Abstract
Cancer is one of the most devastating human diseases that causes a great number of mortalities each year worldwide. Thus, finding and treating cancers early is of increasing interest to the public and presents great opportunity for research. It is well known that the metabolism of cancer cells differs from that of normal tissues. Analysis of volatile organic compounds (VOCs), a group of small molecule metabolites, provides an emerging approach for cancer screening and disease monitoring. VOCs are continuously generated in human body and released through breath, blood, skin, urine and fecal samples, which carry information of the physiological and metabolic status. Furthermore, the development of effective analytical methods for VOCs detection is one of the challenging aspects in cancer research. In this review, the analytical methods such as solid-phase mirco-extraction (SPME) and stir bar sorptive extraction (SBSE) coupled with gas chromatography/mass spectrometry (GC-MS), the application of VOCs in urological cancers diagnosis and potential molecules pathways related to VOCs profile for cancer detection are discussed.
Keywords: Urine, metabolomics, cancer, diagnosis, volatile organic compounds (VOCs)
Introduction
Cancer is one of the most devastating human diseases that causes a vast number of mortalities each year worldwide. Since President Richard Nixon declared war on cancer in 1971, the US spends billions of dollars to develop better drugs and therapies that might control cancer cells, but it has yielded insufficient results: the overall cancer mortality rate in the US has fallen by a scant 8 percent since 1975 as heart disease deaths have dropped by nearly 60 percent in that period, by comparison. While the cure-driven approach has dominated the cancer research, finding and treating cancers early continue to present a great research opportunity for science. It is well accepted that cancer metabolism differs from that of normal tissue. An important hypothesis published in the 1950s by Otto Warburg proposed that cancer cells rely on anaerobic metabolism as the source for energy, even under physiological oxygen levels. As a result, cancer central carbon metabolism has been researched extensively. Cancer is known to involve a wide range of metabolic processes, and many more are still to be unveiled. Studying cancer through metabolomics could reveal new biomarkers for cancer that could be useful for its future prognosis, diagnosis and therapy. Using a metabolomics approach, it is possible to detect a range of metabolites in a single assay and therefore metabolomics can be defined as a holistic and data-driven study of the low molecular weight metabolites present in biological systems.
Among the low molecular weight metabolites, volatile organic compounds (VOCs), the majority being organic in nature, are continuously generated in human body and released through breath, blood, skin, urine and fecal samples (Figure 1) [1-4]. These VOCs carry information of the physiological and metabolic status of the individual [5]. As VOCs are considered the metabolites of biological activities in human body, they exist in our system as a result of pathological processes and a consequence of disease. Recent studies have demonstrated that dogs can differentiate cancer patients from control negative by sniffing their biological samples, such as urine [6-9]. The VOCs emitted from human body can be considered as individual ‘odor-fingerprints’ [10]. Therefore, VOCs could be used as predictive biomarkers for disease detection. In this review, the generation, the analysis and the application of VOCs in urological cancers diagnosis are discussed. Some of the most noteworthy research in the field is highlighted.
Figure 1.
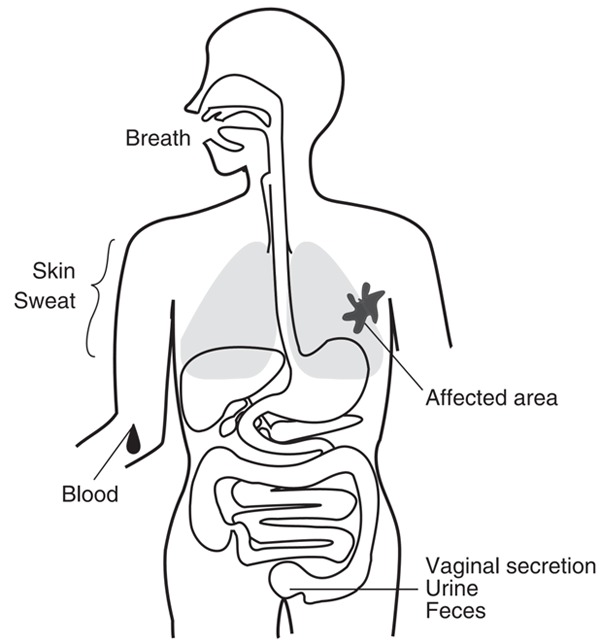
Volatile organic compounds (VOCs) are continuously generated from human body and released through breath, blood, skin, urine and feces. (Source: M. Shirasu and K. Touhara, 2011. Copyright © 2011 The Journal of Biochemistry. [10]).
VOCs emitted from human body
VOCs in blood
Blood directly reflects the internal environment of the body, including nutritional, metabolic and immune status, which highly values the blood samples in disease-specific VOCs studies. The specific VOCs in the blood have been reported to be useful in predicting and diagnosing diseases, such as ovarian cancer, colorectal cancer, lung cancer, and hepatic encephalopathy [11-14]. In the study of Horvath et al. [11], the trained dogs could differentiate ovarian cancer patients from the patients with other gynecological cancers and healthy control subjects through sniffing the blood samples from patients. Wang et al. [12] carried out a study to identify the blood volatile compounds as biomarkers for colorectal cancer by collecting blood samples from 16 colorectal cancer patients and 20 healthy controls. Four metabolic biomarkers were found at significantly higher or lower level in cancer patients. However, obtaining blood samples is invasive and pre-treatment of blood samples is also time-consuming. These factors have limited the use of VOCs in blood for diagnostic tool development. Further studies are needed to evaluate these results and to apply these findings to clinical diagnoses.
VOCs in breath
Exhaled breath contains VOCs that can be attributed to either exogenous or endogenous volatiles [15,16]. Endogenous volatiles consist of blood-borne compounds released to the environment via the lungs and/or compounds made from all classes of symbiotic bacteria. Numerous studies were conducted to investigate the potential of VOCs in breath in diseases diagnosis, especially lung cancer. Collecting breath samples is relatively simple, painless and non-invasive as compared to sampling blood. Phillips et al. [17] collected breath samples from 108 patients and a combination of 22 VOCs in breath samples distinguished between patients with and without lung cancer. In the study of Peng et al. [18], an array of sensors based on gold nanoparticles were shown to be able to rapidly distinguish the breath of lung cancer patients from the breath of healthy individuals by training and optimizing sensors with the VOCs identified through gas chromatography/mass spectrometry (GC-MS). Exogenous volatiles include compounds inhaled from the external environment, such as compounds produced following the oral ingestion of food and compounds derived from smoking cigarettes. It is always challenging to distinguish exogenous compounds of environmental contaminants from endogenously produced VOCs.
VOCs in urine
The VOCs in urine are considered intermediate or end products of metabolic pathways, and may contain a variety of structural motifs, such as ketone, alcohol, furan, pyrrole and sulfide with a particular odor [10]. In some cases, characteristic urine VOCs profile have been directly linked to particular metabolic disorders. Some studies have linked urinary VOCs profiles to infectious diseases [19,20] and different types of cancers, including prostate cancer (PCa) [21], renal cancer (RCa) [22] and bladder cancer (BCa) [23]. Urinary VOC patterns in cancer patients are often different from the patterns in urine samples from control subjects, although the differences depend on cancer types and even cancer stages. Khalid et al. [21] showed that urinary VOCs profile of prostate cancer patients can be discriminated from cancer free controls by using four VOCs, 2,6-dimethyl-7-octen-2-ol, pentanal, 3-octanone, and 2-octanone with accuracy as high as 71%. In an analysis of volatile human urinary metabolome for renal cell carcinoma (RCC), Monteiro et al. [22] reported that the volatile urinary metabolome could discriminate between RCC and control patients with 60.33% of the variability in principal component analysis (PCA). And according to Weber et al. [23], the best diagnostic performance they obtained through the comparison between healthy volunteers and bladder cancer patients was 70% overall accuracy using a gas sensor array and pattern recognition.
These studies have proved the potential in searching for volatile diagnostic biomarkers in the urine of cancer patients. Due to the complexity of urine components, such as metabolites from ingested foods and drinks, and considerable variation among individuals, caution must be taken when determining the source of candidate VOC biomarkers resulting from disease-related changes in metabolism and advanced computer processing of chromatographic data should be involved in identifying the VOC patterns.
VOCs in other biospecimen
VOCs can also be continuously emitted from skin as sweat. Sweat is one of the less employed bio-fluids for discovery of markers. In the research conducted by Calderón-Santiago et al. [24], human sweat was collected and used as clinical sample to develop a screening tool for lung cancer. The five metabolites identified in this study provided 80% specificity and 79% sensitivity to discriminate between patients with lung cancer versus smokers as control individuals. Mi-Jung et al. [25] also applied the analysis of sweat volatile organic compounds in forensic science. Although VOCs in sweat could result from internal hormonal or metabolic changes, many VOCs appear to be derived from symbiotic bacteria that live on the skin surface which then metabolize and transform secreted compounds in sweat and sebum. Any alteration in homeostatic balance due to some inherited metabolic disorder or bacterial infection of the diseased area can induce changes in both the quality and quantity of VOCs. For example, some infectious diseases or cancerous wounds develop characteristic and offensive odors [10]. Therefore, the contamination from the environment must also be taken into consideration, including the interference from the ambient air, humidity and cosmetics.
Human fecal samples represent dietary end-products resulting from digestive and excretory processes and intestinal bacterial metabolism. The investigation of fecal VOCs may reveal potential health consequences and be the best non-invasive way of diagnosing gastrointestinal diseases. Distinct patterns of VOCs have been associated with fecal samples from patients with some types of bacterial infection, such as Vibrio cholera, Clostridium difficile or Campylobacter jejuni infections [26,27]. Batty et al. [28] reported the use of fecal volatile metabolome in screening for colorectal cancer with 78% specificity and 72% sensitivity. VOCs may also be contained in other types of bio-fluids, such as vaginal secretions, which accurately reflect the stages of menstrual cycles.
In summary, VOCs can be emitted from different types of biological fluids of human body and carry “odor fingerprint” of the individuals (Table 1). Pathological processes can influence our daily odor fingerprints by producing new VOCs or by changing the ratio of VOCs that are produced normally [10]. These VOCs could potentially be the markers for clinical diagnosis and therapeutic monitoring of diseases. However, those VOCs may be affected by various factors, such as age, sex, drug therapy, diet and smoking. Therefore, care must be taken when investigating disease related VOCs in clinical samples.
Table 1.
VOCs, as “odor fingerprint”, could be emitted from different types of biological samples of human body
| The origins of odor | Published Paper | Disease Detection | Method | Sample Size | Study Results | |
|---|---|---|---|---|---|---|
|
| ||||||
| # of VOCs | How reliable? | |||||
| Blood | Horvath et al. [11] | Ovarian cancer | Trained dogs | N/A | N/A | Tissue tests: 100% sensitivity and 95% specificity. blood tests: 100% sensitivity and 98% specificity |
| Wang et al. [12] | Colorectal cancer | SPME-GC-MS | 16 cancer patients and 20 healthy controls | 4 | Lower level VOCs (P<0.01): Higher level VOCs (P<0.05): | |
| Breath | Phillips et al. [17] | Lung cancer | GC-MS | 60 cancer patients and 48 non-cancer controls | 22 | 100% sensitivity and 81.3% specificity |
| Peng et al. [18] | Lung cancer | Sensors based on gold nanoparticles | N/A | 42 | Accuracy >86% | |
| Urine | Khalid et al. [21] | Prostate Cancer | SPME-GC-MS | 59 cancer patients and 43 non-cancer controls | 4 | AUC 0.76 |
| Accuracy as high as 74% | ||||||
| Monteiro et al. [22] | Renal cell carcinoma | GC-MS | N/A | N/A | N/A | |
| Weber et al. [23] | bladder cancer | Gas sensor | 30 cancer patients and 59 non-cancer controls | N/A | 70% overall accuracy; 70% sensitivity and 70% specificity | |
| Sweat | Calderón-Santiago et al. [24] | Lung cancer | LC-MS | 41 cancer patients and 55 non-cancer controls | 16 | specificity/sensitivity pair (80 and 79% |
| Feces | Batty et al. [28] | Colorectal cancer | Ion flow tube mass spectrometry (SIFT-MS) | 31 high risk patients and 31 low risk or non-cancer controls | N/A | Accuracy 75% with 78% specificity and 72% sensitivity |
AUC: Area Under the receiver operating characteristic curve.
Extraction and detection of VOCs as potential method for disease diagnosis
VOCs extraction
Since the low concentration of VOCs presents in various biological specimen, the extraction and pre-concentration are crucial for the analysis of VOCs of interest [29] and may affect the reliability and accuracy of the analysis [30].
To increase the reproducibility, selectivity, and extraction capacity of the sample preparation steps, several extraction techniques were developed to facilitate rapid and efficient preparation processes of VOCs [31]. For example, solid-phase micro-extraction (SPME) technique (Figure 2) uses a fine bare fused silica fiber or a fine silica fiber coated with a thin layer of a selective coating (either solid or liquid) to extract organic compounds directly from aqueous samples for instrumental analysis by Gas Chromatography (GC) or Gas Chromatography/Mass Spectrometry (GC-MS) [32]. There are two types of extraction based on the different samples: 1) the direct immersion SPME which is immersing the fiber to extract VOCs in liquid samples, and 2) the headspace SPME by suspending fiber in the headspace above the liquid phase. The analytes are firstly adsorbed during extraction on the surface of the fiber materials as a result of chemical bonding, and then absorbed into the coating materials [33]. There are four types of polymers widely used as the coating materials, polydimethylsiloxane (PDMS), divinylbenzene (DVB), polyacrylate (PA), and polyethyleneglycol (PEG). Those materials could also be used through the combination blended with carboxen (CAR) [34]. After pre-concentrating, the fiber with analytes trapped on its coating materials is injected to the instruments and release analytes through thermal desorption. Deng et al. [13] developed a simple, rapid and sensitive SPME/GC-MS method for the investigation of volatile biomarkers in blood for lung cancer. Poli et al. [30] evaluated the potential of aldehydes from exhaled breath in the diagnosis of non-small cell lung cancer by means of SPME/GC-MS with 93% accuracy and precision between 7.2-15.1%. Monteiro et al. [22] studied the volatile human urinary metabolome difference of RCC and healthy individuals through the headspace SPME sampling coupled with gas chromatography-ion trap/mass spectrometry (GC-IT/MS). Wang et al. [12] analyzed the VOCs in the blood samples from colorectal cancer patients and healthy controls with headspace SPME sampling. Khalid et al. [21] also applied SPME in headspace of urine samples to identify the specific urinary VOCs for the detection of prostate cancer.
Figure 2.
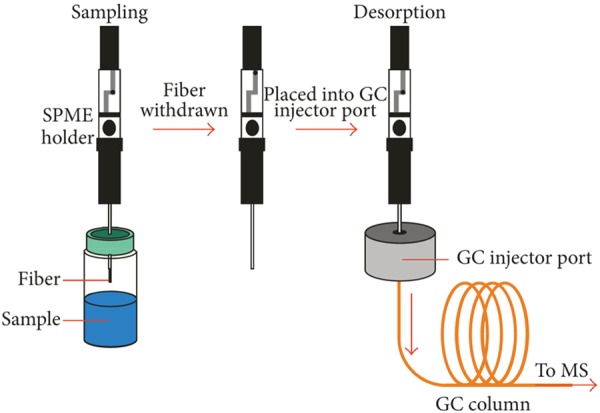
The use of solid phase micro-extraction-gas chromatography-mass spectrometry (SPME-GC-MS) (Source: Kamila Schmidt and Ian Podmore, 2015. Copyright © 2015 The Journal of Biomarkers. [29]).
Similar to the theory of SPME, another novel approach for sample enrichment is referred as stir bar sorptive extraction (SBSE), which was developed by Baltussen et al. [35]. SBSE technique uses stir bars coated with the sorbent PDMS. The results of experiments conducted by Baltussen et al. indicated that the stir bars present higher efficiency than SPME in the pre-concentration of analytes from aqueous samples, with up to a 500-fold increase in sensitivity when stirring between 30 to 60 min. The high efficiency could be contributed to the increased amount of PDMS coated on the stir bars. Furthermore, the volatile compounds can also be easily and conveniently handled due to the absence of drying step. Therefore, SBSE can be applied in the analysis of VOCs in different types of aqueous samples, as well as the biological fluids. Melo et al. [36] carried out an analysis of antidepressants in plasma samples using SBSE and liquid chromatography (LC) with high extraction efficiency. Soini et al. [37] showed a high reproducibility of using SBSE in quantitative comparisons of the urinary profiles with relative standard deviations (RSD) of 1-5% for a wide range of compounds. In one of our studies, SBSE was successfully applied in identifying the specific urinary volatile organic compounds for the diagnosis of prostate cancer [38]. In addition, the coated stir bar could also be used as headspace sorptive extraction (HSSE) [39,40] (Figure 3).
Figure 3.
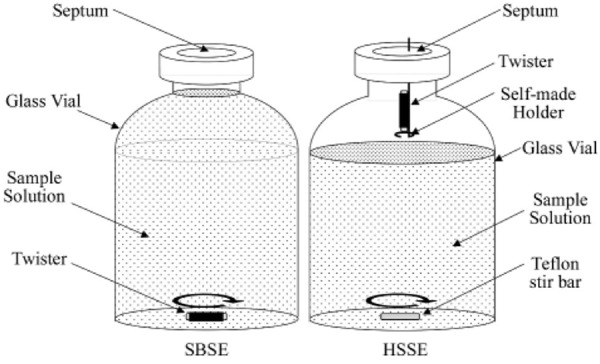
The set-ups of SBSE and HSSE. (Source: Ochiai et al., 2001. Copyright © 2001 The Royal Society of Chemistry. [40]).
Other less common extraction techniques, such as purge and trap [41], single drop micro-extraction [42], were also applied in VOCs extraction. However, their applications in VOCs analysis is relatively limited due to the sensitivity concerns.
VOCs detection
VOCs are easy to be detected by using analytic instruments, such as GC-MS, proton transfer reaction-mass spectrometry (PTR-MS), selected ion flow tube-mass spectrometry (SIFTMS), or gas sensors [21,29,43-45]. As one of the most commonly used analytical technique, GC-MS is widely used in the investigation of VOC biomarkers because of its sensitivity and reliability in analyte identification [12,13,22,30,38]. Studies have shown that GC-MS provides an outstanding sensitivity at ppb (parts per billion) and low ppt (parts per trillion) levels in VOC analysis with the pre-concentration steps [46,47]. Fuchs et al. [46] analyzed aldehydes from the breath samples of lung cancer patients using GC-MS. The concentrations in their study ranged from 7 pmol/l (161 pptV) for butanal to 71 nmol/l (1,582 ppbV) for formaldehyde. In another study using GC-MS conducted by Ligor et al. [47], the limit of detection was in the range of 0.05 to 15.00 ppb. Meanwhile, it provides the most detailed information of VOCs profiles and identifies analytes with most certainty. However, GC-MS instruments are often expensive. Compared to GC-MS, PTR-MS and SIFT-MS do not require a pre-concentration step and can work in real time, which make these two better instant quantification techniques for VOCs analysis [48,49]. Wehinger et al. [48] identified VOCs in the exhaled breath using PTR-MS to discriminate the primary lung cancer patients and controls. As mentioned in this study, even though the technique is simple and time-saving for larger clinical evaluation, it is not possible for PTR-MS to differentiate between compounds with the same molecular mass.
Some other detection techniques are also used in the analysis VOCs emitted from human, such as ion mobility spectrometry (IMS) [50]. Compared with GC-MS, IMS gives a tenfold higher detection rate of VOCs (500 seconds for IMS vs. 1 h for GC-MS per sample). In one study of detecting VOCs in exhaled breath of patients with lung cancer, the IMS was used by Westhoff et al. [50] and a combination of 23 peak regions were identified to discriminate the cancer patients and controls without error.
In addition, several types of electronic noses have been used in the studies of VOCs in cancer [18,23,51]. Natale et al. [51] investigated the possibility of using electronic nose to identify the lung cancer patients from controls. The results in their study indicated a 100% of classification of lung cancer affected patients and 94% of controls. These sensors used in this study showed a good sensitivity towards the compounds identified previously as potential lung cancer markers. However, electronic noses are designed to recognize the VOCs found in established studies but not to identify any unknown VOC patterns. Compared to the mass spectrometry based techniques, the electronic nose is less time consuming and enables the potential of cheap, rapid, simple, and miniature detection devices [52,53]. However, electronic noses are sensitive to moisture, less sensitive, and with poor reproducibility [54,55]. Additionally, electronic noses can only allow the semi-quantitative detection of VOCs [56].
VOCs and urological cancers
Cancer is a leading cause of death and disability globally, impacting more than 14 million people each year [57]. Urological cancers, such as prostate cancer (PCa), renal cancer (RCa), and bladder cancer (BCa), are a major cause of morbidity and mortality worldwide [58]. In 2018, about 164,690 new cases of PCa, 65,340 of RCa, 81,190 of BCa and about 29,430 deaths in PCa, 14,970 in RCa, 17,240 in BCa are estimated in United States according to the American Cancer Society [59]. In the United States, PCa is the most common cancer and the third leading cause of death in men [59]. RCa and BCa also account for more than 2% and 4% of cancer mortality in the United States [59].
Diagnosis and treatment for these urological cancers are associated with different but overlapping clinical challenges [58]. High-throughput genomic screening, proteomic profiling, and metabolomics analysis of related functional protein molecules provide a large amount of informational data and overview of clinical changes of cancer development and progression. The cells, proteins, and metabolites in urine originated from kidney, prostate, and bladder could provide information for biomarkers searching, such as genomics, proteomics, and metabolomics [60-62]. Urine, as a source of excretion from the urological system, is an ideal body fluid for the investigation and detection of biomarkers for those urological cancers. Moreover, urine collection is an easy and non-invasive procedure which increases the feasibility of point-of-care clinical application.
As early diagnosis and treatment of those urological cancers will improve the quality of care and reduce mortality, there is a high demand of reliable, quick and patient-friendly diagnostic method for cancer screening. As aforementioned, several studies have demonstrated that sniffer dogs can differentiate cancer patients from controls by sniffing their urine [6-9]. Cornu et al. [7] reported the trained dog detected PCa by smelling urine with 91% of both sensitivity and specificity. The study from Willis et al. [9] also provided further evidence that volatile compounds found in urine can be identified by trained dogs with 73% sensitivity and 92% specificity. Additionally, VOCs are easy to be detected by using analytic instruments, like gas chromatography-mass spectrometry (GC-MS), or further developed gas sensors [21,43-45]. All of those proved that VOCs, particularly in urine, could be desirable disease markers for their non-invasiveness, easy detection, high sensitivity and high specificity. As one of the most promising metabolomics approaches in cancer detection, the analysis of VOCs can potentially serve as a safe, non-invasive, and specific test for the early detection of those urological cancers.
VOCs in prostate cancer
Currently, PCa are screened by the prostate-specific antigen (PSA) blood test and/or the digital rectal exam (DRE). If the PCa is suspected based on the results of screening tests or other symptoms, further tests, such as prostate biopsy will be required to confirm the diagnosis [63]. Furthermore, techniques used in advanced stages, such as bone scans, computed tomography (CT) scan, and magnetic resonance imaging (MRI), may involve X-rays, magnetic fields, sound waves and radioactive substances which can lead to the second injury of cancer patients [63]. Diagnostic methods which can reduce stress and be more patient-friendly are needed.
Adding to the fact that PSA is not cancer specific, there is no reliable PSA threshold that can accurately distinguish men with or without cancer [64] resulting in over-diagnose of the disease. As high as 80% of men were found PCa negative based on their biopsy results [65]. Therefore, there is a significant interest in finding a more accurate PCa-specific biomarker. Khalid et al. [21] showed the discrimination power of urinary VOCs profile in differentiating PCa patients from controls with 71% accuracy based on only 4 VOCs. In one of our studies [38], the performance of VOCs has been tested and validated with AUC 0.92 (96% sensitivity and 80% specificity). The VOCs based prostate cancer diagnosis tool would be promising in future clinical use.
Several biomarkers have been developed to improve upon the limitations of serum PSA including Iso-PSA, prostate cancer antigen 3 (PCA3), 4Kscore, Prostate Health Index (PHI), TMPRSS2:ERG and ConfirmMDx [66-71]. Among those markers, IsoPSA, PHI and 4Kscore are all PSA-based assay for PCa risk assessment [66,69,71]. PCA3 is a noncoding RNA that is prostate specific and highly overexpressed in prostate cancer [72]. TMPRSS2-ERG gene fusions are reported to be the predominant molecular subtype of prostate cancer [73]. ConfimMDx is an epigenetic test for PCa diagnosis before prostate biopsy [70]. Again, VOCs based prostate cancer diagnosis tool has shown a more satisfactory screening capability for PCa then these methods (Table 2).
Table 2.
Comparison in sensitivity, specificity, and AUC from various biomarkers in prostate cancer diagnosis
VOCs in renal cancer
The most common type of kidney cancer is renal cell carcinoma (RCC) consisting about 90% of kidney cancer cases. RCC is a heterogeneous malignancy, both morphologically and genetically, which is classified into different histologic subtypes, including clear cell RCC (most common one), papillary RCC, chromophobe RCC and other less common subtypes [74-76]. The outcome of RCC is usually unpredictable even after a long period of asymptomatically development and progression [77]. Therefore, its diagnosis is often incidental through the use of medical imagology and is frequently detected at an advanced stage and metastatic when detected clinically [78]. Additionally, RCC is particularly challenging to treat because of its relative insensitivity to radiotherapy and conventional chemotherapy drugs [79]. The early screening of RCC could improve the outcome of diagnosis. However, no early screening method is recommended to screen for kidney cancer clinically in people at average risk or increasing risk.
The potential of urinary VOCs used in RCC diagnose has been highlighted in previous studies [22,80,81]. The purpose of most previous studies were focused on the searching of specific VOCs in RCC patients without further validation [22,80]. In the study reported by Marica Monteiro in 2017 [81], the selected VOCs was validated in different patients group besides the searching of specific VOCs, but the performance of VOCs in differentiating RCC patients and controls was not determined. Besides, two urinary exosomal proteins, AQP-1 and PLIN2 have shown promise as the biomarkers in RCC diagnosis [82]. It should be noted that AQP-1 and PLIN2 can be found in clear cell and papillary RCC but not in the chromophobe subtype of RCC. However, VOCs based screening has great potential to be developed as a more universal screening tool of almost all types of RCC or even specific screening tool for each type of RCC because of the metabolic distinction shown with each selected VOC between cancer patients and controls, Unlike the ELISA detection methods of AQP-1 and PLIN2, the VOCs based diagnostic model could be developed as a high throughput and fast screening method in clinic enabled by high performance GC/MS and statistic assistance.
VOCs in bladder cancer
Bladder cancer (BCa) is the second most common genitourinary malignant disease in United States [83]. And it is also a heterogeneous malignancy, with different histologic subtypes, including transitional cell carcinomas (90%), squamous cell carcinomas (5%), and adenocarcinomas (less than 2%) [84]. The most common symptom of BCa (in 80%-90% of the patients) [85] is hematuria, or blood in the urine, and others including complaints of dysuria (painful urination), increased frequency or urgency of urination, failed attempts to urinate, a mass in the bladder or a ureteral obstruction [86,87]. Intravenous pyelography, cystoscopy, transurethral biopsy, and imaging techniques, such as magnetic resonance imaging and computerized tomography scan, are always involved in the clinical diagnosis procedures of potential BCa [87,88]. Like renal cancer, no early screening method for bladder cancer is recommended in United States [89].
Many studies are attempting to identify genetic and chemical markers in order to complement the use of clinical features and better assess the risk level of BCa [90,91]. The overexpression of the p53 gene, cells containing multiple aneuploid cell lines, and the expression of the Lewis-x blood group antigen were found to be the markers of high risk BCa [90]. Furthermore, nuclear matrix protein 22 (NMP22) and bladder tumour antigen (BTAstat) are more sensitive (50-85% and 50-70%), but less specific (60-70%), than urine cytology, which have been approved by FDA as protein markers of bladder cancer [23,92].
Recently, VOCs are also suggested in different studies that have potential in differentiating patients of BCa from controls. And according to Weber et al. [23], the best diagnostic performance they obtained through the comparison between healthy volunteers and bladder cancer patients was 70% overall accuracy (70% sensitivity and 70% specificity) using a gas sensor array and pattern recognition. The results of another study using gas sensors, reported from Khalid et al. [93], also showed potential of VOCs for the diagnosis of bladder cancer (the best performance: 100% sensitivity and 94.6% specificity). All those studies have revealed the potential of VOCs used in bladder cancer diagnosis.
Potential molecules pathways related to VOCs profile for cancer direction
Androgen signaling and one-carbon metabolism
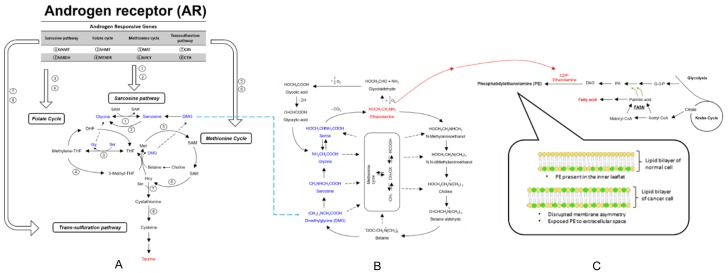
The androgen receptor (AR), is a nuclear receptor that is activated by binding either of the androgenic hormones, testosterone, or dihydrotestosterone in the cytoplasm and then translocating into the nucleus [94,95]. It plays an essential and important role in PCa initiation, progression, and metabolic adaptation that takes place during PCa progression. As a transcription factor, the AR directly affects essential catabolic and biosynthetic pathways through modulating the expression of related effectors and regulators. On the other hand, the AR, as a modulator of the one-carbon metabolism, can also affect epigenetic processes, DNA metabolism, and redox balance indirectly, which are all important factors in tumorigenesis [96].
One-carbon metabolism involves a complex network with two central cycles: (1) the folate cycle and (2) the methionine cycle. One-carbon metabolism also regulates essential processes including DNA synthesis and repair, epigenetic methylation reactions, redox homeostasis, and protein synthesis. The balanced flux through these four pathways, e.g. folate cycle, methionine cycle, transsulfuration pathway, and polyamine synthesis, is essential for cellular homeostasis. (Figure 4A) [96], and disruptions of the balanced flux could contribute to the pathogenesis of many diseases, including cancer [97]. Cancer creates a demand and dependency on one-carbon metabolism. For example, methyl group availability for methyltransferases that modulate gene expression via epigenetic mechanisms is influenced by flux within the folate cycle and methionine cycles [98,99]. Alterations in one-carbon metabolism may contribute to tumorigenesis through fueling DNA synthesis, changing the DNA and histone methylomes, promoting protein translation, driving cell cycle progression, and modulating redox balance. These changes can in turn promote sustained proliferation, induce tumorigenic gene expression changes, contribute to genomic instability, and promote survival-all important processes in tumorigenesis and cancer progression [96].
Figure 4.
A comprehensive illustration of Androgen signaling, one-carbon metabolism, and metabolic phenotype. (A) One-carbon metabolism involves a complex network with four pathways: (1) folate cycle; (2) methionine cycle; (3) transsulfuration pathway; (4) sarcosine pathway. In the prostate, androgens and the AR regulate the activity/expression of several enzymes involved in the one-carbon metabolism pathway. Enzyme abbreviations are as follows: SARDH: Sarcosine Dehydrogenase; SHMT: Serine hydroxymethyltransferase; GNMT: Glycine-N-methyltransferase; MTHFR: Methylene tetrahydrofolate reductase; MAT: Methionine adenosyltransferase; AHCY: S-adenosylhomocysteine hydrolase; CBS: Cystathionine beta-synthase; CTH: cystathione gamma-lyase or gamma-cystathionase. (B) Hypothetical cycle of metabolism involving glycine, serine, ethanolamine, choline, and betaine. [101] (C) Enhanced lipogenesis, arising from increased activities of fatty acid biosynthetic enzymes (including ACC1, FASN, and stearoyl CoA desaturase (SCD1)), is a metabolic hallmark of many cancer cells. [106-108] In addition, the plasma membrane of normal cells is characterized by an asymmetric distribution of various phospholipids over two membrane leaflet. PE resides in the inner leaflet facing the cytosol. The disrupted membrane asymmetry of cancer cell exposes PE to extracellular space. Furthermore, PE is also highly exposed on endothelium cells in tumor vasculature. PA, phosphatidic acid; PC, phosphatidylcholine; DAG, diacylglycerol; CDP-ethanolamine, Cytidine diphosphate ethanolamine.
The progression and metastasis of tumors were associated with metabolite increases in glutathione and cysteine/methionine metabolism pathways. For example, clear cell RCC is characterized by broad shifts in central carbon metabolism, one-carbon metabolism, and antioxidant response, reported by Hakimi et al. [100]. Bridging the gap between the Cancer Genome Atlas (TCGA) transcriptomic profiling and the metabolomic data in their studies, the authors were able to integrate the pathway-level metabolic atlas and to demonstrate discordance between transcriptome and metabolome.
Studies in PCa cell lines demonstrate AR-regulation of one-carbon metabolism enzymes, and altered cellular methylation potential in response to androgens [101-104]. For example, sarcosine, a methylated metabolite of the one-carbon pathway, was found be accumulated in PCa clinical samples [102]. In the prostate, androgens and the AR regulate the activity and/or expression of several enzymes involved in the one-carbon metabolism pathways, specifically enzymes involved in S-adenosyl-methionine (SAM) homeostasis and the entry into the transsulfuration and polyamine synthesis pathways (Figure 4A). Studies directed to identify AR transcriptional networks in different models of PCa have demonstrated an involvement of the AR in global metabolism by directly targeting enzymes involved in several metabolic processes [105-108]. These findings illustrate the role of the AR in PCa tumorigenesis by controlling metabolism, and the value of integrating metabolomic profiling and gene expression analysis for the identification of new biomarkers and therapeutic targets. Also, these observations emphasize the link between the AR and one-carbon metabolism, and the potential effects that changes in AR signaling, that can occur with disease progression, may have on essential cellular processes.
The effect of metabolic phenotype on fatty acid and phospholipid synthesis
The zinc accumulating and citrate synthesizing phenotype is the hallmark of the healthy prostate epithelial cell [109,110]. However, PCa cells reverse this phenotype and adopt a zinc wasting, citrate oxidizing phenotype, thereby representing a major shift in energy metabolism [111]. This shift allows these cells to utilize the Krebs cycle and subsequent oxidative phosphorylation (Figure 4B and 4C). It has long been identified that PCa cells do not conform to the standard Warburg effect seen in most cancers, which described in the early to mid-1900s by Otto Warburg [112]. Malignant cells shift their dominant ATP producing pathway away from oxidative phosphorylation to aerobic glycolysis [112]. Unlike most cancer cells that resort to aerobic glycolysis, prostate cancer cells exhibit a higher level of citric acid cycle activity compared to benign cells [110]. The increased activity of citric acid cycle, essential for the progression of malignancy, was induced by the inability of malignant prostate cells to accumulate high zinc levels, which inhibits citrate oxidation [113].
Another metabolic hallmark of many cancer cells is the enhanced lipogenesis, arising from increased activities of fatty acid biosynthetic enzymes [114-116]. Clear cell RCC (ccRCC) is histologically defined by its lipid and glycogen-rich cytoplasmic deposits [117,118]. In the study of Du et al. [118], the lipid deposition of ccRCC was investigated with focus on the carnitine palmitoyltransferase 1A (CPT1A), as a direct HIF target gene. Prostate cancer cells often utilize lipids derived from androgens through the expression of the AR [119]. However, these cells can also utilize de novo lipid synthesis to produce fatty acids in order to obtain energy. This shift to a lipid-producing phenotype is a key turning point in the progression of prostate cancer. The de novo lipid producers have ability to produce the key energetic molecules for growth without the regulation of androgens (Figure 4C) [120]. Clinically, this is problematic as it represents a disease that is unresponsive to androgen deprivation therapy, known as castration-resistant prostate cancer [121]. These producers include fatty acid synthase (FASN), sterol regulatory element binding protein 1 (SREBP1), and steroyl CoA desaturase. Among them, the enzyme FASN functions to help synthesize long-chain fatty acids. It is believed that unregulated FASN activity within prostate tissue is the beginning of malignant phenotype, and has been argued to be necessary for PCa growth maintenance [122]. The use of lipid by the PCa cells illustrates that these cells bypass potential degenerative pathways, and rather utilize the anabolic pathways in order to maintain energy and growth [123]. A variety of fatty acid moiety were detected in our preliminary study and that supports the importance of specific VOCs in PCa.
Additionally, phospholipids, also as the downstream products of enhanced lipogenesis, in the cancer cell membrane have been found to be abnormal compared with normal cells. The plasma membrane of normal cells is characterized by an asymmetric distribution of various phospholipids over two membrane leaflet. Phosphatidylethanolamine (PE) resides in the inner leaflet facing the cytosol (Figure 4C). The disrupted membrane asymmetry of cancer cell exposes PE to extracellular space, which can serve as a molecular target for anticancer therapy [124]. The increasing need of PE in cancer cell may correlate the excessive consumption of ethanolamine and enhanced lipogenesis.
In conclusion, the use of urinary VOCs has demonstrated a potential application in cancer diagnosis as biomarkers for the assessment or detection of disease. Although the pathways affecting VOCs production are yet to be fully understood, the VOCs identified to be related to cancers may provide valuable information to study the pathways of VOC production in the context of cancer.
Acknowledgements
The authors thank the Wiemer Family Student Endowment for Excellence and Dodson Research Grant provided for Q. Gao. The support by Dr. Keelung Hong Gift Fund for VOC Cancer Research Studies was also acknowledged.
Disclosure of conflict of interest
None.
References
- 1.Gallagher M, Wysocki C, Leyden J, Spielman A, Sun X, Preti G. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780–91. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Holler JS, Needham LL, Patterson DG Jr. Determining volatile organic compounds in human blood from a large sample population by using purge and trap gas chromatography/mass spectrometry. Anal Chem. 1992;64:1021–9. doi: 10.1021/ac00033a011. [DOI] [PubMed] [Google Scholar]
- 3.Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl. 1999;729:75–88. doi: 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 4.de Lacy Costello B, Ratcliffe NM, Smith D. Volatile organic compounds (VOCs) found in urine and stool. The Netherlands: Elsevier, Amsterdam; 2013. [Google Scholar]
- 5.Amann A, Smith D. Volatile biomarkers: non-invasive diagnosis in physiology and medicine. Newnes. 2013 [Google Scholar]
- 6.Balseiro SC, Correia HR. Is olfactory detection of human cancer by dogs based on major histocompatibility complex-dependent odour components? A possible cure and a precocious diagnosis of cancer. Med Hypotheses. 2006;66:270–2. doi: 10.1016/j.mehy.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Cornu JN, Cancel-Tassin G, Ondet V, Girardet C, Cussenot O. Olfactory detection of prostate cancer by dogs sniffing urine: a step forward in early diagnosis. Eur Urol. 2011;59:197–201. doi: 10.1016/j.eururo.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Boedeker E, Friedel G, Walles T. Sniffer dogs as part of a bimodal bionic research approach to develop a lung cancer screening. Interact Cardiovasc Thorac Surg. 2012;14:511–5. doi: 10.1093/icvts/ivr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis CM, Britton LE, Harris R, Wallace J, Guest CM. Volatile organic compounds as biomarkers of bladder cancer: sensitivity and specificity using trained sniffer dogs. Cancer Biomark. 2010-2011;8:145–53. doi: 10.3233/CBM-2011-0208. [DOI] [PubMed] [Google Scholar]
- 10.Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257–66. doi: 10.1093/jb/mvr090. [DOI] [PubMed] [Google Scholar]
- 11.Horvath G, Andersson H, Paulsson G. Characteristic odour in the blood reveals ovarian carcinoma. BMC Cancer. 2010;10:643. doi: 10.1186/1471-2407-10-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Li P, Lian A, Sun B, Wang X, Guo L, Chi C, Liu S, Zhao W, Luo S. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol Ther. 2014;15:200–6. doi: 10.4161/cbt.26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng C, Zhang X, Li N. Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;808:269–77. doi: 10.1016/j.jchromb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg E, Blendis L, Sandler S. A gas chromatographic-mass spectrometric study of profiles of volatile metabolites in hepatic encephalopathy. J Chromatogr. 1981;226:291–9. doi: 10.1016/s0378-4347(00)86063-6. [DOI] [PubMed] [Google Scholar]
- 15.Manolis A. The diagnostic potential of breath analysis. Clinical Chemistry. 1983;29:5–15. [PubMed] [Google Scholar]
- 16.Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl. 1999;729:75–88. doi: 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M, Gleeson K, Hughes JM, Greenberg J, Cataneo RN, Baker L, McVay WP. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet. 1999;353:1930–1933. doi: 10.1016/S0140-6736(98)07552-7. [DOI] [PubMed] [Google Scholar]
- 18.Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Haick H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat Nanotechnol. 2009;4:669–73. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 19.Banday KM, Pasikanti KK, Chan EC, Singla R, Rao KV, Chauhan VS, Nanda RK. Use of urine volatile organic compounds to discriminate tuberculosis patients from healthy subjects. Anal Chem. 2011;83:5526–34. doi: 10.1021/ac200265g. [DOI] [PubMed] [Google Scholar]
- 20.Sethi S, Nanda R, Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev. 2013;26:462–75. doi: 10.1128/CMR.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalid T, Aggio R, White P, De Lacy Costello B, Persad R, Al-Kateb H, Jones P, Probert CS, Ratcliffe N. Urinary volatile organic compounds for the detection of prostate cancer. PLoS One. 2015;10:e0143283. doi: 10.1371/journal.pone.0143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteiro M, Carvalho M, Henrique R, Jeronimo C, Moreira N, de Lourdes Bastos M, de Pinho PG. Analysis of volatile human urinary metabolome by solid-phase microextraction in combination with gas chromatography-mass spectrometry for biomarker discovery: application in a pilot study to discriminate patients with renal cell carcinoma. Eur J Cancer. 2014;50:1993–2002. doi: 10.1016/j.ejca.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Weber CM, Cauchi M, Patel M, Bessant C, Turner C, Britton LE, Willis CM. Evaluation of a gas sensor array and pattern recognition for the identification of bladder cancer from urine headspace. Analyst. 2011;136:359–364. doi: 10.1039/c0an00382d. [DOI] [PubMed] [Google Scholar]
- 24.Calderón-Santiago M, Priego-Capote F, Turck N, Robin X, Jurado-Gámez B, Sanchez JC, De Castro MD. Human sweat metabolomics for lung cancer screening. Anal Bioanal Chem. 2015;407:5381–92. doi: 10.1007/s00216-015-8700-8. [DOI] [PubMed] [Google Scholar]
- 25.Choi MJ, Oh CH. 2nd dimensional GC-MS analysis of sweat volatile organic compounds prepared by solid phase micro-extraction. Technol Health Care. 2014;22:481–8. doi: 10.3233/THC-140807. [DOI] [PubMed] [Google Scholar]
- 26.Garner CE, Smith S, Bardhan P, Ratcliffe NM, Probert C. A pilot study of faecal volatile organic compounds in faeces from cholera patients in bangladesh to determine their utility in disease diagnosis. Trans R Soc Trop Med Hyg. 2009;103:1171–3. doi: 10.1016/j.trstmh.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Garner CE, Smith S, de Lacy Costello B, White P, Spencer R, Probert CS, Ratcliffe NM. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007;21:1675–88. doi: 10.1096/fj.06-6927com. [DOI] [PubMed] [Google Scholar]
- 28.Batty CA, Cauchi M, Lourenco C, Hunter JO, Turner C. Use of the analysis of the volatile faecal metabolome in screening for colorectal cancer. PLoS One. 2015;10:e0130301. doi: 10.1371/journal.pone.0130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt K, Podmore I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J Biomark. 2015;2015:981458. doi: 10.1155/2015/981458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poli D, Goldoni M, Corradi M, Acampa O, Carbognani P, Internullo E, Casalini A, Mutti A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2643–51. doi: 10.1016/j.jchromb.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Analytical Chemistry. 1990;62:2145–2148. [Google Scholar]
- 32.Zhang Z, Pawliszyn J. Headspace solid-phase microextraction. Analytical Chemistry. 1993;65:1843–1852. [Google Scholar]
- 33.Pawliszyn J. Solid phase microextraction: theory and practice. John Wiley & Sons. 1997 [Google Scholar]
- 34.Pawliszyn J. Handbook of solid phase microextraction. Elsevier. 2011 [Google Scholar]
- 35.Baltussen E, Sandra P, David F, Cramers C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: theory and principles. Journal of Microcolumn Separations. 1999;11:737–747. [Google Scholar]
- 36.Melo L, Nogueira A, Lancas F, Queiroz M. Polydimethylsiloxane/polypyrrole stir bar sorptive extraction and liquid chromatography (SBSE/LC-UV) analysis of antidepressants in plasma samples. Anal Chim Acta. 2009;633:57–64. doi: 10.1016/j.aca.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 37.Soini H, Bruce K, Wiesler D, Novotny M. Quantification of volatiles in mammalian urine by stir bar sorptive extraction (SBSE) techniques and gas chromatography [Google Scholar]
- 38.Gao Q, Su X, Annabi MH, Schreiter BR, Prince T, Ackerman A, Morgas S, Mata V, Williams H, Lee WY. Application of urinary volatile organic compounds (VOCs) for the diagnosis of prostate cancer. Clin Genitourin Cancer. 2019;17:183–190. doi: 10.1016/j.clgc.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Tienpont B, David F, Bicchi C, Sandra P. High capacity headspace sorptive extraction. Journal of Microcolumn Separations. 2000;12:577–584. [Google Scholar]
- 40.Ochiai N, Sasamoto K, Takino M, Yamashita S, Daishima S, Heiden A, Hoffman A. Determination of trace amounts of off-flavor compounds in drinking water by stir bar sorptive extraction and thermal desorption GC-MS. Analyst. 2001;126:1652–1657. doi: 10.1039/b102962m. [DOI] [PubMed] [Google Scholar]
- 41.Phillips M. Method for the collection and assay of volatile organic compounds in breath. Anal Biochem. 1997;247:272–8. doi: 10.1006/abio.1997.2069. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Deng C, Yin X, Yao N, Shen X, Zhang X. Gas chromatography-mass spectrometric analysis of hexanal and heptanal in human blood by headspace single-drop microextraction with droplet derivatization. Anal Biochem. 2005;342:318–26. doi: 10.1016/j.ab.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 43.Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Haick H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat Nanotechnol. 2009;4:669–73. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 44.Nakhleh MK, Amal H, Jeries R, Broza YY, Aboud M, Gharra A, Ivgi H, Khatib S, Badarneh S, Har-Shai L, Glass-Marmor L, Lejbkowicz I, Miller A, Badarny S, Winer R, Finberg J, Cohen-Kaminsky S, Perros F, Montani D, Girerd B, Garcia G, Simonneau G, Nakhoul F, Baram S, Salim R, Hakim M, Gruber M, Ronen O, Marshak T, Doweck I, Nativ O, Bahouth Z, Shi DY, Zhang W, Hua QL, Pan YY, Tao L, Liu H, Karban A, Koifman E, Rainis T, Skapars R, Sivins A, Ancans G, Liepniece-Karele I, Kikuste I, Lasina I, Tolmanis I, Johnson D, Millstone SZ, Fulton J, Wells JW, Wilf LH, Humbert M, Leja M, Peled N, Haick H. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano. 2017;11:112–125. doi: 10.1021/acsnano.6b04930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filipiak W, Sponring A, Filipiak A, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. TD-GC-MS analysis of volatile metabolites of human lung cancer and normal cells in vitro. Cancer Epidemiol Biomarkers Prev. 2010;19:182–95. doi: 10.1158/1055-9965.EPI-09-0162. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. Int J Cancer. 2010;126:2663–70. doi: 10.1002/ijc.24970. [DOI] [PubMed] [Google Scholar]
- 47.Ligor T, Ligor M, Amann A, Ager C, Bachler M, Dzien A, Buszewski B. The analysis of healthy volunteers’ exhaled breath by the use of solid-phase microextraction and GC-MS. J Breath Res. 2008;2:046006. doi: 10.1088/1752-7155/2/4/046006. [DOI] [PubMed] [Google Scholar]
- 48.Wehinger A, Schmid A, Mechtcheriakov S, Ledochowski M, Grabmer C, Gastl GA, Amann A. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. International Journal of Mass Spectrometry. 2007;265:49–59. [Google Scholar]
- 49.Smith D, Španěl P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom Rev. 2005;24:661–700. doi: 10.1002/mas.20033. [DOI] [PubMed] [Google Scholar]
- 50.Westhoff M, Litterst P, Freitag L, Urfer W, Bader S, Baumbach JI. Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: results of a pilot study. Thorax. 2009;64:744–748. doi: 10.1136/thx.2008.099465. [DOI] [PubMed] [Google Scholar]
- 51.Di Natale C, Macagnano A, Martinelli E, Paolesse R, D’Arcangelo G, Roscioni C, Finazzi-Agro A, D’Amico A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens Bioelectron. 2003;18:1209–18. doi: 10.1016/s0956-5663(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 52.Hagleitner C, Hierlemann A, Lange D, Kummer A, Kerness N, Brand O, Baltes H. Smart single-chip gas sensor microsystem. Nature. 2001;414:293. doi: 10.1038/35104535. [DOI] [PubMed] [Google Scholar]
- 53.Di Francesco F, Fuoco R, Trivella MG, Ceccarini A. Breath analysis: trends in techniques and clinical applications. Microchemical Journal. 2005;79:405–410. [Google Scholar]
- 54.Oh EH, Song HS, Park TH. Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzyme Microb Technol. 2011;48:427–37. doi: 10.1016/j.enzmictec.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Biasioli F, Yeretzian C, Märk TD, Dewulf J, Van Langenhove H. Direct-injection mass spectrometry adds the time dimension to (B) VOC analysis. TrAC Trends in Analytical Chemistry. 2011;30:1003–1017. [Google Scholar]
- 56.Röck F, Barsan N, Weimar U. Electronic nose: current status and future trends. Chem Rev. 2008;108:705–25. doi: 10.1021/cr068121q. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization. Cancer control: a global snapshot in 2015. World Health Organization; 2016. [Google Scholar]
- 58.Wood SL, Knowles MA, Thompson D, Selby PJ, Banks RE. Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers. Nat Rev Urol. 2013;10:206–18. doi: 10.1038/nrurol.2013.24. [DOI] [PubMed] [Google Scholar]
- 59.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 60.Richter J, Wagner U, Kononen J, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knönagel H, Rist M, Wilber K, Anabitarte M, Hering F, Hardmeier T, Schönenberger A, Flury R, Jäger P, Fehr JL, Schraml P, Moch H, Mihatsch MJ, Gasser T, Kallioniemi OP, Sauter G. High-throughput tissue microarray analysis of cyclin E gene amplification and overexpression in urinary bladder cancer. Am J Pathol. 2000;157:787–94. doi: 10.1016/s0002-9440(10)64592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogers MA, Clarke P, Noble J, Munro NP, Paul A, Selby PJ, Banks RE. Proteomic profiling of urinary proteins in renal cancer by surface enhanced laser desorption ionization and neural-network analysis: identification of key issues affecting potential clinical utility. Cancer Res. 2003;63:6971–83. [PubMed] [Google Scholar]
- 62.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 63.American Cancer Society. Tests for Prostate Cancer. Access at www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/how-diagnosed.html#references on August 11, 2019.
- 64.Moyer VA. Screening for prostate cancer: US preventive services task force recommendation statement. Annals of Internal Medicine. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 65.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 66.Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, Concepcion R, David RD, Deck KB, Dumbadze I. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68:464–70. doi: 10.1016/j.eururo.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 67.Leyten GH, Hessels D, Jannink SA, Smit FP, de Jong H, Cornel EB, de Reijke TM, Vergunst H, Kil P, Knipscheer BC, van Oort IM, Mulders PF, Hulsbergen-van de Kaa CA, Schalken JA. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65:534–42. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 68.Chun FK, de la Taille A, Van Poppel H, Marberger M, Stenzl A, Mulders PF, Huland H, Abbou CC, Stillebroer AB, van Gils MP. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol. 2009;56:659–67. doi: 10.1016/j.eururo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 69.Loeb S, Catalona WJ. The prostate health index: a new test for the detection of prostate cancer. Ther Adv Urol. 2014;6:74–7. doi: 10.1177/1756287213513488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojno KJ, Costa FJ, Cornell RJ, Small JD, Pasin E, Van Criekinge W, Bigley JW, Van Neste L. Reduced rate of repeated prostate biopsies observed in ConfirmMDx clinical utility field study. Am Health Drug Benefits. 2014;7:129–34. [PMC free article] [PubMed] [Google Scholar]
- 71.Klein EA, Chait A, Hafron JM, Kernen KM, Manickam K, Stephenson AJ, Wagner M, Zhu H, Kestranek A, Zaslavsky B. The single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol. 2017;72:942–949. doi: 10.1016/j.eururo.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 72.Hessels D, Smit FP, Verhaegh GW, Witjes JA, Cornel EB, Schalken JA. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin Cancer Res. 2007;13:5103–8. doi: 10.1158/1078-0432.CCR-07-0700. [DOI] [PubMed] [Google Scholar]
- 73.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumours of the urinary system and male genital organs. World Health Organization Classi. 2004 [Google Scholar]
- 75.Ather MH, Masood N, Siddiqui T. Current management of advanced and metastatic renal cell carcinoma. Urol J. 2010;7:1–9. [PubMed] [Google Scholar]
- 76.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 77.Rodrigues D, Monteiro M, Jerónimo C, Henrique R, Belo L, de Lourdes Bastos M, de Pinho PG, Carvalho M. Renal cell carcinoma: a critical analysis of metabolomic biomarkers emerging from current model systems. Transl Res. 2017;180:1–11. doi: 10.1016/j.trsl.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 78.Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23:202–12. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 79.Najjar YG, Rini BI. Novel agents in renal carcinoma: a reality check. Ther Adv Med Oncol. 2012;4:183–94. doi: 10.1177/1758834012443725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang D, Wang C, Pi X, Guo L, Wang Y, Li M, Feng Y, Lin Z, Hou W, Li E. Urinary volatile organic compounds as potential biomarkers for renal cell carcinoma. Biomed Rep. 2016;5:68–72. doi: 10.3892/br.2016.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monteiro M, Moreira N, Pinto J, Pires-Luís AS, Henrique R, Jerónimo C, Bastos ML, Gil AM, Carvalho M, Guedes de Pinho P. GC-MS metabolomics-based approach for the identification of a potential VOC-biomarker panel in the urine of renal cell carcinoma patients. J Cell Mol Med. 2017;21:2092–2105. doi: 10.1111/jcmm.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrissey JJ, Mellnick VM, Luo J, Siegel MJ, Figenshau RS, Bhayani S, Kharasch ED. Evaluation of urine aquaporin-1 and perilipin-2 concentrations as biomarkers to screen for renal cell carcinoma: a prospective cohort study. JAMA Oncol. 2015;1:204–12. doi: 10.1001/jamaoncol.2015.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 84.Mostofi F. Histologic typing of urinary bladder tumors. International Histological Classification of Tumors. 1973:237–256. [Google Scholar]
- 85.Pashos CL, Botteman MF, Laskin BL, Redaelli A. Bladder cancer: epidemiology, diagnosis, and management. Cancer Pract. 2002;10:311–22. doi: 10.1046/j.1523-5394.2002.106011.x. [DOI] [PubMed] [Google Scholar]
- 86.Pow-Sang JM, Seigne JD. Contemporary management of superficial bladder cancer. Cancer Control. 2000;7:335–339. doi: 10.1177/107327480000700402. [DOI] [PubMed] [Google Scholar]
- 87.Cummings KB, Barone J, Ward WS. Diagnosis and staging of bladder cancer. Urol Clin North Am. 1992;19:455–465. [PubMed] [Google Scholar]
- 88.Young MJ, Soloway MS. Office evaluation and management of bladder neoplasms. Urol Clin North Am. 1998;25:603–11. doi: 10.1016/s0094-0143(05)70051-3. [DOI] [PubMed] [Google Scholar]
- 89.CancerNet PDQ, National Cancer Institute. Available at: https://www.cancer.gov/publications/pdq, accessed on August 11, 2019)
- 90.Burchardt M, Burchardt T, Shabsigh A, De La Taille A, Benson MC, Sawczuk I. Current concepts in biomarker technology for bladder cancers. Clin Chem. 2000;46:595–605. [PubMed] [Google Scholar]
- 91.Zlotta AR, Schulman CC. Biological markers in superficial bladder tumors and their prognostic significance. Urol Clin North Am. 2000;27:179–89. xi–xii. doi: 10.1016/s0094-0143(05)70246-9. [DOI] [PubMed] [Google Scholar]
- 92.Poulakis V, Witzsch U, De Vries R, Altmannsberger HM, Manyak M, Becht E. A comparison of urinary nuclear matrix protein-22 and bladder tumour antigen tests with voided urinary cytology in detecting and following bladder cancer: the prognostic value of false-positive results. BJU Int. 2001;88:692–701. doi: 10.1046/j.1464-410x.2001.02355.x. [DOI] [PubMed] [Google Scholar]
- 93.Khalid T, White P, Costello BD, Persad R, Ewen R, Johnson E, Probert CS, Ratcliffe N. A pilot study combining a GC-sensor device with a statistical model for the identification of bladder cancer from urine headspace. PLoS One. 2013;8:e69602. doi: 10.1371/journal.pone.0069602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–97. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- 95.Roy A, Lavrovsky Y, Song C, Chen S, Jung M, Velu N, Bi B, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–52. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 96.Corbin JM, Ruiz-Echevarría MJ. One-carbon metabolism in prostate cancer: the role of androgen signaling. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stover PJ. One-carbon metabolism-genome interactions in folate-associated pathologies. J Nutr. 2009;139:2402–5. doi: 10.3945/jn.109.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mentch SJ, Locasale JW. One-carbon metabolism and epigenetics: understanding the specificity. Ann N Y Acad Sci. 2016;1363:91–8. doi: 10.1111/nyas.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–83. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hakimi AA, Reznik E, Lee CH, Creighton CJ, Brannon AR, Luna A, Aksoy BA, Liu EM, Shen R, Lee W. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell. 2016;29:104–116. doi: 10.1016/j.ccell.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ottaviani S, Brooke GN, O’Hanlon-Brown C, Waxman J, Ali S, Buluwela L. Characterisation of the androgen regulation of glycine N-methyltransferase in prostate cancer cells. J Mol Endocrinol. 2013;51:301–12. doi: 10.1530/JME-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 103.Khan AP, Rajendiran TM, Bushra A, Asangani IA, Athanikar JN, Yocum AK, Mehra R, Siddiqui J, Palapattu G, Wei JT. The role of sarcosine metabolism in prostate cancer progression. Neoplasia. 2013;15:491–501. doi: 10.1593/neo.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Green T, Chen X, Ryan S, Asch AS, Ruiz-Echevarría MJ. TMEFF2 and SARDH cooperate to modulate one-carbon metabolism and invasion of prostate cancer cells. Prostate. 2013;73:1561–1575. doi: 10.1002/pros.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L, Warren A, Scott H, Madhu B, Sharma N. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–33. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barfeld SJ, Itkonen HM, Urbanucci A, Mills IG. Androgen-regulated metabolism and biosynthesis in prostate cancer. Endocr Relat Cancer. 2014;21:T57–66. doi: 10.1530/ERC-13-0515. [DOI] [PubMed] [Google Scholar]
- 107.Tennakoon JB, Shi Y, Han JJ, Tsouko E, White MA, Burns AR, Zhang A, Xia X, Ilkayeva OR, Xin L. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1α-mediated metabolic switch. Oncogene. 2014;33:5251–5261. doi: 10.1038/onc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsouko E, Khan A, White M, Han J, Shi Y, Merchant F, Sharpe M, Xin L, Frigo D. Regulation of the pentose phosphate pathway by an androgen receptor-mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis. 2014;3:e103. doi: 10.1038/oncsis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Costello L, Franklin R, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5:143–153. doi: 10.1016/j.mito.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Costello L, Feng P, Milon B, Tan M, Franklin R. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7:111–7. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Franz MC, Anderle P, Bürzle M, Suzuki Y, Freeman M, Hediger M, Kovacs G. Zinc transporters in prostate cancer. Mol Aspects Med. 2013;34:735–741. doi: 10.1016/j.mam.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Asgari Y, Zabihinpour Z, Salehzadeh-Yazdi A, Schreiber F, Masoudi-Nejad A. Alterations in cancer cell metabolism: the Warburg effect and metabolic adaptation. Genomics. 2015;105:275–281. doi: 10.1016/j.ygeno.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Costello L, Franklin R. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 114.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 115.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–65. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 116.Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31:1509–1515. doi: 10.1093/carcin/bgq131. [DOI] [PubMed] [Google Scholar]
- 117.Nickerson ML, Jaeger E, Shi Y, Durocher JA, Mahurkar S, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko V. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14:4726–34. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun. 2017;8:1769. doi: 10.1038/s41467-017-01965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 120.Deep G, Schlaepfer IR. Aberrant lipid metabolism promotes prostate cancer: role in cell survival under hypoxia and extracellular vesicles biogenesis. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Griffin JE. Androgen resistance-the clinical and molecular spectrum. N Engl J Med. 1992;326:611–8. doi: 10.1056/NEJM199202273260906. [DOI] [PubMed] [Google Scholar]
- 122.Yoshii Y, Furukawa T, Oyama N, Hasegawa Y, Kiyono Y, Nishii R, Waki A, Tsuji AB, Sogawa C, Wakizaka H. Fatty acid synthase is a key target in multiple essential tumor functions of prostate cancer: uptake of radiolabeled acetate as a predictor of the targeted therapy outcome. PLoS One. 2013;8:e64570. doi: 10.1371/journal.pone.0064570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eidelman E, Twum-Ampofo J, Ansari J, Siddiqui MM. The metabolic phenotype of prostate cancer. Front Oncol. 2017;7:131. doi: 10.3389/fonc.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tan LT, Chan KG, Pusparajah P, Lee WL, Chuah LH, Khan TM, Lee LH, Goh BH. Targeting membrane lipid a potential cancer cure? Front Pharmacol. 2017;8:12. doi: 10.3389/fphar.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]