Abstract
Marine natural products have as of now been acknowledged as the most important source of bioactive substances and drug leads. Marine flora and fauna, such as algae, bacteria, sponges, fungi, seaweeds, corals, diatoms, ascidian etc. are important resources from oceans, accounting for more than 90% of the total oceanic biomass. They are taxonomically different with huge productive and are pharmacologically active novel chemical signatures and bid a tremendous opportunity for discovery of new anti-cancer molecules. The water bodies a rich source of potent molecules which improve existence suitability and serve as chemical shield against microbes and little or huge creatures. These molecules have exhibited a range of biological properties antioxidant, antibacterial, antitumour etc. In spite of huge resources enriched with exciting chemicals, the marine floras and faunas are largely unexplored for their anticancer properties. In recent past, numerous marine anticancer compounds have been isolated, characterized, identified and are under trials for human use. In this write up we have tried to compile about marine-derived compounds anticancer biological activities of diverse flora and fauna and their underlying mechanisms and the generous raise in these compounds examined for malignant growth treatment in the course of the most recent quite a long while.
Keywords: Marine, Anti-cancer, Natural products, Corals, Marine herbs, Seaweeds
1. Introduction
The discovery of marine organisms being a source of potential medicinal products dates back to the 1940’s. Marine organisms are a great resource of marine natural products which can be used to treat life threatening diseases/disorders like cancer and acquired immunodeficiency syndrome (AIDS). But the use of these organisms has not been fully explored, as 79% of earth’s surface is surrounded by water. When the food and drug administration’s approval for ziconotide which was isolated from a cone snail in 2004 came through, it was evident that the natural products obtained from the marine sources could be a possible way to obtain new entities of immense therapeutic value. Bioactive compounds are generated by the marine organisms in order to protect themselves from the hazardous effects of light and high oxygen concentrations. Different types of microalgae, fungi, bacteria, actinomycetes, invertebrates and vertebrates present in the marine environment are able generate a substantial amount of bioactive compounds which possess great pharmacological activity. Marine natural products include a wide variety of secondary bioactive metabolites, peptides, sulfated polysaccharides, sterols, carotenoids and derivatives obtained from chitin, chitosan and chitooligosaccharides (Ruiz-Torres et al., 2017). Secondary metabolites like phlorotannins are derived from the polymerization of phloroglucinol. These are mostly present in marine brown algae which possess several biological activities which include antiinflammatory, antidiabetic, radioprotective, antimicrobial and antihypertensive activities. Bioactive peptides are generated as a result of enzymatic hydrolysis of marine products (Kim and Wijesekara, 2010) and also appear as by- products during marine processing (Kim and Mendis, 2006). These bioactive peptides possess dominant anti-coagulant, anti-microbial and anti-oxidant activities besides play an important role in reducing the risk of life threatening cardiovascular diseases (Erdmann et al., 2008). Sulfated polysaccharides obtained from different types of marine algae show a remarkable activity against cancer and AIDS. Plants, fungi, algae and microorganism’s synthesis coloring pigments known as carotenoids which have advantageous effects in preventing diseases like cancer, cardiovascular and some other acute/chronic diseases (Agarwal and Rao, 2000). Sterols are mostly present in the plasma membranes of eukaryotes and are recognized as cholesterol lowering agents (Watson, 2015). All the marine natural products possess enormous pharmacologically important activities with the most important being their activity against cancer and virus. They also have significant anticoagulant and antioxidant activities Fig. 1.
Fig. 1.
Different marine resources having anti-cancer potential.
One of the most leading causes of mortality among humans is cancer and much attention is required to prevent its progression. Several molecules derived from marine sources have proven to be beneficial in combating this disease by either preventing the proliferation of cancerous cells or by being enhancers of apoptosis in cancerous cell lines present in humans. Highly sulfated polysaccharides possess tremendous anti-viral activity (Huheihel et al., 2002). A number of health related problems in humans are caused by the destruction of macromolecules like proteins, membrane lipids and DNA due to reactive oxygen species. This is prevented by anti-oxidants which are present in a large number in marine products. Anti-coagulants inhibit the coagulation of blood necessary in thrombotic disorders. Novel anticoagulant drugs are obtained from marine sources (Church et al., 1989, Matsubara, 2004).
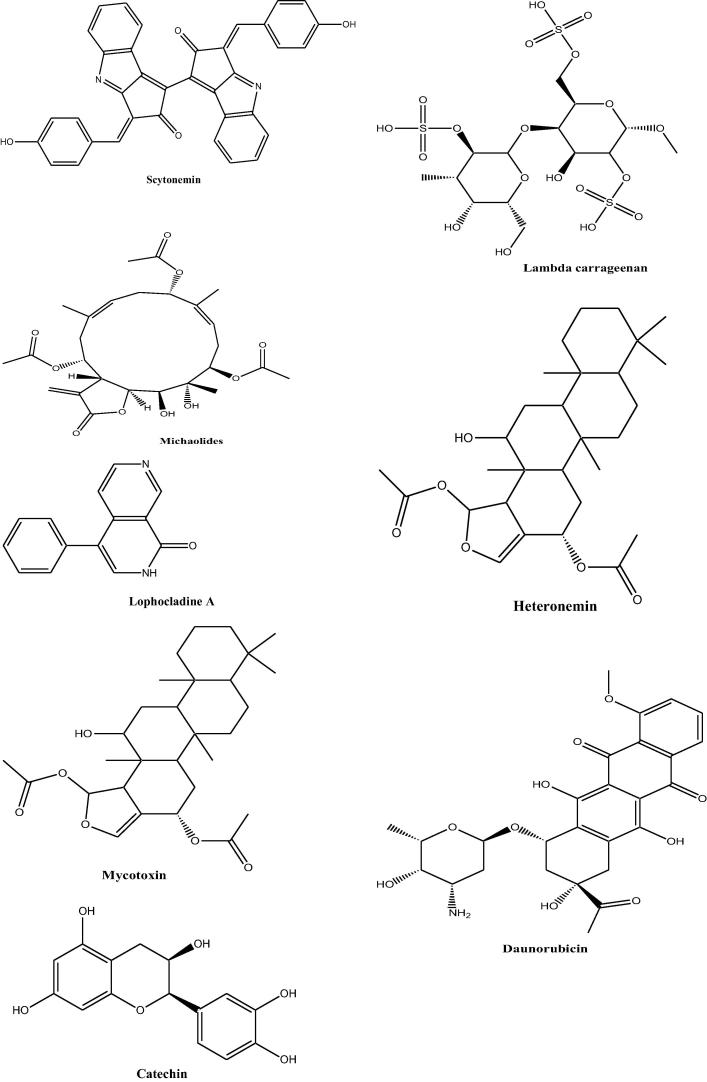
Marine products are a promising source of active ingredients which can be used to treat many diseases Fig. 2. Till until now most the work related to marine natural products has been confined to in-vitro studies or in rodent model systems. And in order to fully utilize the potential of these marine resources, human studies need to be conducted to investigate the role of these natural products in health care. The future role of marine natural products in the human health care system is immense as a vast area of marine resources is yet to be discovered. Following are the various sources from marine exhibiting anticancer activity (Table 1).
Fig. 2.
Structures of some of the marine natural products from various sources.
Table 1.
Listed of compounds approved/clinical trials phase isolated marine sources.
| Clinical Status | Compound Name | Refs. |
|---|---|---|
| FDA-EMA Approved | Cytarabine, ARA-C | Candida et al., 2012 |
| Trabectedin | Gajdos et al., 2011 | |
| Brentuximab vedotin | Hemant and Kritarth (2018) | |
| Phase III | Plitidepsin | Michael et al., 2019 |
| Neovastat® | Falardeau et al., 2001 | |
| Pseudopterosins | Caplan et al., 2016 | |
| Phase I/II | Gemcitabine | Van et al., 2018 |
| Elisidepsin | Petty et al., 2016 | |
| Kahalalide-F | Martín-Algarra et al., 2009 | |
| PM1004 | Mandin and Gottesman (2009) | |
| Phase I | Hemiasterlin | Lesma et al., 2014 |
| Taltobulin | Arie et al. 2005 | |
| Spisulosine | Vilar et al., 2012 | |
| Bryostatin 1 | Clamp et al., 2002 | |
2. Methodology
All the data was achieved by a broad pursuit on electronic databases including: Science Direct, Google, Google Scholar, PubMed, SCOPUS and Taylor and Francis. The search was restricted to English language only. The search terms or keywords used were marine algae, corals reefs, marine herbs, carrageenan polysaccharides and their oligosaccharide derivatives, marine sponges, marine fungi, sea weeds, marine bacteria, marine diatoms and marine ascidiaceans. In almost all cases, the original articles were attained and the relevant date was extracted.
2.1. Marine algae as anti-cancer agents
Marine algae are large group of either unicellular or multicellular eukaryotes belonging to the kingdom Protista. They lack a prominent vascular system and possess different colour pigments like red, blue, brown or gold and chlorophyll pigment for photosynthesis (Ramasubramani et al., 2016). Various algae present in marine water bodies possess several essential nutrients like lipids, minerals, proteins, fiber, fatty acids, polysaccharides, vitamins and many essential amino acids (MacArtain et al., 2007, Cerna, 2011, Misurcova et al., 2012, Tabarsa et al., 2012, Rajapakse and Kim, 2011). Marine algae are a rich source of many bioactive molecules which are reported to have many pharmacological properties including anticancer action (Sithranga and Kathiresan, 2010). Marine algae act as potent antioxidative agents due to their significant reactive oxygen species scavenging activity which in-turn might be responsible for anticarcinogenic property (Lee et al., 2012, Kongara and Karantza, 2012, Park et al., 2011). Secondary bioactive metabolites derived from marine algae include brominated phenols (Liu et al., 2011), nitrogen-containing heterocyclics, kainic acids, guanidine derivatives, phenazine derivatives, amines, sterols (Kim and Ta, 2011) sulfated polysaccharides (Jimenez-Escrig et al., 2011) and prostaglandins (Hsu et al., 2007). All these compounds display a wide range of pharmacological activities like anti-oxidant, immuno-stimulatory and anti-tumour potential (Sithranga Boopathy and Kathiresan, 2010).
2.2. Anti-cancer agents from corals reefs
Coral reefs belong to the phylum Cnidaria and provide a habitat for a wide variety of underwater organisms like fish, sponges, molluscs, echinoderms, crustaceans, etc. Coral reefs have a diverse genetic reservoir and possess medicinal properties and thus can serve as a means for bioprospecting (Cooper et al., 2014). Corals are believed to have many anticarcinogenic properties and can be immensely used as an excellent target for cancer research (Ruiz-Torres et al., 2017). Corals have been reported to have anti-inflammatory, anti-cancer and anti-oxidant activities (Wen-Chi Wei., 2013). The first marine anti-cancer drug found in coral reefs was Cytosar-U® which is used to treat leukemia and lymphoma by killing cancer cells. It works by disrupting DNA synthesis in these cells (Nelson et al., 2016). Dolastatin, isolated from a shell-less mollusk called Dolabela auricularia is an anticancer medicine that is being developed from a coral reef species (Pettit et al., 2011). These are cytotoxic pseudopeptides that prevent abnormal masses of tissues, neoplasms, from forming, causes metaphase arrest in several different types of cancer cells, and induces apoptosis in lymphoma cells (Pettit et al., 2011). This makes them promising for use as potential chemotherapeutics. It has been reported that coral exhibits significant anti-cancer property, various molecules have been isolated from various corals genus (Rajaram et al., 2013). Analogous of nitrogenous diterpene, were isolated from the coral and were evaluated against various human cancer cell line and exhibits 50% inhibition of tumor growth (Altmann, 2001). Corals are known to cause decreased cancer cell growth and survival by activating the proapoptotic cascade leading to apoptosis or cell death. A molecule isolated from Sinularia sp., was known to display anticancer activity via activating various proapoptotic factors (Su et al., 2012). A non-cembranoidal diterpene 5-episinuleptolide acetate isolated from Sinularia sp., causes cytotoxic activity against several cancer cell lines like K562, Molt 4, and HL 60. There is activation of downstream apoptotic pathway via Hsp90 inhibition in HL60 cancer cell line (Huang, 2013). Subergorgia reticulate, a soft coral contains sterols which induce apoptotic cycle in cell and lead to anticancer activity (Byju et al., 2014). Many novel cembranolides like lobomichaolide and michaolides have been isolated from Lobophytum michaelae and are known to inhibit growth of cytomegalovirus and also have pronounced antitumor activities (Wang et al., 2013). Molecule isolated from Sinularia sp., sinularin, demonstrated anticancer capacity by activating various via proapoptotic factors (Hsiao et al., 2016). Different natural sources from environment produce chemical agents to eradicate cancer. Although chemotherapy is associated with different side effects coral reef has been reported as the best anticancer agent with no side effects reported (Cragg, 2007).
2.3. Anticancer activity of marine herbs
Cancer is an abnormal multiplication of cell is an uncontrollable manner which conquers to the other tissues. Cancer is the most threatening disease in the current era. Extensive studies have been carried in order to search for new safe drugs which will have least adverse impact on body. In this ground, researchers have become successful to find some ‘Complementary and Alternative Medicine’ derived from natural resources. Marine origin has always been a most preferred source for drugs as they are the most potent candidates to cure acute and chronic diseases due to their antiinflammatory, anticancer, antimicrobial and neuroprotective effect. It is found that out of 13,000 molecules which were tested for their pharmacologically efficacy, 3000 molecules are having potentiality to act as an active drug (Vignesh et al., 2011). Here, a summarized review is presented on the anticancer activity of marine herbs. Bringmann and his team had found out a derivative, called’ Bryostain’. It was procured from a Bryozoan species called Bugula meritina. This Bryozoan specie was isolated from a marine sponge species. They could derive an alkaloid known as Sorbicillactone A and its analog Sorbicillactone B. It was experimentally proven that these two compounds could fight against leukemia cell. The later one was obtained from salt water culture of Penicillium chrysogenum, isolated from a Mediterranean sponge species (Ircinia fasciculate) (Gerhard et al., 2007). Another interesting field of marine drug is marine blue, green algae also termed as “Cyanobacteria” which is believed to be an effective agent in killing cancer cells by enhancing the apoptotic death. Cell extract of Calothrix isolates were tested against HeLa cancer cell and it came out with positive result (Rodney et al., 1999). A boron containing metabolite called ‘Borophycin’ derived from Nostoc linckia and N. spongiaeforme was successfully used against human epidermoid carcinoma and colorectal adeno carcinoma cell line (Davidson et al., 1995). Again in later stage, Banker and Carmeli stated that, this novel bioactive compound can be derived from both terrestrial strain of Streptomyces antibioticus as well as from marine strain S. griseus (Banker et al., 1998). Another promising sector for anti-carcinogenic component is marine herbs which is widely accepted for its anti-cancer ability. Apart from being an effective source of vitamin, mineral, protein, it constitutes a good amount of (2R,3R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl gallate, and 3,4,5-Trihydroxybenzoic acid (Yoshie et al., 2002). Extensive studies have been going on with different derivatives of marine herbs. One of the most remarkable study was carried out by Vasanthi and his colleague who reported that alcoholic extract of Acanthophora spicifera is tested against Ehrlich’s ascites carcinoma cells in mice. They found that the tumour volume started decreasing and so the viable cell count. Even other marine herbs viz. Ulva reticulata, Gracileria foliifera possess cytotoxic ability (Vasanthi et al., 2004). Rather than these, from brown algae cell wall, one derivative ‘Fucoidan’ was extracted which is a sulfated polysaccharide. It was found to be effective against apoptosis in humanlymphoma HS- Sultancell line (Aisa et al., 2005). Nevertheless, Lophocladia species are another potental candidates as the derivatives (Lophocladine A & B) of this red algae have been found to be active against various cancer cell lines (Grosset et al., 2006: Bentley, 1957). Again alkaloids from marine mangroves showed positive efficacy especially Rhizophrine from Rhizophora mucronata and R. stylosa (Kathiresan et al., 2005). Few years back, a team of researchers found a biological compound termed as herbal-marine compound (HES-A) which is of a marine herbal origin and comprises of different organic and inorganic substances along with aqueous fractions (Moallem et al., 2007). Studies found this compound as an effective candidate against breast cancer (Ahmadi et al., 2005). An aqueous microalgae extract can reduce metastasis in-vivo appears to be related to the preferential killing of suspended cancer cells and the anticolony forming properties of the microalgal extract (Syam et al., 2016). Apart from being a rich source of mineral component (50%), organic component (45%) and aqueous fractions (5%), it also constitutes good amount of highly demanded trace elements like Se, Ni, Zn, Va, Ti (Ahmadi, 2004). Individually all these elements have shown their potency against tumour cells as well as their antimitotic properties in rat models (Zeng et al., 2008). Hence, this HESA is of a great interest among scientists and is believed to come out as a safe drug in near future. As discussed earlier, marine herbs are gaining popularity among researchers due to their anti-cancer properties and is of the keen interest point to the new generation. Years back National cancer institute (NCI), USA, conducted one cytotoxic screening of marine herb Portieria hornemannii and isolated a novel biosynthetic product called Halomon-a penta halogenated monoterpne which has a cytotoxic effect against cancer cells (Faulkner et al., 2002a). Sargassum Polycystum and S. Carpophyllum from South China Sea and North China Sea respectively, are notable for their anticancer action against different cultured cancer cells. Former one is the source of a highly potent sterol called ‘Stigmast’ and from the latter, one two different types of bioactive sterols can be extracted which show encouraging result against several cultured cancer cell line (Tang et al., 2002: Xu et al., 2002). Because of the presence of high amount of polyphenols, alkaloid, polysaccharide, the marine herbal products are being chosen and investigated for new drug discovery. Polyphenols compounds are metabolizing enzymes-xenobiotic which can restrain the development of cancer cell lines. Again Flavonoid is a potent agent to kill cancer cell or prevent aromatase in order to inhibit cancer cell growth (Zhao et al., 2007).
2.4. Carrageenan polysaccharides and their oligosaccharide derivatives as anti-cancer agents
Most abundant form of carbohydrate materials present in nature are polysaccharides which are also known as glycans. Various studies were performed on several algae derived polysaccharides, and rhamnan sulfate based on their biological activities (de Almeida et al., 2011, Lee et al., 2010, Rioux et al., 2010, Li et al., 2008, Larsen et al., 2003). Polysaccharides isolated from the algae are natural polymers that are accessible bounteously in nature harmless, secure and bio-compatible (Guo et al., 1998). These polysaccharides have developed a prominent and broad use in the pharmaceutical and biomedical area. Several studies have recognized that algae are having rich wellspring of bioactive molecules with different pharmacological uses like anticancer, antioxidant, antiobesity, neuroprotective, antimicrobial, antinociceptive, antiinflammatory and antiangiogenic activities (Soheil et al., 2014, Pangestuti and Kim, 2011, Rafael et al., 2011, Carolina et al., 2011, Eom et al., 2012). Carrageenans are naturally happening anionic sulfated polysaccharides, consisting of one of the two galactose or galactose linked with 3,6-anhydrogalactose monosaccharide units which appear as matrix material in excessive quantities by certain red algae (Rhodophyta), for example, Chondrus, Gigartina, Hypnea, and Eucheuma, wherein they help a basic capacity similarity to that of cellulose in plants (Lahaye, 2001) and are different from one another in monosaccharide setup, level of sulfurylation, positions of sulfate groups and molecular weights. They are ordered into three categories dependent on the presence of 3,6-anhydrogalactopyranose and distribution of the sulfate groups on the basic structures as kappa, iota, lambda carrageenan and independently show uncommon antiviral impacts on a few viral agents (Renn, 1997). Carageenans specifically forestall both encompassed and non-wrapped viruses acting transcendently by preventing the binding or internalization of virus into the host cells (Buck et al., 2006, Grassauer et al., 2008). Carrageenans primarily act on human papilloma virus in-vitro by excepting the underlying phase of infection and are likewise gigantically compelling against a progression of sexually transmitted human papilloma infection types that lead to cervical malignant growth and genital moles (Zeitlin et al., 1997, Gonzalez et al., 1987).
2.5. Anti-cancer agents from sponges
Sponges are sessile marine invertebrates belonging to the phylum Porifera and often mentioned as “golf ball sponges or moon sponges” (Szitenberg et al., 2013). Marine porifera mostly belongs to Cinachyrella sp. with characteristic spherical or spirical bodies. Bioactive compounds in sponges have been reported to act as potent agents having antiinflammatory (Costantini et al., 2015), antitumor (Werner et al., 2004) and immunosuppressant activities (Costantino et al., 1999). Some bioactive compounds isolated from sponges are inhibitors of protein kinase C which are exceedingly associated with tumor development and progression. Inhibition of protein kinase C has been engaged with direction in pathogenesis of joint pain and psoriasis and in tumor advancement (Crews et al., 2003). Renieramycin M is the natural molecule isolated from sponges which displays encouraging anticancer activities. Chemically, Renieramycin is a tetrahydroiso-quinoline in nature After various pre-clinical investigations, it was accounted for that renieramycin M actuate apoptosis death in lung carcinomas through p53-subordinate apoptotic pathway. Isolation of Monanchocidin, a polycyclic guanidine alkaloid structure in nature from the marine sponge Monanchora pulchra persuaded cell apoptosis in human cervical cancer mouse epidermal cells and human monocytic leukemia (Muller et al., 2004). (Agustina et al., 2013) reported that Spongistatin-I molecule isolated from Spongia species causes significant cell death in numerous malignant cell by causing cell cycle arrest because of restraint of mitosis and increased binding of vinblastine to tubulin fibrils. Another segment from sponges that has antitumor activity is Heteronemin, that was tested for its pharmacological effects on chronic myelogenous leukemia cells. Heteronemin suppresses the cancerous cells. It influences the cellular forms including apoptosis, cell cycle, mitogen-initiated protein kinases pathway and the nuclear factor kappa B signaling cascade (Azizi et al., 2010). Manzamine A present in a number of marine sponges is recognized to have strong antiinflammatory, antifungal and antitumor activities. 8- hydroxymanzamine A is a Pachypellina species, that is isolated from other form of sponge and exhibits moderate antitumor and antiherpes simplex virus-II activity. It has been reported that the anticancer potency of marine bacteria associated with sponges Jaspis sp. and might be utilized as a chemopreventive agent against cervical cancers (Utami et al., 2014). As per the revealed investigations, the antitumour action of the methanolic extracts of marine sponge Sigmadocia pumila and Holothuria atra (sea cucumber) methanolic extracts was assessed using both in-vitro and in-vivo measures. S. pumila and H. atra demonstrated a high level of antitumour activity against the Human epidermoid larynx carcinoma cell line, human breast cancer, African green monkey kidney normal cell line and human cervical disease cell line. Human breast cancer cell lines and daltons ascites lymphoma cell lines of in-vitro and in-vivo thinks about. Flavonoids and alkaloids present in S. pumila show the decrease of development in tumor cells. (Montaser et al., 2011).
2.6. Marine fungi as anti-cancer agents
Almost 1.5 million to 3 million fungal types exist on the globe (Hawksworth et al., 2012). Numerous bioactives, for example, mycotoxins, antifungal and anticancer agents have been reported from last over 100 years (Frisvad et al., 2004). Current sequencing of complete fungal genomes discovered that a portion of the gene bunches are quiet. This recommends the alternative for some more bioactive molecules (Klejnstrup et al., 2012). In correlation with other natural sources like plants, exceedingly various microorganisms are scarcely found, especially with adoration to their endless capacities as wellsprings of astoundingly bioactive natural products. Of these living beings, growths of fungi tissues of plant in a non-obtrusive relationship (endophytic fungi) have checked evidently valuable and unmatchable as wellsprings of influential bioactive molecules against various sicknesses, for example, malignant growth and related ailments. The kingdom fungi are an outstanding store of numerous bioactive molecules which can demonstrate valuable against several diseases, for example, cancer. Scopararane I, a pimarane-type diterpenes and its anologus are isolated from the culture broth of a marine sediment derived fungus Eutypella sp. FS46 showed moderate cytotoxic activities against MCF-7, NCI-H460 and SF-268 tumour cell lines (Liu et al., 2017). Isolation of varioloid A form the marine alga-derived endophytic fungus Paecilomyces variotii EN-291 exhibited cytotoxicity against A549, HCT116, and HepG2 cell lines (Peng.et al.,2016). Numerous scientists endeavored the linear peptides and a positive assurance prompted the disclosure of eight new simplicilliumtides A-H were isolated from a culture broth of the deep sea derived fungal strain Simplicillium obclavatum EIODSF 020. Out of which simplicilliumtides A, E, G and H exhibits weak anti-cancer activity against HL-60 or K562 cell line (Xiao et al., 2016). The derivatization analogues of azaphilonidal, expedient new anti-cancer molecules penicilazaphilones B and C which revealed anti-cancer against melanoma cells B-16 and human gastric cancer cells SGC-7901 (Zhou et al., 2016). Another molecule from ergochrome class, i.e., secalonic acid D, a mycotoxin, isolated from the mangrove endophytic fungus too shows a good cytotoxic action on HL60 and K562 cells by encouraging leukemia cell apoptosis (Zhang et al., 2009).
2.7. Anti-cancer properties of sea weeds
Seaweeds act as a source of more than 2400 natural products (Manilal et al., 2009). Sea weeds act as source of thermopolysaccharides and phycocolloids like agar, carrageenan and alginates which have been used in a wide range from centuries by mankind because of gelling and emulsifying properties (Laurienzo, 2010, Shalaby, 2011, Stengel et al., 2011, Mohamed et al., 2012). Many biomolecules with therapeutic potential like sulphated polysaccharides, polyphenolic, terpenoids, flavonoids and lipids natured secondary metabolites have been isolated from seaweeds. The secondary bioactive metabolites in seaweeds are recognized for their antioxidant potential (Zaragoza et al., 2008, Souza et al., 2011, Peinado et al., 2014) and antimutagenic activity (Okai et al., 1996). Brown, red and green groups of seaweeds have shown noteworthy anti-proliferative activity against various malignancy cell lines (Yuan and Walsh, 2006, Paul and Kundu, 2013, Murugan and Iyer, 2014). Activation of cytokine mediated pathway for apoptosis is responsible for inhibition of development of cancer cell growth (Shklar, 1998). Seaweeds can also act as a source of many active metabolites and also some functional foods fiber, proteins and minerals. Like other marine products, seaweeds are one of the major source of potent molecules, possesses wide range of pharmacological activities such as antimicrobial (Newman et al., 2004), antiinflammatory (Lindequist et al., 2001) as well as anti-tumor activities which can in turn help in curing many diseases (Zandi et al., 2010). There is a substantial decline in cancer cell progress and proliferation on treatment with Palmaria palmate extracts (Yuan et al., 2005). The alcoholic extract prepared from the red algae, Acanthophora spicifera showed significant antitumor action when tested on Ehrlich’s ascites carcinoma cells (Vasanthi et al., 2004). Beta Polysaccharide extract from of brown seaweeds shows a dose-dependent scavenging of free radicals displaying significant antioxidant potential (Heo et al., 2005) and suppress singed the in-vitro proliferation of selected cancer cell lines (Athukorala et al., 2006). Algae shields the perceptibility of the cell membrane and are vulnerable to lipid peroxidation from free radicals (Yuan et al., 2005).
Halimeda species of seaweed is a source of many bioactive phenolic compounds such as dexcyanidanol, catechuic acid and trihydroxybenzoic acid as reported in (Yoshie et al., 2002). Literature survey revealed that the extract of Ascophyllum species contain higher concentration of polyphenols content when compared with other seaweeds, whereas Ulva species had the least concentration (Xu et al., 2009). Metabolism of many potential carcinogenic agents is altered by these polyphenolic compounds activation of many xenobiotic enzymes (Zhao et al., 2007), disturbance of mitosis at telophase stage, decreasing mitotic index and colony forming units of cancer cells (Gawron et al., 1992). Flavonoids cause inhibition of cancer cell growth by altering hormone production and inhibiting aromatase enzyme.
2.8. Marine bacteria as anti-cancer agents
From hundreds of years’ marine microorganisms have remained one of the preeminent wellspring of anti-infection agents and numerous bioactive metabolites (Saleem et al., 2009). Alkaloids and quinone isolated from marine bacteria may be responsible for their anticancer properties (Solanki et al., 2008). Restraint of topoisomerases prompts to disruption of chromosome and cell structure causing cell cycle arrest (Facompre et al., 2009) mitochondrial damage and empowering the release of cytochrome C and apoptosis inducing factor (Ravikumar et al., 2011). Moreover, quinine derivatives analogues like driamycin, daunorubicin, mitomycin C, streptonigrin, and lapachol shows noteworthy anticancer activity by influencing the pathways of mitochondrial and development of OH− radicals as deadly products in the cell line Various molecules of anthroquinone family resembles parimycin, trioxacarcins and gutingimycin showed antitumor activities (Ravikumar et al., 2011, Gulecha and Shiva kumar, 2011, Kumar et al., 2011). The anticancer action of marine bacterial isolates shows up because of the process of the cell death, antiproliferative and inhibition of angiogenesis (Ravikumar et al., 2011).
2.9. Anti-cancer properties marine diatoms
Diatoms are an essential class of unicellular green growth that produce bioactive polyunsaturated aldehydes (PUAs) that actuate premature births or contortions in the posterity of spineless creatures presented to them amid development (Clementina et al., 2014). Diatoms are the most diverse and spectacular nanostructure cell wall living beings which likewise have different bio-geo chemical properties (Kuppusamya et al., 2017. On the bases of the valve face of the diatom frustules diatoms are principally isolated into two kinds, they are centrales and pennales. The focal valve striae organized essentially in connection to a point and focal areola probably seem symmetrical. The pennales have valve striae orchestrated in connection to a line and will in general seem zygomorphic. The marine derived compound substances are protecting against various chronic and acute bacterial and viral ailments/discorders (Sheppard et al., 2011).
Three different polyunsaturated aldehydes (PUAs) namely 2-trans-4-cis-7-cis-decatrienal, 2-trans-4-trans-7-cis-decatrienal and 2-trans-4-trans-decadienal were isolated from the marine diatoms Thalassiosira rotula, S. costatum and P. delicatissima possess anticancer activity on the human colon adenocarcinoma cell line (Kevin AMA et al., 2018). In another study one clone of S. marinoi (FE60) demonstrated anticancer action on human melanoma A2058 cells, but only when cultured refined in nitrogen-starvation conditions. The other clone FE6 was not dynamic against malignant growth cells (Chiara et al., 2016).
2.10. Anti-cancer agents marine ascidiaceans
Ascidiacea is one of the oldest creatures found in the marine world characterized with more than 2800 species (Shenkar and Swalla, 2011a, Shenkar et al., 2016b). Ascidians are recorded separately in the zoological collection from centuries. It was Aristotle who sorted these odd creatures; their body is pressed inside the shell connected on rocks with two hole separate separated from one another (Voultsiadou et al., 2007). Phylogenetic examinations affirmed that they are individuals from the subphylum Tunicate and are ordered vertebrates (Delsuc et al., 2006). The first list of Mediterranean ascidians (Peres, 1958a, Peres, 1967b) according to the literature survey consist of around 32 species; meanwhile then, the pertinent scientific investigation amplified prominent to 229 ascidian species (Chryssanthi et al., 2016). The majority of them are from the western Mediterranean locale with 165 species, where much more effort has been enthusiastic as opposed to the eastern basin, from which only 86 species have been reported (Coll et al., 2010). Among Mediterranean ascidians, 103 exclusive species are incorporated and the whole territory has been recognized as an area of endemism, at least for this specific taxonomic group (Moreno et al., 2014). Ascidians, the marine invertebrates are productive makers of various bioactive compounds and have anticancer activities. Among the ascidian families Didemnidae and Polycitoridae are maximum manufactures of biologically-active molecules (Watters et al., 1993). The genus Eudistoma, belonging to the most diverse family Polycitoridae and inhabiting mainly tropical regions (Kott, 1990), has been the source of several cytotoxic alkaloids (Jimenez et al., 2012), including eudistomins, eilatin, staurosporine derivatives, methyleudistomins and pibocin (Makarieva et al., 2001, Menna et al., 2011). Metabolites for example, halocynthiaxanthin and fucoxanthinol from Halocynthia roretzi (Konish et al., 2006), meridianins, brominated 3-(2-aminopyrimidine)-indoles from Aplidium meridianum (Gompel et al., 2004), and compounds from Diplosoma virens (Ogi et al., 2008) 283 and Policlinum indicum (Rajesh et al., 2010) have novel modes of action of inducing apoptosis and cell-cycle arrest. Apoptosis, a strongly regulated and structurally-distinct form of programmed cell death, has been recognized as an important target for therapeutic intervention and rational drug discovery (Nicholson et al., 2000) and cancer treatment strategies are directed towards reconstituting the tumor cell’s ability to undergo apoptosis (Ashkenazi et al., 1999).
3. Marine drugs approved and under clinical trials
Marine derived drugs have shown to be an intriguing source of bioactive moleclues with exceptional and remarkable synthetic highlights on which the molecular modeling and chemical synthesis of new medications can be based with more noteworthy adequacy and particularity for the therapeutics (Mayer et al., 2017). The identification of marine origin compounds for the treatment of malignant growth has seen a huge increment in the course of the most recent couple of decades. In the course of the most recent 30 years, extraordinary endeavors have been made, appearing and encouraging outcomes, since it has been characterized the significant propensities in secondary metabolism of a few classes of marine living beings. Just over the most recent 20 years, around 18,000 new marine compounds were isolated and six out of the nine marine-origin medications as of now used in clinical treatment were approved (Celso et al., 2018). Some marine origin compounds have given promising outcomes in their preclinical stages and have being elevated to clinical preliminaries or even affirmed by regulatory agencies. The quantity of recently approved medications from marine origin will keep on expanding, since 28 marine or marine-origin medications are at present in clinical trials (John et al., 2018). Cytarabine was the first marine bioactive compound approved by the United States (US) Food and Drug Administration (FDA) in 1969, followed by trabectedin etc. (Lotte-Van et al., 2018) (Table 2).
Table 2.
Listed of moleclues isolated from various marine sources.
| Source | Compounds isolated | Refs. |
|---|---|---|
| Marine Algae | Nitrogen-containing heterocyclics, kainic acids, guanidine derivatives, phenazine derivatives, amines, sterols | Kim and Ta, 2011 |
| Sulfated polysaccharides | Jimenez-Escrig et al., 2011 | |
| Prostaglandins | Hsu et al., 2007 | |
| Corals Reefs | Cytosar-U® | Nelson et al., 2016 |
| Dolastatin | Pettit et al., 2011 | |
| Nitrogenous diterpene | Cooper et al., 2011; Altmann, 2001 | |
| Non-cembranoidal diterpene 5-episinuleptolide acetate | Huang, 2013 | |
| Sterols | Byju et al., 2014 | |
| Cembranolides like lobomichaolide and michaolides | Wang et al., 2013 | |
| Marine Herbs | Bryostain, Sorbicillactone A and Sorbicillactone B | Gerhard et al., 2007 |
| Borophycin | Davidson et al., 1995 | |
| (2R,3R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl gallate, and 3,4,5-Trihydroxybenzoic acid | Yoshie et al., 2002 | |
| Fucoidan | Aisa et al., 2005 | |
| Lophocladine A & B | Grosset et al., 2006: Bentley, 1957 | |
| Halomon-A penta halogenated monoterpne | Faulkner et al., 2002a | |
| Stigmast | Tang et al., 2002: Xu et al., 2002 | |
| Marine Sponges | A tetrahydroiso-quinoline Renieramycin m, A polycyclic guanidine alkaloid monanchocidin, |
Muller et al., 2004 |
| Spongistatin-I | Agustina et al., 2013 | |
| Heteronemin | Azizi et al., 2010 | |
| Manzamine A, 8- hydroxymanzamine A |
Utami et al., 2014 | |
| Marine Fungi | A pimarane-type diterpenes, Scopararane I | Liu et al., 2017 |
| Varioloid A | Peng.et al.,2016 | |
| Simplicilliumtides A-H | Xiao et al., 2016 | |
| Azaphilonidal, penicilazaphilones B and C | Zhou et al., 2016 | |
| Secalonic acid D | Zhang et al., 2009 | |
| Sea Weeds | Dexcyanidanol, catechuic acid and trihydroxybenzoic acid | Yoshie et al., 2002 |
| Marine Bacteria | Quinine derivatives analogues like driamycin, daunorubicin, mitomycin C, streptonigrin, and lapachol, Anthroquinone family resembles parimycin, trioxacarcins and gutingimycin |
Ravikumar et al., 2011, Gulecha and Shiva kumar, 2011, Kumar et al., 2011 |
| Marine Ascidiaceans | Eudistomins, eilatin, staurosporine derivatives, methyleudistomins and pibocin | Makarieva et al., 2001, Menna et al., 2011 |
| Halocynthiaxanthin and fucoxanthinol | Konish et al., 2006 | |
| Meridianins, brominated 3-(2-aminopyrimidine)-indoles | Gompel et al., 2004 | |
| Marine diatoms | 2-trans-4-cis-7-cis-decatrienal, 2-trans-4-trans-7-cis-decatrienal, 2-trans-4-trans-decadienal, 2-trans-4-trans-octadienal, 2-trans-4-trans-heptadienal | Kevin AMA et al., 2018 |
4. Conclusion
In spite of the extraordinary potential for sourcing new prescriptions from marine natural products, very few compounds have actually been used for treatment of cancer. There have been very few available comprehensive reports on biological evaluation of marine natural resources. The current discussion demonstrates the worth of vast marine water bodies as an essential asset for the finding of novel anticancer specialists. Not much work has stood attentive on assessment /evaluation of individual molecules from marine water bodies’ flora and fauna. Thus, it’s obvious and comprehensive isolation of cancer leading molecules and the development of their derivatives are important, and will make way for better perspectives for the design and development of new pharmacological and therapeutic agents.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal S., Rao A.V. Carotenoids and chronic diseases. Drug Metabol. Drug Interact. 2000;17:189–210. doi: 10.1515/dmdi.2000.17.1-4.189. [DOI] [PubMed] [Google Scholar]
- Agustina R., Alam G., Syukur R., Lethe C., Rahim A. Ekstraksi dan Fraksinasi Senyawa Bioaktif Antimitosis dari Spons Callispongia hispidoconulosa. Majalah Farmasi dan Farmakologi. 2013;17:21–24. [Google Scholar]
- Ahmadi A., Mohagheghi M.A., Fazeli M.S., Nahavandian B., Bashardoost N., Musavi J.A., Gharipoor M. HESA-A, a new treatment for breast cancer and choroidal metastasis. Med. Sci. Monit. 2005;11:300–303. [PubMed] [Google Scholar]
- Ahmadi A. Research Division, Tehran, Islamic Republic of Iran. 2004. Analysis of HESA-A, report of Atomic Energy Organization of Iran (AEOI) [Google Scholar]
- Aisa Y., Miyakawa Y., Nakazato T., Shibata H., Saito K., Ikeda Y., Kizaki M. Fucoidan induces apoptosis of human HS-Sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005;78:7–14. doi: 10.1002/ajh.20182. [DOI] [PubMed] [Google Scholar]
- Altmann K.H. Microtubule-stabilizing agents: a growing class of important anticancer drugs. Curr. Opin. Chem. Biol. 2001;5:424–431. doi: 10.1016/s1367-5931(00)00225-8. [DOI] [PubMed] [Google Scholar]
- Arie Z., Joshua K., Sylvia M., Loganzo Frank. Hybrids of the Hemiasterlin analogue taltobulin and the dolastatins are potent antimicrotubule agents. J. Am. Chem. Soc. 2005;127(50):17667–17671. doi: 10.1021/ja053663v. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A.P.R.C., Fong S., Leung S., Lawrence D.A., Marsters S.A., Blackie C., Chang L., McMurtrey A.E., Hebert A., DeForge L., Koumenis I.L., Lewis D., Harris L. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Cli. Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athukorala Y., Jung W.K., Vasanthan T., Jeon Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohyd. Polym. 2006;66:184–191. [Google Scholar]
- Azizi E., Namazi A., Kaabinejadian S., Fouladdel S., Rezaei P., Ramezani M. Molecular analysis of Men1expression in MCF7, 47D and MDA-MB 468 breast cancer cell lines treated with Adriamycin using RT-PCR and immunocytochemistry DARU. J. Pharm. Sci. 2010;1:17–22. [PMC free article] [PubMed] [Google Scholar]
- Banker R., Carmeli S. Tenuecyclamides A-D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue. J. Nat. Prod. 1998;61:1248–1251. doi: 10.1021/np980138j. [DOI] [PubMed] [Google Scholar]
- Bentley K.W. Interscience; New York, NY, USA: 1957. The Alkaloids. [Google Scholar]
- Buck C.B., Thompson C.D., Roberts J.N., Muller M., Lowy D.R., Schiller J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;7:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byju K., Anuradha V., Vasundhara G., Nair S.M., Kumar N.C. In-vitro and in silico studies on the anticancer and apoptosis-inducing activities of the sterols identified from the soft coral, subergorgia reticulata. Pharmacogn. Mag. 2014;10:S65–S71. doi: 10.4103/0973-1296.127345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candida N., Alfredo C., Patrizia R. Anticancer drug discovery from the marine environment. Recent Pat. Anti-Cancer Drug Discov. 2012;7:218–232. doi: 10.2174/157489212799972963. [DOI] [PubMed] [Google Scholar]
- Caplan S.L., Zheng B., Dawson-Scully K., White C.A., West L.M. Pseudopterosin A: protection of synaptic function and potential as a neuromodulatory agent. Mar. Drugs. 2016;14: 3:55. doi: 10.3390/md14030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolina B.B.M., Everton T.S., Aline C.Q., Daysianne P.L., Morgana V.A., Luiz H.A.C., George E.C.M., Joao X.A., Jr, Jose M.B.F., Barbara V.O.S. Antinociceptive and anti-inflammatory activity from algae of the Genus Caulerpa. Mar. Drugs. 2011;9:307–318. doi: 10.3390/md9030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celso A., Joana S., Susete P., Helena G., Maria C.A., Luis M.B., Rui P. From marine origin to therapeutics: the antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018;9:1–24. doi: 10.3389/fphar.2018.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerna M. Seaweed proteins and amino acids as nutraceuticals. Adv. Food Nutr. Res. 2011;64:297–312. doi: 10.1016/B978-0-12-387669-0.00024-7. [DOI] [PubMed] [Google Scholar]
- Chiara L., Jeanette H.A., Espen H., Marte A., Laura E., Francesco E., Helland Kirsti, Kine O.H., Giovanna R., Adrianna I. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities front. Mar. Sci. 2016;10:1–12. [Google Scholar]
- Chryssanthi A., Vasilis G., Nicolas B. Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist. Biodivers. Data J. 2016;4:e9273. doi: 10.3897/BDJ.4.e9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church F.C., Meade J.B., Treanor E.R., Whinna H.C. Antithrombin activity of Fucoidan. The interaction of fucoidan with heparin cofactor II, antithrombin III, and thrombin. J. Biol. Chem. 1989;264:3618–3623. [PubMed] [Google Scholar]
- Clamp A., Jayson G.C. The clinical development of the bryostatins. Anticancer Drugs. 2002;13(7):673–683. doi: 10.1097/00001813-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Clementina S., Alessandra B., Elena E., Giovanna R., Anna P., Raffaella C., Maria F., Adrianna I. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS ONE. 2014;9(7):e101220. doi: 10.1371/journal.pone.0101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M., Piroddi C., Steenbeek J., Kaschner K., Rais Lasram F.B., Aguzzi J., Ballesteros E., Bianchi C.N., Corbera J., Dailianis T. The biodiversity of the Mediterranean sea: estimates, patterns, and threats. PLoS ONE. 2010;5(8):e11842. doi: 10.1371/journal.pone.0011842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E.L., Hirabayashi K., Strychar K.B., Sammarco P.W. Corals and their potential applications to integrative medicine. Evid. Based Complem. Alternat. Med. 2014;184959:1–9. doi: 10.1155/2014/184959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini S., Romano G., Rusolo F., Capone F., Guerriero E., Colonna G., Ianora A., Ciliberto G., Costantini M. Anti-inflammatory effects of a methanol extract from the marine sponge geodiacydonium on the human breast cancer MCF-7 cell line. Mediat. Inflamm. 2015;204975:1–9. doi: 10.1155/2015/204975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino V., Fattorusso E., Mangoni A., Di Rosa M., Ianaro A. Glycolipids from sponges. VII. simplexides, novel immunosuppressive glycolipids from the Caribbean sponge Plakortis simplex. Bioorg. Med. Chem. Lett. 1999;9:271–276. doi: 10.1016/s0960-894x(98)00719-7. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Crews P., Gewick W.H., Schmitz F.J., France D., Bai B.W.K. Molecular approaches to discover marine natural products anticancer leads-an update from drug discovery group collaboration. Pharmaco. Biol. 2003;41:39–52. [Google Scholar]
- Davidson B.S. New dimensions in natural products research: cultured marinemicroorganisms. Curr. Opin. Biotechnol. 1995;6:284–291. [Google Scholar]
- de Almeida C.L.F., Heloina de S.F., Gedson R.M., Camila de A.M., Narlize S.L., Petronio F.A., Luis C.R., Maria de Fatima V., Barbosa-Filho J.M., Leonia M.B. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011;12:4550–4573. doi: 10.3390/ijms12074550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsuc F., Brinkmann H., Chourrout D., Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Eom S.H., Kim Y.M., Kim S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012;50:3251–3255. doi: 10.1016/j.fct.2012.06.028. [DOI] [PubMed] [Google Scholar]
- Erdmann K., Cheung B.W.Y., Schroder H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008;19:643–654. doi: 10.1016/j.jnutbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Facompre M., Tardy C., Bal-Mahieu C., Colson P., Pere Z.C., Manzanares I. A novel potent inhibitor of topoisomerase I. Cancer Res. 2009;63:7392–7399. [PubMed] [Google Scholar]
- Falardeau P., Champagne P., Poyet P., Hariton C., Dupont E. Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Semin. Oncol. 2001;28(6):620–625. doi: 10.1016/s0093-7754(01)90035-1. [DOI] [PubMed] [Google Scholar]
- Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004;49:201–241. [Google Scholar]
- Gajdos C., Elias A. Trabectedin: safety and efficacy in the treatment of advanced sarcoma. Clin. Med. Insights. Oncol. 2011;5:35–43. doi: 10.4137/CMO.S4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron A., Kruk I. Cytotoxic effect of xanthotoxol (8-hydroxypsoralen) on TCTC cells in vitro. Pol. J. Pharmacol. Pharm. 1992;44:51–57. [PubMed] [Google Scholar]
- Gerhard B., Tobias A.M.G., Gerhard L., Stefanie S., Rudiger S., Jutta W., Kerstin N., Johannes F.I. Large-scale biotechnological production of the antileukemic marine natural product sorbicillactone A. Mar. Drugs. 2007;5:23–30. doi: 10.3390/md502023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompel M., Leost M., Joffe E.B.D., Puricelli L., Franco L.H., Palermo J., Meijer L. Meridianins, a new family of protein kinase inhibitors isolated from the ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004;14:1703–1707. doi: 10.1016/j.bmcl.2004.01.050. [DOI] [PubMed] [Google Scholar]
- Gonzalez M.E., Alarcon B., Carrasco L. Polysaccharides as antiviral agents: antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987;31:1388–1393. doi: 10.1128/aac.31.9.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008;5:107. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H., Goeger D.E., Hills P., Mooberry S.L., Ballantine D.L., Murray T.F., Valeriote F.A., Gerwick W.H. Lophocladines, bioactive alkaloids from the red alga Lophocladia sp. J. Nat. Prod. 2006;69:640–644. doi: 10.1021/np050519e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulecha V., Shiva kumar T. Anticancer activity of tephrosia purpurea and ficus religiosa using MCF-7 cell lines. Asian Pac. J. Trop. Med. 2011;4:526–529. doi: 10.1016/S1995-7645(11)60139-9. [DOI] [PubMed] [Google Scholar]
- Guo J.H., Skinner G.W., Harcum W.W., Barnum P.E. Pharmaceutical applications of naturally occurring watersoluble polymers. Pharm. Sci. Technol. Today. 1998;6:254–261. [Google Scholar]
- Hawksworth D.L. Global species numbers of fungi: are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers. Conserv. 2012;21:2425–2433. [Google Scholar]
- Hemant R.K., Kritarth N.M.S. Marine pharmacology: potential, challenges, and future in India. J. Med. Sci. 2018;38(2):49–53. [Google Scholar]
- Heo S.J., Park P.J., Park E.J., Kim S.E., Jeon Y.J. Antioxidant activity of enzymatic extracts from a brown seaweed Ecklonia cava by electron spin resonance spectrometry and comet assay. Eur. Food Res. Technol. 2005;221:41–47. [Google Scholar]
- Hsiao K.Y., Wu Y.J., Liu Z.N., Chuang C.W., Huang H.H., Kuo S.M. Anticancer effects of sinulariolide-conjugated hyaluronan nanoparticles on lung adenocarcinoma cells. Molecules. 2016;21:297. doi: 10.3390/molecules21030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu B.Y., Tsao C.Y., Chiou T.K., Hwang P.A., Hwang D.F. HPLC determination for prostaglandins from seaweed Gracilaria gigas. Food Contr. 2007;18:639–645. [Google Scholar]
- Huang K.J., Chen Y.C., El-Shazly M., Du Y.C., Su J.H., Tsao C.W., Yen W.H., Chang W.B., Su Y.D., Yeh Y.T. 5-Episinuleptolide acetate, a norcembranoidal diterpene from the formosan softcoral Sinularia sp., induces leukemia cell apoptosis through Hsp90 inhibition. Molecules. 2013;18:2924–2933. doi: 10.3390/molecules18032924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huheihel M., Ishanu V., Tal J., Arad S.M. Activity of Porphyridium Sp. Polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods. 2002;50:189–200. doi: 10.1016/s0165-022x(01)00186-5. [DOI] [PubMed] [Google Scholar]
- Jimenez P.C., Wilke D.V., Ferreira E.G., Takeara R., De Moraes M.O., Silveira E.R., da Cruz Lotufo T.M., Lopes N.P., Costa-Lotufo L.V. Structure elucidation and anticancer activity of 7-oxostaurosporine derivatives from the Brazilian endemic tunicate Eudistoma vannamei. Mar Drugs. 2012;10:1092–1102. doi: 10.3390/md10051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Escrig A., Gomez-Ordonez E., Ruperez P. Seaweed as a source of novel nutraceuticals: sulfated polysaccharides and peptides. Adv. Food Nutr. Res. 2011;64:325–337. doi: 10.1016/B978-0-12-387669-0.00026-0. [DOI] [PubMed] [Google Scholar]
- Miller J.H., Field J.J., Kanakkanthara A., Owen J.G., Singh A.J., Northcote P.T. Marine invertebrate natural products that target microtubules. J. Nat. Prod. 2018;81(3):691–702. doi: 10.1021/acs.jnatprod.7b00964. [DOI] [PubMed] [Google Scholar]
- Kathiresan K., Qasim S.Z. Hindustan Publishing Corporation; New Delhi, India: 2005. Biodiversity of Mangrove Ecosystems. [Google Scholar]
- Kevin A.M.A., Chiara L., Giovanna R., Adrianna I. Marine microalgae with anti-cancer properties. Mar. Drugs. 2018;16(5):165. doi: 10.3390/md16050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.K., Mendis E. Bioactive compounds from marine processing by products-a review. Food Res. Int. 2006;39:383–393. [Google Scholar]
- Kim S.K., Ta Q.V. Potential beneficial effects of marine algal sterols on human health. Adv. Food Nutr. Res. 2011;64:191–198. doi: 10.1016/B978-0-12-387669-0.00014-4. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Wijesekara I. Development and biological activities of marine-derived bioactive peptides: a review. J. Funct. Foods. 2010;2:1–9. [Google Scholar]
- Klejnstrup M.L., Frandsen H.R.J.N., Nielsen D.K., Mortensen M.T., Larsen U.H., Nielsen T.O. Genetics of polyketide metabolism in Aspergillus nidulans. Metabolites. 2012;2:100–133. doi: 10.3390/metabo2010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongara S., Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konish I., Hosokawa M., Sashima T., Kobayashi H., Miyashita K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol., C Toxicol., Pharmacol. 2006;142:53–59. doi: 10.1016/j.cbpc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kott P. The Australian ascidiacea. Part 2. Aplousobranchia (1) Mem. Qd Mus. 1990;29:1–266. [Google Scholar]
- Kumar R.S., Rajkapoor B., Perumal P. In-vitro and in-vivo anticancer activity of indigofera cassiodies Rottl. Ex DC. Asian Rac. J. Trop. Med. 2011;4:379–385. doi: 10.1016/S1995-7645(11)60108-9. [DOI] [PubMed] [Google Scholar]
- Kuppusamya P., Soundharrajana I., Srigopalrama S., Yusoffa M.M., Maniamb G.P., Govindanb N., Choia K.C. Potential pharmaceutical and biomedical applications of Diatoms microalgae – an overview. Indian J. Geomarine Sci. 2017;46:663–667. [Google Scholar]
- Lahaye M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001;13:173–184. [Google Scholar]
- Larsen B., Salem D.M.S.A., Sallam M.A.E., Mishrikey M.M., Beltagy A.I. Characterization of the alginates from algae harvested at the Egyptian Red Sea coast. Carbohydr Polym. 2003;338:2325–2336. doi: 10.1016/s0008-6215(03)00378-1. [DOI] [PubMed] [Google Scholar]
- Laurienzo P. Marine polysaccharides in pharmaceutical applications: an overview. Mar. Drugs. 2010;8:2435–2465. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.B., Koizumi S., Hayashi K., Hayashi T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010;81:572–577. [Google Scholar]
- Lee J.C., Son Y.O., Pratheeshkumar P., Shi X. Oxidative stress and metal carcinogenesis. Free Radic. Biol. Med. 2012;53:742–757. doi: 10.1016/j.freeradbiomed.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Lesma G., Sacchetti A., Bai R., Basso G., Bortolozzi R., Hamel E., Viola G. Hemiasterlin analogues incorporating an aromatic, and heterocyclic type C-terminus: design, synthesis and biological evaluation. Mol. Divers. 2014;18(2):357–373. doi: 10.1007/s11030-014-9507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Lu F., Wei X., Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindequist U., Schweder T. Marine biotechnology. In: Rehm H.J., Reed G., editors. Biotechnology. Wiley- VCH, Weinheim. 2001. pp. 441–484. [Google Scholar]
- Liu M., Hansen P.E., Lin X. Bromophenols in marine algae and their bioactivities. Mar. Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang L., Chen Y., Li S., Tan G., Sun Z., Pan Q., Ye W., Li H., Zhang W. Cytotoxic pimarane-type diterpenes from the marine sediment-derived fungus Eutypella sp. FS46. Nat. Prod. Res. 2017;31:404–410. doi: 10.1080/14786419.2016.1169418. [DOI] [PubMed] [Google Scholar]
- Lotte-Van A., Jan H.M.S., Jos H.B. Review of chromatographic bioanalytical assays for the quantitative determination of marine-derived drugs for cancer treatment. Mar. Drugs. 2018;16(7):246. doi: 10.3390/md16070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArtain P., Gill C.I., Brooks M., Campbell R., Rowland I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007;65:535–543. doi: 10.1301/nr.2007.dec.535-543. [DOI] [PubMed] [Google Scholar]
- Makarieva T.N., Dmitrenok A.S., Dmitrenok P.S., Grebnev B.B., Stonik V., Pibocin B. The first N-O-methylindole marine alkaloid, a metabolite from the Far-Eastern ascidian Eudistoma species. J. Nat. Prod. 2001;64:1559–1561. doi: 10.1021/np010161w. [DOI] [PubMed] [Google Scholar]
- Mandin P., Gottesman S.A. Genetic approach for finding small rnas regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol. Microbiol. 2009;72:551–565. doi: 10.1111/j.1365-2958.2009.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manilal A., Sujith S., Kiran G.S., Selvin J., Shakir C., Gandhimathi R., Panikkar M.V.N. Bio-potentials of seaweeds collected from Southwest coast of India. J. Mar. Sci. Technol. 2009;17:67–73. [Google Scholar]
- Martín-Algarra Salvador. Phase II study of weekly Kahalalide F in patients with advanced malignant melanoma. Eur. J. Cancer. 2009;45(5):732–735. doi: 10.1016/j.ejca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Matsubara K. Recent advances in marine algal anticoagulants. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2004;2:13–19. doi: 10.2174/1568016043477314. [DOI] [PubMed] [Google Scholar]
- Mayer A., Rodriguez A., Taglialatela Scafati O., Fusetani N. Marine pharmacology in 2012–2013: marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs. 2017;15:273. doi: 10.3390/md15090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menna M., Fattorusso E., Imperatore C. Alkaloids from marine ascidians. Molecules. 2011;16:8694–8732. [Google Scholar]
- Michael L., Alexander E., Richard G. Plitidepsin: a potential new treatment for relapsed/refractory multiple myeloma. Drug Eval. 2019;15:2. [Google Scholar]
- Misurcova L., Skrovankova S., Samek D., Ambrozova J., Machu L. Health benefits of algal polysaccharides in human nutrition. Adv. Food Nutr. Res. 2012;66:75–145. doi: 10.1016/B978-0-12-394597-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Moallem S., Ahmadi A., Moshafi M., Taghavi M. Evaluation of fetal toxicity of HESA-A, a natural anticancer agent, in mice. J. Kerman Med. Sci. 2007;14:124–133. [Google Scholar]
- Mohamed S., Hashim S.N., Rahman H.A. Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012;23:83–96. [Google Scholar]
- Montaser R., Luesch H. Marine natural products: a new wave of drugs? Future Med. Chem. 2011;3:1475–1489. doi: 10.4155/fmc.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T.R., de Faria S.B., Rocha R.M. Biogeography of Atlantic and Mediterranean scidians. Mar. Biol. 2014;161:2023–2033. [Google Scholar]
- Muller W.E., Grebenjuk V.A., Le Pennec G., Schroder H., Brummer F., Hentschel U., Muller I.M., Breter H. Sustainable production of bioactive compounds by sponges–cell culture and gene cluster approach: a review. Mar. Biotechnol. 2004;6:105–117. doi: 10.1007/s10126-002-0098-6. [DOI] [PubMed] [Google Scholar]
- Murugan K., Iyer V.V. Antioxidant and antiproliferative activities of extracts of selected red and brown seaweeds from the Mandapam Coast of Tamil Nadu. J. Food Biochem. 2014;38:92–101. [Google Scholar]
- Nelson G.M.G., Ramesh D., Sunena C., Robert K., Alexander K. Marine invertebrate metabolites with anticancer activities: solutions to the “supply problem”. Mar. Drugs. 2016;14:98. doi: 10.3390/md14050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004;67:1216–1238. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- Ogi T., Taira J., Margiastuti P., Ueda K. Cytotoxic metabolites from the Okinawan ascidian Diplosoma Virens. Molecules. 2008;13:595–602. doi: 10.3390/molecules13030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okai Y., Higashi-Okai K., Yano Y., Otani S. Identification of antimutagenic substances in an extract of edible red alga, Porphyra tenera (Asadusa-nori) Cancer Lett. 1996;100:235–240. doi: 10.1016/0304-3835(95)04101-x. [DOI] [PubMed] [Google Scholar]
- Pangestuti R., Kim S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods. 2011;3:255–266. [Google Scholar]
- Park S.H., Ozden O., Jiang H., Cha Y.I., Pennington J.D., Aykin-Burns N., Spitz D.R., Gius D., Kim H.S. Sirt3, mitochondrial ROS, ageing, and carcinogenesis. Int. J. Mol. Sci. 2011;12:6226–6239. doi: 10.3390/ijms12096226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Kundu R. Antiproliferative activity of methanolic extracts from two green algae, Enteromorpha intestinalis and Rizoclonium riparium on HeLa cells. DARU. 2013;21:72. doi: 10.1186/2008-2231-21-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado I., Giro N.J., Koutsidis G., Ames J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014;66:36–44. [Google Scholar]
- Peng Z., Li Xiao-Ming, Xin-Xin M., Mandi Attila, Kurtan Tibor, Bin-Gui W. Varioloid A, a new indolyl-6,10b-dihydro-5aH-[1]benzofuro[2,3-b]indole derivative from the marine alga-derived endophytic fungus Paecilomyces variotii EN-291. Beilstein J. Org. Chem. 2016;12:2012–2018. doi: 10.3762/bjoc.12.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres J.M. Origine et affinités du peuplement en ascidies de la Méditerranée. Rapports et Procès Verbaux de la CIESM. 1958;14:493–502. [Google Scholar]
- Peres J.M. The Mediterranean Benthos. Oceanogr. Mar. Biol. Annu. Rev. 1967;5:449–533. [Google Scholar]
- Pettit G.R., Hogan F., Toms S. Antineoplastic agents. 592. Highly effective cancer cell growth inhibitory structural modifications of dolastatin 10. J. Nat. Prod. 2011;74:962–968. doi: 10.1021/np1007334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty R., Anthoney A., Metges J.P. Phase Ib/II study of elisidepsin in metastatic or advanced gastroesophageal cancer (IMAGE trial) Cancer Chemother. Pharmacol. 2016;77:819. doi: 10.1007/s00280-016-2991-0. [DOI] [PubMed] [Google Scholar]
- Rafael B.G.C., Leandro S.C., Gabriel P.F., Leonardo T.D.B.N., Nednaldo D., Sara L.C., Mariana S.S.P.C., Luciana G., Hugo A.O.R. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs. 2011;9:124–138. doi: 10.3390/md9010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N., Kim S.K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011;64:17–28. doi: 10.1016/B978-0-12-387669-0.00002-8. [DOI] [PubMed] [Google Scholar]
- Rajaram S., Ramulu U., Ramesh D., Srikanth D., Bhattacharya P., Prabhakar P., Kalivendi S.V., Babu K.S., Venkateswarlu Y., Navath S. Anti-cancer evaluation of carboxamides of furano- esquiterpene carboxylic acids from the soft coral Sinularia kavarattiensis. Bioorg. Med. Chem. Lett. 2013;23:6234–6238. doi: 10.1016/j.bmcl.2013.09.093. [DOI] [PubMed] [Google Scholar]
- Rajesh R.P., Ramasamy M.S., Murugan A. Anticancer activity of the ascidian Polyclinum indicum against cervical cancer cells (HeLa) medicated through apoptosis induction. J. Med. Chem. 2010;6:396–405. doi: 10.2174/157340610793564009. [DOI] [PubMed] [Google Scholar]
- Ramasubramani R., Praveen R., Sathyanarayanan K.S. Study on the strength properties of marine algae concrete. Rasayan J. Chem. 2016;4:706–715. [Google Scholar]
- Ravikumar S., Gnanadesigan M., Thajuddin N., Chakkaravarthi V.S.D., Banerjee B.M. Anticancer property of sponge associated actinomycetes along Palk Strait. J. Pharm. Res. 2011;2010(3):2415–2417. [Google Scholar]
- Renn D. Biotechnology and the red seaweed polysaccharide industry: status, needs and prospects. Trends Biotechnol. 1997;15:9–14. [Google Scholar]
- Rioux L.E., Turgeon S.L., Beaulieu M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry. 2010;71:1586–1595. doi: 10.1016/j.phytochem.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Rodney W.R., Jennifer M.R., Anthony C.W., Nola M.C., Julie K., Kiaran K., Kevin J.S., Geoffrey D.S. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron. 1999;55:13513–13520. [Google Scholar]
- Ruiz-Torres V., Encinar J.A., Herranz-Lopez M., Perez-Sanchez A., Galiano V., Barrajon-Catalan E., Micol V. An updated review on marine anticancer compounds: the use of virtual screening for the discovery of small-molecule cancer drugs. Molecules. 2017;22:1037. doi: 10.3390/molecules22071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem B.N., Rekha R., Komala M., Ruby S. Production of extracellular anti-leukaemic enzyme L-asparaginase from Marine actinomycetes by solid-state and submerged fermentation: purification and characterization. Trop. J. Pharm. Res. 2009;8:353–360. [Google Scholar]
- Shalaby E. Algae as promising organisms for environment and health. Plant Signal Behav. 2011;6:1338–1350. doi: 10.4161/psb.6.9.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkar N, Gittenberger A, Lambert G, Rius M, Moreira Da Rocha R, Swalla BJ, Turon X. 2016b. Ascidiacea World Database. http://www.marinespecies.org/ascidiacea/.
- Shenkar N., Swalla B.J. Global diversity of Ascidiacea. PLoS ONE. 2011;6:e20657. doi: 10.1371/journal.pone.0020657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard V.C., Scheffel A., Poulsen N., Kroger N. Live diatom silica immobilization of multimeric and redox-active enzymes. Appl. Environ. Microbiol. 2011;78:211–218. doi: 10.1128/AEM.06698-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklar G. Mechanisms of cancer inhibition by antioxidant nutrients. Oral Oncol. 1998;34:24–29. doi: 10.1016/s1368-8375(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Sithranga Boopathy N., Kathiresan K. Anticancer drugs from marine flora: an overview. J Oncol. 2010;214186:1–18. doi: 10.1155/2010/214186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soheil Z.M., Hamed K., Ramin K., Mahboubeh R., Mohammad F., Keivan Z., Habsah A.K. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci. World J. 2014;768323:1–10. doi: 10.1155/2014/768323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki R., Khanna M., Lal R. Bioactive compounds from marine actinomycetes. Indian J. Microbiol. 2008;48:410–431. doi: 10.1007/s12088-008-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza B.W., Cerqueira M.A., Martins J.T., Quintas M.A., Ferreira A.C., Teixeira J.A., Vicente A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011;59:55895594. doi: 10.1021/jf200999n. [DOI] [PubMed] [Google Scholar]
- Stengel D.B., Connan S., Popper Z.A. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011;29:483–501. doi: 10.1016/j.biotechadv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Su T.R., Lin J.J., Chiu C.C., Chen J.Y., Su J.H., Cheng Z.J., Hwang W.I., Huang H.H., Wu Y.J. Proteomic investigation of anti-tumor activities exerted by sinularin against A2058 melanoma cells. Electrophoresis. 2012;33:1139–1152. doi: 10.1002/elps.201100462. [DOI] [PubMed] [Google Scholar]
- Syam P.S., Amal E., Poul H., Sorensen Yuzhuo W, Hongwei C. An aqueous extract of marine microalgae exhibits antimetastatic activity through preferential killing of suspended cancer cells and anticolony forming activity. Evid.-Based Complem. Alternat. Med. 2016:1–8. doi: 10.1155/2016/9730654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szitenberg A., Becking L.E., Vargas S., Fernandez J.C., Santodomingo N., Worheide G., Ilan M., Kelly M., Huchon D. Phylogeny of Tetillidae (Porifera, Demospongiae, Spirophorida) based on threemolecular markers. Mol. Phylogenet Evol. 2013;67:509–519. doi: 10.1016/j.ympev.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Tabarsa M., Rezaei M., Ramezanpour Z., Waaland J.R. Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J. Sci. Food Agric. 2012;92:2500–2506. doi: 10.1002/jsfa.5659. [DOI] [PubMed] [Google Scholar]
- Tang H.F., Yi Y.H., Yao X.S., Xu Q.Z., Zhang S.Y., Lin H.W. Bioactive steroids from the brown alga Sargassum carpophyllum. J. Asian Nat. Prod. Res. 2002;4:95–105. doi: 10.1080/10286020290027362. [DOI] [PubMed] [Google Scholar]
- Utami A.W.A., Wahyudi A.T., Batubara I. Toxicity, anticancer and antioxidant activity of extracts from marine bacteria associated with sponge Jaspis sp. Int. J. Pharm. Biol. Sci. 2014;5:917–923. [Google Scholar]
- Van C., Hidalgo M., Canon J.L., Macarulla T., Bazin I., Poddubskaya E., Manojlovic N., Radenkovic D., Verslype C., Raymond E., Cubillo A., Schueler A., Zhao C., Hammel P. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int. J. Cancer. 2018;15:2053–2064. doi: 10.1002/ijc.31603. [DOI] [PubMed] [Google Scholar]
- Vasanthi H.R., Rajamanickam G.V., Saraswathy A. Tumoricidal effect of the red algae Acanthophora spicifera on Ehrlich’s ascites carcinoma in mice, Seaweed Res. UtilNet. 2004;25:217–224. [Google Scholar]
- Vignesh S., Raja A., James R.A. Marine drugs: implication and future studies. Int. J. Pharmacol. 2011;7:22–30. [Google Scholar]
- Vilar E., Grünwald V., Schoffski P. A phase I dose-escalating study of ES-285, a marine sphingolipid-derived compound, with repeat dose administration in patients with advanced solid tumors. Invest. New Drugs. 2012;30:299. doi: 10.1007/s10637-010-9529-9. [DOI] [PubMed] [Google Scholar]
- Voultsiadou E., Vafidis D. Marine invertebrate diversity in Aristotle’s zoology. Contribut. Zool. 2007;76:103–120. [Google Scholar]
- Wang S.K., Hsieh M.K., Duh C.Y. New diterpenoids from soft coral Sarcophyton ehrenbergi. Mar. Drugs. 2013;11:4318–4327. doi: 10.3390/md11114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. Biological membranes. Essays Biochem. 2015;59:43–69. doi: 10.1042/bse0590043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters D.J., Van den Brenk A.L. Toxins from ascidians. Toxicon. 1993;31:1349–1372. doi: 10.1016/0041-0101(93)90202-t. [DOI] [PubMed] [Google Scholar]
- Wei Wen-Chi, Sung Ping-Jyun, Duh Chang-Yih, Chen Bo-Wei, Sheu Jyh-Horng, Yang Ning-Sun. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs. 2013;11:4083–4126. doi: 10.3390/md11104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E.G.M., Heinz C.S., Matthias W., Sanja P.O., Renato B., Isabel M.M. Traditional and modern biomedical prospecting: part II-the benefits approaches for a sustainable exploitation of biodiversity (secondary metabolites and biomaterials from sponges) Evid. Based Complem. Alternat. Med. 2004;2:133–144. doi: 10.1093/ecam/neh030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Xiao-Yong Z., Xu-Hua N., Jie W., Zhong-Hui H., Shu-Hua Q. Eight linear peptides from the deep-sea-derived fungus Simplicillium obclavatum EIODSF 020. Tetrahedron. 2016;72:3092–3097. [Google Scholar]
- Xu S.H., Ding L.S., Wang M.K., Peng S.L., Liao X. Studies on the chemical constituents of the Algae Sargassum polycystum. Chin. J. Org. Chem. 2002;22:138–140. [Google Scholar]
- Xu W.H., Ding Y., Jacob M.R., Agarwal A.K., Clark A.M., Ferreira D., Liang Z.S., Li X.C. Puupehanol, a sesquiterpenehydroquinone derivative from the marine sponge Hyrtios sp. Bioorg. Med. Chem. Lett. 2009;19:6140–6143. doi: 10.1016/j.bmcl.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie Y., Wang W., Hsieh Y.P., Suzuki T. Compositional difference of phenolic compounds between two seaweeds Halimeda spp. J. Tokyo Univ. Fish. 2002;88:21–24. [Google Scholar]
- Yuan Y.V., Carrington M.F., Walsh N.A. Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro. Food Chem. Toxicol. 2005;43:1073–1081. doi: 10.1016/j.fct.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Yuan Y.V., Walsh N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Zandi K., Ahmadzadeh S., Tajbakhsh S., Rastian Z., Yousefi F., Farshadpour F., Sartavi K. Anticancer activity of Sargassum oligocystum water extract against human cancer cell lines. Eur. Rev. Med. Pharmacol. Sci. 2010;14:669–673. [PubMed] [Google Scholar]
- Zaragoza M., Lopez D.P., Saiz M., Poquet M., Perez J., Puig- Parellada P., Marmol F., Simonetti P., Gardana C., Lerat Y. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008;56:7773–7780. doi: 10.1021/jf8007053. [DOI] [PubMed] [Google Scholar]
- Zeitlin L., Whaley K.J., Hegarty T.A., Moench T.R., Cone R.A. Tests of vaginal microbicides in the mouse genital herpes model. Contraception. 1997;56:329–335. doi: 10.1016/s0010-7824(97)00154-6. [DOI] [PubMed] [Google Scholar]
- Zeng H., Combs G.F., Jr. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008;19:1–7. doi: 10.1016/j.jnutbio.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Tao L.Y., Liang Y.J. Secalonic acid D induced leukemia cell apoptosis and cell cycle arrest of G1 with involvement of GSK-3b/b-catenin/c-Myc pathway. Cell Cycle. 2009;8:2444–2450. doi: 10.4161/cc.8.15.9170. [DOI] [PubMed] [Google Scholar]
- Zhao M., Yang B., Wang J., Liu Y., Yu L., Jiang Y. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int. J. Immunopharmacol. 2007;7:162–166. doi: 10.1016/j.intimp.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zhou S.L., Wang M., Zhao H.G., Huang Y.H., Lin Y.Y., Tan G.H., Chen S.L. Penicilazaphilone C, a new antineoplastic and antibacterial azaphilone from the Marine Fungus Penicillium sclerotiorum. Arch. Pharm. Res. 2016;12:1621–1627. doi: 10.1007/s12272-016-0828-3. [DOI] [PubMed] [Google Scholar]