Abstract
Respiratory complications are potential causes of death in patients with spinal cord injury (SCI). Nocturnal swallowing could be related to transient arousals and could lead to fragmented sleep in SCI patients. However, the impact of nocturnal swallowing on breathing and sleep physiology in SCI is unknown. The objectives of this study were 1) to determine whether nocturnal swallowing is more common in SCI than in able-bodied (AB) subjects, 2) to determine the role of nocturnal swallowing on arousal threshold (ArTh) in SCI individuals with sleep-disordered breathing (SDB), and 3) to determine the effect of continuous positive airway pressure (CPAP) treatment on nocturnal swallowing. A total of 16 SCI and 13 AB subjects with SDB completed in-laboratory polysomnography with a pharyngeal catheter. A swallowing event (SW) was defined as a positive spike in pharyngeal pressure and was used to calculate the swallow index (SI) defined as a number of SW/total sleep time. Each SW was assessed for a relationship to the sleep stages and respiratory cycle phases, and associated arousals and ArTh were calculated. SI was higher in the SCI group compared with AB subjects during wake and different sleep stages (P < 0.05). SWs were found to be significantly higher in the late expiratory phase in the group with SCI compared with the other respiratory phases and were eliminated by CPAP (P < 0.05). ArTh for the subjects with SCI was significantly lower (P < 0.05) compared with the AB subjects. Nocturnal swallowing is more common in SCI than in AB individuals who have SDB, particularly during the expiratory phase. The ArTh is significantly lower in SCI (indicating increased arousal propensity), which may contribute to the mechanism of sleep disturbances in SCI.
NEW & NOTEWORTHY Nocturnal swallowing is common in patients with chronic spinal cord injury (SCI) and is associated with frequent arousals from sleep. The lower arousal threshold during sleep in SCI may contribute to the mechanism of sleep disturbances that are commonly found in cervical and high thoracic SCI. Continuous positive airway pressure may play a therapeutic role in alleviating nocturnal swallowing, which may contribute to reduced risk of aspiration.
Keywords: arousal, arousal threshold, sleep, sleep-disordered breathing, spinal cord injury, swallowing
INTRODUCTION
Swallowing is a normal physiologic function that requires the coordination of multiple muscles located within the oral cavity, pharynx, larynx, and esophagus. These muscle movements are controlled by several cranial (V, VII, and IX–XII) and cervical spine nerves (C1–C3) and are coordinated within the brain stem (27). Swallowing and respiration share the pharynx as a common channel; therefore, coordination between the two functions is critical to preventing aspiration (12, 16). Evidence in the literature suggests that individuals with spinal cord injury (SCI) may be at higher risk of aspiration and related morbidity and mortality (3). In fact, respiratory complications are common causes of death in patients with SCI (5, 8, 30).
Swallowing dysfunction is common in patients with neuromuscular disease (4) and may contribute to aspiration risk and respiratory complications in individuals with SCI (5). Likewise, gastresophageal reflux disease (GERD) and dysphagia may also contribute to increased aspiration risk in this population (9, 13). Treatment with continuous positive airway pressure (CPAP) has been shown to reduce GERD severity in individuals with and without sleep-disordered breathing (SDB), likely because of the attenuation of the magnitude of negative intrathoracic pressure or the salutary effect of CPAP on the lower esophageal sphincter making it less susceptible to reflux (10, 29).
Because swallowing occurs primarily during wakefulness, swallowing during sleep may require transient arousals and therefore, leads to fragmented sleep in patients with SCI. The effect of abnormal nocturnal swallowing on sleep and breathing—and the interactive effect of CPAP particularly in patients with SCI—remains unknown. The purpose of this study was to test the hypotheses that individuals with SCI 1) will experience more frequent nocturnal swallowing and a lower arousal threshold (ArTh) compared with healthy control adults, and 2) the application of nasal CPAP will elevate the ArTh. Results of this study have previously been reported in the form of an abstract (23).
METHODS
Participants.
This study used data obtained from the parent ongoing project investigating SDB in individuals with chronic SCI (24–26). The experimental protocol was approved by the Human Investigation Committee of the John D. Dingell Veterans Affairs Medical Center and Wayne State University (Detroit, MI), and written informed consent was obtained.
Inclusion and exclusion criteria.
We included adults with chronic SCI (more than 6 mo) and a comparator group of able-bodied (AB) participants. The chronic SCI group included cervical (C4–C7) and high thoracic levels of SCIs (T1–T6).
Participants were excluded from the study for any of the following: 1) history of cardiac disease, including advanced heart failure, peripheral vascular disease, or stroke; 2) history of head trauma resulting in neurological symptoms or loss of consciousness; 3) advanced lung, liver, or chronic kidney disease; 4) current ventilator dependency or tracheostomy tube; 5) pregnant or lactating women; or 6) other illness that would interfere with completion of the study.
Protocol.
Each participant had an initial screening visit to complete brief medical history and a physical exam. The second visit consisted of an overnight polysomnography (PSG). Zolpidem was administered orally 30 min before the beginning of recordings for the patients who had difficulty sleeping.
PSG.
Participants were asked to arrive at the sleep laboratory in the evening to be instrumented and prepared for PSG. Grass Comet PSG system (AS-40 Model) or the Heritage II PSG System (Grass Technologies Corp., Warwick, RI) and the SomnoStar (CareFusion, San Diego, CA) were used to record PSG with patients in a supine position. The channels included an electrocardiogram, electroencephalogram, electrooculogram, and chin electromyogram (EMG) using the International 10–20 system of electrode placement (electroencephalogram: C3–A2 and C4–A2; electrooculogram: O–A2). Subjects were required to wear a nasal mask (Respironics Profile Lite, Respironics Inc., Murrysville, PA) connected to a pneumotachometer (Model 3700-A, Hans Rudolph Inc., Shawnee, KS) that measured airflow. To minimize leaks, the mask was adjusted and glued in place when required. Supraglottic airway pressure was measured with a pressure-tipped catheter (Millar Instruments, Houston, TX) placed through one nostril and extended down into the hypopharynx at least 2 cm caudal to the visible base of the tongue and superior to the epiglottis. Tidal volume was determined via integration of the pneumotachometer flow signal. Pulse oximetry was measured via ear probe (Biox 3740, Datex-Ohmeda Inc., Madison, WI). By using a PowerLab Data Acquisition System (Model 16-SP, ADInstruments Inc., Colorado Springs, CO) ventilation data from the pneumotachometer, supraglottic catheter, and pulse oximeter were digitized and analyzed. The recording was done on subjects while breathing spontaneously on room air.
Data analysis.
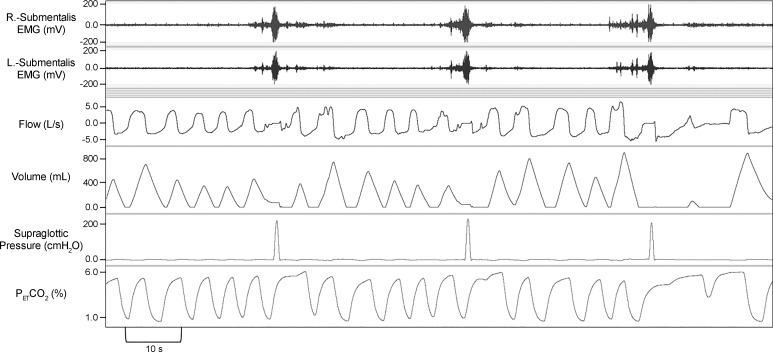
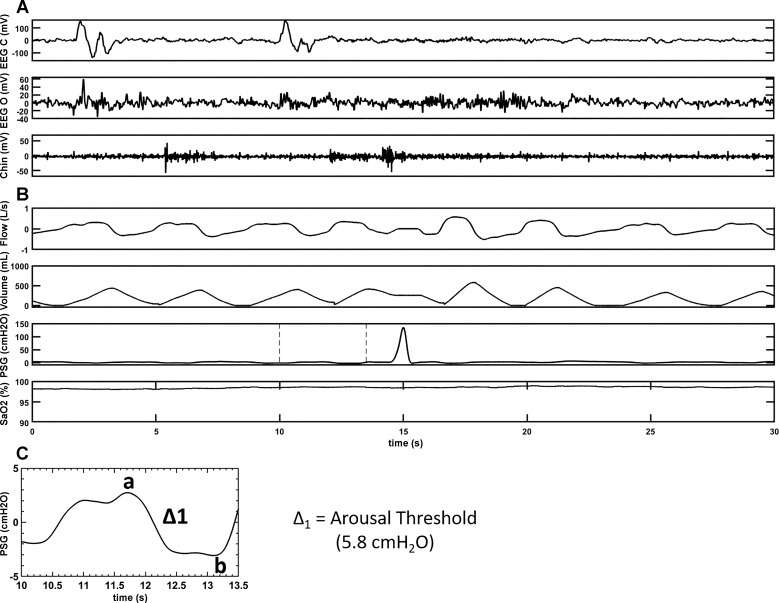
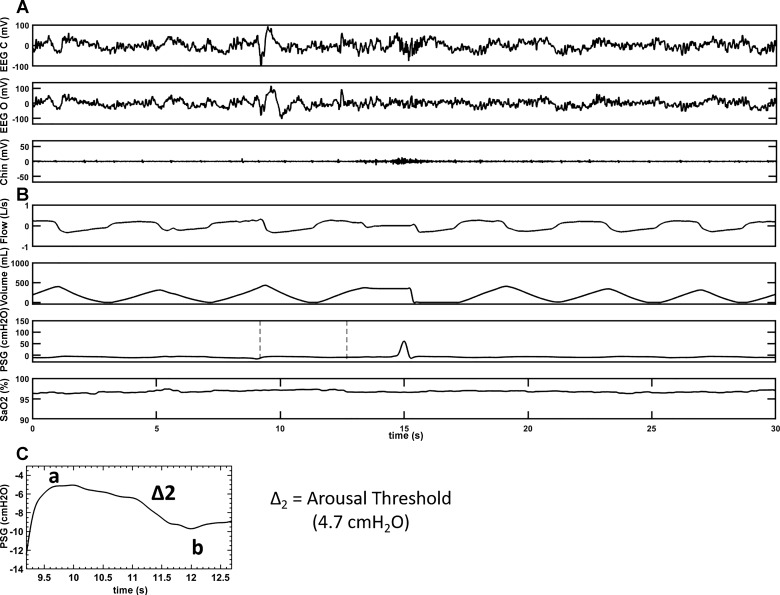
PSGs were scored using the American Academy of Sleep Medicine 2012 recommended criteria (1). Swallowing events (SW) were defined as a positive spike in the supraglottic pressure as was reported in the literature previously (7). Eriksson et al. (7) showed pressure changes during contraction at the upper margin of the tongue base associated with the medial and inferior portion of the pharyngeal constrictor muscle in a paralyzed volunteer. To assess that swallows are represented by a spike in supraglottic pressure, we performed an additional study during wakefulness in the supine position. A simultaneous recording of supraglottic pressure and submental EMG during swallowing was obtained (depicted in Fig. 1). All subjects were first analyzed for a total number of swallows during wake and sleep periods. Each SW was then assessed for the respiratory phases: beginning to peak inspiratory phase, peak to end inspiratory phase, beginning to peak expiratory phase, peak to end-expiratory phase, and sleep stages [rapid eye movement (REM), N1, N2, N3, and wake], arousals, chin muscle activation, and associated respiratory events (hypopnea, obstructive apnea, and central apnea). Arousals that occurred within 0.5 to 1 s preceding or following the SW were determined. In a subset of participants (six SCI and eight AB) arousals preceding the swallow (AS) occurring during sleep were used to calculate the delta supraglottic pressure (ΔPSG), a surrogate of the ArTh. ΔPSG was defined as the magnitude of negative pressure generated in the upper airway (UA) during a respiratory effort, relative to the resting pressure in the UA [the delta pressure swing on supraglottic pressure before an inspiratory effort as depicted point (a) of maximal respiratory effort to point (b); as shown in Figs. 2 and 3] for the breath before arousal. The narrower the delta of supraglottic pressures the lower the ArTh (lower ArTh indicates increased arousal propensity), and vice versa. Respiratory arousals (RAs) were defined as arousals that are related to the respiratory events but not associated with swallows and were identified. With total sleep time (TST), we then calculated swallow index (SI: total number of SW during sleep/TST), swallow arousal index (AS/TST), and respiratory arousal index (RAs/TST).
Fig. 1.
A representative polygraph record in an able-bodied subject during wake demonstrating alignment of supraglottic pressure and submentalis electromyogram (EMG) with instructed swallowing. Swallows are represented by a spike in supraglottic pressure. A disruption of the flow signal can be observed during expiratory phase at the time of the swallows. ; end-tidal pressure of carbon dioxide.
Fig. 2.
A representative polygraph record of a swallow with an AS during sleep in an able-bodied subject. A: brain electrical activity during sleep in two separate EEG channels, EEG(c) [records brain activity from electrode attached to the scalp near central (top) portion of the brain] and EEG(o) [records brain activity from electrode attached to the scalp near occipital (back) portion of the brain], respectively. A also shows muscle tension in the body during sleep via activity in the chin channel. B: respiratory parameters (flow, volume, and SaO2) during sleep along with the supraglottic pressure channel, which is the main channel to depict swallow (a positive spike on the supraglottic pressure channel) during sleep. C: magnification of the highlighted portion of the PSG channel of B to calculate the delta pressure change (Δ1 = a–b) for the breath before arousal preceding a swallow. A and B are representatives of a sleep segment recorded on two separate sleep systems, which are time matched. AS, arousal preceding the swallow; EEG(c), electroencephalogram (central); EEG(o), electroencephalogram (occipital); SaO2, oxygen saturation.
Fig. 3.
A representative polygraph record of a swallow with an AS during sleep in an SCI subject. A: brain electrical activity during sleep in two separate EEG channels, EEG(c) [records brain activity from electrode attached to the scalp near central (top) portion of the brain] and EEG(o) [records brain activity from electrode attached to the scalp near occipital (back) portion of the brain], respectively. A also shows measure of muscle tension in the body during sleep via activity in the chin channel. B: respiratory parameters (flow, volume, and SaO2) during sleep along with the supraglottic pressure channel, which is the main channel to depict swallow (a positive spike on the supraglottic pressure channel) during sleep. C: magnification of the highlighted portion of the supraglottic pressure channel of B to calculate the delta pressure change (Δ2 = a–b) for the breath before AS. A and B are representatives of a sleep segment recorded on two separate sleep systems, which are time-matched. AS arousal preceding the swallow; SCI, spinal cord injury; EEG(c), electroencephalogram (central); EEG(o), electroencephalogram (occipital); SaO2, oxygen saturation.
To determine the effect of eliminating respiratory events using CPAP therapy on the number or pattern of SW, a subanalysis was performed on six SCI and eight AB subjects to identify swallows on CPAP segments (segment of sleep on CPAP is sufficient to eliminate respiratory events) and later compared for SI for the sleep segment with CPAP and without CPAP in both SCI and AB groups.
Three secondary analyses were performed: 1) to determine if swallowing is more common in participants with SCI compared with AB subjects, SI during sleep was compared between participants in each group; 2) to determine if swallowing occurred more during inspiratory or late expiratory phase of respiration, which may contribute to high risk of aspiration, SW in each respiratory phase were compared within each group and between the two groups; and 3) to determine the potential role of ArTh in the contribution of sleep disturbance, ΔPSG was compared in both the subjects with SCI and AB subjects. We calculated ΔPSG for the breath before the AS as shown in Figs. 2 and 3. The ΔPSG (for combined AS and RA) was compared in eight AB participants and six SCI participants.
Statistical analysis.
Two-way repeated measures ANOVA (Sigma Plot 12.1) was performed to determine differences within the group (SCI and AB) and between the groups for nocturnal SW and related ventilatory parameters including ArTh. When appropriate, post hoc pairwise multiple comparisons were made using the Student-Newman-Keuls method. When data were not normally distributed, the appropriate nonparametric analysis was employed. T-tests were used to compare all demographic data between AB and SCI. All data are reported as mean ± SD, and significance was set at P < 0.05.
RESULTS
We studied 29 participants [16 SCI (13 men, 3 women; age 42.5 ± 16.2 yr; body mass index 25.7 ± 5.8 kg/m2) and 13 AB subjects (7 men, 6 women; age 45.4 ± 14.4 yr; body mass index 29.0 ± 3.3 kg/m2)] with SDB and comparable demographics as presented in Table 1 completed in-laboratory polysomnography with the pharyngeal catheter. A summary of sleep characteristics is shown in Table 2.
Table 1.
Patient demographics
| Able-Bodied | SCI | |
|---|---|---|
| Age, yr | 45.4 ± 14.4 | 42.5 ± 16.2 |
| BMI, kg/m2 | 29.0 ± 3.3 | 25.7 ± 5.8 |
| Sex, M/F | 7:6 | 13:3 |
| NC, cm | 37.4 ± 2.7 | 38.2 ± 3.1 |
| Level of injury (C/T) | 8:8 | |
| ASIA: A, % | 62.5 | |
| ASIA: C, % | 18.6 | |
| ASIA: D, % | 6.2 | |
| Ic, % | 12.5 | |
| Zolpidem, Y/N | 0:13 | 8:8 |
All values mean ± SD; n = 13 able-bodied, 16 SCI. ASIA, American Spinal Injury Association; ASIA: A, complete lack of motor and sensory function below the level of injury (including the anal area); BMI, body mass index; ASIA: C, some muscle movement is spared below the level of injury but 50% of the muscles below the level of injury cannot move against gravity; C/T, cervical/thoracic; ASIA: D, most (more than 50%) of the muscles that are spared below the level of injury are strong enough to move against gravity; Ic, incomplete; NC, neck circumference; Zolpidem, Y/N, yes/no (whether a participant required Zolpidem for sleep or not).
Table 2.
Sleep characteristics
| Able-Bodied | SCI | |
|---|---|---|
| W, h | 0.9 ± 0.51 | 1.02 ± 1.04 |
| N1, h | 0.9 ± 0.56 | 0.62 ± 0.52 |
| N2, h | 1.78 ± 1.09 | 1.35 ± 0.66 |
| N3, h | 0.62 ± 0.81 | 0.55 ± 0.68 |
| R, h | 0.21 ± 0.23 | 0.17 ± 0.40 |
| TST, h | 3.2 ± 2.1 | 2.7 ± 1.3 |
| SI, swallows/h | 3.3 ± 4.5 | 5.1 ± 5.0 |
| Total AI, arousals/h | 34.6 ± 18.5 | 38.1 ± 25.3 |
| SAI, arousals with swallow/h | 2.3 ± 2.5 | 4.6 ± 5.1 |
| AHI, events/h | 15.5 ± 13.2 | 27.7 ± 25.9 |
| ODI, desaturation/h | 1.93 ± 3.1 | 12.4 ± 17.1* |
| OAI, events/h | 5.0 ± 6.5 | 9.2 ± 20.9 |
All values mean ± SD; n = 13 able-bodied, 16 SCI. AHI, apnea-hypopnea index; N1, non-REM stage 1; N2, non-REM stage 2; N3, non-REM stage 3; OAI, obstructive apnea index; ODI, oxygen desaturation index; R, REM; REM, rapid eye movement; SAI, swallow arousal index; SCI, spinal cord injury; SI, swallow index; Total AI, total arousal index; TST, total sleep time; W, wake.
P < 0.05 for SCI vs. AB.
SW and sleep stages.
The analysis for SI for SCI and AB groups is summarized according to wake-sleep stages in Fig. 4. The SI was higher in the SCI group compared with AB during wake and different sleep stages (P < 0.05).
Fig. 4.
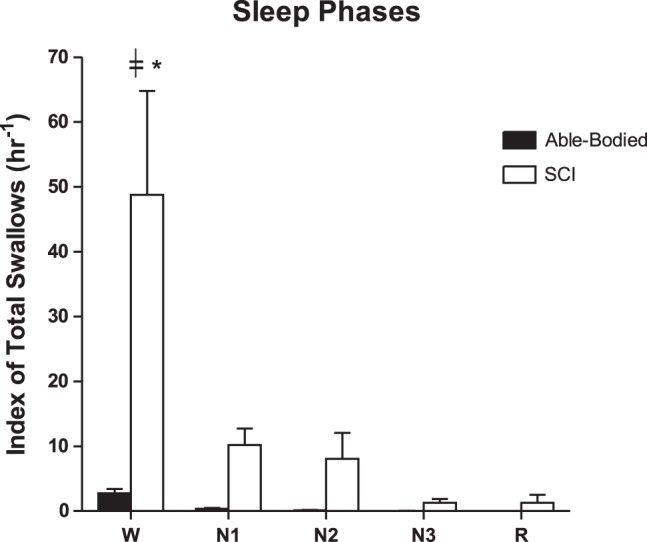
A representative summary of the index of total swallows (hr−1) for each sleep phase (W, N1, N2, N3, and R) in the subjects with SCI (n = 16) vs. able-bodied (AB; n = 13) subjects. *Number of swallows in the W phase is significantly higher in the subjects with SCI compared with the AB subjects (P < 0.05). ╪Wake phase is significantly higher (P < 0.001) than other event phases. The statistical test used is two-way repeated measures ANOVA (F = 76.1, P = 0.001 for sleep phase factor). All representative data are mean ± SE. N1, non-REM stage1; N2, non-REM stage2; N3, non-REM stage3; R, REM; REM, rapid eye movement; SCI, spinal cord injury; W, Wake.
Respiratory phases of SW.
As demonstrated in Fig. 5 the majority of SW occurred at end expiration (phase 4 of respiration; P < 0.05) in the SCI group. However, there was no significant difference between the two groups (P > 0.05) or within the different respiratory phases within the AB group (P > 0.05).
Fig. 5.
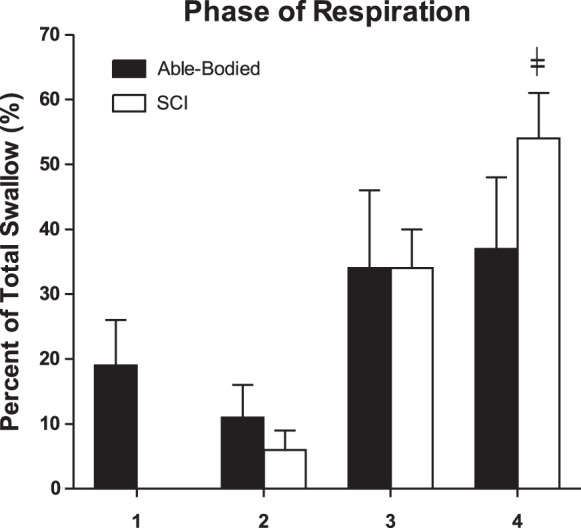
A representative summary of the percent of total swallows (%) for each phase of respiration (1–4) in the subjects with SCI (n = 16) vs. able-bodied (n = 13) subjects. ╪Number of swallows is significantly higher for the phase 4 (late expiratory phase; P < 0.05), but no significant difference is noted between the subjects with SCI and able-bodied subjects. The statistical test used is two-way repeated measures ANOVA (F = 15.7, P < 0.05 for respiratory cycle phase factor). All representative data are mean ± SE. 1, beginning of inspiration to peak of inspiration; 2, peak of inspiration to end of inspiration; 3, beginning of expiration to peak of expiration; 4, peak of expiration to end of expiration; SCI, spinal cord injury.
Relationship between ΔPSG and arousals related to SW.
The summary data of ΔPSG for both the SCI and AB subjects is depicted in Fig. 6. The mean ΔPSG was significantly narrower in the SCI group in comparison to the AB group (P < 0.05). No significant correlation was found between arousal index and SI (P > 0.05) for either the SCI and AB subjects.
Fig. 6.
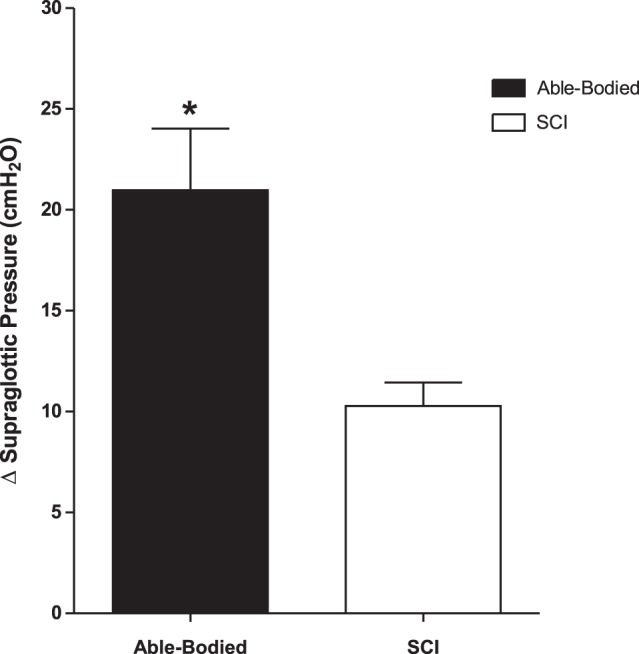
A summary of the delta (Δ) supraglottic pressure in the SCI (n = 6) vs. able-bodied (n = 8) subjects. *Δ supraglottic pressure is significantly reduced in the SCI subjects compared with able-bodied subjects (P < 0.05). Statistical test used is unpaired t-test (t = 2.9, P < 0.05). All representative data are mean ± SE. SCI, spinal cord injury.
Effect of CPAP on sleep and swallowing.
To determine the effect of CPAP on nocturnal swallowing, we studied sleep segments on CPAP vs. off CPAP in the SCI and AB subjects. We noted that CPAP was associated with decreased SI in the SCI but not in the AB group; SI during sleep on CPAP was significantly lower (P < 0.05) when compared with the period of sleep without CPAP in the same SCI group (Table 3).
Table 3.
Subanalysis (SI for sleep segments with CPAP vs. without CPAP in SCI and able-bodied subjects)
| CPAP (swallows/h) | No CPAP (swallows/h) | P Value | |
|---|---|---|---|
| Able-bodied | 2.35 ± 2.22 | 4.38 ± 5.55 | 0.36 |
| SCI (C/T) | 0.29 ± 0.70* | 5.68 ± 2.74 | 0.006 |
All values are mean ± SD; n = 8 able-bodied, 6 SCI. CPAP, continuous positive airway pressure; SCI (C/T), cervical/thoracic spinal cord injury; SI, swallow index.
The statistical test used is two-way paired t-test.
P < 0.05 for SI with CPAP in SCI group.
DISCUSSION
Our study demonstrated several significant findings regarding the frequency and pattern of nocturnal swallowing in individuals with SCI and SDB in comparison to the AB controls: 1) nocturnal swallowing in SCI subjects was more common than AB controls, during wake and sleep; 2) swallowing was predominantly observed during the late expiratory phase; 3) swallowing frequency during wakefulness was significantly higher in the SCI subjects compared with the AB subjects; 4) in comparison to AB controls, ΔPSG before arousal was narrower in SCI and therefore ArTh was lower, which indicates increased arousal propensity.
Characteristics of swallowing during sleep.
A tight temporal coordination between pharyngeal-laryngeal swallowing dynamics and respiration is usually maintained in healthy adults to prevent aspiration (14, 16, 17) via vagal glottal closure reflexes (32). This preferential coupling of swallowing has been shown previously to increase during expiration relative to other phases of respiration during water swallows and tachypnea (33). We noted that swallowing during supine sleep occurred predominantly in the expiratory phases of the respiratory cycle in both groups (AB and SCI). However, more than 50% of the swallowing occurred at end expiration in the SCI group (phase 4, Fig. 4). This is in contrast to previous studies during wakefulness in healthy, seated volunteers, which demonstrated that swallowing occurred predominantly during midexpiratory phases (20). In addition, swallowing in awake healthy volunteers is associated with a transient respiratory pause or apnea associated with active breath holding and increased abdominal EMG activity (12). These physiological associations preserve lung volume and minimize the risk of aspiration following a swallow. This coordination of respiration and swallowing is affected by aging and comorbidities (33). For instance, age, head and neck cancer, and chronic obstructive pulmonary disease alter this temporal coordination of swallowing (19). Furthermore, swallowing has been found to be significantly impaired in patients with central nervous system diseases such as stroke, Parkinsonism, and dementia, etc. (13). It is important, however, to note the lack of uniformity in the literature regarding the determination of respiratory phase (12, 16, 27, 28). In the present study, the respiratory phase was determined using pneumotachography, the gold standard method for detection of flow, as well as measurement of pharyngeal pressure, which allows for a precise determination of the timing of swallowing within the respiratory cycle.
Our study also demonstrated an association between nocturnal swallowing and arousal. Specifically, swallowing was either preceded or followed by an arousal in most instances in both the SCI and AB subjects. However, the ArTh (for AS during sleep) was significantly lower (narrowed ΔPSG; P < 0.05) in the SCI group compared with the AB subjects. Reduced ArTh may contribute to sleep fragmentation in this population. Conversely, higher ArTh may mitigate the effect of GERD by enhancing clearance of acid reflux on the UA, particularly when esophageal acid clearance is significantly prolonged.
Our study demonstrated that the application of CPAP was associated with significant decrease in SI in the SCI group. The mechanism for the reduced swallows during the application of CPAP could be related to the effect of positive pressure on 1) lung inflation, which inhibits the swallowing reflex and/or 2) on elevating ArTh. First, the effect of lung inflation on swallowing is demonstrated by prolonged swallowing latency following a bolus of distilled water and the decreased number of swallows (21). Furthermore, there is evidence that changes in lung volume induced by the application of negative extrathoracic pressure can alter the timing of swallowing in reference to the respiratory cycle (15). Second, CPAP may decrease swallowing by altering the ArTh. Previous studies have shown that the ArTh is one of the physiological traits that contribute to the pathogenesis of obstructive sleep apnea (OSA) and can be a useful therapeutic target in one-third of patients with OSA (5). However, CPAP elevated the ArTh in patients with severe OSA during non-REM sleep (11), which may explain the reduced frequency of swallows on CPAP in SCI.
Clinical and therapeutic implications.
Our findings have significant implications related to the effect of swallowing in patients with SCI. First, sleep fragmentation because of frequent swallowing associated arousal perpetuates breathing instability via episodes of hyperpnea, hypocapnia, and recurrent central apnea/hypopnea. Recurrent central apnea or hypopnea may be associated with UA narrowing or closure (31). Therefore, elevated ArTh may contribute to the pathogenesis of SDB in this population. Second, decreased swallow frequency while on CPAP may provide another potential CPAP therapeutic benefit by interrupting the vicious cycle of swallowing-arousal-hyperpnea-hypopnea. Finally, decreasing swallowing-related arousals may mitigate heart rate variability, a common consequence of respiratory vents (26). Overall, it is plausible that decreasing swallowing-related arousals with CPAP therapy may represent a heretofore unrecognized benefit of positive airway pressure therapy in patients with SDB.
Methodological considerations.
Our laboratory has extensive experience in measuring UA mechanics in healthy volunteers and in patients with SCI (24–26, 31). Nevertheless, several considerations may influence the interpretation of the findings. First, the sample size of this study is relatively small, which may limit the ability to interpret negative results due to lack of sufficient power. This may explain some of the results, particularly in the AB group, and the effect of CPAP application on ArTh and swallows. Second, some of the participants in the SCI group were studied during sleep after the administration of Zolpidem because of difficulty sleeping with heavy instrumentation. Zolpidem is less likely to explain the reduced ArTh observed in the SCI group compared with AB group given that the hypnotic effect of this drug has been shown to elevate rather than reduce the ArTh during sleep (2). Third, we assessed swallowing using a standard pharyngeal pressure catheter method; however, the swallow pressure signal was directly validated with submental EMG signal (Fig. 1). Future studies are needed to assess the role of UA muscles and their function in the pathogenesis of swallowing in SCI.
In summary, nocturnal swallowing is commonly found in patients with SCI and occurs predominantly during the late expiratory phase. The lower ArTh associated with swallowing in patients with SCI may contribute to the genesis of sleep disturbances in this group of patients.
GRANTS
This work was supported by Career Development Award no. 1IK2CX000547 (to A. Sankari) from the Clinical Science Research & Development Service of the Veterans Affairs (VA) Office of Research and Development from the United States Department of Veterans Affairs, Merit Review Award no. 1I01CX001040 from the Clinical Science Research & Development Service of the VA Office of Research and Development, and Department of Veterans Affairs and the National Heart, Lung, and Blood Institute Award no. R01HL130552 (to M. Badr). The authors are also supported by the National Heart, Lung, and Blood Institute Award no. R21HL140447 (to A. Sankari), Department of Defense no. SC150201 (to M. Badr), and the Rehabilitation Research & Development Service of the VA Office of Research and Development, Department of Veterans Affairs no. 1I01RX002116 (to M. Badr).
DISCLAIMERS
There was no off-label or investigational use. The opinions expressed in this article reflect those of the authors and do not necessarily represent official views of the Department of Veterans Affairs (VA). This is not a clinical trial study, and therefore no clinical trial registration was obtained.
DISCLOSURES
Patent pending to assess the role of autonomic heart rate alterations during sleep in diagnosis of sleep-disordered breathing. The authors have indicated no financial conflicts of interest.
AUTHOR CONTRIBUTIONS
A.S. and M.S.B. conceived and designed research; A.T.B. performed experiments; A.R. and A.S. analyzed data; A.R., A.S., and M.S.B. interpreted results of experiments; A.R. and S.V. prepared figures; A.R. drafted manuscript; A.R., A.S., S.V., and M.S.B. edited and revised manuscript; A.R., A.S., and M.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Nicole Nickert, Sukanya Pranathiageswaran, and Geoffrey Ginter for technical assistance. The authors thank all the subjects in this study.
REFERENCES
- 1.Berry R, Brooks R, Gamaldo C, Harding S, Marcus C, Vaughn B. The AASM Manual for The Scoring of Sleep and Associated Events: Rules, Terminology And Technical Specifications, Version 2.0. Darien, Illinois: American Academy of Sleep Medicine, 2012, p. 47. [Google Scholar]
- 2.Carberry JC, Fisher LP, Grunstein RR, Gandevia SC, McKenzie DK, Butler JE, Eckert DJ. Role of common hypnotics on the phenotypic causes of obstructive sleep apnoea: paradoxical effects of zolpidem. Eur Respir J 50: 1701344, 2017. doi: 10.1183/13993003.01344-2017. [DOI] [PubMed] [Google Scholar]
- 3.Chaw E, Shem K, Castillo K, Wong SL, Chang J. Dysphagia and associated respiratory considerations in cervical spinal cord injury. Top Spinal Cord Inj Rehabil 18: 291–299, 2012. doi: 10.1310/sci1804-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donner MW, Silbiger ML. Cinefluorographic analysis of pharyngeal swallowing in neuromuscular disorders. Am J Med Sci 251: 600–616, 1966. doi: 10.1097/00000441-196605000-00013. [DOI] [PubMed] [Google Scholar]
- 5.DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 74: 248–254, 1993. [PubMed] [Google Scholar]
- 6.Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, Bakker JP, Genta PR, Owens RL, White DP, Wellman A, Malhotra A. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med 190: 1293–1300, 2014. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson LI, Sundman E, Olsson R, Nilsson L, Witt H, Ekberg O, Kuylenstierna R. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology 87: 1035–1043, 1997. doi: 10.1097/00000542-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 43: 408–416, 2005. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaude GS. Pulmonary manifestations of gastroesophageal reflux disease. Ann Thorac Med 4: 115–123, 2009. doi: 10.4103/1817-1737.53347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green BT, Broughton WA, O’Connor JB. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continuous positive airway pressure. Arch Intern Med 163: 41–45, 2003. doi: 10.1001/archinte.163.1.41. [DOI] [PubMed] [Google Scholar]
- 11.Haba-Rubio J, Sforza E, Weiss T, Schröder C, Krieger J. Effect of CPAP treatment on inspiratory arousal threshold during NREM sleep in OSAS. Sleep Breath 9: 12–19, 2005. doi: 10.1007/s11325-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 12.Hårdemark Cedborg AI, Sundman E, Bodén K, Hedström HW, Kuylenstierna R, Ekberg O, Eriksson LI. Co-ordination of spontaneous swallowing with respiratory airflow and diaphragmatic and abdominal muscle activity in healthy adult humans. Exp Physiol 94: 459–468, 2009. doi: 10.1113/expphysiol.2008.045724. [DOI] [PubMed] [Google Scholar]
- 13.Hadjikoutis S, Pickersgill TP, Dawson K, Wiles CM. Abnormal patterns of breathing during swallowing in neurological disorders. Brain 123: 1863–1873, 2000. doi: 10.1093/brain/123.9.1863. [DOI] [PubMed] [Google Scholar]
- 14.Kelly BN, Huckabee M-L, Jones RD, Carroll GJ. The influence of volition on breathing-swallowing coordination in healthy adults. Behav Neurosci 121: 1174–1179, 2007. doi: 10.1037/0735-7044.121.6.1174. [DOI] [PubMed] [Google Scholar]
- 15.Kijima M, Isono S, Nishino T. Modulation of swallowing reflex by lung volume changes. Am J Respir Crit Care Med 162: 1855–1858, 2000. doi: 10.1164/ajrccm.162.5.2005106. [DOI] [PubMed] [Google Scholar]
- 16.Klahn MS, Perlman AL. Temporal and durational patterns associating respiration and swallowing. Dysphagia 14: 131–138, 1999. doi: 10.1007/PL00009594. [DOI] [PubMed] [Google Scholar]
- 17.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol (1985) 76: 714–723, 1994. doi: 10.1152/jappl.1994.76.2.714. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Harris B, McFarland D, Hill EG, Strange CB, Focht KL, Wan Z, Blair J, McGrattan K. Respiratory-swallow training in patients with head and neck cancer [PMID: 25498307]. [PMCID: PMC4410058]. Arch Phys Med Rehabil 96: 885–893, 2015. doi: 10.1016/j.apmr.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarland DH, Martin-Harris B, Fortin AJ, Humphries K, Hill E, Armeson K. Respiratory-swallowing coordination in normal subjects: lung volume at swallowing initiation. Respir Physiol Neurobiol 234, Suppl C: 89–96, 2016. doi: 10.1016/j.resp.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino T, Sugimori K, Kohchi A, Hiraga K. Nasal constant positive airway pressure inhibits the swallowing reflex. Am Rev Respir Dis 140: 1290–1293, 1989. doi: 10.1164/ajrccm/140.5.1290. [DOI] [PubMed] [Google Scholar]
- 23.Rizwan AS, Sankari A, Bascom AT, Safwan Badr M. Nocturnal Swallowing in Patients with Chronic Spinal Cord Injury (2015 ed) Sleep, 2015, p. 2. [Google Scholar]
- 24.Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med 10: 65–72, 2014. doi: 10.5664/jcsm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sankari A, Martin JL, Bascom AT, Mitchell MN, Badr MS. Identification and treatment of sleep-disordered breathing in chronic spinal cord injury. Spinal Cord 53: 145–149, 2015. doi: 10.1038/sc.2014.216. [DOI] [PubMed] [Google Scholar]
- 26.Sankari A, Pranathiageswaran S, Maresh S, Hosni AM, Badr MS. Characteristics and consequences of non-apneic respiratory events during sleep. Sleep 40: 2017. doi: 10.1093/sleep/zsw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selley WG, Flack FC, Ellis RE, Brooks WA. Respiratory patterns associated with swallowing: Part 1. The normal adult pattern and changes with age. Age Ageing 18: 168–172, 1989. doi: 10.1093/ageing/18.3.168. [DOI] [PubMed] [Google Scholar]
- 28.Shaw SM, Martino R. The normal swallow: muscular and neurophysiological control. Otolaryngol Clin North Am 46: 937–956, 2013. doi: 10.1016/j.otc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd KL, Holloway RH, Hillman DR, Eastwood PR. The impact of continuous positive airway pressure on the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 292: G1200–G1205, 2007. doi: 10.1152/ajpgi.00476.2006. [DOI] [PubMed] [Google Scholar]
- 30.Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord 38: 604–610, 2000. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- 31.Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. Am J Respir Crit Care Med 179: 313–319, 2009. doi: 10.1164/rccm.200805-741OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaker R, Ren J, Kern M, Dodds WJ, Hogan WJ, Li Q. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol 262: G621–G628, 1992. doi: 10.1152/ajpgi.1992.262.4.G621. [DOI] [PubMed] [Google Scholar]
- 33.Shaker R, Li Q, Ren J, Townsend WF, Dodds WJ, Martin BJ, Kern MK, Rynders A. Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol Gastrointest Liver Physiol 263: G750–G755, 1992. doi: 10.1152/ajpgi.1992.263.5.G750. [DOI] [PubMed] [Google Scholar]