Abstract
Objective
To prepare a novel nanoemulsion- Carbopol® 934 gel for Eugenol, in order to prevent the periodontitis.
Material and methods
Spontaneous emulsification method was used for the preparation of nanoemulsion in which it contain Eugenol (oil phase), Tween-80 (surfactant), and PEG (co-surfactant). To the development of best nanoemulsion, three-factor three-level central composite design was used in which %oil; %Smix and % water were optimized as independent variables. An optimized–nanoemulsion were converted to nanoemulsion–Carbopol® 934 gel.
Results
5.5% oil, 35.5% Smix and 59.0% water were optimized as independent and dependent variables. Finally dependent variables optimized as a particle size (nm), PDI and %transmittance were observed 79.92 ± 6.33 nm, 0.229 ± 0.019, and 98.88 ± 1.31% respectively. The values of final results for dependent variables like particle size (nm), PDI and % transmittance were evaluated as 79.92 ± 6.33 nm, 0.229 ± 0.019, and 98.88 ± 1.31%, respectively. TEM and SEM showed a spherical shape of developed nanoemulsion with refractive index (1.63 ± 0.038), zeta potential (−19.16 ± 0.11), pH (7.4 ± 0.06), viscosity (34.28 ± 6 cp), and drug content of 98.8 ± 0.09%. After that a final optimized EUG–NE–Gel was assessed on the basis of their pH measurement, drug content, syringeability, and mucoadhesion on the goat buccal mucosa. Optimized EUG-NE-Gel (Tween-80 and Carbopol® 934 used) showed the results, to improve the periodontal drug delivery of EUG in future.
Conclusion
EUG-NE-Gel showed a significant role in anti-inflammatory activity, analgesic, and anesthetic, antibacterial, and treatment of periodontal disease.
Keywords: Eugenol, Nanoemulsion–gel, Mucoadhesion, Anti-inflammatory & periodontal disease
1. Introduction
Now a day, the major cause of tooth loss is due to periodontitis infections and it is very common disease (Liu et al., 2012). In the present days, world populations (75%) suffer from serious periodontal disease (i.e. gingivitis) and also suffer from chronic periodontitis (20–30%) (Javed and Kohli, 2010). Periodontitis causes inflammation in gums tissues results gingival bleeding, periodontal pocket development, damage the connective tissue attached with teeth, and also alveolar bone resorption (Xiao et al., 2012). Periodontitis caused by the bacterial plaque presence after that it may starts the inflammatory reactions against pre–disposed hosts. It is mainly responsible factors for the development of periodontal disease, and breaking of connective tissues of teeth (Ozdemir et al., 2012). Inflammatory responses for host causes edema, infiltration of leukocyte; it releases inflammatory mediators which ultimately results development of periodontal pocket, detached connective tissue, & alveolar bone resorption, finally it causes teeth loss (Samejima et al., 1990, Botelho et al., 2007). EPD in Wistar rats is a not similar part of ligature histology, and it is analysed (Semenoff et al., 2008).
Eugenol is the major active component of clove oil found in Eugenia aromatica, it showed local anesthetic, analgesic, anti-bacterial effects, and anti-inflammatory (Srivastava et al., 2016b, Ahmad et al., 2018a). Eugenol is a principal inhibitor of COX-2 not for COX-1, proved through molecular studies for anti-inflammatory activity in cell culture of mice macrophage (Hong et al., 2002). Jadhav et al., 2004 reported that eugenol contain anti–inflammatory activity through cyclooxygenase–II enzyme inhibitor, analgesic activity because of capsaicin receptor selective binding, and also exhibited antibacterial activity on Gram negative and Gram positive bacteria. Eugenol exhibit anti-nociceptive action; it is also used in topical applications in the combination of prilocaine/ lidocaine maybe contain synergistic effects in decreasing pain (Goswami, 2013). Eugenol (≤5%) is safe for topical application if applied drug concentration (8%) causes local irritation (Opdyke, 1979).
Nanoemulsions are used as a lipid based drug delivery systems and have a thermodynamically stable system which is exhibit the mixture of surfactant; oil; cosurfactant, and water contain nanometers droplet size (Shakeel et al., 2010). Due to their nanosized droplets range and thermodynamic stability, nanoemulsions showed various applications over topically used unstable dispersions. For the enhancement of transdermal permeation, nanoformulation (e.g. Nanoemulsions) is great approach in comparison of conventional topical formulations like gels and emulsions (Shakeel et al., 2010, Ahmad et al., 2016, Ahmad et al., 2018a, Ahmad et al., 2018c, Ee et al., 2008). In a one report nanoemulsion gel (based on acaprylic acid) enhanced the meloxicam permeation and penetration transdermally due to diminish the skin barrier effects (Khurana et al., 2013).
Nanoemulsions exhibit various advantages over conventional formulations like enhancement of drug solubility, permeability to periodontal mucosa; decrease the dose with side effects reduction. Nano–droplet and surfactant reacts to outer membrane of microbes, breakdown the microbe’s membrane, and killed the microorganism. Nanoemulsions contain a broad spectrum action to bacteria, e.g. Staphylococcus aureus, Salmonella typhae, and Escherichia coli (Hamouda et al., 1999a, Hamouda et al., 1999b, Lee et al., 2010, Srivastava et al., 2016a, Srivastava et al., 2016b). Nanoemulsions are safe for periodontal disease treatment because of particular toxicity to the concentration of microbes, and also nonirritant to mucous membranes due to low concentration of surfactant/detergent in nanoemulsion. Therefore, in this research for the development of novel nanoformulation, EUG is chosen as oil phase.
Mechanical properties and drug–release of the nano–formulation are most important parameters for the development of clinical efficacy for the treatment to patients. Therefore, an ideal nano–formulation must be required that simply inserted into the periodontal pocket with controlled release of the drug into the Gingival crevicular fluid (GCF) that contain more retention into pocket in the definite time period (without any application of mechanical bonding to the tooth surfaces). Developed nanoemulsion should be non-irritant, non-toxic, and biodegradable. Carbopol–934P has been chosen for periodontal delivery. Carbopol–934P interacted CP 934P reacted to mucin which is coated on epithelial cells and tooth surfaces via selected interfacial forces. It is called as mucoadhesion which is specific type of bioadhesion (Jones et al., 1996, Bruschi et al., 2007). In situ gelling system and thermorevesible accepted as a different type of technical method which delivers the drugs directly into the pocket for sustained/controlled release form. In this research study eugenol is selected as drug and oil phase both i.e. contain double benefit with avoid first pass metabolism and their side effects based on dose related.
Hence, in this research work defines the development, evaluation, and characterization of nanoemulsion–gel of EUG used in the treatment of periodontitis and periodontal pocket delivery. Eugenol nanoemulsion optimized on the basis of independent variables and also characterized by particle size, PDI, and transmittance with evaluated by Central composite design. The EUG–NE–Gel characterized through various parameters e.g. gelling capacity, pH, syringeability, mucoadhesion, irritancy studies, and in vitro drug release.
2. Materials and methods
2.1. Materials
Eugenol was procured from Santa Cruz Biotechnology (USA). Tween 80, Polyethylene Glycol (PEG), and other surfactants samples were used as a gift samples by Sun Pharma (Gurgaon, Haryana, India). Deionized water (DI) was more purified from Milli-Q water purification system (Millipore, Bedford, MA, USA) was used for purification of water to deionized water (DI). Methanol, Ethanol, Acetonitrile and other chemicals HPLC/MS grade (99.9% purity) were procured from Sigma Aldrich (Steinheim, Germany). Carbopol® 934 grades was used and purchased from Loba Chemie Pvt. Ltd., Mumbai, India.
2.2. Excipients screening
Surfactant, co-surfactant, and Oil (i.e. Eugenol) was selected on drug solubility and stability of nanoemulsion development. Here, EUG is selected oil phase and active ingredient for activity. Tween–80 (T–80), Tween–20 (T–20), P (Peceol), L (Labrafil), LS (Labrasol), PEG–400 (Polyethylene glycol), E (ethanol), were selected as surfactants. Maximum amount of the drug (i.e. EUG) is mixed with surfactant (as a solvent 1 mL) in the MCT (microcentrifuge tube) after that vortexed it properly at 25 ± 1 °C for 72 h, through Remi CM-101 cyclomixer. After 72 h, the undissolved drug was separated through centrifugation (Remi R8C Laboratory Centrifuge) at 3000 rpm for 10 min from the mixtures. 10 μL of the supernatant of drug was taken in a MCT and made up the volume upto 1 mL with the help of methanol. Before filtration, it is vortexed and filter by nylon filter (0.22 μm). Made the different dilution of filtered supernatant and their concentration of drug in oil/surfactants was measured by already developed calibration curve at absorbance 280 nm (Ahmad et al., 2018a, Ahmad et al., 2018c). First, determined the solubility of drug in different excipients and followed by stability study of the established nanoemulsion.
2.3. Nanoemulsion preparation & pseudo–ternary phase–diagram
Water titration method or spontaneous emulsification method was applied to prepare nanoemulsion. Solubility of Eugenol was performed, oil phase was chosen as Eugenol. Surfactant & co–surfactant were chosen as Tween 80 and PEG (Fig. 1). Aqueous phase was distilled water. Pseudoternary phase diagrams were plotted as per the use of water titration or spontaneous emulsification method for the measurement of zone of nanoemulsion. Co–surfactant and Surfactant (Smix) were properly mixed in different ratios (2:1, 1:1, 3:1, 1:2, 5:1, and 4:1) (Fig. 2). Concentrations of surfactant were increased with respect to co–surfactant vice versa for Smix for Phase Diagrams Study. Definite Smix ratio & oil were mixed well in various volume ratios (1:9 to 9:1) range. 16–different combinations of oil and Smix (1:3, 1:3.5, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, & 9:2) were plotted upto higher ratio included whole study to study all over the boarders phases which is formed in the phase diagrams. Every ratio of oil & Smix were moderate stirred with aqueous phase, and their evaluation was based on transparent and simply flowable nanoemulsion. Nanoemulsion was physically pointed on three component phase diagram in which one axis is oil phase, another is aqueous phase, and third one was surfactant and co-surfactant mixture (Smix ratio), it is fixed.
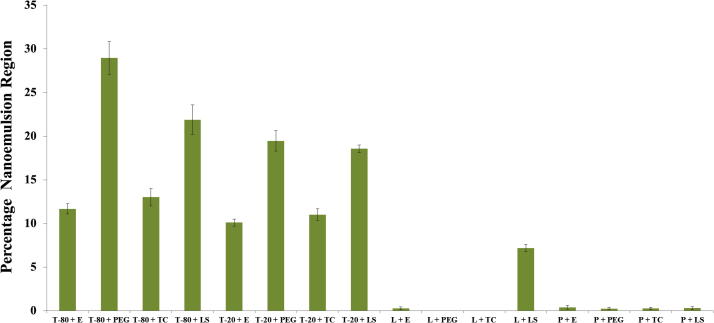
Fig. 1.
Percentage nanoemulsion region obtained for different Surfactant and Co-surfactants.
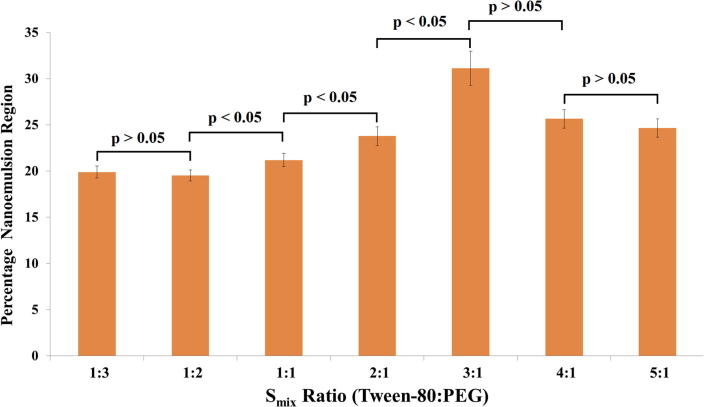
Fig. 2.
Percentage nanoemulsion region obtained for different Smix ratios.
2.4. Preparation of eugenol–nanoemulsion
Spontaneous emulsification method was used to prepare the EUG-NE (Bouchemal et al., 2004, Aqil et al., 2016). Eugenol oil mixed to the mixture of Smix (surfactant: Tween 80 & co–surfactant: PEG-400) in a suitable ratio was added and properly mixed and stirred on magnetic stirrer for complete homogenization at room temperature. The measured quantity of water added drop by drop with continuous mixing.
2.5. EUG–NE: Thermodynamic stability testing
Physical thermodynamic stability tests were executed to overcome metastable nano–formulations problem (Ahmad et al., 2018a).
2.6. Centrifugation study
All prepared nano–formulations were centrifuged at 4000 rpm for 40 min and evaluated for cracking or creaming, phase separation. The nano–formulations have not shown instability (cracking or creaming, phase separation) were chosen to heating–cooling cycle measurement.
2.7. Heating–cooling cycle
Stability of nanoemulsions was observed on the basis of effect of temperature on it. 6–cycles were performed to observe their stability in between the 4 °C to 40 °C at every point of refrigerator temperature not <48 h. All nano–formulations were stable at the mentioned temperatures followed by the result of freeze–thaw stress test.
2.8. Freeze–thaw cycle
3–freeze–thaw cycles were performed for all nano–formulations in between temperatures the −21 °C to +25 °C which are stored at each temperature for not <48 h. All nano–formulations passed tests for thermodynamic stability. It was taken for more studies.
2.9. Experimental design of Eugenol-nanoemulsion
Design expert ® Software (version 11.0.4, Stat-Ease, Minneapolis, USA) and surface methodology were used to optimization of EUG–loaded–nanoemulsion. Central composite design (CCD) and Box-Benhnken design (BBD) software were used to design the formulation. CCD is chosen as compared to Box-Benhnken design as; BBD suggests formulation with only low, mid and high value for independent variables on other side CCD gives an idea about two more values like +α and −α in which rotatability requirements of design are enclosed.
Current study selected central composite design as an optimization tool. The particle size (nm), polydispersity index (PDI), and transmittance (%); the independent variables (IV) selected were water (%), Smix (%), and oil (%) which have a potential affect. Oil and surfactant (high and low level) were chosen from ternary phase diagram whereas, −α and +α levels the medium selection given through software. Seventeen–randomized formulations run were recommended through the CCD on the basis of first run values feed in the software. On the basis of seventeen– formulation runs, 8 were factorial points, 3 were center points, and finally 6 were axial points. Constraints have been applied due to the requirements on the basis of suggested best nanoformulation. A quadratic polynomial equation has been given by software for 3–factor 3–level which is as follows (Eq. (1a)):
| (1a) |
2.10. Characterization of emulsions
Particle Size, PDI, zeta potential, SEM, and TEM were measured for test samples on the basis of Ahmad et al. method (Ahmad et al., 2016).
2.11. %Transmittance
Percent Transmittance was measured for test samples on the basis of Ahmad et al. method (Ahmad et al., 2016, Ahmad et al., 2018a).
2.12. Preparation of Eugenol–nanoemulsion–gel
Nanoemulsions have low viscosity in comparison of gels. The prepared formulation should be more retained on application site for a long time until the maximum penetration of drug takes place. For the gum tissues, the viscosity is the most important parameter to permeate and retain the drug for a maximum time. Therefore, we selected the Carbopol® 934 to enhance the more viscosity of prepared nanoemulsion. It will increase the better retention of the nanoformulation (nanoemulsion–based–gel) on the gum tissues (Hosny and Banjar, 2013, Zheng et al., 2016). Carbopol is reported as non–irritant–gelling agent and safest; till date no one reported about their sensitivity on topical delivery in human beings (Zheng et al., 2016, Ahad et al., 2017).
Therefore, we prepared a nanoemulsion–gel after that it is best to evaluate in vitro and in vivo characterization. First, Carbopol (1.25%; w/w) was dispersed in water and after that keep aside for a time. In this way, polymer chains will swell fully and also hydrated completely. For the further improvement of this gel, PEG–400 added followed by triethanolamine vigorous stirring while a transparent gel was formed with alkaline nature. EUG–NE was added to this prepared–gel base followed by continuous stirring until the EUG–NE–Gel nano–formulation (Jaiswal et al., 2016, Zheng et al., 2016, Ahad et al., 2017).
2.13. Evaluation of Eugenol-nanoemulsion-gel
2.13.1. pH of Eugenol-nanoemulsion-gel
EUG–NE–Gel (1.0 g) was weighted & dissolved in Milli–Q–water (100 mL). pH of EUG–NE–Gel was estimated by keeping a pH meter electrode into the gel and keep it to equilibrate for 1–2 min (Al-Suwayeh et al., 2014, Zheng et al., 2016).
2.13.2. Viscosity of EUG–NE–Gel
Viscosity of optimized Eugenol–nanoemulsion–gel was identified using Brookfield R/S plus cone (25 °C) and plate Rheometer with spindle C15–1 (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA), at 50–60 rpm of spindle of Rheometer for 80 s (Srivastava et al., 2016a, Srivastava et al., 2016b).
2.13.3. Homogeneity test
The homogeneity test of EUG–NE–Gel was observed through visual inspection to inspect particles or lumps are visible. We divided into grades like A+: Good, A: Fair, A− Poor. Further, EUG–NE–Gel (little amount) were pressed in–between the index finger and the thumb, to determine the consistency of EUG–NE–Gel which is homogeneous or non–homogenous (Ahad et al., 2017).
2.13.4. Spreadability
EUG–NE–Gel spreadability was calculated through keeping 0.6 g EUG–NE–Gel in the circle (2 cm diameter) properly marked on the glass plate. Second glass–plate was put on the first one and also weighed (0.6 kg) was put on upper side of glass plate (5–6 min), and also enhance the diameter after spreading was noted (Shinde et al., 2012).
The spread (%) through area is calculated by formula below:
Where, ø = 2 cm & A2: final spreading area.
2.13.5. Drug content
EUG–NE–Gel (1 g) was dissolved into methanol (100 mL) (vesicles lysed) (Ahad et al., 2017), and sonicated the solution, filtered, and injected into UHPLC–PDA (Ahmad et al., 2018b). For UHPLC-PDA, Water ACQUITY UPLCTM (Waters Corp., MA, USA) with a Photodiode Array detector i.e. PDA (Synapt; Waters, Manchester, UK) at λmax 280 nm, and binary system of solvent delivery was used whereas for chromatographic separation, ACQUITY UPLCTM BEH C-18 column (1.7 µm, 2.1 × 100 mm) was utilized. The mobile phase consisted of degassed HPLC grade solvents with isocratic elution i.e. v/v/v Acetonitrile:Water:0.1% Formic Acid (60:40:0.01%) and flow rate (0.25 mL/min) as well as injection volume (10 µL/min) for a total run time of 6.0 mins.
2.13.6. Ex vivo mucoadhesive strength measurement
EUG–NE–Gel mucoadhesive strength was measured through texture analyzer [TA-XT plus, Stable Micro Systems, Surrey, UK] on goat buccal mucosa. Buccal mucosa of goat was freshly removed and keeps in SGCF (simulated gingival crevicular fluid) (freshly prepared isotonic phosphate buffer solution: PBS: pH = 7.4) at 4–5 °C. The goat buccal mucosa tissue was properly dipped & keeps in ice–cold PBS. Thickness of slice tissues maintained in between 2 and 2.2 mm. The experiment was carried out at defined temperatures (4.0, 25.0 and 37.0 °C) within 2.0 h of incision of nasal mucosa. For the application, gel (50.0 mg) applied on goat buccal membrane surface (i.e. mucosal side) in the area (2.41 ± 0.01 cm2). EUG–NE–Gel (25 g) fixed and applied to make an adhesion bonding to EUG–NE–Gel with goat buccal mucosa. When pre–load–time completed, it was released and after that force has been given to the probe to separate the membrane. The detachment force was written like mucoadhesive strength (n = 3). The entire test was performed showed the significance p < 0.05.
2.13.7. In vitro drug release kinetics of EUG–NE–Gel
Dialysis bag method was used in this study. Periodontal sol (400 mg) having drug (2%) kept in a dialysis bag (MWCO 6000–8000) cut-off. SGCF soaked previously PBS (pH 7.4 as same as periodontal diseases pH) for 48 h which is put in a vessel (100 mL SGCF) and also maintained at 37 ± 0.05 °C with stirring continued at 100 rpm. Samples collected at 0.50; 1.0; 2.0; 3.0; 4.0; 5.0; 6.0; 7.0; 8.0; 12.0; 24.0 h to analyse the EUG quantity by the previously reported UHPLC method (Ahmad et al., 2018b).
2.14. HET-CAM method for irritation test
Optimized EUG–NE–Gel used to evaluate toxicity test or irritation test on Hen’s egg test-chorioallantoic membrane (HET-CAM) with the help of previously reported method (Gilleron et al., 1996, Dahl, 2007, Srivastava et al., 2016b). Finally, irritancy potential was determined by formula:
Where,
H = time (hemorrhage appeared)
V = time (vasoconstriction occurred)
C = time (coagulation of protein or blood)
Score (6–eggs) has been calculated for every material. Test materials classified as follows (Luepke, 1985, Vinardell and Mitjans, 2006):
| S.N. | Category | Score |
|---|---|---|
| 1. | Non-irritant | 0.00–0.90 |
| 2. | Slightly irritant | 1.00–4.90 |
| 3. | Moderately irritant | 5.00–8.90 |
| 4. | Strongly irritant | 9.00–21.00. |
2.14.1. In vivo study
Male Wistar rats (2–months old & 200–250 g weight) chosen to perform in vivo study. The protocol used for the experiment was approved by the Institutional Review Board of Animal Research, Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia. All rats were adapted for before one week and in–housed under normal laboratory condition with chow food and water available ad libitum.
2.15. Induction of experimental periodontitis and treatment
24–Wistar rats used in the current study; out of them, 18 were infected with ligature-induced EPD. [Group A] The remaining six rats were used in the normal group i.e., no ligature induction to periodontal diseases. To induce periodontitis, 18 rats (200–250 g) were initially anesthetized with an i.m. injection of ketamine (90 mg/kg b.w.) & xylazine (10 mg/kg b.w.) (Branco-de-Almeida et al., 2012). Experimental periodontitis were induced by the kept a non-absorbable sterile surgical silk ligature (3/0), the method adopted by Srivastava et al. (2016a).
All EPD rats were fed with sucrose solution (10%w/v) with 8 weeks duration time. All rats divided into IV–groups (Every group contain 6–rats).
Group A: Group Control [no induction of periodontal diseases]
Group B: [EPD]: [ligature-induced periodontal disease: No pharmacological treatment]
Group C: treated with Doxycycline;
Group D: treated with EUG–NE–Gel.
After 11th day of treatments, the effects of the different treatment groups were examined on various parameters (Liu et al., 2012). Comparison of different clinical parameters liked tooth mobility (TM), Gingival index (GI), and ABL with histological changes can give an idea about to understand for inflammatory condition in periodontitis and their classification mentioned in Table 6A, Table 6B (Xu and Wei, 2006). The current study was also revealed a reduction of proinflammatory cytokines such as IL-1β and TNF-α on the gingival tissue of Wistar rats treated with Doxycycline and EUG–NE–Gel. The role of IL-1β and TNF-α, on periodontal disease, has been discussed earlier (Lima et al., 2004, Botelho et al., 2010).
Table 6A.
Gingival index (GI) classification based as per the inflammation of the gingival tissue.
| S.N. | Observation | Score/Grade |
|---|---|---|
| 1. | Normal Gingival | 0 |
| 2. | Mild Inflammation: slight edema, minor change in color, and absence of bleeding on probing | 1 |
| 3. | Moderate Inflammation: edema, glazing, redness, and bleeding on probing | 2 |
| 4. | Severe Inflammation: extreme redness, presence of ulcer, edema, and severe bleeding. | 3 |
Table 6B.
Tooth mobility (TM) classification based as per the mobility of second molar teeth (ligated).
| S.N. | Observation | Score/Grade |
|---|---|---|
| 5. | No Mobility | 0 |
| 6. | Slight Mobility | 1 |
| 7. | Moderate Mobility | 2 |
| 8. | Severe Mobility | 3 |
2.16. Measurements of ABL
The rats euthanized on eleven–day of periodontitis induction. Their maxillae removed and fixed with neutral formalin (10%). Both maxillaries were defleshed, properly cleaned after that put in 1 M-NaOH solution (25 °C) for 1 h to remove all soft tissue debris. Jaws stained from Loeffler’s methylene blue (1%w/v) (SD fine chemical Limited, Mumbai, India) for identification of the cemento–enamel–junction. The horizontal ABL was measured by using the similar method as explained by Samejima et al. (1990). All the Measurements were taken every root axis for 3–roots from 1st teeth, two roots from 2nd & 3rd molar teeth. The loss of alveolar bone was calculated through sum of buccal tooth surfaces & right maxilla value subtracted (unlighted control) from the left (mL) (Botelho et al., 2010). Cemento–enamel junction & alveolar bone distance was calculated with the help of an eyepiece micrometer in a dissecting microscope (20.0× magnifications (Xu and Wei, 2006).
2.17. Histological analysis
The rats were euthanized under anesthesia. The dental–alveolar segment having soft tissue was fixed in 10% v/v formalin solution and demineralized with nitric acid (7%) for 24 h (Botelho et al., 2010). We dehydrated these specimens; dipped in paraffin after that sectioning molar in a mesiodistal plane for hematoxylin with eosin (HE) staining. Sections of 6 mm thicknesses were evaluated using light microscopy (40× magnifications). The stained sections were analyzed by the parameters like ABL and inflammatory cell infiltration in gingival tissue. Alveolar bone specimens from Group A (control) (no ligature) were also measured to analyze the results from ligature Groups D (Xu and Wei, 2006).
2.18. IL-1β and TNF-α detection in gingival tissue
The gingival tissue part from area adjacent the lower left molars (between first and second) of different groups was collected at 11th day after periodontitis induction. The tissue of groups was collected, homogenized, and processed (Safieh-Garabedian et al., 1995, Srivastava et al., 2016a). The detection of IL-1β and TNF-α concentrations was calculated through ELISA (enzyme-linked immune sorbent assay) RayBio (New Delhi, India) as earlier described by Cunha et al. (1993). Briefly, 96-well micro–titer plates were coated over–night at 4 °C with an antibody against rat IL-β or TNF-α (100 mg/mL). The standard and test samples at different dilutions were added in duplicate and incubated at 4 °C for 24 h after blocking the plates. The plates were washed again 3–times with the buffer. After then 100 lL of biotin antibody anti-rat TNF-α and anti-rat IL-1β were added to the plate wells and incubated for 1 h at 25 ± 2 °C. The plates were further washed three times and added 100 lL of Streptavidin solution and incubated 45 min at 25 ± 2 °C. We further added 100 lL of TMB (3,30,5,50-tetramethylbenzidine) one-step substrate reagent to each well and incubated for 30 min. The enzyme reaction was stopped with 50 lL stop solutions (0.2 M sulfuric acid) and the absorbance was measured at 450 nm. The results were reported as mean ± standard error mean for six animals. The least measurable dose of IL-1β and TNF-α were 80 pg/mL and 25 pg/mL, respectively, as per manual.
2.19. Antibacterial activity
In vitro, Evaluation of antibacterial activity of Doxycycline and EUG–NE–Gel concentration were measured by using the agar diffusion method (the cup plate method). Escherichia coli and Staphylococcus aureus bacteria were used in the study. The culture media for antibacterial assay was nutrient agar media. Above selected microbes grow actively in broth culture and showed turbidity (109 CFU/mL). Sterilized molten nutrient agar was dipped into sterilized petri dishes and leave for solidify. The plates were swabbed with the 100 mL culture of the microorganisms. Uniform-sized cups of 6-mm diameter were aseptically punched into the seeded agar medium using a sterilized well bore at a equidistant position. The prepared gel samples were filled into the cylinder cup and incubated at 37° ± 0.5 °C for 48 h. The diameter (mm) of the zone of growth inhibition was estimated as the diameter (mm). Three times (n = 3) performed all tests.
2.20. Statistical analysis
Results calculated and exhibited as mean ± standard error of mean (SEM). Student’s–t–test used significant observation on the unpaired observations via ANOVA (p–value < 0.05).
3. Result and discussion
3.1. Excipients screening
In this study, Eugenol was used in place of oil or oil phase. Surfactant (Tween 80) and co–surfactant (PEG) mixtures was found highest percentage yield (28.96 ± 1.88%) for Smix combinations (Fig. 1). Tween-80 contains HLB–value 15.0 and after that PEG exhibited a highest solubilizing capacity. Low molecular weight Tween–80 reduced particle size more efficiently in comparison of other polymeric surfactants (Fig. 1) (Ahmad et al., 2018a). Tween–80 chosen as a surfactant on the basis of showed properties. For the selection of co–surfactants, PEG combined with Tween–80 showed a very clear nanoemulsion.
This observation supported previously reported data of Chen et al., 2015, Aqil et al., 2016 showed the a clear microemulsion when they used hydrophilic co–surfactant. We observed very low zeta potential (−19.16 ± 0.095) with the expectation of decrease of nanoemulsion droplets due to the use of non-ionic surfactant (McClements and Xiao, 2012).
3.2. Preparation of placebo nanoemulsion: Application of pseudo–ternary–phase diagram
Pseudo–Ternary–Phase Diagram has been made for different Smix ratio. We have also made a plot for different Smix ratio that displays nanoemulsion region (%) column charts with the help of cut & weigh method (Fig. 2). On the basis of values 3:1 ratio is statistically significant with the comparison of nanoemulsion areas of test samples. On the basis of plotted graph showed lowest region 1:2, and highest region 3:1. Surfactant quantity increased from Smix 1:2 to Smix 3:1 due to this reason enlarge nanoemulsion region of Tween–80 (HLB value: 15). This indicates HLB value increase parallel with increment of Tween–80 quantity. It is opposite of the previously reported trend, Enhanced the quantity of Tween–80 affected the droplet size i.e. breakup and disruption of droplet (Jafari et al., 2008). Pseudo–Ternary–Phase–Diagram can be used for estimation of lower and greater % of oil & Smix also.
We have tried various combinations of Smix, oil, and water after that selected best of two formulations finally. We discarded few formulations due to higher % of oil than water as a result of w/o nanoemulsion production. Lowest percentage of oil has been used, if more than 10.0% of oil used, it forms the microemulsion as Tadros et al. (2004), reported previously. The %age of Oil [5.5%] and %age of surfactants [35.5%] is best optimized for nanoemulsion.
Different % transmittance of dispersions values mentioned in Table 1. There are so many surfactants to emulsify Eugenol oil (Clove oil), it was already reported like PEG and Tween–80 was used as co–surfactant and surfactant for preparation of nanoemulsion. Therefore, we used these two surfactants.
Table 1.
Thermodynamic stability test of randomly selected oil:Smix: distilled water combinations based on Pseudo ternary phase diagram of Smix ratio 3:1.
| Code (Formulation) | Oil (%v/v) | Smix (%v/v) | Distilled water (% v/v) | Centrifugation cycle | Heating–cooling cycle | Freeze–thaw cycle |
|---|---|---|---|---|---|---|
| E1 | 5 | 35 | 60 | √ | √ | √ |
| E2 | 5.5 | 37.5 | 57 | √ | √ | √ |
| E3 | 6 | 38.5 | 55.5 | √ | √ | √ |
| E4 | 6.5 | 34.5 | 59.0 | √ | √ | √ |
| E5 | 7.0 | 39.5 | 53.5 | √ | √ | √ |
| E6 | 7.5 | 39.5 | 53.0 | √ | √ | √ |
| E7 | 8.0 | 33.5 | 58.5 | √ | √ | √ |
| E8 | 8.5 | 33.0 | 58.5 | √ | √ | √ |
| E9 | 9.0 | 31.5 | 59.5 | √ | √ | √ |
| E10 | 9.5 | 31 | 59.5 | √ | √ | √ |
| E11 | 10.0 | 29 | 61 | √ | √ | √ |
3.3. Thermodynamic stability tests
Stress testing is necessary to avoid the metastable formulations. Most of the selected formulations have been included due to o/w nanoemulsion region in the pseudoternary phase diagrams (Smix 3:1). A Smix 3:1 combination has been indicated maximum nanoemulsion area with maximum thermodynamic stability which is based on freeze–thaw cycle, heating–cooling cycle, and centrifugation. In this ratio of Smix 3:1 combination of surfactants was not showed any phase separation, creaming/cracking, turbidity. Thermodynamic stability is the most important parameter which is related to the long shelf life of the prepared nanoemulsion in comparison of normal emulsions. Different composition of all tried nanoformulations mentioned in the Table 1. We used Design Expert design software to try these different compositions [oil (5.0–10%), surfactant (29–40%), water (40–60%)] as trials for the optimization of nanoemulsion which is based on thermodynamic studies.
In addition, despite low zeta potential (−19.16 ± 0.12 mV), EUG-NE5 showed a stability up to 14 weeks and this may be attributed to the presence of non-ionic surfactant i.e. Tween-80 which helps in nanoemulsion stabilization (Ahmad et al., 2018a).
3.4. Design for experiment: Fitting of model and EUG-NE optimization
For the optimization of prepared nanoemulsion, Design Expert Software (3–level, 3–factor) was used to find out the polynomial equation and to examine the all dependent variables quadratic responses. All dependent & independent variables presented in Table 2. For the optimization of prepared nanoemulsion were produced total seventeen runs as mentioned in Table 3. Fig. 3 exhibited prepared 3–dimensional plots on the basis of Y1, Y2 and Y3 responses. All these responses mentioned in the plots helped to evaluate the factors interaction effects and also helpful to determine three factors response effects at single time. All the predicted values based on the experimental values were co–related with each other as shown in Fig. 4.
Table 2.
Variables in “Design Expert” software for preparation and optimization of Eugenol-Nanoemulsion (EUG-NE).
| Factors | Levels |
||
|---|---|---|---|
| Independent Variables | Low | Medium (0) | High |
| X1 = Oil (Eugenol) (% v/v) | 5.0 | 7.5 | 10 |
| X2 = Smix (% v/v) | 30 | 35 | 40 |
| X3 = Water (% v/v) | 40 | 50 | 60 |
| Dependent variables | |||
| Y1 = Particle size (nm) | Minimize | ||
| Y2 = Polydispersity Index (PDI) | Minimize | ||
| Y3 = Transmittance (%) | Enhance | ||
Table 3.
Nanoemulsion suggested by “Design Expert” software at independent variables and their responses.
| Formulation code | Independent variables | Dependent variables |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coded factors |
Observed responses |
Predicted responses |
|||||||
| X1 | X2 | X3 | Y1 | Y2 | Y3 | Y1 | Y2 | Y3 | |
| ENE1 | 5 | 30 | 40 | 209.47 ± 12.78 | 0.511 ± 0.029 | 71.10 ± 1.59 | 212.87 | 0.513 | 69.66 |
| ENE2 | 10 | 30 | 40 | 223.99 ± 13.87 | 0.449 ± 0.028 | 64.33 ± 1.51 | 219.17 | 0.461 | 66.09 |
| ENE3 | 5 | 40 | 40 | 280.99 ± 15.89 | 0.459 ± 0.029 | 60.97 ± 1.49 | 278.51 | 0.474 | 60.10 |
| ENE4 | 10 | 40 | 40 | 313.01 ± 18.27 | 0.572 ± 0.034 | 58.63 ± 1.48 | 318.68 | 0.581 | 57.70 |
| ENE5 | 5.5 | 35.5 | 59 | 79.92 ± 6.33 | 0.229 ± 0.019 | 98.88 ± 1.31 | 78.65 | 0.237 | 99.75 |
| ENE6 | 10 | 30 | 60 | 141.01 ± 9.83 | 0.264 ± 0.026 | 81.44 ± 1.01 | 148.75 | 0.271 | 81.88 |
| ENE7 | 5 | 40 | 60 | 98.32 ± 8.16 | 0.281 ± 0.027 | 96.46 ± 1.16 | 108.92 | 0.280 | 93.69 |
| ENE8 | 10 | 40 | 60 | 149.62 ± 8.64 | 0.404 ± 0.035 | 90.10 ± 0.98 | 151.50 | 0.411 | 90.40 |
| Axial Points | |||||||||
| ENE9 | 3.2955 | 35 | 50 | 56.23 ± 5.10 | 0.249 ± 0.019 | 95.98 ± 1.17 | 52.48 | 0.251 | 98.26 |
| ENE10 | 11.7045 | 35 | 50 | 97.32 ± 7.12 | 0.319 ± 0.021 | 93.69 ± 1.03 | 93.58 | 0.318 | 92.48 |
| ENE11 | 7.5 | 26.5910 | 50 | 269.27 ± 16.15 | 0.459 ± 0.030 | 60.18 ± 1.03 | 268.13 | 0.465 | 59.89 |
| ENE12 | 7.5 | 43.4090 | 50 | 332.27 ± 19.61 | 0.558 ± 0.039 | 56.66 ± 1.16 | 325.64 | 0.550 | 59.02 |
| ENE13 | 7.5 | 35 | 33.1821 | 310.98 ± 16.45 | 0.602 ± 0.049 | 53.27 ± 0.98 | 312.42 | 0.587 | 54.06 |
| ENE14 | 7.5 | 35 | 66.8179 | 119.44 ± 9.62 | 0.261 ± 0.029 | 94.89 ± 1.31 | 110.59 | 0.265 | 95.59 |
| Centre Points | |||||||||
| ENE15 | 7.5 | 35 | 50 | 130.97 ± 7.69 | 0.3180 ± 0.018 | 90.15 ± 1.10 | 136.13 | 0.320 | 90.02 |
| ENE16 | 7.5 | 35 | 50 | 135.92 ± 8.95 | 0.3200 ± 0.017 | 90.15 ± 0.96 | 136.13 | 0.320 | 90.02 |
| ENE17 | 7.5 | 35 | 50 | 135.92 ± 8.95 | 0.3200 ± 0.017 | 90.15 ± 0.96 | 136.13 | 0.320 | 90.02 |
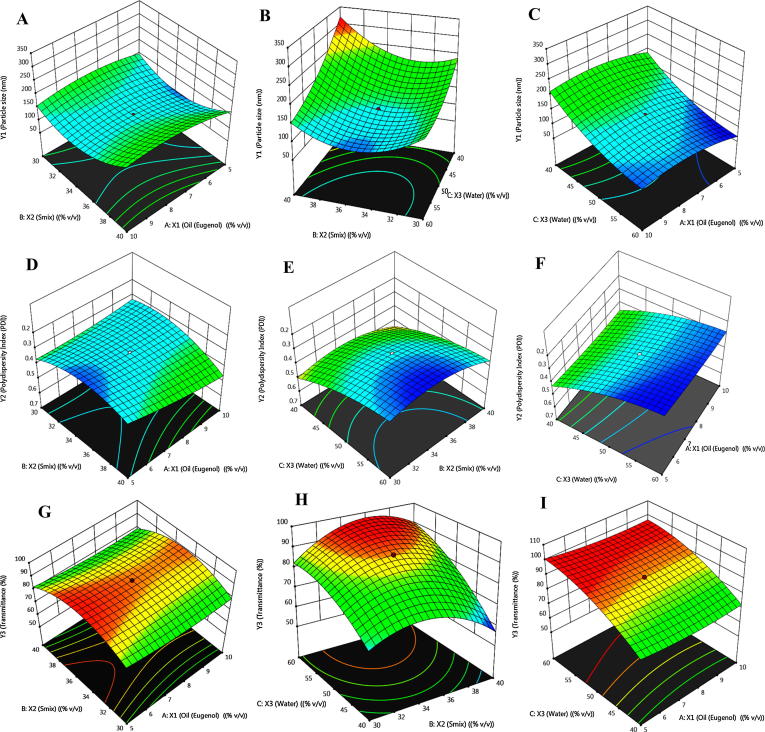
Fig. 3.
3-D response surface plots showing the interaction eff.
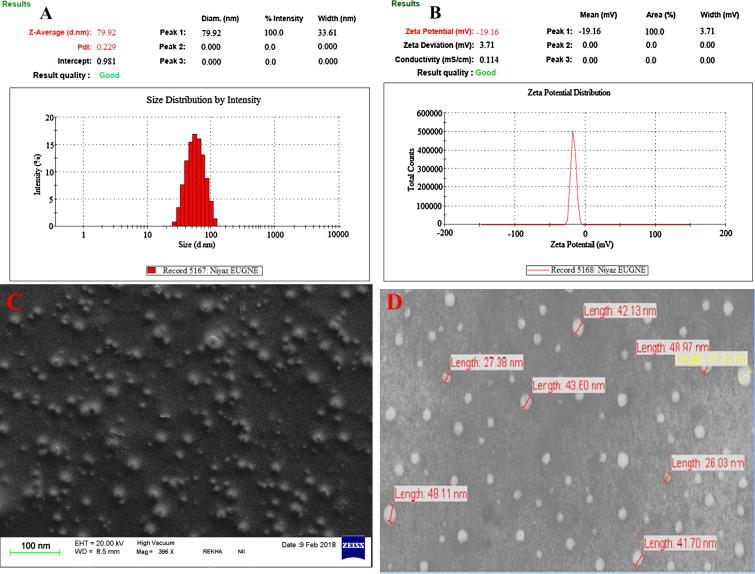
Fig. 4.
Dynamic light scattering techniques for determining th.
On the basis of above mentioned studies, the results come from independent variables for example %oil, % water, and %Smix. The data obtained as particle size [Y1 = 56.23–332.27 nm], PDI [Y2 = 0.229–0.602], and %Transmittance [Y3 = 53.27–98.88%] in Table 3. Finally, obtained data loaded in the software to find out the optimization of polynomial quadratic models (p < 0.001) for all 3–dependent variables i.e. DV. There was some data find out not significant (p > 0.05) i.e. “not fit to” for all 3–dependent variables i.e. DV. “R2 values predicted” from all 3–DV were agreed to fit the “R2 values adjusted” in Table 3. The model fitted in the proposed equations mentioned in Table 4. X3 (% water), X2 (%Smix), and X1(%oil) individually data were shown in Table 1 such as Eq. (1). Individually %oil & %Smix data obtained which showed a positive effect from proposed equation. It is also obtained a positive effect on % Transmittance with hydrodynamic diameter (Y1). Correspondingly, independent variables combination exhibited a positive effect for %oil ∗ % transmittance (X1X3) and % oil ∗ %Smix (X1X2) in 3–dimensional plot for particle size in Fig. 3A–C. Hydrodynamic diameter reduced gradually for the concentration of Smix and water (%). It was observed detrimental effect i.e. an enhancement of oil concentration parallel to the hydrodynamic diameter in nano–formulation.
Table 4.
Results of regression analysis for responses Y1 (Particle Size, nm), Y2 (Transmittance %) and Y3 (PDI).
| Quadratic model | R2 | Adjusted R2 | Predicted R2 | Standard Deviation (SD) | % Coefficient of Variation (CV) |
|---|---|---|---|---|---|
| Response (Y1) | 0.9968 | 0.9939 | 0.9709 | 6.58 | 3.76 |
| Response (Y2) | 0.9967 | 0.9937 | 0.9651 | 0.0019 | 2.40 |
| Response (Y3) | 0.9942 | 0.9890 | 0.9492 | 1.66 | 2.05 |
Y1 = 136.13 + 12.22 * X1 + 17.10 * X2 − 60.00 * X3 + 8.47 * X1X2 + 0.6020 * X1X3 − 24.19 * X2X3 − 22.31 * X12 + 56.84 * X22 + 26.65 X32 (Eq. (1))
Y2 = +32.01 + 0.0198 * X1 + 0.0251 * X2 −0.0959 * X3 + 0.0398 * X1X2 + 0.0059 * X1X3 + 0.0049 * X2X3 − 0.0125 * X12 + 0.0663 * X22 + 0.0375 X32 (Eq. (2))
Y3 = + 90.02 − 1.72 * X1 − 0.2606 * X2 + 12.35 * X3 + 0.2931 * X1X2 − 0.2239 * X1X3 + 4.23 * X2X3 − 1.89 * X12 − 10.81 * X22 − 5.37 X32 (Eq. (3))
PDI effect exhibited in Eq. (3) & Fig. 3D–F. Enhancement of PDI was detected an enhancement of % Smix and % water was just opposing to the increase in concentration of oil i.e. a very good favor for PDI. A combined independent variables [i.e. %oil ∗ % water, %oil ∗ % Smix, and %Smix * % water] given a positive magnitude. It also proved a strong dominancy for % water on %Smix and % Oil. It can be accredited to a reduction in particle size (i.e. hydrodynamic diameter) consequently favors highest drug release observed just because of % water.
Likewise, a positive enhancement of %transmittance was obtained with a reduction in % oil and %Smix which is a reduction of certain detrimental effect limit with the reduction of % oil in the prepared formulation in Eq. (2) (Fig. 3G–I). Additionally, a positive effect was shown on %transmittance because of combined effect of % oil ∗ %Smix (X1X2). Though, a significant effect was find out for % oil ∗ % water (X1X3) as same as significant on %Smix ∗ % water (X2X3).
Constraints were utilized for independent variables so as to find out a final optimized nanoformulation (Table 2, Table 3) whereas % oil and % water (independent variables) were put in the range of constraint utilized for %Smix i.e. “decrease”. Instead of dependent variables “decrease” was marked for particle size and “highest” was marked for PDI and %transmittance.
Optimized EUG-NE was expected through PDI with the final composition of oil (5.5%), Smix (35.5%), and water (59%) for 3–dependent variables on the basis of constraints and quadratic equations. Additionally, the particle size (79.92 ± 6.33 nm), PDI (0.229 ± 0.019), and %transmittance (98.88 ± 1.31%) was expected data for the optimized EUG-NE with 0.9968 R2 value. EUG-NE was formulated based on the experimental level with 3–EUG–NE nanoformulations so as to validate properly based on parameters i.e. transmittance (%), particle size, & PDI in Table 5A.
Table 5A.
Best optimized and predicted batch of EUG-NE with independent variables, and dependent variables.
| Batch | Independent variables |
Dependent variables |
||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| Predicted | 5.5 | 35.510 | 59.00 | 78.65 | 0.237 | 99.75 |
| Optimized | 5.5 | 35.5 | 59.00 | 79.92 ± 6.33 | 0.229 ± 0.019 | 98.88 ± 1.31 |
In addition, despite low zeta potential (−19.16 ± 0.12 mV), EUG-NE5 showed a stability up to 14 weeks and this may be attributed to the presence of non-ionic surfactant i.e. Tween-80 which helps in nanoemulsion stabilization (Ahmad et al., 2018a).
3.5. Nanoemulsion characterization: Hydrodynamic diameter, PDI, and %transmittance
“Central composite design” predicted particle size (78.65 nm) of prepared nanoemulsion and their observed size was 79.92 ± 6.33 nm in Fig. 4A as well as zeta potential (−19.16 ± 0.12 mV) which is good correlation of monomodal distribution of droplet size. Moreover, Flux = 12.31 ± 1.48 μg/cm2/h and their PDI (0.229 ± 0.019) was also taken for final prepared and optimized EUG-NE (Tables 5A and 5B, Fig. 4).
Table 5B.
Some other characterized parameters of EUG-NE.
| Flux (μg/cm2/h) | Zeta Potential (mV) | Refractive index | Viscosity (centipoise) | pH | Drug content (%) |
|---|---|---|---|---|---|
| 12.31 ± 1.48 | −19.16 ± 0.12 | 1.63 ± 0.038 | 34 ± 5 cp | 7.4 ± 0.06 | 98.38 ± 0.09% |
3.6. Nanoemulsion characterization: Zeta potential
Zeta potential of optimized EUG–NE was −19.16 ± 0.12 mV (Fig. 4B). It was previously described oil droplets stabilized by non-ionic surfactant and also their magnitude of droplet charge (Ghosh et al., 2013).
3.7. Surface morphology of nanoemulsion characterization
TEM and SEM were used for surface appearance and shape of EUG–NE. It was observed round and smooth appearance by SEM (Fig. 4C and D) followed by round shaped contain <100.0 nm particle size with the help of TEM (Fig. 4D).
3.8. pH, viscosity, refractive index (RI), and drug content
RI was found 1.63 ± 0.038 with a clear and less dense optimized EUG-NE. Furthermore, pH and viscosity was detected 7.4 ± 0.06 and 34 ± 5 cp followed by drug content (98.38 ± 0.09%) of optimized EUG-NE (Table 5B).
3.9. EUG–NE–Gel preparation
EUG–NE converted into gel form with the help of Carbopol® 934 (used as a gelling agent) to treat periodontitis. Eugenol–Nanoemulsion–Carbopol® 934 gel (EUG–NE–Gel) has been optimized and selected for the treatment of periodontitis and gum tissues. Zeta potential, PDI, and vesicles size of optimized EUG–NE–Gel were determined −19.16 ± 0.12 mV, 0.229 ± 0.019, and 79.92 ± 6.33 nm (Ahad et al., 2017, Srivastava et al., 2016a, Srivastava et al., 2016b). First optimized EUG–NE was not so much viscous in comparison of after prepared EUG–NE–Gel for the application of site for a long time action. Hence, optimized EUG–NE was converted into EUG–NE–Gel with the help of Carbopol (gelling agent) to increase more permeability of EUG–NE in the rats. Optimized Eugenol–Nanoemulsion– Carbopol® 934 gel (EUG–NE–Gel) exhibited a smooth texture followed by homogeneous and pleasant appearance.
3.10. EUG–NE–Gel: Spreadability, viscosity, pH and homogeneity
EUG–NE–Gel contains pH range (6.71 ± 0.06 to 6.97 ± 0.07) which is very closely to neutral pH effective for the delivery of periodontal drug delivery having no irritation; results obtained from in situ NE–Gel. It is most important parameter for the drug permeation which is related to viscosity of NE–Gel. Viscosity of NE–Gel formulation showed its consistency. This is the most characteristic of gel should be selected for the skin or gum tissues in thin layers (Srivastava et al., 2016a, Srivastava et al., 2016b). Optimized Eugenol–Nanoemulsion–Carbopol gel (EUG–NE–Gel) exhibited a smooth texture followed by homogeneous and pleasant appearance. EUG–NE–Gel have not showed any gritty particles and also not showed any signs of phase separation with the standards.
Characteristics of spreadability of any gel showed very less time to spreading and also show consistency. It was also reported before any gels have been affected by spreading ability of gel formulations (Varshosaz et al., 2002). Optimized EUG–NE–Gel was found very good spreading diameter (5.83 ± 0.06 cm) in this study.
3.11. Drug content
It was investigated uniform distribution of EUG in EUG–NE–Gel. EUG in EUG–NE–Gel was calculated 98.38 ± 0.09%. The observed results of EUG in the EUG–NE–gel due to the consistently of Carbopol® 934 which is significant to no drug loss.
3.12. Syringeability, transition of sol–gel, and mucoadhesive strength studies
The temperature range of sol–gel transition observed 37.65 ± 0.41 °C. Gelling time was 39.67 ± 0.58 s. Syringeability test was also passed. Goat buccal mucosa was selected for mucoadhesive strength studies i.e. 32.87 ± 0.92 g (Srivastava et al., 2016b).
3.13. Irritation studies
In vitro Irritation studies, HET–CAM membrane is a very sensitive membrane which is selected for irritation of EUG–NE–Gel potential. EUG–NE–Gel scores were determined 0.59 ± 0.087. Optimized EUG–NE–Gel showed irritation potential on the mucous membranes. Isotonic solution of NaCl and 0.1 N sodium hydroxide scored 0.44 ± 0.062. In vitro toxicity studies showed no toxicity which is very significant to prepared EUG–NE–Gel. It was also suitable for intra-pocket delivery (Figures not shown).
3.14. In vitro drug release studies
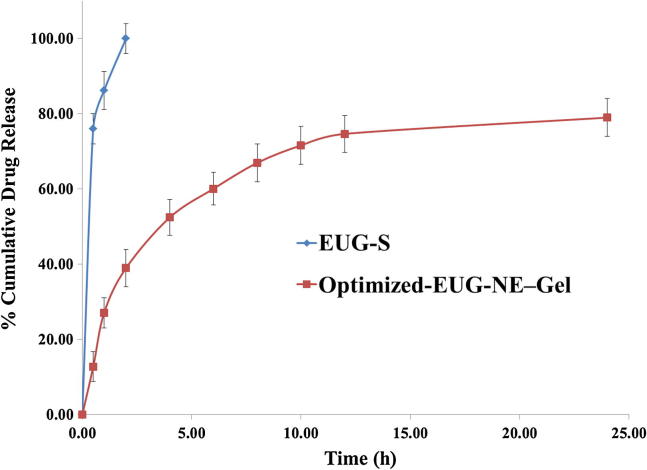
Fig. 5, shows a control release state after 12–24 h for EUG from EUG–NE–Gel i.e. 74.68% attaining up to 79.01%.
Fig. 5.
The cumulative percentage release of Eugenol from EUG.
3.15. Tooth Mobility (TM), Gingival Index (GI) and Measurement of Alveolar Bone Loss (ABL) in rats
Tooth Mobility (TM) treatment group was significantly lower compared with Group A (p < 0.05) (Table 1). Tooth Mobility in Group A was increased to 3.710 ± 0.098; after treatment, it was reduced to 0.461 ± 0.109 in Group B with a mean percentage of bone reductions of 87.05% (Table 7). Gingival Index was significantly lower compared with Group A (Table 7). Gingival Index in Group A rats was increased to 3.561 ± 0.030; after treatment, it was reduced to 0.299 ± 0.016 in Group B with a mean percentage of bone reductions of 89.91%, (Table 7). Alveolar Bone Loss (ABL) in treated group was significantly lower compared with Group A (p < 0.05) (Table 1). The Alveolar Bone Loss (mm) in Group A was increased to 4.67 ± 0.65 mm; after treatment, it was reduced to 1.12 ± 0.09 mm in Group B with a mean percentage of bone reductions of 76.86% (Fig. 6, Table 7).
Table 7.
Visual observation from experimental groups in rats (treated/non-treated group).
| Treatment–group/evaluation parameters | Gingival Index (score unit) mean ± S.D | Tooth mobility (score unit) mean ± S.D | Bone resorption (mm) Mean ± S.D |
|---|---|---|---|
| Group A (EPD without treatment) | 3.561 ± 0.030 | 3.710 ± 0.098 | 4.67 ± 0.65 |
| Group B (EPD + EUG–NE–Gel) | 0.299 ± 0.016* | 0.461 ± 0.109* | 1.12 ± 0.09* |
| Group C (control) | 0.0 ± 0.0 | 0.099 ± 0.009 | 0.0 ± 0.0 |
Statistics: one-way ANOVA analysis all groups compared with Group 1.
p < 0.05 was considered less significant difference compared with EPD+EUG–NE–Gel and non-treated groups.
Fig. 6.
Histological results of the periodontium of rats in di.
3.16. Histopathological analysis
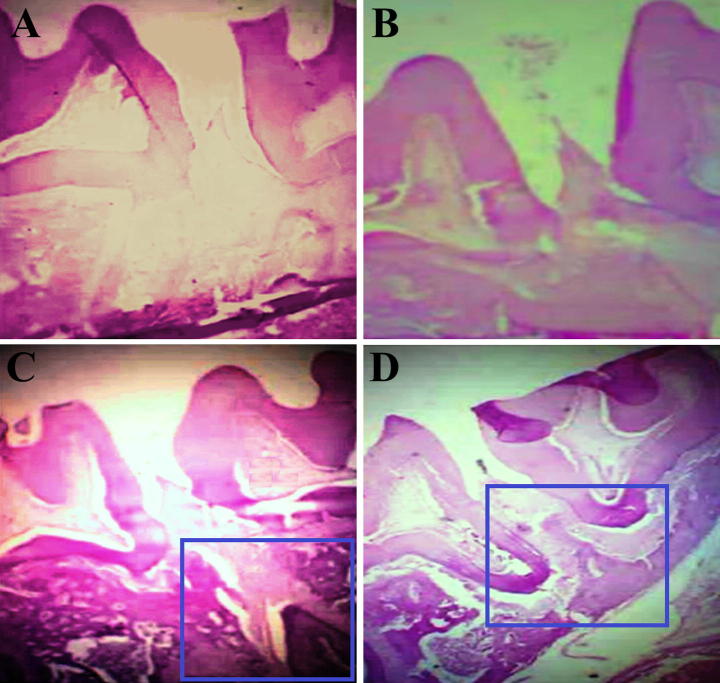
Rat’s periodontium histopathological results were presented in Fig. 6 for ligature–induced periodontitis. The distance of cementoenamel junction (CEJ)–bone and resorption of septal bone might be utilized for measurement of bone loss in periodontitis (Xie et al., 2011). All the figures showed the results found that no clear differentiation of CEJ–bone distance of any treatment groups. The distance of CEJ–Bone was not found in between the treatment groups. It was observed that there was no septal bone i.e. alveolar bone destruction in which Fig. 6A as a control and Fig. 6B as a pure eugenol if we were determine the septal bone resorption. It was observed that activity of pure eugenol was reduced may be due to the gingival crevicular fluid washing rapidly. There was another results found that septal bone have minimal or no destruction when it was treated with Doxycycline (Fig. 6C) and EUG–NE–Gel (Fig. 6D). Therefore, we concluded, it may be due to the resorption of septal bone prevented by EUG–NE–Gel in periodontitis.
3.17. TNF-α and IL-1β detection in gingival tissue
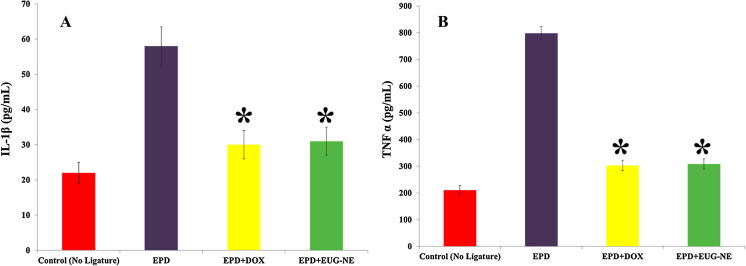
Group A is used as control i.e. no ligature. The level of TNF-α and IL-1β in Group B [i.e. EPD] rats was increased to 798.01 ± 17.23 and 58.02 ± 4.01 respectively. After treatment of EPD rats, the level of TNF-α and IL-1β showed that Group C [i.e. EOD + DOX] had more significant reduction of cytokine levels [TNF-α (303.90 ± 18.19) and IL-1β (29.98 ± 2.09)]; Group D [EUG–NE–Gel] had significant reduction of cytokine levels [TNF-α (309.67 ± 19.09) and IL-1β (31.17 ± 3.11)], respectively in gingival tissue of Wistar rats subjected to EPD (Fig. 7). The cytokine levels of Group D were closed to the value of the control group [TNF-α (211.38 ± 17.49) and IL-1β (21.98 ± 3.01)], suggests that developed EUG–NE–Gel nano–formulation effectively treats periodontitis. The reduction in TNF-α 59.45% & IL-1β 48.01% were observed in Group D (Fig. 7).
Fig. 7.
Comparative effects of 5.5% w/w nanoemulsion-gel of E.
3.18. Antibacterial activity
The zone of growth inhibition of S. aureus and E. coli was studied by the agar-cup diffusion method. The Doxycycline-Gel showed more significant (p < 0.05) antibacterial effect on microbes S. aureus and E. coli having the zone of growth inhibition 9.94 ± 0.29 mm and 8.10 ± 0.31 mm, respectively. The zone of growth inhibition of S. aureus and E. coli in EUG–NE–Gel was found to be 8.82 ± 0.28 mm and 7.58 ± 0.31 mm, respectively. The zone of growth inhibition of S. aureus and E. coli in EUG–S was found to be 4.89 ± 0.31 mm and 5.01 ± 0.21 mm, respectively. The EUG–NE–Gel showed more antibacterial activity in comparison with eugenol alone and least difference in Doxycycline treated gel because in EUG–NE–Gel, eugenol was in nano–droplet size, which can be easily fused with the outer membrane of the microbes, and surfactants of the EUG–NE–Gel can disrupt the external membrane and kill the microbes (Hamouda et al., 1999a, Hamouda et al., 1999b, Lee et al., 2010).
4. Conclusion
Preparation of EUG-NE–Gel used effectively with help of optimization through “Quality by Design”, chemical engineering, and ultrasonic tailoring. Different Smix ratios plotted with the help of Pseudo–Ternary–Phase–Diagrams which have been suggested 3:1 Smix ratio contain maximum nanoemulsion region. In this case, spontaneous emulsification method was applied to prepare fine nanoemulsion. TEM and SEM were used for the confirmation of their size of globule and smooth surface. The cytokine levels of the group treated with EUG–NE–Gel were closed to the value of the control group suggests that developed EUG–NE–Gel nano–formulation effectively treats periodontitis. The histopathology of the periodontium showed that Group D had a highly significant decrement in resorption of alveolar bones, infiltration of inflammatory cell, and cementum (p < 0.05). The outcomes may be due to arbitrate by its inhibitory effect on the periodontal microbes and its modulator role in the inflammatory mechanism. EUG–NE–Gel nano–formulation have potential antibacterial, analgesic, and anesthetic properties to treat periodontal disease and further studies are necessary to further more explanation for its effectiveness in clinical situation.
Conflict of interests
All authors have no conflict in between. During this research study, the authors have not received any grants.
Acknowledgments
It’s my pleasure to give a chance to collaborate this study between Jamia Hamdard (Hamdard University), New Delhi, India and Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. I am very thankful to Prof. (Dr.) Farhan Jalees Ahmad.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahad A., Al-Saleh A.A., Al-Mohizea A.M., Al-Jenoobi F.I., Raish M., Yassin A.E.B., Alam M.A. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol® gel under Dermaroller® on rats with methyl prednisolone acetate-induced hypertension. Biomed. Pharmacother. 2017;89:177–184. doi: 10.1016/j.biopha.2017.01.164. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Ahmad R., Alam M.A., Ahmad F.J. Quantification and brain targeting of eugenol-loaded surface modified nanoparticles through intranasal route in the treatment of cerebral ischemia. Drug Res. (Stuttg) 2018 doi: 10.1055/a-0596-7288. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Ahmad R., Alam M.A., Samim M., Iqbal Z., Ahmad F.J. Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int. J. Biol. Macromol. 2016;88:320–332. doi: 10.1016/j.ijbiomac.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Ahmad R., Naqvi A.A., Alam M.A., Ashafaq M., Abdur Rub R., Ahmad F.J. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif. Cells Nanomed. Biotechnol. 2018;46(4):717–729. doi: 10.1080/21691401.2017.1337024. [DOI] [PubMed] [Google Scholar]
- Ahmad N., Alam M.A., Ahmad F.J., Sarafroz M., Ansari K., Sharma S., Amir M. Ultrasonication techniques used for the preparation of novel Eugenol-Nanoemulsion in the treatment of wounds healings and anti-inflammatory. J. Drug Deliv. Sci. Technol. 2018;46:461–473. [Google Scholar]
- Al-Suwayeh S.A., Taha E.I., Al-Qahtani F.M., Ahmed M.O., Badran M.M. Evaluation of skin permeation and analgesic activity effects of carbopol lornoxicam topical gels containing penetration enhancer. Sci. World J. 2014;2014:127495. doi: 10.1155/2014/127495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqil M., Kamran M., Ahad A., Imam S.S. Development of clove oil based nanoemulsion of olmesartan for transdermal delivery: Box-Behnken design optimization and pharmacokinetic evaluation. J. Mol. Liq. 2016;214:238–248. [Google Scholar]
- Botelho M.A., Martins J.G., Ruela R.S., Queiroz D.B., Ruela W.S. Nanotechnology in ligature-induced periodontitis: protective effect of a doxycycline gel with nanoparticules. J. Appl. Oral Sci. 2010;18(4):335–342. doi: 10.1590/S1678-77572010000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho M.A., Rao V.S., Carvalho C.B., Bezerra-Filho J.G., Fonseca S.G., Vale M.L., Montenegro D., Cunha F., Ribeiro R.A., Brito G.A. Lippia sidoides and Myracrodruon urundeuva gel prevents alveolar bone resorption in experimental periodontitis in rats. J. Ethnopharmacol. 2007;113(3):471–478. doi: 10.1016/j.jep.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Bouchemal K., Briançon S., Perrier E., Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int. J. Pharm. 2004;280(1–2):241–251. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Branco-de-Almeida L.S., Franco G.C., Castro M.L., Dos Santos J.G., Anbinder A.L., Cortelli S.C., Kajiya M., Kawai T., Rosalen P.L. Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. J. Periodontol. 2012;83(5):664–671. doi: 10.1902/jop.2011.110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschi M.L., Jones D.S., Panzeri H., Gremião M.P., de Freitas O., Lara E.H. Semisolid systems containing propolis for the treatment of periodontal disease: in vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J. Pharm. Sci. 2007;96(8):2074–2089. doi: 10.1002/jps.20843. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Yang J., Sun H. Improved antioxidant capacity of optimization of a self-microemulsifying drug delivery system for resveratrol. Molecules. 2015;20(12):21167–21177. doi: 10.3390/molecules201219750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F.Q., Boukili M.A., da Motta J.I., Vargaftig B.B., Ferreira S.H. Blockade by fenspiride of endotoxin-induced neutrophil migration in the rat. Eur. J. Pharmacol. 1993;238(1):47–52. doi: 10.1016/0014-2999(93)90503-a. [DOI] [PubMed] [Google Scholar]
- Dahl J.E. Potential of dental adhesives to induce mucosal irritation evaluated by the HET-CAM method. Acta Odontol. Scand. 2007;65(5):275–283. doi: 10.1080/00016350701589286. [DOI] [PubMed] [Google Scholar]
- Ee S.L., Duan X., Liew J., Nguyen D. Droplet size and stability of nanoemulsions produced by the temperature phase inversion method. Chem. Eng. J. 2008;140:626–631. [Google Scholar]
- Gilleron L., Coecke S., Sysmans M., Hansen E., Van Oproy S., Marzin D., Van Cauteren H., Vanparys P. Evaluation of a modified HET-CAM assay as a screening test for eye irritancy. Toxicol. In Vitro. 1996;10(4):431–446. doi: 10.1016/0887-2333(96)00021-5. [DOI] [PubMed] [Google Scholar]
- Goswami D.S. Permeation enhancer for TDDS from natural and synthetic sources: a review. J. Biomed. Pharmaceut. Res. 2013;2:19–29. [Google Scholar]
- Ghosh V., Mukherjee A., Chandrasekaran N. Ultrasonic emulsification of foodgrade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013;20(1):338–344. doi: 10.1016/j.ultsonch.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Hamouda T., Cao Z., Tonda R. A novel surfactant nanoemulsion with broad spectrum sporicidal activity against bacillus species. J. Infect. Dis. 1999;180:1939–1949. doi: 10.1086/315124. [DOI] [PubMed] [Google Scholar]
- Hamouda T., Hayes M.M., Cao Z., Tonda R., Johnson K., Wright D.C., Brisker J., Baker J.R., Jr. A novel surfactant nanoemulsion with broad spectrum sporicidal activity against bacillus species. J. Infect. Dis. 1999;180(6):1939–1949. doi: 10.1086/315124. [DOI] [PubMed] [Google Scholar]
- Hong C.H., Hur S.K., Oh O.J., Kim S.S., Nam K.A., Lee S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J. Ethnopharmacol. 2002;83(1–2):153–159. doi: 10.1016/s0378-8741(02)00205-2. [DOI] [PubMed] [Google Scholar]
- Hosny K.M., Banjar Z.M. The formulation of a nasal nanoemulsion zaleplon in situ gel for the treatment of insomnia. Expert Opin. Drug Deliv. 2013;10(8):1033–1041. doi: 10.1517/17425247.2013.812069. [DOI] [PubMed] [Google Scholar]
- Jadhav B.K., Khandelwal K.R., Ketkar A.R., Pisal S.S. Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases. Drug Dev. Ind. Pharm. 2004;30:195–203. doi: 10.1081/ddc-120028715. [DOI] [PubMed] [Google Scholar]
- Jafari S.M., Assadpoor E., He Y., Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008;22(7):1191–1202. [Google Scholar]
- Jaiswal M., Kumar A., Sharma S. Nanoemulsions loaded Carbopol® 934 based gel for intranasal delivery of neuroprotective Centella asiatica extract: in–vitro and ex–vivo permeation study. J. Pharm. Investig. 2016;46:79–89. [Google Scholar]
- Javed S., Kohli K. Local delivery of minocycline: a therapeutic paradigm in periodontal diseases. Curr. Drug Deliv. 2010;7:398–406. doi: 10.2174/156720110793566290. [DOI] [PubMed] [Google Scholar]
- Jones D.S., Woolfson A.D., Djokic J., Coulter W.A. Development and mechanical characterization of bioadhesive semisolid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm. Res. 1996;13:1734–1738. doi: 10.1023/a:1016413428473. [DOI] [PubMed] [Google Scholar]
- Khurana S., Jain N.K., Bedi P.M. Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci. 2013;92:383–392. doi: 10.1016/j.lfs.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Lee V.A., Karthikeyan R., Rawls H.R., Amaechi B.T. Anticariogenic effect of a cetylpyridinium chloride containing nanoemulsion. J. Dent. 2010;38:742–749. doi: 10.1016/j.jdent.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima V., Vidal F.D., Rocha F.A., Brito G.A., Ribeiro R.A. Effects of tumour necrosis factor-alpha inhibitors pentoxifylline and thalidomide on alveolar bone loss in short-term experimental disease in rats. J. Periodontol. 2004;75(1):162–168. doi: 10.1902/jop.2004.75.1.162. [DOI] [PubMed] [Google Scholar]
- Liu R., Li N., Liu N., Zhou X., Dong Z.M., Wen X.J., Liu L.C. Effects of systemic ornidazole, systemic and local compound ornidazole and pefloxacin mesylate on experimental periodontitis in rats. Med. Sci. Monit. 2012;18(3):BR95-102. doi: 10.12659/MSM.882514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luepke N.P. Hen’s egg chorioallantoic membrane for irritation potential. Food Chem. Toxicol. 1985;23:287–291. doi: 10.1016/0278-6915(85)90030-4. [DOI] [PubMed] [Google Scholar]
- McClements D.J., Xiao H. Potential biological fate of ingested nanoemulsions: influence of particle characteristics. Food Funct. 2012;3(3):202–220. doi: 10.1039/c1fo10193e. [DOI] [PubMed] [Google Scholar]
- Opdyke D.L.J. Elsevier; 1979. Monographs on Fragrance Raw Materials. 247–249, 376. [DOI] [PubMed] [Google Scholar]
- Ozdemir H., Kara M.I., Erciyas K., Ozer H., Ay S. Preventive effects of thymoquinone in a rat periodontitis model: a morphometric and histopathological study. J. Periodontal Res. 2012;47(1):74–80. doi: 10.1111/j.1600-0765.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- Safieh-Garabedian B., Poole S., Allchorne A., Winter J., Woolf C.J. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995;115(7):1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima Y., Ebisu S., Okada H. Effect of infection with Eikenella corrodens on the progression of ligature-induced periodontitis in rats. J. Periodontal Res. 1990;25:308–315. doi: 10.1111/j.1600-0765.1990.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Semenoff T.A., Semenoff-Segundo A., Bosco A.F., Nagata M.J., Garcia V.G., Biasoli E.R. Histometric analysis of ligature-induced periodontitis in rats: a comparison of histological section planes. J. Appl. Oral Sci. 2008;16(4):251–256. doi: 10.1590/S1678-77572008000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel F., Ramadan W., Faisal M.S., Rizwan M., Faiyazuddin M., Mustafa G., Shafiq S. Transdermal and topical delivery of anti-inflammatory agents using nanoemulsion/microemulsion: an updated review. Curr. Nanosci. 2010;6:184–198. [Google Scholar]
- Shinde U., Pokharkar S., Modani S. Design and evaluation of microemulsion gel system of nadifloxacin. Indian J. Pharm. Sci. 2012;74:237–247. doi: 10.4103/0250-474X.106066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M., Kohli K., Ali M. Formulation development of novel in situ nanoemulgel (NEG) of ketoprofen for the treatment of periodontitis. Drug Deliv. 2016;23(1):154–166. doi: 10.3109/10717544.2014.907842. [DOI] [PubMed] [Google Scholar]
- Srivastava M., Neupane Y.R., Kumar P., Kohli K. Nanoemulgel (NEG) of Ketoprofen with eugenol as oil phase for the treatment of ligature-induced experimental periodontitis in Wistar rats. Drug Deliv. 2016;23(7):2228–2234. doi: 10.3109/10717544.2014.958625. [DOI] [PubMed] [Google Scholar]
- Tadros T., Izquierdo P., Esquena J., Solans C. Formation and stability of nanoemulsions. Adv. Colloid Interface Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Varshosaz J., Tavakoli N., Saidian S. Development and physical characterization of a periodontal bioadhesive gel of metronidazole. Drug Deliv. 2002;9(2):127–133. doi: 10.1080/10426500290095601. [DOI] [PubMed] [Google Scholar]
- Vinardell M.P., Mitjans M. The chorioallantoic membrane test as a model to predict the potential human eye irritation induced by commonly used laboratory solvents. Toxicol. In Vitro. 2006;20:1066–1070. doi: 10.1016/j.tiv.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Xiao X., Li Y., Zhang G., Gao Y., Kong Y., Liu M., Tan Y. Detection of bacterial diversity in rat’s periodontitis model under imitational altitude hypoxia environment. Arch. Oral Biol. 2012;57(1):23–29. doi: 10.1016/j.archoralbio.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Xie R., Kuijpers-Jagtman A.M., Maltha J.C. Inflammatory responses in two commonly used rat models for experimental tooth movement: comparison with ligature induced periodontitis. Arch. Oral Biol. 2011;56(2):159–167. doi: 10.1016/j.archoralbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Xu Y., Wei W.A. Comparative study of systemic subantimicrobial and topical treatment of minocycline in experimental periodontitis of rats. Arch. Oral Biol. 2006;51:794–803. doi: 10.1016/j.archoralbio.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ouyang W.Q., Wei Y.P., Syed S.F., Hao C.S., Wang B.Z., Shang Y.H. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: a skin permeation study. Int. J. Nanomed. 2016;11:5971–5987. doi: 10.2147/IJN.S119286. [DOI] [PMC free article] [PubMed] [Google Scholar]