Abstract
We aimed to extend our knowledge on the relationship between physical fitness (PF) and both white matter microstructure and cognition through in-depth investigation of various cognitive domains while accounting for potentially relevant nuisance covariates in a well-powered sample. To this end, associations between walking endurance, diffusion-tensor-imaging (DTI) based measures of fractional anisotropy (FA) within brain white matter and cognitive measures included in the NIH Toolbox Cognition Battery were investigated in a sample of n = 1206 healthy, young adults (mean age = 28.8; 45.5% male) as part of the human connectome project. Higher levels of endurance were associated with widespread higher FA (pFWE < 0.05) as well as with enhanced global cognitive function (p < 0.001). Significant positive relationships between endurance and cognitive performance were similarly found for almost all cognitive domains. Higher FA was significantly associated with enhanced global cognitive function (p < 0.001) and FA was shown to significantly mediate the association between walking endurance and cognitive performance. Inclusion of potentially relevant nuisance covariates including gender, age, education, BMI, HBA1c, and arterial blood pressure did not change the overall pattern of results. These findings support the notion of a beneficial and potentially protective effect of PF on brain structure and cognition.
Subject terms: Risk factors, Cognitive neuroscience
Introduction
Over recent years increased attention has been dedicated to the relationship between physical fitness (PF) and brain physiology. PF has repeatedly shown protective effects on diverse mental health outcomes including decreased risk of future dementia1, decreased stress-related2 and depressive symptoms2,3. A better understanding of the precise relationships between PF as a modifiable lifestyle factor and healthy brain physiology would provide insights of crucial relevance for the future development of preventive efforts for various neuropsychiatric disorders.
More specifically, meta-analytic evidence supports the notion of a positive relationship between measures of physical activity and brain functioning as measured by cognitive performance across a wide range of cognitive domains including executive function, attention and working memory4,5. Importantly, these associations have been demonstrated across the life-span5,6 in healthy samples as well as in samples showing differing degrees of cognitive impairment (mild cognitive impairment, dementia)6–8.
Corresponding findings from neuroimaging research on positive associations between PF and white matter (WM) structure9,10 as well as gray matter volume11,12 appear to further support the notion of neuroprotective effects of physical activity and fitness. However, considerably less is known on the positive association of PF with white matter microstructure13,14 as assessed by diffusion tensor imaging (DTI), and studies combining PF, DTI and cognition are rare: In older adults (>60 years), white matter microstructure was positively associated with cardiorespiratory fitness and working memory performance15. The aforementioned correlational studies are furthermore supplemented by findings of prospective associations between PF, brain structure and cognition from the few existing longitudinal studies: Findings from one of the few randomized controlled trials (RCT) involving 120 participants on this topic reported increased hippocampal gray matter volume and increased cognitive performance compared to the control group following a 12-month aerobic walking intervention16. Similarly, another RCT adds further weight to this notion by showing that improvements in PF after a 12-month exercise intervention were associated with increased temporal and prefrontal white matter integrity as assessed via fractional anisotropy (FA) as well as with increased memory performance in an elderly sample (age 55–80; n = 70)17.
In sum, previous neurobiological research has demonstrated a positive relationship between PF and both brain structure and cognitive function. Yet, some heterogeneity in the literature must be acknowledged: As pointed out by a recent review and meta-analysis the association between aerobic exercise and cognitive performance varied substantially with effect sizes ranging from g = −0.015 to 0.275 for executive function and from g = 0.052 to 0.146 for attention and processing speed depending on the selected studies and the type of assessment instrument18. It thus appears highly relevant to further clarify the contribution of PF on distinct cognitive domains in a well powered sample. Furthermore, the observed heterogeneity might partially be due to the potentially undiscovered contribution of relevant covariates. Since the majority of previous studies did not control for further socioeconomic, cardiovascular and metabolic risk factors in a standardized manner, the possibility that reported associations between PF and brain structure and cognition in previous research might be subject to bias or spurious correlations cannot be suspended up to now. This notion appears particularly relevant since several variables closely related to PF such as body mass index (BMI)19,20, blood pressure21 as well as metabolic serum markers22 but also presence and history of neuropsychiatric disorders23–25 have been shown to contribute to variation in both brain structural integrity and cognitive function. Moreover, the majority of previous studies have investigated single cognitive domains only, while studies investigating associations between PF, white matter integrity and multiple differential cognitive domains simultaneously are rare which makes it difficult to delineate and to compare domain specific contributions of PF. Therefore, the aim of the present work was to overcome limitations of previous research (a) by validation of previous findings on the association between PF, brain structural integrity and cognition in a large sample of healthy, young adults (b) by simultaneously investigating the contribution of PF to a wider range of cognitive domains, (c) by accounting for potentially relevant covariates and (d) by performing mediation analyses to investigate whether the PF-cognition association is mediated by white matter microstructure.
Material and Methods
Participants
We investigated open-access brain imaging data from the Human Connectome Project (HCP) WU-Minn HCP 1200 Subjects Data Release26 (for further information on details of data acquisition and processing in this sample please see: https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release). Exclusion criteria were neurodevelopmental disorders (e.g., autism), documented neuropsychiatric disorders (e.g., schizophrenia or depression), neurologic disorders (e.g., Parkinson’s disease), diabetes or high blood pressure26. Per HCP protocol, all subjects gave written informed consent to the Human Connectome Project consortium. All subject recruitment procedures and informed consent forms, including consent to share data, were approved by the Washington University Institutional Review Board27. All experiments were performed in accordance with relevant guidelines and regulations. All 1206 subjects were included in the present study. Analyses were performed with the maximum number of available data for each analysis. We report the respective n for each analysis. The mean age of the sample was 28.8 years, 45.5% of all participants were male. BMI was calculated as the body weight (in kilogram) divided by squared body height (in meters) (masskg/heightm2). BMI was available for n = 1200, the mean was 27.09. Total education years, a proxy for socioeconomic status, were calculated as the years of completed education (range 11–17 years, mean = 14.87 years). Subjects were primarily recruited in Missouri; additional recruiting efforts were made to ensure that participants generally reflect the ethnic and racial composition of the U.S. population. For data acquisition participants visited the Washington University, St. Louis, Missouri, twice with a fixed order of magnetic resonance imaging (MRI) scanning and extensive behavioral assessment on each day26.
Endurance
Physical fitness was operationalized as a result on a walking endurance test. Endurance data was available for n = 1204 subjects. Endurance was assessed using the 2-min walk test as part of the Motor domain of the official NIH toolbox for the Assessment of Neurological and Behavioral Function28. In a single trial, subjects were asked to walk as fast as they could for 2 minutes on a 50-foot (out and back) course. After two minutes distance was measured in feet and inches. The raw scores were normed to a scale score with mean = 100, SD = 15. Extensive reliability and validity tests were performed for this test during implementation of the NIH toolbox29, showing a good test-retest reliability (intraclass correlation (ICC) > 0.80) and very high external validity, for instance a high correlation with the 6 minute walk test (r > 0.96). Performance on these walking tests have been associated with functional, morbidity and mortality outcomes across all ages both in clinical as well as healthy populations30.
Cognitive performance
Cognitive measures were available for n = 1187 subjects. All tests and cognitive outcome parameters included in the HCP dataset were included in the study. The NIH Cognition Total Composite Score (“global cognition score”) is calculated as the average of subtest-scores contained in the NIH Toolbox Cognition Battery (Flanker, Dimensional Change Card Sort, Picture Sequence Memory, List Sorting and Pattern Comparison, Picture Vocabulary and Reading Recognitions) and reflects overall cognitive performance28,31,32. For further information on all cognitive measures see Supplementary Methods 1.
DTI data acquisition
The following methods (Section 2.4 and 2.5) have been described in detail in our previous work19. In brief, DTI data was available for n = 1050 in the HCP sample. Data for the HCP was acquired on a customized Siemens 3 T “Connectome Skyra” housed at Washington University in St. Louis, using a standard 32-channel Siemens receive head coil and a “body” transmission coil designed by Siemens specifically for the smaller space available using the special gradients of the WU-Minn and MGH-UCLA Connectome scanners33,34.
A full diffusion MRI session includes 6 runs (each approximately 9 minutes and 50 seconds), representing 3 different gradient tables, with each table acquired once with right-to-left and left-to-right phase encoding polarities, respectively. Each gradient table includes approximately 90 diffusion weighting directions plus 6 b = 0 acquisitions interspersed throughout each run. Diffusion weighting consisted of 3 shells of b = 1000, 2000, and 3000 s/mm2 interspersed with an approximately equal number of acquisitions on each shell within each run (Sequence: Spin-echo EPI, TR 5520 ms, TE 89,5 ms, flip angle 78 deg, refocusing flip angle 160 deg, FOV 210 × 180 (RO × PE), matrix 168 × 144 (RO × PE), slice thickness 1.255 mm, 111 slices, 1.25 mm isotropic voxels, multiband factor 3, echo spacing 0.78 ms, BW 1488 Hz/Px, phase partial fourier 6/8, b-values 1000, 2000, 3000 s/mm2)34,35.
DTI data preprocessing and analysis
Diffusion data accessible from the HCP was preprocessed with their MR Diffusion Pipeline36: The diffusion preprocessing pipeline does the following: normalizes the b0 image intensity across runs; removes EPI distortions, eddy-current-induced distortions, and subject motion; corrects for gradient-nonlinearities; registers the diffusion data with the structural; brings it into 1.25 mm structural space; and masks the data with the final brain mask: 1. Basic preprocessing: Intensity normalization across runs, preparation for later modules. 2. ‘TOPUP’ algorithm for EPI distortion correction. 3. ‘EDDY’ algorithm for eddy current and motion correction. 4. Gradient nonlinearity correction, calculation of gradient bvalue/bvector deviation. 5. Registration of mean b0 to native volume T1w with FLIRT BBR + bbregister and transformation of diffusion data, gradient deviation, and gradient directions to 1.25 mm structural space. The brain mask is based on FreeSurfer segmentation.
Tract-based spatial statistics (TBSS)37 is a well-established analysis method for DTI imaging and was applied as described extensively in a recent study19. Briefly, standard TBSS preprocessing was performed37: The FA images were registered to the FMRIB58 FA template and averaged to create a mean FA image. A WM skeleton was created with an FA threshold of 0.2 and overlaid onto each subject’s registered FA image. Individual FA values were warped onto this mean skeleton mask by searching perpendicular from the skeleton for maximum FA values.
To test for statistical significance, we used the non-parametric permutation testing implemented in FSL’s ‘randomize’ with 5000 permutations. Threshold-Free Cluster Enhancement (TFCE)38 was used to correct for multiple comparisons. This allows to estimate cluster sizes corrected for the family-wise error (FWE; p < 0.05, 5000 permutations). MNI coordinates and cluster size at peak voxel were derived with FSL Cluster and the corresponding WM tract retrieved from the ICBM-DTI-81 white-matter atlas39.
Statistical analyses
-
In order to investigate whether associations between endurance and cognitive measures exist, we performed hierarchical linear regressions using SPSS (IBM Version 25). As baseline, the global cognition score was regressed on endurance. Control variables (sex, age, years of completed formal education, BMI, HbA1c and systolic blood pressure) were then successively incorporated to extend the regressions in order to check if any of the control variables significantly weaken the estimated observations. Subjects with missing values for any of the control variables were dropped beforehand to ensure a continuous sample throughout all hierarchical model specifications. The resulting sample consisted of n = 801 observations. The results are reported in Supplementary Results 1.
In the next step, we investigated whether endurance has a specific impact on any of the domains of cognition. Thus, we linearly regressed scores of each cognitive domain on endurance as the sole independent variable (“baseline”) as well as on the full model containing all above-mentioned covariates. The results are reported in Table 1.
Second, we investigated whether endurance has any impact on FA across the entire WM skeleton using general linear models within FSL. We accounted for the effects of nuisance covariates that could influence WM structure: age40 and sex41. Combined endurance and DTI data were available for a sample of n = 1048 subjects. To further test for the specificity of endurance-related effects, we extracted a mean FA value from all significant voxels and used this value in a baseline regression of endurance on FA within SPSS. In analogy to 1., we employed hierarchical regression specifications, subsequently adding control variables (age, sex, education years, BMI, HbA1c and systolic blood pressure) to the model. The results are reported in Supplementary Results 1.
We sought to investigate the relationship between cognition and endurance-related white matter microstructure. In line with the analysis in steps 1 and 2 above, hierarchical regressions within SPSS were performed using global cognition as the dependent variable and the extracted FA value as the regressor of interest, subsequently adding the above-mentioned covariates in the same order. The results are reported in Supplementary Results 1. Furthermore, additional regression analyses between FA and all available measures of cognitive domains were carried out to further delineate the contribution of specific domains to the observations made in step 1. above. Thus, we linearly regressed these subscores on FA as the sole independent variable (“baseline”) as well as on the full model containing all above-mentioned covariates. The results are reported in Table 1.
To directly test the presence of an indirect effect of walking endurance on cognitive performance through white matter microstructure, we carried out a mediation analysis with walking endurance as predictor variable (X), white matter microstructure as mediator (M) and global cognitive performance as outcome variable (Y). For this analysis step, a bootstrapping approach as implemented in the SPSS macro PROCESS was applied (http://www.processmacro.org) which has been demonstrated to provide reliable results in neuroimaging research42–44. PROCESS estimates direct and indirect effects between a defined set of variables by applying an ordinary least squares path analytic framework. Inference of indirect (mediated) effects is assessed through bootstrap confidence intervals. Significance of an indirect effects is assumed if the 95% confidence interval (95%-CI) does not include zero. The number of bootstrap samples was set to n = 5000. Unstandardized regression coefficients (coeff) and standard errors (SE) are presented for each effect. To enhance comparability with the literature, mediation analyses were repeated using standardized (z-transformed) variables in order to obtain standardized regression coefficients (Std coeff). In line with all other analyses steps the mediation analysis was first conducted without any covariates and secondly repeated by including age, sex, BMI, education, HbA1c and systolic blood pressure as nuisance regressors in the model.
Table 1.
Regression analyses of endurance and FA with cognitive subscores.
| Baseline regression Endurance |
Full model regression Endurancea | Baseline regression FA |
Full model regression FAa |
||
|---|---|---|---|---|---|
|
Picture Sequence Memory: [non-verbal] episodic memory |
ß | 0.510** | 0.109** | 0.158** | 0.119** |
| p-value | 0.000 | 0.002 | 0.000 | 0.001 | |
| df | 1187 | 800 | 1048 | 714 | |
|
Dimensional Change Card Sort Test: executive function & cognitive flexibility |
ß | 0.574** | 0.155** | 0.054 | 0.038 |
| p-value | 0.000 | 0.000 | 0.080 | 0.316 | |
| df | 1187 | 800 | 1048 | 714 | |
|
Flanker Inhibitory Control and Attention Test: executive function |
ß | 0.524** | 0.132** | 0.021 | 0.020 |
| p-value | 0.000 | 0.000 | 0.503 | 0.593 | |
| df | 1187 | 800 | 1048 | 714 | |
|
Penn Progressive Matrices, total correct responses: fluid intelligence |
ß | 0.735** | 0.171** | 0.071* | 0.073 |
| p-value | 0.000 | 0.000 | 0.021 | 0.051 | |
| df | 1187 | 800 | 1048 | 714 | |
|
Oral Reading Recognition Test: reading decoding skills |
ß | 0.518** | 0.119** | 0.084** | 0.065 |
| p-value | 0.000 | 0.001 | 0.007 | 0.083 | |
| df | 1178 | 800 | 1044 | 714 | |
|
Picture Vocabulary Test: vocabulary knowledge |
ß | 0.711** | 0.116** | 0.087** | 0.071 |
| p-value | 0.000 | 0.001 | 0.005 | 0.059 | |
| df | 1187 | 800 | 1048 | 714 | |
| Pattern Comparison Processing Speed Test | ß | 0.574** | 0.145** | 0.072* | 0.057 |
| p-value | 0.000 | 0.000 | 0.020 | 0.126 | |
| df | 1187 | 800 | 1048 | 714 | |
|
Delay Discounting, 40k: Self-regulation/Impulsivity |
ß | 0.210** | 0.083* | 0.082** | 0.035 |
| p-value | 0.000 | 0.019 | 0.008 | 0.353 | |
| df | 1179 | 800 | 1045 | 714 | |
|
Variable Short Penn Line Orientation, total correct items: spatial orientation processing |
ß | 0.448** | 0.129** | 0.016 | −0.002 |
| p-value | 0.000 | 0.000 | 0.608 | 0.947 | |
| df | 1178 | 800 | 1045 | 714 | |
|
Short Penn Continuous Performance Test, specificity of right decisions: Sustained attention |
ß | 0.222** | 0.085* | 0.096** | 0.023 |
| p-value | 0.000 | 0.016 | 0.002 | 0.541 | |
| df | 1179 | 800 | 1045 | 714 | |
|
Penn Word Memory Test: verbal episodic memory |
ß | 0.312** | 0.083* | 0.131** | 0.117** |
| p-value | 0.000 | 0.018 | 0.000 | 0.002 | |
| df | 1179 | 800 | 1045 | 714 | |
| List Sorting Working Memory Test | ß | 0.582** | 0.064 | 0.059 | 0.098** |
| p-value | 0.000 | 0.070 | 0.056 | 0.009 | |
| df | 1187 | 800 | 1048 | 714 | |
aFull regression model controls for age, sex, education years, BMI HbA1c, Systolic Blood Pressure.
Endurance: This test measures sub-maximal cardiovascular endurance by recording the distance that the participant is able to walk on a 50-foot (out and back) course in 2 minutes. The participant’s raw score is the distance in feet and inches walked in 2 minutes. The raw scores are normed to a scale score with mean = 100, 1 SD = 15.
FA: Fractional anisotropy, mean value extracted from the FA-global cognition association results mask.
ß: Standardized ß-Coefficients from regression analyses with the cognitive scores as the dependent variables.
df: degrees of freedom, all available data was used for the respective analyses leading to different degrees of freedom for different models.
For more information on the cognitive subscores see Supplementary Methods 1.
Results
Endurance – cognition
Endurance was positively associated with the global cognition score (degrees of freedom (df) = 800, standardized coefficient ß = 0.338, p < 0.001, explained variance of the model R2 = 0.115; Supplementary Fig. 1). After adding age, sex, education years, BMI, HbA1c and systolic blood pressure as regressors, endurance was still positively associated with cognition (df = 793, ß = 0.229, p < 0.001, R2 = 0.225). While the (subsequent) inclusion of additional regressors improved the share of variance explained by the model, only the inclusion of years of education had a significant impact on the estimated effect of endurance on cognition. For all details on the hierarchical regression analyses, please see Supplementary Results 1. The regressions of all cognition subscores on endurance revealed significant associations of the latter with all cognitive measures except for the list sorting working memory task which marginally failed to reach significance in the full model controlling for all covariates (ß = 0.064, p = 0.070). Most pronounced associations between endurance and cognitive performance emerged for cognitive flexibility (ß = 0.155, p < 0.001), fluid intelligence (ß = 0.171, p < 0.001) and cognitive processing speed (ß = 0.155, p < 0.001) when correcting for age, sex, education years, BMI, HbA1c and systolic blood pressure. For more details on the association with all subtests see Table 1.
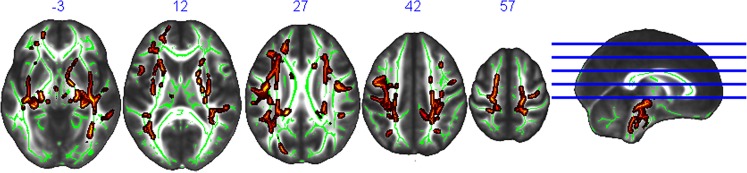
Endurance – white matter
We found a significant positive association (pFWE < 0.05; cluster size k: 27313 in 4 clusters) between FA and endurance in large, widespread clusters, including the genu of the corpus callosum, the bilateral longitudinal superior fascicle, the bilateral internal and external capsule, the bilateral uncinate fascicle, the corticospinal tract and the cerebellar peduncles among others (Table 2, Fig. 1, Supplementary Fig. 2). Hierarchical regression analyses revealed a positive association between endurance and extracted FA values even after correcting for all additional regressors (df = 723, ß = 0.145, p < 0.001, R2 = 0.155). For all details on the hierarchical regression analyses, please see Supplementary Results 2.
Table 2.
Positive association of Fractional Anisotropy with Endurance.
| Voxels | p FWE | MNI X (mm) | MNI Y (mm) | MNI Z (mm) |
|---|---|---|---|---|
| 26380 | 0.002 | 8 | −40 | −36 |
| 685 | 0.037 | −19 | 44 | 11 |
| 185 | 0.040 | −20 | −81 | 24 |
| 63 | 0.049 | −15 | 42 | 29 |
| Probabilities of affected tracts in percent | ||||
| Region | Laterality | Probability | ||
| Middle cerebellar peduncle: | 4.25 | |||
| Pontine crossing tract (a part of MCP) | 1.04 | |||
| Body of corpus callosum | 1.60 | |||
| Splenium of corpus callosum | 0.30 | |||
| Corticospinal tract | R | 1.16 | ||
| Corticospinal tract | L | 1.18 | ||
| Medial lemniscus | R | 0.65 | ||
| Medial lemniscus | L | 0.68 | ||
| Inferior cerebellar peduncle | R | 0.30 | ||
| Inferior cerebellar peduncle | L | 0.46 | ||
| Superior cerebellar peduncle | R | 0.91 | ||
| Superior cerebellar peduncle | L | 0.77 | ||
| Cerebral peduncle | R | 1.45 | ||
| Cerebral peduncle | L | 1.88 | ||
| Anterior limb of internal capsule | R | 0.95 | ||
| Anterior limb of internal capsule | L | 0.33 | ||
| Posterior limb of internal capsule | R | 0.85 | ||
| Posterior limb of internal capsule | L | 0.36 | ||
| Retrolenticular part of internal capsule | R | 0.87 | ||
| Retrolenticular part of internal capsule | L | 0.41 | ||
| Anterior corona radiata | R | 0.59 | ||
| Anterior corona radiata | L | 0.62 | ||
| Superior corona radiata | R | 1.85 | ||
| Superior corona radiata | L | 1.76 | ||
| Posterior corona radiata | R | 1.16 | ||
| Posterior corona radiata | L | 1.25 | ||
| Posterior thalamic radiation (include optic radiation) | R | 1.08 | ||
| Posterior thalamic radiation (include optic radiation) | L | 0.62 | ||
| Sagittal stratum (include inferior longitudinal fasciculus and inferior fronto-occipital fasciculus) | R | 1.32 | ||
| Sagittal stratum (include inferior longitudinal fasciculus and inferior fronto-occipital fasciculus) | L | 0.42 | ||
| External capsule | R | 2.34 | ||
| External capsule | L | 2.31 | ||
| Fornix (cres)/Stria terminalis | R | 0.76 | ||
| Fornix (cres)/Stria terminalis | L | 0.86 | ||
| Superior longitudinal fasciculus | R | 1.52 | ||
| Superior longitudinal fasciculus | L | 2.48 | ||
| Superior fronto-occipital fasciculus (could be a part of anterior internal capsule) | R | 0.13 | ||
| Superior fronto-occipital fasciculus (could be a part of anterior internal capsule) | L | 0.01 | ||
| Uncinate fasciculus | L | 0.10 | ||
| Tapetum | R | 0.01 | ||
| Unclassified | 58.18 | |||
Negative correlation.
No significant results.
On the top dimensions of clusters (number of voxels) and localization of signal peaks (MNI coordinates) are given for regions showing maximal differences of tract-based spatial statistics values (signal peak). Below are the white matter tracts in the cluster based on the JHU ICBM-DTI-81 White-Matter Labels (as implemented in FSL).
Probabilities of affected tracts: It gives the (average) probability of all significant voxels being a member of the different labelled regions within the atlas (JHU ICBM-DTI-81 White-Matter), calculated with the FSL tool “atlasquery”.
Figure 1.
Positive Association of Endurance with Fractional Anisotropy. Top: Axial slices with corresponding y-axis values (MNI) are presented. Red-yellow areas represent voxels (using FSL’s “fill” command for better visualization), where a significant positive association between Endurance and Fractional Anisotropy was detected (pFWE < 0.05, corrected for age and sex). Sagittal view with blue lines indicating axial slices on the left.
White matter – cognition
FA and global cognition were positively associated (df = 723, ß = 0.132, p < 0.001, R2 = 0.017), even after correcting for all additional regressors (df = 716, ß = 0.116, p < 0.001, R2 = 0.183). For all details on the hierarchical regression analyses, please see Supplementary Results 3. The regression analyses of all subscores revealed the strongest association for non-verbal episodic memory (ß = 0.119, p = 0.001) and verbal episodic memory (ß = 0.117, p = 0.002) and a trend in fluid intelligence (ß = 0.021, p = 0.051) when correcting for age, sex, education years, BMI, HbA1c and systolic blood pressure. For more details on the association with different subtests see Table 1.
Mediation analysis
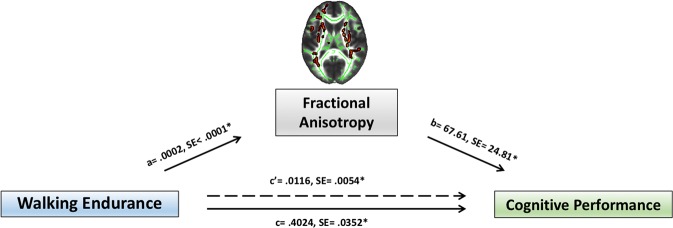
In line with analyses steps 3.1–3.3 the mediation model confirmed a significant association between walking endurance and FA (Std coeff = 0.1217, coeff = 0.0002, SE < 0.0001, 95%-CI = 0.0001 to 0.0003, t = 3.93, p = 0.0001) as well as a significant association between FA and global cognitive performance (Std coeff = 0.0780; coeff = 67.61, SE = 24.81, 95%-CI = 18.93 to 116.29, t = 2.73, p = 0.0065). The mediation model furthermore yielded a significant positive indirect (mediated) effect of walking endurance on cognitive performance through FA (indirect effect: Std coeff = 0.0095; coeff = 0.0116, SE = 0.0054, 95%-CI = 0.0025 to 0.0236) (see Fig. 2). Moreover, a significant direct effect of walking endurance on cognitive performance could also be detected (direct effect: Std coeff = 0.3302; coeff = 0.4024, SE = 0.0352, 95%-CI = 0.3334 to 0.4714, t = 11.44, p < 0.001). The mediation model including age, sex, BMI, HbA1c, education years and blood pressure as additional nuisance covariates confirmed these results and similarly yielded a significant indirect (mediated) effect of walking endurance on cognitive performance through FA (indirect effect: Std coeff = 0.0129; coeff = 0.0157, SE = 0.0079, 95%-CI = 0.0026 to 0.0339).
Figure 2.
Fractional anisotropy mediates the association between walking endurance and cognitive performance. Depiction of the applied mediation model: Unstandardized coefficients and standard errors for each path of the mediation model are presented. Note that c represents the direct effect and c‘ the indirect effect. * indicates significance at p < 0.05.
Discussion
With the present work we provide evidence for a positive relationship between PF and both white matter microstructure as well as cognitive performance in a large sample of healthy young adults. The observed positive association between PF and cognitive function extended to nearly all cognitive domains with most pronounced associations for fluid intelligence, cognitive flexibility and processing speed. Our finding of a significant mediation effect of PF on cognitive performance through white matter microstructure furthermore points to a crucial role of brain structural alterations in the association between PF and cognition. Importantly, the observed significant associations between PF, brain structure and cognition withstood correction for a wide range of potentially relevant nuisance covariates, which makes it unlikely that the observed pattern of results was biased by the presence of common cardiovascular or metabolic risk factors. A setup of hierarchical regressions revealed that while regressors such as age, sex, BMI and HbA1c were associated with cognition and FA in the respective models, only the inclusion of education years substantially weakened the estimated effect of endurance on cognition and FA. The present study thus supports the concept of a robust positive relationship between PF and preserved brain structural integrity as well as cognitive performance in a wide range of cognitive domains.
The presented positive association between PF as operationalized through walking endurance and overall cognitive function is in line with reports on a similar positive relationship between physical activity or fitness and cognition from the literature including meta-analyses4,8,16. Two aspects related to the association between PF and cognitive performance in the present study warrant further discussion: First, the present findings were based on analyses in a relatively young sample of healthy adults and thus demonstrate that associations between PF and cognitive performance are already present during early adulthood. This finding is supported by previous research reporting a corresponding positive relationship between PF and cognition across the life-span that appears to be similarly present in children and adolescents as well as in older healthy adults5,6.
Second, the present study included data on a broad spectrum of cognitive domains that were simultaneously investigated and revealed positive associations between endurance and cognitive performance in a wide range of cognitive domains with strongest effect sizes for associations with cognitive flexibility, processing speed and fluid intelligence. This finding appears to be in line with previous meta-analytic evidence on similar associations between physical activity and executive function and cognitive flexibility5. Of note, while the significant associations between endurance and cognition were demonstrated for nearly all cognitive domains and could be demonstrated even when controlling for the presence of further metabolic risk factors, the association between endurance and performance in the list sorting working memory task marginally failed to reach significance when controlling for further covariates. Importantly, this finding might suggest that in comparison to other cognitive domains working memory performance in young adults is more likely to be influenced by other metabolic risk factors such as HbA1c, BMI or blood pressure, a finding that is in line with a previously reported pronounced association between BMI and working memory performance45.
Another important finding of the present work was the positive relationship between endurance and fractional anisotropy as a measure of white matter integrity. While evidence on grey and white matter volume associations with PF are well-established11, investigations into PF-FA relationship is scarce. However, these results correspond to previous studies demonstrating positive correlations between physical exercise and white matter microstructure15,17. This association may be mediated by several neurobiological pathways (for reviews see9,46): higher PF might lead to better WM microstructure via increased cerebrovascular health and perfusion47,48, neuroplasticity-related effects (e.g. BDNF excretion)49, oligodendrocyte proliferation50 and neuroprotective effects via down-regulation of the inflammatory system51,52.
Importantly, additional investigation of cognitive domains furthermore indicated that the observed significant relationship between cognitive performance and PF-associated white matter microstructure was primarily driven by associations between episodic memory and white matter microstructure supporting previous studies into white matter integrity associations with this specific cognitive domain53,54. Also, this suggests white matter integrity as one potential means by which physical exercise may augment episodic memory. In contrast, other cognitive measures such as performance in attention related tasks did not show significant associations with white matter microstructure in PF-related white matter tracts in the present study. This finding is of relevance for future studies that seek to investigate neural correlates of cognition and might furthermore explain the heterogeneous findings for the association between cognition and white matter microstructure from the literature55.
Taken together, while we fully acknowledge the cross-sectional character of the present study, the observed pattern of results appears to support the notion of a beneficial effect of PF on cognitive function, possibly mediated by its effect on white matter integrity. This is supported by our mediation analysis that elucidates a possible neurobiological pathway from PF to cognitive performance via white matter microstructure. This notion is supported by the few available experimental studies indicating that physical exercise leads to increases in memory performance and brain structural integrity16. This concept might be of relevance for a wide range of domains in health and life sciences including prevention, clinical care and neurobiological research. Along with previous findings, our findings point to the potential of PF as a modifiable factor that might be applied as an intervention in prevention and clinical care.
Limitations of the present work include its cross-sectional design which prevents us from inferring on causal relationships. In line with this notion, our results, especially findings from the mediation model should be interpreted with caution since they do not allow to draw any causal conclusions. Furthermore, we must acknowledge that PF was measured using a single variable while the amount, intensity and type of physical activity as well as the extent of regular physical activity or previous physical exercise of the participants was not assessed in the present study. However, the results underline the applicability of the 2-min walking test as a proxy for PF in young, healthy adults. Strengths of our study include its comparatively large and well characterized sample size and the inclusion of a wide range of relevant covariates in all main analyses. In addition, the availability of measures from several cognitive domains allowed us to delineate domain specific associations between cognition and PF as well as between cognition and white matter microstructure.
In sum, our work demonstrates that higher PF is associated with preserved white matter microstructure and better performance in a wide range of cognitive domains. The present findings thus support previous evidence for a beneficial contribution of PF on brain structure and cognitive function. The inclusion of several distinct cognitive domains and the fact that all main analyses accounted for a wide range of relevant covariates extends the present understanding of the association between PF, brain structure and cognition and supports the concept of a particularly robust association between PF and brain physiology. Future studies should aim at investigating effects of physical exercise and fitness on brain structure and cognition in longitudinal and interventional studies to ultimately clarify a potential causal effect of PF on healthy brain ageing.
Supplementary information
Acknowledgements
This work was funded by the German Research Foundation (DFG, Grant FOR2107 DA1151/5-1 and DA1151/5-2 to UD; SFB-TRR58, Projects C09 and Z02 to UD;), the Interdisciplinary Centre for Clinical Research (IZKF) of the Medical Faculty of Münster (Grant Dan3/012/17 to UD and SEED 11/18 to NO), and the Deanery of the Medical Faculty of the University of Münster. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Author Contributions
N.O. and J.R. wrote the manuscript. S.M., R.R., U.D. have made substantial contributions to the analysis and interpretation of data. S.M., R.R., U.D., J.G., V.E., M.R. and A.J. have substantively revised it. All authors have approved the final article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49301-y.
References
- 1.Guure CB, Ibrahim NA, Adam MB, Said SM. Impact of Physical Activity on Cognitive Decline, Dementia, and Its Subtypes: Meta-Analysis of Prospective Studies. Biomed Res. Int. 2017;2017:9016924. doi: 10.1155/2017/9016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chekroud SR, et al. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: a cross-sectional study. The Lancet Psychiatry. 2018;5:739–746. doi: 10.1016/S2215-0366(18)30227-X. [DOI] [PubMed] [Google Scholar]
- 3.Pinto Pereira SM, Geoffroy M-C, Power C. Depressive Symptoms and Physical Activity During 3 Decades in Adult Life. JAMA Psychiatry. 2014;71:1373. doi: 10.1001/jamapsychiatry.2014.1240. [DOI] [PubMed] [Google Scholar]
- 4.de Greeff JW, Bosker RJ, Oosterlaan J, Visscher C, Hartman E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: a meta-analysis. J. Sci. Med. Sport. 2018;21:501–507. doi: 10.1016/j.jsams.2017.09.595. [DOI] [PubMed] [Google Scholar]
- 5.Álvarez-Bueno C, et al. The Effect of Physical Activity Interventions on Children’s Cognition and Metacognition: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:729–738. doi: 10.1016/j.jaac.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br. J. Sports Med. 2018;52:154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 7.Song D, Yu DSF, Li PWC, Lei Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018;79:155–164. doi: 10.1016/j.ijnurstu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Du Z, et al. Physical activity can improve cognition in patients with Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging. 2018;13:1593–1603. doi: 10.2147/CIA.S169565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sexton CE, et al. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. 2016;131:81–90. doi: 10.1016/j.neuroimage.2015.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampaio-Baptista C, Johansen-Berg H. White Matter Plasticity in the Adult Brain. Neuron. 2017;96:1239–1251. doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol. Aging. 2014;35:S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papenberg G, et al. Physical activity and inflammation: effects on gray-matter volume and cognitive decline in aging. Hum. Brain Mapp. 2016;37:3462–3473. doi: 10.1002/hbm.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gons R. A. R., Tuladhar A. M., de Laat K. F., van Norden A. G. W., van Dijk E. J., Norris D. G., Zwiers M. P., de Leeuw F.-E. Physical activity is related to the structural integrity of cerebral white matter. Neurology. 2013;81(11):971–976. doi: 10.1212/WNL.0b013e3182a43e33. [DOI] [PubMed] [Google Scholar]
- 14.Hayes Scott M., Salat David H., Forman Daniel E., Sperling Reisa A., Verfaellie Mieke. Cardiorespiratory fitness is associated with white matter integrity in aging. Annals of Clinical and Translational Neurology. 2015;2(6):688–698. doi: 10.1002/acn3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberlin Lauren E., Verstynen Timothy D., Burzynska Agnieszka Z., Voss Michelle W., Prakash Ruchika Shaurya, Chaddock-Heyman Laura, Wong Chelsea, Fanning Jason, Awick Elizabeth, Gothe Neha, Phillips Siobhan M., Mailey Emily, Ehlers Diane, Olson Erin, Wojcicki Thomas, McAuley Edward, Kramer Arthur F., Erickson Kirk I. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. NeuroImage. 2016;131:91–101. doi: 10.1016/j.neuroimage.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voss MW, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum. Brain Mapp. 2013;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith Patrick J., Blumenthal James A., Hoffman Benson M., Cooper Harris, Strauman Timothy A., Welsh-Bohmer Kathleen, Browndyke Jeffrey N., Sherwood Andrew. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Psychosomatic Medicine. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Repple Jonathan, Opel Nils, Meinert Susanne, Redlich Ronny, Hahn Tim, Winter Nils R., Kaehler Claas, Emden Daniel, Leenings Ramona, Grotegerd Dominik, Zaremba Dario, Bürger Christian, Förster Katharina, Dohm Katharina, Enneking Verena, Leehr Elisabeth J., Böhnlein Joscha, Karliczek Greta, Heindel Walter, Kugel Harald, Bauer Jochen, Arolt Volker, Dannlowski Udo. Elevated body-mass index is associated with reduced white matter integrity in two large independent cohorts. Psychoneuroendocrinology. 2018;91:179–185. doi: 10.1016/j.psyneuen.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Cook RL, et al. Relationship between Obesity and Cognitive Function in Young Women: The Food, Mood and Mind Study. J. Obes. 2017;2017:5923862. doi: 10.1155/2017/5923862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maillard P, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfaro FJ, et al. White matter microstructure and cognitive decline in metabolic syndrome: a review of diffusion tensor imaging. Metabolism. 2018;78:52–68. doi: 10.1016/j.metabol.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Repple J, et al. A voxel-based diffusion tensor imaging study in unipolar and bipolar depression. Bipolar Disord. 2017;19:23–31. doi: 10.1111/bdi.12465. [DOI] [PubMed] [Google Scholar]
- 24.Opel N, et al. Differing brain structural correlates of familial and environmental risk for major depressive disorder revealed by a combined VBM/pattern recognition approach. Psychol. Med. 2016;46:277–290. doi: 10.1017/S0033291715001683. [DOI] [PubMed] [Google Scholar]
- 25.McGrath JJ, et al. The association between family history of mental disorders and general cognitive ability. Transl. Psychiatry. 2014;4:e412–e412. doi: 10.1038/tp.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Essen DC, et al. The WU-Minn Human Connectome Project: An overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasser Matthew F, Smith Stephen M, Marcus Daniel S, Andersson Jesper L R, Auerbach Edward J, Behrens Timothy E J, Coalson Timothy S, Harms Michael P, Jenkinson Mark, Moeller Steen, Robinson Emma C, Sotiropoulos Stamatios N, Xu Junqian, Yacoub Essa, Ugurbil Kamil, Van Essen David C. The Human Connectome Project's neuroimaging approach. Nature Neuroscience. 2016;19(9):1175–1187. doi: 10.1038/nn.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gershon R. C., Wagster M. V., Hendrie H. C., Fox N. A., Cook K. F., Nowinski C. J. NIH Toolbox for Assessment of Neurological and Behavioral Function. Neurology. 2013;80(Issue 11, Supplement 3):S2–S6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuben DB, et al. Motor assessment using the NIH Toolbox. Neurology. 2013 doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuben D. B., Magasi S., McCreath H. E., Bohannon R. W., Wang Y.-C., Bubela D. J., Rymer W. Z., Beaumont J., Rine R. M., Lai J.-S., Gershon R. C. Motor assessment using the NIH Toolbox. Neurology. 2013;80(Issue 11, Supplement 3):S65–S75. doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weintraub S, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S49–S53. doi: 10.1212/WNL.0b013e31827b915c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heaton RK, et al. Reliability and validity of composite scores from the NIH toolbox cognition battery in adults. J. Int. Neuropsychol. Soc. 2014;20:588–598. doi: 10.1017/S1355617714000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Essen DC, et al. The Human Connectome Project: A data acquisition perspective. Neuroimage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feinberg DA, et al. Multiplexed echo planar imaging for sub-second whole brain fmri and fast diffusion imaging. PLoS One. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sotiropoulos SN, et al. Effects of image reconstruction on fiber orientation mapping from multichannel diffusion MRI: Reducing the noise floor using SENSE. Magn. Reson. Med. 2013;70:1682–1689. doi: 10.1002/mrm.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 39.Pekar JJ, et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2007;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochunov P, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: Tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opel N, Redlich R, Kaehler C, Grotegerd D, Dohm K, Heindel W, Kugel H, Thalamuthu A, Koutsouleris N, Arolt V, Teuber A, Wersching H, Baune B T, Berger K, Dannlowski U. Prefrontal gray matter volume mediates genetic risks for obesity. Molecular Psychiatry. 2017;22(5):703–710. doi: 10.1038/mp.2017.51. [DOI] [PubMed] [Google Scholar]
- 43.Opel Nils, Redlich Ronny, Dohm Katharina, Zaremba Dario, Goltermann Janik, Repple Jonathan, Kaehler Claas, Grotegerd Dominik, Leehr Elisabeth J, Böhnlein Joscha, Förster Katharina, Meinert Susanne, Enneking Verena, Sindermann Lisa, Dzvonyar Fanni, Emden Daniel, Leenings Ramona, Winter Nils, Hahn Tim, Kugel Harald, Heindel Walter, Buhlmann Ulrike, Baune Bernhard T, Arolt Volker, Dannlowski Udo. Mediation of the influence of childhood maltreatment on depression relapse by cortical structure: a 2-year longitudinal observational study. The Lancet Psychiatry. 2019;6(4):318–326. doi: 10.1016/S2215-0366(19)30044-6. [DOI] [PubMed] [Google Scholar]
- 44.Mackey Scott, Chaarani Bader, Kan Kees-Jan, Spechler Philip A., Orr Catherine, Banaschewski Tobias, Barker Gareth, Bokde Arun L.W., Bromberg Uli, Büchel Christian, Cattrell Anna, Conrod Patricia J., Desrivières Sylvane, Flor Herta, Frouin Vincent, Gallinat Jürgen, Gowland Penny, Heinz Andreas, Ittermann Bernd, Paillère Martinot Marie-Laure, Artiges Eric, Nees Frauke, Papadopoulos-Orfanos Dimitri, Poustka Luise, Smolka Michael N., Jurk Sarah, Walter Henrik, Whelan Robert, Schumann Gunter, Althoff Robert R., Garavan Hugh. Brain Regions Related to Impulsivity Mediate the Effects of Early Adversity on Antisocial Behavior. Biological Psychiatry. 2017;82(4):275–282. doi: 10.1016/j.biopsych.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 45.Alarcón Gabriela, Ray Siddharth, Nagel Bonnie J. Lower Working Memory Performance in Overweight and Obese Adolescents Is Mediated by White Matter Microstructure. Journal of the International Neuropsychological Society. 2015;22(3):281–292. doi: 10.1017/S1355617715001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Sayes Jenin, Harasym Diana, Turco Claudia V., Locke Mitchell B., Nelson Aimee J. Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. The Neuroscientist. 2018;25(1):65–85. doi: 10.1177/1073858418771538. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt Reinhold, Petrovic Katja, Ropele Stefan, Enzinger Christian, Fazekas Franz. Progression of Leukoaraiosis and Cognition. Stroke. 2007;38(9):2619–2625. doi: 10.1161/STROKEAHA.107.489112. [DOI] [PubMed] [Google Scholar]
- 48.Bullitt E., Rahman F.N., Smith J.K., Kim E., Zeng D., Katz L.M., Marks B.L. The Effect of Exercise on the Cerebral Vasculature of Healthy Aged Subjects as Visualized by MR Angiography. American Journal of Neuroradiology. 2009;30(10):1857–1863. doi: 10.3174/ajnr.A1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson Kirk I., Miller Destiny L., Roecklein Kathryn A. The Aging Hippocampus. The Neuroscientist. 2011;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto Yoko, Tsunekawa Yuji, Nomura Tadashi, Suto Fumikazu, Matsumata Miho, Tsuchiya Shigeru, Osumi Noriko. Differential Proliferation Rhythm of Neural Progenitor and Oligodendrocyte Precursor Cells in the Young Adult Hippocampus. PLoS ONE. 2011;6(11):e27628. doi: 10.1371/journal.pone.0027628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson Richard J., Lowder Thomas W., Spielmann Guillaume, Bigley Austin B., LaVoy Emily C., Kunz Hawley. Exercise and the aging immune system. Ageing Research Reviews. 2012;11(3):404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Chupel, M. U. et al. Strength training decreases inflammation and increases cognition and physical fitness in older women with cognitive impairment. Front. Physiol., 10.3389/fphys.2017.00377 (2017). [DOI] [PMC free article] [PubMed]
- 53.Ngo Chi T., Alm Kylie H., Metoki Athanasia, Hampton William, Riggins Tracy, Newcombe Nora S., Olson Ingrid R. White matter structural connectivity and episodic memory in early childhood. Developmental Cognitive Neuroscience. 2017;28:41–53. doi: 10.1016/j.dcn.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lancaster Melissa A., Seidenberg Michael, Smith J. Carson, Nielson Kristy A., Woodard John L., Durgerian Sally, Rao Stephen M. Diffusion Tensor Imaging Predictors of Episodic Memory Decline in Healthy Elders at Genetic Risk for Alzheimer’s Disease. Journal of the International Neuropsychological Society. 2016;22(10):1005–1015. doi: 10.1017/S1355617716000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anatürk M, Demnitz N, Ebmeier KP, Sexton CE. A systematic review and meta-analysis of structural magnetic resonance imaging studies investigating cognitive and social activity levels in older adults. Neurosci. Biobehav. Rev. 2018;93:71–84. doi: 10.1016/j.neubiorev.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.