Abstract
Preeclampsia is a hypertensive disorder of pregnancy characterized by new-onset hypertension, proteinuria, and edema occurring after 20 wk of gestation, with a prevalence of ~7–10% of pregnancies in the United States and ~8 million pregnancies worldwide. Despite the postpartum remission of preeclamptic symptoms, women who have had preeclampsia are two to four times more likely to develop cardiovascular disease (CVD) and are significantly more likely to die of CVD compared with women with a history of normal pregnancy. Although the relation between history of preeclampsia and elevated CVD risk is well documented, the mechanism(s) underlying this association remains unclear. One hypothesis explaining this association is that the initial vascular damage and dysfunction sustained during the preeclamptic pregnancy persist chronically. Indeed, even in the absence of, or in advance of, overt CVD women who have had preeclampsia have compromised vascular endothelial function. Emerging mechanistic studies in these women have provided some insight into the underlying mechanisms of this persistent vascular dysfunction and have begun to identify potential therapeutic targets for the prevention or mitigation of CVD progression in this vulnerable population. This review summarizes the existing literature examining vascular function and dysfunction in women with a history of preeclampsia and highlights future directions for mechanistic investigations and development of novel intervention strategies aimed at halting or slowing the progression of CVD in these women.
Keywords: cardiovascular disease risk, endothelium, preeclampsia, vascular dysfunction
INTRODUCTION
Preeclampsia is a hypertensive disorder of pregnancy that affects ~7–10% of all pregnancies in the United States and ~8 million pregnancies worldwide (91). Preeclampsia is diagnosed after the 20th week of pregnancy by new-onset hypertension (i.e., systolic and diastolic blood pressures ≥140 and ≥90 mmHg, respectively) with either proteinuria or, in the absence of proteinuria, new onset of thrombocytopenia, renal insufficiency, abnormal liver function, pulmonary edema, and/or cerebral or visual symptoms (6). Despite the remission of clinical symptoms of preeclampsia postpartum, otherwise healthy women who develop preeclampsia during pregnancy are at a significantly (2–4 times) greater risk for the development of cardiovascular disease (CVD) (12). These women develop primary hypertension at a younger age (~30–40 yr of age vs. ~50–60 yr in women who have a normal pregnancy) and with greater frequency than women who have healthy pregnancies (2, 35, 44, 63), and they are significantly more likely to die of stroke, myocardial infarction, and end-stage renal disease (5, 7, 52). Although the association between history of preeclampsia and elevated CVD risk is well established, the mechanisms underlying this increased risk remain unclear.
Over the past decade, studies characterizing vascular function in women who have had preeclampsia have consistently found a residual degree of vascular dysfunction postpartum (38). These findings suggest that residual effects of the preeclamptic pregnancy undermine normal vascular function in these women, even in the absence of clinical symptoms of CVD, and it may be that this underlying dysfunction leads to the accelerated development of overt CVD in women who have had preeclampsia. More recently, studies have sought to delineate the mechanistic underpinnings of this persistent vascular dysfunction, shedding light on potential therapeutic targets for the prevention or mitigation of CVD progression in these women. However, additional research is necessary to fully understand the signaling cascade(s) associated with this dysfunction and how best to target them clinically. Therefore, the purpose of this review is to summarize the existing literature examining vascular function and dysfunction in women with a history of preeclampsia and to highlight future directions for mechanistic investigations and development of novel intervention strategies aimed at halting or slowing the progression of CVD in these women.
VASCULAR DYSFUNCTION DURING PREECLAMPSIA
Although a comprehensive discussion of the maternal vascular dysfunction that occurs during preeclampsia is outside the scope of this review, a general understanding of the underlying mechanisms causing this dysfunction provides a foundation for the literature examining persistent postdelivery vascular dysfunction in these women. While the initiating factor(s) causing preeclampsia remain elusive, placental ischemia resulting from insufficient cytotrophoblast invasion of the uterine spiral arteries with impaired placental blood flow is central to the disorder (58, 62, 69). As a result of underperfusion of the placenta, placental ischemia/hypoxia triggers the release of antiangiogenic factors [soluble fms-like tyrosine kinase-1 (sFlt-1), soluble endoglin], inflammatory mediators, and reactive oxygen species, which enter the maternal circulation. These factors are thought to mediate the multifaceted maternal syndrome through their effects on systemic maternal endothelial function (18, 93). The clinical symptoms of preeclampsia (hypertension, proteinuria, headaches/blurred vision, edema, etc.) may all be explained by acute vascular injury during the preeclamptic pregnancy. Indeed, women who have preeclampsia have blunted conduit artery endothelium-dependent vasodilation measured by flow-mediated dilation during the affected pregnancy (41, 68, 94).
The mechanisms mediating vascular dysfunction during preeclampsia have received a great deal of attention, and the development of animal models of preeclampsia facilitated a number of vascular investigations that were not possible in pregnant humans (60). These studies suggest that oxidative stress, immune activation and inflammation (54, 56), and subsequent changes in endothelin-1 (ET-1) (4, 59, 66, 73, 89) and angiotensin II (ANG II) (25, 55, 95) signaling play a major role in the vascular dysfunction observed during preeclampsia. These studies have been supported by human studies that have demonstrated elevated agonistic antibody for the ANG II type 1 receptor (AT1R-AA) (82, 92), elevated ET-1 (14), and elevated inflammatory markers (23, 88) and measures of oxidative stress (27) in the plasma of women during a preeclamptic pregnancy. Taken together, the extant literature demonstrates that an imbalance between endothelium-derived relaxing factors and endothelium-derived contracting factors results in a proconstrictor state of endothelial dysfunction in preeclampsia. For a more detailed discussion of these mechanisms, the reader is referred to one of these excellent reviews on the topic (18, 36, 93).
VASCULAR DYSFUNCTION AFTER PREECLAMPSIA
Epidemiological evidence identifying preeclampsia as an independent risk factor for CVD death (hazard ratio 2.14), further elevated with early-onset preeclampsia (diagnosed ≤34 wk gestation; hazard ratio 9.54), suggests that persistent cardiovascular dysfunction after the affected pregnancy is directly related to the severity of the preeclampsia (63, 72). Women who have a history of preeclampsia have subclinical atherosclerosis and are much more likely to be treated for primary hypertension ≥10 yr after the affected pregnancy, a trend that persists throughout and after the menopausal transition (20, 34, 61). Aside from elevated incidence of primary hypertension, it is important to note that women with a history of preeclampsia also have a significantly higher incidence of type 2 diabetes and hypercholesterolemia as well as increased prothrombotic risk factors years after the affected pregnancy (79, 86). These associations persist even when adjusting for confounding conditions before pregnancy, and it is likely that these elevations in traditional CVD risk factors further contribute to endothelial dysfunction and CVD risk in these women across the life span.
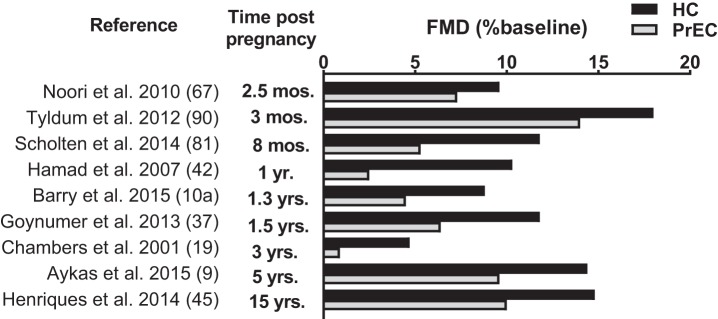
Recently, Barnes et al. demonstrated that cerebral vascular reactivity is attenuated in postmenopausal women with a history of preeclampsia (10). Although the mechanisms contributing to this dysfunction have not been identified, vascular dysfunction is evident in the cerebral microvascular bed years beyond the affected pregnancy. In studies that have assessed arterial stiffness in women with a history of preeclampsia, pulse wave velocity and the augmentation index were elevated in these women compared with age- and parity-matched control subjects (21, 28, 29, 57, 70, 98). Similarly, studies assessing structural changes in the vessels of women who have had preeclampsia demonstrate an increase in carotid intima-media thickness, an early measure of atherosclerosis (3, 9, 15, 37). Collectively, it is abundantly clear that changes in microvascular function as well as macrovascular stiffness and structure persist after a preeclamptic pregnancy. Studies of endothelial function in women with a history of preeclampsia have primarily been conducted in large conduit arteries assessed noninvasively by brachial artery flow-mediated dilation or invasively through arterial administration of the endothelium-dependent agonist acetylcholine. Overwhelmingly, these studies demonstrate attenuated endothelium-dependent vasodilation in women who have had preeclampsia (1, 9, 19, 42). This dysfunction is present immediately after delivery (1–3 mo postpartum) (64, 67, 90), persists throughout the years following the pregnancy (19, 37, 42), and is even detectable 15 yr postpartum (45) (Fig. 1).
Fig. 1.
Measures of flow-mediated dilation (FMD) in otherwise healthy women who have had preeclampsia (PrEC) and control subjects who have had a healthy pregnancy (HC) by time since pregnancy. Overwhelmingly, women who have had preeclampsia demonstrate a significantly reduced FMD response, indicative of reduced endothelial function. This attenuation is present immediately after delivery (≤2.5 mo) and persists up to 15 yr after the affected pregnancy.
Interestingly, although attenuations in vascular function can be detected in women with prior preeclampsia compared with parity-matched control subjects across the life span, the differences in function between these groups are most pronounced in younger women (38, 94). For example, large differences in endothelial function are detectable between formerly preeclamptic and control women tested in their 30s and 40s, whereas smaller, although still significant, differences between these groups are detected later in life. This may be explained by the damage that is present immediately after complicated pregnancy, during a women’s reproductive years, a period when traditional CVD risk factors are generally low and women are relatively protected from CVD (13). Over time, the immergence of traditional atherosclerotic risk factors and/or the initiation of therapeutic treatments for overt CVD may begin to close the gap in vascular function between groups.
Complementary to direct measures of cardiovascular function, measures of serum biomarkers of dysregulated angiogenesis and inflammation in women with a history of preeclampsia substantiate the increased risk of CVD morbidity and mortality in these women. Because they are elevated and have been shown to play a role in vascular dysfunction during preeclampsia, vascular endothelial growth factor (VEGF) and sFlt-1 have been the most commonly assessed markers of angiogenesis in this cohort. The results of these analyses have been equivocal, with some studies reporting elevated serum biomarkers and others failing to detect differences between previously preeclamptic women and matched control subjects. In a recent meta-analysis, Grand’Maison et al. found that mean concentrations of sFlt-1 were modestly higher in women with a history of hypertensive pregnancy, whereas there was no difference from matched control subjects in plasma VEGF (38). This same meta-analysis found no group differences in markers of vascular inflammation (soluble ICAM-1, soluble VCAM-1) examined in the pooled analysis; however, the authors noted that there were not enough studies in this area to assess publication bias (38). The authors hypothesized that these serum biomarkers, which are expressed in the placental tissue rather than the endothelium, likely contribute to endothelial damage during the preeclamptic pregnancy but do not remain significantly elevated after the affected pregnancy. It is possible that the vascular damage sustained during the preeclamptic pregnancy persists and contributes to its own cascade in the progression of CVD in these women.
POTENTIAL MECHANISMS CONTRIBUTING TO VASCULAR DYSFUNCTION AFTER PREECLAMPSIA
Studies examining the mechanisms underlying vascular dysfunction in women who have had preeclampsia are sparse. However, in light of the sobering epidemiological evidence supporting the increased risk of CVD morbidity and mortality in this population, these questions have recently received new attention in the form of mechanistic in vivo studies aimed at identifying aberrant mechanisms and potential therapeutic targets.
Reductions in Nitric Oxide-Mediated Dilation
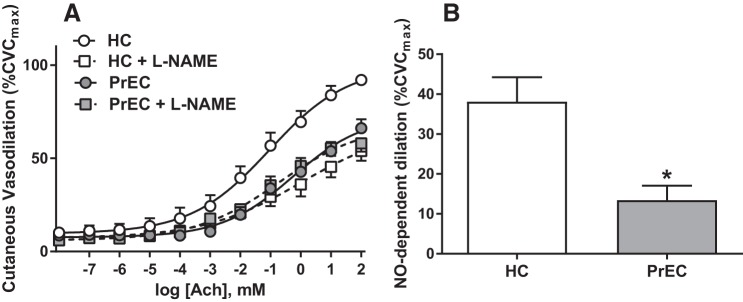
Our laboratory recently performed a series of studies examining microvascular function in vivo in postpartum women who had preeclampsia. Utilizing the cutaneous circulation as a model for microcirculatory vascular function (47), we demonstrated that women who recently had preeclampsia (tested an average of 8 mo postpartum) had attenuated endothelium-dependent vasodilatory responses to acetylcholine despite the remission of overt clinical symptoms of preeclampsia (84). Furthermore, we demonstrated that this attenuation in endothelium-dependent vasodilation was mediated, at least in part, by reductions in nitric oxide-dependent dilation (Fig. 2). However, although functional nitric oxide-dependent dilation was attenuated, we did not find biochemical changes in endothelial nitric oxide synthase or vasodilator-stimulated phosphoprotein (VASP) expression or phosphorylation, suggesting that mechanisms secondary to nitric oxide production and diffusion to the vascular smooth muscle (such as increased vasoconstrictor tone, exaggerated inflammatory signaling, elevated reactive oxygen species, etc.) contribute to endothelial dysfunction in these women (84).
Fig. 2.
Endothelial dysfunction measured in the cutaneous microvasculature of women who have had preeclampsia. Compared with women who had a healthy pregnancy (HC), women who had preeclampsia (PrEC) have an attenuated cutaneous vasodilation response to the endothelium-dependent agonist acetylcholine (ACh). A: there were no differences between the groups in response to ACh with coinfusion of the nonspecific nitric oxide (NO) synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME). B: calculated % NO-dependent vasodilation at the highest dose of ACh was attenuated in PrEC. *P < 0.05 vs. HC. CVCmax, maximum cutaneous vascular conductance. These data demonstrate an attenuated endothelial function in the microvasculature of otherwise healthy women who have had preeclampsia. Reprinted with permission from Stanhewicz et al., Hypertension 70: 382–389, 2017 (84).
Angiotensin II Signaling
Given that both ANG II and ET-1 signaling pathways have been implicated in the vascular dysfunction observed both in preeclamptic women and in animal models of preeclampsia, investigations of these pathways in postpartum women have logically followed. Initial studies of ANG II sensitivity demonstrated that women with a history of preeclampsia had an exaggerated pressor response to systemic infusion of ANG II (77). Directly translating the mechanistic findings from animal studies to our human model, we found that women who have had preeclampsia had an increased microvascular vasoconstrictor sensitivity to ANG II (84) (Fig. 3). Furthermore, this increase in vasoconstrictor sensitivity was specific to ANG II-dependent mechanisms, as formerly preeclamptic women did not demonstrate an increased sensitivity to the adrenergic agonist norepinephrine (84). Additionally, we found that women who had preeclampsia also had increased protein expression of the ANG II type-1 receptor (AT1R) and pharmacological inhibition of AT1R with losartan improved endothelium-dependent dilation and nitric oxide-dependent dilation in these women (Fig. 3) (84). Interestingly, women who had preeclampsia did not have elevated circulating ANG II; however, plasma AT1R-AA has been shown to remain elevated after preeclampsia (49). It is uncertain how these autoantibodies interact with, and/or contribute to, increased sensitivity of the AT1R and tonic constrictor tone in this group of women. Collectively, these findings suggest that dysregulation of the ANG II signaling cascade contributes to persistent microvascular dysfunction in women who have had preeclampsia and raise a number of follow-up questions regarding the interplay of circulating neurohumoral factors and intracellular mechanisms specific to ANG II in the accelerated progression of CVD in these women.
Fig. 3.
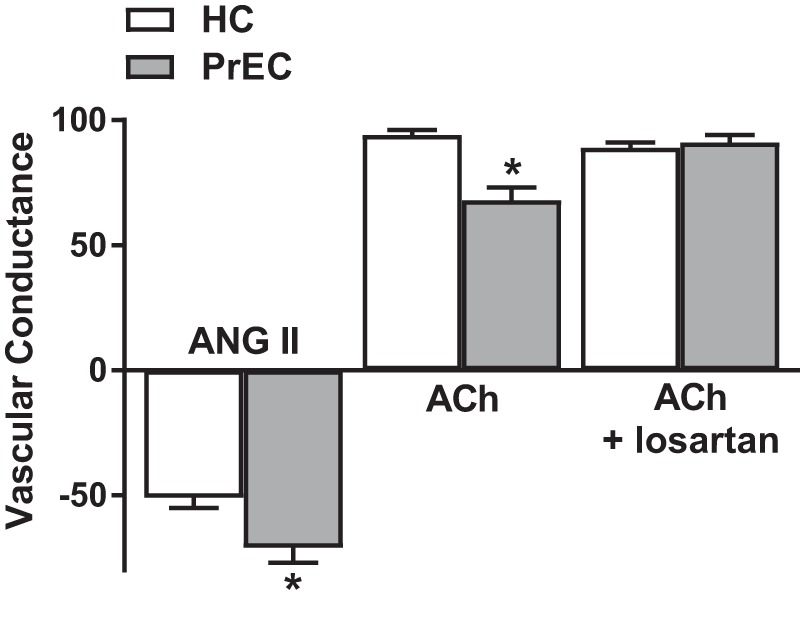
Increased sensitivity to angiotensin II (ANG II) contributes to endothelial dysfunction in women who have had preeclampsia. Compared with women who had a healthy pregnancy (HC), women who had preeclampsia (PrEC) have an exaggerated vasoconstriction response to ANG II (10−4 M) measured in the cutaneous microvasculature. Coinfusion of the ANG II type 1 receptor antagonist losartan with the endothelium-dependent agonist acetylcholine (ACh, 10−1 M) improved the microvascular dilation response in PrEC. but had no effect on HC. *P < 0.05 vs. HC. These data demonstrate that increased sensitivity to ANG II contributes to endothelial dysfunction in women who have had preeclampsia. Adapted with permission from Stanhewicz et al., Hypertension 70: 382–389, 2017 (84).
Endothelin-1 Signaling
Similar to ANG II, both animal and human studies have identified a critical role for the endothelin system in vascular dysfunction as well as in the clinical symptoms observed during a preeclamptic pregnancy. ET-1 is a potent vasoconstrictor that is produced by the endothelium, binds to membrane-bound ET-1 receptors located on the endothelial and vascular smooth muscle cells, and induces an overall increase in vascular tone. The vasoconstrictor effects of ET-1 are mediated by its binding to the membrane-bound endothelin type A (ETAR) and type B (ETBR) receptor subtypes on the vascular smooth muscle. ETBR are also expressed on the vascular endothelium and, upon ET-1 binding, induce the release of endothelium-derived vasodilators such as nitric oxide. As such, the net effect of ET-1-mediated vascular tone derives from the balance of vascular smooth muscle ETAR- and ETBR-mediated vasoconstriction and endothelial ETBR-mediated vasodilation. ET-1 also induces the production of growth factors, reactive oxygen species, and inflammatory mediators, which contribute to vascular pathology associated with overactivity of the ET-1 system.
In healthy premenopausal women, a greater proportion of endothelial ETBR relative to vascular smooth muscle ET-1 receptors favors more dilation to counteract the overall proconstrictor tone. However, animal studies also suggest that, in addition to increased circulating ET-1 in preeclamptic women, dysregulation of the receptor subtypes likewise contributes to an increased vasoconstrictor sensitivity to ET-1 (59). Recently, our laboratory examined vasoconstrictor responses to ET-1 in the cutaneous microvasculature of women who had preeclampsia. In agreement with the animal findings, we found that formerly preeclamptic women had an exaggerated vasoconstrictor response to ET-1, mediated by dysregulation of the ETBR (83). When compared with women who had a normal pregnancy, women with a history of preeclampsia had reduced ETBR-mediated dilation, and, in fact, ETBR signaling contributed to vasoconstriction, presumably through increased ETBR protein expression in the vascular smooth muscle (Fig. 4). Furthermore, we found that pharmacological ETBR blockade with BQ-788 augmented endothelium-dependent dilation in women with a history of preeclampsia. Taken together, these findings suggest that the increased constrictor tone mediated by ETBR is also contributing to reduced endothelium-dependent dilation in women who have had a preeclamptic pregnancy (Fig. 5). Although not the primary focus of that investigation, ETAR-mediated vasoconstriction was attenuated in women who had preeclampsia compared with women who had a healthy pregnancy (Fig. 4; P = 0.02 preeclampsia vs. healthy pregnancy at ET-1 + BQ-788 site). However, we did not find differences in ETAR protein expression between groups. Animal models of preeclampsia have suggested that exaggerated ETAR-mediated constriction contributes to the vascular dysfunction during preeclamptic pregnancy (4, 89). To date, no human studies of the role of ETAR in vascular function after preeclamptic pregnancy have been conducted.
Fig. 4.
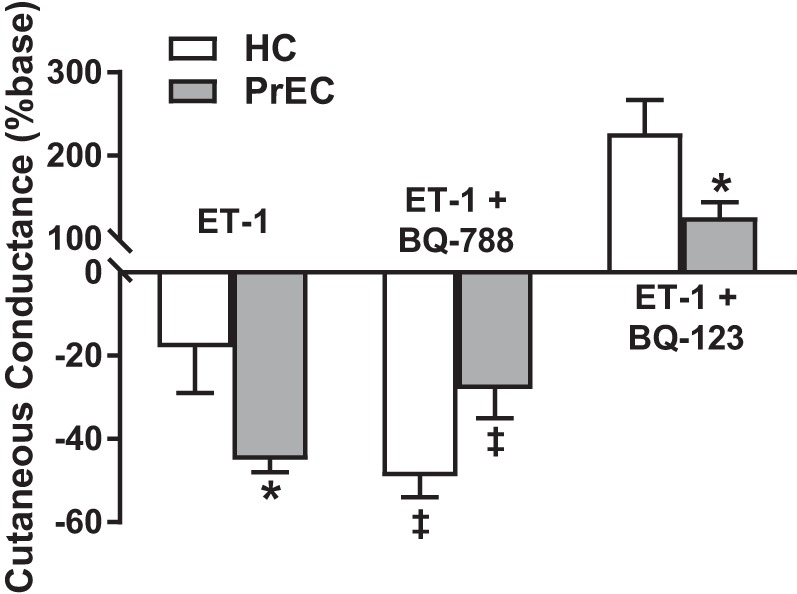
Dysregulation of endothelin receptor subtype B (ETBR) contributes to exaggerated vasoconstrictor tone in women who have had preeclampsia. Compared with women who had a healthy pregnancy (HC), women who had preeclampsia (PrEC) have an exaggerated vasoconstriction response to endothelin (ET-1, 10−8 M) measured in the cutaneous microvasculature. Coinfusion of the ETBR-antagonist BQ-788 augments vasoconstriction in HC, demonstrating the vasodilatory role of ETBR in healthy premenopausal women. However, coinfusion of BQ-788 attenuates the vasoconstriction response in PrEC, suggesting that ETBR contributes to constriction in women who have had preeclampsia. ETBR-mediated dilation, measured as the increase in cutaneous conductance during coinfusion of the ETAR-antagonist BQ-123, was attenuated in PrEC compared with HC. *P < 0.05 vs. HC; ‡P < 0.05 vs. ET-1 alone within group. These data demonstrate that dysregulation of the ETBR contributes to microvascular dysfunction in women who have had preeclampsia. Adapted with permission of Portland Press, from Stanhewicz et al., Clin Sci (Lond) 131: 2777–2789, 2017 (83).
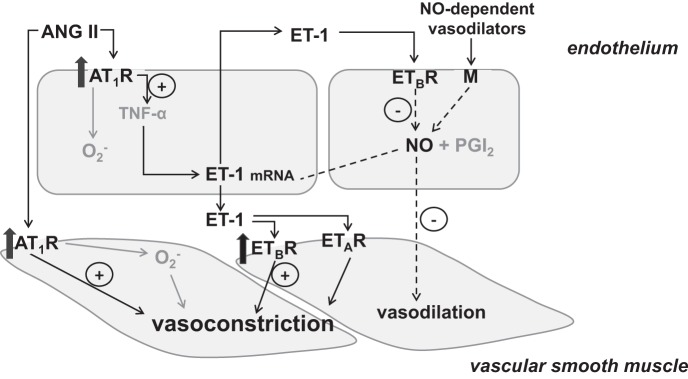
Fig. 5.
Schematic representation of the mechanisms contributing to microvascular dysfunction in women who have had preeclampsia. An increase in angiotensin II (ANG II) sensitivity and increased endothelin-1 (ET-1) production, coupled with attenuated ET-1-mediated vasodilation, result in persistent vascular smooth muscle vasoconstriction and attenuated vasodilation responses to endothelium-dependent agonists. AT1R, ANG II type 1 receptor; TNF-α, tumor necrosis factor-α; ETAR, endothelin receptor type A; ETBR, endothelin receptor type B; M, membrane-bound receptor; NO, nitric oxide.
The mechanism(s) mediating the increase in vascular smooth muscle ETBR protein expression during preeclamptic pregnancy is not fully elucidated; however, it has been suggested that elevated inflammatory signaling during the pregnancy is a likely contributor. Recently, Sun et al. utilized the reduced uterine perfusion pressure (RUPP) rat model to examine changes in ETBR expression during preeclampsia (87). These authors found that, compared with normal pregnant rats, RUPP rats had a significant redistribution of ETBR expression in the intima and media of the aorta, although there was no significant difference in ETAR expression between the two groups. ETBR upregulation in vascular smooth muscle cells enhanced cellular contraction and contributed to hypertension in the RUPP rats, and the TNF-α plasma levels in RUPP rats were twofold higher than those in control rats. The authors concluded that the expression of ETBR was upregulated in vascular smooth muscle cells through the NF-κB pathways in RUPP rats and that redistribution of ETBR between the media and intima played an important role in the pathogenesis of hypertension in preeclampsia (87). These findings agree with data from our laboratory group suggesting that ETBR expression and location play a role in mediating vascular dysfunction after a preeclamptic pregnancy (83) and provide evidence that elevated inflammatory cytokines during the preeclamptic pregnancy contribute to this change.
Increased Sympathetic Nervous System Activity
Women with preeclampsia exhibit elevated sympathetic activation at the time of diagnosis (31, 40, 78), indicating that an excessive increase in sympathetic activity during pregnancy (i.e., beyond the normal increase that occurs with healthy pregnancy) (17, 40, 51) is likely a key contributor to hypertensive disorders of pregnancy. In nonpregnant populations, hyperactivity of sympathetic outflow to the peripheral vasculature at rest and/or during sympathetic stimuli has been associated with hypertension (30) and heart failure (39). As such, it stands to reason that if elevated sympathetic outflow persists postpartum in women who had preeclampsia, this may be another mechanism through which CVD risk may be elevated in this population. Stickford et al. recently investigated sympathetic neural and cardiovascular responses during a sympathetic stimulus (static handgrip exercise) in women with a history of hypertensive pregnancy and in a control group with a history of normal pregnancy (85). Although there were no differences between groups in resting sympathetic nervous system activity, women with a history of hypertensive pregnancy had exaggerated reactivity to the static handgrip stimulus, suggesting impaired neurocardiovascular control in these women (85). Interestingly, the study by Stickford et al. tested women within ~10 yr of the affected pregnancy and well before the menopausal transition. After menopause, these differences in sympathetic responses to static handgrip were not apparent (71), and hypertensive pregnancy disorders were not associated with increased sympathetic outflow assessed 40 yr after the affected pregnancy (22). This handful of studies suggests that altered sympathetic nervous system activity may also contribute to cardiovascular dysfunction before the menopausal transition in postpartum women who had preeclampsia.
THE POSSIBILITY OF PRECEDING VASCULAR DYSFUNCTION—THE “STRESS TEST HYPOTHESIS”
One major limitation of the existing studies of vascular dysfunction after a preeclamptic pregnancy is a lack of knowledge about the vascular health and function of the women before the affected pregnancy. This limitation introduces the question of whether these women were already predisposed to vascular complications and the substantial cardiovascular strain of the pregnancy simply uncovers this underlying weakness. This hypothesis, often referred to as the “stress test hypothesis,” is supported by the fact that preexisting cardiovascular and/or metabolic disorders such as overweight or obesity, preexisting high blood pressure, elevated blood cholesterol, physical inactivity, and type 2 diabetes increase the risk for preeclampsia, suggesting that some degree of preexisting vascular dysfunction may contribute to the development of preeclampsia during pregnancy. Similarly, while delivery induces remission of most gestational disorders, this remission may be only transient (75, 97). Opponents of this hypothesis point to the fact that women with no preexisting conditions still develop preeclampsia and, although a prior pregnancy complicated by preeclampsia is a risk factor, not all women who have preeclampsia in an early pregnancy develop it in subsequent pregnancies. The question of “which came first” remains a point of debate.
The majority of investigations that have attempted to answer the question of whether preexisting vascular dysfunction precedes and predicts preeclampsia in otherwise healthy women have performed vascular measures (typically brachial artery flow-mediated dilation) during early pregnancy (~20 wk of gestation) before the clinical diagnosis of preeclampsia. Overwhelmingly, these studies demonstrate a decrease in endothelial function in women who go on to develop preeclampsia (33, 53, 67, 76), with a confirmatory meta-analysis of these investigations reporting an overall standardized mean difference of −0.84 (95% confidence interval: −1.19, −0.50) compared with women who had uncomplicated pregnancies (94). However, it is important to note that although these women are not yet clinically symptomatic they are already pregnant and the initiating factors of preeclampsia begin very early in pregnancy at the time of placentation. As such, it stands to reason that by the time these vascular measures were made, although the clinical symptoms of preeclampsia may not have presented, the women were likely exposed to the antiangiogenic, oxidative, and inflammatory mediators released by the placenta for many weeks. Although these studies suggest that endothelial dysfunction precedes the clinical symptomology of preeclampsia during the pregnancy, they do not settle the debate about preexisting dysfunction prior to the affected pregnancy.
The relatively low incidence of preeclampsia (~7–10% of pregnancies) and the challenges in predicting who will become preeclamptic in any given pregnancy combine to make prospective studies of vascular health in women who may become pregnant and then subsequently develop preeclampsia difficult and costly. To date, only one study has examined the relation between prepregnancy indicators of cardiovascular risk and pregnancy complications. Using data from 359 women in the Cardiovascular Risk in Young Finns Study that were linked with the national birth registry, Harville et al. examined the association between flow-mediated dilation, carotid intima-media thickness, and Young’s elastic modulus and carotid artery distensibility and pregnancy outcome (43). There were no significant relationships between any of these prepregnancy vascular measures and hypertensive disorders of pregnancy, suggesting that any associations between prepregnancy vascular function and the likelihood of developing pregnancy complications were likely to be small (43). Although these findings seemingly refute the stress test hypothesis, only prospective studies emphasizing vascular measurement before pregnancy can fully elucidate the role of preceding vascular health and CVD risk in the development of preeclampsia and future CVD incidence in women of childbearing age.
CLINICAL APPLICATIONS AND FUTURE QUESTIONS
In light of the increased risk of incident CVD in women who have had preeclampsia, the American Heart Association and American Stoke Association have recognized history of preeclamptic pregnancy as a risk factor for CVD and stroke in later life (16, 65). As such, not only is there a basic science need to identify mechanism-specific intervention strategies to slow or prevent the progression of CVD in these women, but we can expect clinical practitioners to begin looking to the scientific community to inform their treatment approach. Although inhibitors of the ET-1 and renin-angiotensin systems are contraindicated during pregnancy because of their teratogenic effects on the offspring, the mechanistic studies described above suggest that inhibitors of the ANG II system (specifically AT1R blockers) or inhibitors of the ET-1 system (specifically ETBR blockers) are putative mechanism-specific strategies that may be applied after delivery. However, the efficacy of these treatments has not yet been tested in either systemic or chronic treatment regimens.
Aside from the mechanisms that have already been explored in postpartum women who had preeclampsia, other treatment strategies may be informed by interventions that are currently being explored to treat or prevent vascular dysfunction during a preeclamptic pregnancy (Table 1). Low-dose aspirin is commonly prescribed to prevent pregnancy-related vascular disorders, and although it is widely used and considered safe at lower doses (75–150 mg/day), controversy exists as to the efficacy of this treatment for the primary prevention of preeclampsia (8, 96). Statins have recently received attention for their prophylactic use in pregnant women at high risk for preeclampsia. Statin medications have a number of pleiotropic effects aside from their role in lowering cholesterol and have been shown to prevent or reverse vascular dysfunction in animal models of preeclampsia (11, 32, 48, 74). In a double-blind placebo-controlled pilot study of 10 mg daily pravastatin for the prevention of preeclampsia in high-risk pregnancy, none of the 10 patients assigned to the pravastatin treatment developed preeclampsia compared with 4 of the 10 assigned to placebo (24) and no identifiable safety risks were associated with the pravastatin treatment. While the sample sizes were small, this study provided preliminary evidence to support larger trials of pravastatin treatment in this population. Both low-dose aspirin and statins are commonly prescribed for the primary and secondary prevention of CVD in men and nonpregnant women, but their efficacy for the treatment or prevention of CVD in women who had preeclampsia has not been explored.
Table 1.
Proposed therapeutic strategies for improving vascular function in women who have had preeclampsia
| Intervention | Proposed Vascular Mechanism of Action | Efficacy in Reducing Incidence of Preeclampsia in Human Trials | Effect on Endothelial Function After Pregnancy in Human Studies | References |
|---|---|---|---|---|
| Low-dose aspirin | ↓Inflammation | ↓Relative risk in high-risk pregnancy | Unknown | (8, 96) |
| ↓Platelet activation | ||||
| ↓Angiogenic imbalance during preeclampsia | ||||
| Statin | ↓Inflammation and ROS production | ↓Relative risk in high-risk pregnancy | Unknown | (24) |
| ↑NO synthesis and bioavailability | ||||
| Vitamin D | ↓Inflammation | ↓Relative risk in high-risk pregnancy | Unknown | (50) |
| Calcium | ↓PTH and renin release | ↓Relative risk in high-risk pregnancy | Unknown | (46) |
| ↓Vasoconstriction | ||||
| AT1R inhibition | Inhibit exaggerated AT1R-mediated constriction | Unknown | ↑Endothelium-dependent dilation acutely | (84) |
| ETBR inhibition | Inhibit ETBR-mediated constriction | Unknown | ↑Endothelium-dependent dilation acutely | (83) |
| Aerobic exercise | ↓Inflammation | No effect | ↑Endothelium-dependent dilation | (26, 80, 81) |
| ↑NO synthesis and bioavailability | ↓Retrograde shear rate |
AT1R, angiotensin II type 1 receptor; ETBR, endothelin type B receptor; NO, nitric oxide; PTH, parathyroid hormone; ROS, reactive oxygen species.
Nutritional interventions for the prevention of preeclampsia in high-risk pregnancy have also gained recent attention. Meta-analyses of both vitamin D supplementation (50) and calcium supplementation (≥1 g/day) (46) during high-risk pregnancy show a reduction in the relative risk of preeclampsia with either supplement (pooled odds ratios of 0.81 and 0.45, respectively). Similarly, there is an inverse relation between plasma 25-hydroxy vitamin D concentrations and incidence of preeclampsia (50). Although these findings are promising from a pregnancy perspective, the mechanism of action of these supplements is purely speculative. Presumably, vitamin D supplementation attenuates inflammation, whereas calcium supplementation may reduce parathyroid hormone and renin release, in turn reducing smooth muscle contractility. However, these mechanisms remain unexplored, and the therapeutic potential of these supplements in preventing the progression of CVD after the affected pregnancy is unknown.
Although pharmacological treatments for the prevention of CVD progression in women who had preeclampsia are appealing because of their ease of administration and mechanism specificity, questions about the cost-benefit ratio of putting an otherwise healthy young woman on a vascular medication for the rest of her life must be carefully weighed and considered. It is also important to consider that women with a history of preeclampsia are more likely to be overweight or obese and are more likely to develop other traditional CVD risk factors such as type 2 diabetes and hypercholesterolemia. In weighing treatment and/or prevention strategies for the development of overt CVD, a more comprehensive approach to this constellation of risk factors may be most appropriate. Scholten et al. recently demonstrated that 12 wk of aerobic exercise training improved endothelial function and autonomic control, and decreased vascular wall thickness and retrograde shear rate, in women who had preeclampsia (80, 81). These findings, coupled with the promising findings of nutritional approaches to prevent preeclampsia in high-risk pregnancy, suggest that perhaps lifestyle modifications that have been shown to be efficacious in improving vascular health through a variety of beneficial mechanisms in other populations may also be effective here. These interventional approaches could provide cost-effective strategies devoid of side effects for lifelong mitigation of CVD risk in women who have had preeclampsia.
Perspectives and Significance
It is now abundantly clear that despite the remission of clinical symptoms postpartum, women who have had preeclamptic pregnancies are significantly more likely to develop, and ultimately die from, CVD compared with women who have had uncomplicated pregnancies. In light of this risk, women who have had preeclampsia represent an important at-risk cohort that requires early mechanism-specific intervention strategies to prevent or mitigate CVD morbidity and mortality. Multiple studies have demonstrated that women with a history of preeclampsia have impaired endothelial function and/or increased arterial stiffness lasting months, years, and even decades after the affected pregnancy. However, only recently have a few mechanistic studies begun to examine the underlying etiology of that persistent vascular dysfunction. Those studies suggest that alterations in ANG II and ET-1 signaling, as well as changes in sympathetic nervous system control of the vasculature, contribute to subclinical vascular dysfunction in the early postpartum years and likely accelerate the development of overt CVD in women who have had preeclampsia.
Although these studies lay the groundwork for testing mechanism-specific intervention strategies, examinations of associated intervention strategies from a systemic and/or long-term perspective are lacking. Emerging animal and integrative human data from studies of preeclampsia during pregnancy point to additional potential mechanisms, such as immune dysregulation, chronic inflammatory signaling, and increased reactive oxygen signaling, that warrant consideration in the context of persistent vascular dysfunction after delivery. Although recent advances have begun to uncover the underlying mechanisms contributing to persistent vascular dysfunction in women who have had preeclampsia, a number of complementary mechanisms as well as interventional approaches remain currently unexplored.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-138133.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
A.E.S. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol 286: H1389–H1393, 2004. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol 63: 1815–1822, 2014. doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 3.Akhter T, Larsson M, Wikström AK, Naessen T. Thicknesses of individual layers of artery wall indicate increased cardiovascular risk in severe pre-eclampsia. Ultrasound Obstet Gynecol 43: 675–680, 2014. doi: 10.1002/uog.13289. [DOI] [PubMed] [Google Scholar]
- 4.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37: 485–489, 2001. doi: 10.1161/01.HYP.37.2.485. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Alvarez B, Martell-Claros N, Abad-Cardiel M, García-Donaire JA. [Hypertensive disorders during pregnancy: cardiovascular long-term outcomes]. Hipertens Riesgo Vasc 34: 85–92, 2017. doi: 10.1016/j.hipert.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS, Olafsson O. Cardiovascular death in women who had hypertension in pregnancy: a case-control study. BJOG 112: 286–292, 2005. doi: 10.1111/j.1471-0528.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 8.Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V. Aspirin for prevention of preeclampsia. Drugs 77: 1819–1831, 2017. doi: 10.1007/s40265-017-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aykas F, Solak Y, Erden A, Bulut K, Dogan S, Sarli B, Acmaz G, Afsar B, Siriopol D, Covic A, Sharma S, Johnson RJ, Kanbay M. Persistence of cardiovascular risk factors in women with previous preeclampsia: a long-term follow-up study. J Investig Med 63: 641–645, 2015. doi: 10.1097/JIM.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 10.Barnes JN, Harvey RE, Miller KB, Jayachandran M, Malterer KR, Lahr BD, Bailey KR, Joyner MJ, Miller VM. Cerebrovascular reactivity and vascular activation in postmenopausal women with histories of preeclampsia. Hypertension 71: 110–117, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Barry DR, Utzschneider KM, Tong J, Gaba K, Leotta DF, Brunzell JD, Easterling TR. Intraabdominal fat, insulin sensitivity, and cardiovascular risk factors in postpartum women with a history of preeclampsia. Am J Obstet Gynecol 213: 104.e1–104.e11, 2015. doi: 10.1016/j.ajog.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, Gilbert JS. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension 61: 1103–1110, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974, 2007. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UK, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics—2018 Update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018.] doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 14.Bernardi FC, Vuolo F, Petronilho F, Michels M, Ritter C, Dal-Pizzol F. Plasma nitric oxide, endothelin-1, arginase and superoxide dismutase in the plasma and placentae from preeclamptic patients. An Acad Bras Cienc 87: 713–719, 2015. doi: 10.1590/0001-3765201520140069. [DOI] [PubMed] [Google Scholar]
- 15.Blaauw J, Souwer ET, Coffeng SM, Smit AJ, van Doormaal JJ, Faas MM, van Pampus MG. Follow up of intima-media thickness after severe early-onset preeclampsia. Acta Obstet Gynecol Scand 93: 1309–1316, 2014. doi: 10.1111/aogs.12499. [DOI] [PubMed] [Google Scholar]
- 16.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council for High Blood Pressure Research . Guidelines for the Prevention of Stroke in Women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45: 1545–1588, 2014. [Errata in Stroke 45: e95, 2014 and Stroke 45: e214, 2014.] doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter JR. Sympathetic neural tone: large and in charge throughout pregnancy. J Physiol 590: 3411–3412, 2012. doi: 10.1113/jphysiol.2012.238980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 10: 466–480, 2014. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA 285: 1607–1612, 2001. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 20.Christensen M, Kronborg CS, Eldrup N, Rossen NB, Knudsen UB. Preeclampsia and cardiovascular disease risk assessment—do arterial stiffness and atherosclerosis uncover increased risk ten years after delivery? Pregnancy Hypertens 6: 110–114, 2016. doi: 10.1016/j.preghy.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Collén AC, Hellgren M, Gustafsson H, Johansson MC, Manhem K. Cardiovascular and metabolic characteristics 40 years after hypertensive pregnancies: a long-term follow-up study of mothers. J Hypertens 31: 758–765, 2013. doi: 10.1097/HJH.0b013e32835e2a9b. [DOI] [PubMed] [Google Scholar]
- 22.Collén AC, Manhem K, Sverrisdóttir YB. Sympathetic nerve activity in women 40 years after a hypertensive pregnancy. J Hypertens 30: 1203–1210, 2012. doi: 10.1097/HJH.0b013e3283531ed2. [DOI] [PubMed] [Google Scholar]
- 23.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 40: 102–111, 1998. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 24.Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, Easterling TR, Haas DM, Haneline LS, Caritis SN, Venkataramanan R, West H, D’Alton M, Hankins G; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network . Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 214: 720.e1–720.e17, 2016. doi: 10.1016/j.ajog.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham MW Jr, Williams JM, Amaral L, Usry N, Wallukat G, Dechend R, LaMarca B. Agonistic autoantibodies to the angiotensin II type 1 receptor enhance angiotensin II-induced renal vascular sensitivity and reduce renal function during pregnancy. Hypertension 68: 1308–1313, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva SG, Hallal PC, Domingues MR, Bertoldi AD, Silveira MF, Bassani D, da Silva IC, da Silva BG, Coll CV, Evenson K. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act 14: 175, 2017. doi: 10.1186/s12966-017-0632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demir ME, Ulas T, Dal MS, Eren MA, Aydogan H, Yalcin S, Camuzcuoglu A, Hilali NG, Aksoy N, Buyukhatipoglu H. Oxidative stress parameters and ceruloplasmin levels in patients with severe preeclampsia. Clin Ter 164: e83–e87, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Ehrenthal DB, Goldstein ND, Wu P, Rogers S, Townsend RR, Edwards DG. Arterial stiffness and wave reflection 1 year after a pregnancy complicated by hypertension. J Clin Hypertens (Greenwich) 16: 695–699, 2014. doi: 10.1111/jch.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elvan-Taşpinar A, Bots ML, Franx A, Bruinse HW, Engelbert RH. Stiffness of the arterial wall, joints and skin in women with a history of pre-eclampsia. J Hypertens 23: 147–151, 2005. doi: 10.1097/00004872-200501000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Esler M, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J Hypertens Suppl 8: S53–S57, 1990. [PubMed] [Google Scholar]
- 31.Fischer T, Schobel HP, Frank H, Andreae M, Schneider KT, Heusser K. Pregnancy-induced sympathetic overactivity: a precursor of preeclampsia. Eur J Clin Invest 34: 443–448, 2004. doi: 10.1111/j.1365-2362.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 32.Fox KA, Longo M, Tamayo E, Kechichian T, Bytautiene E, Hankins GD, Saade GR, Costantine MM. Effects of pravastatin on mediators of vascular function in a mouse model of soluble Fms-like tyrosine kinase-1-induced preeclampsia. Am J Obstet Gynecol 205: 366.e1–366.e5, 2011. doi: 10.1016/j.ajog.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 33.García RG, Celedón J, Sierra-Laguado J, Alarcón MA, Luengas C, Silva F, Arenas-Mantilla M, López-Jaramillo P. Raised C-reactive protein and impaired flow-mediated vasodilation precede the development of preeclampsia. Am J Hypertens 20: 98–103, 2007. doi: 10.1016/j.amjhyper.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Garovic VD, Milic NM, Weissgerber TL, Mielke MM, Bailey KR, Lahr B, Jayachandran M, White WM, Hodis HN, Miller VM. Carotid artery intima-media thickness and subclinical atherosclerosis in women with remote histories of preeclampsia: results from a Rochester Epidemiology Project-based study and meta-analysis. Mayo Clin Proc 92: 1328–1340, 2017. doi: 10.1016/j.mayocp.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP, van der Vlugt MJ, Janssen MC, Heidema WM, Scholten RR, Spaanderman ME. Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound Obstet Gynecol 49: 143–149, 2017. doi: 10.1002/uog.17343. [DOI] [PubMed] [Google Scholar]
- 36.Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med 21: 88–97, 2015. doi: 10.1016/j.molmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Goynumer G, Yucel N, Adali E, Tan T, Baskent E, Karadag C. Vascular risk in women with a history of severe preeclampsia. J Clin Ultrasound 41: 145–150, 2013. doi: 10.1002/jcu.21962. [DOI] [PubMed] [Google Scholar]
- 38.Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of vascular dysfunction after hypertensive disorders of pregnancy: a systematic review and meta-analysis. Hypertension 68: 1447–1458, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07907. [DOI] [PubMed] [Google Scholar]
- 39.Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell’Oro R, Bolla G, Mancia G. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension 49: 535–541, 2007. doi: 10.1161/01.HYP.0000255983.32896.b9. [DOI] [PubMed] [Google Scholar]
- 40.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation 104: 2200–2204, 2001. doi: 10.1161/hc4301.098253. [DOI] [PubMed] [Google Scholar]
- 41.Guimarães MF, Brandão AH, Rezende CA, Cabral AC, Brum AP, Leite HV, Capuruço CA. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch Gynecol Obstet 290: 441–447, 2014. doi: 10.1007/s00404-014-3220-x. [DOI] [PubMed] [Google Scholar]
- 42.Hamad RR, Eriksson MJ, Silveira A, Hamsten A, Bremme K. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens 25: 2301–2307, 2007. doi: 10.1097/HJH.0b013e3282ef5fc0. [DOI] [PubMed] [Google Scholar]
- 43.Harville EW, Juonala M, Viikari JS, Kähönen M, Raitakari OT. Vascular ultrasound measures before pregnancy and pregnancy complications: a prospective cohort study. Hypertens Pregnancy 36: 53–58, 2017. doi: 10.1080/10641955.2016.1237643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haukkamaa L, Salminen M, Laivuori H, Leinonen H, Hiilesmaa V, Kaaja R. Risk for subsequent coronary artery disease after preeclampsia. Am J Cardiol 93: 805–808, 2004. doi: 10.1016/j.amjcard.2003.11.065. [DOI] [PubMed] [Google Scholar]
- 45.Henriques AC, Carvalho FH, Feitosa HN, Macena RH, Mota RM, Alencar JC. Endothelial dysfunction after pregnancy-induced hypertension. Int J Gynaecol Obstet 124: 230–234, 2014. doi: 10.1016/j.ijgo.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev 6: CD001059, 2014. doi: 10.1002/14651858.CD001059.pub4. [DOI] [PubMed] [Google Scholar]
- 47.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 48.Huai J, Yang Z, Yi YH, Wang GJ. Different effects of pravastatin on preeclampsia-like symptoms in different mouse models. Chin Med J (Engl) 131: 461–470, 2018. doi: 10.4103/0366-6999.225058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 49: 612–617, 2007. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 50.Hyppönen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, Bánhidy F, Lawlor D, Czeizel AE. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab 63: 331–340, 2013. doi: 10.1159/000358338. [DOI] [PubMed] [Google Scholar]
- 51.Jarvis SS, Shibata S, Bivens TB, Okada Y, Casey BM, Levine BD, Fu Q. Sympathetic activation during early pregnancy in humans. J Physiol 590: 3535–3543, 2012. doi: 10.1113/jphysiol.2012.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jónsdóttir LS, Arngrímsson R, Geirsson RT, Sigvaldason H, Sigfússon N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand 74: 772–776, 1995. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 53.Kamat R, Jain V, Bahl A. Serial estimation of flow mediated dilatation in women at risk of hypertensive disorders of pregnancy. Int J Cardiol 149: 17–22, 2011. doi: 10.1016/j.ijcard.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 54.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol 62: 105–120, 2010. [PMC free article] [PubMed] [Google Scholar]
- 55.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54: 905–909, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 52: 1161–1167, 2008. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lampinen KH, Rönnback M, Kaaja RJ, Groop PH. Impaired vascular dilatation in women with a history of pre-eclampsia. J Hypertens 24: 751–756, 2006. doi: 10.1097/01.hjh.0000217859.27864.19. [DOI] [PubMed] [Google Scholar]
- 58.Matijevic R, Johnston T. In vivo assessment of failed trophoblastic invasion of the spiral arteries in pre-eclampsia. Br J Obstet Gynaecol 106: 78–82, 1999. doi: 10.1111/j.1471-0528.1999.tb08089.x. [DOI] [PubMed] [Google Scholar]
- 59.Mazzuca MQ, Li W, Reslan OM, Yu P, Mata KM, Khalil RA. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension 64: 632–643, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta 32: 413–419, 2011. doi: 10.1016/j.placenta.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 61.McDonald SD, Ray J, Teo K, Jung H, Salehian O, Yusuf S, Lonn E. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis 229: 234–239, 2013. doi: 10.1016/j.atherosclerosis.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 62.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 101: 669–674, 1994. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 63.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the Child Health and Development Studies cohort. Hypertension 56: 166–171, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mori T, Watanabe K, Iwasaki A, Kimura C, Matsushita H, Shinohara K, Wakatsuki A. Differences in vascular reactivity between pregnant women with chronic hypertension and preeclampsia. Hypertens Res 37: 145–150, 2014. doi: 10.1038/hr.2013.131. [DOI] [PubMed] [Google Scholar]
- 65.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK; American Heart Association . Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 57: 1404–1423, 2011. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55: 394–398, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 122: 478–487, 2010. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira OP, Araujo Júnior E, Lima JW, Salustiano EM, Ruano R, Martins WP, Costa FS. Flow-mediated dilation of brachial artery and endothelial dysfunction in pregnant women with preeclampsia: a case control study. Minerva Ginecol 67: 307–313, 2015. [PubMed] [Google Scholar]
- 69.Ong SS, Baker PN, Mayhew TM, Dunn WR. Remodeling of myometrial radial arteries in preeclampsia. Am J Obstet Gynecol 192: 572–579, 2005. doi: 10.1016/j.ajog.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 70.Pàez O, Alfie J, Gorosito M, Puleio P, de Maria M, Prieto N, Majul C. Parallel decrease in arterial distensibility and in endothelium-dependent dilatation in young women with a history of pre-eclampsia. Clin Exp Hypertens 31: 544–552, 2009. doi: 10.3109/10641960902890176. [DOI] [PubMed] [Google Scholar]
- 71.Ranadive SM, Harvey RE, Lahr BD, Miller VM, Joyner MJ, Barnes JN. Sympathetic responsiveness is not increased in women with a history of hypertensive pregnancy. Am J Physiol Regul Integr Comp Physiol 312: R49–R54, 2017. doi: 10.1152/ajpregu.00379.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet 366: 1797–1803, 2005. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 73.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension 47: 615–618, 2006. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 74.Saad AF, Kechichian T, Yin H, Sbrana E, Longo M, Wen M, Tamayo E, Hankins GD, Saade GR, Costantine MM. Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod Sci 21: 138–145, 2014. doi: 10.1177/1933719113492207. [DOI] [PubMed] [Google Scholar]
- 75.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 325: 157–160, 2002. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savvidou MD, Hingorani AD, Tsikas D, Frölich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 361: 1511–1517, 2003. doi: 10.1016/S0140-6736(03)13177-7. [DOI] [PubMed] [Google Scholar]
- 77.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 55: 1239–1245, 2010. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia—a state of sympathetic overactivity. N Engl J Med 335: 1480–1485, 1996. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 79.Scholten RR, Hopman MT, Sweep FC, Van de Vlugt MJ, Van Dijk AP, Oyen WJ, Lotgering FK, Spaanderman ME. Co-occurrence of cardiovascular and prothrombotic risk factors in women with a history of preeclampsia. Obstet Gynecol 121: 97–105, 2013. doi: 10.1097/AOG.0b013e318273764b. [DOI] [PubMed] [Google Scholar]
- 80.Scholten RR, Spaanderman ME, Green DJ, Hopman MT, Thijssen DH. Retrograde shear rate in formerly preeclamptic and healthy women before and after exercise training: relationship with endothelial function. Am J Physiol Heart Circ Physiol 307: H418–H425, 2014. doi: 10.1152/ajpheart.00128.2014. [DOI] [PubMed] [Google Scholar]
- 81.Scholten RR, Thijssen DJ, Lotgering FK, Hopman MT, Spaanderman ME. Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. Am J Obstet Gynecol 211: 516.e1–516.e11, 2014. doi: 10.1016/j.ajog.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 82.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 55: 386–393, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clin Sci (Lond) 131: 2777–2789, 2017. doi: 10.1042/CS20171292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension 70: 382–389, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stickford AS, Okada Y, Best SA, Parker RS, Levine BD, Fu Q. Sympathetic neural and cardiovascular responses during static handgrip exercise in women with a history of hypertensive pregnancy. Clin Auton Res 26: 395–405, 2016. doi: 10.1007/s10286-016-0372-8. [DOI] [PubMed] [Google Scholar]
- 86.Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, Rich-Edwards JW. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med 169: 224–232, 2018. doi: 10.7326/M17-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun Y, Zhang X, Chen Z, Xu M, Ou M. Reduction of uterine perfusion pressure induced redistribution of endothelin receptor type-B between the intima and media contributes to the pathogenesis of pregnancy-induced hypertension. Cell Physiol Biochem 44: 1715–1725, 2017. doi: 10.1159/000485777. [DOI] [PubMed] [Google Scholar]
- 88.Szarka A, Rigó J Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11: 59, 2010. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN Jr, Lamarca B, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol 204: 330.e1–330.e4, 2011. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tyldum EV, Backe B, Støylen A, Slørdahl SA. Maternal left ventricular and endothelial functions in preeclampsia. Acta Obstet Gynecol Scand 91: 566–573, 2012. doi: 10.1111/j.1600-0412.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 91.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 7: 467–474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warrington JP, George EM, Palei AC, Spradley FT, Granger JP. Recent advances in the understanding of the pathophysiology of preeclampsia. Hypertension 62: 666–673, 2013. doi: 10.1161/HYPERTENSIONAHA.113.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD. Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension 67: 415–423, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58: 77–84, 2011. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol 126: 1242–1250, 2015. doi: 10.1097/AOG.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 97.Williams D. Pregnancy: a stress test for life. Curr Opin Obstet Gynecol 15: 465–471, 2003. doi: 10.1097/00001703-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 98.Yuan LJ, Xue D, Duan YY, Cao TS, Yang HG, Zhou N. Carotid arterial intima–media thickness and arterial stiffness in pre-eclampsia: analysis with a radiofrequency ultrasound technique. Ultrasound Obstet Gynecol 42: 644–652, 2013. doi: 10.1002/uog.12409. [DOI] [PubMed] [Google Scholar]