Abstract
Background
Complex insomnia, the comorbidity of chronic insomnia and obstructive sleep apnea (OSA), is a common sleep disorder, but the OSA component, whether presenting overtly or covertly, often goes unsuspected and undiagnosed due to a low index of suspicion. Among complex insomniacs, preliminary evidence demonstrates standard CPAP decreases insomnia severity. However, CPAP causes expiratory pressure intolerance or iatrogenic central apneas that may diminish its use. An advanced PAP mode—adaptive servo-ventilation (ASV)—may alleviate CPAP side-effects and yield superior outcomes.
Methods
In a single-site protocol investigating covert complex insomnia (ClinicalTrials.gov identifier: NCT02365064), a low index of suspicion for this comorbidity was confirmed by exclusion of 455 of 660 eligible patients who presented during the study period with overt OSA signs and symptoms. Ultimately, stringent inclusion/exclusion criteria to test efficacy yielded 40 adult, covert complex insomnia patients [average Insomnia Severity Index (ISI) moderate–severe 19.30 (95% CI 18.42–20.17)] who reported no definitive OSA symptoms or risks and who failed behavioral or drug therapy for an average of one decade. All 40 were diagnosed with OSA and randomized (using block randomization) to a single-blind, prospective protocol, comparing CPAP (n = 21) and ASV (n = 19). Three successive PAP titrations fine-tuned pressure settings, facilitated greater PAP use, and collected objective sleep and breathing data. Patients received 14 weeks of treatment including intensive biweekly coaching and follow-up to foster regular PAP use in order to accurately measure efficaciousness. Primary outcomes measured insomnia severity and sleep quality. Secondary outcomes measured daytime impact: OSA-induced impairment, fatigue severity, insomnia impairment, and quality of life. Performance on these seven variables was assessed using repeated measures ANCOVA to account for the multiple biweekly time points.
Findings
At intake, OSA diagnosis and OSA as a cause for insomnia were denied by all 40 patients, yet PAP significantly decreased insomnia severity scores (p = 0.021 in the primary ANCOVA analysis). To quantify effect sizes, mean intake vs endpoint analysis was conducted with ASV yielding nearly twice the effects of CPAP [− 13.2 (10.7–15.7), Hedges' g = 2.50 vs − 9.3 (6.3–12.3), g = 1.39], and between mode effect size was in the medium-large range 0.65. Clinically, ASV led to remission (ISI < 8) in 68% of cases compared to 24% on CPAP [Fisher's exact p = 0.010]. Two sleep quality measures in the ANCOVA analysis again demonstrated superior significant effects for ASV compared to CPAP (both p < 0.03), and pre- and post-analysis demonstrated substantial effects for both scales [ASV (g = 1.42; g = 1.81) over CPAP (g = 1.04; g = 0.75)] with medium size effects between modes (0.54, 0.51). Measures of impairment, residual objective sleep breathing events, and normalized breathing periods consistently demonstrated larger beneficial effects for ASV over CPAP.
Interpretation
PAP therapy was highly efficacious in decreasing insomnia severity in chronic insomnia patients with previously undiagnosed co-morbid OSA. ASV proved superior to CPAP in this first efficacy trial to compare advanced to traditional PAP modes in complex insomnia. Future research must determine the following: pathophysiological mechanisms to explain how OSA causes chronic insomnia; general population prevalence of this comorbidity; and, cost-effectiveness of ASV therapy in complex insomnia. Last, efforts to raise awareness of complex insomnia are urgently needed as patients and providers appear to disregard both overt and covert signs and symptoms of OSA in the assessment of chronic insomnia.
Keywords: Efficacy trial, Insomnia, Sleep quality, Sleep fragmentation, Obstructive sleep apnea, Upper airway resistance syndrome, Complex insomnia, Continuous positive airway pressure, Adaptive servo-ventilation, Apnea, Hypopnea, Respiratory effort-related arousals
Research in context
Evidence before this study
Nearly 50-years of research in the field of sleep medicine have been unable to discover a universally accepted theory on the etiology and fundamental nature of the most common sleep complaint: chronic insomnia. Numerous attempts to identify definitive causality have instead elucidated multiple and diverse precipitants for chronic insomnia, leading to a consensus model defining unwanted bouts of sleeplessness as a psychophysiological condition. The most effective treatment has been established with cognitive-behavioral therapy for insomnia (CBT-I); yet, pharmacotherapy including OTC remedies are selected or prescribed more frequently. Notwithstanding, Guilleminault et al. (1973) published the first case reports 46 years ago describing chronic insomnia as a medical disorder fueled by abnormal respiration during sleep (sleep-disordered breathing). Currently, a PubMed search including terms “Insomnia”, “Sleep Apnea”, and “PAP” reveals more than a dozen review articles describing relationships between co-morbid insomnia and obstructive sleep apnea (aka “complex insomnia”) as well as fourteen treatment studies where CPAP therapy and other sleep breathing treatments were associated with significant decreases in insomnia severity. This evidence indicates chronic insomnia is caused in part by chronic sleep fragmentation objectively induced by underlying (and typically unrecognized) obstructive sleep apnea. When attempting to use CPAP, however, insomniacs face additional challenges due to their anxious, poorly tolerated, and sometimes traumatizing reactions to fixed pressurized air (CPAP). Advanced PAP therapy (e.g. ASV, adaptive servo-ventilation), with dual and variable pressure settings combined with “smart” algorithms, may alleviate side-effects related to CPAP intolerance.
Added value of this study
The current efficacy research trial demonstrates how: 1) providers and patients frequently overlook the link between sleep apnea and insomnia; 2) PAP therapy unequivocally and rapidly reverses objective sleep fragmentation, thereby improving sleep quality; 3) marked improvements in sleep quality lead to marked insomnia improvements; 4) ASV PAP therapy is more efficacious than CPAP through its capacity to provide significantly higher inspiratory pressures and significantly lower expiratory pressures, which result in greater normalization of sleep breathing as well as greater patient comfort; and 5) ASV PAP therapy demonstrates superior efficacy to CPAP in treating complex insomnia based on levels of success (insomnia remission rates) nearly equivalent to gold standard CBT-I. Clinically, these chronic insomnia disorder patients had been failing several insomnia treatments for a decade, on average, and neither they nor their doctors or therapists imagined they had been suffering from sleep apnea. Taken together, this efficacy trial provides new and additional evidence to categorize abnormal sleep respiration as a causative factor triggering insomnia through sleep fragmentation effects.
Implications of all the available evidence
The link between chronic, objective, sleep apnea-induced sleep fragmentation (i.e. degraded sleep quality) and chronic insomnia appears more prevalent than previously recognized. In this protocol, 455 of 660 eligible chronic insomnia patients were excluded from the study because they overtly suffered signs and symptoms of undiagnosed and untreated OSA, apparently for one decade. Another variant, covert complex insomnia, is even more difficult to diagnose due to the absence of sleep breathing signs or symptoms, such as a lack of obesity, sleepiness, and witnessed apneas. Overall, many chronic insomnia patients may be receiving inadequate or inappropriate treatment from medications that do not treat a key pathophysiological component of their condition. Last, among these types of patients failing conventional therapies, treatment with any PAP mode decreases insomnia; but, notably, treatment with the advanced PAP mode known as ASV proved most efficacious among a population historically intolerant to or susceptible to failing CPAP.
Alt-text: Unlabelled Box
1. Introduction
Most medical and mental health professionals identify insomnia, the most common sleep condition affecting 25% of adults intermittently and 10% regularly [1], as a psychological disorder with clinical pathophysiological effects managed with behavioral therapies or pharmacotherapy [1], [2]. As such, over-the-counter (OTC) agents are widely available but with scant evidence of efficacy [3]. Prescription sedatives are extensively marketed and dispensed, yet efficacy and safety questions persist regarding long-term use [1], [3]. The American Academy of Sleep Medicine (AASM) recommends gold standard cognitive-behavioral therapy for insomnia (CBT-I) [3], [4], which navigates insomniacs through reframing steps to adjust thinking (cognitive) and actions (behavioral) to “stop losing sleep over losing sleep.” CBT-I is highly efficacious with limited accessibility [5], [6], although newer internet programs are expanding CBT-I resources [7]. However, CBT-I may cause adverse effects from sleep restriction [8].
In sharp contrast to this standard perspective on the most common sleep disorder, a newer physiological conceptualization of insomnia, originally termed “complex insomnia” in 2001, has been defined as the comorbidity of chronic insomnia and covert obstructive sleep apnea (OSA) [9]. This comorbidity was discovered much earlier by Guilleminault et al. [10] in five insomnia patients with co-occurring, previously unsuspected sleep-disordered breathing [10]. Conventional wisdom typically dispels this co-occurrence as an epiphenomenon based on the coincidental high prevalence of both disorders [11], [12], [13]. In contrast, complex insomnia theory proposes OSA, or its variant upper airway resistance syndrome (UARS), directly causes chronic insomnia in a sizeable proportion of patients unaware of their OSA/UARS [11], [13], [14], [15]; the theory posits sleep breathing events are critical pathophysiological factors triggering chronic, pervasive, objective sleep fragmentation (i.e. poor sleep quality), which ultimately fuels unwanted sleeplessness [9].
In this new model, chronic insomnia is in part a medical disorder of abnormal sleep respiration [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], but the true prevalence is unknown as no general population studies have been conducted. Nonetheless, extant literature studying numerous cohorts suggests co-morbid insomnia and objectively diagnosed OSA/UARS are quite common [11], [13], [14], [15]. Among patients presenting with primary insomnia, investigated within or outside sleep center populations and grouped by patient characteristics or co-occurring health conditions, OSA/UARS prevalence was as follows: active duty military (38%) [30], chronic kidney disease (51%) [31], crime victims (91%) [9], disaster survivors (95%) [32], elderly (29–43%) [33], [34], hypnotic failure (71–91%) [35], [36], [37], postmenopausal women (83%) [38], primary care patients (23%–34%) [39], [40], [41], PTSD patients (76%) [42], rape survivors (90%) [43], and veterans (46%) [44].
The potential clinical relevance of the complex insomnia theory has been further elaborated upon in 14 peer-reviewed treatment studies, all demonstrating significant improvements with medium to large effects in chronic insomnia patients' outcomes following OSA therapies. Modalities included CPAP [16], [17], [18], [19], [20], [21], [22], [23], auto-bilevel PAP (ABPAP) [24], [25], [26], adaptive servo-ventilation (ASV) [25], [26], [29], oral airway surgery (e.g. T & A, UPPP) [21], oral appliance therapy [28], bariatric surgery [16], and nasal dilator strips that expand cross-sectional volume of nasal airflow [27]. This evidence informs the theory that treatment of OSA/UARS decreases insomnia severity by reversing sleep fragmentation triggered by objectively diagnosable sleep breathing events, a well-described therapeutic process that enhances sleep consolidation (greater sleep quality) [45].
Unfortunately, current professional attitudes about CPAP, the gold standard OSA treatment, appear to presume nearly automatic rejection by insomnia patients [20], [46], [47], [48]. Even among most sleep medicine specialists, CPAP is believed to be a nonstarter for insomniacs due to older evidence showing CPAP worsens insomnia [49]; and, other evidence shows insomnia makes CPAP adherence more difficult [48]. However, more recent evidence suggests advanced PAP modes (ABPAP, ASV) are associated with greater use among insomnia patients who previously failed CPAP [50].
In general, continuous pressure (fixed CPAP) failures are often the result of recurring or persistent subjective or objective expiratory pressure intolerance (EPI)—the unpleasant and sometimes traumatizing sensation of breathing out against pressurized airflow (described as “drowning in air”). EPI (also described as claustrophobic-like) emerges among insomnia patients attempting CPAP and may be associated with iatrogenic central sleep apneas (CSA) [29]. Both side-effects (EPI, CSA) are observed among individuals who reject CPAP [25]. Advanced PAP may prove more efficacious than CPAP by providing dual pressures with lower exhalation settings to yield a comfort effect; some advanced PAP modes (e.g. ASV) deliver dual pressures using proprietary volume-based algorithms coupled with a respiratory back-up rate to eliminate CSA [51]. No studies, however, have compared the efficacy of CPAP to advanced PAP therapy in chronic insomnia patients.
The current randomized controlled trial (RCT) evaluated adult complex insomnia patients randomized to CPAP or ASV. We hypothesized ASV would prove more efficacious than CPAP in significantly decreasing insomnia severity by restoring sleep quality, subjectively and objectively. Our secondary hypothesis predicted superior ASV efficacy to CPAP in decreasing daytime impairment, improving quality of life, and enhancing various objective measures of sleep and breathing.
2. Methods
2.1. Efficacy Trial Requirements for Sample Selection Based on Complex Insomnia Presentation
An efficacy protocol was applied [52], [53], [54], because no standard treatment of complex insomnia has been established. Moreover, no comparative studies have tested CPAP versus advanced PAP for complex insomnia patients. Last, beyond general difficulties in adhering to PAP therapy, complex insomniacs have manifested greater barriers to regular use of PAP and therefore require considerably more hands-on support to achieve a reasonable degree of adherence sufficient to test efficacy [14], [15], [26], [29], [50], [55].
Complex insomnia presents in two distinct forms: covert and overt. In covert complex insomnia, described as occult [34], covert [9], or veiled [11] during the past two decades by several research groups [9], [34], [41], [44], minimal or no signs and symptoms of a sleep breathing disorder are reported. Instead, insomnia complaints are usually intense or urgent, leading primary care providers, mental health professionals, and some sleep medicine specialists to bypass other potential etiologies to explain sleeplessness [49]. These patients may never be sent for sleep center referrals but are almost invariably offered pharmacotherapy as well as psychotherapy (rarely CBT-I). These covert cases were selected for the current protocol, because they closely match the common clinical presentation of those patients who appear to be “pure” insomniacs (psychologically driven) seen in mental health and primary care clinics. They also provide an additional masking advantage to the protocol, because they themselves never suspect the presence of a sleep breathing disorder or imagine it as a cause of their sleeplessness.
In contrast, overt presentations of complex insomnia are more common and invariably include risks or symptoms like witnessed apneas, obesity, and daytime sleepiness [56]. However, many healthcare professionals managing large clinical caseloads of insomnia patients remain unaware that these risks portend a link to a physical cause for sleeplessness [12], [56]. Thus, current practices may have created an aversive filter that prevents the recognition of the need for sleep testing in either overt or covert forms of complex insomnia. Nonetheless, overt complex insomnia patients were excluded from this protocol, because their awareness of breathing symptoms or OSA risks could have led to bias against our specific aim to work with patients who never suspected the presence of a sleep breathing disorder. Parenthetically, by restricting the sample to covert complex insomnia, the protocol indirectly provided an opportunity to confirm this general problem of low index of suspicion, because many overt cases would be excluded in our recruitment process.
2.2. IRB Approval, Intake, & Inclusion/Exclusion Criteria
The study was approved by Presbyterian Healthcare Institutional Review Board and listed at https://clinicaltrials.gov/ct2/show/NCT02365064 (February, 2015, after which recruitment began) and followed CONSORT guidelines. Patients completed a standard online intake for Maimonides Sleep Arts & Sciences (Albuquerque, NM). The first author identified potentially eligible patients following intake review; these patients then learned of a randomized controlled trial of two unspecified treatments for chronic insomnia. Primary inclusion criteria were adult chronic insomnia disorder patients who believed their sleep problems were due to psychological, psychiatric, behavioral, or environmental factors and affirmed classic features of insomnia (racing thoughts at bedtime, clock-watching, anxiety and fear in the bedroom, poor sleep hygiene, lying awake in bed, “losing sleep over losing sleep” aka psychophysiological conditioning, or regular use of OTC or prescription sleep aids). Patients echoed common presentations of chronic insomnia observed at mental health, primary care, and sleep clinics [1], [3]; none believed sleep breathing symptoms or disorders caused or contributed to their insomnia prior to being objectively diagnosed with sleep-disordered breathing. Main exclusion criteria (Fig. 1) focused on obvious risks for a sleep breathing disorder: obesity, excessive daytime sleepiness, witnessed apneas, and past evaluations at sleep centers. Patients without psychological features of insomnia [1] were excluded. Co-morbid sleep disorders, notably restless legs syndrome (RLS) and periodic limb movement disorder (PLMD), were major exclusion criteria, but due to their inconsistent diagnostic manifestations, some of these patients needed to be excluded post-randomization [57] (see Section 2.5). Last, due to recent controversies on the use of ASV in congestive heart failure patients [58], cardiac patients were excluded.
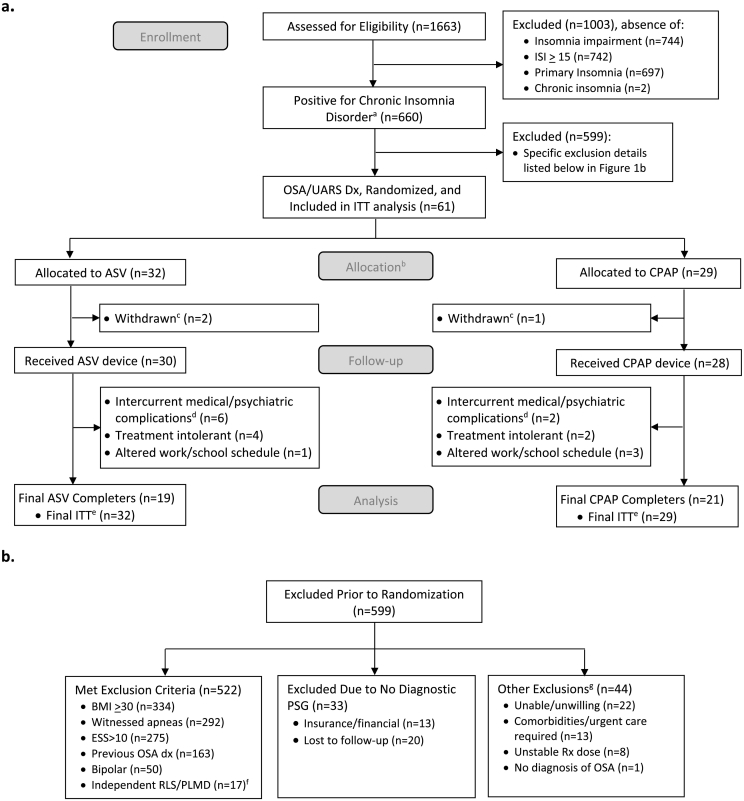
Fig. 1.
Flowchart showing a) eligibility and final sample of chronic insomnia disorder patients presenting with chief complaint of chronic psychophysiological insomnia attributed to behavioral, psychological, psychiatric, and environmental factors, and b) excluded patients' details.
Abbreviations: ISI — Insomnia Severity Index; ITT — intent to treat; Dx — diagnosis; BMI — body mass index; ESS — Epworth Sleepiness Scale; PSG — Polysomnography Sleep Test; Rx — prescription; OSA — obstructive sleep apnea; UARS — upper airway resistance syndrome; ASV — adaptive servo-ventilation; CPAP — continuous positive airway pressure; RLS — restless leg syndrome; PLMD — periodic limb movement disorder.
Footnote:
aChronic insomnia disorder as defined by the AASM: chief complaint of insomnia, ISI ≥ 15, impairment due to insomnia, and duration of at least 6 months.
bRandomization reset halfway through sample collection due to exclusion of ASV participants secondary to disproportionate leg movement disorders in this group.
cWithdrawn patients after randomization and completion of first exposure to PAP treatment; chose not to pursue research without offering explanation.
dIntercurrent medical/psychiatric patients developed medical or psychiatric issues that required immediate attention and subsequent removal from the study due to treatment plan.
eFinal ITT included all eligible randomized patients, total n = 61.
fThe 17 exclusions were eligible for the study and completed the informed consent and randomization process. However, during a titration study they exhibited independent RLS/PLMD and were excluded as post-randomization exclusions.
gUnable/unwilling: patients with scheduling conflicts or no interest in participation; comorbidities/urgent care required: patients with multiple health issues that may have confounded research or patients who needed immediate treatment of their sleep-disordered breathing; unstable Rx dose: patients working with doctors to titrate medication dosage; no diagnosis of OSA: did not meet requirements for OSA/UARS.
2.3. Procedure
2.3.1. Pre-Research Diagnosis & Informed Consent
Timeline of the protocol (Fig. 2) begins with patient referral to the sleep center. No recruitment advertising was conducted; primary care physicians were apprised of the research and instructed not to divulge the protocol to candidates, who were informed of a “chronic insomnia research study.” Pre-research, diagnostic PSG was completed. During results review, eligible patients learned for the first time about their co-occurring OSA (apnea-hypopnea index (AHI) > 5) or UARS, the latter diagnosed when respiratory effort-related arousals (RERAs) (aka flow limitation events) yielded a respiratory disturbance index (RDI) > 15 when AHI < 5. Discussion of the protocol and informed consent followed. A theory was offered about problematic breathing during sleep causing EEG sleep fragmentation, resulting in chronic insomnia. At consent, no discussion occurred about psychological aspects of insomnia. Interventions were described as two types of PAP, but specifics were omitted. Patients were instructed and needed to agree to forego new sleep or psychotropic medications; established sleep aids and psychotropic drugs at current dosages were permitted for protocol entirety. Extensive time commitments and tasks in the 15-week protocol were reviewed. All these steps were completed prior to randomization, thus neither the principal investigator nor the two co-investigators were aware of any PAP mode assignments prior to a patient completing the consent step.
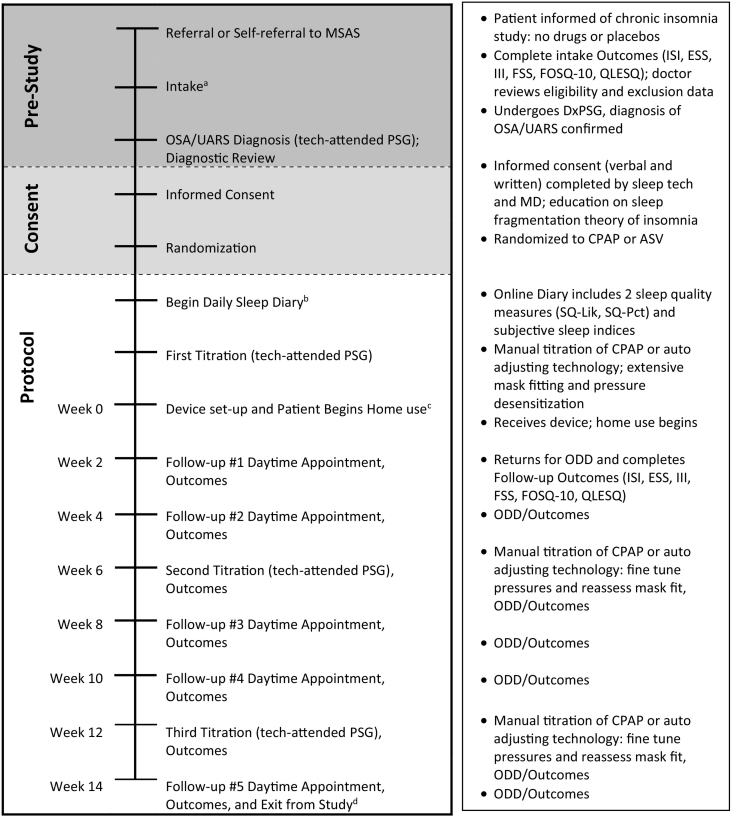
Fig. 2.
Protocol timeline: patient pathway from referral through study exit.
Abbreviations: MSAS — Maimonides Sleep Arts & Sciences; OSA — obstructive sleep apnea; UARS — upper airway resistance syndrome; PSG — Polysomnography Sleep Test; ISI — Insomnia Severity Index; ESS — Epworth Sleepiness Scale; III — Insomnia Impairment Index; FSS — Fatigue Severity Scale; FOSQ-10 — Functional Outcomes of Sleep Questionnaire Short Form; QLESQ — Quality of Life Enjoyment and Satisfaction Questionnaire Summary; DxPSG — Diagnostic Polysomnography Sleep Test; MD — medical director; CPAP — continuous positive airway pressure; ASV — adaptive servo-ventilation; SQ-Lik — sleep quality (Likert scale); SQ-Pct — sleep quality (percent scale); ODD — objective data download.
Footnote:
aIntake included ISI, ESS, III, FSS, FOSQ-10, QLESQ. Potentially eligible patients were informed “the protocol does not involve drugs, placebos or deception; it is a randomized controlled trial of two different techniques to treat chronic insomnia,” but no further details were provided about treatment.
bOnline sleep diary completed daily via home computer or smart phone throughout the study and included two questions regarding subjective sleep quality, SQ-Lik and SQ-Pct, as well as subjective sleep indices. Pre-treatment sleep diary completed for 7 consecutive days prior to first titration.
cPatients encouraged to use PAP nightly; the protocol was designed for patients to quickly attain or approach standard PAP compliance metrics to ensure final results would measure efficacy among two samples of regular users. Adaptation to CPAP and ASV may follow different courses, because CPAP users must contend with EPI and central apneas from the outset; whereas ASV patients may experience immediate alleviation of EPI as well as fewer or no central apneas. However, ASV patients may struggle with the constant auto-adjusting changes embedded within this technology, which makes some individuals feel as if they are losing control over their breathing.
dCompletion of protocol granted patient ownership of the PAP device and transportation reimbursement ($85).
2.4. Protocol
2.4.1. Randomization, PAP Technology and Masking
After consent, random assignment was conducted using non-stratified block randomization of block size 4, with equal weights for each arm (CPAP or ASV). Research staff were unaware of the randomization schedule and only learned of PAP mode assignment after consent. Patients then completed one-week prospective sleep diaries followed by first overnight in-lab titration. All patients were PAP naïve and therefore unable to detect mode distinctions between fixed pressurized air (CPAP) and ASV, the latter technology providing higher inspiratory pressures to more effectively titrate RERAs while maintaining lower expiratory pressure to prevent expiratory pressure intolerance [59], [60]. AASM mandates RERAs should be eliminated during a titration [45]. Patients were set up with ResMed AirCurve™ devices, masked by blocking LCD visualization using thick black paper and tape, with built-in capacity to apply either ASV or CPAP [61].
2.4.2. Repeat Titrations, Follow-up, and Support
A specific aim prevented over-titration to avoid harm from intolerable pressurized air (EPI). As stated, AASM requires titration to optimal settings, including virtual elimination of residual RERA breathing events (normalized airflow signal) [45], but when pressures provoke discomfort or intolerance, settings are lowered temporarily. Pressures were then gradually increased during the entire protocol to reach optimal settings. To resolve these conflicting objectives, we worked to normalize the airflow signal as much as possible while recognizing efforts to eliminate RERAs may provoke EPI or frank central apneas (Fig. 2).
Follow-up occurred daily with sleep tracking logs to gather standard sleep indices (see abbreviations in Section 2.4.3) as well as sleep quality assessments. Additional follow-up occurred at biweekly intervals (4 clinic visits interspersed with 2 lab nights, Fig. 2) with collection of follow-up measurements and objective data downloads (ODD). ODD captured residual breathing events [apneas, hypopneas, flow limitation (i.e. “flattening” frequency mild/moderate/severe)], mask leak, use hours, and average pressurized air levels [53]. Final follow-up measurements occurred two weeks after last titration. All daily and biweekly measures were collected electronically by direct patient input and locked into a database to ensure data integrity.
For this efficacy research, given the degree of difficulty in adhering to PAP and unique barriers associated with PAP attempts in chronic insomnia patients [48], unlimited additional support from research staff was available by email, telephone, or walk-in appointments. Overall, the support was extensive to promote regular use, so that efficacy of each mode could be determined. This lengthy, intensive protocol accommodated the natural slow trajectory of incremental adaptation in first-time PAP users as well as the gradual improvement in outcomes over many weeks duration.
2.4.3. Measurements
Primary subjective instruments comprised the Insomnia Severity Index (ISI) and two self-rating sleep quality measures: SQ-Lik, based on an 8-point Likert scale and SQ-Pct, a 0 to 100% rating with higher scores indicative of better sleep quality for both measures. The main secondary measures included three impairment indexes [Insomnia Impairment Index (III), Fatigue Severity Scale (FSS), Functional Outcomes of Sleep Questionnaire Short Form (FOSQ-10)] and one quality of life scale [Quality of Life Enjoyment and Satisfaction Questionnaire Summary Form (QLESQ)]. (See supplement for detailed descriptions.) Objective measurements focused on respiratory disturbance indexes, sleep indices [sleep onset latency (SOL), wake after sleep onset (WASO), and sleep efficiency (SE)], and sleep architecture. Normalized sleep breathing time (NSBT) measured by percent and in minutes, measured at all four PSGS (diagnostic and three titrations), was calculated by subtracting time spent during breathing events (apneas, hypopneas, FLEs) and other arousals from total sleep time. PSGs were scored by an independent, registered, 20-year experienced sleep technologist, blinded to protocol and PAP mode.
2.5. Power Analysis, Sample Size Estimates and Re-randomization
We hypothesized large to very large effects in ASV users compared to medium to large effects in CPAP users for primary outcomes, thus we anticipated an average net difference between modes of ~ 0.80 effect size for insomnia and sleep quality measures. Two-tailed significance testing at α = 0.05 with 90% power and n = 26 per group (total sample of 52) yielded a minimal detectable difference of 4 units on the ISI scale for mean group change. Sleep quality change estimates were similar. We sought to recruit 75 patients for 52 patients to complete the protocol, but due to partially unexpected findings regarding RLS and PLMD [62], [63], coupled with the expected majority of patients manifesting overt signs and symptoms of OSA, many individuals were excluded (Fig. 1). After eliminating the 17 post-randomization exclusions due to RLS/PLMD status, the eligible, protocol-directed cohort totaled 61 individuals, and as described subsequently comprised groups of 32 ASV and 29 CPAP, thus virtually no imbalance existed in the treatment arms.
To clarify the post-randomization exclusion [57] of patients with RLS/PLMD, it is important to note the condition must be deemed an independent, co-morbid state [63], [64]. However, actual PAP use provides the only confirmation of an independent limb movement disorder during sleep (PLMD) [63] because secondary limb movements typically resolve with PAP [63], [64]. Thus, patients manifesting RLS/PLMD on diagnostic studies were enrolled given the likelihood the problem would dissipate. Also, patients reporting no limb movements and demonstrating no movements on diagnostic studies may subsequently manifest independent leg jerks on a PAP titration [62], [64]. Leg jerks triggering EEG arousal activity despite normalized breathing are deemed independent [62], [63], [64]. Thus, any case of independent PLMD required a post-randomization exclusion from the protocol as they exhibited this additional co-morbid condition not under study in this protocol. For no discernible reason, more ASV patients were affected and excluded due to RLS/PLMD, thus block randomization was repeated and re-weighted at study midpoint to balance the sample.
2.6. Final Sample & Data Analysis
During the 3.5 year recruitment process, 660 adult patients presented to the sleep center with a chief complaint of chronic insomnia and met nosology for a chronic insomnia disorder (Fig. 1a) [1]; 455 patients were acutely excluded due to high suspicions for a sleep breathing disorder (obesity, excessive daytime sleepiness, witnessed apneas, past sleep center encounters, or past OSA/UARS diagnosis) (Fig. 1b). Ironically, among these 455 excluded patients, 75% reported no awareness of sleep breathing as a cause of their nocturnal awakenings, and 65% denied sleep breathing treatments as relevant to their sleep problems. Remarkably, 163 of these 455 had already been diagnosed with sleep apnea yet none reported awareness of any relationship between sleep breathing and insomnia; the remaining 292 chronic insomnia patients were ultimately diagnosed with OSA/UARS. See Fig. 1b for additional exclusions.
As noted above, the final sample comprised 61 patients of which 40 (19 ASV and 21 CPAP) completed the full protocol (see Fig. 1a, for post-randomization exclusions and other patients who withdrew from the study). These 40 completers comprised the final sample from which the efficacy analysis was conducted [52], [53], [54].
Sociodemographics (Table 1a, Table 1b) and baseline subjective and objective sleep variables (Table 2a, Table 2b) were analyzed with two-tailed t-test or Fisher's exact test, as appropriate, for all 61 randomized individuals including comparisons for completers vs non-completers and separately for the two modes. To compare treatment modalities over the course of the study (multiple time points) for the main outcomes, repeated measures ANCOVA using linear mixed models accounted for within-subject correlations, with correlation structure chosen by minimizing AIC (Akaike Information) and checked by regression diagnostics and variograms; in all cases either AR(1) or compound symmetry was selected. Nonlinear trends were modeled by fitting week of study using restricted cubic splines with 4 knots [65]. A modality × week interaction allowed different trends for the two modes. Calculations were with R [66] using rms [67] and nlme [68] packages. Significance testing was by Wald χ2 tests at the 0.05 level.
Table 1a.
Comparison of sociodemographics and insomnia chronicity for total randomized sample (n = 61) by mode and completion status: % or mean(95% confidence interval)a.
| Total (n = 61) |
ASV (n = 32) |
CPAP (n = 29) |
p-valueb, gc | Completers (n = 40) |
Non-completers (n = 21) |
p-valueb, gc | |
|---|---|---|---|---|---|---|---|
| Sociodemographics | |||||||
| Female (%) | 68.9 (55.7–80.1) | 71.9 (53.3–86.3) | 65.5 (45.7–82.1) | 0.782, 0.74 (0.25–2.20) | 65.0 (48.3–79.4) | 76.2 (52.8–91.8) | 0.561, 1.72 (0.52–5.70) |
| Age | 45.44 (41.51–49.37) | 45.89 (40.26–51.52) | 44.95 (39.14–50.76) | 0.814, 0.06 | 48.38 (43.66–53.11) | 39.84 (32.94–46.73) | 0.041, 0.57 |
| BMI | 24.81 (23.94–25.69) | 25.07 (23.76–26.38) | 24.53 (23.32–25.75) | 0.545, 0.16 | 24.97 (23.82–26.13) | 24.50 (23.05–25.95) | 0.595, 0.14 |
| Caucasian (%) | 70.5 (57.4–81.5) | 65.6 (46.8–81.4) | 75.9 (56.5–89.7) | 0.414, 0.61 (0.20–1.86) | 75.0 (58.8–87.3) | 61.9 (38.4–81.9) | 0.378, 1.85 (0.59–5.74) |
| Hispanic (%) | 21.3 (11.9–33.7) | 25.0 (11.5–43.4) | 17.2 (5.8–35.8) | 0.541, 1.60 (0.46–5.60) | 17.5 (7.3–32.8) | 28.6 (11.3–52.2) | 0.341, 0.53 (0.15–1.85) |
| College degree (%) | 54.1 (40.8–66.9) | 56.3 (37.7–73.6) | 51.7 (32.5–70.6) | 0.800, 1.20 (0.44–3.29) | 55.0 (38.5–70.7) | 52.4 (29.8–74.3) | 1.000, 1.11 (0.39–3.20) |
| Married (%) | 54.1 (40.8–66.9) | 56.3 (37.7–73.6) | 51.7 (32.5–70.6) | 0.800, 1.20 (0.44–3.29) | 52.5 (36.1–68.5) | 57.1 (34.0–78.2) | 0.791, 0.83 (0.29–2.40) |
| Insomnia chronicity | |||||||
| Duration (years) | 10.26 (8.41–12.10) | 11.24 (8.75–13.72) | 9.18 (6.32–12.03) | 0.268, 0.28 | 9.80 (7.48–12.13) | 11.12 (7.84–14.39) | 0.503, 0.18 |
t-test confidence interval for continuous variables.
p-value is Fisher's exact t-test for dichotomous variables, t-test for continuous variables.
Odds ratio (95% confidence interval) calculated for dichotomous variables; Hedges' g calculated for continuous variables.
Table 1b.
Comparison of sociodemographics and insomnia chronicity for completers (n = 40) by mode: % or mean(95% confidence interval)a.
| Completers (n = 40) |
ASV (n = 19) |
CPAP (n = 21) |
p-valueb, gc | |
|---|---|---|---|---|
| Sociodemographics | ||||
| Female (%) | 65.0 (48.3–79.4) | 73.7 (48.8–90.9) | 57.1 (34.0–78.2) | 0.333, 0.48 (0.13–1.82) |
| Age | 48.38 (43.66–53.11) | 52.03 (44.94–59.12) | 45.09 (38.54–51.64) | 0.140, 0.47 |
| BMI | 24.97 (23.82–26.13) | 24.78 (22.76–26.80) | 25.15 (23.81–26.50) | 0.745, 0.10 |
| Caucasian (%) | 75.0 (58.8–87.3) | 73.7 (48.8–90.9) | 76.2 (52.8–91.8) | 1.000, 0.88 (0.21–3.66) |
| Hispanic (%) | 17.5 (7.3–32.8) | 15.8 (3.4–39.6) | 19.0 (5.4–41.9) | 1.000, 0.80 (0.15–4.13) |
| College degree (%) | 55.0 (38.5–70.7) | 52.6 (28.9–75.6) | 57.1 (34.0–78.2) | 1.000, 0.83 (0.24–2.90) |
| Married (%) | 52.5 (36.1–68.5) | 57.9 (33.5–79.7) | 47.6 (25.7–70.2) | 0.545, 1.51 (0.43–5.28) |
| Insomnia chronicity | ||||
| Duration (years) | 9.80 (7.48–12.13) | 11.23 (8.12–14.34) | 8.52 (4.93–12.10) | 0.244, 0.37 |
t-test confidence interval for continuous variables.
p-value is Fisher's exact t-test for dichotomous variables, t-test for continuous variables.
Odds ratio (95% confidence interval) calculated for dichotomous variables; Hedges' g calculated for continuous variables.
Table 2a.
Comparison of baseline characteristics for subjective/objective primary and secondary measures, % or mean (95% confidence interval)a, by total randomized sample (n = 61).
| Total (n = 61) |
ASV (n = 32) |
CPAP (n = 29) |
p-value, g | Completers (n = 40) |
Non-completers (n = 21) |
p-value, g | |
|---|---|---|---|---|---|---|---|
| Primary measures | |||||||
| ISI | 19.97 (19.08–20.85) | 19.91 (18.63–21.19) | 20.03 (18.73–21.34) | .887, 0.03 | 19.30 (18.40–20.20) | 21.24 (19.31–23.17) | .069, 0.57 |
| SQ-Lik | 2.98 (2.73–3.22) | 3.05 (2.70–3.41) | 2.90 (2.54–3.25) | .532, 0.15 | 3.08 (2.76–3.40) | 2.78 (2.39–3.16) | .218, 0.031 |
| SQ-Pct (%) | 51.20 (47.20–55.20) | 52.84 (48.02–57.66) | 49.40 (42.61–56.18) | .394, 0.22 | 52.01 (47.16–56.86) | 49.66 (42.04–57.28) | .594, 0.15 |
| Secondary measures | |||||||
| Questionnaires | |||||||
| ESS | 5.64 (4.85–6.43) | 6.31 (5.43–7.19) | 4.90 (3.54–6.25) | .073, 0.46 | 5.70 (4.73–6.67) | 5.52 (4.05–7.00) | .838, 0.06 |
| III | 21.13 (18.82–23.45) | 21.91 (18.42–25.39) | 20.28 (17.09–23.47) | .486, 0.18 | 19.20 (16.87–21.53) | 24.81 (19.83–29.79) | .043, 0.64 |
| FSS | 41.39 (37.87–44.92) | 43.88 (38.99–48.76) | 38.66 (33.43–43.88) | .141, 0.38 | 40.55 (36.44–44.66) | 43.00 (35.90–50.10) | .540, 0.17 |
| FOSQ-10 | 13.99 (13.12–14.87) | 13.60 (12.30–14.89) | 14.43 (13.21–15.65) | .344, 0.24 | 14.91 (14.08–15.74) | 12.24 (10.37–14.12) | .011, 0.83 |
| QLESQ (%) | 58.26 (53.66–62.85) | 57.70 (51.34–64.06) | 58.87 (51.82–65.91) | .802, 0.06 | 60.22 (54.36–66.09) | 54.51 (46.81–62.20) | .230, 0.32 |
| Sleep diary | |||||||
| SOL | 58.86 (46.11–71.60) | 62.83 (45.68–79.99) | 54.47 (34.49–74.45) | .517, 0.17 | 58.49 (40.70–76.28) | 59.56 (42.57–76.55) | .929, 0.02 |
| WASO | 74.08 (59.44–88.71) | 61.82 (44.93–78.70) | 87.60 (62.94–112.27) | .078, 0.45 | 79.33 (59.82–98.83) | 64.08 (41.89–86.26) | .293, 0.26 |
| SE (%) | 73.55 (69.85–77.25) | 75.07 (70.00–80.14) | 71.87 (66.19–77.55) | .392, 0.22 | 73.95 (69.34–78.56) | 72.77 (66.03–79.52) | .767, 0.08 |
| PSG sleep indices | |||||||
| SOL | 28.19 (20.22–36.17) | 28.10 (17.27–38.94) | 28.29 (15.81–70.78) | .981, 0.01 | 25.32 (15.33–35.32) | 33.66 (19.59–47.73) | .324, 0.26 |
| WASO | 65.98 (52.57–79.39) | 65.62 (49.84–81.40) | 66.38 (43.08–89.68) | .956, 0.01 | 71.79 (54.38–89.19) | 54.92 (33.33–76.52) | .217, 0.32 |
| SE (%) | 77.18 (73.28–81.07) | 76.98 (72.58–81.37) | 77.39 (70.47–84.31) | .916, 0.03 | 76.48 (71.06–81.91) | 78.50 (73.31–83.68) | .584, 0.13 |
| N1 (%) | 6.85 (4.96–8.74) | 5.11 (3.19–7.03) | 8.76 (5.40–12.12) | .053, 0.50 | 6.26 (4.14–8.38) | 7.96 (4.01–11.92) | .438, 0.23 |
| N2 (%) | 58.40 (55.19–61.60) | 55.60 (51.54–59.67) | 61.48 (56.44–66.52) | .066, 0.47 | 61.05 (57.03–65.06) | 53.35 (48.33–58.37) | .017, 0.63 |
| N3 (%) | 16.32 (13.54–19.11) | 19.33 (15.27–23.39) | 13.00 (9.37–16.64) | .022, 0.60 | 14.24 (11.05–17.42) | 20.30 (14.99–25.61) | .050, 0.57 |
| REM (%) | 18.44 (16.33–20.54) | 19.95 (17.27–22.63) | 16.77 (13.40–20.13) | .132, 0.39 | 18.46 (15.73–21.19) | 18.40 (14.84–21.95) | .977, 0.01 |
| REM (min) | 62.13 (53.31–70.95) | 64.42 (52.86–75.98) | 59.59 (45.41–73.77) | .589, 0.14 | 62.39 (50.84–73.94) | 61.62 (47.13–76.11) | .932, 0.02 |
| PSG breathing indices | |||||||
| AHI | 15.88 (11.33–20.43) | 13.95 (8.60–19.31) | 18.01 (10.19–25.83) | .378, 0.23 | 19.16 (13.52–24.80) | 9.64 (2.04–17.24) | .044, 0.54 |
| CAI | 0.33 (0.18–0.48) | 0.35 (0.17–0.53) | 0.31 (0.04–0.58) | .784, 0.06 | 0.13 (0.02–0.24) | 0.71 (0.35–1.07) | .004, 1.05 |
| RDI | 36.51 (31.43–41.58) | 33.13 (25.62–40.64) | 40.23 (33.30–47.17) | .164, 0.36 | 34.05 (29.26–38.84) | 41.18 (29.10–53.27) | .265, 0.36 |
Abbreviations: ISI — Insomnia Severity Index; SQ-Lik — Sleep quality Likert scale; SQ-Pct — Sleep quality percent scale; ESS — Epworth Sleepiness Scale; III — Insomnia Impairment Index; FSS — Fatigue Severity Scale; FOSQ-10 — Functional Outcomes of Sleep Questionnaire Short Form; QLESQ — Quality of Life Enjoyment and Satisfaction Questionnaire Summary; SOL — sleep onset latency; WASO — wake after sleep onset; SE — sleep efficiency; N1 — non-REM stage 1; N2 — non-REM stage 2; N3 — non-REM stage 3; REM — rapid eye movement sleep; AHI — Apnea Hypopnea Index; RDI — Respiratory Disturbance Index.
t-test confidence interval for continuous variables.
Table 2b.
Comparison of baseline characteristics for subjective/objective primary and secondary measures, % or mean (95% confidence interval)a, by completers (n = 40).
| Completers (n = 40) |
Intake CPAP (n = 21) |
Intake ASV (n = 19) |
p-value, g | |
|---|---|---|---|---|
| Primary measures | ||||
| ISI | 19.30 (18.40–20.20) | 19.48 (18.30–20.65) | 19.11 (17.61–20.60) | 0.683, 0.13 |
| SQ-Lik | 3.08 (2.76–3.40) | 2.95 (2.53–3.38) | 3.23 (2.70–3.75) | 0.395, 0.27 |
| SQ-Pct (%) | 52.01 (47.16–56.86) | 49.47 (42.53–56.41) | 54.82 (47.62–62.02) | 0.270, 0.35 |
| Secondary measures | ||||
| Questionnaires | ||||
| ESS | 5.70 (4.73–6.67) | 5.52 (3.89–7.16) | 5.89 (4.74–7.05) | 0.706, 0.12 |
| III | 19.20 (16.87–21.53) | 18.00 (15.23–20.77) | 20.53 (16.47–24.58) | 0.280, 0.34 |
| FSS | 40.55 (36.44–44.66) | 36.81 (30.47–43.15) | 44.68 (39.67–49.70) | 0.052, 0.62 |
| FOSQ-10 | 14.91 (14.08–15.74) | 15.12 (14.08–16.17) | 14.68 (13.27–16.09) | 0.597, 0.16 |
| QLESQ (%) | 60.22 (54.36–66.09) | 61.56 (53.58–69.55) | 58.74 (49.32–68.16) | 0.633, 0.15 |
| Sleep diary | ||||
| SOL | 58.49 (40.70–76.28) | 57.40 (29.91–84.88) | 59.69 (34.88–84.51) | 0.898, 0.04 |
| WASO | 79.33 (59.82–98.83) | 90.57 (59.83–121.30) | 66.90 (41.92–91.88) | 0.225, 0.38 |
| SE (%) | 73.95 (69.34–78.56) | 71.87 (65.48–78.25) | 76.26 (69.10–83.43) | 0.342, 0.30 |
| PSG sleep indices | ||||
| SOL | 25.32 (15.33–35.32) | 26.76 (11.77–41.75) | 23.73 (9.22–38.25) | 0.764, 0.09 |
| WASO | 71.79 (54.38–89.19) | 68.71 (40.67–96.75) | 75.18 (52.68–97.68) | 0.712, 0.12 |
| SE (%) | 76.48 (71.06–81.91) | 77.50 (68.69–86.31) | 75.36 (68.45–82.26) | 0.695, 0.12 |
| N1 (%) | 6.26 (4.14–8.38) | 7.47 (4.18–10.76) | 4.92 (2.11–7.73) | 0.230, 0.38 |
| N2 (%) | 61.05 (57.03–65.06) | 62.28 (55.70–68.85) | 59.68 (54.74–64.62) | 0.521, 0.20 |
| N3 (%) | 14.24 (11.05–17.42) | 13.19 (8.70–17.67) | 15.39 (10.47–20.32) | 0.491, 0.22 |
| REM (%) | 18.46 (15.73–21.19) | 17.07 (13.04–21.10) | 19.99 (16.07–23.90) | 0.286, 0.34 |
| REM (min) | 62.39 (50.84–73.94) | 60.89 (43.58–78.21) | 64.05 (47.27–80.84) | 0.786, 0.08 |
| PSG breathing indices | ||||
| AHI | 19.16 (13.52–24.80) | 22.83 (12.64–33.01) | 15.11 (10.69–19.52) | 0.169, 0.43b |
| CAI | 0.13 (0.02–0.24) | 0.28 (0.10–0.30)c | 0.83 (0.10–1.70)c | 0.281, 0.36 |
| RDI | 34.05 (29.26–38.84) | 38.79 (30.56–47.02) | 28.81 (24.88–32.75) | 0.033, 0.69b |
Abbreviations: ISI — Insomnia Severity Index; SQ-Lik — Sleep quality Likert scale; SQ-Pct — Sleep quality percent scale; ESS — Epworth Sleepiness Scale; III — Insomnia Impairment Index; FSS — Fatigue Severity Scale; FOSQ-10 — Functional Outcomes of Sleep Questionnaire Short Form; QLESQ — Quality of Life Enjoyment and Satisfaction Questionnaire Summary; SOL — sleep onset latency; WASO — wake after sleep onset; SE — sleep efficiency; N1 — non-REM stage 1; N2 — non-REM stage 2; N3 — non-REM stage 3; REM — rapid eye movement sleep; AHI — Apnea Hypopnea Index; RDI — Respiratory Disturbance Index.
t-test confidence interval for continuous variables.
The statistical significance and effect sizes for both AHI and RDI are not clinically relevant because in both instances they reflect patients with similar clinical severity levels of sleep-disordered breathing.
Due to high prevalence of “0s” in data, Exact Wilcoxon Sign Ranked Test was used for pseudo median and confidence intervals.
To describe a clinical perspective on the overall changes in outcomes as well as to calculate pre- and post-treatment effect sizes (Hedges' g) for all primary and secondary variables and for objective and subjective sleep measures, additional analysis included the following: between group comparisons at baseline using 2-sample t-tests; within-group comparisons of change from baseline to end of study using paired t-tests; and, comparison of change scores from baseline to end of study using 2-sample t-tests. Last, due to small sample sizes, Fisher's exact test was used for dichotomous variables of changes in insomnia disorder status (remission rates) and changes in flattening index severity (ODD flow limitation respiratory patterns).
All primary and secondary variables as well as objective and subjective sleep variable data are presented as means with 95% confidence intervals. Assumptions were checked using standard diagnostics. To correct for severe non-normal distributions, exact Wilcoxon signed-rank tests with Hodges-Lehmann estimates and confidence intervals were used as appropriate [69], [70].
3. Results
3.1. Sociodemographics and Baseline Characteristics
Among 61 eligible participants, there were 32 randomized to ASV and 29 randomized to CPAP with no systematic statistical differences between the two groups for sociodemographic variables (Table 1a, Table 1b) or baseline primary and secondary measures or baseline objective and subjective sleep measures (Table 2a, Table 2b). When comparing the 40 who completed the study and the 21 participants who did not, the 21 non-completers were noticeably younger with less severe OSA without other systematic differences between groups (Table 1a, Table 2a). There were also no sociodemographic differences based on PAP mode among the completers of the study (Table 1b); these 40 individuals were predominately middle-aged [mean (95% CI), 48.38 (43.66–53.11)], married (52.5%), Caucasian (75.0%) or Hispanic (17.5%), females (65.0%) with normal BMI [24.97 (23.82–26.13)] and a college degree (55.0%) (Table 1b).
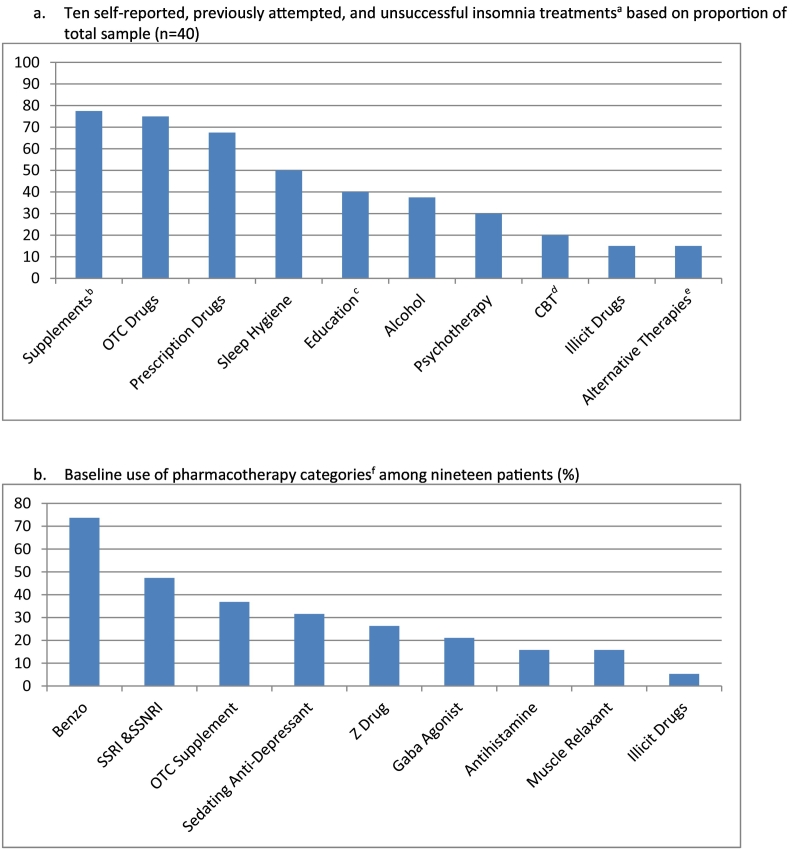
The final sample of 40 completers suffered moderately severe insomnia [ISI 19.30 (18.40–20.20)] (Table 2a, Table 2b). Average intake subjective sleep indices corroborated one-week pre-treatment sleep-log values (SOL ~ 62 min, WASO ~ 90 min, SE% ~ 72%), consistent with moderately severe insomnia. Sleep quality ratings ranged fair to poor. No statistically significant differences by PAP mode were noted in baseline outcomes, sleep indices, or diagnostic breathing event indexes (Table 2b). Patients suffered from insomnia for an average of 10-years [9.80 (95% CI 7.56–12.1); range: 0.5–20.9 years] and reported multiple failed attempts [mean 4.48 (3.61–5.34)] at insomnia treatment, comprising ten different approaches (Fig. 3a). Among the category of failed sleep aids, nine different drug types were attempted (Fig. 3b). No patient had been offered CBT-I. Diagnoses included 90.0% OSA and 10.0% UARS. Enrollment dates: February 2015 to August 2018.
Fig. 3.
Categories of failed a) all insomnia treatments and b) pharmacologic insomnia treatments at intake.
Abbreviations: OTC — over the counter; CBT — cognitive-behavioral therapy; SSRI — selective serotonin reuptake inhibitor; SSNRI — selective serotonin and norepinephrine reuptake inhibitor.
Footnote:
aPatients suffered from insomnia for an average of 10 years and reported multiple attempts at insomnia treatment [mean 4.48 (3.61–5.34)].
bOTC supplements and herbal remedies.
cEducation from books, articles, magazines, etc.
dCBT does not refer to CBT-I. No patient was introduced to or attempted CBT-I.
eMeditation, acupuncture, other alternative therapies.
fBenzo: lorazepam, alprazolam, clonazepam, triazolam, temazepam; SSRI & SSNRI: sertraline, escitalopram, fluoxetine, duloxetine, paroxetine; OTC supplement: melatonin, 5-htp; Sedating anti-depressant: mirtazapine, doxepin, trazodone; Z drug: eszopiclone, zolpidem; Gaba agonist: gabapentin; Antihistamine: diphenhydramine, chlorpheniramine, doxylamine; Muscle relaxant: baclofen, chlorzoxazone; Illicit drugs: tetrahydrocannabinol (THC).
All subsequent analysis includes the 40 completers; afterwards, intent-to-treat (ITT) analysis includes 61 total completers and non-completers [52], [53], [54].
3.2. Primary Outcomes: Insomnia Severity and Sleep Quality Ratings
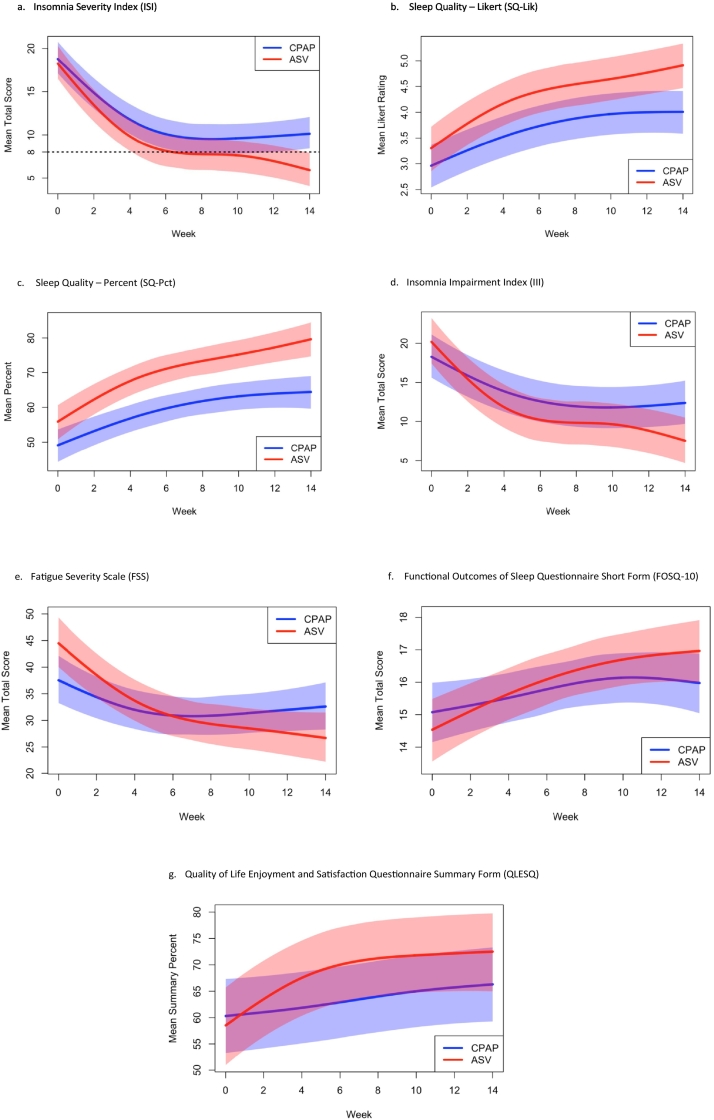
ISI decreased significantly in the entire sample [11.15 (9.17–13.13)]. Between groups, ASV proved superior to CPAP [Wald χ2 (4 df) = 11.50, p = 0.021] as estimated mean ISI for ASV was consistently lower than CPAP (Fig. 4a), with statistically significant separation between modes by week 11. Within-group effect sizes for decrease from baseline to final follow-up were markedly different [ASV, 13.21 (10.72–15.70), g = 2.50; CPAP, 9.29 (6.29–12.28), g = 1.39], leading to exit ISI scores averaging subthreshold insomnia diagnostic levels for ASV [5.89 (4.14–7.65)] compared to average persisting borderline insomnia for CPAP [10.19 (7.61–12.77)]. The between group effect size for difference in change scores was in the medium-large range (g = 0.65). Clinically, 68% of ASV users no longer met the validated scale criteria for an insomnia disorder (ISI < 8), whereas only 24% of CPAP users attained this remission status, Fisher's exact p = 0.010.
Fig. 4.
Improvement in (a) insomnia, (b, c) sleep quality, (d–f) daytime impairment, and (g) quality of life over 14 weeks of therapy by mode.
a. Insomnia Severity Index (ISI)
b. Sleep quality – Likert (SQ-Lik)
c. Sleep quality – percent (SQ-Pct)
d. Insomnia Impairment Index (III)
e. Fatigue Severity Scale (FSS)
f. Functional Outcomes of Sleep Questionnaire Short Form (FOSQ-10)
g. Quality of Life Enjoyment and Satisfaction Questionnaire Summary Form (QLESQ)
Estimated trajectories for primary outcomes of each mode are shown along with 95% confidence intervals for average scores at each time point. Overlap in the band indicates intersection of confidence intervals. (a) Average ISI scores for ASV patients drop below clinical threshold for insomnia (ISI = 8) by week 7 and statistical separation between modes occurred at week 11. (b) Mean SQ-Lik scores were consistently higher for ASV shortly after baseline, with statistically significant separation by the third week. (c) Mean SQ-Pct scores were consistently higher for ASV reaching nearly 80% at final follow-up with significant separation between the modes by the second week. (d) III mirrored the changes in ISI but significant separation between the modes did not occur until week 13. (e–g) No statistical separation between modes occurred, although there was a trend for between mode separation in QLESQ scores.
Marked improvement in scores were evident in both sleep quality scales [improvement: SQ-Lik 1.34 (0.99–1.69) and SQ-Pct 19.63 (14.04–25.23)]. Between groups analysis showed superiority of ASV over CPAP for changes in SQ-Lik [Wald χ2 (4 df) = 11.42, p = 0.022] (Fig. 4b) and SQ-Pct [Wald χ2 (4 df) = 50.61, p < 0.0001] (Fig. 4c), as estimated mean ASV values were consistently higher, with statistically significant separation by the third and second weeks respectively (or two months earlier than separation for insomnia). Within groups, ASV yielded very large effects in improvement from baseline to final follow-up [SQ-Lik 1.65 (1.10–2.19), g = 1.42 and SQ-Pct 24.33% (17.99–30.66), g = 1.81] compared to large effects for CPAP [1.06 (0.61–1.52), g = 1.04 and 15.39% (6.27–24.51), g = 0.75]. The between group effect sizes for difference in change were SQ-Lik = 0.54 and SQ-Pct = 0.51. As a clinical distinction to gauge changes in sleep quality, ASV patients at exit averaged “Good” sleep quality compared to “Adequate” for CPAP patients, consistent with differences in endpoint scores [4.87 (4.44–5.30) vs 4.01 (3.52–4.51) and 79.14% (74.94–83.35) vs 64.86% (57.08–72.64) respectively].
There were no significant differences in primary outcome change scores within PAP mode between those using prescription sleep aids and those not using such aids (Supplement).
3.3. Secondary Outcomes: Impairment and Quality of Life
III scores decreased for the entire sample [mean change 9.28 (6.61–11.94)]. Between groups analysis revealed ASV superiority to CPAP [Wald χ2 (4 df) = 14.87, p = 0.005] (Fig. 4d) with statistically significant separation between modes by week 13; this change occurred 2 weeks after the separation in ISI scores attained significance. Although III showed a large effect for improvement within subjects for CPAP [6.00 (3.07–8.93), g = 0.91], the ASV effect was larger [12.89 (8.63–17.16), g = 1.43]. The between group effect size for difference in change was large (g = 0.88).
FSS and FOSQ-10 scores improved significantly for the entire sample [FSS 11.13 (7.09–15.16); FOSQ-10 1.63 (0.74–2.52)], but there were no significant differences between modes for FSS [Wald χ2 (4 df) = 7.08, p = 0.132] (Fig. 4e) or FOSQ-10 scores [Wald χ2 (4 df) = 3.06, p = 0.548] (Fig. 4f). Of potential clinical import, albeit speculatively, when comparing improvement within each mode, ASV demonstrated greater observed effects to CPAP (large vs small to medium-sized) for FSS [ASV 18.11 (13.10–23.11), g = 1.71; CPAP 4.81 (− 0.27 to 9.89), g = 0.42] and for FOSQ-10 [ASV 2.44 (1.08–3.80), g = 0.85; CPAP 0.90 (− 0.30 to 2.10), g = 0.34]. The between group effect sizes for difference in change were large (g = 1.21 and g = 0.72 respectively). Baseline imbalances may be an alternate explanation for these findings.
Quality of life scores as measured by the QLESQ significantly increased by nearly 10% for the entire sample [mean 9.33 (4.46–14.20)]. A trend was noted for ASV compared to CPAP for improvement in QLESQ scores [Wald χ2 (4 df) = 8.64, p = 0.071] (Fig. 4g). Within-subjects effect sizes for both groups from baseline to final follow-up were different with ASV resulting in large effects and CPAP small to medium effects [ASV 14.00 (6.94–21.07), g = 0.94; CPAP 5.10 (− 1.65 to 11.85), g = 0.34]. Between group effect size for difference in change was large (g = 0.70).
3.4. Intent-to-Treat Analyses
Intent-to-treat analysis included the 40 completers and the additional 21 non-completers (13 ASV, 8 CPAP). At baseline (Table 2a), there were no differences in primary variables (insomnia and sleep quality) between the two groups aside from the 21 non-completers who were noticeably younger with less severe OSA as previously mentioned. Non-completer follow-up data were mostly missing at most time points due to very early to early withdrawal from the protocol (see Fig. 1 for explanations). The ITT patients (61 total) were analyzed for the 7 main primary and secondary scales described above. A sensitivity analysis assessed whether results changed with inclusion of the 21 additional patients. LME methods were repeated by adding the sparse data from the 21 withdrawn patients; there was no attempt to impute missing values, because none of the non-completers had appreciable time in treatment. Neither statistical significance nor direction of effect changed, and qualitative behavior as seen in Fig. 4a–g was essentially unchanged.
3.5. Objective Respiratory Event Indexes and Data Downloads
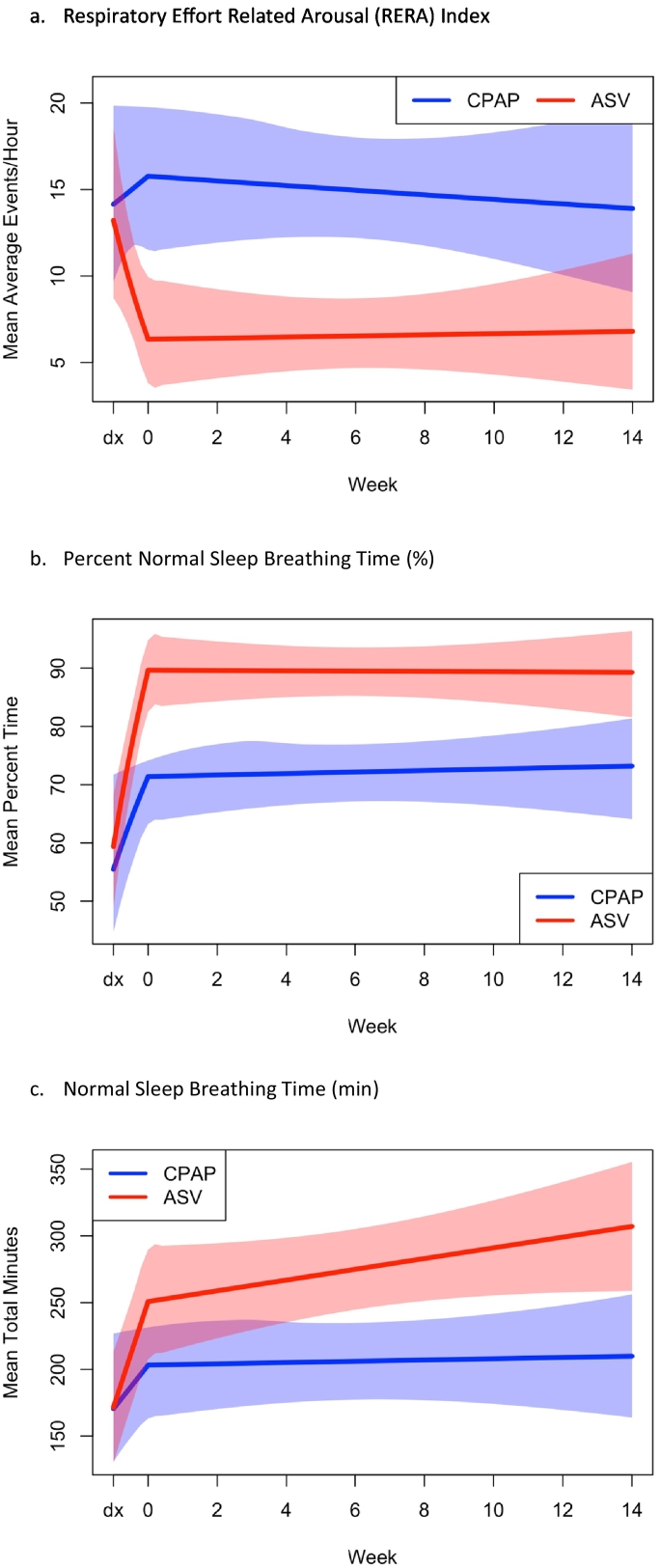
Objective respiratory data by mode is available in Table 3a, Table 3b, Table 3c. On the final titration and corroborated on final ODD, AHI residual breathing events were similar between groups [AHI-titration: ASV 0.53 (0.20–9.30) vs CPAP 2.25 (0.60–3.65); AHI-ODD: ASV 0.57 (0.22–0.93) vs CPAP 1.60 (0.33–2.88)]. In contrast, final titration RERAs per hour were significantly fewer for ASV compared to CPAP [7.71 (4.24–11.18) vs 17.32 (10.41–24.24), p = 0.016, g = 0.79]. Moreover the ANCOVA analysis for all time points in the study demonstrated a significantly greater reduction of RERAs in the ASV group compared to CPAP (Wald χ2 (3df) = 22.5, p < 0.0001, Fig. 5a). The within-group changes for RERAs were (ASV: 5.99 (1.95–10.03), p < 0.0001, g = 0.70; CPAP: − 1.36 (− 8.37 to 11.08) p = 0.774, g = 0.06); between group differences were non-significant albeit medium effect sizes were observed [7.35 (− 3.01 to 17.70); p = 0.16, g = 0.44]. This finding was somewhat corroborated using final ODD flattening frequency (ODD surrogate for RERAS), which revealed a trend (Wald χ2 = 3.879, p = 0.062) for the comparison between ASV and CPAP [mild/moderate residual flattening: 74% ASV, 43% CPAP vs. moderate/severe residual flattening: 26% ASV, 57% CPAP].
Table 3a.
Completer intake, final outcome, and change scores for subjective/objective primary and secondary measures, mean (95% confidence interval)a, for CPAP.
| Intake CPAP (n = 21) |
Final/exit CPAP (n = 21) |
Change CPAP (n = 21)b |
Within-subjects CPAP; p, g | |
|---|---|---|---|---|
| Primary measures | ||||
| ISI | 19.48 (18.30–20.65) | 10.19 (7.61–12.77) | 9.29 (6.29–12.28) | < 0.0001, 1.39 |
| SQ-Lik | 2.95 (2.53–3.38) | 4.01 (3.52–4.51) | 1.06 (0.61–1.52) | < 0.0001, 1.04 |
| SQ-Pct (%) | 49.47 (42.53–56.41) | 64.86 (57.08–72.64) | 15.39 (6.27–24.51) | 0.002, 0.75 |
| Secondary measures | ||||
| Questionnaires | ||||
| ESS | 5.52 (3.89–7.16) | 4.86 (3.42–6.30) | 0.67 (− 0.69 to 2.02) | 0.316, 0.22 |
| III | 18.00 (15.23–20.77) | 12.00 (8.57–15.43) | 6.00 (3.07–8.93) | 0.0003, 0.91 |
| FSS | 36.81 (30.47–43.15) | 32.00 (25.79–38.21) | 4.81 (− 0.27 to 9.89) | 0.062, 0.42 |
| FOSQ-10 | 15.12 (14.08–16.17) | 16.02 (14.79–17.26) | 0.90 (− 0.30 to 2.10) | 0.132, 0.34 |
| QLESQ (%) | 61.56 (53.58–69.55) | 66.67 (58.81–74.53) | 5.10 (− 1.65 to 11.85) | 0.131, 0.34 |
| Sleep diary | ||||
| SOL | 57.40 (29.91–84.88) | 31.06 (11.37–50.75) | 26.33 (14.30–38.36) | 0.0002, 0.98 |
| WASO | 90.57 (59.83–121.30) | 40.78 (25.23–56.32) | 49.79 (18.50–81.08) | 0.0034, 0.71 |
| SE (%) | 71.87 (65.48–78.25) | 84.02 (79.86–88.17) | 12.15 (5.83–18.47) | 0.0007, 0.86 |
| PSG sleep indices | ||||
| SOL | 26.76 (11.77–41.75) | 32.85 (10.82–54.87) | 6.09 (− 26.29 to 14.12)‡ | 0.537, 0.13 |
| WASO | 68.71 (40.67–96.75) | 62.99 (41.29–84.68) | 5.72 (− 21.01 to 32.46) | 0.660, 0.10 |
| SE (%) | 77.50 (68.69–86.31) | 76.75 (67.98–85.52) | 0.75 (− 8.34 to 6.84) ‡ | 0.838, 0.04 |
| N1 (%) | 7.47 (4.18–10.76) | 6.17 (3.69–8.64) | 1.31 (− 2.25 to 4.86) | 0.453, 0.16 |
| N2 (%) | 62.28 (55.70–68.85) | 57.08 (51.96–62.20) | 5.20 (− 1.94 to 12.33) | 0.144, 0.33 |
| N3 (%) | 13.19 (8.70–17.67) | 20.73 (16.35–25.10) | 7.54 (1.99–13.10) | 0.010, 0.61 |
| REM (%) | 17.07 (13.04–21.10) | 16.03 (12.63–19.44) | 1.04 (− 2.22 to 4.30) ‡ | 0.514, 0.14 |
| REM (min) | 60.89 (43.58–78.21) | 53.76 (39.80–67.71) | 7.13 (− 6.70 to 20.96) ‡ | 0.295, 0.23 |
| REMCI | 12.28 (8.10–16.46) | 12.33 (9.44–15.21) | 0.05 (− 4.46 to 4.60) | 0.981, 0.01 |
| PSG breathing indices | ||||
| AHI | 22.83 (12.64–33.01) | 2.25 (0.60–3.65)c | 19.48 (9.66–29.30) | 0.0005, 0.89 |
| CAI | 0.28 (0.10–0.30)c | 0.40 (0.10–0.70)c | 0.10 (− 0.25 to 0.30)c | < 0.0001, 0.19c |
| RDI | 38.79 (30.56–47.02) | 20.67 (12.63–28.70) | 18.12 (10.33–25.92) | < 0.0001, 1.04 |
| RERAI | 15.97 (10.88–21.05) | 17.32 (10.41–24.24) | 1.36 (− 8.37 to 11.08) ‡ | 0.774, 0.06 |
| NSBT (%) | 50.26 (39.97–60.54) | 68.11 (56.76–79.47) | 17.86 (4.45–31.26) | 0.012, 0.59 |
| NSBT (min) | 170.15 (125.74–214.55) | 208.51 (162.83–254.20) | 38.36 (3.32–80.05) | 0.069, 0.41 |
Abbreviations: ASV — adaptive servo-ventilation; CPAP — continuous positive airway pressure; ISI — Insomnia Severity Index; SQ-Lik — Sleep quality Likert scale; SQ-Pct — Sleep quality percent scale; ESS — Epworth Sleepiness Scale; III — Insomnia Impairment Index; FSS — Fatigue Severity Scale; FOSQ-10 — Functional Outcomes of Sleep Questionnaire Short Form; QLESQ — Quality of Life Enjoyment and Satisfaction Questionnaire Summary; SOL — sleep onset latency; WASO — wake after sleep onset; SE — sleep efficiency; N1 — non-REM stage 1; N2 — non-REM stage 2; N3 — non-REM stage 3; REM — rapid eye movement sleep; REMCI — REM consolidation index; AHI — Apnea Hypopnea Index; RDI — Respiratory Disturbance Index; RERAI — Respiratory Effort-Related Arousal event Index; NSBT — normal sleep breathing time.
t-test confidence interval.
All changes are in expected direction (improvement) except those identified by ‡.
Due to high prevalence of “0s” in data, Exact Wilcoxon Sign Ranked Test.
Table 3b.
Completer intake, final outcome, and change scores for subjective/objective primary and secondary measures, mean (95% confidence interval)a, for ASV.
| Intake ASV (n = 19) |
Final/exit ASV (n = 19) |
Change ASV (n = 19)b |
Within-subjects ASV; p, g | |
|---|---|---|---|---|
| Primary measures | ||||
| ISI | 19.11 (17.61–20.60) | 5.89 (4.14–7.65) | 13.21 (10.72–15.70) | < 0.0001, 2.50 |
| SQ-Lik | 3.23 (2.70–3.75) | 4.87 (4.44–5.30) | 1.65 (1.10–2.19) | < 0.0001, 1.42 |
| SQ-Pct (%) | 54.82 (47.62–62.02) | 79.14 (74.94–83.35) | 24.33 (17.99–30.66) | < 0.0001, 1.81 |
| Secondary measures | ||||
| Questionnaires | ||||
| ESS | 5.89 (4.74–7.05) | 4.74 (3.03–6.44) | 1.16 (− 0.65 to 2.97) | 0.196, 0.30 |
| III | 20.53 (16.47–24.58) | 7.63 (4.81–10.45) | 12.89 (8.63–17.16) | < 0.0001, 1.43 |
| FSS | 44.68 (39.67–49.70) | 26.58 (19.90–33.26) | 18.11 (13.10–23.11) | < 0.0001, 1.71 |
| FOSQ-10 | 14.68 (13.27–16.09) | 17.12 (15.81–18.43) | 2.44 (1.08–3.80) | 0.0014, 0.85 |
| QLESQ (%) | 58.74 (49.32–68.16) | 72.74 (65.09–80.40) | 14.00 (6.94–21.07) | 0.0006, 0.94 |
| Sleep diary | ||||
| SOL | 59.69 (34.88–84.51) | 33.94 (18.72–49.16) | 25.75 (12.67–38.83) | 0.0006, 0.93 |
| WASO | 66.90 (41.92–91.88) | 32.00 (19.31–44.68) | 34.90 (11.00–58.81) | 0.0066, 0.69 |
| SE (%) | 76.26 (69.10–83.43) | 84.73 (80.03–89.43) | 8.47 (3.36–13.57) | 0.0026, 0.78 |
| PSG sleep indices | ||||
| SOL | 23.73 (9.22–38.25) | 16.56 (8.25–24.86) | 7.17 (− 5.79 to 20.14) | 0.260, 0.26 |
| WASO | 75.18 (52.68–97.68) | 75.31 (46.10–104.51) | 0.12 (− 29.95 to 29.70) ‡ | 0.993, 0.00 |
| SE (%) | 75.36 (68.45–82.26) | 78.21 (70.72–85.70) | 2.85 (− 4.88 to 10.59) | 0.448, 0.17 |
| N1 (%) | 4.92 (2.11–7.73) | 3.57 (2.31–4.82) | 1.35 (− 0.77 to 3.47) | 0.197, 0.30 |
| N2 (%) | 59.68 (54.74–64.62) | 55.24 (46.40–64.09) | 4.44 (− 3.95 to 12.83) | 0.281, 0.25 |
| N3 (%) | 15.39 (10.47–20.32) | 21.30 (14.36–28.24) | 5.91 (5.15–12.33) | 0.069, 0.43 |
| REM (%) | 19.99 (16.07–23.90) | 19.90 (16.19–23.61) | 0.09 (− 4.29 to 4.47) ‡ | 0.966, 0.01 |
| REM (min) | 64.05 (47.27–80.84) | 67.13 (50.36–83.90) | 3.08 (− 14.26 to 20.41) | 0.713, 0.08 |
| REMCI | 17.64 (12.31–22.98) | 18.17 (13.25–23.10) | 0.53 (− 6.96 to 8.03) | 0.883, 0.03 |
| PSG breathing indices | ||||
| AHI | 15.11 (10.69–19.52) | 0.53 (0.20–9.30)c | 14.01 (9.20–18.82) | < 0.0001, 1.37 |
| CAI | 0.83 (0.10–1.70)c | 0d | 0.83 (0.1–1.70)c | 0.063c,e |
| RDI | 28.81 (24.88–32.75) | 8.81 (4.88–12.73) | 20.01 (14.46–25.55) | < 0.0001, 1.70 |
| RERAI | 13.70 (10.93–16.47) | 7.71 (4.24–11.18) | 5.99 (1.95–10.03) | < 0.0001, 0.70 |
| NSBT (%) | 56.30 (46.38–66.21) | 88.89 (83.08–94.70) | 32.60 (20.67–44.52) | < 0.0001, 1.29 |
| NSBT (min) | 175.38 (136.51–214.24) | 303.48 (259.56–347.40) | 128.11 (81.68–174.54) | < 0.0001, 1.30 |
Abbreviations: ASV — adaptive servo-ventilation; CPAP — continuous positive airway pressure; ISI — Insomnia Severity Index; SQ-Lik — Sleep quality Likert scale; SQ-Pct — Sleep quality percent scale; ESS — Epworth Sleepiness Scale; III — Insomnia Impairment Index; FSS — Fatigue Severity Scale; FOSQ-10 — Functional Outcomes of Sleep Questionnaire Short Form; QLESQ — Quality of Life Enjoyment and Satisfaction Questionnaire Summary; SOL — sleep onset latency; WASO — wake after sleep onset; SE — sleep efficiency; N1 — non-REM stage 1; N2 — non-REM stage 2; N3 — non-REM stage 3; REM — rapid eye movement sleep; REMCI — REM consolidation index; AHI — Apnea Hypopnea Index; RDI — Respiratory Disturbance Index; RERAI — Respiratory Effort-Related Arousal event Index; NSBT — normal sleep breathing time.
t-test confidence interval.
All changes are in expected direction (improvement) except those identified by ‡.
Due to high prevalence of “0s” in data, Exact Wilcoxon Sign Ranked Test.
All values were "0".
No g value reported due to high incidence of “0s” in data.
Table 3c.
Completer intake, final outcome, and change scores for subjective/objective primary and secondary measures, mean (95% confidence interval)a, for between mode (CPAP and ASV) comparison.
.
| Change between subjectsb | Between subjects (p-value, g) | |
|---|---|---|
| Primary measures | ||
| ISI | − 3.92 (− 7.70 to − 0.15) | 0.042, 0.65 |
| SQ-Lik | 0.58 (− 0.10 to 1.27) | 0.094, 0.54 |
| SQ-Pct (%) | 8.94 (− 1.85 to 19.72) | 0.101, 0.51 |
| Secondary measures | ||
| Questionnaires | ||
| ESS | − 0.49 (− 2.68 to 1.70) | 0.652, 0.14 |
| III | − 6.89 (− 11.92 to − 1.87) | 0.009, 0.88 |
| FSS | − 13.30 (− 20.20 to − 6.39) | 0.0004, 1.21 |
| FOSQ-10 | 1.67 (0.13–3.21)c | 0.030, 0.72c |
| QLESQ (%) | 10.71 (0.00–21.43)c | 0.034, 0.70c |
| Sleep diary | ||
| SOL | 0.58 (− 16.60 to 17.77)† | 0.946, 0.02 |
| WASO | 14.89 (− 23.29 to 53.06) | 0.434, 0.24 |
| SE (%) | 3.68 (− 11.55 to 4.19)† | 0.349, 0.29 |
| PSG sleep indices | ||
| SOL | 13.26 (− 36.62 to 10.10) | 0.257, 0.35 |
| WASO | 5.84 (− 32.90 to 44.59)† | 0.762, 0.10 |
| SE (%) | 3.61 (− 6.88 to 14.09) | 0.490, 0.22 |
| N1 (%) | 0.05 (− 4.08 to 3.99) | 0.981, 0.01 |
| N2 (%) | 0.75 (− 9.91 to 11.41)† | 0.887, 0.04 |
| N3 (%) | 1.64 (− 9.85 to 6.58)† | 0.689, 0.13 |
| REM (%) | 0.95 (− 4.34 to 6.24) | 0.718, 0.11 |
| REM (min) | 10.21 (− 11.27 to 31.69) | 0.341, 0.30 |
| REMCI | 0.48 (− 8.05 to 9.01) | 0.909, 0.04 |
| PSG breathing indices | ||
| AHI | 5.47 (− 5.24 to 16.18)† | 0.305, 0.31 |
| CAI | 0.18 (− 0.44 to 0.08) | 0.163, 0.46 |
| RDI | 1.88 (11.17–7.41) | 0.683, 0.13 |
| RERAI | 7.35 (− 3.01 to 17.70) | 0.157, 0.44 |
| NSBT (%) | 14.74 (− 2.62 to 32.10) | 0.094, 0.53 |
| NSBT (min) | 89.74 (29.38–150.11) | 0.005, 0.94 |
Abbreviations: ASV — adaptive servo-ventilation; CPAP — continuous positive airway pressure; ISI — Insomnia Severity Index; SQ-Lik — Sleep quality Likert scale; SQ-Pct — Sleep quality percent scale; ESS — Epworth Sleepiness Scale; III — Insomnia Impairment Index; FSS — Fatigue Severity Scale; FOSQ-10 — Functional Outcomes of Sleep Questionnaire Short Form; QLESQ — Quality of Life Enjoyment and Satisfaction Questionnaire Summary; SOL — sleep onset latency; WASO — wake after sleep onset; SE — sleep efficiency; N1 — non-REM stage 1; N2 — non-REM stage 2; N3 — non-REM stage 3; REM — rapid eye movement sleep; REMCI — REM consolidation index; AHI — Apnea Hypopnea Index; RDI — Respiratory Disturbance Index; RERAI — Respiratory Effort-Related Arousal event Index; OSA — obstructive sleep apnea; UARS — upper airway resistance syndrome; NSBT — normal sleep breathing time.
t-test confidence interval.
All improvements are as expected (greater improvement in ASV) except those identified by †.
Due to high prevalence of “0s” in data, Exact Wilcoxon Sign Ranked Test was used for pseudo median and confidence intervals.
Fig. 5.
Improvement in (a) Respiratory Effort-Related Arousal (RERA) Index, (b) percent Normal Sleep Breathing Time, and (c) Normal Sleep Breathing Time in minutes, over 14 weeks of therapy by mode.
Estimated trajectories for outcomes of each mode are shown along with 95% confidence intervals for average scores at each time point. Overlap in the band indicates intersection of confidence intervals. (a) RERA index improved for the ASV group at first encounter with PAP therapy and remained low for the duration of the study whereas the CPAP group increased in RERA index at first encounter with PAP and then gradually decreased back to baseline RERA index. (b) Percent time with Normal Sleep Breathing improved at first encounter with PAP and remained relatively consistent for the remainder of the study with ASV demonstrating nearly 20% more time with normal sleep breathing throughout treatment. (c) Minutes spent with Normal Sleep Breathing improved for both groups at first encounter with PAP therapy and continued to improve for the ASV group.
Additional objective respiratory parameters were analyzed based on normal sleep breathing time (NSBT). Consistent with other breathing improvements noted above, repeated measure ANCOVA revealed improvement in the percent normal sleep breathing time for ASV over CPAP (Wald χ2 = 37.9, p < 0.0001, Fig. 5b) as well as for normal sleep breathing time in minutes (Wald χ2 = 17.2, p < 0.0001, Fig. 5c). Using pre- and post-test analysis for percent time spent in NSBT, ASV demonstrated a trend in improvement compared to CPAP [32.60% (20.67–44.52) vs 17.86% (4.45–321.26), p = 0.094, g = 0.53] and based on final titration data, there was a 20% difference in groups [88.89% (83.08–94.70) vs 68.11% (56.76–79.47) (t-test p = 0.002, g = 1.02). Pre- and post-test for NSBT minutes showed greater improvement in ASV over CPAP [128.11 (81.68–174.54) vs 38.36 (− 3.32 to 80.08) p = 0.005, g = 0.94], which yielded a large effect size as exemplified by the nearly 100 min of greater normal sleep breathing time for ASV compared to CPAP on final titration [303.48 (259.56–347.40) vs. 208.51 (162.83–254.20)] (t-test p = 0.003. g = 0.97).
ODD revealed no significant difference in compliance at final follow-up [68.4% ASV users, average nightly use 5.76 (4.64–6.87) vs 57.1% CPAP users, 5.39 (4.33–6.44), Wald χ2 = 0.541, p = 0.462]. Throughout the 14-week treatment phase, ASV users averaged 35 min more than CPAP users, which was not significant but with a small to medium effect size (p = 0.354; g = 0.29). Comparison of average pressures at final follow-up [CPAP 10.92 (9.76–12.09) and ASV IPAP 14.87 (13.33–16.42), ASV EPAP 8.02 (6.79–9.24)] showed significant differences: CPAP vs ASV IPAP (p = 0.001, g = 1.34) and CPAP vs ASV EPAP (p < 0.001, g = 1.11). Between mode leak levels were similar [ASV 8.17 (4.05–12.29) vs CPAP 7.36 (4.32–10.40), p = 0.639, g = 0.15].
3.6. Prospective Sleep Indices and Objective Sleep Architecture
For both modes, daily online sleep diary metrics showed significant improvements or medium to large effects from baseline to exit for SOL, WASO, and SE%; whereas, rare sleep architecture changes occurred. Between PAP mode changes were non-significant (Table 3a, Table 3b, Table 3c).
3.7. Adverse Effects
Side-effects were consistent with those commonly seen with regular PAP use. The most common subjective side-effects to PAP therapy were mask leak (90.0%), dry mouth (87.5%), skin irritation (70.0%), mask related insomnia (60.0%), mouth leak (57.5%), congestion (57.5%), skin crease from mask (52.5%), nostril tickle/burn (50.0%), aerophagia (40.0%), and eye irritation (37.5%). There were no systematic differences between modes; and, despite the high frequency of complaints, these issues were not sufficiently distressing to prevent the final sample from completing the protocol. The most common reported adverse effects among the six patients who received a device but did not complete the study due to intolerance (Fig. 1), were mask related insomnia (5 of 6), pressure related insomnia (4 of 6), and no appreciable benefit from PAP (3 of 6). Another complication manifested in the form of persistent EPI among CPAP patients compared to its near absence in ASV, consistent with greater or enhanced ASV comfort effects from lower exhalation pressures.
4. Discussion
In a randomized controlled trial on the efficacy of two PAP therapy modes, insomnia severity markedly decreased by targeting poor sleep quality (i.e. eradicating sleep breathing induced sleep fragmentation) in covert complex insomnia patients. The results from this sample support the older and now re-emerging theory categorizing chronic insomnia as a medical disorder of pervasive, respiratory-induced, objective sleep fragmentation. Successful PAP treatment of chronic insomnia confirmed this underlying medical etiology, which had gone undetected by patients and providers for an average of one decade. Moreover, our novel design, the first to our knowledge to compare two PAP modes in chronic insomnia patients, demonstrated superior ASV efficacy over CPAP based on significantly greater improvements in insomnia and sleep quality.
Remarkably, two-thirds of ASV users no longer met insomnia disorder criteria compared to only one-quarter on CPAP at the close of the study. Both daily sleep quality measures not only confirmed ASV superiority but also of singular import, between mode separation occurred far earlier in the protocol for sleep quality than for insomnia outcomes, consistent with the theory that poor sleep quality fueled insomnia in this sample of patients. Greater ASV treatment gains further substantiated this medical disorder theory, because more efficacious treatment of all sleep breathing events achieved better results, albeit it must be noted that continued use of the PAP device would be required to maintain these improvements.
Objectively, a significantly higher proportion of sleep time spent with normalized airflow on ASV compared to CPAP provides evidence of a dose–response relationship, and as noted above, this near normalization of airflow occurred instantaneously during the very first exposure to ASV (Fig. 5), whereas CPAP demonstrated very minor improvements throughout the entire protocol. In other words, the larger treatment effects with ASV compared to CPAP resulted in significantly lower residual RERAs and flattening frequencies, presumably due to ASV delivery of significantly higher inspiratory pressures and significantly lower expiratory pressures. Physiologically, as hypothesized, ASV appeared to protect against EPI while providing more efficacious treatment of RERAs. Overall, these technological advantages yielded greater patient comfort, superior objective responses, and easier adaptability than traditional CPAP.
In sum, OSA/UARS manifested as a pivotal factor in insomnia improvement for 82.5% of 40 chronic, covert complex insomnia cases (18 cases of insomnia remission plus 15 cases achieving sub-clinical status of ISI < 12) in contrast to the traditional etiologies of behavioral, psychological, psychiatric, or environmental factors for which they had undergone numerous prior unsuccessful treatments. Changes in secondary outcomes of impairment and quality of life also support concurrent validity, but statistical results were less robust due to the small sample and baseline imbalances, albeit effect size differences between PAP modes ranged from medium to large. Still, it cannot go without saying that if these patients were treated with CBT-I earlier in their average decade-long chronicity, they may have gained similar, or perhaps greater insomnia improvements (though sleep quality gains might have lagged) despite the presence of co-morbid OSA [71]. Future investigations must clarify the timing for PAP or CBT-I as first-line therapy for complex insomnia patients or whether concurrent administration is worthwhile [12], [55], [72].
Clinically, it is imperative to emphasize that the complex insomnia theory advocating sleep breathing treatments is at variance with standard insomnia therapies; nevertheless, exploring these divergent (psychological vs physiological) frameworks is of no small clinical importance given the wide swath of medical and psychiatric disorders affected by chronic insomnia [2], [73], [74], [75]. Regrettably, both clinical considerations and research investigations on complex insomnia have been limited. As a prime example, routine clinical use of polysomnography (PSG) sleep testing to evaluate insomnia is discouraged by the AASM [76], an entity which has not recommended broad research into this comorbidity. Yet, PSG could prove valuable in objectively measuring sleep consolidation in a patient on prescription sedatives while simultaneously testing for OSA/UARS. A larger and yet rarely cited barrier stems from health insurers or government regulations hindering clinical practices by indirectly stifling insomnia care through coverage determinations rejecting PSG for chronic insomnia. Such policies guide healthcare professionals toward expedient treatments, such as sleep hygiene or, more commonly, pharmacotherapy, either of which potentially offering some sleep consolidation gains. However, and specific to research, most insomnia clinical trials are dedicated to drug solutions [77], despite the high rates of OSA in patients failing hypnotics and the growing concerns about prescribing such medications [37], [49].
Understandably, covert complex insomnia is difficult to detect, especially in the context of past insomnia research in which breathing was never assessed or not assessed with nasal cannula pressure transducer [78], the technology required to diagnose UARS [79]. However, it remains puzzling so many patients among the large numbers in our excluded sample had never been diagnosed or even considered for diagnostic testing despite their undisguised (overt) risks or symptoms of sleep-disordered breathing. Hypothetically, so many medical and mental health providers adhere to conventional wisdom explicating insomnia as a psychophysiological condition, we speculate the ofttimes desperate complaint of sleeplessness itself steers healthcare providers away from any consideration of sleep breathing comorbidity. Notwithstanding, based on our work and several other research groups [13], [14], [17], [21], [42], [55], [80], [81], this comorbidity (whether overt or covert) appears notably more prevalent than formerly recognized in clinical practices. Though recent reviews on insomnia diagnosis and treatment mention the possibility of a sleep breathing etiology [1], [14], [82], we do not sense that recurrent failures of pharmacotherapy or other treatments or even recent concerns about black box warnings (warnings directed at Ambien, Lunesta and Sonata regarding parasomnia side-effects among patients using these common sedatives) [83] lead to more comprehensive sleep medicine assessments. Instead, we speculate these patients frequently appear to be categorized as “treatment resistant,” when the reality may be closer to “misdiagnosis” or “missed-diagnosis” [12], [35], [36], [37], [49].
While our results require replication with larger samples, research must determine the following: (1) the prevalence of complex insomnia among treatment-seeking insomnia patients; (2) a clearer pathophysiological explanation for how sleep breathing disorders cause or aggravate insomnia; and (3) the most efficacious and cost-effective treatment regimens [12], [55]. Given the entrenched process whereby insomnia complaints eclipse any OSA suspicions even in the presence of obesity, sleepiness, and classic sleep breathing symptoms, at minimum we recommend consideration of intake screening questions focusing on nonrestorative sleep to facilitate proper assessment. With the advent of home sleep testing (HST) early evaluation is feasible [84], although RERA measurement on HST may be inconsistent [85], a potential disadvantage given the crucial importance of this subtle sleep breathing event in our treatment paradigm [86]. Finally, as chronic insomnia has been linked to so many conditions such as depression [87], [88], suicide, [89], [90], [91], and cardiovascular disease including hypertension, myocardial infarction, congestive heart failure, cardiac arrhythmias, and the metabolic syndrome [2], as well as costing tens of billions of dollars annually in healthcare expenses, decreased productivity, sick days and accidents [2], [92], it might prove informative to explore whether past research has been unknowingly influenced by the effects of undetected OSA or UARS among various insomnia cohorts [93].
Limitations consist of two main categories. First was the sample size, a result of the extremely narrow inclusion criteria weighted against enrollment of patients suspected of suffering from a sleep breathing disorder coupled with the necessity to conduct an efficacy trial as a preliminary approach to the treatment of complex insomnia. Moreover, we favored external validity as it relates to covert complex insomnia, because of prevailing views consistently discounting the comorbidity of these two disorders as merely an epiphenomenon [11], [12], [13]. By studying a sample of patients who would never have been recommended for PSG and whose tests might have returned negative due to the failure to use appropriate respiratory sensor technology, we attempted to replicate the experiences of many insomniacs who are rarely offered a comprehensive sleep evaluation let alone a night in a sleep laboratory. The most clinically relevant limitation is the well-described problem regarding difficulties adapting to PAP therapy, another major factor necessitating an efficacy trial. Despite considerable coaching and repeated laboratory titrations, several patients struggled to achieve standard adherence. Then again, some coaching aspects were restricted so as to not reveal mode or not to discuss PAP delivery of pressures. These issues are crucial to the practicality of using PAP for complex insomnia and clearly point to a likely need to research alternative sleep breathing treatments (e.g. oral appliance therapy, ENT surgery, or even nasal strips or other nasal dilators). Moreover, head-to-head studies may reveal CBT-I as effective as PAP in decreasing insomnia severity when accounting for dropouts; albeit, the sequelae from untreated OSA may reveal deficiencies in first-line CBT-I. And, it is essential to point out that in contrast to CBT-I, PAP most likely would require regular application to maintain therapeutic effects. Other limitations were the absence of a placebo control and use of a single-blind design, which could be addressed in future experimental designs. The problem of co-morbid PLMD must also be addressed through larger samples.
In conclusion, our study supports the original 1973 [10], subsequent 2001 [9], and currently re-emerging theory elucidating chronic insomnia as a medical disorder of compromised sleep breathing [13], [14], [17], [21], [42], [55], [80], [81]. And, regardless of the apparent superiority of ASV over CPAP, a main import of the study was the demonstration of consistent therapeutic effects: when OSA/UARS is treated, sleep quality (a complaint about not sleeping well) rapidly improves, leading to downstream effects on both insomnia (a complaint about not sleeping) and ostensibly impairment (a complaint about daytime effects of poor sleep quality). These intricate pathophysiological mechanisms continue to be overlooked by many healthcare providers who encounter chronic insomniacs. Education to increase awareness on how sleep breathing events induce chronic sleep fragmentation must be disseminated throughout medical and mental health professional communities. Doing so will diminish chronic insomnia patients' exposure to ineffective and sometimes harmful medications as well as numerous, non-insomnia-focused psychological therapies, all of which result in variable impact on chronic, pathophysiological sleep fragmentation. Correct diagnosis may also lead to reduced morbidity and mortality from untreated OSA/UARS [94]. Placing sleep-disordered breathing at or near the top rung of the differential diagnosis in the evaluation of a chronic insomnia disorder is a vital step toward helping patients who suffer, unwittingly, from a medical disorder of objectively measurable nonrestorative sleep due to abnormal sleep respiration.
Contributors
BK derived hypotheses, created study design and protocol, analyzed data, and wrote the manuscript. NDM and VAU helped with study design and protocol, data collection and analysis, table and figure preparation, literature review, and manuscript preparation. JK helped create study design and protocol and manuscript preparation. RMS analyzed data, produced graphs, and helped with manuscript preparation. All authors read and approved the final version of the paper.
Funding
The study was funded by ResMed Science Center, San Diego, California, USA.
Role of Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Declaration of Competing Interest
Authors NDM, VAU, JK, and RMS report grants from ResMed Sciences during the conduct of the study. Additionally, author JK is the CEO of the commercial sleep center, Maimonides Sleep Arts & Sciences, where the study was conducted in part; Barry and Jessica Krakow are married.
Author BK reports: 6 main activities related to his work in sleep medicine.
For websites, Dr. Krakow owns and operates 6 sites that provide education and offer products and services for sleep disorders patients: www.nightmaretreatment.com, www.ptsdsleepclinic.com, www.sleeptreatment.com, www.sleepdynamictherapy.com, www.soundsleepsoundmind.com, and www.nocturiacures.com.
For other professional services, he is the medical director of a national DME company Classic SleepCare for which his sole functions are consultation and QA; he has neither patient encounters nor does he benefit from the sale of any DME equipment. For intellectual property, Dr. Krakow markets and sells 3 books for sleep disorders patients: Insomnia Cures, Turning Nightmares into Dreams, and Sound Sleep, Sound Mind. For clinical services, he owns and operates one commercial sleep center: Maimonides Sleep Arts & Sciences, Ltd. For educational and consulting services: Dr. Krakow conducts CME/CEU educational programs for medical and mental health providers to learn about sleep disorders. Sometimes these programs involve the attendee paying a fee directly to Maimonides Sleep Arts & Sciences. Other times, he conducts the workshops at other locations, which may be paid for by vendors such as Respironics and ResMed or other institutions such as the AMEDDC&S, VAMC, and regional sleep center conferences. He is also president and principal investigator of a non-profit sleep research center, the Sleep & Human Health Institute (www.sleepingresearch.org, www.shhi.org) that occasionally provides consultation services or receives grants for pilot studies, the most recent: ResMed ~$400,000 January 2015 (funding for this randomized control trial of treatment in insomnia patients). Recently, and after this research had been conducted, he provided a brief consultation to ASOCorp, a medical supply company that manufactures nasal strips.
Acknowledgements
We thank the following practitioners for their dedication in referring to our protocol: David Leech, DO; Lewis Nemes, PhD; James Tryon, MD; and Jana Weisborn, CNP. And, we are grateful to Dominic Melendrez, RPSGT for his independent and masked scoring of all sleep studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.06.011.
Appendix A. Supplementary data
Supplement includes detailed description of the measures used in this study and well as data analysis regarding sleep aids and psychotropic medication.
References
- 1.Morin C.M., Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. March 24. [DOI] [PubMed] [Google Scholar]
- 2.Riemann D., Nissen C., Palagini L., Otte A., Perlis M.L., Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–558. doi: 10.1016/S1474-4422(15)00021-6. May. [DOI] [PubMed] [Google Scholar]
- 3.Schutte-Rodin S., Broch L., Buysse D., Dorsey C., Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. October 15. [PMC free article] [PubMed] [Google Scholar]
- 4.Morgenthaler T., Kramer M., Alessi C., Friedman L., Boehlecke B., Brown T. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. November. [PubMed] [Google Scholar]
- 5.Bramoweth A.D., Germain A., Youk A.O., Rodriguez K.L., Chinman M.J. A hybrid type I trial to increase veterans' access to insomnia care: study protocol for a randomized controlled trial. Trials. 2018;19(1):73. doi: 10.1186/s13063-017-2437-y. January 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manber R., Carney C., Edinger J., Epstein D., Friedman L., Haynes P.L. Dissemination of CBTI to the non-sleep specialist: protocol development and training issues. J Clin Sleep Med. 2012;8(2):209–218. doi: 10.5664/jcsm.1786. April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen H., Batterham P.J., Gosling J.A., Ritterband L.M., Griffiths K.M., Thorndike F.P. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333–341. doi: 10.1016/S2215-0366(15)00536-2. April. [DOI] [PubMed] [Google Scholar]
- 8.Kyle S.D., Miller C.B., Rogers Z., Siriwardena A.N., Macmahon K.M., Espie C.A. Sleep restriction therapy for insomnia is associated with reduced objective total sleep time, increased daytime somnolence, and objectively impaired vigilance: implications for the clinical management of insomnia disorder. Sleep. 2014;37(2):229–237. doi: 10.5665/sleep.3386. February 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krakow B., Melendrez D., Pedersen B., Johnston L., Hollifield M., Germain A. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11):948–953. doi: 10.1016/s0006-3223(00)01087-8. June 1. [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C., Eldridge F.L., Dement W.C. Insomnia with sleep apnea: a new syndrome. Science. 1973;181(4102):856–858. doi: 10.1126/science.181.4102.856. August 31. [DOI] [PubMed] [Google Scholar]
- 11.Lavie P. Insomnia and sleep-disordered breathing. Sleep Med. 2007;8(Suppl. 4):S21–S25. doi: 10.1016/S1389-9457(08)70005-4. December. [DOI] [PubMed] [Google Scholar]
- 12.Al-Jawder S.E., Bahammam A.S. Comorbid insomnia in sleep-related breathing disorders: an under-recognized association. Sleep Breath. 2012;16(2):295–304. doi: 10.1007/s11325-011-0513-1. June. [DOI] [PubMed] [Google Scholar]
- 13.Beneto A., Gomez-Siurana E., Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13(4):287–293. doi: 10.1016/j.smrv.2008.09.006. August. [DOI] [PubMed] [Google Scholar]
- 14.Luyster FS, Buysse DJ, Strollo PJ, Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med 2010 April 15;6(2):196–204. [PMC free article] [PubMed]
- 15.Wickwire E.M., Collop N.A. Insomnia and sleep-related breathing disorders. Chest. 2010;137(6):1449–1463. doi: 10.1378/chest.09-1485. June. [DOI] [PubMed] [Google Scholar]
- 16.Krakow B., Lowry C., Germain A., Gaddy L., Hollifield M., Koss M. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291–298. doi: 10.1016/s0022-3999(00)00147-1. November. [DOI] [PubMed] [Google Scholar]
- 17.Bjornsdottir E., Janson C., Sigurdsson J.F., Gehrman P., Perlis M., Juliusson S. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901–1909. doi: 10.5665/sleep.3226. December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glidewell R.N., Renn B.N., Roby E., Orr W.C. Predictors and patterns of insomnia symptoms in OSA before and after PAP therapy. Sleep Med. 2014;15(8):899–905. doi: 10.1016/j.sleep.2014.05.001. August. [DOI] [PubMed] [Google Scholar]
- 19.Guilleminault C., Palombini L., Poyares D., Chowdhuri S. Chronic insomnia, premenopausal women and sleep disordered breathing: part 2. Comparison of nondrug treatment trials in normal breathing and UARS postmenopausal women complaining of chronic insomnia. J Psychosom Res. 2002;53(1):617–623. doi: 10.1016/s0022-3999(02)00463-4. July. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen X.L., Chaskalovic J., Rakotonanahary D., Fleury B. Insomnia symptoms and CPAP compliance in OSAS patients: a descriptive study using data mining methods. Sleep Med. 2010;11(8):777–784. doi: 10.1016/j.sleep.2010.04.008. September. [DOI] [PubMed] [Google Scholar]
- 21.Guilleminault C., Davis K., Huynh N.T. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31(11):1527–1533. doi: 10.1093/sleep/31.11.1527. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krakow B., Melendrez D., Lee S.A., Warner T.D., Clark J.O., Sklar D. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8(1):15–29. doi: 10.1007/s11325-004-0015-5. March. [DOI] [PubMed] [Google Scholar]
- 23.Pien G.W., Ye L., Keenan B.T., Maislin G., Bjornsdottir E., Arnardottir E.S. Changing faces of OSA: treatment effects by cluster designation in the Icelandic sleep apnea cohort. Sleep. 2018;43(3):1–13. doi: 10.1093/sleep/zsx201. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krakow B., Ulibarri V.A., McIver N.D., Nadorff M.R. A novel therapy for chronic sleep-onset insomnia: a retrospective, nonrandomized controlled study of auto-adjusting, dual-level, positive airway pressure technology. Prim Care Companion CNS Disord. 2016;18(5) doi: 10.4088/PCC.16m01980. September 29. [DOI] [PubMed] [Google Scholar]
- 25.Krakow B., Ulibarri V.A., Foley-Shea M.R., Tidler A., McIver N.D. Adherence and subthreshold adherence in sleep apnea subjects receiving positive airway pressure therapy: a retrospective study evaluating differences in adherence versus use. Respir Care. 2016;61(8):1023–1032. doi: 10.4187/respcare.04538. [April 26] [DOI] [PubMed] [Google Scholar]
- 26.Krakow B., McIver N.D., Ulibarri V.A., Nadorff M.R. Retrospective, nonrandomized controlled study on autoadjusting, dual-pressure positive airway pressure therapy for a consecutive series of complex insomnia disorder patients. Nat Sci Sleep. 2017;9:81–95. doi: 10.2147/NSS.S120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krakow B., Melendrez D., Sisley B., Warner T.D., Krakow J., Leahigh L. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: a randomized controlled trial. Sleep Breath. 2006;10(1):16–28. doi: 10.1007/s11325-005-0037-7. March. [DOI] [PubMed] [Google Scholar]
- 28.Nikolopoulou M., Byraki A., Ahlberg J., Heymans M.W., Hamburger H.L., De L.J. Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnoea syndrome: a randomised, placebo-controlled trial on self-reported symptoms of common sleep disorders and sleep-related problems. J Oral Rehabil. 2017;44(6):452–460. doi: 10.1111/joor.12505. June. [DOI] [PubMed] [Google Scholar]
- 29.Krakow B., Ulibarri V.A., Romero E.A., Thomas R.J., McIver N.D. Adaptive servo-ventilation therapy in a case series of patients with co-morbid insomnia and sleep apnea. Journal of Sleep Disorders: Treatment and Care. 2013;2(1):1–10. [Google Scholar]
- 30.Mysliwiec V., Gill J., Lee H., Baxter T., Pierce R., Barr T.L. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549–557. doi: 10.1378/chest.13-0088. August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad S., Gupta M., Gupta R., Dhyani M. Prevalence and correlates of insomnia and obstructive sleep apnea in chronic kidney disease. N Am J Med Sci. 2013;5(11):641–646. doi: 10.4103/1947-2714.122306. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krakow B., Haynes P.L., Warner T.D., Santana E., Melendrez D., Johnston L. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257–268. doi: 10.1023/B:JOTS.0000029269.29098.67. June. [DOI] [PubMed] [Google Scholar]
- 33.Gooneratne N.S., Gehrman P.R., Nkwuo J.E., Bellamy S.L., Schutte-Rodin S., Dinges D.F. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166(16):1732–1738. doi: 10.1001/archinte.166.16.1732. September 18. [DOI] [PubMed] [Google Scholar]
- 34.Lichstein K.L., Riedel B.W., Lester K.W., Aguillard R.N. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67(3):405–410. doi: 10.1037//0022-006x.67.3.405. June. [DOI] [PubMed] [Google Scholar]
- 35.Krakow B., Ulibarri V.A., Romero E.A. Patients with treatment-resistant insomnia taking nightly prescription medications for sleep: a retrospective assessment of diagnostic and treatment variables. Prim Care Companion J Clin Psychiatry. 2010;12(4) doi: 10.4088/PCC.09m00873bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krakow B., Ulibarri V.A., Romero E. Persistent insomnia in chronic hypnotic users presenting to a sleep medical center: a retrospective chart review of 137 consecutive patients. J Nerv Ment Dis. 2010;198(10):734–741. doi: 10.1097/NMD.0b013e3181f4aca1. October. [DOI] [PubMed] [Google Scholar]
- 37.Krakow B., Ulibarri V.A., McIver N.D. Pharmacotherapeutic failure in a large cohort of patients with insomnia presenting to a sleep medicine center and laboratory: subjective pretest predictions and objective diagnoses. Mayo Clin Proc. 2014;89(12):1608–1620. doi: 10.1016/j.mayocp.2014.04.032. December. [DOI] [PubMed] [Google Scholar]
- 38.Guilleminault C., Palombini L., Poyares D., Chowdhuri S. Chronic insomnia, postmenopausal women, and sleep disordered breathing: part 1. Frequency of sleep disordered breathing in a cohort. J Psychosom Res. 2002;53(1):611–615. doi: 10.1016/s0022-3999(02)00445-2. July. [DOI] [PubMed] [Google Scholar]
- 39.Arroll B., Fernando A., III, Falloon K., Goodyear-Smith F., Samaranayake C., Warman G. Prevalence of causes of insomnia in primary care: a cross-sectional study. Br J Gen Pract. 2012;62(595):e99–103. doi: 10.3399/bjgp12X625157. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailes S., Rizzo D., Baltzan M., Grad R., Pavilanis A., Creti L. Manifestations of insomnia in sleep apnea: implications for screening and treatment. Behav Sleep Med. 2016;14(4):429–441. doi: 10.1080/15402002.2015.1017098. July. [DOI] [PubMed] [Google Scholar]
- 41.Glidewell R.N., Roby E.K., Orr W.C. Is insomnia an independent predictor of obstructive sleep apnea? J Am Board Fam Med. 2012;25(1):104–110. doi: 10.3122/jabfm.2012.01.110123. January. [DOI] [PubMed] [Google Scholar]
- 42.Mysliwiec V., Capaldi V.F., Gill J., Baxter T., O'Reilly B.M., Matsangas P. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475–482. doi: 10.7205/MILMED-D-14-00197. April. [DOI] [PubMed] [Google Scholar]
- 43.Krakow B., Melendrez D., Johnston L., Warner T.D., Clark J.O., Pacheco M. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis. 2002;190(7):442–452. doi: 10.1097/00005053-200207000-00004. July. [DOI] [PubMed] [Google Scholar]
- 44.Fung C.H., Martin J.L., Dzierzewski J.M., Jouldjian S., Josephson K., Park M. Prevalence and symptoms of occult sleep disordered breathing among older veterans with insomnia. J Clin Sleep Med. 2013;9(11):1173–1178. doi: 10.5664/jcsm.3162. November 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kushida C.A., Chediak A., Berry R.B., Brown L.K., Gozal D., Iber C. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. April 15. [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace D.M., Sawyer A.M., Shafazand S. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath. 2018;22(1):5–15. doi: 10.1007/s11325-017-1605-3. March. [DOI] [PubMed] [Google Scholar]
- 47.Wohlgemuth W.K., Chirinos D.A., Domingo S., Wallace D.M. Attempters, adherers, and non-adherers: latent profile analysis of CPAP use with correlates. Sleep Med. 2015;16(3):336–342. doi: 10.1016/j.sleep.2014.08.013. March. [DOI] [PubMed] [Google Scholar]
- 48.Wickwire E.M., Smith M.T., Birnbaum S., Collop N.A. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772–776. doi: 10.1016/j.sleep.2010.03.012. September. [DOI] [PubMed] [Google Scholar]
- 49.Li Y., Li Z., Lei F., Du L., Tang X. Persistent insomnia despite long-term nightly use of sleeping pills. J Clin Sleep Med. 2013;9(8):834–836. doi: 10.5664/jcsm.2936. August 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krakow B., Ulibarri V.A., McIver N.D., Yonemoto C., Tidler A., Obando J. Reversal of PAP failure with the REPAP protocol. Respir Care. 2017;62(4):396–408. doi: 10.4187/respcare.05032. April. [DOI] [PubMed] [Google Scholar]
- 51.Morgenthaler T.I., Kuzniar T.J., Wolfe L.F., Willes L., McLain W.C., III, Goldberg R. The complex sleep apnea resolution study: a prospective randomized controlled trial of continuous positive airway pressure versus adaptive servoventilation therapy. Sleep. 2014;37(5):927–934. doi: 10.5665/sleep.3662. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gartlehner G., Hansen R.A., Nissman D., Lohr K.N., Carey T.S. A simple and valid tool distinguished efficacy from effectiveness studies. J Clin Epidemiol. 2006;59(10):1040–1048. doi: 10.1016/j.jclinepi.2006.01.011. October. [DOI] [PubMed] [Google Scholar]
- 53.Singal A.G., Higgins P.D., Waljee A.K. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5 doi: 10.1038/ctg.2013.13. (January 2: e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorpe K.E., Zwarenstein M., Oxman A.D., Treweek S., Furberg C.D., Altman D.G. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62(5):464–475. doi: 10.1016/j.jclinepi.2008.12.011. May. [DOI] [PubMed] [Google Scholar]
- 55.Sweetman A.M., Lack L.C., Catcheside P.G., Antic N.A., Chai-Coetzer C.L., Smith S.S. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med Rev. 2017;33:28–38. doi: 10.1016/j.smrv.2016.04.004. June. [DOI] [PubMed] [Google Scholar]
- 56.Krakow B., Ulibarri V.A. Prevalence of sleep breathing complaints reported by treatment-seeking chronic insomnia disorder patients on presentation to a sleep medical center: a preliminary report. Sleep Breath. 2013;17(1):317–322. doi: 10.1007/s11325-012-0694-2. March. [DOI] [PubMed] [Google Scholar]
- 57.Fergusson D., Aaron S.D., Guyatt G., Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652–654. doi: 10.1136/bmj.325.7365.652. September 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cowie M.R., Woehrle H., Wegscheider K., Angermann C., d'Ortho M.P., Erdmann E. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. September 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pepin J.D., Woehrle H., Liu D., Shao S., Armitstead J.P., Cistulli P.A. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med. 2018;14(1):57–63. doi: 10.5664/jcsm.6880. January 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su M., Zhang X., Huang M., Ding N. Adaptive pressure support servoventilation: a novel treatment for residual sleepiness associated with central sleep apnea events. Sleep Breath. 2011;15(4):695–699. doi: 10.1007/s11325-010-0424-6. December. [DOI] [PubMed] [Google Scholar]
- 61.Liu D., Armitstead J., Benjafield A., Shao S., Malhotra A., Cistulli P.A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751–760. doi: 10.1016/j.chest.2017.06.010. October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aritake-Okada S., Namba K., Hidano N., Asaoka S., Komada Y., Usui A. Change in frequency of periodic limb movements during sleep with usage of continuous positive airway pressure in obstructive sleep apnea syndrome. J Neurol Sci. 2012;317(1–2):13–16. doi: 10.1016/j.jns.2012.03.013. June 15. [DOI] [PubMed] [Google Scholar]
- 63.Baran A.S., Richert A.C., Douglass A.B., May W., Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26(6):717–720. doi: 10.1093/sleep/26.6.717. September. [DOI] [PubMed] [Google Scholar]
- 64.American Academy of Sleep Medicine . International classification of sleep disorders. 2nd ed. American Academy of Sleep Medicine; Darien, IL: 2005. Sleep related movement disorders: periodic limb movement disorder; pp. 182–186. [Google Scholar]
- 65.Harrell FE Jr. Regression modeling strategies with applications to linear models, logistic and ordinal regression, and survival analysis. 2 ed. Switzerland: Springer International Publishing; 2015.
- 66.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. A language and environment for statistical computing. [Google Scholar]
- 67.Harrell FE Jr. Regression modeling strategies: R package version 5.1–2. 2018.
- 68.Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team . 2018. Linear and nonlinear mixed effects models. R package version 3; pp. 1–137. [Google Scholar]
- 69.Hollander M., Wolfe D.A. John Wiley & Sons; New York: 1973. Nonparametric statistical inference. [Google Scholar]
- 70.Exact rank tests: exact distributions for rank and permutation tests. R package version 0. 2017. pp. 8–29. computer program. [Google Scholar]
- 71.Sweetman A., Lack L., Lambert S., Gradisar M., Harris J. Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med. 2017;39:38–46. doi: 10.1016/j.sleep.2017.09.003. November. [DOI] [PubMed] [Google Scholar]
- 72.Brock M.S., Mysliwiec V. Comorbid insomnia and sleep apnea: a prevalent but overlooked disorder. Sleep Breath. 2018;22(1):1–3. doi: 10.1007/s11325-018-1628-4. March. [DOI] [PubMed] [Google Scholar]
- 73.Palmer C.A., Alfano C.A. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. doi: 10.1016/j.smrv.2015.12.006. February. [DOI] [PubMed] [Google Scholar]
- 74.Sivertsen B., Lallukka T., Salo P., Pallesen S., Hysing M., Krokstad S. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT study in Norway. J Sleep Res. 2014;23(2):124–132. doi: 10.1111/jsr.12102. April. [DOI] [PubMed] [Google Scholar]
- 75.Wu J.Q., Appleman E.R., Salazar R.D., Ong J.C. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. doi: 10.1001/jamainternmed.2015.3006. September. [DOI] [PubMed] [Google Scholar]
- 76.Littner M., Hirshkowitz M., Kramer M., Kapen S., Anderson W.M., Bailey D. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26(6):754–760. doi: 10.1093/sleep/26.6.754. September. [DOI] [PubMed] [Google Scholar]
- 77.Insomnia Clinical Trials 2018. https://www.centerwatch.com/clinical-trials/listings/condition/189/insomnia/
- 78.McCall W.V., Erman M., Krystal A.D., Rosenberg R., Scharf M., Zammit G.K. A polysomnography study of eszopiclone in elderly patients with insomnia. Curr Med Res Opin. 2006;22(9):1633–1642. doi: 10.1185/030079906X112741. September. [DOI] [PubMed] [Google Scholar]
- 79.Hosselet J., Ayappa I., Norman R.G., Krieger A.C., Rapoport D.M. Classification of sleep-disordered breathing. Am J Respir Crit Care Med. 2001;163(2):398–405. doi: 10.1164/ajrccm.163.2.9808132. February. [DOI] [PubMed] [Google Scholar]
- 80.Crawford M.R., Turner A.D., Wyatt J.K., Fogg L.F., Ong J.C. Evaluating the treatment of obstructive sleep apnea comorbid with insomnia disorder using an incomplete factorial design. Contemp Clin Trials. 2016;47:146–152. doi: 10.1016/j.cct.2015.12.017. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ong J.C., Crawford M.R., Kong A., Park M., Cvengros J.A., Crisostomo M.I. Management of obstructive sleep apnea and comorbid insomnia: a mixed-methods evaluation. Behav Sleep Med. 2017;15(3):180–197. doi: 10.1080/15402002.2015.1087000. May. [DOI] [PubMed] [Google Scholar]
- 82.Riemann D., Baglioni C., Bassetti C., Bjorvatn B., Dolenc G.L., Ellis J.G. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594. December. [DOI] [PubMed] [Google Scholar]
- 83.FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines: FDA Drug Safety Communication. [4-30-2019].
- 84.Gay P.C., Selecky P.A. Are sleep studies appropriately done in the home? Respir Care. 2010;55(1):66–75. January. [PubMed] [Google Scholar]
- 85.Malhotra R.K., Kirsch D.B., Kristo D.A., Olson E.J., Aurora R.N., Carden K.A. Polysomnography for obstructive sleep apnea should include arousal-based scoring: an American academy of sleep medicine position statement. J Clin Sleep Med. 2018;14(7):1245–1247. doi: 10.5664/jcsm.7234. [July 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arora N., Meskill G., Guilleminault C. The role of flow limitation as an important diagnostic tool and clinical finding in mild sleep-disordered breathing. Sleep Sci. 2015;8:134–142. doi: 10.1016/j.slsci.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baglioni C., Spiegelhalder K., Lombardo C., Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. doi: 10.1016/j.smrv.2009.10.007. August. [DOI] [PubMed] [Google Scholar]
- 88.Sivertsen B., Salo P., Mykletun A., Hysing M., Pallesen S., Krokstad S. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74(7):758–765. doi: 10.1097/PSY.0b013e3182648619. September. [DOI] [PubMed] [Google Scholar]
- 89.McCall W.V., Black C.G. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013;15(9):389. doi: 10.1007/s11920-013-0389-9. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pigeon W.R. Diagnosis, prevalence, pathways, consequences & treatment of insomnia. Indian J Med Res. 2010;131:321–332. February. [PMC free article] [PubMed] [Google Scholar]
- 91.Trockel M., Karlin B.E., Taylor C.B., Brown G.K., Manber R. Effects of cognitive behavioral therapy for insomnia on suicidal ideation in veterans. Sleep. 2015;38(2):259–265. doi: 10.5665/sleep.4410. February 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kessler R.C., Berglund P.A., Coulouvrat C., Hajak G., Roth T., Shahly V. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34(9):1161–1171. doi: 10.5665/SLEEP.1230. September 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vozoris N.T. Sleep apnea-plus: prevalence, risk factors, and association with cardiovascular diseases using United States population-level data. Sleep Med. 2012;13(6):637–644. doi: 10.1016/j.sleep.2012.01.004. June. [DOI] [PubMed] [Google Scholar]
- 94.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. March 19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement includes detailed description of the measures used in this study and well as data analysis regarding sleep aids and psychotropic medication.