NiO catalyzer at 550 °C.
Fig. A5.
ion chromatogram of CCB original powder at 700 °C.
Fig. A6.
Ion chromatograms of CCB treatment with the Ag catalyzer at 700 °C.
Fig. A7.
Ion chromatograms of CCB treatment with the NiO catalyzer at 700 °C.
Fig. A8.
Ion chromatograms of CCB treatment with the Ag and NiO catalyzer at 700 °C.
3.2.1. 550 °C pyrolyzates of CCB
At 550 °C, a total of 60 peaks were retrieved from 79 peaks in A sample (Fig. A1 and Table A1). A large amount of chemicals were detected in f. For example, Acetic acid (9.16%) is a good fixation fluid for fixed chromosomes (Zheng et al., 2018). Phenol, 2-methoxy- (3.21%) is used for the synthesis of dyes and is also an analytical reagent (Cheng et al., 2017). beta.-D-Glucopyranose, 1,6-anhydro- (2.96%) is a biochemical reagent. Benzaldehyde, 4-hydroxy-3,5-dimethoxy- (1.70%) used in pesticide chemistry and organic synthesis industry. Eicosanoic acid (0.90%) is used to produce washing powder, photographic material, lubricating oil and so on. 1-Eicosene (3.51%) and 1-Hexacosanol (0.70%) are organic materials (Lu et al., 2017).
A contains some compounds that can be used in the pharmaceutical industry. 9,12-Octadecadienoic acid (Z, Z) - (3.49%) is a lipid-lowering drug and can also be used as a raw material for the treatment of atherosclerosis (Yang et al., 2016). beta.-Sitosterol (0.50%) has the functions of lowering cholesterol, relieving cough, eliminating phlegm, inhibiting tumor and repairing tissue. Octacosanol (0.28%) has the effect of anti-fatigue, reducing blood lipid and enhancing sexual function, and can be used to treat Parkinson's disease in the early stage of old age. Creosol (1.02%) is an intermediate for manufacturing pharmaceutical products.
Bioenergy components were detected in A. Furfural (7.08%) is a flammable liquid, which is explosive when mixed with air and combustible when exposed to open flame. It may have the potential of bioenergy (Lopes et al., 2017). Catechol (2.48%) is hot and flammable, and its reaction with oxidant is intense and explosive (Maier et al., 2018).
A contains some food additive and aroma substances. 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- (1.67%) are used as flavoring and sweetener synergistic agents. They are mainly used to make smoke, cream, hard candy and apricot essence (Qin et al., 2017). 2-Methoxy-4-vinylphenol (1.38%) and Penol, 2,6-dimethoxy-(1.46%) can be used in the food additive and spices industry. 1,2-Benzenediol, 4-methyl- (0.96%) can make apple flavors.
A small amount of cosmetic ingredients were found in A. GeraNiOl (0.26%) is a natural perfume, widely used in the preparation of cosmetics for daily use. D-Limonene (0.54%) it can be used as a fresh head spice for cosmetic and soap use (Xu et al., 2018).
In total, 69 compounds were identified from 84 peaks in B sample. Some chemical raw materials were found in B sample. 2-Cyclopenten-1-one (2.23%) and Creosol (1.56%) are intermediates in organic synthesis (Scognamiglio et al., 2012). Ethanone, 1- (4-hydroxy-3,5-dimethoxyphenyl) - (0.76%) are commonly used additive in plant tissue culture. Mequinol (1.86%) is mainly used as polymerization inhibitor, ultraviolet ray inhibitor, dye intermediate, antioxidant, plasticizer and so on for vinyl plastic monomer. Catechol (2.13%) is an important chemical intermediate, which can be used to manufacture rubber hardeners, plating additive, skin antiseptics, fungicides, hair dyes, insecticides and so on.
B sample contains medicinal compounds. 2-Cyclopenten-1-one, 3-methyl- (0.72%) is the raw material for manufacturing pharmaceutical products. Acetic acid (13.01%) has the effect of anti bacterial and fungal infection. Phytol (0.31%) is used for the synthesis of vitamin E and vitamin K1 (Liu et al., 2018a, Liu et al., 2018b).
A small amount of chemicals can be detected in B sample and can be used as bioenergy sources. For example, Pyridine (1.13%) is a flammable liquid. It can be exploded with air and is the raw material for making explosives.
A number of bioactive ingredients which can be used as food additive, fragrances and spices have been found in B sample. 1,2-Cyclopentanedione, 3-methyl- (3.02%), a flavoring and sweetening synergist, is widely used in ice cream, pastries and sweets. It is also used in food flavors. It can also be used to modulate special flavors, such as maple maple syrup, chocolate and caramel. Phenol, 4-ethyl-2-methoxy- (1.75%) are used as food additive and fragrance bodies. 3,5-Dimethoxy-4-hydroxytoluene (0.93%) and Phenol, 2,6-dimethoxy-4-(2-propenyl) - (0.86%) can be used as seasonings by writers in meat products, snacks, and cheese (Cheng et al., 2018).
B sample contains a small amount of cosmetic active ingredients. 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z) - (1.48%) are used for the preparation of cosmetics for daily use, such as violet, orange blossom, Magnolia and cloves. Mequinol (1.86%) is used to synthesize cosmetic antioxidant BHA.
At 550 °C, 71 chemical substances were found in 87 peaks of C sample. C sample contains some chemicals that can be used in the chemical industry. 1-Octadecene (2.98%) is a comparative sample of gas chromatography and is used in organic synthesis to produce surfactants, dyes and polymers 2-Cyclopenten-1-one (2.57%) is a chemical raw material. Boron, trihydro (pyridine) - (T-4) - (0.87%) is used in reduction and borohydride reactions to produce other chemical products. Furfural (3.73%) is used as industrial solvent, raw material for organic synthesis, and can be used in synthetic resin, pesticides, rubber and coatings and many other chemical products.
Biopharmaceutical active substances were identified in C sample. Phenol, 2-methoxy-4- (1-propenyl) - (0.99%) is applied to dental drugs. Phenol, 2-methoxy- (5.02%) is used in medicine to make calcium guaiacol sulfonate (Pardo-Garcia et al., 2017). Catechol (3.90%) can be used to produce antitussin, Ding Zixiang phenol, berberine and isoproterenol.
Bioenergetic components were found in C sample. Cyclohexanone (4.44%) is a flammable liquid, explosive mixture with air, and a viscous solvent for piston-type aviation lubricants (Lim et al., 2018). 1-Tetradecene (0.77%) is liquid fuel.
Some food additive and spices were identified in C sample. Tetradecanal (0.41%) is an edible spice that is used to mix peach, fat and milk flavors. The degradation products of Neophytadiene (1.34%) have an important contribution to tobacco aroma (He et al., 2018). 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl) - (1.86%) can release volatile aromatic compounds, which contribute to the aroma of barrel wine (Truong et al., 2017). 2-Furancarboxaldehyde, 5-methyl- (1.24%) are food flavors for tobacco flavors.
Chemical substances that can be used in cosmetics are detected in C samples. Docosanoic acid (0.90%) is the raw material for making cosmetics. N-Hexadecanoic acid (2.11%) has special aroma and can be used to produce soap (Chen et al., 2017).
At 550 °C, a total of 82 components were detected in 93 peaks of D sample. There are many chemicals in D sample. Cyclopentadecane (2.19%) is a kind of chemical pigment. 1,2-Benzenediol, 3-methoxy- (0.64%) and Apocynin (1.04%) are used in organic synthesis (Rahman et al., 2017). 9-Tricosene (Z) - (0.46%) is used as sex attractant for female and male housefly, which interferes with mating. 1,2-Benzenediol, 4-methyl- (1.58%) can be used as photosensitive materials for germicidal and mildew-proof, and can synthesize antibacterial agents, antioxidants, high-efficiency polymerization inhibitors (Wang et al., 2018).
Some bioactive components that can be applied to medicine have been found in D sample. 4-Pyridinol (0.41%) is used to synthesize diuretic drug or other intermediate (Ge et al., 2016) Tetradecanoic acid (0.52%) and Hexadecanoic acid, 2-hydroxy-1 - (hydroxymethyl) ethyl ester (0.60%) are the raw materials for the synthesis of pharmaceutical products.
Bioactive components were detected in D sample. For example, Furfural (6.85%) and Cyclopentene (0.79%) are flammable and explosive liquids, and their vapors and air easily form explosive mixtures, which have the potential of bioenergy.
Some food additive, flavors and spices were found in D sample. Catechol (2.64%) can be used to make spices. Phenol, 2-methoxy- (3.85%) are mainly used to make coffee, vanilla, tobacco and other flavors. They are used in perfume industry to make vanillin and artificial musk. D-Limonene (1.33%) is used to blend orange blossom essence, citrus oil essence, and so on. It can be used as a substitute for lemon essential oil (Wang et al., 2018).
D sample contains a small amount of cosmetic substances. Such as 1,2-Cyclopentanedione, 3-methyl- (1.89%), n-Hexadecanoic acid (1.50%).
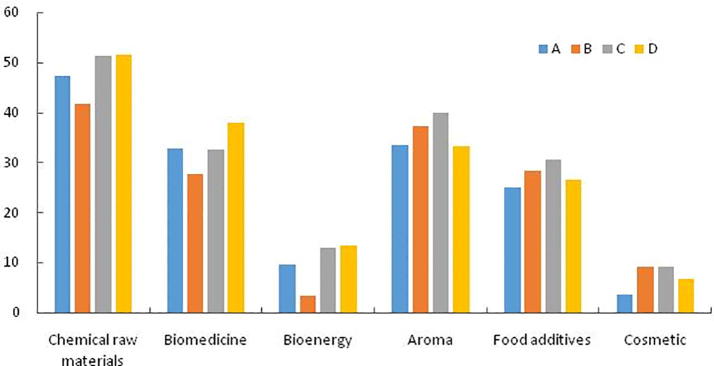
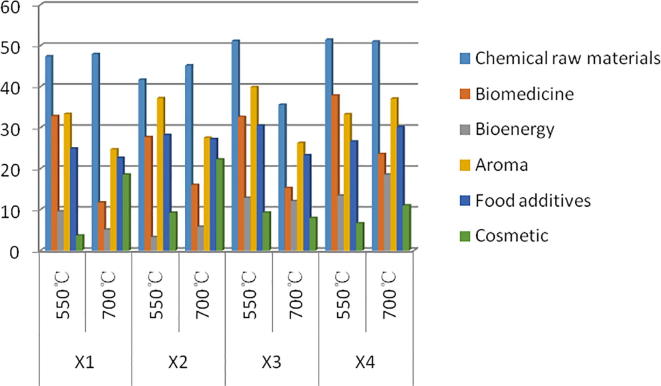
According to Fig. 3, we can see that the highest content of all samples is chemical raw materials, the second highest is biomedicine and spices, followed by less bioenergy and cosmetics. Compared with the chemical raw materials and bioenergy content in each sample, the overall trend is roughly the same, that is, the content of D is the highest, there is no significant difference with the content of C, and the content of B is the lowest (D > C > A > B). The results show that the addition of NiO catalyst and (1/2Ag and 1/2NiO) catalyst alone can increase the content of chemical raw materials and bioenergy in the samples. D have the highest bioactive components, followed by A (D > A > C > B), indicating that the mixture of the two catalysts could effectively improve the bioactive components in the samples. The highest content of spices in C is more than B (C > B > A > D), indicating that two kinds of catalysts added to Ag and NiO alone could effectively improve the content of flavors and fragrances, but the catalytic effect of NiO catalyst was better. C had the highest content of food additive in all samples and A had the lowest content (C > B > D > A), indicating that the addition of catalyst could increase the content of food additive in the samples. The effect of using catalyst alone was better than mixture of the two catalysts. The content of B and C in cosmetics was the highest, followed by D, but higher than A, indicating that the addition of catalyst can increase the content of cosmetic components in the sample. The effect of Ag catalyst on chemical raw materials, biomedicine and bioenergy is not as high as that of raw powder without any catalyst. However, adding Ag catalyst in samples can increase the content of functional substances such as food additive and cosmetics.
Fig. 3.
Comparison of functional categories of 550 °C Py-GC/MS (the same substance may be repeated for various purposes).
3.2.2. 700 °C pyrolyzates of CCB
At 700 °C, 64 chemical substances were retrieved from 90 peaks of A sample. Abundant chemical substances were found in A. Cyclotetradecane (1.33%) is mainly used for organic synthesis, and can also be used as solvent and standard hydrocarbon. 1-Eicosene (2.29%) and Hexanedioic acid, bis (2-methylpropyl) ester (0.63%) are chemicals used in organic synthesis (Lu et al., 2017). N-Hexadecanoic acid (7.47%) is used as a precipitant, chemical reagent, waterproofing agent and defoamer, as well as to determine the hardness of water. 2-Dodecen-1-yl (-) succinic anhydride (8.34%) was used as curing agent for epoxy resin. Addition of 2-Dodecen-1-yl (-) succinic anhydride (8.34%) into solvent-based adhesive prevented the corrosion of the adhesive to the iron packaging and did not cause the color change of the adhesive (Chinisaz et al., 2017). Hexadecane (1.34%) can be used to produce various maleic anhydride copolymers. A contains some biopharmaceutical ingredients. Styrene (0.21%) is the original medicine of cough and expectorant in Changning. 9-Octadecenoic acid, (E) - (0.31%) for medical research. P-Cymene (0.26%) is a kind of expectorant, antitussive and antitussive drug, and is also an intermediate in the manufacture of other pharmaceutical products (Granato et al., 2017).
A small amount of bioactive components were found in A. Toluene (0.78%) mixture of steam and air forms explosive material and can be used to produce ladder explosives. 1-Decene (1.01%) is a flammable liquid that can be mixed with air and can be used as a liquid fuel. Some food additive, fragrances and spices were detected in A. 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- (0.20%) is a fragrance and sweetener synergist, mainly used to make smoked tobacco, butter, hard candy and apricot essence. 1-Octadecene (1.64%) is used in organic synthesis to produce spices. .gamma.-Sitosterol (1.10%) is a spice that exists in cigarette smoke. A contains cosmetic active ingredients. Eugenol (2.00%) is used as a modifier and perfumery fixative. It is perfumed with colored soap and can be prepared and blended with cosmetics (Hu et al., 2018). Octadecanoic acid (1.92%) is the raw material for making cosmetics.
A total of 68 chemical constituents were identified in 90 peaks of B sample. Chemical raw materials were found in B sample. Erucic acid (0.85%) is mainly used as an intermediate of fine chemicals to produce various surfactants, lubricants, plasticizers, emulsifiers and other chemical products (Sissener et al., 2018). Cyclopentadecane (2.78%) is a kind of chemical pigment. 9-Octadecenamide (Z) - (2.34%) is a chemical additive which must be added to low density polyethylene (LDPE) film material. It can also be used as antistatic agent, demoulding agent, pigment, dye and other dispersants (Xu et al., 2018). 1-Nonene (0.24%) is used in organic synthesis to produce nonylbenzene and nonylphenol petroleum products additive. Oleic acid (9.05%) can be used as pesticide emulsifier, printing and dyeing auxiliaries, industrial solvents, metal mineral flotation agent, also can be used as copy paper and type wax paper raw materials (Burgess et al., 2018). B sample contains substances that can be used in biomedicine. Phenol, 2-methyl- (0.29%) is a medical disinfectant. Phenol, 2,6-dimethyl- (0.08%) can be used to prepare antiarrhythmic drugs. Naphthalene, 2-methyl-(0.10%) can produce vitamin K3, oral contraceptives, and many other bioactive pharmaceutical products (Liu et al., 2018a, Liu et al., 2018b). Bioenergy components were found in B sample. P-Xylene (0.47%) is an additive for aviation power fuel (Ni et al., 2017). 9-Hexadecenoic acid (0.57%) is an ideal raw material for biodiesel. Bioactive ingredients that can be used as food additive, flavors and fragrances and cosmetics were found in B sample. Oxacyclohexadecan-2-one (1.74%) has good musk smell and setting effect. It is suitable for scent flavors such as flowers and wines. It can also be used as tobacco, vanillin, fennel and other fragrances. It is widely used in perfumes, hair and cosmetics. Oleic Acid (9.05%) is the raw material for making spices and soap, and is also used in sugar industry. Tridecanoic acid (1.37%) is used in the production of soap, detergent, cosmetic surfactant, ointment cream, food additive, spices industry has many applications. 1-Dodecene (0.21%) is used to produce flavors and fragrances. Phenol, 3-methyl- (0.36%) edible spices that are allowed to be used for the preparation of other spices.
67 compounds were identified in 83 peaks of C sample. Some chemicals were found in C sample. Pentadecane (0.65%) is used in organic synthesis and can be used as a certified reference material for chromatographic analysis (Sugawara et al., 2018). Heptacosane, 1-chloro- (0.70%), 1-Docosene (2.30%) and Oxirane, hexadecyl- (0.46%) are organic raw materials for the synthesis of other chemical products. Phenol, 3-methyl-(0.36%) is used as analytical reagent and organic synthesis intermediates for the production of pesticides, resin plasticizers, film and other chemical industries. It contains bioactive ingredients in C. Acetic acid, phenyl ester (2.75%) is used to treat acute and chronic jaundice hepatitis and cholecystitis (Hackl et al., 2015). 9-Octadecenoic acid, (E) - (0.11%) for medical research. Bioenergetic components were detected in C. For example, Dibutyl phthalate (1.34%), Limonene (4.33%) and Phenol, 3-methyl - (3.47%) are flammable liquids, which may explode when mixed with air and have potential as liquid fuels (Evageliou et al., 2017). Some food additive and aroma substances were found in C. For example, 2-Methoxy-4-vinylphenol (4.08%), Phenol, 2,6-dimethoxy-4-(2-propenyl) - (2.34%) and n-Hexadecanoic acid (1.74%). N-Hexadecanoic acid has special aroma and taste, which can be used to prepare various edible flavors, defoamers and other food additive (Chen et al., 2018).
A small amount of cosmetic ingredients were found in C. Such as n-Hexadecanoic acid (1.74%), Octadecanoic acid (0.65%). 70 chemical substances were found in 82 peaks of D sample. D is rich in chemical raw materials. Bicyclo [4.2.0] octa-1,3,5-triene (1.50%) has excellent electrical insulation properties and can be widely used in high-tech electronic fields. Benzene, 1-butynyl- (3.57%) and 1-Pentadecene (1.03%) are chemical intermediates. Benzenemethanol, 4-hydroxy- (2.63%) are used for peptide synthesis. 9-Octadecen-1-ol, Z-(0.46%) is used in the manufacture of special surfactants, oil additive, detergents, plasticizers (Peng et al., 2017). Hexadecanoic acid, methyl ester (0.69%) is used as a stationary liquid for gas chromatography and as an organic synthesis of other chemicals. Biopharmaceutical substances were discovered in D. Phenol (2.61%) and Benzene, 1,3-dimethyl- (2.31%) are the raw material of synthetic medicine products (Pinheiro et al., 2018). P-Cresol (4.13%) is an important basic raw material for the production of pharmaceutical TMP (Usha et al., 2018). Bioactive components found in D. For example, 1-Tetradecene (2.28%) and 1-Dodecene (1.03%) can be used as liquid fuels. Phenol (2.61%) is the raw material for producing explosives. D contains some food additive, flavors and fragrances, cosmetics active ingredients. Such as Eugenol (1.04%), Phenol, 2-methyl-(1.57%), Phenol, 2,6-dimethoxy-(2.98%), 1,2-Cyclopentanedione, 3-methyl-(2.21%).
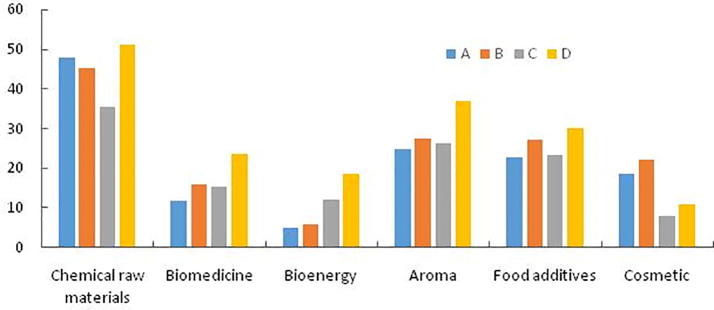
All samples contain a large number of chemical raw materials, in addition to biomedicine, bioenergy, spices, food additive, cosmetics and other active ingredients. D has the highest content of chemical raw materials, A takes the second place, C is the lowest (D > A > B > C), indicating that the mixture of the two catalysts (Ag, NiO) can effectively increase the content of chemical raw materials in the sample. The content of D is the highest and A is the lowest in biomedicine, spices and food additive. It shows that the content of these three functional substances can be effectively increased by adding catalyst. The effect of mixing the two catalysts is the best, the effect of adding Ag catalyst is the second, and the effect of adding NiO catalyst is the same. That's the worst. The contents of flavors and food additive in A, B and C were similar, but the content of flavors in D was significantly higher than that of food additive. The content of bioenergy in D was the highest and that in A was the lowest (D > C > B > A). The results showed that the bioenergy content in the sample treated with catalyst was higher than that without catalyst. The bioenergy content in the sample treated with catalyst could be increased by adding catalyst, and the effect of mixing the two catalysts was better. The content of cosmetics was the highest in B, followed by A (B > A > D > C), indicating that adding Ag catalyst could increase the content of cosmetic ingredients (Fig. 4).
Fig. 4.
Comparison of functional categories of 700 °C Py-GC/MS (the same substance may be repeated for various purposes).
3.2.3. Comparison of 550 °C and 700 °C pyrolyzates of CCB
All samples contain a large amount of chemical materials at two temperatures. The content of chemical raw materials in A and B samples is higher at 700 °C, but it is opposite in C and D. The contents of biomedical components in these four samples at different temperatures were higher than those at 550 °C. The results indicated that the pyrolysis samples at 550 °Cwas beneficial to the formation of bioactive components. The content of bioenergy in A and C samples are higher at 550 °C, whereas in B and D samples, the content of bioenergy is higher at 700 °C. The content of flavoring substances and food additive in A, B and C is higher at 550 °C, but the content of these two functional substances is higher at 700 °C in D. The content of cosmetics in C is higher at 550 °C, but it is higher than that in the other three samples (A, B, D) at 700 °C. In A, the content of cosmetic components at 550 °C is about 20%, B is about 41% and D is about 60%. Compared with the samples treated with different temperatures and catalysts, the contents of chemical raw materials and bio-medicines in D samples at 550 °C were the highest, the contents of bioenergy in D samples at 700 °C were the highest, the contents of spices and food additive in C samples at 550 °C were the highest, and B samples at 700 °C were the highest. Cosmetics contain the highest content of cosmetics. On the whole, the total amount of bioactive components was higher at 550 °C (Fig. 5).
Fig. 5.
Comparison of functional categories of PY GC/MS at different temperatures (550 °C, 700 °C) (the same substance may be repeated for various purposes).
4. Conclusion and discussion
The pyrolysis of CCB (cold-acclimated CCB in North China) raw powder treated with nanocatalysis (Ag, NiO, 1/2Ag + 1/2NiO) at two different temperatures (550 °C, 700 °C) .Overall, the total amount of bioactive substances was higher at 550 °C. A large number of chemical materials were detected in all samples under two temperature conditions. In the mixture of two catalysts (1/2Ag + 1/2NiO) the content of the chemical raw materials is highest at 550 °C. The highest content of bioenergy was found in the mixture of the two nanocatalysis (1/2Ag + 1/2NiO) at 700 °C. The relative content of biomedicine in each sample was higher at 550 °C than that at 700 °C, and the highest content was found in the mixture of the two nanocatalysis at 550 °C. The content of spices and food additive is higher in the samples added by NiO catalysis at 550 °C, and higher in the samples mixed by the two catalysts at 700 °C. It can draw a conclusion that the best condition is that the samples treated by NiO catalysis at 550 °C. The content of active components in cosmetics is the highest in samples treated with Ag nanocatalysis at 700 °C. The nano-catalyst and pyrolysis temperature can be selected according to the demand.
We can do further research to figure out these pyrolysis products which have high content but unknown function. such as 2-Cyclopenten-1-one, 2-hydroxy-, (Z)-3-(pentadec-8-en-1-yl) phenol. At 550 °C, The relative contents of bioenergy, biomedicine and chemical components are the highest by 1/2Ag + 1/2NiO nanocatalysis, the relative contents of spices, food additive and cosmetics components are the highest by NiO nanocatalysis. However, At 700 °C, The relative contents of bioenergy, biomedicine, chemical, spices and food additive are the highest by 1/2Ag + 1/2NiO nanocatalysis. The relative content of cosmetics is the highest by Ag nanocatalysis. Whether the mixed use of the two nanocatalysts can improve the catalytic effect and the relationship between the relative content of Nanocatalysts and various bioactive components affected by temperature can be further verified. At present, only the pyrolysis products at 550 °C and 700 °C have been analyzed. For further study, other catalysts and different temperatures can be selected, such as Fe2O3, ZnS and so on. At the same time, the pyrolysis temperature with the fastest weight loss can be tried in TG analysis, such as about 330 °C in this paper.
Acknowledgements
This project was supported by the talent project (Dangquan Zhang) of Henan Agriculture University, China, and the Project of Henan Provincial Science Research, China (192102110174).
Footnotes
Peer review under responsibility of King Saud University.
Appendix.
See Table A1, Table A2, Table A3, Table A4, Table A5, Table A6, Table A7, Table A8 and Fig. A1, Fig. A2, Fig. A3, Fig. A4, Fig. A5, Fig. A6, Fig. A7, Fig. A8.
Table A1.
Components of the raw CCB powder at 550 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 3.124 | 9.16 | Acetic acid |
| 2 | 4.891 | 4.23 | Furfural |
| 3 | 4.975 | 2.86 | Furfural |
| 4 | 6.040 | 0.80 | 2H-Pyran, 5,6-dihydro-2-methyl- |
| 5 | 6.438 | 3.80 | 2-Cyclopenten-1-one, 2-hydroxy- |
| 6 | 6.928 | 2.30 | 2-Furancarboxaldehyde, 5-methyl- |
| 7 | 7.887 | 0.55 | o-Cymene |
| 8 | 7.955 | 0.54 | D-Limonene |
| 9 | 8.026 | 1.67 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- |
| 10 | 8.960 | 3.21 | Phenol, 2-methoxy- |
| 11 | 10.319 | 0.33 | Bicyclo[2.2.1]heptane, 1,7,7-trimethyl- |
| 12 | 10.525 | 1.02 | Creosol |
| 13 | 10.794 | 2.48 | Catechol |
| 14 | 11.209 | 0.69 | 5-Hydroxymethylfurfural |
| 15 | 11.359 | 0.26 | GeraNiOl |
| 16 | 11.605 | 0.45 | 1,2-Benzenediol, 3-methoxy- |
| 17 | 11.740 | 0.25 | Phenol, 4-ethyl-2-methoxy- |
| 18 | 11.807 | 0.15 | Ethanone, 1-(2,5-dihydroxyphenyl)- |
| 19 | 11.914 | 0.47 | Cyclopropane, nonyl- |
| 20 | 12.091 | 0.96 | 1,2-Benzenediol, 4-methyl- |
| 21 | 12.279 | 1.38 | 2-Methoxy-4-vinylphenol |
| 22 | 12.768 | 1.46 | Phenol, 2,6-dimethoxy- |
| 23 | 13.271 | 0.20 | Cyclopropane, nonyl- |
| 24 | 13.514 | 1.07 | Phenol, 2-methoxy-4-(1-propenyl)-, (Z)- |
| 25 | 14.004 | 0.53 | 3,5-Dimethoxy-4-hydroxytoluene |
| 26 | 14.110 | 1.03 | trans-Isoeugenol |
| 27 | 14.600 | 0.97 | Apocynin |
| 28 | 15.102 | 1.16 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 29 | 15.311 | 2.96 | .beta.-D-Glucopyranose, 1,6-anhydro- |
| 30 | 15.897 | 0.58 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- |
| 31 | 16.483 | 0.55 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 32 | 16.546 | 0.52 | Benzenepropanol, 4-hydroxy-3-methoxy- |
| 33 | 16.658 | 1.70 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- |
| 34 | 16.953 | 0.35 | 2-Allyl-1,4-dimethoxy-3-methyl-benzene |
| 35 | 17.108 | 1.66 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 36 | 17.608 | 0.69 | 3,7-Benzofurandiol, 2,3-dihydro-2,2-dimethyl- |
| 37 | 17.823 | 0.81 | 1-(1-Hydroxybutyl)-2,5-dimethoxybenzene |
| 38 | 18.042 | 0.29 | Cyclotetradecane |
| 39 | 18.506 | 1.10 | Neophytadiene |
| 40 | 19.035 | 0.22 | (1R,3aS,5aS,8aR)-1,3a,5a-Trimethyl-4-methylenedecahydrocyclopenta[c]pentalene |
| 41 | 19.100 | 0.45 | Cyclotridecane |
| 42 | 19.236 | 0.20 | Diepicedrene-1-oxide |
| 43 | 19.823 | 2.19 | n-Hexadecanoic acid |
| 44 | 20.100 | 0.75 | 1-Eicosene |
| 45 | 21.056 | 0.61 | 1-Eicosene |
| 46 | 21.474 | 1.36 | 9,12-Octadecadienoic acid (Z,Z)- |
| 47 | 21.534 | 1.70 | 9,12-Octadecadienoic acid (Z,Z)- |
| 48 | 21.710 | 0.66 | Octadecanoic acid |
| 49 | 21.969 | 0.96 | 1-Eicosene |
| 50 | 22.139 | 0.35 | Cyclopentadecanone, 2-hydroxy- |
| 51 | 22.272 | 0.43 | 9,12-Octadecadienoic acid (Z,Z)- |
| 52 | 22.462 | 0.55 | Diepicedrene-1-oxide |
| 53 | 22.599 | 0.23 | 2-Dodecen-1-yl(-)succinic anhydride |
| 54 | 22.867 | 3.37 | 3-Tridecylphenol |
| 55 | 23.072 | 1.56 | Linoelaidic acid |
| 56 | 23.461 | 0.90 | Eicosanoic acid |
| 57 | 23.694 | 0.70 | 1-Hexacosanol |
| 58 | 23.912 | 0.36 | 2,2-Dimethyl-3-vinyl-bicyclo[2.2.1]heptane |
| 59 | 23.996 | 0.52 | 2-Methyl-Z,Z-3,13-octadecadienol |
| 60 | 24.172 | 0.30 | Z-7-Tetradecenoic acid |
| 61 | 24.453 | 5.12 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 62 | 24.551 | 5.67 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 63 | 24.819 | 0.75 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 64 | 24.946 | 0.35 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 65 | 25.075 | 1.46 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 66 | 25.287 | 0.62 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 67 | 25.429 | 1.03 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 68 | 25.651 | 0.53 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 69 | 25.956 | 1.13 | 3-((4Z,7Z)-Heptadeca-4,7-dien-1-yl)phenol |
| 70 | 26.055 | 1.24 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 71 | 26.135 | 2.45 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 72 | 26.415 | 0.59 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 73 | 26.585 | 0.64 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 74 | 26.760 | 1.20 | 1-Eicosene |
| 75 | 27.532 | 1.13 | 1-Tetracosene |
| 76 | 28.135 | 0.37 | Pregn-5-en-3-ol, 21-bromo-20-methyl-, (3.beta.)- |
| 77 | 29.350 | 1.40 | Stigmasta-3,5-diene |
| 78 | 29.772 | 0.28 | Octacosanol |
| 79 | 31.990 | 0.50 | .beta.-Sitosterol |
Table A2.
Components of CCB treatment with the Ag catalyzer at 550 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 3.284 | 6.43 | Acetic acid |
| 2 | 3.443 | 2.50 | Acetic acid |
| 3 | 3.504 | 4.07 | Acetic acid |
| 4 | 4.406 | 1.13 | Pyridine |
| 5 | 5.275 | 2.23 | 2-Cyclopenten-1-one |
| 6 | 5.923 | 1.83 | Cyclopent-4-ene-1,3-dione |
| 7 | 6.675 | 4.67 | 2-Cyclopenten-1-one, 2-hydroxy- |
| 8 | 7.064 | 1.39 | 2-Furancarboxaldehyde, 5-methyl- |
| 9 | 7.160 | 0.72 | 2-Cyclopenten-1-one, 3-methyl- |
| 10 | 7.872 | 0.60 | Benzene, 4-ethyl-1,2-dimethyl- |
| 11 | 7.945 | 0.82 | D-Limonene |
| 12 | 8.194 | 3.02 | 1,2-Cyclopentanedione, 3-methyl- |
| 13 | 8.902 | 1.86 | Mequinol |
| 14 | 9.020 | 3.36 | Phenol, 2-methoxy- |
| 15 | 10.554 | 1.56 | Creosol |
| 16 | 10.943 | 2.13 | Catechol |
| 17 | 11.378 | 1.48 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- |
| 18 | 11.757 | 1.75 | Phenol, 4-ethyl-2-methoxy- |
| 19 | 11.907 | 0.75 | 3-Trifluoroacetoxytetradecane |
| 20 | 12.326 | 4.77 | 2-Methoxy-4-vinylphenol |
| 21 | 12.836 | 2.57 | Phenol, 2,6-dimethoxy- |
| 22 | 13.275 | 0.33 | 1-Heptadecene |
| 23 | 13.548 | 0.91 | Phenol, 2-methoxy-4-(1-propenyl)-, (Z)- |
| 24 | 14.055 | 0.93 | 3,5-Dimethoxy-4-hydroxytoluene |
| 25 | 14.147 | 1.76 | trans-Isoeugenol |
| 26 | 14.560 | 0.71 | 1-Tridecene |
| 27 | 14.658 | 0.67 | Ethanone, 1-[4-(methylthio)phenyl]- |
| 28 | 14.754 | 0.39 | 2,5-Dimethoxyethylbenzene |
| 29 | 15.155 | 1.35 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 30 | 15.924 | 0.86 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- |
| 31 | 15.996 | 0.26 | 4-Propyl-1,1′-diphenyl |
| 32 | 16.503 | 0.72 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 33 | 16.582 | 0.62 | Benzenepropanol, 4-hydroxy-3-methoxy- |
| 34 | 16.693 | 1.17 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- |
| 35 | 17.142 | 2.07 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 36 | 17.237 | 0.39 | Tetradecanal |
| 37 | 17.477 | 0.76 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- |
| 38 | 17.634 | 0.58 | 4-Hydroxy-2-methoxycinnamaldehyde |
| 39 | 17.732 | 0.55 | 3,7-Benzofurandiol, 2,3-dihydro-2,2-dimethyl- |
| 40 | 17.840 | 1.09 | 3,5-Dimethoxy-4-hydroxyphenylacetic acid |
| 41 | 18.049 | 0.49 | Cyclopentadecane |
| 42 | 18.249 | 0.31 | 2(3H)-Naphthalenone, 4,4a,5,6,7,8-hexahydro-1-methoxy- |
| 43 | 18.510 | 1.28 | Neophytadiene |
| 44 | 18.952 | 0.47 | 11-Hexadecen-1-ol, acetate, (Z)- |
| 45 | 19.044 | 0.21 | 1,4-Methanoazulen-7-ol, decahydro-4,8,8,9-tetramethyl-, (+)- |
| 46 | 19.100 | 0.21 | n-Pentadecanol |
| 47 | 19.625 | 0.64 | Culmorin |
| 48 | 19.845 | 2.02 | n-Hexadecanoic acid |
| 49 | 20.102 | 0.89 | 1-Heneicosyl formate |
| 50 | 20.427 | 0.32 | Naphthalene, decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-, [4aR-(4a.alpha.,7.alpha.,8a.beta.)]- |
| 51 | 20.888 | 0.32 | Diepicedrene-1-oxide |
| 52 | 21.061 | 0.60 | 10-Heneicosene (c,t) |
| 53 | 21.215 | 0.31 | Phytol |
| 54 | 21.514 | 1.61 | 9,12-Octadecadienoic acid (Z,Z)- |
| 55 | 21.678 | 0.54 | 9,12-Octadecadienoic acid (Z,Z)- |
| 56 | 21.972 | 0.49 | 1-Octadecene |
| 57 | 22.021 | 0.50 | Nonadecane |
| 58 | 22.268 | 0.34 | cis-7,cis-11-Hexadecadien-1-yl acetate |
| 59 | 22.465 | 0.42 | 2,5-Furandione, 3-dodecyl- |
| 60 | 22.659 | 0.38 | Androstane, (5.alpha.)- |
| 61 | 22.846 | 0.90 | 3-Tridecylphenol |
| 62 | 23.065 | 0.80 | Linoelaidic acid |
| 63 | 23.454 | 0.71 | Eicosanoic acid |
| 64 | 23.694 | 0.53 | cis-1-Chloro-9-octadecene |
| 65 | 23.831 | 0.17 | 2-Methyl-Z,Z-3,13-octadecadienol |
| 66 | 23.903 | 0.25 | (E)-15,16-Dinorlabda-8(17),12-dien-14-al |
| 67 | 24.006 | 0.39 | cis-7,cis-11-Hexadecadien-1-yl acetate |
| 68 | 24.308 | 0.24 | 2,5-Furandione, 3-dodecyl- |
| 69 | 24.452 | 2.95 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 70 | 24.549 | 3.57 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 71 | 24.815 | 0.55 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 72 | 25.075 | 1.19 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 73 | 25.428 | 0.63 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 74 | 25.958 | 1.30 | 3-((4Z,7Z)-Heptadeca-4,7-dien-1-yl)phenol |
| 75 | 26.058 | 1.00 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 76 | 26.142 | 2.02 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 77 | 26.410 | 0.51 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 78 | 26.584 | 0.35 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 79 | 26.762 | 0.98 | cis-1-Chloro-9-octadecene |
| 80 | 27.530 | 0.74 | 1-Tetracosene |
| 81 | 28.134 | 0.24 | Pregn-5-en-3-ol, 21-bromo-20-methyl-, (3.beta.)- |
| 82 | 28.993 | 0.37 | Pregn-5-en-3-ol, 21-bromo-20-methyl-, (3.beta.)- |
| 83 | 29.352 | 1.14 | Stigmasta-3,5-diene |
| 84 | 31.989 | 0.32 | .beta.-Sitosterol |
Table A3.
Components of CCB treatment with the NiO catalyzer at 550 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 3.487 | 5.44 | Acetic acid |
| 2 | 3.619 | 5.70 | Acetic acid |
| 3 | 4.550 | 0.87 | Boron, trihydro(pyridine)-, (T-4)- |
| 4 | 5.011 | 3.73 | Furfural |
| 5 | 5.381 | 2.57 | 2-Cyclopenten-1-one |
| 6 | 6.743 | 4.44 | Cyclohexanone |
| 7 | 7.112 | 1.24 | 2-Furancarboxaldehyde, 5-methyl- |
| 8 | 7.458 | 1.65 | exo-Norbornyl alcohol |
| 9 | 7.857 | 2.00 | o-Cymene |
| 10 | 7.944 | 0.89 | D-Limonene |
| 11 | 8.236 | 2.94 | 1,2-Cyclopentanedione, 3-methyl- |
| 12 | 8.906 | 1.99 | Phenol, 2-methoxy- |
| 13 | 9.038 | 3.03 | Phenol, 2-methoxy- |
| 14 | 10.503 | 0.87 | Creosol |
| 15 | 10.564 | 0.89 | Creosol |
| 16 | 10.952 | 1.56 | Catechol |
| 17 | 11.060 | 2.34 | Catechol |
| 18 | 11.384 | 1.41 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- |
| 19 | 11.908 | 0.77 | 1-Tetradecene |
| 20 | 12.335 | 4.53 | 2-Methoxy-4-vinylphenol |
| 21 | 12.848 | 2.55 | Phenol, 2,6-dimethoxy- |
| 22 | 13.276 | 0.33 | 1-Heptadecene |
| 23 | 13.555 | 0.99 | Phenol, 2-methoxy-4-(1-propenyl)- |
| 24 | 14.067 | 1.00 | 3,5-Dimethoxy-4-hydroxytoluene |
| 25 | 14.156 | 1.75 | trans-Isoeugenol |
| 26 | 14.563 | 0.60 | 1-Tridecene |
| 27 | 14.669 | 0.74 | Apocynin |
| 28 | 15.001 | 1.13 | 5-tert-Butylpyrogallol |
| 29 | 15.172 | 1.86 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 30 | 15.707 | 0.45 | 3,7-Benzofurandiol, 2,3-dihydro-2,2-dimethyl- |
| 31 | 15.933 | 0.86 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- |
| 32 | 16.004 | 0.28 | 4-Propyl-1,1′-diphenyl |
| 33 | 16.511 | 0.73 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 34 | 16.595 | 0.60 | Benzenepropanol, 4-hydroxy-3-methoxy- |
| 35 | 16.707 | 1.20 | 3,5-Dimethoxy-4-(isopropyl)oxybenzaldehyde |
| 36 | 17.151 | 1.99 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 37 | 17.240 | 0.41 | Tetradecanal |
| 38 | 17.490 | 1.00 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- |
| 39 | 17.643 | 0.63 | 4-Hydroxy-2-methoxycinnamaldehyde |
| 40 | 17.851 | 1.13 | 2′,4′-Dihydroxyacetophenone oxime |
| 41 | 18.051 | 0.51 | 1-Hexadecanol, 2-methyl- |
| 42 | 18.510 | 1.34 | Neophytadiene |
| 43 | 19.101 | 0.43 | 1-Nonadecene |
| 44 | 19.300 | 0.18 | Longipinane, (E)- |
| 45 | 19.631 | 0.70 | 1,7-Hexadecadiene |
| 46 | 19.858 | 2.11 | n-Hexadecanoic acid |
| 47 | 20.103 | 0.98 | 1-Octadecene |
| 48 | 20.430 | 0.33 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (Z,E)- |
| 49 | 20.890 | 0.34 | Diepicedrene-1-oxide |
| 50 | 21.063 | 0.67 | 1-Octadecene |
| 51 | 21.536 | 2.08 | Linoelaidic acid |
| 52 | 21.679 | 0.62 | (Z)-18-Octadec-9-enolide |
| 53 | 21.827 | 0.22 | 7-Pentadecyne |
| 54 | 21.973 | 1.09 | 1-Eicosene |
| 55 | 22.269 | 0.40 | 9,12-Octadecadienoic acid (Z,Z)- |
| 56 | 22.462 | 0.46 | E-11-Hexadecenal |
| 57 | 22.605 | 0.16 | Caparratriene |
| 58 | 22.775 | 0.20 | 2,5-Furandione, 3-dodecyl- |
| 59 | 22.986 | 0.18 | 2-Dodecen-1-yl(-)succinic anhydride |
| 60 | 23.105 | 0.34 | (7R,8S)-cis-anti-cis-7,8-Epoxytricyclo[7.3.0.0(2,6)]dodecane |
| 61 | 23.154 | 0.25 | Oxirane, hexadecyl- |
| 62 | 23.456 | 0.60 | Eicosanoic acid |
| 63 | 23.693 | 0.58 | 1-Octadecene |
| 64 | 23.833 | 0.19 | Oxacyclotetradecan-2-one |
| 65 | 24.004 | 0.25 | 1,3,12-Nonadecatriene |
| 66 | 24.095 | 0.13 | 2(1H)-Naphthalenone, octahydro-4a-methyl-7-(1-methylethyl)-, (4a.alpha.,7.beta.,8a.beta.)- |
| 67 | 24.289 | 0.41 | 2,5-Furandione, 3-dodecyl- |
| 68 | 24.446 | 1.73 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 69 | 24.531 | 1.73 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 70 | 25.078 | 0.90 | Docosanoic acid |
| 71 | 25.308 | 0.38 | 1-Chloroeicosane |
| 72 | 25.429 | 0.54 | Cyclopentadecanone, 4-methyl- |
| 73 | 25.606 | 0.10 | 2,5-Furandione, 3-dodecyl- |
| 74 | 25.962 | 1.37 | 3-((4Z,7Z)-Heptadeca-4,7-dien-1-yl)phenol |
| 75 | 26.061 | 1.11 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 76 | 26.152 | 2.12 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 77 | 26.407 | 0.42 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 78 | 26.770 | 0.74 | 1-Octadecene |
| 79 | 26.899 | 0.21 | 2-Dodecen-1-yl(-)succinic anhydride |
| 80 | 27.528 | 0.97 | 1-Hexacosene |
| 81 | 27.906 | 0.44 | Trifluoroacetic acid, pentadecyl ester |
| 82 | 28.402 | 0.18 | 2- Chloropropionic acid, octadecyl ester |
| 83 | 29.360 | 1.27 | Stigmasta-3,5-diene |
| 84 | 30.443 | 0.31 | 2- Chloropropionic acid, octadecyl ester |
| 85 | 31.994 | 0.35 | .beta.-Sitosterol |
| 86 | 33.413 | 0.11 | 1,6,10,14,18,22-Tetracosahexaen-3-ol, 2,6,10,15,19,23-hexamethyl-, (all-E)-(.+/-.)- |
| 87 | 35.235 | 0.14 | 1-Nonadecene |
Table A4.
Components of CCB treatment with the Ag and NiO catalyzer at 550 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 2.895 | 6.04 | Acetic acid |
| 2 | 3.206 | 8.38 | Acetic acid |
| 3 | 4.888 | 4.82 | Furfural |
| 4 | 5.057 | 2.03 | Furfural |
| 5 | 5.748 | 1.54 | Cyclopent-4-ene-1,3-dione |
| 6 | 6.087 | 0.79 | Cyclopentene |
| 7 | 6.499 | 3.11 | Cyclohexanone |
| 8 | 6.959 | 1.09 | 2-Furancarboxaldehyde, 5-methyl- |
| 9 | 7.045 | 0.83 | 1,2-Pentadiene |
| 10 | 7.258 | 1.48 | Cyclopentane, butyl- |
| 11 | 7.950 | 1.33 | D-Limonene |
| 12 | 8.069 | 1.89 | 1,2-Cyclopentanedione, 3-methyl- |
| 13 | 8.966 | 3.85 | Phenol, 2-methoxy- |
| 14 | 9.908 | 0.41 | 4-Pyridinol |
| 15 | 10.244 | 0.29 | 2,3-Dihydroxybenzaldehyde |
| 16 | 10.323 | 0.40 | Bicyclo[2.2.1]heptane, 1,7,7-trimethyl- |
| 17 | 10.524 | 1.00 | Creosol |
| 18 | 10.831 | 2.64 | Catechol |
| 19 | 11.372 | 0.50 | GeraNiOl |
| 20 | 11.629 | 0.64 | 1,2-Benzenediol, 3-methoxy- |
| 21 | 11.745 | 0.38 | Phenol, 4-ethyl-2-methoxy- |
| 22 | 11.820 | 0.21 | 3,4-Dihydroxyacetophenone |
| 23 | 11.909 | 0.27 | 2-Tetradecene, (E)- |
| 24 | 12.118 | 1.58 | 1,2-Benzenediol, 4-methyl- |
| 25 | 12.295 | 1.96 | 2-Methoxy-4-vinylphenol |
| 26 | 12.791 | 2.07 | Phenol, 2,6-dimethoxy- |
| 27 | 13.273 | 0.25 | 1-Heptadecene |
| 28 | 13.526 | 1.03 | Phenol, 2-methoxy-4-(1-propenyl)-, (Z)- |
| 29 | 14.021 | 0.59 | 3,5-Dimethoxy-4-hydroxytoluene |
| 30 | 14.123 | 1.33 | trans-Isoeugenol |
| 31 | 14.621 | 1.04 | Apocynin |
| 32 | 15.122 | 1.58 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 33 | 15.669 | 0.40 | 4-(1-Hydroxyallyl)-2-methoxyphenol |
| 34 | 15.906 | 0.71 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- |
| 35 | 16.378 | 0.48 | Phenylamine, N,4,5-trimethyl-2-nitro- |
| 36 | 16.489 | 0.62 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 37 | 16.559 | 0.58 | Benzenepropanol, 4-hydroxy-3-methoxy- |
| 38 | 16.667 | 1.12 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- |
| 39 | 16.957 | 0.40 | 2-Allyl-1,4-dimethoxy-3-methyl-benzene |
| 40 | 17.122 | 2.18 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 41 | 17.463 | 1.08 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- |
| 42 | 17.726 | 0.52 | Tetradecanoic acid |
| 43 | 18.045 | 0.57 | Cyclopentadecane |
| 44 | 18.509 | 1.18 | Neophytadiene |
| 45 | 18.951 | 0.40 | 11-Hexadecen-1-ol, acetate, (Z)- |
| 46 | 19.038 | 0.21 | 1,4-Methanoazulen-7-ol, decahydro-4,8,8,9-tetramethyl-, (+)- |
| 47 | 19.377 | 0.44 | Neoclovene oxide |
| 48 | 19.607 | 0.84 | (R)-(-)-14-Methyl-8-hexadecyn-1-ol |
| 49 | 19.836 | 1.50 | n-Hexadecanoic acid |
| 50 | 20.097 | 0.93 | Aromadendrene oxide-(2) |
| 51 | 20.426 | 0.51 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (Z,E)- |
| 52 | 20.568 | 0.50 | 1,2-Longidione |
| 53 | 21.058 | 0.43 | Cyclopentadecane |
| 54 | 21.531 | 2.69 | Linoelaidic acid |
| 55 | 21.702 | 0.66 | Octadecanoic acid |
| 56 | 21.898 | 0.57 | Cyclohexene, 4-pentyl-1-(4-propylcyclohexyl)- |
| 57 | 21.971 | 0.60 | Cyclopentadecane |
| 58 | 22.132 | 0.24 | Thunbergol |
| 59 | 22.267 | 0.69 | 9,12-Octadecadienoic acid (Z,Z)- |
| 60 | 22.440 | 0.52 | 13-Octadecenal, (Z)- |
| 61 | 22.590 | 0.19 | 2-Cyclohexen-1-one, 4-(3-hydroxybutyl)-3,5,5-trimethyl- |
| 62 | 22.764 | 0.25 | E-11-Hexadecenal |
| 63 | 23.153 | 0.27 | Z,E-3,13-Octadecadien-1-ol |
| 64 | 23.288 | 0.53 | 2- Chloropropionic acid, hexadecyl ester |
| 65 | 23.455 | 0.71 | Eicosanoic acid |
| 66 | 23.688 | 0.59 | Cyclopentadecane |
| 67 | 23.902 | 0.26 | (E)-15,16-Dinorlabda-8(17),12-dien-14-al |
| 68 | 24.003 | 0.29 | 2,5-Furandione, 3-dodecyl- |
| 69 | 24.100 | 0.29 | 2,5-Furandione, 3-dodecyl- |
| 70 | 24.273 | 0.30 | 2,5-Furandione, 3-dodecyl- |
| 71 | 24.427 | 1.17 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 72 | 24.512 | 1.16 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 73 | 24.672 | 0.60 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
| 74 | 25.075 | 0.75 | Docosanoic acid |
| 75 | 25.290 | 0.46 | 9-Tricosene, (Z)- |
| 76 | 25.427 | 0.69 | Oxacyclopentadecan-2-one |
| 77 | 25.668 | 0.50 | 2(1H)-Naphthalenone, octahydro-4a-methyl-7-(1-methylethyl)-, (4a.alpha.,7.beta.,8a.beta.)- |
| 78 | 25.959 | 1.57 | 3-((4Z,7Z)-Heptadeca-4,7-dien-1-yl)phenol |
| 79 | 26.058 | 1.54 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 80 | 26.150 | 3.23 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 81 | 26.404 | 0.65 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 82 | 26.770 | 1.07 | 9-Hexacosene |
| 83 | 27.526 | 1.50 | 1-Hexacosene |
| 84 | 27.660 | 0.25 | 13-Tetradecen-1-ol acetate |
| 85 | 27.905 | 0.57 | Trifluoroacetic acid, pentadecyl ester |
| 86 | 28.290 | 0.56 | 17-Pentatriacontene |
| 87 | 28.403 | 0.25 | 1-Octadecene |
| 88 | 29.234 | 0.29 | Tetracosane |
| 89 | 29.358 | 1.26 | Stigmasta-3,5-diene |
| 90 | 30.277 | 0.25 | 2- Chloropropionic acid, octadecyl ester |
| 91 | 30.444 | 0.24 | Octacosanol |
| 92 | 30.846 | 0.39 | Ergost-7-en-3-ol, (3.beta.)- |
| 93 | 31.991 | 0.44 | .beta.-Sitosterol |
Table A5.
Components of the raw CCB powder at 700 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 2.805 | 3.22 | Acetic acid |
| 2 | 3.820 | 0.78 | Toluene |
| 3 | 4.805 | 0.96 | Furfural |
| 4 | 5.614 | 0.26 | 1-Nonene |
| 5 | 5.685 | 0.21 | Styrene |
| 6 | 6.451 | 0.47 | 2-Cyclopenten-1-one, 2-hydroxy- |
| 7 | 6.900 | 0.21 | 2-Furancarboxaldehyde, 5-methyl- |
| 8 | 7.012 | 0.07 | 1,4-Pentadiene |
| 9 | 7.147 | 0.11 | 4-Cyclopentene-1,3-dione, 4-propyl- |
| 10 | 7.285 | 0.30 | 1-Decene |
| 11 | 7.910 | 0.26 | p-Cymene |
| 12 | 8.086 | 0.20 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- |
| 13 | 8.191 | 0.09 | Benzyl alcohol |
| 14 | 8.276 | 0.14 | Benzene, 1-propynyl- |
| 15 | 8.511 | 0.13 | Phenol, 2-methyl- |
| 16 | 8.924 | 0.70 | 1-Decene |
| 17 | 9.980 | 0.20 | Phenol, 2,3-dimethyl- |
| 18 | 10.292 | 0.12 | Phenol, 3-ethyl- |
| 19 | 10.474 | 0.26 | 1-Dodecene |
| 20 | 10.556 | 0.09 | 1H-Indene, 1-methylene- |
| 21 | 10.686 | 0.23 | Cyclohexene, 1-methyl-4-(1-methylethylidene)- |
| 22 | 11.926 | 0.49 | 1-Tridecene |
| 23 | 12.327 | 1.03 | 2-Methoxy-4-vinylphenol |
| 24 | 13.292 | 0.68 | 1-Tetradecene |
| 25 | 14.158 | 2.00 | Eugenol |
| 26 | 14.585 | 0.55 | 1-Tridecene |
| 27 | 15.662 | 1.36 | Glutaric acid, isobutyl 2-pentyl ester |
| 28 | 15.731 | 0.87 | .beta.-D-Glucopyranose, 1,6-anhydro- |
| 29 | 16.897 | 0.63 | Hexanedioic acid, bis(2-methylpropyl) ester |
| 30 | 16.970 | 1.28 | Trichloroacetic acid, pentadecyl ester |
| 31 | 17.349 | 1.46 | 1-Dodecanol, 3,7,11-trimethyl- |
| 32 | 18.074 | 1.05 | 1-Octadecene |
| 33 | 18.738 | 1.02 | 14-Pentadecenoic acid |
| 34 | 18.870 | 2.18 | 2-(Pentyloxycarbonyl)benzoic acid |
| 35 | 19.059 | 0.57 | Cyclododecane, ethyl- |
| 36 | 19.125 | 0.58 | 1-Octadecene |
| 37 | 20.038 | 7.47 | n-Hexadecanoic acid |
| 38 | 20.127 | 2.56 | 4-Methyl-2,7-dioxa-tricyclo[4.4.0.0(3,8)]decane |
| 39 | 20.627 | 1.33 | Cyclotetradecane |
| 40 | 20.698 | 1.18 | Octadec-9-enoic acid |
| 41 | 21.088 | 1.15 | 1-Nonadecene |
| 42 | 21.265 | 0.81 | Oxirane, tetradecyl- |
| 43 | 21.735 | 7.15 | Oleic Acid |
| 44 | 21.884 | 1.92 | Octadecanoic acid |
| 45 | 22.011 | 1.94 | Cyclopentadecane |
| 46 | 22.217 | 1.42 | Cyclopropaneoctanal, 2-octyl- |
| 47 | 22.390 | 0.65 | Cyclohexene, 1-pentyl-4-(4-propylcyclohexyl)- |
| 48 | 22.659 | 1.23 | 2,5-Furandione, 3-dodecyl- |
| 49 | 22.952 | 4.51 | 3-Tridecylphenol |
| 50 | 23.361 | 1.39 | Z,Z-11,13-Hexadecadien-1-ol acetate |
| 51 | 23.569 | 1.93 | 9-Octadecenamide, (Z)- |
| 52 | 23.734 | 2.13 | 1-Nonadecene |
| 53 | 23.974 | 2.05 | 2(1H)-Naphthalenone, octahydro-4a-methyl-7-(1-methylethyl)-, (4a.alpha.,7.beta.,8a.beta.)- |
| 54 | 24.214 | 0.61 | 2-Dodecen-1-yl(-)succinic anhydride |
| 55 | 24.346 | 0.85 | 2,5-Furandione, 3-dodecyl- |
| 56 | 24.507 | 1.48 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 57 | 24.584 | 2.04 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 58 | 24.971 | 1.20 | 2- Chloropropionic acid, hexadecyl ester |
| 59 | 25.133 | 0.93 | 2-Dodecen-1-yl(-)succinic anhydride |
| 60 | 25.329 | 2.54 | 1-Nonadecene |
| 61 | 25.600 | 0.40 | 2-Dodecen-1-yl(-)succinic anhydride |
| 62 | 25.747 | 0.75 | 2-Dodecen-1-yl(-)succinic anhydride |
| 63 | 25.824 | 0.31 | 22-Tricosenoic acid |
| 64 | 26.103 | 2.29 | 1-Eicosene |
| 65 | 26.191 | 1.01 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 66 | 26.299 | 0.72 | 22-Tricosenoic acid |
| 67 | 26.469 | 0.86 | 2-Dodecen-1-yl(-)succinic anhydride |
| 68 | 26.644 | 0.83 | 2-Dodecen-1-yl(-)succinic anhydride |
| 69 | 26.804 | 3.09 | 1-Nonadecene |
| 70 | 27.138 | 1.16 | 2-Dodecen-1-yl(-)succinic anhydride |
| 71 | 27.395 | 0.80 | 2-Dodecen-1-yl(-)succinic anhydride |
| 72 | 27.558 | 1.34 | Hexadecane |
| 73 | 27.703 | 0.44 | Cyclopentadecanone, 2-hydroxy- |
| 74 | 27.950 | 0.46 | Cyclopentadecanone, 2-hydroxy- |
| 75 | 28.029 | 0.66 | 2-Dodecen-1-yl(-)succinic anhydride |
| 76 | 28.189 | 0.58 | 2-Dodecen-1-yl(-)succinic anhydride |
| 77 | 28.276 | 0.32 | Pentafluoropropionic acid, tetradecyl ester |
| 78 | 28.353 | 0.61 | (Z)-Decyl icos-9-enoate |
| 79 | 28.458 | 0.35 | Cyclopentadecanone, 2-hydroxy- |
| 80 | 29.425 | 1.81 | Stigmasta-3,5-diene |
| 81 | 29.565 | 0.31 | 9-Octadecenoic acid, (E)- |
| 82 | 29.925 | 0.50 | 2-Dodecen-1-yl(-)succinic anhydride |
| 83 | 30.179 | 0.90 | i-Propyl 9-octadecenoate |
| 84 | 30.525 | 0.44 | Cyclopentadecanone, 2-hydroxy- |
| 85 | 30.630 | 0.51 | i-Propyl 9-octadecenoate |
| 86 | 30.858 | 0.43 | Cyclohexene, 4-(4-ethylcyclohexyl)-1-pentyl- |
| 87 | 31.271 | 0.88 | i-Propyl 9-octadecenoate |
| 88 | 31.668 | 0.27 | 2-Dodecen-1-yl(-)succinic anhydride |
| 89 | 31.826 | 0.57 | Cyclopentadecanone, 2-hydroxy- |
| 90 | 32.127 | 1.10 | .gamma.-Sitosterol |
Table A6.
Components of CCB treatment with the Ag catalyzer at 700 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 4.919 | 1.25 | Toluene |
| 2 | 5.562 | 0.81 | Furfural |
| 3 | 5.871 | 0.50 | Benzene, 1,3-dimethyl- |
| 4 | 5.979 | 0.47 | p-Xylene |
| 5 | 6.167 | 0.24 | 1-Nonene |
| 6 | 6.263 | 0.37 | Styrene |
| 7 | 6.440 | 0.32 | Bicyclo[2.1.0]pentane |
| 8 | 6.963 | 0.07 | 1,4-Pentadiene, 2,3,3-trimethyl- |
| 9 | 7.291 | 0.16 | 2-Cyclopenten-1-one, 3-methyl- |
| 10 | 8.117 | 0.11 | p-Cymene |
| 11 | 8.213 | 0.16 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- |
| 12 | 8.467 | 0.13 | Indene |
| 13 | 8.610 | 0.29 | Phenol, 2-methyl- |
| 14 | 8.958 | 0.36 | Phenol, 3-methyl- |
| 15 | 9.039 | 0.55 | 1-Undecene |
| 16 | 9.415 | 0.08 | Phenol, 2,6-dimethyl- |
| 17 | 10.035 | 0.30 | Phenol, 2,3-dimethyl- |
| 18 | 10.331 | 0.16 | Phenol, 2-ethyl- |
| 19 | 10.401 | 0.14 | 1,11-Dodecadiene |
| 20 | 10.516 | 0.21 | 1-Dodecene |
| 21 | 11.288 | 0.05 | Phenol, 2-ethyl-5-methyl- |
| 22 | 11.607 | 0.10 | 3-Ethylphenol, methyl ether |
| 23 | 11.829 | 0.16 | cis-9-Tetradecen-1-ol |
| 24 | 11.938 | 0.20 | 1-Tridecene |
| 25 | 12.200 | 0.10 | Naphthalene, 2-methyl- |
| 26 | 12.323 | 0.22 | 2-Methoxy-4-vinylphenol |
| 27 | 13.195 | 0.25 | 1,12-Tridecadiene |
| 28 | 13.294 | 0.31 | 1-Tetradecene |
| 29 | 14.143 | 0.36 | Eugenol |
| 30 | 14.582 | 0.34 | 1-Tridecene |
| 31 | 15.532 | 6.20 | .beta.-D-Glucopyranose, 1,6-anhydro- |
| 32 | 16.966 | 0.51 | Trichloroacetic acid, tridecyl ester |
| 33 | 17.813 | 0.81 | Cyclododecane, ethyl- |
| 34 | 18.371 | 0.99 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- |
| 35 | 19.255 | 1.00 | Panaxjapyne A |
| 36 | 19.715 | 1.99 | Cyclopentadecanone, 2-hydroxy- |
| 37 | 19.972 | 7.05 | n-Hexadecanoic acid |
| 38 | 20.524 | 0.76 | Solavetivone |
| 39 | 20.676 | 1.18 | Solavetivone |
| 40 | 20.862 | 1.37 | Tridecanoic acid |
| 41 | 21.152 | 0.57 | 9-Hexadecenoic acid |
| 42 | 21.257 | 0.80 | 13-Oxabicyclo[9.3.1]pentadecane |
| 43 | 21.329 | 0.98 | 2,2-Dimethyl-3-vinyl-bicyclo[2.2.1]heptane |
| 44 | 21.715 | 9.05 | Oleic Acid |
| 45 | 21.865 | 2.66 | Octadecanoic acid |
| 46 | 22.033 | 1.74 | Oxacyclohexadecan-2-one |
| 47 | 22.209 | 1.07 | Cyclopropaneoctanal, 2-octyl- |
| 48 | 22.301 | 0.58 | 2-Dodecen-1-yl(-)succinic anhydride |
| 49 | 22.378 | 0.75 | Cyclohexene, 1-pentyl-4-(4-propylcyclohexyl)- |
| 50 | 22.459 | 0.58 | Cyclohexene, 1-pentyl-4-(4-propylcyclohexyl)- |
| 51 | 22.649 | 1.34 | cis-9-Hexadecenal |
| 52 | 22.940 | 5.84 | 3-Tridecylphenol |
| 53 | 23.558 | 2.34 | 9-Octadecenamide, (Z)- |
| 54 | 23.728 | 2.46 | 1-Octadecene |
| 55 | 23.973 | 1.14 | 2(1H)-Naphthalenone, octahydro-4a-methyl-7-(1-methylethyl)-, (4a.alpha.,7.beta.,8a.beta.)- |
| 56 | 24.072 | 1.04 | 9,17-Octadecadienal, (Z)- |
| 57 | 24.214 | 0.73 | 9-Octadecenal, (Z)- |
| 58 | 24.341 | 0.98 | 2- Chloropropionic acid, hexadecyl ester |
| 59 | 24.500 | 1.86 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 60 | 24.577 | 2.50 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 61 | 24.966 | 0.85 | Erucic acid |
| 62 | 25.130 | 1.10 | 2-Heptadecenal |
| 63 | 25.325 | 2.21 | 9-Tricosene, (Z)- |
| 64 | 25.475 | 0.82 | Octacosanol |
| 65 | 25.599 | 0.74 | Cyclopentadecanone, 2-hydroxy- |
| 66 | 25.823 | 0.39 | 2-Dodecen-1-yl(-)succinic anhydride |
| 67 | 26.102 | 2.78 | Cyclopentadecane |
| 68 | 26.188 | 1.34 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 69 | 26.298 | 0.91 | Cyclopentadecanone, 2-hydroxy- |
| 70 | 26.468 | 1.37 | Cyclopentadecanone, 2-hydroxy- |
| 71 | 26.802 | 2.99 | 1-Nonadecene |
| 72 | 27.007 | 0.84 | Cyclopentadecanone, 2-hydroxy- |
| 73 | 27.133 | 0.71 | Cyclopentadecanone, 2-hydroxy- |
| 74 | 27.256 | 0.47 | Cyclopentadecanone, 2-hydroxy- |
| 75 | 27.392 | 1.18 | Cyclopentadecanone, 2-hydroxy- |
| 76 | 27.556 | 1.54 | 1-Hexacosene |
| 77 | 27.701 | 0.52 | Cyclopentadecanone, 2-hydroxy- |
| 78 | 27.794 | 0.96 | Cyclopentadecanone, 2-hydroxy- |
| 79 | 27.947 | 0.48 | Cyclopentadecanone, 2-hydroxy- |
| 80 | 28.025 | 0.70 | Cyclopentadecanone, 2-hydroxy- |
| 81 | 28.183 | 0.72 | Cyclopentadecanone, 2-hydroxy- |
| 82 | 28.274 | 0.36 | 2-Dodecen-1-yl(-)succinic anhydride |
| 83 | 28.349 | 0.70 | (Z)-Decyl icos-9-enoate |
| 84 | 28.455 | 0.43 | Cyclopentadecanone, 2-hydroxy- |
| 85 | 29.419 | 2.09 | Stigmasta-3,5-diene |
| 86 | 29.564 | 0.41 | Cyclopentadecanone, 2-hydroxy- |
| 87 | 30.376 | 0.69 | Cyclopentadecanone, 2-hydroxy- |
| 88 | 30.520 | 0.51 | Cyclopentadecanone, 2-hydroxy- |
| 89 | 30.849 | 0.41 | i-Propyl 9-octadecenoate |
| 90 | 32.112 | 1.07 | .gamma.-Sitosterol |
Table A7.
Components of t CCB treatment with the NiO catalyzer at 700 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 3.774 | 2.92 | Toluene |
| 2 | 5.179 | 2.86 | Acetic acid |
| 3 | 6.287 | 3.07 | 2-Cyclopenten-1-one |
| 4 | 7.976 | 4.33 | Limonene |
| 5 | 8.443 | 2.75 | Acetic acid, phenyl ester |
| 6 | 9.017 | 1.21 | 1,2-Cyclopentanedione, 3-methyl- |
| 7 | 9.362 | 1.84 | Phenol, 2-methoxy- |
| 8 | 9.561 | 3.47 | Phenol, 3-methyl- |
| 9 | 10.118 | 0.84 | 4,7-Methano-1H-inden-1-ol, 3a,4,7,7a-tetrahydro-, acetate |
| 10 | 11.881 | 1.75 | 2-Cyclohexen-1-one, 5-methyl-2-(1-methylethyl)- |
| 11 | 12.548 | 2.93 | 2-Methoxy-4-vinylphenol |
| 12 | 12.701 | 1.15 | 2-Methoxy-4-vinylphenol |
| 13 | 12.795 | 1.38 | 2-Isopropylidene-3-methylhexa-3,5-dienal |
| 14 | 13.103 | 3.84 | Phenol, 2-methoxy-3-(2-propenyl)- |
| 15 | 13.790 | 1.96 | Phenol, 2-methoxy-5-(1-propenyl)-, (E)- |
| 16 | 14.443 | 2.92 | trans-Isoeugenol |
| 17 | 14.628 | 0.99 | Cyclododecane |
| 18 | 14.720 | 0.65 | Pentadecane |
| 19 | 15.068 | 1.09 | 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene |
| 20 | 15.592 | 0.55 | 10-Methyltricyclo[4.3.1.1(2,5)]undecan-10-ol |
| 21 | 16.140 | 2.34 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- |
| 22 | 16.350 | 0.72 | 2(3H)-Naphthalenone, 4,4a,5,6,7,8-hexahydro-1-methoxy- |
| 23 | 16.689 | 1.10 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 24 | 16.762 | 0.58 | 1,9-Tetradecadiene |
| 25 | 16.836 | 0.78 | 1,9-Tetradecadiene |
| 26 | 16.934 | 0.81 | 5-Dodecenol |
| 27 | 17.015 | 2.00 | 1-Heptadecene |
| 28 | 17.312 | 1.28 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 29 | 17.384 | 2.01 | 1-Dodecanol, 3,7,11-trimethyl- |
| 30 | 17.638 | 0.62 | Sesquirosefuran |
| 31 | 17.754 | 0.83 | 3-Oxabicyclo[4.1.0]heptan-2-one, 4,4,7,7-tetramethyl- |
| 32 | 17.948 | 1.16 | Heptadecanal |
| 33 | 18.035 | 0.62 | Oleyl alcohol , acetate |
| 34 | 18.114 | 1.78 | 1-Octadecene |
| 35 | 18.474 | 0.38 | Sesquirosefuran |
| 36 | 18.563 | 0.75 | Neophytadiene |
| 37 | 18.905 | 1.34 | Dibutyl phthalate |
| 38 | 19.087 | 0.46 | Oxirane, hexadecyl- |
| 39 | 19.160 | 1.57 | 1-Nonadecene |
| 40 | 19.363 | 0.55 | Methyl tetrahydroionol |
| 41 | 19.473 | 0.75 | Pentadecanoic acid, 14-methyl-, methyl ester |
| 42 | 19.632 | 0.32 | Cyclohexane, 1,1,3-trimethyl-2,3-epoxy-2-(3-methylcyclobuten-2-yl-1)-4-acetyloxy- |
| 43 | 19.729 | 0.65 | Cyclohexane, 1,1,3-trimethyl-2,3-epoxy-2-(3-methylcyclobuten-2-yl-1)-4-acetyloxy- |
| 44 | 19.971 | 1.74 | n-Hexadecanoic acid |
| 45 | 20.158 | 1.98 | Cycloeicosane |
| 46 | 20.386 | 0.43 | Cyclopentane, (2-hexyloctyl)- |
| 47 | 20.489 | 0.40 | Cyclohexane, 1,1,3-trimethyl-2,3-epoxy-2-(3-methylcyclobuten-2-yl-1)-4-acetyloxy- |
| 48 | 20.939 | 0.91 | p-Menth-8(10)-en-9-ol, cis- |
| 49 | 21.112 | 2.48 | Z-5-Nonadecene |
| 50 | 21.728 | 3.00 | 9,17-Octadecadienal, (Z)- |
| 51 | 21.873 | 0.65 | Octadecanoic acid |
| 52 | 22.029 | 2.30 | 1-Docosene |
| 53 | 22.378 | 0.39 | cis-7,cis-11-Hexadecadien-1-yl acetate |
| 54 | 22.467 | 0.26 | 2- Chloropropionic acid, hexadecyl ester |
| 55 | 22.529 | 0.49 | Cyclopropaneoctanal, 2-octyl- |
| 56 | 22.655 | 0.66 | Cyclopentadecanone, 2-hydroxy- |
| 57 | 22.943 | 2.11 | 3-Tridecylphenol |
| 58 | 23.568 | 1.01 | 9-Octadecenamide, (Z)- |
| 59 | 23.735 | 0.89 | 1-Nonadecene |
| 60 | 23.982 | 0.98 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalene-2,3-diol |
| 61 | 24.217 | 0.36 | Cyclopentadecanone, 2-hydroxy- |
| 62 | 24.351 | 0.42 | 2- Chloropropionic acid, hexadecyl ester |
| 63 | 24.514 | 0.86 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 64 | 24.592 | 1.32 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 65 | 24.975 | 0.60 | 2- Chloropropionic acid, hexadecyl ester |
| 66 | 25.138 | 0.52 | 2- Chloropropionic acid, hexadecyl ester |
| 67 | 25.326 | 0.75 | Cyclooctacosane |
| 68 | 25.481 | 0.37 | Octacosanol |
| 69 | 25.600 | 0.45 | Cyclopentadecanone, 2-hydroxy- |
| 70 | 26.106 | 0.76 | 17-Pentatriacontene |
| 71 | 26.199 | 0.94 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 72 | 26.464 | 0.55 | 9-Hexacosene |
| 73 | 26.809 | 1.52 | 1-Nonadecene |
| 74 | 27.557 | 0.70 | Heptacosane, 1-chloro- |
| 75 | 27.710 | 0.25 | 2-Dodecen-1-yl(-)succinic anhydride |
| 76 | 28.186 | 0.30 | 1H-Indene, 5-butyl-6-hexyloctahydro- |
| 77 | 29.427 | 0.80 | Stigmasta-3,5-diene |
| 78 | 30.769 | 0.11 | 9-Octadecenoic acid, (E)- |
| 79 | 30.957 | 0.60 | Cyclohexene, 4-(4-ethylcyclohexyl)-1-pentyl- |
| 80 | 31.838 | 0.34 | 11-Tricosene |
| 81 | 32.130 | 0.44 | .gamma.-Sitosterol |
| 82 | 32.715 | 0.13 | Cyclopentadecanone, 2-hydroxy- |
| 83 | 35.444 | 0.31 | Octacosanol |
Table A8.
Components of CCB treatment with the Ag and NiO catalyzer at 700 °C.
| No. | Retention time | Relative content | Compounds name |
|---|---|---|---|
| (min) | (%) | ||
| 1 | 4.342 | 2.58 | Acetic acid |
| 2 | 5.354 | 2.31 | Benzene, 1,3-dimethyl- |
| 3 | 5.614 | 1.08 | 1-Nonene |
| 4 | 5.717 | 1.50 | Bicyclo[4.2.0]octa-1,3,5-triene |
| 5 | 7.274 | 1.89 | 1-Decene |
| 6 | 7.908 | 1.44 | Phenol |
| 7 | 7.975 | 1.17 | Phenol |
| 8 | 8.746 | 2.21 | 1,2-Cyclopentanedione, 3-methyl- |
| 9 | 8.934 | 1.57 | Phenol, 2-methyl- |
| 10 | 9.025 | 1.00 | 9-Oxabicyclo[6.1.0]non-6-en-2-one |
| 11 | 9.279 | 4.13 | p-Cresol |
| 12 | 10.002 | 3.57 | Benzene, 1-butynyl- |
| 13 | 10.266 | 1.40 | Phenol, 2,3-dimethyl- |
| 14 | 10.385 | 0.86 | trans-8-oxabicyclo[4.3.0]nonane |
| 15 | 10.513 | 1.03 | 1-Dodecene |
| 16 | 10.755 | 2.04 | Creosol |
| 17 | 12.514 | 2.34 | 2-Methoxy-4-vinylphenol |
| 18 | 12.791 | 2.63 | Benzenemethanol, 4-hydroxy- |
| 19 | 13.055 | 2.98 | Phenol, 2,6-dimethoxy- |
| 20 | 13.246 | 0.89 | 7-Methyl-1,6-octadiene |
| 21 | 13.344 | 2.28 | 1-Tetradecene |
| 22 | 13.717 | 1.04 | Eugenol |
| 23 | 14.331 | 2.44 | Phenol, 2-methoxy-4-(1-propenyl)- |
| 24 | 14.646 | 1.03 | 1-Pentadecene |
| 25 | 15.588 | 0.67 | 10-Methyltricyclo[4.3.1.1(2,5)]undecan-10-ol |
| 26 | 15.870 | 1.81 | Cetene |
| 27 | 16.085 | 2.05 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- |
| 28 | 16.658 | 1.44 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 29 | 16.768 | 0.60 | 1,5-Dodecadiene |
| 30 | 16.835 | 0.69 | 8-Dodecen-1-ol, (Z)- |
| 31 | 17.023 | 1.54 | 1-Heptadecene |
| 32 | 17.273 | 1.48 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol |
| 33 | 17.950 | 1.07 | 2(1H)-Benzocyclooctenone, decahydro-10a-methyl-, trans- |
| 34 | 18.043 | 0.70 | 1,13-Tetradecadiene |
| 35 | 18.123 | 1.68 | 1-Octadecene |
| 36 | 18.569 | 0.70 | Neophytadiene |
| 37 | 18.909 | 1.25 | 2-(Heptyloxycarbonyl)benzoic acid |
| 38 | 19.010 | 0.61 | 9,12-Octadecadienoic acid (Z,Z)- |
| 39 | 19.096 | 0.46 | 9-Octadecen-1-ol, (Z)- |
| 40 | 19.169 | 2.03 | 1-Nonadecene |
| 41 | 19.483 | 0.69 | Hexadecanoic acid, methyl ester |
| 42 | 19.742 | 0.78 | 2(1H)-Naphthalenone, octahydro-4a-methyl-7-(1-methylethyl)-, (4a.alpha.,7.beta.,8a.beta.)- |
| 43 | 20.011 | 1.83 | n-Hexadecanoic acid |
| 44 | 20.104 | 0.44 | 1,19-Eicosadiene |
| 45 | 20.170 | 1.98 | Cycloeicosane |
| 46 | 20.887 | 0.59 | Tetrahydroionone |
| 47 | 21.124 | 2.29 | Z-5-Nonadecene |
| 48 | 21.352 | 0.47 | Solavetivone |
| 49 | 21.724 | 2.90 | Oleic Acid |
| 50 | 21.871 | 0.58 | Octadecanoic acid |
| 51 | 22.040 | 2.38 | Carbonic acid, octadecyl 2,2,2-trichloroethyl ester |
| 52 | 22.388 | 0.34 | 9,12-Octadecadienoic acid (Z,Z)- |
| 53 | 22.484 | 0.30 | 2,5-Furandione, 3-dodecyl- |
| 54 | 22.542 | 0.49 | Cyclopropaneoctanal, 2-octyl- |
| 55 | 22.671 | 0.64 | 2,5-Furandione, 3-dodecyl- |
| 56 | 22.957 | 2.22 | 3-Tridecylphenol |
| 57 | 23.375 | 0.71 | 2-Dodecen-1-yl(-)succinic anhydride |
| 58 | 23.580 | 0.83 | 9-Octadecenamide, (Z)- |
| 59 | 23.753 | 1.32 | Cyclotetracosane |
| 60 | 23.993 | 0.68 | 2- Chloropropionic acid, hexadecyl ester |
| 61 | 24.235 | 0.37 | 2- Chloropropionic acid, hexadecyl ester |
| 62 | 24.535 | 1.13 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 63 | 24.612 | 1.52 | (Z)-3-(pentadec-8-en-1-yl)phenol |
| 64 | 24.986 | 0.62 | Cyclopentadecanone, 2-hydroxy- |
| 65 | 25.339 | 0.78 | 1-Nonadecene |
| 66 | 25.492 | 0.38 | Octacosanol |
| 67 | 25.610 | 0.39 | Octacosanol |
| 68 | 25.761 | 0.28 | 17-(1,5-Dimethylhexyl)-10,13-dimethyl-4,5,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthrene |
| 69 | 26.117 | 0.71 | cis-1-Chloro-9-octadecene |
| 70 | 26.207 | 0.93 | (Z)-3-(Heptadec-10-en-1-yl)phenol |
| 71 | 26.477 | 0.55 | 2-Dodecen-1-yl(-)succinic anhydride |
| 72 | 26.820 | 1.51 | 1-Nonadecene |
| 73 | 27.411 | 0.50 | Undec-10-ynoic acid, heptadecyl ester |
| 74 | 27.568 | 0.62 | Hexadecane |
| 75 | 28.363 | 0.48 | Octacosanol |
| 76 | 29.310 | 0.22 | Eicosane |
| 77 | 29.448 | 0.76 | Stigmasta-3,5-diene |
| 78 | 30.375 | 0.30 | Trifluoroacetic acid, pentadecyl ester |
| 79 | 30.982 | 0.52 | Cyclohexene, 4-(4-ethylcyclohexyl)-1-pentyl- |
| 80 | 31.860 | 0.27 | Pentadec-7-ene, 7-bromomethyl- |
| 81 | 32.151 | 0.34 | .gamma.-Sitosterol |
| 82 | 35.450 | 0.16 | Octacosanol |
References
- Almeida H.N., Calixto G.Q., Chagas B.M.E., Melo D.M.A., Resende F.M., Melo M.A.F., Braga R.M. Characterization and pyrolysis of Chlorella vulgaris and Arthrospira platensis: potential of bio-oil and chemical production by Py-GC/MS analysis. Environ. Sci. Pollut. Res. Int. 2017;24(16):14142–14150. doi: 10.1007/s11356-017-9009-2. [DOI] [PubMed] [Google Scholar]
- Burgess B., Melis M., Scoular K., Driver M., Schaich K.M., Keller K.L., Tomassini B.I., Tepper B.J. Effects of CD36 genotype on oral perception of oleic acid supplemented safflower oil emulsions in two ethnic groups: a preliminary study. J. Food Sci. 2018;83(5):1373–1380. doi: 10.1111/1750-3841.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Wang Y., Cao P., Liu Y. Effect of temperature on thermal oxidation of palmitic acid studied by combination of EPR spin trapping technique and SPME-GC-MS/MS. Food Chem. 2017;234:439–444. doi: 10.1016/j.foodchem.2017.04.135. [DOI] [PubMed] [Google Scholar]
- Chen W.H., Wang C.W., Kumar G., Rousset P., Hsieh T.H. Effect of torrefaction pretreatment on the pyrolysis of rubber wood sawdust analyzed by Py-GC/MS. Bioresour. Technol. 2018;259:469–473. doi: 10.1016/j.biortech.2018.03.033. [DOI] [PubMed] [Google Scholar]
- Cheng H., Wu S., Huang J., Zhang X. Direct evidence from in situ FTIR spectroscopy that o-quinonemethide is a key intermediate during the pyrolysis of guaiacol. Anal. Bioanal. Chem. 2017;409(10):2531–2537. doi: 10.1007/s00216-017-0194-0. [DOI] [PubMed] [Google Scholar]
- Cheng X., Yang T., Wang Y., Zhou B., Yan L., Teng L., Wang F., Chen L., He Y., Guo K., Zhang D. New method for effective identification of adulterated Camellia oil basing on Camellia oleifera-specific DNA. Arab. J. Chem. 2018;11(6):815–826. [Google Scholar]
- Chinisaz M., Ebrahim-Habibi A., Dehpour A.R., Yaghmaei P., Parivar K., Moosavi-MovahediA A. Structure and function of anhydride-modified forms of human insulin: in silico, in vitro and in vivo studies. Eur. J. Pharm. Sci. 2017;96:342–350. doi: 10.1016/j.ejps.2016.09.030. [DOI] [PubMed] [Google Scholar]
- De F.E.L.P., Shabudin S.V., Claúdio A.F.M., Válega M., Domingues F.M.J., Freire C.S.R., Silvestre A.J.D., Freire M.G. Aqueous solutions of surface-active ionic liquids: remarkable alternative solvents to improve the solubility of triterpenic acids and their extraction from biomass. ACS Sustain Chem Eng. 2017;5(8):7344–7351. doi: 10.1021/acssuschemeng.7b01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney S.P., Nethercott M.J., Mays C.J., Winquist N.T., Arthur D., Calahan J.L., Sethi M., Pardue D.S., Kim J., Amidon G., Munson E.J. Characterization of synthesized and commercial forms of magnesium stearate using differential scanning calorimetry, thermogravimetric analysis, powder X-ray diffraction, and solid-state NMR spectroscopy. J. Pharm. Sci. 2017;106(1):338–347. doi: 10.1016/j.xphs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Evageliou V., Saliari D. Limonene encapsulation in freeze dried gellan systems. Food Chem. 2017;223:72–75. doi: 10.1016/j.foodchem.2016.12.030. [DOI] [PubMed] [Google Scholar]
- Gao W., Baig A.Q., Ali H., Sajjad W., Farahani M.R. Margin based ontology sparse vector learning algorithm and applied in biology science. Saudi J. Biol. Sci. 2017;24(1):132–138. doi: 10.1016/j.sjbs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S., Li L., Wang L., Jiang T., Peng W.X. Understanding the Bioconversion of Quercus baronii Wood during the Artificial Cultivation of Lentinus edodes. BioResources. 2016;11(3):7654–7671. [Google Scholar]
- Ge S., Wang L., Ma J., Jiang S., Peng W.X. Biological analysis on extractives of bayberry fresh flesh by GC–MS. Saudi J. Biol. Sci. 2018;36(14):816–818. doi: 10.1016/j.sjbs.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez X., Meredith W., Fernández C., Sánchez-García M., Díez-Antolíne R., Garzón-Santos J., Snape C.E. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: application of Py-GC/MS. Environ. Sci. Pollut. Res. Int. 2018;25(25):25600–25611. doi: 10.1007/s11356-018-2644-4. [DOI] [PubMed] [Google Scholar]
- Granato A.V., Santos A.G., Dos S.E.N. P-Cymene as solvent for olefin metathesis: matching efficiency and sustainability. ChemSusChem. 2017;10(8):1832–1837. doi: 10.1002/cssc.201700116. [DOI] [PubMed] [Google Scholar]
- Guo S., Geng Z., Zhang W., Liang J., Wang C., Deng Z., Du S. The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016;17(11):1836. doi: 10.3390/ijms17111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Cui M., Deng M., Liu X., Huang X., Zhang X., Luo L. Molecular differentiation of five Cinnamomum camphora chemotypes using desorption atmospheric pressure chemical ionization mass spectrometry of raw leaves. Sci. Rep. 2017;7:4–9. doi: 10.1038/srep46579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl M.W., Lakemeyer M., Dahmen M., Glaser M., Pahl A., Lorenz-Baath K., Menzel T., Sievers S., Böttcher T., Antes I., Waldmann H., Sieber S.A. Phenyl esters are potent inhibitors of Caseinolytic Protease P and reveal a stereogenic switch for deoligomerization. J. Am. Chem. Soc. 2015;137(26):8475–8483. doi: 10.1021/jacs.5b03084. [DOI] [PubMed] [Google Scholar]
- He H., Qin J., Cheng X., Xu K., Teng L., Zhang D. Effects of exogenous 6-BA and NAA on growth and contents of medicinal ingredient of Phellodendron chinense seedlings. Sci. Saudi J Biol. 2018;25(6):1189–1195. doi: 10.1016/j.sjbs.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Li H., Huang S., Wang C., Sun W.J., Mo H.Z., Shi Z.Q., Chen J. Eugenol confers cadmium tolerance via intensifying endogenous hydrogen sulfide signaling in Brassica rapa. J. Agric. Food Chem. 2018;66(38):9914–9922. doi: 10.1021/acs.jafc.8b03098. [DOI] [PubMed] [Google Scholar]
- Jiang H., Wang J., Song L., Cao X., Yao X., Tang F., Yue Y. GC×GC-TOFMS analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora L. Presl and their insecticidal and repellent activities. Molecules. 2016;21(4):423. doi: 10.3390/molecules21040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.R., Fu C.S., Yang W.J., Wang X.L., Feng D., Wang X.N., Ren D.M., Lou H.X., Shen T. Investigation of constituents from Cinnamomum camphora (L.) J. Presl and evaluation of their anti-inflammatory properties in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2018:37–47. doi: 10.1016/j.jep.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Li Z., Han C., Wei D. Empirical research on the relationship between natural gas consumption and economic growth in the northeast asia. Energy Environ. 2018;29(2):216–231. [Google Scholar]
- Lim C.H., Lee Y.H., Kim Y.S., Choi H.S., Seo D.S. Assessment of cyclohexanone toxicity in inhalation-exposed F344 rats and B6C3F1 mice: applications in occupational health. Inhal. Toxicol. 2018;1–8 doi: 10.1080/08958378.2018.1512689. [DOI] [PubMed] [Google Scholar]
- Liu L., Cheng X., Zhao W., Wang Y., Dong X., Chen L., Zhang D., Peng W.X. Systematic characterization of volatile organic components and pyrolyzates from Camellia oleifera seed cake for developing high value-added products. Arab. J. Chem. 2018;11(6):802–814. [Google Scholar]
- Liu X., Wu S., Zhang D., Shen J., Han W., Sun X., Li J., Wang L. Simultaneous pyridine biodegradation and nitrogen removal in an aerobic granular system. J. Environ. Sci. 2018;67:318–329. doi: 10.1016/j.jes.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Lopes D.S.T., Santo R., Reis A., Passarinho P.C. Effect of furfural on Saccharomyces carlsbergensis growth, physiology and ethanol production. Appl. Biochem. Biotechnol. 2017;182(2):708–720. doi: 10.1007/s12010-016-2356-5. [DOI] [PubMed] [Google Scholar]
- Lu J., Lv Y., Ji Y., Tang X., Qi Z., Li L. Resonant absorption induced fast melting studied with mid-IR QCLs. Rev. Sci Instrum. 2017;88(2):023108. doi: 10.1063/1.4975401. [DOI] [PubMed] [Google Scholar]
- Maier G.P., Bernt C.M., Butler A. Catechol oxidation: considerations in the design of wet adhesive materials. Biomater. Sci. 2018;6(2):332–339. doi: 10.1039/c7bm00884h. [DOI] [PubMed] [Google Scholar]
- Ni L., Xin J., Dong H., Lu X., Liu X., Zhang S.A. Simple and mild approach for the synthesis of p-Xylene from bio-based 2,5-dimethyfuran by using metal triflates. Chem. Sus. Chem. 2017;10(11):2394–2401. doi: 10.1002/cssc.201700020. [DOI] [PubMed] [Google Scholar]
- Pardo-Garcia A.I., Wilkinson K.L., Culbert J.A., Lloyd N.D.R., Alonso G.L., Salinas M.R. Accumulation of guaiacol glycoconjugates in fruit, leaves and shoots of Vitis vinifera cv. Monastrell following foliar applications of guaiacol or oak extract to grapevines. Food Chem. 2017;15:217. doi: 10.1016/j.foodchem.2016.08.090. [DOI] [PubMed] [Google Scholar]
- Peng, W., Wang, L.M., Mirzaee, H., Ahmadi, M.J., Esfahani, S., Fremaux., 2017. Hydrogen and syngas production by catalytic biomass gasification. Energy Convers. Manage. vol. 135, pp. 270–273.
- Pinheiro P.F., Menini L.A.P., Bernardes P.C., Saraiva S.H., Carneiro J.W.M., Costa A.V., Arruda T.R., Lage M.R., Gonçalves P.M., Bernardes C.O., Alvarenga E.S., Menini L. Semisynthetic phenol derivatives obtained from natural phenols: antimicrobial activity and molecular properties. J. Agric. Food Chem. 2018;66(1):323–330. doi: 10.1021/acs.jafc.7b04418. [DOI] [PubMed] [Google Scholar]
- Qin J., Wang Y., He G., Chen L., He H., Cheng X., Xu K., Zhang D.Q. High-efficiency Micropropagation of dormant buds in spine base of red pitaya (Hylocereus polyrhizus) for industrial breeding. Int. J. Agric. Biol. 2017;19(1):193–198. [Google Scholar]
- Rahman M.M., Muse A.Y., Khan D.M.I.O., Ahmed I.H., Subhan N., Reza H.M., Alam M.A., Nahar L., Sarker S.D. Apocynin prevented inflammation and oxidative stress in carbon tetra chloride induced hepatic dysfunction in rats. Biomed. Pharmacother. 2017;92:421–428. doi: 10.1016/j.biopha.2017.05.101. [DOI] [PubMed] [Google Scholar]
- Scognamiglio J., Jones L., Letizia C.S., Api A.M. Fragrance material review on 3-methyl-2-(n-pentanyl)-2-cyclopenten-1-one. Food Chem. Toxicol. 2012;50(SI3):S653–S657. doi: 10.1016/j.fct.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Sissener N.H., Ørnsrud R., Sanden M., Frøyland L., Remø S., Lundebye A.K. Erucic Acid (22:1n–9) in fish feed, farmed, and wild fish and seafood products. Nutrients. 2018;10(10):1443. doi: 10.3390/nu10101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara, T., Chinze, I.M., Numano, S., Kitazaki, C., Asayama, M., 2018. Flocculation and pentadecane production of a novel filamentous cyanobacterium Limnothrix sp. strain SK1-2-1. Biotechnol. Lett. vol. 40, 5, pp. 829–836. [DOI] [PubMed]
- Truong L.T.B., Abd E.A.M., Kim H.J., Rahman M.M., Kim S.W., Shin H.C., Shim J.H. Application of a solvent-free solid injection technique coupled with GC-MS for discrimination between the secondary metabolites of wild and cultivated South Korean medicinal foods. Biomed. Chromatogr. 2017;31(6):e3896. doi: 10.1002/bmc.3896. [DOI] [PubMed] [Google Scholar]
- Usha S.P., Gupta B.D. Urinary p-cresol diagnosis using nanocomposite of ZnO/MoS and molecular imprinted polymer on optical fiber based lossy mode resonance sensor. Biosens. Bioelectron. 2018;101:135–145. doi: 10.1016/j.bios.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Wang X., Li G., Shen W. Protective effects of D-Limonene against transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Exp. Ther Med. 2018;15(1):699–706. doi: 10.3892/etm.2017.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., He G., Qin J., Cheng X., He H., Zhang D.Q., Peng W.X. High-efficient extraction of principal medicinal components from fresh Phellodendron bark (cortex phellodendri) Saudi J. Biol. Sci. 2018;25(4):811–815. doi: 10.1016/j.sjbs.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Zhang H., Peng K., Chen L., He H., Huang X., Qin J., Zhang D.Q. Differential gene regulation of lipid synthesis in the developing seeds of two biodiesel tree species, Jatropha and Vernicia. Int. J. Agric. Biol. 2016;18(6):1143–1152. [Google Scholar]
- Zhai Y., Dai Q., Jiang K., Zhu Y., Xu B., Peng C., Wang T., Zeng G. Traffic-related heavy metals uptake by wild plants grow along two main highways in Hunan Province, China: effects of soil factors, accumulation ability, and biological indication potential. Environ. Sci. Pollut. Res. Int. 2016;23(13):13368–13377. doi: 10.1007/s11356-016-6507-6. [DOI] [PubMed] [Google Scholar]
- Zheng H., Jing L., Yao N., Yang Q.S., Peng H.S., Shen Y., Huang L.Q. Cloning and expression analysis of 1-deoxy-D-xylulose-5-phosphate reductoisomerase gene (CcDXR1) in Cinnamomum camphora L.) Presl. Yao Xue Xue Bao. 2016;51(9):1494–1501. [PubMed] [Google Scholar]
- Zheng Y., Wang J., Bai X., Chang Y., Mou J., Song J., Wang M. Improving the acetic acid tolerance and fermetation of Acetobacter pasteurianus by nucleotide excision repair protein UvrA.Appl. Microbiol. Biotechnol. 2018;102(15):6493–6502. doi: 10.1007/s00253-018-9066-6. [DOI] [PubMed] [Google Scholar]