Abstract
Posture and movement planning, preparation, and execution of a goal-directed reaching movement are impaired in individuals with stroke. No studies have shown whether the deficits are generally impaired or are specific to the lesioned hemisphere/paretic arm. This study utilized StartReact (SR) responses elicited by loud acoustic stimuli (LAS) to investigate the preparation and execution of anticipatory postural adjustments (APAs) and reach movement response during both paretic and nonparetic arm reaching in individuals with stroke and in age-matched healthy controls. Subjects were asked to get ready after receiving a warning cue and to reach at a “go” cue. An LAS was delivered at –500, –200, and 0 ms relative to the go cue. Kinetic, kinematic, and electromyographic data were recorded to characterize APA-reach movement responses. Individuals with stroke demonstrated systemwide deficits in posture and in movement planning, preparation, and execution of APA-reach sequence as shown by significant reduction in the incidence of SR response and impaired APA-reach performance, with greater deficits during paretic arm reaching. Use of trunk compensation strategy as characterized by greater involvement of trunk and pelvic rotation was utilized by individuals with stroke during paretic arm reaching compared with nonparetic arm reaching and healthy controls. Our findings have implications for upper extremity and postural control, suggesting that intervention should include training not only for the paretic arm but also for the nonparetic arm with simultaneous postural control requirements to improve the coordination of the APA-reach performance and subsequently reduce instability while functional tasks are performed during standing.
NEW & NOTEWORTHY Our study is the first to show that nonparetic arm reaching also demonstrates impairment in posture and movement planning, preparation, and execution when performed during standing by individuals with stroke. In addition, we found compensatory trunk and pelvic rotations were used during a standing reach task for the paretic arms. The findings have clinical implications for upper extremity and postural rehabilitation, suggesting that training should include the nonparetic arms and incorporate simultaneous postural control demands.
Keywords: motor preparation, postural control, reach, StartReact, stroke
INTRODUCTION
Stroke is the leading cause of long-term disability among adults with ~795,000 people having a new or recurrent stroke each year in the US (Mozaffarian et al. 2016). A majority of stroke survivors have multifactorial impairments such as limited arm/hand function and impaired postural control in legs and trunk, which lead to difficulty in performing daily activities such as goal-directed reaching movements in standing and, subsequently, falls (Nakayama et al. 1994; Tyson et al. 2006). The impairments can limit functional recovery and the performance of activities of daily living and can negatively affect the quality of life.
Goal-directed reaching movements during standing involve posture and movement planning, preparation, and execution. A balance perturbation from a voluntary reaching movement normally involves a proactive postural adjustment to counter in advance the movement-related postural disturbance, typically referred to as an anticipatory postural adjustment (APA). For forward reaching in standing, the typical APA events preceding a voluntary movement are characterized by inhibition of tonic soleus (SOL) activity accompanied by tibialis anterior (TA) burst that initially moves the center of pressure (CoP) posteriorly, followed by bursts of soleus activity to move the CoP anteriorly as the hand/arm reaches forward (Leonard et al. 2009), as well as activation of erector spine (ES) to stabilize the trunk (Stapley et al. 1999). The APAs from legs and trunk are not only responsible for maintaining postural stability but also provide the dynamic support to improve the performance of transporting the arm/hand to the target in terms of force or velocity (Kaminski 2007; Massion et al. 2004). Following stroke, reduced and delayed APAs muscle activation patterns in leg muscles and altered CoP parameters have been commonly reported during nonparetic arm pointing, raising, and load-dropping tasks in standing (Garland et al. 1997, 2009; Slijper et al. 2002). Similarly, disrupted trunk muscle activation patterns (Dickstein et al. 2004; Pereira et al. 2014) and greater trunk rotation displacement (Cirstea et al. 2003; Robertson and Roby-Brami 2011) were also reported in individuals with stroke compared with healthy subjects during sitting reach movement. However, it is not clear how the trunk contributes to the APA-reach sequence during standing in terms of biomechanical characteristics following stroke. Because the APAs that precede and accompany a focal arm movement need to be appropriately directed, timed, and scaled throughout the whole movement, especially in the planning and execution stages (Stapley et al. 1999), the effects of a stroke on these stages of movement are important to determine.
From a neurophysiological standpoint, a loud acoustic stimulus (LAS) can be used to probe the state of central neuronal excitability reflecting movement planning and preparation when delivered before a prepared goal-directed action (Alibiglou and MacKinnon 2012; Valls-Solé et al. 1999). In a simple reaction time (RT) paradigm, when the intended action is known in advance, an LAS applied before the imperative signal can trigger the prepared movement with earlier onset latency (<100 ms) but with comparable spatial and temporal kinematics of the voluntary movement sequence (Valls-Solé et al. 1999), referred to as a StartReact (SR) response. Based on the short reaction times and the coexpression of classical startle reflex detected by ocular and neck (sternocleidomastoid) muscle activation, it has been proposed that the pontomedullary reticular formation (PMRF) that mediates the classical startle reflexes is involved in the early release of the prepared movement sequence (Valls-Solé et al. 1999). Studies have found that transcortical pathway may also be involved in the triggering process of SR responses, based on the evidence of delaying SR responses by suprathreshold transcranial magnetic stimulation (TMS) over the primary motor cortex (Alibiglou and MacKinnon 2012) and change of cortical excitability in response to an LAS during movement preparation phase (Marinovic et al. 2014).
Limited studies have shown intact SR responses with comparable muscle activation patterns during an elbow flexion movement (Honeycutt and Perreault 2012), a hand extension task (Honeycutt et al. 2015), and a reaching task during sitting with the paretic arm in individuals with stroke compared with healthy controls (Marinovic et al. 2016). However, inappropriate muscle activity can result from an unsuppressed classical startle reflex that could interfere with the movement. This effect increased with increasing levels of upper extremity impairment following stroke (Honeycutt and Perreault 2012, 2014). Whereas these studies suggested the ability of movement planning and preparation for a single joint movement in sitting may be preserved in individuals with stroke, our preliminary study found that persons after stroke had abnormal posture and abnormal movement planning and preparation as shown by an absence of SR responses during rapid paretic arm reaching in standing (McCombe Waller et al. 2016). The different demands for posture and task complexity may explain the differences between these studies. However, no studies have shown if the abnormal posture and abnormal movement planning and preparation are systemwide problems in individuals with stroke (i.e., occur bilaterally) or if these are specific to the lesioned hemisphere only.
To further address these issues, the purposes of this study were to address 1) posture and movement planning, preparation, and execution as measured by the incidence, onset, and magnitude of SR responses, 2) the APA-reach sequence in people with stroke, and 3) the role played by the trunk in APA-reach sequence as measured by the trunk rotation accompanying paretic and nonparetic arm reaching during standing in individuals with chronic hemiparesis and in healthy controls. Our hypotheses were 1) compared with healthy controls, there would be posture and movement planning and preparation deficits as shown by a reduced incidence, magnitude, and delayed onset of the SR responses and impaired APA-reach sequence in persons after stroke with greater deficits for paretic arm reaching, and 2) compared with healthy controls, increased trunk compensation as shown by greater trunk rotation would be found with more compensation during paretic arm reaching.
METHODS
Subjects
Data were collected from 10 individuals with chronic stroke [7 men, 3 women, mean age = 69.13 yr (SD 7.61); Table 1] and 10 age-matched healthy controls [6 men, 4 women; mean age = 66.01 yr (SD 6.38)]. Inclusion criteria for subjects with stroke were 1) unilateral cortical or white matter subcortical stroke, 2) age 40 yr and older, 3) ≥6 mo after ischemic stroke or ≥12 mo after hemorrhagic stroke and completed standard inpatient and outpatient therapy, 4) residual arm hemiparesis as indicated by Fugl-Meyer Upper Extremity (FM-UE; Gladstone et al. 2002; Sanford et al. 1993) assessment score between 20 and 65, 5) the ability to perform reaching movements with the paretic arm while standing without an assistive device for a minimum of 10 min (for participation in testing), and 6) the cognitive ability to understand, follow verbal instructions, and give informed consent. Individuals with different lesion locations were included because no specific effects of lesion locations on SR responses were observed in our previous study (McCombe Waller et al. 2016). Exclusion criteria for subjects with stroke were 1) stroke involving bilateral hemisphere, brain stem, or cerebellum and 2) any medical condition precluding participation in testing, such as acute cardiac or respiratory conditions limiting activity and other health conditions significantly affecting balance control and UE movement function beyond the effects of stroke, such as other neurological conditions or peripheral neuropathies. Inclusion criteria for healthy controls were 1) age-matched to the stroke subjects and without a history of stroke or any known neurological conditions and 2) the cognitive ability to follow two-step commands and give informed consent. Exclusion criteria for healthy controls were the same as the second exclusion criterion in stroke subjects. All participants gave written informed consent to participate, and the study was approved by the Institutional Review Board at the University of Maryland Baltimore.
Table 1.
Demographic characteristics
| Age, yr | Sex | Time Poststroke, yr | Paresis | Lesion Location | Dominant Side | FM-UE (/66) | FM-LE (/34) | CB&M (/96) | |
|---|---|---|---|---|---|---|---|---|---|
| Stroke subject | |||||||||
| 1 | 75.73 | M | 14.00 | R | Mixed | R | 49 | 31 | 58 |
| 2 | 63.36 | M | 5.91 | R | Mixed | R | 39 | 25 | 48 |
| 3 | 77.63 | M | 20.51 | L | Cortical | L | 33 | 19 | 30 |
| 4 | 62.58 | F | 7.55 | L | Mixed | R | 30 | 17 | 27 |
| 5 | 68.14 | M | 8.81 | L | Mixed | R | 62 | 26 | 30 |
| 6 | 70.33 | F | 16.40 | R | Subcortical | R | 26 | 21 | 16 |
| 7 | 74.23 | F | 51.26 | L | Subcortical | L | 36 | 19 | 17 |
| 8 | 64.10 | M | 0.97 | R | Subcortical | R | 65 | 34 | 78 |
| 9 | 55.99 | M | 2.26 | R | Subcortical | R | 65 | 34 | 75 |
| 10 | 79.29 | M | 1.29 | L | Subcortical | R | 55 | 29 | 31 |
| Group | |||||||||
| Stroke | 69.13 (7.61) | 7M/3F | 12.90 (15.00) | 5R/5L | 1 Cortical/5 Subcortical/ 4 Mixed | 8R/2L | 46.00 (15.06) | 25.50 (6.36) | 41.00* (22.61) |
| Control | 66.01 (6.38) | 6M/4F | 9R/1L | 76.70* (11.11) |
Values are means (SD). FM, Fugl-Meyer Assessment. CB&M, Community Balance and Mobility Scale; LE, lower extremity; UE, upper extremity. Mixed lesion location indicates cortical and subcortical.
P < 0.05.
Loud Acoustic Stimulus
An LAS was used to examine the movement planning and preparation of APAs and goal-directed reaching movement for the paretic and nonparetic arms in stroke subjects and for the nondominant and dominant arms in healthy controls during standing. A computer-generated analog tone (1 kHz, 40 ms) was used to create the LAS (Campbell et al. 2012; Carlsen and MacKinnon 2010; Carlsen et al. 2011; MacKinnon et al. 2013). The tone was amplified and presented by a horn speaker (HS-17T; MG Electronics) placed 30 cm behind the head of the subject. The intensity of the LAS near the subject’s ears was ~123 dB. Sound intensity was measured with a digital sound meter (Extech 407730; FLIR Commercial Systems) placed 30 cm from the speaker before each testing to make sure the intensity was consistent for all subjects.
Protocol
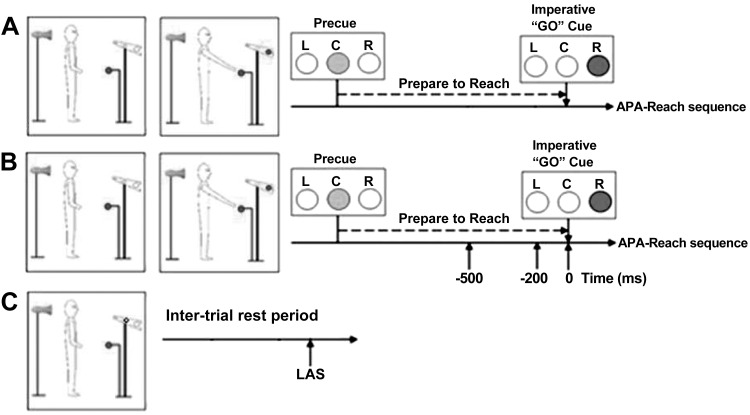
Each subject participated in two sessions: paretic and nonparetic arm sessions for stroke subjects and dominant and nondominant arm sessions for healthy controls. Subjects were notified as to which arm they were going to reach before each session. The order of sessions was randomized. In each session, subjects stood on two separate force platforms with their arms relaxed on the sides and feet placed a natural and comfortable distance apart. An outline of each foot was drawn on paper taped to the force platforms to ensure consistent foot placement across trials and between sessions. A visually cued delayed-response paradigm was used to examine the transition from a stationary standing posture to the rapid initiation of reaching. Task instruction stimuli were presented to the subject using a horizontal bank of three LED lights (left-center-right) placed at eye level 3 m in front of the subject. A precue (center) light was presented, followed by the imperative “go” cue (left or right) light with an interstimulus delay of 2.5 s. The right go cue light was illuminated after the precue (center) light when the reach arm was the right arm (Fig. 1), and the left go cue light was illuminated after the precue (center) light when the reach arm was the left arm. The target ball was placed at 65% of the subject’s height at the midline, and the target distance was 10 cm beyond the subjects’ maximal reach distance of the paretic arm for stroke subjects and the nondominant arm for healthy controls. In the standing reach task, subjects were instructed to reach for a target ball “as quickly as possible” in response to the go cue, but not to initiate the reach before the go cue. In each session, subjects performed 65 trials including 3 conditions. In condition 1, control reach trials (45 trials) consisted of standing reach movements performed with no LAS presented. In condition 2, LAS reach trials (3 time points × 5 trials, 15 trials) consisted of standing reach movement performed with the LAS presented at one of the three time points: – 500, – 200, or 0 ms relative to the go cue. These time points were selected on the basis of past normative studies showing progressive increases in the incidence and magnitude of SR responses during this time window reflecting motor preparation (MacKinnon et al. 2007). In condition 3, control LAS trials (5 trials) were collected in which an LAS was delivered during an intertrial standing rest period without reach and without the presence of the precue and go cue, serving as a control condition for LAS trials to verify that SR responses did not occur in the absence of movement planning and to confirm that the LAS could elicit a classical startle reflex. The number of trials with LAS (15 LAS trials and 5 control LAS trials) was kept at 33% of all trials to ensure that subjects did not habituate to the stimulus (Carlsen et al. 2003; Siegmund et al. 2001). The order of presentation of LAS and control reach trials was partly randomized with the exceptions that the LAS was not presented during the first five trials and that no more than two trials with LAS (LAS reach trials and control LAS trials) were presented in a row.
Fig. 1.
Example of a right arm reaching session. A: a control reach trial. B: a loud acoustic stimulus (LAS) reach trial. C: a control LAS trial. APA, anticipatory postural adjustments; C, center; L, left; R, right.
Data Collection
Kinetic data including ground reaction forces and moment were collected from two force platforms (AMTI, Watertown, MA) placed beneath the right and left feet at a collection frequency of 600 Hz. These data were filtered with a low-pass, 4th-order Butterworth digital filter with a cutoff frequency at 10 Hz (Hermens et al. 2000; Roy et al. 2007). Kinematic data were collected at 120 Hz, using a 10-camera motion analysis system (Vicon, Los Angeles, CA). These data were filtered with a low-pass, 4th-order Butterworth digital filter with a cutoff frequency at 10 Hz. Reflective markers were placed bilaterally on the foot (toe between 2nd and 3rd metatarsal and calcaneus), ankle (lateral malleoli), knee (lateral femoral condyle), shank (in line with medial knee and ankle markers), thigh (in line with greater trochanter of the femur and lateral knee), pelvis (anterior superior iliac spine and sacrum), shoulders (acromion), elbow (lateral epicondyle), upper arm (middle part of the humerus, in line with acromion and lateral elbow), wrist (radius and ulnar styloids), and head (glabellum and mastoid process) (Eames et al. 1999). Kinematic computations of joint centers were performed using a model (Eames et al. 1999) written in commercially available software (BodyBuilder; Vicon, Centennial, CO). The muscle activity was recorded bilaterally from the reaching arm muscle including anterior deltoid (AD), leg muscles including TA and SOL, and back muscle (ES at level of L3) with a wireless electromyography (EMG) system TeleMyo direct transmission system (Noraxon, Scottsdale, AZ) using bipolar surface Ag-AgCl electrodes. Following skin preparation, electrodes were placed longitudinally to the muscle fibers at standard recording sites to minimize skin impedance and maximize signal conduction (Hermens et al. 2000; Roy et al. 2007). All electrodes placements followed the recommendations of SENIAM (https://www.seniam.org). Raw EMG signal was sampled at 1,500 Hz. The data were bandpass filtered between 30 and 500 Hz with a 5th-order Butterworth filter using the MATLAB program filtfilt, full-wave rectified, and low-pass filtered (10 Hz, 4th-order Butterworth) for smoothing purposes. Kinetic, kinematic, and EMG data were collected for 10 s per trial and synchronized through an external trigger.
Data Analysis
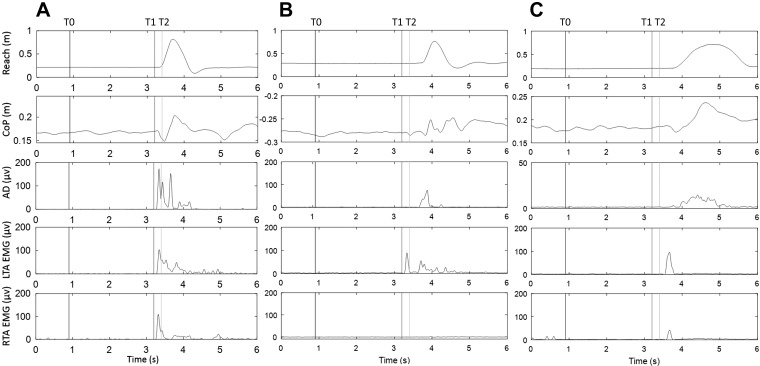
Custom-written MATLAB programs (The MathWorks, Natick, MA) were used to process kinetic, kinematic, and EMG data, and all data were verified by visual inspection. Movement planning and preparation were examined using the presence of SR responses. The incidence of SR responses as an index of response occurrence was reported during the movement preparation period when the LAS was applied before the go cue (i.e., time points −500 and –200 ms). For the trials where the LAS was presented at the go signal, the SR responses were not determined, because the responses evoked by the LAS were possibly intermingled with the responses to the imperative go signal. To be considered an SR response in the APA or reach, the occurrence of components of APA or reach was required to be met within one of the following time windows: 1) between the LAS and the go cue or 2) within an early onset of <3 SD from the average onset in the control reach condition (MacKinnon et al. 2007). The components for an APA response were 1) an initial posterior shift in the CoP and 2) an early EMG burst in TA before the onset of reach. In some subjects, a clear suppression of SOL activity was observed. However, this was not consistent in all subjects; therefore, a suppression of SOL activity was not considered as a required component to identify an APA. The components for a reach response were 1) an anterior movement of hand and 2) an EMG burst in AD. CoP-related parameters were derived from the forces and moments. Reach and APA onsets were determined with a threshold equivalent to 5% peak velocity. The onset times of muscle activation were calculated based on changes of >3 SD for at least 100 ms from the mean signal recorded before the go cue or LAS and a continuous increase of muscle activity were seen. The onset times were verified by visual inspection (Hodges and Bui 1996). For LAS trials, the onset was expressed relative to the timing of LAS. For the control reach condition, the onset was expressed relative to the imperative go cue. SR responses were further categorized to full or partial SR APA-reach responses. Full SR APA-reach response required a completed SR response in both APA and reach, whereas partial SR APA-reach response required an SR in either APA or reach (see Fig. 2).
Fig. 2.
Example of full, partial, and absent StartReact (SR) responses. A: full SR response with a completed anticipatory postural adjustment (APA)-reach sequence as shown by anterior displacement of the hand (1st window), posterior center of pressure (CoP) displacement (2nd window), and corresponding muscle activation (3rd–5th windows) after the loud acoustic stimulus (LAS) and before the go cue. B: partial SR response with SR response only in APA as shown by posterior CoP displacement and tibialis anterior (TA) activation between the LAS and go cue. C: absent SR response with neither SR response in APA onset nor SR response in reach. T0 represents the timing of the warning cue. T1 represents the timing of the LAS. T2 represents the timing of the go cue. Note that the LAS is at −200 ms relative to the go cue. AD, anterior deltoid; LTA, left TA; RTA, right TA.
Outcome Measures
Movement planning and preparation were assessed using the incidence of SR responses. Reach onsets were determined from the wrist joint center movement in the anterior-posterior direction. Reach peak velocity was determined as the maximum velocity of the reaching movement defined by the wrist joint center. APA onset was determined by the onset of the posterior CoP displacement. APA magnitudes were determined by the maximum posterior CoP displacements from the onset of the CoP normalized by reach distance. APA peak velocity was determined as the maximum velocity of the posterior CoP movement. Temporal coordination of APA and reach was determined by the lag between APA and reach onsets. Trunk rotation was determined from the angular displacement of the line connecting both shoulders in the horizontal plane in the direction of the reach at maximum reach normalized by reach distance (i.e., angular displacement divided by the reaching distance, rad/m). Pelvic rotation was determined from the angular displacement of the line connecting both pelvic joint centers in the horizontal plane in the direction of the reach at maximum reach normalized by reach distance. Trunk and pelvic rotation onset were determined by the onset of the trunk and pelvic rotation, respectively. Trunk-pelvic rotation difference was determined from the difference between horizontal trunk and pelvic angular displacement at maximum reach normalized by reach distance. Fugl-Meyer assessment was used to confirm eligibility and characterize the level of motor impairment in UE and lower extremity in stroke subjects (Gladstone et al. 2002; Sanford et al. 1993). The Community Balance and Mobility Scale (CB&M) was used to characterize balance function and mobility in both stroke subjects and healthy controls (Balasubramanian 2015; Howe et al. 2006; Knorr et al. 2010). A higher of CB&M score indicates better balance and mobility function.
Statistical Analyses
A linear mixed-effects model using group (healthy controls vs. stroke group), paretic arm (paretic arms in the stroke group vs. nonparetic arms in the stroke group and both arms in healthy controls), LAS condition (LAS at − 500, − 200, and 0 ms relative to the go, and control reach) as fixed factors and subjects as a random factor was performed to test the difference in 1) arm reaching in healthy controls vs. nonparetic arm reaching in the stroke group, 2) paretic vs. nonparetic arm reaching in the stroke group, and 3) arm reaching in healthy controls vs. paretic arm reaching in the stroke group, adjusting for LAS condition. Because the difference between the dominant and nondominant arms is not the main interest of this study, the dominant and nondominant arms were treated as the same in this model. The model included the main effects of group, paretic arm, LAS condition, group × LAS condition interaction, paretic arm × LAS condition interaction, and a random intercept for subjects. The interaction term that had the least significant effect was excluded first, and then the model was reexamined to see if the remaining interaction term became significant. If the remaining interaction did not reach significance, it was removed. If the remaining interaction reached significance, it stayed in the model. Stratified analyses were administered when there was an interaction effect to determine any differences of interest. Before analysis, proportion variables (e.g., incidence of SR response) were converted for normality using an arcsine square root transformation. The Bonferroni test was used for all post hoc comparisons. Between-group differences in demographic data were tested by independent Student t-tests for continuous variables and by χ2 tests for dichotomous variables. All statistical analyses were performed using SPSS (v.22; IBM, Armonk, NY). All statistical tests were made at a significance level of P < 0.05.
RESULTS
Table 1 presents demographic characteristics of the study participants. Age and sex were comparable in both groups. The stroke group had significantly lower CB&M scores, indicating impaired balance function and mobility compared with healthy controls [t(18) = 4.481, P = 0.001]. One healthy control (male, age = 81 yr) was excluded from the analysis because the subject’s movement onset time and velocity deviated more than 3 SD from that of other healthy controls.
SR Response Incidence When the LAS Was Applied Before the Go Cue
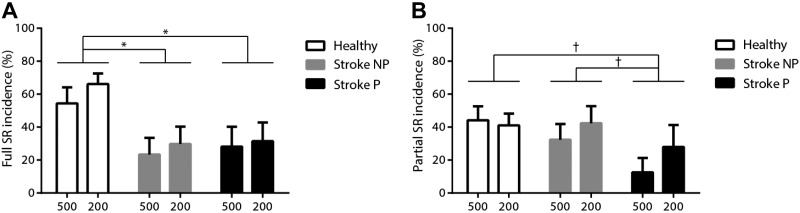
A higher incidence of SR responses was seen in most of the healthy controls (66%, averaging dominant and nondominant arms), whereas in most of the individuals with stroke, there were comparatively few or no SR responses (nonparetic vs. paretic arm: 38% vs. 32%). The incidence of SR responses for LAS trials at − 500 and – 200 ms for each individual with stroke is presented in Table 2. Comparisons of the paretic and nonparetic arms, and with both arms in healthy controls, showed that when the LAS was presented before the go cue, reduced full SR incidence was found in both paretic (P = 0.016) and nonparetic arm reaching (P = 0.008) with no significant difference between the paretic and nonparetic arms in individuals with stroke (Fig. 3A). There was a trend toward reduced partial SR incidence during paretic arm reaching compared with nonparetic arm reaching (P = 0.063) in the stroke group and with arm reaching in healthy controls (P = 0.066) (Fig. 3B).
Table 2.
Occurrence and incidence of full and partial SR responses for LAS trials before the go cue for individuals with stroke
| Paretic Arm |
Nonparetic Arm |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −500 ms |
−200 ms |
%SR Response |
−500 ms |
−200 ms |
%SR Response |
|||||||
| Subject | Full SR | Partial SR | Full SR | Partial SR | Full SR | Partial SR | Full SR | Partial SR | Full SR | Partial SR | Full SR | Partial SR |
| 1 | 3 | 0 | 2 | 1 | 55* | 11* | 3 | 1 | 2 | 2 | 50 | 30 |
| 2 | 0 | 0 | 0 | 2 | 0 | 20 | 1 | 1 | 1 | 4 | 20 | 50 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 1 | 50 | 20 |
| 4 | 0 | 1 | 1 | 0 | 10 | 10 | 0 | 2 | 0 | 2 | 0 | 40 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 2 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 1 | 0 | 0 | 0 | 10 | 0 | 0 | 3 | 0 | 0 | 0 | 30 |
| 8 | 2 | 0 | 1 | 1 | 30 | 10 | 0 | 0 | 1 | 1 | 10 | 10 |
| 9 | 0 | 3 | 0 | 5 | 0 | 80 | 1 | 0 | 2 | 2 | 30 | 20 |
| 10 | 3 | 0 | 4 | 0 | 70 | 0 | 0 | 1 | 0 | 1 | 0 | 20 |
Value are StartReact (SR) responses, determined as the percentage of total number of full SR response occurrences at −500 and –200 ms relative to onset divided by the total number of trials for each individual. There were 10 loud acoustic stimulus (LAS) trials (5 trials per time point) for each arm.
There was 1 missing trial due to false start.
Fig. 3.
Mean (±SE) incidence of full StartReact (SR) response (A) and partial SR response (B) when the loud acoustic stimulus (LAS) was at − 500 and – 200 ms relative to the go cue in healthy controls and during the nonparetic (NP) and paretic (P) arm reaching in individuals with stroke. *P < 0.05; †P < 0.1. There were no significant group × LAS condition or paretic arm × LAS condition interactions.
APA-Reach Performance
Paretic vs. nonparetic arm within the stroke group.
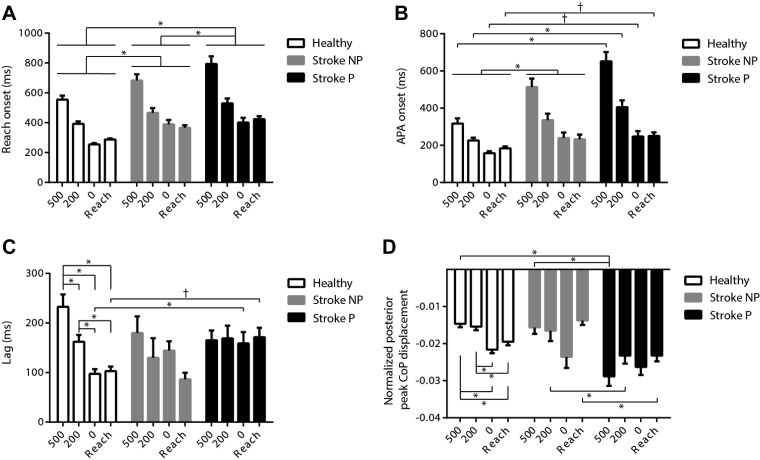
Comparison between the paretic and the nonparetic arm performances showed greater deficits during paretic arm reaching, with later reach onset (P = 0.001) (Fig. 4A) and arm muscle activation onset in AD (P < 0.001), longer movement time (P < 0.001), and slower reach peak velocity (P < 0.001) across all LAS conditions. For the APA-reach sequence, no significant difference in the timing interval between APA and reach onsets was found between the paretic and the nonparetic arm reaching (Fig. 4C). For APA variables, although there was no significant difference in APA onsets (Fig. 4B), later ipsilateral TA onsets were found during the paretic arm reaching at LAS time point −200 ms and in the control reach condition compared with the nonparetic arm reaching (P = 0.028). During the paretic arm reaching, the APA velocity was faster across all LAS conditions (P = 0.015), and APA amplitudes were greater at LAS time points –500 (P < 0.001) and –200 ms (P = 0.038) and in the control reach condition (P < 0.001) compared with the nonparetic arm reaching (Fig. 4D).
Fig. 4.
Mean (±SE) reach onset (A), anticipatory postural adjustment (APA) onset (B), lag between APA and reach onsets (C), and APA amplitude (D) across conditions [loud acoustic stimuli (LAS) at −500, −200, and 0 ms relative to the go, and the control reach condition] in healthy controls and during the nonparetic (NP) and paretic (P) arm reaching in individuals with stroke. *P < 0.05; †P < 0.1. In A, there were no significant group × LAS condition or paretic arm × LAS condition interactions. In B, there was a significant paretic arm × LAS condition interaction but no significant group × LAS condition interaction. In C, there was a significant group × LAS condition interaction but no significant paretic arm × LAS condition interaction. In D, there were significant group × LAS condition and paretic arm × LAS condition interactions.
Nonparetic arm movement in the stroke group vs. arm movement in healthy controls.
Comparison of the nonparetic arm in the stroke group with both arms in the healthy control group showed the nonparetic arm also demonstrated altered APA-reach performance compared with healthy controls. During the nonparetic arm reaching, later reach onset (P = 0.032) (Fig. 4A), later muscle activation onset in AD (P = 0.043), slower reach peak velocity (P = 0.011), and later APA onset (P = 0.007) (Fig. 4B) were observed across all LAS conditions. A later contralateral TA onset was also found during the nonparetic arm reaching in the stroke group compared with arm reaching in healthy controls in the control reach condition (P = 0.001). The effects of LAS timing were found only in the controls for the APA amplitude (P < 0.001) (Fig. 4D) and the time interval between APA and reach onsets (Fig. 4C). In healthy controls, the LAS at −500 ms was associated with a smaller APA amplitude and longer lag between APA and reach onsets compared with other conditions. In healthy controls, as the stimulation timing got closer to the go cue, the APA amplitude was gradually increased and the lag was shortened (APA amplitude: −500 vs. 0 ms, P < 0.001; −500 ms vs. control reach, P < 0.001; −200 vs. 0 ms, P < 0.001; −200 ms vs. control reach, P = 0.001; lag: −500 vs. −200 ms, P = 0.003; −500 vs. 0 ms, P < 0.001; −500 ms vs. control reach, P < 0.001; −200 vs. 0 ms, P = 0.001; −200 ms vs. control reach, P = 0.022).
Paretic arm movement in the stroke group vs. arm movement in healthy controls.
Comparisons between the paretic arm in the stroke group and both arms in the healthy control group indicated general impairments in reach performance during the paretic arm reaching compared with arm reaching in healthy controls, reflected in significantly later reach onset (P = 0.002) (Fig. 4A), later muscle activation onset in AD (P = 0.002), longer reach movement time (−500 ms, P = 0.013; −200 ms, P = 0.007; 0 ms, P = 0.002; control reach, P = 0.002), and slower reach peak velocity (P < 0.001) across LAS conditions. The temporal APA-reach sequence differed during paretic arm reaching compared with reaching in healthy controls as shown by a longer onset timing lag between APA and reach onsets when the LAS was at 0 ms (P = 0.033) and a marginal difference for the control reach condition (P = 0.063) (Fig. 4C). For APA-related variables, during the paretic arm reaching, significantly later onsets in APA were observed when the LAS was delivered before the go (−500 ms, P = 0.002; −200 ms, P = 0.001), and a trend toward later onsets was observed when the LAS was at the go cue (P = 0.089) and for the control reach condition (P = 0.074), compared with healthy controls (Fig. 4B). Later ipsilateral and contralateral TA onsets were also found during the paretic arm reaching compared with arm reaching in healthy controls in the control reach condition (P = 0.003 and P = 0.013, respectively). The APA amplitudes (P < 0.001) (Fig. 4D) and velocity (P = 0.029) were greater during the paretic arm reaching compared with arm reaching in healthy controls at LAS time point −500 ms.
Trunk Performance
Paretic vs. nonparetic arm movement in the stroke group.
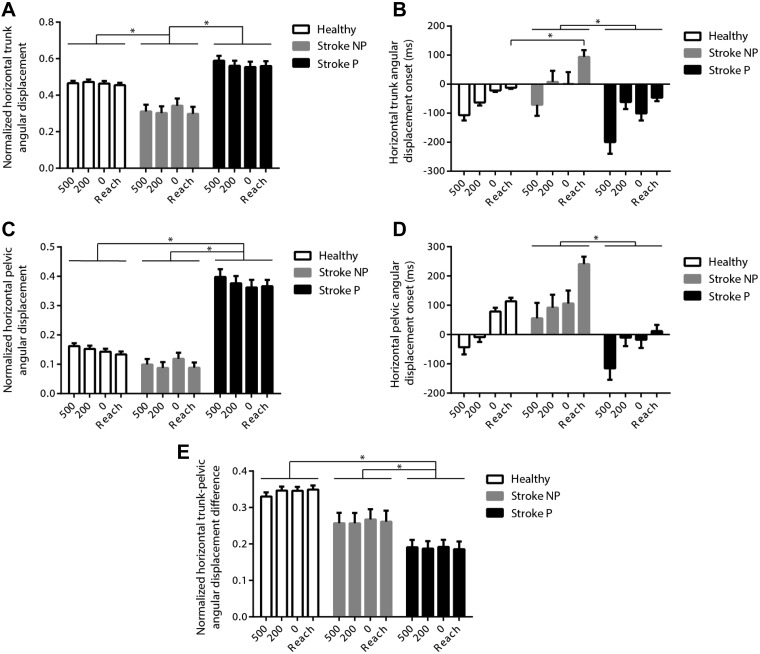
Comparisons between the paretic and nonparetic arm reaching demonstrated greater trunk rotation (P < 0.001) (Fig. 5A), earlier trunk rotation onset (P < 0.001) (Fig. 5B), greater pelvic rotation (P < 0.001) (Fig. 5C), earlier pelvic rotation onset (P < 0.001) (Fig. 5D), and reduced trunk-pelvic rotation difference (P < 0.001) (Fig. 5E) during paretic arm reaching.
Fig. 5.
Mean (±SE) normalized trunk rotation (A), trunk rotation onset (B), pelvic rotation (C), pelvic rotation onset (D), and trunk-pelvic rotation difference (E) across conditions [loud acoustic stimuli (LAS) at −500, −200, and 0 ms relative to the go, and the control reach condition)] in healthy controls and during the nonparetic (NP) and paretic (P) arm reaching in individuals with stroke. *P < 0.05. Note that trunk and pelvic onsets are presented relative to reach onsets. In A, C, D, and E, there were no significant group × LAS condition or paretic arm × LAS condition interactions. In B, there was a significant group × LAS condition interaction but no significant paretic arm × LAS condition interaction.
Nonparetic arm movement in the stroke group vs. arm movement in healthy controls.
Comparisons between the nonparetic arm reaching in individuals with stroke and arm reaching in healthy controls showed there was significantly reduced trunk rotation (P = 0.038) (Fig. 5A) and later muscle activation onset in ipsilateral (P = 0.007) and contralateral ES (P = 0.014) during the nonparetic arm reaching. In the control reach condition, healthy controls had earlier trunk rotation onset compared with individuals in the stroke group performing nonparetic arm reaching (P = 0.026) (Fig. 5B).
Paretic arm movement in the stroke group vs. arm movement in healthy controls.
Comparisons between the paretic arm reaching in the stroke group and arm reaching in healthy controls showed greater trunk rotation, greater pelvic rotation (P < 0.001) (Fig. 5C), and reduced trunk-pelvic rotation difference (P = 0.004) (Fig. 5E) during the paretic arm reaching. Later trunk muscle activation onsets in ipsilateral (P = 0.006) and contralateral ES (P = 0.002) were found across all LAS conditions during the paretic arm reaching compared with arm reaching in healthy controls.
DISCUSSION
This study examined posture and movement planning, preparation, and execution during a standing reach task in the paretic and nonparetic arms in individuals with stroke and in both arms of age-matched healthy controls. There are three main findings from this study. First, impaired movement planning and preparation measured by the incidence of SR responses is a general problem in the central nervous system (CNS) in persons after stroke, which involves both the lesioned and contralesional side. Second, although individuals with stroke demonstrated better control in APA and reach performance during nonparetic compared with paretic arm reaching movement, the impairments were still present for nonparetic arm movements as shown by later APA, later reach onsets, and slower reach velocity compared with reaching movements in the healthy controls. Third, individuals with stroke tended to use trunk and pelvic compensations as shown by earlier trunk and pelvic rotation onset and greater trunk and pelvic rotation to transport the paretic arm toward the target.
Abnormal Movement Planning and Preparation in Individuals with Stroke
SR responses triggered by the LAS have been used widely to reflect the status of movement planning and preparation (Carlsen et al. 2011; Marinovic and Tresilian 2016). Studies in healthy subjects have shown that the triggering of an SR response by an LAS depends on a planned motor action that is known in advance (Carlsen et al. 2011). This is supported by the higher incidence of SR responses for a simple RT task compared with a choice RT task, since in the simple reaction task, the required motor action has been preinstructed and the action can be planned and prepared in advance. This also has been illustrated by the absence of SR responses when the LAS is presented alone without preparation for movement triggered by an impending imperative cue (MacKinnon et al. 2007; Valls-Solé et al. 1999). Another factor affecting the absence or presence of SR responses is the timing of the LAS relative to an upcoming reaction stimulus cue. Moreover, in the early stage of preparatory period, the incidence of SR responses is lower and gradually increases when the LAS timing approaches the onset of the imperative cue, because the movement is progressively planned and prepared in anticipation of the cue to move (MacKinnon et al. 2007, 2013). In the present study, the incidence of SR responses in the healthy controls was comparable to that of our previous study (McCombe Waller et al. 2016). We did not observe a gradual increase in the incidence of SR responses as the LAS approached the go cue, which may be due to the limitation in our experimental design in which only two LAS time points before the go (e.g., −500 and −200 ms) were used.
In individuals with stroke, a mainly absent SR response during paretic arm reaching has been shown when the LAS was presented before the go signal compared with arm reaching in healthy controls, which indicated impairments in movement planning and preparation of the APA-reach sequence (McCombe Waller et al. 2016). Our current study further demonstrated that the movement planning and preparation ability for a standing reach movement was also impaired during preparation for the nonparetic arm reaching in individuals with stroke. This indicated a more pervasive general problem with movement planning and preparation after stroke that was not confined to movements mainly involving the lesioned side. There are at least two possible reasons for the marked absence of SR responses following stroke: impaired movement planning ability or insufficient movement preparation. The SR responses triggered by LAS are thought to reflect the changes in central neuronal excitability during movement planning and preparation of an intended motor action (Carlsen et al. 2011). In this study, we were not able to tease out whether individuals with stroke had deficits in one or both of the proposed deficits because the absent SR responses may be due to an inability to plan the movement appropriately, insufficient preparation for the upcoming motor action, or both. Future studies may manipulate the motor preparatory activity to discern whether individuals with stroke demonstrate higher incidence of SR responses when they are highly prepared and a lower incidence of SR responses when they are not prepared for the motor action. One way to manipulate the motor preparatory activity is by using an anticipation-timing task to provide temporal information about when the motor action is required to be executed. For example, in a study where the subjects were asked to perform a wrist flexion when a clock hand reached to a target, an LAS was presented at 1,500, 500, and 150 ms before the time of responding (Carlsen and MacKinnon 2010). The LAS applied at 150 ms before the time of responding resulted in SR responses in 98% of trials, whereas the LAS applied at 1,500 and 500 ms before the time of responding resulted in only 0% and 18% of SR responses, respectively. A similar paradigm can be utilized to maximize the level of motor preparatory activity (i.e., when the clock hand is closer to the target time) and examine whether at this stage stroke subjects still demonstrate marked absence of SR responses. If this is the case, it is possible that the absence of SR responses is due to the inability to plan the movement following stroke. If stroke subjects show an increase in the incidence of SR response when the preparatory activity level is assumed to be high as the clock hand gets closer to the target time, the absence of SR responses found in our study may be due to insufficient preparation for the upcoming movement. In addition, the foreperiod (i.e., the interval between the warning and the go cue) and intertrial interval may also contribute to the level of motor preparatory activity (Leow et al. 2018). On the other hand, we observed a heightened classical startle reflex as shown by task-inappropriate flexor activity triggered by the LAS in the paretic elbows during both the paretic and nonparetic arm reaching. Resembling the findings from previous studies (Honeycutt and Perreault, 2014; McCombe Waller et al. 2016), the interference from a hypermetric classical startle reflex likely resulting from cortical damage and/or the imbalanced strengthening of reticulospinal connections to elbow flexors during recovery may impede arm extension (Zaaimi et al. 2012), which in turn influences the release of the SR responses.
Two categories of SR responses were elicited during the preparation period when the LAS was presented in advance of the go cue. We based these categories on the completeness of a triggered SR response: full and partial SR responses. Full SR responses (i.e., APA and reach) represented complete movement planning elements, whereas partial SR responses (i.e., SR responses in APA or SR responses in reach only) represented incomplete movement planning elements for the APA-reach sequence. Although individual differences were observed in each group, generally we found that healthy controls had a higher incidence of full SR responses compared with reduced incidence of SR responses during the paretic and nonparetic arm movements seen in the stroke group. This suggests that healthy controls had a better ability to assemble a “complete” APA-reach sequence. There was no difference in the incidence of full SR between the paretic and the nonparetic movements, but there was a trend toward a higher incidence of partial SR responses during nonparetic arm reaching. This may indicate that when preparing the nonparetic arm movements, the CNS may have a greater degree of movement planning ability although the triggered planned movement was not a complete APA-reach sequence. Another possible explanation is that lesion locations may contribute to different SR responses. We observed that subject 2 and 4 with cortical and subcortical white matter stroke had a higher incidence of partial SR responses during the nonparetic arm reaching, whereas subject 9 with subcortical stroke had a higher incidence of partial SR responses during the paretic arm reaching than other subjects with cortical stroke. Future studies may use multiple time points before the go cue and a larger sample size to examine the relevance of full and partial SR responses to motor impairments or functions, as well as different types of lesion locations in individuals with stroke.
Deficits in APA-Reach Sequence Following Stroke
We found larger APA amplitudes and greater APA velocity during the paretic arm reaching compared with the nonparetic arm reaching and compared with arm reaching in healthy controls. The amplitude of APAs is known to be scaled with the movement speed and amplitude to counterbalance the postural perturbation from the upcoming arm movement and/or to assist in the reaching performance in healthy subjects (Bouisset et al. 2000; Horak et al. 1984; Shiratori and Aruin 2007). Because individuals with stroke performed the reach movements at slower speeds and shorter distances compared with the healthy controls, larger APA amplitudes and velocity were not produced to counteract the perturbations from faster or larger reaching movements. It is plausible that during forward reaching, larger APA amplitudes, as measured by posterior CoP displacement, can create a greater moment arm (i.e., the distance between the CoP and center of mass) to produce forward angular momentums to assist in moving the body forward to the target (Stapley et al. 1998). Following stroke, an alternative APA strategy may be used to generate sufficient momentum to compensate for the limitations in the paretic arm movement and assist in the forward reaching movement. In terms of the temporal coordination of the APA-reach sequence, there was a trend toward a longer time lag between the onsets of APA and paretic arm reaching in the control reach condition, and a longer lag when the LAS was presented at the go cue, compared with time lags in healthy controls, indicating altered temporal coordination between postural and goal-directed movement systems. This resembled our previous finding, which demonstrated a longer lag between the onsets of muscle activation for the APA and reach (McCombe Waller et al. 2016).
Consistent with previous findings, deficits in APAs as measured by later APA onset were found during the nonparetic arm reaching, suggesting difficulties with postural control during the nonparetic as well as paretic arm reaching (Garland et al., 1997, 2003; Gray et al. 2012). Additional impairments in the nonparetic arm reach included later reach onset and slower reach velocity, whereas the temporal coordination between the reach and APA onset was not different from that in the healthy controls. Although no prior studies have demonstrated impaired nonparetic reaching performance in standing following stroke, impaired nonparetic arm reaching during sitting following stroke has been shown. According to the theory based on hemispheric specialization for movement control (Mani et al. 2013; Sainburg and Duff 2006), the dominant hemisphere is responsible for predicting limb and task dynamics, whereas the nondominant hemisphere is responsible for specifying limb position through impedance control mechanisms (Mutha et al. 2012). Therefore, damage in the left hemisphere impairs reaching movement trajectory, whereas damage in the right hemisphere impairs the end-point control of the reach (Schaefer et al. 2009). On inspection of our data, although we did not find any specific pattern between dominant vs. nondominant side-affected stroke performance or left vs. right hemisphere-affected stroke, our outcome variables were not designed to distinguish hemispheric specialization for movement control limit. Thus we cannot rule out the possibility that hemispheric specialization may cause a differential effect on movement control of reaching.
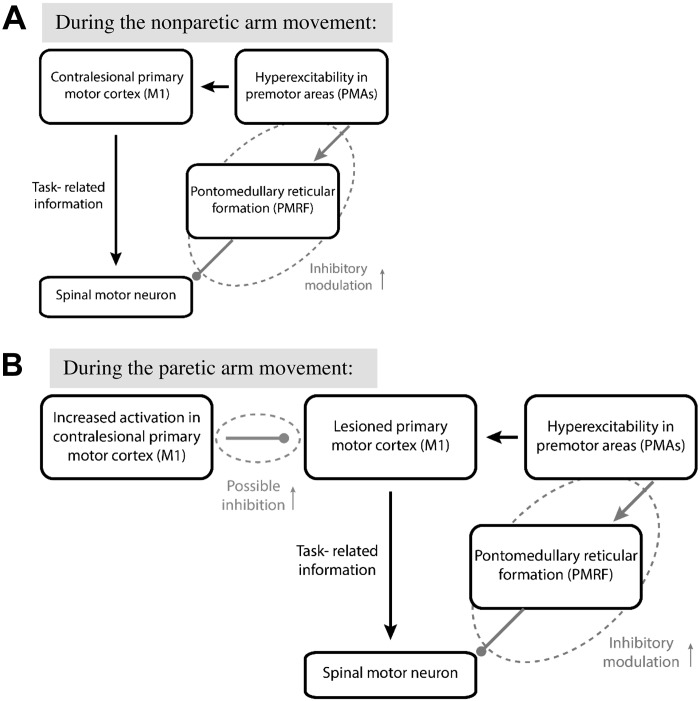
A Conceptual Model for Abnormal Posture and Abnormal Movement Planning and Preparation Following Stroke
It has been proposed that premotor areas (PMAs) of cortex are responsible for selecting appropriate neural networks for APAs, whereas the contralateral primary motor cortex (M1) is responsible for arm movement, and these signals are organized together at the subcortical level (Massion 1992; Massion et al. 2004). Therefore, the integration of subcortical postural control and cortically mediated goal-directed reaching systems requires optimal modulation of neuronal excitability between subcortical and cortical levels. One recent study in healthy subjects also suggested the involvement of both cortical and subcortical structures in mediating the SR responses by showing that the task requiring combined proximal and distal UE movement is more susceptible to SR responses than a simple distal UE movement (Castellote and Kofler 2018). A model for abnormal posture and abnormal movement planning and preparation in individuals with chronic stroke is presented in Fig. 6A. During movement planning and preparation, normally two primary pathways affect spinal motoneuronal circuitry. One pathway delivers task-related information from PMAs to M1 and subsequently to the spinal cord over the corticospinal pathway. The other cortico-reticulospinal pathway from PMAs to spinal cord via PMRF applies inhibitory modulation to prevent premature release of movement and specifies magnitude scaling of the motor action (Cohen et al. 2010). Following stroke, PMAs such as premotor cortex or supplementary motor areas show increased excitability (Ward et al. 2003) leading to excessive inhibition via the cortico-reticulospinal pathway that consequently impairs posture and movement planning, preparation, and execution. This view is supported by absent or reduced SR responses and impairments in APA-reach movement performance in the nonparetic arm in our study and also by impaired UE and balance performance on the nonparetic side found in the literature (Geurts et al. 2005; Yarosh et al. 2004). However, the temporal coordination between the APA and goal-directed reaching systems is preserved during the nonparetic reaching, although both APA and reach onsets were delayed. During the preparation for the paretic arm reaching movement, it is possible that the neuronal networks responsible for the paretic arm movements involve increased inhibition from contralesional to ipsilesional M1 areas (Murase et al. 2004) (Fig. 6B). Thus the neuronal excitability between the cortical and subcortical levels would be impaired and subsequently affect the posture and movement planning, preparation, and execution for the paretic arm reaching to a greater extent compared with the nonparetic arm reaching. This is supported by a trend toward a lower incidence of partial SR responses, altered temporal coupling between APA and reach onsets, and more impaired APA-reach performance during the paretic arm reaching compared with the nonparetic arm reaching.
Fig. 6.
Conceptual model for abnormal movement planning and preparation following stroke [model is adapted from Cohen et al. (2010)]. During movement planning and preparation, normally two primary pathways affect spinal motoneuronal circuitry. The first pathway delivers task-related information from premotor areas (PMAs) to primary motor cortex (M1) and subsequently to spinal cord over the corticospinal pathway. The second cortico-reticulospinal pathway from PMAs to spinal cord via the pontomedullary reticular formation (PMRF) applies inhibitory modulation to prevent premature release and specify magnitude scaling of the motor action. A: after stroke, hyperexcitability in PMAs leads to excessive inhibitory input via the cortico-reticulospinal pathway (“inhibitory modulation;” dashed ellipse) and consequently impairs movement planning preparation and performance as shown by absent/reduced StartReact (SR) response during the nonparetic arm reaching. B: during the paretic arm reaching, there is possible inhibition (small dashed ellipse) from increased activation in contralesional M1 on the ipsilesional M1. Thus movement preparation and performance is impaired to a greater extent compared with that during the nonparetic arm reaching movement.
Trunk and Pelvic Compensation in Individuals with Stroke
A compensatory strategy was shown by a greater pelvic rotation and reduced trunk-pelvic rotation difference during the reaching movement execution in those with stroke during the paretic arm reaching movements compared with reaching movement in healthy controls. This is consistent with previous studies that examined the trunk contribution during paretic reaching while sitting (Levin et al. 2002, 2009). We further demonstrated that stroke subjects utilized whole body rotation to compensate for the deficits in elbow extension and shoulder flexion as indicated by large pelvic rotations with a small difference between trunk and pelvic rotation during paretic arm reaching. On the other hand, healthy controls appeared to stabilize their pelvis and utilize mostly trunk rotation while reaching forward to the target. Moreover, with the same target distance, stroke subjects recruited greater and earlier trunk and pelvic rotation during the paretic arm reaching compared with the nonparetic arm reaching. As proposed by Latash and Anson (1996), in individuals with abnormal neuromusculoskeletal systems such as chronic stroke, the CNS takes into account the changed state of the central and peripheral motor systems and then chooses a new optimal movement strategy leading to altered or compensatory movement patterns. However, this does not mean that it is impossible for individuals with stroke to demonstrate a relatively normal movement pattern (Latash and Anson 1996). In fact, many studies have demonstrated that the use of limiting trunk movement (“trunk restraint”) decreases compensatory trunk strategies and improves reaching during sitting in people with stroke (de Oliveira Cacho et al. 2015; Pain et al. 2015; Woodbury et al. 2009). Our findings have clinical implications that the development of therapeutic interventions to avoid the excessive use of a trunk and pelvic compensation strategy in individuals with stroke during a goal-directed functional task, especially in standing, may be a useful approach.
Although we found reduced trunk rotation during the nonparetic arm reaching after stroke compared with reaching in controls, this is possibly due to the limitation of the current experimental design in which the target distances were set according to the maximal reach distance of the nondominant arms in healthy controls and the paretic arms in individuals with stroke. Because the maximal reach distance of the paretic arms was shorter, the target distance for the nonparetic arm reaching may be not equally challenging as for the healthy controls. Therefore, the reasons for the difference in trunk involvement between the healthy controls and the stroke subjects during the nonparetic arm reaching remains unclear. Future studies may examine the trunk contribution between the nonparetic arm reaching in individuals with stroke and arm reaching in healthy subjects by using a standardized target distance based on the nonparetic arm reaching ability.
Conclusions
The present study showed that posture and movement planning, preparation, and execution of both APAs and goal-directed reach were generally impaired in individuals with stroke not only during paretic arm reaching but also during nonparetic arm reaching. Specifically, altered APA-reach performance during the nonparetic arm reaching, as shown by later APA-reach sequence and slower reach velocity, was observed compared that during arm reaching in the controls. Individuals with stroke utilized increased trunk and pelvic rotation compensation during paretic arm reaching. Such difficulties with planning and executing a complex antigravity reaching arm movement while standing may subsequently lead to loss of balance and limited functional performance during activities of daily living, and negatively impact the quality of life. These findings have implications for upper extremity and postural rehabilitation in individuals with stroke, suggesting that in addition to training for the paretic arm, intervention strategies should also include training of the nonparetic arms with simultaneous postural control demands such as in whole body reaching training and should emphasize movement speed to facilitate the integration of both cortically mediated goal-directed reaching and subcortically mediated postural control systems.
GRANTS
This study was supported by American Heart Association Award 16PRE29970004 and the University of Maryland Claude D. Pepper-OAIC NIH/NIA grant P30AG028747.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.-l.Y. conceived and designed research; C.-l.Y. and S.M.W. performed experiments; C.-l.Y. analyzed data; C.-l.Y. interpreted results of experiments; C.-l.Y. prepared figures; C.-l.Y. drafted manuscript; C.-l.Y., M.W.R., and S.M.W. edited and revised manuscript; C.-l.Y., R.A.C., L.M., M.W.R., and S.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We especially thank all the subjects who participated in this study.
REFERENCES
- Alibiglou L, MacKinnon CD. The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex. J Physiol 590: 919–936, 2012. doi: 10.1113/jphysiol.2011.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian CK. The community balance and mobility scale alleviates the ceiling effects observed in the currently used gait and balance assessments for the community-dwelling older adults. J Geriatr Phys Ther 38: 78–89, 2015. doi: 10.1519/JPT.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Richardson J, Zattara M. Are amplitude and duration of anticipatory postural adjustments identically scaled to focal movement parameters in humans? Neurosci Lett 278: 153–156, 2000. doi: 10.1016/S0304-3940(99)00912-X. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Chua R, Inglis JT, Carpenter MG. Startle induces early initiation of classically conditioned postural responses. J Neurophysiol 108: 2946–2956, 2012. doi: 10.1152/jn.01157.2011. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Startle response is dishabituated during a reaction time task. Exp Brain Res 152: 510–518, 2003. doi: 10.1007/s00221-003-1575-5. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, MacKinnon CD. Motor preparation is modulated by the resolution of the response timing information. Brain Res 1322: 38–49, 2010. doi: 10.1016/j.brainres.2010.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35: 366–376, 2011. doi: 10.1016/j.neubiorev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Castellote JM, Kofler M. StartReact effects in first dorsal interosseous muscle are absent in a pinch task, but present when combined with elbow flexion. PLoS One 13: e0201301, 2018. doi: 10.1371/journal.pone.0201301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Ptito A, Levin MF. Arm reaching improvements with short-term practice depend on the severity of the motor deficit in stroke. Exp Brain Res 152: 476–488, 2003. doi: 10.1007/s00221-003-1568-4. [DOI] [PubMed] [Google Scholar]
- Cohen O, Sherman E, Zinger N, Perlmutter S, Prut Y. Getting ready to move: transmitted information in the corticospinal pathway during preparation for movement. Curr Opin Neurobiol 20: 696–703, 2010. doi: 10.1016/j.conb.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Cacho R, Cacho EW, Ortolan RL, Cliquet A Jr, Borges G. Trunk restraint therapy: the continuous use of the harness could promote feedback dependence in poststroke patients: a randomized trial. Medicine (Baltimore) 94: e641, 2015. doi: 10.1097/MD.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein R, Shefi S, Marcovitz E, Villa Y. Anticipatory postural adjustment in selected trunk muscles in post stroke hemiparetic patients. Arch Phys Med Rehabil 85: 261–267, 2004. doi: 10.1016/j.apmr.2003.05.011. [DOI] [PubMed] [Google Scholar]
- Eames MH, Cosgrove A, Baker R. Comparing methods of estimating the total body centre of mass in three-dimensions in normal and pathological gaits. Hum Mov Sci 18: 637–646, 1999. doi: 10.1016/S0167-9457(99)00022-6. [DOI] [Google Scholar]
- Garland SJ, Gray VL, Knorr S. Muscle activation patterns and postural control following stroke. Mot Contr 13: 387–411, 2009. doi: 10.1123/mcj.13.4.387. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Stevenson TJ, Ivanova T. Postural responses to unilateral arm perturbation in young, elderly, and hemiplegic subjects. Arch Phys Med Rehabil 78: 1072–1077, 1997. doi: 10.1016/S0003-9993(97)90130-1. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Willems DA, Ivanova TD, Miller KJ. Recovery of standing balance and functional mobility after stroke. Arch Phys Med Rehabil 84: 1753–1759, 2003. doi: 10.1016/j.apmr.2003.03.002. [DOI] [PubMed] [Google Scholar]
- Geurts AC, de Haart M, van Nes IJ, Duysens J. A review of standing balance recovery from stroke. Gait Posture 22: 267–281, 2005. doi: 10.1016/j.gaitpost.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 16: 232–240, 2002. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Gray V, Rice CL, Garland SJ. Factors that influence muscle weakness following stroke and their clinical implications: a critical review. Physiother Can 64: 415–426, 2012. doi: 10.3138/ptc.2011-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol 101: 511–519, 1996. [DOI] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ. Planning of ballistic movement following stroke: insights from the startle reflex. PLoS One 7: e43097, 2012. doi: 10.1371/journal.pone.0043097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ. Deficits in startle-evoked arm movements increase with impairment following stroke. Clin Neurophysiol 125: 1682–1688, 2014. doi: 10.1016/j.clinph.2013.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Tresch UA, Perreault EJ. Startling acoustic stimuli can evoke fast hand extension movements in stroke survivors. Clin Neurophysiol 126: 160–164, 2015. doi: 10.1016/j.clinph.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Esselman P, Anderson ME, Lynch MK. The effects of movement velocity, mass displaced, and task certainty on associated postural adjustments made by normal and hemiplegic individuals. J Neurol Neurosurg Psychiatry 47: 1020–1028, 1984. doi: 10.1136/jnnp.47.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JA, Inness EL, Venturini A, Williams JI, Verrier MC. The Community Balance and Mobility Scale–a balance measure for individuals with traumatic brain injury. Clin Rehabil 20: 885–895, 2006. doi: 10.1177/0269215506072183. [DOI] [PubMed] [Google Scholar]
- Kaminski TR. The coupling between upper and lower extremity synergies during whole body reaching. Gait Posture 26: 256–262, 2007. doi: 10.1016/j.gaitpost.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Knorr S, Brouwer B, Garland SJ. Validity of the Community Balance and Mobility Scale in community-dwelling persons after stroke. Arch Phys Med Rehabil 91: 890–896, 2010. doi: 10.1016/j.apmr.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Latash ML, Anson JG. What are “normal movements” in atypical populations? Behav Brain Sci 19: 55–106, 1996. doi: 10.1017/S0140525X00041467. [DOI] [Google Scholar]
- Leonard JA, Brown RH, Stapley PJ. Reaching to multiple targets when standing: the spatial organization of feedforward postural adjustments. J Neurophysiol 101: 2120–2133, 2009. doi: 10.1152/jn.91135.2008. [DOI] [PubMed] [Google Scholar]
- Leow LA, Uchida A, Egberts JL, Riek S, Lipp OV, Tresilian J, Marinovic W. Triggering mechanisms for motor actions: the effects of expectation on reaction times to intense acoustic stimuli. Neuroscience 393: 226–235, 2018. doi: 10.1016/j.neuroscience.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair 23: 313–319, 2009. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- Levin MF, Michaelsen SM, Cirstea CM, Roby-Brami A. Use of the trunk for reaching targets placed within and beyond the reach in adult hemiparesis. Exp Brain Res 143: 171–180, 2002. doi: 10.1007/s00221-001-0976-6. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Allen DP, Shiratori T, Rogers MW. Early and unintentional release of planned motor actions during motor cortical preparation. PLoS One 8: e63417, 2013. doi: 10.1371/journal.pone.0063417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol 97: 4368–4379, 2007. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain 136: 1288–1303, 2013. doi: 10.1093/brain/aws283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinovic W, Brauer SG, Hayward KS, Carroll TJ, Riek S. Electric and acoustic stimulation during movement preparation can facilitate movement execution in healthy participants and stroke survivors. Neurosci Lett 618: 134–138, 2016. doi: 10.1016/j.neulet.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Tresilian JR. Triggering prepared actions by sudden sounds: reassessing the evidence for a single mechanism. Acta Physiol (Oxf) 217: 13–32, 2016. doi: 10.1111/apha.12627. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Tresilian JR, de Rugy A, Sidhu S, Riek S. Corticospinal modulation induced by sounds depends on action preparedness. J Physiol 592: 153–169, 2014. doi: 10.1113/jphysiol.2013.254581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol 38: 35–56, 1992. doi: 10.1016/0301-0082(92)90034-C. [DOI] [PubMed] [Google Scholar]
- Massion J, Alexandrov A, Frolov A. Why and how are posture and movement coordinated? Prog Brain Res 143: 13–27, 2004. doi: 10.1016/S0079-6123(03)43002-1. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Yang CL, Magder L, Yungher D, Gray V, Rogers MW. Impaired motor preparation and execution during standing reach in people with chronic stroke. Neurosci Lett 630: 38–44, 2016. [Erratum in Neurosci Lett 633: 290, 2016.] doi: 10.1016/j.neulet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Executive Summary: Heart Disease and Stroke Statistics–2016 Update: a report from the American Heart Association. Circulation 133: 447–454, 2016. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55: 400–409, 2004. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Haaland KY, Sainburg RL. The effects of brain lateralization on motor control and adaptation. J Mot Behav 44: 455–469, 2012. doi: 10.1080/00222895.2012.747482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch Phys Med Rehabil 75: 852–857, 1994. doi: 10.1016/0003-9993(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Pain LM, Baker R, Richardson D, Agur AM. Effect of trunk-restraint training on function and compensatory trunk, shoulder and elbow patterns during post-stroke reach: a systematic review. Disabil Rehabil 37: 553–562, 2015. doi: 10.3109/09638288.2014.932450. [DOI] [PubMed] [Google Scholar]
- Pereira S, Silva CC, Ferreira S, Silva C, Oliveira N, Santos R, Vilas-Boas JP, Correia MV. Anticipatory postural adjustments during sitting reach movement in post-stroke subjects. J Electromyogr Kinesiol 24: 165–171, 2014. doi: 10.1016/j.jelekin.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Robertson JV, Roby-Brami A. The trunk as a part of the kinematic chain for reaching movements in healthy subjects and hemiparetic patients. Brain Res 1382: 137–146, 2011. doi: 10.1016/j.brainres.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Roy SH, De Luca G, Cheng MS, Johansson A, Gilmore LD, De Luca CJ. Electro-mechanical stability of surface EMG sensors. Med Biol Eng Comput 45: 447–457, 2007. doi: 10.1007/s11517-007-0168-z. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Duff SV. Does motor lateralization have implications for stroke rehabilitation? J Rehabil Res Dev 43: 311–322, 2006. doi: 10.1682/JRRD.2005.01.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther 73: 447–454, 1993. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia 47: 2953–2966, 2009. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T, Aruin A. Modulation of anticipatory postural adjustments associated with unloading perturbation: effect of characteristics of a motor action. Exp Brain Res 178: 206–215, 2007. doi: 10.1007/s00221-006-0725-y. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535: 289–300, 2001. doi: 10.1111/j.1469-7793.2001.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slijper H, Latash ML, Rao N, Aruin AS. Task-specific modulation of anticipatory postural adjustments in individuals with hemiparesis. Clin Neurophysiol 113: 642–655, 2002. doi: 10.1016/S1388-2457(02)00041-X. [DOI] [PubMed] [Google Scholar]
- Stapley P, Pozzo T, Grishin A. The role of anticipatory postural adjustments during whole body forward reaching movements. Neuroreport 9: 395–401, 1998. doi: 10.1097/00001756-199802160-00007. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Pozzo T, Cheron G, Grishin A. Does the coordination between posture and movement during human whole-body reaching ensure center of mass stabilization? Exp Brain Res 129: 134–146, 1999. doi: 10.1007/s002210050944. [DOI] [PubMed] [Google Scholar]
- Tyson SF, Hanley M, Chillala J, Selley A, Tallis RC. Balance disability after stroke. Phys Ther 86: 30–38, 2006. doi: 10.1093/ptj/86.1.30. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516: 931–938, 1999. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain 126: 1430–1448, 2003. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury ML, Howland DR, McGuirk TE, Davis SB, Senesac CR, Kautz S, Richards LG. Effects of trunk restraint combined with intensive task practice on poststroke upper extremity reach and function: a pilot study. Neurorehabil Neural Repair 23: 78–91, 2009. doi: 10.1177/1545968308318836. [DOI] [PubMed] [Google Scholar]
- Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol 92: 3276–3285, 2004. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135: 2277–2289, 2012. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]