Abstract
Relaxed skeletal muscle has an inbuilt resistance to movement. In particular, the resistance manifests itself as a substantial stiffness for small movements. The stiffness is impermanent, because it forms only when the muscle is stationary for some time and is reduced upon active or passive movement. Because the resistance to movement increases with time at rest and is reduced by movement, this behavior has become known as muscle thixotropy. In this short review, we describe the phenomenon of thixotropy and illustrate its significance in postural control with particular emphasis on human standing. We show how thixotropy came to be unambiguously associated with muscle mechanics and we review present knowledge of the molecular basis of thixotropic behavior. Specifically, we examine how recent knowledge about titin, and about the control of cross-bridge cycling, has impacted on the role of non-cross-bridge mechanisms and cross-bridge mechanisms in explaining thixotropy. We describe how thixotropic changes in muscle stiffness that occur during transitions from posture to movement can be tracked by analyzing physiological tremor. Finally, because skeletal muscle contains sensory receptors, and because some of these receptors are themselves thixotropic, we outline some of the consequences of muscle thixotropy for proprioception.
Keywords: movement, muscle thixotropy, posture, relaxed muscle
INTRODUCTION
Any posture of the body and limbs is defended against external and internal disturbances. This resistance is known as muscle tone. Muscle tone is modulated by the nervous system, but part is intrinsic to cellular-level physiology. Assessment of muscle tone attracted clinical attention because it can be altered (usually increased) by disease, and one aim of therapy is to restore it to normal levels. Following a number of attempts to measure human muscle tone it slowly became clear that it is more than a simple springlike resistance. First, resistance is much greater when the size of the deformation is small, reducing with larger movements. Second, it emerged that this high resistance to small movements is evanescent—melting away with repeated movements and reforming if the limb remains stationary. Because the high stiffness is temporarily reduced by movement and redevelops with time at rest, the behavior has become known as muscle thixotropy. Since it gives muscle nonlinear, time-dependent properties, thixotropy has important consequences for the physiology of posture, movement, and, because muscles contain important sensory receptors, proprioception.
THIXOTROPY: UNDERLYING CONCEPT AND APPLICATION TO MUSCLE
Most fluids produce a viscous resistance to movement; the force depends on the rate of deformation. In some of these fluids, the viscosity decreases when they are stirred or made to flow rapidly, a phenomenon known as shear thinning. Meyer et al. (63) have shown that muscle viscosity is pseudoplastic (effectively shear thinning). Such changes can occur extremely quickly. Thixotropy is a special form of this behavior, and describes a situation where the viscosity returns only gradually to its original level following a perturbation. Thixotropic substances are thus history-dependent and have a “memory time” (62).
Although the term “thixotropy” was introduced to rheology by Freundlich (25) who wrote a book with that title, it was coined by Thomas Peterfi (68). Peterfi was a biologist who invented the term to describe the behavior of oocyte cytoplasm which changed from a semisolid (gel) to a fluid (sol) when agitated by the movement of spermatozoa, preceding fertilization. The name is derived from a combination of the Greek words θίξις (thixis: stirring, shaking) and τρέπω (trepo: turning or changing). Thixotropy is often seen in substances that form weak bonds between their constituent molecules which are destroyed by agitation and reform progressively (flocculate) when at rest. Very many common materials are thixotropic, for example, clay, blood, and ketchup. Most are colloidal suspensions (“structured liquids”) and are conveniently characterized by their viscosity.
Muscles exhibit similar behavior; their mechanical properties are changed by movement and recover with time after the movement stops. Buchthal and Kaiser (11) were among the first to measure this behavior and described muscle as thixotropic even though the most striking effect in muscle is a history-dependent reduction in stiffness (change in force proportional to change in length) as opposed to viscosity (force proportional to velocity). Such changes are clearly observed for very slow stretches where the contribution of viscosity will be minimal. However, time- and history-dependent changes in viscosity will occur (63). Consequently, muscle thixotropy can be parsimoniously described as a temporary reduction in the resistance of muscles to externally applied, or internally generated, movement.
POSTURAL THIXOTROPY
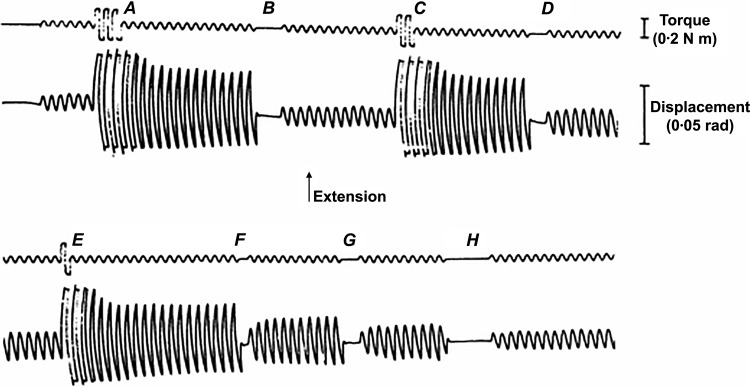
Thixotropy can be demonstrated in humans by driving the hand with a small sinusoidal torque, producing a small (~1 degree) flexion/extension oscillation at the wrist. The size of this oscillation depends critically on preceding movements of the hand. It becomes larger following brief perturbations, but if the torque is removed for a few seconds and then reapplied, it becomes small again (Fig. 1). Active or passive hand movements produce the effect. These sequences can be endlessly repeated and are not associated with EMG activity. They occur in subjects who are anesthetized with neuromuscular blockade (48, 51) implying that the behavior reflects musculoskeletal properties rather than the nervous system. Neither are the effects associated with a particular muscle length because the behavior persists when the mean position of the wrist is adjusted by adding biasing torque. Similar behavior has been demonstrated at many other joints (89). The musculoskeletal system thus limits the movements induced by small forces. Large forces exceed the short-range resistance and produce a temporary self-sustaining decrease in resistance which reappears only with time at rest. Thixotropy describes the history dependence of the resistance.
Fig. 1.
Thixotropy. The relaxed hand is oscillated by 2.5 Hz sinusoidally varying torque. A, C, and E: large perturbations in which 3, 2, and 1 cycle, respectively, cause the same subsequent increase in amplitude. B, D, F, G, and H: driving torque is removed for 3, 2, 1, 2, and 5 cycles, respectively. Removal for 3 or 5 cycles (B or H) causes a reduction in amplitude to original value. Removal for 1 or 2 cycles (D, F, and G) causes a partial reduction. All changes can be sustained indefinitely. [Redrawn from Lakie et al. (50) with permission.]
It took decades of research to determine the physiological basis of this behavior. Denny-Brown (21) showed that relaxed cat muscles exhibited a nonlinear stiffness that depended on time at rest. Subsequent experiments then demonstrated similar properties in isolated muscles and isolated muscle fibers (24, 34, 52, 53). Finally, Wiegner (90) analyzed relaxed rat ankle joints and compared the thixotropic properties of intact joints, blood-perfused soleus muscles, isolated soleus muscles, and ankle joints isolated by severing all muscular attachments. Based on the range of action, the elastic modulus, and the time course of redevelopment, Wiegner concluded that thixotropy was primarily due to the history dependence of muscle’s short-range stiffness. Postural thixotropy is thus a property of muscle rather than of joints.
IMPORTANCE OF RELAXED MUSCLE FOR POSTURAL CONTROL
Posture is characterized by low levels of active force generation. Typically, only relatively few motor units, recruited at submaximal rates, are required to counteract the effect of gravity and the resistance of the unstimulated fibers in the agonist and antagonist muscles. It is difficult to measure the relative contributions of the active and inactive fibers to overall muscle stiffness during posture. However, it is a consistent finding that relaxed muscles make a large contribution to joint stiffness. For example, Hunter and Kearney (37) investigated the stiffness of human leg muscles at different levels of muscle activation. For the calf muscles they found that stiffness was almost exactly linearly related to the degree of activation. However, in every individual, the relaxed stiffness (zero activation) was substantial ranging from 16 to 55 N·m/rad. In human standing, the necessary stiffness for each ankle is typically around 300 N·m/rad. Accordingly, 5–20% of stiffness can be generated by relaxed calf muscle fibers so they are thus of real importance during standing. For other postural tasks where a single limb, rather than the entire body, must be balanced, the contribution of relaxed fibers is potentially even greater.
The first study linking the biophysics of relaxed muscle to the sliding filament mechanism was performed by D.K. Hill in 1968 (34). He applied ramp stretches to isolated frog muscles and showed that force changed proportionally with length when muscles were stretched or shortened by up to ~0.2% muscle length. The linear relationship between force and length is characteristic of an elastic stiffness so Hill attributed the behavior to a short-range elastic component (SREC). If the muscle was extended beyond its “elastic limit,” force remained roughly constant and was only minimally dependent on the stretch velocity. This phase of the response was described as a frictional resistance.
Hill interpreted these results in terms of cross-bridges linking the actin and myosin filaments in relaxed muscle. The SREC reflected the combined stiffness of these links so that small length changes produced proportional changes in force. A stretch larger than the muscle’s elastic limit broke the original cross-bridges, but these were replaced almost immediately by newly forming links. (If this continual reattachment had not occurred, force would have fallen toward zero beyond the elastic limit instead of remaining constant during a sustained stretch). The end result was that relaxed muscle developed a self-resetting short-range stiffness whenever thick and thin filaments overlapped.
TIME AND MOVEMENT DEPENDENCE OF SHORT-RANGE STIFFNESS IN RELAXED MUSCLE
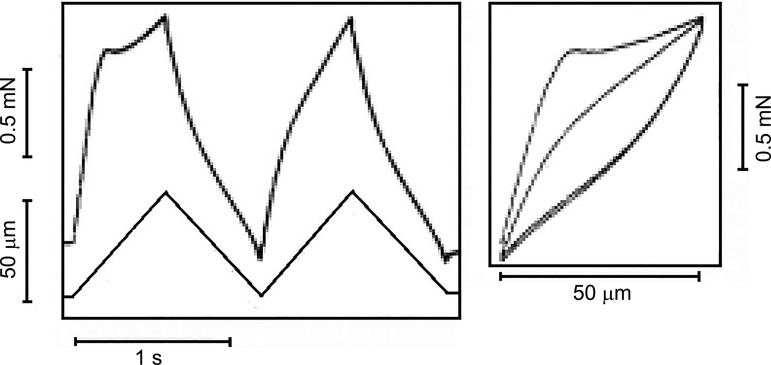
Hill knew that the stiffness of relaxed muscle depended on the time at rest because he commented (p. 645 of Ref. 34) that each test stretch had to be preceded by a larger conditioning stretch followed by a wait of ~30 s if consistent results were to be obtained. However, he did not explicitly focus on the history dependence of relaxed muscle. As a result, Lännergren (52) was the first, to our knowledge, to demonstrate that the SREC was history dependent, and Herbst (31) published the first force-length plot (Fig. 2, right panel). Herbst’s figure is particularly interesting because it shows that when a relaxed muscle is subjected to repeated stretch/release cycles, the force responses are identical during the shortening phases of the motion. All of the history dependence arises in the initial phase of the stretches. More specifically, it is the stiffness of the SREC that is reduced by motion. This is the fundamental basis of muscle thixotropy.
Fig. 2.
Muscle thixotropy. Isolated, relaxed extensor brevis profundus digiti IV muscle (frog) was stretched by 50 µm. The first stretch encountered an initial resistance to stretch [the short-range elastic component (SREC)]. When a second stretch was immediately applied, the initial resistance was greatly reduced. The slope of the line (right panel) is the stiffness. The stiffness of the SREC is less in the second (and all subsequent) stretches unless time is allowed between stretches. The initial resistance is regained only by waiting for a few seconds between stretches. [Redrawn from Herbst (31) with permission from Springer Nature. Copyright 1976 Springer-Verlag.]
MOLECULAR MECHANISMS UNDERPINNING SHORT-RANGE STIFFNESS IN RELAXED MUSCLE
As discussed above, Hill (34) had proposed that the short-range stiffness of relaxed muscle was due to a small number of cross-bridges that linked the actin and myosin filaments in relaxed muscle. Several additional papers support Hill’s hypothesis (15, 31,) but there is also mounting evidence for non-cross-bridge mechanisms (7, 41, 70). Some of the more recent experimental findings are summarized below, starting with non-cross-bridge mechanisms.
Non-Cross-Bridge Mechanisms
Titin, the giant protein that spans half the length of the sarcomere, dominates the passive-length tension curve of an isolated skeletal fiber (28). In skeletal muscle, the I-band region of the molecule is extensible and consists of multiple immunoglobulin-like segments and a PEVK region. When fibers are stretched, titin molecules elongate by straightening the interdomain linkers in the Ig regions and lengthening the PEVK segment.
Titin had been known to exhibit history-dependent properties for some time (42) but Mártonfalvi et al. (59) advanced the field in 2014 by showing that single titin molecules exhibit thixotropic behavior at forces low enough to be relevant to relaxed muscle. The authors speculated that the low-force history dependence might reflect rupture of transient electrostatic interactions within the PEVK region. If this is true, it might be possible to disrupt the interactions by adding glutamate-rich polyE fragments of the PEVK region to the bathing solutions. Mártonfalvi et al. tested this hypothesis and showed that the fragments decreased the history dependence of isolated titin molecules and reduced the short-rage stiffness of relaxed psoas fibers in a concentration-dependent manner. These data suggest that intra-titin interactions could contribute to the short-range properties of relaxed muscle.
Titin has also been shown to bind to actin (43, 93), and some data suggest that the interaction may be at least partially regulated by Ca2+ (84). Transient bonds between titin and actin could produce history-dependent properties by changing which regions of titin are free to extend during stretch. This type of mechanism could, in principle, explain multiple facets of muscle mechanics including short-range stiffness and residual force enhancement (66), but there is controversy about how best to interpret many of the published experiments (19, 32, 74, 75).
Some of the most exciting new data relate to myosin-binding protein-C. These molecules are localized to distinct stripes on the thick filaments and help regulate cross-bridge cycling (1). Early data suggested that phosphorylating myosin binding protein-C increased contractility by allowing myosin heads to move closer to actin (30) but it is now clear that the NH2 terminus of myosin binding protein-C can also bind directly to the thin filament (65, 86). In short, myosin binding protein-C can form cross-links between the thick and thin filaments. These would probably be difficult to distinguish from conventional actin-myosin cross-bridges in purely mechanical experiments.
Regulation Of Cross-Bridge Cycling
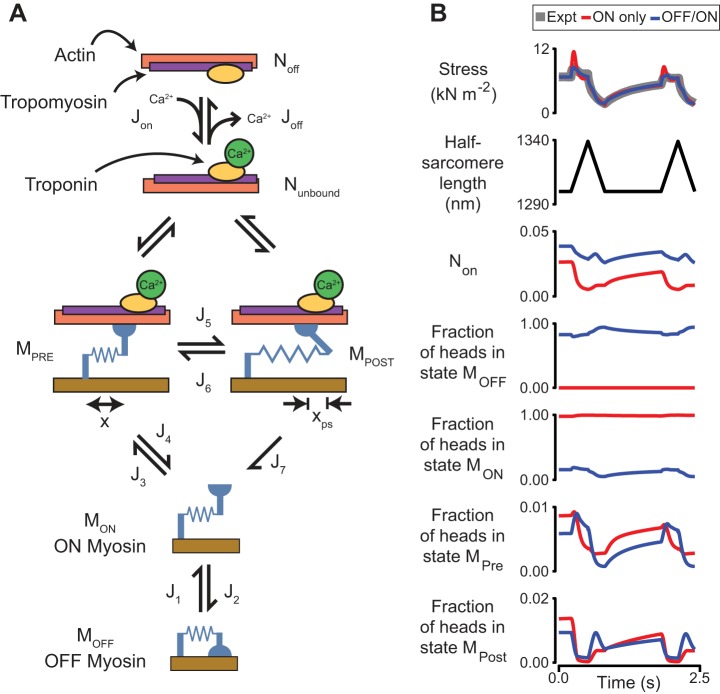
Until a few years ago, cross-bridge cycling in striated muscle was thought to be regulated primarily by the number of binding sites on actin that were activated. It is now clear that that number of recruitable heads is also under dynamic control. More specifically, thick filaments transition between an OFF state (where myosin heads are prevented from binding to actin) and an ON state (where actin-myosin binding is permitted) (38) (Fig. 3A). The OFF state of myosin is often referred to as the superrelaxed state (67, 91) and may correspond to the Interacting Heads Motif that has been observed using cryoelectron microscopy (3).
Fig. 3.
A: schematic of a kinetic scheme showing myosin heads coupled to Ca2+-dependent binding sites. Myosin heads in the ON state can bind to actin but heads in the OFF state are unable to interact with the thin filament. Heads attach to actin in the MPRE state and can develop force by undergoing a power-stroke transition to the MPOST state. xps indicates the working distance of the power stroke. J terms show fluxes between different states. B: best-fits of two simulations to force recorded from a permeabilized rat soleus fiber at low Ca2+ activation (pCa 6.5). Data are replotted from Fig. 8 of Ref. 17. In the “On Only” simulation (shown in red), myosin heads are prevented from entering the OFF state (i.e., J2 = 0). This mimics the field’s understanding of muscle before the discovery of the OFF state of myosin. In the “OFF/ON” simulation (shown in blue), the rate of the OFF to ON transition increases with force. Non shows the proportion of bound heads. Calculations were performed using MyoSim (13) and are based on methods described in detail in Ref. 14.
The OFF state predominates in relaxed muscle, but pulse-chase experiments with fluorescently-labeled ATP (35, 91) and fluorescent polarization measurements (26) show that some myosin heads remain in the ON state where they can interact with actin. The transitions between the OFF and ON states are regulated by multiple mechanisms. For example, interactions with myosin binding protein-C stabilize the OFF state (61) whereas phosphorylation of regulatory light chain shifts the equilibrium toward the ON state (40). The transition is also sensitive to mechanics (27, 55, 77) with recent simulations modeling length-dependent activation in myocardium, suggesting that the rate of the OFF to ON transition increases with muscle force (14). A similar mechanism in skeletal muscle would have significant implications for short-range stiffness and thixotropy in skeletal muscle.
D. K. Hill (34) pointed out that cross-bridges that detach during a length change must be replaced quickly if they are to sustain force beyond the elastic limit. This conflicts with the low attachment rate required to produce the slow time course of thixotropic stiffness recovery. Filament registration effects may help to satisfy these differing requirements (15), but OFF to ON transitions could also play an important role.
Figure 3B shows thixotropic behavior (gray) measured in a rat muscle fiber at a low level of Ca2+ activation and the best-attained fits for two related models. In the first model, shown in red, myosin heads cannot enter the OFF state. This mimics the field’s understanding of muscle before the discovery of the OFF state and all detached heads are therefore potentially able to bind to actin. With this model, the only way of producing a slow recovery between stretches is for myosin to have a low attachment rate. Force thus declines during the stretch as cross-bridges detach faster than they are replaced. The best-fit simulation is a compromise where force overestimates the experimental data during the short-range response and underestimates the data during the stretch plateau.
In the second model, shown in blue, heads transition between the OFF and ON states with the OFF to ON rate increasing with force (14). The flux through the J3 transition (cross-bridge formation) thus depends on the number of heads in the ON state as well as on the rate at which individual heads attach. When force is low, only a few heads are in the ON state, so the rate of cross-bridge formation remains low. However, stretching the muscle increases force and accelerates the OFF to ON transition. This pulls more heads into the ON state, thereby increasing the rate of cross-bridge formation. Put simply, these simulations fit the experimental data better because the force-dependent OFF to ON transition allows quick replacement of cross-bridges during stretch and slow recovery of force between stretches.
Current Understanding of Molecular Mechanisms in Relaxed Muscle
Our current view is that thixotropy in relaxed muscle is not due to a single molecular mechanism. The short-range stiffness of relaxed muscle reflects contributions from cross-bridges and from non-cross-bridge mechanisms. The relative size of the components may depend on the type of muscle and the physiological conditions. Cross-bridges may dominate in some muscles under some circumstances without excluding the importance of non-cross-bridge mechanisms in others.
THIXOTROPY IN CONTRACTING MUSCLE
The short-range stiffness rises by ~2 orders of magnitude when muscles are activated due to the increased number of attached cross-bridges (12). Only a few papers have attempted to measure thixotropy in contracting muscle (16, 17) with more attention being focused on other history-dependent phenomena such as residual force enhancement (18, 33) and residual force depression (54). One recent study performed by Altman et al. (4) applied sinusoidal length changes to permeabilized psoas fibers and demonstrated clear thixotropic behavior for oscillations faster than 1 Hz. Slower oscillations induced the opposite effect; the force response increased over successive cycles. This phenomenon is called rheopexy and had not previously been reported in muscle fibers. The molecular basis remains unclear but may reflect Ca2+-dependent changes in titin stiffness (76).
THE CONSEQUENCES OF MUSCLE THIXOTROPY FOR POSTURE
For postural maintenance small forces must be economically maintained for very long periods. Fish and birds may use aero- or hydrodynamic forces or buoyancy to offset gravity. For land-living animals, without lockable joints, any gravitational forces must be countered by muscle force. Muscle fibers with similar properties are aggregated into motor units. Slow motor units are used to sustain small forces. In some species there are extremely slow fibers which develop a sustained contraction as the result of a single depolarization (tonus bundles). In mammalian species even the slowest muscle fibers are relatively fast and to sustain posture they must be repetitively activated. In humans, the twitch time difference between fast and slow fibers is actually rather slight (35 vs. 95 ms) (82), considering that movement may be over in a fraction of a second and that posture may endure for many minutes. There is a growing awareness that motor unit size and type are not the only determinants of muscle behavior and there are other complementary muscle mechanisms, such as thixotropy, stretch force enhancement, and “sticking” of muscles, which aid the economical preservation of posture (39, 83, 88).
THE IMPLICATIONS OF MUSCLE THIXOTROPY FOR MOVEMENT AND NEURAL CONTROL
For skeletal muscle the input is action potentials and the output is force. The relationship between stimulation (natural or artificial) and force output has been extensively studied in vitro and in vivo. There has been comparatively little investigation into the relationship between stimulation and movement. Because the resistance of muscle to applied force depends on the history of movement, there is a variable relationship between action potentials and limb movement, and this is now considered.
Axelson and Hagbarth (5, 6) measured the EMG required to drive repetitive wrist dorsiflexions and demonstrated that the neural activity for a given motion increased with the time at rest. This shows that during voluntary movements, the central nervous system takes account of, and compensates for, the history dependence of the resistance. The nervous system may “know” the movement history of the muscles and make appropriate prejudged adjustments to its output in an open-loop way. However, it seems more likely that the output is automatically regulated by a feedback mechanism which acts to close the loop. As movement (of more than a small size) reduces stiffness and reduced stiffness assists movement, there is evidently positive feedback. Thus, for movement of more than a critical, small size, stiffness may tend to collapse toward a single value implying that most movements may automatically generate a low and relatively unchanging resistance (87).
PHYSIOLOGICAL TREMOR TRACKS MUSCLE THIXOTROPY IN POSTURE AND MOVEMENT
Recent work has shown that it is possible to assess muscle thixotropy by analyzing tremor. Because the normal rate of firing of most motor neurons is too low to produce a fused tetanus, muscle produces a pulsatile force. This combines with the underdamped resonance of the limb to produce a background “dither” or tremor which is most easily measured as a limb acceleration signal. Tremor is normally small, but the relationship between the EMG driving the motion and the amplitude of the tremor can be changed by factors that alter the contractile characteristics of the muscle—for example, increased by muscle heating or by β2 agonists and decreased by cooling or hypoxia (45). Tremor size reflects effectiveness with which muscle converts electrical input into movement. Tremor size is increased during and after active or passive limb movements (85). Because the EMG changes only slightly, and the tremor size increases a lot, this increase is attributed to thixotropic alterations in passive muscle stiffness (49, 78).
The frequency of physiological tremor is also affected by thixotropy. Limbs are underdamped (damping ratio of ~30% of critical damping) and thus have a natural or resonant frequency at which they can easily be induced to oscillate (2, 51, 64). The resonant frequency (rf) (for example, ~2 Hz for the hand, for large movements) depends on the stiffness of the tendons and muscles (k) and the mass (m) of the parts that move and is given by
As the mass of the limb is effectively unchanging, the resonant frequency provides an index of stiffness, increasing as the square root of k. Movements that do not exceed the short-range stiffness are associated with higher resonant frequencies because k is larger. For example, the resonant frequency of the hand increases to ~8 Hz for tremor-sized movements (51). Similarly, resonant frequencies are reduced following perturbations (46) and increase with time at rest (46, 78) due to thixotropic changes in muscle stiffness.
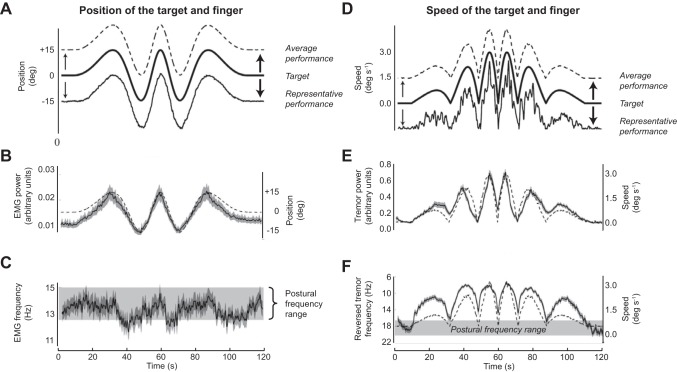
Figure 4 shows data from experiments where participants performed a simple tracking task to follow a target on a screen using vertically orientated extension/flexion movements at the metacarpophalangeal joint of the splinted middle finger. The target transitioned from a static position to slow movements of increasing speed and then symmetrically back to a static position. The key findings are that tremor power (Fig. 4E) was very clearly directly correlated to speed of movement, and tremor frequency (Fig. 4F) was inversely correlated to speed of movement. Neither of these relationships could be explained by alterations in the driving EMG (Fig. 4, B and C) so altered muscle properties are responsible. When the finger is nearly static, tremor power is low and frequency is high; when it moves, tremor power increases and frequency reduces. The frequency change is smaller than might be assumed. Muscle stiffness may reduce by a factor of ~15 with movement (57), and this would imply a nearly fourfold reduction in peak tremor frequency. However, the muscle lies in series with a compliant tendon which means that the effective reduction in stiffness is much less (for explanation, see 49). These movements are actually rather slow (maximum 3 deg/s), and Fig. 4F shows that even extremely slow movement is sufficient to lower tremor frequency from its higher postural range. To reattain the postural range requires a second or so at rest.
Fig. 4.
Participants use flexion/extension of the middle finger to track a target on a screen in a simple slow task. Upward movement of the finger (extension) is up. A: target position, finger position (representative subject), finger position (average, all subjects). B: rEMG of extensor muscle (means ± SD). C: peak frequency of rEMG spectrum (means ± SD). D: target speed, finger speed (representative subject), finger speed (average, all subjects). E: peak power of finger acceleration spectrum (means ± SD). F: peak frequency of finger acceleration spectrum. Note frequency scale is inverted (means ± SD). In B, the faint line shows average finger position, and in E and F it shows finger speed to emphasize how they correlate with the measured variables. In A and D, the traces are offset for clarity. [Modified from Vernooij et al. (88) under Creative Commons Attribution License 4.0.]
It is often said that “posture follows movement like a shadow.” These results suggest that the intrinsic thixotropic properties of muscle may be the explanation. Manmade servo-systems often incorporate a brake to assist them when stationary, so that they can economically maintain a fixed position and generate rapid movement, two goals which have diametrically opposed requirements. Muscle thixotropy, which makes muscles increasingly stiff as movement stops, may be Nature’s version of the brake. Animals can voluntarily move at different speeds, and by definition posture is an attempt to move at zero speed. The nervous system may need a dedicated neural pathway to maintain zero velocity [a “postural system” (44)]. Alternatively, thixotropic changes in muscle stiffness, revealed here by changes in tremor, may render such a dedicated system unnecessary because it provides skeletal muscle with an inbuilt automatic braking system.
Most movement is “fluid,” and free from obvious jerks or discontinuities. Such behavior is often achieved in robotic systems by adding considerable damping. Damping generates a force which acts to keep velocities low. In effect it is as if the moving system is immersed in treacle. Damping can be viscous in which case the absorbed force increases proportionately with speed of movement. Consequently for intentional rapid movements considerable force will be uselessly dissipated. Skeletal muscle operates differently. As Hill (34) showed, the response of passive muscle to long-range length change is frictionlike, so for long-range movement, muscle approximates to a friction damper which will absorb approximately the same moderate amount of force at all velocities. Posture is stabilized by a substantial temporary, springlike stiffness when stationary, rather than by energy-wasting, dissipative damping.
MUSCLE THIXOTROPY CAN AID STABILIZATION IN STANDING
Human bipedal standing is one form of postural activity. Anteroposterior sway at the ankle is small and slow. If relaxed muscle had zero stiffness, the intrinsic stiffness of the ankle would be very low, because small sways of the body, corresponding to small rotations of the ankle, would pull on tendons and stretch muscles, generating no tension. However, studies have shown that there is a substantial stiffness of the relaxed or slightly active ankle joint (37, 57). Although this intrinsic ankle stiffness is not adequate to prevent the body toppling due to gravity, it is a considerable benefit to standing in that it confers stability and increases time for the nervous system to react (56). Ankle stiffness has an inverse correlation with physiological sway size and is thixotropic, being temporarily reduced by up to 43% by artificial disturbances which increase the size of sway (81). Thixotropic muscle stiffening may permit a virtuous circle, where skilled neural control produces small sway size, thus increasing mechanical ankle stiffness. In turn, the increased stiffness facilitates the task of the nervous system.
THE CONSEQUENCES OF MUSCLE THIXOTROPY FOR PROPRIOCEPTION
Muscle spindles provide a signal corresponding to the state of parent muscle fibers. They lie strictly in parallel with parent muscles, span all or some of their length (73), and are intimately involved in signaling limb position and joint angle. Despite early ideas to the contrary, it is abundantly clear that they provide both unconscious, and reportable, sensation. The spindle has polar regions which contain myofibrils and a motor nerve supply. These regions are themselves thixotropic and stiffen at rest and after activation. Consequently the transmission of spindle elongation to the equatorial sensory primary ending rises considerably when resting following movement and this is one reason for the exquisite short-range sensitivity which is present at any length of the spindle primary ending [up to 2 orders of magnitude greater than long-range sensitivity (60)].
Considerable evidence (9, 10, 36) has suggested that this “self-resetting,” “plastic property” could be explained by history-dependent alterations in the stiffness of the polar muscular parts of the spindle [reviewed by Proske et al. (71)]. Before the role of thixotropy in skeletal muscle became widely known, it was proposed as an explanation for the behavior of intrafusal muscle. This proposal has been amply confirmed. The discharge of muscle spindle endings is affected both by previous stretch of the parent muscle (23) and by previous fusimotor activation (58, 73, 79, 92). Muscle spindle discharge can remain elevated long after a voluntary movement. It has recently been shown that thixotropic stiffening of polar regions may also allow muscle spindles to signal changes in passive intramuscular force, unlike tendon organs which signal mainly active force (8).
The sensitivity of a muscle spindle to joint rotation is affected in opposing ways by muscle thixotropy. By temporarily stiffening the polar regions of the spindle, thixotropy makes the primary endings extremely sensitive (60). However, by stiffening the parent muscle, thixotropy makes it very stiff relative to the tendon so that joint rotation stretches the tendon rather than altering the length of the spindle, greatly reducing its sensitivity. Possibly, these two effects offset each another, so that the sensitivity of spindles to joint rotation becomes more linear. It has been suggested (20, 22) that muscle spindles in quiescent muscles could serve as useful joint angle indicators, because their output is not contaminated by active alterations in muscle length. Although using inactive muscle length to faithfully signal joint angle seems attractive, thixotropic changes in quiescent muscle stiffness following larger joint movements might mean that the relationship between joint angle and muscle length/spindle output is very variable.
Unsurprisingly, muscle thixotropy has been shown to have significant effects on proprioception, with perception of joint position being affected by whether the muscle had previously contracted or was passively held in a lengthened or shortened position (69, 72, 73). It remains to be established how much of this effect is due to thixotropic changes in the spindle and/or to thixotropic changes in the parent muscles. Mechanical inputs such as sustained vibration applied to the muscle tendon have been shown to decrease resting spindle firing rate as well as to reduce sensitivity to passive stretch immediately after the cessation of vibration (79). The recovery time is compatible with thixotropic stiffening of muscle and/or spindles. These changes in spindle activity might account for the kinesthetic illusions that follow vibration (80).
CONCLUSIONS AND UNANSWERED QUESTIONS REGARDING MUSCLE THIXOTROPY
Muscle thixotropy describes the way in which the stiffness of muscle reduces during, and for some time after, movement. The resistance to movement that redevelops progressively at rest has practical consequences: for example, it can provide substantial stabilizing force in human standing. It can be observed by applying external forces to limbs, or by analyzing internally generated tremor. It introduces a degree of ambiguity into sensory signals derived from muscle and tendon receptors. There are several plausible biophysical mechanisms which may contribute to the phenomenon. There remain a number of other unresolved questions, such as these, for example.
1) How Do Active Fibers Contribute to Thixotropy in Typical Posture?
Only a fraction of motor units are recruited during typical posture. However, active fibers are ~2 orders of magnitude stiffer than relaxed fibers and could make an important contribution to overall muscle stiffness. We are not aware of any studies that have attempted to partition thixotropic responses into its active and relaxed components. Until data become available, the potential importance of a small number of active fibers should not be underestimated.
2) Does Slack Influence Physiological Function?
A consequence of thixotropy and short range stiffness is that muscles become stiff at any length. In this situation thixotropic stiffening can be used as a “trick” to stick the muscle at a length that is shorter or longer than usual. This implies that muscles could fall slack when their origins are approximated by passive shortening. This has been observed by ultrasonic imaging in some muscles (83). This slack may persist (29) or a slow process such as filamentary resting tension (34) may act to pull the muscle tendon unit into a tensed state. The outcome may depend critically on the particular muscle and its length. However, it seems that prolonged muscle slackness is an unusual situation because in a resting limb small joint rotations always encounter a disproportionately high initial resistance. If slackness was a general phenomenon the initial resistance would be nil, rising only when the slackness was taken up.
3) Why Is Muscle Stiffness Reduced After a Contraction?
Muscle stiffness is an asset during posture but is likely to impede movement. The stiffness of a muscle is temporarily reduced after a twitch contraction (47, 52). It is not clear whether the reduction in stiffness is an active process (perhaps related to latency relaxation) or whether it is a consequence of interfilamentary movement during the contraction. That is, is the stiffness actively “switched off” or does it disappear purely as a consequence of movement and bond breakage? Length-clamp type experiments might help to resolve the issue.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L. and K.S.C. conceived and designed research; M.L. and K.S.C. prepared figures; M.L. and K.S.C. drafted manuscript; M.L. and K.S.C. edited and revised manuscript; M.L. and K.S.C. approved final version of manuscript.
REFERENCES
- 1.Ackermann MA, Kontrogianni-Konstantopoulos A. Myosin binding protein-C: a regulator of actomyosin interaction in striated muscle. J Biomed Biotechnol 2011: 636403, 2011. doi: 10.1155/2011/636403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal GC, Gottlieb GL. Compliance of the human ankle joint. J Biomech Eng 99: 166–170, 1977. doi: 10.1115/1.3426285. [DOI] [Google Scholar]
- 3.Alamo L, Wriggers W, Pinto A, Bártoli F, Salazar L, Zhao FQ, Craig R, Padrón R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol 384: 780–797, 2008. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altman D, Minozzo FC, Rassier DE. Thixotropy and rheopexy of muscle fibers probed using sinusoidal oscillations. PLoS One 10: e0121726, 2015. doi: 10.1371/journal.pone.0121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelson HW, Hagbarth K-E. Human motor control consequences of thixotropic changes in muscular short-range stiffness. J Physiol 535: 279–288, 2001. doi: 10.1111/j.1469-7793.2001.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axelson HW, Hagbarth K-E. Human motor compensations for thixotropy-dependent changes in resting wrist joint position after large joint movements. Acta Physiol Scand 179: 389–398, 2003. doi: 10.1046/j.0001-6772.2003.01217.x. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P, Nagy A, Kengyel A, Szatmári D, Mártonfalvi Z, Huber T, Kellermayer MSZ. Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J 93: 2102–2109, 2007. doi: 10.1529/biophysj.107.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum KP, Lamotte D’Incamps B, Zytnicki D, Ting LH. Force encoding in muscle spindles during stretch of passive muscle. PLOS Comput Biol 13: e1005767, 2017. doi: 10.1371/journal.pcbi.1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MC. The responses of frog muscle spindles and fast and slow muscle fibres to a variety of mechanical inputs. J Physiol 218: 1–17, 1971. doi: 10.1113/jphysiol.1971.sp009601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown MC, Goodwin GM, Matthews PBC. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol 205: 677–694, 1969. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchthal F, Kaiser E. The Rheology of the Cross Striated Muscle Fibre with Particular Reference to Isotonic Conditions. Royal Danish Science Society Biological Bulletin Vol. 21, no. 7. Copenhagen, Denmark: Munksgaard, 1951. [Google Scholar]
- 12.Campbell KS. Short-range mechanical properties of skeletal and cardiac muscles. Adv Exp Med Biol 682: 223–246, 2010. doi: 10.1007/978-1-4419-6366-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell KS. Dynamic coupling of regulated binding sites and cycling myosin heads in striated muscle. J Gen Physiol 143: 387–399, 2014. doi: 10.1085/jgp.201311078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell KS, Janssen PML, Campbell SG. Force-dependent recruitment from the myosin OFF state contributes to length-dependent activation. Biophys J 115: 543–553, 2018. doi: 10.1016/j.bpj.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell KS, Lakie M. A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. J Physiol 510: 941–962, 1998. doi: 10.1111/j.1469-7793.1998.941bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell KS, Moss RL. A thixotropic effect in contracting rabbit psoas muscle: prior movement reduces the initial tension response to stretch. J Physiol 525: 531–548, 2000. doi: 10.1111/j.1469-7793.2000.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell KS, Moss RL. History-dependent mechanical properties of permeabilized rat soleus muscle fibers. Biophys J 82: 929–943, 2002. doi: 10.1016/S0006-3495(02)75454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell SG, Campbell KS. Mechanisms of residual force enhancement in skeletal muscle: insights from experiments and mathematical models. Biophys Rev 3: 199–207, 2011. doi: 10.1007/s12551-011-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornachione AS, Leite F, Bagni MA, Rassier DE. The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms. Am J Physiol Cell Physiol 310: C19–C26, 2016. doi: 10.1152/ajpcell.00156.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day J, Bent LR, Birznieks I, Macefield VG, Cresswell AG. Muscle spindles in human tibialis anterior encode muscle fascicle length changes. J Neurophysiol 117: 1489–1498, 2017. doi: 10.1152/jn.00374.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denny-Brown D. On the nature of postural reflexes. Proc R Soc Lond, B 104: 252–301, 1929. doi: 10.1098/rspb.1929.0010. [DOI] [Google Scholar]
- 22.Di Giulio I, Maganaris CN, Baltzopoulos V, Loram ID. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol 587: 2399–2416, 2009. doi: 10.1113/jphysiol.2009.168690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edin BB, Vallbo AB. Stretch sensitization of human muscle spindles. J Physiol 400: 101–111, 1988. doi: 10.1113/jphysiol.1988.sp017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flitney FW, Hirst DG. Proceedings: Short-range elastic properties of contracting frog’s muscle. J Physiol 239: 119P–121P, 1974. [PubMed] [Google Scholar]
- 25.Freundlich H. Thixotropy. Paris: Hermann, 1935. [Google Scholar]
- 26.Fusi L, Huang Z, Irving M. The conformation of myosin heads in relaxed skeletal muscle: implications for myosin-based regulation. Biophys J 109: 783–792, 2015. doi: 10.1016/j.bpj.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusi L, Percario V, Brunello E, Caremani M, Bianco P, Powers JD, Reconditi M, Lombardi V, Piazzesi G. Minimum number of myosin motors accounting for shortening velocity under zero load in skeletal muscle. J Physiol 595: 1127–1142, 2017. doi: 10.1113/JP273299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granzier HL, Labeit S. Titin and its associated proteins: the third myofilament system of the sarcomere. Adv Protein Chem 71: 89–119, 2005. doi: 10.1016/S0065-3233(04)71003-7. [DOI] [PubMed] [Google Scholar]
- 29.Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles. J Neurophysiol 56: 451–461, 1986. doi: 10.1152/jn.1986.56.2.451. [DOI] [PubMed] [Google Scholar]
- 30.Gruen M, Prinz H, Gautel M. cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on-off fashion. FEBS Lett 453: 254–259, 1999. doi: 10.1016/S0014-5793(99)00727-9. [DOI] [PubMed] [Google Scholar]
- 31.Herbst M. Studies on the relation between latency relaxation and resting cross-bridges of frog skeletal muscle. Pflugers Arch 364: 71–76, 1976. doi: 10.1007/BF01062914. [DOI] [PubMed] [Google Scholar]
- 32.Herzog W. Letter to the Editor: Comments on Cornachione et al. (2016): “The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms”. Am J Physiol Cell Physiol 311: C158–C159, 2016. doi: 10.1152/ajpcell.00373.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzog W, Lee EJ, Rassier DE. Residual force enhancement in skeletal muscle. J Physiol 574: 635–642, 2006. doi: 10.1113/jphysiol.2006.107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill DK. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol 199: 637–684, 1968. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J 100: 1969–1976, 2011. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt CC, Kuffler SW. Further study of efferent small-nerve fibers to mammalian muscle spindles; multiple spindle innervation and activity during contraction. J Physiol 113: 283–297, 1951. doi: 10.1113/jphysiol.1951.sp004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter IW, Kearney RE. Dynamics of human ankle stiffness: variation with mean ankle torque. J Biomech 15: 747–752, 1982. doi: 10.1016/0021-9290(82)90089-6. [DOI] [PubMed] [Google Scholar]
- 38.Irving M. Regulation of contraction by the thick filaments in skeletal muscle. Biophys J 113: 2579–2594, 2017. doi: 10.1016/j.bpj.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanenko Y, Gurfinkel VS. Human Postural Control. Front Neurosci 12: 171, 2018. doi: 10.3389/fnins.2018.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kampourakis T, Sun YB, Irving M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc Natl Acad Sci USA 113: E3039–E3047, 2016. doi: 10.1073/pnas.1602776113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellermayer MSZ, Bianco P, Martonfalvi Z, Nagy A, Kengyel A, Szatmari D, Huber T, Linari M, Caremani M, Lombardi V. Muscle thixotropy: more than just cross-bridges? Biophys J 94: 329–330, 2008. doi: 10.1529/biophysj.107.122309. [DOI] [Google Scholar]
- 42.Kellermayer MSZ, Smith SB, Bustamante C, Granzier HL. Mechanical fatigue in repetitively stretched single molecules of titin. Biophys J 80: 852–863, 2001. doi: 10.1016/S0006-3495(01)76064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res 89: 874–881, 2001. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- 44.Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci 8: 498–504, 2005. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- 45.Lakie MD, Hayes NR, Combes N, Langford N. Is postural tremor size controlled by interstitial potassium concentration in muscle? J Neurol Neurosurg Psychiatry 75: 1013–1018, 2004. doi: 10.1136/jnnp.2003.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakie M, Robson LG. Thixotropic changes in human muscle stiffness and the effects of fatigue. Q J Exp Physiol 73: 487–500, 1988. doi: 10.1113/expphysiol.1988.sp003169. [DOI] [PubMed] [Google Scholar]
- 47.Lakie M, Robson LG. Thixotropy: the effect of stimulation in frog muscle. Q J Exp Physiol 73: 627–630, 1988. doi: 10.1113/expphysiol.1988.sp003183. [DOI] [PubMed] [Google Scholar]
- 48.Lakie M, Scott DB, Walsh EG, Wright GW. Resonance at the wrist in anaesthetized subjects. J Physiol 338: 32P, 1982. [Google Scholar]
- 49.Lakie M, Vernooij CA, Osborne TM, Reynolds RF. The resonant component of human physiological hand tremor is altered by slow voluntary movements. J Physiol 590: 2471–2483, 2012. doi: 10.1113/jphysiol.2011.226449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lakie M, Walsh EG, Wright GW. Passive wrist movements: a large thixotropic effect. J Physiol 300: 36–37P, 1979. [Google Scholar]
- 51.Lakie M, Walsh EG, Wright GW. Resonance at the wrist demonstrated by the use of a torque motor: an instrumental analysis of muscle tone in man. J Physiol 353: 265–285, 1984. doi: 10.1113/jphysiol.1984.sp015335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lännergren J. The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. J Gen Physiol 58: 145–162, 1971. doi: 10.1085/jgp.58.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lännergren J, Noth J. The effect of bathing solution tonicity on resting tension in frog muscle fibers. J Gen Physiol 62: 737–755, 1973. doi: 10.1085/jgp.62.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HD, Herzog W. Force depression following muscle shortening of voluntarily activated and electrically stimulated human adductor pollicis. J Physiol 551: 993–1003, 2003. doi: 10.1113/jphysiol.2002.037333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, Piazzesi G, Lombardi V, Irving M. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 528: 276–279, 2015. doi: 10.1038/nature15727. [DOI] [PubMed] [Google Scholar]
- 56.Loram ID, Gawthrop PJ, Lakie M. The frequency of human, manual adjustments in balancing an inverted pendulum is constrained by intrinsic physiological factors. J Physiol 577: 417–432, 2006. doi: 10.1113/jphysiol.2006.118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loram ID, Maganaris CN, Lakie M. The passive, human calf muscles in relation to standing: the short range stiffness lies in the contractile component. J Physiol 584: 677–692, 2007. doi: 10.1113/jphysiol.2007.140053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol 440: 497–512, 1991. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mártonfalvi Z, Bianco P, Linari M, Caremani M, Nagy A, Lombardi V, Kellermayer M. Low-force transitions in single titin molecules reflect a memory of contractile history. J Cell Sci 127: 858–870, 2014. doi: 10.1242/jcs.138461. [DOI] [PubMed] [Google Scholar]
- 60.Matthews PBC. Muscle spindles: their messages and their fusimotor supply . In: Handbook of Physiology. The Nervous System. Motor Control, edited by Brookhart JM, Mountcastle VB, Brooks VB. Bethesda, MD: Am. Physiol. Soc., 1981, vol. II, pt. I, p. 189–228. doi: 10.1002/cphy.cp010206. [DOI] [Google Scholar]
- 61.McNamara JW, Li A, Smith NJ, Lal S, Graham RM, Kooiker KB, van Dijk SJ, Remedios CGD, Harris SP, Cooke R. Ablation of cardiac myosin binding protein-C disrupts the super-relaxed state of myosin in murine cardiomyocytes. J Mol Cell Cardiol 94: 65–71, 2016. doi: 10.1016/j.yjmcc.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mewis J, Wagner NJ. Thixotropy. Adv Colloid Interface Sci 147-148: 214–227, 2009. doi: 10.1016/j.cis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Meyer GA, McCulloch AD, Lieber RL. A nonlinear model of passive muscle viscosity. J Biomech Eng 133: 091007, 2011. doi: 10.1115/1.4004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milner TE, Cloutier C. Damping of the wrist joint during voluntary movement. Exp Brain Res 122: 309–317, 1998. doi: 10.1007/s002210050519. [DOI] [PubMed] [Google Scholar]
- 65.Mun JY, Previs MJ, Yu HY, Gulick J, Tobacman LS, Beck Previs S, Robbins J, Warshaw DM, Craig R. Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism. Proc Natl Acad Sci USA 111: 2170–2175, 2014. doi: 10.1073/pnas.1316001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishikawa K. Eccentric contraction: unraveling mechanisms of force enhancement and energy conservation. J Exp Biol 219: 189–196, 2016. doi: 10.1242/jeb.124057. [DOI] [PubMed] [Google Scholar]
- 67.Nogara L, Naber N, Canton M, Reggiani C, Pate E, Cooke R. Destabilizing the super relaxed state of skeletal muscle myosin to treat obesity and type 2 diabetes. Biophys J 110: 304a, 2016. doi: 10.1016/j.bpj.2015.11.1633. [DOI] [Google Scholar]
- 68.Peterfi T. Die Abhebung der Befruchtungsmembran bei Seeigeleiern. Arch Entwicklungsmech Org 112: 660–695, 1927. doi: 10.1007/BF02253780. [DOI] [PubMed] [Google Scholar]
- 69.Proske U, Gandevia SC. The kinaesthetic senses. J Physiol 587: 4139–4146, 2009. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Proske U, Morgan DL. Do cross-bridges contribute to the tension during stretch of passive muscle? J Muscle Res Cell Motil 20: 433–442, 1999. doi: 10.1023/A:1005573625675. [DOI] [PubMed] [Google Scholar]
- 71.Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol 41: 705–721, 1993. doi: 10.1016/0301-0082(93)90032-N. [DOI] [PubMed] [Google Scholar]
- 72.Proske U, Tsay A, Allen T. Muscle thixotropy as a tool in the study of proprioception. Exp Brain Res 232: 3397–3412, 2014. doi: 10.1007/s00221-014-4088-5. [DOI] [PubMed] [Google Scholar]
- 73.Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Prog Neurobiol 60: 85–96, 2000. doi: 10.1016/S0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 74.Rassier DE. Reply to Letter to the Editor: Comments on Cornachione et al. (2016): “The increase in non- cross-bridge forces after stretch of activated striated muscle is related to titin isoforms”. Am J Physiol Cell Physiol 311: C160–C161, 2016. doi: 10.1152/ajpcell.00159.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rassier DE. Reply to “Letter to the Editor: Titin-actin interaction: the report of its death was an exaggeration”. Am J Physiol Cell Physiol 310: C623, 2016. doi: 10.1152/ajpcell.00365.2015. [DOI] [PubMed] [Google Scholar]
- 76.Rassier DE, Leite FS, Nocella M, Cornachione AS, Colombini B, Bagni MA. Non-crossbridge forces in activated striated muscles: a titin dependent mechanism of regulation? J Muscle Res Cell Motil 36: 37–45, 2015. doi: 10.1007/s10974-014-9397-6. [DOI] [PubMed] [Google Scholar]
- 77.Reconditi M, Caremani M, Pinzauti F, Powers JD, Narayanan T, Stienen GJ, Linari M, Lombardi V, Piazzesi G. Myosin filament activation in the heart is tuned to the mechanical task. Proc Natl Acad Sci USA 114: 3240–3245, 2017. doi: 10.1073/pnas.1619484114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reynolds R, Lakie M. Postmovement changes in the frequency and amplitude of physiological tremor despite unchanged neural output. J Neurophysiol 104: 2020–2023, 2010. doi: 10.1152/jn.00513.2010. [DOI] [PubMed] [Google Scholar]
- 79.Ribot-Ciscar E, Rossi-Durand C, Roll J-P. Muscle spindle activity following muscle tendon vibration in man. Neurosci Lett 258: 147–150, 1998. doi: 10.1016/S0304-3940(98)00732-0. [DOI] [PubMed] [Google Scholar]
- 80.Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47: 177–190, 1982. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- 81.Sakanaka TE, Lakie M, Reynolds RF. Sway-dependent changes in standing ankle stiffness caused by muscle thixotropy. J Physiol 594: 781–793, 2016. doi: 10.1113/JP271137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sica REP, McComas AJ. Fast and slow twitch units in a human muscle. J Neurol Neurosurg Psychiatry 34: 113–120, 1971. doi: 10.1136/jnnp.34.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stubbs PW, Walsh LD, D’Souza A, Héroux ME, Bolsterlee B, Gandevia SC, Herbert RD. History-dependence of muscle slack length following contraction and stretch in the human vastus lateralis. J Physiol 596: 2121–2129, 2018. doi: 10.1113/JP275527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stuyvers BD, Miura M, Jin J-P, ter Keurs HEDJ. Ca(2+)-dependence of diastolic properties of cardiac sarcomeres: involvement of titin. Prog Biophys Mol Biol 69: 425–443, 1998. doi: 10.1016/S0079-6107(98)00018-2. [DOI] [PubMed] [Google Scholar]
- 85.Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol 469: 673–691, 1993. doi: 10.1113/jphysiol.1993.sp019837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Dijk SJ, Bezold KL, Harris SP. Earning stripes: myosin binding protein-C interactions with actin. Pflugers Arch 466: 445–450, 2014. doi: 10.1007/s00424-013-1432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vernooij CA, Lakie M, Reynolds RF. The complete frequency spectrum of physiological tremor can be recreated by broadband mechanical or electrical drive. J Neurophysiol 113: 647–656, 2015. doi: 10.1152/jn.00519.2014. [DOI] [PubMed] [Google Scholar]
- 88.Vernooij CA, Reynolds RF, Lakie M. Physiological tremor reveals how thixotropy adapts skeletal muscle for posture and movement. R Soc Open Sci 3: 160065, 2016. doi: 10.1098/rsos.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walsh EG. Muscles, Masses, and Motion. London: MacKeith Press, 1996. [Google Scholar]
- 90.Wiegner AW. Mechanism of thixotropic behavior at relaxed joints in the rat. J Appl Physiol (1985) 62: 1615–1621, 1987. doi: 10.1152/jappl.1987.62.4.1615. [DOI] [PubMed] [Google Scholar]
- 91.Wilson C, Naber N, Pate E, Cooke R. The myosin inhibitor blebbistatin stabilizes the super-relaxed state in skeletal muscle. Biophys J 107: 1637–1646, 2014. doi: 10.1016/j.bpj.2014.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson LR, Gandevia SC, Burke D. Discharge of human muscle spindle afferents innervating ankle dorsiflexors during target isometric contractions. J Physiol 504: 221–232, 1997. doi: 10.1111/j.1469-7793.1997.221bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamasaki R, Berri M, Wu Y, Trombitás K, McNabb M, Kellermayer MSZ, Witt C, Labeit D, Labeit S, Greaser M, Granzier H. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J 81: 2297–2313, 2001. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]