Abstract
Cancer and cardiovascular disease are major causes of morbidity and mortality worldwide. Older cancer patients often wrestle with underlying heart disease during cancer therapy, whereas childhood cancer survivors are living long enough to face long-term unintended cardiac consequences of cancer therapies, including anthracyclines. Although effective and widely used, particularly in the pediatric population, anthracycline-related side effects including dose-dependent association with cardiac dysfunction limit their usage. Currently, there is only one United States Food and Drug Administration-approved drug, dexrazoxane, available for the prevention and mitigation of cardiotoxicity related to anthracycline therapy. While aerobic exercise has been shown to reduce cardiovascular complications in multiple diseases, its role as a therapeutic approach to mitigate cardiovascular consequences of cancer therapy is in its infancy. This systematic review aims to summarize how aerobic exercise can help to alleviate unintended cardiotoxic side effects and identify gaps in need of further research. While published work supports the benefits of aerobic exercise, additional clinical investigations are warranted to determine the effects of different exercise modalities, timing, and duration to identify optimal aerobic training regimens for reducing cardiovascular complications, particularly late cardiac effects, in cancer survivors exposed to anthracyclines.
Keywords: aerobic exercise, anthracyclines, cancer, cardiotoxicity
cancer survivors are living longer because treatment options including anthracycline (AC)-based chemotherapeutic agents are more effective. The five-year survival rate of children with a history of cancer has risen to 80% and is continually increasing (48). According to a 2014 study by the American Cancer Society, nearly 14.5 million children and adults are long-term cancer survivors (>5 yr), and this number is predicted to grow to 19 million by 2024 (1). As this population steadily increases, long-term secondary side effects associated with cancer therapy become more significant.
The discovery of ACs is one of the most important advances in cancer chemotherapy because of their effectiveness. Although ACs have become the backbone of many chemotherapeutic regimens for cancer (59), there is a dose-dependent association between AC and risk of cardiotoxicity (18). To date, AC-induced cardiotoxicity most commonly presents with left ventricular dysfunction (47), which can occur virtually any time during AC infusion and up to years or decades later. Frequently, patients who undergo treatment with ACs may receive additional radiation therapy, which also precipitates damage of nearby vascular and soft tissues that cannot be shielded from the radiation beam. In recent years, lower doses of radiation have been used because of greater awareness of long-term consequences of radiotherapy (restrictive cardiomyopathies, early valvular degeneration, coronary disease). In those receiving both local radiation and systemic AC, cardiotoxic properties are additive. The reader is referred to other excellent reviews with in-depth discussions of cardiac damage secondary to radiotherapy (15, 23, 35).
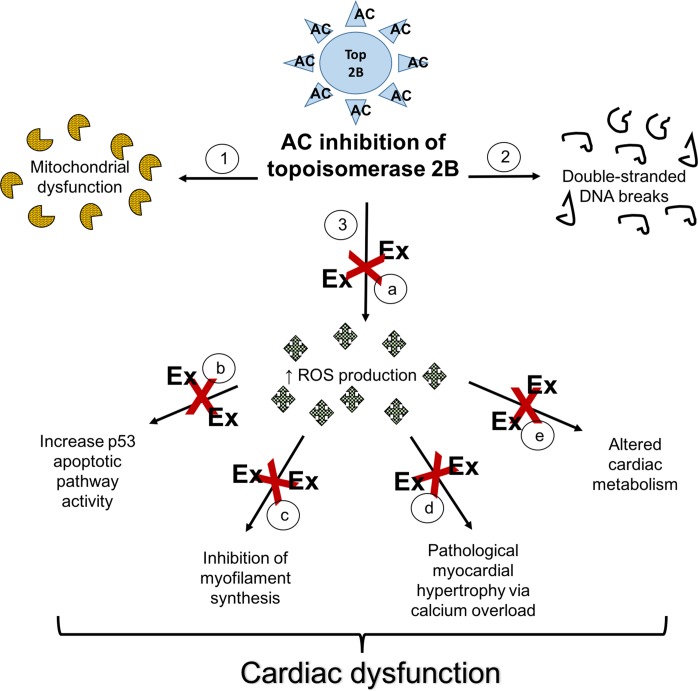
Although the mechanism by which ACs induce cardiotoxicity is unclear, two mainstream hypotheses have been proposed (Fig. 1). The reactive oxygen species (ROS) hypothesis suggests that increased concentration of ROS leads to increased expression of proteins involved in cardiac autophagy, pathological myocardial hypertrophy via calcium overload, and inhibition of myoprotein synthesis (47, 51). The topoisomerase (Top) 2B hypothesis on the other hand suggests that ACs bind to Top 2B, distorting its structure and preventing Top 2B from unwinding DNA (58). This action causes double-stranded DNA breaks, activates apoptotic pathways, and causes cardiomyocyte death (58). In fact, the Top 2B hypothesis could potentially initiate the pathway of the ROS hypothesis where inactivated Top 2B reduces antioxidant gene transcription and leads to increased ROS production.
Fig. 1.
Proposed mechanisms of anthracycline-induced cardiotoxicity and exercise attenuation of anthracycline-induced myocardial injury. Potential mechanisms of anthracycline (AC)-induced cardiotoxicity (1–3) include AC inhibition of topoisomerase 2B to cause mitochondrial dysfunction (1), double-stranded DNA breaks (2), and increased production of reactive oxidative species (ROS) (3). Increased ROS leads to myocyte apoptosis, decrease myofilament synthesis, myocardial hypertrophy, and altered cardiac metabolism. Exercise (red X) modulates AC-induced myocardial injury by attenuation of ROS production (through increasing antioxidants and 70-kDa heat shock protein expression) (a), decreasing apoptosis (inhibition of p53 expression, decrease activation of p38 kinases) (b), increase myofilament/cardiomyocyte proliferation (c), physiological myocardial hypertrophy (increase in calcium handling to prevent calcium overload) (d), and alter cardiac energy metabolism (increase AMP-activated kinase) (e).
Strategies to prevent AC-induced cardiotoxicity are only moderately successful and include strategies aimed at 1) treating preexisting cardiovascular risk factors such as hypertension and 2) modulation of the AC infusion through the use of pegylated liposomal formulations (59), concurrent treatment with the cardioprotectant dexrazoxane (8), or use of AC derivatives such as tetrahydropyranyl-adriamycin (49). However, the limited success of these strategies led to renewed interest in alternate or complementary approaches.
It is known from observational cohort studies that cancer survivors have low physical activity levels (60), and in survivors of Hodgkin’s lymphoma, increased exercise activity is associated with lower risk of cardiovascular events in a dose-dependent manner (30). The American College of Sports Medicine (ACSM) suggests that exercise training can lead to improvements in aerobic fitness, muscle strength, quality of life, and cancer-related fatigue in several cancer survivor groups (44). Although light to moderate intensity resistance exercise training programs have been recommended to certain cancer patients and associated benefits such as improved muscular function and body composition have been reported (53), both preclinical models and clinical studies suggest that aerobic exercise may mitigate and prevent the effects of AC-induced cardiotoxicity (12, 13, 34, 43, 46, 51, 55). One recent study in particular (7) has demonstrated the role of AMP-kinase pathway activation as a mechanism of action for the benefits of exercise.
The purpose of this paper is to conduct a systematic review of published preclinical and clinical evidence relating to the effects of aerobic exercise on AC-induced cardiotoxicity and to identify research gaps that could lead to preventive strategies for improving cardiovascular health in cancer survivors exposed to ACs. While preclinical models can help address specific mechanistic questions, human clinical data are necessary to translate results of preclinical trials into applicable prescriptions, which can then be implemented in team-based, patient-centered models of healthcare delivery.
Literature Search
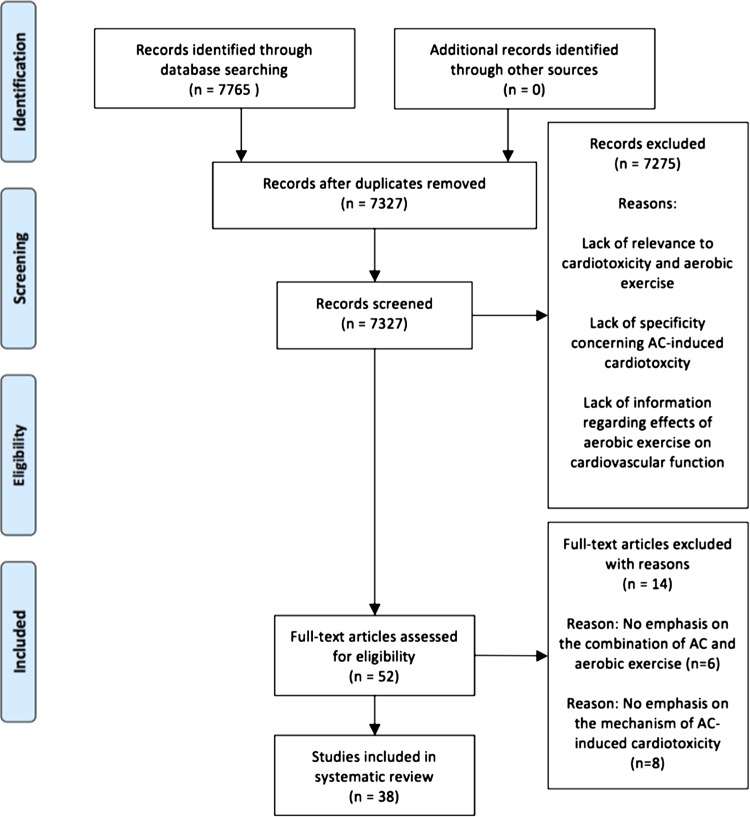
A literature search using PubMed (2005–2015) with the search terms “cancer,” “cardiotoxicity,” “ACs,” and “exercise” was performed. The search was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations (24). Forty-six articles were found. Using combinations of the keywords returned >7500 additional articles. The date last searched was December 17, 2015. Two authors reviewed the titles and abstracts for appropriateness. Thirty-eight articles were included (Fig. 2). The selected articles included preclinical studies, observational studies on humans and animals, and systematic reviews. Studies included both childhood and adult survivors of all cancer types. Only studies that focused on 1) how aerobic exercise affects cardiovascular function, 2) the mechanisms by which ACs induce cardiotoxicity, and 3) the effects of aerobic exercise on AC treatments were included. All specific types of AC-class drugs were combined under the general term AC. We evaluated the efficacy of aerobic exercise by using each individual study’s primary measures on whether aerobic exercise had a significant effect on cardiotoxicity. The Cochrane collaboration tool (24) was used to evaluate the risk of bias in the selected articles. Given the inclusion criteria, exercise intervention studies with unblinded subjects could potentially cause an inevitable detection bias in the individual studies. Since few human studies investigate the role of exercise or exercise capacity in adult cancer survivors exposed to ACs, our data summary will focus on the childhood cancer survivor cohort.
Fig. 2.
Flow diagram of included and excluded studies. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart format was adapted from Moher et al. (38).
Aerobic Exercise Has Cardioprotective Effects
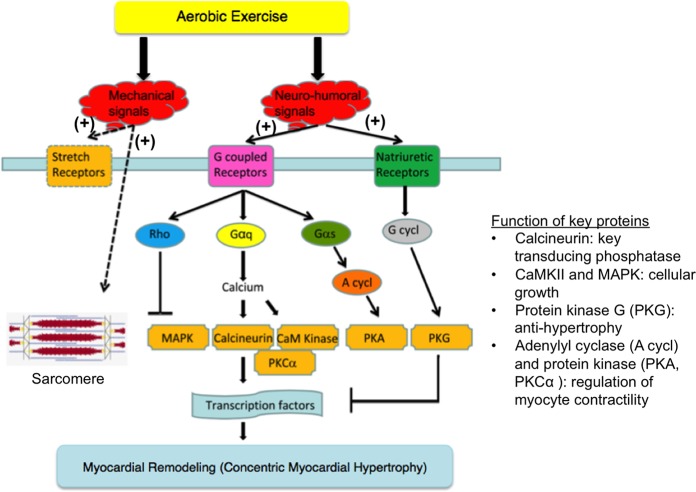
The cardioprotective benefits of aerobic exercise have been well documented in many patient populations (21, 57). Aerobic exercise provides cardioprotection by 1) stimulating antioxidants and reducing elevated ROS levels, which can cause significant cardiac damage (31); 2) reducing proapoptotic molecular species and signaling (3); 3) stimulation of myofilament synthesis (5); 4) facilitating physiological concentric hypertrophy in myocardial remodeling (33); and 5) altering cardiac metabolism through AMP-mediated kinases (10) (Fig. 1). Cardiomyocytes may become more resistant to ROS-induced apoptotic stimuli after short-term aerobic exercise through equal upregulation of biochemical alterations in cardiac mitochondria (31). In patients with chronic heart failure, aerobic exercise has been shown to increase peak cardiac output via a combination of increased heart rate and stroke volume (37). Furthermore, aerobic exercise alters mechanical and neurohormonal signals, which can result in physiological cardiac hypertrophy and is mediated in part by increased activation of phosphatidylinositide 3-kinases within the myocardium (36). Mechanical signals stimulate stretch receptors to act on sarcomeres, whereas neurohormonal signals activate G-coupled receptors and natriuretic receptors to cause myocardial remodeling in the form of concentric myocardial hypertrophy (Fig. 3) (33). Activation of G-coupled receptors alters G-protein and Rho family protein signaling, which subsequently modulates phospholipases and cyclases to alter cellular transcription factors. Activation of kinases regulate myocyte contractility and cellular growth. In exercise-induced myocardial hypertrophy, the cardiac myocytes enlarge to compensate for increased workload (33). This form of ventricular remodeling is typically not accompanied by fibrosis or reactivation of fetal gene expression (markers which are more frequently associated with pathological remodeling). In addition, endothelial dysfunction, which is frequently considered an initiating event in cardiovascular disease, has been observed in cancer survivors (39). ACs can cause endothelial cell apoptosis and, through ROS generation, disrupt the delicate balance between nitric oxide and superoxide, which lead to endothelial dysfunction (52) of both the myocardial microvascular system and the epicardial coronary artery system (32, 62). Aerobic exercise may alleviate endothelial dysfunction in cancer survivors (55) and potentially avert the doxorubicin-induced injury (29). For these reasons, aerobic exercise has been explored as one potential preventive strategy to reduce cardiotoxic effects of ACs.
Fig. 3.
Pathway for physiological myocardial remodeling via aerobic exercise. Mechanical and neurohormonal signals triggered by aerobic exercise can initiate remodeling through activation of G proteins (Gαq, Gαs) and Rho family proteins, which modulate phospholipases and cyclases (G cycl, A cycl). This leads to downstream alteration of various kinases (CaMKIII, MAPK, PKA, PKG) and phosphatases (calcineurin), which in turn modify transcription rates and promotes physiological concentric myocardial hypertrophy. Different from pathophysiological hypertrophy, exercise-induced myocardial remodeling typically does not induce fibrosis or reactivation of fetal gene expression. This illustration was adapted from Kehat et al. (33) and used with permission.
Although the cardioprotective benefits of exercise are well validated, there remains a “risk-benefit paradox” in exercise. Vigorous exercise is defined as 6 METS (metabolic equivalent tasks) or more, with vigorous aerobic exercise sometimes being quantified as an oxygen uptake of 21 ml·kg−1·min−1. However, vigorous exercise needs to also be considered in the context of relative intensity because the latter is a better indicator of risks associated with higher-intensity exercise. Certainly, it would be plausible that prolonged strenuous exercise would increase the risk of exercise-related adverse events, particularly in those who are unfit, elderly, or chronically ill and who are not well conditioned. Data have also emerged to suggest a point of diminishing returns. At the higher end of exercise activity, there may be a potential attenuation of benefit. Additional detail relating to exercise assessment and prescription is available in the ACSM’s exercise guidelines for cancer survivors (44).
AC Exposure Impairs Exercise Capacity Through Both Cardiac and Noncardiac Effects
Exercise capacity can be detected and quantified using a variety of parameters. Outlined in Table 1 are cross-sectional analyses of exercise capacity in childhood cancer survivors. Some studies (11, 56) used maximum oxygen consumption (V̇o2max), which is the gold standard marker of aerobic exercise performance (56), while others used measures of echo-derived cardiac function (16, 20, 27) and related biomarkers, i.e., brain natriuretic peptide (BNP) (42, 49). AC treatment can impair aerobic exercise capacity through a decrease in myocardial function (20, 27, 42, 49) or through noncardiac effects such as reducing oxygen consumption (11, 56) and impairment of skeletal contractile function (57a).
Table 1.
Effect of AC exposure on exercise capacity in cancer patients and survivors
| Author N | Chest Radiation | Cumulative AC Dose (mg/m2)/Type | Reference Group | Evaluation Parameter | Outcome Parameter |
|---|---|---|---|---|---|
| Shimomura et al. (49) N = 61 CCS* |
Unspecified | 120 to 740/THP Mean: 299 ± 192 |
Age-matched healthy controls | BNP levels | BNP levels were significantly positively, correlated with THP dosage. |
| De Caro et al. (11) N = 55 CCS* |
N = 21 1,633 ± 735 cGy |
100 to 500/mixed AC type Median: 240 Mean: 258.4 ± 87.7 |
Age- and sex-matched controls | V̇o2max | V̇o2max is lower in AC-treated patients but V̇o2max can be influenced by various factors. |
| Tham et al. (56) N = 30 CCS* |
N = 5 Unspecified dose |
197.2 ± 84.3/type not stated | Healthy control | V̇o2max | Peak V̇o2 negatively correlated with AC dosage. |
| Gallucci et al. (16) N = 30 adults* |
N = 30 Unspecified dose |
360 to 540/mixed AC type Mean: unspecified |
Self | Echo-assessed left ventricular ejection fraction | AC induced damage to myocytes by blunting contractile response. |
| Guimaraes-Filho et al. (20) N = 27 CCS* |
Unspecified | 100/mixed AC type Mean: unspecified |
Healthy control | Systolic reserve: echo-assessed end-systolic stress index and LV end-systolic wall stress |
AC treatment associated with smaller systolic reserves. |
| Jarfelt et al. (27) N = 23 CCS* |
N = 11 Cranial radiation* 18–24 Gy |
120–400/mixed AC type Median: 120 |
Healthy Controls | Echo-assessed LV ejection fraction, fractional shortening and stroke volume | Subclinical LV dysfunction detected during exercise associated with AC dosage. |
| Pinarli et al. (42) N = 34 CCS* |
N = 2 Mean, 2,220 cGy |
90 to 490/mixed AC type Mean: 259 ± 127 |
Healthy Controls | BNP levels, Echo-assessed E-to-A ratio, and wall stress | AC treatment led to abnormal diastolic filling patterns, higher BNP levels, and increased wall stress. |
BNP, brain natriuretic peptide; CCS, childhood cancer survivors; LV, left ventricle (ventricular); mixed anthracycline (AC) type, multiple AC-class drugs including doxorubicin, daunorubicin, idarubicin, and epirubicin were used; THP, pirarubicin; V̇o2, oxygen consumption; V̇o2max, maximum oxygen consumption; Gy, gray; cGy, centigray.
All studies were considered to have level-II evidence based on “Levels of Evidence for Clinical Studies” guidelines by Elsevier (14).
Adverse effects of ACs on exercise capacity: a focus on the heart.
AC treatment can disrupt the contractile function of cardiomyocytes (16), leading to impairment in both systolic function [reflected as a decrease in ejection fraction (EF)] and diastolic function (20, 27, 42) (Table 1). Both systolic and diastolic impairment may occur at rest or with exercise. Notably, cardiomyocyte contractile dysfunction may not affect overall cardiac function at rest but be diminished in response to exercise (16). This is reflected as a lower EF at peak exercise in survivors treated with ACs (16).
Examples of AC-induced impairment may also include lower resting EF, increased wall stress, and decreased stroke volume (27, 42). One study (27) (Table 1) identified the presence of subclinical cardiac dysfunction using two-dimensional echocardiography in asymptomatic survivors of acute lymphoblastic leukemia treated with ACs. The study discovered that survivors had significantly lower EF both at rest and after a maximal exercise stress test (27). In addition, the study found that survivors also exhibited diastolic dysfunction in the form of increased left ventricular filling pressure (27). As demonstrated by the cancer survivors’ lower maximal metabolic equivalent during the exercise stress test, it is possible that AC-induced systolic and diastolic dysfunction may diminish an individual’s capacity for exercise even when AC dosages are relatively low (120–400 mg/m2) (27).
Furthermore, impaired cardiac function can be reflected as an increase in serum BNP. BNP is circulating protein secreted by the ventricles in response to an increase in ventricular volume and pressure (41, 43). Widely used in the clinical setting, BNP has been used as an early sign of heart failure and is particularly useful in patients who are asymptomatic or have atypical symptoms (42). In cancer survivors treated with ACs, BNP can serve as a surrogate for heart failure (42). In one study, young asymptomatic patients with solid tumors treated with an AC derivative called pirarubicin underwent exercise stress testing and pre- and post-BNP assessments (49) (Table 1). BNP levels were found to be significantly correlated with cumulative dosage of pirarubicin (49). When compared with controls, patients with pirarubicin dosage above 300 mg/m2 had a 1.7-fold increase in total BNP levels prestress testing. Notably, BNP levels were 18-fold higher after the exercise test, suggesting the presence of subclinical myocardial impairment or exercise-induced increases in myocardial wall stress (49). Similarly, Pinarli et al. (42) found that BNP levels were significantly higher after exercise testing in individuals treated with ACs compared with healthy controls. However, this study did not find a significant correlation between cumulative dosage of ACs and BNP levels.
Adverse effects of ACs on exercise capacity: a focus on skeletal muscle.
Peak V̇o2 obtained during cardiopulmonary exercise testing is an objective measure of exercise capacity. Asymptomatic cancer survivors with normal left ventricular ejection fraction sometimes have reduced peak V̇o2 levels (56). The reduction in V̇o2 is directly correlated with higher cumulative doses of ACs (56). While AC treatment may be a primary cause for significantly lower V̇o2 levels in cancer survivors, other factors including cancer-related deconditioning, and psychological perception could also affect exercise capacity (11). In cancer survivors with subclinical cardiac dysfunction, decreased V̇o2max may also result from deconditioning even though they are able to participate in aerobic exercise (11). In these cases, an aerobic exercise regimen after therapy can offset deconditioning.
Additionally, ACs can impair contractile function of skeletal muscles, leading to peripheral muscle weakness. An experimental study using ex vivo mice skeletal muscles showed that AC treatment impaired both relaxation and contractile velocity of skeletal muscle especially at higher forces (57a). These effects are related to AC’s impairment of calcium reuptake into the sarcoplasmic reticulum or the mitochondria, resulting in additional time needed for skeletal muscles to return to a relaxed state (57a). Calcium reuptake impairment also accounts for decreased maximal contractile velocity at higher forces since there is insufficient storage of calcium (57a). Both AC-induced impairment in skeletal muscle contractile function and cardiopulmonary impairment may contribute to the decline in activity of cancer survivors. However, a combination of weight training and aerobic exercises may help with muscle strengthening and improved cardiopulmonary function.
Rationale for Timing of Aerobic Exercise to Mitigate AC-Induced Cardiotoxicity
Aerobic exercise before AC treatment.
To date, the majority of published studies incorporated aerobic exercise before AC treatment (4, 9, 19, 26, 28, 50, 51, 61) and are summarized in Table 2. These studies were conducted in rodent and one murine model and highlight two potential mechanisms by which aerobic exercise mitigates AC-induced cardiotoxicity: reduction of AC-induced oxidative stress (4, 9, 50, 51, 61) and accelerated clearance of AC from myocardial tissue (19, 28).
Table 2.
Cardioprotective effects of aerobic exercise in rodent and murine models exposed to ACs
| Author | Animal Species/Age | Anthracycline Dose (mg/kg)/Type | Exercise Protocol | Outcome Parameter |
|---|---|---|---|---|
| Smuder et al. (51) | Rats/6 mo old | 20b/DOX | Treadmill, 60 min/day, 5 consecutive days | Aerobic exercise regulates catalase protein levels and increases removal rate of ROS. |
| Ashrafi and Roshan (4) | Rats/8 wk old | 10 or 20b/DOX | Treadmill, 25–39 min, 5 days/wk, 3 wk | Aerobic exercise upregulates antioxidant defenses. |
| Chicco et al. (9) | Rats/unspecified | 15b/DOX | 20–60 min/day, 5 days/wk, 12 wk | Aerobic exercise attenuates AC-induced myocardial lipid peroxidation. |
| Gibson et al. 2013 (19) | Rats/10 wk old | 15b/DOX | Voluntary wheel running, 14 wk | Aerobic exercise counteracts AC-induced upregulation of autophagy system markers. |
| Hydock et al. (26) | Rats/unspecified | 10b/DOX | Voluntary wheel running or treadmill, 20–60 min/day, 5 days/wk. Both 10 wk | Aerobic exercise preserves β-MHC expression ratio for >10 days. |
| Jensen et al. (28) | Rats/10 to 11 wk old | Bolus: 10b/DOX | Voluntary wheel running, 10 wk, or treadmill, 20–60 min/day, 5 days/wk, 10 wk | Aerobic exercise preserved cardiac function and reduced AC accumulation in LV. |
| Shirinbayan and Roshan (50) | Rats/10 wk old | 10 or 20b/DOX | Treadmill, 25–39 min/day, 5 days/wk, 3 wk | Aerobic exercise associated with increased expression of HSP70. |
| Wonders et al. (61) | Rats/unspecified | Bolus: 15b/DOX | Treadmill, 2 wk acclimation protocol, single acute bout, 60 min | Single acute bout of aerobic exercise preserved LV function via mitigation of AC-induced oxidative stress. |
| Hayward et al. (22) | Rats/25 days old | IP injection: 14c/DOX | Voluntary wheel running, 10 wk | Aerobic exercise preserved function of remaining cardiomyocytes. |
| Sturgeon et al. (54) | Mice/6–8 wk | Bolus: 4c/DOX | Modality not stated, 45 min/day, 5 days/wk, 2 wk | Aerobic exercise upregulates Akt signaling (pathway involved in cellular survival and growth), but no significant cardioprotection. |
| Hydock et al. (25) | Rats/unspecified | Bolus: 15a,c/DOX | Voluntary wheel running, 10 wk | Low intensity and low volume of exercise adequate to provide cardioprotection. |
DOX, doxorubicin; HSP70, 70-kDa heat shock protein; MHC, myosin heavy chain; ROS, reactive oxygen species, IP, intraperitoneal.
Exercise intervention before AC treatment;
exercise intervention concomitant with AC treatment;
exercise intervention after AC treatment.
mitigation of ac induced oxidative stress.
Several studies noted that aerobic exercise intervention before AC treatment provides cardioprotection by attenuating AC-induced oxidative stress (4, 9, 50, 51, 61). Shirinbayan and Roshan (50) injected male Wistar rats with 20 mg/kg of AC after a 3-wk pretreatment treadmill running intervention and discovered that AC-treated rats exhibited elevated expression of a 70-kDa heat shock protein (HSP70). HSP70 is a molecular chaperone and plays a crucial role in the adaptive response to myocardial stress. HSP70, in its inducible form, protects cells from apoptosis during stress including oxidative stress, radiation, and chemical toxins. However, the mechanisms relating to how HSP70 protects stress-induced injury remains unclear and may relate to competitive binding with Fas-associated factor 1 (17), a factor that acts upstream on caspase-8 to cause cell death. Based on what is known about HSP70, Shirinbayan and Roshan (50) postulated that aerobic exercise-induced increase expression of HSP70 may modify the myocardium’s response to stress-induced injury and thereby facilitates reduction of AC-induced oxidative stress. Others have hypothesized that HSP70 works by attenuating mitochondrial dysfunction (2).
In addition to upregulation of heat shock protein expression, aerobic exercise has been found to reduce AC-induced oxidative stress by increasing antioxidant production in cardiac mitochondria (4, 51). Male Wistar rats placed on a 3-wk treadmill program before AC treatment exhibited higher levels of antioxidants (superoxidase dismutases and apelin) (4). These antioxidants provide cardioprotective benefits by inhibiting AC-induced ROS (4).
Smuder et al. (51) also demonstrated that increased antioxidant levels due to pretreatment aerobic exercise alleviated AC-induced oxidative stress (Table 2). One major consequence of cardiac damage resulting from AC-induced oxidative stress is heightened activation of autophagic signaling, the pathway that controls cellular degradation (autophagy). Whereas a constant low level of autophagic signaling is required to ensure proper breakdown of damaged or defective organelles and proteins, long-term elevated signaling can lead to the degradation of important organelles and proteins and also stimulate apoptotic pathways. These authors found that Sprague-Dawley rats pretreated with 5 consecutive days of treadmill running before AC treatment showed lower levels of the autophagic signaling markers. Based on these findings, Smuder et al. suggested that pretreatment with aerobic exercise could potentially increase expression of antioxidants that counteract elevated ROS levels and reduce cardiac damage resulting from AC-induced oxidative stress. This effect would then limit excessive activation of the autophagic signaling pathway.
Importantly, data suggest that even a single acute bout of pretreatment exercise may be sufficient to mitigate AC-induced oxidative stress. Wonders et al. (61) discovered that a single 60-min acute bout of treadmill running before AC treatment completely preserved cardiac LV end-systolic pressure and partially preserved cardiac left ventricular diastolic pressure. The preservation of LV pressure was associated with a decrease in two key markers of LV oxidative stress: malondialdehyde and 4-hydroxy-alkenals. They concluded that the acute bout of aerobic exercise preserved cardiac function by decreasing AC-induced oxidative stress. Such a short exercise intervention has appeal for patients recently diagnosed with cancer who are anxious to get started on chemotherapy.
increased clearance of ac from cardiac tissue.
Studies have also found that aerobic exercise before AC treatment is beneficial because exercise may inhibit AC accumulation and increase the clearance rate of AC from cardiac tissue without sacrificing their therapeutic benefits (19, 28). For example, a study (28) (Table 2) in Sprague-Dawley rats found that preconditioning with either treadmill running or wheel running for 10 wk decreased AC accumulation in the left ventricle. Because the ATP-binding cassette (ABC) transporters (proteins that transport substrates against their concentration gradient) have a high affinity for AC and thus would be efficient in removing AC from cardiac tissue, these authors posited that aerobic exercise facilitates AC clearance through upregulation of ABC transporters. Therefore, aerobic exercise may promote cardiac function through upregulation of ABC transporters to both inhibit AC accumulation and enhance AC clearance from the LV (28).
While these studies show promise for using pretreatment aerobic exercise to mitigate AC-induced cardiotoxicity, the authors made no definitive conclusions about the mechanisms by which aerobic exercise provides cardioprotective benefits in humans. Additionally, although these exercise studies (4, 9, 19, 26, 28, 50, 51, 61) demonstrate that pretreatment with aerobic exercise prevents AC-induced cardiotoxicity, the studies were conducted in small animal models. The authors suggested a host of other potential mechanisms including the ability of aerobic exercise to alter AC metabolism (28), but generalization to human cancer survivors is challenging and further confirmation in human models of disease is needed. To date, however, little is known about whether AC-induced cardiotoxicity is less frequent in those who exercise regularly before being diagnosed and treated with ACs.
Aerobic exercise concomitant with and/or after AC treatment.
There is less research clarifying the effects of aerobic exercise on AC-induced cardiotoxicity concomitant with and/or after AC treatment. Some studies (22, 25, 54) have aimed to address this gap, and the findings are summarized in Table 2. Rather than focusing on different mechanisms by which aerobic exercise could provide cardioprotective benefits, these studies evaluated the efficacy of using aerobic exercise to mitigate AC-induced cardiotoxicity when prescribed concomitant with and/or after AC treatment. The studies either implemented aerobic exercise during (22, 54) AC treatments exclusively or during and after AC treatment (25) using rodent models.
One study (54) (Table 2) used low-intensity treadmill walking concomitant with weekly AC injections to determine the effects of exercise on both AC-induced cardiotoxicity and AC’s ability to inhibit tumor growth. While concomitant low-intensity aerobic exercise was more effective at inhibiting tumor growth, it did not mitigate AC-induced cardiac fibrosis and cardiac wasting (54). Furthermore, low-intensity aerobic exercise during treatment did not provide sufficient stimulation of cardiac hypertrophy biosignaling pathways to activate cardioprotective mechanisms (54).
In contrast, Hayward et al. (22) discovered that voluntary aerobic exercise increased cardioprotection against delayed-onset cardiotoxicity in juvenile male Sprague-Dawley rats. The wheel-running exercise regimen was initiated during the first of seven consecutive daily AC doses and continued for 10 wk thereafter. While aerobic exercise did not mitigate the AC-associated decrease in growth rate, it preserved cardiac function (22). Therefore, it is possible that there is a threshold that must be reached before aerobic exercise will preserve and even enhance the function of preexisting cardiomyocytes (22).
Similarly, another group found that voluntary wheel running during and after AC treatment minimized AC-induced cardiac damage in female Sprague-Dawley rats (25). In this study, rats were separated into either a control or one of two experimental groups; one group was injected with daily doses of AC for 15 consecutive days and another with weekly doses for 6 wk. Concurrently, both groups had 24-h access to a running wheel for 10 wk (25). Although animals injected with ACs ran a shorter total distance over the 10 wk than controls, the distance was adequate to confer cardioprotective benefits (25). Overall, aerobic exercise modified cardiac hemodynamics by decreasing maximal mitral and aortic blood flow velocities, left ventricular pressure, and changes in the myosin heavy chain (MHC) isoforms (25). These findings support the hypothesis that aerobic exercise serves as a powerful stimulus to stimulate and preserve α-MHC expression, which is usually reduced secondary to oxidative damage caused by ACs.
While a smaller number of studies explored the effects of aerobic exercise on AC-induced cardiotoxicity when prescribed concomitant and/or after AC treatment, the findings from these studies provide valuable insight into the use of aerobic exercise to help cancer survivors who are currently undergoing or have previously undergone AC treatment.
Duration of Exercise-Mediated Cardioprotection in AC-Induced Cardiotoxicity
One key question regarding aerobic exercise as a means to protect against AC-induced cardiotoxicity is the duration of exercise training and the subsequent longevity of the cardioprotective benefits. Hydock et al. (26) demonstrated that a relatively long duration of training resulted in a prolonged period of protection. These authors assessed the effects of aerobic exercise on AC-induced alteration of MHC isoform ratio as a surrogate for systolic dysfunction. The study found that 10 wk of pretreatment aerobic exercise consisting of either voluntary wheel running or progressive treadmill regimen minimized AC-induced damage on the cardiomyocytes and maintained proper MHC isoform ratio for 4 wk after the last AC treatment dosage (26).
More relevant to human cancer patients is how short of a period of aerobic training is sufficient for protection, since few cancer patients or their doctors would want to delay treatment for 10 wk for any reason. Results from different studies reveal a wide range therapeutic exercise regimens. There is evidence that pretreatment with treadmill running for 3 wk enhances myocardial tolerance for increased oxidative stress for at least the first 48 h after each bout of exercise (4). Chicco et al. (9) demonstrated increased cardioprotective benefits, in the form of increased expression of HSP72, were sustained for at least 6 days after cessation of aerobic exercise. As noted above, even a single bout of exercise training has been shown to be beneficial in an animal model of AC therapy.
Future Directions
Based on our systematic review of the published literature between 2005 and 2015, there is strong evidence highlighting the role of aerobic exercise to mitigate and possibly prevent AC-induced cardiotoxicity. However, many of the studies were conducted in childhood cancer survivors and rodent models. Because childhood cancer survivors and adult cancer survivors face somewhat different cardiotoxic effects in regard to chronicity, relative lifespan, and underlying comorbidities, exercise studies in both childhood and adult cancer survivors would be helpful in defining more individualized risk burden. Additionally, questions concerning the optimal timing for incorporation of aerobic exercise and the modality or intensity of exercise remain. While specific mechanisms by which aerobic exercise provides cardioprotective benefits in rodent models are recognized, whether the same mechanisms apply in humans remain inconclusive.
Timing of exercise.
In our systematic review, fewer studies explored the effects of aerobic exercise either concomitant or after AC treatment alone–the use of aerobic exercise “concomitant with” or “after” treatment is rarely exclusive of each other. Because there is little consensus on the effects of concomitant or posttreatment aerobic exercise on AC-induced cardiotoxicity, future studies may consider focusing on the separate role of concomitant or posttreatment aerobic exercise in mitigating AC-induced cardiotoxicity.
Exercise modality and intensity.
Because many of the studies used rodent models, the methods of exercise were limited to treadmill or wheel running or swimming. However, apparent from the study findings (4, 9, 19, 22, 25, 26, 28, 50, 51, 54, 61) summarized in Table 2, aerobic exercise is able to prevent or attenuate AC-induced cardiac damage regardless of exercise modality. Since the eventual goal is to incorporate aerobic exercise alongside cancer treatment for humans, research may be conducted on whether different exercise modalities (i.e., running, walking, biking, rowing) offer different levels of cardioprotective benefits and clinical trials be performed in humans. Investigations could also explore the efficacy of utilizing alternate forms of exercise, including resistance training and interval training as means to counteract AC-induced cardiotoxicity.
In addition, exercise intensity levels varied significantly between studies. While aerobic exercise has been demonstrated to be safe in childhood cancer survivors (6), additional research may focus on barriers to implementing and sustaining exercise regimens. Childhood cancer survivors are at high risk for long-term CV-related morbidity and mortality because they have a longer lifespan whereby nontraditional risk of AC and/or radiation exposure may potentiate other factors of cardiometabolic health leading to premature cardiovascular disease. Furthermore, while low-intensity aerobic exercise is generally considered safe, future studies may consider focusing on the efficacy of high-intensity aerobic exercise in this target population.
Mechanisms of cardioprotective benefits.
Currently, multiple mechanisms have been postulated using animal models. However, most authors in these studies claim that the mechanisms by which aerobic exercise mitigates AC-induced cardiotoxicity remain unclear. Understanding the exact mechanisms by which aerobic exercise induces cardioprotective benefits in humans, while challenging, would also be beneficial toward developing additional methods aimed at preventing AC-induced cardiotoxicity. In both clinical and research practices, cardiac tissue biopsies and invasive techniques in human studies would pose obstacles. However, techniques leveraging peripheral leukocytes from blood samples and next generation gene expression analysis may help provide additional surrogate markers for underlying mechanisms when performed in the context of an intervention study. AC exposure has been associated with the presence of diffuse myocardial fibrosis (56). While clinical imaging techniques are limited in its ability to identify mechanisms at the molecular level, with cardiovascular imaging techniques such as cardiac magnetic resonance, one could assess for augmentation of diffuse myocardial fibrosis with exercise.
Conclusion
This review highlights the potential for using aerobic exercise to mitigate the adverse cardiovascular effects of ACs. We found that although AC treatment can decrease exercise capacity, aerobic exercise prescription before, concomitant, and after AC treatment provides cardioprotective benefits, which can mitigate or even prevent AC-induced cardiotoxicity. Both preclinical and clinical data are essential to understanding and improving personalized care of cancer survivors exposed to ACs. However, because the majority of current studies have been confined to rodent models, future studies should also focus on determining the specific mechanisms by which aerobic exercise confers cardioprotective benefits and the efficacy of different exercise timing, modalities, and intensities in humans.
In general, there are noncardiac limitations for exercise training in cancer survivors and cancer patients undergoing active cancer therapy or whose cancers are not in remission. A Cochrane analysis identified that many childhood cancer survivors suffer from motor function disabilities, reduced aerobic capacity, and decreased muscle strength (6). These findings highlight the clinical need for a combined aerobic and strength-training regimen in this population. Acutely or during active cancer therapy, tumors located in the musculoskeletal system may cause severe pain that can limit exercise tolerance (45). Similarly, patients whose lungs or thoracic cavity are affected by cancer frequently experience shortness of breath due to tumor infiltration of lung tissue or compressive effects (45), both of which can limit exercise tolerance. In addition, one challenge for designing a precise aerobic exercise prescription in AC-induced cardiotoxicity is the variable effect of ACs on each patient’s physiological response to exercise. While exercise in the acute setting may be limiting, exercise training after therapy to improve cardiac reserve may prevent future long-term, late cardiac effects that can occur years to decades later.
GRANTS
This work was supported by a pilot grant from the Jonsson Comprehensive Cancer Center Foundation and National Center for Advancing Translational Sciences Clinical and Translational Sciences Institute grant UL1TR000124.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.J.C., P.T.W., and K.-L.N. analyzed data; J.J.C., P.T.W., and K.-L.N. prepared figures; J.J.C., P.T.W., H.R.M., and K.-L.N. drafted manuscript; J.J.C., P.T.W., H.R.M., and K.-L.N. edited and revised manuscript; J.J.C., P.T.W., H.R.M., and K.-L.N. approved final version of manuscript.
REFERENCES
- 1.American Cancer Society Cancer treatment & survivorship: facts & figures 2014–2015 [Online]. 2015. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf [1 Jan. 2015].
- 2.Ascensão A, Ferreira R, Magalhães J. Exercise-induced cardioprotection–biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol : 16–30, 2007. doi: 10.1016/j.ijcard.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 3.Ascensão A, Magalhães J, Soares J, Ferreira R, Neuparth M, Marques F, Oliveira P, Duarte J. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol : H722–H731, 2005. doi: 10.1152/ajpheart.01249.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ashraf J, Roshan VD. Is short-term exercise a therapeutic tool for improvement of cardioprotection against DOX-induced cardiotoxicity? An experimental controlled protocol in rats. Asian Pac J Cancer Prev : 4025–4030, 2012. doi: 10.7314/APJCP.2012.13.8.4025. [DOI] [PubMed] [Google Scholar]
- 5.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell : 1072–1083, 2010. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev : CD008796. 2016. doi: 10.1002/14651858.CD008796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadeddu C, Mercurio V, Spallarossa P, Nodari S, Triggiani M, Monte I, Piras R, Madonna R, Pagliaro P, Tocchetti CG, Mercuro G. Preventing antiblastic drug-related cardiomyopathy: old and new therapeutic strategies. J Cardiovasc Med , Suppl 1, e64–e75, 2016. doi: 10.2459/JCM.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 8.Calvé A, Haddad R, Barama SN, Meilleur M, Sebag IA, Chalifour LE. Cardiac response to doxorubicin and dexrazoxane in intact and ovariectomized young female rats at rest and after swim training. Am J Physiol Heart Circ Physiol : H2048–H2057, 2012. doi: 10.1152/ajpheart.01069.2011. [DOI] [PubMed] [Google Scholar]
- 9.Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol : 182–189, 2006. doi: 10.1097/01.fjc.0000199682.43448.2d. [DOI] [PubMed] [Google Scholar]
- 10.Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab : E629–E636, 2003. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- 11.De Caro E, Smeraldi A, Trocchio G, Calevo M, Hanau G, Pongiglione G. Subclinical cardiac dysfunction and exercise performance in childhood cancer survivors. Pediatr Blood Cancer : 122–126, 2011. doi: 10.1002/pbc.22606. [DOI] [PubMed] [Google Scholar]
- 12.Dolinsky VW, Rogan KJ, Sung MM, Zordoky BN, Haykowsky MJ, Young ME, Jones LW, Dyck JR. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol Metab : E243–E253, 2013. doi: 10.1152/ajpendo.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisele JC, Schaefer IM, Randel Nyengaard J, Post H, Liebetanz D, Brüel A, Mühlfeld C. Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic Res Cardiol : 12–21, 2008. doi: 10.1007/s00395-007-0684-x. [DOI] [PubMed] [Google Scholar]
- 14.Elsevier Levels of Evidence for Primary Research 1 [Online]. 2005. https://www.elsevier.com/__data/promis_misc/Levels_of_Evidence.pdf [1 Jan. 2016].
- 15.Filopei J, Frishman W. Radiation-induced heart disease. Cardiol Rev : 184–188, 2012. doi: 10.1097/CRD.0b013e3182431c23. [DOI] [PubMed] [Google Scholar]
- 16.Gallucci G, Coccaro M, Storto G, Lapadula L, Tartarone A, Nappi A, Cammarota A, Buonerba C, Di Lorenzo G, Fusco V, Aieta M. The clinical impact of a cardiologic follow-up in breast cancer survivors: an observational study. Int J Immunopathol Pharmacol : 1221–1227, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Liu W, Huang L, Zhang T, Mei Z, Wang X, Gong J, Zhao Y, Xie F, Ma J, Qian L. HSP70 inhibits stress-induced cardiomyocyte apoptosis by competitively binding to FAF1. Cell Stress Chaperones : 653–661, 2015. doi: 10.1007/s12192-015-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol : 3777–3784, 2008. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson NM, Greufe SE, Hydock DS, Hayward R. Doxorubicin-induced vascular dysfunction and its attenuation by exercise preconditioning. J Cardiovasc Pharmacol : 355–360, 2013. doi: 10.1097/FJC.0b013e31829c9993. [DOI] [PubMed] [Google Scholar]
- 20.Guimaraes-Filho FV, Tan DM, Braga JCF, Rodrigues A, Waib PH, Matsubara BB. Ventricular systolic reserve in asymptomatic children previously treated with low doses of anthracyclines: a longitudinal, prospective exercise echocardiography study. Pediatr Blood Cancer : 548–552, 2012. doi: 10.1002/pbc.24000. [DOI] [PubMed] [Google Scholar]
- 21.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A; American College of Sports Medicine; American Heart Association . Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation : 1081–1093, 2007. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 22.Hayward R, Lien CY, Jensen BT, Hydock DS, Schneider CM. Exercise training mitigates anthracycline-induced chronic cardiotoxicity in a juvenile rat model. Pediatr Blood Cancer : 149–154, 2012. doi: 10.1002/pbc.23392. [DOI] [PubMed] [Google Scholar]
- 23.Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart : 252–258, 2009. doi: 10.1136/hrt.2008.149088. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ : d5928, 2011. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hydock DS, Lien CY, Jensen BT, Parry TL, Schneider CM, Hayward R. Rehabilitative exercise in a rat model of doxorubicin cardiotoxicity. Exp Biol Med (Maywood) : 1483–1492, 2012. doi: 10.1258/ebm.2012.012137. [DOI] [PubMed] [Google Scholar]
- 26.Hydock DS, Lien CY, Jensen BT, Schneider CM, Hayward R. Exercise preconditioning provides long-term protection against early chronic doxorubicin cardiotoxicity. Integr Cancer Ther : 47–57, 2011. doi: 10.1177/1534735410392577. [DOI] [PubMed] [Google Scholar]
- 27.Jarfelt M, Kujacic V, Holmgren D, Bjarnason R, Lannering B. Exercise echocardiography reveals subclinical cardiac dysfunction in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer : 835–840, 2007. doi: 10.1002/pbc.21289. [DOI] [PubMed] [Google Scholar]
- 28.Jensen BT, Lien CY, Hydock DS, Schneider CM, Hayward R. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J Cardiovasc Pharmacol : 263–269, 2013. doi: 10.1097/FJC.0b013e3182982ce0. [DOI] [PubMed] [Google Scholar]
- 29.Jones L, Dolinsky V, Haykowsky M, Patterson I, Jones L, Allen J, Scott J, Rogan K, Khouri M, Hornsby W, Young M, Peppercorn J, Kimmick G, Dyck J. Effects of aerobic training to improve cardiovascular function and prevent cardiac remodeling after cytotoxic therapy in early breast cancer. Cancer Res , Suppl 8: 5024, 2011. doi: 10.1158/1538-7445.AM2011-5024. [DOI] [Google Scholar]
- 30.Jones LW, Liu Q, Armstrong GT, Ness KK, Yasui Y, Devine K, Tonorezos E, Soares-Miranda L, Sklar CA, Douglas PS, Robison LL, Oeffinger KC. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol : 3643–3650, 2014. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol : H928–H935, 2008. doi: 10.1152/ajpheart.01231.2007. [DOI] [PubMed] [Google Scholar]
- 32.Kaye DM, Jennings G, Angus JA. Evidence for impaired endothelium dependent vasodilation in experimental left ventricular dysfunction. Clin Exp Pharmacol Physiol : 709–719, 1994. doi: 10.1111/j.1440-1681.1994.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 33.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiologic stimulation. Circulation : 2727–2735, 2011. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkham AA, Davis MK. Exercise Prevention of Cardiovascular Disease in Breast Cancer Survivors. J Oncol : 917606, 2015. doi: 10.1155/2015/917606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, Cosyns B, Coucke P, Dulgheru R, Edvardsen T, Gaemperli O, Galderisi M, Griffin B, Heidenreich PA, Nieman K, Plana JC, Port SC, Scherrer-Crosbie M, Schwartz RG, Sebag IA, Voigt J-U, Wann S, Yang PC; European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; Society for Cardiovascular Magnetic Resonance; Society of Cardiovascular Computed Tomography . Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging : 721–740, 2013. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 36.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA : 612–617, 2007. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mezzani A, Corrà U, Giannuzzi P. Central adaptations to exercise training in patients with chronic heart failure. Heart Fail Rev : 13–20, 2008. doi: 10.1007/s10741-007-9053-y. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement (Reprinted from Annals of Internal Medicine). Phys Ther : 873–880, 2009. [PMC free article] [PubMed] [Google Scholar]
- 39.Mulrooney DA, Blaes AH, Duprez D. Vascular injury in cancer survivors. J Cardiovasc Transl Res : 287–295, 2012. doi: 10.1007/s12265-012-9358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichon MF, Cvitkovic F, Hacene K, Delaunay J, Lokiec F, Collignon MA, Pecking AP. Drug-induced cardiotoxicity studied by longitudinal B-type natriuretic peptide assays and radionuclide ventriculography. In Vivo : 567–576, 2005. [PubMed] [Google Scholar]
- 42.Pinarli FG, Oğuz A, Tunaoğlu FS, Karadeniz C, Gökçora N, Elbeg S. Late cardiac evaluation of children with solid tumors after anthracycline chemotherapy. Pediatr Blood Cancer : 370–377, 2005. doi: 10.1002/pbc.20281. [DOI] [PubMed] [Google Scholar]
- 43.Ryerson AB, Border WL, Wasilewski-Masker K, Goodman M, Meacham L, Austin H, Mertens AC. Assessing anthracycline-treated childhood cancer survivors with advanced stress echocardiography. Pediatr Blood Cancer : 502–508, 2015. doi: 10.1002/pbc.25328. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz K, Courneya K, Matthews C, Demark-Wahnefried W, Galvao D, Pinto B, Irwin M, Wolin K, Segal R, Lucia A, Schneider C, von Gruenigen V, Schwartz A. American College of Sports Medicine . American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exerc : 1409–1426, 2010. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz BAL, Edition T, Durstine JL, Moore GE, Painter PL, Roberts SO. Employ exercise as a tool for helping cancer patients. In: ACSM’s Exercise Management for Persons With Chronic Diseases and Disabilities (3rd ed.). Champaign, IL: American College of Sports Medicine, 2009, p. 2–5. [Google Scholar]
- 46.Scott JM, Koelwyn GJ, Hornsby WE, Khouri M, Peppercorn J, Douglas PS, Jones LW. Exercise therapy as treatment for cardiovascular and oncologic disease after a diagnosis of early-stage cancer. Semin Oncol : 218–228, 2013. doi: 10.1053/j.seminoncol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation : 642–650, 2011. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol : 122–128, 2007. doi: 10.1007/s12012-007-0006-4. [DOI] [PubMed] [Google Scholar]
- 49.Shimomura Y, Baba R, Watanabe A, Horikoshi Y, Asami K, Hyakuna N, Iwai A, Matsushita T, Yamaji K, Hori T, Tsurusawa M; Japanese Childhood Cancer and Leukemia Study Group (JCCLSG) . Assessment of late cardiotoxicity of pirarubicin (THP) in children with acute lymphoblastic leukemia. Pediatr Blood Cancer : 461–466, 2011. doi: 10.1002/pbc.23012. [DOI] [PubMed] [Google Scholar]
- 50.Shirinbayan V, Roshan VD. Pretreatment effect of running exercise on HSP70 and DOX-induced cardiotoxicity. Asian Pac J Cancer Prev : 5849–5855, 2012. doi: 10.7314/APJCP.2012.13.11.5849. [DOI] [PubMed] [Google Scholar]
- 51.Smuder AJ, Kavazis AN, Min K, Powers SK. Doxorubicin-induced markers of myocardial autophagic signaling in sedentary and exercise trained animals. J Appl Physiol (1985) : 176–185, 2013. doi: 10.1152/japplphysiol.00924.2012. [DOI] [PubMed] [Google Scholar]
- 52.Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MA, Papadimitriou C. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev : 473–483, 2012. doi: 10.1016/j.ctrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc : 2080–2090, 2013. doi: 10.1249/MSS.0b013e31829a3b63. [DOI] [PubMed] [Google Scholar]
- 54.Sturgeon K, Schadler K, Muthukumaran G, Ding D, Bajulaiye A, Thomas NJ, Ferrari V, Ryeom S, Libonati JR. Concomitant low-dose doxorubicin treatment and exercise. Am J Physiol Regul Integr Comp Physiol : R685–R692, 2014. doi: 10.1152/ajpregu.00082.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sturgeon KM, Ky B, Libonati JR, Schmitz KH. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res Treat : 219–226, 2014. doi: 10.1007/s10549-013-2808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS, Thompson RB. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson : 48, 2013. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA III, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Heart Association Council on Clinical Cardiology; American College of Sports Medicine . Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation : 2358–2368, 2007. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 57a.van Norren K, van Helvoort A, Argilés JM, van Tuijl S, Arts K, Gorselink M, Laviano A, Kegler D, Haagsman HP, van der Beek EM. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer : 311–314, 2009. doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol : 938–945, 2014. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 59.Volkova M, Russell R 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev : 214–220, 2011. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson CL, Stratton K, Leisenring WL, Oeffinger KC, Nathan PC, Wasilewski-Masker K, Hudson MM, Castellino SM, Stovall M, Armstrong GT, Brinkman TM, Krull KR, Robison LL, Ness KK. Decline in physical activity level in the Childhood Cancer Survivor Study cohort. Cancer Epidemiol Biomarkers Prev : 1619–1627, 2014. doi: 10.1158/1055-9965.EPI-14-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wonders KY, Hydock DS, Schneider CM, Hayward R. Acute exercise protects against doxorubicin cardiotoxicity. Integr Cancer Ther : 147–154, 2008. doi: 10.1177/1534735408322848. [DOI] [PubMed] [Google Scholar]
- 62.Wu S, Ko YS, Teng MS, Ko YL, Hsu LA, Hsueh C, Chou YY, Liew CC, Lee YS. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: in vitro and in vivo studies. J Mol Cell Cardiol : 1595–1607, 2002. doi: 10.1006/jmcc.2002.2110. [DOI] [PubMed] [Google Scholar]