Abstract
Sickle cell disease (SCD) is caused by a mutation of the β-globin gene (Ingram VM. Nature 180: 326–328, 1957), which triggers the polymerization of deoxygenated sickle hemoglobin (HbS). Approximately 100,000 SCD patients in the United States and millions worldwide (Piel FB, et al. PLoS Med 10: e1001484, 2013) suffer from chronic hemolytic anemia, painful crises, multisystem organ damage, and reduced life expectancy (Rees DC, et al. Lancet 376: 2018–2031, 2010; Serjeant GR. Cold Spring Harb Perspect Med 3: a011783, 2013). Hematopoietic stem cell transplantation can be curative, but the majority of patients do not have a suitable donor (Talano JA, Cairo MS. Eur J Haematol 94: 391–399, 2015). Advanced gene-editing technologies also offer the possibility of a cure (Goodman MA, Malik P. Ther Adv Hematol 7: 302–315, 2016; Lettre G, Bauer DE. Lancet 387: 2554–2564, 2016), but the likelihood that these strategies can be mobilized to treat the large numbers of patients residing in developing countries is remote. A pharmacological treatment to increase fetal hemoglobin (HbF) as a therapy for SCD has been a long-sought goal, because increased levels of HbF (α2γ2) inhibit the polymerization of HbS (Poillin WN, et al. Proc Natl Acad Sci USA 90: 5039–5043, 1993; Sunshine HR, et al. J Mol Biol 133: 435–467, 1979) and are associated with reduced symptoms and increased lifespan of SCD patients (Platt OS, et al. N Engl J Med 330: 1639–1644, 1994; Platt OS, et al. N Engl J Med 325: 11–16, 1991). Only two drugs, hydroxyurea and l-glutamine, are approved by the US Food and Drug Administration for treatment of SCD. Hydroxyurea is ineffective at HbF induction in ~50% of patients (Charache S, et al. N Engl J Med 332: 1317–1322, 1995). While polymerization of HbS has been traditionally considered the driving force in the hemolysis of SCD, the excessive reactive oxygen species generated from red blood cells, with further amplification by intravascular hemolysis, also are a major contributor to SCD pathology. This review highlights a new class of drugs, lysine-specific demethylase (LSD1) inhibitors, that induce HbF and reduce reactive oxygen species.
Keywords: fetal hemoglobin, LDS1 inhibitors, mitochondria, reactive oxygen species, sickle cell disease
BACKGROUND
Sickle cell disease (SCD) is a qualitative hemoglobinopathy. In the United States, 1 of every 400 African Americans is born with SCD. Approximately 100,000 Americans have SCD (18). The causative mutation is an A-T transversion in the sixth codon of the β-globin gene, leading to the substitution of glutamic acid for valine, which results in the formation of the abnormal sickle hemoglobin (HbS) (22, 51). After deoxygenation in red blood cells (RBCs), HbS forms polymers, causing the RBCs to become deformed (sickled) and adherent, leading to vasoocclusive events.
Initially, the polymerization of HbS was considered to be the only driving force of hemolysis in SCD. However, over decades, the importance of the accumulation of reactive oxygen species (ROS) inside the RBC and in the vasculature has been established (83). The combination of vasoocclusion and ROS accumulation is involved in chronic organ damage and vasculopathies (49). Increased intracellular ROS accumulation has been linked to the exposure of phosphatidylserine (PS) and to a reduction in flippase activity, which activates the interaction of RBCs with other cells (42). Previous studies provide insights into the relative contributions of RBC ROS-induced membrane damage and biophysical alterations, as shown by a decrease in the deformability of SCD RBCs with the progression of disease severity (4). The presence of oxidized HbS and deoxygenated polymerization of HbS in the RBC create a cycle of ROS, membrane damage, hemolysis, and cellular adhesion. Membrane damage to SCD RBCs and reticulocytes by ROS causes vascular endothelial damage through abnormal cellular adhesion and the release of intravascular hemolytic products (27). SCD reticulocytes have increased adherence to endothelial cells, while ROS generated by hemolysis activate monocytes, polymorphonuclear neutrophils, and platelets in the vascular lumen, resulting in inflammation, which damages vascular endothelial cells. Damage to vascular endothelial cell leads to vascular narrowing, causing ischemia, reperfusion, infarction, and, ultimately, multiple organ failures (2, 14, 23, 62). While bone marrow transplantation can be a curative therapy for SCD, the vast majority of African Americans do not have suitable family donors. In addition, bone marrow transplantation must be performed in specialized facilities and, therefore, is not a practical option for most patients worldwide. Therefore, current treatment modalities at a majority of institutions focus on the administration of disease-modulating drugs, control of infections, and pain management (23).
Only two drugs, hydroxyurea (HU) and l-glutamine, have been approved by the US Food and Drug Administration for treatment of SCD. HU, an inhibitor of ribonucleotide reductase, has multiple effects, including induction of fetal hemoglobin (HbF), nitric oxide, and glutathione peroxidase 1 activity and alteration of RBC-endothelial cell interactions. HbF (α2γ2) inhibits the polymerization of HbS, and elevated levels of HbF are associated with less severe illness and longer survival (56, 78). Therefore, a pharmacological therapy for induction of HbF has long been sought for SCD therapy. It is only effective in ~50% of patients, and the distribution of HbF is not pancellular (6, 32, 76). HbF is mainly distributed in a fraction of the RBCs, F cells, which contain HbF. Other drugs that are being developed for HbF induction include inhibitors of epigenetic-modifying enzymes that exist as components of corepressor complexes involved in repression of the γ-globin gene. The targets of the pharmacological inhibitors include histone deacetylases (HDACs), DNA methyltransferase 1 (DNMT1), protein arginine N-methyltransferase 5 (PRMT5), euchromatic histone lysine methyltransferase 2 (EHMT2/G9a), and lysine-specific demethylase 1 [LSD1 (KDMA1)].
While the mechanism of l-glutamate is not completely understood, it is postulated to affect RBC ROS levels that may damage the SCD RBCs and vessels. Oxidative stress occurs when an increase in oxidants without a similar increase in antioxidants triggers a cascade of oxidative reactions that, ultimately, lead to cell death due to damage to lipids, proteins, and DNA. Several mechanisms are responsible for increased oxidative stress in SCD: 1) high levels of intracellular RBC ROS (25, 45), 2) restoration of oxygen-rich blood after ischemic injuries that generate superoxides (3, 46), and 3) the NADPH-mediated oxidative burst produced by activated neutrophils. Excessive ROS in the bloodstream leads to multiple pathophysiological outcomes, including endothelial damage (17, 26), accelerated hemolysis, hypercoagulability (73), and SCD-related vasoocclusion (50).
Multiple mechanisms are responsible for RBC intracellular generation of ROS. NADPH oxidases have been identified as a major source of ROS in SCD RBCs. The other known sources of ROS in RBCs in SCD are 1) HbS autoxidation, 2) the Fenton reaction, and 3) low levels of antioxidants, such as selenium and glutathione peroxidase 1, SOD, and catalase (9, 47). In addition, Jagadeeswaran et al. (24) recently discovered that abnormally retained mitochondria in the SCD RBC are a source of ROS. An increased proportion of RBCs that retain mitochondria was observed in patients with SCD and in a transgenic mouse model of SCD (24). ROS levels were higher in RBCs with retained mitochondria than RBCs without mitochondria. During normal terminal differentiation of RBCs, mitochondria are eliminated through a process known as “mitophagy.” Mouse models with deletions in mitophagy genes exhibited reduced survival of RBCs and severe anemia due to lack of mitochondrial clearance (12, 44, 67). Mitochondria generate ROS, primarily superoxide (O2·−), by the respiratory chain; O2·− is transformed into H2O2, and, then, in the presence of ferrous ions, the more damaging hydroxyl radical (OH·) is formed (40).
Recently, targeting of intracellular ROS has been suggested as a possible therapeutic strategy for SCD. Keleku-Lukwete et al. (28) crossed a mouse model of SCD with a mouse that overexpressed the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of the antioxidant cell-defense system, and showed amelioration of tissue damage. Previous studies suggest that Nrf2 could reduce the ROS levels not only by transcriptionally upregulating antioxidant enzymes and NADPH-generating pentose pathway enzymes, but also by lowering the levels of NADPH oxidase (Nox2) (30). Recent studies also showed that administration of dimethyl fumarate, a drug approved for the treatment of multiple sclerosis, to SCD mice decreased hepatic necrosis, inflammatory cytokines, and irregularly shaped RBCs and increased HbF (5).
This review focuses on LSD1 inhibitors in two preclinical models: 1) the SCD mouse model and 2) the baboon model. Testing in these models has shown that LSD1 (KDM1A) inhibitors have two key mechanisms: 1) induction of high levels of HbF in nonhuman primates and 2) reduction of the abnormal presence of mitochondria in the mature RBC of SCD mice (24b). Both the SCD mouse model and the baboon model are necessary, because each has limitations. The SCD mouse model demonstrates SCD pathology, but the human γ-globin gene is regulated as an embryonic, rather than the fetal-stage, gene in the mouse (69) and, therefore, is not efficiently induced by pharmacological agents. In contrast, nonhuman primates such as the baboon are considered to be excellent animal models to test the activity of HbF-inducing drugs, because the structure and developmental regulation of the β-like globin gene locus are highly conserved between baboon and human, and effects in the baboon are highly predictive of results in patients (1, 43). The baboon model, however, does not have HbS and, therefore, does not exhibit SCD pathology.
LSD1 (KDMA1) Plays a Crucial Role in Repression of γ-Globin
Extensive efforts to elucidate the mechanism of γ-globin repression during adult erythropoiesis have identified four trans-acting factors, the orphan nuclear receptors TR2/TR4, BCL11A, PRMT5, and LRF (ZBTB7A), that repress the γ-globin gene (35, 39, 41, 59, 68, 81, 82). TR2/TR4, the first repressor characterized, recruits a multiprotein corepressor complex, DRED, to the direct repeat (DR) elements within the γ-globin promoter (10). Biochemical purification of DRED showed that it consisted of a tetrameric core element containing TR2/TR4, DNMT1, and LSD1 with additional proteins, including HDACs, the G9A histone methyltransferase, and CoREST associated with the core to form the larger DRED complex (10). BCL11A, initially identified by genome-wide associated studies (35, 41, 68), was confirmed to be a γ-globin repressor by transgenic knockout mouse experiments that showed that deletion of BCL11A prevented repression of mouse embryonic globin and the human γ-globin gene during development (68). BCL11A also binds to the γ-globin promoter (8, 37, 38) and recruits a multiprotein corepressor complex containing HDACs, DNMT1, and LSD1 to repress γ-globin expression (85). Recruitment of corepressor complexes also facilitates γ-globin repression by ZBTB7A and PRMT5 (39, 59).
Thus recruitment of multiprotein corepressor complexes containing enzymes that modify the epigenome is essential to the mechanism of γ-globin repression. The temporal order of enzymatic action to establish repressive epigenetic modifications of the γ-globin gene during erythroid differentiation and the possible codependence of their activities is unknown. These enzymes maintain the high levels of DNA methylation, low levels of histone H3K4 methylation and histone acetylation, and high levels of histone H3K9 methylation that are characteristic of repressed genes and are targets for therapeutic interventions to increase HbF (15, 32, 79). The use of HDAC inhibitors is limited by their toxicity, while the pharmacological G9A inhibitors (7, 31, 61) developed thus far have poor bioavailability. Our work shows that inhibitors of DNMT and LSD1 are potent activators of HbF and among the most promising agents in development to increase HbF levels for therapy of SCD (19, 43, 75).
Reexpression of γ-Globin by LSD1 (KDM1A) Inhibitors
LSD1 (KDM1A) demethylates mono- and dimethylated histone H3K4 residues. Deletion of LSD1 in mice is lethal (84). The functional role of LSD1 in hematopoiesis has been investigated using tetracycline-inducible knockdown (74) and targeted deletion strategies (29). Knockdown of LSD1 expanded progenitor cells and compromised terminal hematopoietic differentiation, leading to granulocytopenia, anemia, and thrombocytopenia. In contrast, LSD1 knockdown increased the numbers of monocytes. Increased expression of key hematopoietic genes, including Gfi1b, HoxA9, and Meis1, was observed. Targeted deletion experiments also showed that LSD1 was required for the terminal differentiation of multiple blood cell lineages and impaired differentiation of stem cells. LSD1 was shown to act at both promoters and enhancers of various genes expressed in stem and progenitor cells to silence their expression during hematopoiesis (29).
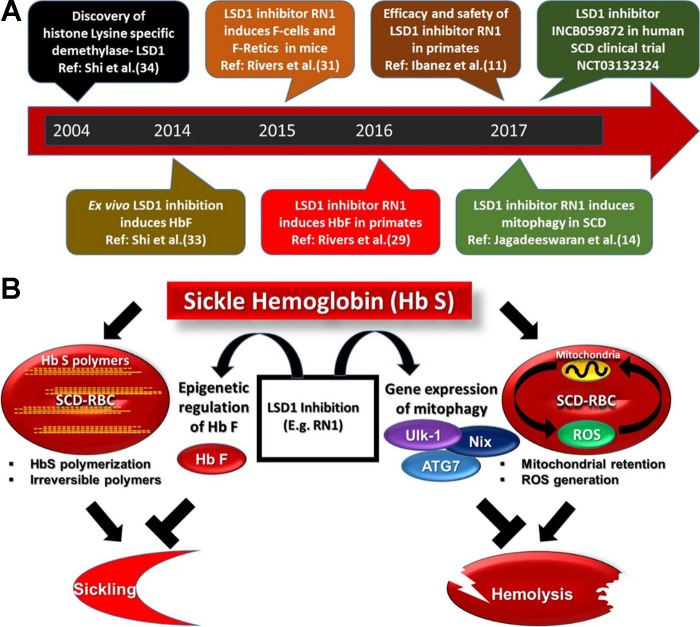
Shi et al. (71) initially identified LSD1 as a therapeutic target for reactivation of HbF through experiments using RNA interference strategies and pharmacological inhibitors in cultured human erythroid progenitor cells. LSD1 knockdown in cultured human erythroid cells enhanced γ-globin mRNA synthesis in cultured human erythroid progenitor cells. Administration of tranylcypromine (TCP) increased HbF from 4.6% to 31% of total hemoglobin and γ-globin mRNA expression 9.4-fold at the highest dose tested. Induction of HbF with TCP was superior to HU and comparable to the DNMT1 inhibitor decitabine (DAC). In addition, TCP increased γ-globin expression in β-globin yeast artificial chromosome (YAC) transgenic mice (72). A timeline of the key progress in the development of LSD1 inhibitor for SCD is shown in Fig. 1A.
Fig. 1.
A: timeline of selected key events in lysine-specific demethylase (LSD1) research and development in therapeutically targeting LSD1 inhibitors in sickle cell disease (SCD). B: the LSD1 inhibitor RN-1 has 2 distinct mechanisms. One addresses sickle hemoglobin (HbS) polymerization-mediated sickling, and the other addresses red blood cell (RBC) reactive oxygen species (ROS) generation-induced hemolysis. HbF, fetal hemoglobin; Retics, reticulocytes.
Following this report, Rivers et al. (65) demonstrated that 2-(1R,2S)-2-{[4-(benzyloxy)phenyl]cyclopropylamino}-1-(4-methylpiperazin-1-yl)ethanone, HCl (RN-1), a LSD1 inhibitor with increased potency and specificity (Table 1), induced F cells and γ-globin mRNA in humanized SCD mice to levels similar to DAC, the most powerful HbF-inducing drug known, and to higher levels than either TCP or HU. F cells on day 11 were increased in mice treated with DAC (11.8 ± 2.6, P < 0.005), HU (6.2 ± 10.88, P < 0.03), and RN-1 at 2.5 mg/kg (7.76 ± 1.13, P < 0.005) and 5 mg/kg (12.5 ± 1.85, P < 0.005) compared with control mice (4.8 ± 0.6). No increase in F cell levels was observed in mice treated with TCP (4.9 ± 0.95). Increased levels of γ-globin mRNA (γ/γ + β-fold change) were observed on day 11 in mice treated with DAC (4.24 ± 1.4, P < 0.001), HU (1.8 ± 0.6, P < 0.02), and RN-1 at 2.5 mg/kg (1.78 ± 0.98, P < 0.001) and 5 mg/kg (4.34 ± 1.36, P < 0.005) compared with controls. No increase in γ-globin mRNA was observed in mice treated with TCP. On day 11, levels of F cells (P < 0.001) and γ-globin mRNA (P < 0.02) were significantly higher in mice treated with 5 mg/kg RN-1 than in those treated with HU.
Table 1.
IC50 of TCP and RN-1
| IC50, µM |
|||
|---|---|---|---|
| Compound | LSD1 | MAO-A | MAO-B |
| TCP | 2–100 | 0.48 | 4.881 |
| RN-1 | 0.01–0.07 | 0.51 | 2.785 |
MAO, monoamine oxidase; RN-1, 2-(1R,2S)-2-{[4-(benzyloxy)phenyl]cyclopropylamino}-1-(4-methylpiperazin-1-yl)ethanone, HCl; TCP, tranylcypromine.
In an additional study in humanized SCD mice, Cui et al. (11) demonstrated similar HbF-inducing effects of RN-1 and also observed that the liver and spleen of SCD mice treated with RN-1 did not exhibit the necrotic lesions that are usually associated with SCD. They also reported reduced reticulocytosis and increased total hemoglobin and RBC lifespan in the SCD mice treated with RN-1. RN-1 (3 or 10 mg·kg body wt−1·day−1) was administered to SCD mice for 5 days/wk for 4 consecutive weeks. In untreated SCD mice, HbF was 0.32% of total hemoglobin, while treatment with 10 mg/kg RN-1 increased HbF to 1.2% of total hemoglobin. The increased RBC life span and decreased organ pathology were attributed to induction of mouse embryonic globin genes.
Efficacy and Safety of LSD1 Inhibitor RN-1 in Primates
While treatment of humanized SCD mice with RN-1 increased γ-globin mRNA, F cells, and F reticulocytes (11, 65), the levels of HbF were low, because the human γ-globin gene is not efficiently reactivated in this mouse model. Therefore, we tested the effect of RN-1 in baboons, considered to be the best animal model to study the effect of HbF-inducing drugs due to the conservation of the structure and pattern of developmental expression of the β-like globin genes among simian primates. Experiments performed in baboons have been directly translated to clinical trials in SCD patients (1, 43). RN-1 treatment of anemic baboons stimulated high levels of γ-globin synthesis, HbF, F cells, and F reticulocytes (63). In addition, RN-1 treatment restored high levels of HbF and caused synthesis of the individual 5′-Iγ- and 3′-Vγ-globin chains in the ratio characteristic of fetal development. Chromatin immunoprecipitation experiments showed that RN-1 treatment increased levels of histone H3K4me2, H3K4me3, and H3K9 acetylation (H3K9ac) associated with the γ-globin promoter region.
These experiments were followed by long-term (>265 days) administration of RN-1 to two normal baboons to evaluate relative safety and effectiveness (20). HbF and F cells were maintained at high levels with minimal hematological toxicity. Decreases in hemoglobin were observed during menstrual bleeding in one animal and following an accidental laceration of the perineal swelling. In vitro platelet activation assays showed that platelet function was inhibited in the treated baboons. Importantly, inhibitors of platelet function are in clinical trials in patients with SCD (48, 77). The level of inhibition of platelet function targeted by these studies appears similar to that in baboons treated with RN-1.
The LSD1 Inhibitor RN-1 Reduces Mitochondria-Containing RBCs and ROS in a SCD Mouse Model
Treatment with the LSD1 inhibitor RN-1 decreased the level of mitochondria-retaining RBCs, reduced ROS, and enhanced RBC lifespan in SCD mice. Gene expression analysis of SCD mice treated with RN-1 showed a significant (>2-fold) upregulation in the expression of key mitophagy genes, including Ulk-1, also known as ATG1, and the erythroid-specific mitophagy gene ATG7, compared with untreated SCD mice (24). ATG1 and ATG7 are key effector molecules in mitochondrial autophagy in mammalian hematopoietic cells, and their loss in erythroid cells leads to the incomplete removal of mitochondria and severe anemia in vivo (44). RN-1 reduces levels of RBCs with abnormally retaining mitochondria, thereby reducing ROS. This effect leads to an increased RBC lifespan.
LSD1 Inhibitors in Combination with Other Drugs
While relatively long-term administration of RN-1 to baboons was accomplished with minimal hematological toxicity, the therapeutic window does appear rather narrow, with increased doses producing neutropenia and thrombocytopenia due to the requirement for LSD1 activity during terminal hematopoietic differentiation. Induction of HbF by other drugs, such as DAC, can also be associated with dose-related hematological toxicities. Therefore, the best strategy may be development of combinatorial drug regimens targeting different epigenetic-modifying enzymes present in the corepressor complexes that are recruited to the γ-globin gene. This strategy will increase HbF expression in a combinatorial or synergistic manner while minimizing hematological toxicities and, thus, maximizing the therapeutic index. Although the use of combinatorial drug regimens has been proposed to increase HbF, few combinations have been tested in vivo (13, 52). We have shown that HU and RN-1 administered together induced a combinatorial increase in γ-globin expression in SCD mice (24a). In the same study, administration of RN-1 and HU in combination reduced the peripheral red blood sickle cells and spleen size in a SCD mouse model.
While other studies showed that the combination of HU and erythropoietin (Epo) administered to SCD patients was no more effective than HU alone (36), our laboratory showed that the combination of stem cell factor (SCF), Epo, and HU led to combinatorial increases in HbF synthesis in baboons (33). In baboons, administration of SCF, Epo, and HU in combination increased γ-globin chain synthesis to high levels (0.4–0.5 γ/γ + β-fold change), suggesting that the effect may be mediated by a CD117+ cell. We have observed that RN-1 increases the number of CD117+ bone marrow cells. This suggests that perturbation of erythroid differentiation by RN-1 may increase the number of HU-responsive cells. Experiments in cultured human erythroid progenitor cells showed that the KDM1A inhibitor TCP and DEC, in combination, increased HbF expression in a synergistic manner (71). Experiments in SCD mice also showed that RN-1 and DEC administered together increased HbF expression in an additive manner, although cytotoxicity was increased (24a). These results suggest that drug combinations should be further explored.
Future LSD1 Inhibitors
Recently, we showed that oral administration of an improved LSD1 inhibitor, OG-S1335, developed by Oryzon Genomics, with increased potency and specificity compared with RN-1, increased F reticulocytes and γ-globin mRNA in normal and anemic baboons (64). Another orally available LSD1 inhibitor, developed by Incyte, has entered clinical trials (ClinicalTrials.gov registry no. NCT03132324) in patients with SCD. The development of new LSD1 inhibitors with increased specificity and limited access to the central nervous system, as well as the development of targeted delivery systems to direct delivery of the drug to erythroid progenitor cells to limit adverse effects on other hematopoietic lineages, would be highly desirable.
DISCUSSION
The LSD1 inhibitor RN-1 may be an effective therapeutic agent for SCD that targets SCD pathology through two mechanisms of action. 1) RN-1 is a potent inducer of HbF in the baboon model. The effect of RN-1 on HbF levels appears to be similar to that of DAC, the most potent inducer of HbF thus far identified. The drug was safely administered for >265 days in two normal baboons at a dose that increased and maintained elevated HbF levels. 2) In a SCD mouse model, RN-1 increased RBC survival by reducing the number of RBCs with abnormal mitochondria retention, leading to reduced ROS. Further studies on mitochondrial function in SCD RBCs and related complications will provide insight into specific complications of SCD that could be ameliorated by LSD1 inhibitors. In conclusion, our studies have shown that LSD1 inhibitors may be highly effective therapeutic agents that act through a dual mechanism to attack SCD pathology. Further development of these drugs for SCD therapy should be encouraged.
Perspectives and Significance
The study of LSD1 inhibitors in SCD mice led to our discovery of abnormal mitochondrial retention in SCD. We conclude that LSD1 inhibitors increase RBC survival secondary to reduced mitochondrial ROS. An additional novel mechanism could be reduction of increased oxygen consumption by the retained mitochondria. Mitochondria are known to consume oxygen to produce ATP. Their retention in RBCs could lead to a hypoxic intracellular environment that causes HbS to deoxygenate and, subsequently, polymerize.
We have also observed that RN-1 increases expression of mitophagy regulation genes, suggesting that expression of mitophagy genes may be suppressed in SCD. These results should be addressed by future studies to compare mitophagy gene expression during normal and SCD erythropoiesis. Further studies of LSD1 effects could shift the current therapy paradigm and clinical practice to include the use of promitophagy drugs in SCD.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants R03 HL-135453, K01 HL-103172, and U01 HL-117658.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.R., R.J., and D.L. drafted the manuscript; R.J prepared figure; A.R., R.J., and D.L. edited manuscript; A.R., R.J., and D.L. approved a final version of the manuscript.
REFERENCES
- 1.Akpan I, Banzon V, Ibanez V, Vaitkus K, DeSimone J, Lavelle D. Decitabine increases fetal hemoglobin in Papio anubis by increasing γ-globin gene transcription. Exp Hematol 38: 989–993.e1, 2010. doi: 10.1016/j.exphem.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslan M, Freeman BA. Redox-dependent impairment of vascular function in sickle cell disease. Free Radic Biol Med 43: 1469–1483, 2007. doi: 10.1016/j.freeradbiomed.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98: 15215–15220, 2001. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barodka VM, Nagababu E, Mohanty JG, Nyhan D, Berkowitz DE, Rifkind JM, Strouse JJ. New insights provided by a comparison of impaired deformability with erythrocyte oxidative stress for sickle cell disease. Blood Cells Mol Dis 52: 230–235, 2014. doi: 10.1016/j.bcmd.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Belcher JD, Chen C, Nguyen J, Zhang P, Abdulla F, Nguyen P, Killeen T, Xu P, O’Sullivan G, Nath KA, Vercellotti GM. Control of oxidative stress and inflammation in sickle cell disease with the Nrf2 activator dimethyl fumarate. Antioxid Redox Signal 26: 748–762, 2017. doi: 10.1089/ars.2015.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR; Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia . Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med 332: 1317–1322, 1995. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Skutt-Kakaria K, Davison J, Ou YL, Choi E, Malik P, Loeb K, Wood B, Georges G, Torok-Storb B, Paddison PJ. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes Dev 26: 2499–2511, 2012. doi: 10.1101/gad.200329.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Luo HY, Steinberg MH, Chui DH. BCL11A represses HBG transcription in K562 cells. Blood Cells Mol Dis 42: 144–149, 2009. doi: 10.1016/j.bcmd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 64: 72–80, 2012. doi: 10.1002/iub.584. [DOI] [PubMed] [Google Scholar]
- 10.Cui S, Kolodziej KE, Obara N, Amaral-Psarris A, Demmers J, Shi L, Engel JD, Grosveld F, Strouboulis J, Tanabe O. Nuclear receptors TR2 and TR4 recruit multiple epigenetic transcriptional corepressors that associate specifically with the embryonic β-type globin promoters in differentiated adult erythroid cells. Mol Cell Biol 31: 3298–3311, 2011. doi: 10.1128/MCB.05310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui S, Lim KC, Shi L, Lee M, Jearawiriyapaisarn N, Myers G, Campbell A, Harro D, Iwase S, Trievel RC, Rivers A, DeSimone J, Lavelle D, Saunthararajah Y, Engel JD. The LSD1 inhibitor RN-1 induces fetal hemoglobin synthesis and reduces disease pathology in sickle cell mice. Blood 126: 386–396, 2015. doi: 10.1182/blood-2015-02-626259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW 2nd, Yin XM. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 285: 27879–27890, 2010. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fard AD, Hosseini SA, Shahjahani M, Salari F, Jaseb K. Evaluation of novel fetal hemoglobin inducer drugs in treatment of β-hemoglobinopathy disorders. Int J Hematol Oncol Stem Cell Res 7: 47–54, 2013. [PMC free article] [PubMed] [Google Scholar]
- 14.George A, Pushkaran S, Konstantinidis DG, Koochaki S, Malik P, Mohandas N, Zheng Y, Joiner CH, Kalfa TA. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 121: 2099–2107, 2013. doi: 10.1182/blood-2012-07-441188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginder GD. Epigenetic regulation of fetal globin gene expression in adult erythroid cells. Transl Res 165: 115–125, 2015. doi: 10.1016/j.trsl.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman MA, Malik P. The potential of gene therapy approaches for the treatment of hemoglobinopathies: achievements and challenges. Ther Adv Hematol 7: 302–315, 2016. doi: 10.1177/2040620716653729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation 11: 129–151, 2004. doi: 10.1080/mic.11.2.129.151. [DOI] [PubMed] [Google Scholar]
- 18.Hulihan M, Hassell KL, Raphael JL, Smith-Whitley K, Thorpe P. CDC grand rounds: improving the lives of persons with sickle cell disease. MMWR Morb Mortal Wkly Rep 66: 1269–1271, 2017. doi: 10.15585/mmwr.mm6646a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibanez V, Vaitkus K, Rivers A, Molokie R, Cui S, Engel JD, DeSimone J, Lavelle D. Efficacy and safety of long-term RN-1 treatment to increase HbF in baboons. Blood 129: 260–263, 2017. doi: 10.1182/blood-2016-10-746727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibanez V, Vaitkus K, Rivers A, Molokie R, Cui S, Engel JD, DeSimone J, Lavelle D. Efficacy and safety of long-term RN-1 treatment to increase HbF in baboons. Blood 129: 260–263, 2017. doi: 10.1182/blood-2016-10-746727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature 180: 326–328, 1957. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- 22.Ingram VM. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature 178: 792–794, 1956. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- 23.Jagadeeswaran R, Rivers A. Evolving treatment paradigms in sickle cell disease. Hematology Am Soc Hematol Educ Program 2017: 440–446, 2017. doi: 10.1182/asheducation-2017.1.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagadeeswaran R, Vazquez BA, Thiruppathi M, Ganesh BB, Ibanez V, Cui S, Engel JD, Diamond AM, Molokie RE, DeSimone J, Lavelle D, Rivers A. Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp Hematol 50: 46–52, 2017. doi: 10.1016/j.exphem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Jagadeeswaran R, Agarwal Y, Ibanez V, Ruiz MA, Vaitkus K, DeSimone J, Lavelle D, Rivers A. Investigation of two combination HbF induction regimens, RN-1 and hydroxyurea versus RN-1 and decitabine, in a humanized sickle cell mouse model. Blood 126: 3386, 2015. [Google Scholar]
- 24b.Jagadeeswaran R. Ibanez V, Ruiz MA, Vaitkus K, DeSimone J, Lavelle D, Rivers A. RN-1, an LSD-1 inhibitor, induces Hb F in the baboon (P. anubis) and reduces ROS in a SCD mouse model. 20th Biennial Hemoglobin Switch Meeting Pacific Grove, CA, September 14–18, 2016. [Google Scholar]
- 25.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100: 879–887, 2002. doi: 10.1182/blood.V100.3.879. [DOI] [PubMed] [Google Scholar]
- 26.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol 84: 618–625, 2009. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 127: 750–760, 2017. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keleku-Lukwete N, Suzuki M, Otsuki A, Tsuchida K, Katayama S, Hayashi M, Naganuma E, Moriguchi T, Tanabe O, Engel JD, Imaizumi M, Yamamoto M. Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci USA 112: 12169–12174, 2015. doi: 10.1073/pnas.1509158112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerenyi MA, Shao Z, Hsu YJ, Guo G, Luc S, O’Brien K, Fujiwara Y, Peng C, Nguyen M, Orkin SH. Histone demethylase Lsd1 represses hematopoietic stem and progenitor cell signatures during blood cell maturation. eLife 2: e00633, 2013. doi: 10.7554/eLife.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovac S, Angelova PR, Holmström KM, Zhang Y, Dinkova-Kostova AT, Abramov AY. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta 1850: 794–801, 2015. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krivega I, Byrnes C, de Vasconcellos JF, Lee YT, Kaushal M, Dean A, Miller JL. Inhibition of G9a methyltransferase stimulates fetal hemoglobin production by facilitating LCR/γ-globin looping. Blood 126: 665–672, 2015. doi: 10.1182/blood-2015-02-629972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavelle D, Engel JD, Saunthararajah Y. Fetal hemoglobin induction by epigenetic drugs. Semin Hematol 55: 60–67, 2018. doi: 10.1053/j.seminhematol.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavelle D, Molokie R, Ducksworth J, DeSimone J. Effects of hydroxurea, stem cell factor, and erythropoietin in combination on fetal hemoglobin in the baboon. Exp Hematol 29: 156–162, 2001. doi: 10.1016/S0301-472X(00)00654-8. [DOI] [PubMed] [Google Scholar]
- 34.Lettre G, Bauer DE. Fetal haemoglobin in sickle-cell disease: from genetic epidemiology to new therapeutic strategies. Lancet 387: 2554–2564, 2016. doi: 10.1016/S0140-6736(15)01341-0. [DOI] [PubMed] [Google Scholar]
- 35.Lettre G, Sankaran VG, Bezerra MA, Araújo AS, Uda M, Sanna S, Cao A, Schlessinger D, Costa FF, Hirschhorn JN, Orkin SH. DNA polymorphisms at the BCL11A, HBS1L-MYB, and β-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci USA 105: 11869–11874, 2008. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little JA, McGowan VR, Kato GJ, Partovi KS, Feld JJ, Maric I, Martyr S, Taylor JG 6th, Machado RF, Heller T, Castro O, Gladwin MT. Combination erythropoietin-hydroxyurea therapy in sickle cell disease: experience from the National Institutes of Health and a literature review. Haematologica 91: 1076–1083, 2006. [PMC free article] [PubMed] [Google Scholar]
- 37.Liu N, Hargreaves VV, Zhu Q, Kurland JV, Hong J, Kim W, Sher F, Macias-Trevino C, Rogers JM, Kurita R, Nakamura Y, Yuan GC, Bauer DE, Xu J, Bulyk ML, Orkin SH. Direct promoter repression by BCL11A controls the fetal to adult hemoglobin switch. Cell 173: 430–442.e17, 2018. doi: 10.1016/j.cell.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martyn GE, Wienert B, Yang L, Shah M, Norton LJ, Burdach J, Kurita R, Nakamura Y, Pearson RCM, Funnell APW, Quinlan KGR, Crossley M. Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat Genet 50: 498–503, 2018. doi: 10.1038/s41588-018-0085-0. [DOI] [PubMed] [Google Scholar]
- 39.Masuda T, Wang X, Maeda M, Canver MC, Sher F, Funnell AP, Fisher C, Suciu M, Martyn GE, Norton LJ, Zhu C, Kurita R, Nakamura Y, Xu J, Higgs DR, Crossley M, Bauer DE, Orkin SH, Kharchenko PV, Maeda T. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science 351: 285–289, 2016. doi: 10.1126/science.aad3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushita M, Noguchi H, Lu YF, Tomizawa K, Michiue H, Li ST, Hirose K, Bonner-Weir S, Matsui H. Photo-acceleration of protein release from endosome in the protein transduction system. FEBS Lett 572: 221–226, 2004. doi: 10.1016/j.febslet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H, Best S, Spector TD, Farrall M, Lathrop M, Thein SL. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet 39: 1197–1199, 2007. doi: 10.1038/ng2108. [DOI] [PubMed] [Google Scholar]
- 42.Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 5: 84, 2014. doi: 10.3389/fphys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molokie R, Lavelle D, Gowhari M. Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: a randomized phase 1 study. PLoS Med 14: e1002382, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci USA 107: 832–837, 2010. doi: 10.1073/pnas.0913170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagababu E, Fabry ME, Nagel RL, Rifkind JM. Heme degradation and oxidative stress in murine models for hemoglobinopathies: thalassemia, sickle cell disease and hemoglobin C disease. Blood Cells Mol Dis 41: 60–66, 2008. doi: 10.1016/j.bcmd.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 158: 893–903, 2001. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natta CL, Chen LC, Chow CK. Selenium and glutathione peroxidase levels in sickle cell anemia. Acta Haematol 83: 130–132, 1990. doi: 10.1159/000205188. [DOI] [PubMed] [Google Scholar]
- 48.Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev 30: 245–256, 2016. doi: 10.1016/j.blre.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nur E, Biemond BJ, Otten HM, Brandjes DP, Schnog JJ; CURAMA Study Group . Oxidative stress in sickle cell disease: pathophysiology and potential implications for disease management. Am J Hematol 86: 484–489, 2011. doi: 10.1002/ajh.22012. [DOI] [PubMed] [Google Scholar]
- 50.Nur E, Brandjes DP, Schnog JJ, Otten HM, Fijnvandraat K, Schalkwijk CG, Biemond BJ; CURAMA Study Group . Plasma levels of advanced glycation end products are associated with haemolysis-related organ complications in sickle cell patients. Br J Haematol 151: 62–69, 2010. doi: 10.1111/j.1365-2141.2010.08320.x. [DOI] [PubMed] [Google Scholar]
- 51.Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia: a molecular disease. Science 110: 543–548, 1949. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 52.Perrine SP, Pace BS, Faller DV. Targeted fetal hemoglobin induction for treatment of β-hemoglobinopathies. Hematol Oncol Clin North Am 28: 233–248, 2014. doi: 10.1016/j.hoc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med 10: e1001484, 2013. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 330: 1639–1644, 1994. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 55.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 325: 11–16, 1991. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 56.Poillon WN, Kim BC, Rodgers GP, Noguchi CT, Schechter AN. Sparing effect of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S at physiologic ligand saturations. Proc Natl Acad Sci USA 90: 5039–5043, 1993. doi: 10.1073/pnas.90.11.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rank G, Cerruti L, Simpson RJ, Moritz RL, Jane SM, Zhao Q. Identification of a PRMT5-dependent repressor complex linked to silencing of human fetal globin gene expression. Blood 116: 1585–1592, 2010. doi: 10.1182/blood-2009-10-251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 376: 2018–2031, 2010. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 61.Renneville A, Van Galen P, Canver MC, McConkey M, Krill-Burger JM, Dorfman DM, Holson EB, Bernstein BE, Orkin SH, Bauer DE, Ebert BL. EHMT1 and EHMT2 inhibition induces fetal hemoglobin expression. Blood 126: 1930–1939, 2015. doi: 10.1182/blood-2015-06-649087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Repka T, Hebbel RP. Hydroxyl radical formation by sickle erythrocyte membranes: role of pathologic iron deposits and cytoplasmic reducing agents. Blood 78: 2753–2758, 1991. [PubMed] [Google Scholar]
- 63.Rivers A, Vaitkus K, Ibanez V, Ruiz MA, Jagadeeswaran R, Saunthararajah Y, Cui S, Engel JD, DeSimone J, Lavelle D. The LSD1 inhibitor RN-1 recapitulates the fetal pattern of hemoglobin synthesis in baboons (P. anubis). Haematologica 101: 688–697, 2016. doi: 10.3324/haematol.2015.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivers A, Vaitkus K, Jagadeeswaran R, Ibanez V, Ruiz MA, Ciceri F, Cavalcanti F, Molokie RE, Lavelle D. Oral administration of the LSD1 inhibitor OG-S1335 increases fetal hemoglobin in humanized transgenic sickle cell disease mice and in baboons. Blood 130: 356–356, 2017. [Google Scholar]
- 65.Rivers A, Vaitkus K, Ruiz MA, Ibanez V, Jagadeeswaran R, Kouznetsova T, DeSimone J, Lavelle D. RN-1, a potent and selective lysine-specific demethylase 1 inhibitor, increases γ-globin expression, F reticulocytes, and F cells in a sickle cell disease mouse model. Exp Hematol 43: 546–553.e1, 2015. doi: 10.1016/j.exphem.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 454: 232–235, 2008. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HK, Hirschhorn JN, Cantor AB, Orkin SH. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322: 1839–1842, 2008. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 69.Sankaran VG, Xu J, Ragoczy T, Ippolito GC, Walkley CR, Maika SD, Fujiwara Y, Ito M, Groudine M, Bender MA, Tucker PW, Orkin SH. Developmental and species-divergent globin switching are driven by BCL11A. Nature 460: 1093–1097, 2009. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med 3: a011783, 2013. doi: 10.1101/cshperspect.a011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi L, Cui S, Engel JD, Tanabe O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat Med 19: 291–294, 2013. doi: 10.1038/nm.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119: 941–953, 2004. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Singer ST, Ataga KI. Hypercoagulability in sickle cell disease and β-thalassemia. Curr Mol Med 8: 639–645, 2008. doi: 10.2174/156652408786241366. [DOI] [PubMed] [Google Scholar]
- 74.Sprüssel A, Schulte JH, Weber S, Necke M, Händschke K, Thor T, Pajtler KW, Schramm A, König K, Diehl L, Mestdagh P, Vandesompele J, Speleman F, Jastrow H, Heukamp LC, Schüle R, Dührsen U, Buettner R, Eggert A, Göthert JR. Lysine-specific demethylase 1 restricts hematopoietic progenitor proliferation and is essential for terminal differentiation. Leukemia 26: 2039–2051, 2012. doi: 10.1038/leu.2012.157. [DOI] [PubMed] [Google Scholar]
- 75.Steinberg MH, Chui DH, Dover GJ, Sebastiani P, Alsultan A. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 123: 481–485, 2014. doi: 10.1182/blood-2013-09-528067. [DOI] [PubMed] [Google Scholar]
- 76.Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ; Multicenter Study of Hydroxyurea . Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Blood 89: 1078–1088, 1997. [PubMed] [Google Scholar]
- 77.Styles L, Heiselman D, Heath LE, Moser BA, Small DS, Jakubowski JA, Zhou C, Redding-Lallinger R, Heeney MM, Quinn CT, Rana SR, Kanter J, Winters KJ. Prasugrel in children with sickle cell disease: pharmacokinetic and pharmacodynamic data from an open-label, adaptive-design, dose-ranging study. J Pediatr Hematol Oncol 37: 1–9, 2015. doi: 10.1097/MPH.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 78.Sunshine HR, Hofrichter J, Eaton WA. Gelation of sickle cell hemoglobin in mixtures with normal adult and fetal hemoglobins. J Mol Biol 133: 435–467, 1979. doi: 10.1016/0022-2836(79)90402-9. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki M, Yamamoto M, Engel JD. Fetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathies. Mol Cell Biol 34: 3560–3569, 2014. doi: 10.1128/MCB.00714-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Talano JA, Cairo MS. Hematopoietic stem cell transplantation for sickle cell disease: state of the science. Eur J Haematol 94: 391–399, 2015. doi: 10.1111/ejh.12447. [DOI] [PubMed] [Google Scholar]
- 81.Tanabe O, Katsuoka F, Campbell AD, Song W, Yamamoto M, Tanimoto K, Engel JD. An embryonic/fetal β-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer. EMBO J 21: 3434–3442, 2002. doi: 10.1093/emboj/cdf340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanabe O, McPhee D, Kobayashi S, Shen Y, Brandt W, Jiang X, Campbell AD, Chen YT, Chang C, Yamamoto M, Tanimoto K, Engel JD. Embryonic and fetal β-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J 26: 2295–2306, 2007. doi: 10.1038/sj.emboj.7601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voskou S, Aslan M, Fanis P, Phylactides M, Kleanthous M. Oxidative stress in β-thalassaemia and sickle cell disease. Redox Biol 6: 226–239, 2015. doi: 10.1016/j.redox.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I, Liu F, Taylor H, Lozach J, Jayes FL, Korach KS, Glass CK, Fu XD, Rosenfeld MG. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature 446: 882–887, 2007. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- 85.Xu J, Bauer DE, Kerenyi MA, Vo TD, Hou S, Hsu YJ, Yao H, Trowbridge JJ, Mandel G, Orkin SH. Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc Natl Acad Sci USA 110: 6518–6523, 2013. doi: 10.1073/pnas.1303976110. [DOI] [PMC free article] [PubMed] [Google Scholar]