Abstract
One of the mechanisms responsible for blood pressure (BP) regulation is thought to be oxidative stress. In this review, we highlight preclinical studies that strongly support a role for oxidative stress in development and maintenance of hypertension in male animals, based on depressor responses to antioxidants, particularly tempol and apocynin. In females, oxidative stress seems to be important in the initial development of hypertension. However, whether maintenance of hypertension in females is mediated by oxidative stress is not clear. In clinical studies, pharmacological intervention to reduce BP with antioxidants has conflicting results, mostly negative. This review will discuss the uncertainties regarding blood pressure control and oxidative stress and potential reasons for these outcomes.
Introduction
There is abundant evidence that there is gender/sexual dimorphism in the incidence and regulation of blood pressure (BP) in humans and experimental animal models, respectively. Younger women have a lower prevalence of hypertension than men (21), but after menopause, the prevalence of hypertension increases in women. The Center for Disease Control reported that in 2011–2014, the percentage of men, aged 65–74 yr, in the U.S. who were hypertensive was 64.1%, and the percentage of age-matched women with hypertension was 63.4% (9). However, of those hypertensives, the percentage who had uncontrolled hypertension with medication was 38.2% in men and 44.5% in women. With aging, the percentages increase so that after the age of 75 yr, 72.9% of men and 79.9% of women were hypertensive, but, alarmingly, the percentage of these hypertensives whose BP was uncontrolled was 46.5% in men but 61.5% in women (9). The reasons for these gender differences in BP incidence and control are not apparent but suggest that perhaps the mechanisms responsible for hypertension in men and women may be different.
In 2015, the results of the Systolic Blood Pressure Intervention Trial (SPRINT) were published, and in the fall of 2017, the American College of Cardiology/American Heart Association (ACC/AHA) based its recommendations for BP treatment and control on these data (52, 79). The SPRINT studies showed that intensive (<120 mmHg) systolic BP (SBP) targets were associated with 27% lower all-cause mortality than the standardized targets (<140 mmHg) in individuals at high cardiovascular disease risk but without diabetes mellitus, stroke, or heart failure (67). The SPRINT trial was criticized because the average age of participants was almost 70 yr, thus making its findings somewhat questionable for younger individuals. In addition, only 38% of the individuals in the trial were women (67, 81).
In 2018, Shapiro and colleagues reevaluated the data from SPRINT to expand the primary outcomes and adverse events (69). They separated the SBP data into tertiles and noted that women fell into the higher SBP tertile at baseline, were older, and had higher cardiovascular risk (69). Ochoa-Jimenez and colleagues also reevaluated the data from SPRINT based on gender and determined that there was no evidence that intensive antihypertensive therapy in women provided lower risk of composite outcomes (myocardial infarction, acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes) (44). Indeed, they found that men were protected from the composite risk factors with intensive antihypertensive treatment (44). These data are surprising since, based on the higher cardiovascular risk and higher baseline BP in women, intensive antihypertensive therapy should have been protective against adverse cardiovascular outcomes. These data also suggest that the mechanisms responsible for hypertension and cardiovascular disease in men and women may be different.
Oxidative stress has been implicated as being a mediator of hypertension since most studies of individuals who have lower BP also have higher levels of endogenous antioxidants such as vitamins E and C (27). In addition, data from animal studies, mainly performed in males, strongly show a correlation between oxidative stress and hypertension (18, 61, 62). Despite these observations, treatment of hypertensive individuals with antioxidants has failed to reduce their BP (22, 73). This review will elaborate on the animal studies and discuss the hypothesis that the role of oxidative stress in BP control may be different for men and women.
Mechanisms for Production of Oxidative Stress
Oxidative stress is defined as an imbalance between the production of reactive oxygen species (ROS) and antioxidants (57). There are excellent reviews written recently on oxidative stress, its causes, and the consequences to different organ systems (8, 16, 38, 42, 49). ROS are naturally produced by mitochondrial energy production, but also by enzymes such as NADPH oxidase and xanthine oxidase, as well as interactions with other oxidants. ROS play important roles in mediating intracellular signaling mechanisms, actions that may be either beneficial or deleterious (49, 65). For example, in endothelial cells, superoxide can be dismutated to produce hydrogen peroxide, which can act as a second messenger and can activate proteins such as ion channels, tyrosine kinase receptors, and phosphatases to accomplish cellular functions (49).
As shown in FIGURE 1A, the antioxidant defense system is made up of adequate levels of superoxide dismutase (SOD), catalase, and glutathione peroxidase, the situation that presumably occurs with normotension. If there are adequate levels of SOD, superoxide will bind superoxide dismutase (SOD) to form H2O2, which is subsequently metabolized by catalase or glutathione peroxidase to water. However, if SOD level is not adequate or superoxide levels are significantly high, superoxide will bind NO. The rate constant for the NO-superoxide reaction is as large or larger than the rate constant for superoxide and superoxide dismutase or for NO and heme compounds (5, 33, 34). Thus superoxide, if present, preferentially binds NO and produces peroxynitrite. The loss of NO bioavailability when it binds to superoxide causes vasoconstriction.
FIGURE 1.
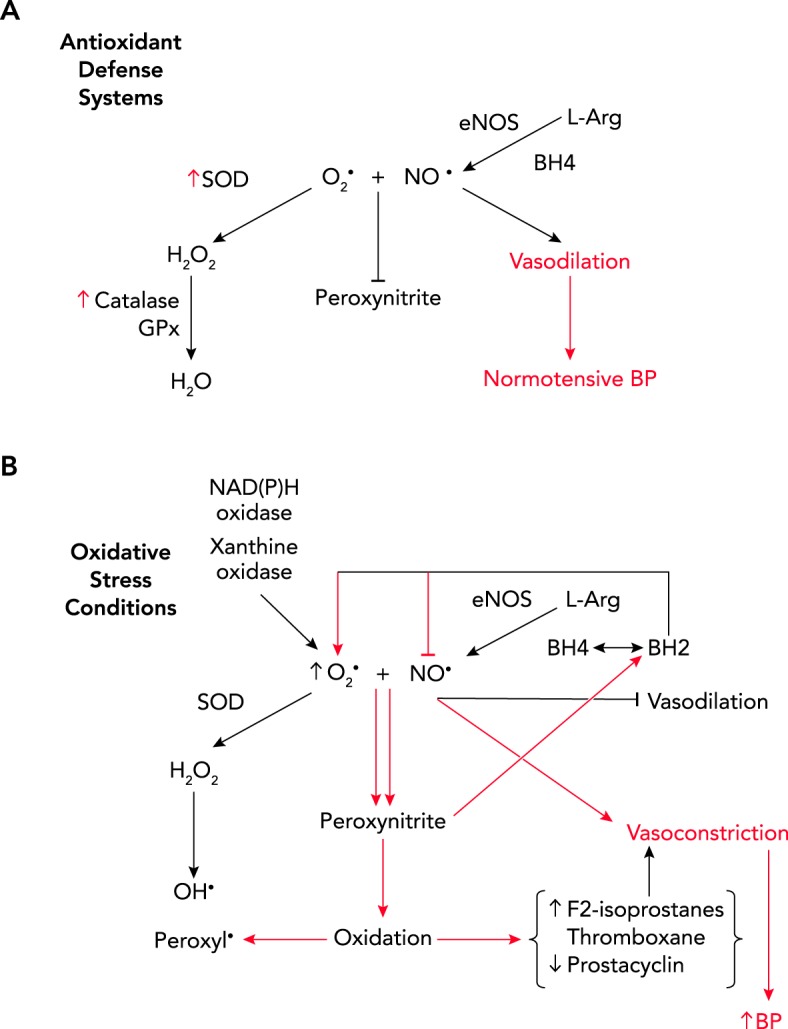
Standard antioxidant defense system
A: if superoxide dismutase is at adequate levels, superoxide will be dismutated to hydrogen peroxide (H2O2) and then to water (H2O) via catalase and glutathione peroxidase (GPx), leaving nitric oxide (NO), produced by endothelial NO synthase (eNOS) from l-arginine (L-Arg) to be bioavailable for vasodilation and normalization of blood pressure (BP). B: oxidative stress conditions. If SOD is not at adequate levels or superoxide levels are high due to activation of NADPH oxidase or xanthine oxidase, superoxide will preferentially bind to NO to produce peroxynitrite. Peroxynitrite can cause oxidation of tetrahydrobiopterin to dihydrobiopterin, causing eNOS to produce superoxide rather than NO, the so-called “uncoupling” of eNOS. The loss of NO bioavailability causes vasoconstriction. The potent oxidation by peroxynitrite also causes increases in lipid oxidation and formation of vasoconstrictors F2-isoprostane and thromboxane, inactivation of vasodilator prostacyclin, and increase in hydroxyl and peroxyl radicals, all leading to increases in BP.
Peroxynitrite is a vasodilator, but within a short amount of time the vasculature develops tachyphylaxis to peroxynitrite, and thus vasoconstriction occurs, especially in the kidney (57) (see FIGURE 1B). Beckman and colleagues showed that peroxynitrite and peroxynitrous acid were also continuous sources of active hydroxyl radical (5). Peroxyl radicals are thought to affect other intracellular signaling processes as well (40, 41). However, because it is such a potent oxidant, peroxynitrite has been shown to oxidize the vasodilator, prostacyclin, inactivating it and increasing synthesis of the vasoconstrictor, thromboxane, both potentially leading to increase in BP (57). As discussed below, peroxynitrite can also oxidize lipids to produce F2-isoprostanes, also potent vasoconstrictors (57).
As mentioned, NADPH oxidase is one of the major sources of superoxide anion (FIGURE 1B). Seven isoforms of NADPH oxidase have been identified in animals: Nox1-5 and Duox (dual oxidase) 1 and 2 (see Ref. 38 for a comprehensive review). Xanthine oxidase is another oxidant that is produced intracellularly to produce ROS and H2O2. Thus these are two enzymes that play important roles in producing ROS and oxidative stress in cells.
Oxidative Stress as a Mediator of Hypertension
Oxidative stress was implicated as playing a role in mediating hypertension in 1991, when Nakazono and colleagues reported that infusion of a fusion-protein that consisted of human Cu/Zn-SOD and a COOH-terminal basic peptide with high affinity for heparan sulfate (HB-SOD) was able to reduce BP in male spontaneously hypertensive rats (SHR) (41). They also found that oxypurinol, an inhibitor of xanthine oxidase, reduced the BP in male SHR, thus implicating oxidate stress in the hypertension of male SHR (41).
In 1996, Rajagopalan and colleagues showed that treatment of male rats with high doses (0.7 mg · kg–1 · day–1) of angiotensin (Ang) II increased BP and caused an increase in vascular superoxide mediated by NADPH oxidase (51). In contrast, norepinephrine that increased BP to similar levels had no effect on superoxide levels. When those investigators gave a liposome encapsulated superoxide dismutase, vascular dysfunction and constriction were abolished (46). These investigators went on to show that superoxide likely interfered with or “degraded” vascular NO to increase BP (28).
Since these seminal studies, numerous investigators have determined that, as shown in FIGURE 2A, Ang II activates NADPH oxidase and thus increases superoxide, which binds NO, produced by endothelial NO synthase (eNOS), leading to synthesis of peroxynitrite and loss of NO bioavailability causing vasoconstriction. Furthermore, tetrahydrobiopterin (BH4), a cofactor for eNOS, is reduced in the presence of ROS to dihydrobiopterin, leading to eNOS synthesizing superoxide rather than NO (11, 58, 84) (see FIGURE 2B). This situation is called the “uncoupling” of eNOS (11) and is thought to be an important mechanism for increasing ROS in endothelial cells and thus promoting oxidative stress leading to hypertension. Peroxynitrite is also capable of inactivating eNOS. Chen and colleagues reported that peroxynitrite dose dependently reduced eNOS activity by destabilizing the eNOS dimer (10). Addition of BH4 only partially reversed the destabilization (10). These investigators proposed that peroxynitrite is able to inactivate eNOS in part by oxidation of BH4 but also by destroying the heme/heme center in the enzyme (10).
FIGURE 2.
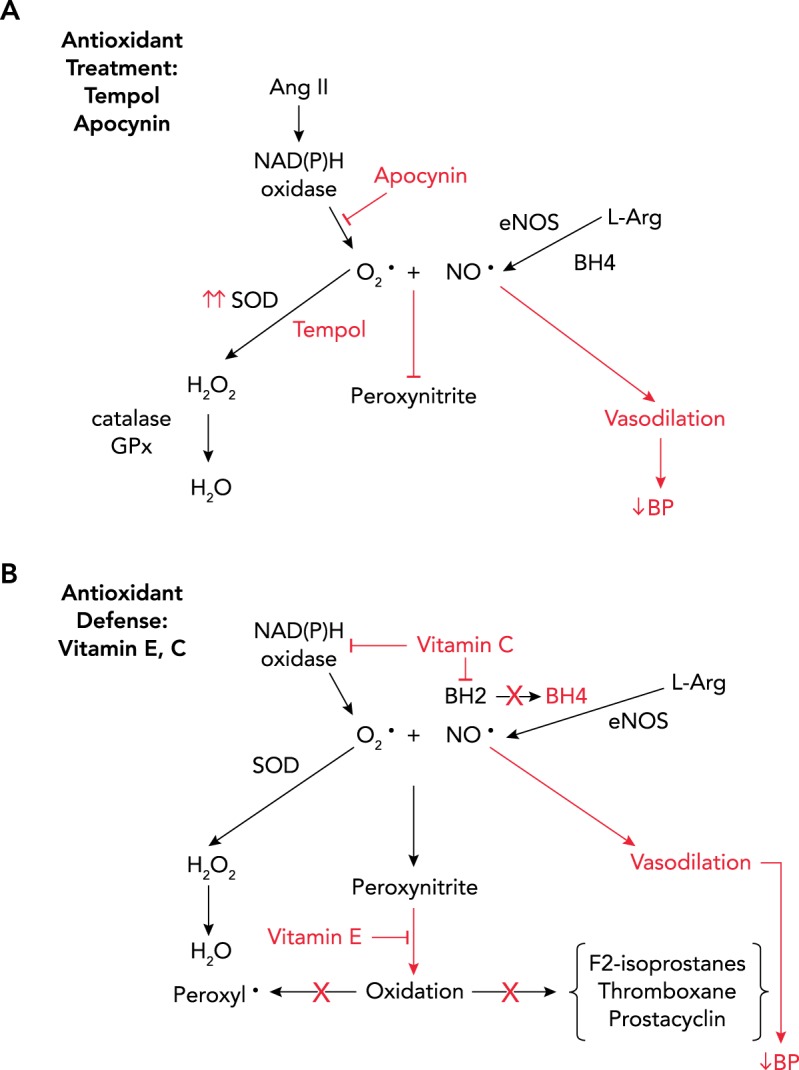
Antioxidant treatment with tempol and apocynin
A: antioxidant treatment with tempol and apocynin. Apocynin is an inhibitor of NADPH oxidase, and tempol is a SOD mimetic, both reducing the levels of superoxide blocking peroxynitrite synthesis and restoring NO bioavailability to cause vasodilation and reduce BP. B: antioxidant treatment with vitamins E and C. Vitamin C has been shown to inhibit NADPH oxidase and prevent oxidation of BH4, thus reducing the amount of superoxide and peroxynitrite produced, allowing NO to be bioactive to accomplish vasodilation and reduction of BP. Vitamin E inactivates peroxynitrite and prevents formation of peroxyl and hydroxyl radicals, and prevents oxidation of lipids (reducing F2-isoprostane), preventing synthesis of thromboxane and inhibition of prostacyclin. Taken together, these effects would reduce BP if these oxidants are involved in the maintenance of hypertension.
In experimental models of hypertension, the depressor responses to many antioxidants have been studied, mainly in male animals. These antioxidants include vitamins E and C; tempol, a superoxide dismutase mimetic; apocynin, an NADPH oxidase inhibitor; allopurinol, a xanthine oxidase inhibitor; N-acetylcysteine, a reduced glutathione precursor; and BH4 (38). For the purposes of this review, we will discuss the sex differences in the depressor responses to tempol and apocynin along with vitamins E and C since these studies are the most prevalent.
Sex Differences in the Depressor Responses to Antioxidants in SHR
The SHR is a model of essential hypertension in humans and one in which males have higher BP than females as young adults (53), but both sexes are hypertensive compared with normotensive Sprague-Dawley or WKY rats. The role of oxidative stress in mediating their hypertension has been studied more in SHR than in any other rat model. Tempol, the SOD mimetic (FIGURE 2A) given to adult male SHR reduced their BP (15, 18, 61, 62), but tempol at similar doses had no effect on BP in adult females (18). Gillis and colleagues also reported that tempol increased BH4 levels in male SHR but not in females (20). Apocynin, which inhibits NADPH oxidase (see FIGURE 2A), also reduced BP in male SHR but not in females (59). Interestingly, tempol reduced BP in males to similar levels as BP in untreated females, perhaps suggesting that the difference in BP between male and female SHR is due to higher levels of oxidative stress in males.
In support of this contention, most biomarker data suggest that oxidative stress is in fact higher in male SHR than in females. In renal tissue, lucigenin chemiluminescence studies showed that both basal levels of ROS and NADPH-stimulated levels of ROS, an index of NADPH oxidase activity, were higher in male SHR than in females (59). Renal expression of antioxidant enzymes, Mn-SOD, Cu, Zn-SOD, glutathione peroxidase, and catalase, were higher in the renal cortex of male SHR than in females (59), and urinary hydrogen peroxide excretion was also higher in male SHR than in females (70). In contrast, total antioxidant capacity (TAC), which is a measure of the ability of plasma to reduce ROS, was similar in male and female SHR (59), whereas plasma SOD activity was reported to be higher in females than in males (39).
Another biomarker for ROS is F2-isoprostane (8-iso-PGF2α), which is produced by the non-cyclooxygenase-mediated conversion of arachidonic acid, and reflects levels of lipid peroxidation (37) (see FIGURE 1B). In plasma, there were no differences in F2-isoprostane between adult male and female SHR (31, 59). In whole kidney tissue, F2-isoprostane levels were 30% higher in male SHR than in females, and urinary excretion of the metabolite of F2-isoprostane, 15-F2t-isoprostane, an indicator of whole body F2-isoprostane, was twofold higher in males (59). F2-isoprostane is a potent renal vasoconstrictor (3), and blockade of the thromboxane receptor, which is thought to be activated by F2-isoprostane (4, 75), reduces BP in male SHR but not in females (1). In contrast, inhibition of thromboxane synthesis, which is independent of F2-isoprostanes, has no effect on BP in either male or female SHR (1), showing that it is likely not thromboxane that is activating the thromboxane receptor to increase BP in male SHR, but F2-isoprostane (13).
Taken together, the biomarker data suggest that male SHR have higher levels of oxidative stress that contribute to their hypertension than females, and thus one would expect that antioxidants would cause a better depressor response in males than in females.
Does Increasing Oxidative Stress in SHR Increase BP?
The studies discussed thus far have focused on the measurement of biomarkers of ROS and the consequences to the BP of reducing the endogenous oxidative stress levels by antioxidants. Whether increasing oxidative stress exogenously also impacts BP was also studied in male and female SHR. We hypothesized that increasing oxidative stress by infusing the pro-oxidant molsidomine to increase superoxide to higher levels would increase BP, particularly in male SHR (19). Prior to molsidomine treatment, renal ROS levels, as measured by lucigenin chemiluminescence and urinary excretion of nitrate/nitrite, an index of systemic NO, were both higher in male SHR than WKY, and, in response to molsidimine, lucigenin chemiluminescence and nitrate/nitrite excretion increased in both rat strains (19). In contrast, BP increased with molsidomine in male SHR but decreased in male WKY (19). In response to the increase in oxidative stress caused by molsidomine, renal catalase and glutathione peroxidase expression increased three- to fourfold in WKY males but not in SHR (19). Taken together, these data suggest that, in male SHR, the inability to upregulate antioxidant enzymes in response to the increase in exogenous ROS with molsidomine may have contributed to the increase in their BP.
Interestingly, molsidomine failed to increase BP in female SHR (59). Based on these data, we hypothesized that perhaps higher levels of endogenous NO in female SHR prevented molsidomine from increasing BP. Estradiol has been shown to increase NO synthase expression (80), and Chen and colleagues found that NOS1 and 3 were higher in renal microvessels of female SHR than in males (12). Furthermore, Sullivan and colleagues reported that female SHR had higher renal inner medullary NOS activity and cGMP levels than males (71).
Thus, to test the hypothesis that higher renal NO bioavailability in females contributes to the lack of a pressor response to molsidomine, we gave female SHR the NO synthase inhibitor nitro-l-arginine methyl esther (l-NAME) in the presence of molsidomine. Molsidomine increased both nitrate/nitrite excretion and F2-isoprostane (30), as expected. Although l-NAME alone increased BP in female SHR, molsidomine alone had no effect on BP (30), as we previously found (59). Molsidomine + l-NAME also failed to increase BP to a higher level than with l-NAME alone, despite a further increase in F2-isoprostane with molsidomine and a decrease in nitrate/nitrite excretion in response to l-NAME (30). Taken with the previous studies, these data show that neither increasing nor decreasing superoxide affected BP in female SHR.
Role of Age in the Depressor Response to Tempol in SHR
All the previous studies described above were performed in male and female SHR that were adults. In another study, we determined the effect of chronic tempol started in prepubertal males and females to determine the role that oxidative stress may play in mediating the development of hypertension in SHR (18). Tempol was begun at 6 wk of age in both male and female SHR and continued until adulthood at 15 wk of age. We had shown previously that testosterone levels reach a peak in male SHR at 10 wk of age, the time when BP levels also reach a peak (53). With tempol treatment, BP in both male and female SHR was prevented from increasing to hypertensive levels (18).
We also tested the effect of long-term antioxidants, tempol or vitamins E and C, on the BP in postcycling SHR females compared with age-matched males. With cessation of estrus cycling (by 12 mo of age), advanced aging to 16–18 mo is associated with increases in BP in female SHR to levels that are similar to or higher than in males (17). To address the role that oxidative stress may play in mediating the increase in BP in aging SHR, males and females, 10 mo of age, were given tempol or vitamins E and C and were treated for 8 mo (17). At 18 mo of age, BP was not attenuated with chronic tempol treatment in female SHR compared with untreated females, nor was F2-isoprostane excretion affected (17). In contrast, tempol lowered BP in male SHR, as we and others had previously seen in younger males (15, 18, 61, 62). Surprisingly to us, chronic vitamins E and C treatment did prevent the increase in BP and lowered F2-isoprostane excretion in aging female SHR, but similar treatment had no effect in aged male SHR (17).
Why vitamins E and C were successful in reducing BP in aging female SHR, whereas only tempol was successful in reducing BP in male SHR, was not clear from these studies. Plasma levels of endogenous vitamins E and C have been shown to be inversely associated with mortality, cardiovascular disease, and cancer (27, 77). Vitamin E is a potent peroxyl radical scavenger that prevents the damage caused by free radicals in the membrane (64, 74) (see FIGURE 2B). The α-tocopherol radical reacts with vitamin C, which allows the α-tocopherol to return to its reduced state (74). Therefore, one of the roles of vitamin C is to regenerate the potency of vitamin E, and thus the two vitamins should be administered in combination to produce the maximal antioxidant-mediated effect.
The effects of vitamin C are not so straightforward. Vitamin C protects against lipid peroxidation by acting as a scavenger of ROS, but vitamin C at millimolar concentrations can also produce superoxide and hydrogen peroxide (48). Vitamin C prevents BH4 from being oxidized by ROS (45), thus protecting eNOS, and can downregulate NADPH oxidase (68) (FIGURE 2B). Vitamin C has also been shown to prevent peroxidase-induced NO consumption, especially in inflammatory conditions (54).
Taken together, the data intriguingly pose the possibility that the mechanisms responsible for hypertension in aging male and female SHR are different. The data suggest that, in males, increasing SOD activity in the form of tempol prevents superoxide from interacting with NO, thus reinstating the bioactivity of NO to cause vasodilation resulting in lower BP. In contrast, the data suggest that it may be peroxyl radicals, rather than superoxide and loss of NO vasodilation, that contribute to the higher BP in aging female SHR since vitamin E is known to reduce peroxyl radicals (64, 74). Removal of peroxynitrite then could also prevent the loss of prostacyclin and the increase in thromboxane, and protect BH4 from inactivation. Future studies are needed to test these hypotheses in the context of sex differences in the molecular mechanisms of hypertension.
Sex Differences in the Response to Ang II
Based on previous studies that Ang II increases oxidative stress and BP (51), we tested the hypothesis that Ang II would have similar effects on BP and oxidative stress biomarkers in male and female Sprague-Dawley rats (60). To test this hypothesis, a slow pressor dose of Ang II (150 ng · kg–1 · min–1, sc) was infused in male and female rats in the presence of enalapril, the Ang-converting enzyme inhibitor, to block endogenous Ang II synthesis (60). During baseline and before enalapril treatment, BP was lower in female rats than in males (60), and enalapril lowered BP by similar amounts in both males and females. Ang II + enalapril caused a greater pressor response in females than in males. Despite the higher BP developed in females, Ang II caused an increase in urinary F2-isoprostane in males only. When animals were placed on high salt diet with Ang II and enalapril, urinary F2-isoprostane continued to increase in both males and females, but BP continued to increase only in males. Thus Ang II-mediated increase in BP was independent of oxidative stress in females but not in males.
Bhaatia and colleagues evaluated the pressor response to Ang II in male and female SHR given apocynin, the NADPH oxidase inhibitor (6). Ang II infusion (200 ng · kg–1 · min–1, sc) caused an increase in BP in both males and females that was associated with an increase in F2-isoprostane in males but not in females (6). Apocynin begun 7 days before Ang II prevented the pressor response to Ang II in male SHR but not in females (6). These data suggest that Ang II hypertension in male SHR is more dependent on oxidative stress in the form of superoxide than in females, supported Rajagolan and colleagues, who showed that Ang II caused an increase in NADPH oxidase-mediated superoxide (51).
Oxidative Stress in Other Models of Hypertension
Dahl Salt-Sensitive Rats
Many investigators have studied the role of oxidative stress in male Dahl salt-sensitive (DS) rats (76). Meng and colleagues reported that tempol reduced BP in male DS rats on high salt diet but not on low salt diet (36). Zicha and colleagues suggested that the deficiency in NO production in the DS rat was mediated by superoxide (83). Tempol also decreased the levels of angiotensinogen in DS rats on high salt diet, suggesting that this may contribute to the depressor response to tempol (26), although renin levels would be expected to be reduced in response to the high salt diet. Whether ROS contribute to the salt-sensitive hypertension in female DS has not been reported to our knowledge.
Models of Preeclampsia and Developmental Programming of Hypertension
Reduced uterine perfusion pressure (RUPP), a model of preeclampsia in women, is produced by placing metal clips on the uterine arteries and on the abdominal aorta on day 13 of pregnancy to reduce the blood flow to the developing placentae in the rat (29) and mouse (24). BP subsequently increases until delivery and then returns to near prepregnant levels after delivery, mimicking the timeline for preeclampsia in women. Tempol given to the dam at the time of clip placement surgery (gestational day 13), prevented the development of hypertension in later pregnancy (gestational day 19) and reduced excretion of F2-isoprostanes (43, 63). However, to our knowledge, there have been no studies in which tempol has been given to RUPP dams to determine whether BP could be attenuated once the hypertension has developed.
The offspring of RUPP females exhibit intrauterine growth restriction (IUGR) (24, 29, 47), as commonly found also in offspring of women who have preeclampsia. The male offspring of a RUPP pregnancy develop increased BP with adulthood, whereas the female offspring do not (46). Tempol reduced the BP in male RUPP offspring but, as might be expected, failed to have any effect on the normotensive female offspring (46). These data suggest that development of the elevated BP in male offspring is mediated at least in part by oxidative stress.
Oxidative Stress and a Model of Lupus Erythematosus
The female NZBWF1 mouse, a model of the autoimmune disease lupus erythematosus, develops elevated BP by 40 wk of age, along with increases in markers of oxidative stress (32). Beginning at 20 wk of age, treatment with tempol and apocynin prevented the development of hypertension in these females (32). No studies have been done to our knowledge in which tempol has been given to female NZBWF1 mice that were already hypertensive to determine whether the established hypertension could be attenuated.
The Immune System and Oxidative Stress-Mediated Hypertension
The question as to the roles that inflammation and activation of the immune system play in mediating hypertension has been one of significant study over the past several years. Recent reviews by Norlander (42), Small (66), and their colleagues support that differences in immunity and autoimmunity contribute to hypertension. NADPH oxidases and the subsequent increase in ROS are thought to be critically important to immune cell recruitment to the kidney and vasculature, and immune cells themselves produce and release ROS (38), setting up a vicious cycle. Recent studies have shown that the immunosuppressant, mycophenolate mofetil (MMF), which also has antioxidant capacity, reduces BP in male SHR (72), male DS rats (14), and males with Ang II hypertension (56). Tipton and colleagues also reported that MMF reduced BP in female SHR (72). However, whether MMF reduced the BP by reducing oxidative stress in female SHR is not clear, since oxidative stress biomarkers were not measured in this study. It is possible then that it was the reduction in inflammation that caused the reduction in BP in female SHR. Interestingly, vitamins C and E are thought to protect against inflammation as well (48, 74), and perhaps this is another mechanism by which the vitamins reduced the BP in old female SHR rather than or in addition to affecting oxidative stress.
How do These Preclinical Studies Relate to Human Studies?
Despite the positive depressor results of tempol and apocynin in male animals, most studies in hypertensive humans in which antioxidant supplements have been given showed no effect on BP (69, 73). Hodgson and colleagues performed ambulatory BP measurements in cohorts of diabetic, hypertensive men and women who were given vitamin E (α-tocopheral) for 6 wk, or hypertensive-only individuals who received vitamin C and/or grape seed polyphenols for 6 wk (22). Compared with pre-study ambulatory BP and compared with placebo, neither vitamin E alone nor vitamin C with or without polyphenols reduced daytime or nighttime systolic or diastolic BP, or pulse pressure variation (22).
Why are the results of studies in humans so different from those in animal models? As mentioned above, NO can combine with superoxide to reduce NO bioavailability and thus cause vasoconstriction in the renal microvasculature, and thereby increase BP. We and others showed in young adult male SHR that blockade of NO synthase with l-NAME prevented tempol from reducing BP (61, 82). Similar findings were made by other investigators in rats given l-NNA and the antioxidant, resveratrol (2). Thus a “healthy” NO system is imperative for antioxidants to be able to reduce BP. Indeed, acutely administered intravenous vitamin C was able to reduce BP in a cohort of men and women who were normotensive (55) and likely had adequate NO production capacity. However, most clinical trials in hypertensive humans have been done in individuals in their late 50s or older, since this is the population that develops essential hypertension. At this age, the individuals likely also have endothelial dysfunction with reductions in levels of NO, perhaps due to increased oxidative stress but also due to reductions in synthesis with aging and a lack of the ability to increase NO in response to blocking superoxide or peroxynitrite production with antioxidants. This is especially the case for women who are postmenopausal, as in most of the clinical trials with antioxidants. In addition, as we hypothesized in the differential responses between aging male and female SHR with chronic tempol and vitamins E and C treatment (17), it is possible that different oxidants may affect the BP in men and women differently, and thus clinical studies are needed to identify the oxidants and then develop guidelines for specific antioxidants for each gender that also take into consideration their age.
Another reason for the failure of clinical trials to show that antioxidants reduce BP may be that many studies do not evaluate the data separately for men and women, and it is possible that hypertension in men may actually respond to most antioxidants, whereas hypertension in women does not, provided the men have adequate endothelial function and the ability to produce NO. Thus clinical studies should be powered to separately evaluate the data in men and women to determine whether there are gender differences in the depressor responses to antioxidant therapy.
Another confounder for determining the role of oxidative stress in mediating hypertension or any chronic disease is the lack of appropriate, consistent measurements of ROS in human studies. Most animal studies determine the efficacy of antioxidants to actually reduce ROS, as measured by various tissue, circulating, and urinary biomarkers. In contrast, clinical studies show divergent results for biomarkers of oxidative stress in men and women. For example, in some studies, men exhibited lower levels of urinary F2-isoprostane (33, 35) but higher levels of urinary 15-F2t-isoprostanes (the F2-isoprostane metabolite) and thiobarbituric acid-reactive substances (such as malondialdehyde) than women (23, 50). Conversely, in a study of 121 men and 177 women, aged 19–78 yr, plasma malondialdehyde and F2-isoprostanes were found to be significantly higher in women than in men (7). Furthermore, in conditions in which oxidative stress is expected to be elevated, the markers of ROS in humans are not always increased. For example, in a study reported by Keaney et al., in which there was a positive correlation between markers of oxidative stress and body mass index, there was no positive correlation with aging and oxidative stress, as defined by F2-isoprostane (25). In fact, aging was associated with less oxidative stress (25). These data are in contrast with those of another study in which F2-isoprostanes increased with aging in both men and women (78). In both studies, F2-isoprostanes were measured by gas chromatography/mass spectroscopy, in the same laboratory, which rules out the method of measurement as the source of the discrepancy. Thus specific, more consistent measures of ROS or ROS biomarkers in human studies are needed to determine the importance of oxidative stress in mediating cardiovascular diseases and essential hypertension in particular.
Summary
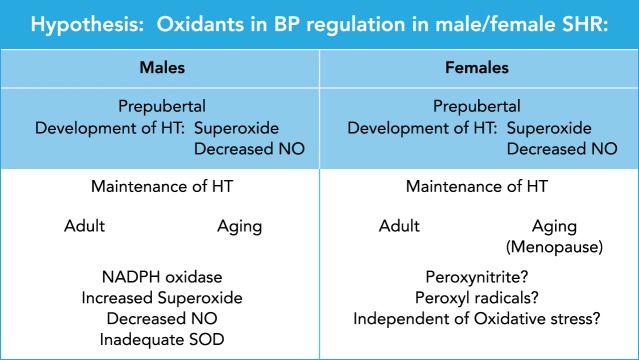
As shown in FIGURE 3, data in animal models suggest that both development and maintenance of hypertension are mediated by oxidative stress in male models at any age (SHR, Dahl SS, male offspring of RUPP dams, Ang II hypertension), as determined by responses to tempol and apocynin. In females, the data are not consistent. Development of hypertension in female SHR is mediated by ROS that can be blocked by chronic tempol when the drug is started prepubertally. These data are consistent with other models of hypertension in females (RUPP and NZBWF1) in which tempol is able to attenuate the development of hypertension. However, maintenance of hypertension in female SHR is refractory to both tempol and apocynin, and antioxidant treatment for maintenance of hypertension has not been tested in the other models (RUPP and NZBWF1). For exacerbation of existing hypertension with aging (similar to postmenopausal hypertension) in the SHR, females remain refractory to tempol, but chronic vitamins E and C are succesful in attenuating the hypertension, in contrast to age-matched males in which vitamins had no effect on their BP. These data strongly support that maintenance of hypertension in females may be mediated by different oxidant pathways than in males, such as peroxynitrite and peroxyl radicals, if in fact oxidative stress contributes to their hypertension at all. Future animal studies are needed to definitively determine the role of oxidative stress and the type of oxidative pathways involved, if any, in mediating the hypertension in females.
FIGURE 3.
Hypothesis: oxidants in BP regulation in male and female SHR
In male SHR, the data are conclusive that both development and maintenance of hypertension, regardless of the age of the animals, can be ameliorated by tempol, SOD mimetic, and apocynin, the NADPH oxidase inhibitor. Taken together, these data suggest that hypertension in males is mediated by increased superoxide and loss of bioavailable NO, perhaps due to inadequate SOD. In females, the data suggest that prepubetal development of hypertension may be superoxide mediated since tempol is able to prevent the increase in BP with adulthood. Maintenance of hypertension in females either in adults or following cessation of estrous cycling (menopause) is refactory to tempol (adult and postcycling) but is attenuated with chronic vitamins E and C (postcycling), suggesting that, if the hypertension in mediated by oxidative stress, the oxidants may be peroxynitrite mediated. However, it is also possible that vitamins E and C affected BP via reductions in inflammation rather than oxidative stress per se and that BP in females, regardless of age, plays no role in their hypertension.
Thus depressor responses to antioxidants in animals and humans are likely age specific, antioxidant specific, treatment time specific, targeted oxidant pathway specific, and sex and gender specific. For essential hypertensive humans, it remains unclear whether oxidative stress is a cause or consequence of hypertension, and whether the cause or consequence is different for the different genders.
Acknowledgments
This work is supported by National Heart, Lung, and Blood Institute Grants R01 HL-66072, R01 HL-67028, and P01 HL-51971. Research reported in this publication was supported by the National Institute of General Medical Sciences under Award No. P20 GM-121334 and P20 GM-104357. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
No conflicts of interest, financial or otherwise, are declared by the author(s).
J.F.R. and L.L.Y.C. analyzed data; J.F.R. and L.L.Y.C. interpreted results of experiments; J.F.R. and L.L.Y.C. drafted manuscript; J.F.R., D.G.R., and L.L.Y.C. edited and revised manuscript; J.F.R., D.G.R., and L.L.Y.C. approved final version of manuscript; L.L.Y.C. performed experiments; L.L.Y.C. prepared figures.
References
- 1.Acosta Cazal MC, Fortepiani LA, Santacruz F, Reckelhoff JF. Gender difference in response to thromboxane A2/prostaglandin H2 receptor antagonism in spontaneously hypertensive rats. Gend Med 1: 100–105, 2004. doi: 10.1016/S1550-8579(04)80015-9. [DOI] [PubMed] [Google Scholar]
- 2.Aydin M, Gungor B, Akdur AS, Aksulu HE, Silan C, Susam I, Cabuk AK, Cabuk G. Resveratrol did not alter blood pressure in rats with nitric oxide synthase-inhibited hypertension. Cardiovasc J Afr 28: 141–146, 2017. doi: 10.5830/CVJA-2016-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badr KF, Abi-Antoun TE. Isoprostanes and the kidney. Antioxid Redox Signal 7: 236–243, 2005. doi: 10.1089/ars.2005.7.236. [DOI] [PubMed] [Google Scholar]
- 4.Bauer J, Ripperger A, Frantz S, Ergün S, Schwedhelm E, Benndorf RA. Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. Br J Pharmacol 171: 3115–3131, 2014. doi: 10.1111/bph.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87: 1620–1624, 1990. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia K, Elmarakby AA, El-Remessy AB, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 302: R274–R282, 2012. doi: 10.1152/ajpregu.00546.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol 156: 274–285, 2002. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 8.Carmargo LL, Harvey AP, Rios FJ, Tsiropoulou S, Da Silva RNO, Cao Z, Graham D, McMAster C, Burchmore RJ, Hartley RC, Bulleid N, Montezano AC, Touyz RM. Vascular Nos (NADPH oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension 72: 235–246, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Disease Control : High Blood Pressure Facts. https://www.cdc.gov/bloodpressure/facts.htm.
- 10.Chen W, Druhan LJ, Chen CA, Hemann C, Chen YR, Berka V, Tsai AL, Zweier JL. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry 49: 3129–3137, 2010. doi: 10.1021/bi9016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen DD, Chen LY, Xie JB, Shu C, Yang T, Zhou S, Yuan H, Chen AF. Tetrahydrobiopterin regulation of eNOS redox function. Curr Pharm Des 20: 3554–3562, 2014. doi: 10.2174/13816128113196660747. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Sullivan JC, Edwards A, Layton AT. Sex-specific computational models of the spontaneously hypertensive rat kidneys: factors affecting nitric oxide bioavailability. Am J Physiol Renal Physiol 313: F174–F183, 2017. doi: 10.1152/ajprenal.00482.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cracowski JL, Devillier P, Durand T, Stanke-Labesque F, Bessard G. Vascular biology of the isoprostanes. J Vasc Res 38: 93–103, 2001. doi: 10.1159/000051036. [DOI] [PubMed] [Google Scholar]
- 14.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dornas WC, Silva M, Tavares R, de Lima WG, dos Santos RC, Pedrosa ML, Silva ME. Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: a meta-analysis. J Hypertens 33: 14–23, 2015. doi: 10.1097/HJH.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 16.Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertrán E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, My-Chan Dang P, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Förstermann U, Giniatullin R, Giricz Z, Görbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustín P, Hillion M, Huang J, Ilikay S, Jansen-Dürr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz LO, Korac B, Korkmaz KS, Koziel R, Kračun D, Krause KH, Křen V, Krieg T, Laranjinha J, Lazou A, Li H, Martínez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milković L, Miranda-Vizuete A, Mojović M, Monsalve M, Mouthuy PA, Mulvey J, Münzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavićević A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Antón N, Rodríguez-Mañas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schröder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanić V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentová K, Van Breusegem F, Varisli L, Veal EA, Yalçın AS, Yelisyeyeva O, Žarković N, Zatloukalová M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt HHHW, Di Lisa F, Daiber A. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol 13: 94–162, 2017. doi: 10.1016/j.redox.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortepiani LA, Zhang H, Racusen L, Roberts LJ II, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension 41: 640–645, 2003. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- 18.Fortepiani LA, Reckelhoff JF. Treatment with tetrahydrobiopterin reduces blood pressure in male SHR by reducing testosterone synthesis. Am J Physiol Regul Integr Comp Physiol 288: R733–R736, 2005. doi: 10.1152/ajpregu.00500.2004. [DOI] [PubMed] [Google Scholar]
- 19.Fortepiani LA, Reckelhoff JF. Increasing oxidative stress with molsidomine increases blood pressure in genetically hypertensive rats but not normotensive controls. Am J Physiol Regul Integr Comp Physiol 289: R763–R770, 2005. doi: 10.1152/ajpregu.00526.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gillis EE, Brinson KN, Rafikova O, Chen W, Musall JB, Harrison DG, Sullivan JC. Oxidative stress induces BH4 deficiency in male, but not female, SHR. Biosci Rep 38: BSR20180111, 2018. doi: 10.1042/BSR20180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health, United States, 2016: With chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics, 2017. [PubMed] [Google Scholar]
- 22.Hodgson JM, Croft KD, Woodman RJ, Puddey IB, Bondonno CP, Wu JH, Beilin LJ, Lukoshkova EV, Head GA, Ward NC. Effects of vitamin E, vitamin C and polyphenols on the rate of blood pressure variation: results of two randomised controlled trials. Br J Nutr 112: 1551–1561, 2014. doi: 10.1017/S0007114514002542. [DOI] [PubMed] [Google Scholar]
- 23.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol 22: 438–442, 2002. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 24.Intapad S, Warrington JP, Spradley FT, Palei AC, Drummond HA, Ryan MJ, Granger JP, Alexander BT. Reduced uterine perfusion pressure induces hypertension in the pregnant mouse. Am J Physiol Regul Integr Comp Physiol 307: R1353–R1357, 2014. doi: 10.1152/ajpregu.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ; Framingham Study . Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23: 434–439, 2003. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 26.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun 315: 746–750, 2004. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwabara A, Nakade M, Tamai H, Tsuboyama-Kasaoka N, Tanaka K. The association between vitamin E intake and hypertension: results from the re-analysis of the National Health and Nutrition Survey. J Nutr Sci Vitaminol (Tokyo) 60: 239–245, 2014. doi: 10.3177/jnsv.60.239. [DOI] [PubMed] [Google Scholar]
- 28.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95: 588–593, 1997. doi: 10.1161/01.CIR.95.3.588. [DOI] [PubMed] [Google Scholar]
- 29.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am J Physiol Heart Circ Physiol 295: H466–H474, 2008. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Ruiz AF, Iliescu R, Reckelhoff JF. Refractory blood pressure in female SHR to increased oxidative stress is not mediated by NO or by upregulation of renal antioxidant enzymes. Am J Physiol Regul Integr Comp Physiol 298: R266–R271, 2010. doi: 10.1152/ajpregu.00471.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathis KW, Venegas-Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59: 673–679, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem 244: 6056–6063, 1969. [PubMed] [Google Scholar]
- 34.McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968-1988). Free Radic Biol Med 5: 363–369, 1988. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 35.Melton CD, Luo R, Wong BJ, Spasojevic I, Wagenknecht LE, D’Agostino RB Jr, Il’yasova D. Urinary F2-isoprostanes and the risk of hypertension. Ann Epidemiol 27: 391–396, 2017. doi: 10.1016/j.annepidem.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD Jr. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension 41: 1346–1352, 2003. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 37.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers 10, Suppl 1: S10–S23, 2005. 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 38.Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 31: 631–641, 2015. doi: 10.1016/j.cjca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Morales RC, Bahnson ES, Havelka GE, Cantu-Medellin N, Kelley EE, Kibbe MR. Sex-based differential regulation of oxidative stress in the vasculature by nitric oxide. Redox Biol 4: 226–233, 2015. doi: 10.1016/j.redox.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustafa AG, Alfaqih MA, Al-Shboul O, Al-Dwairi A. Scavenging of lipid peroxyl radicals protects plasma lipids and proteins from peroxynitrite. Biomed Rep 9: 421–426, 2018. doi: 10.3892/br.2018.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA 88: 10045–10048, 1991. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med 205: 21–33, 2018. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol 302: R1197–R1201, 2012. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochoa-Jimenez R, Viquez-Beita K, Daluwatte C, Zusterzeel R. Sex differences of patients with systemic hypertension (from analysis of the Systolic Blood Pressure Intervential Trial [SPRINT]). Am J Cardiol 122: 985–993, 2018. doi: 10.1016/j.amjcard.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 45.Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care 18: 460, 2014. doi: 10.1186/s13054-014-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojeda NB, Hennington BS, Williamson DT, Hill ML, Betson NE, Sartori-Valinotti JC, Reckelhoff JF, Royals TP, Alexander BT. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 60: 114–122, 2012. doi: 10.1161/HYPERTENSIONAHA.112.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ojeda NB, Intapad S, Alexander BT. Sex differences in the developmental programming of hypertension. Acta Physiol (Oxf) 210: 307–316, 2014. doi: 10.1111/apha.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis 22: 463–493, 2016. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panieri E, Santoro MM. ROS signaling and redox biology in endothelial cells. Cell Mol Life Sci 72: 3281–3303, 2015. doi: 10.1007/s00018-015-1928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powers RW, Majors AK, Lykins DL, Sims CJ, Lain KY, Roberts JM. Plasma homocysteine and malondialdehyde are correlated in an age- and gender-specific manner. Metabolism 51: 1433–1438, 2002. doi: 10.1053/meta.2002.35587. [DOI] [PubMed] [Google Scholar]
- 51.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EPR III, Polonsky T, Thompson-Paul AM, Vupputuri S. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPCN/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: e116–e135, 2018. doi: 10.1161/HYP.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 53.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998. doi: 10.1161/01.HYP.31.1.435. [DOI] [PubMed] [Google Scholar]
- 54.Rees MD, Maiocchi SL, Kettle AJ, Thomas SR. Mechanism and regulation of peroxidase-catalyzed nitric oxide consumption in physiological fluids: critical protective actions of ascorbate and thiocyanate. Free Radic Biol Med 72: 91–103, 2014. doi: 10.1016/j.freeradbiomed.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 55.Ried K, Travica N, Sali A. The acute effect of high-dose intravenous vitamin C and other nutrients on blood pressure: a cohort study. Blood Press Monit 21: 160–167, 2016. doi: 10.1097/MBP.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 57.Romero JC, Reckelhoff JF. State-of-the-art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 34: 943–949, 1999. doi: 10.1161/01.HYP.34.4.943. [DOI] [PubMed] [Google Scholar]
- 58.Santhanam AV, d’Uscio LV, Smith LA, Katusic ZS. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice. J Neurochem 122: 1211–1218, 2012. doi: 10.1111/j.1471-4159.2012.07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol 34: 938–945, 2007. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 60.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 51: 1170–1176, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 61.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998. doi: 10.1161/01.HYP.32.1.59. [DOI] [PubMed] [Google Scholar]
- 62.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999. doi: 10.1161/01.HYP.33.1.424. [DOI] [PubMed] [Google Scholar]
- 63.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem 275: 13049–13055, 2000. doi: 10.1074/jbc.275.17.13049. [DOI] [PubMed] [Google Scholar]
- 65.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell 163: 560–569, 2015. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Small HY, Migliarino S, Czesnikiewicz-Guzik M, Guzik TJ. Hypertension: focus on autoimmunity and oxidative stress. Free Radic Biol Med 125: 104–115, 2018. doi: 10.1016/j.freeradbiomed.2018.05.085. [DOI] [PubMed] [Google Scholar]
- 67.SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2106, 2015. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorriento D, De Luca N, Trimarco B, Iaccarino G. The antioxidant therapy: New insights in the treatment of hypertension. Front Physiol 9: 258, 2018. doi: 10.3389/fphys.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shapiro BP, Ambrosius WT, Blackshear JL, Cushman WC, Whelton PK, Oparil S, Beddhu S, Dwyer JP, Gren LH, Kostis WJ, Lioudis M, Pisoni R, Rosendorff C, Haley WE; SPRINT Research Group . Impact of intensive versus standard blood pressure management by tertiles of blood pressure in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 71: 1064–1074, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292: R764–R768, 2007. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 298: R61–R69, 2010. doi: 10.1152/ajpregu.00526.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Togliatto G, Lombardo G, Brizzi MF. The future challenge of reactive oxigen species (ROS) in hypertension: from bench to bed side. Int J Mol Sci 18: 1988, 2017. doi: 10.3390/ijms18091988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43: 4–15, 2007. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsou PS, Amin MA, Campbell PL, Zakhem G, Balogh B, Edhayan G, Ohara RA, Schiopu E, Khanna D, Koch AE, Fox DA. Activation of the thromboxane A2 receptor by 8-isoprostane inhibits the pro-angiotenic effect of vascular endothelial growth factor in scleroderma. J Invest Dermatol 135: 3153–3162, 2015. doi: 10.1038/jid.2015.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang SM, Fan JH, Taylor PR, Lam TK, Dawsey SM, Qiao YL, Abnet CC. Association of plasma vitamin C concentrtion to total and cause-soecific mortality: a 16 year prospective study in China. J Epidemiol Community Health 72: 1076–1082, 2018. doi: 10.1136/jech-2018-210809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ward WF, Qi W, Van Remmen H, Zackert WE, Roberts LJ II, Richardson A. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. J Gerontol A Biol Sci Med Sci 60: 847–851, 2005. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- 79.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPCN/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: e13–e115, 2018. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 80.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA 91: 5212–5216, 1994. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wenger NK, Ferdinand KC, Bairey Merz CN, Walsh MN, Gulati M, Pepine CJ; American College of Cardiology Cardiovascular Disease in Women Committee . Women, Hypertension, and the Systolic Blood Pressure Intervention Trial. Am J Med 129: 1030–1036, 2016. doi: 10.1016/j.amjmed.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 82.Yanes L, Romero D, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, Bell W, Zhang H, Reckelhoff JF. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the RAS, and oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R903–R908, 2005. doi: 10.1152/ajpregu.00530.2004. [DOI] [PubMed] [Google Scholar]
- 83.Zicha J, Dobesová Z, Kunes J. Relative deficiency of nitric oxide-dependent vasodilation in salt-hypertensive Dahl rats: the possible role of superoxide anions. J Hypertens 19: 247–254, 2001. doi: 10.1097/00004872-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal 14: 1769–1775, 2011. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]