Abstract
This study investigated for the first time the outcome of ingestion of calcium carbide-ripened fruit on some female reproductive parameters. A set of unripe mature bananas ripened with calcium carbide (CCRB) and another set ripened via non-artificial means (NARB) were fed orally to prepubertal female mice for three days using the uterotrophic assay procedure. A distilled water group and oestradiol group (10 mg/kg) were also assigned. Food intake, body weights, vaginal openings and cytology were analysed. Samples of blood, uteri, ovaries and cervices were additionally collected and analysed. Increased serum oestrogen level and uterus weight were detected in the CCRB and oestradiol treated groups. Histopathology showed increased numbers of myometrial cells, presence of secondary follicles and regressing corpus lutea as well as thickened cervix epithelia which were evidence of oestrogenic disruptions. This study has shown that consumption of fruits ripened with calcium carbide negatively alters the female reproductive physiology, accelerates puberty onset and increases serum oestrogen levels. Caution must therefore be exercised by fruit sellers in the use of calcium carbide and policies set in place for strict regulation of its use worldwide.
Keywords: Physiology, Toxicology, Environmental toxicology, Nutrition, Pharmacology, Reproductive system, Banana, Calcium carbide, Female reproduction, Oestrogen, Ovary, Uterus
1. Introduction
Several fruits are very often artificially ripened and banana which is the fruit utilized in this study is one of those fruits. Some fruits like banana are climacteric and when ripe are consumed raw. Banana is one of many fruits that constitute major starchy foods in Sub-Saharan Africa and Asia where it supplies over 25% of their carbohydrate needs [1]. Most fruits are perishable with a short half-life when harvested. This has led to significant post-harvest losses (up to 50% losses) as a result of quality deterioration and handling [2, 3]. Due to the high post-harvest losses, commercial farmers harvest fruits when green but mature. The harvested fruits are then artificially ripened when needed (usually at the markets prior to retailing) with the aid of artificial ripening agents [4, 5]. The process from storage to transporting of fruits from the farm to retailers can take several days. During this time and process, the fruits may become over ripened, inedible and lack appeal to consumers. Some of the fruits can also get damaged or even destroyed during transportation. All of these result in economic losses for the fruit sellers and farmers which causes the fruit sellers to induce artificial ripening before retailing [6].
Several types of artificial ripening agents are in use. Wholesalers in some countries have been reported to sell fruits ripened with calcium carbide and treated with formalin to avoid financial losses [7]. While some post-harvest ripening chemicals are legalized for use in some countries, there are established allowable limits [8]. A very small concentration (1 ppm) of ethylene in air is sufficient to promote the fruit ripening process [9]. In resource poor settings, the expensive cost of acquiring these agents has made the sellers resort to the use of cheaply acquired calcium carbide which is also an easily accessible artificial ripening agent, to activate the fruit ripening process [2].
The use of calcium carbide in fruit ripening is growing rapidly in different parts of the world [10]. It is usually employed in different ways. The calcium carbide powder may be kept in the same vicinity as the fruits such that when in contact with moisture, acetylene gas is released to induce ripening. In other instances, calcium carbide is applied directly on the fruits to induce ripening [8]. It has also been reported that most of the ripening agents utilized by the fruit-sellers are of industrial grade, and are often collected from unauthorized sources [8]. Use of calcium carbide have been reported to impact ripened colour on immature fruits and promote an increase in the shelf life. It can also maintain the ripened colour for an extended period of time [11]. The optimum dose of calcium carbide required to induce ripening of mangoes for instance to achieve overall acceptability was found to be 1 g/kg fruit. At this level of calcium carbide, no difference was observed in the total soluble solids content, titratable acidity and taste between artificially and naturally ripened fruits [12].
Calcium carbide of industrial grade has been reported to contain traces of arsenic and phosphorus hydride, which are harmful to health [11]. Calcium carbide is an alkaline compound reported to irritate the stomach lining on ingestion [13] and have potential carcinogenic and neurological actions [7]. There have been concerns about the harmful effects of environmental agents which exert oestrogenic actions on reproduction. These agents do not affect adult reproduction alone but also affect reproductive organs and systems that mature by regulation of the gonadal hormones [14]. Therefore the effects of these environmental agents such as artificial ripening agents on pubertal maturation as well as overall endocrine disruption is also of concern both for immediate and long term harmful effects. Environmental agents (such as artificial ripening agents) have been reported to play a role in accelerated and delayed pubertal maturation of both males and females [15, 16]. These agents are often referred to as endocrine disruptors which act through interference with the hormonal balance in the body by binding strongly to oestrogen and androgen receptors [17] and act as agonists or antagonists.
While studies have been done to evaluate toxicological effects associated with calcium carbide, effects on the female reproductive system is yet to be studied and characterized. Food safety is an important and growing challenge. This study therefore compares the effect of consumption of calcium-carbide ripened fruit with naturally ripened fruit (banana was used in this study) on the female reproductive system.
2. Material and methods
2.1. Fruit collection and preparation
Unripe but mature bunches of banana (2) were purchased from the local market near the University of Benin premises in Benin City Nigeria. One bunch was placed in a clean polythene bag together with wrapped calcium carbide (10 g) and kept in dry cupboard. The amount of calcium carbide used and the process were determined from interaction with the retail fruit sellers. The second bunch of bananas was similarly placed in a polythene bag in a separate cupboard but without calcium carbide. The fruits were monitored daily for signs of ripening indicated by colour changes in the peel [4]. The ripening stage was assessed using an industrial ripening scale occurring from 1-7. When the banana finger is hard and completely green it is ascribed scale 1; when green but has some traces of yellow it is ascribed scale 2; when there are more yellow colour but still more green than yellow it is ascribed scale 3; when there are more yellow than green it is ascribed scale 4; when there occurs more yellow but with traces of green it is ascribed scale 5; when the banana finger has a completely yellow colour it is ascribed scale 6 and when it is yellow but with black spots it is ascribed scale 7 [18].
2.2. Experimental protocol
A scale of 6 was considered ripe for this study. The ripe bananas were then mashed into a smooth paste with the aid of a clean laboratory mortar and pestle. This was done separately for each of the calcium carbide ripened bananas (CCRB) and the non-artificially ripened bananas (NARB). The resulting paste was constituted in distilled water and doses of 1, 10 and 100 mg/kg were obtained.
2.3. Animals
Virgin immature albino female mice (5–9 g) were utilized in this study. The young animals were purchased from Benin City, Edo State and maintained at the Animal Unit of the Department of Pharmacology & Toxicology, Faculty of Pharmacy, University of Benin, Nigeria. The animals were acclimatized to the laboratory environment for one week prior to the start of the experiment when they were exactly18 d. They were housed in cages at an environmentally controlled room temperature of 27 ± 4 °C and natural lighting conditions. Ethical consent was obtained prior to start of the experiments from the Faculty of Pharmacy Ethics Committee, University of Benin, Nigeria (EC/FP/018/18). The animals were handled as much as possible according to standards of the Public Health Service policy on humane care and use of laboratory animals [19, 20]. Animals were maintained on standard diet of animal pellets and clean tap water provided ad libitum.
2.4. Uterotrophic assay
Immature female mice (5–9 g), 18-d old and without evidence of any disease or physical abnormalities were utilized [21] and placed in experimental groups. The experimental groups included the CCRB group, the NARB group, the control distilled water group and the oestradiol group. The CCRB and NARB groups were further subdivided into 3 subgroups according to the doses of mashed banana administered which included doses of 1, 10 and 100 mg/kg. The control group received 0.2 mL of distilled water and was considered the vehicle control group; the oestradiol group (which was the positive control group) received 17α-ethinyloestradiol (10 mg/kg p.o.). Water was supplied from stainless steel containers only. The banana paste and drug doses were administered orally with the aid of a feeding syringe at 9–10 a.m. daily for three consecutive days [21]. All animals were observed for mortality, morbidity, and general clinical signs such as changes in behaviour, occurrence of secretions and excretions. Day 1 was the day administration began and day 4 was the day after the last administration and was also the day the animals were euthanized.
2.5. Body weight and food consumption measurement
Body weights were measured prior to treatment and thereafter was observed daily and on the day after treatment. The amount of food consumed during the treatment period were measured per cage of 6 animals each by weighing the feeders. The food consumption results were expressed in grams per cage of 6 animals per day.
2.6. Measurement of vaginal opening
The animals were observed for vaginal opening daily during the course of treatment. This has been reported to occur in mice from 35 days of age under the influence of oestrogen [22]. The percentage of vaginal opening was calculated using the formula [22]:
Animals showing complete canalization and patency (vaginal opening) and those that did not were identified. Increase in vaginal opening was regarded as an indication of oestrogenic activity.
2.7. Vaginal smear collection and observation
In groups where vaginal opening was observed, vaginal smears were obtained via lavage in the morning by 9.30 am prior to fruit or drug administration. Sterile saline (0.1 mL) was carefully expelled into the vaginal opening and re-drawn up into the pipette tip [23]. The fluid was then expelled onto a clean glass slide and allowed to evaporate at room temperature. After lavage fluid had evaporated, cells were fixed with ethanol for 5 min and stained with gentian violet which was gently rinsed off and allowed to dry. Cell morphology was observed and noted at 40× magnification.
2.8. Blood sample collection and measurement
Blood samples were collected under anaesthesia (diethyl ether - inhalation) via cardiac puncture prior to organ isolation. The blood samples obtained were placed in lithium-heparin sample bottles for serum oestrogen levels assessment.
2.9. Uterus, ovary and cervix isolation
Twenty-four hours after the last treatment, the mice were humanely killed first via diethyl ether anaesthesia inhalation and then careful exsanguination. The whole uterus was isolated carefully but rapidly to avoid desiccation [24], excess fat and connective tissue were also removed and the whole uterus weighed. The ovaries were then removed at the oviduct to avoid luminal fluid loss from the uterine horns. Each uterus was transferred to a pre-weighed container with saline dampened filter paper. The uterus with luminal fluid was weighed (wet uterine weight). The uterine horns were then cut longitudinally, placed on lightly moistened filter paper and gently pressed with a second piece of lightly moistened filter paper to remove the luminal fluid. The uterus, without the luminal contents was also weighed (blotted uterine weight). After weighing, the uterus, ovary, and cervix were fixed in 10% formal-saline for histopathologic examination after Haematoxylin & Eosin (H&E)-staining.
2.10. Histopathological analysis
The isolated organs were kept in 10% neutral buffer formalin, but the uterus was kept separately in Bouin's fluid and were all submitted for histopathology [25]. The organs were subsequently cut into short segments using the paraffin technique as described [26]. Briefly, sections of 5 μm thicknesses were cut and stained using routine haematoxylin and eosin method. All organs were observed and measured on haematoxylin and eosin stained slides, and 3 randomly chosen areas of the sections were measured per slide. The fixed tissue sections were processed for histopathological examination. The tissue sections were washed in tap water for 30 min, and later dehydrated in graded changes of equal volumes of chloroform; xylene mixture and cleared in two changes of pure xylene. The sections were impregnated in two changes of molten paraffin wax at 60 °C to remove the clearing agents, and embedded in the molten paraffin enblocked in a mould. The blocks were allowed to solidify. Solid blocks of tissues in paraffin wax were sectioned to the required thickness of 5 μm, using microtome (Behr Manning Troy, N.Y). The embedded specimens were cut into thin paraffin ribbons and smeared on the slide and stained with haematoxylin (Sigma, U.S.A) and eosin (Sigma, USA) following a standard staining procedure [27]. The prepared slides and processed specimens on the slides were examined with an Olympus optical microscope (Germany). Photomicrographs of the tissues were captured with a digital camera, 14 mega pixels attached to the microscope and connected to a computer by a USB cord.
2.11. Data analysis
Data are represented either as the mean ± standard error of mean (S.E.M.) or as standalone measurements without mean computations in cases where the animals were simply counted or data represented a collective output of a group. The student t-test or one-way analysis of variance (one factor ANOVA) with Tukey multiple comparison post hoc testing for group differences was employed where appropriate. Differences were considered to be significant when p-values were less than 0.05.
3. Results
3.1. Fruit ripening
The calcium carbide ripened fruit took 2 days to get ripe while the non-artificially ripened fruit took 4 days to get ripe under the same conditions.
3.2. Behavioural changes
No change in behaviour was observed in the animals during the period of administration.
3.3. Body weight analysis
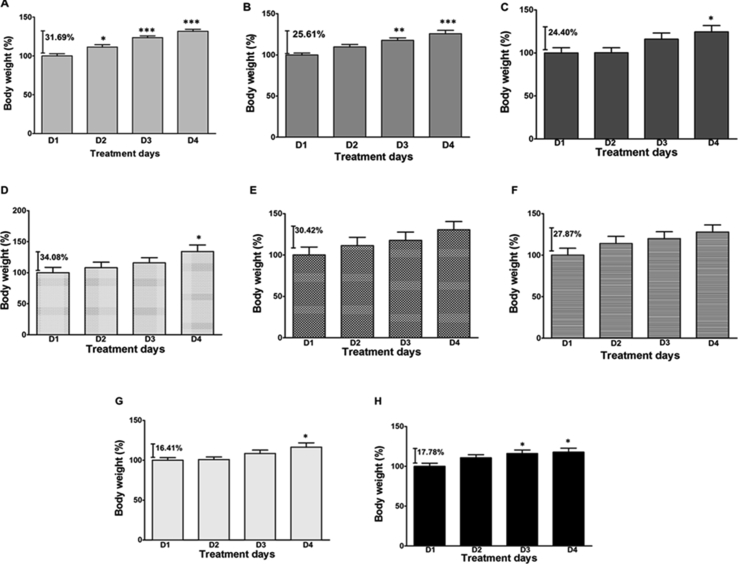
From the body weight assessment, the CCRB groups experienced daily increases. CCRB 1 mg/kg group was significantly increased (p < 0.05; p < 0.01; F = 25.71; df total = 23) compared to their body weights on day 1; CCRB 10 mg/kg group was significantly increased (p < 0.01; p < 0.001; F = 11.84; df total = 23) compared to their body weights on day 1; CCRB 100 mg/kg group was significantly increased (p < 0.05; F = 3.33; df total = 23) compared to their body weights on day 1 (Fig. 1A). CCRB at 1 and 10 mg/kg caused more significant increases (p < 0.01; F = 25.71 for CCRB 1 mg/kg and p < 0.01; F = 11.84 for CCRB 10 mg/kg; df total = 23) in body weights than the oestradiol group at the dose used in this study (Fig. 1A). The NARB groups however experienced a more steady change in body weight throughout the course of the study slightly similar to what was observed in the distilled water group (p < 0.05; F = 3.54; df total = 23) (Fig. 1). The NARB group showed a significant increase in body weight (p < 0.05; F = 2.58; df total = 23) after 3 d of administration as seen with the distilled water group (Fig. 1D–F)). The oestradiol group began showing a significant increase (p < 0.05; F = 3.42; df total = 23) in body weight from the day 3 of administration (Fig. 1). Overall, the CCRB group experienced greater percentage increase in body weight and this was followed closely by the NARB group. The oestradiol group gave the least percentage increase in body weight and was followed closely by the distilled water group (Fig. 1).
Fig. 1.
Bar graph showing the body weights of animals treated with CCRB, NARB, oestradiol and water. A general increase in body weights were observed in all groups in the course of the study. CCRB groups (A-1 mg/kg; B – 10 mg/kg; C-100 mg/kg) showed greater percentage increase in body weights and was followed closely by NARB groups (D-1 mg/kg; E -10 mg/kg; F – 100 mg/kg), G- Distilled water group and E – Oestradiol group, 10 mg/kg. *p < 0.05; **p < 0.01; ***p < 0.001 compared to D1 of the study. n = 6 animals.
3.4. Food intake analysis
The animals’ food intake per group was found to remain reasonably steady in the distilled water group throughout the period of study (Table 1). CCRB 1 mg/kg showed an increase on day 3 which dropped by about 1 g on day 4 but the food intake on day 4 was still higher than on day 2. CCRB 10 and 100 mg/kg showed steady increases from day 2 to day 4. NARB 1 mg/kg increased on day 3 and had a slight decrease on day 4 by about 1 g. NARB 10 mg/kg similarly increased on day 3 and dropped slightly by about 0.3 g on day 4. NARB 100 mg/kg however showed a decrease in food intake from day 2 to day 4. An overall increase in food intake was observed in both the CCRB and NARB groups except for NARB 100 mg/kg (Table 1) with CCRB groups showing a greater overall increase in food intake (Table 1). The oestradiol group was also observed to exhibit an increase in food intake on day 3 which dropped on day 4 (Table 1).
Table 1.
Table showing food intake analysis during administration of calcium carbide and non-artificially ripened banana.
| Groups | Food intake (g) |
||
|---|---|---|---|
| Day 2 | Day 3 | Day 4 | |
| Distilled water | 6.23 | 6.10 | 6.20 |
| CCRB 1 mg/kg | 5.00 | 7.60 | 6.62 |
| CCRB 10 mg/kg | 4.55 | 5.20 | 6.35 |
| CCRB 100 mg/kg | 4.30 | 4.21 | 5.23 |
| NARB 1 mg/kg | 5.25 | 6.22 | 7.20 |
| NARB 10 mg/kg | 4.30 | 6.50 | 6.20 |
| NARB 100 mg/kg | 6.00 | 5.10 | 4.11 |
| Oestradiol 10 mg/kg | 8.32 | 9.20 | 7.10 |
n = 6 animals.
3.5. Vaginal opening analysis
Animals in the distilled water and NARB group experienced no vaginal opening during the study (Table 2). However, 4 animals from the CCRB 1 mg/kg exhibited opening after 2 days of administration with all 6 animals showing opening after 3 days of administration (Table 2). Similarly, 5 animals from the oestradiol group showed opening after 2 days of administration while all 6 animals showed opening after 3 days of administration (Table 2).
Table 2.
Percentage of mice with vaginal opening for calcium carbide and non-artificially ripened banana.
| Groups | Percentage of animals with vaginal opening (%) |
|||
|---|---|---|---|---|
| Day 1/18 D old | Day 2/19 D old | Day 3/20 D old | Day 4/21 D old | |
| Control distilled water | 0.00 | 0.00 | 0.00 | 0.00 |
| CCRB 1 mg/kg | 0.00 | 0.00 | 66.67 | 100.00 |
| CCRB 10 mg/kg | 0.00 | 0.00 | 0.00 | 0.00 |
| CCRB 100 mg/kg | 0.00 | 0.00 | 0.00 | 0.00 |
| NARB 1 mg/kg | 0.00 | 0.00 | 0.00 | 0.00 |
| NARB 10 mg/kg | 0.00 | 0.00 | 0.00 | 0.00 |
| NARB 100 mg/kg | 0.00 | 0.00 | 0.00 | 0.00 |
| Oestradiol 10 mg/kg | 0.00 | 0.00 | 83.33 | 100.00 |
n = 6 animals.
3.6. Vaginal cytology analysis
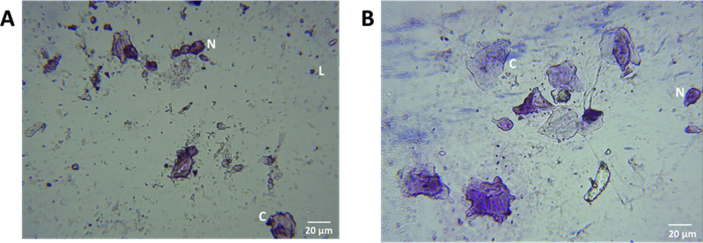
Only animals treated with oestradiol (Fig. 2A) and CCRB 1 mg/kg (Fig. 2B) showed vaginal opening on days 3 and 4 and allowed for collection of vaginal cells on day 4. Assessment of cells taken from both groups showed a dominance of nucleated epithelial cells, few leukocytes and few cornified squamous epithelial cells (Fig. 2).
Fig. 2.
Representative vaginal cytology of CCRB 1 mg/mL (A) and oestradiol treated groups (B) at x 400 magnification. Nucleated epithelial cells (N) and cornified cells (C) are observed in both groups with few leukocytes (L).
3.7. Serum oestrogen analysis
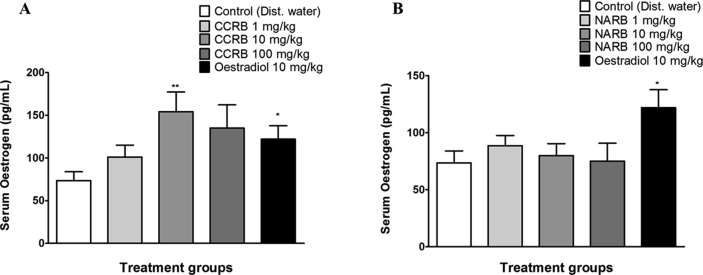
The serum oestrogen level in the CCRB treated groups showed an increase with the CCRB 10 mg/kg group showing a significant increase (p < 0.01; F = 4.88; df = 10) compared to the distilled water group (Fig. 3A). This was also similarly observed with the oestradiol group which showed a significant increase (p < 0.05; F = 2.31; df = 10) in serum oestrogen compared to the distilled water group (Fig. 3A). For the NARB group there was no significant difference in the serum oestrogen levels compared to the distilled water group (Fig. 3B).
Fig. 3.
Bar graphs showing the serum oestrogen levels of CCRB, NARB, oestradiol and water treated groups. CCRB (A) and oestradiol treated groups showed an increase in serum oestrogen levels. CCRB 100 mg/kg showed a significant increase (p < 0.01) while oestradiol showed a significant increase (p < 0.05) compared to the distilled water group. No significant change was observed with the NARB treated groups (B) while oestradiol showed a significant increase (p < 0.05) compared to the distilled water group. *p < 0.05; **p < 0.01 compared to the control; n = 6 animals. Dist. water = distilled water.
3.8. Organ weight analysis
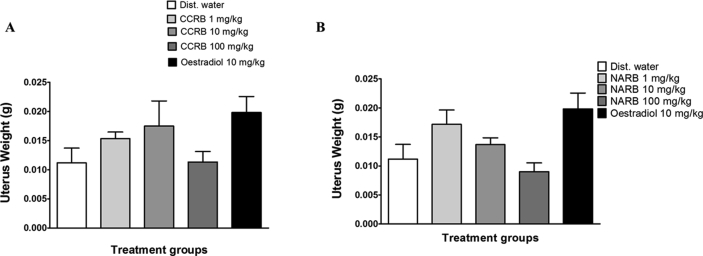
An increase in uterine weight was observed in the CCRB treated animals compared to the distilled water group (Fig. 4A). However, it was not statistically significant. Though a non-significant moderate increase was observed in uterine weights of NARB 1 mg/kg, NARB 10 mg/kg did not exhibit a change in uterine weight compared to the distilled water group while NARB 100 mg/kg exhibited a slight decrease in uterine weight (Fig. 4B). The oestradiol treated groups showed an increase in uterine weights similar to that observed with the CCRB treated groups (Fig. 4).
Fig. 4.
Bar graphs showing the uterus weights of CCRB (A) and NARB (B) treated groups. CCRB and oestradiol treated groups showed a non-significant increase in uterine weights compared to the distilled water group; while the NARB showed a decrease in weights of uterus except for NARB 1 mg/kg where a non-significant increase was observed. n = 6 animals. Dist. Water = distilled water.
3.9. Histological analysis
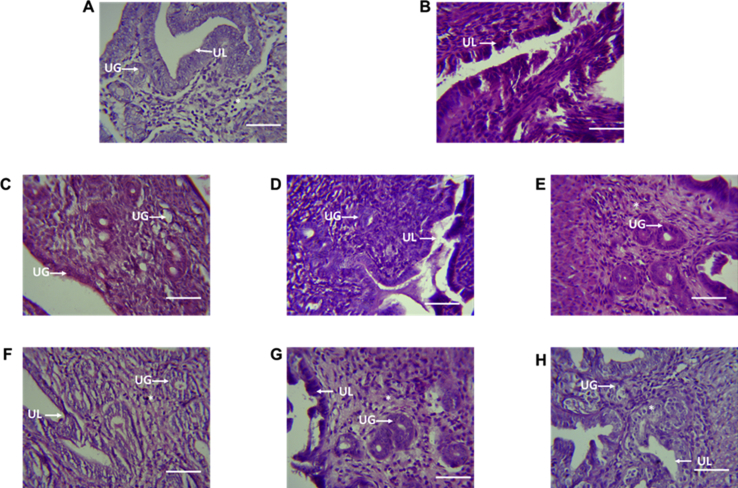
Histopathological analysis of the uterus revealed congestion of the lumen epithelium in the CCRB and oestradiol treated group (Fig. 5). Narrowing of the uterine lumen was also observed in the CCRB and oestradiol treated groups (Fig. 5). Developing uterine glands were seen in the NARB treated groups (Fig. 5) but they are mostly immature and quiescent. Normal differentiation of myometrial cells and well defined uterine lumen were observed in both the NARB and distilled water treated groups (Fig. 5).
Fig. 5.
Uterus (mouse, H & E ×400). Representative histological features of the uterus from CCRB, NARB, oestradiol and distilled water treated mice. (A) Mice administered water showed immature quiescent uterus with developing lumen and glands (UG) while oestradiol treated mice (B) showed distorted luminal epithelium. CCRB treated mice 1 mg/kg (C), 10 mg/kg (D) and 100 mg/kg (E) showed congestion of the lumen (UL). NARB treated mice 1 mg/kg (F), 10 mg/kg (G) and 100 mg/kg (H) showed largely normal quiescent uterus. Scale represents 20 μm.
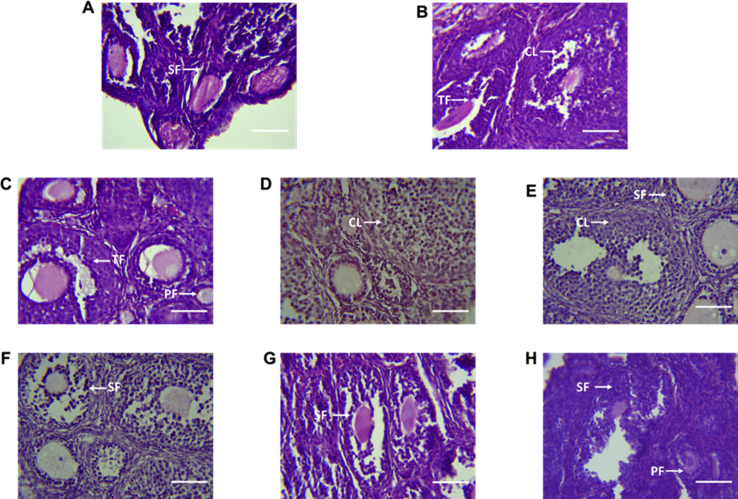
Histopathological analysis of the ovaries revealed the presence of mainly tertiary and secondary follicles in the CCRB and oestradiol treated groups (Fig. 6). Regressing corpus lutea and dense granulosa cells were also observed in the CCRB and oestradiol treated groups (Fig. 6). On the other hand, mainly primary follicles and few secondary follicles were observed in the NARB and distilled water treated groups (Fig. 6).
Fig. 6.
Ovary (mouse, H & E ×400). Representative histological features of the ovary from CCRB, NARB, oestradiol and distilled water treated mice. Mice administered water (A) showed presence of secondary follicles (SF) and primordial follicles (not shown). Oestradiol treated animals (B) presence of tertiary follicles (TF) and mature corpora lutea (CL). CCRB treated mice 1 mg/kg (C), 10 mg/kg (D) and 100 mg/kg (E) showed both tertiary (TF) and secondary follicles (SF). Mature and regressing corpus luteum (CL) can also be observed in the CCRB groups. NARB treated mice 1 mg/kg (E), 10 mg/kg (F) and 100 mg/kg (G) showed mainly secondary follicles (SF) and primary follicles (pf). Scale represents 20 μm.
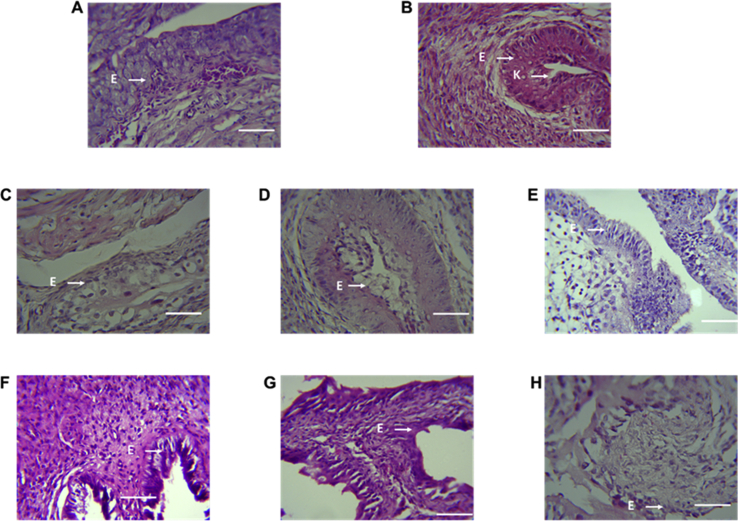
Analysis of the cervix revealed the presence of keratin and several layers of epithelia in the CCRB and oestradiol treated groups (Fig. 7) while the epithelium in the NARB and distilled water treated groups were mostly immature and composed of plump to cuboidal cells (Fig. 7). Cells with dysplastic changes were observed in the CCRB 100 mg/kg group (Fig. 7).
Fig. 7.
Cervix (mouse, H & E ×400). Representative histological features of the cervix from CCRB, NARB, oestradiol and distilled water treated mice. Mice administered water (A) showed histologically immature and quiescent cervix with plump and cuboidal epithelial cells in stratum germinativum. Oestradiol treated animals showed abundant luminal keratin characteristic of oestrus (B). CCRB treated mice 1 mg/kg (C), 10 mg/kg (D) and 100 mg/kg (E) showed the stratum germinativum, stratum granulosum, and stratum corneum with small numbers of luminal luminal keratin consistent with oestrus. NARB treated mice 1 mg/kg (F), 10 mg/kg (G) and 100 mg/kg (H) normal quiescent cervical epithelium. Scale represents 20 μm.
4. Discussion
The uterotrophic assay utilized in this study was developed to screen for estrogenic compounds. It is based on the ability of estrogenic compounds to increase uterine weight also known as a uterotrophic response [28]. For the uterotrophic response, oestrogen causes water imbibition in the uterus and results in an initial increase in weight followed by weight gain due to tissue growth [29]. Agonists and antagonists of oestrogen, function as ligands to oestrogen receptors α and β where they may activate or inhibit, respectively, the receptors’ transcriptional action. However, this may potentially result in adverse health hazards, such as reproductive and developmental effects [30].
The current study utilized immature animals with intact hypothalamic-pituitary-gonadal (HPG) axis and will therefore show the contribution of the HPG axis as well as the contribution of the oestrogen receptor to the fruit and drugs under study. As females approach puberty, they undergo silent cycles that do not lead to vaginal opening or ovulation. However, when a chemical or diet stimulates the HPG axis at about the prepubertal age it would result in precocious puberty and early ovulation leading to vaginal opening [21].
In this study, the fruit ripened with calcium carbide took a shorter period for complete ripening compared to fruits with no artificial ripening agent. This confirms its use by fruit sellers to hasten ripening in fruits. The observation that body weights were much more increased in the CCRB and NARB groups compared to other groups may have resulted from the added banana meal fed to these set of animals and may not be directly related to hormonal influence. A high fat or energy dense diet can induce a state of increased energy which can lead to increased body weight [31] which may explain the increased body weight observed in animals that received the fruit banana in this study. Notably, the CCRB animals displayed the largest weight gain compared to the NARB animals suggesting that an additional component besides just being an energy dense fruit was responsible for the weight gain in the CCRB groups. The increased weight gain in the CCRB group may have been as a result of the increased food intake observed in the CCRB group which had one of the largest food intake in the animals under study. The oestradiol group showed the highest food intake and was closely followed by the CCRB group, though the percentage body weight increase in the CCRB group was higher than the oestradiol group. Oestradiol is generally known to cause a reduction in food intake and consequently a reduction in weight [32, 33, 34]. Recent evidence however suggests that there may be some instances where oestradiol increased food intake and weight gain [35]. It would seem that in such instance, a disruption in ovarian cyclicity occurs with oestrogen which therefore leads to an increase in food intake and body weight [36]. Further experiments are however required to confirm this possibility. Nonetheless, it can be inferred that oestradiol administration in this study disrupted ovarian cycling leading to increases in food intake and body weight. The possible presence of oestradiol in CCRB may have also accounted for the larger increase in food intake and body weight seen with the CCRB group over and beyond what was observed in the NARB group. The food intake and body weight pattern observed in the distilled water group reflected the normal growth and meal pattern that occurs in the animals in the absence of regulating factors.
The incidence of vaginal opening was significantly increased at CCRB 1 mg/kg and on oestradiol administration also suggesting the oestrogenic potential of CCRB. Though oestradiol showed a greater incidence of accelerating vaginal opening in this study than CCRB. In rodents and animals, it has been reported that puberty occurs due to sensitivity of the central gonadotropin releasing hormone (GnRH) pulse generator to inhibition by circulating gonadal steroids principally 17β-oestradiol [37]. It is also reported that oestradiol exerts positive neurotropic effects which contribute to the maturation of the GnRH pulse generator during puberty [38]. Exposure of prepubertal animals to oestrogen has been shown to accelerate vaginal opening [39] supporting the oestrogenic potential of CCRB. Since the rupture of the vaginal membrane is sensitive to oestradiol levels, vaginal opening can be used as an index for puberty onset determination and achievement of reproductive competence in rodents as seen in this study. For animals with vaginal opening, cytology showed the animals to be in the pro-oestrus stage of the oestrus cycle which is under the influence of rising oestrogen [23]. This finding also supports the oestrogenic potential of CCRB. As further support to the oestrogenic potential of CCRB, an increase in serum oestrogen levels was observed in animals administered CCRB as opposed to animals fed with NARB and those administered water only. This increase in oestrogen correlates with the pro-oestrus stage in the CCRB animals. The increase in uterine wet weight seen in CCRB fed animals also supports the oestrogenic potential of CCRB. The uterotrophic assay is traditionally validated and used to establish the oestrogenic activity of sex steroids and suspected environmental oestrogens [40, 41]. The present study therefore reveals that CCRB is able to induce uterotrophic effects, also known as, increase in wet weight of the uterus, though the effect was more pronounced at the median dose used 10 mg/kg. NARB on the other hand did not produce uterotrophic effects. The water imbibition induced by oestrogen results in a prompt increase in uterine wet weight. The mechanisms that themselves lead to water imbibition are poorly understood however, the physiologic processes are well described and include regulation of inflammation and congestion in several cell types (endothelial, stromal, and endometrial cells) within the uterus [42]. It has also been reported that the vascular endothelial growth factor (VEGF - a signalling factor) contributes to the regulation of uterine fluid accumulation [43, 44]. All of these occur through the classical oestrogen signalling via interaction with the oestrogen nuclear receptors.
Under the influence of oestrogens, the area of the epithelium within the uterus is increased [22] and endometrial proliferation is induced [45]. This was evident in the histological uterus tissue assessment of the CCRB and oestradiol treated groups. VEGF is primarily expressed in the luminal epithelium of the murine uterus [44]. During the late phase of the oestrous cycle, the response to VEGF peaks after 24–72 h and includes a sequence of epithelial cell proliferation and differentiation [46] under the influence of oestrogen. Uterine epithelial cell proliferation is regulated by the D-type cyclins and the cyclin-dependent kinases [47]. In the uterus, both oestradiol have been shown to mobilize cyclin D1 from the cytoplasm to the nucleus prior to epithelial cell proliferation [47]. The epithelial layer transforms into columnar secretory cells with abundant mitosis [48]. The later phase also involves the induction of target genes, such as lactoferrin [49]. In the absence of oestrogen or responsiveness to oestrogens, the animals will fail to produce a proliferative epithelial response [49]. These actions may therefore contribute to the epithelial proliferative effect detected with CCRB in the present study and can also explain the lack of uterotrophic effect seen with NARB treated groups.
Further histologic observations of the reproductive organs showed the presence of regressing corpus lutea and secondary follicles in the ovaries of CCRB and oestradiol treated groups. In follicular development, primordial follicles are dominant in the prepubertal stage. After the formation of primordial follicles they are activated via the phosphatidylinositol 3-kinase pathway and transition to primary follicles. A process associated with morphological and proliferative changes in the granulosa cells and growth of the oocyte. Primordial follicles are dominant in the prepubertal stage with increase in primary follicles occurring as puberty approaches and at puberty [50, 51]. Further proliferation of granulosa cells results in multilaminar secondary follicles [50]. On stimulation by follicle-stimulating hormone, the granulosa cells undergo rapid proliferation coincident with reorganization around the antrum, a fluid-filled space. These differentiation and proliferative changes signal the final growth stage prior to ovulation. Follicles at each of these later stages (primary and secondary follicular stages) that are not selected for further growth are then scheduled for atresia [52, 53]. The presence of developing corpora lutea in the NARB groups is indicative of sexual maturation [54]. The corpus luteum is a transient endocrine gland formed by residual granulosa theca cells. The corpus luteum (CL) is formed following ovulation, though the real stimulus for luteinisation is the preovulatory luteinizing hormone (LH) surge from the hypophysis [55]. Preovulatory surge of LH from the pituitary gland induces the activation of LH receptor (LH-R) on the follicular cells which is necessary for ovulation to occur. Simultaneously, LH induces the transformation of ovulated follicle cells into the CL, a process known as luteinisation [56]. The steroidogenic cells of CL, regardless of their cellular origin, provide the required progesterone levels to initiate uterine quiescence and glandularization in preparation and establishment of pregnancy [57].
The histology of the cervix corresponds with the changes observed in the uterus and ovaries. The cervix of the NARB and distilled water group appeared largely immature with that of the CCRB and oestradiol group showing signs of epithelial differentiation into the different cell layers. Epithelial differentiation in the cervix and vagina have been reported to occur at and after puberty and coincides with the formation and development of the CL in the ovaries [58].
Interestingly, the oestrogenic effects of CCRB were more pronounced in the 1 and 10 mg/kg groups and not in the 100 mg/kg CCRB group suggesting an inverted-dose relationship in this study. Central oestrogen receptor specifically, ERβ has been shown to be involved in early puberty onset with the involvement of Kiss1 expression [59]. Early activation of ERβ by oestradiol in prepubertal animals with intact HPG leads to increased expression of Kiss1 which then leads to early release of LH which mediates early onset of puberty [60]. However ERα is also present but develops much later in prepubertal animals and it is suggested to act in an opposing fashion to ERβ [61, 62, 63]. The ratio of both oestrogen receptors is therefore important in regulating the signalling activity of oestrogen. The presence of ERβ in prepubertal mice also reduces the number of gonadotropin releasing hormone (GnRH) neurons present. It is therefore possible that higher concentrations of oestrogen in prepubertal animals will reduce or blunt the responsiveness of GnRH neurons and oestrogen receptors and this may be observed as a reduced activity and lower response compared to lower concentrations of oestrogen. This is also a function of the negative feedback mechanism in place with the GnRH neurons and can contribute to the oestrogenic activity seemingly more prominent at the lower doses in this study. Higher doses of oestradiol are generally reported to suppress GnRH and LH secretion [64]. It is unclear at this the time the exact concentrations of oestrogens that may have been present in the different quantities of banana fruit utilized in this study. However, the content of oestradiol consumed by the animals would have increased with increasing quantities of banana fruit administered. This increase may therefore have resulted in high concentrations of oestrogen being delivered to the animal's body and consequently a blunting of oestrogenic response characterized by the lack of vaginal opening and the increased concentrations of oestrogen measured in serum in the CCRB groups.
5. Conclusions
This study has shown that consumption of fruits ripened with calcium carbide alters the reproductive physiology in female mice. This was clearly observed in the onset of puberty acceleration observed in immature mice fed with CCRB. Fruits ripened with calcium carbide have also been shown in this study to have oestrogenic potential observed in the increased levels of serum oestrogen and the hypertrophy of the uterus typical of oestrogen agonists. It can be inferred from this study that calcium carbide ripened fruits act by interaction with nuclear oestrogenic receptors which are primarily responsible for the uterotrophic effect observed in this study. We therefore suggest that fruits ripened with calcium carbide produce oestrogen agonistic activities via interaction with oestrogenic receptors as a key event in the female reproductive system and can lead to key relationships such as downstream activation of Kiss1 expression and GnRH modulation. Further molecular studies are needed to confirm this. Nonetheless, caution is advised to fruit sellers utilizing calcium carbide for fruit ripening and also to consumers of calcium carbide ripened fruits. Future studies on effect of calcium carbide fruits on fertility and on development of reproductive tumours are suggested.
Declarations
Author contribution statement
Enitome E. Bafor: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Emmanuella Greg-Egor: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Osemelomen Omoruyi, Ejiroghene Ochoyama, Glory U. Omogiade: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge Mr. Kadiri for his technical assistance and Miss Faith Ukpebor for her assistance during this study.
References
- 1.Adeniji T., Sanni L., Barimalaa I., Hart A. Nutritional and anti-nutritional composition of flour made from plantain and banana hybrid pulp and peel mixture, Niger. Food J. 2007;25:68–72. [Google Scholar]
- 2.Ajayi A.R., Mbah G.O. Identification of indigenous ripening technologies of banana and plantain fruits among women-marketers in Southeastern Nigeria. J. Agric. Food Environ. Ext. 2007;6:60–66. [Google Scholar]
- 3.Zewter A., Woldetsadik K., Workneh T.S. Effect of 1-methylcyclopropene, potassium permanganate and packaging on quality of banana. Afr. J. Agric. Res. 2012;7:2425–2437. [Google Scholar]
- 4.Sogo-Temi C.M., Idowu O.A., Idowu E. Effect of biological and chemical ripening agents on the nutritional and chemical ripening agents on the nutritional and metal composition of banana (Musa spp) J. Appl. Sci. Environ. Manag. 2014;18:243–246. [Google Scholar]
- 5.Singal S., Kumud M., Thakral S. Application of apple as ripening agent for banana. Indian J. Nat. Prod. Resour. 2012;3:61–64. [Google Scholar]
- 6.Mursalat M., Rony A.H., Hasnat A., Rahman Z., Islam M.,M.N., Khan M.S. A critical analysis of artificial fruit ripening: scientific, legislative and socio-economic aspects. Chem. Eng. Sci. Mag. 2013;4:6–12. [Google Scholar]
- 7.Fattah S.A., Ali M.Y. Carbide ripened fruits a recent health hazard. Faridpur Med. Coll. J. 2010;5:37. [Google Scholar]
- 8.Hossain M.F., Akhtar S., Anwar M. Health hazards posed by the consumption of artificially ripened fruits in Bangladesh. Int. Food Res. J. 2015;22:1755–1760. [Google Scholar]
- 9.Brady C.J. Fruit ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1987;38:155–178. 38.060187.001103. [Google Scholar]
- 10.Islam G.M.R., Hoque M.M. Food safety regulation in Bangladesh, chemical hazard and some perception to overcome the dilemma. Int. Food Res. J. 2013;20:1. [Google Scholar]
- 11.Ur-Rahman A., Chowdhury F.R., Alam M.B. Artificial ripening: what we are eating. J. Med. 2008;9:42–44. [Google Scholar]
- 12.Amarakoon R., Illeperuma D.C.K., Sarananda K.H. Effect of calcium carbide treatment on ripening and quality of Velleicolomban and Willard mangoes. Trop. Agric. Res. 1999;11:54–60. [Google Scholar]
- 13.Siddiqui W., Dhua R.S. Eating artificially ripened fruits is harmful. Curr. Sci. 2010:1664–1668. [Google Scholar]
- 14.Golub M.S., Hogrefe C.E., Germann S.L., Lasley B.L., Natarajan K., Tarantal A.F. Effects of exogenous estrogenic agents on pubertal growth and reproductive system maturation in female rhesus monkeys. Toxicol. Sci. 2003 doi: 10.1093/toxsci/kfg090. [DOI] [PubMed] [Google Scholar]
- 15.Blanck H.M., Marcus M., Tolbert P.E., Rubin C., Henderson A.K., Hertzberg V.S., Zhang R.H., Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11:641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Den Hond E., Roels H.A., Hoppenbrouwers K., Nawrot T., Thijs L., Vandermeulen C., Winneke G., Vanderschueren D., Staessen J.A. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: sharpe and Skakkebaek’s hypothesis revisited. Environ. Health Perspect. 2002;110:771–776. doi: 10.1289/ehp.02110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabb M.M., Blumberg B. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol. 2006;20:475–482. doi: 10.1210/me.2004-0513. [DOI] [PubMed] [Google Scholar]
- 18.Forster M., Rodríguez E.R., Martín J.D., Romero C.D. Distribution of nutrients in edible banana pulp, Food Technol. Biotechnology. 2003;4:167–171. [Google Scholar]
- 19.National Research Council . Guid. Care Use Lab. Anim. eighth ed. National Academies Press; 2010. Guide for the care and use of laboratory animals; p. 118. [Google Scholar]
- 20.NIH . 2015. Public Health Service Policy on Humane Care and Use of Laboratory Animals.http://grants.nih.gov/grants/olaw/references/PHSPolicyLabAnimals.pdf [Google Scholar]
- 21.OECD . OECD Publishing; Paris: 2007. Test No. 440: Uterotrophic Bioassay in Rodents: A Short-Term Screening Test for Oestrogenic Properties. [Google Scholar]
- 22.Markey C.M., Michaelson C.L., Veson E.C., Sonnenschein C., Soto A.M. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphonel A. Environ. Health Perspect. 2001;109:55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean A.C., Valenzuela N., Fai S., Bennett S.A.L. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. 2012;67:4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thigpen J.E., Li L.A., Richter C.B., Lebetkin E.H., Jameson C.W. The mouse bioassay for the detection of estrogenic activity in rodent diets: II. Comparative estrogenic activity of purified, certified and standard open and closed formula rodent diets. Lab. Anim. Sci. 1987;37:602–605. [PubMed] [Google Scholar]
- 25.Dar S.H., Qureshi S., Palanivelu M., Muthu S., Mehrotra S., Jan M.H., Chaudhary G.R., Kumar H., Saravanan R., Narayanan K. Evaluating a murine model of endometritis using uterine isolates of Escherichia coli from postpartum buffalo, Iran. J. Vet. Res. 2016;17:171–176. [PMC free article] [PubMed] [Google Scholar]
- 26.Kluwe W.M. Renal function tests as indicators of kidney injury in subacute toxicity studies. Toxicol. Appl. Pharmacol. 1981;57:414–424. doi: 10.1016/0041-008x(81)90239-8. [DOI] [PubMed] [Google Scholar]
- 27.Mahé A., Faye O., Thiam N’Diaye H., Ly F., Konaré H., Kéita S., Traoré A.K., Hay R. Definition of an algorithm for the management of common skin diseases at primary health care level in sub-Saharan Africa. Trans. R. Soc. Trop. Med. Hyg. 2005;99:39–47. doi: 10.1016/j.trstmh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Reel J.R., Lamb J.C., IV, Neal B.H. Survey and assessment of mammalian estrogen biological assays for hazard characterization. Fundam. Appl. Toxicol. 1996;34:288–305. doi: 10.1006/faat.1996.0198. [DOI] [PubMed] [Google Scholar]
- 29.Jones R.C., Edgren R.A. The effects of various steroid on the vaginal histology in the rat. Fertil. Steril. 1973;24:284–291. doi: 10.1016/s0015-0282(16)39613-3. [DOI] [PubMed] [Google Scholar]
- 30.Kanno J., Onyon L., Haseman J., Fenner-Crisp P., Ashby J., Owens W. The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 1. Environ. Health Perspect. 2001;109:785–794. doi: 10.1289/ehp.01109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraborty T.R., Donthireddy L., Adhikary D., Chakraborty S. Long-term high fat diet has a profound effect on body weight, hormone levels, and estrous cycle in mice. Med. Sci. Monit. 2016;22:1601–1608. doi: 10.12659/MSM.897628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ter Haar M.B. Circadian and estrual rhythms in food intake in the rat. Horm. Behav. 1972;3:213–220. doi: 10.1016/0018-506x(72)90034-7. [DOI] [PubMed] [Google Scholar]
- 33.Blaustein J.D., Wade G.N. Ovarian influences on the meal patterns of female rats. Physiol. Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 34.Gong E.J., Garrel D., Calloway D.H. Menstrual cycle and voluntary food intake. Am. J. Clin. Nutr. 1989;49:252–259. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- 35.Boswell K.J., Reid L.D., Caffalette C.A., Stitt K.T., Klein L.A., Lacroix A.M., Reid M.L. Estradiol increases consumption of a chocolate cake mix in female rats. Pharmacol. Biochem. Behav. 2006;84:84–93. doi: 10.1016/j.pbb.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Butera P.C., Wojcik D.M., Clough S.J. Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physiol. Behav. 2010;99:142–145. doi: 10.1016/j.physbeh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulin H.E., Grumbach M.M., Kaplan S.L. Changing sensitivity of the pubertal gonadal hypothalamic feedback mechanism in man. Science. 1969;166:1012–1013. doi: 10.1126/science.166.3908.1012. [DOI] [PubMed] [Google Scholar]
- 38.Ojeda S.R., Skinner M.K. Puberty in the rat. In: Neill J.D., editor. Knobil Neill’s Physiol. Reprod. third ed. Elsevier; St. Louis: 2006. pp. 2061–2126. [Google Scholar]
- 39.Navarro V.M., Fernández-Fernández R., Castellano J.M., Roa J., Mayen A., Barreiro M.L., Gaytan F., Aguilar E., Pinilla L., Dieguez C., Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J. Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans J.S., Varney R.F., Koch F.C. The mouse uterine weight method for the assay of estrogens. Endocrinology. 1941;28:747–752. [Google Scholar]
- 41.Gellert R.J., Lewis J., Petra P.H. Neonatal treatment with sex steroids: relationship between the uterotropic. Endocrinology. 1977;100:520–528. doi: 10.1210/endo-100-2-520. [DOI] [PubMed] [Google Scholar]
- 42.Kurita T., Lee K., Saunders P.T., Cooke P.S., a Taylor J., Lubahn D.B., Zhao C., Mäkelä S., a Gustafsson J., Dahiya R., Cunha G.R. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol. Reprod. 2001;64:272–283. doi: 10.1095/biolreprod64.1.272. [DOI] [PubMed] [Google Scholar]
- 43.Rockwell L.C., Pillai S., Olson C.E., Koos R.D. Inhibition of vascular endothelial growth factor/vascular permeability factor action blocks estrogen-induced uterine edema and implantation in rodents. Biol. Reprod. 2002;67:1804–1810. doi: 10.1095/biolreprod.102.006700. [DOI] [PubMed] [Google Scholar]
- 44.Kazi A.A., Jenny J.M., Kood R.D. Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor α and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol. Endocrinol. 2009;19:2006–2019. doi: 10.1210/me.2004-0388. [DOI] [PubMed] [Google Scholar]
- 45.Young S.L. Oestrogen and progesterone action on endometrium: a translational approach to understanding endometrial receptivity. Reprod. Biomed. Online. 2013;27:10. doi: 10.1016/j.rbmo.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couse J.F., Korach K.S. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H., McElrath T., Tong W., Pollard J.W. The molecular basis of tamoxifen induction of mouse uterine epithelial cell proliferation. J. Endocrinol. 2005;184:129–140. doi: 10.1677/joe.1.05987. [DOI] [PubMed] [Google Scholar]
- 48.Pollard J.W., Pacey J., V Cheng S., Jordan E.G. Estrogens and cell death in murine uterine luminal epithelium. Cell Tissue Res. 1987;249:533–540. doi: 10.1007/BF00217324. [DOI] [PubMed] [Google Scholar]
- 49.Couse J.F., Curtis S.W., Washburn T.F., Lindzey J., Golding T.S., Lubahn D.B., Smithies O., Korach K.S. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 50.Tawakal N., Sultana F., Tawakal N., Umar F.K. Morphometric and histological changes in ovaries of prepubertal rats after gamma irradiation. Proc. (Shaikh Zayed Postgrad. Med. Institute) 2010;24:17–23. [Google Scholar]
- 51.Tingen C.M., Bristol-Gould S.K., Kiesewetter S.E., Wellington J.T., Shea L., Woodruff T.K. Prepubertal primordial follicle loss in mice is not due to classical apoptotic Pathways1. Biol. Reprod. 2009;81:16–25. doi: 10.1095/biolreprod.108.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirshfield A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 53.Bristol-Gould S.K., Kreeger P.K., Selkirk C.G., Kilen S.M., Mayo K.E., Shea L.D., Woodruff T.K. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev. Biol. 2006;298:149–154. doi: 10.1016/j.ydbio.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 54.Rosa-E-Silva A., Guimaraes M.A., Padmanabhan V., Lara H.E. Prepubertal administration of estradiol valerate disrupts cyclicity and leads to cystic ovarian morphology during adult life in the rat: role of sympathetic innervation. Endocrinology. 2003;144:4289–4297. doi: 10.1210/en.2003-0146. [DOI] [PubMed] [Google Scholar]
- 55.Richards J.S. Ovulation: new factors that prepare the oocyte for fertilization. Mol. Cell. Endocrinol. 2005;234:75–79. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Russell D.L., Robker R.L. Molecular mechanisms of ovulation: Co-ordination through the cumulus complex. Hum. Reprod. Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 57.Attardi B., Klatt B., Hoffman G.E., Smith M.S. Facilitation or inhibition of the estradiol-induced gonadotropin surge in the immature rat by progesterone: regulation of GnRH and LH messenger RNAs and activation of GnRH neurons. J. Neuroendocrinol. 1997;9:589–599. doi: 10.1046/j.1365-2826.1997.00610.x. [DOI] [PubMed] [Google Scholar]
- 58.Picut C.A., Remick A.K., Asakawa M.G., Simons M.L., Parker G.A. Histologic features of prepubertal and pubertal reproductive development in female sprague-dawley rats. Toxicol. Pathol. 2014;42:403–413. doi: 10.1177/0192623313484832. [DOI] [PubMed] [Google Scholar]
- 59.Naulé L., Robert V., Parmentier C., Martini M., Keller M., Cohen-Solal M., Hardin-Pouzet H., Grange-Messent V., Franceschini I., Mhaouty-Kodja S. Delayed pubertal onset and prepubertal Kiss1 expression in female mice lacking central oestrogen receptor beta. Hum. Mol. Genet. 2015;24:7326–7328. doi: 10.1093/hmg/ddv430. [DOI] [PubMed] [Google Scholar]
- 60.Boehm U., Radovick S., Wolfe A., Levine J.E., Mayer C., Acosta-Martinez M., Dubois S.L. Timing and completion of puberty in female mice depend on estrogen receptor -signaling in kisspeptin neurons. Proc. Natl. Acad. Sci. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paech K., Webb P., Kuiper G.G.J.M., Nilsson S., Gustafsson J.Å., Kushner P.J., Scanlan T.S. Differential ligand activation of estrogen receptors ERα and ERrβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 62.Pettersson K., Delaunay F., Gustafsson J.A. Estrogen receptor β acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 63.Titolo D., Cai F., Belsham D.D. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) α to ERβ in clonal hypothalamic neurons. Mol. Endocrinol. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 64.Shughrue P.J., Roger Askew G., Dellovade T.L., Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]