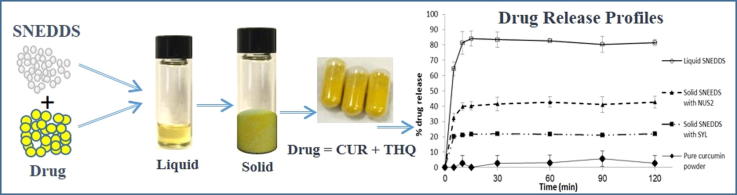
Graphical abstract
Curcumin (CUR) and Thymoquinone (THQ) were formulated using self-nanoemulsifying drug delivery systems (SNEDDS) as combined dosage form. The solubility, stability and drug release in dissolution media were significantly improved by the SNEDDS formulation compare to the raw drug powder.
Schematic Diagram: Dosage form design and CUR & THQ release after oral administration of lipid-based SNEDDS formulations.
Keywords: Self-nanoemulsifying drug delivery systems (SNEDDS), Solubility improvement, Curcumin, Thymoquinone, Combined therapeutic effects
Abstract
Background
Curcumin and Thymoquinone are very well-known phytochemicals for their potent anti-inflammatory and anticancer properties. The major challenges for curcumin is its poor aqueous solubility and erratic oral bioavailability.
Objective
To develop a novel liquid self-nanoemulsifying drug delivery system (SNEDDS) containing curcumin and thymoquinone and further converted into a solid dosage form using adsorbents Syloid® and Neusilin® as the solid carrier.
Methods
The characterization of the liquid and solid SNEDDS was performed by particle size & zeta potential analysis, scanning electron microscopy, differential scanning calorimetry, fourier transform infrared spectroscopy and X-ray powder diffraction. The drug loading, and in vitro release studies were carried out to investigate the efficiency of curcumin release from SNEDDS.
Results
The liquid SNEDDS containing black seed oil showed excellent self-emulsification performance with transparent appearance. The results of characterization studies showed that solidification using 50% (w/w) Syloid® and Neusilin® in the liquid formulation yield free flowing powder with no agglomeration but Neusilin® produced smooth granules than Syloid® and kept the drugs stable in amorphous state. In vitro dissolution studies indicated that liquid SNEDDS formulations of F4 and its solid SNEDDS using Neusilin® provided high dissolution efficiency and reproducibility for curcumin and thymoquinone. However, Neusilin® showed higher rate of dissolution (more than 65%, p < 0.05) compared to Syloid® for curcumin.
Conclusions
Curcumin loaded-SNEDDS formulation containing thymoquinone in liquid & solid dosage forms were successfully developed with an increased drug loading and dissolution rate, which could be the potential combined delivery system for various anti-inflammatory and anti-cancer treatments.
1. Introduction
Curcumin (CUR), a yellow colored turmeric spice (Curcuma longa) that belongs to the ginger family (Zingiberaceae). Besides its identity as coloring additive and preservative in foods, turmeric is used to treat as antibacterial and balancing blood sugar levels in human subjects. It has been used in many other common illnesses including stomach upset, stomach ulcers, dysentery, jaundice, arthritis, wounds, acnes, and skin/eye infections (Corson and Crews, 2007). CUR has been shown to have wide range of pharmacological activities including anti-inflammatory (Satoskar et al., 1986), anti-cancer (Kuttan et al. 1985), anti-oxidant (Sharma, 1976, ToDA et al., 1985), wound healing (Sidhu, et al., 1998) and anti-microbial effects (Negi et al., 1999) (Egan et al., 2004).
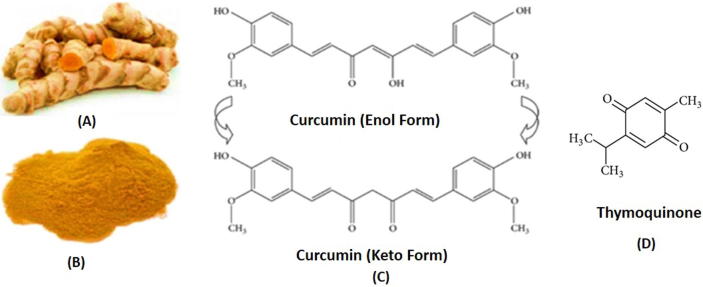
Curcumin (1,7-Bis(4-hydroxy-3-methoxy phenyl)-1-6- heptadiene-3,5-dione) is a low molecular weight hydrophobic polyphenol that can exist in different tautomeric forms, functionalized with methoxy and hydroxy groups (shown in Fig. 1A–C) (Payton et al., 2007).
Fig. 1.
Curcumin crude stem (A), raw powder (B) and chemical structure of CUR in Enol/Keto form (C) and THQ (D).
In patients undergoing surgery, oral application of CUR reduces post-operative inflammation (Satoskar et al., 1986, Kumar et al., 2002). CUR has also potent antiproliferative effects against a variety of tumors in vitro. It also enhances the antitumor effects of several classic chemotherapeutic drugs, such as doxorubicin, cisplatin, and paclitaxel (Bava et al., 2005).
Thymoquinone (THQ in Fig. 1D), is a phytochemical compound found in the plant Nigella sativa Linn (Ranunculaceae). It is an herbaceous plant widely used in indigenous system of traditional medicine for treatment of numerous disorders for over 2000 years (Zhang, 2013). Black seed oil (containing THQ) have been used widely in traditional Arabic medicine for the effective treatment of arthritis, lung diseases and hypercholesterolemia. The plant Nigella sativa has some reported pharmacological properties such as hypotensive, uricosuric, choleretic, anti-nociceptive, anti-diabetic, anti-histaminic, anti-oxidant, anti-inflammatory, anti-microbial, anti-tumor and immunomodulatory effects (Baetta and Corsini, 2011).
The combination therapy using CUR and THQ could produce greater therapeutic effect as well as reducing their toxicity (Woo et al., 2012) (Akhondian et al., 2011). Co-administration of black seeds (thymoquinone) and turmeric (curcumin) showed enhanced efficacy in preventing inflammations, cancers and metabolic syndrome in fructose-fed rats. The current study will demonstrate the therapeutic superiority of the combination of CUR and THQ at low possible doses against individual single unit dose and with improved treatment features (Decalf et al., 2016).
Almost 75% drugs in the current market, which are water insoluble and or poorly water soluble require innovative formulation design techniques in order to maximize bioavailability and drug exposure (Mohsin, 2012, Shahba et al., 2012, Mohsin et al., 2016). Nonetheless, a great number of the drug compounds get diminished in the GI tract after oral administration due to their poor aqueous solubility and sometimes due to high lipophilicity (Devraj et al., 2013). Lipid-surfactant based self-emulsifying formulations are constantly remaining as the first choice to overcome the limitations, and unpredicted oral bioavailability of such poorly water soluble lipophilic compounds (Mohsin, 2012, Juan et al., 2013, Obitte et al., 2014).
Among the self-emulsifying formulation systems, self-nanoemulsifying drug delivery systems (SNEDDS) are relatively more in demand technologically, which have shown to reduce the slow, and incomplete drug dissolution and facilitate the formation of its solubilized phase that are highly potent for systemic absorption (Umeyor et al., 2016a, Umeyor et al., 2016b). Primarily, SNEDDS is a liquid isotropic mixtures of active pharmaceutical drug in a combination of lipids, surfactants and water soluble co-solvents, which can be solidified as solid SNEDDS. (Zschiesche et al., 2002, Juan et al., 2013). SNEDDS can produce ultrafine emulsions (droplet size between 10 and 200 nm) upon gentle agitation in aqueous phase, such as the upper part of intestinal content. However, the most risk factor of developing formulations with the model drug CUR was its stability concern in the dosage form, where it was reported that CUR is not stable in liquid lipid systems (Pathak and Udupa, 2010).
One of the most important aspects of lipid-based SNEDDS is the most recent conversion technique, in which liquid formulation can be developed into solid dosage form (Shazly and Mohsin, 2015, Anthony et al., 2016, Shahba et al., 2018). This technique is getting more attention, where liquid lipid formulations have been converted in several recent studies utilizing adsorbent method, solid lipid nanoparticles technique and fluid bed coating techniques. Among all the solidification techniques, adsorbent method is the most economic, simple, and takes considerably less amount of time for development. The materials that are suitably used for adsorption on the surface are mainly silica substances and have high capacity to adsorb lipids (oils) to produce free flowing powder. This free flowing powder can be compressed directly into tablet by one step process or encapsulated into hard gelatin capsules. In the current investigations, adsorption method was taken into consideration to achieve the enhanced solubility and bioavailability with low production cost, convenience of process control, high stability and reproducibility, and better patient compliance of CUR dosage form.
Therefore, the aim of the current studies was to load a maximal amount of CUR in the most suitable liquid SNEDDS containing THQ, which was later converted into solid dosage form to increase its stability and reduce the impact of its in vitro dissolution time and carry the drug in solubilized form (avoiding precipitation) before entering to the systemic circulation for absorption.
2. Materials and methods
2.1. Materials
Curcumin (CUR, 99.5% pure) was purchased from Enzo life Sciences, (Lausen, Switzerland) and THQ (purity >99.8%) from Sigma Aldrich Company St Louis, MO, USA. Sodium chloride (NaCl), and Hydrochloric acid (HCl) were obtained from (BDH laboratories Ltd. UK). The high purity water was obtained through a Milli-Q Integral Water Purification System (Millipore, Bedford, MA). Imwitor 988 (I988, medium chain mono- and diglycerides), and HCO40 (PEG-40-hydrogenated castor oil) were obtained as gift from Nikko Chemicals Co. (Tokyo, Japan). Black seed oil (BSO) was collected directly from naturally obtained Nigella Sativa seeds by cold pressing (100% pure, no added solvents). The amount of thymoquinone was available in the BSO depending on the ratio used in the formulations. CrEL (Cremophor EL), and CrRH40 (Cremophor RH40) were purchased from BASF, Germany. Transcutol P (TcP) was kindly supplied by Gattefossé, France. Neusilin® US2 grade substance as adsorbent (fine powder of Magnesium Alumino Metasilicate) was obtained from Fuji Chemical Industry, Japan. Another adsorbent Syloid® 244 FP silica material was purchased from W. R. Grace & Co., USA. All other chemicals and solvents used in the studies were analytically pure and or HPLC grade, respectively.
2.2. Black seed oil (therapeutically active excipient)
The black seed oil is used for the first time to design SNEDDS which is chemically rich (Goreja, 2003). The fixed oil (>30% solvent free) extracted from the pure seeds has the active principles include thymoquinone (THQ), thymohydroquinone, dithymoquinone, thymol, carvacrol, nigellicine, nigellidine and -hedrin (Randhawa and Al-Ghamdi, 2002). The most of the prime actions have been recognized with use of THQ (2-isopropyl-5-methylbenzo-1, 4-quinone, approximately 4.59 mg/g content of BSO) (Fig. 1D). THQ is the most bioactive component and a hydrophobic molecule, thus its solubility is a challenge (Ali and Blunden, 2003, Salem, 2005) (549–740 µg/ml in aqueous solutions) (Salmani et al., 2014) to make it available for systemic circulation and cause limitations in drug formulation. THQ, as a naturally derived compound not only shows anti-oxidant and anti-inflammatory properties but also a vast array of other benefits. Although, it has lately received particular consideration but has been extensively explored in the current studies for its therapeutic properties.
2.3. Methods
2.3.1. Development of self-nanoemulsifying lipid formulations (SNEDDS)
The compositions of SNEDDS were prepared using different natural/ semi synthetic oils, hydrophilic surfactants and water-soluble co-solvents. The whole range of SNEDDS within lipid formulations were studied using only six components; Black seed oil (BSO), Imwitor 988 (I988), Cremophor EL (CrEL), CrRH40 (Cremophor RH40), Hydrogenated castor oil (HCO40), Transcutol P (TcP), and by changing just one excipient at a time (Table 1). As mentioned above black seed oil (BSO) contained the known phytochemical THQ (4.59 mg/g), which was quantified in the SNEDDS formulation along with model compound CUR.
Table 1.
The composition of excipients used at different ratios (% w/w) in the development of lipid formulation systems.
| Formulation | % BSO | % I988 | % HCO40 | % TcP | % CrEL | % CrRH40 |
|---|---|---|---|---|---|---|
| F1 | 21 | 9 | 70 | – | – | – |
| F2 | 21 | 9 | – | – | 70 | – |
| F3 | 21 | 9 | – | – | – | 70 |
| F4 | 12 | 12 | – | 6 | – | 70 |
** BSO: Black Seed Oil, I988: Imwitor 988, CrEL: Cremophor EL, Cr RH40: Cremophor RH40, TcP: Transcutol P, HCO40: hydrogenated castor oil.
2.3.2. Droplet size distribution, polydispersity index (PDI) and zeta potential analysis of liquid SNEDDS
The droplet size analysis was important for the current studies as the self-emulsifying efficiency is strongly associated with the mean droplet size and the clarity of the produced emulsion (Atef and Belmonte, 2008). The mean droplet size distribution and polydispersity index of SNEDDS in aqueous media was measured using Zetasizer (NanoZS, Malvern Instruments, UK) particle sizing instruments utilizing laser diffraction analysis (Agarwal et al., 2009). To avoid multiple scattering effects in the measurements, the formulations were diluted at a ratio of 1:1000 V/V (SNEDDS: Milli-Q water) and mixed for 1 min before analysis at room temperature (22 °C). Each sample was measured 10 times within one run using disposable capillary cuvette (DTS1060, Malvern Instruments, UK) equipped with electrodes. The particle size was calculated from the average volume size distribution.
The zeta potential (surface charge) were measured using plain folded capillary zeta cells by a Malvern Zetasizer Nano ZS90 (Malvern Instruments, UK) at 22 °C. All experiments in particle sizing analysis were performed at least three times and the average value were considered with good agreement found between measurements.
2.3.3. CUR solubility studies
Anhydrous formulation represents the pre-concentrate, which can have maximum drug loading capacity for dosage form design. The loading of CUR within the representative four SNEDDS was determined using the simple “shake flask” drug solubility method. Samples for solubility experiment were prepared at room temperature (22 °C ± 1 °C) with excess CUR in the SNEDDS. After 7-days incubation in dry heat incubator at 37 °C, the samples were removed and centrifuged (at 13,000 rpm for 10 min) to separate excess solid drug from dissolved drug. Then, an aliquot of the supernatant was taken by weight (approx. 50 mg) and diluted in 25 ml of methanol. The amount of CUR solubilized was analyzed using a validated ultra-high-performance liquid chromatography (UHPLC) method published by our group (Mohsin Kazi, 2017). THQ concentration which was already present in the SNEDDS were also determined along with CUR by the similar simultaneous UHPLC method analysis. Three replicate samples were considered for analysis of each formulation system.
2.3.4. Solidification of SNEDDS
It was hypothesized that free flowing powders may be obtained from liquid SNEDDS formulations by adsorption onto solid carriers. The adsorption is a simple quick process and just involves addition of the liquid formulation onto suitable carriers by mixing in a vortex device (for approx. 3–5 min). After solidification, the resulting powder may be filled directly into hard gelatin capsules. A significant benefit of the adsorption technique is good content uniformity. SNEDDS can be adsorbed at high levels (up to 70% w/w) onto suitable carriers (Witek et al., 1999). Solid carriers can be micro porous inorganic substances, high surface area colloidal inorganic adsorbent substances, cross linked polymers or nanoparticle adsorbents, for example, silica, silicates, magnesium trisilicate, magnesium hydroxide, talcum, crospovidone, cross-linked sodium carboxymethyl cellulose, cross linked polymethyl methacrylate (Carli and Chiellini, 2002), like Syloid® 244 FP and Neusilin® US2 (commercial adsorbent that are very common to use as solid carriers).
In the current study, Neusilin® US2 (NUS2) and Syloid® 244 FP (SYL) were chosen as adsorbent for the conversion of liquid SNEDDS. Optimised formulations of liquid SNEDDS were converted to solid SNEDDS by using NUS2 and SYL to get the advantage of the solid dosage form as free flowing powder. The prepared liquid formulation of CUR was added drop-wise on a solid adsorbent carrier in glass mortar. The mixture was physically mixed until uniform free-flowing powder was obtained. Then the powder was filled in a “00” size fish gelatin capsules for further studies.
2.3.5. Solid state characterization of solid SNEDDS
2.3.5.1. Differential scanning calorimetry (DSC)
The thermochemical properties of the pure CUR, CUR loaded solid SNEDDS were characterized by differential scanning calorimetry (DSC) using DSC-60, Shimadzu, Kyoto, Japan. Microbalance (Sartorius) was used to weigh small quantity (2 mg) of test samples and placed into an aluminum pan with a lid and then the pan was sealed. An empty aluminum pan sealed with its lid was used as a control for all the samples. The temperature ramp speed and the heat flow were set at 10 °C/ min and recorded from 40 to 250 °C, respectively. Each sample was purged with pure dry nitrogen at a flow rate of 70 ml/ min.
2.3.5.2. Field emission scanning electron microscopy (FESEM)
High-resolution images of CUR solid SNEDDS were produced using a field emission scanning electron microscope (Jeol JSM7600F). Samples were analyzed at different magnification ranged (2000x and 1000x) with immediate data capture of the images onto a personal computer. The solid CUR samples were scattered on double-side adhesive carbon tape, which was then attached to FESEM specimen mounts. The specimens were sputter-coated for 2 min to obtain uniform coating on the sample by Auto fine coater (Jeol JFC-1600).
2.3.5.3. Fourier transform infrared spectroscopy (FT-IR)
FT-IR studies were done to assess whether any possible interaction is existing among drug CUR, oil, surfactant and co-solvents. The complexation and chemical properties of powdered samples was performed by Fourier Transform Infrared spectroscopy (FT-IR Spectrum BX from Perkin Elmer LLC, USA). A suitable amount of pure CUR powder, CUR loaded solid SNEDDS-SYL and CUR loaded solid SNEDDS-NUS2 powder were equipped by compressing the powders for 5 min at 5 bars on a KBr press and the spectra were scanned on the wavenumber range of 400–4400 cm−1.
2.3.5.4. X-ray diffraction study (XRPD)
Powder X-ray diffraction of the sample, was evaluated by Ultima IV diffractometer (Rigaku, College of Pharmacy, King Saud University, Saudi Arabia) over the 3–60° 2θ range at a scan speed of 0.5 deg./min. The tube anode was Cu with Ka = 0.1540562 nm monochromatized with a graphite crystal. The pattern was collected at 40 kV of tube voltage and 40 mA of tube current in step scan mode (step size 0.02°, counting time 1 s per step).
2.3.5.5. In vitro dissolution studies
The final SNEDDS formulations were selected according to optimal solubilization capacity of the CUR. The release studies of the representative SNEDDS formulations were performed using automated dissolution tester with an automated sample collector, dissolution apparatus II (paddle type, Model UDT-814, LOGAN Inst. Corp., USA) with 500 ml of pH 1.2 solution as acid medium to simulate stomach medium at 37 ± 0.5 °C. The rotation speed of the paddle was adjusted to 50 rpm. Then, the drug loaded formulations (encapsulated in fish gelatin capsules, size “00”) were dropped into dissolution medium using capsule sinkers to prevent the capsules from floating in the medium. On the other hand, the capsule was filled with solid SNEDDS powder (prepared with NUS2 and SYL at 5 mg dose of solubilized CUR), and the pure CUR only powder contained 5 mg of CUR. At predetermined time intervals an aliquot (2 ml) of the sample was collected and analyzed for CUR contents by UHPLC. An equivalent volume (2 ml of the fresh dissolution medium solution), was immediately added to the vessel which was withdrawn due to sampling. Samples were collected periodically after 5, 10, 15, 30, 45, 60, 90, and 120 min and replaced by freshly prepared dissolution medium. The dissolution studies were carried out in triplicates.
2.4. Dynamic dispersion studies
CUR was dissolved in the representative F4-SNEDDS at a dose equivalent to 60% of its maximum loading capacity estimated in the relevant anhydrous formulation. The formulation which has high drug loading capacity from the study of the equilibrium solubility was included in the corresponding dynamic dispersion studies to investigate whether the model drug will precipitate during dispersion in bulk aqueous media and the precipitation rate. 500 mg of formulation was dropped into 50 ml aqueous media (1 in 100 dilution) using milli-Q water & Fasted State Simulated Intestinal Fluid (FaSSIF) and kept in a dry heat incubator at 37 °C for 24 h with occasional shaking. During this 24 h incubating period, 1 ml of the dispersed sample from each container was withdrawn periodically (at certain intervals from 0 to 24 h), and centrifuged for 5 min at 13,000 g. A 100 µl aliquot of the resulting supernatant was assayed by the developed UHPLC method to quantify the drug concentration which was remained in solution during aqueous dispersion. The experiments were performed in triplicates.
3. Results and discussion
Over the years, several studies have been conducted to improve the solubility and absorption of CUR from the intestine after oral administration of different doses using rat models (Ravindranath and Chandrasekhara, 1980, Ravindranath and Chandrasekhara, 1981). Most of the studies showed that oral administration of CUR in rats resulted in approximately 80% being excreted in the feces (Wahlström and Blennow, 1978) (Holder et al., 1978) suggesting very poor absorption of CUR from the intestine. Few other studies have confirmed the presence of different metabolites of CUR, which has been shown to be bio-transformed to dihydrocurcumin and tetrahydrocurcumin (Pan et al., 1999). In another study, the main biliary metabolites of CUR are found as glucuronide conjugates of tetrahydrocurcumin and hexahydrocurcumin (Holder et al., 1978). Therefore, an effort was given to the efficient formulation development so that high amount of CUR along with THQ can be loaded and maintained in solubilized form in intestinal contents for better absorption.
3.1. SNEDDS development and their assessment
The excipients used to design SNEDDS in this research and the way in which they were blended together to represent various formulation systems are shown in Table 1.
Table 1 shows that four formulations were developed using various concentrations of oils, surfactants and or cosolvents. The first three formulations (F1-F3) contained 21% BSO each, whereas formulation F4 has 12% BSO. Among all the formulations, F4 has less THQ quantity due to the low amount of BSO present in the formulation. F1-F3 formulations contained high amount of (70%) surfactant without any co-solvent but F4 contained 6% co-solvent (TcP) at the expense of oil%.
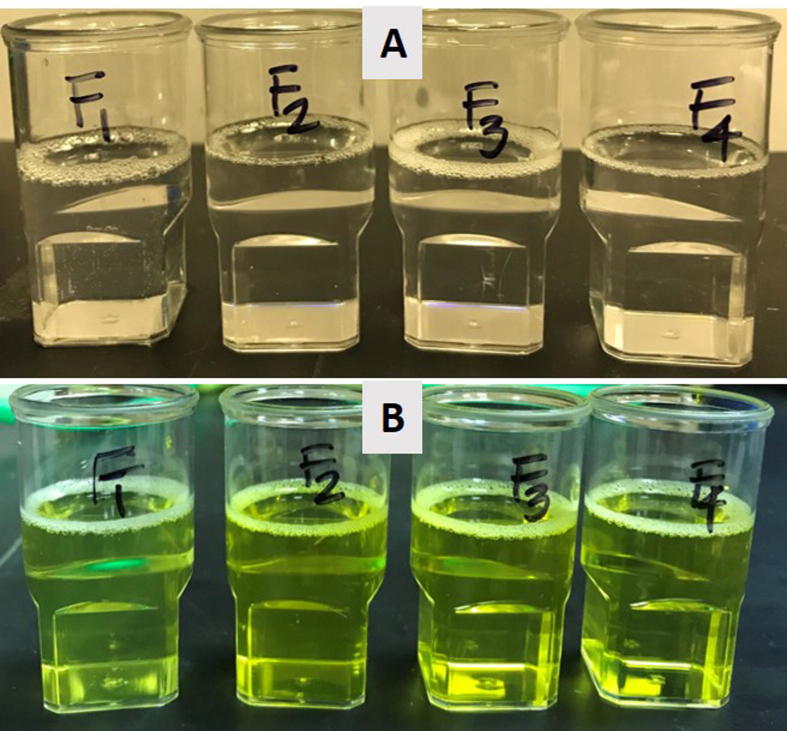
Within the context of self-emulsifying efficiency, visual assessment is a smart preliminary evaluation method to sensor and reduce the excess usage of chemicals due to the trial & errors. In the current study, visual assessment helped to determine the self-emulsification properties of the formulation labeled as SNEDDS (Kommuru et al., 2001). The following factors for visual assessments were taken into consideration during the optimization of SNEDDS: miscibility of the oil/surfactant mixture, homogeneity and appearance upon aqueous dilution (ratio maintained, formulation: water 1:1000). Within the scope of the current research work, efficient formulations were labeled if the formulations were homogeneous and have clear appearance.
Table 2 showed the efficiency assessment results where providentially all the four formulations were transparent after aqueous dilution. Therefore, these formulations were considered as SNEDDS due to their “transparent” appearances and passed to further experimental studies.
Table 2.
Performance of self-emulsifying lipid-based formulations in terms of homogeneity, appearance and self-emulsification capability.
| Formulation | Compositions (w/w) | Homogeneity | Appearance |
|---|---|---|---|
| F1 | {BSO:I988 (7:3)/HCO40}[3/7] | Yes | Transparent |
| F2 | {BSO:I988 (7:3)/CrEL}[3/7] | Yes | Transparent |
| F3 | {BSO:I988 (7:3)/CrRH40}[3/7] | Yes | Transparent |
| F4 | {BSO:I988:TcP (2:2:1)/CrRH40}[3/7] | Yes | Transparent |
Oils were important ingredient of the system to solubilize large amount of CUR and may also facilitate the transport via intestinal lymphatic system (depending on the lipophilicity), and therefore increase drug absorption from the gastro intestinal tract (GIT). Long and medium chain triglyceride (LCT/MCT) oils from natural sources or slightly modified with varying degree of saturation have been commonly used to design SNEDDS system. The surfactant is an essential excipient to stabilize SNEDDS and make it possible to uptake large amounts of drug compounds as a result of improved solvent capacity.
It is worth mentioning that initially only the visual observation may be enough for an experienced formulator to differentiate good and poor formulations. The picture in Fig. 2 shows that all four formulations were producing transparent dispersion when diluted with water at maximum 1 in 1000 dilution level.
Fig. 2.
Appearance of the blank SNEDDS (A) (THQ naturally present) and CUR loaded formulations (SNEDDS) (B) after aqueous dispersion with water.
3.2. Particle sizing, PDI and zeta potential measurement
The variety of components used in SNEDDS may affect the size of droplet upon aqueous dispersion in presence of the stomach contents. Therefore, the droplet size analysis of the formulation was essential within the scope of the study. Table 3 summarised the mean droplet size and monodispersity of the SNEDDS formulations which were critical factors to their stability. In the study, the droplet size measurement and PDI values suggest that water soluble (polar) excipients in the formulation can produce relatively the lower nanodroplets upon aqueous dispersion. Hence, the droplet sizes of all the formulations were less than 26 nm with widely monodispersed in the aqueous media (polydispersity values of less than 0.2).
Table 3.
Mean particle size, polydispersity index (PDI) and zeta potential values of the CUR-THQ loaded different liquid SNEDDS formulations.
| Formulation | Particle size (d.nm) | PDI* | Zeta potential (mV) |
|---|---|---|---|
| [BSO:I988 (7:3)/HCO40 [3:7] | 25.41 ± 9.20 | 0.189 ± 0.055 | −9.19 ± 1.18 |
| [BSO:I988 (7:3)/CrEL [3:7] | 20.46 ± 7.13 | 0.187 ± 0.015 | −19.88 ± 2.57 |
| [BSO:I988 (7:3)/CrRH40 [3:7] | 18.34 ± 8.22 | 0.147 ± 0.087 | −14.2 ± 1.02 |
| [BSO:I988:TcP (2:2:1)/CrRH40 [3:7] | 19.81 ± 3.09 | 0.134 ± 0.050 | −23.15 ± 2.69 |
PDI – “Polydispersity Index” is a measure of the heterogeneity of particle sizes in a mixture.
The zeta potential absolute values of all four CUR loaded SNEDDS were recorded for F1: −9.19 mV, F2: −19.88 mV, F3: −14.20 mV and F4: −23.15 mV, respectively (shown in Table 3). The negative value of the surface charge could be attributed to the presence of lipid excipients in the formulation. The higher surface charge suggests the stabilization of the SNEDDS for long periods of time.
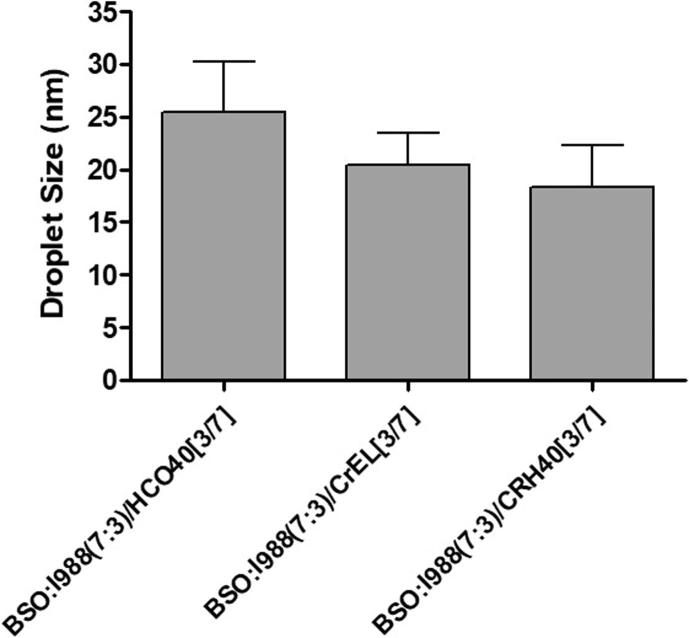
3.3. The droplet size of the SNEDDS: Effect of surfactant
Fig. 3 is showing the particle size of three SNEDDS formulations (F1-F3) containing different surfactants with same lipid compositions. CUR loaded SNEDDS using surfactant Cremophor RH40 (HLB ≈ 14–16) showed smaller particle sizes than other SNEDDS containing Cremophor EL (HLB ≈ 12–14) and HCO40 (HLB ≈ 12.5). The mean particle diameters of the SNEDDS composed of Black Seed Oil (BSO) and Imwitor 988 with Cremophor RH40 was the lowest of 18.34 nm. On the other hand, the SNEDDS composed of BSO and Imwitor 988 with surfactants Cremophor EL and HCO40 were higher in particle diameter of 20.46 and 25.4 nm, respectively. The mean particle diameters of all the SNEDDS in Fig. 3 suggested that if the SNEDDS formulation contains hydrophilic surfactant with higher HLB value, it could reduce the particle size substantially. However, the formulators should be aware of using high concentration of hydrophilic surfactants in the SNEDDS as it may increase stomach irritation.
Fig. 3.
Effect of surfactant on the particle size of CUR-THQ loaded SNEDDS.
3.4. Physical stability study
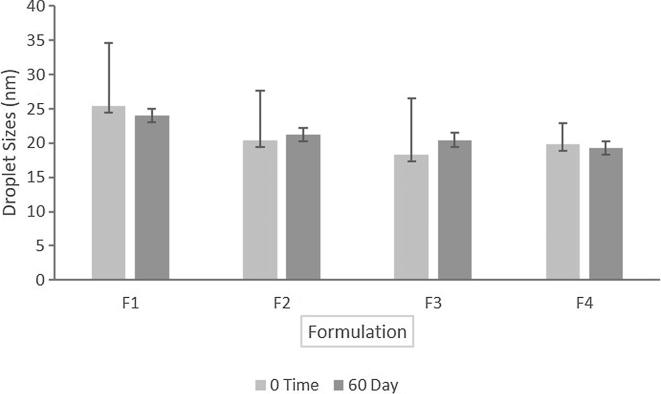
The time course-dependent change in the average droplet sizes of the representative lipid SNEDDS containing CUR 60% of the equilibrium solubility were assessed during sample storage for 60 days at room temperature (22 ◦C). The results are presented in Fig. 4. The average particle sizes at day 60 were 24.01 nm for F1, 21.20 nm for F2, 20.47 nm for F3 and 19.30 nm for F4, respectively. The results suggested no significant changes in droplet sizes at two different storage conditions due to the stable dispersion in aqueous media.
Fig. 4.
Particle sizes (nm) of the SNEDDS formulations at zero time and 60th day of storage at room temperature.
3.5. Equilibrium solubility of CUR in SNEDDS and drug stability
The therapeutic efficiency of CUR is very limited due to its poor aqueous solubility and low oral bioavailability. In this study, we tried to develop SNEDDS with new mixing ratio, which might improve the solubility and oral absorption of CUR. During the initial SNEDDS screening process for CUR, the following important parameters were taken into considerations: (1) the selection of simple, orally safe, and compatible formulation compositions, (2) good solubility of CUR in various components, and (3) the efficient droplet size after forming nanoemulsifying systems (Zhang et al., 2008). It was evident that selection of oil, surfactant and co-solvent as well as the mixing ratio of oil to surfactant/co-solvent could play a vital role on the performance of SNEDDS formulation.
The solubility of CUR in anhydrous SNEDDS formulations were shown in Table 4. The components used in the SNEDDS were able to solubilize the maximum amount of drug and possessed a high efficiency in self-emulsification approach.
Table 4.
Equilibrium solubility of CUR and THQ in various self-nanoemulsifying formulations (SNEDDS) at 0 months and 3 months.
| No. | Composition | Solubility at 0 month |
Solubility at 3 months |
||
|---|---|---|---|---|---|
| Solubility of CUR (mg/g) | THQ found (mg/g) | CUR (mg/g) | THQ (mg/g) | ||
| F1 | BSO:I988 (7:3)/HCO40 [3:7] | 11.097 ± 1.232 | 0.914 ± 0.075 | 10.988 ± 0.985 | 0.914 ± 0.023 |
| F2 | BSO:I988 (7:3)/CrEL [3:7] | 7.966 ± 0.980 | 0.911 ± 0.066 | 8.010 ± 0.555 | 0.911 ± 0.099 |
| F3 | BSO:I988 (7:3)/CrRH40 [3:7] | 10.095 ± 0.755 | 0.860 ± 0.102 | 9.791 ± 1.011 | 0.860 ± 0.125 |
| F4 | BSO:I988:TcP(2:2:1)/CrRH40 [3:7] | 23.396 ± 1.246 | 0.542 ± 0.081 | 21.785 ± 1.081 | 0.532 ± 0.009 |
Amongst all the anhydrous formulations, BSO: I988 (7:3) with HCO40 at ratio [3:7] developed as F1 formulation provided 11.1 mg/g solubility of CUR with clear appearance upon aqueous dilution. Formulations (F2 and F3), which contained the same oil combinations as F1 but replaced with surfactant Cremophor EL and Cremophor RH 40 at ratio [3:7], respectively showed the comparably lower solubility (7.97 mg and 10.09 mg) in anhydrous formulations. These formulations also yielded a transparent clear appearance when dispersed in aquesous media. On the other hand, BSO, I988, & TcP at (2:2:1) ratio with 70% CrRH40 as surfactant have high equilibrium solubility of 23.40 mg and also produced a transparent clear appearance.
From the overall solubility results, it was confirmed that among all SNEDDS formulations, the higher drug solubility and better aqueous dispersibility was reported for F4 formulation [Transparent appearance, BSO: I988: TcP (2:2:1)/ CrRH40 (3/7)] and chosen for further experimental studies of solidification and characterization of SNEDDS, in vitro dissolution and dispersion for CUR. It was also established that CUR prefers the SNEDDS which contains more polar glycerides and water soluble surfactants.
The solubility experiment of CUR and THQ was conducted at initial and after 3 months’ incubation period to establish the drug stability profile in SNEDDS. If the clear picture is appeared on the stability concern of the SNEDDS then it could be easy to develop the dosage form for particular drug. The concentration of CUR and THQ in all the SNEDDS were not reduced more than 5% which suggested that the SNEDDS were stable for several months in liquid form (Table 4).
3.5.1. Estimation of THQ in the blank and CUR loaded SNEDDS
Thymoquinone (THQ) was the main component of Black Seed Oil (BSO) which was used in all the SNEDDS formulations. Table 5 summarized the concentration of THQ available in the blank formulations and combined with CUR in the SNEDDS. The pure BSO oil (contains 4.59 mg/g THQ) is nondispersible in aqueous media therefore it was used partly to have the efficient SNEDDS. The THQ concentration was almost similar in all blank formulations due to the same fraction of BSO used (SNEDDS F1, F2 & F3 systems comprised 210 mg/g BSO, THQ ≈ 0.914 – 0.860 mg/g), except F4, which has low amount of BSO (120 mg/g, THQ ≈ 0.539 mg/g, P < 0.05) in the SNEDDS. The % THQ was not significantly different from blank SNEDDS compared with the CUR loaded SNEDDS (Table 5). The highest % THQ was obtained from F4 blank and CUR loaded formulation of 97.86%, and 98.40%, respectively. However, it was slightly higher compared to other three formulations (F1, F2, & F3) which were contributed to 95.24%, 94.41%, & 90.47% THQ in the blank SNEDDS and 94.82%, 94.51% & 89.22% THQ in the CUR loaded SNEDDS, respectively. The overall data from the THQ concentrations in blank and CUR loaded SNEDDS suggests that there was no interaction recorded between THQ and CUR in the combined dosage form. Thus the combined dosage form of THQ-CUR could have the potential therapeutic benefit if loaded with SNEDDS.
Table 5.
% THQ concentration in blank and CUR loaded self-nanoemulsifying formulations. Pure Black Seed Oil (BSO) naturally contains 4.59 mg/g (0.46%) of THQ.
| Formulations | BSO (mg) in SNEDDS | THQ found in blank SNEDDS (mg/g) |
THQ found with CUR (mg/g) |
||
|---|---|---|---|---|---|
| THQ (mg/g) | %THQ | THQ (mg/g) | %THQ | ||
| BSO:I988 (7:3)/HCO40 [3:7] | 210 | 0.918 ± 0.010 | 95.24 | 0.914 ± 0.075 | 94.82 |
| BSO:I988 (7:3)/CrEL [3:7] | 210 | 0.910 ± 0.091 | 94.41 | 0.911 ± 0.066 | 94.51 |
| BSO:I988 (7:3)/CrRH40 [3:7] | 210 | 0.872 ± 0.086 | 90.47 | 0.860 ± 0.102 | 89.22 |
| BSO:I988:TcP(2:2:1)/CrRH40[3:7] | 120 | 0.539 ± 0.070 | 97.86 | 0.542 ± 0.081 | 98.40 |
3.6. In vitro dissolution studies of liquid and solid SNEDDS
It is well-known that CUR and THQ as pure powder are poorly soluble in water and aqueous solution containing acidic pH. The in vitro dissolution experiments were conducted for pure CUR powder, liquid SNEDDS and solid SNEDDS containing CUR and THQ using two adsorbents such as Syloid® 244 FP and Neusilin® US2 to investigate the drug release profiles.
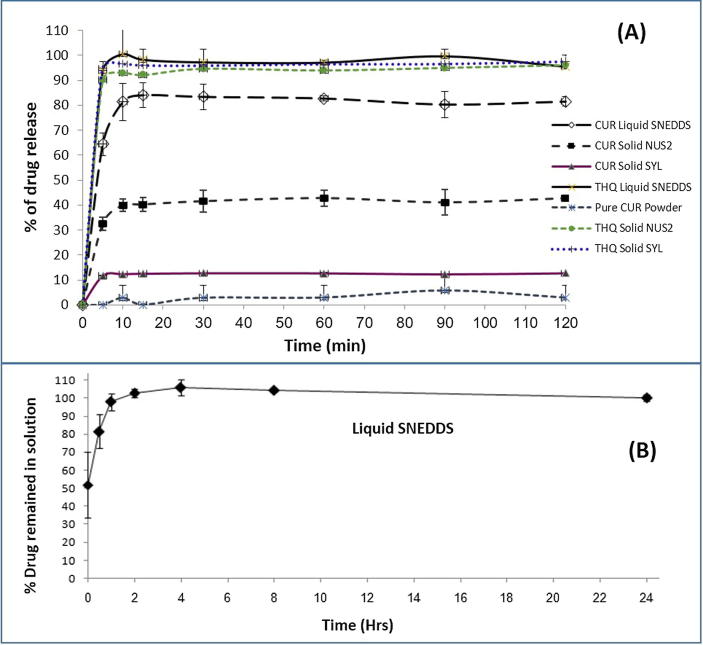
The release profiles as shown in Fig. 5A, pure CUR powder showed very low release (less than 2%) even after 120 min in gastric media (pH 1.2). The release of CUR from the liquid SNEDDS showed the best dissolution performance as approximately 85% of CUR from the liquid SNEDDS was released in the medium within 10 min. Upon aqueous dilution, liquid SNEDDS dispersed very quickly to form nanodroplets in the dissolution medium. It was also apparent that release of CUR from liquid SNEDDS was independent of pH under the condition of dissolution. Solidification of SNEDDS showed different results compared to the liquid SNEDDS for CUR. Solid SNEDDS using SYL could only release 20% CUR during 120 min dissolution times. The solid SNEDDS using NUS2 has almost 40% release, which suggest that NUS2 has better performance than SYL for CUR.
Fig. 5.
In vitro dissolution profiles (A) of the representative CUR and THQ loaded liquid F4 SNEDDS (F4- BSO: I988: TcP (2:2:1)/CrRH40 (3/7)), solid F4 SNEDDS with NUS2, solid F4 SNEDDS with SYL and pure CUR powder in gastric media (pH 1.2), (B) % Cur in solution during 24 hrs time after 1:100 dilution in the gastric media (FaSSIF at pH 5.0) (CUR was dissolved at 60% of the equilibrium solubility in the anhydrous F4). The formulation represents only liquid F4 SNEDDS. Data are presented as mean ± SD, (n = 3).
On the other hand, the liquid and solid SNEDDS containing THQ had superior release profiles compared to CUR. Almost 99% THQ was released within the first 10 mins in the dissolution media from the liquid SNEDDS. Similarly, the higher release profiles of THQ were observed with the solid SNEDDS using SYL and NUS2 as 96.45% and 92.92%, respectively. The overall release profiles suggested that both CUR and THQ has the highest release profiles from liquid SNEDDS but CUR has poor release from solid SNEDDS compared to THQ (P <0.05).
The reason for poor release of CUR from the solid SNEDDS is the use of adsorbent in the liquid SNEDDS. The adsorbent SYL and NUS2 is porous alumino metasilicate, which may keep the drug CUR (externally loaded) in their pores and suppress the release. Whereas this was not occurred in case of THQ as it was internally (naturally) present in BSO. In the future, to have better release profiles of CUR, it is necessary to close the pores of the adsorbent.
3.7. Dynamic in vitro dispersion studies
In order to investigate how long, the CUR concentration remains in solution or if any quick precipitation is likely to occur for the representative liquid F4 SNEDDS formulation, dynamic dispersion tests in vitro were carried out and compared with equilibrium solubility experiments. The dispersion test was carried out using only liquid F4 SNEDDS containing CUR at an amount equivalent to 60% (14.04 mg) of the equilibrium solubility. Fig. 5B showed the % CUR that was remaining in solution at 0, 0.5, 1, 2, 4, 8 and 24 h after dispersion of the formulation in fasted state intestinal media (FaSSIF at pH 5.0) at 37 °C. It was also indicated the concentration of CUR which was maintained in a supersaturated state immediately after the dilution had taken place. In Fig. 5B, the percent of the original dose remaining in solution was plotted against time. The result from the study showed that F4 (Which contain BSO and I988 with Cremophor RH40) maintained at least 98% of the dose (CUR concentration) in solution.
Lipid based formulation within oral drug delivery systems has been disadvantaged by the lack of in vitro data describing the relationship between formulation and performance in vivo. SNEDDS that disperse within few seconds to give an ultrafine emulsion or microemulsion are well-designed in appearance, but a more important characteristic is the ability of the formulation to keep the drug in solution throughout its transit through the gastrointestinal tract. To achieve this goal, the drug must be keep in solution during dispersion, which typically takes place in the stomach, and also during digestion and subsequent to digestion in the small intestine. In this study we focused on the dispersion phase in intestinal media (FaSSIF) and examine how precipitation rate is influenced by the different excipients used in the formulation.
A recent study by Dai et al., has put an insight on the correlation between drug precipitation and in vivo bioavailability (Dai et al., 2007) indicating the potential significance of this approach. From the past experimental studies, we strongly believe that in vitro digestion testing should be used in parallel with dispersion/precipitation testing to predict the in vivo performance of SNEDDS formulations. The two in vitro dynamic techniques, when used in parallel offer the formulator a great advantage of anticipating the likelihood of drug precipitation in both the stomach and intestine (Cuiné et al., 2007).
3.8. Characterization studies
3.8.1. Solid SNEDDS
3.8.1.1. Differential scanning calorimetry (DSC)
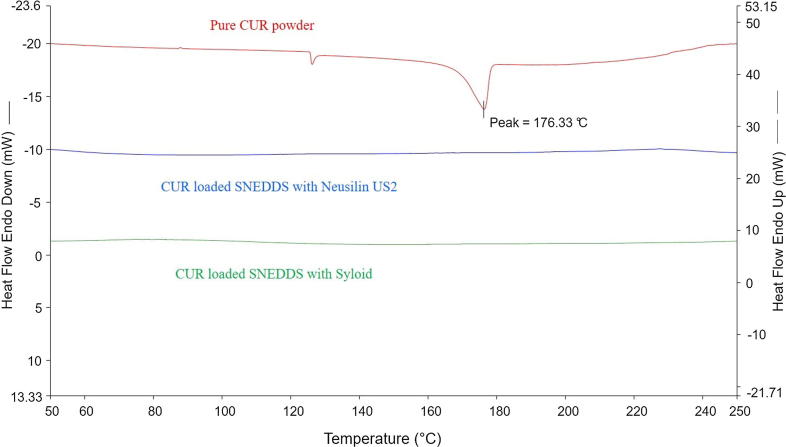
Thermodynamical techniques were applied for determining the thermal stress of pure drug and medicinal compounds of the excipients as well as their interactions during the formulation development process. Polymorphic transformation in lipid-based drug delivery systems, could take place during the conversion of solid dosage form (i.e., solid SNEDDS), thus may exhibit significant effects on drug loading and delivery. Fig. 6 shows the DSC thermograms of pure CUR powder, CUR solid SNEDDS (F4) with NUS2, and CUR solid SNEDDS (F4) with SYL. Pure CUR powder showed a single sharp endothermic melting peak at about 176 °C corresponding to its melting point, indicating its characteristic crystalline nature. As observed in Fig. 6 the DSC trace of CUR solid SNEDDS with NUS2 and CUR solid SNEDDS with SYL did not contain any endothermic peak (the endothermic peak of crystalline drug was disappeared). This suggests the conversion of crystalline CUR to the amorphous CUR which could be attributed to complete dissolution of the drug in the solid SNEDDS of F4. This phenomenon was also true for all the CUR loaded solid SNEDDS formulations investigated in the current studies.
Fig. 6.
Differential scanning calorimetric thermogram of F4 SNEDDS formulation: (A) Pure CUR powder, (B) CUR SEDDS with NUS2, and (C) CUR SNEDDS with SYL.
3.8.1.2. Scanning electron microscopy (SEM)
SEM pictures of pure CUR powder, CUR solid SNEDDS with NUS2, and CUR solid SNEDDS with SYL are given in Fig. 7 which illustrated the arrangement of pure CUR powder, CUR loaded SNEDDS with NUS2 and with SYL for F4 SNEDDS. The image of pure CUR was observed to be irregular in shape while CUR loaded SNEDDS using both NUS2 & SYL was observed to be discrete, spherical, and regular in shape. However, there was a large particle evidenced due to aggregation of solidified SNEDDS formulation.
Fig. 7.
Scanning electron microscopy images of F4 Formulation: (A) Pure CUR powder, (B) CUR loaded SNEDDS with NUS2, and (C) CUR loaded SNEDDS with SYL.
3.8.1.3. X-ray powder diffraction (XRD)
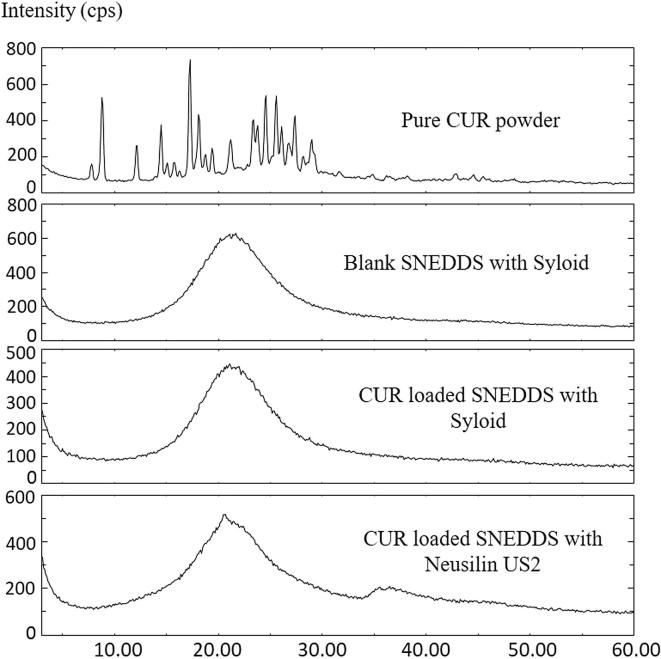
XRD analysis is a distinctive method in determining the crystallinity of a drug compound with proper interpretation before and after loading in the SNEDDS formulation, thus allows the identification of the drug crystalline changes. Fig. 8 shows the X-ray diffraction (XRD) pattern of pure CUR powder; solid blank SNEDDS with SYL (without CUR), solid blank SNEDDS with NUS2 (without CUR), solid CUR loaded SNEDDS using SYL and solid CUR loaded SNEDDS using NUS2. Pure CUR manifested the distinct peaks at 2θ: 7.8°, 8.8°, 12.1°, 14.5°, 15.8°, 17.3°, 18.1°, 19.4°, 21.2°, 23.3°, 24.6°, 25.6°, 26.1°, 27.4°, 28.2°, 29°, 42.8° indicating the highly crystalline form of the drug. The XRD pattern of solid blank SNEDDS using NUS2 & SYL without CUR showed peaks at about 20.2°, 21.6°, and 22.3°, indicating the crystalline nature of the lipid. The characteristic peaks of CUR in the XRD pattern of CUR loaded SNEDDS indicated that CUR was not in crystalline nature in SNEDDS and the amorphous state would contribute to the higher drug up taking capacity of SNEDDS. Furthermore, the major peak of drug loaded lipid did not shift but had a reduced intensity as compared to the free lipid. This may be attributed to the incorporation of CUR between the parts of the lipid components, leading to a change in the crystallinity of the CUR loaded SNEDDS.
Fig. 8.
X-ray powder diffraction of F4 Formulation: (A) Pure CUR powder; (B) SYL solid SNEDDS without CUR, (C) CUR solid SNEDDS with SYL, and (D) CUR solid SNEDDS with NUS2.
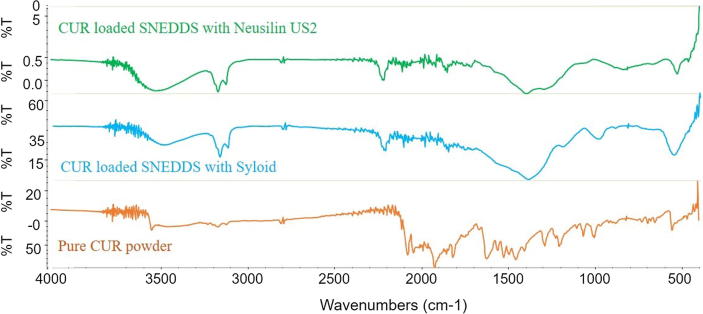
3.8.1.4. Fourier transform-infrared spectroscopy (FT-IR)
The FT-IR spectra of pure CUR powder, CUR loaded solid SNEDDS using SYL and NUS2 were shown in Fig. 9. In FT-IR analysis, the infrared spectrum of different chemical bonds or functional groups were located in a different wavenumber which were in the state of vibration. So the presence of peak at particular wavenumber can directly reflect the presence of a specific chemical bond (Wan et al., 2012). In contrast, if specific interactions took place between CUR and SNEDDS formulation, new peaks or a shift of existing peaks would appear. The overall FT-IR results proved no chemical interaction between drug and its carriers (representative SNEDDS). Similar conclusion was drawn for other formulations of similar nature.
Fig. 9.
FT-IR spectra of pure CUR powder, solid CUR loaded SNEDDS with SYL and solid CUR loaded SNEDDS with NUS2.
4. Conclusions
SNEDDS formulation of CUR containing 12% black seed oil, 12% Imwitor 988, and 6% Transcutol P with 70% surfactant Cremophor RH40 was successfully developed and optimized based on lower droplet size (nanometer range with good polydispersity), higher solubility and therefore increased dissolution rate. The representative liquid SNEDDS was successfully solidified and encapsulated using hard gelatin capsules. The optimized F4 liquid and solid CUR loaded SNEDDS formulation showed higher percent cumulative CUR release as compared to the pure drug powder without any significant change in the observed physical parameters. The present investigation confirmed that the developed SNEDDS of CUR & THQ formulation was superior to CUR marketed products with respect to in vitro dissolution profile and could be used as a potential nanocarrier system to deliver high amount of CUR and THQ to the systemic circulation with enhanced bioavailability.
Acknowledgments
Acknowledgement
The author would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding through research group number (RG#1435-017).
Declaration of Competing Interest
The author reports no declarations of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal V., Siddiqui A., Ali H., Nazzal S. Dissolution and powder flow characterization of solid self-emulsified drug delivery system (SEDDS) Int. J. Pharm. 2009;366(1):44–52. doi: 10.1016/j.ijpharm.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Akhondian J., Kianifar H., Raoofziaee M., Moayedpour A., Toosi M.B., Khajedaluee M. The effect of thymoquinone on intractable pediatric seizures (pilot study) Epilepsy Res. 2011;93(1):39–43. doi: 10.1016/j.eplepsyres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Ali B., Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytotherapy Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- Attama Anthony A., Ebele F.C.K., Onuigbo B., Nnamani Petra O., Obitte Nicholas, Finke Jahn H., Pretor Sascha, Müller-Goymann Christel C. Solid lipid nanoparticles encapsulating a fluorescent marker (coumarin 6) and antimalarials – artemether and lumefantrine: evaluation of cellular uptake and antimalarial activity. Eur. J. Nanomed. 2016;8(3):129–138. [Google Scholar]
- Atef E., Belmonte A.A. Formulation and in vitro and in vivo characterization of a phenytoin self-emulsifying drug delivery system (SEDDS) Eur. J. Pharm. Sci. 2008;35(4):257–263. doi: 10.1016/j.ejps.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Baetta R., Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs. 2011;71(11):1441–1467. doi: 10.2165/11591400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bava S.V., Puliappadamba V.T., Deepti A., Nair A., Karunagaran D., Anto R.J. Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-κB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J. Biol. Chem. 2005;280(8):6301–6308. doi: 10.1074/jbc.M410647200. [DOI] [PubMed] [Google Scholar]
- Carli F., Chiellini E. Google Patents; 2002. Pharmaceutical composition comprising a water/oil/water double microemulsion incorporated in a solid support. [Google Scholar]
- Chukwuebuka Umeyor A.A., Uronnachi Emmanuel, Agbo Chinazom, Reginald-Opara Joy, Kenechukwu Franklin. Formulation of gentamicin as surface modified self-nanoemulsifying formulations (SNEFs) improves its anti-pneumococcal activity. Eur. J. Nanomed. 2016;8(2) [Google Scholar]
- Corson T.W., Crews C.M. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130(5):769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuiné J.F., Charman W.N., Pouton C.W., Edwards G.A., Porter C.J. Increasing the proportional content of surfactant (Cremophor EL) relative to lipid in self-emulsifying lipid-based formulations of danazol reduces oral bioavailability in beagle dogs. Pharm. Res. 2007;24(4):748–757. doi: 10.1007/s11095-006-9194-z. [DOI] [PubMed] [Google Scholar]
- Dai W.G., Dong L.C., Shi X., Nguyen J., Evans J., Xu Y., Creasey A.A. Evaluation of drug precipitation of solubility-enhancing liquid formulations using milligram quantities of a new molecular entity (NME) J. Pharm. Sci. 2007;96(11):2957–2969. doi: 10.1002/jps.20886. [DOI] [PubMed] [Google Scholar]
- Decalf J., Tarbell K.V., Casrouge A., Price J.D., Linder G., Mottez E., Sultanik P., Mallet V., Pol S., Duffy D., Albert M.L. Inhibition of DPP4 activity in humans establishes its in vivo role in CXCL10 post-translational modification: prospective placebo-controlled clinical studies. EMBO Mol. Med. 2016;8(6):679–683. doi: 10.15252/emmm.201506145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devraj R., Williams H.D., Warren D.B., Mohsin K., Porter C.J., Pouton C.W. In vitro assessment of drug-free and fenofibrate-containing lipid formulations using dispersion and digestion testing gives detailed insights into the likely fate of formulations in the intestine. Eur. J. Pharm. Sci. 2013;49(4):748–760. doi: 10.1016/j.ejps.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Egan M.E., Pearson M., Weiner S.A., Rajendran V., Rubin D., Glöckner-Pagel J., Canny S., Du K., Lukacs G.L., Caplan M.J. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304(5670):600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- Goreja W. Karger Publishers; 2003. Black Seed: Nature's Miracle Remedy. [Google Scholar]
- Holder G.M., Plummer J.L., Ryan A.J. The metabolism and excretion of curcumin (1, 7-bis-(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione) in the rat. Xenobiotica. 1978;8(12):761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- Juan H., Jing T., Hua-Wan Y. P-gp induction by curcumin: an effective antidotal pathway. J. Bio-equiv. Availab. 2013;5(6):236–241. [Google Scholar]
- Kommuru T., Gurley B., Khan M., Reddy I. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q 10: formulation development and bioavailability assessment. Int. J. Pharm. 2001;212(2):233–246. doi: 10.1016/s0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- Kumar V., Lewis S.A., Mutalik S., Shenoy D.B., Udupa N. Biodegradable microspheres of curcumin for treatment of inflammation. Indian J. Physiol. Pharmacol. 2002;46(2):209–217. [PubMed] [Google Scholar]
- Kuttan R., Bhanumathy P., Nirmala K., George M. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29(2):197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- Mohsin K. Design of lipid-based formulations for oral administration of poorly water-soluble drug fenofibrate: effects of digestion. AAPS PharmSciTech. 2012;13(2):637–646. doi: 10.1208/s12249-012-9787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin K., Alamri R., Ahmad A., Raish M., Alanazi F.K., Hussain M.D. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int. J. Nanomed. 2016;11:2829–2838. doi: 10.2147/IJN.S104187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi M., Shariare M.H., Al-bgomi M., Hussain M.D., Alanazi F.K. Simultaneous determination of curcumin (Cur) and thymoquinone (THQ) in lipid based self-nanoemulsifying systems and its application to the commercial product using UHPLC-UV-Vis spectrophotometer. Curr. Pharm. Anal. 2017;13 [Google Scholar]
- Negi P., Jayaprakasha G., JaganMohanRao L., Sakariah K. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J. Agric. Food. Chem. 1999;47(10):4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- Obitte N.C., Ofokansi K.C., Kenechukwu F.C. Development and evaluation of novel self-nanoemulsifying drug delivery systems based on a homolipid from capra hircus and its admixtures with melon oil for the delivery of indomethacin. J Pharm (Cairo) 2014;2014:340486. doi: 10.1155/2014/340486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M.-H., Huang T.-M., Lin J.-K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999;27(4):486–494. [PubMed] [Google Scholar]
- Pathak S.M., Udupa N. Pre-clinical evidence of enhanced oral bioavailability of the P-glycoprotein substrate talinolol in combination with morin. Biopharm. Drug Dispos. 2010;31(2–3):202–214. doi: 10.1002/bdd.703. [DOI] [PubMed] [Google Scholar]
- Payton F., Sandusky P., Alworth W.L. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007;70(2):143–146. doi: 10.1021/np060263s. [DOI] [PubMed] [Google Scholar]
- Randhawa M.A., Al-Ghamdi M.S. A review of the pharmacothera peutic effects of Nigella sativa. Pak. J. Med. Res. 2002;41(2):77–83. [Google Scholar]
- Ravindranath V., Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16(3):259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- Ravindranath V., Chandrasekhara N. Metabolism of curcumn-studies with [3H] curcumin. Toxicology. 1981;22(4):337–344. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005;5(13):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Salmani J.M.M., Asghar S., Lv H., Zhou J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules. 2014;19(5):5925–5939. doi: 10.3390/molecules19055925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoskar R., Shah S., Shenoy S. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol., Therapy, Toxicol. 1986;24(12):651–654. [PubMed] [Google Scholar]
- Shahba A.A.-W., Ahmed A.R., Alanazi F.K., Mohsin K., Abdel-Rahman S.I. Multi-layer self-nanoemulsifying pellets: an innovative drug delivery system for the poorly water-soluble drug cinnarizine. AAPS PharmSciTech. 2018:1–16. doi: 10.1208/s12249-018-0990-7. [DOI] [PubMed] [Google Scholar]
- Shahba A.A., Mohsin K., Alanazi F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: design, optimization, and in-vitro assessment. AAPS PharmSciTech. 2012;13(3):967–977. doi: 10.1208/s12249-012-9821-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma O. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976;25(15):1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- Shazly G., Mohsin K. Dissolution improvement of solid self-emulsifying drug delivery systems of fenofi brate using an inorganic high surface adsorption material. Acta Pharmaceutica. 2015;65(1):29–42. doi: 10.1515/acph-2015-0003. [DOI] [PubMed] [Google Scholar]
- Sidhu G.S., Singh A.K., Thaloor D., Banaudha K.K., Patnaik G.K., Srimal R.C., Maheshwari R.K. Enhancement of wound healing by curcumin in animals. Wound Repair Regeneration. 1998;6(2):167–177. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- ToDA S., Miyase T., Arichi H., Tanizawa H., Takino Y. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem. Pharm. Bull. 1985;33(4):1725–1728. doi: 10.1248/cpb.33.1725. [DOI] [PubMed] [Google Scholar]
- Umeyor C., Attama A., Uronnachi E., Kenechukwu F., Nwakile C., Nzekwe I., Okoye E., Esimone C. Formulation design and in vitro physicochemical characterization of surface modified self-nanoemulsifying formulations (SNEFs) of gentamicin. Int. J. Pharm. 2016;497(1–2):161–198. doi: 10.1016/j.ijpharm.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Wahlström B., Blennow G. A study on the fate of curcumin in the rat. Basic Clin. Pharmacol. Toxicol. 1978;43(2):86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Wan S., Sun Y., Qi X., Tan F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS Pharmscitech. 2012;13(1):159–166. doi: 10.1208/s12249-011-9732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek A., Hopkala H., Matysik G. TLC-densitometric determination of bisoprolol, labetalol and propafenone, as dabsyl derivatives, in pharmaceutical preparations. Chromatographia. 1999;50(1–2):41–44. [Google Scholar]
- Woo C.C., Kumar A.P., Sethi G., Tan K.H.B. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012;83(4):443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Zhang P., Liu Y., Feng N., Xu J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2008;355(1):269–276. doi: 10.1016/j.ijpharm.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zheng Y., Zhang L., Wang Q., Zhang D. Stability of nanosuspensions in drug delivery. J. Control. Release. 2013;172(3):1126–1141. doi: 10.1016/j.jconrel.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Zschiesche M., Lemma G.L., Klebingat K.J., Franke G., Terhaag B., Hoffmann A., Gramatté T., Kroemer H.K., Siegmund W. Stereoselective disposition of talinolol in man. J. Pharm. Sci. 2002;91(2):303–311. doi: 10.1002/jps.10054. [DOI] [PubMed] [Google Scholar]