Abstract
Momordica charantia is used in folk medicine to manage diabetes mellitus. In this study, we investigated the possible herb-drug interaction between M. charantia fruit extract (MCFE) and glibenclamide (GLB) in streptozotocin-diabetic rats. Rats were divided into 7 groups. The 1st group received 3% Tween 80. The 2nd–5th groups were diabetic rats received vehicle, GLB (5 mg/kg), MCFE (250 and 500 mg/kg), respectively. The 6th–7th groups administered GLB plus MCFE (250 and 500 mg/kg), respectively. After 8 weeks, fasting blood glucose (FBG), insulin and glycosylated hemoglobin (HbA1c) levels were assessed. Histopathological and immunohistochemical examinations of the pancreases were done. Quantitative RT-PCR was used to analyze hepatic mRNA expression of insulin receptor (INR), glucose transporter 2 (Slc2a2) and peroxisome proliferator-activated receptor α (PPAR-α) genes. All medicaments greatly reduced FBG in diabetic rats when compared with diabetic control group. GLB plus MCFE combination was better than GLB alone in improving levels of insulin and HbA1c. All medicaments restored insulin content of pancreatic β-cells and reduced glucagon and somatostatin of alpha and delta endocrine cells. Moreover, GLB plus MCFE-500 was the most efficient in restoring INR, Slc2a2 and PPAR-α mRNA expression to their normal levels. In conclusion, MCFE in combination with GLB gives greater glycemic improvement than GLB monotherapy.
Keywords: Momordica charantia, Streptozotocin, Glibenclamide, INR, Slc2a2, PPAR-α
1. Introduction
Diabetes mellitus (DM) is a chronic condition of metabolic disorder characterized by increased blood glucose levels. The disease has a serious impact on health and quality of life of diabetic patients. Oral antidiabetic drugs are used to control diabetes. GLB is one of the oral antidiabetic agents, which acts by blocking ATP-sensitive potassium channels in β-cells of pancreas. This blocking induces depolarization of cell membrane, which causes voltage dependent calcium channels to open and causes an increase in intracellular Ca++ in the β-cells to stimulate insulin secretion from the pancreas, thus it is widely used in the treatment of diabetes (Nalwaya, 2008).
Nowadays, herbal drugs are highly reputable in the management of DM. A previous study has reported more than 300 herbal species, which possess antidiabetic activity (Ur-Rahman and Zaman, 1989).
Momordica charantia L. (Cucurbitaceae) is known as bitter melon in English and karela in Hindi (Grover and Yadav, 2004). It has attracted great interest for its different pharmacological effects, such as anti-diabetic, antioxidant, anti-inflammatory, hypotriglyceridemic and immune stimulating activities (Zhu et al., 2012). Some reports have shown that leaves and fruits of the plant had rich phenolics and exhibited potent antioxidant effect (Kubola and Siriamornpun, 2008). A great number of diabetics turn to self-medication using a combination of an oral antidiabetic drug and a herb. Although, a great number of reports indicate that using of oral antidiabetic agents together with a herb with antidiabetic activity intensifies the development of interactions (Miller, 1998). The interaction can be valuable or sometimes harmful. A valuable effect can be additive blood sugar lowering effect but this effect can be harmful also when the sugar level goes down the normal level. Since there is a potential for the combined use of GLB and MCFE by diabetic patients, the study is planned to evaluate the effect of MCFE on glycemic regulation achieved by GLB in STZ induced diabetic rats.
2. Materials and methods
2.1. Plant material and extraction
Fresh fruits of Momordica charantia L. were purchased from the local market in Al-Kharj city, Saudi Arabia. The identity of the fruits was confirmed by Dr. Mohammad Atiqur Rahman, taxonomist of the Medicinal, Aromatic and Poisonous Plants Research Center (MAPPRC), College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. The fresh fruits (5000 g) were macerated and extracted to exhaustion by percolation at room temperature with 90% ethanol (12 L), and the extract was evaporated under reduced pressure to leave 163.10 g of the total M. charantia extract (MCFE).
2.2. LC-MS study of the extract
ESI-MS in both positive and negative modes was carried out using XEVO TQD triple quadruple mass spectrometer (Waters Corporation, Milford, MA01757, USA). LC preformed on ACQUITY UPLC - BEH C18 1.7 µm - 2.1 × 50 mm Column at flow rate of 0.2 mL\min. Initial mobile phases composed of 90% water containing 0.1% formic acid/10% methanol containing 0.1% formic acid in gradient system ended with 10% water containing 0.1% formic acid/90% methanol containing 0.1% formic acid. Run time was 32 min. The sample (100 μg/mL) solution was prepared using high performance liquid chromatography (HPLC) analytical grade MeOH and volume of 10 μL was injected into the UPLC instrument. The parameters for analysis were as follows: source temperature 150 °C, cone voltage 30 eV, capillary voltage 3 kV, desolvation temperature 440 °C, cone gas flow 50 L/h, and desolvation gas flow 900 L/h. Mass spectra were detected in the ESI positive and negative ion mode between m/z 100–1000. The peaks and spectra were processed using the Maslynx 4.1 software and tentatively identified by comparing its mass spectrum with reported data for known components of MCFE.
2.3. Animals
The male Wister rats, aged 4 months (body weight: 260 ± 10 g), were obtained from the Animal House Colony at the National Research Centre (NRC), Egypt. The animals were housed under standard conditions of natural 12 h light and dark cycle and free access to feed and water. The experimental procedure complied with the National Institutes of Health Guide for the Care and were performed according to the protocol approved by the Institutional Animal Care and Use Committee at Cairo University (approval number: CU-II-F-14-18) and NRC Ethics Committee (approval number: MREC-17-142).
2.4. Acute toxicity study
Acute toxicity study for MCFE was carried in adult male Wister rats (n = 6) according to OECD guidelines (OECD, 2001). Rats were kept fasting providing only water, after which MCFE was administered orally by gastric tube in different gradual doses (1000–5000 mg/kg). The control rats treated with the vehicle (3% v/v Tween 80 in distilled water) and kept under the same conditions. Rats were kept under observation for symptoms of toxicity and/or mortalities after 0.5 h of extract administration and periodically during the first 24 h, then daily for a total of 14 days.
2.5. Induction of experimental diabetes
Diabetes was induced in overnight fasted rats following intraperitoneal injection of STZ (Sigma- Aldrich Corp, St. Louis, MO, USA) at a dose of 60 mg/kg body weight (Mehenni et al., 2016), dissolved in 0.1 M citrate buffer, pH 4.5. For vehicle control rats, only citrate buffer was administered. Three days later, diabetes was confirmed by determination of FBG levels in blood samples collected from the tail vein using a blood glucose meter (Accu-Check Performa, Roche Diagnostic, Germany). Only rats with blood glucose level >250 mg/dL were considered diabetic and included in the study.
2.6. Experimental design
GLB and the tested extract were suspended in 3% Tween 80. Three days after STZ injection, rats were randomly assigned into seven groups (n = 6).
-
1.
Normal control group (NC): Non-diabetic rats treated with vehicle (3% Tween 80, 5 mL/kg).
-
2.
Diabetic control group (DC): STZ-diabetic rats treated with vehicle (3% Tween 80, 5 mL/kg).
-
3.
GLB group: STZ-diabetic rats treated with GLB (5 mg/kg).
-
4.
MCFE-250 group: STZ-diabetic rats treated with MCFE (250 mg/kg).
-
5.
MCFE-500 group: STZ-diabetic rats treated with MCFE (500 mg/kg).
-
6.
GLB + MCFE-250 group: STZ-diabetic rats treated with GLB (5 mg/kg) plus MCFE (250 mg/kg).
-
7.
GLB + MCFE-500 group: STZ-diabetic rats treated with GLB (5 mg/kg) plus MCFE (500 mg/kg).
Doses of MCFE were selected following Hossain et al (Hossain et al., 2014). GLB and the tested extract were administered orally, once daily using oral tube for 8 weeks. Body weights of all the experimental animals were monitored at the beginning of the experiment (0-time) and at the ends of the 2nd, 4th and 8th week of treatments.
2.7. Estimation of biochemical parameters
Blood samples were withdrawn through the retro-orbital venous plexus under mild ketamine anesthesia from the overnight fasted animals into sampling tubes at weeks 0, 2, 4 and 8 post-medication. Blood samples were centrifuged at 5000 rpm for 20 min to separate serum. The FBG levels in serum were estimated at 0, 2, 4 and 8 weeks post-medication using the commercially available kits (Spinreact, Spain) while serum insulin levels were determined by using ELISA kits (Cobas, Belgium) according to the manufacturer’s manual.
At the end of the experiment, blood sample was obtained from the retro-orbital region of each rat into tubes containing EDTA as anticoagulant and used for estimation of total hemoglobin (Hb) and glycosylated hemoglobin (HbA1c) using the commercially available kits (QCA, Spain). Another blood sample was obtained to estimate the serum levels of lipid profile such as [triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C)], markers of liver injury [(alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (γ-GT) and total bilirubin (BRN)] according to the instructor manual of commercially available kits.
2.8. Tissue collection
Immediately following blood collection at the end of the experimental period, all animals were euthanized with an intraperitoneal overdose of pentobarbital sodium. Pancreas of each rat was separated from the surrounding tissue.
2.9. Oxidative stress and lipid peroxidation markers in the pancreatic tissues
A portion of pancreas was weighted and washed with ice-cold saline immediately, and kept at −80 °C until analysis. The pancreas tissues were homogenized separately in 0.1 M Tris-HCl (pH 7.4). Pancreas homogenates were centrifuged at 1700 rpm for 10 min and the supernatants were collected and maintained at −80 °C until subsequent biochemical analysis. Activities of the antioxidant enzymes as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) and levels of reduced glutathione (GSH) and malondialdehyde (MDA) in pancreatic homogenates were estimated using the corresponding assay kits purchased from Biodiagnostic (Egypt) according to the standard procedures in the manufacturer's instructions.
2.10. Histopathological examination of pancreas
Pancreatic tissues from each group were harvested and fixed in 10% neutral buffered formalin and routinely processed for paraffin embedding to obtain 4 µm sections according to the methods described by Bancroft and Gamble (Bancroft and Gamble, 2008).
2.11. Immunohistochemical analysis of insulin, glucagon and somatostatin
The immunohistochemical analysis of the insulin, glucagon and somatostatin content of the pancreatic islets were performed according to the methods described by Jevdjovic et al (Jevdjovic et al., 2005). After deparaffinization, rehydration, blocking of the endogenous peroxidase activity and antigenic retrieval, the tissue sections were incubated for 3 h with mouse monoclonal anti-insulin (18–0066; Zymed, San Francisco, CA) at a dilution of 1:50, sheep polyclonal anti-glucagon antibody (ab36232; Cambridge, UK) at a dilution of 1:100 and rabbit polyclonal anti-somatostatin antibody (ab108456; Cambridge, UK) at a dilution of 1:50. The tissue sections were incubated with a biotinylated goat anti rabbit antibody (Thermo scientific, USA) and rabbit anti-sheep antibody (ab6747; Cambridge, UK) for 10 min. The sections were incubated finally with Streptavidin peroxidase (Thermo scientific, USA), 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma) and counterstained with haematoxylin. In each field, the immunopositive areas were analyzed by Lieca Qwin 500 Image Analyzer (Leica, Cambridge, England) in 10 microscopic fields under high-power field (X400) microscope. Percentage of positive stained area (%) was calculated as mean of 10 fields/slide.
2.12. Real time-PCR for gene expression analysis
Total RNA was purified from liver samples using Qiagen Rneasy Mini Kit following the manufacturer's protocol. The purified RNA was reverse transcribed into cDNA and used for PCR with primers specific for Peroxisome Proliferator-Activated Receptor α (PPAR-α), Slc2a2 gene (coding glucose transporter 2, GLUT2) and insulin receptor gene (INR) as shown in Table 1. mRNA expression levels of the target genes were assessed using real time-PCR standardized by co-amplification with beta actin as a housekeeping gene, which served as an internal control. cDNA was added to a SYBR Green qPCR Master Mix (Qiagen) containing 30 pg/ml of each primer. The cDNA was amplified by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 15 s and extension at 72 °C for 45 s. During the first cycle, the 95 °C step was extended to 1 min. The β actin gene was amplified in the same reaction to serve as the reference gene.
Table 1.
Oligonucleotides primers sequences.
| Gene | Primer sequence | Accession | |
|---|---|---|---|
| PPAR-α | Forward | TTCGGAAACTGCAGACCT | NM_013196.1 |
| Reverse | TTAGGAACTCTCGGGTGAT | ||
| INSR | Forward | TTTGTCATGGATGGAGGCTA | XM_006248753.2 |
| Reverse | CCTCATCTTGGGGTTGAACT | ||
| Slc2a2 | Forward | TCTGTGCTGCTTGTGGAG | XM_006232207.2 |
| Reverse | ACTGACGAAGAGGAAGATGG | ||
| Β-actin | Forward | ATGGTGGGTATGGGTCAG | NM_031144.3 |
| Reverse | CAATGCCGTGTTCAATGG | ||
2.13. Statistical analysis
Results were presented as mean ± SEM. Statistical analysis of all the data obtained was evaluated using one-way ANOVA followed by Dunnett’s multiple comparison tests (SPSS Program; Version 11.5). A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. LC-MS study of the extract
The LC-MS study of the MCFE in the positive and negative modes; enable the identification of the key phytochemicals and their semiquantitative estimated concentrations base on the peak area of each compound. Results are depicted in Fig. 1 and Table 2.
Fig. 1.
A: LC-MS spectrum of Momordicin I, B: LC-MS spectrum of Momordicin II in positive mode.
Table 2.
Identified compounds in MCFE and their relative percentage obtained from LC-MS.
| Name | Molecular weight | M+ and/or M− | Percentage | Retention time |
|---|---|---|---|---|
| Momordicin II | 634 | 657a | 1.37 | 14.43 |
| Momordicin I | 472 | 473 | 1.73 | 16.73 |
| Charantin | 473 | 496a | 0.51 | 19.73 |
| Momordicophenoide A | 432 | 431/433 | 0.6 | 22.99 |
| Linolenic or α-oleostearic acid | 278 | 279 | 1.66 | 25.25 |
| Oleic acid | 282 | 281 | 4.28 | 26.76 |
| Stearic acid | 284 | 283 | 8.34 | 27.54 |
M+ + Na.
3.2. Acute toxicity study
The rats treated with doses up to 5 g/kg of MCFE did not show any symptom of toxic reaction or lethality during 14 days of observation. The obtained findings assure the non-toxic nature of the extract.
3.3. Effect on body weight
At the end of the 2nd, 4th and 8th week of medication period, the weights of DC rats were significantly decreased compared with those of NC rats (Table 3) The diabetic groups that received GLB, MCFE-250, MCFE-500 and GLB plus MCFE-250 combination did not show significant weight gain at the end of the 2nd week of medications compared to DC group. However, the same groups exhibited marked (P ≤ 0.05) improvement in weight gain after 4 and 8 weeks of medications compared to DC rats. The GLB plus MCFE-500 combinations showed better effect on body weight of diabetic rats at the end of the 2nd, 4th and 8th week treatment. Interestingly, body weights in the diabetic groups received MCFE-500 were higher than those in rats treated with GLB monotherapy allover the experimental period. This combination therapy was able to successfully normalize the body weight of diabetic rats after 4 weeks medication (P ≤ 0.05).
Table 3.
Effect of GLB, MCFE and their combination on body weights of STZ-diabetic rats.
| Treatment groups | Body weight (g) |
|||
|---|---|---|---|---|
| 0-time | 2 weeks | 4 weeks | 8 weeks | |
| NC | 267.1 ± 11.7 | 314.5 ± 10.2b | 357.9 ± 9.4b | 403.7 ± 10.4b |
| DC (STZ) | 261.5 ± 10.4 | 267.6 ± 11.8a | 283.1 ± 12.5a, c | 300.9 ± 11.8a, c |
| GLB | 268.6 ± 9.5 | 300.6 ± 13.5 | 320.6 ± 10.2a, b | 353.7 ± 11.2a, b |
| MCFE-250 | 269.5 ± 10.8 | 298.7 ± 14.3 | 318.4 ± 9.1a, b | 347.1 ± 13.2a, b |
| MCFE-500 | 267.2 ± 12.3 | 303.1 ± 12.5 | 321.8 ± 10.7a, b | 362.9 ± 12.7a, b |
| GLB + MCFE-250 | 268.3 ± 11.2 | 304.8 ± 12.7 | 326.1 ± 10.2a, b | 370.4 ± 10.4a, b |
| GLB + MCFE-500 | 269.6 ± 9.5 | 312.7 ± 13.3b | 352.5 ± 10.0b, c | 397.2 ± 10.3b, c |
Values are expressed as mean ± SE (n = 6).
Multiple group comparisons were performed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test at p ≤ 0.05.
P ≤ 0.05, statistically significant from the normal control (NC) group.
P ≤ 0.05, statistically significant from the diabetic control (DC) group.
P ≤ 0.05, statistically significant from GLB group.
3.4. Effect on fasting blood glucose levels
Table 4 illustrates the effects of administration of GLB and MCFE monotherapies or their combination on FBG levels in STZ-diabetic rats. After 2, 4 and 8 weeks of medication, the FBG levels were significantly lower in the GLB monotherapy group compared to the values of DC rats at the corresponding time. The antidiabetic effect of GLB monotherapy was superior to that of MCFE-250. On the other hand, MCFE-500 monotherapy or the GLB plus MCFE-250 combination were able to reduce the elevated FBG level at the 2nd week (54.09% and 56.61%, respectively), 4th week (58.89% and 60.24%, respectively) and 8th week (61.49% and 62.54%, respectively) of treatments in comparison to their day-0 values but these reduction values were insignificant compared to GLB-treated group. Importantly, the rats treated with the GLB plus MCFE-500 combination achieved a more significant reduction in the FBG level compared with that for the rats that received GLB monotherapy. This combination brought back the FBG level to normal value (106.1 ± 6.06 mg/dL) after 2 weeks treatment.
Table 4.
Effect of GLB, MCFE and their combination on serum levels of FBG in STZ-diabetic rats.
| Treatment groups | FBG (mg/dL) |
|||
|---|---|---|---|---|
| 0-time | 2 week | 4 weeks | 8 weeks | |
| NC | 96.3 ± 5.43b, c | 95.7 ± 5.57b, c | 95.2 ± 5.32b, c | 95.3 ± 6.67b, c |
| DC (STZ) | 349.2 ± 7.57a | 351.5 ± 18.72a, c | 364.8 ± 16.35a, c | 365.8 ± 17.71a, c |
| GLB | 339.4 ± 9.87a | 166.3 ± 8.15a, b | 162.6 ± 8.23a, b | 143.5 ± 7.34a, b |
| MCFE-250 | 347.1 ± 5.08a | 192.7 ± 9.22a, b | 189.1 ± 9.08a, b | 166.8 ± 8.70a, b |
| MCFE-500 | 345.9 ± 7.66a | 158.8 ± 9.63a, b | 142.2 ± 7.32a, b | 133.2 ± 6.72a, b |
| GLB + MCFE-250 | 347.8 ± 8.97a | 150.9 ± 5.30a, b | 138.3 ± 7.57a, b | 130.3 ± 6.46a, b |
| GLB + MCFE-500 | 358.5 ± 6.98a | 119.6 ± 7.15a,b, c | 106.1 ± 6.06b, c | 100.50 ± 5.89b, c |
Values are expressed as mean ± SE (n = 6).
Multiple group comparisons were performed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test at p ≤ 0.05.
P ≤ 0.05, statistically significant from the normal control (NC) group.
P ≤ 0.05, statistically significant from the diabetic control (DC) group.
P ≤ 0.05, statistically significant from GLB group.
3.5. Effect on fasting blood insulin levels
Table 5 summarizes the levels of insulin in serum of medicated rats at the end of the two, four and eight-week treatment periods. At these periods, the serum insulin levels of DC rats were significantly lower. The diabetic rats in the GLB monotherapy group showed significantly (P ≤ 0.05) elevated levels of insulin (5.6 ± 0.33, 5.9 ± 0.20 and 6.1 ± 0.21 U/L, respectively) compared to DC rats. The STZ-rats exposed to MCFE-250, MCFE-500 and GLB plus MCFE-250 combination had markedly (P ≤ 0.05) increased insulin levels comparable to those induced by GLB monotherapy. Although the serum insulin level of diabetic rats exposed to GLB plus MCFE-250 combination did not reach statistical significance for the comparison with GLB monotherapy, a statistically significant difference was observed in favor of GLB plus MCFE-500 combination therapy at the end of the 2nd, 4th and 8th week of medication. The present results revealed that the highest percentage elevation in blood insulin level in diabetic rats was exhibited by GLB plus MCFE-500 combination after 2, 4 and 8 weeks of treatment. At these times, the blood insulin levels of diabetic rats exposed to this combination (6.5 ± 0.22, 7.0 ± 0.49 and 7.1 ± 0.44 U/L, respectively) are comparable to those of NC rats (8.7 ± 0.47, 8.6 ± 0.50 and 8.6 ± 0.59 U/L, respectively).
Table 5.
Effect of GLB, MCFE and their combination on serum levels of insulin in STZ-diabetic rats.
| Treatment groups | Insulin (U/L) |
|||
|---|---|---|---|---|
| 0-time | 2 week | 4 weeks | 8 weeks | |
| NC | 8.5 ± 0.15b, c | 8.7 ± 0.47b, c | 8.6 ± 0.50b, c | 8.6 ± 0.59b, c |
| DC (STZ) | 3.3 ± 0.12a | 3.2 ± 0.20a, c | 3.2 ± 0.26a, c | 3.1 ± 0.20a, c |
| GLB | 3.5 ± 0.10a | 5.6 ± 0.33a, b | 5.9 ± 0.20a, b | 6.1 ± 0.21a, b |
| MCFE-250 | 3.3 ± 0.13a | 5.2 ± 0.37a, b | 5.4 ± 0.28a, b | 5.5 ± 0.28a, b |
| MCFE-500 | 3.3 ± 0.10a | 5.8 ± 0.39a, b | 6.0 ± 0.30a, b | 6.2 ± 0.28a, b |
| GLB + MCFE-250 | 3.4 ± 0.11a | 6.0 ± 0.30a, b | 6.3 ± 0.25a, b | 6.5 ± 0.20a, b |
| GLB + MCFE-500 | 3.4 ± 0.10a | 6.5 ± 0.22a, b, c | 7.0 ± 0.49b, c | 7.1 ± 0.44b, c |
Values are expressed as mean ± SE (n = 6).
Multiple group comparisons were performed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test at p ≤ 0.05.
P ≤ 0.05, statistically significant from the normal control (NC) group.
P ≤ 0.05, statistically significant from the diabetic control (DC) group.
P ≤ 0.05, statistically significant from GLB group.
3.6. Effect on total hemoglobin (Hb) and glycosylated hemoglobin (HbA1c) levels
DC rats showed significant (P ≤ 0.05) reduction in Hb (10.7 ± 0.16 mg/dL) and increase (P ≤ 0.05) in HbA1c (13.2 ± 0.27%) levels in blood when compared to normal levels (14.3 ± 0.39 mg/dL and 7.1 ± 0.18%, respectively). Over the 8-week treatment period, HbA1c levels improved from 13.2 ± 0.27% in DC rats to 8.8 ± 0.16% with GLB monotherapy, to 9.2 ± 0.22% with MCFE-250 monotherapy and to 8.5 ± 0.34% with MCFE-500 monotherapy (Table 6). The end of treatment mean ± SEM of HbA1c was 8.1 ± 0.35% for GLB plus MCFE-250 combination and 7.5 ± 0.28% for GLB plus MCFE-500 combination. The GLB plus MCFE-500 combination exhibited improvement in the blood level of HbA1c, compared to the diabetic control and GLB monotherapy groups, and nearly normalized the level of both Hb and HbA1c.
Table 6.
Effect of GLB, MCFE and their combination on blood levels of total Hb and HbA1c in STZ-diabetic rats.
| Treatment groups | Total hemoglobin (mg/dL) | HbA1c (%) |
|---|---|---|
| NC | 14.3 ± 0.39b, c | 7.1 ± 0.18b, c |
| DC (STZ) | 10.7 ± 0.16a, c | 13.2 ± 0.27a, c |
| GLB | 12.9 ± 0.33a, b | 8.8 ± 0.16a, b |
| MCFE-250 | 12.4 ± 0.32a, b | 9.2 ± 0.22a, b |
| MCFE-500 | 13.0 ± 0.34a, b | 8.5 ± 0.34a, b |
| GLB + MCFE-250 | 13.2 ± 0.30a, b | 8.1 ± 0.35a, b |
| GLB + MCFE-500 | 13.9 ± 0.30b, c | 7.5 ± 0.28b, c |
Values are expressed as mean ± SE (n = 6).
Multiple group comparisons were performed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test at p ≤ 0.05.
P ≤ 0.05, statistically significant from the normal control (NC) group.
P ≤ 0.05, statistically significant from the diabetic control (DC) group.
P ≤ 0.05, statistically significant from GLB group.
3.7. Effect on serum lipid profile
Table 7 shows serum lipid profile in the control and experimental groups. DC rats showed a significant (P ≤ 0.05) elevation in the blood concentrations of TG (46.4 ± 3.24 mg/dL), TC (64.8 ± 3.15 mg/dL) and LDL-C (26.6 ± 0.87 mg/dL) in comparison with the values of NC rats (28.8 ± 1.12 mg/dL, 42.2 ± 1.27 mg/dL and 15.8 ± 0.66 mg/dL, respectively). On the other hand, HDL-C was significantly reduced (14.4 ± 0.58 mg/dL) when compared to 25.5 ± 1.76 mg/dL of NC group. Administration of GLB, MCFE-250 and MCFE-500 monotherapies and the GLB plus MCFE-250 combination significantly (P ≤ 0.05) decreased the levels of TG, TC and LDL-C in diabetic rats compared to DC group but levels remained significantly elevated compared with NC animals. Additionally, the levels of HDL-C were significantly increased (19.44%, 24.31%, 30.56% and 26.39%, respectively) compared to DC group. Significant improvements were also observed in the serum lipid profile for the GLB plus MCFE-500 combination therapy group. Administering this combination to diabetic rats tends to bring serum lipid profile to normal values.
Table 7.
Effect of GLB, MCFE and their combination on lipid profile in blood of STZ-diabetic rats.
| Treatment groups | TG (mg/dL) | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) |
|---|---|---|---|---|
| NC | 28.8 ± 1.12b, c | 42.2 ± 1.27b, c | 25.5 ± 1.76b, c | 15.8 ± 0.66b, c |
| DC (STZ) | 46.4 ± 3.24a, c | 64.8 ± 3.15a, c | 14.4 ± 0.58a, c | 26.6 ± 0.87a, c |
| GLB | 37.6 ± 2.14a, b | 54.2 ± 1.78a, b | 17.2 ± 0.65a, b | 21.9 ± 0.95a, b |
| MCFE-250 | 35.5 ± 2.06a, b | 51.8 ± 1.52a, b | 17.9 ± 0.46a, b | 20.2 ± 0.67a, b |
| MCFE-500 | 32.4 ± 1.10a, b | 48.6 ± 1.86a, b | 18.8 ± 0.49a, b | 19.2 ± 0.82a, b |
| GLB + MCFE-250 | 34.6 ± 2.02a, b | 49.5 ± 1.56a, b | 18.2 ± 0.58a, b | 19.4 ± 0.75a, b |
| GLB + MCFE-500 | 30.7 ± 1.37b, c | 45.4 ± 1.74b, c | 21.4 ± 0.93b, c | 17.5 ± 0.72b, c |
Values are expressed as mean ± SE (n = 6).
Multiple group comparisons were performed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test at p ≤ 0.05.
P ≤ 0.05, statistically significant from the normal control (NC) group.
P ≤ 0.05, statistically significant from the diabetic control (DC) group.
P ≤ 0.05, statistically significant from GLB group.
3.8. Effect on liver function biomarkers
Table 8 depicts the levels of liver function biomarkers (ALT, AST, ALP, γ-GT and BRN) in the serum of rats. Significantly (P ≤ 0.05) elevated levels of ALT (113.0 ± 6.68 U/L), AST (172.7 ± 8.17 U/L), ALP (342.3 ± 14.42 U/L), γ-GT (28.5 ± 1.67 U/L) and BRN (1.43 ± 0.10 mg/dL) were observed in serum of DC rats compared to NC group (52.2 ± 3.51 U/L, 68.3 ± 4.74 U/L, 144.5 ± 8.95 U/L, 13.8 ± 0.97 U/L and 0.55 ± 0.03 mg/dL, respectively). After administration of GLB, MCFE-250 and MCFE-500 monotherapies, the enzyme activities and BRN levels in serum were reduced toward the standard levels but the values still significant in comparison with those in NC rats. Similarly, the serum levels of ALT, AST, ALP, γ-GT and BRN were significantly (P ≤ 0.05) reduced in the diabetic rats exposed to the GLB plus MCFE-250 combination (32.21%, 45.28%, 27.46%, 31.58% and 47.55%, respectively) when compared to those of DC rats. The GLB plus MCFE-500 combination exhibited improvements in the liver function biomarkers compared to DC and GLB monotherapy groups, and nearly normalized the levels of liver function biomarkers.
Table 8.
Effect of GLB, MCFE and their combination on Liver function biomarkers in blood of STZ-diabetic rats.
| Treatment groups | ALT (U/L) | AST (U/L) | ALP (U/L) | γ-GT (U/L) | BRN (mg/dL) |
|---|---|---|---|---|---|
| NC | 52.2 ± 3.51b, c | 68.3 ± 4.74b, c | 144.5 ± 8.95b, c | 13.8 ± 0.97b, c | 0.55 ± 0.03b, c |
| DC (STZ) | 113.0 ± 6.68a, c | 172.7 ± 8.17a, c | 342.3 ± 14.42a, c | 28.5 ± 1.67a, c | 1.43 ± 0.10a, c |
| GLB | 88.7 ± 4.79a, b | 107.3 ± 6.37a, b | 286.5 ± 12.57a, b | 23.2 ± 1.61a, b | 0.92 ± 0.06a, b |
| MCFE-250 | 82.8 ± 4.89a, b | 98.5 ± 5.51a, b | 274.8 ± 12.53a, b | 22.6 ± 1.79a, b | 0.84 ± 0.06a, b |
| MCFE-500 | 78.5 ± 4.62a, b | 97.3 ± 5.66a, b | 259.5 ± 11.42a, b | 20.2 ± 1.27a, b | 0.78 ± 0.05a, b |
| GLB + MCFE-250 | 76.6 ± 4.98a, b | 94.5 ± 5.16a, b | 248.3 ± 12.09a, b | 19.5 ± 1.35a, b | 0.75 ± 0.05a, b |
| GLB + MCFE-500 | 63.5 ± 3.95b, c | 84.5 ± 5.95b, c | 172.2 ± 9.39b, c | 17.3 ± 1.29b, c | 0.67 ± 0.05b, c |
Values are expressed as mean ± SE (n = 6).
Multiple group comparisons were performed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test at p ≤ 0.05.
P ≤ 0.05, statistically significant from the normal control (NC) group.
P ≤ 0.05, statistically significant from the diabetic control (DC) group.
P ≤ 0.05, statistically significant from GLB group.
3.9. Effect on oxidative stress and lipid peroxidation markers in the pancreatic tissues
Table 9 illustrates the effect of GLB and MCFE on oxidative stress and lipid peroxidation markers in the pancreatic homogenates of rats. The pancreatic homogenates of DC rats showed significant (P ≤ 0.05) reduction in the levels of SOD, GPx, CAT and GSH (24.8 ± 1.80 U/mg protein, 1.9 ± 0.12 U/mg protein, 4.8 ± 0.22 U/mg protein and 3.5 ± 0.22 µmol/g tissue, respectively) along with elevation in the level of MDA (43.1 ± 2.85 nmol/g tissue) as compared to normal control group (52.4 ± 3.18 U/mg protein, 6.6 ± 0.61 U/mg protein, 11.3 ± 0.92 U/mg protein, 9.6 ± 0.71 µMol/g tissue and 25.6 ± 1.15 nmol/g tissue, respectively). As depicted in Table 9, the diabetic groups exposed to GLB, MCFE-250, MCFE-500 monotherapies or GLB plus MCFE combinations showed significantly (P ≤ 0.05) increased levels of SOD, GPx, CAT and GSH, whereas MDA levels were significantly (P ≤ 0.05) decreased as compared with DC rats. Interestingly, the GLB plus MCFE-500 combination exhibited improvements in the pancreatic levels of SOD, GPx, CAT, GSH and MDA, compared to DC values, and nearly normalized the levels of SOD, CAT and MDA.
Table 9.
Effect of GLB, MCFE and their combination on oxidative stress and lipid peroxidation parameters in pancreatic tissues of STZ-diabetic rats.
| Treatment groups | SOD (U/mg protein) | GPx (U/mg protein) | CAT (U/mg protein) | GSH (µmol/g tissue) | MDA (nmol/g tissue) |
|---|---|---|---|---|---|
| NC | 52.4 ± 3.18b, c | 6.6 ± 0.61b, c | 11.3 ± 0.92b, c | 9.6 ± 0.71b, c | 25.6 ± 1.15b, c |
| DC (STZ) | 24.8 ± 1.80a | 1.9 ± 0.12a | 4.8 ± 0.22a | 3.5 ± 0.22a | 43.1 ± 2.85a |
| GLB | 29.2 ± 2.84a, b | 3.0 ± 0.28a, b | 5.7 ± 0.42a, b | 5.4 ± 0.29a, b | 33.6 ± 2.36a, b |
| MCFE-250 | 36.1 ± 1.96a, b | 3.1 ± 0.29a, b | 6.4 ± 0.33a, b | 5.8 ± 0.33a, b | 32.4 ± 2.14a, b |
| MCFE-500 | 37.5 ± 3.39a, b | 3.3 ± 0.38a, b | 6.9 ± 0.48a, b | 6.0 ± 0.48a, b | 31.7 ± 2.11a, b |
| GLB + MCFE-250 | 39.0 ± 2.80a, b | 3.4 ± 0.39a, b | 7.3 ± 0.64a, b | 6.1 ± 0.58a, b | 30.8 ± 2.00a, b |
| GLB + MCFE-500 | 43.8 ± 2.89b, c | 3.6 ± 0.47b, c | 8.8 ± 0.72b, c | 6.5 ± 0.60b, c | 26.5 ± 2.01b, c |
Values are expressed as mean ± SE (n = 6).
Multiple group comparisons were performed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test at p ≤ 0.05.
P ≤ 0.05, statistically significant from the normal control (NC) group.
P ≤ 0.05, statistically significant from the diabetic control (DC) group.
P ≤ 0.05, statistically significant from GLB group.
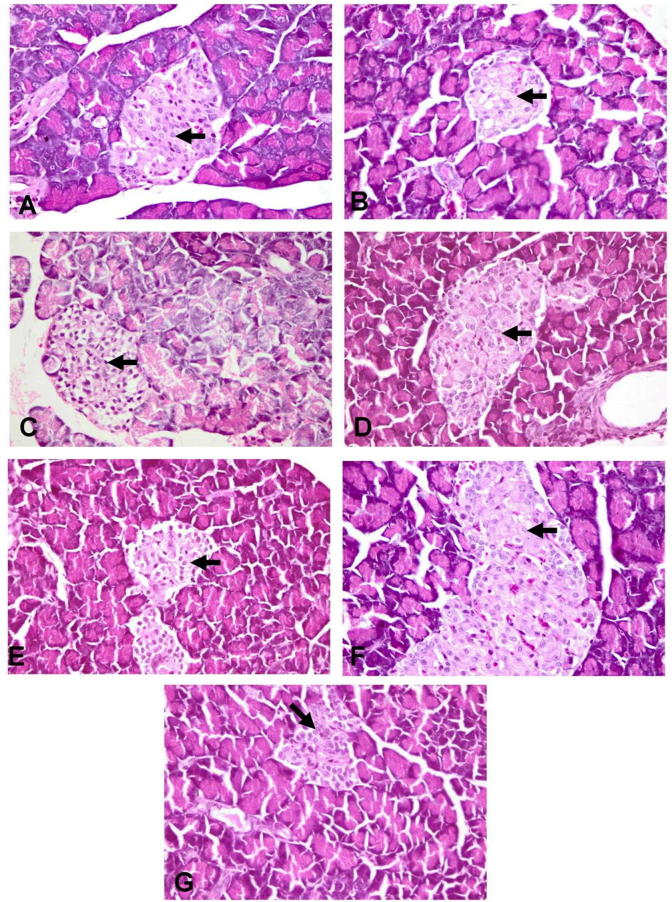
3.10. Histopathological examination of pancreases
The NC group revealed normal pancreatic acini and islets of Langerhans that is characterized by central core of β-cells with large mantle of α and δ endocrine cells in the peripheral zone (Fig. 2-A). In DC group, the islets of Langerhans showed massive reduction in the number of β-cells (Fig. 2-B) with marked increase in the number of α and δ endocrine cells with disruption in the appearance of the islets. Papillary hyperplasia of the epithelial lining pancreatic duct was also observed. The groups treated with MCFE-500, GLB plus MCFE-250, GLB plus MCFE-500 showed moderate to marked improvement in the previously described histopathological lesions of the pancreatic tissue (Fig. 2-E, F, G). The groups treated with GLB and MCFE-250 showed the slight to moderate enhancement in the histopathological lesions induced by STZ (Fig. 2-C, D).
Fig. 2.
Photomicrographs of rats’ pancreas (stained with H&E X 400), (A) normal control showing normal histological architecture of the pancreas with centrally located β-cells (arrow); (B) diabetic control showing distortion of the islet with marked necrosis (arrow), vacuolation and decrease in number of β-cells; (C) GLB showing marked elevation in the number of β-cells with Karyopyknosis of some cells (arrow); (D) MCFE-250 and (E) MCFE-500 showing moderate increase in the number of the β-cells with necrosis of some cells (arrow); (F) GLB plus MCFE-250 and (G) GLB plus MCFE-500 showing marked increase in the centrally located β-cells (arrow) with mild vacuolization of its cytoplasm.
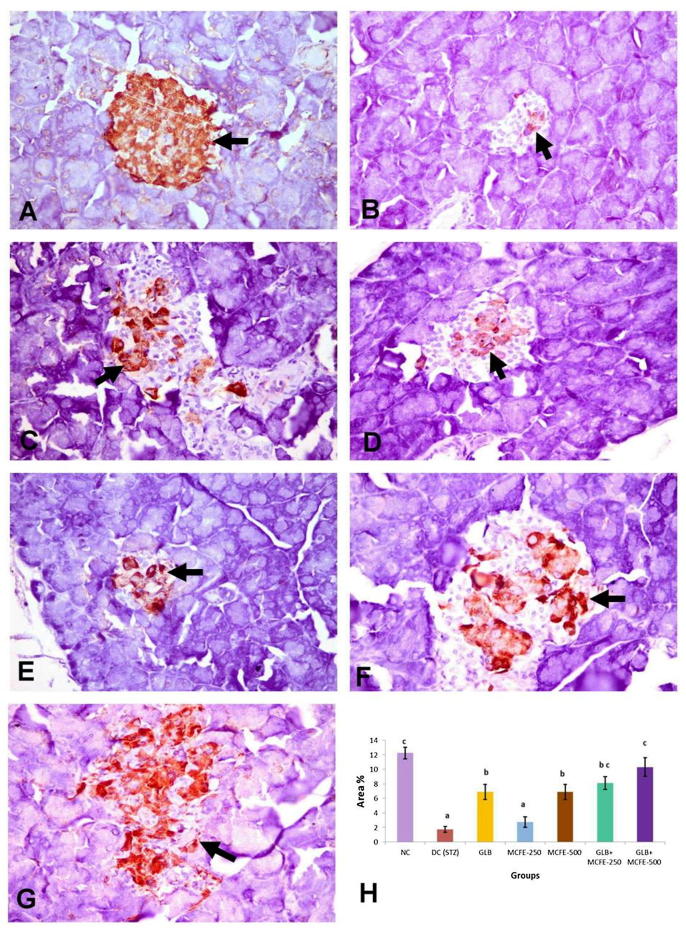
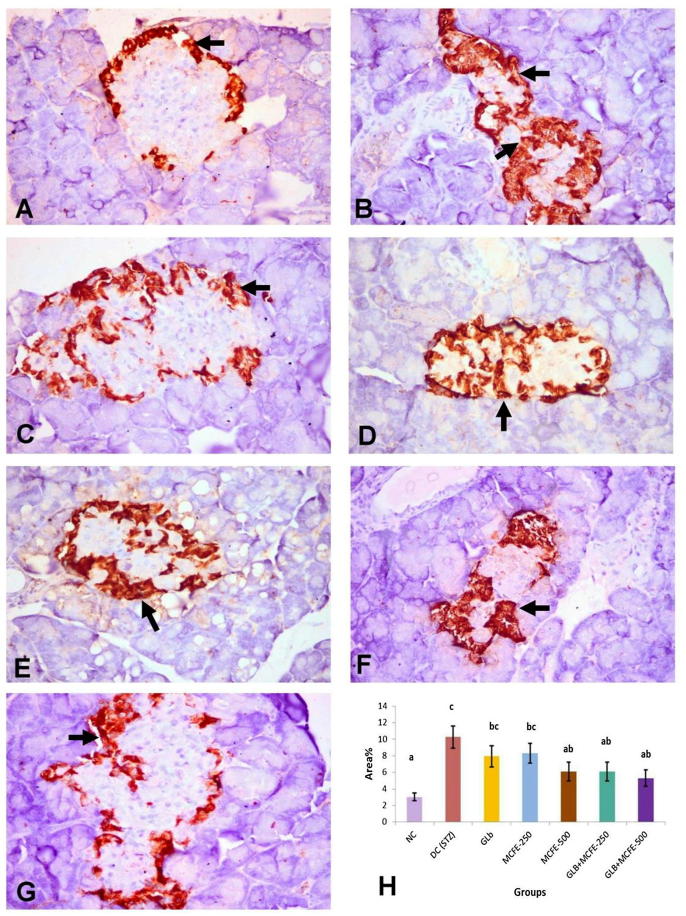
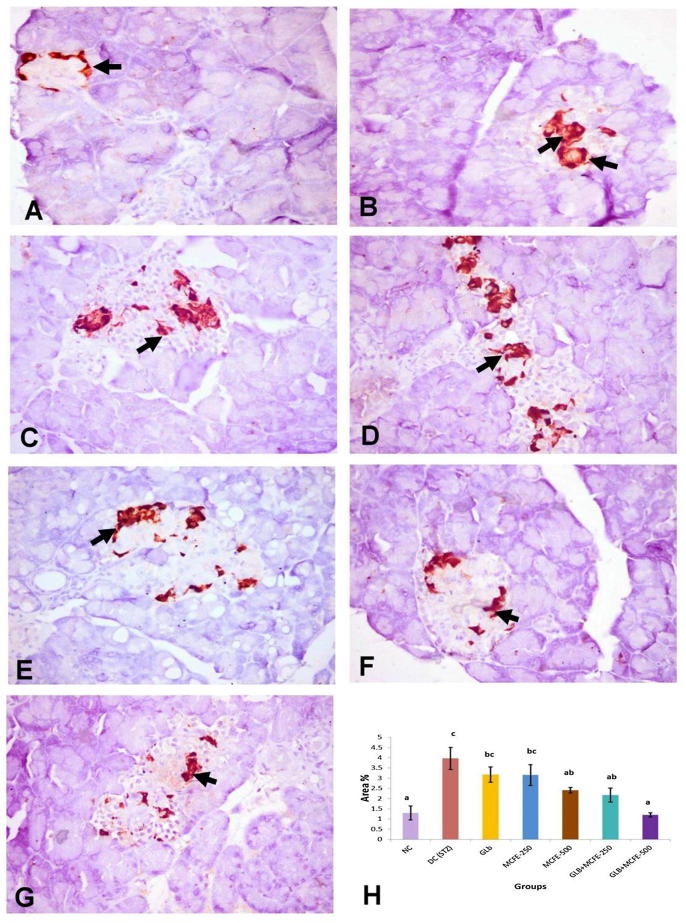
3.11. Immunohistochemical analysis of insulin, glucagon and somatostatin protein expression
The NC group showed diffuse distribution of positive anti-insulin immunostaining all over the pancreatic islets (Fig. 3-A). Anti-glucagon immunostaining was observed in α-cells located in the peripheral zone of the pancreatic islet (Fig. 4-A). Anti-somatostatin protein expression localized in δ-cells which forming incomplete ring in the peripherally of the islet of Langerhans (Fig. 5-A). The DC group revealed marked reduction in the insulin content of β-cells (Fig. 3-B) with marked elevation of the glucagon (Fig. 4-B) and somatostatin (Fig. 5-B) contents of the pancreatic islets with central in addition to peripheral distribution in the pancreatic islet. On one hand, the groups treated with GLB, MCFE-500, GLB plus MCFE-250 and GLB plus MCFE-500, the insulin contents of the β-cells were significantly increase compared to DC group (Fig. 3-C, E, F, G). Moreover, the glucagon content (Fig. 4- C, E, F, G) and the somatostatin content (Fig. 5-C, E, F, G) of their pancreatic islets; showed significance reduction when compared to DC group. On the other hand, the group treated with MCFE-250 showed no significance difference in the insulin content when compared with DC group (Fig. 3-D), while the glucagon (Fig. 4-D) and somatostatin contents (Fig. 5-D) of their pancreatic islets showed significant decrease when compared with DC group.
Fig. 3.
Representive anti-insulin immunohistochemistry in the islets of langerhans in the different experimental groups (X400), (A) normal control showing normal distribution of the insulin that occupies the center of the islet (arrow); (B) diabetic control showing marked reduction in the insulin content (arrow) of the β-cells in the islets of langerhans; (C) GLB, (D) MCFE-250 and (E) MCFE-500 showing moderate increase in insulin content of the β-cells (arrow); (F) GLB plus MCFE-250 and (G) GLB plus MCFE-500 showing marked increase in insulin content of the centrally located β-cells; (H) The bar chart represents anti-insulin immunopositive staining expressed as area %. Values with different superscripts are significantly different (p ≤ 0.05).
Fig. 4.
Representive anti-glucagon immunohistochemistry in the islets of langerhans in the different experimental groups (X400), (A) normal control showing normal distribution of gulcagon in peripherally located α-cells of islets (arrow); (B) diabetic control showing marked increase of the glucagon content of the α-cells in both central (arrow) and peripheral areas (C) GLB and (D) MCFE-250 showing mild elevation in glucagon content of the α-cells (arrow); (E) MCFE-500; (F) GLB plus MCFE-250 and (G) GLB plus MCFE-500 showing marked reduction in glucagon content of the α-cells in central area (arrow); (H) The bar chart represents anti-glucagon immunopositive staining expressed as area %. Values with different superscripts are significantly different (p ≤ 0.05).
Fig. 5.
Representive anti-somatostatin immunohistochemistry in the islets of langerhans in different experimental groups (X400). (A) Normal control showing incomplete ring of somatostatin content of δ-cells in the peripheral area of the islet (arrow). (B) Diabetic control showing marked elevation of somatostatin content of δ-cells occupying the peripheral and central area of the islet of langerhans (arrow). (C and D) GLB and MCFE-250, respectively, showing moderate increase in somatostatin content of δ-cells (arrow) in the central area of the islet. (E, F and G) MCFE-500, GLB plus MCFE-250 and GLB plus MCFE-500, respectively, showing mild decrease in somatostatin content of δ-cells (arrow) of the islet, compared with diabetic control group. (H) The bar chart represents anti-somatostatin immunopositive staining expressed as area %. Values with different superscripts are significantly different (p ≤ 0.05).
3.12. Real Time-PCR for gene expression analysis
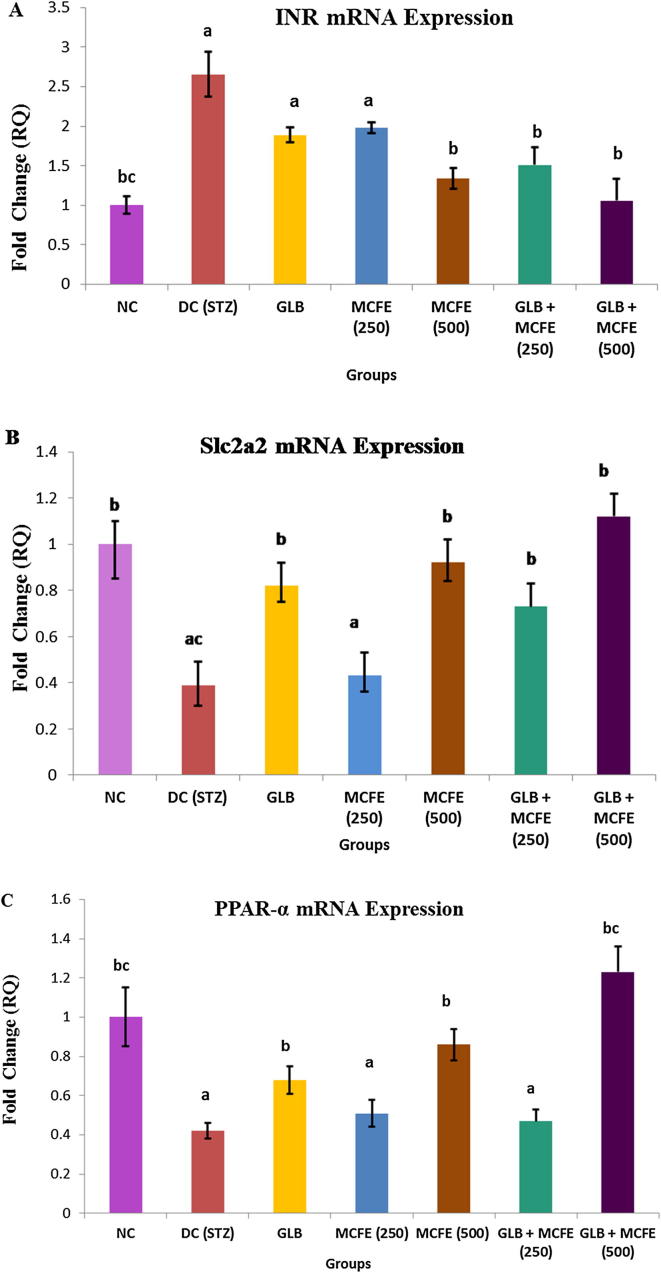
As shown in Fig. 6, the expression levels of the selected target genes (INR, Slc2a2, PPAR.) were altered in diabetic rats livers, compared with those in the control group (P ≤ 0.05). INR mRNA expression was markedly increased in diabetic control group to about 2.6 fold of its normal expression level. Following the administration of MCFE-500 or GLB plus MCFE-250 for 8 weeks, the expression of INR gene decreased significantly to 1.3 and 1.5 folds compared with DC group. GLB plus MCFE-500 nearly normalize the hepatic INR expression (Fig. 6-A). Slc2a2 gene expression decreased significantly in the STZ-induced diabetic group to about 39% of its normal expression level. Treatment of diabetic rats with GLB, MCFE-500, GLB plus MCFE-250 or GLB plus MCFE-500 combinations restored Slc2a2 mRNA level to 82%, 92%, 73% and 1.12% of its normal level, respectively (Fig. 6-B). Results of PPAR-α mRNA expression indicated that STZ resulted in a significant reduction in the liver PPAR-α expression (0.47 fold of its normal level). However, administration of GLB, MCFE-500, or GLB plus MCFE-500 combination enhanced the liver PPAR-α expression (0.68, 0.86 and 1.23, respectively) compared with DC group (Fig. 6-C).
Fig. 6.
Effect of GLB, MCFE and their combinations on gene expression in liver tissue of STZ-diabetic rats. (A) Insulin receptor (INR), (B) (Slc2a2) (coding GLUT2), (C) PPAR-ɑ. Values are expressed as mean ± SE. aP ≤ 0.05, statistically significant from the NC group. bP ≤ 0.05, statistically significant from the DC group. cP ≤ 0.05, statistically significant from the GLB group.
4. Discussion
LC-MS study of MCFE in positive and negative modes allows the identification of numerous compounds in the extract with their semiquantitative relative quantities by correlating the peak area of each component to the total area of the extract. The antidiabetic effect of M. charantia was correlated to terpenoids and saponins (Jia et al., 2017). The major saponins; momordicin I and momordicin II could be identified in the MCFE in relative amounts of 1.73 and 1.37%, respectively. The minor saponin momordicophenoide A was also identified (Jia et al., 2017). Both stearic acid (8.34%) and oleic acid (4.28%) are the most abundant components in the MCFE as both bulbs and seeds are rich in fatty components (Table 2).
The acute toxicity testing did not show any detectable signs of toxicity in rats’ administered MCFE at doses up to 5 g/kg. Consequently, it is proposed that oral median lethal dose (LD50) of the tested extract was higher than 5000 mg/kg b.wt. No mortality was observed during the test period. Since substances possessing LD50 higher than 50 mg/kg are non-toxic (Buck et al., 1976), the tested extracts were considered safe.
M. charantia is one of the most promising plants to treat DM (Joseph and Jini, 2013). Simultaneous use of herb and drug can interact with each other and may result in herb drug interaction. In the current study, the efficacy of combining GLB with MCFE was assessed to determine if there was any pharmacological benefit over GLB alone.
Diabetic rats gained significantly less weight during the course of the study as compared to NC rats. Low body weight in diabetes might be a result of tissue proteins breakdown, severe muscle degeneration and increased muscle wasting (Singh et al., 2008). Interestingly, we noted that the GLB plus MCFE-500 combination was more potent for improving body weight than the GLB monotherapy in diabetic rats. In this study, DC rats exhibited high FBG level and low insulin level compared with NC rats. The serum levels of glucose and insulin reflect the glycemic state of these animals. Insulin level in diabetic rats in this study, imply that some insulin secretory β-cells are intact and still have the potential of insulin synthesis and secretion. Treatment of diabetic rats with GLB reduced the blood glucose level and increased insulin level compared with DC group. However, these levels were not returned to the normal state. GLB improves hyperglycemia mostly by blocking ATP-sensitive potassium channels in β-pancreatic cells, which results in depolarizing cell membrane, opening voltage-dependent Ca++ channel. Accordingly, the level of intracellular Ca++ in the β-pancreatic cells increases and results in stimulation of insulin release, that is to say, the release of preformed insulin (Serrano-Martín et al., 2006). Therefore, the GLB requires the presence of a critical mass of β-cells with insulin secretory capacity in order to acts. In addition, the serum levels of glucose and insulin were not significantly different among the GLB, MCFE-250, MCFE-500 and GLB plus MCFE-250 groups. Further, the efficacy of MCFE-500 in reducing the blood glucose levels is much higher than that of the GLB. Several studies have established that M. charantia has a powerful antidiabetic effect through cell-based assays, animal models and human clinical trials (Jia et al., 2017). Of the five diabetic-treatment groups, the GLB plus MCFE-500 combination was found to be the most effective in decreasing FBG and elevating insulin levels throughout the experimental period. The blood glucose and insulin levels, which were monitored for 8 weeks after the induction of diabetes, showed more stability and were within the normal physiological ranges following administration of GLB plus MCFE-500 combination. Notably, the beneficial effect on glycaemic control observed for GLB plus MCFE-500 combination occurs without an increased risk of hypoglycaemia. Various mechanisms have been reported in the literature to explain the possible antidiabetic effects of MCFE. These mechanisms include the recovery of partially destroyed β-cells in the pancreas (Ahmed et al., 2001) or displaying insulin-like properties (Chen et al., 2003). Fernandes et al. (2007) proposed that the mechanism of M. charantia extracts as an antidiabetic might be owed to stimulating insulin release by the β-cells of the pancreas, diminshing glycogenesis in liver tissue, enhancing the utilization of peripheral glucose and increasing serum protein levels. Other studies considered the activation of the AMP-activated protein kinase system as another possible mechanism for antidiabetic action of M. charantia (Cheng et al., 2008). Nevertheless, some studies have also ascribed the antidiabetic effect of M. charantia to an extra-pancreatic effect, which includes increased GLUT4 transporter protein of muscles and increased glucose consumption in both liver and muscle (Sarkar et al., 1996).
The antihyperglycemic differences between GLB and MCFE may be due to the bioactive compounds of M. charantia. The major constituents of M. charantia, which are accountable for the antidiabetic effect, are glycosides, saponins, alkaloids, triterpenes, steroids and polyphenolic compounds (Joseph and Jini, 2013). Individually, isolated phytochemicals (charantin, a polypeptide-p, momordin, oleanolic acid 3-O-monodesmoside, and oleanolic acid 3-O-glucuronide) of M. charantia have shown glucose lowering activity (Grover and Yadav, 2004). Pitiphanpong et al. (2007) revealed that charantin might be used to control DM and can possibly be a replacing treatment. Several studies have reported that charantin is more effective than the oral hypoglycemic agent tolbutamide (Joseph and Jini, 2013). Two compounds were isolated as saponins from M. charantia; Momordicine II and 3-hydroxycucurbita-5, 24-dien-19-al-7, 23-di-O-β-glucopyranoside (4). These compounds revealed marked insulin releasing effect in MIN6 β-cells at two different concentrations 10 and 25 µg/mL (Keller et al., 2011). Another compound cucurbitanes that was isolated from M. charantia has also been reported to be responsible for the anti-diabetic activity (Chen et al., 2005). Fasting blood glucose levels points to an additive effect of the co-administration of GLB and MCFE-500 since these levels were not recovered to the normal values by the treatment of GLB or MCFE-500 alone.
In diabetes, high blood glucose levels react with hemoglobin to form glycated heamoglobin (HbA1c). Consequently, the total Hb level is diminished in diabetics. The rate of glycosylation is directly correlated with the blood glucose level. HbA1c is formed extensively and irreversibly over a period of time. Therefore, HbA1c is considered as an outstanding marker of overall glycemic control. In our study, untreated diabetic rats showed significant decrease in Hb and significant increase in HbA1c levels indicating poor glycemic control. According to the glucose-lowering effect, MCFE-250 and MCFE-500 monotherapies and GLB plus MCFE-250 combination significantly reduced HbA1c levels compared to DC rats but did not significantly improve the rate of glycosylation compared with GLB treatment alone. Blood Hb and HbA1c levels were effectively controlled in the rats that received GLB plus MCFE-500 combination more than that seen in animals treated with GLB alone. Both, Hb and HbA1c levels were reversed to normal range following 8 weeks administration of GLB plus MCFE-500 combination. The present results reveal that, GLB, MCFE and their combinations protect against heamoglobin glycation in decreasing order of GLB + MCFE-500 > GLB + MCFE-250 > MCFE-500 > GLB > MCFE-250. This effect might be due to the effective control of hyperglycemia by GLB plus MCFE-500 combination. This also indicates that the combination might be very effective for long-term control of DM and preventing further complications in diabetic individuals.
A well-known fact, that inadequate control of glucose in DM results in disturbance in the serum lipid profile (Shah and Khan, 2014). Under normal circumstances, insulin leads to lipoprotein lipase (LPL) activation and hydrolyzes lipoprotein bound TG leading to free fatty acids production (Ng et al., 1987). In diabetic state LPL is not initiated because of insulin inadequacy, resulting in hypertriglyceridemia (Pushparaj et al., 2007). The results of the current study indicated dyslipidemia in STZ-diabetic rats evidenced by elevated serum TG, TC and LDL-C coupled with reduced level of HDL-C compared to NC group. These findings are consistent with those obtained by other researchers (Verma et al., 2013). The increased level of serum TG could contribute to reduced level of blood insulin. Additionally, the elevated level of LDL-C found in DC rats may be attributed to cholesterol-mediated down-regulation of LDL receptors (Mustad et al., 1997). Decreased level of HDL-C following STZ administration may be owed to the acceleration of apoA-I clearance from the plasma due to high cholesterol levels (Mustad et al., 1997). Good glycemic control could result in improvement in lipoprotein abnormalities (Mullugeta et al., 2012). The direct relationship of glycemic control with dyslipidemia has also been confirmed by Mohammadi et al, (2009), who observed that the TG, LDL-C, and total serum lipids levels of poorly controlled diabetic children were higher than those of the control group. In our study, administration of GLB reversed all the changes in the lipid profile of diabetic rats that might be related to its ability to reduce sugar level. MCFE-250, MCFE-500 and GLB plus MCFE-250 treatments not only lowered the levels of TC and LDL-C, but also reduced TG and enhanced the levels of HDL-C in serum of diabetic rats. Interestingly the GLB plus MCFE-500 combination had a superior effect on regulating serum lipid than GLB monotherapy. Following oral administration of GLB plus MCFE-500 combination, lipid profile levels reverted back to those seen in normal rats which may be indicative of the stronger antidiabetic role played by the combination therapy. This effect might be owed to increased insulin secretion from pancreatic β-cells that further stimulate fatty acid synthesis and incorporation of fatty acids into TG in the liver and adipose tissue as well. Reductions in serum lipids, particularly of the TG and LDL-C to normal levels are considered as beneficial for the long-term prognosis of diabetic patients (Chattopadhyay and Bandyopadhyay, 2005). Since the combination of MCFE-500 with GLB produced further improvement in the lipid profile than that produced by GLB or MCFE per se, it is suggested that MCFE may be acting by some different mechanism than that of GLB on lipid metabolic pathways. The study of Gadang et al., (2011) proposed that M. charantia ameliorates lipid profile and serum glucose levels by modulating PPAR-γ gene expression. Saponins may possibly be the main active compounds to induce this contribution (Zhu et al., 2012). In addition, Popovich et al, (2010) found that M. charantia triterpenoid extract reduces lipid accumulation and adiponectin expression in 3T3-L1 cells.
Hyperglycaemia affects the metabolism of lipids, carbohydrates and proteins and can lead to liver damage. In the current study, the activities of the liver marker enzymes (AST, ALT, ALP and γ-GT) and level of BRN in serum were significantly elevated after STZ administration in comparison with normal controls, denoting hepatic injury. These results suggested that membrane permeability and transport function have been altered due to the liver injury, leading to leakage of these enzymes. The significant changes in liver enzymes is in agreement with the fact that T2DM is associated with the elevation of liver marker enzymes (Ghimire et al., 2018). Ghosh and Suryawanshi (2001) demonstrated that insulin deficiency during DM, contributes to increased serum levels of transaminase enzymes because sufficient amounts of amino acids stimulate the occurrence of gluconeogenesis and ketogenesis. Protective effects of GLB, MCFE-250, MCFE-500 and GLB plus MCFE-250 combination against diabetes-associated liver injury were confirmed by the decrease in the serum activities of AST, ALT, ALP and γ-GT but their levels still above the normal values. Treatment with GLB plus MCFE-500 combination significantly reversed the increased levels of liver marker enzymes and BRN. The activities of those hepatotoxicity markers in the combination-treated group remained almost the same as normal, indicating stimulation of insulin secretion into the circulation and a resulting hepatoprotective effect.
Abundant clinical evidence demonstrated that diabetes correlated closely with excessive generation of free radicals and oxidative stress (Long et al., 2018). In this respect, tissues have possesses an antioxidant defense system including enzymatic and non-enzymatic antioxidants to keep themselves against oxidative stress. The enzymatic antioxidants such as SOD, GPx and CAT are crucial components of the antioxidant defense system in the body. They have been considered as primary enzymes since they were involved in the direct elimination of reactive oxygen species. GSH is non-enzymatic antioxidant found in almost all forms of aerobic life and plays a vital role in maintaining cellular antioxidant capacity. The decreased activities of oxidative stress biomarkers in STZ-diabetic rats have been reported (Ravi et al., 2004). Similar results have been obtained in the present study. In the diabetic state, oxidative stress induction in the pancreas might be due to a hypoxic state as diabetes results in HbA1c formation that interferes with oxygen delivery at the pancreas. In this study, Oral administration of GLB to STZ-induced diabetic rats resulted in increased levels of SOD, GPx, CAT and GSH in their pancreatic tissues. This may be attributed to the antidiabetic effect of GLB. Further, treatment of diabetic rats with MCFE-250 and MCFE-500 resulted in the elevation of the antioxidant enzymes and GSH levels. The protective effect of both doses of MCFE and GLB plus MCFE-250 combination against oxidative stress in the pancreatic tissues of diabetic rats was superior to that seen in the group of rats that received GLB. Jia et al. (2017) reported that administration M. charantia alcoholic extract for eight successive weeks exerted antioxidant potentials by reversing the oxidant/antioxidant imbalance. In addition, several studies have demonstrated that M. charantia is a good natural source of antioxidants. The active compounds mainly include saponins, polysaccharides and phenolics (Lucas et al., 2010). In our study, the attenuation of oxidative stress by MCFE in STZ-diabetic rats might be mediated by MCFE’s antioxidant properties. The anti-oxidant efficacy of GLB plus MCFE-500 combination was more beneficial in diabetic rats than treatment with either GLB or GLB plus MCFE-250 combination. After oral administration of GLB plus MCFE-500 combination to diabetic rats, the altered levels of SOD, GPx, CAT and GSH in their pancreatic tissues were brought back to normal. These results suggest that GLB and MCE-500 have a synergistic effect against oxidative stress in STZ diabetes.
MDA is considered a good biomarker of the lipid peroxidation process. Results from previous studies showed that MDA levels in plasma and tissue are elevated in STZ-induced diabetic rats (Nakhaee et al. 2009). Similarly, the present study showed that STZ exposure significantly enhanced lipid peroxidation in the pancreatic tissues of experimental animals compared to normal group. The pancreatic levels of MDA were not significantly different among the GLB, MCFE-250, MCFE-500 and GLB plus MCFE-250 groups. Additionally, eight-week GLB plus MCFE-500 administration has successfully normalized the disordered level of MDA in the pancreas of diabetic rats. These results suggest that GLB and MCFE-500 have a synergistic effect on reducing lipid peroxidation in the pancreas of STZ diabetic rats. Improvement in the level of MDA in the pancreatic tissues may directly or indirectly protects β-cells against lipid peroxidation.
STZ uptake into the pancreatic β-cells islets induces β-cells toxicity by many mechanisms such as nitric oxide donation and free radicals production resulted in marked reduction of intra cellular insulin content of these cells (Nugent et al., 2008) and increase the glucagon and somatostatin content of α- and δ-cells located in the peripheral and central zones of the pancreatic islets. In our study, the immunohistochemical staining of insulin was dramatically decrease in the diabetic control group that was associated with vacuolation, apoptosis and necrosis of β-cells but, these abnormal histopathological lesions were markedly decreased in the groups with different medicaments specially groups treated with the combination of GLB plus MCEF. Therefore, the combination of GLB plus MCEF may protect the β-cells of the islets from toxicity produced by STZ which appeared in the form of reducing the hyperglycemia in these groups.
In the current study, liver INR mRNA expression was significantly increased in STZ- diabetic rats. Former studies showed that changes in IR expression partially contribute to the modulation of insulin binding in the liver of rats with experimental induction of insulin deficiency (Morakinyo et al., 2018). INR expression was significantly reduced by MCFE-500 and combination of GLB + MCFE-250. Interestingly, INR expression is nearly normalized in GLB plus MCFE-500 treated group. These results indicated an improved cellular sensitivity to insulin and, consequently, the regulation of glucose uptake and metabolism in liver. In the same context, hepatic glucose utilization is regulated by Slc2a2 which is a membrane bound, insulin-independent glucose transporter with a high glucose Michaelis constant (Km), mainly expressed in the liver. Defects in the Slc2a2-encoding gene potentially alter glucose homeostasis (Zhou et al, 2016). In line with the results from earlier studies, DC rats exhibited a significant decrease in the Slc2a2 mRNA level. Slc2a2 expression is corrected to near normal levels in groups treated with MCFE-500 or GLB plus MCFE-250 (Al-Shaqha et al., 2015).
PPARα isotype is one of PPARs family members of nuclear receptors that predominantly expressed in liver and play a central role in glucose and lipid homeostasis by regulation of their related genes expression (Berger and Moller, 2002). PPARα controls gluconeogenesis, fatty acid oxidation, ketone body biosynthesis and lipoprotein metabolism. PPARα activation in the liver is efficient to counteract metabolic hepatic disorders and indirectly alleviates insulin resistance (Berger and Moller, 2002). Concerted activation of PPAR subtypes has been proposed as an alternative, synergistic therapeutic approach in diabetes. Previous studies reported a down-regulation of the PPAR-α mRNA expression in the liver of diabetic rats (Kaviarasan and Pugalendi, 2009). A high glucose level alone is a direct cause of PPARα down-regulation in diabetic conditions (Hu et al., 2013).
In our study, treatment with GLB, MCFE-500, or GLB plus MCFE-500 combination enhanced the PPAR-α mRNA levels in the liver. Moreover, PPAR-α upregulation at the transcriptional level in MCEF-treated rats might be responsible for its influence on lipid profile. Targeting PPARα may offer a new therapeutic strategy for diabetes (Kaviarasan and Pugalendi, 2009).
5. Conclusion
In conclusion, these results indicate that combining MCFE with GLB therapy provided additional benefits in the form of reduced glycemic load and improvement in the lipid profile. Moreover, current study demonstrates that the pathway mediating the synergy between MCFE with GLB therapy involves up-regulation of hepatic PPARα expression. These observations suggest that MCFE is an attractive therapeutic option for the treatment of diabetic patients who have already undergone treatment with GLB. Further studies are needed to determine the ingredients of the extract.
Declaration of Competing Interest
The authors declare that; there is no conflict of interest.
Acknowledgment
The authors are thankful to Deanship of Scientific Research (DSR), Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia for providing the fund to carry out this study under research grants No. 6610/03/2018.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed I., Lakhani M.S., Gillett M., John A., Raza H. Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diabetes Res. Clin. Pract. 2001;51:155–161. doi: 10.1016/s0168-8227(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Al-Shaqha W., Khan M., Salam N., Azzi A., Chaudhary A. Anti-diabetic potential of Catharanthus roseus Linn. and its effect on the glucose transport gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats. BMC Compl. Altern. M. 2015;15:379. doi: 10.1186/s12906-015-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J.D., Gamble M. Elsevier Health Sciences; UK: 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- Berger J., Moller D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Buck W., Osweiter G., Van Gelder A. second ed. Kendall/Hunt publishing Co; Iowa: 1976. Clinical and Diagnostic Veterinary Toxicology; p. 5211. [Google Scholar]
- Chattopadhyay R., Bandyopadhyay M. Effect of Azadirachta indica leaf extract on serum lipid profile changes in normal and streptozotocin induced diabetic rats. Afr. J. Biochem. Res. 2005;8:101–104. [Google Scholar]
- Chen J.C., Chiu M.H., Nie R.L., Cordell G.A., Qiu S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- Chen Q., Chan L.L., Li E.T. Bitter melon (Momordica charantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet. J. Nutr. 2003;133:1088–1093. doi: 10.1093/jn/133.4.1088. [DOI] [PubMed] [Google Scholar]
- Cheng H., Huang H., Chang C., Tsai C., Chou C. A cell-based screening identifies compounds from the stem of Momordica charantia that overcome insulin resistance and activate AMP-activated protein kinase. J. Agric. Food Chem. 2008;27:6835–6843. doi: 10.1021/jf800801k. [DOI] [PubMed] [Google Scholar]
- Fernandes N.P., Lagishetty C.V., Panda V.S., Naik S.R. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Compl. Altern. Med. 2007;7:29. doi: 10.1186/1472-6882-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadang V., Gilbert W., Hettiararchchy N., Horax R., Katwa L., Devareddy L. Dietary bitter melon seed increases peroxisome proliferator-activated receptor-γ gene expression in adipose tissue, down-regulates the nuclear factor-κB expression, and alleviates the symptoms associated with metabolic syndrome. J. Med. Food. 2011;14:86–93. doi: 10.1089/jmf.2010.0010. [DOI] [PubMed] [Google Scholar]
- Ghimire S., Shakya S., Shakya J., Acharya P., Pardhe B.D. Abnormal liver parameters among individuals with type 2 Diabetes Mellitus nepalese population. Biochem. Pharmacol. (Los Angel) 2018;7:243. [Google Scholar]
- Ghosh S., Suryawanshi S. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J. Exp. Biol. 2001;39(8):748–759. [PubMed] [Google Scholar]
- Grover J., Yadav S. Pharmacological actions and potential uses of Momordica charantia: a review. J. Ethnopharmacol. 2004;93:123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Hossain M., Mostofa M., Abdul Awal M., Chowdhury E., Sikder M. Histomorphological and morphometric studies of the pancreatic islet cells of diabetic rats treated with aqueous extracts of Momordica charantia (karela) fruits. Asian Pacific J. Trop. Disease. 2014;4(2):S698–S704. [Google Scholar]
- Hu Y., Chen Y., Ding L., He X., Takahashi Y., Gao Y., Shen W., Cheng R., Chen Q., Qi X., Boulton M.E., Ma J.X. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. USA. 2013;110(38):15401–15406. doi: 10.1073/pnas.1307211110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevdjovic T., Maake C., Zwimpfer C., Krey G., Eppler E., Zapf J., Reinecke M. The effect of hypophysectomy on pancreatic islet hormone and insulin-like growth factor I content and mRNA expression in rat. Histochem. Cell Biol. 2005;123:179–188. doi: 10.1007/s00418-005-0760-y. [DOI] [PubMed] [Google Scholar]
- Jia S., Shen M., Zhang F., Xie J. Recent advances in Momordica charantia: functional components and biological activities. Int. J. Mol. Sci. 2017;18(12):2555. doi: 10.3390/ijms18122555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013;3(2):93–102. [Google Scholar]
- Kaviarasan K., Pugalendi K.V. Influence of flavonoid-rich fraction from Spermacoce hispida seed on PPAR-alpha gene expression, antioxidant redox status, protein metabolism and marker enzymes in high-fat-diet fed STZ diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2009;20(2):141–158. doi: 10.1515/jbcpp.2009.20.2.141. [DOI] [PubMed] [Google Scholar]
- Keller A.C., Ma J., Kavalier A., He K., Brillantes A.M.B., Kennelly E.J. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine. 2011;19:32–37. doi: 10.1016/j.phymed.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubola J., Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008;110:881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Long L., Qiu H., Cai B., Chen N., Lu X., Zheng S., Ye X., Li Y. Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget. 2018;9(4):5321–5336. doi: 10.18632/oncotarget.23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E.A., Dumancas G.G., Smith B.J., Clarke S.L., Arjmandi B.H. Health benefits of bitter melon (Momordica charantia) Foods Promot. Health. 2010;35:525–549. [Google Scholar]
- Mehenni C., Atmani-Kilani D., Dumarcay S., Perrin D., Gerardin P., Atmani D. Hepatoprotective and antidiabetic effects of Pistacia lentiscus leaf and fruit extracts. J. Food Drug Anal. 2016;24:653–669. doi: 10.1016/j.jfda.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions. Arch. Intern. Med. 1998;158(20):2200–2211. doi: 10.1001/archinte.158.20.2200. [DOI] [PubMed] [Google Scholar]
- Mohammadi H., Abdelouahed E., Hassar M., Bouchrif B., Qarbal B., Dahbi F., Hilal L., Ghalim N. Glycaemic control, HbA1c, and lipid profile in children with type 1 diabetes mellitus. Eur. J. Sci. Res. 2009;29(2):289–294. [Google Scholar]
- Morakinyo A., Samuel T., Adekunbi D. Magnesium upregulates insulin receptor and glucose transporter-4 in streptozotocin-nicotinamide-induced type-2 diabetic rats. Endocr. Regul. 2018;52(1):6–16. doi: 10.2478/enr-2018-0002. [DOI] [PubMed] [Google Scholar]
- Mullugeta Y., Chawla R., Kebede T., Worku Y. Dyslipidemia associated with poor glycemic control in Type 2 Diabetes Mellitus and the protective effect of metformin supplementation. Indian J. Clin. Biochem. 2012;27(4):363–369. doi: 10.1007/s12291-012-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustad V.A., Etherton T.D., Cooper A.D., Mastro A.M., Pearson T.A., Jonnalagadda S.S., Kris-Etherton P.M. Reducing saturated fat intake is associated with increased levels of LDL receptors on mononuclear cells in healthy men and women. J. Lipid Res. 1997;38(3):459–468. [PubMed] [Google Scholar]
- Nakhaee A., Bokaeian M., Saravani M., Farhangi A., Akbarzadeh A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J. Clin. Biochem. 2009;24(4):419–425. doi: 10.1007/s12291-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalwaya N. Spectrophotometric estimation of glibenclamide solid dosage form. Ind. Pharmacist. 2008;7(77):114–118. [Google Scholar]
- Ng T.B., Li W., Yeung H. Effects of ginsenosides, lectins and Momordica charantia insulin-like peptide on corticosterone production by isolated rat adrenal cells. J. Ethnopharmacol. 1987;21:21–29. [PubMed] [Google Scholar]
- Nugent D.A., Smith D.M., David M., Jones H.B. A review of islet of Langerhans degeneration in rodent models of type 2 diabetes. Toxicol. Pathol. 2008;36(4):529–551. doi: 10.1177/0192623308318209. [DOI] [PubMed] [Google Scholar]
- OECD, 2001. OECD Guideline for Testing of Chemicals: Acute Oral Toxicity-Acute Toxic Class Method. Guideline No. 423, Organization for Economic Co-operation and Development, 2001, pp. 1–14. <http://ntp.niehs.nih.gov/iccvam/SuppDocs/FedDocs/OE CD/OECD_GL423.pdf>.
- Pitiphanpong J., Chitprasert S., Goto M., Jiratchariyakul W., Sasaki M., Shotipruk A. New approach for extraction of charantin from Momordica charantia with pressurized liquid extraction. Sep. Purif. Technol. 2007;52:416–422. [Google Scholar]
- Popovich D.G., Li L., Zhang W. Bitter melon (Momordica charantia) triterpenoid extract reduces preadipocyte viability, lipid accumulation and adiponectin expression in 3T3-L1 cells. Food Chem. Toxicol. 2010;48:1619–1626. doi: 10.1016/j.fct.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Pushparaj P., Low H., Manikandan J., Tan B., Tan C. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007;111(2):430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Ravi K., Ramachandran B., Subramanian S. Protective effect of Eugenia jambolana seed kernel on tissue antioxidants in streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2004;27:1212–1217. doi: 10.1248/bpb.27.1212. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Pranava M., Marita R. Demonstration of hypoglycemic action of Momordica charantia in a validated animal model of diabetes. Pharmacol. Res. 1996;33:1–4. doi: 10.1006/phrs.1996.0001. [DOI] [PubMed] [Google Scholar]
- Serrano-Martín X., Payares G., Mendoza-Le A. Glibenclamide, a blocker of K+ATP channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2006;50(12):4214–4216. doi: 10.1128/AAC.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N., Khan M. Antidiabetic effect of Sida cordata in alloxan induced diabetic rats. Biomed Res. Int. 2014;2014:671294. doi: 10.1155/2014/671294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Gupta M., Sirohi P., Varsha Effects of alcoholic extract of Momordica charantia (Linn.) whole fruit powder on the pancreatic islets of alloxan diabetic albino rats. J. Environ. Biol. 2008;29(1):101–106. [PubMed] [Google Scholar]
- Ur-Rahman A., Zaman K. Medicinal plants with hypoglycemic activity. J. Ethnopharmacol. 1989;26(1):1–55. doi: 10.1016/0378-8741(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Verma P., Itankar P., Arora S. Evaluation of antidiabetic antihyperlipidemic and pancreatic regeneration, potential of aerial parts of Clitoria ternatea. Rev. Bras. Farmacogn. (Braz. J. Pharmacogn.) 2013;23:819–829. [Google Scholar]
- Zhou K., Yee S., Seiser E., van Leeuwen N., Tavendale R., Bennett A., Groves C., Coleman R., van der Heijden A., Beulens J.W., de Keyser C.E. Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat. Genet. 2016;48(9):1055–1059. doi: 10.1038/ng.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Dong Y., Qian X., Cui F., Guo Q., Zhou X., Wang Y., Zhang Y., Xiong Z. Effect of superfine grinding on antidiabetic activity of bitter melon powder. Int. J. Mol. Sci. 2012;13:14203–14218. doi: 10.3390/ijms131114203. [DOI] [PMC free article] [PubMed] [Google Scholar]