Abstract
Chronic tinnitus has been associated with brain structural changes in both the auditory system as well as limbic system. While there is considerable inconsistency across brain structural findings, growing evidence suggests that distress and other non-auditory symptoms modulate effects. In this study we addressed this issue, testing the hypothesis that limbic changes in tinnitus relate to both disease-related distress as well as co-morbid psychopathology. We obtained high-resolution structural magnetic resonance imaging (MRI) scans from a total of 125 subjects: 59 patients with bilateral chronic tinnitus (29 with a co-morbid psychiatric condition, 30 without), 40 healthy controls and 26 psychiatric controls with depression/anxiety disorders (without tinnitus). Voxel-based morphometry with the CAT12 software package was used to analyse data. First, we analysed data based on a 2 × 2 factorial design (tinnitus; psychiatric co-morbidity), showing trend-level effects for tinnitus in ROI analyses of the anterior cingulate cortex and superior/transverse temporal gyri, and for voxel-based analysis in the left parahippocampal cortex. Multiple regression analyses showed that the parahippocampal finding was mostly predicted by tinnitus rather than (dimensional) psychopathology ratings. Comparing only low-distress tinnitus patients (independent of co-morbid conditions) with healthy controls also showed reduced left parahippocampal grey matter. Our findings demonstrate that depression and anxiety (not only subjective distress) are major modulators of brain structural effects in tinnitus, calling for a stronger consideration of psychopathology in future neurobiological and clinical studies of tinnitus.
Keywords: Anxiety, Co-morbidity, Depression, Distress, Limbic system, Tinnitus, Voxel-based morphometry (VBM)
Highlights
-
•
Chronic tinnitus is associated with high psychiatric co-morbidity and distress.
-
•
Parahippocamal grey matter is associated with tinnitus rather than distress.
-
•
Psychiatric co-morbidity modulates tinnitus-related structural patterns.
1. Introduction
Tinnitus is characterised by phantom perceptions of humming or ringing in one or both ears. It affects about one third of the general population in its acute form (life-time risk) and about 5–15% chronically for more than three months, (Baguley et al., 2013; McCormack et al., 2016; Rosing et al., 2016; Swan et al., 2017). Chronic tinnitus can cause considerable distress and impairment in activities of daily life and has high rates of psychiatric comorbidity: about 25% of chronic tinnitus patients suffer from anxiety and/or depression correlated with higher tinnitus-related distress. They are also more likely to suffer from insomnia and incapacity to work (Bhatt et al., 2017; Gul et al., 2015; Zirke et al., 2013).
Current models of the generation and perpetuation of chronic tinnitus implicate not only cochlear dysfunction but also involvement of cortical and subcortical brain areas over the course of disease (Eggermont, 2003; Rauschecker et al., 2010). Noise-induced or degenerative hearing impairment are assumed as a common origin of chronic tinnitus (Kim et al., 2015; Theodoroff et al., 2015). While cochlear alterations might lead to tinnitus at first, it is assumed that altered input causes reshaping of the auditory pathway leading up to subtle reorganisation of cortical auditory areas (Langers et al., 2012). This mechanism is reflected in the close relationship of Tinnitus pitch and frequency of maximum hearing loss (Schecklmann et al., 2012b). However, even auditory neurectomy only improves tinnitus severity in 45% of cases, while the rest might report reporting worsening or unchanged discomfort (House and Brackmann, 1981). Consecutive factors in the maintenance of tinnitus are (additional) structural and functional changes in brain areas related to emotion processing, possibly due to conscious or subliminal negative evaluation of the constant noise and therefore inability to habituate (Goebel, 2015). The affected areas overlap with structural and functional findings in depression or anxiety patients (Goodkind et al., 2015), which corresponds to the high comorbidity with these illnesses as well.
There is now increasing evidence from neuroimaging studies of tinnitus to support this model of alterations at both auditory sensory and limbic areas (Adjamian et al., 2014; Simonetti and Oiticica, 2015). However, different areas of increased or decreased grey matter volume (GMV) have been reported across studies: GMV in Heschl's gyrus and superior temporal gyrus showed changes in both directions in tinnitus patients compared to healthy controls (Boyen et al., 2013; Husain et al., 2011; Mahoney et al., 2011; Muhlau et al., 2006), whereas inferior colliculus volume (Landgrebe et al., 2009) was decreased and medial geniculate body volume was increased (Muhlau et al., 2006) in patients. As for non-auditory limbic brain structures, decreased GMV has been reported in ventromedial (vmPFC) and dorsomedial (dmPFC) prefrontal cortices, nucleus accumbens, anterior (ACC) and posterior cingulate cortices, hippocampus and supramarginal gyrus (Landgrebe et al., 2009; Leaver et al., 2011; Leaver et al., 2012; Mahoney et al., 2011). However, alterations in some of the areas were modulated by hearing loss (Boyen et al., 2013; Husain et al., 2011; Melcher et al., 2013). In a few studies, regional volumes were associated with comorbid depressive and anxiety symptoms, such as the insula, cerebellum and ACC (Leaver et al., 2012; Schecklmann et al., 2012a).
The model of altered sensory cortical/limbic interaction has also received support from functional neuroimaging studies, which additionally implicate areas involved in (emotional) memory and cognition. Resting state functional magnetic resonance imaging (rs fMRI) has shown several areas of increased connectivity in tinnitus patients including brain stem, basal ganglia, hippocampus and parahippocampal gyri, inferior parietal gyri, as well as the cerebellum. Lower connectivity values were shown for primary auditory cortex, prefrontal areas, left fusiform gyrus and bilateral occipital regions (Simonetti and Oiticica, 2015).
A limitation of several previous studies might have been the assessment of emotional distress and psychiatric comorbidities, the latter often being defined as an exclusion criterion for tinnitus patients (Boyen et al., 2013; Husain et al., 2011; Landgrebe et al., 2009; Leaver et al., 2011; Schecklmann et al., 2012a). This might have impeded discriminating tinnitus effects (in a narrow sense) from psychiatric comorbidity, and also differences in selection biases across (chronic) tinnitus populations. However, psychiatric comorbidity might not only be essential in developing more effective treatment programmes, but also in integrating converging or overlapping biological pathways leading to chronic tinnitus. Disentangling brain structures affected in chronic tinnitus with and without manifest psychiatric co-morbidities might also affect identification of new target areas for repetitive transcranial magnetic stimulation (rTMS) for therapeutic interventions (Lehner et al., 2016; Wang et al., 2015).
In the present study, we aimed to address the problem of overlapping vs. distinct brain structural alterations associated with distress and psychiatric co-morbidity. We recruited chronic tinnitus patients with different ranges of psychological distress, including also more disabled patients with psychiatric co-morbidities, using both psychiatrically healthy controls as well as psychiatric controls (without tinnitus) matched for psychopathology. Our first goal was to replicate previous results comparing GMV between low-distress tinnitus patients (a cohort most similar to that in previous tinnitus imaging studies) and healthy controls. We then used all four cohorts in a 2 × 2 factorial design to contrast effects of tinnitus vs. those of psychiatric disorders/co-morbidity, testing for the main effect of tinnitus vs. distress.
2. Methods
2.1. Participants
We included a total of 125 subjects, which fell into four cohorts (matched for age and gender): low-distress chronic tinnitus, high-distress chronic tinnitus, healthy controls and psychiatric controls. All participants gave written informed consent to a study protocol approved by the local Ethics Committee of Jena University Medical School. This protocol included audiometry and structural brain imaging, as well as psychometric measures of distress/psychiatric symptoms and comprehensive psychiatric evaluation using the German version of the Structured Clinical Interview for DSM-IV Axis I Disorders (SKID-I) (Lobbestael et al., 2011) (administered by a psychiatric resident, B.B.). Resulting DSM-IV diagnoses were confirmed by two different psychiatrists (B.B. and I.N.) based on structured clinical interviews (using SKID-I interviews by a resident psychiatrist, B.B.) in all participants of the study.
For assessment of psychological symptoms common in tinnitus, all study participants completed well established self-ratings as well as rater-based evaluations of symptoms: Depressive symptoms were measured using Beck's depression inventory, BDI-2 (Beck and Steer, 1984), the Hospital anxiety and depression scale, HADS-D (Herrmann, 1997; Hinz and Brahler, 2011), and Hamilton depression scale, HAM-D (Bailey and Coppen, 1976; Schwab et al., 1967; Worboys, 2013). Anxiety was measured using the Hospital anxiety and depression scale (Herrmann, 1997; Hinz and Brahler, 2011) (HADS-A) and Hamilton anxiety scale, HAM-A (Gjerris et al., 1983). Somatoform symptoms were assessed using the SOMS-2 Screening for somatoform symptoms (Rief et al., 2001; Rief and Hiller, 1999). In addition, all participants completed the Symptom checklist 90 revised (SCL-90-R), which can be divided into ten subscales (Derogatis et al., 1974; Schmitz et al., 2000), reflecting a range of psychopathologies and general psychological distress. An overview of demographic, audiometric and psychometric characteristics of all four groups is given in Table 1.
Table 1.
Demographic and audiometric data on the four groups.
| Tinnitus only (n = 30) | Tinnitus & psychiatric comorbidity (n = 29) | Healthy controls (n = 40) | Psychiatric controls (n = 26) | Between-subject-effects –ANOVA p (F) | |
|---|---|---|---|---|---|
| Mean age (SD) | 51.9 (±12.5) | 49.3 (±12.3) | 45.7 (±15.7) | 44.3 (±11.2) | |
| Gender | 13 f, 17 m | 14 f, 15 m | 20 f, 20 m | 15 f, 11 m | |
| IQ | 113.3 (±15.9) | 107.9 (±14.6) | 117.7 (±16.7) | 110.8 (±14.7) | |
| Handedness | 0.7 (±0.6) | 0.7 (±0.5) | 0.8 (±0.4) | 0.7 (±0.5) | |
| TQ | 41.6 (±13.1) | 42.8 (±18.6) | n.a. | n.a. | |
| Mean left 4-PTA (SD) | 24.4 (±11.9) | 25.5 (±14.7) | 11 (±5.2) | 10.6 (±5.3) | |
| Mean right 4-PTA (SD) | 22.2 (±9.1) | 23.2 (±14.5) | 8.8 (±4) | 9 (±5) | |
| Mean BDI-2 (SD) | 9.2 (±6.4) | 15.7 (±10) | 2.5 (±5.1) | 22.4 (±11.2) | 0.0001 (34.63) |
| Mean HAM-A (SD) | 9.1 (±4.4) | 12.8 (±7.2) | 0.7 (±1.2) | 13 (±7.6) | 0.0001 (37.22) |
| Mean HADS-D (SD) | 2.3 (± 2.2) | 6.9 (±4.7) | 1.1 (±2.1) | 9.9 (±4) | 0.0001 (44.51) |
| Mean HADS-A (SD) | 4.9 (±3.1) | 7.6 (±3.9) | 2 (±2.3) | 9.3 (±3.4) | 0.0001 (32.8) |
| SOMS-2 SI (DSM-IV) (SD) | 65.8 (±25.3) | 80.8 (±20.6) | 43.7 (±20.1) | 77.4 (±18.7) | 0.0001 (22.05) |
| SCL-90-R SOM (SD) | 0.4 (±0.4) | 0.7 (±0.7) | 0.2 (±0.3) | 0.6 (±0.5) | 0.0001 (7.52) |
| SCL-90-R OBS (SD) | 0.5 (±0.4) | 1 (±0.8) | 0.2 (±0.3) | 1.1 (±0.6) | 0.0001 (20.58) |
| SCL-90-R INT (SD) | 0.3 (±0.4) | 0.7 (±0.8) | 0.1 (±0.2) | 0.8 (±0.8) | 0.0001 (11.26) |
| SCL-90-R DEP (SD) | 0.4 (±0.3) | 0.9 (±0.7) | 0.1 (±0.3) | 1.3 (±0.8) | 0.0001 (28.16) |
| SCL-90-R ANX (SD) | 0.2 (±0.3) | 0.6 (±0.6) | 0.1 (±0.2) | 1 (±0.6) | 0.0001 (22.82) |
| SCL-90-R HOS (SD) | 0.2 (±0.3) | 0.6 (±0.7) | 0.1 (±0.3) | 0.5 (±0.5) | 0.0001 (7.07) |
| SCL-90-R PHO (SD) | 0.1 (±0.2) | 0.3 (±0.4) | 0.1 (±0.3) | 0.6 (±0.7) | 0.0001 (7.87) |
| SCL-90-R PAR (SD) | 0.3 (±0.4) | 0.6 (±0.7) | 0.1 (±0.3) | 0.7 (±0.8) | 0.0001 (9.06) |
| SCL-90-R PSY (SD) | 0.2 (±0.3) | 0.4 (±0.4) | 0.1 (±0.2) | 0.5 (±0.5) | 0.0001 (11.16) |
| SCL-90-R Z (SD) | 3.9 (±3.1) | 6.7 (±5.5) | 1.1 (±1.2) | 6.9 (±5.7) | 0.0001 (8.15) |
SD – standard deviation; f-female<, m-male; IQ – intelligence quotient; handedness ranging from −1 (purely left-handed) to 1 (purely right-handed); TQ – tinnitus questionnaire; 4-PTA- pure tone average from frequencies 500 Hz, 1 kHz, 2 kHz and 4 kHz; HAM-A – Hamilton anxiety scale; HADS – D – Hospital anxiety and depression scale, depression subscale; HADS – A – Hospital anxiety and depression scale, anxiety subscale; SOMS-2 SI – Screening for Somatoform Disorders, Somatization Index (according to DSM-IV criteria); SCL-90-R subscales: SOM – Somatization, OBS – obsessive-compulsive, INT – interpersonal sensitivity, DEP – depression, ANX – anxiety, HOS – hostility, PHO – phobic anxiety, PAR – paranoid ideation, PSY – psychoticism, Z - subscale for sleep disturbances.
General exclusion criteria for all participants were history of (other) major neurological as well as untreated major general medical conditions. All participants completed the MWT-B, a German inventory similar to the NART (Antretter et al., 2013), to estimate IQ and confirm the inclusion criterion of IQ higher 80. Handedness was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971).
The two tinnitus groups (total of n = 59 patients) included patients with chronic, bilateral tinnitus with a duration of more than three months. Patients were recruited from the specialised interdisciplinary tinnitus entre (Ivansic et al., 2017) of the Department for Otorhinolaryngology in Jena, were they had undergone extensive medical investigation before taking part in the study. Most of the tinnitus patients suffered from hearing loss (4-pure tone average (4-PTA) > 25 dB in pure tone audiogram). We assessed tinnitus distress using the well-established German version of the tinnitus questionnaire (TQ) (Goebel and Hiller, 1994). TQ values under 46 points indicated low-distress tinnitus (n = 37 of the sample) whereas higher scores defined high-distress patients.
The total tinnitus cohort included subgroups with acute or history of major comorbid psychiatric disease (n = 29) (other than minor symptoms of adjustment or distress), as well as without psychiatric comorbidity (n = 30). The former included mostly affective disorders (22 patients: 9 with current depressive episode, 9 with history of depressive disorder, currently remitted, 2 dysthymia, 2 with current minor depression/adjustment disorder), in addition one had post-traumatic stress disorder, one was addicted to analgesics, and two reported harmful alcohol use (without dependence). Of these 29 tinnitus patients, 11 received antidepressant medication (either selective serotonin inhibitors, SSRI, or combined serotonin-norepinephrine inhibitors, SNRI), in one case <2 weeks, in the other cases >2 weeks.
A healthy control group (n = 40) was recruited from the community via press releases and contacted the laboratory through email or phone. They neither had a current or a history of DSM-IV axis I psychiatric disorders, no first-degree relative with a known psychiatric disorder, nor tinnitus (other than sensations lasting up to few seconds sporadically) or hearing impairment with >25 dB (4-PTA) measured by pure tone audiogram. None of the healthy controls received psychotropic medication.
Finally, the psychiatric control group (n = 26) included patients with an affective or anxiety disorder, but no other medical history of hearing loss (4-PTA < 25 dB). This cohort, included for the 2 × 2 factorial design of the study, was intended to match as closely as possible the clinical psychiatric phenotype of the tinnitus subgroup with psychiatric co-morbidities, since these are most common co-morbidities among chronic tinnitus patients (Bhatt et al., 2017; Hiller and Goebel, 1992; Zirke et al., 2013). Patients were recruited from in- or out-patient units of the Department of Psychiatry and Psychotherapy in Jena. Among psychiatric controls 23 had a depressive disorder (22 with major depression and current depressive episode, 1 remitted), 2 had agoraphobia with panic disorder and one had a depressive episode with somatoform pain disorder. Within this group 24 of the 26 patients received antidepressants (SSRI or SNRI): 4 for <2 weeks, the other 20 for >2 weeks.
2.2. Magnetic resonance imaging (MRI)
We obtained high-resolution T1-weighted structural MRI scans on a 3 Tesla Siemens Prisma fit system (Siemens, Erlangen, Germany) using a standard quadrature head coil and an axial 3-dimensional magnetization prepared rapid gradient echo (MP-RAGE) sequence (TR 2300 ms, TE 2.07 ms, α 9°, 192 contiguous sagittal slices, in-plane field of view 256 mm, voxel resolution 1 × 1 × 1 mm; acquisition time 5:21 min). Tinnitus patients received additional ear protection with earplugs and earmuffs, which made MRI noise tolerable for all of them. None of the study participants had a structural pathology, as assessed by a radiologist, and all were visually inspected to confirm absence of artefacts.
2.3. Voxel-based morphometry
We used the CAT12 toolbox (http://www.neuro.uni-jena.de/cat; Structural Brain Mapping Group, Jena University Hospital, Jena, Germany) implemented in SPM12 (Statistical Parametric Mapping, Institute of Neurology, London, UK) for voxel-based morphometry (VBM) analysis of imaging data. All T1-weighted images were corrected for bias – field inhomogeneities, then segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) (Ashburner and Friston, 2005) and spatially normalised using the DARTEL algorithm (Ashburner, 2007). The segmentation process was further extended by accounting for partial volume effects (Tohka et al., 2004), applying adaptive maximum a posteriori estimations (Rajapakse et al., 1997). After pre-processing and in addition to visual checks for artefacts, all scans passed an automated quality check protocol. Scans were smoothed with a Gaussian kernel of 8 mm (FWHM). For exclusion of artefacts on the grey/white matter border (i.e. incorrect voxel classification), we applied an absolute grey matter threshold of 0.1.
For subsequent analyses, we considered previous studies of tinnitus for anatomical hypotheses (Adjamian et al., 2014; Adjamian et al., 2009; Simonetti and Oiticica, 2015), and performed regional analyses using small volume correction for bilateral anterior and posterior cingulate cortices, hippocampus, insula, medial frontal cerebrum, nucleus accumbens, parahippocampal gyri, superior and transverse temporal and supramarginal gyri, applying an image calculated according to the SPM12 Neuromorphometrics atlas. In addition, whole-brain voxel-wise analyses are reported.
2.4. Statistics
For statistical analysis of grey matter maps, we applied the general linear model (GLM) approach implemented in SPM12. We performed whole-brain and region-of-interest (ROI) analyses and included total intracranial volume (TIV), age and gender as nuisance variables in order to remove the related variance in all our analyses.
First, we sought to replicate former findings of brain structural changes in tinnitus patients with low distress, comparing the low-distress tinnitus subgroup with TQ ratings lower than 46 (n = 37) with the 40 age and gender matched healthy controls using a two-sample t-test.
Second, we tested our main hypothesis applying a 2 × 2 factorial ANOVA with the factors tinnitus and psychiatric co-morbidity across the four groups, i.e.: tinnitus patients with (n = 29) and without (n = 30) psychiatric comorbidity, healthy controls (n = 40) and psychiatric controls (n = 26). All groups were matched for age and gender and the tinnitus groups showed no significant difference of mean 4-PTA bilaterally (p > .1).
From these two analyses we extracted mean cortical volumes of significant clusters for subsequent analysis and applied automatic linear modelling in SPSS (IBM SPSS Statistics, version 23) to determine variables best predicting these structural variations: age, gender, diagnosis of chronic bilateral tinnitus, psychiatric diagnosis, IQ, handedness, psychometric measures (BDI-2, HADS-D, HAM-D, HADS-A, HAM-A, SCL-90-R subscales, SOMS-2), bilateral 4-PTA, TIV.
For psychometric characterization of the four groups, we calculated a multifactorial ANOVA to compare all self- and rater-assessment scales between all groups using SPSS Statistics.
3. Results
3.1. Comparison of psychometric data across groups
The MANOVA revealed significant group differences regarding the psychometric data (Pillai's trace: p < .0001, F = 5.105).
Tinnitus patients with psychiatric comorbidity showed significantly higher values in all psychopathology scales (except for the somatization subscale of SCL-90-R) compared to tinnitus patients without psychiatric comorbidity. They mostly did not differ from psychiatric controls except for HADS-D, BDI-2 and SCL-90-R anxiety subscales, in which psychiatric controls scored higher.
Tinnitus patients without psychiatric comorbidity had significantly more symptoms than healthy controls except for SCL-90-R hostility, phobia and psychotic subscale. They also had no significant differences regarding somatoform symptoms (according to SOMS-2 and SCL-90-R somatization subscale) to psychiatric controls, but had otherwise significantly less psychiatric symptoms of all other scales.
Tinnitus patients with psychiatric comorbidity and psychiatric controls had throughout significantly higher scale values than healthy controls. Between- subject-effects are listed in Table 1.
3.2. Effects of tinnitus vs. psychiatric co-morbidity on brain structure (2 × 2 factorial ANOVA)
Testing the main hypothesis of our study by comparing effects of tinnitus vs. psychiatric co-morbidity, we identified diverging effects of the factors tinnitus vs. the psychiatric morbidity factor across several brain regions.
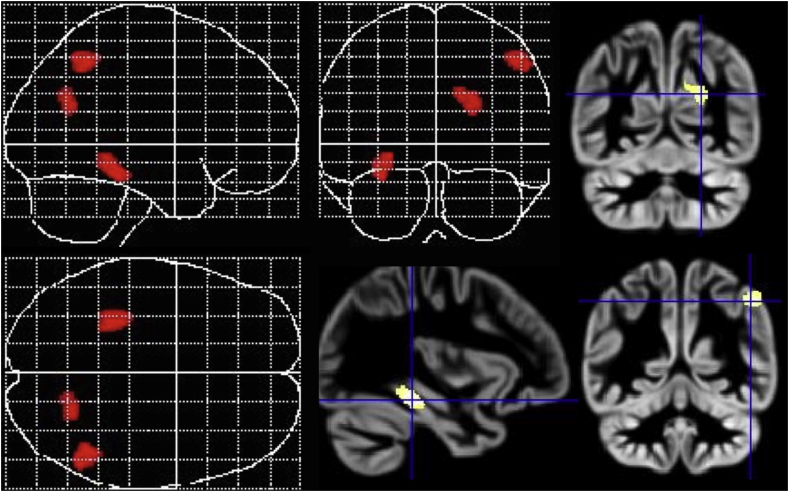
Testing for the main effect of tinnitus (equalling a comparison of tinnitus patients to both the healthy and the psychiatric control groups), we found a trend at corrected threshold levels for decreased GMV related to presence of tinnitus in the ROI analyses for ACC, superior and transverse temporal gyri (p < .1, FWE-corrected), and a significant decrease in ROI analysis for the parahippocampal cortex (p = .012, FWE-corrected), with whole-brain level effects at p < .001 (uncorrected) mainly in left parahippocampal and fusiform gyri, as well as right inferior parietal gyrus and precuneus (Table 3, Fig. 2).
Table 3.
Overview of significant decreased GMV in tinnitus patients vs. controls (main effect of tinnitus).
| Anatomical region | Co-ordinates | k | p (uncorrected peak-level) | T |
|---|---|---|---|---|
| Left parahippocampal and fusiform g. | −33;−34;−15 | 352 | 0.0001 | 4.01 |
| Right inferior parietal g. | 46;−52;48 | 307 | 0.0001 | 3.84 |
| Right precuneus | 22;−62;24 | 244 | 0.0001 | 3.59 |
| 14;−64;30 | 3.5 | |||
| ROI: bilateral parahippocampal g. | −32;−34;−14 | 77 | 0.012 (FWE-corrected) | 3.93 |
| ROI: bilateral ACC | 3;44;4 | 50 | 0.041 (FDR-corrected) | 3.26 |
| 14;39;0 | 3.24 | |||
| 8;44;0 | 3.23 | |||
| −9;40;15 | 5 | 3.25 | ||
| ROI: bilateral superior temporal g. | −54;−14;−3 | 30 | 0.067 (FDR-corrected) | 3.34 |
| −52;−12;−8 | 3.25 | |||
| 50;−40;24 | 3 | 3.2 | ||
| −46;−45;16 | 4 | 3.2 | ||
| −50;−45;16 | 1 | 3.18 | ||
| ROI: bilateral transverse temporal g. | 45;−12;8 | 1 | 0.056 (FWE-corrected) | 3.18 |
k – number of voxels; expected voxels per cluster k = 158.37.
Fig. 2.
Main effect of tinnitus diagnosis in the 2 × 2 factorial ANOVA presented as maximum intensity projections and section overlays on an average image of all participants.
Main effect of psychiatric diagnosis showed significantly increased GMV in whole-brain analyses in psychiatrically ill patients (apart from chronic tinnitus; p < .001) in several very small clusters. Only one cluster exceeded expected cluster size of 158.37 with a size of k = 260 and was located in right superior temporal gyrus (co-ordinates: 46; −20; −4 and 42; −26; 0; T = 3.5; p < .001 uncorrected at peak-level).
3.3. Multiple regression analyses
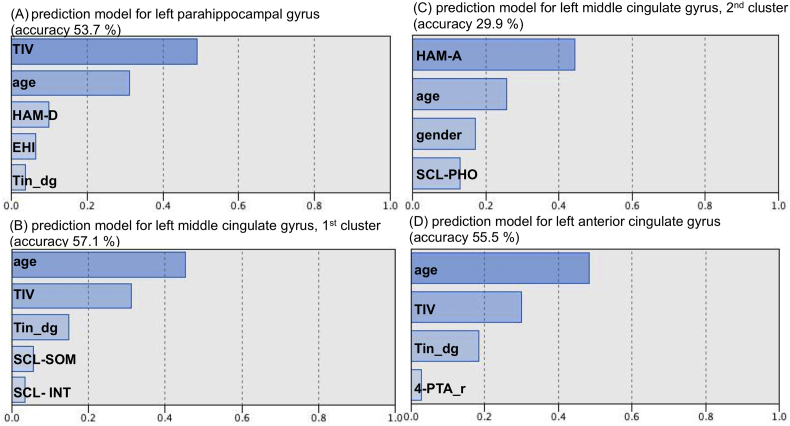
The automatic linear modelling procedure identified 4–7 factors predicting about 50% of each cluster (Fig. 3, Fig. 4). All of the clusters showed high associations with age and TIV. Apart from those two factors, areas extracted from the two-sample t-test produced the following factors: left parahippocampal gyrus was mostly predicted by HAM-D, less by handedness and tinnitus diagnosis. Left middle cingulate gyrus was predicted by tinnitus diagnosis and to a lesser extent by somatoform and social anxiety symptoms (as derived from SCL-90-R subscales). The model for the other peak defined next to age and gender anxiety symptoms (HAM-A and SCL-90-R-phobia subscale) as the most predictive factors. Left ACC was more predicted by tinnitus diagnosis and right 4-PTA.
Fig. 3.
Bar graphs illustrating predictive values of the four to five main factors contributing to GMV decrease in low-distress tinnitus patients compared to healthy controls in (A) left parahippocampal gyrus (accuracy 53.7%), (B) and (C) left middle cingulate gyrus (accuracy 57.1% resp. 29.9%) and (D) left ACC (accuracy 55.5%). TIV – total intracranial volume, HAM-D – Hamilton depression inventory, EHI – Edinburgh handedness inventory, Tin_dg – tinnitus diagnosis, SCL – symptom checklist 90 revised (SOM – subscale for somatoform symptoms, INT – subscale for symptoms of social insecurity, PHO – phobic symptoms), HAM-A – Hamilton anxiety inventory, 4-PTA_r – right-sided 4- pure tone audiogram.
Fig. 4.
Bar graphs illustrating predictive values of the four to eight main factors contributing to GMV decrease as the main effect of tinnitus (with and without psychiatric comorbidity) compared to controls with and without psychiatric illness in (A) left parahippocampal gyrus (accuracy 44.3%), (B) right inferior parietal gyrus (accuracy 43.9%) and (C) right precuneus (accuracy 33.4%). TIV – total intracranial volume, Tin_dg – tinnitus diagnosis, SCL – symptom checklist 90 revised (PSY – subscale for psychotic symptoms, PAR – subscale for paranoid symptoms, OBS – subscale for obsessive-compulsive symptoms, Z – subscale for sleep disturbances), BDI-2 – Beck's depression inventory, HAM-A – Hamilton anxiety inventory, HADS-A – anxiety subscale of hospital anxiety and depression scale.
For our main hypotheses tested in the 2 × 2 ANOVA design, the left parahippocampal cluster extracted from these analyses was mainly predicted by tinnitus diagnosis and less by psychometric measures (SCL-90-R psychoticism and paranoia subscales and BDI-2). The right inferior parietal cluster was mainly predicted by tinnitus diagnosis, anxiety measures (HAM-A, HADS-A) but also obsessive-compulsive symptoms and sleep disturbance (SCL-90-R). GMV variation in precuneus was mostly predicted by tinnitus diagnosis (next to age, gender and TIV).
3.4. Low-distress tinnitus vs. healthy controls (two-sample t-test)
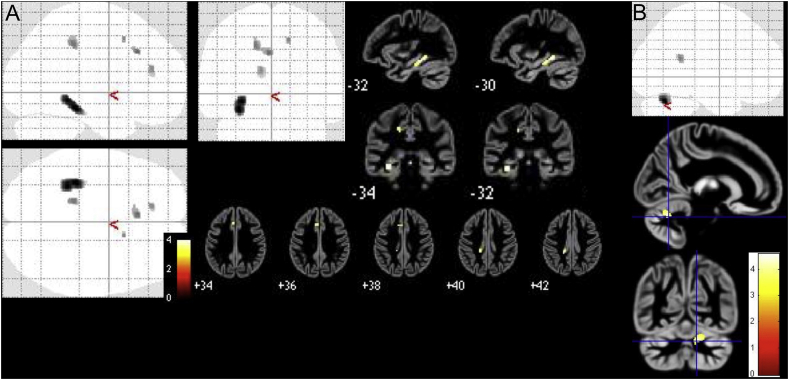
Low-distress tinnitus patients had decreased GMV in anterior and posterior cingulate gyri and parahippocampal gyri compared to healthy controls (small-volume correction, p < .1, FWE-corrected), while whole-brain analyses showed reduced GMV in patients in the left parahippocampal and fusiform gyri, left anterior and middle cingulate cortex, as well as right superior frontal gyrus (p < .001, uncorrected).
The low-distress tinnitus patients also showed higher GMV in right cerebellum and superior and middle temporal gyri (p < .001, uncorrected) with only trend-level significance FWE-corrected levels for the superior temporal gyrus when applying small volume correction (Table. 2, Fig. 1).
Table 2.
Overview of significant GMV differences between low-distress tinnitus patients and healthy controls.
| Anatomical region | Co-ordinates | k | p (uncorrected peak-level) | T |
|---|---|---|---|---|
| Low-distress tinnitus < healthy controls | ||||
| Left parahippocampal and fusiform g. | −33;−33;−15 | 319 | 0.0001 | 4.05 |
| −30;−40;−9 | 4.02 | |||
| Left middle cingular g. | −4;24;34 | 40 | 0.0001 | 3.57 |
| −16;−34;40 | 63 | 0.0001 | 3.53 | |
| Right superior frontal g. | 14;12;46 | 7 | 0.0001 | 3.48 |
| Left anterior cingulate g. | −12;38;20 | 33 | 0.0001 | 3.45 |
| ROI: bilateral ACC | −12;38;20 | 33 | 0.096 (FWE-corr.) | 3.45 |
| ROI: bilateral PCC | −16;-34;40 | 25 | 0.084 (FWE-corr.) | 3.53 |
| ROI: bilat. parahipp. g. | −32;−32;−16 | 72 | 0.019 (FWE-corr.) | 4.01 |
| −30;−38;−10 | 3.78 | |||
| Low-distress tinnitus > healthy controls | ||||
| Right cerebellum (area 6) | 12;−63;−28 | 242 | 0.0001 | 4.58 |
| Right sup. and mid. temporal g. | 68;−46;18 | 67 | 0.0001 | 3.70 |
| ROI: right superior temporal g. | 68;−46;18 | 42 | 0.091 (FWE-corr.) | |
k – number of voxels; ROI: “region of interest” (result of small volume correction); FWE: “family-wise-error” (method of correction for multiple comparisons).
Fig. 1.
Results of two-sample t-test between low-distress tinnitus patients and healthy controls presented as maximum intensity projections and overlays on an average image of all participants, (A) significant clusters of decreased GMV in patients, (B) significant clusters of increased GMV in patients.
4. Discussion
In this study, we aimed to address the problem of psychiatric co-morbidity on the brain structural changes induced by tinnitus.
While our ROI-based analysis of brain areas implicated in tinnitus (esp. parahippocampal cortex, ACC and superior temporal/transverse temporal cortices) did show main effects (ROI, FWE-corrected) of tinnitus in the parahippocampal cortex and trend-level findings in the ACC and superior/transverse temporal cortices, several other findings at whole-brain level (e.g. in the precuneus) did not survive more conservative correction for multiple comparisons. The parahippocampal grey matter reduction was also seen in a comparison analysis of tinnitus patients without psychiatric co-morbidity vs. healthy controls, thus substantiating our findings.
Our findings confirm the clinical impression that tinnitus patients suffer from psychological distress nearly as much as psychiatric patients. And even if they do not meet diagnostic criteria for psychiatric co-morbidity they are more distressed than healthy controls. Our main finding regarding brain structural associations of this distress in tinnitus patients emphasizes the importance of limbic structures (anterior and posterior cingulate gyri and parahippocampal gyri). These changes appear to be multifactorial as they are predicted by several parameters (age, gender, diagnosis, auditory function, psychometric data), which is in line with recent studies of MR morphometry in tinnitus. They have increasingly acknowledged that multiple factors appear to influence both the magnitude as well as the location of changes throughout the cortex and subcortical structures (Leaver et al., 2016; Leaver et al., 2012). In particular, this has been the case for attempting to segregate effects of hearing loss vs. tinnitus (Allan et al., 2016; Boyen et al., 2013; Husain et al., 2011; Yoo et al., 2016), as well as (subjective) distress (Leaver et al., 2012; Schecklmann et al., 2013), which differs considerably across tinnitus populations. In view of the increasing divergence of both structural and functional MR findings in tinnitus, it has been argued that considering failures to replicate previous findings (Melcher, 2013) as well as to appreciate the heterogeneity of patient cohorts and the potential of subgroup analyses (Schmidt et al., 2018) are important moves to clarify neural network changes.
Our findings add another layer to this ongoing research by addressing the problem of co-morbid psychiatric disorders. Three main findings emerge from our results. First, we identify co-morbid mental disorder as a major factor influencing the pattern of grey matter alterations associated with tinnitus. Importantly, this does not overlap with mere levels of distress, but might be an independent source of biological heterogeneity. Second, our findings highlight the problem of this variable in recruitment of tinnitus patients: participation criteria that are focused on excluding tinnitus patients with co-morbid mental disorders might be a source of selection bias, which then again influences sample composition and thus magnitudes and patterns of structural (and/or functional) changes. Finally, our multiple regression analyses demonstrate not only the magnitude of effects related to tinnitus (as compared to age or other variables), but also the differential contribution of symptom profiles on effect sizes.
Several regional structural findings merit particular attention in reference to the current literature. Grey matter volume reduction in the (left) parahippocampal cortex is among the main regional findings of this study, with effects in both the 2 × 2 factorial analysis as well as the comparison of low-distress tinnitus vs. healthy controls (albeit at different thresholds of significance). This area has repeatedly been implicated in studies of tinnitus, both structural (Schmidt et al., 2018; Simonetti and Oiticica, 2015) as well as functional (Chen et al., 2017a).
In current models of tinnitus, the parahippocampal cortex has been implicated in memory mechanisms related to phantom percept persistence (Langguth et al., 2012). While recent functional studies have tied parahippocampal connectivity to distress (Mohan et al., 2018) or disease duration (Lv et al., 2017) as well as possibly predictive of training therapies aimed at reducing distress (Kim et al., 2016), the structural findings rather appear to indicate a primary network role unrelated to the clinical expression of distress. This is indeed in line with our multiple regression findings. A most recent study has provided initial evidence for genetic modulation, with effects in this region being particularly present in Met allele carriers of the COMT Val158Met polymorphism (Vanneste et al., 2018), but these findings have not yet been extended to larger samples.
There are also multiple links to disturbed parahippocampal function in tinnitus. A most recent functional MRI study found short-range temporal lobe connectivity including the parahippocampal cortex to be altered in chronic (rather than acute) tinnitus patients (Chen et al., 2017b; Zheng et al., 2019). Already a previous meta-analysis of fMRI studies has highlighted the role of this region in tinnitus (Chen et al., 2017a). While these rs fMRI studies also provide a link to default mode network dysfunction, the role of other structures like the precuneus is still not being fully understood (Schmidt et al., 2017). A functional study has suggested that the precuneus shows increased connectivity in recent-onset compared to long-term tinnitus (Carpenter-Thompson et al., 2015). However, it is unclear, whether that transition might then be accompanied by secondary structural changes. Parietal cortex plasticity has, for example, been documented in targeted interventions for tinnitus (Stein et al., 2015).
While our study is the first to explicitly address psychiatric co-morbidity as a factor in structural changes in tinnitus, we also need to consider that this might limit comparability to previous VBM studies. Indeed, several previous studies have not included tinnitus patients with psychiatric co-morbidity. While this might allow for more homogeneous samples, it is not congruent with the clinical realities of tinnitus not only being associated with subjective distress but also manifest psychopathologies (Durai and Searchfield, 2016; Ivansic et al., 2017; Jacques et al., 2013; Pattyn et al., 2016; Trevis et al., 2016; Ziai et al., 2017). Even though our study aimed at addressing this factor, we need to consider the limitation that our choice of psychiatric controls mostly included depression/anxiety disorder patients (to match this particular tinnitus sample), so expansion to other psychopathology dimensions is warranted. A further limitation to be noted is that several of our findings (as indicated above) did not withstand more conservative statistical corrections. This might not only be related to sample size (which was nevertheless larger than in several other VBM studies), but also the larger heterogeneity in our tinnitus samples.
Psychiatric co-morbidity, although in many cases correlated with, is, however, not identical to subjective distress. While loudness might be conceptualised as a dimensional parameter to be considered in statistical models, psychiatric co-morbidities are often treated as categorical variables. Incorporating both would therefore necessitate not only more complex clinical assessments, but also larger samples to dissect variance related to the different sources. As shown in our multiple regression analyses, which differentiate the impact of the various dimensions of psychopathologies (depressive, anxious, somatisation etc.), the combination of dimensional and categorical approaches leads to a more detailed analysis of the associations of regional brain structural changes with clinical facets. Adding a further level of complexity, it also remains unclear whether co-morbid psychiatric disorders exert additive effects (with or without interactions) on brain parameters (Langguth et al., 2011).
Our findings demonstrate that the current pathophysiogical models of chronic tinnitus need to take into account not only the dimension of subjective distress (Rauschecker et al., 2010; Rauschecker et al., 2015; Schecklmann et al., 2013; Schmidt et al., 2018). Both (initial) hearing loss as well as subsequent gating processes (Rauschecker et al., 2015) and development of subjective distress might interact with (genetic or developmental) liability of depression or anxiety disorders to generate a complex interaction of auditory cortical, ACC, parahippocampal and other regional changes. The identification of the differential associations might, however, be a crucial step in identifying neural targets of interventions such as transcranial magnetic stimulation (rTMS). There is increasing evidence that regional stimulation might improve tinnitus, but the optimal target (or multiple targets) of stimulation is still under debate (Chung et al., 2012; Lehner et al., 2016; Lehner et al., 2013; To et al., 2018; Wang et al., 2015). While some research suggests that there might be target areas like the DLPFC which are relevant to multiple disorders (tinnitus, depression etc.), it is unclear whether subgroups of (chronic) tinnitus patients might benefit from different stimulation sites. A recent combined rTMS and VBM/fMRI study has also demonstrated reversibility of structural effects following a treatment course of rTMS in chronic tinnitus (Poeppl et al., 2018).
In summary, our findings provide some support for previous studies implicating the (left) parahippocampal cortex as one of several key areas in chronic tinnitus, which is not related merely to tinnitus distress. Psychiatric co-morbidity is a potentially significant modulator of brain structural changes in tinnitus, and might have been underestimated in previous studies, due to sample selection. The dissection of differential regional effects of the complex tinnitus network holds the potential to provide improved selection of brain stimulation sites in experimental treatments of tinnitus.
Acknowledgments
The project (“TiPs”-study) was in in part funded by IZKF Jena (junior scientist grant to B.B.) and by a project of the German Research Foundation / Deutsche Forschungsgemeinschaft(grant to C.D. DFG Do 711/10-1). We are grateful to Marlen Hagemann for conducting pure tone audiograms for all participants, as well as collection and tabulation of tinnitus questionnaires. We also thank Ines Krumbein for overseeing most of the MRI measurements. Also, we would like to thank Prof. Heinrich Sauer for his support for the project.
References
- Adjamian P., Sereda M., Hall D.A. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hear. Res. 2009;253:15–31. doi: 10.1016/j.heares.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Adjamian P., Hall D.A., Palmer A.R., Allan T.W., Langers D.R. Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci. Biobehav. Rev. 2014;45:119–133. doi: 10.1016/j.neubiorev.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan T.W., Besle J., Langers D.R., Davies J., Hall D.A., Palmer A.R., Adjamian P. Neuroanatomical alterations in tinnitus assessed with magnetic resonance imaging. Front. Aging Neurosci. 2016;8:221. doi: 10.3389/fnagi.2016.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antretter E., Dunkel D., Haring C. The assessment of cognitive abilities in psychiatric patients: are widely used psychological tests still up-to-date? Psychiatr. Prax. 2013;40:120–129. doi: 10.1055/s-0032-1332988. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baguley D., McFerran D., Hall D. Tinnitus. Lancet. 2013;382:1600–1607. doi: 10.1016/S0140-6736(13)60142-7. [DOI] [PubMed] [Google Scholar]
- Bailey J., Coppen A. A comparison between the Hamilton rating scale and the Beck inventory in the measurement of depression. Br. J. Psychiatry. 1976;128:486–489. doi: 10.1192/bjp.128.5.486. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A. Internal consistencies of the original and revised Beck depression inventory. J. Clin. Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Bhatt J.M., Bhattacharyya N., Lin H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope. 2017;127:466–469. doi: 10.1002/lary.26107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyen K., Langers D.R., de Kleine E., van Dijk P. Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear. Res. 2013;295:67–78. doi: 10.1016/j.heares.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Carpenter-Thompson J.R., Schmidt S., McAuley E., Husain F.T. Increased frontal response may underlie decreased tinnitus severity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Wang F., Wang J., Bo F., Xia W., Gu J.P., Yin X. Resting-state brain abnormalities in chronic subjective tinnitus: a meta-analysis. Front. Hum. Neurosci. 2017;11:22. doi: 10.3389/fnhum.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Xia W., Chen H., Feng Y., Xu J.J., Gu J.P., Salvi R., Yin X. Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Hum. Brain Mapp. 2017;38:2384–2397. doi: 10.1002/hbm.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.K., Tsai C.H., Lin Y.C., Chen J.M., Tsou Y.A., Wang C.Y., Lin C.D., Jeng F.C., Chung J.G., Tsai M.H. Effectiveness of theta-burst repetitive transcranial magnetic stimulation for treating chronic tinnitus. Audiol. Neurotol. 2012;17:112–120. doi: 10.1159/000330882. [DOI] [PubMed] [Google Scholar]
- Derogatis L.R., Lipman R.S., Rickels K., Uhlenhuth E.H., Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav. Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- Durai M., Searchfield G. Anxiety and depression, personality traits relevant to tinnitus: a scoping review. Int. J. Audiol. 2016;55:605–615. doi: 10.1080/14992027.2016.1198966. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J. Central tinnitus. Auris Nasus Larynx. 2003;30:S7–S12. doi: 10.1016/s0385-8146(02)00122-0. [DOI] [PubMed] [Google Scholar]
- Gjerris A., Bech P., Bojholm S., Bolwig T.G., Kramp P., Clemmesen L., Andersen J., Jensen E., Rafaelsen O.J. The Hamilton Anxiety Scale. Evaluation of homogeneity and inter-observer reliability in patients with depressive disorders. J. Affect. Disord. 1983;5:163–170. doi: 10.1016/0165-0327(83)90009-5. [DOI] [PubMed] [Google Scholar]
- Goebel G. Tinnitus and psychiatric comorbidities. HNO. 2015;63:272–282. doi: 10.1007/s00106-014-2977-3. [DOI] [PubMed] [Google Scholar]
- Goebel G., Hiller W. The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO. 1994;42:166–172. [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y.V., Roach E.L., Korgaonkar M.S., Grieve S.M., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul A.I., Ozkiris M., Aydin R., Simsek G., Saydam L. Coexistence of anxiety sensitivity and psychiatric comorbidities in patients with chronic tinnitus. Neuropsychiatr. Dis. Treat. 2015;11:413–418. doi: 10.2147/NDT.S77786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C. International experiences with the hospital anxiety and depression scale—a review of validation data and clinical results. J. Psychosom. Res. 1997;42:17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- Hiller W., Goebel G. A psychometric study of complaints in chronic tinnitus. J. Psychosom. Res. 1992;36:337–348. doi: 10.1016/0022-3999(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Hinz A., Brahler E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J. Psychosom. Res. 2011;71:74–78. doi: 10.1016/j.jpsychores.2011.01.005. [DOI] [PubMed] [Google Scholar]
- House J.W., Brackmann D.E. Tinnitus: surgical treatment. CIBA Found. Symp. 1981;85:204–216. doi: 10.1002/9780470720677.ch12. [DOI] [PubMed] [Google Scholar]
- Husain F.T., Medina R.E., Davis C.W., Szymko-Bennett Y., Simonyan K., Pajor N.M., Horwitz B. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 2011;1369:74–88. doi: 10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivansic D., Dobel C., Volk G.F., Reinhardt D., Muller B., Smolenski U.C., Guntinas-Lichius O. Results of an interdisciplinary day care approach for chronic tinnitus treatment: a prospective study introducing the Jena interdisciplinary treatment for tinnitus. Front. Aging Neurosci. 2017;9:192. doi: 10.3389/fnagi.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques D., Nozeret Y., Zdanowicz N., Reynaert C., Garin P., Gilain C. Tinnitus and psychiatric comorbidities in liaison psychiatry analysis of three years in an audiophonology centre. Psychiatr. Danub. 2013;25(Suppl. 2):S102–S104. [PubMed] [Google Scholar]
- Kim H.J., Lee H.J., An S.Y., Sim S., Park B., Kim S.W., Lee J.S., Hong S.K., Choi H.G. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Jang J.H., Lee S.Y., Han J.J., Koo J.W., Vanneste S., De Ridder D., Song J.J. Neural substrates predicting short-term improvement of tinnitus loudness and distress after modified tinnitus retraining therapy. Sci. Rep. 2016;6 doi: 10.1038/srep29140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M., Langguth B., Rosengarth K., Braun S., Koch A., Kleinjung T., May A., de Ridder D., Hajak G. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage. 2009;46:213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Langers D.R., de Kleine E., van Dijk P. Tinnitus does not require macroscopic tonotopic map reorganization. Front. Syst. Neurosci. 2012;6:2. doi: 10.3389/fnsys.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Landgrebe M., Kleinjung T., Sand G.P., Hajak G. Tinnitus and depression. World J. Biol. Psychiatry. 2011;12:489–500. doi: 10.3109/15622975.2011.575178. [DOI] [PubMed] [Google Scholar]
- Langguth B., Schecklmann M., Lehner A., Landgrebe M., Poeppl T.B., Kreuzer P.M., Schlee W., Weisz N., Vanneste S., De Ridder D. Neuroimaging and neuromodulation: complementary approaches for identifying the neuronal correlates of tinnitus. Front. Syst. Neurosci. 2012;6:15. doi: 10.3389/fnsys.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A.M., Renier L., Chevillet M.A., Morgan S., Kim H.J., Rauschecker J.P. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A.M., Seydell-Greenwald A., Turesky T.K., Morgan S., Kim H.J., Rauschecker J.P. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front. Syst. Neurosci. 2012;6:21. doi: 10.3389/fnsys.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A.M., Seydell-Greenwald A., Rauschecker J.P. Auditory-limbic interactions in chronic tinnitus: challenges for neuroimaging research. Hear. Res. 2016;334:49–57. doi: 10.1016/j.heares.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner A., Schecklmann M., Poeppl T.B., Kreuzer P.M., Vielsmeier V., Rupprecht R., Landgrebe M., Langguth B. Multisite rTMS for the treatment of chronic tinnitus: stimulation of the cortical tinnitus network—a pilot study. Brain Topogr. 2013;26:501–510. doi: 10.1007/s10548-012-0268-4. [DOI] [PubMed] [Google Scholar]
- Lehner A., Schecklmann M., Greenlee M.W., Rupprecht R., Langguth B. Triple-site rTMS for the treatment of chronic tinnitus: a randomized controlled trial. Sci. Rep. 2016;6 doi: 10.1038/srep22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbestael J., Leurgans M., Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clin. Psychol. Psychother. 2011;18:75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- Lv H., Zhao P., Liu Z., Li R., Zhang L., Wang P., Yan F., Liu L., Wang G., Zeng R., Li T., Dong C., Gong S., Wang Z. Abnormal regional activity and functional connectivity in resting-state brain networks associated with etiology confirmed unilateral pulsatile tinnitus in the early stage of disease. Hear. Res. 2017;346:55–61. doi: 10.1016/j.heares.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Mahoney C.J., Rohrer J.D., Goll J.C., Fox N.C., Rossor M.N., Warren J.D. Structural neuroanatomy of tinnitus and hyperacusis in semantic dementia. J. Neurol. Neurosurg. Psychiatry. 2011;82:1274–1278. doi: 10.1136/jnnp.2010.235473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Somerset S., Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016;337:70–79. doi: 10.1016/j.heares.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Melcher J.R. Comment on Schecklmann et al.: a call to consider both “negative” and “positive” results in brain research on tinnitus. Brain Struct. Funct. 2013;218:1071. doi: 10.1007/s00429-013-0567-x. [DOI] [PubMed] [Google Scholar]
- Melcher J.R., Knudson I.M., Levine R.A. Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear. Res. 2013;295:79–86. doi: 10.1016/j.heares.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Mohan A., De Ridder D., Idiculla R., C D.S., Vanneste S. Distress-dependent temporal variability of regions encoding domain-specific and domain-general behavioral manifestations of phantom percepts. Eur. J. Neurosci. 2018;48:1743–1764. doi: 10.1111/ejn.13988. [DOI] [PubMed] [Google Scholar]
- Muhlau M., Rauschecker J.P., Oestreicher E., Gaser C., Rottinger M., Wohlschlager A.M., Simon F., Etgen T., Conrad B., Sander D. Structural brain changes in tinnitus. Cereb. Cortex. 2006;16:1283–1288. doi: 10.1093/cercor/bhj070. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pattyn T., Van Den Eede F., Vanneste S., Cassiers L., Veltman D.J., Van De Heyning P., Sabbe B.C.G. Tinnitus and anxiety disorders: a review. Hear. Res. 2016;333:255–265. doi: 10.1016/j.heares.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Poeppl T.B., Langguth B., Lehner A., Frodl T., Rupprecht R., Kreuzer P.M., Landgrebe M., Schecklmann M. Brain stimulation-induced neuroplasticity underlying therapeutic response in phantom sounds. Hum. Brain Mapp. 2018;39:554–562. doi: 10.1002/hbm.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse J.C., Giedd J.N., Rapoport J.L. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans. Med. Imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Rauschecker J.P., Leaver A.M., Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J.P., May E.S., Maudoux A., Ploner M. Frontostriatal gating of tinnitus and chronic pain. Trends Cogn. Sci. 2015;19:567–578. doi: 10.1016/j.tics.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief W., Hiller W. Toward empirically based criteria for the classification of somatoform disorders. J. Psychosom. Res. 1999;46:507–518. doi: 10.1016/s0022-3999(99)00023-9. [DOI] [PubMed] [Google Scholar]
- Rief W., Hessel A., Braehler E. Somatization symptoms and hypochondriacal features in the general population. Psychosom. Med. 2001;63:595–602. doi: 10.1097/00006842-200107000-00012. [DOI] [PubMed] [Google Scholar]
- Rosing S.N., Schmidt J.H., Wedderkopp N., Baguley D.M. Prevalence of tinnitus and hyperacusis in children and adolescents: a systematic review. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M., Lehner A., Poeppl T.B., Kreuzer P.M., Hajak G., Landgrebe M., Langguth B. Cluster analysis for identifying sub-types of tinnitus: a positron emission tomography and voxel-based morphometry study. Brain Res. 2012;1485:3–9. doi: 10.1016/j.brainres.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Schecklmann M., Vielsmeier V., Steffens T., Landgrebe M., Langguth B., Kleinjung T. Relationship between audiometric slope and tinnitus pitch in tinnitus patients: insights into the mechanisms of tinnitus generation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M., Lehner A., Poeppl T.B., Kreuzer P.M., Rupprecht R., Rackl J., Burger J., Frank E., Hajak G., Langguth B., Landgrebe M. Auditory cortex is implicated in tinnitus distress: a voxel-based morphometry study. Brain Struct. Funct. 2013;218:1061–1070. doi: 10.1007/s00429-013-0520-z. [DOI] [PubMed] [Google Scholar]
- Schmidt S.A., Carpenter-Thompson J., Husain F.T. Connectivity of precuneus to the default mode and dorsal attention networks: a possible invariant marker of long-term tinnitus. Neuroimage Clin. 2017;16:196–204. doi: 10.1016/j.nicl.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S.A., Zimmerman B., Bido Medina R.O., Carpenter-Thompson J.R., Husain F.T. Changes in gray and white matter in subgroups within the tinnitus population. Brain Res. 2018;1679:64–74. doi: 10.1016/j.brainres.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Hartkamp N., Kiuse J., Franke G.H., Reister G., Tress W. The symptom check-List-90-R (SCL-90-R): a German validation study. Qual. Life Res. 2000;9:185–193. doi: 10.1023/a:1008931926181. [DOI] [PubMed] [Google Scholar]
- Schwab J.J., Bialow M.R., Clemmons R.S., Holzer C.E. Hamilton rating scale for depression with medical in-patients. Br. J. Psychiatry. 1967;113:83–88. doi: 10.1192/bjp.113.494.83. [DOI] [PubMed] [Google Scholar]
- Simonetti P., Oiticica J. Tinnitus neural mechanisms and structural changes in the brain: the contribution of neuroimaging research. Int. Arch. Otorhinolaryngol. 2015;19:259–265. doi: 10.1055/s-0035-1548671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Engell A., Junghoefer M., Wunderlich R., Lau P., Wollbrink A., Rudack C., Pantev C. Inhibition-induced plasticity in tinnitus patients after repetitive exposure to tailor-made notched music. Clin. Neurophysiol. 2015;126:1007–1015. doi: 10.1016/j.clinph.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Swan A.A., Nelson J.T., Swiger B., Jaramillo C.A., Eapen B.C., Packer M., Pugh M.J. Prevalence of hearing loss and tinnitus in Iraq and Afghanistan veterans: a chronic effects of neurotrauma consortium study. Hear. Res. 2017;349:4–12. doi: 10.1016/j.heares.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Theodoroff S.M., Lewis M.S., Folmer R.L., Henry J.A., Carlson K.F. Hearing impairment and tinnitus: prevalence, risk factors, and outcomes in US service members and veterans deployed to the Iraq and Afghanistan wars. Epidemiol. Rev. 2015;37:71–85. doi: 10.1093/epirev/mxu005. [DOI] [PubMed] [Google Scholar]
- To W.T., De Ridder D., Hart J., Jr., Vanneste S. Changing brain networks through non-invasive neuromodulation. Front. Hum. Neurosci. 2018;12:128. doi: 10.3389/fnhum.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J., Zijdenbos A., Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Trevis K.J., McLachlan N.M., Wilson S.J. Psychological mediators of chronic tinnitus: the critical role of depression. J. Affect. Disord. 2016;204:234–240. doi: 10.1016/j.jad.2016.06.055. [DOI] [PubMed] [Google Scholar]
- Vanneste S., Alsalman O., De Ridder D. COMT and the neurogenetic architecture of hearing loss induced tinnitus. Hear. Res. 2018;365:1–15. doi: 10.1016/j.heares.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Wang H., Li B., Feng Y., Cui B., Wu H., Shi H., Yin S. A pilot study of EEG source analysis based repetitive transcranial magnetic stimulation for the treatment of tinnitus. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worboys M. The Hamilton rating scale for depression: the making of a “gold standard” and the unmaking of a chronic illness, 1960–1980. Chronic Illn. 2013;9:202–219. doi: 10.1177/1742395312467658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H.B., De Ridder D., Vanneste S. The importance of aging in gray matter changes within tinnitus patients shown in cortical thickness, surface area and volume. Brain Topogr. 2016;29:885–896. doi: 10.1007/s10548-016-0511-5. [DOI] [PubMed] [Google Scholar]
- Zheng W., Peng Z., Pengfei Z., Jing L., Heyu D., Hongxia Y., Yawen L., Zhengyu Z., Shusheng G., Zhenghan Y., Han L., Zhenchang W. Long-term reactions to pulsatile tinnitus are marked by weakened short-range functional connectivity within a brain network in the right temporal lobe. J. Magn. Reson. Imaging. 2019;49:1629–1637. doi: 10.1002/jmri.26545. [DOI] [PubMed] [Google Scholar]
- Ziai K., Moshtaghi O., Mahboubi H., Djalilian H.R. Tinnitus patients suffering from anxiety and depression: a review. Int. Tinnitus J. 2017;21:68–73. doi: 10.5935/0946-5448.20170013. [DOI] [PubMed] [Google Scholar]
- Zirke N., Seydel C., Arsoy D., Klapp B.F., Haupt H., Szczepek A.J., Olze H., Goebel G., Mazurek B. Analysis of mental disorders in tinnitus patients performed with composite international diagnostic interview. Qual. Life Res. 2013;22:2095–2104. doi: 10.1007/s11136-012-0338-9. [DOI] [PubMed] [Google Scholar]