Abstract
Programmed cell death (PCD) refers to the way in which cells die depending on specific genes encoding signals or activities. Apoptosis, autophagy, and pyroptosis are all mechanisms of PCD. Among these mechanisms, pyroptosis is mediated by the gasdermin family, accompanied by inflammatory and immune responses. The relationship between pyroptosis and cancer is complex, and the effects of pyroptosis on cancer vary in different tissues and genetic backgrounds. On one hand, pyroptosis can inhibit the occurrence and development of tumors; on the other hand, as a type of proinflammatory death, pyroptosis can form a suitable microenvironment for tumor cell growth and thus promote tumor growth. In addition, the induction of tumor pyroptosis is also considered a potential cancer treatment strategy. Studies have shown that DFNA5 (nonsyndromic hearing impairment protein 5)/GSDME (Gasdermin-E) mRNA methylation results in lower expression levels of DFNA5/GSDME in most tumor cells than in normal cells, making it difficult to activate the pyroptosis in most tumor cells. During the treatment of malignant tumors, appropriate chemotherapeutic drugs can be selected according to the expression levels of DFNA5/GSDME, which can be upregulated in tumor cells, thereby increasing the sensitivity to chemotherapeutic drugs and reducing drug resistance. Therefore, induced pyroptosis may play a predominant role in the treatment of cancer. Here, we review the latest research on the anti- and protumor effects of pyroptosis and its potential applications in cancer treatment.
Subject terms: Cancer, Cancer
Facts
1. Pyroptosis, a lytic, inflammatory type of regulated cell death that requires membrane-damaging gasdermin proteins, characterized by the swelling and lysis of cells, and release of many proinflammatory factors.
2. The inflammasome, caspase and gasdermin family are play key roles in pyroptosis.
3. Pyroptosis, its associated signaling pathways and the release of various inflammatory mediators are closely related to the tumorigenesis and drug resistance of tumors.
4. Triggering tumor (especially apoptosis resistance) pyroptosis holds great therapeutic potential for cancer treatment.
Open questions
1. Does pyroptosis play differential roles in normal and tumor tissues?
2. What are the key signals that initiate pyroptosis?
3. What are the key signaling pathways impacted by pyroptosis in tumors?
4. How can pyroptosis be manipulated to drive tumor fate?
Introduction
The dynamic balance between cell proliferation, differentiation and death maintains ontogeny, homeostasis and pathological processes in multicellular organisms. Cell death are mainly divided into two categories, necrosis and programmed cell death (PCD). Apoptosis is a type of PCD involving the automatic self-destruction of cells controlled by genes, the cell membrane remains intact, and generally not inducing inflammation. Necrosis is a passive type of cell death caused by pathological stimuli. The cell membrane permeability of necrotic cells increases, causing the cells to swell and eventually breakdown to release the cellular contents, leading to inflammatory reaction1. Pyroptosis is a new procedural and inflammatory death discovered after apoptosis and necrosis. Similar to apoptosis, pyroptotic cells undergo nuclear condensation and chromatin DNA fragmentation, and TUNEL staining is positive2,3. Similar to necrosis, during pyroptosis, the formation of the pores disrupts the balance of ion gradients on both sides of the cell membrane, leading to water inflow, cell swelling, cell membrane rupture, and the release of proinflammatory mediators, including IL-1β, IL-18, ATP, and HMGB14, which induce inflammatory responses, thus pyroptosis is also known as inflammatory “necrosis”5,6.
A close relationship between pyroptosis and various human diseases, especially malignant tumors. Pyroptosis may play a dual role in the pathogenesis of tumors. On one hand, the multiple signaling pathways and inflammatory mediators released during pyroptosis are closely related to the tumorigenesis as well as to their drug resistance to chemotherapeutic drugs7–9. On the other hand, as a type of death, pyroptosis can inhibit the occurrence and development of tumors7,10. The role of pyroptosis in tumor has become increasingly prominent as research has advanced. This review will summarize and discuss the potential effects of pyroptosis on cancer and the role of pyroptosis in anticancer therapy.
Discovery of the cell pyroptosis phenomenon
The term pyroptosis combines the Greek roots ‘pyro’ and ‘ptosis’, which mean fever and falling, respectively, to define a newly discovered inflammatory PCD11. As early as 1990s, scientists discovered that Shigella flexneri or Salmonella infection of mouse macrophages or human monocytes cause cell death12,13. In 1997, Arturo Zychlinsky found that Shigella dysenteriae could activate caspase-1 in host cells14. In 1999, the Arturo Zychlinsky laboratory found that knocking out caspase-1 could block the cell death caused by Salmonella15. In 2001, the laboratories of Lawrence H. Boise and Brad Cookson gradually elucidated that the macrophage death caused by bacterial infection was a death mode completely different from apoptosis and named it caspase-1-dependent programmed necrosis11,16.
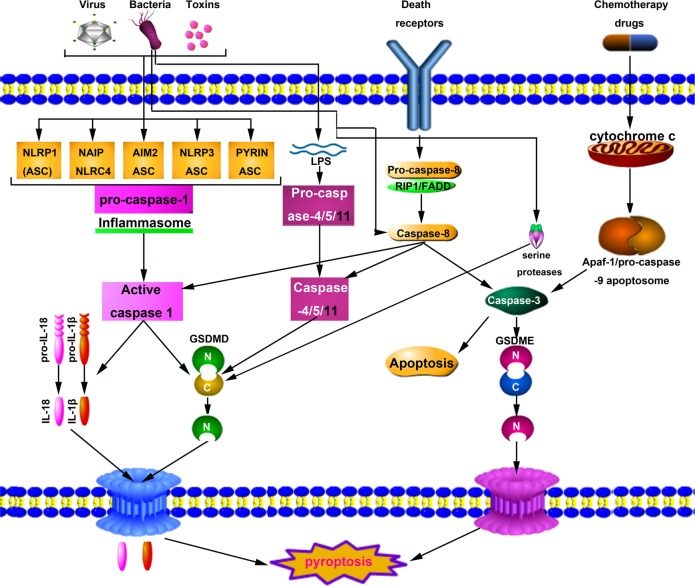
But until recently, a new gasdermin-D (GSDMD) protein has been discovered and identified, which normally in a state of auto-inhibition. After caspase cleavage, GSDMD releases the N-terminal fragment (GSDMD-cNT), in turn, the cells swell until they rupture17,18, indicating that GSDMD is the executor of pyroptosis. Like GSDMD, other members of the gasdermin family include GSDMA, GSDMB, GSDMC, DFNA5/GSDME, and DFNB59 also have membrane perforation activity and induce pyroptosis18,19. Wang et al. confirmed that the N-terminal domain of GSDME combines with 4,5-diphosphate phosphatidylinositol [PI (4,5) P2], leading to the perforation of liposomes and loss of their phospholipid contents, which is consistent with the mechanism of pyroptosis caused by the GSDMD-cNT20. Therefore, the Feng Shao group redefined pyroptosis as gasdermin family-mediated programmed necrosis21. More recently, Kambara et al. reported that neutrophil elastase (NE) cleaved GSDMD and this cleavage induced neutrophil pyroptosis22. In 2018, the Nomenclature Committee on Cell Death (NCCD) proposed defining pyroptosis as a form of regulated cell death (RCD) that critically depends on the formation of plasma membrane pores by members of the gasdermin protein family, often (but not always) as a consequence of inflammatory caspase activation23. Figure 1 summarizes the pyroptosis history to date.
Fig. 1.
Timeline summary of the history of pyroptosis
Signaling transduction of pyroptosis
Inflammasome activation is the basis of pyroptosis
Pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs) or nonpathogen-related damage-associated molecular patterns (DAMPs), which initiate pyroptosis. Studies have shown that PRRs related to pyroptosis include Toll-like receptors (TLRs), intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and AIM2-like receptors (ALRs)24. PRRs recognize PAMPs or DAMPs that are specific to each inflammasome and initiate assembly to recruit and ultimately facilitate caspase-1 dimerization and activation25,26. For example, NLRPlb can detect lethal toxins from Bacillus anthracis and components of Toxoplasma gondii. Many stimulants, such as bacteria, viruses, fungi, uric acid, and ATP, can activate NLRP3 inflammasomes, while flagellin and type III secretory system proteins can be recognized by the NLRC4, and AIM2 inflammasomes primarily recognize double-stranded DNA contained in bacteria or viruses27. When PRRs are stimulated, caspase-1 was recruited directly or via ASC to form caspase-1-dependent inflammasome25,28. After assembly of the inflammasome, caspase-1 becomes self-activated and changes from a proenzyme to protease, which plays further physiological role.
Activation mechanism of caspase-1
Caspase-1 exist as a proenzyme in the resting state and is an essential component of the inflammasome, which is formed by different PRRs through (or without) ASC and caspase-1 under the stimulation of specific PAMPs and DAMPs. The inflammasome contains an NLR and the adaptor protein ASC, associating via caspase recruitment domain interactions with procaspase-1, then undergoes autocleavage to form active caspase-1. Caspase-1 not only can mediate the maturation and secretion of proinflammatory cytokines (interleukin-1β (IL-1β) and IL-18) but also initiate the pyroptosis29. Joosten et al. found that IL-1 recognizes antigens and induces inflammatory responses. In addition, IL-1 stimulates the activation of primary T cells and memory T cells30. IL-18 promotes the synthesis of interferon in Th1 lymphocytes, natural killer cells, and cytotoxic T cells; promotes the differentiation of Th2 cells; and increases local inflammatory responses31. Fink et al. found that caspase-1 activation induced the cellular formation of many small pores on the cell membrane. These pores induce communication between the internal and external sides of the membrane, and both sides rapidly lose their ion gradient. Because a large amount of water enters the cell, cell swelling, and the cell eventually dies, allowing the cytoplasmic contents to escape4. Therefore, the RCD mediated by caspase-1 is also known as the classical pyroptosis pathway.
Activation mechanism of caspase-4/5/11
Nonclassical pyroptosis pathways also exist, such as cytoplasmic lipopolysaccharide (LPS) directly activates caspase-4/5/11 to mediate pyroptosis32. Caspase-4/5/11 can be directly stimulated by intracellular Gram-negative bacterial LPS to activate and hydrolyze the own protease activity. Activated caspase-4/5/11 can also act on GSDMD and produce the same cleavage effect as caspase-1, leading to the formation of cell membrane pores. Activated caspase-4/5/11 can physically interact with caspase-1 to promote its activation in the presence of NLRP3 and ASC33–36. Caspase-1 cleaves precursors of IL-1β and IL-18 to form active IL-1β and IL-18, which can be released through channels formed by GSDMD-cNT and cause pyroptosis34,36. Notably, in the nonclassical pyroptosis, only the cleavage of the IL-1β and IL-18 precursors is dependent on caspase-1. For the cleavage of GSDMD, caspase-1 is not required33.
GSDMD is a common substrate for caspase-1/4/5/11
Although the inflammatory caspases initiates the pyroptosis, its specific mechanism is not well understood. Recent studies have found that the GSDMD is a common substrate for caspase-1/4/5/1117,33,37, and a highly conserved caspase-1/4/5/11 cleavage site exists in the hinge region of GSDMD17,33,37–39. At this cleavage site, if the wild-type “FLTD” sequence is mutated to “LTA”, the cells are no longer able to respond to pedestal proteins and LPS. The overexpression of GSDMD-cNT alone can cause very strong pyroptosis, while the overexpression of the C-terminal domain and full-length GSDMD do not cause pyroptosis17,18,40. When activated caspase-1/4/5/11 cleaves the hinge region between the N- and C-terminal domains to produce GSDMD-cNT, the autoinhibition activity of C-terminal domain is relieved, and the lethal activity of the N-terminal domain is released, causing pyroptosis. GSDMD-cNT bind with phosphatidylinositol, phosphatidic acid, and phosphatidylserine on the inner surface of cell membranes and form pores in the lipid bilayer, which is the basis for the interleukins secretion17,18,40,41.
‘Alternative pathway’ of pyroptosis
In 2017, Rogers et al. found that activated caspase-3 can cleave DFNA5/GSDME, to generate N-terminal fragment (GSDME-NT) and induce cell pyroptosis after caspase-3 successfully induces apoptosis42. Wang et al. also verified the cleavage and activation of GSDME by caspase-3 and further confirmed that the pyroptosis is a mechanism underlying the toxic side effects of some chemotherapeutic drugs20. These authors showed that the caspase-1 cleavage site on the GSDMD could be replaced by the caspase-3 cleavage site in HeLa cells. The combined use of TNF-α and cycloheximide activates caspase-3, which further cleaves the GSDMD to produce GSDMD-cNT and induce the apoptosis-to-pyroptosis switch. These authors also found that the expression of wild-type DFNA5/GSDME in HeLa cells reversed caspase-3 activation, causing an apoptosis-to-pyroptosis switch. Wang et al. identified a specific caspase-3 cleavage region between the C- and N-terminal domains of DFNA5/GSDME and established that cells undergo apoptosis after mutation in this cutting region. These researchers further confirmed that the GSDME-NT share the same mechanism caused by GSDMD-cNT20. Recent studies have indicated that apoptotic caspase-8 can also induce the cleavage of GSDMD and DFNA5/GSDME, thus inducing pyroptosis during Yersinia infection, which indicates that apoptosis and pyroptosis may share many signal pathways43–45. It is interesting to note that serine proteases, including NE and cathepsin G (CatG), can cleave GSDMD independently of caspase activity to generate a fully active NE-derived N-terminal fragment (GSDMD-eNT) and the signature N-terminal domain GSDMD-p30 to induce pyroptosis in neutrophils22,46 (Fig. 2).
Fig. 2. Schematic representation of pyroptosis pathways.
The canonical pathway upon sensing DAMPs, PAMPs or other cytosolic disturbances results in the recruitment and activation of caspase-1 either directly or through recruitment of the receptor protein ASC. Caspase-1 successively promotes maturation of the precursors of IL-1β and IL-18 into mature forms and cleaves GSDMD. The pore form domain (PFD) of GSDMD interacts with the plasma membrane to form GSDMD pores, resulting in the release of intracellular contents, including IL-1β and IL-18. The noncanonical pathway is initiated by the caspase-11 self-detection of cytosolic LPS in Gram-negative bacteria. Activated caspase-11 (caspase-4 or caspase-5 in humans) successively cleaves GSDMD and induces pyroptosis. The other pathway of pyroptosis can be engaged through mechanisms such as CASP8-GSDMD and CASP3-GSDME. In turn, activated caspase-3 cleaves GSDME to produce GSDMD-cNT, which forms pores in the plasma membrane and activates pyroptosis. The bacteria are recognized by TLR4, which signals via RIP1 to form a cell death complex consisting of RIP1, caspase-8, and FADD. Both this complex cleavage of GSDMD and activation through caspase 1/11 and GSDME cleavage via caspase-3 lead to cell membrane permeabilization and subsequent pyroptosis. In addition, neutrophil elastase (NE) is able to cleave GSDMD independently of caspase activity
Role of pyroptosis in tumorigenesis and metastasis
The tumorigenesis is related to various factors, including the activity of proto- and antioncogenes, the immune microenvironment, oxidative stress and chronic inflammation. The long-term exposure of tissues and/or cells to the inflammatory environment increases the risk of cancer. The activation of pyroptosis leads to the release of the inflammatory mediators IL-1 and IL-18, which could promote the occurrence of cancer in many ways. Studies utilizing Nlrp3−/− and caspase-1−/− mice have shown that mice lacking active inflammasomes are more sensitive to azoxymethane/dextran sulfate sodium (AOM/DSS)-induced colitis-associated colon cancer (CAC) than control mice47–50. These studies indicated that pyroptosis may play a dual role in promoting and inhibiting tumor cell growth in different tumor cells. However, the specific mechanism of pyroptosis and its rele in tumorigenesis deserve further study.
Pyroptosis and hepatocellular carcinoma (HCC)
Wei et al. found that the expression of NLRP3 in HCC tissues was significantly downregulated or even completely absent, and its expression was negatively correlated with the pathological grade and clinical stage of HCC, indicating that the NLRP3 inflammasome was involved in the progression of HCC51. Furthermore, they found that 17β-estradiol exerted anticancer effects, which attributed to its ability to trigger pyroptosis via activation of NLRP3 inflammasome52. The AIM2 inflammasome can weaken the activation of S6K1 by targeting mTOR, thus inhibiting the growth of cancer cells, and the accumulation of the AIM2 inflammasome can cause HCC cells pyroptosis, exerting antitumor effects53,54. Caspase-1 was significantly reduced in HCC tissues, and the caspase-1, IL-1β, and IL-18 expression were lower in HCC tissues than these in adjacent normal tissues55,56. The expression of DFNA5/GSDME in HCC cells is significantly lower than that in normal cells and upregulating DFNA5/GSDME expression inhibited cell proliferation, indicated that DFNA5/GSDME may be an antioncogene57. In addition, the lncRNA CCND2-AS1 involved in improper regulation of pyroptosis in HCC, showing a unique feature of HCC58 (Table 1).
Table 1.
Expression of pyroptosis core proteins in cancer and their impacts on cancer
| Expression level | Tumor type | Prognosis | Ref (s) | |
|---|---|---|---|---|
| NLRP3 | Low protein level | Hepatocellular carcinoma (HCC) | NLRP3 deficiency is significantly correlated with advanced stages and poor pathological differentiation. | 51 |
| AIM2 | Low protein level | Hepatocellular carcinoma (HCC) | Low protein level of AIM2 promotes HCC progression. | 53 |
| Caspase-1 | Low protein level | Hepatocellular carcinoma (HCC) | Not determined. | 56 |
| DFNA5/GSDME | Low protein level | Hepatocellular carcinoma (HCC) | DFNA5 may function as a tumor suppressor gene with an important role in HCC. | 57 |
| GSDMB | High protein level | Breast cancer (BC) | GSDMB induces invasion, tumor progression and metastasis in MCF7 cells. | 59 |
| GSDME | Not determined | Breast cancer (BC) | Not determined. | 20 |
| NLRP3 | Low protein level | Colorectal cancer (CRC) | The NLRP3 inflammasome functions as a negative regulator of intestinal tumorigenesis. | 49 |
| NLRP1 | Low protein level | Colorectal cancer (CRC) | The NLRP1 inflammasome functions as a negative regulator of intestinal tumorigenesis. | 68, 69 |
| AIM2 | Low protein level | Colorectal cancer (CRC) | Lack of AIM2 expression is closely associated with poor outcomes in colorectal cancer. | 66 |
| GSDMA | High protein level | Colorectal cancer (CRC) | GSDMA is overexpressed in carcinoma. | 72 |
| GSDMC | High protein level | Colorectal cancer (CRC) | GSDMC functions as an oncogene, promoting cell proliferation in colorectal carcinogenesis. | 73 |
| GSDMD | High protein level | Colorectal cancer (CRC) | GSDMD is downregulated at both the mRNA and protein levels in carcinoma. | 72 |
| GSDME | High protein level | Colorectal cancer (CRC) | GSDME may be a promising biomarker for the detection of colorectal cancer. | 74 |
| GSDMC | High protein level | skin cancer | GSDMC plays an important role in promoting proliferation in colorectal tumorigenesis in vivo. | 73 |
| GSDME | Low protein level | skin cancer | A decreased DNFA5 mRNA expression level is associated with increased etoposide resistance in melanoma cells. | 77 |
| NLRC4 | Low protein level | Gastric cancer (GC) | The NLRC4 expression level in gastric cancer cells is higher than that in normal gastric epithelial cells. | 79 |
| GSDMA | Low protein level | Gastric cancer (GC) | GSDMA is downregulated in gastric cancer cells and is thought to be a tumor suppressor gene. | 81, 82 |
| GSDMB | High protein level | Gastric cancer (GC) | GSDMB is increased in gastric cancer cells and is thought to be a tumor suppressor gene. | 82, 89 |
| GSDMC | Low protein level | Gastric cancer (GC) | GSDMC is downregulated in gastric cancer cells and is thought to be a tumor suppressor gene. | 73, 82 |
| GSDMD | Low protein level | Gastric cancer (GC) | GSDMD expression is decreased in GC, and the decreased expression of GSDMD could markedly promote the proliferation of tumors in vivo and in vitro. | 82 |
| GSDME | Low protein level | Gastric cancer (GC) | GSDME may be a tumor suppressor gene. | 73 |
| GSDMD | High protein level | Lung cancer | High GSDMD expression indicates a poor prognosis in LUAD. | 91 |
| GSDME | High protein level | Lung cancer | GSDME overexpression leads to enhanced drug sensitivity in vivo and in vitro. | 91 |
| GSDME | High protein level | esophageal squamous | GSDME is more highly expressed in esophageal squamous cell carcinoma than in normal adjacent tissues. | 104 |
| GSDML | Unknown | gastric, liver and colon carcinomas | The GSDML protein splicing variants range in molecular weight from 35 to 50 kDa, and the expression profile varies between tumor and nontumor. | 84 |
Pyroptosis and breast cancer (BC)
In BC, a high level of GSDMB is associated with a low survival rate and a high metastasis rate. For HER2-positive BC, overexpression of GSDMB predicts low reactivity to HER2-targeted treatment59. Therefore, GSDMB may become a new marker for BC and participate in the evaluation of prognosis. DFNA5/GSDME, initially termed ICERE-1, is overexpressed in ER-negative cell lines and may participate in tumorigenesis specific to hormonally unresponsive BC60. Interestingly, this methylation was detected in only estrogen receptor-positive cell lines61,62. Moreover, DFNA5 methylation was found to be associated with lymph node metastasis62. A study comparing paclitaxel (PTX) drug sensitivity before and after low DFNA5/GSDME expression in MCF-7 cells showed that low DFNA5/GSDME expression reduces the sensitivity of MCF-7 cells to PTX drugs, i.e., the decreased-GSDME increases the resistance of MCF-7 cells to PTX63. p53 can induce DFNA5/GSDME expression via a specific p53 binding site in intron 1 of DFNA564. As a member of the p53 family, P63γ also increases DFNA5 levels, suggesting that DFNA5 is a transcriptional target of the p53 family61 (Table 1).
Pyroptosis and intestinal cancer
Studies on Nlrp3−/− or caspase-1−/− mice have shown that mice lacking active inflammasomes are more sensitive to AOM/DSS-induced CAC than control mice47–50. Study showed that AIM2 inflammasome-mediated pyroptosis plays a key role in radiation-induced gastrointestinal syndrome65. Dihlmannd et al. reported that the expression of AIM2 was decreased in 67.4% of colorectal tumors (CRC) cells and absentd in 9.18% of CRC cells. After adjusting for factors such as gender, tumor stage, age, tumor grade, tumor site and chemotherapy, the mortality of 5a patients with AIM2 deficiency increased66. These results indicate that the AIM2 inflammasome is closely related to CRC and/or pyroptosis65–67. Studies have reported that the expression of NLRP1 in CRC tissues was decreased compared with normal tissues, and Nlrp1b−/− mice showed a higher tumor incidence than control mice68. The levels of the NLRP1 inflammasome in CRC tissues are lower than those in adjacent tissues. Stage III and IV CAC patients have lower NLRP1 inflammasome than stage I and II CAC patients. Survival analysis have revealed that lower NLRP1 are correlated with a shorter patient survival period69. In addition, compared to wild-type (WT) littermates, Casp11−/− mice are highly vulnerable to the AOM/DSS model of CAC70. GSDMA was not expressed in normal colorectal epithelial cells but was gradually overexpressed in carcinoma cells, while GSDMD exhibited the opposite trend71,72. Gsdmc is not detected in normal colorectal tissues but is present in CRC tissues. Miguchi et al. found that inactivation mutations of Tgfbr2 often occur, which upregulates Gsdmc expression, induces tumor cell proliferation and promotes tumorigenesis. Therefore, Gsdmc is an oncogene that may act as a new therapeutic target for CRC treatment with the Tgfbr2 mutation73. Recently, Ibrahim et al. identified two combinations of CpGs that can accurately distinguish between CRC and normal tissues regardless of age and stage, suggesting that GSDME may be a promising biomarker for CRC detection74 (Table 1).
Pyroptosis and skin cancer
GSDMC is not detectable in normal epithelial cells but is present in malignant melanoma, which may be related to the invasion and metastasis of these cancer cells73,75. DFNA5/GSDME expression in the nonresistant MeWo cell line was markedly increased compared with that in the etoposide-resistant MeWo ETO 1 cell line76. In etoposide-resistant melanoma cells, knockout of DFNA5/GSDME increased cell resistance to etoposide, while upregulation of DFNA5/GSDME expression increased the cell sensitivity to etoposide, suggesting that decreased-DNFA5 is related to the increase in etoposide resistance in melanoma cells77. Eukaryotic elongation factor-2 kinase (eEF-2K) is a negative regulator of protein synthesis that plays an important role in autophagy and pyroptosis of tumor cells under various conditions. In melanoma cells, silencing eEF-2K promoted doxorubicin-induced pyroptosis, thus sensitizing melanoma cells to doxorubicin78.
Pyroptosis and gastric cancer (GC)
The NLRC4 inflammasome is involved in aseptic and autologous inflammation, and its expression in GC is higher than that in normal gastric epithelial cells79. In macrophages, activated-NLRC4 inflammasomes can activate caspase-1, causing pyroptosis80. GSDMA is downregulated in GC and is considered as an antioncogene81,82. GSDMB is not detected in most normal gastric tissue but is expressed in a few normal gastric tissue. Most precancerous samples show moderate GSDMB expression, and most cancer samples show high-level of GSDMB, which overexpression may associate with tumor invasion82,83. Compared with normal gastric and esophageal tissues, GSDMC is downregulated in GC and esophageal cancer cells and may function as a cancer suppressor gene73,82. In addition, the regulation of GSDML splice variant transcription and translation may alter in the gastrointestinal tract cancers84. GSDMD was expressed at low levels in GC cell lines and models81,82. Further studies showed that decreased-GSDMD regulated cell cycle-related proteins expression by activating the STAT3 and PI3K/PKB signal pathways, accelerating S/G2 phase transformation and promoting tumor cell growth. Compared with normal nude mice, the tumor volume in nude mice with low-level GSDMD was larger after the implantation of GC cells, suggesting that the GSDMD level may be related to the GC occurrence85. The silencing of DFNA5/GSDME was first reported in primary GC and GC cell lines86. The methylation pattern of DFNA5 was also studied in 89 primary cancer tissues, of which 52% showed abnormal DFNA5 promoter methylation87. Transfection of DFNA5/GSDME in these cancer cell lines decreased the number of colonies and cell growth inhibition compared to those in cells transfected with empty vector62,88. Moreover, the fact that p53 modulated the expression of DFNA5/GSDME strongly suggests that DFNA5 is a tumor suppressor gene64. Recent studies have shown that chemotherapeutic drugs can convert caspase-3-dependent apoptosis to pyroptosis through DFNA5/GSDME89. DFNA5/GSDME can be downregulated due to promoter methylation. Treatment with the decitabine can induce the upregulation of DFNA5/GSDME expression in tumor cells, causing pyroptosis and making these cells more sensitive to chemotherapeutic drugs20,90 (Table 1).
Pyroptosis and lung cancer (LC)
In non-small cell lung cancer (NSCLC), higher GSDMD expression is related to invasive features, including more advanced tumor-node-metastasis stages and larger tumor sizes. GSDMD-silenced NSCLC cells show decreased epidermal growth factor receptor signaling, increased caspase 3 decomposition and enhanced apoptosis, resulting in the suppression of tumor growth in transplanted mice. Notably, the activation of NLRP3/caspase-1 signaling induces apoptosis rather than pyroptosis in tumor cells lacking GSDMD. GSDMD-deficiency activates the division of caspase-3 and promotes cancer cells death through the mitochondrial apoptosis pathway91. Xi et al. found that GSDMD contributes to cytotoxic T lymphocytes-mediated killing in lung squamous cell carcinoma and lung adenocarcinoma92. Lu et al. showed that knockout of DFNA5/GSDME can induce the apoptosis-to-pyroptosis switch, supporting the notion that the DFNA5/GSDME level determines the death mode of caspase-3-activated cells. In LC, loss of the DFNA5/GSDME gene promotes drug resistance, while overexpression of DFNA5/GSDME leads to increased drug sensitivity93 (Table 1).
Pyroptosis and cervical cancer (CC)
Studies shown that the NLRP3 inflammasome participates in the innate immune response to CC and its expression is widely present in tumor cells94,95. NLRP3 inflammasome activation can be achieved via a half-ion channel, lysosomal rupture and reactive oxygen species (ROS). In CC, the NLRP3 inflammasome is mainly activated by the ROS to induce the pyroptosis95. In HPV-infected CC cells, AIM2 can play a tumor inhibitory role by stimulating pyroptosis96. CC cells release more IL-18 and IL-1β than normal cervical epithelial cells97. However, some studies have found that the removal of proinflammatory factors produced by pyroptosis can inhibit the growth of CC cells and simultaneously weaken the bodily immune effect on tumor cells98–102. Pyroptosis has a dual effect of promoting and inhibiting CC, but the mechanism of proinflammatory factors produced by pyroptosis in CC cells remains to be studied.
Pyroptosis and other cancers
Nadatani et al. reported that the expression of pro-IL-18, pro-IL-1β, and NLRP3 in Barrett’s esophageal cancer cells treated with LPS was increased, and the levels of mitochondrial ROS, caspase-1, IL-18, IL-1β, lactate dehydrogenase (LDH), and pyroptosis were also increased. These results indicated that the NLRP3 inflammasome activation of caspase-1 induces the secretion of proinflammatory factors and pyroptosis. The application of a caspase-1 inhibitor can interfere with the expression of NLRP3 and block the production of IL-1β and IL-18 and the release of LDH induced by LPS103. In addition, the level of GSDME was more highly in esophageal squamous cell carcinoma (ESCC) than in normal adjacent tissues104. miR-214 suppresses cell growth and metastasis by modulating caspase-1-mediated cell pyroptosis in glioma cells105. A study reported that the level of MST1 is decreased in pancreatic ductal adenocarcinoma (PDAC) cells, and the restored expression of mammalian STE20-like kinase 1 (MST1) promotes PDAC cells death and suppres the proliferation, migration and invasion of PDAC cells via pyroptosis mediated by ROS106.
Manipulating pyroptosis for therapeutic benefit
Pyroptosis is closely related to many human diseases6,107–109, including tumors56. In recent years, researchers have attempted to combine pyroptosis with various tumor treatments and to treat tumors by regulating pyroptosis and inhibiting the proliferation, migration and invasion of tumor cells.
Drug-regulated pyroptosis of tumor cells
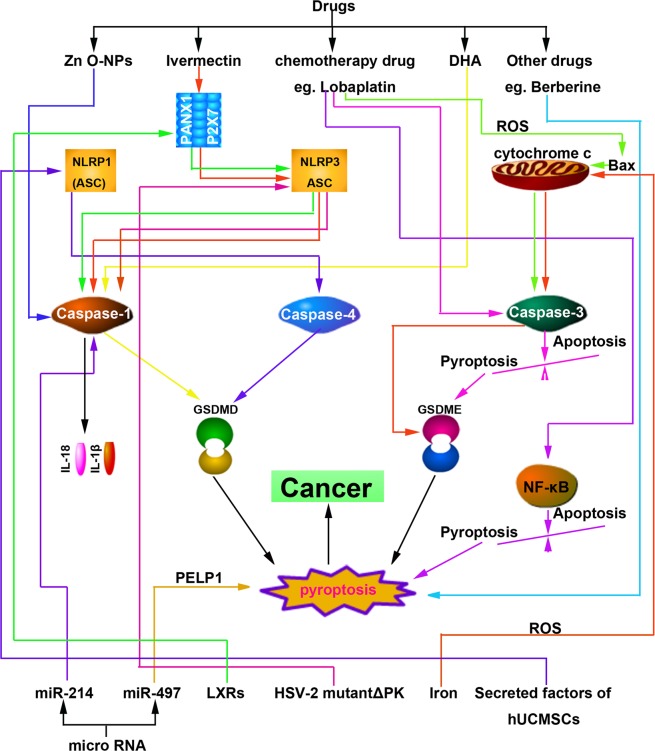
Song et al. found that the level of IL-1β, caspase-1, and LDH were positively correlated with dose and time after treating A549 cells with Zinc oxide nanoparticles (Zn O-NPs), suggesting that Zn O-NPs can activate pyroptosis in A549 cells110. Ivermectin selectively inhibits the growth of T cell factor (TCF)-dependent transplanted tumors111. Dobrin et al. treated the tri-negative BC cells with ivermectin and found that ivermectin could activate the pannexin-1 channel and induce the overexpression of P2X4/P2X7 receptor. The overexpression of P2X7 receptors can release ATP, thereby enhancing the cytotoxicity caused by ivermectin, which eventually resulting in the apoptosis, necrosis or pyroptosis of BC cells112. In LC treatment, the thiopyran derivative L61H10 has good antitumor activity through an apoptosis-to-pyroptosis switch113. Nathalia et al. found that omega-3 docosahexaenoic acid can induce the pyroptosis of tri-negative BC cells and the caspase-1 inhibitor can protect BC cells from omega-3 docosahexaenoic acid-induced-pyroptosis114. Chu et al. found that berberine can reduce the viability and invasiveness of cancer cells by inducing the pyroptosis of liver cancer cells56. Anthocyanin can accelerate the death of oral squamous cell carcinoma cells by inducing pyroptosis and inhibiting tumor progression115. RIG-I agonists activate the pyroptosis of tumor cells, induce the expression of inflammatory cytokines, recruit leukocyte chemokines, introduce leukocytes into the tumor microenvironment, and reduce the growth and metastasis of tumors116. Val-boroPro can activate the ‘inflammasome’ sensor protein CARD8, which successively activates procaspase-1 to mediate pyroptosis in primary acute myeloid leukemia (AML) samples and most AML cell lines, suggesting that Val-boroPro-induced-pyroptosis is suitable for the treatment of AML117,118. Recently, 13d, a derivative of EF24, has been shown to be a potent antitumor agent for LC therapy that functions via the apoptosis-to-pyroptosis switch119 (Fig. 3).
Fig. 3. Therapeutic targets of pyroptosis core proteins in cancer.
Drugs (chemotherapy drugs, new material, traditional Chinese medicines, etc.), miRNAs, receptor proteins, secreted factors of human umbilical cord mesenchymal stem cells, etc. can all modulate pyroptosis target core proteins in canonical, noncanonical, and other pathways for therapeutic benefit
Wang et al. found that chemotherapy drugs induced tumor cells with high-level GSDME pyroptosis due to the caspase-3 activation20. In tumor cell lines with low-level GSDME, the expression of GSDME was upregulated in the corresponding cell lines after treatment with the distamine, and the sensitivity of tumor cells to chemotherapy drugs was also increased, which made these cells more prone to pyroptosis. Notably, only 1/10 of tumor cells detected by the researchers had high-level GSDME, while 3/5 of human primary normal cells were found to have high-level GSDME. These high-level GSDME cells undergo pyroptosis after chemotherapeutic drugs treatment. Researchers further confirmed that GSDME-mediated pyroptosis is likely a mechanism underlying the toxic side effects of chemotherapeutic drugs20. In addition, chemotherapeutic drugs were found to convert caspase-3-dependent apoptosis into pyroptosis via GSDME20,89. In CAC cells, lobaplatin induces pyroptosis and caspase-3/9 activation downstream of the ROS/JNK/BAX mitochondrial apoptosis pathway, which is mediated by GSDME120. Chen et al. reported that PTX induced pyroptosis in A549 cells is closely related to the levels of active caspase-3 and GSDME-NT. Compared with PTX, the cisplatin-induced pyroptosis of NSCLC and ESCC cells was high, suggesting that cisplatin may have additional advantages in the treatment of GSDME-overexpression cancer subtypes104,121. Gsdme−/− mice were not affected by tissue damage or weight loss induced by chemotherapeutic drugs20. Intraperitoneal injection of cisplatin or 5-FU resulted in severe small intestinal injury and immune cell infiltration in Gsdme+/+ mice, whereas in Gsdme−/− mice, the signs of tissue damage were reduced122. In addition, Gsdme−/− mice showed reduced lung injury and inflammatory response to cisplatin or bleomycin122. These observations confirm the key role of GSDME-mediated pyroptosis in promoting the harmful effects of chemotherapy and provide new insights into cancer treatment (Fig. 3).
miRNA-regulated tumor pyroptosis
MicroRNAs (miRNA)s are noncoding single-stranded RNA containing approximately 22 nucleotides that can regulate the expression of multiple target genes. Some miRNAs, which functions similar to those of tumor-suppressor genes, can downregulate the expression of oncogenes and inhibit the growth of tumors. Jiang et al. demonstrated that miR-214 could decrease the expression of caspase-1 and inhibit tumor proliferation, migration and invasion of glioma cells. After administration of a caspase-1 inhibitor, the above mentioned abilities of tumor cells were recovered, suggesting that miR-214 can induce the pyroptosis of glioma cells by regulating the caspase-1, thereby inhibiting tumor growth105. Proline-, glutamic acid- and leucine-rich protein-1 (PELP1), a scaffolding oncogene, is highly correlated with cancer progression and outcomes for patients with advanced ESCC. Wang et al. reported that upregulating miR-497 can downregulate PELP1 and eventually induce ESCC pyroptosis, which may serve as an alternative treatment for chemo- and radiotherapy for refractory ESCC or other cancers sharing the same pyroptosis mechanisms123 (Fig. 3).
Receptor protein-mediated tumor pyroptosis
Liver X receptors (LXRs) are members of the nuclear receptor family and play a key role in the inflammatory response. Studies have shown that LXRs are expressed in many cancer tissues and participate in various anticancer mechanisms. Derangere et al. found that plentiful caspase-1 was activated after LXRβ agonist T0901317 treatment, while apoptosis-related caspase-3/8/9 were not detected124. Further detection showed that numerous NLRP3 inflammasomes formed, and the cell death was related to the activation of the P2X7 receptor pathway and the release of ATP/ROS. Therefore, Derangere et al. believe that the LXR ligand and LXR receptor combination can open the pannexin-1 channel and release ATP, and high extracellular ATP participates in the activation of P2X7 receptor and caspase-1, inducing the formation of NLRP3 inflammasomes and thereby promoting the nonclassical pyroptosis of CAC cells124. Furthermore, the genetic and pharmacological inactivation of LXR in murine bone marrow-derived macrophages enhanced the inhibitory effects of radiation therapy on tumor growth through the induction of pyroptosis and activation of the inflammatory cascade125 (Fig. 3).
Other ways to regulate tumor cell pyroptosis
A recent study shown that the factors secreted from human umbilical cord mesenchymal stem cells can induce MCF7 cell pyroptosis126. Further experiments showed that the expression of NLRP1 and CAPS4 and the pathways associated with inflammation were significantly changed126. Colunga et al. found that calpain, caspase-7 and caspase-3 were activated in numerous malignant melanoma cells infected with the herpes simplex virus type 2 (HSV-2) mutant △PK (HSV-2 mutant △PK) to promote the oncolytic effect of ΔPK. The researchers also found that △PK could increase the level of heat shock proteins, such as Beclin-1 and H11/Hsp B8; the corresponding immunohistochemical results showed that under the effect of △PK, TNF-α was activated and participated in the formation of the NLRP3 inflammasome mediated by caspase-1, which successively led to the induction of both apoptosis and pyroptosis127. Zhou et al. found that Tom20, an outer mitochondrial membrane protein, can be oxidized by elevated ROS, facilitating Bax recruitment to mitochondria and stimulating caspase-3/GSDME-mediated pyroptosis after iron treatment. Iron may be a potential candidate for the treatment of melanoma because it activates ROS to induce DFNA5/GSDME-dependent pyroptosis and specifically induces high levels of DFNA5/GSDME expression in melanoma cells. Further studies have shown that the use of iron supplements in patients with iron deficiency can maximize the antitumor effect of clinical ROS-induced drugs, thereby inhibiting the growth and metastasis of xenografted melanoma cells through DFNA5/GSDME-dependent pyroptosis128. In addition, simvastatin exerts an antitumor effect by inducing pyroptosis in NSCLC129 (Fig. 3).
Conclusion and perspectives
Pyroptosis is a type of RCD mediated by the gasdermin family, and the inflammasome plays an important role in pyroptosis20. The occurrence of tumor cell pyroptosis in vivo suggests the potential role of pyroptosis in the regulation of tumorigenesis. Triggering the apoptosis of cancer cells has been designed and applied to eliminate malignant cells130. However, because one characteristic of tumors is escaping apoptosis, inducing pyroptosis is particularly important in the treatment of antiapoptotic tumors. Based on the theories of inflammation-cancer transformation and chronic inflammation-induced cell carcinogenesis, pyroptosis, as a mode of proinflammatory death, can form a microenvironment suitable for tumor cell growth. The factors related to pyroptosis have dual mechanisms of promoting and inhibiting tumorigenesis. Further exploration of the pyroptosis mechanism in different tumor cells, as well as of the proteins related to upstream and downstream of signal pathways, can provide new ideas for the treatment of related tumors.
The newly developed tumor pyroptosis therapeutic strategy shows great potential. Numerous reports have shown that chemotherapy drugs, miRNA, etc., can induce tumor pyroptosis, thereby inhibiting the malignant progression of tumors. The gasdermin family is an important group of proteins mediating pyroptosis. However, methods for preventing excessive inflammation responses by downregulating GSDMD to avoid endotoxin shock still need further study. The methylation of DFNA5/GSDME mRNA results in lower DFNA5/GSDME expression in many types of tumor cells than in normal cells62,74,87–89,131, which makes activating pyroptosis difficult in most tumor cells. In the chemotherapeutic treatment of malignant tumors, appropriate chemotherapeutic drugs can be selected according to the level of DFNA5/GSDME expression, which can be upregulated in tumor cells, thereby enhancing chemotherapeutic drug sensitivity and reducing drug resistance. For example, demethylating drugs, such as distamine, are combined with chemotherapy drugs, and drug combinations will be a focus of future research. In addition, the relationship between other gasdermin family proteins and tumors should also be actively researched to provide new directions for the chemotherapeutic treatment of tumors. Therefore, more experiments and clinical trials are needed to explore the potential application of anticancer therapy based on pyroptosis.
In conclusion, increasing evidence shows that pyroptosis plays a dual antitumor and tumor-promoting role during tumor progression. With the development of pyroptosis in many fields of human diseases and the gradual analysis of its mechanism, pyroptosis, similar to apoptosis and autophagy, will have an inestimable impact on the diagnosis and treatment of cancer.
Acknowledgements
This review was funded by the National Natural Science Foundation of China (no. 31702263) and the China Postdoctoral Science Foundation (no. 2017M622346).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by G. Raschellà
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thi H, Hong S. Inflammasome as a therapeutic target for cancer prevention and treatment. J. Cancer Prev. 2017;22:62. doi: 10.15430/JCP.2017.22.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection. Biochim Biophys. Acta Rev. Cancer. 2019;1872:1–10. doi: 10.1016/j.bbcan.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Wei Z, Sun Y, Li G, Liu Z, Jiao S. Advances of research in cancer-associated inflammation and tumor microenvironments. Chin. J. Clin. Oncol. 2018;45:1117–1121. [Google Scholar]
- 10.Nagarajan K, Soundarapandian K, Thorne RF, Li D, Li D. Activation of pyroptotic cell death pathways in cancer: alternative therapeutic approach. Transl. Oncol. 2019;12:925–931. doi: 10.1016/j.tranon.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 12.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl Acad. Sci. USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 14.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect. Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hersh D, et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbio.l. 2001;9:64–67. doi: 10.1016/S0966-842X(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 19.Martirosyan A, Gorvel JP. Brucella evasion of adaptive immunity. Future Microbiol. 2013;8:147–154. doi: 10.2217/fmb.12.140. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Kambara H, et al. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 2018;22:2924–2936. doi: 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Van Opdenbosch N, et al. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat. Commun. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karki R, Kanneganti T. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer. 2019;19:197. doi: 10.1038/s41568-019-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Sun B. Advances in inflammasome and inflammasome-related diseases. Chin. J. Immunol. 2015;31:721727. [Google Scholar]
- 28.Man SM, et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl Acad. Sci. USA. 2014;111:7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joosten LA, Netea MG, Dinarello CA. Interleukin-1beta in innate inflammation, autophagy and immunity. Semin. Immunol. 2013;25:416–424. doi: 10.1016/j.smim.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 33.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 34.Kang SJ, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 2000;149:613–622. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/S0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 36.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 37.Agard NJ, Maltby D, Wells JA. Inflammatory stimuli regulate caspase substrate profiles. Mol. Cell Proteomics. 2010;9:880–893. doi: 10.1074/mcp.M900528-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2007;14:66. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- 39.Crawford ED, Wells JA. Caspase substrates and cellular remodeling. Annu. Rev. Biochem. 2011;80:1055–1087. doi: 10.1146/annurev-biochem-061809-121639. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aglietti RA, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers C, et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarhan J, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruhl S, et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956–960. doi: 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- 45.Orning P, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgener SS, et al. Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation. Cell Rep. 2019;27:3646–3656. doi: 10.1016/j.celrep.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl Acad. Sci. USA. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Q, et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94:52–62. doi: 10.1038/labinvest.2013.126. [DOI] [PubMed] [Google Scholar]
- 52.Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol. Res. 2019;27:827. doi: 10.3727/096504018X15462920753012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma X, et al. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget. 2016;7:36185–36197. doi: 10.18632/oncotarget.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao W, Yang J, Liu W, Wang Y, Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl Acad. Sci. USA. 2016;113:E4857–E4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen YF, Qi HY, Wu FL. Euxanthone exhibits anti-proliferative and anti-invasive activities in hepatocellular carcinoma by inducing pyroptosis: preliminary results. Eur. Rev. Med Pharm. Sci. 2018;22:8186–8196. doi: 10.26355/eurrev_201812_16511. [DOI] [PubMed] [Google Scholar]
- 56.Chu Q, et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget. 2016;7:84658–84665. doi: 10.18632/oncotarget.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang CJ, et al. The expression and regulation of DFNA5 in human hepatocellular carcinoma DFNA5 in hepatocellular carcinoma. Mol. Biol. Rep. 2013;40:6525–6531. doi: 10.1007/s11033-013-2581-8. [DOI] [PubMed] [Google Scholar]
- 58.Falcon T, et al. Analysis of the Cancer Genome Atlas Data reveals novel putative ncRNAs targets in hepatocellular carcinoma. Biomed. Res. Int. 2018;2018:2864120. doi: 10.1155/2018/2864120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hergueta-Redondo M, et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS ONE. 2014;9:e90099. doi: 10.1371/journal.pone.0090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson DA, Weigel RJ. Characterization of a gene that is inversely correlated with estrogen receptor expression (ICERE-1) in breast carcinomas. Eur. J. Biochem. 1998;252:169. doi: 10.1046/j.1432-1327.1998.2520169.x. [DOI] [PubMed] [Google Scholar]
- 61.Fujikane T, et al. Genomic screening for genes upregulated by demethylation revealed novel targets of epigenetic silencing in breast cancer. Breast Cancer Res. Treat. 2010;122:699–710. doi: 10.1007/s10549-009-0600-1. [DOI] [PubMed] [Google Scholar]
- 62.Kim MS, et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem. Biophys. Res. Commun. 2008;370:38–43. doi: 10.1016/j.bbrc.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Y, et al. GSDME influences sensitivity of breast cancer MCF-7 cells to paclitaxel by regulating cell pyroptosis. Chin. J. Cancer Biother. 2019;26:146–151. [Google Scholar]
- 64.Masuda Y, et al. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. J. Hum. Genet. 2006;51:652. doi: 10.1007/s10038-006-0004-6. [DOI] [PubMed] [Google Scholar]
- 65.Hu B, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765–768. doi: 10.1126/science.aaf7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dihlmann S, et al. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int. J. Cancer. 2014;135:2387–2396. doi: 10.1002/ijc.28891. [DOI] [PubMed] [Google Scholar]
- 67.He L, et al. Nucleic acid sensing pattern recognition receptors in the development of colorectal cancer and colitis. Cell. Mol. Life Sci. 2017;74:2395–2411. doi: 10.1007/s00018-017-2477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams TM, et al. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J. Immunol. 2015;194:3369–3380. doi: 10.4049/jimmunol.1402098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen C, et al. DAC can restore expression of NALP1 to suppress tumor growth in colon cancer. Cell Death Dis. 2015;6:e1602. doi: 10.1038/cddis.2014.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flood B, et al. Caspase-11 regulates the tumour suppressor function of STAT1 in a murine model of colitis-associated carcinogenesis. Oncogene. 2019;38:2658–2674. doi: 10.1038/s41388-018-0613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Y, Chen Y, Lin C, Hu G. Biological functions and clinical significance of the newly identified long noncoding RNA RP185F18.6 in colorectal cancer. Oncol. Rep. 2018;40:2648–2658. doi: 10.3892/or.2018.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orita H, et al. The efficacy of Gasdermin gene family for tumor marker in colorectal cancer. Cancer Res. 2015;75:3424. [Google Scholar]
- 73.Miguchi M, et al. Gasdermin C is upregulated by inactivation of transforming growth factor beta receptor Type II in the presence of mutated apc, promoting colorectal cancer proliferation. PLoS ONE. 2016;11:e166422. doi: 10.1371/journal.pone.0166422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ibrahim J, et al. Methylation analysis of Gasdermin E shows great promise as a biomarker for colorectal cancer. Cancer Med. 2019;8:2133–2145. doi: 10.1002/cam4.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watabe K, et al. Structure, expression and chromosome mapping of MLZE, a novel gene which is preferentially expressed in metastatic melanoma cells. Jpn J. Cancer Res. 2001;92:140–151. doi: 10.1111/j.1349-7006.2001.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grottke C, Mantwill K, Dietel M, Schadendorf D, Lage H. Identification of differentially expressed genes in human melanoma cells with acquired resistance to various antineoplastic drugs. Int. J. Cancer. 2000;88:535–546. doi: 10.1002/1097-0215(20001115)88:4<535::AID-IJC4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 77.Lage H, Helmbach H, Grottke C, Dietel M, Schadendorf D. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells. FEBS Lett. 2001;494:54–59. doi: 10.1016/S0014-5793(01)02304-3. [DOI] [PubMed] [Google Scholar]
- 78.Yu P, et al. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacol. Sin. 2019;40:1237–1244. doi: 10.1038/s41401-019-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee BL, et al. ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci. Rep. 2018;8:3788. doi: 10.1038/s41598-018-21998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freeman L, et al. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017;214:1351–1370. doi: 10.1084/jem.20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiu S, Liu J, Xing F. ‘Hints’ in the killer protein gasdermin D: unveiling the secrets of gasdermins driving cell death. Cell Death Differ. 2017;24:588–596. doi: 10.1038/cdd.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saeki N, et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer. 2009;48:261–271. doi: 10.1002/gcc.20636. [DOI] [PubMed] [Google Scholar]
- 83.Komiyama H, et al. Alu-derived cis-element regulates tumorigenesis-dependent gastric expression of GASDERMIN B (GSDMB) Genes Genet. Syst. 2010;85:75–83. doi: 10.1266/ggs.85.75. [DOI] [PubMed] [Google Scholar]
- 84.Carl-Mcgrath S, Schneider-Stock R, Ebert M, Roecken C. Differential expression and localisation of gasdermin-like (GSDML), a novel member of the cancer-associated GSDMDC protein family, in neoplastic and non-neoplastic gastric, hepatic, and colon tissues. Pathology. 2008;40:13–24. doi: 10.1080/00313020701716250. [DOI] [PubMed] [Google Scholar]
- 85.Wang WJ, et al. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J. Dig. Dis. 2018;19:74–83. doi: 10.1111/1751-2980.12576. [DOI] [PubMed] [Google Scholar]
- 86.Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm. Genome. 2000;11:718–724. doi: 10.1007/s003350010138. [DOI] [PubMed] [Google Scholar]
- 87.Akino K, et al. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci. 2007;98:88–95. doi: 10.1111/j.1349-7006.2006.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim MS, et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene. 2008;27:3624. doi: 10.1038/sj.onc.1211021. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, et al. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem. Biophys. Res. Commun. 2018;495:1418–1425. doi: 10.1016/j.bbrc.2017.11.156. [DOI] [PubMed] [Google Scholar]
- 90.Feng S, Fox D, Man SM. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J. Mol. Biol. 2018;430:3068–3080. doi: 10.1016/j.jmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Gao J, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in nonsmall cell lung cancer. Oncol. Rep. 2018;40:1971–1984. doi: 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xi G, et al. GSDMD is required for effector CD8(+) T cell responses to lung cancer cells. Int. Immunopharmacol. 2019;74:105713. doi: 10.1016/j.intimp.2019.105713. [DOI] [PubMed] [Google Scholar]
- 93.Lu H, et al. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clin. Cancer Res. 2018;24:6066–6077. doi: 10.1158/1078-0432.CCR-18-1478. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H, Li L, Liu L. FcgammaRI (CD64) contributes to the severity of immune inflammation through regulating NF-kappaB/NLRP3 inflammasome pathway. Life Sci. 2018;207:296–303. doi: 10.1016/j.lfs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 95.Hoseini Z, et al. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell Physiol. 2018;233:2116–2132. doi: 10.1002/jcp.25930. [DOI] [PubMed] [Google Scholar]
- 96.So D, et al. Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene. 2018;37:5191–5204. doi: 10.1038/s41388-018-0339-4. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Z, Zhou G, Ren Y, Yang Y, Zhang G. Correlation between IL-1β, IL-6, IL-17 expression, HPV infection and cervical cancer. Practical J. Cancer. 2017;32:358–360. [Google Scholar]
- 98.Janowski AM, Kolb R, Zhang W, Sutterwala FS. Beneficial and detrimental roles of NLRs in carcinogenesis. Front. Immunol. 2013;4:370. doi: 10.3389/fimmu.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drexler SK, Yazdi AS. Complex roles of inflammasomes in carcinogenesis. Cancer J. 2013;19:468–472. doi: 10.1097/PPO.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dunn JH, Ellis LZ, Fujita M. Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer Lett. 2012;314:24–33. doi: 10.1016/j.canlet.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 102.Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nadatani Y, et al. NOD-like receptor protein 3 inflammasome priming and activation in Barrett’s epithelial cells. Cell Mol. Gastroenterol. Hepatol. 2016;2:439–453. doi: 10.1016/j.jcmgh.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu M, et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. Ebiomedicine. 2019;41:244–255. doi: 10.1016/j.ebiom.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang Z, et al. miRNA-214 inhibits cellular proliferation and migration in glioma cells targeting caspase 1 involved in pyroptosis. Oncol. Res. 2017;25:1009–1019. doi: 10.3727/096504016X14813859905646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cui J, et al. MST1 suppresses pancreatic cancer progression via ROS-induced pyroptosis. Mol. Cancer. Res. 2019;17:1316–1325. doi: 10.1158/1541-7786.MCR-18-0910. [DOI] [PubMed] [Google Scholar]
- 107.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chien H, Dix RD. Evidence for multiple cell death pathways during development of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression: apoptosis, necroptosis, and pyroptosis. J. Virol. 2012;86:10961–10978. doi: 10.1128/JVI.01275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song J, Du L, Feng Y, Wu W, Yan Z. Pyroptosis induced by zinc oxide nanoparticles in A549 cells. Wei Sheng Yan Jiu. 2013;42:273–276. [PubMed] [Google Scholar]
- 111.Melotti A, et al. The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol. Med. 2014;6:1263–1278. doi: 10.15252/emmm.201404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Draganov D, et al. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci. Rep. 2015;5:16222. doi: 10.1038/srep16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen L, et al. A thiopyran derivative with low murine toxicity with therapeutic potential on lung cancer acting through a NF-kappaB mediated apoptosis-to-pyroptosis switch. Apoptosis. 2019;24:74–82. doi: 10.1007/s10495-018-1499-y. [DOI] [PubMed] [Google Scholar]
- 114.Pizato N, et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci. Rep. 2018;8:1952. doi: 10.1038/s41598-018-20422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yue E, et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine. 2019;56:286–294. doi: 10.1016/j.phymed.2018.09.223. [DOI] [PubMed] [Google Scholar]
- 116.Elion DL, et al. Therapeutically active RIG-I agonist induces immunogenic tumor cell killing in breast cancers. Cancer Res. 2018;78:6183–6195. doi: 10.1158/0008-5472.CAN-18-0730. [DOI] [PubMed] [Google Scholar]
- 117.Okondo MC, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 2017;13:46. doi: 10.1038/nchembio.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson DC, et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat. Med. 2018;24:1151. doi: 10.1038/s41591-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen L, et al. Design and optimize N-substituted EF24 as effective and low toxicity NF-kappaB inhibitor for lung cancer therapy via apoptosis-to-pyroptosis switch. Chem. Biol. Drug Des. 2019;94:1368–1377. doi: 10.1111/cbdd.13514. [DOI] [PubMed] [Google Scholar]
- 120.Yu J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193. doi: 10.1038/s41419-019-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang CC, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24:312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 122.Yu X, He S. GSDME as an executioner of chemotherapy-induced cell death. Sci. China Life. Sci. 2017;60:1291–1294. doi: 10.1007/s11427-017-9142-2. [DOI] [PubMed] [Google Scholar]
- 123.Wang L, et al. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett. 2019;450:22–31. doi: 10.1016/j.canlet.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 124.Derangere V, et al. Liver X receptor beta activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21:1914–1924. doi: 10.1038/cdd.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tabraue C, et al. LXR signaling regulates macrophage survival and inflammation in response to ionizing radiation. Int. 2019;104:913–923. doi: 10.1016/j.ijrobp.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 126.Jiao Y, et al. Pyroptosis of MCF7 cells induced by the secreted factors of hUCMSCs. Stem Cells Int. 2018;2018:5912194. doi: 10.1155/2018/5912194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Colunga AG, Laing JM, Aurelian L. The HSV-2 mutant DeltaPK induces melanoma oncolysis through nonredundant death programs and associated with autophagy and pyroptosis proteins. Gene Ther. 2010;17:315–327. doi: 10.1038/gt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou B, et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171–1185. doi: 10.1038/s41422-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang F, et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int. J. Biol. Sci. 2018;14:406–417. doi: 10.7150/ijbs.23542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fulda S. Targeting apoptosis for anticancer therapy. Semin. Cancer Biol. 2015;31:84–88. doi: 10.1016/j.semcancer.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 131.Croes L, et al. Large-scale analysis of DFNA5 methylation reveals its potential as biomarker for breast cancer. Clin. Epigenetics. 2018;10:51. doi: 10.1186/s13148-018-0479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]