Abstract
Upon its secretion from pancreatic β-cells, insulin reaches the liver through the portal circulation to exert its action and eventually undergo clearance in the hepatocytes. In addition to insulin secretion, hepatic insulin clearance regulates the homeostatic level of insulin that is required to reach peripheral insulin target tissues to elicit proper insulin action. Receptor-mediated insulin uptake followed by its degradation constitutes the basic mechanism of insulin clearance. Upon its phosphorylation by the insulin receptor tyrosine kinase, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) takes part in the insulin-insulin receptor complex to increase the rate of its endocytosis and targeting to the degradation pathways. This review summarizes how this process is regulated and how it is associated with insulin-degrading enzyme in the liver. It also discusses the physiological implications of impaired hepatic insulin clearance: Whereas reduced insulin clearance cooperates with increased insulin secretion to compensate for insulin resistance, it can also cause hepatic insulin resistance. Because chronic hyperinsulinemia stimulates hepatic de novo lipogenesis, impaired insulin clearance also causes hepatic steatosis. Thus impaired insulin clearance can underlie the link between hepatic insulin resistance and hepatic steatosis. Delineating these regulatory pathways should lead to building more effective therapeutic strategies against metabolic syndrome.
Introduction
Insulin is cleared mainly in liver and kidney, with the former contributing to a greater extent than the latter in removing endogenously released hormone. Skeletal muscle, adipocytes, and other extra-hepatic insulin target tissues/cells contribute to peripheral insulin clearance (169). Following its pulsatile secretion from pancreatic β-cells (171), insulin is transported rapidly via the portal vein to reach the hepatocytes by crossing the fenestrations in the liver sinusoidal endothelium (134).
Under normal physiological conditions, up to 80% of secreted insulin is cleared during its first passage through the liver (50), and the half-life of insulin in the portal circulation is ~3–5 min (52). Receptor-mediated insulin uptake followed by insulin degradation in hepatocytes constitutes the basic mechanism of hepatic insulin clearance (204). Unprocessed insulin is delivered into the systemic circulation to exert its action in target peripheral tissues and brain, and to undergo further receptor-mediated uptake and degradation in the periphery (204). Unprocessed insulin can also return to the liver via the hepatic artery for a second round of insulin degradation (204). Contrary to the liver, insulin transport across endothelial cells of peripheral tissues is the rate-limiting step for insulin uptake (12). In this manner, endothelial cells in extra-hepatic insulin target tissues contribute to the regulation of systemic insulin homeostasis and action (107, 116).
The association between Type 2 diabetes (T2D) and impaired insulin clearance was initially reported in 1949 (22). Since then, several studies have identified defective insulin clearance as a critical factor in the pathogenesis of hyperinsulinemia in metabolic syndrome (97, 166), particularly among Hispanics and African Americans (77, 113, 125). Reduced insulin extraction has also been detected in aging (134) and in other metabolic abnormalities irrespective of ethnicity, including obesity (100, 102, 127, 206), non-alcoholic steatohepatitis (21, 109, 128), and polycystic ovarian syndrome (36). Moreover, hepatic, but not extra-hepatic, insulin clearance is lower in African Americans than in European American women (165), pointing to the dominant role of hepatic insulin clearance in maintaining insulin sensitivity in the African-American population. In Japanese T2D patients, however, peripheral rather than hepatic insulin clearance appears to be more adversely affected (154). Collectively, these studies link impaired insulin clearance to insulin resistance and other manifestations of metabolic syndrome.
Despite the wealth of evidence supporting a strong association between reduced insulin clearance and metabolic disorders, the cause-effect relationship between insulin resistance and its hallmark, hyperinsulinemia, remains obscure (189). Chronic hyperinsulinemia emerges from increased insulin secretion (68) as well as reduced insulin clearance (7, 77, 100, 109) to compensate for peripheral insulin resistance, in particular in individuals with obesity (69, 96, 98). Several epidemiological studies have suggested that reduced insulin clearance in obese subjects is more relevant than the increase in insulin secretion to compensate for peripheral insulin resistance (58, 59). In obese patients, reduced insulin clearance seems to be secondary to insulin resistance rather than to robust visceral obesity (101, 206). Consistently, a weight loss by ~10% of initial body weight increases insulin clearance and restores insulin sensitivity (96).
On the other hand, impaired insulin clearance can initiate hyperinsulinemia-driven systemic insulin resistance (16, 39, 170). Supportive evidence of the causative role of chronic hyperinsulinemia in the pathogenesis of secondary insulin resistance in obesity and T2D is mounting (131, 175, 201). Mechanistically, this could be mediated, at least in part, by desensitization and lysosomal downregulation of the insulin receptor by chronically elevated levels of insulin (106). Such a paradigm is supported by the systemic insulin resistance state that develops in response to impairing hepatic insulin clearance in mice with liver-specific inactivation (173) and deletion (75) of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), a glycoprotein that plays a central role in promoting hepatic insulin clearance (157, 173). In this review, we will describe how CEACAM1-dependent insulin clearance pathways regulate homeostatic insulin levels and, subsequently, insulin sensitivity. We will also describe how CEACAM1 coordinates its effect with insulin-degrading enzyme (IDE) to regulate intracellular insulin trafficking and targeting to the degradation process to remove insulin from the circulation.
Role of CEACAM1 in Receptor-Mediated Insulin Endocytosis
CEACAM1: General Structure
CEACAM1 is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily of the CEA family of cell adhesion molecules (91, 143, 158). Before its nomenclature was revised in 1999 (14), CEACAM1 had been referred to as BGP1, CD66a, C-CAM1, Ecto-ATPase, and pp120/HA4 (93, 142).
CEACAM1 is ubiquitously expressed in particular in the liver and to a lower extent in the kidney, main sites of insulin clearance (93). The structure as well as the cellular and tissue distribution of CEACAM1 protein are highly conserved among species (33, 42, 174, 179). In humans and rodents, CEACAM1 is expressed as two alternatively spliced isoforms with CEACAM1–4L containing a long (L), highly conserved cytoplasmic tail of 71 amino acids (based on the rat sequence) (152), and the short (CEACAM1–4S) containing only 10 amino acids of the intracellular tail and lacking critical phosphorylation sites: Ser503, Tyr488, and Tyr513 residues (FIGURE 1). Genomic and cDNA analyses revealed that these isoforms result from the alternative splicing of exon 7 of the Ceacam1 gene (comprised of nine exons) (140). Recent studies have shown that alternative splicing is regulated by interferon regulatory factor 1 (44). CEACAM1–4L and CEACAM1–4S contain four immunoglobulin (IgG)-like structures in their extracellular domains that foster the cell adhesion property of CEACAM1, which is chiefly regulated by the NH2-terminal IgV-like loop (93). The other common pair of CEACAM1 in humans and rodents is CEACAM1–2L and CEACAM1–2S, which possess two IgG loops in their extracellular domains emerging from alternative splicing of exons 3 and 4 (150).
FIGURE 1.
Regulation of Insulin Signaling by CEACAM1
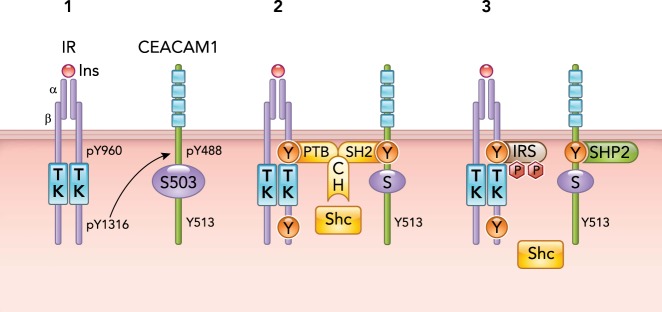
Insulin (Ins) binding to the α-subunit of its receptor (IRα) activates its tyrosine kinase (TK) to catalyze its autophosphorylation on tyrosine 960 (pY960) in the juxtamembrane domain and on tyrosine 1316 (pY1316) on the COOH-terminus tail of the β-subunit (IRβ) of the receptor (1). CEACAM1 phosphorylation on Y488 requires phosphorylation on Y1316 in IRβ (1). pY488 on CEACAM1 binds to the SH2 domain of Shc, which, in turn, allows the binding of the PTB domain of Shc to pY960 in IRβ. In this manner, Shc mediates the formation of a complex between IR and CEACAM1 (2). CEACAM1 binds to SHP2 to sequester it. This protects IRS against the phosphatase and prolongs its activation. This also releases Shc from pY960 in IRβ (3).
CEACAM1-L contains in its cytoplasmic tail a basally phosphorylated Ser503 residue and two tyrosine residues (Tyr488 and Tyr513) that could be phosphorylated by Src-kinase and dephosphorylated by the inhibitory SH2-containing tyrosine phosphatases 1 and 2 (SHP-1 and SHP-2) (24, 139, 146). One of these tyrosine residues (Tyr488) undergoes phosphorylation by ligand-stimulated insulin receptor (IR) and epidermal growth factor receptor (EGFR) tyrosine kinases (2, 147). Tyr488 phosphorylation site of CEACAM1-L mediates several of its functions, including regulation of insulin and fatty acid metabolism in liver (71, 142, 149, 173) and inhibition of cell proliferation in response to insulin and EGF (2, 172), among others (13, 80, 93). Whereas the short isoform of CEACAM1 (CEACAM1-S) is devoid of these phosphorylation sites, it can still undergo phosphorylation by protein kinases to regulate cytoskeletal dynamics (54, 55, 104). In addition to the relative distribution of the long vs. the short isoform, the differential distribution to the lateral vs. the apical domains of the plasma membrane in polarized epithelial cells determines the ultimate function of CEACAM1 (199, 200). Under normal physiological conditions, CEACAM1-S is exclusively expressed on the apical domain, whereas CEACAM1-L is expressed on the lateral and apical domains of polarized epithelial cells (199, 200). A main mediator of CEACAM1’s regulatory role in insulin and fatty acid metabolism is the phosphorylation state of its long isoform, which is, in turn, regulated by an interplay of several kinases and phosphatases (142, 153). Consistently, experiments in transfected cell systems ruled out a significant role for CEACAM1-S in receptor-mediated insulin uptake (34, 71). Thus we will focus this review on the role of the long isoform in insulin clearance and fatty acid synthesis in hepatocytes and how this is mediated by its phosphorylation by the IR tyrosine kinase.
CEACAM1 Phosphorylation and Regulation of Insulin Signaling
Endogenous insulin is secreted from pancreatic β-cells into the portal vein in acute pulses to rapidly reach the sinusoidal domain of the hepatocyte via fenestrae in liver capillaries. Insulin binding activates the tyrosine kinase of the receptor to initiate a chain of phosphorylation/dephosphorylation reactions, starting with the autophosphorylation of the receptor by its own tyrosine kinase in the cytosolic tail of its β-subunits (IRβ) (41, 82, 164, 184, 213). In addition to the receptor, several other substrates have been identified, including insulin receptor substrate (IRS) family of proteins and Src-homology-2-containing protein (Shc) (185), which undergo phosphorylation in a spatiotemporal manner to avoid competition for binding to Tyr960 in the juxtamembrane domain of IRβ. The perinuclear distribution of Shc facilitates the relative delay in its binding to Tyr960 in IRβ subunit.
Expressed at a very high level on the sinusoidal surface membrane of hepatocytes, CEACAM1-L (from here on referred to as CEACAM1 for simplicity, unless otherwise mentioned) undergoes rapid phosphorylation by IR in intact cells (147). Phosphoamino acid analysis in H35 rat hepatoma cells indicated that CEACAM1 is basally and constitutively phosphorylated on serine and that this phosphorylation decreases significantly upon insulin treatment to be replaced by tyrosine phosphorylation (162). Site-directed mutagenesis revealed that Ser503 is the main site of basal serine phosphorylation (147). Neither the serine kinase that catalyzes this reaction nor the potential serine phosphatase that reverses its activity in the presence of insulin has been clearly identified, but Ser503 is located in a potential cAMP-dependent protein kinase phosphorylation site (Lys-Arg-Pro-Thr-Ser503) (124, 192), and the phosphatase activity appears to require an intact Tyr513 in the cytosolic tail of CEACAM1 (141).
In contrast to most other substrates, CEACAM1 is not phosphorylated by the IR’s close relative, the insulin-like growth factor receptor 1 (IGF-1R) (144). This differential phosphorylation is mediated by the non-conserved Tyr1316 in the COOH-terminus tail of IRβ, in contrast to the other substrates, the phosphorylation of which requires the conserved Tyr960 in the juxtamembrane domain of this subunit. This differential dependence on key tyrosine residues in IRβ precludes a direct competition for phosphorylation by IR between CEACAM1 and IRS proteins, and allows their simultaneous phosphorylation by the activated receptor (147).
Site-directed mutagenesis revealed that Tyr488 (Asp-Asp-Val-Ser-Tyr488), located downstream of two acidic amino acids (159), is the main site of CEACAM1 phosphorylation by the IR tyrosine kinase (FIGURE 1). This requires an intact Ser503 in its cytoplasmic tail, as demonstrated by the inability of insulin to stimulate Tyr488 phosphorylation when Ser503 is mutated to Ala (S503A mutant) (147). Even though Tyr513 is also located downstream of glutamic acid (Glu-Thr-Val-Tyr513), it does not undergo phosphorylation by IR (147). In response to insulin, phosphorylated Tyr488 binds to the Src Homology 2 (SH2) domain of Shc (24) and brings it closer to the juxtamembrane region to bind to Tyr960 of the receptor via its NH2-terminal phosphotyrosine-binding domain (PTB) (FIGURE 1) (172). CEACAM1 interaction bestows on Shc the ability to compete with IRS for binding to Tyr960 of IR and decrease PI3Kinase/Akt activation in response to insulin. In addition, CEACAM1 binding to Shc sequesters this main coupler of Grb2 adaptor to the receptor and reduces the Ras/MAPKinase activity and, subsequently, the mitogenic effect of insulin (172). Thus, by binding to Shc, CEACAM1 phosphorylation by the receptor can regulate both arms of the insulin signaling pathways. CEACAM1 phosphorylation on Tyr488 can also regulate insulin signaling through its binding to SH2-containing phosphatases, such as SHP1 (46, 139) and SHP2/Syp (146, 153). We have shown that this interaction sequesters SHP2 and reduces its binding to IRS proteins, prolonging the activation of the IRS/PI3Kinase pathway in response to insulin (FIGURE 1) (146). Because of the opposing effect of CEACAM1 binding to Shc and SHP2 on IRS/PI3Kinase activation, it is conceivable that its interactions with these downstream signaling molecules are tightly regulated by the metabolic demand on the cell, as discussed below.
CEACAM1 Increases Receptor-Mediated Insulin Endocytosis and Targeting to the Degradation Process
It has been suggested that the internalization of the insulin-IR complex is a two-step process. The first step involves redistribution of the receptor from the microvilli to the nonvillous membrane of the cell, whereas the second step consists of anchoring the receptor in clathrin-coated pits (29). Activation of IR by insulin is required for the rapid internalization of the insulin-IR complex into clathrin-coated vesicles to be ultimately targeted to the degradation process (27, 28, 78, 130, 218). Whereas insulin undergoes degradation, the insulin receptor may either recycle back to the plasma membrane or translocate into lysosomes for degradation. The fate of the insulin receptor depends on the duration and saturating doses of insulin (45). Under normal physiological conditions, the internalized receptor is recycled to the plasma membrane (221), as opposed to being targeted to lysosomal degradation under conditions of prolonged hyperinsulinemia (70). Furthermore, non-receptor-mediated processes, such as pinocytosis, are more relevant in chronic hyperinsulinemic states (86).
Although there is a general agreement that the juxtamembrane domain of the receptor plays an important role in insulin endocytosis (10, 83, 99), the underlying mechanisms remain controversial despite ample investigations on the role of the two Tyr-centered sequences around Tyr953 (Gly-Pro-Leu-Tyr953) and Tyr960 (Asn-Pro-Glu-Tyr960) located in tight β-turn structures that conform to the internalization signaling motif. Moreover, the COOH-terminus tail of IRβ appears to play a significant role in stabilizing the receptor to prevent its targeting to the lysosomal compartment (56, 119). Thus we posited that, in light of its regulation by Tyr1316 in the COOH-terminus tail of IRβ, CEACAM1 phosphorylation plays a significant role in the intracellular trafficking of the insulin-IR complex, including lysosomal exclusion of the receptor under normo-insulinemic conditions.
Whereas Tyr960 of the juxtamembrane domain of IRβ is required for phosphorylation of IRS proteins, studies in transfected Chinese Hamster ovary cells ruled out a role for IRS-1 in insulin-receptor endocytosis (29). In contrast, co-transfecting CEACAM1-L, but not CEACAM1-S or the phosphorylation-defective mutants of CEACAM1 (Y488F, S503A, and Y488F/Y513F), with human IR-A form (hIR-A) in NIH-3T3 fibroblasts increased the rate of uptake of 125[I]Insulin via its receptor and its degradation by two- to threefold relative to cells expressing hIR-A alone (71). On the other hand, inhibiting CEACAM1 expression in H4-II-E rat hepatoma cells by antisense mRNA transfection reduced receptor-mediated insulin internalization and degradation (71). Thus phosphorylation of CEACAM1 regulates receptor-mediated insulin uptake followed by its degradation (34).
Mutating Tyr960 to Phe in hIR-A did not affect CEACAM1 phosphorylation but abolished its stimulating effect on insulin endocytosis and degradation in NIH-3T3 fibroblasts (145), pointing to a role for IRβ's Tyr960 in mediating CEACAM1-dependent insulin endocytosis pathways. This is consistent with a model locating phosphorylated CEACAM1 in the insulin-IR endocytosis complex, the formation of which is mediated by the binding of Shc to phosphorylated Tyr488 of CEACAM1 via its SH2 domain and to phosphorylated Tyr960 of IRβ through its PTB domain (FIGURES 1 AND 2). This complex is targeted to clathrin-coated pits/vesicles via the collagen homology domain of Shc (FIGURE 2), which is known to associate with adaptor protein-2 (AP2) in the adaptin structure of the clathrin coat of endocytosis vesicles to participate in ligand-stimulated internalization of growth factor receptors (155).
FIGURE 2.
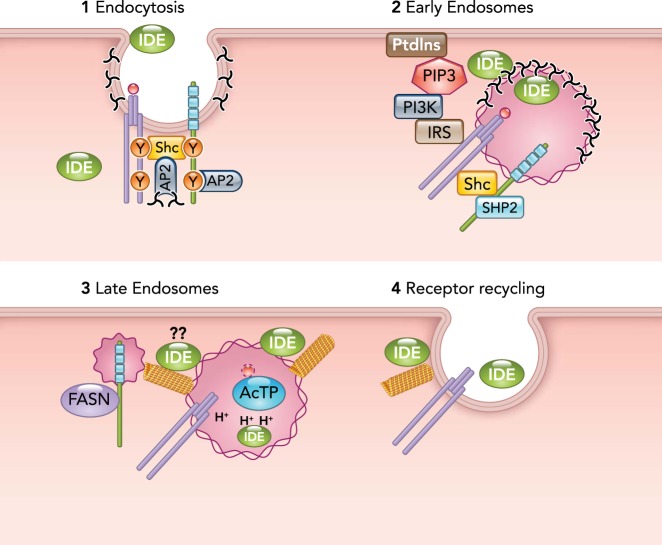
Coordinated regulation of receptor-mediated insulin intracellular trafficking by CEACAM1 and IDE
Step 1: insulin binding to its receptor activates it to form a complex with CEACAM1 via Shc (as described in the legend to FIGURE 1). Together with Y513 in CEACAM1, the collagen homology domain of Shc targets the complex to the AP2 adaptin in the clathrin-coated pits. Step 2: the complex is internalized inside the early endosomes, and the extracellular IDE becomes sequestered into the vesicular lumen. Progressive SHP2 binding to pY488 on CEACAM1 destabilizes the complex and causes a progressive loss of Shc to allow IRS binding to pY960. This leads to increased IRS phosphorylation and activation of PI3Kinase that, in turn, mediates the recruitment of cytosolic IDE to the outer membrane and maintainance of CEACAM1 at the endosomes. Luminal IDE degrades insulin. Step 3: as the endosomes mature into late endosomes and acidify (H+), IDE is inactivated. FASN binding to pY488 pulls off CEACAM1 from the insulin-IR complex to destabilize it and allow insulin to dissociate from its receptor to be degraded by the acidic thiol protease (AcTP). Step 4: the receptor is recycled back to the membrane during exocytosis, resulting from microtubular polymerization, which is possibly regulated by IDE at the outer membrane of the vesicle. Whether this IDE mediates the formation of the CEACAM1/FASN vesicle remains to be tested.
Additionally, Tyr513 of CEACAM1, located within the Tyr-x-x-Φ sequence (Tyr513-Ser-Val-Val) that is shared by proteins sorted into clathrin-coated vesicles (178, 205) targets CEACAM1 to AP2-associated clathrin-coated vesicles at the plasma membrane of polarized Madin-Darby canine kidney (MDCK) epithelial cells (199) (FIGURE 2, step 1). In contrast, Tyr488 fails to target CEACAM1 directly to clathrin-coated vesicles despite its localization in an AP2-targeted tyrosine-centered motif (Tyr488-Ser-Val-Leu) (199).
In addition to playing a key role in the inward routing of CEACAM1 into endocytosis vesicles, Tyr513, but not Tyr488, plays a dominant role in targeting the newly synthesized glycoprotein from the trans-Golgi network (TGN) via AP1-associated vesicles to its final destination on the lateral domain of the plasma membrane in MDCK cells (199). Tyrosine phosphorylation of this residue increases PI3Kinase activity that leads to endosomal accumulation of CEACAM1 and its rapid disappearance from the lateral membrane of MDCK (199). Additionally, Ser503 plays an essential role in regulating the posttranslational maturation of the glycosylated moieties of CEACAM1 during its vectorial transport from TGN to the plasma membrane (35), as shown by the higher diversion of the S503A CEACAM1 mutant to the lysosomal compartments of transfected NIH-3T3 cells (35), and of wild-type CEACAM1 when MDCK cells were treated with Staurosporine, a Ser/Thr kinase inhibitor (199). Thus it is conceivable that being subjected continuously to clathrin-mediated transport to and from the plasma membrane in a manner depending on key amino acid residues in its cytosolic tail, CEACAM1 serves as a faithful chaperone to the insulin-IR complex during its intracellular trafficking.
That CEACAM1 phosphorylation is required to mediate its stimulatory effect on insulin endocytosis and degradation is further supported by the observations that 1) CEACAM1 fails to regulate receptor-mediated IGF-1 endocytosis (145) and 2) blunting the cell adhesion property of CEACAM1 by mutating Arg98 in the NH2-terminal IgV-like ectoplasmic domain to Ala (193) without modulating its phosphorylation state does not adversely affect the ability of CEACAM1 to stimulate insulin endocytosis in NIH-3T3 fibroblasts (195).
To undergo degradation, insulin has to dissociate from its receptor in the acidic environment of late endosomes (11). This requires destabilization of the receptor-Shc-CEACAM1 complex. As we have shown (149), fatty acid synthase (FASN) binds to phosphorylated Tyr488, which would, in turn, pull CEACAM1 off the complex to destabilize it and allow the separation of insulin from its receptor. The pathways mediating the vesicular separation of the CEACAM1-FASN complex are not known but are consistent with CEACAM1 not being involved in receptor-mediated insulin retro-endocytosis (120), which appears to be regulated mainly by a protein kinase C (PKC)-mediated process (72).
In agreement with the observation that insulin endocytosis is not required for its signaling but mainly for its removal (130), our studies summarized above demonstrated that insulin-induced phosphorylated CEACAM1 increases the cellular uptake of insulin, likely by stabilizing the insulin-IR complex via its binding to Shc, which together with Tyr513 targets the complex to the endocytosis vesicles to increase the rate of insulin degradation (130, 218). Because Tyr1316 in the COOH-terminus tail of IRβ is required for CEACAM1 phosphorylation (196), it is likely that the association of phosphorylated CEACAM1 with the insulin-IR complex mediates the stabilizing effect of the COOH-terminus tail of IRβ to prevent receptor’s targeting to lysosomes under normo-insulinemic conditions.
CEACAM1 Links Insulin Removal with Low Fatty Acid Synthesis in Hepatocytes
Under non-fasting conditions, insulin receptor (210), CEACAM1 (93), and FASN (95, 188) are highly expressed in hepatocytes, a major site of insulin clearance from the portal circulation (93, 142). In human adult hepatocytes, the insulin receptor gene is expressed as two alternatively spliced isoforms that differ by the absence (IR-A) or presence (IR-B) of a 12-amino acid segment encoded by exon 11 at the COOH terminus of the α-subunit of the receptor (60, 137, 187, 212). IR-A is less abundant but has a higher affinity for insulin than IR-B. This differential binding affinity to insulin may represent a mechanism whereby hepatic insulin receptors are less sensitive to insulin-induced receptor downregulation to protect the liver against the physiologically higher levels of insulin in the portal vein (208). We have shown that CEACAM1 preferentially promotes insulin endocytosis via IR-A isoform without affecting its recycling to the surface membrane despite being equally phosphorylated by both receptor isoforms (120). This is consistent with CEACAM1 promoting insulin uptake by IR-A to target insulin to the degradation process and protect the expression of the low abundant high-affinity receptor (IR-A) against insulin-induced downregulation, in particular when plasma insulin levels are high. By regulating the relative distribution of IR-A and IR-B in hepatocytes, CEACAM1-dependent pathways contribute to maintaining insulin sensitivity in the liver.
Moreover, we have shown that, in response to acute insulin, internalized CEACAM1 binds to FASN on its phosphorylated Tyr488 residue to separate FASN from the insulin-IR complex and suppress its enzymatic activity. The physiological implication of this phospho-CEACAM1/FASN interaction is not only to destabilize the insulin-IR complex in late endosomes but also to restrict hepatic de novo lipogenesis in the face of the higher levels of hepatic than extra-hepatic lipogenic gene expression resulting from the physiologically higher insulin concentration in the portal than in the systemic circulation (208). Thus we presented the first line of evidence that, in contrast to its chronic stimulation of lipogenic gene transcription (156), acutely released pulses of insulin from pancreatic β-cells suppress FASN activity in the hepatocyte in a manner depending on insulin-mediated phosphorylation of CEACAM1 and internalization. Using Ob/Ob obese mice (149), we have demonstrated that, under conditions of hyperinsulinemia, when the pulsatility of insulin release diminishes (129), and insulin signaling and CEACAM1 phosphorylation are compromised, cellular insulin uptake and its acute effect on FASN activity are blunted, giving way to the long-term positive effect of chronically elevated levels of insulin on lipogenesis that are mediated by the sterol regulatory element binding protein (SREBP1c), a master transcriptional regulator of lipogenic genes (156), and the upstream stimulatory factor (USF-1) (216). This intertwined modulatory effect of CEACAM1 on insulin and lipid metabolism is consistent with a physiological model whereby CEACAM1-dependent pathways protect the liver against the physiologically high levels of insulin in the portal vein by increasing insulin uptake via its high-affinity IR-A receptor to suppress de novo lipogenesis and remove insulin while maintaining homeostatic levels of IR-A in hepatocytes. Disturbance in this system leads to impaired insulin clearance with exaggerated hyperinsulinemia that could lead to downregulation of the insulin receptor and hepatic steatosis.
Insulin Regulates Its Hepatic Clearance
Several studies have shown that insulin regulates its synthesis from pancreatic β-cells (76). Even though CEACAM1 is expressed in these endocrine cells, insulin secretion is not compromised in mice with global null deletion of Ceacam1 gene (Cc1–/–) (43). Thus CEACAM1 plays a minor role in the regulation of insulin secretion and regulates homeostatic insulin levels mainly by promoting insulin clearance in hepatocytes.
As stated above, hepatic insulin clearance is intimately associated with insulin signaling by its dependence on receptor-mediated insulin delivery to the intracellular compartments where it is degraded. Whereas insulin action is mediated by insulin signaling pathways, the process is itself regulated by homeostatic circulating levels of insulin, which is in turn determined, not only by insulin secretion from pancreatic β-cells, but also by hepatic insulin clearance.
Insulin clearance in hepatocytes is initiated by the activation of the insulin receptor by acute release of insulin pulses from pancreatic β-cells, which in turn phosphorylates CEACAM1 to mediate insulin targeting to its degradation process. Additionally, acute insulin pulses during the first few hours of refeeding following an overnight fast induce the transcriptional activity of Ceacam1 promoter (140) to stimulate CEACAM1 expression (176). Thus, by inducing CEACAM1 transcription and phosphorylation, insulin stimulates its own clearance in hepatocytes, which in turn regulates its homeostatic levels to secure the propagation of its multiple functions in peripheral target cells.
The physiological implication of how insulin regulates its own clearance to coordinate with secretion its homeostatic levels is supported by several studies. To mention a few, fenofibrate maintains insulin sensitivity in mice fed a high-fat diet despite its negative effect on insulin secretion by exerting a parallel downregulatory effect on insulin clearance to maintain normo-insulinemia (177). Exenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist, maintains physiological insulin level, despite its stimulatory effect on insulin secretion by causing a parallel increase in insulin clearance in mice fed a high-fat diet (74). The inability of fenofibrate (177) and exenatide (74) to reverse the effect of high-fat diet in Cc1–/– mice demonstrates the dependence of their concomitant modulation of insulin secretion and clearance on hepatic CEACAM1-dependent pathways. Fenofibrate regulates Ceacam1 transcription by activating the peroxisome proliferator-activated receptor α (PPARα) (177), and exenatide binds to the PPRE response element in the Ceacam1 promoter to activate PPARγ to stimulate Ceacam1 transcription (74). Additionally, exenatide stimulates CEACAM1 expression in vivo through its ability to release insulin, which, in turn, stimulates Ceacam1 transcription (140).
Moreover, at fasting when insulin release is low, CEACAM1 expression is reduced by a PPARα-dependent mechanism to upregulate FASN activity and, in turn, reduce the level of malonyl-CoA and its inhibitory effect on fatty acids β-oxidation (176). Low level of CEACAM1 at fasting limits insulin extraction in the face of its low production to maintain homeostatic insulin levels. This is consistent with the observation that selective reduction in β-cell mass restricts pulsatility of insulin secretion followed by a compensatory reduction of postprandial hepatic insulin extraction in minipigs (105). Collectively, these findings emphasize the central role that hepatic insulin clearance plays in coordinating with insulin secretion the regulation of insulin homeostasis and, ultimately, its function (82).
Impaired Insulin Clearance, Hyperinsulinemia, and Insulin Resistance
The hallmark of insulin resistance is hyperinsulinemia. Mechanistically, the cause-effect relationship between these two parameters remains elusive.
As stated above, elevated plasma insulin levels can be caused by a compensatory increase in insulin secretion from β-cells and reduced insulin clearance. Several studies in humans (17) and animals (7, 103) have shown that reduced insulin clearance can cooperate with increased insulin secretion to properly regulate glucose homeostasis. In this regard, reduced insulin extraction alleviates the burden of pancreatic β-cells and limits the need for a compensatory increase in insulin synthesis and release. Because hepatic insulin clearance depends on receptor-mediated entry of insulin, blunting insulin signaling can restrict insulin uptake and its degradation, leading to reduced insulin clearance and providing a mechanistic underpinning for the negative effect of hepatic insulin resistance on insulin clearance.
On the other hand, impaired insulin clearance leads to hyperinsulinemia, which in turn, causes insulin resistance through several mechanisms. These include 1) desensitization and lysosomal downregulation of the insulin receptor leading to its reduced expression on the surface membrane (106), 2) limiting the pulsatility of insulin release (129, 151, 186), and 3) decreasing brown adipogenesis and, consequently, energy expenditure (175).
Hyperinsulinemia Causes Insulin Resistance: A Lesson from Animal Models of Impaired CEACAM1-Dependent Pathways
That impaired insulin clearance causes secondary insulin resistance is supported by our studies on genetically modified mouse models targeting the Ceacam1 gene globally or in a liver-specific manner. Using these mice, we have demonstrated that 1) CEACAM1 is a key player in promoting insulin clearance (43, 75, 173, 217), 2) by promoting insulin clearance, CEACAM1 regulates insulin sensitivity and links insulin to lipid metabolism (89, 149), and 3) reduced hepatic CEACAM1 level/function impairs insulin clearance to cause hepatic insulin resistance independently of lipolysis (75, 181).
Cc1–/– mice with global null deletion (43) and AlbCre+Cc1fl/fl mice with liver-specific deletion (75) of the Ceacam1 gene display chronic hyperinsulinemia caused by impaired insulin clearance at 2 mo of age without significant changes in insulin secretion, plasma glucagon levels, and mass of pancreatic β- or α-cells. Hepatic insulin resistance does not develop in both groups until ~6–7 mo of age, when both null deletions are propagated on a pure inbred C57BL/6J background (43, 75, 173, 217). Whereas Cc1–/– mice develop hepatic insulin resistance with compromised ability of insulin to suppress hepatic gluconeogenesis, they do not develop fasting hyperglycemia, consistent with their intact pancreatic β-cell function (43).
Mutant mice with global (43, 217) or liver-specific deletion of Ceacam1 gene (75) and L-SACC1 mice harboring a liver-specific overexpression of the S503A dominant-negative phosphorylation-defective CEACAM1 mutant (173) also manifest hepatic steatosis concomitantly with hepatic insulin resistance, resulting from increased hepatic de novo lipogenesis and reduced fatty acid β-oxidation. Increased hyperinsulinemia-driven de novo lipogenesis [resulting from SREBP1c activation and the loss of the counter-regulatory effect of insulin on FASN activity (149)] tips the balance in favor of reesterification and redistribution of VLDL-triacylglycerol preferentially to white adipose tissue when mice are propagated on the C57BL/6J genetic background (75, 149, 157). This leads to visceral obesity and systemic insulin resistance in association with hyperinsulinemia-driven downregulation of the insulin receptor and compromised insulin signaling in extra-hepatic tissues such as hypothalamus, aorta, and heart, among others (181, 182). The progressive manifestation of metabolic abnormalities in AlbCre+Cc1fl/fl mice with liver-specific deletion of Ceacam1 gene (75) demonstrates that hyperinsulinemia-driven blunted insulin signaling elevates hypothalamic FASN activity, which, together with hyperleptinemia, contributes to hyperphagia and, subsequently, to systemic insulin resistance and an increase in lipolysis (75). This provides evidence that hepatic insulin resistance in Ceacam1-null mutants develops independently of lipolysis, mainly as a consequence of hyperinsulinemia caused by impaired hepatic CEACAM1-dependent insulin clearance pathways.
The fact that loss of CEACAM1 in liver plays a critical role in the metabolic abnormalities of Cc1–/– mice was further demonstrated by the restoration of normal metabolic phenotype, including hyperphagia, upon exclusive transgenic restoration of CEACAM1 in the liver and recovering normal insulin clearance. Given the role of CEACAM1 in suppressing lipogenesis (149), it is possible that loss of hepatic CEACAM1 induced hepatic de novo lipogenesis, which could in turn be associated with hepatic insulin resistance (163), independently of insulin clearance (110). Mechanistically, increased hepatic lipid production could also be followed by lipid redistribution to white adipose tissue and lipolysis. Lipolysis-derived free fatty acids, initially reaching the liver via the portal vein, could in turn cause systemic insulin resistance (161) that triggers compensatory increase in insulin secretion and hyperinsulinemia. This possible scenario would rule out a key role for impaired insulin clearance in the pathogenesis of insulin resistance in Cc1–/– mice. However, blunting lipolysis by nicotinic acid treatment fails to restore insulin clearance and reverse hyperinsulinemia and systemic insulin resistance in Cc1–/– mice (180). Nicotinic acid also fails to reverse hyperinsulinemia-driven downregulation of the insulin receptor level and activation in hepatic and extra-hepatic tissues (such as white adipose tissue and hypothalamus) in Cc1–/– mice (181). This gain-of-function model (Cc1–/–xliver+ rescue mice) substantiates the findings of the liver-specific loss-of-function model (AlbCre+Cc1fl/fl mice) that lipolysis is the consequence rather than the trigger of systemic insulin resistance in Cc1–/– mice and that hyperinsulinemia stemming from impaired CEACAM1-dependent hepatic insulin clearance pathways plays a causative role in the pathogenesis of insulin resistance in Ceacam1 mutants.
Altered CEACAM1 Expression in Metabolic Diseases
In addition to insulin resistance, visceral obesity, and hepatic steatosis, L-SACC1 and Cc1–/– mice develop features of non-alcoholic fatty liver disease (NAFLD) with hepatic inflammation (89, 148) and cardiovascular abnormalities such as hypertension and endothelial and cardiac dysfunction (94, 182). Rescuing CEACAM1 expression in the liver reverses these cardio-metabolic abnormalities (181, 182), strengthening the notion that reduction in hepatic CEACAM1 levels impairs insulin clearance to cause hyperinsulinemia, followed by cardio-metabolic anomalies. Consistently, hepatic CEACAM1 is downregulated and insulin clearance is impaired in several rat models of obesity, insulin resistance, and hepatic steatosis [such as obese Zucker hyperphagic rats without diabetes (fa/fa) or with diabetes (Zucker Diabetic Fatty rats; ZDF), and obese spontaneous hypertensive Koletsky rats (f/f)] (90). Similarly, rats selectively bred for low aerobic running capacity (LCR) exhibit all features of metabolic syndrome, including hyperinsulinemia, insulin resistance, visceral obesity, hypertension, endothelial dysfunction, and NAFLD, compared with age-matched high-capacity runners (HCR) (18, 202). Interestingly, this selection for low aerobic capacity lowers hepatic Ceacam1 mRNA (215) and protein levels (18), and, subsequently, hepatic insulin clearance in LCR relative to HCR. Whereas high-intensity interval endurance training for 8 wk restores the cardiovascular abnormalities in LCR, it fails to reduce hepatic de novo lipogenesis of LCR and restore its hepatic CEACAM1 levels to the level of HCR (87). In contrast, caloric restriction reverses all of the metabolic abnormalities in parallel to restoring hepatic CEACAM1 levels and insulin clearance (18).
We (90) and others (115) have shown that hepatic CEACAM1 is reduced in obese human subjects with insulin resistance and hepatic steatosis and that the severity of fat deposition in the liver is linear with the degree of the loss of hepatic CEACAM1 levels (115). Moreover, CEACAM1 protein content is remarkably reduced in primary hepatocytes derived from steatotic livers of obese humans relative to age-matched lean subjects, demonstrating an intrinsic loss of hepatic CEACAM1 in obesity (90). In light of the reduction of Ceacam1 transcription by fatty acid-activated PPARα (176, 177), lower CEACAM1 levels in hepatocytes from obese humans could result from the rise in plasma free fatty acids that are mobilized during lipolysis. Increased lipolysis-driven hepatic fatty acid β-oxidation in humans with uncomplicated obesity (81), and its role in regulating hepatic de novo lipogenesis (108, 203), propose an important role for the loss of hepatic CEACAM1 in the regulation of lipid homeostasis in hepatocytes. In fact, we have shown that high-fat feeding of normal C57BL/6 mice progressively represses Ceacam1 mRNA and protein levels in the hepatocyte by fatty acids’ activation of PPARα-dependent mechanisms (176) to provide a positive feedback mechanism on fatty acid β-oxidation and to reduce the burden of excessive energy supply by limiting its adverse effect on insulin sensitivity (180). However, when more than half of hepatic CEACAM1 is lost, insulin clearance followed by chronic hyperinsulinemia and hepatic steatosis develops (180). This is consistent with reduction of hepatic insulin clearance in dogs (214) and rats (161, 220) by the rise of free fatty acids in the portal circulation. In further support of the critical role of CEACAM1 along this adipocyte-hepatocyte axis in mediating dietary-induced insulin resistance, forced transgenic CEACAM1 overexpression (5) and adenoviral-mediated redelivery of CEACAM1 to the liver protect the liver against diet-induced impairment of insulin clearance and associated metabolic abnormalities (118, 180).
Molecular Mechanism of Insulin Degradation: The Role of Insulin-Degrading Enzyme (IDE) Revisited
Insulin-degrading enzyme (IDE; EC 3.4.99.45), is the most-abundant protease that degrades insulin in the cytosol, in particular in kidney (47, 48), although insulin-degrading enzyme activity also has been reported in lysosomes and membrane fractions. It has been proposed that cytosolic IDE, and other insulin proteases, may initiate the “specific” cleavage of intact insulin, yielding different intermediate products, which synergistically are degraded by non-specific membrane-associated proteases that fail to act on intact insulin (47, 48, 88, 92).
Insulin-Degrading Enzyme: General Information
IDE, also known as insulin protease, insulinase, insulysin, insulin-glucagon protease, neutral thiol protease, metalloendoprotease, or peroxisomal protease, is a ubiquitously expressed and well-conserved Zn2+ metallo-endopeptidase (8, 22, 49, 50). In rat liver, IDE is present in parenchymal cells, particularly near the portal tract, and in the epithelium of the bile duct (4).
IDE has a high affinity for insulin (Km ~0.1 µM) and degrades insulin into several fragments (49, 50). However, despite its comparable affinity, proinsulin is a poor substrate of IDE (51). IDE can also degrade insulin growth factor (IGF) I and II in in vitro systems more slowly than insulin (3, 132). Thus proinsulin and IGF I could in principal serve as competitive IDE inhibitors (133). Albeit with lower affinity for insulin, IDE can also degrade glucagon (6, 47), amylin (15), β-amyloid (Aβ) peptide (112), Aβ precursor protein intracellular domain (APP-AICD) (53), amyloid Bri (ABri) and amyloid Dan (ADan) (135), cytochrome c (211), atrial natriuretic peptide (138), calcitonin and β-endorphin (67), transforming growth factor-α (TGF-α) (73), oxidized hemoglobin (61), growth hormone-releasing factor (206), and chemokine ligand (CCL)3 and CCL4 (123).
Molecular Mechanisms Underlying IDE Action
Receptor-bound insulin is internalized into hepatocytes resulting in its degradation or release of the intact or partially degraded hormone into circulation (85). IDE can degrade insulin in various cellular compartments, including the plasma membrane (219). However, most of the receptor-bound insulin is internalized into early/sorting endosomes where degradation is rapidly initiated in their neutral non-acidic environment mostly by IDE (84, 219). Subsequent maturation of late endosomes and their acidification allow dissociation of insulin from its receptor to undergo degradation (84) with no significant transfer to lysosomes (160). However, this proteolytic activity is not attributed to IDE but rather to acidic thiol protease (9), because cellular acidosis inactivates IDE by modulating its oligomerization state (79). In addition to intact insulin that escapes endosomal degradation, partially degraded insulin fragments are completely degraded in lysosomes (10, 11, 50). Degradation of internalized insulin may also occur in the cytosol, nucleus, and Golgi, proposing multiple intracellular pathways for insulin degradation.
The role of IDE in the endosomal degradation of internalized insulin remains controversial, suggesting a role for an alternative insulin-specific endosomal protease distinct from IDE that regulates hepatic insulin degradation (11). The rationale for this dispute is based on several observations. First, changes in endosomal pH produce different insulin degradation products, implying engagement of several enzymes with different pH-dependent enzymatic activities (37). Second, insulin dissociation from its receptor and its full degradation require an acidic pH that inactivates IDE (79). Third, IDE is mostly cytosolic, whereas endosomal degradation of insulin is membrane-associated, which implies that different proteases, including IDE, gain access inside the lumen of vesicles. It has been reported that a targeting sequence at the COOH terminus and an alternative splice isoform with extended NH2 terminus serve to direct IDE to peroxisomes and mitochondria, respectively (117, 136). However, there are no identified specific sequences in IDE that could target it into the lumen of endosomes. It might be possible that plasma membrane-associated IDE is internalized together with the insulin receptor into the lumen of vesicles. Alternatively, proteolytic vesicles containing IDE might fuse with early endosomes. Interestingly, phosphatidylinositol (3-5)-triphosphate (PIP3), a second messenger produced by the intracellular insulin signaling pathway, stimulates the recruitment of IDE to early endosomes (194). However, this fails to explain how IDE gains access inside late endosomes. Some studies have proposed that this occurs during the micro-autophagy of cytosolic proteins due to IDE’s weak interaction with phosphatidylinositol phosphates (183, 194). On the other hand, insulin signaling-mediated localization of IDE to early endosomes could represent a way in which IDE regulates the sorting process of these vesicles, which, in turn, determines whether endocytosed insulin receptors are targeted to lysosomal degradation or plasma membrane recycling. However, this non-proteolytic function of IDE has not been confirmed experimentally. Together, this suggests that IDE may exert proteolytic as well as non-proteolytic activities in regulating hepatic insulin metabolism.
IDE and Insulin Clearance
IDE has been proposed to be a major enzyme involved in regulating insulin signaling and clearance (8, 49). Earlier studies demonstrated that ablation of Ide gene leads to hyperinsulinemia, supporting the notion of a physiological role for IDE in insulin clearance (1, 63). However, we recently demonstrated that liver-specific deletion of the Ide gene in mice (L-IDE-KO) does not result in hyperinsulinemia, as expected from deleting a protein that is postulated to play a major role in hepatic insulin clearance (207). Instead, it impairs insulin signaling by downregulating the surface membrane expression of the insulin receptor. These findings are consistent with a non-proteolytical function of IDE in the regulation of insulin action.
A novel insight on the role of renal IDE in regulating systemic insulin clearance has recently been reported (122). Insulin increases the association of IDE with sorting nexin 5 (SNX5), a cytoplasmic and a membrane-associated protein that regulates intracellular trafficking in the brush-border membrane of proximal tubules in human and rodent kidneys (122). Renal-selective depletion of SNX5 by the subcapsular infusion of Snx5-specific siRNA reduces IDE levels by ~40% and leads to a rise in circulating insulin levels with reduction in urinary insulin excretion. Moreover, silencing of Snx5 in mice decreased the expression level of the insulin receptor in renal proximal tubule cells, a major site of IDE action (121). Taken together, these data question the current notion that the main function of renal IDE in regulating systemic insulin clearance through its protease activity and prompt the formulation of a new paradigm whereby IDE regulates renal insulin clearance via a non-proteolytical function (i.e., regulating the level of insulin receptors on the brush border membrane of proximal tubule cells). Further research is warranted to fully elucidate the molecular mechanisms underlying the regulation of systemic insulin clearance by IDE.
Role of IDE in Insulin Clearance in Obesity
Controversy surrounds the impact of obesity on hepatic IDE levels and insulin clearance. Feeding female Swiss mice (10–12 wk old) a cafeteria diet for 8 wk causes an ~22% increase in body weight, glucose intolerance, and insulin resistance, and a reduction in insulin clearance in association with reduced hepatic protein levels of IDE (19). Interestingly, hepatic IDE mRNA levels from the canonical catalytic efficient 15a-IDE spliceoform and the catalytic inefficient 15b-IDE spliceoform were significantly reduced by the cafeteria diet (19, 62). In contrast, feeding female Wistar rats (10–12 wk old) a cafeteria diet for 17 wk causes hyperinsulinemia with insulin resistance in parallel to an increase in IDE mRNA levels and IDE activity, suggesting enhanced plasma insulin clearance (30). Feeding C57BL/6 mice (6–8 wk of age) a high-fat diet (58% kcal in fat) for 6 mo caused higher hepatic IDE protein levels and activity (209). Male Wistar rats fed a high-fructose diet (20% fructose in drinking water) for 6 wk developed obesity, hyperinsulinemia, hyperglucagonemia, hyperglycemia, and insulin resistance (57). However, hepatic IDE protein levels were significantly higher than in control rats (57). This was attributed to either a hyperinsulinemia-driven negative feedback mechanism or to hyperglucagonemia inducing hepatic IDE expression (57).
The discrepancies between these studies could arise from the use of different experimental models, as well as to the length and composition of diet. Nonetheless, no conclusive information could be drawn concerning the cause-effect relationship between IDE and impaired insulin clearance in the setting of obesity and diabetes.
Role of IDE in Insulin Clearance in Diabetes
Compelling evidence has demonstrated an association between reduced IDE levels and lower insulin clearance in Type 2 diabetics (19, 168, 206). Other studies have shown lower IDE activity and insulin degradation in both subcutaneous and visceral fat depots, with a greater extent in the visceral depot, of pre-diabetics and Type 2 diabetics compared with non-diabetic patients (65).
Conversely, IDE activity was increased in T2D patients treated with sulfonylureas (197). In human islets, IDE protein levels were higher in T2D patients treated with insulin than in those treated with oral hypoglycemic drugs (66). In contrast, well controlled Type 1 diabetics did not show changes in IDE activity (206). Moreover, the route of insulin administration can affect the response of IDE activity in diabetic patients, with subcutaneous but not intravenous injections being associated with changes in IDE activity (206).
The potential use of IDE inhibitors for the treatment of T2D has attracted a lot of attention in recent decades. However, despite the discovery of a variety of compounds with diverse chemical structures, none of them demonstrated valid therapeutic use due to toxicity, low potency or selectivity (see Ref. 167 for a comprehensive review). For instance, long-term inhibition of IDE may cause chronic hyperinsulinemia, resulting in secondary insulin resistance and impaired insulin secretion. Furthermore, hyperinsulinemia could be related to the upregulation of cell proliferation in tissues such as liver. In addition to insulin, IDE degrades glucagon, amylin, and Aβ. It is uncertain whether inhibiting IDE could increase hepatic glucose output and glucose intolerance due to altered insulin-to-glucagon ratio. Acute inhibition of IDE by 6bK seems to predict this unfavorable scenario (126). Likewise, deposition of amylin in pancreas and of Aβ in the brain could, respectively, cause cytotoxicity in pancreatic β-cells in parallel to reduced insulin secretion and to brain damage due to neuroinflammation. In summary, whether acute or long-term inhibition of IDE activity constitutes an effective strategy to regulate insulin clearance remains to be established.
Proposed Coordination of Insulin Trafficking and Metabolism by CEACAM1 and IDE-Dependent Pathways
Activation of the insulin receptor and intracellular signal transduction are intimately associated with a highly regulated receptor-mediated insulin internalization and removal process. Signal transduction by receptor-bound insulin is not confined to the cell surface but continues upon the entry of the endocytosis complex into early endosomes, with the α-subunit of the receptor being in the vesicular lumen and its tyrosine kinase on IRβ exposed to substrates in the cytoplasm. This feature allows the insulin receptor to continue to target its downstream substrates to transmit insulin signaling while it carries its cargo for removal from the circulation.
CEACAM1 has been assigned a major role in promoting the rate of uptake of insulin via its receptor and its translocation to the degradation process in hepatocytes as well as in isolated renal proximal tubule cells (5). Insulin degradation has been ascribed to proteolytic enzymes, among which IDE has been postulated to play a major role, in particular in kidneys (50). Intact insulin clearance in L-IDE KO mice has challenged this paradigm and revealed a non-proteolytic activity of IDE involving maintenance of insulin signaling (207). Although IDE may play a cooperative role with CEACAM1 in targeting the insulin-IR complex through its intracellular trajectory, it appears to contribute to a minor extent to insulin degradation in the early endosomes in hepatocytes. The novel conceptual model of the coordinated regulation of insulin metabolism by CEACAM1 and IDE is captured in FIGURE 2. According to this model, insulin binding to its receptor leads to a conformational change that induces autophosphorylation of the insulin receptor. Alhtough it has not been demonstrated, it is possible that cytosolic IDE participates in a conformational change that favors this step, similar to its interaction with the ~50 amino acid cytoplasmic tail of the Type A scavenger receptor A (26), and with androgen and glucocorticoid receptors (111).
Nonetheless, insulin-activated IR-A phosphorylates CEACAM1 that, in turn, takes part in the formation of a stable insulin-IR complex via Shc, which, together with Tyr513 in the COOH-terminus tail of CEACAM1, targets the complex to AP2-associated clathrin-coated pits and early endosomes (FIGURE 2, step 1). It is possible that IDE located extracellularly and on the plasma membrane is sequestered inside the vesicle during this process (25).
In parallel, insulin induces the production of PIP3 from phosphatidylinositol by PI3Kinase to stimulate IDE recruitment to the outer layer of the early/sorting endosomes (194) and to retain CEACAM1 in endosomes (199). Activation of PI3Kinase likely results from the progressive binding of SHP2 to CEACAM1, an event that sequesters the phosphatase and prevents dephosphorylation of IRS (146). In this manner, CEACAM1 contributes to the recruitment of a cytosolic IDE pool to the outer side of early endosomes while prolonging insulin signaling via the IRS/PI3Kinase pathway as a compensatory mechanism to insulin degradation by vesicular IDE (FIGURE 2, step 2). Binding of CEACAM1 to SHP2 requires the progressive loss of Shc-CEACAM1 interaction and, subsequently, progressive detachment of CEACAM1 from the insulin-IR complex by binding to FASN on Tyr488 residue. It is possible that IDE in the outer layer of the vesicle contributes to microtubule reorganization and polymerization to form the CEACAM1-FASN vesicle. This remains to be investigated. Nonetheless, as the insulin-IR complex is destabilized in part by the detachment of CEACAM1, and as the endosome matures and undergoes acidification (FIGURE 2, step 3; H+), insulin dissociates from its receptor to be degraded by the acidic thiol protease (FIGURE 2, step 3; AcTP) and/or by the aspartic acid protease cathepsin D (9), but not by IDE that is inactivated by the acidic milieu of late endosomes (FIGURE 2, step 3).
Insulin receptor, free of its hormone and of CEACAM1, is recycled back to the plasma membrane (FIGURE 2, step 4). The pool of IDE-bound to the outer side of late endosomes might interact with α-synuclein and/or other proteins involved in regulating vesicular trafficking of IR-A back to the plasma membrane. That a non-proteolytical activity of IDE is involved in vesicular trafficking is supported by the observation that IDE binds to the COOH terminus of α-synuclein, an amyloidogenic protein, to reduce the formation of α-synuclein oligomers (190, 191), thereby increasing tubulin polymerization and microtubule-dependent trafficking for proper autophagy (32, 64, 114). In fact, depletion of IDE from pancreatic β-cells is associated with increased levels of α-synuclein, in parallel to reduced tubulin polymerization, autophagy, and microtubule-dependent recruitment of secretory granules containing insulin (198). Moreover, insulin increases the association of SNX5 with IDE in the plasma membrane of renal proximal tubule cells (122), and depletion of renal SNX5 results in reduced expression of the insulin receptor and lower signal transduction (122). Because IDE is the primary enzyme responsible for insulin degradation in kidney, the dynamic interaction between IDE and SNX5 regulates systemic circulating insulin levels (122). Thus IDE might similarly regulate the levels of α-synuclein oligomers in hepatocytes to facilitate microtubule polymerization and trafficking of late endosomes (with their cargo) to the plasma membrane.
The proposed model compartmentalizes the coordinated functions of CEACAM1 and IDE in regulating insulin disposal. CEACAM1 regulates insulin action by promoting receptor-mediated insulin endocytosis and targeting to the degradation process. Although IDE initiates the degradation of insulin in early endosomes, its main effect on insulin action appears to be mediated by its non-proteolytic activity on the intracellular trafficking of the insulin-IR complex and, in particular, on insulin receptor recycling to the plasma membrane, an important step in insulin retro-endocytosis. Reduced expression level and phosphorylation of the insulin receptor (and CEACAM1) on the membrane of hepatocytes derived from L-IDE-KO mice and their hepatic insulin resistance are consistent with the significant non-proteolytic regulation of insulin action by IDE (207). Low IR level in hepatocytes isolated from these mice could also stem from increased trafficking of IR-A to lysosomes. More studies are needed to delineate the underlying mechanisms.
Concluding Remarks
Insulin secretion and clearance coordinate their regulation of insulin homeostasis and action. In this respect, the liver is equipped with multiple mechanisms that protect it against the high levels of insulin in the portal vein. This protective repertoire is mediated by the pulsatility of insulin release and by the high level of hepatocytic CEACAM1 that mediates excess insulin removal upon its phosphorylation by ligand-activated insulin receptor to maintain normo-insulinemia. Through targeting the insulin-IR complex to the intracellular degradation process, CEACAM1 also mediates a negative effect of the acute pulses of insulin on FASN activity. This restricts hepatic de novo lipogenesis and limits hepatic steatosis in the face of the higher level of insulin in the portal than systemic circulation. Thus, when hyperinsulinemia develops and pulsatility of insulin release diminishes, CEACAM1 phosphorylation is compromised to impair insulin clearance, causing hepatic insulin resistance (downregulation of the insulin receptor by lysosomal degradation) and giving way to the lipogenic effect of chronically elevated levels of insulin. Through substrate redistribution to white adipose tissue, elevated hepatic de novo lipogenesis triggers visceral obesity followed by systemic insulin resistance. Thus we posit that insulin resistance is not selective with respect to insulin’s ability to acutely reduce hepatic gluconeogenesis, as has been widely accepted (23, 38), but it also includes its inability to acutely suppress hepatic de novo lipogenesis. Nonetheless, our studies show that impaired CEACAM1-dependent insulin clearance pathways link hyperinsulinemia-driven systemic insulin resistance to hepatic steatosis (as in NAFLD).
Mechanistically, hepatic insulin clearance is mediated by receptor-mediated insulin uptake and degradation in hepatocytes, a process that is upregulated by CEACAM1. Devoid of enzymatic activity, CEACAM1 does not engage in the proteolytic degradation pathway, per se. Instead, it acts as a chaperone that delivers the complex to late endosomes where insulin ultimately dissociates from its receptor to be fully degraded. Whereas IDE catalyzes insulin degradation in early endosomes, its proteolytic activity is inhibited in the acidic environment of late endosomes, giving way to resident acidic proteases to degrade insulin. Normo-insulinemia in mice with liver-specific deletion of the Ide gene rules out a major role for IDE in insulin degradation. Instead, it proposes a non-proteolytic regulation of insulin action by IDE, namely via promoting the surface content of insulin receptor in hepatocytes. This is consistent with disagreement over the therapeutic effect of IDE inhibition as a strategy to ameliorate insulin resistance. In light of the undisputed critical role of CEACAM1 in regulating insulin action by promoting insulin clearance, we propose that therapy based on inducing CEACAM1 is likely to be more beneficial in the prevention as well as in the treatment of insulin resistance and hepatic steatosis. In support of this notion, hyperinsulinemia in patients with NAFLD appears to be more strongly associated with impaired insulin clearance than with increased insulin secretion (21). Upregulation of CEACAM1 transcription by agonists of PPARγ (pioglitazone) and GLP-1R (exenatide) (74) strengthened the rationale for the use of these drugs in the treatment of T2D and NAFLD (20, 31, 40). Studies are underway to document changes in hepatic CEACAM1 levels in patients with metabolic syndrome, but reduced insulin clearance is emerging as a major risk factor for this disease, including NAFLD (21, 77, 113, 166). This provides impetus to carry out further studies to validate reduced hepatic CEACAM1 level as a biomarker for metabolic syndrome, with an overarching goal to target insulin clearance as a therapeutic strategy against this complex disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-DK-054254, R01-DK-083850, and R01-HL-112248) to S.M.N., and from Ministerio de Economía, Industria y Competitividad (SAF2014-58702-C2-2-R and SAF2016-77871-C2-2-R) to G.P. The work was also supported by the EFSD European Research Programme on New Targets for Type 2 Diabetes supported by an educational research grant from MSD to G.P.
No conflicts of interest, financial or otherwise, are declared by the author(s).
S.M.N. and G.M.P. interpreted results of experiments; S.M.N. and G.P. prepared figures; S.M.N. and G.P. drafted manuscript; S.M.N. and G.P. edited and revised manuscript; S.M.N. and G.P. approved final version of manuscript.
References
- 1.Abdul-Hay SO, Kang D, McBride M, Li L, Zhao J, Leissring MA. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. PLoS One 6: e20818, 2011. doi: 10.1371/journal.pone.0020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou-Rjaily GA, Lee SJ, May D, Al-Share QY, Deangelis AM, Ruch RJ, Neumaier M, Kalthoff H, Lin SH, Najjar SM. CEACAM1 modulates epidermal growth factor receptor–mediated cell proliferation. J Clin Invest 114: 944–952, 2004. doi: 10.1172/JCI200421786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Affholter JA, Cascieri MA, Bayne ML, Brange J, Casaretto M, Roth RA. Identification of residues in the insulin molecule important for binding to insulin-degrading enzyme. Biochemistry 29: 7727–7733, 1990. doi: 10.1021/bi00485a022. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama H, Shii K, Yokono K, Yonezawa K, Sato S, Watanabe K, Baba S. Cellular localization of insulin-degrading enzyme in rat liver using monoclonal antibodies specific for this enzyme. Biochem Biophys Res Commun 155: 914–922, 1988. doi: 10.1016/S0006-291X(88)80583-7. [DOI] [PubMed] [Google Scholar]
- 5.Al-Share QY, DeAngelis AM, Lester SG, Bowman TA, Ramakrishnan SK, Abdallah SL, Russo L, Patel PR, Kaw MK, Raphael CK, Kim AJ, Heinrich G, Lee AD, Kim JK, Kulkarni RN, Philbrick WM, Najjar SM. Forced hepatic overexpression of CEACAM1 curtails diet-induced insulin resistance. Diabetes 64: 2780–2790, 2015. doi: 10.2337/db14-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansorge S, Bohley P, Kirschke H, Langner J, Wiederanders B. The insulin and glucagon degrading proteinase of rat liver: a metal-dependent enzyme. Biomed Biochim Acta 43: 39–46, 1984. [PubMed] [Google Scholar]
- 7.Asare-Bediako I, Paszkiewicz RL, Kim SP, Woolcott OO, Kolka CM, Burch MA, Kabir M, Bergman RN. Variability of directly measured first-pass hepatic insulin extraction and its association with insulin sensitivity and plasma insulin. Diabetes 67: 1495–1503, 2018. doi: 10.2337/db17-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Authier F, Posner BI, Bergeron JJ. Insulin-degrading enzyme. Clin Invest Med 19: 149–160, 1996. [PubMed] [Google Scholar]
- 9.Authier F, Rachubinski RA, Posner BI, Bergeron JJ. Endosomal proteolysis of insulin by an acidic thiol metalloprotease unrelated to insulin degrading enzyme. J Biol Chem 269: 3010–3016, 1994. [PubMed] [Google Scholar]
- 10.Backer JM, Kahn CR, Cahill DA, Ullrich A, White MF. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor beta-subunit. J Biol Chem 265: 16450–16454, 1990. [PubMed] [Google Scholar]
- 11.Backer JM, Kahn CR, White MF. The dissociation and degradation of internalized insulin occur in the endosomes of rat hepatoma cells. J Biol Chem 265: 14828–14835, 1990. [PubMed] [Google Scholar]
- 12.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 32: 643–671, 2013. doi: 10.1007/s10555-013-9444-6. [DOI] [PubMed] [Google Scholar]
- 14.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlsson A, Kuroki M, Lin SH, Lucka L, Najjar SM, Neumaier M, Obrink B, Shively JE, Skubitz KM, Stanners CP, Thomas P, Thompson JA, Virji M, von Kleist S, Wagener C, Watt S, Zimmermann W. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res 252: 243–249, 1999. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 15.Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J Biol Chem 275: 36621–36625, 2000. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- 16.Bojsen-Møller KN, Lundsgaard AM, Madsbad S, Kiens B, Holst JJ. Hepatic Insulin clearance in regulation of systemic insulin concentrations-role of carbohydrate and energy availability. Diabetes 67: 2129–2136, 2018. doi: 10.2337/db18-0539. [DOI] [PubMed] [Google Scholar]
- 17.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 32: 438–446, 1983. doi: 10.1016/0026-0495(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 18.Bowman TA, Ramakrishnan SK, Kaw M, Lee SJ, Patel PR, Golla VK, Bourey RE, Haram PM, Koch LG, Britton SL, Wisløff U, Lee AD, Najjar SM. Caloric restriction reverses hepatic insulin resistance and steatosis in rats with low aerobic capacity. Endocrinology 151: 5157–5164, 2010. doi: 10.1210/en.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandimarti P, Costa-Júnior JM, Ferreira SM, Protzek AO, Santos GJ, Carneiro EM, Boschero AC, Rezende LF. Cafeteria diet inhibits insulin clearance by reduced insulin-degrading enzyme expression and mRNA splicing. J Endocrinol 219: 173–182, 2013. doi: 10.1530/JOE-13-0177. [DOI] [PubMed] [Google Scholar]
- 20.Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: A call to action. Diabetes Care 40: 419–430, 2017. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 21.Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F, Hecht J, Cusi K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 59: 2178–2187, 2014. doi: 10.1002/hep.26988. [DOI] [PubMed] [Google Scholar]
- 22.Broh-Kahn RH, Mirsky IA. The inactivation of insulin by tissue extracts; the effect of fasting on the insulinase content of rat liver. Arch Biochem 20: 10–14, 1949. [PubMed] [Google Scholar]
- 23.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7: 95–96, 2008. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Brümmer J, Neumaier M, Göpfert C, Wagener C. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene 11: 1649–1655, 1995. [PubMed] [Google Scholar]
- 25.Bulloj A, Leal MC, Xu H, Castaño EM, Morelli L. Insulin-degrading enzyme sorting in exosomes: a secretory pathway for a key brain amyloid-beta degrading protease. J Alzheimers Dis 19: 79–95, 2010. doi: 10.3233/JAD-2010-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caravaggio JW, Hasu M, MacLaren R, Thabet M, Raizman JE, Veinot JP, Marcel YL, Milne RW, Whitman SC. Insulin-degrading enzyme deficiency in bone marrow cells increases atherosclerosis in LDL receptor-deficient mice. Cardiovasc Pathol 22: 458–464, 2013. doi: 10.1016/j.carpath.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Carpentier JL. Insulin receptor internalization: molecular mechanisms and physiopathological implications. Diabetologia 37, Suppl 2: S117–S124, 1994. doi: 10.1007/BF00400835. [DOI] [PubMed] [Google Scholar]
- 28.Carpentier JL, Gorden P, Amherdt M, Van Obberghen E, Kahn CR, Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest 61: 1057–1070, 1978. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpentier JL, Paccaud JP, Backer J, Gilbert A, Orci L, Kahn CR, Baecker J. Two steps of insulin receptor internalization depend on different domains of the beta-subunit. J Cell Biol 122: 1243–1252, 1993. doi: 10.1083/jcb.122.6.1243. An erratum for this article was published in J Cell Biol 123: 1047, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castell-Auví A, Cedó L, Pallarès V, Blay M, Ardévol A, Pinent M. The effects of a cafeteria diet on insulin production and clearance in rats. Br J Nutr 108: 1155–1162, 2012. doi: 10.1017/S0007114511006623. [DOI] [PubMed] [Google Scholar]
- 31.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023, 2012. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Jin J, Davis J, Zhou Y, Wang Y, Liu J, Lockhart PJ, Zhang J. Oligomeric alpha-synuclein inhibits tubulin polymerization. Biochem Biophys Res Commun 356: 548–553, 2007. doi: 10.1016/j.bbrc.2007.02.163. [DOI] [PubMed] [Google Scholar]
- 33.Cheung PH, Thompson NL, Earley K, Culic O, Hixson D, Lin SH. Cell-CAM105 isoforms with different adhesion functions are coexpressed in adult rat tissues and during liver development. J Biol Chem 268: 6139–6146, 1993. [PubMed] [Google Scholar]
- 34.Choice CV, Howard MJ, Poy MN, Hankin MH, Najjar SM. Insulin stimulates pp120 endocytosis in cells co-expressing insulin receptors. J Biol Chem 273: 22194–22200, 1998. doi: 10.1074/jbc.273.35.22194. [DOI] [PubMed] [Google Scholar]
- 35.Choice CV, Poy MN, Formisano P, Najjar SM. Comparison of the intracellular trafficking of two alternatively spliced isoforms of pp120, a substrate of the insulin receptor tyrosine kinase. J Cell Biochem 76: 133–142, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 36.Ciampelli M, Fulghesu AM, Cucinelli F, Pavone V, Caruso A, Mancuso S, Lanzone A. Heterogeneity in beta cell activity, hepatic insulin clearance and peripheral insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod 12: 1897–1901, 1997. doi: 10.1093/humrep/12.9.1897. [DOI] [PubMed] [Google Scholar]
- 37.Clot JP, Janicot M, Fouque F, Desbuquois B, Haumont PY, Lederer F. Characterization of insulin degradation products generated in liver endosomes: in vivo and in vitro studies. Mol Cell Endocrinol 72: 175–185, 1990. doi: 10.1016/0303-7207(90)90142-U. [DOI] [PubMed] [Google Scholar]
- 38.Cook JR, Langlet F, Kido Y, Accili D. Pathogenesis of selective insulin resistance in isolated hepatocytes. J Biol Chem 290: 13972–13980, 2015. doi: 10.1074/jbc.M115.638197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61: 4–13, 2012. doi: 10.2337/db11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Ann Intern Med 165: 305–315, 2016. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 41.Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 56: 949–964, 2013. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniels E, Letourneau S, Turbide C, Kuprina N, Rudinskaya T, Yazova AC, Holmes KV, Dveksler GS, Beauchemin N. Biliary glycoprotein 1 expression during embryogenesis: correlation with events of epithelial differentiation, mesenchymal-epithelial interactions, absorption, and myogenesis. Dev Dyn 206: 272–290, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 43.DeAngelis AM, Heinrich G, Dai T, Bowman TA, Patel PR, Lee SJ, Hong EG, Jung DY, Assmann A, Kulkarni RN, Kim JK, Najjar SM. Carcinoembryonic antigen-related cell adhesion molecule 1: a link between insulin and lipid metabolism. Diabetes 57: 2296–2303, 2008. doi: 10.2337/db08-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dery KJ, Silver C, Yang L, Shively JE. Interferon regulatory factor 1 and a variant of heterogeneous nuclear ribonucleoprotein L coordinately silence the gene for adhesion protein CEACAM1. J Biol Chem 293: 9277–9291, 2018. doi: 10.1074/jbc.RA117.001507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signalling. Mol Cell Biochem 182: 59–63, 1998. doi: 10.1023/A:1006883311233. [DOI] [PubMed] [Google Scholar]
- 46.Dubois MJ, Bergeron S, Kim HJ, Dombrowski L, Perreault M, Fournès B, Faure R, Olivier M, Beauchemin N, Shulman GI, Siminovitch KA, Kim JK, Marette A. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med 12: 549–556, 2006. doi: 10.1038/nm1397. [DOI] [PubMed] [Google Scholar]
- 47.Duckworth WC. Insulin and glucagon degradation by the kidney. I. Subcellular distribution under different assay condition. Biochim Biophys Acta 437: 518–530, 1976. doi: 10.1016/0304-4165(76)90020-9. [DOI] [PubMed] [Google Scholar]
- 48.Duckworth WC. Insulin and glucagon degradation by the kidney. II. Characterization of the mechanisms at neutral pH. Biochim Biophys Acta 437: 531–542, 1976. doi: 10.1016/0304-4165(76)90021-0. [DOI] [PubMed] [Google Scholar]
- 49.Duckworth WC. Insulin degradation: mechanisms, products, and significance. Endocr Rev 9: 319–345, 1988. doi: 10.1210/edrv-9-3-319. [DOI] [PubMed] [Google Scholar]
- 50.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 19: 608–624, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Duckworth WC, Heinemann MA, Kitabchi AE. Purification of insulin-specific protease by affinity chromatography. Proc Natl Acad Sci USA 69: 3698–3702, 1972. doi: 10.1073/pnas.69.12.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eaton RP, Allen RC, Schade DS. Hepatic removal of insulin in normal man: dose response to endogenous insulin secretion. J Clin Endocrinol Metab 56: 1294–1300, 1983. doi: 10.1210/jcem-56-6-1294. [DOI] [PubMed] [Google Scholar]
- 53.Edbauer D, Willem M, Lammich S, Steiner H, Haass C. Insulin-degrading enzyme rapidly removes the beta-amyloid precursor protein intracellular domain (AICD). J Biol Chem 277: 13389–13393, 2002. doi: 10.1074/jbc.M111571200. [DOI] [PubMed] [Google Scholar]
- 54.Edlund M, Öbrink B. Evidence for calmodulin binding to the cytoplasmic domains of two C-CAM isoforms. FEBS Lett 327: 90–94, 1993. doi: 10.1016/0014-5793(93)81046-3. [DOI] [PubMed] [Google Scholar]
- 55.Edlund M, Wikström K, Toomik R, Ek P, Öbrink B. Characterization of protein kinase C-mediated phosphorylation of the short cytoplasmic domain isoform of C-CAM. FEBS Lett 425: 166–170, 1998. doi: 10.1016/S0014-5793(98)00222-1. [DOI] [PubMed] [Google Scholar]
- 56.Ellis L, Clauser E, Morgan DO, Edery M, Roth RA, Rutter WJ. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell 45: 721–732, 1986. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- 57.Elseweidy MM, Amin RS, Atteia HH, Ali MA. Vitamin D3 intake as regulator of insulin degrading enzyme and insulin receptor phosphorylation in diabetic rats. Biomed Pharmacother 85: 155–159, 2017. doi: 10.1016/j.biopha.2016.11.116. [DOI] [PubMed] [Google Scholar]
- 58.Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metab 294: E568–E575, 2008. doi: 10.1152/ajpendo.00560.2007. [DOI] [PubMed] [Google Scholar]
- 59.Erdmann J, Mayr M, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Weight-dependent differential contribution of insulin secretion and clearance to hyperinsulinemia of obesity. Regul Pept 152: 1–7, 2009. doi: 10.1016/j.regpep.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Escribano O, Beneit N, Rubio-Longás C, López-Pastor AR, Gómez-Hernández A. The role of insulin receptor isoforms in diabetes and its metabolic and vascular complications. J Diabetes Res 2017: 1403206, 2017. doi: 10.1155/2017/1403206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fagan JM, Waxman L. Purification of a protease in red blood cells that degrades oxidatively damaged haemoglobin. Biochem J 277: 779–786, 1991. doi: 10.1042/bj2770779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farris W, Leissring MA, Hemming ML, Chang AY, Selkoe DJ. Alternative splicing of human insulin-degrading enzyme yields a novel isoform with a decreased ability to degrade insulin and amyloid beta-protein. Biochemistry 44: 6513–6525, 2005. doi: 10.1021/bi0476578. [DOI] [PubMed] [Google Scholar]
- 63.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA 100: 4162–4167, 2003. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem 281: 36303–36316, 2006. doi: 10.1074/jbc.M607031200. [DOI] [PubMed] [Google Scholar]
- 65.Fawcett J, Sang H, Permana PA, Levy JL, Duckworth WC. Insulin metabolism in human adipocytes from subcutaneous and visceral depots. Biochem Biophys Res Commun 402: 762–766, 2010. doi: 10.1016/j.bbrc.2010.10.104. [DOI] [PubMed] [Google Scholar]
- 66.Fernández-Díaz CM, Escobar-Curbelo L, López-Acosta JF, Lobatón CD, Moreno A, Sanz-Ortega J, Perdomo G, Cózar-Castellano I. Insulin degrading enzyme is up-regulated in pancreatic β cells by insulin treatment. Histol Histopathol 33: 1167–1180, 2018. [DOI] [PubMed] [Google Scholar]
- 67.Fernández-Gamba A, Leal MC, Morelli L, Castaño EM. Insulin-degrading enzyme: structure-function relationship and its possible roles in health and disease. Curr Pharm Des 15: 3644–3655, 2009. doi: 10.2174/138161209789271799. [DOI] [PubMed] [Google Scholar]
- 68.Ferrannini E. The stunned beta cell: a brief history. Cell Metab 11: 349–352, 2010. doi: 10.1016/j.cmet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G; European Group for the Study of Insulin Resistance (EGIR) . Insulin resistance and hypersecretion in obesity. J Clin Invest 100: 1166–1173, 1997. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flier JS. Insulin receptors and insulin resistance. Annu Rev Med 34: 145–160, 1983. doi: 10.1146/annurev.me.34.020183.001045. [DOI] [PubMed] [Google Scholar]
- 71.Formisano P, Najjar SM, Gross CN, Philippe N, Oriente F, Kern-Buell CL, Accili D, Gorden P. Receptor-mediated internalization of insulin. Potential role of pp120/HA4, a substrate of the insulin receptor kinase. J Biol Chem 270: 24073–24077, 1995. doi: 10.1074/jbc.270.41.24073. [DOI] [PubMed] [Google Scholar]
- 72.Formisano P, Oriente F, Miele C, Caruso M, Auricchio R, Vigliotta G, Condorelli G, Beguinot F. In NIH-3T3 fibroblasts, insulin receptor interaction with specific protein kinase C isoforms controls receptor intracellular routing. J Biol Chem 273: 13197–13202, 1998. doi: 10.1074/jbc.273.21.13197. [DOI] [PubMed] [Google Scholar]
- 73.Garcia JV, Gehm BD, Rosner MR. An evolutionarily conserved enzyme degrades transforming growth factor-alpha as well as insulin. J Cell Biol 109: 1301–1307, 1989. doi: 10.1083/jcb.109.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghadieh HE, Muturi HT, Russo L, Marino CC, Ghanem SS, Khuder SS, Hanna JC, Jash S, Puri V, Heinrich G, Gatto-Weis C, Lee KY, Najjar SM. Exenatide induces carcinoembryonic antigen-related cell adhesion molecule 1 expression to prevent hepatic steatosis. Hepatol Commun 2: 35–47, 2017. doi: 10.1002/hep4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghadieh HE, Russo L, Muturi HT, Ghanem SS, Manaserh IH, Noh HL, Suk S, Kim JK, Hill JW, Najjar SM. Hyperinsulinemia drives hepatic insulin resistance in male mice with liver-specific Ceacam1 deletion independently of lipolysis. Metabolism S0026-0495(19)30023-X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldfine AB, Kulkarni RN. Modulation of β-cell function: a translational journey from the bench to the bedside. Diabetes Obes Metab 14, Suppl 3: 152–160, 2012. doi: 10.1111/j.1463-1326.2012.01647.x. [DOI] [PubMed] [Google Scholar]
- 77.Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab 301: E402–E408, 2011. doi: 10.1152/ajpendo.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorden P, Carpentier J-L, Freychet P, LeCam A, Orci L. Intracellular translocation of iodine-125-labeled insulin: direct demonstration in isolated hepatocytes. Science 200: 782–785, 1978. doi: 10.1126/science.644321. [DOI] [PubMed] [Google Scholar]
- 79.Grasso G, Satriano C, Milardi D. A neglected modulator of insulin-degrading enzyme activity and conformation: The pH. Biophys Chem 203-204: 33–40, 2015. doi: 10.1016/j.bpc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 6: 433–446, 2006. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 81.Groop LC, Bonadonna RC, Shank M, Petrides AS, DeFronzo RA. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest 87: 83–89, 1991. doi: 10.1172/JCI115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol 19: 31–44, 2018. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haft CR, Klausner RD, Taylor SI. Involvement of dileucine motifs in the internalization and degradation of the insulin receptor. J Biol Chem 269: 26286–26294, 1994. [PubMed] [Google Scholar]
- 84.Hamel FG, Mahoney MJ, Duckworth WC. Degradation of intraendosomal insulin by insulin-degrading enzyme without acidification. Diabetes 40: 436–443, 1991. doi: 10.2337/diab.40.4.436. [DOI] [PubMed] [Google Scholar]
- 85.Hamel FG, Peavy DE, Ryan MP, Duckworth WC. HPLC analysis of insulin degradation products from isolated hepatocytes. Effects of inhibitors suggest intracellular and extracellular pathways. Diabetes 36: 702–708, 1987. doi: 10.2337/diab.36.6.702. [DOI] [PubMed] [Google Scholar]
- 86.Harada S, Loten EG, Smith RM, Jarett L. Nonreceptor mediated nuclear accumulation of insulin in H35 rat hepatoma cells. J Cell Physiol 153: 607–613, 1992. doi: 10.1002/jcp.1041530323. [DOI] [PubMed] [Google Scholar]
- 87.Haram PM, Kemi OJ, Lee SJ, Bendheim MO, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81: 723–732, 2009. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hare JF. A novel proteinase associated with mitochondrial membranes. Biochem Biophys Res Commun 83: 1206–1215, 1978. doi: 10.1016/0006-291X(78)91523-1. [DOI] [PubMed] [Google Scholar]
- 89.Heinrich G, Ghadieh HE, Ghanem SS, Muturi HT, Rezaei K, Al-Share QY, Bowman TA, Zhang D, Garofalo RS, Yin L, Najjar SM. Loss of hepatic CEACAM1: A unifying mechanism linking insulin resistance to obesity and non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 8: 8, 2017. doi: 10.3389/fendo.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heinrich G, Muturi HT, Rezaei K, Al-Share QY, DeAngelis AM, Bowman TA, Ghadieh HE, Ghanem SS, Zhang D, Garofalo RS, Yin L, Najjar SM. Reduced hepatic carcinoembryonic antigen-related cell adhesion molecule 1 level in obesity. Front Endocrinol (Lausanne) 8: 54, 2017. doi: 10.3389/fendo.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hinoda Y, Neumaier M, Hefta SA, Drzeniek Z, Wagener C, Shively L, Hefta LJ, Shively JE, Paxton RJ. Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immunologically crossreactive with carcinoembryonic antigen. Proc Natl Acad Sci USA 85: 6959–6963, 1988. doi: 10.1073/pnas.85.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hjelle JT, Oparil S, Peterson DR. Subcellular sites of insulin hydrolysis in renal proximal tubules. Am J Physiol 246: F409–F416, 1984. [DOI] [PubMed] [Google Scholar]
- 93.Horst AK, Najjar SM, Wagener C, Tiegs G. CEACAM1 in liver injury, metabolic and immune regulation. Int J Mol Sci 19: E3110, 2018. doi: 10.3390/ijms19103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang J, Ledford KJ, Pitkin WB, Russo L, Najjar SM, Siragy HM. Targeted deletion of murine CEACAM 1 activates PI3K-Akt signaling and contributes to the expression of (Pro)renin receptor via CREB family and NF-κB transcription factors. Hypertension 62: 317–323, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iritani N. Nutritional and hormonal regulation of lipogenic-enzyme gene expression in rat liver. Eur J Biochem 205: 433–442, 1992. doi: 10.1111/j.1432-1033.1992.tb16797.x. [DOI] [PubMed] [Google Scholar]
- 96.Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab 278: E501–E508, 2000. doi: 10.1152/ajpendo.2000.278.3.E501. [DOI] [PubMed] [Google Scholar]
- 97.Jones CN, Pei D, Staris P, Polonsky KS, Chen YD, Reaven GM. Alterations in the glucose-stimulated insulin secretory dose-response curve and in insulin clearance in nondiabetic insulin-resistant individuals. J Clin Endocrinol Metab 82: 1834–1838, 1997. doi: 10.1210/jcem.82.6.3979. [DOI] [PubMed] [Google Scholar]
- 98.Jung SH, Jung CH, Reaven GM, Kim SH. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia 61: 681–687, 2018. doi: 10.1007/s00125-017-4511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaburagi Y, Momomura K, Yamamoto-Honda R, Tobe K, Tamori Y, Sakura H, Akanuma Y, Yazaki Y, Kadowaki T. Site-directed mutagenesis of the juxtamembrane domain of the human insulin receptor. J Biol Chem 268: 16610–16622, 1993. [PubMed] [Google Scholar]
- 100.Kaga H, Tamura Y, Takeno K, Kakehi S, Funayama T, Furukawa Y, Nishitani-Yokoyama M, Shimada K, Daida H, Aoki S, Giacca A, Kanazawa A, Kawamori R, Watada H. Correlates of insulin clearance in apparently healthy non-obese Japanese men. Sci Rep 7: 1462, 2017. doi: 10.1038/s41598-017-01469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim MK, Reaven GM, Chen YD, Kim E, Kim SH. Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity (Silver Spring) 23: 2430–2434, 2015. doi: 10.1002/oby.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim MK, Reaven GM, Kim SH. Dissecting the relationship between obesity and hyperinsulinemia: Role of insulin secretion and insulin clearance. Obesity (Silver Spring) 25: 378–383, 2017. doi: 10.1002/oby.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim SP, Ellmerer M, Kirkman EL, Bergman RN. Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab 292: E1581–E1589, 2007. doi: 10.1152/ajpendo.00351.2006. [DOI] [PubMed] [Google Scholar]
- 104.Kirshner J, Schumann D, Shively JE. CEACAM1, a cell-cell adhesion molecule, directly associates with annexin II in a three-dimensional model of mammary morphogenesis. J Biol Chem 278: 50338–50345, 2003. doi: 10.1074/jbc.M309115200. [DOI] [PubMed] [Google Scholar]
- 105.Kjems LL, Kirby BM, Welsh EM, Veldhuis JD, Straume M, McIntyre SS, Yang D, Lefèbvre P, Butler PC. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes 50: 2001–2012, 2001. doi: 10.2337/diabetes.50.9.2001. [DOI] [PubMed] [Google Scholar]
- 106.Knutson VP, Ronnett GV, Lane MD. Rapid, reversible internalization of cell surface insulin receptors. Correlation with insulin-induced down-regulation. J Biol Chem 258: 12139–12142, 1983. [PubMed] [Google Scholar]
- 107.Kolka CM, Bergman RN. The barrier within: endothelial transport of hormones. Physiology (Bethesda) 27: 237–247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koopmans SJ, Kushwaha RS, DeFronzo RA. Chronic physiologic hyperinsulinemia impairs suppression of plasma free fatty acids and increases de novo lipogenesis but does not cause dyslipidemia in conscious normal rats. Metabolism 48: 330–337, 1999. doi: 10.1016/S0026-0495(99)90081-1. [DOI] [PubMed] [Google Scholar]
- 109.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 135: 122–130, 2008. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 110.Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab 293: E1709–E1715, 2007. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 111.Kupfer SR, Wilson EM, French FS. Androgen and glucocorticoid receptors interact with insulin degrading enzyme. J Biol Chem 269: 20622–20628, 1994. [PubMed] [Google Scholar]
- 112.Kurochkin IV, Goto S. Alzheimer’s beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett 345: 33–37, 1994. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- 113.Lee CC, Haffner SM, Wagenknecht LE, Lorenzo C, Norris JM, Bergman RN, Stefanovski D, Anderson AM, Rotter JI, Goodarzi MO, Hanley AJ. Insulin clearance and the incidence of Type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care 36: 901–907, 2013. doi: 10.2337/dc12-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur J Neurosci 24: 3153–3162, 2006. doi: 10.1111/j.1460-9568.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 115.Lee W. The CEACAM1 expression is decreased in the liver of severely obese patients with or without diabetes. Diagn Pathol 6: 40, 2011. doi: 10.1186/1746-1596-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee WL, Klip A. Endothelial transcytosis of insulin: Does it contribute to insulin resistance? Physiology (Bethesda) 31: 336–345, 2016. [DOI] [PubMed] [Google Scholar]
- 117.Leissring MA, Farris W, Wu X, Christodoulou DC, Haigis MC, Guarente L, Selkoe DJ. Alternative translation initiation generates a novel isoform of insulin-degrading enzyme targeted to mitochondria. Biochem J 383: 439–446, 2004. doi: 10.1042/BJ20041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lester SG, Russo L, Ghanem SS, Khuder SS, DeAngelis AM, Esakov EL, Bowman TA, Heinrich G, Al-Share QY, McInerney MF, Philbrick WM, Najjar SM. Hepatic CEACAM1 over-expression protects against diet-induced fibrosis and inflammation in white adipose tissue. Front Endocrinol (Lausanne) 6: 116–122, 2015. doi: 10.3389/fendo.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levy-Toledano R, Accili D, Taylor SI. Deletion of C-terminal 113 amino acids impairs processing and internalization of human insulin receptor: comparison of receptors expressed in CHO and NIH-3T3 cells. Biochim Biophys Acta 1220: 1–14, 1993. doi: 10.1016/0167-4889(93)90090-C. [DOI] [PubMed] [Google Scholar]
- 120.Li Calzi S, Choice CV, Najjar SM. Differential effect of pp120 on insulin endocytosis by two variant insulin receptor isoforms. Am J Physiol 273: E801–E808, 1997. [DOI] [PubMed] [Google Scholar]
- 121.Li F, Yang J, Jones JE, Villar VA, Yu P, Armando I, Felder RA, Jose PA. Sorting nexin 5 and dopamine d1 receptor regulate the expression of the insulin receptor in human renal proximal tubule cells. Endocrinology 156: 2211–2221, 2015. doi: 10.1210/en.2014-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li F, Yang J, Villar VAM, Asico LD, Ma X, Armando I, Sanada H, Yoneda M, Felder RA, Jose PA, Wang X. Loss of renal SNX5 results in impaired IDE activity and insulin resistance in mice. Diabetologia 61: 727–737, 2018. doi: 10.1007/s00125-017-4482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liang WG, Ren M, Zhao F, Tang WJ. Structures of human CCL18, CCL3, and CCL4 reveal molecular determinants for quaternary structures and sensitivity to insulin-degrading enzyme. J Mol Biol 427, 6 Pt B: 1345–1358, 2015. doi: 10.1016/j.jmb.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin S-H, Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem 264: 14408–14414, 1989. [PubMed] [Google Scholar]
- 125.Lorenzo C, Hanley AJ, Wagenknecht LE, Rewers MJ, Stefanovski D, Goodarzi MO, Haffner SM. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: the insulin resistance atherosclerosis study. Diabetes Care 36: 101–103, 2013. doi: 10.2337/dc12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maianti JP, McFedries A, Foda ZH, Kleiner RE, Du XQ, Leissring MA, Tang WJ, Charron MJ, Seeliger MA, Saghatelian A, Liu DR. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature 511: 94–98, 2014. doi: 10.1038/nature13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marini MA, Frontoni S, Succurro E, Arturi F, Fiorentino TV, Sciacqua A, Perticone F, Sesti G. Differences in insulin clearance between metabolically healthy and unhealthy obese subjects. Acta Diabetol 51: 257–261, 2014. doi: 10.1007/s00592-013-0511-9. [DOI] [PubMed] [Google Scholar]
- 128.Matsubayashi Y, Yoshida A, Suganami H, Ishiguro H, Yamamoto M, Fujihara K, Kodama S, Tanaka S, Kaku K, Sone H. Role of fatty liver in the association between obesity and reduced hepatic insulin clearance. Diabetes Metab 44: 135–142, 2018. doi: 10.1016/j.diabet.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 129.Matveyenko AV, Liuwantara D, Gurlo T, Kirakossian D, Dalla Man C, Cobelli C, White MF, Copps KD, Volpi E, Fujita S, Butler PC. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes 61: 2269–2279, 2012. doi: 10.2337/db11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McClain DA. Mechanism and role of insulin receptor endocytosis. Am J Med Sci 304: 192–201, 1992. doi: 10.1097/00000441-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 131.Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, Bamji SX, Clee SM, Johnson JD. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab 16: 723–737, 2012. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 132.Misbin RI, Almira EC. Degradation of insulin and insulin-like growth factors by enzyme purified from human erythrocytes. Comparison of degradation products observed with A14- and B26-[125I]monoiodoinsulin. Diabetes 38: 152–158, 1989. doi: 10.2337/diab.38.2.152. [DOI] [PubMed] [Google Scholar]
- 133.Misbin RI, Almira EC, Duckworth WC, Mehl TD. Inhibition of insulin degradation by insulin-like growth factors. Endocrinology 113: 1525–1527, 1983. doi: 10.1210/endo-113-4-1525. [DOI] [PubMed] [Google Scholar]
- 134.Mohamad M, Mitchell SJ, Wu LE, White MY, Cordwell SJ, Mach J, Solon-Biet SM, Boyer D, Nines D, Das A, Catherine Li SY, Warren A, Hilmer SN, Fraser R, Sinclair DA, Simpson SJ, de Cabo R, Le Couteur DG, Cogger VC. Ultrastructure of the liver microcirculation influences hepatic and systemic insulin activity and provides a mechanism for age-related insulin resistance. Aging Cell 15: 706–715, 2016. doi: 10.1111/acel.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morelli L, Llovera RE, Alonso LG, Frangione B, de Prat-Gay G, Ghiso J, Castaño EM. Insulin-degrading enzyme degrades amyloid peptides associated with British and Danish familial dementia. Biochem Biophys Res Commun 332: 808–816, 2005. doi: 10.1016/j.bbrc.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 136.Morita M, Kurochkin IV, Motojima K, Goto S, Takano T, Okamura S, Sato R, Yokota S, Imanaka T. Insulin-degrading enzyme exists inside of rat liver peroxisomes and degrades oxidized proteins. Cell Struct Funct 25: 309–315, 2000. doi: 10.1247/csf.25.309. [DOI] [PubMed] [Google Scholar]
- 137.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J 9: 2409–2413, 1990. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Müller D, Schulze C, Baumeister H, Buck F, Richter D. Rat insulin-degrading enzyme: cleavage pattern of the natriuretic peptide hormones ANP, BNP, and CNP revealed by HPLC and mass spectrometry. Biochemistry 31: 11138–11143, 1992. doi: 10.1021/bi00160a026. [DOI] [PubMed] [Google Scholar]
- 139.Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, Qiao SW, Chen Z, Glickman J, Najjar SM, Nakajima A, Neel BG, Blumberg RS. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity 25: 769–781, 2006. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 140.Najjar SM, Boisclair YR, Nabih ZT, Philippe N, Imai Y, Suzuki Y, Suh DS, Ooi GT. Cloning and characterization of a functional promoter of the rat pp120 gene, encoding a substrate of the insulin receptor tyrosine kinase. J Biol Chem 271: 8809–8817, 1996. doi: 10.1074/jbc.271.15.8809. [DOI] [PubMed] [Google Scholar]
- 141.Najjar SM. pp120, a substrate of the insulin receptor tyrosine kinase, is associated with phosphatase activity. Biochem Biophys Res Commun 247: 457–461, 1998. doi: 10.1006/bbrc.1998.8822. [DOI] [PubMed] [Google Scholar]
- 142.Najjar SM. Regulation of insulin action by CEACAM1. Trends Endocrinol Metab 13: 240–245, 2002. doi: 10.1016/S1043-2760(02)00608-2. [DOI] [PubMed] [Google Scholar]
- 143.Najjar SM, Accili D, Philippe N, Jernberg J, Margolis R, Taylor SI. pp120/ecto-ATPase, an endogenous substrate of the insulin receptor tyrosine kinase, is expressed as two variably spliced isoforms. J Biol Chem 268: 1201–1206, 1993. [PubMed] [Google Scholar]
- 144.Najjar SM, Blakesley VA, Li Calzi S, Kato H, LeRoith D, Choice CV. Differential phosphorylation of pp120 by insulin and insulin-like growth factor-1 receptors: role for the C-terminal domain of the beta-subunit. Biochemistry 36: 6827–6834, 1997. doi: 10.1021/bi962634h. [DOI] [PubMed] [Google Scholar]
- 145.Najjar SM, Choice CV, Soni P, Whitman CM, Poy MN. Effect of pp120 on receptor-mediated insulin endocytosis is regulated by the juxtamembrane domain of the insulin receptor. J Biol Chem 273: 12923–12928, 1998. doi: 10.1074/jbc.273.21.12923. [DOI] [PubMed] [Google Scholar]
- 146.Najjar SM, Ledford KJ, Abdallah SL, Paus A, Russo L, Kaw MK, Ramakrishnan SK, Muturi HT, Raphael CK, Lester SG, Heinrich G, Pierre SV, Benndorf R, Kleff V, Jaffa AA, Lévy E, Vazquez G, Goldberg IJ, Beauchemin N, Scalia R, Ergün S. Ceacam1 deletion causes vascular alterations in large vessels. Am J Physiol Endocrinol Metab 305: E519–E529, 2013. doi: 10.1152/ajpendo.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Najjar SM, Philippe N, Suzuki Y, Ignacio GA, Formisano P, Accili D, Taylor SI. Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry 34: 9341–9349, 1995. doi: 10.1021/bi00029a009. [DOI] [PubMed] [Google Scholar]
- 148.Najjar SM, Russo L. CEACAM1 loss links inflammation to insulin resistance in obesity and non-alcoholic steatohepatitis (NASH). Semin Immunopathol 36: 55–71, 2014. doi: 10.1007/s00281-013-0407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Najjar SM, Yang Y, Fernström MA, Lee SJ, Deangelis AM, Rjaily GA, Al-Share QY, Dai T, Miller TA, Ratnam S, Ruch RJ, Smith S, Lin SH, Beauchemin N, Oyarce AM. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab 2: 43–53, 2005. doi: 10.1016/j.cmet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 150.Nédellec P, Turbide C, Beauchemin N. Characterization and transcriptional activity of the mouse biliary glycoprotein 1 gene, a carcinoembryonic antigen-related gene. Eur J Biochem 231: 104–114, 1995. doi: 10.1111/j.1432-1033.1995.tb20676.x. [DOI] [PubMed] [Google Scholar]
- 151.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med 318: 1225–1230, 1988. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 152.Öbrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol 9: 616–626, 1997. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Obrink B, Sawa H, Scheffrahn I, Singer BB, Sigmundsson K, Sundberg U, Heymann R, Beauchemin N, Weng G, Ram P, Iyengar R. Computational analysis of isoform-specific signal regulation by CEACAM1-A cell adhesion molecule expressed in PC12 cells. Ann N Y Acad Sci 971: 597–607, 2002. doi: 10.1111/j.1749-6632.2002.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 154.Ohashi K, Fujii M, Uda S, Kubota H, Komada H, Sakaguchi K, Ogawa W, Kuroda S. Increase in hepatic and decrease in peripheral insulin clearance characterize abnormal temporal patterns of serum insulin in diabetic subjects. NPJ Syst Biol Appl 4: 14, 2018. doi: 10.1038/s41540-018-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Okabayashi Y, Sugimoto Y, Totty NF, Hsuan J, Kido Y, Sakaguchi K, Gout I, Waterfield MD, Kasuga M. Interaction of Shc with adaptor protein adaptins. J Biol Chem 271: 5265–5269, 1996. doi: 10.1074/jbc.271.9.5265. [DOI] [PubMed] [Google Scholar]
- 156.Osborne TF. Sterol regulatory element-binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J Biol Chem 275: 32379–32382, 2000. doi: 10.1074/jbc.R000017200. [DOI] [PubMed] [Google Scholar]
- 157.Park SY, Cho YR, Kim HJ, Hong EG, Higashimori T, Lee SJ, Goldberg IJ, Shulman GI, Najjar SM, Kim JK. Mechanism of glucose intolerance in mice with dominant negative mutation of CEACAM1. Am J Physiol Endocrinol Metab 291: E517–E524, 2006. doi: 10.1152/ajpendo.00077.2006. [DOI] [PubMed] [Google Scholar]
- 158.Paxton RJ, Mooser G, Pande H, Lee TD, Shively JE. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci USA 84: 920–924, 1987. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol 200: 62–81, 1991. doi: 10.1016/0076-6879(91)00127-I. [DOI] [PubMed] [Google Scholar]
- 160.Pease RJ, Smith GD, Peters TJ. Characterization of insulin degradation by rat-liver low-density vesicles. Eur J Biochem 164: 251–257, 1987. doi: 10.1111/j.1432-1033.1987.tb11018.x. [DOI] [PubMed] [Google Scholar]
- 161.Pereira S, Park E, Mori Y, Haber CA, Han P, Uchida T, Stavar L, Oprescu AI, Koulajian K, Ivovic A, Yu Z, Li D, Bowman TA, Dewald J, El-Benna J, Brindley DN, Gutierrez-Juarez R, Lam TK, Najjar SM, McKay RA, Bhanot S, Fantus IG, Giacca A. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress. Am J Physiol Endocrinol Metab 307: E34–E46, 2014. doi: 10.1152/ajpendo.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Perrotti N, Accili D, Marcus-Samuels B, Rees-Jones RW, Taylor SI. Insulin stimulates phosphorylation of a 120-kDa glycoprotein substrate (pp120) for the receptor-associated protein kinase in intact H-35 hepatoma cells. Proc Natl Acad Sci USA 84: 3137–3140, 1987. doi: 10.1073/pnas.84.10.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510: 84–91, 2014. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest 106: 165–169, 2000. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Piccinini F, Polidori DC, Gower BA, Bergman RN. Hepatic but not extrahepatic insulin clearance Is lower in African American than in European American women. Diabetes 66: 2564–2570, 2017. doi: 10.2337/db17-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pivovarova O, Bernigau W, Bobbert T, Isken F, Möhlig M, Spranger J, Weickert MO, Osterhoff M, Pfeiffer AF, Rudovich N. Hepatic insulin clearance is closely related to metabolic syndrome components. Diabetes Care 36: 3779–3785, 2013. doi: 10.2337/dc12-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pivovarova O, Höhn A, Grune T, Pfeiffer AF, Rudovich N. Insulin-degrading enzyme: new therapeutic target for diabetes and Alzheimer’s disease? Ann Med 48: 614–624, 2016. doi: 10.1080/07853890.2016.1197416. [DOI] [PubMed] [Google Scholar]
- 168.Pivovarova O, von Loeffelholz C, Ilkavets I, Sticht C, Zhuk S, Murahovschi V, Lukowski S, Döcke S, Kriebel J, de las Heras Gala T, Malashicheva A, Kostareva A, Lock JF, Stockmann M, Grallert H, Gretz N, Dooley S, Pfeiffer AF, Rudovich N. Modulation of insulin degrading enzyme activity and liver cell proliferation. Cell Cycle 14: 2293–2300, 2015. doi: 10.1080/15384101.2015.1046647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: Results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes 65: 1556–1564, 2016. doi: 10.2337/db15-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care 35: 2438–2442, 2012. doi: 10.2337/dc12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pørksen N, Munn S, Steers J, Vore S, Veldhuis J, Butler P. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol 269: E478–E488, 1995. [DOI] [PubMed] [Google Scholar]
- 172.Poy MN, Ruch RJ, Fernstrom MA, Okabayashi Y, Najjar SM. Shc and CEACAM1 interact to regulate the mitogenic action of insulin. J Biol Chem 277: 1076–1084, 2002. doi: 10.1074/jbc.M108415200. [DOI] [PubMed] [Google Scholar]
- 173.Poy MN, Yang Y, Rezaei K, Fernström MA, Lee AD, Kido Y, Erickson SK, Najjar SM. CEACAM1 regulates insulin clearance in liver. Nat Genet 30: 270–276, 2002. doi: 10.1038/ng840. [DOI] [PubMed] [Google Scholar]
- 174.Prall F, Nollau P, Neumaier M, Haubeck HD, Drzeniek Z, Helmchen U, Löning T, Wagener C. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J Histochem Cytochem 44: 35–41, 1996. doi: 10.1177/44.1.8543780. [DOI] [PubMed] [Google Scholar]
- 175.Rajan S, Shankar K, Beg M, Varshney S, Gupta A, Srivastava A, Kumar D, Mishra RK, Hussain Z, Gayen JR, Gaikwad AN. Chronic hyperinsulinemia reduces insulin sensitivity and metabolic functions of brown adipocyte. J Endocrinol 230: 275–290, 2016. doi: 10.1530/JOE-16-0099. [DOI] [PubMed] [Google Scholar]
- 176.Ramakrishnan SK, Khuder SS, Al-Share QY, Russo L, Abdallah SL, Patel PR, Heinrich G, Muturi HT, Mopidevi BR, Oyarce AM, Shah YM, Sanchez ER, Najjar SM. PPARalpha (peroxisome proliferator-activated receptor alpha) activation reduces hepatic CEACAM1 protein expression to regulate fatty acid oxidation during fasting-refeeding transition. J Biol Chem 291: 8121–8129, 2016. doi: 10.1074/jbc.M116.714014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ramakrishnan SK, Russo L, Ghanem SS, Patel PR, Oyarce AM, Heinrich G, Najjar SM. Fenofibrate decreases insulin clearance and insulin secretion to maintain insulin sensitivity. J Biol Chem 291: 23915–23924, 2016. doi: 10.1074/jbc.M116.745778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol 13: 444–453, 2001. doi: 10.1016/S0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 179.Robitaille J, Izzi L, Daniels E, Zelus B, Holmes KV, Beauchemin N. Comparison of expression patterns and cell adhesion properties of the mouse biliary glycoproteins Bbgp1 and Bbgp2. Eur J Biochem 264: 534–544, 1999. doi: 10.1046/j.1432-1327.1999.00660.x. [DOI] [PubMed] [Google Scholar]
- 180.Russo L, Ghadieh HE, Ghanem SS, Al-Share QY, Smiley ZN, Gatto-Weis C, Esakov EL, McInerney MF, Heinrich G, Tong X, Yin L, Najjar SM. Role for hepatic CEACAM1 in regulating fatty acid metabolism along the adipocyte-hepatocyte axis. J Lipid Res 57: 2163–2175, 2016. doi: 10.1194/jlr.M072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Russo L, Muturi HT, Ghadieh HE, Ghanem SS, Bowman TA, Noh HL, Dagdeviren S, Dogbey GY, Kim JK, Heinrich G, Najjar SM. Liver-specific reconstitution of CEACAM1 reverses the metabolic abnormalities caused by its global deletion in male mice. Diabetologia 60: 2463–2474, 2017. doi: 10.1007/s00125-017-4432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Russo L, Muturi HT, Ghadieh HE, Wisniewski AM, Morgan EE, Quadri SS, Landesberg GP, Siragy HM, Vazquez G, Scalia R, Gupta R, Najjar SM. Liver-specific rescuing of CEACAM1 reverses endothelial and cardiovascular abnormalities in male mice with null deletion of Ceacam1 gene. Mol Metab 9: 98–113, 2018. doi: 10.1016/j.molmet.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20: 131–139, 2011. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806, 2001. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 185.Sasaoka T, Rose DW, Jhun BH, Saltiel AR, Draznin B, Olefsky JM. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem 269: 13689–13694, 1994. [PubMed] [Google Scholar]
- 186.Satin LS, Butler PC, Ha J, Sherman AS. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Aspects Med 42: 61–77, 2015. doi: 10.1016/j.mam.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Seino S, Bell GI. Alternative splicing of human insulin receptor messenger RNA. Biochem Biophys Res Commun 159: 312–316, 1989. doi: 10.1016/0006-291X(89)92439-X. [DOI] [PubMed] [Google Scholar]
- 188.Semenkovich CF. Regulation of fatty acid synthase (FAS). Prog Lipid Res 36: 43–53, 1997. doi: 10.1016/S0163-7827(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 189.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31, Suppl 2: S262–S268, 2008. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 190.Sharma SK, Chorell E, Steneberg P, Vernersson-Lindahl E, Edlund H, Wittung-Stafshede P. Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner. Sci Rep 5: 12531, 2015. doi: 10.1038/srep12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sharma SK, Chorell E, Wittung-Stafshede P. Insulin-degrading enzyme is activated by the C-terminus of α-synuclein. Biochem Biophys Res Commun 466: 192–195, 2015. doi: 10.1016/j.bbrc.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 192.Sippel CJ, Fallon RJ, Perlmutter DH. Bile acid efflux mediated by the rat liver canalicular bile acid transport/ecto-ATPase protein requires serine 503 phosphorylation and is regulated by tyrosine 488 phosphorylation. J Biol Chem 269: 19539–19545, 1994. [PubMed] [Google Scholar]
- 193.Sippel CJ, Shen T, Perlmutter DH. Site-directed mutagenesis within an ectoplasmic ATPase consensus sequence abrogates the cell aggregating properties of the rat liver canalicular bile acid transporter/ecto-ATPase/cell CAM 105 and carcinoembryonic antigen. J Biol Chem 271: 33095–33104, 1996. doi: 10.1074/jbc.271.51.33095. [DOI] [PubMed] [Google Scholar]
- 194.Song ES, Jang H, Guo HF, Juliano MA, Juliano L, Morris AJ, Galperin E, Rodgers DW, Hersh LB. Inositol phosphates and phosphoinositides activate insulin-degrading enzyme, while phosphoinositides also mediate binding to endosomes. Proc Natl Acad Sci USA 114: E2826–E2835, 2017. doi: 10.1073/pnas.1613447114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Soni P, Al-Hosaini KA, Fernström MA, Najjar SM. Cell adhesion properties and effects on receptor-mediated insulin endocytosis are independent properties of pp120, a substrate of the insulin receptor tyrosine kinase. Mol Cell Biol Res Commun 1: 102–108, 1999. doi: 10.1006/mcbr.1999.0116. [DOI] [PubMed] [Google Scholar]
- 196.Soni P, Lakkis M, Poy MN, Fernström MA, Najjar SM. The differential effects of pp120 (Ceacam 1) on the mitogenic action of insulin and insulin-like growth factor 1 are regulated by the nonconserved tyrosine 1316 in the insulin receptor. Mol Cell Biol 20: 3896–3905, 2000. doi: 10.1128/MCB.20.11.3896-3905.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Standl E, Kolb HJ. Insulin degrading enzyme activity and insulin binding of erythrocytes in normal subjects and Type 2 (non-insulin-dependent) diabetic patients. Diabetologia 27: 17–22, 1984. doi: 10.1007/BF00253495. [DOI] [PubMed] [Google Scholar]
- 198.Steneberg P, Bernardo L, Edfalk S, Lundberg L, Backlund F, Ostenson CG, Edlund H. The Type 2 diabetes-associated gene ide is required for insulin secretion and suppression of α-synuclein levels in β-cells. Diabetes 62: 2004–2014, 2013. doi: 10.2337/db12-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. tk;2Sundberg U, Beauchemin N, Öbrink B. The cytoplasmic domain of CEACAM1-L controls its lateral localization and the organization of desmosomes in polarized epithelial cells. J Cell Sci 117: 1091–1104, 2004. doi: 10.1242/jcs.00944. [DOI] [PubMed] [Google Scholar]
- 200.Sundberg U, Öbrink B. CEACAM1 isoforms with different cytoplasmic domains show different localization, organization and adhesive properties in polarized epithelial cells. J Cell Sci 115: 1273–1284, 2002. [DOI] [PubMed] [Google Scholar]
- 201.Templeman NM, Clee SM, Johnson JD. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia 58: 2392–2402, 2015. doi: 10.1007/s00125-015-3676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, Chen H, Monks BR, Chen J, Rabinowitz JD, Birnbaum MJ. Direct hepatocyte insulin signaling is required for lipogenesis but Is dispensable for the suppression of glucose production. Cell Metab 23: 1154–1166, 2016. doi: 10.1016/j.cmet.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tokarz VL, MacDonald PE, Klip A. The cell biology of systemic insulin function. J Cell Biol 217: 2273–2289, 2018. doi: 10.1083/jcb.201802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol 9: 129–161, 1993. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 206.Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr 22: 487–493, 2003. doi: 10.1080/07315724.2003.10719326. [DOI] [PubMed] [Google Scholar]
- 207.Villa-Pérez P, Merino B, Fernández-Díaz CM, Cidad P, Lobatón CD, Moreno A, Muturi HT, Ghadieh HE, Najjar SM, Leissring MA, Cózar-Castellano I, Perdomo G. tk;2Liver-specific ablation of insulin-degrading enzyme causes hepatic insulin resistance and glucose intolerance, without affecting insulin clearance in mice. Metabolism 88: 1–11, 2018. doi: 10.1016/j.metabol.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ward GM, Walters JM, Aitken PM, Best JD, Alford FP. Effects of prolonged pulsatile hyperinsulinemia in humans. Enhancement of insulin sensitivity. Diabetes 39: 501–507, 1990. doi: 10.2337/diab.39.4.501. [DOI] [PubMed] [Google Scholar]
- 209.Wei X, Ke B, Zhao Z, Ye X, Gao Z, Ye J. Regulation of insulin degrading enzyme activity by obesity-associated factors and pioglitazone in liver of diet-induced obese mice. PLoS One 9: e95399, 2014. doi: 10.1371/journal.pone.0095399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wei Y, Gokhale RH, Sonnenschein A, Montgomery KM, Ingersoll A, Arnosti DN. Complex cis-regulatory landscape of the insulin receptor gene underlies the broad expression of a central signaling regulator. Development 143: 3591–3603, 2016. doi: 10.1242/dev.138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Werlen RC, Offord RE, Rose K. Preparation and characterization of novel substrates of insulin proteinase (EC 3.4.99.45). Biochem J 302: 907–911, 1994. doi: 10.1042/bj3020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Westermeier F, Sáez T, Arroyo P, Toledo F, Gutiérrez J, Sanhueza C, Pardo F, Leiva A, Sobrevia L. Insulin receptor isoforms: an integrated view focused on gestational diabetes mellitus. Diabetes Metab Res Rev 32: 350–365, 2016. doi: 10.1002/dmrr.2729. [DOI] [PubMed] [Google Scholar]
- 213.White MF. Insulin signaling in health and disease. Science 302: 1710–1711, 2003. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 214.Wiesenthal SR, Sandhu H, McCall RH, Tchipashvili V, Yoshii H, Polonsky K, Shi ZQ, Lewis GF, Mari A, Giacca A. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 48: 766–774, 1999. doi: 10.2337/diabetes.48.4.766. [DOI] [PubMed] [Google Scholar]
- 215.Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 216.Wong RH, Chang I, Hudak CS, Hyun S, Kwan HY, Sul HS. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell 136: 1056–1072, 2009. doi: 10.1016/j.cell.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xu E, Dubois MJ, Leung N, Charbonneau A, Turbide C, Avramoglu RK, DeMarte L, Elchebly M, Streichert T, Lévy E, Beauchemin N, Marette A. Targeted disruption of carcinoembryonic antigen-related cell adhesion molecule 1 promotes diet-induced hepatic steatosis and insulin resistance. Endocrinology 150: 3503–3512, 2009. doi: 10.1210/en.2008-1439. [DOI] [PubMed] [Google Scholar]
- 218.Yamada K, Carpentier JL, Cheatham B, Goncalves E, Shoelson SE, Kahn CR. Role of the transmembrane domain and flanking amino acids in internalization and down-regulation of the insulin receptor. J Biol Chem 270: 3115–3122, 1995. doi: 10.1074/jbc.270.7.3115. [DOI] [PubMed] [Google Scholar]
- 219.Yonezawa K, Yokono K, Shii K, Hari J, Yaso S, Amano K, Sakamoto T, Kawase Y, Akiyama H, Nagata M, Baba S. Insulin-degrading enzyme is capable of degrading receptor-bound insulin. Biochem Biophys Res Commun 150: 605–614, 1988. doi: 10.1016/0006-291X(88)90436-6. [DOI] [PubMed] [Google Scholar]
- 220.Yoshii H, Lam TK, Gupta N, Goh T, Haber CA, Uchino H, Kim TT, Chong VZ, Shah K, Fantus IG, Mari A, Kawamori R, Giacca A. Effects of portal free fatty acid elevation on insulin clearance and hepatic glucose flux. Am J Physiol Endocrinol Metab 290: E1089–E1097, 2006. doi: 10.1152/ajpendo.00306.2005. [DOI] [PubMed] [Google Scholar]
- 221.Zapf A, Hsu D, Olefsky JM. Comparison of the intracellular itineraries of insulin-like growth factor-I and insulin and their receptors in Rat-1 fibroblasts. Endocrinology 134: 2445–2452, 1994. doi: 10.1210/endo.134.6.8194471. [DOI] [PubMed] [Google Scholar]