Abstract
Background
Because ACO (Asthma-COPD-Overlap) does not fill out asthma or COPD (Chronic Obstructive Pulmonary Disease) criteria, such patients are poorly evaluated. The aim of this study was to screen asthma and COPD for an alternative diagnosis of ACO, then to determine subgroups of patients, using cluster analysis.
Material and methods
Using GINA-GOLD stepwise approach, asthmatics and COPD were screened for ACO. Clusterization was then performed employing Multiple Correspondent Analysis (MCA) model, encompassing 9 variables (age, symptoms onset, sex, BMI (Body Mass Index), smoking, FEV-1, dyspnea, exacerbation, comorbidity). Finally, clusters were compared to determine phenotypes.
Results
MCA analysis was performed on 172 ACO subjects. To better distinguish clusters, the analysis was then focused on 55 subjects, having at least one cosine squared >0.3. Six clusters were identified, allowing the description of 4 phenotypes. Phenotype A represented overweighed heavy smokers, with an early onset and a severe disease (27% of ACO patients). Phenotype B gathered similar patients, with a late onset (29%). Patients from Phenotypes C-D were slighter smokers, presenting a moderate disease, with early and late onset respectively (respectively 13% and 31%).
Conclusions
By providing evidences for clusters within ACO, our study confirms its heterogeneity, allowing the identification of 4 phenotypes. Further prospective studies are mandatory to confirm these data, to determine both specific management requirements and prognostic value.
Keywords: ACO, Asthma, COPD, Cluster, Phenotype, Heterogeneity, MCA
1. Introduction
Obstructive bronchial diseases concern a high proportion of general population. In France, at least 4 million patients are concerned by asthma [1], and COPD accounts for 8% of the population [2]. Classifying obstructive airway diseases as either asthma or COPD is based on a largely accepted paradigm. However, the recognition of patients sharing characteristics of both entities has been underlined as early as 1955 [3], preceding the concept of ACO. In 2005, Guerra et al. [4] described an association of asthma and COPD. Then Gibson et al. [5] reported subgroups of patients, sharing characteristics of asthma and COPD, suggesting the awareness to revisit the classification of bronchial obstructive diseases. An additional interest for this overlapping syndrome rose up from 2013, achieving 108 PubMed publications in 2017. The term ACOS (Asthma-COPD-Overlap-Syndrome), subsequently modified for ACO in GINA recommendations 2017 was introduced in GINA-GOLD recommendations in 2014. Its recognition among asthma and COPD patients is based on a GINA-GOLD stepwise approach [6]. ACO patients are defined as having an equal number of asthma and COPD features, among 11 items, belonging to a check-list of symptoms, clinical course, lung function and chest X-ray characteristics. This proposition has led to display ACO in recent clinical guidelines [7].
This stepwise approach employs a large list of criteria, which generates a huge heterogeneity among these patients. The incomplete reversibility of bronchial obstruction is the unique mandatory characteristic, shared by the overall ACO population [6]. In this context, Miravitlles summarized in 5 commandments what ACO is and is not [8], however a single definition of ACO is not realistic [9]. This heterogeneity explains the discrepancies of ACO prevalences, severity and prognosis between groups of patients [[10], [11], [12], [13], [14]].
Consequently, it seems inconsistent to propose a unique medical care program for these patients. Beside the well-known guidelines for asthma and COPD treatment, no strong recommendations have been developed for ACO [15,16] and in routine, ACO management is mainly based on physicians’ clinical judgment.
To provide new data on ACO and to describe subgroups of patients, we firstly analyzed COPD and asthma populations, using the GINA-GOLD approach [6]. Then, using a strategy of clusterization, employing 9 clinical and categorical variables, the aim of the study was to determine subgroups of ACO patients and to validate ACO phenotypes.
2. Methods
2.1. Study design and subjects
This retrospective study was performed from October 2017 to January 2018, analyzing medical records from 7 medical centers of respiratory diseases (Croix-Rousse Hospital, Hospices Civils de Lyon, Saint-Etienne University Hospital, Bourg-en-Bresse, Annecy, Villefranche-sur-Saône and Saint-Chamond Hospitals and the private Parot office, Lyon). Physicians of each center were implicated both in the study design and the analysis of medical data.
Asthmatics and COPD patients were systematically screened for an alternative diagnosis of ACO, applying the GINA GOLD stepwise approach [6,17]. A score between −3 and +3 was required to select a subject as ACO, in the presence of a post-bronchodilator FEV-1/FVC ≤0,7. Patients with bronchiectasis were excluded.
Each patient selected as ACO was then evaluated by a descriptive questionnaire. Anthropometric data, occupational exposure, length and cumulative tobacco smoking, personal comorbidities (cardio-vascular diseases, diabetes, osteoporosis, mood disorders, sleep apnea syndrome, cancer and GERD) were collected. In addition, clinical expression of ACO (dyspnea scale, number of exacerbation and hospitalization for acute respiratory event in the past 12 months, age of onset of respiratory symptoms), respiratory functional tests (post bronchodilator FEV-1, RV and KCO, emphysema on thoracic CT-scan, blood eosinophilia and respiratory disease-related treatments were also gathered.
The Ethics Committee of Saint-Etienne University Hospital, approved the study in September 2017 (reference IRBN452017/CHUSTE), which was registered to National Comity of Ethic and Liberty in October 2017.
2.2. Variables
To perform the cluster analysis, 9 categorical variables were chosen for their clinical relevance: age (median age as cut off), BMI (30 kg/m2 as cut off), sex, dyspnea assessed by mMRC scale (0–1 or ≥ 2), age of onset of respiratory symptoms (before or after 40 years-old), exacerbation per year (0–1 or ≥ 2), number of comorbidities (0–1 or ≥ 2), post-bronchodilator FEV-1 (median FEV1 as cut off), tobacco pack-year (PY) (median as cut off). Variables were used as categorical with threshold (mentioned above) to increase their clinical value.
2.3. Statistical analysis
In the absence of prior-hypothesis, Multiple Correspondence Analysis (MCA), a descriptive and exploratory data-driven analysis was chosen to analyze the relationships between the 9 above clinical variables, in multi-way tables containing measure of correspondence between rows and columns.
Patients’ data were integrated altogether in a multivariate analysis. Results are presented on a graphical display, that represents configuration of points in projection planes. The nine-dimensional analysis is represented in a 2-dimensional plan by the statistical program. Two sets of data were generated: the first representing the 9 categorical variables (rows), and the second representing the patients (columns).
Clusterization was performed by the visual interpretation of proximities of points. Finally, clinical, functional, biological and C-scan data of the apparent clusters were compared using parametric and non-parametric tests (ANOVA, Kruskal-Wallis test, test, Chi-square test, Fisher's exact test).
3. Results
3.1. Patients’ characteristics
Around 1500 patients were analyzed for ACO characterization, leading to identify 176 relevant patients. Among them, 172 ACO subjects were finally included (11% of asthma and COPD cohorts) and 4 were excluded in the absence of available data.
Patients’ characteristics are represented in Table 1. Interestingly, the ACO population was composed of a majority of male, overweight, with a history of smoking and 54% of them presenting respiratory symptoms before 40-year-old. A large majority of subjects (70%) was frequent exacerbators, reporting at least 2 exacerbations per year. Half had a dyspnea score higher than 1 and two third had at least 2 comorbidities. The mean serum eosinophilia was 0.46 G/L (median 0.33 G/L)
Table 1.
Characteristics of the 172 ACO subjects.
| Male/Female (%) | 93/79 (54/46) |
| Age (median (25th-75th percentile)) [years] | 62 (53,5–71,5) |
|
| |
| BMI (median (25th-75th percentile)) [kg/m2] | 27 (24–30) |
| <30 (%) | 68 |
| ≥30 (%) | 32 |
|
| |
| Exacerbation per patient per year | |
| 0-1 (%) | 30 |
| ≥2 (%) | 70 |
|
| |
| Smoking exposure PY (median (25th-75th percentile)) | 30 (15–40) |
|
| |
| mMRC dyspnea score | |
| 0-1 (%) | 49 |
| 2-3-4 (%) | 51 |
|
| |
| Number of Comorbidities | |
| 0-1 (%) | 34 |
| ≥2 (%) | 66 |
|
| |
| Post-bronchodilator FEV-1 (median (25th-75th percentile)) [% of predicted value] | 64 (50–78) |
|
| |
| Age of onset of respiratory symptoms (median (25th-75th percentile)) | 33,5 (12,5–50) |
| <40 year-old (%) | 54 |
| ≥40 year-old (%) | 46 |
BMI: Body Mass Index; PY: pack year; mMRC: modified Medical Research Council; FEV-1: Forced Expiratory Volume in one second.
3.2. Variables multiple correspondence analysis
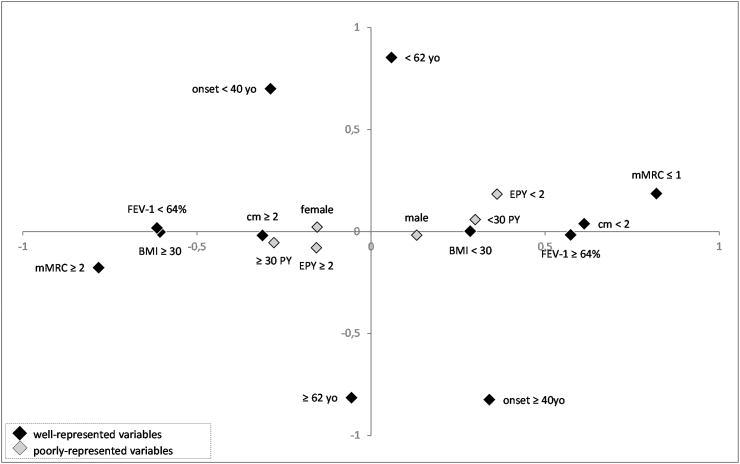
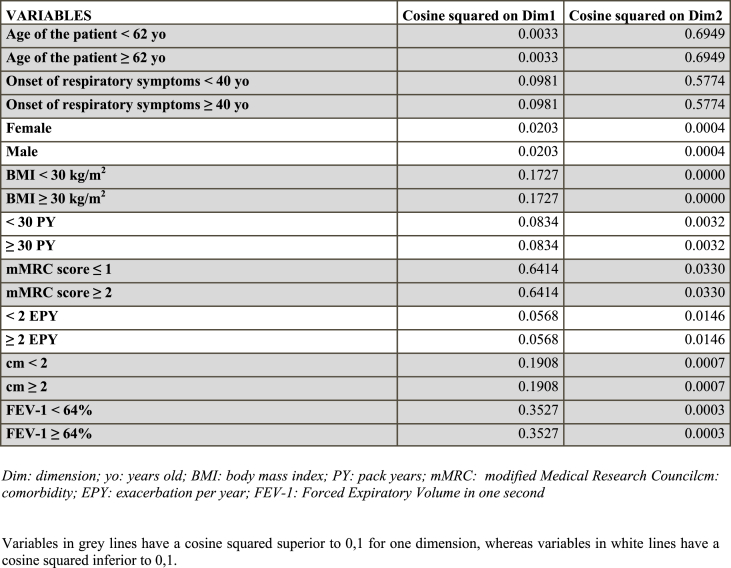
Results are shown using a two-dimensional presentation (Fig. 1). For a better understanding, dots representing patients were removed, allowing better identification of variables only, which are categorical and represented by 2 points. The quality of each dot on each dimension is defined as the square of the cosines of the angle that the dot forms with the axis of the dimension (Table 2). Closer to 1 the cosine squared is, better the variable is related to an axis or dimension.
Fig. 1.
Representation of the 9 categorical variables using Multiple Component Analysis. Categorical variables are represented by diamonds. Grey diamonds represent variables with low values of square cosines, and black diamonds represent variables with high values of square cosines. BMI: Body Mass Index; mMRC: modified Medical Research Council; PY: pack year; FEV-1: Forced Expiratory Volume in 1 second; < 62 yo: age inferior to 62 years old; ≥62 yo: minimum age of 62 years old; < 40 yo: Onset of respiratory symptoms before 40 years old; ≥ 40 yo: Onset of respiratory symptoms from 40 year olds; < 30 PY: tobacco consumption inferior to 30 pack-year; ≥ 30 PY: tobacco consumption of at least 30 pack year; < 2 EPY: less than 2 exacerbations per year; ≥ 2 EPY: at least 2 exacerbations per year; cm < 2: less than 2 comorbidities; cm ≥ 2: at least 2 comorbidities.
Table 2.
Multiple correspondence analysis of clinical values of the 172 ACO subjects.
BMI, mMRC score, comorbidity, FEV-1 are better represented on abscissa (Dimension 1) as their cosine square is higher for Dimension 1 than Dimension 2. By contrast, the age of onset of respiratory symptoms, and the age of subjects are better represented on ordinate (Dimension 2). Sex, Smoking history (PY), and exacerbations are less relevant, associated with low cosine-squared values (<0,1) for both dimensions.
The first dimension (axis of abscissas) appears to be linked to the severity of ACO. Low FEV-1, high mMRC score, and numerous comorbidities, which are assigned to more severe traits of the disease, are ranged between −1 and 0 values. By contrast, low mMRC score, high FEV-1, the absence or the presence of few comorbidities, are ranged between 0 and + 1 values. Additionally, obesity is also close to −1, whereas BMI <30kg/m2 is closer to 1. The second dimension (axis of ordinate) was used to qualify age of patients and age of disease onset. Similarly, older age and later age of onset of respiratory symptoms were ranged between −1 and 0. By contrast, younger age and earlier onset of respiratory symptoms were ranged between 0 and + 1 values (Fig. 1).
3.3. ACO cluster analysis
Using a similar statistical program, 172 ACO patients were analyzed on a 2-dimensional map. Each dot is determined by an original profile of the 9 clinical values and is representative of a single patient. The distance between each dot is indicative of clinical similarity (data not shown).
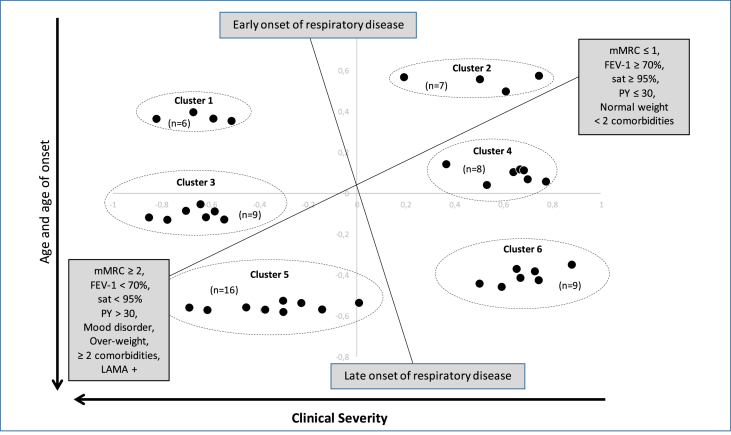
To accurate ACO clusterization, and facilitate identification of subgroups of patients, solely ACO patients having a cosine-square > 0,3 for dimension 1 or dimension 2 (55 patients) were selected for further analysis (Fig. 2). As some patients shared identical coordinates on the 2 dimensional-plan, the number of dots is inferior to 55.
Fig. 2.
Visual representation of clustering analysis.
mMRC: modified Medical Research Council; PY: pack year; FEV-1: Forced Expiratory Volume in 1 second; 55 ACO patients are represented on the two-dimensional graph. Dots were gathered together if close enough, creating 6 ellipses which represent 6 clusters of patients.
After comparing clusters with the whole collected data, clusters determined different phenotypes different in term of age, age of onset of respiratory symptoms, dyspnea (mMRC), FEV-1 (% of predicted value), oxygen saturation (sat), smoking history (PY: pack year), BMI (Body Mass Index), number of comorbidity, frequency of mood disorder and LAMA (long acting muscarinic antagonist) prescription.
3.4. ACO phenotypes
Six clusters of patients were individualized, as shown on Fig. 2 and were compared for the baseline characteristics, to determine relevant phenotypes (Table 3).
Table 3.
Clusterization of selected ACO patients.
| Patients | Cluster 1 (n = 6) | Cluster 2 (n = 7) | Cluster 3 (n = 9) | Cluster 4 (n = 8) | Cluster 5 (n = 16) | Cluster 6 (n = 9) | p-value |
|---|---|---|---|---|---|---|---|
| Age (year-old)a | 52,70 | 50,00 | 68,70 | 55,5 | 70,9 | 69 | <0.0001 |
| Male proportionb | 33,3 | 100 | 55,6 | 62,5 | 37,5 | 55,6 | NS |
| BMI (kg/m2)a | 33,30 | 24,40 | 29,30 | 24,6 | 25,9 | 24,3 | <0.04 |
| Smoking exposure (PY)a | 40,30 | 10,20 | 70,20 | 17,3 | 35,1 | 28,5 | 0.0005 |
| Current Smokerb | 50 | 28,6 | 33,3 | 25 | 37,5 | 22,2 | NS |
| Rural life styleb | 16,70 | 28,60 | 33,30 | 37,5 | 31,3 | 22,2 | NS |
| Occupationnal exposureb | 33,30 | 42,90 | 33,30 | 37,5 | 12,5 | 55,6 | NS |
| Age of symptoms onseta | 13,30 | 17,40 | 10,90 | 46,1 | 51,2 | 53,8 | <0.0001 |
| Number of exacerbation/yeara | 4,20 | 1,60 | 3,60 | 1,8 | 2,9 | 3,1 | NS |
| Dyspnea score (mMRC)a | 2,20 | 0,90 | 2,80 | 0,5 | 2,4 | 0,7 | <0.0001 |
| Number of Hospitalization during the last yeara | 0,60 | 0,00 | 0,60 | 0,6 | 0,9 | 0,3 | NS |
| Number of Comorbiditiesa | 3,00 | 0,90 | 3,30 | 0,9 | 2,6 | 1,2 | 0.0002 |
| Cardiovascularb | 50 | 14,3 | 55,6 | 25 | 62,5 | 22,2 | NS |
| Osteoporosisb | 33,3 | 14,3 | 55,6 | 0 | 31,3 | 22,2 | NS |
| Diabetesb | 50 | 14,3 | 44,4 | 12,5 | 25 | 11,1 | NS |
| GERDb | 66,7 | 28,6 | 77,8 | 12,5 | 43,8 | 22,2 | NS |
| Sleep Apneab | 33,3 | 0 | 11,1 | 25 | 18,8 | 22,2 | NS |
| Mood disorderb | 50 | 14,3 | 77,8 | 12,5 | 43,8 | 0 | <0.004 |
| Cancerb | 16,7 | 0 | 11,1 | 0 | 31,3 | 22,2 | NS |
| FEV-1 (% of predicted value)a | 50,30 | 72,60 | 41,90 | 85,6 | 61,8 | 83,7 | <0.0001 |
| FEV-1 Reversibility (%)a | 11,40 | 4,10 | 8,80 | 5,4 | 6,3 | 4,5 | NS |
| KCO (% of predicted value)a | 68,00 | 90,00 | 88,00 | 88,8 | 72,5 | 86 | NS |
| O2 saturation (%)a | 94,0 | 97,3 | 93,0 | 95,8 | 93,8 | 96,3 | <0.05 |
| RV (% of predicted value)a | 170,20 | 169,00 | 163,30 | 146 | 154,2 | 137,1 | NS |
| Serum Eosinophilia (Giga/l)a | 0,20 | 0,70 | 0,60 | 0,3 | 0,4 | 0,7 | NS |
| Emphysema on CT scanb | 66,7 | 33,3 | 62,5 | 28,6 | 60 | 28,6 | NS |
| ICSb | 100 | 71,4 | 88,9 | 87,5 | 93,8 | 88,9 | NS |
| LABAb | 100 | 85,7 | 88,9 | 75 | 93,8 | 55,6 | NS |
| LAMAb | 100 | 42,9 | 88,9 | 50 | 93,8 | 88,9 | <0.02 |
| Oral corticosteroidsb | 16,7 | 0 | 44,4 | 12,5 | 12,5 | 33,3 | NS |
mMRC: modified Medical Research Council, GERD: gastroesophageal reflux; ICS: inhaled corticosteroid treatment; LABA: Long acting beta agonist; LAMA: long acting muscarinic antagonist.
Average.
Percentage of patients presenting this characteristic.
Subjects of clusters 1, 2 and 4 were younger than those of clusters 3, 5 and 6 (52.7, 50 and 55.5 vs 68.7, 70.9, and 69 ys-old respectively, p < 0.0001). In addition, the onset of respiratory symptoms occurred earlier for patients from clusters 1, 2 and 3, than for patients from clusters 4, 5, 6 (13.3, 17.4 and 10.9 vs 46.1, 51.2 and 53.8 years old respectively, p < 0.0001).
When compared to patients from clusters 2, 4 and 6, patients from clusters 1, 3 and 5 appeared to be more frequently overweight (33.3, 29.3 and 25.9 vs 24.4, 24.6 and 24.3 Kg/m2 respectively, p < 0.04). They accumulated a higher smoking history (40.3, 70.2 and 35.1 vs 10.2, 17.3, 28.5 PY respectively, p = 0.0005). They displayed more clinical characteristics of disease severity, with a higher dyspnea score on mMRC scale (2.2, 2.8 and 2.4 vs 0.9, 0.5 and 0.7 respectively, p < 0.0001), a lower oxygen saturation (94, 93 and 93.8% vs 97.3, 95.8 and 96.3% respectively, p < 0.05), more comorbidities (3, 3.3 and 2.6 vs 0.9, 0.9 and 1.2 respectively, p = 0.0002), with more frequent mood disorders (50, 77.8 and 43.8% vs 14, 12.5 and 0% respectively, p < 0.004), a lower FEV-1 (50.3, 41.9 and 61.8% vs 72.6, 85.6 and 83.7 respectively, p < 0.0001). Although ICS-LABA (Inhaled corticosteroid- Long-acting beta2-agonist) were equally prescribed, LAMA (Long-acting-muscarinic-antagonist) employment was more frequent within clusters 1, 3 and 5 than clusters 2, 4 and 6 (100, 88.9, 93.8% vs 42.9, 50 and 88.9% respectively, p < 0.02). Interestingly, neither the number of exacerbation, nor the number of hospitalization were different between clusters. In addition, emphysema lesion on CT-scan, KCO and functional parameters of lung distention were similar between groups, as well as blood eosinophilia.
Finally, these comparisons of clusters, using the whole collected data led to the identification of 6 different phenotypes, different for age, age of onset of respiratory symptoms, dyspnea, oxygen saturation, FEV-1, smoking, number of comorbidity, frequency of mood disorder, and BMI.
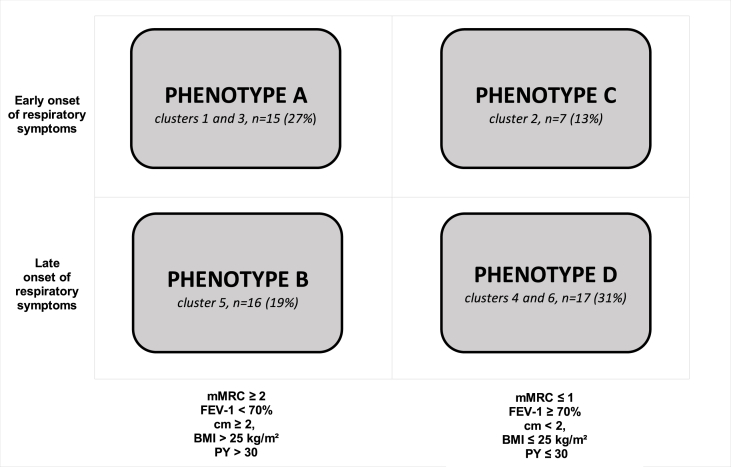
3.5. Proposition of simplification toward 4 ACO phenotypes
To be more convenient for clinical practice, we switched the previous “6 phenotypes” version toward a “4 phenotypes” proposition. Closed phenotypes 1 and 3, 4 and 6 were gathered, leading to creat a four-cell graph, representing 4 ACO phenotypes A, B, C and D (Fig. 3).
Fig. 3.
Final phenotypic expression of ACO patients. mMRC: modified Medical Research Council; PY: pack year; FEV-1: Forced Expiratory Volume in 1 second; cm < 2: less than 2 comorbidities; cm ≥ 2: at least 2 comorbidities; BMI: Body Mass Index; ≤ 30 PY: tobacco consumption inferior to 30 pack-year; > 30 PY: tobacco consumption of at least 30 pack year.
Patients belonging to phenotypes A and B were heavy smokers, more symptomatic, overweighed, with a lower FEV-1 and more comorbidities. By contrast, patients from phenotypes C and D were less symptomatic, with a mMRC grade 0–1, a FEV-1 > 70% of predicted value, less comorbidities, without overweight and with a lower tobacco consumption. Phenotypes A, C and phenotypes B, D had an early and late onset of respiratory symptoms respectively (Fig. 3).
4. Discussion
ACO subjects have been identified from a large multicenter cohort of around 1500 asthma and COPD patients, using the classical GINA GOLD stepwise approach [6]. A MCA analysis was then performed on 172 ACO subjects to get a clusterization of this population. To better distinguish clusters, we focused on 55 subjects from the analysis, having at least one cosine squared >0.3. In this way, six clusters were identified, allowing a more convenient description of 4 phenotypes. Phenotype A represented overweighted heavy smokers, with an early onset and a severe disease. Phenotype B gathered similar patients, with a late onset of the disease. Patients from Phenotypes C-D were slighter smokers, presenting a moderate disease, with early and late onset of the disease respectively.
The prevalence of ACO in asthma and in COPD cohorts is highly variable, reported from 5.2 to 35.4% [[17], [18]]. Theses divergences might be explained by the diversity of study designs. Using the GINA GOLD stepwise approach, we have identified an identical ACO prevalence of 11% from asthma and COPD cohorts, both sampled from respiratory clinics.
Although asthma and COPD are distinct diseases, the existence of overlapping diseases, sharing characteristics of both diseases is more commonly accepted, leading to preliminary guidelines [6]. ACO is characterized by persistent airflow limitation, consistent with COPD, with several features of asthma [6]. When compared to asthma and COPD, ACO is also associated to more rapid decline in lung function, more frequent exacerbations, increase health care resource utilization, worsening quality of life and higher mortality rates [[19], [20], [21]]. However, no consensus exists to accurately determine ACO. In context, the GINA GOLD stepwise methodology, that we have employed, is an accepted tool to determine the ACO population. This strategy of ACO identification is based on the determination of an equal number of asthma and COPD characteristics. This methodology by itself may generate a huge heterogeneity in the ACO population [6], allowing to presume the presence of numerous subgroups of patients. The presence of multiples phenotypes of ACO is also supported by the heterogeneity and the description of numerous clusters of patients in COPD and asthma [[22], [23], [24]].
Clusterization of asthma patients is proposed since many years, especially in severe asthma. Five clusters have been proposed by Moore et al. [22], confirming the heterogeneity of the disease, supporting different pathophysiologic networks. This strategy is supposed to be helpful to determine therapeutic options. In addition, beside the well-known demonstration of asthma heterogeneity, Haldar et al. [23] have shown that the individualization of 5 clusters in primary- and secondary-care asthma population was of interest to guide the treatment, using both symptoms and eosinophilic inflammation. By contrast, the determination of COPD phenotypes using cluster analysis has been less developed. Using this methodology, Burgel et al. [24] have displayed, four clinical phenotypes. This description underlined the importance of clinical phenotypes, independently of the classical severity of the disease, based on the unique FEV-1 value.
To our knowledge, ACO phenotypes description using a cluster analysis has not been reported. Characterization of ACO in this way is of interest for several reasons. Firstly, classical criteria employed to determine ACO are numerous and suggestive of a tremendous clinical heterogeneity, suggesting the presence of subgroups of patients associated to specific clinical characteristics. The determination of subgroups of patients, using clinical characteristics seems much more pertinent than the presence of a persistent airflow limitation to approach outcomes of ACO patients. The identification of such subgroups might be associated with the presence of specific comorbidities and linked to a prognostic value. Indeed, phenotypes A and B appear to be more severe, combining more respiratory symptoms, higher smoking habits, more comorbidities and higher lung function deterioration than phenotypes C and D. The analysis of the age of the disease onset appears also relevant, leading to distinguish 2 groups of clusters. Although patients from clusters 1, 2 and 3 (phenotypes A and C) enter earlier in the disease, those from clusters 4, 5 and 6 (phenotypes B and D) develop symptoms after 40-year-old. This discrepancy is a hallmark of ACO, illustrating also its huge heterogeneity, combining characteristics of asthma and COPD, each of them starting at different time-points. Beside the classical early and more delayed onsets of asthma and COPD respectively, the onset of both diseases is known to be also associated to large fluctuations [23,25]. Finally, the individualization of distinct phenotypes in ACO suggests the presence of specific pathophysiologic pathways. This aspect has been largely demonstrated in severe asthma, allowing the recognition of Th2 high/low-associated inflammatory profiles and the development of specific therapeutic options [26]. In ACO, this aspect might be also relevant, since recommendations for ACO treatment remains speculative [6]. Indeed, ACO patients being currently excluded from clinical trials, robust evidences of treatment effectiveness are lacking. We speculate that ACO clinical trials might be designed using such phenotypic classification to gain new insights and to determine the good treatment for the good ACO patient.
The multi-centered recruitment of ACO within a huge population of asthma and COPD patients is a strength of the current study, leading an analysis based on a large cohort of ACO. As previously shown, the employment of MCA for transforming variables included in the cluster analysis is a powerful approach [24].
Our study has several limitations. First, the recruitment of ACO patients was based on a retrospective analysis of medical records. However, asthma and COPD medical records from 7 medical centers were systematically reviewed. The retrospective analysis of comorbidities may be particularly criticized, lacking of exhaustiveness. Similarly, atopic traits of patients were missing from many data files. The final limit is the descriptive aspect of the data-driven methodology. Visual interpretation leads to subjectivity in the clusters description even if biases are avoided by the absence of any prior hypothesis. Also, we chose as representative patients those who had a cosine squared >0,3, which is arbitrary. However, it permitted to obtain enough patients to create and compare clusters. Furthermore, it allowed to distinguish subgroups on the graph, avoiding no interesting dots.
To conclude, although ACO patients do not fulfill overall criteria for asthma or COPD, their individualization remains poorly performed. As asthma and COPD, ACO is supposed to be also a heterogeneous disease. Employing the GINA GOLD stepwise approach, then a cluster analysis, we reported the description of 4 phenotypes in ACO patients, based on clinical features. This approach might be powerful to determine phenotype-related specific outcomes, comorbidities and mortality in ACO. However, these preliminary results required to be confirmed by subsequent and larger prospective studies. The aims of such future study are firstly to validate these phenotypes, then to determine specific pathophysiologic pathway and finally to design specific clinical trial to ameliorate ACO health cares.
Declaration of interest
Dr Lainez reports grants from BOEHRINGER INGELHEIM, during the conduct of the study; Dr. Court-Fortune reports grants from BOEHRINGER INGELHEIM, during the conduct of the study; personal fees from BOEHRINGER INGELHEIM, personal fees from PFIZER, personal fees from NOVARTIS, outside the submitted work; Dr. Vercherin has nothing to disclose; Dr. Falchero has nothing to disclose; Dr. Didi has nothing to disclose; Dr Beynel has nothing to disclose; Dr. Froudarakis has nothing to disclose; Dr. Piperno reports personal fees from ASTRA ZENECA, personal fees from NOVARTIS, personal fees from VIVISOL, personal fees from ELIA MEDICAL, outside the submitted work; Dr. Devouassoux reports grants from BOEHRINGER INGELHEIM, during the conduct of the study; personal fees and non-financial support from GSK, personal fees and non-financial support fromASTRA ZENECA, personal fees and non-financial support from NOVARTIS, grants, personal fees and non-financial support from CHIESI, personal fees and other from MENARINI, outside the submitted work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.100929.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Afrite A., Allonier C., Com-Ruelle L., Le Guen N. IRDS Publications; 2011. L’asthme en France en 2006 : prévalence, contrôle et déterminants; p. rap1820. [Google Scholar]
- 2.Landis S.H. Continuing to confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012-2013. Int. J. Chronic Obstr. Pulm. Dis. 2014;9:597–611. doi: 10.2147/COPD.S61854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lister W.A., Asthma Chronic bronchitis and emphysema. Lancet. 1955;269:733–737. doi: 10.1016/s0140-6736(55)92435-1. [DOI] [PubMed] [Google Scholar]
- 4.Guerra S. Overlap of asthma and chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2005 Jan;11:7–13. doi: 10.1097/01.mcp.0000146780.33963.bf. [DOI] [PubMed] [Google Scholar]
- 5.Gibson P.G., Simpson J.L. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 6.Diagnosis of diseases of chronic airflow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS), GINA Report, Global Strategy for Asthma Management and Prevention. 2017:7–10. http://www.ginasthma.org [Google Scholar]
- 7.Woodruff P.G. ATS-NHLBI asthma COPD overlap (ACO) workshop report. Am. J. Respir. Crit. Care Med. 2017;196:375–381. doi: 10.1164/rccm.201705-0973WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miravitlles M. Diagnosis of asthma-COPD overlap: the five commandments. Eur. Respir. J. 2017;49:1700506. doi: 10.1183/13993003.00506-2017. pii. [DOI] [PubMed] [Google Scholar]
- 9.Bonten T.N. Defining asthma-COPD overlap syndrome: a population-based study. Eur. Respir. J. 2017;49 doi: 10.1183/13993003.02008-2016. [DOI] [PubMed] [Google Scholar]
- 10.Alshabanat A., Zafari Z., Albanyan O., Dairi M., FitzGerald J. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriksen A.H., Langhammer A., Steinshamn S., Mai X.M., Brumpton B.M. The prevalence and symptom profile of asthma-COPD overlap: the HUNT study. COPD. 2017;0:1–9. doi: 10.1080/15412555.2017.1408580. [DOI] [PubMed] [Google Scholar]
- 12.Kumbhare S., Pleasants R., Ohar J.A., Strange C. Characteristics and prevalence of asthma/chronic obstructive pulmonary disease overlap in the United States. Ann Am Thorac Soc. 2016;13:803–810. doi: 10.1513/AnnalsATS.201508-554OC. [DOI] [PubMed] [Google Scholar]
- 13.Van Boven J.F., Roman-Rodriguez M., Palmer J.F., Pons N.T., Cosio B.G., Soriano J.B. Comorbidome, pattern and impact of asthma-COPD overlap syndrome (ACOS) in real-life. Chest. 2016;149:1011–1020. doi: 10.1016/j.chest.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Guerriero M., Caminati M., Viegi G., Senna G., Pomari C. Prevalence and features of asthma-chronic obstructive pulmonary disease overlap in Northern Italy general population. J. Asthma. 2018;8:1–7. doi: 10.1080/02770903.2018.1424190. [DOI] [PubMed] [Google Scholar]
- 15.Hines K.L., Peebles R.S., Jr. Management of the asthma-COPD overlap syndrome: a review of the evidence. Curr. Allergy Asthma Rep. 2017;15 doi: 10.1007/s11882-017-0683-4. [DOI] [PubMed] [Google Scholar]
- 16.Ding B., Small M. Treatment trends in patients with asthma-COPD overlap syndrome in a COPD cohort: findings from a real-world survey. International Journal of COPD. 2017;12:1753–1763. doi: 10.2147/COPD.S136314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garelli V., Petitpierre N., Nicod L.P. Rev. Med. Suisse. 2015;11:2145–2150. [PubMed] [Google Scholar]
- 18.Gibson P.G., McDonald V.M. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70:683–691. doi: 10.1136/thoraxjnl-2014-206740. [DOI] [PubMed] [Google Scholar]
- 19.Ding B., Enstone A. Asthma and chronic obstructive pulmonary disease overlap syndrome (ACOS): structured and literature review and physician insights. Expert Rev. Respir. Med. 2016;10:363–371. doi: 10.1586/17476348.2016.1144476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerhardsson de Verdier M., Andersson M., Kern D.M., Zhou S., Tunceli O. Asthma and chronic obstructive pulmonary disease overlap syndrome: doubled costs compared with patients with asthma alone. Value Health. 2015;18:759–766. doi: 10.1016/j.jval.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Piras B., Miravittles M. The overlap syndrome: the (missing) link between asthma and COPD. Multidiscip Respir Med. 2012;7:8. doi: 10.1186/2049-6958-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore W.C. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am. J. Respir. Crit. Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haldar P., Pavord I.D., Shaw D.E., Berry M.A., Thomas M., Brightling C.E., Wardlaw A.J., Green R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008;178:21824. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgel P.R. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur. Respir. J. 2010;36:531–539. doi: 10.1183/09031936.00175109. [DOI] [PubMed] [Google Scholar]
- 25.Lange P. Lung-function trajectories leading to chronic obstructive pulmonary disease. N. Engl. J. Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 26.Wenzel S.E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.