Abstract
Formation of the metanephric kidney requires coordinated interaction among the stroma, ureteric bud, and cap mesenchyme. The transcription factor Foxd1, a specific marker of renal stromal cells, is critical for normal kidney development. The prorenin receptor (PRR), a receptor for renin and prorenin, is also an accessory subunit of the vacuolar proton pump V-ATPase. Global loss of PRR is embryonically lethal in mice, indicating an essential role of the PRR in embryonic development. Here, we report that conditional deletion of the PRR in Foxd1+ stromal progenitors in mice (cKO) results in neonatal mortality. The kidneys of surviving mice show reduced expression of stromal markers Foxd1 and Meis1 and a marked decrease in arterial and arteriolar development with the subsequent decreased number of glomeruli, expansion of Six2+ nephron progenitors, and delay in nephron differentiation. Intrarenal arteries and arterioles in cKO mice were fewer and thinner and showed a marked decrease in the expression of renin, suggesting a central role for the PRR in the development of renin-expressing cells, which in turn are essential for the proper formation of the renal arterial tree. We conclude that stromal PRR is crucial for the appropriate differentiation of the renal arterial tree, which in turn may restrict excessive expansion of nephron progenitors to promote a coordinated and proper morphogenesis of the nephrovascular structures of the mammalian kidney.
Keywords: kidney development, nephrogenesis, nephrovascular development, prorenin receptor, renal stroma
INTRODUCTION
Kidney development begins at approximately embryonic day (E)10.5 in mice, when the ureteric bud (UB) invades the surrounding metanephric mesenchyme (8). Further kidney development is driven by eliciting reciprocal inductive interactions between self-renewing Six2+;Cited1+ nephron progenitor cells (NPCs) of the cap mesenchyme (CM) and the UB (17). NPCs give rise to all the components of nephron epithelia proximal to the UB-derived collecting duct (22). Foxd1, a member of the forkhead family of transcription factors, is a specific marker of renal stromal cells in mice (9). Foxd1+ stromal progenitor cells (SPCs) surround the CM from E10.5 to E11.5 (9). As renal development proceeds, Foxd1+ SPCs differentiate into the mural cells of the renal arterial tree (renin cells, smooth muscle cells, perivascular fibroblasts, and pericytes) and glomerular mesangium (32). Foxd1+ SPCs remain at the periphery of the CM and are also observed between UB branches and induced nephrons. Deletion of the transcription factor Foxd1 in mice results in smaller pelvic kidneys fused in the midline with expansion of NPCs and a severe deficit in nephron differentiation (9, 19) as well as an anomalous distribution of the renal arterial tree, with multiple subcapsular and capsular arterioles entering the kidney in the reverse centripetal rather than in the normal centrifugal fashion (32).
The prorenin receptor (PRR) is a receptor for prorenin and renin encoded by the ATP6AP2 (ATPase-associated protein 2) gene (subsequently referred to as PRR) located on the X chromosome in humans and mice (25). PRR is also an accessory protein of the vacuolar proton pump V-ATPase (23). V-ATPases are expressed in intracellular compartments of virtually all cell types and play important roles in protein trafficking and degradation via acidification of intracellular organelles (6). Global PRR knockout is embryonically lethal in mice, indicating an essential role of the PRR in embryonic development (33). In humans, PRR mutations are associated with a high blood pressure, left ventricular hypertrophy and X-linked mental retardation (10, 11, 28). Recent data demonstrate that PRR is critical for normal kidney development and function. In this regard, targeted PRR deletion in Six2+ nephron progenitors in mice results in podocyte foot process effacement and small cystic kidneys at birth (37). PRR ablation in the UB using the Hoxb7 promoter driving cre recombinase leads to kidney hypoplasia, polyuria, and a reduced capacity to acidify the urine (35). However, the role of stromal PRR in kidney development remains unknown.
Therefore, in this study, to investigate the role of the PRR in the differentiation of Foxd1+ stromal progenitors, we generated mice with conditional deletion (cKO) of the PRR in Foxd1+ cells.
MATERIALS AND METHODS
Conditional deletion of PRR from stromal progenitors.
To investigate the role of the PRR in stromal cells, we used Foxd1-eGFP-Cre (Foxd1GC) mice (14). In these animals, Cre recombinase, driven by the Foxd1 promoter, is expressed in Foxd1+ stromal progenitors which give rise to all the mural cells of the kidney vasculature including renin cells, mesangial cells, and resident fibroblasts. To delete PRR in Foxd1+ stromal progenitors, Foxd1-eGFP-Cre+ mice were crossed with PRRflox/flox mice. PRR-floxed mice were provided by Dr. Atsuhiro Ichihara (Keio University, Tokyo, Japan) (27). The resulting Foxd1Cre+/PRRflox/flox mice represent stromal Foxd1+ progenitor-specific PRR-knockout mice (cKO). Control mice consisted of Foxd1Cre−/PRRflox/flox littermates (Con). All experiments involving mice were approved by Tulane Institutional Animal Care and Use Committee.
Genotyping.
Mice were genotyped from tail genomic DNA by standard PCR performed in an Eppendorf thermocycler using Taq polymerase (Promega, Madison, WI). The primers used to detect Foxd1-cre in genomic DNA are 5′-TCTGGTCCAAGAATCCGAAG-3′ (forward) and 5′-GGGAGGATTGGGAAGACAAT-3′ (reverse), which showed a cre-positive band at 450 base pairs (bp), whereas cre-negative mice had no band. The primers used to detect PRR are 5′-CACATTGCGTCAGCTCCGTAA-3′ (forward) and 5′-CTCACCAGGGATGTGTCGAAT-3′ (reverse), which showed a single PRR/Flox-positive band at 600 bp in cKO, a single 300-bp band in Con and two bands at 600 and 300 bp in heterozygous (Het) mice. The PCR annealing temperature was 58°C for 45 s.
Quantitative reverse transcription-polymerase chain reaction.
Quantitative reverse transcription-polymerase chain reaction was performed with Mx3000P equipment (Stratagene, La Jolla, CA), using MxPro QPCR software (Stratagene) as previously described (35). mRNA was extracted from snap-frozen E18.5 cKO and Con kidneys (n = 3 mice/group) and from P60 Het and Con kidneys (n = 3 mice/group). The quantity of each target mRNA was normalized by that of GAPDH mRNA expression. RNA samples were analyzed in triplicate in each run. PCR reaction was performed twice.
Immunohistochemistry and histopathology.
Whole intact E15.5-P60 kidneys and E18.5 lung from cKO and Con mice were fixed in 4% PFA at 4°C and paraffin embedded. Kidneys were sectioned at 4 μm and processed for hematoxylin and eosin staining and immunostaining. The number of glomeruli in each of three consecutive sections adjacent to the longitudinal midplane was counted, and the mean number of glomeruli per section per kidney was calculated. All counts were performed in a blinded fashion. Immunostaining was performed by the immunoperoxidase technique, using 4-μm sections with Vectastain Elite kit (Vector Laboratories, Burlingame, CA). Primary antibodies included anti-PRR (1:200; Santa Cruz Biotechnology), anti-Jagged1 (1:100; Santa Cruz Biotechnology), anti-Sall1 (1:100; Abcam), anti-Pax2 (1:100; Invitrogen), anti-WT1 (1:100; Abcam), anti-Six2 (1:100; Proteintech), Foxd1 (1:500; Abcam), Meis1 (1:100, Thermofisher), anti-SMA (1:100; Abcam), anti-Pecam-1 (1:1,000; rabbit polyclonal IgG, M-20R), anti-p57kip2 (1:500; Thermo Scientific), anti-renin (1:500 rabbit anti-mouse renin polyclonal antibody; 6), and Lotus Tetragonolobus Lectin (LTL; 1:400; Vector Laboratories). For immunofluorescence studies, secondary antibodies were detected with Alexa Fluor dyes (Invitrogen). Specificity of immunostaining was documented by the omission of the primary antibody. The intensity of immunofluorescence in E18.5 kidney sections (n = 3 mice/group) was determined using Slide book 4.0 software (Intelligent Imaging Innovations, Denver, CO). Image analysis of Pecam expression was performed using the public domain, free software Fiji (https://fiji.sc), a Java-based image processing program. The plug-in Image-based Tool for Counting Nuclei (ITCN), which was installed in Fiji, and is accessible at https://imagej.nih.gov/ij/plugins/itcn.html was used to count the number of cells. First, the images in TIF format were processed and converted to 8-bit images. The inputs in the ITCN used for estimation of cell diameter, minimum distance between cells, and level of threshold were set up to 7, 2.5, and 2 pixels, respectively, according to the best output obtained. Afterward, cells were counted and the data displayed according to the average of cell number per kidney image (16.09 sq. in. taken at ×20). Six images per kidney section were analyzed from WT (n = 4) and KO (n = 4) mice. For experiments involving embryonic kidneys, embryo sex was not identified. P60 Het and Con mice were females.
Cell proliferation and apoptosis assays.
Cell proliferation and apoptosis was examined in E17.5 kidney sections from cKO and Con mice (n = 3 kidneys/genotype, 3 sections/kidney), as previously described (35). Anti-phospho-histone H3 (pH3) and anti-cleaved caspase-3 antibodies were used (1:50; Cell Signaling Technology, Danvers, MA). The number of proliferating and apoptotic cells per kidney section was counted manually in a blinded fashion.
Blood pressure measurement.
Conscious tail-cuff mean (MAP), systolic, and diastolic arterial blood pressure were measured in Het (n = 3) and Con (n = 4) mice at 2 mo of age (P60), using a Visitech BP2000 system (Visitech Systems, Apex, NC). Both Het and Con mice were females. After 3 days of animal conditioning training, three consecutive cycles (10 recordings/mouse/cycle) of blood pressure readings were obtained on same day. Mean blood pressure values were calculated for each animal and used for statistical analysis.
Statistics.
Statistical analyses were carried out upon all biological replicates with Student’s t-test or a one-way ANOVA, followed by Bonferroni test. Data are presented as means ± SE. A P value of <0.05 was considered statistically significant.
RESULTS
Reduced PRR gene dosage in Foxd1+ stromal progenitors results in early postnatal death, reduced nephron number at birth, and delayed nephrogenesis.
cKO and Het mice were born at the expected Mendelian ratio and did not show any overt malformations, and their kidneys were grossly indistinguishable from Con mice on postnatal day 0 (P0; Fig. 1). Foxd1Cre-eGFP is active in the stroma of cKO and Het kidneys on E15.5 (Fig. 2). Most cKO mice died of unknown causes within 24–48 h after birth. Kidney weight (11.2 ± 0.3 vs. 11.5 ± 0.4 mg, P = 0.56) did not differ in surviving mutant and Con mice at P0. Total kidney PRR/GAPDH mRNA levels were reduced by 30% in cKO compared with Con mice at E18.5, consistent with likely PRR deletion in the stroma. We also observed a marked reduction in the intensity of PRR immunofluorescence in whole kidney sections (in particular in stromal Meis1+ and Foxd1+ cells) of cKO compared with Con kidneys at E18.5 (36 ± 1.7 vs. 56 ± 3.0; P < 0.01; Fig. 3). Histological examination revealed that cKO kidneys were indistinguishable from Con kidneys at E15.5 (Fig. 1). UB tree morphology appeared normal in cKO kidneys on the basis of anti-pancytokeratin staining of ureteric buds, and UB tip number did not differ in cKO and Con mice (22.3 ± 0.9 vs. 20.7 ± 1.2, P = 0.32) on E15.5 (Fig. 2). Although cKO kidneys were grossly indistinguishable from Con kidneys on E18.5, glomerular number per kidney section was decreased in cKO compared with Con mice at P0 (Fig. 1). In addition, cKO kidneys showed a delay in nephrogenesis with AN apparent absence of S-shaped and mature nephrons in the outer cortex. Given that Foxd1-positive mesenchymal cells of the developing lung are a source of endothelial progenitors that are likely critical to patterning the vasculature (34) and that PRR protein is expressed in the fetal mouse lung (36), we examined whether abnormal lung development may account for early neonatal death in cKO mice. At E18.5, cKO lungs had poorly developed bronchial tree with narrow airway lumens (Fig. 2).
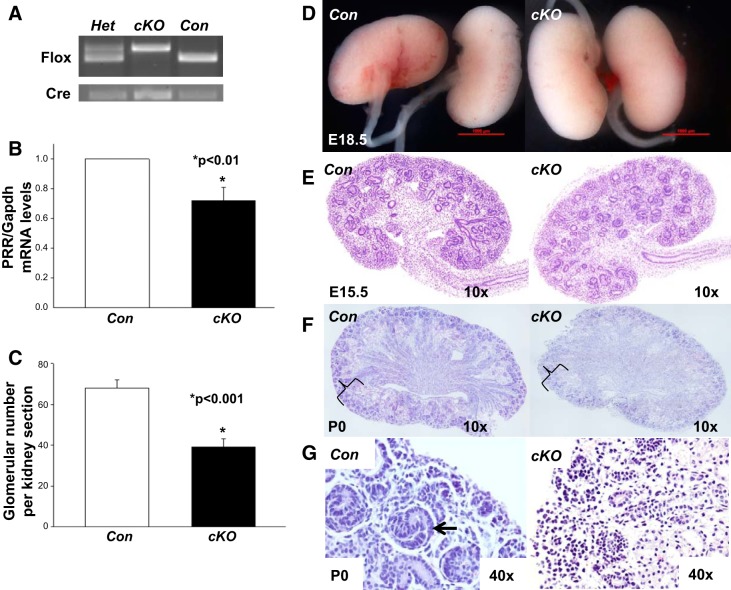
Fig. 1.
Stromal prorenin receptor (PRR) is crucial for normal nephrogenesis. A: image of gel electrophoresis showing target genotypes. B: PRR/GAPDH mRNA levels are reduced in the whole conditional deletion (cKO) compared with control (Con) kidney on postnatal day 0 (P0). C: glomerular number/kidney section is reduced in cKO compared with Con kidney at P0. D: macroscopic views of Con and cKO kidneys at embryonic day 18.5 (E18.5), showing similar kidney size. E–G: hematoxylin and eosin-stained kidney sections. E: at E15.5, the overall structure of a cKO kidney appears similar to the one of the littermate Con. F and G: at P0, cKO kidney shows marked decrease in the thickness of the cortex with reduction in nephron number (braces in F) and delayed nephrogenesis in cKO compared with Con kidneys (G). Arrow in G depicts S-shaped nephron. Scale bar, 1,000 µM.
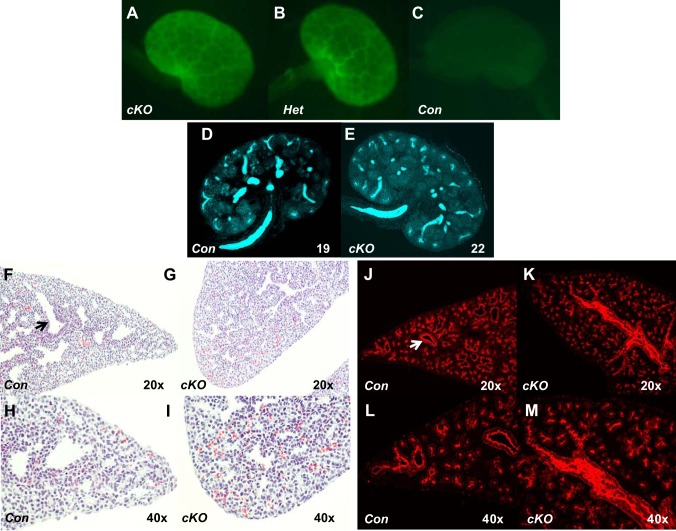
Fig. 2.
Stromal prorenin receptor (PRR) is dispensable for the normal ureteric bud (UB) branching but important for normal lung development. A–C: Foxd1+ cells are observed in embryonic day (E)15.5 conditional deletion (cKO) and heterozygous (Het) kidneys as a honeycomb pattern that interdigitates between the forming nephron progenitor units from Cre-enhanced green fluorescent protein (eGFP) expression (green staining). Control (Con) kidney (Foxd1Cre−/PRRflox/flox) is eGFP negative. D and E: images of whole E15.5 kidneys show the number of UB tips (19 vs. 22, blue staining, anti-pancytokeratin) in E15.5 cKO and Con kidneys. F–M: on E18.5, Con lung has a well-developed aiway tree with widely opened lumens (arrows in F and J). cKO lung has poorly developed bronchial tree with narrow airway lumens; hematoxylin and eosin stain (F–I) and anti-pancytokeratin stain, red (J–M).
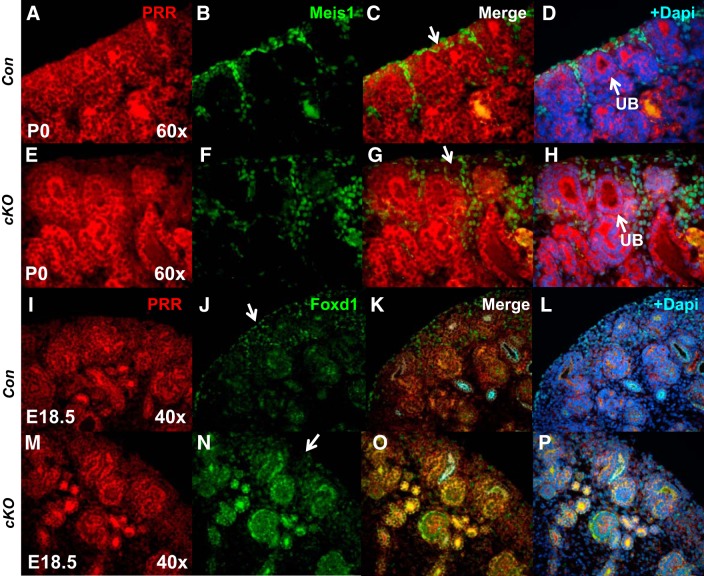
Fig. 3.
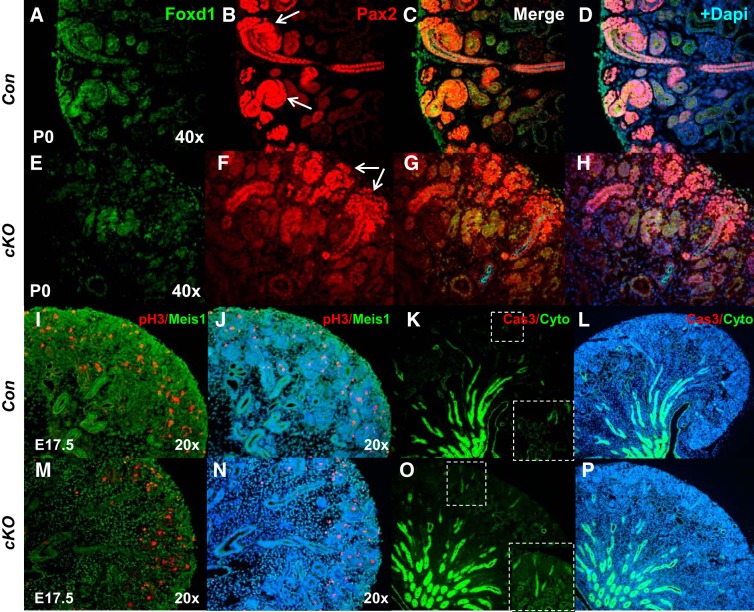
Stromal prorenin receptor (PRR) is essential for normal expression of stromal markers Meis1 and Foxd1. A–H: presence of PRR in the stroma of the control kidneys can be shown by the overlap of green and red (more yellowish in the overlap) immunofluorescence, and the lack of red in the green cells (lack of overlap) indicated the deletion of PRR in the Foxd1+ cells and Meis1+ cells that derive from the Foxd1+ cells. Apparent absence of PRR immunofluorescence in subcapsular stroma in conditional deletion (cKO) mice (arrow in G) and altered spatial expression of stromal marker Meis1 in cKO compared with Con kidneys. I–P: decreased expression of Foxd1 in the subcapsular stroma of cKO kidneys (arrow in N). E18.5, embryonic day 18.5; P0, postnatal day 0; UB, ureteric bud.
Deletion of PRR in Foxd1+ stromal progenitors impairs nephrogenesis program.
Recent studies in mice showed an essential role for the renal stroma in nephrogenesis, UB branching morphogenesis, and smooth muscle formation during kidney development (20, 21, 32). We first explored whether loss of PRR in Foxd1+ cells alters expression of stromal markers, homeobox protein Meis1 (Meis1), and Foxd1. Whereas Foxd1 is normally expressed in capsular and cortical stroma, Meis1 is present in stromal pericytes and fibroblasts (2, 19). Immunofluorescence analysis showed altered spatial expression of Meis1 and Foxd1 in cKO compared with Con kidneys with loose distribution of Meis1+ cells and lack of Foxd1 fluorescence in subcapsular stroma in cKO kidneys (Fig. 3). These findings are consistent with mispatterning of the renal stromal structures in cKO mice.
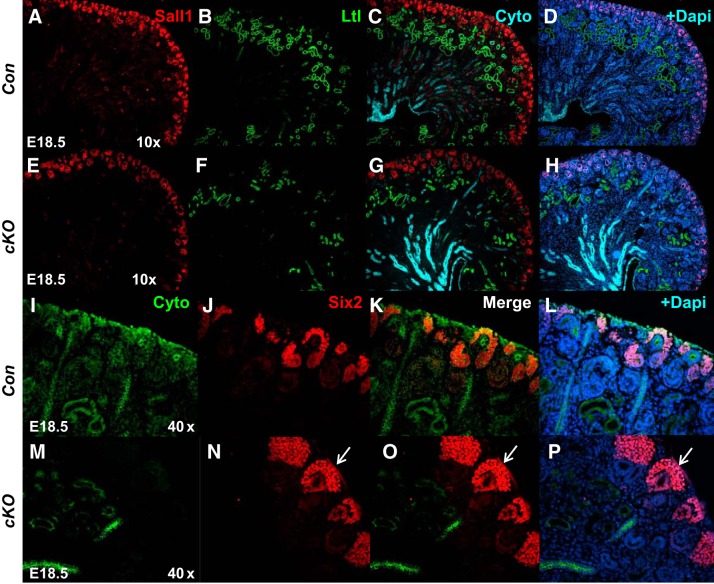
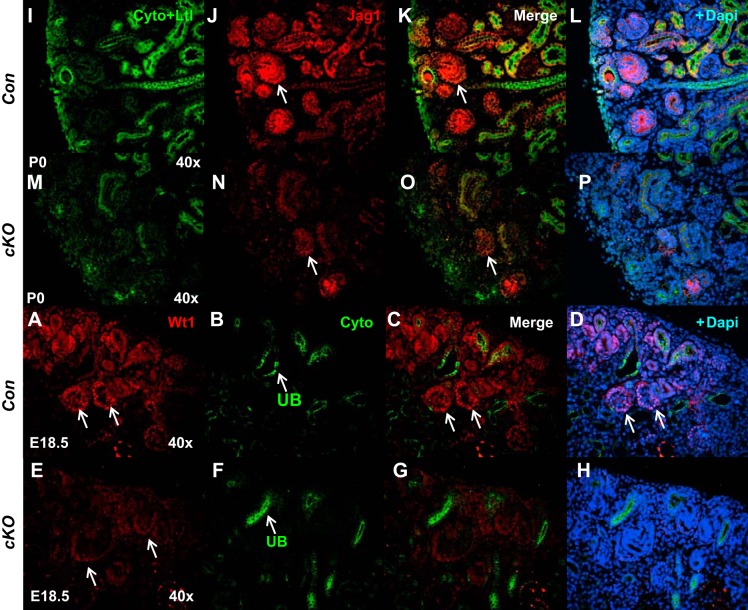
To more clearly define the effect on nephrogenesis, immunofluorescence was performed for markers specific to nephron progenitors and developing nephron structures. We first examined expression of nuclear factor Sall1 present in both nephron and stromal progenitors (15). Reduction in the intensity of Sall1 immunofluorescence in the nephrogenic zone and subcapsular and cortical/medullary stroma was observed in cKO compared with Con kidneys (2.3 ± 0.2 vs. 5.7 ± 0.3; P < 0.01; Fig. 4). We next examined the nephron progenitor caps by immunohistochemical staining for Six2 and Pax2. Six2 staining revealed that cKO kidneys have thicker nephron progenitor layers and widened progenitor caps compared with controls (Fig. 4). Immunofluorescence demonstrated increased intensity of Six2 staining in cKO compared with Con kidneys (6.8 ± 0.5 vs. 2.9 ± 0.3; P < 0.01). There was also reduced formation of proximal tubules in cKO kidneys, as noted by lotus tetragonolobus lectin (LTL) staining (2.1 ± 0.2 vs. 4.9 ± 0.3; P < 0.01) and an overall reduction of cytokeratin+ epithelial/tubular structures (Fig. 4). We also observed loose and diffuse expression of Pax2, a member of the “paired-box” (PAX) family of transcription factors, in the mutant cap mesenchyme, a reduced number of vesicles, the earliest epithelial derivatives of nephron progenitors, and more mature nephrons in cKO mice (Fig. 5). In accord with Pax2 findings, reduced expression of Jagged 1 (Jag1; 4.8 ± 0.4 vs. 14.4 ± 1.7, P < 0.01), another renal vesicle marker, was seen in mutant kidneys (Fig. 6). There were also fewer Wt1-positive glomeruli present in cKO kidneys (1.6 ± 0.1 vs. 6.0 ± 0.3, P < 0.01) (Fig. 6). Thus, formation of renal vesicles and nephron segmentation is impaired in cKO mice. Because partial block in nephrogenesis in mutants could be caused by alterations in either cell proliferation or apoptosis, we next analyzed these processes on E17.5. No significant difference was noted in the number of pH3- (82 ± 10 vs. 69 ± 7.5, P = 0.31) or caspase 3-positive cells (26 ± 4 vs. 23 ± 4.5, P = 0.33) per whole kidney midplane section in cKO compared with Con kidneys.
Fig. 4.
Deletion of prorenin receptor (PRR) in Foxd1+ stromal progenitors impairs nephrogenesis program. A–H: immunofluorescence shows reduction in the expression of Sall1 (nephron and stromal progenitor marker; red) in the nephrogenic zone, subcapsular, and cortical/medullary stroma in conditional deletion (cKO) mice (arrow in E); reduction in the abundance of Ltl (Lotus tetragonolobus lectin; proximal tubular marker, green staining) in cKO (F) compared with control (Con; B) kidneys. I–P: marked expansion of Six2+ nephron progenitor domain in cKO (red staining, arrows in N–P) compared with Con (J–L) kidneys. Cyto, anti-pancytokeratin; E18.5, embryonic day 18.5.
Fig. 5.
Stromal prorenin receptor (PRR) regulates spatial expression of mesenchymal and stromal markers. A–H: immunofluorescence shows altered spatial expression of Foxd1 in conditional deletion (cKO) compared with control (Con) kidneys. Loose and diffuse expression of Pax2 in the cap mesenchyme and nascent nephrons of cKO kidney (G, arrows). I–P: loose distribution of Meis1+ cells in cKO kidneys. No change in the expression of ureteric bud marker cytokeratin (Cyto), cell proliferation [phospho-histone H3 (pH3)], or cell death [caspase-3 (Cas3)] in embryonic day 17.5 (E17.5) cKO vs. Con kidneys. Immunoflurescence for pH3 (red; I, J, M, and N) and activated Cas3 (red; K, L, O, and R). Blue staining in D, H, J, L, N, and P: DAPI. Insets in right lower corners of K and O show high power images of areas shown by dashed line insets.
Fig. 6.
Deletion of prorenin receptor (PRR) in Foxd1+ stromal progenitors delays nephron differentiation. A–H: WT1 immunostaining (red) is present in podocytes on control (Con) mice (arrows in A, C, and D). Reduced expression of WT1 (red) in deeper, more differentiated nephrons in conditional deletion (cKO) mice (arrows in E). I–P: decreased expression of Jag1 (Jagged1) in renal vesicles of cKO (arrows in N and O) compared with Con (arrows in J and K) mice. Cyto, anti-pancytokeratin, marker of ureteric bud (UB, arrows in B and F); Ltl, lotus tetragonolobus lectin, marker of proximal tubule.
Stromal PRR is crucial for normal development of the renal vasculature.
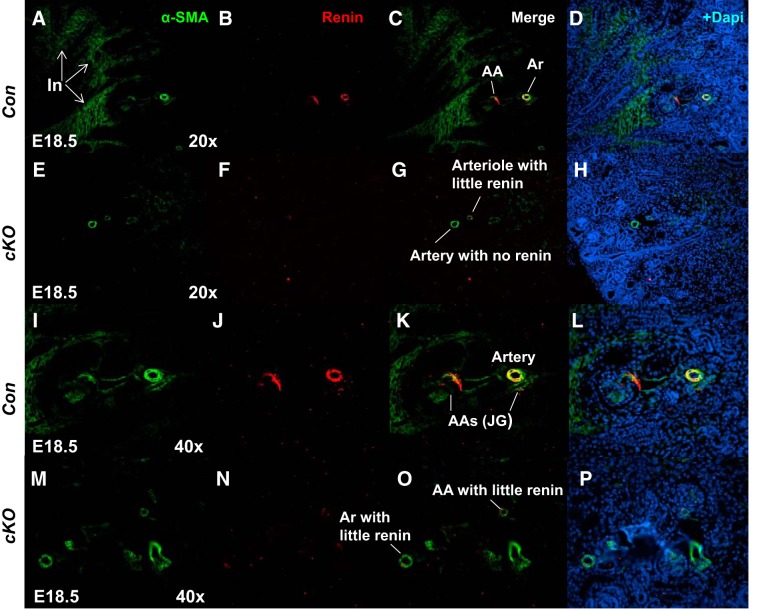
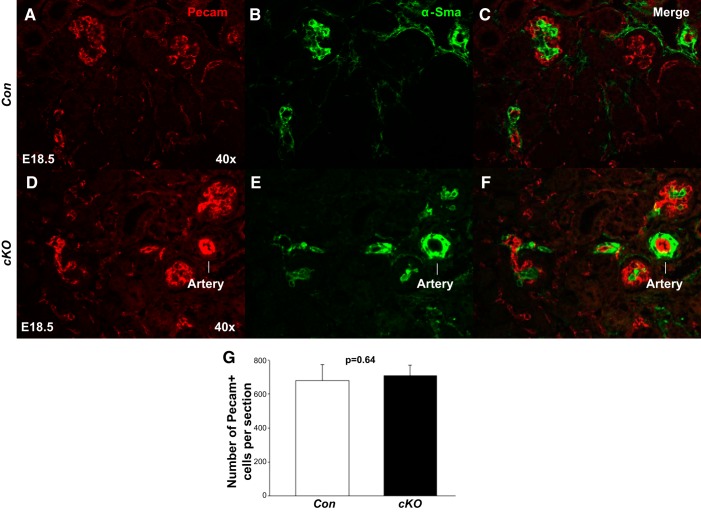
Because Foxd1+ stromal progenitor population gives rise to vascular smooth muscle (VSMC) and renin-producing cells (18, 21, 27), we performed immunohistochemistry for α-smooth muscle actin (α-SMA; VSMC marker) and renin [marker of juxtaglomerular (JG) cells in adult mammals] on E18.5. There was a marked decrease in the expression of α-SMA (2.4 ± 0.2 vs. 9.3 ± 1.5, P < 0.01) and renin (3.9 ± 0.3 vs. 19.3 ± 0.9, P < 0.01) proteins in the cKO vasculature (Fig. 7). The cKO kidneys seem to have fewer and thinner intrarenal arteries and decreased expression of α-SMA in the developing interstitium (Fig. 7). The glomeruli also appear to be less developed with reduced expression of α-SMA in the developing mesangium of cKO mice. These findings suggest that stromal PRR is also a positive regulator of angiogenic vessel growth in the developing interstitium as well as of renin expression in JG cells. To test whether arrest in nephron differentiation coupled with expansion of the Six2+ stem cell population in E18.5 cKO mice is due to lack of capillary development angiogenesis in the nephrogenic zone, we examined the expression of the vascular endothelial marker Pecam on E18.5. We did not observe a difference in Pecam expression in cKO and Con kidneys (710 ± 59 vs. 680 ± 92; P = 0.64) (Fig. 8). These findings suggest that arrest in nephrogenesis on E18.5 in cKO kidneys is not due to defects in Pecam+ microvasculature formation in the nephrogenic zone but may be due to an arrest in arteriolar development.
Fig. 7.
Stromal prorenin receptor (PRR) is critical for proper maintenance of the kidney vasculature. A–P: in the control (Con) embryonic day 18.5 (E18.5) kidney; both α-smooth muscle actin (α-SMA; vascular smooth muscle cell marker) and renin [marker of juxtaglomerular (JG) cells in adult mammals] immunofluorescence is present in afferent arterioles (AA) and JG cells. There is marked decrease in the expression of renin protein in the conditional deletion (cKO) vasculature. The cKO kidneys show fewer and thinner intrarenal arteries and decreased expression of α-SMA in the developing interstitium (In). Glomeruli of cKO mice show reduced expression of α-SMA in the developing mesangium. Ar, arteriole.
Fig. 8.
Stromal prorenin receptor (PRR) is dispensable for the formation of Pecam+ microvasculature. A–F: in the embryonic day 18.5 (E18.5) kidney, we did not observe changes in the immunofluorescent expression of Pecam (endothelial marker) in the vasculature of the conditional deletion (cKO) compared with control (Con) mice. G: bar graph shows no difference in the number of Pecam+ cells/kidney section in cKO and Con mice.
Reduced PRR gene dosage in stromal progenitors does not result in development of hypertension during later life.
Because cKO mice exhibit reduced nephron number at birth, a known determinant of susceptibility to hypertension both in animals and humans (1, 39), we evaluated the effect of reduced stromal PRR gene dosage in Het mice on blood pressure at 2 mo of age. Het mice were used because cKO mice died within 24–48 h after birth. We first determined whole kidney PRR mRNA levels and glomerular number in Het and Con mice on P60. Both PRR/GAPDH mRNA ratio (0.82 ± 0.02 vs. 1.0 ±0.00, P < 0.01) and the number of glomeruli per kidney section (126 ± 2.9 vs.146 ± 5.7, P < 0.05) was reduced in Het compared with Con kidneys. However, conscious tail-cuff mean (92 ± 6.4 vs. 88 ± 2.3, P = 0.59), systolic (133 ± 11 vs. 124 ± 3.5, P = 0.44), and diastolic (68 ± 5 vs. 69 ± 3.1, P = 0.91) arterial blood pressure did not differ in Het and Con mice. Thus, reduction in PRR gene dosage in stromal progenitors in Het kidneys is sufficient to decrease glomerular number but does not alter blood pressure. It is possible that more severe glomerular deficit is required to impact blood pressure postnatally.
DISCUSSION
The present study demonstrates that cortical stromal PRR is essential for nephrogenesis, stromal, and nephrovascular development. Foxd1+ stromal progenitors give rise to VSMCs, renin cells, glomerular mesangial cells, pericytes and perivascular fibroblasts, and a subset of peritubular endothelium (13, 32). The Foxd1+ cortical renal stroma develops immediately adjacent to the nephron progenitors and provides critical signals to nephron progenitors as they self-renew and differentiate into functional nephrons (20). Expansion of nephron progenitors, aberrant nephrogenesis, and abnormal vascular development is a consistent feature in stromal defect mouse models (4, 13, 19, 32). Several stromal mediators, including Fat4/Yap and Decorin-BMP/Smad and Sall1 pathways participate in stromal-to-cap mesenchyme signaling to regulate nephrogenesis (4, 5, 26). Foxd1 per se directs the origin, number, and orientation of the renal arterial tree (7, 32). The cause of early postnatal death of cKO mice is uknown. Possible causes of death may include heart failure, anemia, and aberrant patterning of the pulmonary vasculature or bronchial tree (34). Latter possibilities are supported by lethal heart failure observed in mice with cardiomyocyte-specific ablation of the PRR and deregulated production of renal erythropoietin production within the Foxd1+ stroma-derived cell population (16, 18). Because cKO lung had poorly developed bronchial tree with narrow airway lumens, pulmonary tissue dysplasia may contribute in part to early neonatal death in cKO mice.
Our present findings demonstrate that PRR cKO kidneys have reduced glomerular numbers at birth with apparent partial block in nephrogenesis. These changes are accompanied by aberrant expression of stromal markers Meis1 and Foxd1, consistent with mispatterning of the renal stromal structures in cKO mice. Expansion of Six2+ nephron progenitor caps in cKO kidneys is associated with an apparent reduction in the number of Jag1+/Pax2+ renal vesicles, which are the differentiating nascent nephrons, intensity of Sall1 immunostaining in nephrogenic zone, subcapsular and cortical stroma, decreased formation of Ltl+ proximal tubules, and fewer Wt1-positive glomeruli present. The impaired formation of renal vesicles and nephron segmentation in cKO mice are not due to detectable changes in cell proliferation or apoptosis at total kidney tissue level. In addition to the observed changes in nephrogenic lineage, we identified an important role of the stromal PRR in renal vascular development and formation of renin-positive cells of the JG apparatus that modulates blood pressure and fluid electrolyte homeostasis in the mature kidney (30). The cKO kidneys showed fewer and thinner intrarenal arteries, which was probably due to the marked decrease in the expression of renin protein in the cKO vasculature and of α-SMA in the developing interstitium. These findings suggest that stromal PRR is a positive regulator of angiogenic vessel growth in the developing interstitium as well as of renin expression in JG cells.
Several potential mechanisms by which cortical stromal PRR inhibits nephron progenitor cell expansion and promotes nephron differentiation in PRR-cKO mice can be considered. First, stromal PRR may modulate molecular signals sent from the renal stroma that are paramount to the normal nephrogenesis and stromal development. These signals may include stromal factors Foxd1 and Meis1, expression of which is altered in PRR-cKO kidneys. This possibility is supported by similarity in renal phenotypes observed in PRR-cKO and Foxd1−/− mice. Conditional ablation of renal stromal Foxd1-expressing cells in mice with Diphtheria toxin results in thickened nephron progenitor caps, cortical regions devoid of nephron progenitors, and aberrant development of the renal vasculature (13, 32). Cap mesenchyme cells in Foxd1−/− kidneys are trapped in the Cited1+ compartment, indicating that the initial step in nephron progenitor differentiation is inhibited, which is due in part to inhibition of Bmp/Smad signaling by proteoglycan-encoding Decorin (5, 26). Atypical cadherin Fat4 represents another stromal factor shown to promote nephron differentiation and restrict progenitor expansion (4). Aberrant expansion of nephron progenitors, which is associated with reduced expression of Fat4 and upregulated Decorin, is also observed in mice deficient in stromal Sall1, a member of zinc finger transcription factors (26). Thus, the apparent reduction in the intensity of stromal Sall1 immunostaining observed in PRR-cKO mice suggests that Sall1-mediated regulation of Decorin and Fat4 might at least partially underlie the pathogenesis. Because defects in kidney development have been observed in Meis1-mutant embryos, aberrant Meis1 expression in PRR-cKO kidneys may also contribute to the observed phenotype (12). Renin cells have been associated with the branching and development of the renal arterial tree (29). Furthermore, mice with deletion of renin in Foxd1+ stromal cells and all their vascular descendants display abnormalities in the renal vasculature as well as in the renal parenchyma (32). Therefore, the renal vascular defects present in PRR-cKO mice in this study may be due to lack of appropriate differentiation of renin cells, which are also precursor cells for a subset of vascular smooth muscle cells of the renal arterial tree (31), which in turn may be essential for the coordinated differentiation of the epithelial nephron. We have also shown previously that Foxd1 is an upstream positive regulator of renin during early metanephric development (38). Arrested nephrogenesis in cKO mice may also be due to hypoxia from reduced oxygen delivery via the developing renal arterial tree, as mediated by the Foxd1+ expressing stroma in the periphery. Although our findings suggest that defects in Pecam+ microvasculature formation in the nephrogenic zone do not play a role in this process, hypoxia could explain in part the lack of appropriate renal arteriolar development, the phenotype observed in cKO mice.
Besides being a receptor for prorenin and renin, PRR is an accessory subunit of the vacuolar proton pump V-ATPase and a key player in V-ATPase regulation (3). V-ATPase acidifies endosomes and lysosomes, creating an acidic environment in the lumen of these vesicles essential for protein trafficking, recycling, and degradation through pH-dependent enzymes. It is possible that PRR, as a V-ATPase subunit, modulates specific V-ATPase functions in stromal cells during kidney development and that loss of PRR/V-ATPase function in the stroma may result in defective nephrogenesis and formation of the renal arteries. This possibility is supported by the findings that UB PRR regulates the expression of V-ATPase subunit-α4, which is essential for normal kidney morphogenesis and for function of collecting duct cells involved in acid base homeostasis (35).
Collectively, these findings demonstrate that stromal PRR is crucial for normal progression of nephrogenesis, differentiation of renin-positive cells, and the proper formation of the renal arterial tree. From the present findings and considering the similarities with the Foxd1 knockout mice phenotype, we hypothesize that the alterations in kidney vascular and nephron progenitor compartments seen in the PRR-cKO mutants likely result from disrupted molecular signals that are being produced by the renal stroma to pattern the developing nephrons and kidney vasculature.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-0916373 and DK-116196 to M. L. S. Sequeira-Lopez and a Tulane Bridge Fund grant to I. V. Yosypiv.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.V.Y. conceived and designed research; I.V.Y., M.L.S.S.-L., R.S., and A.D.G.M. analyzed data; I.V.Y. and M.L.S.S.-L. interpreted results of experiments; I.V.Y. and A.D.G.M. prepared figures; I.V.Y. and M.L.S.S.-L. edited and revised manuscript; I.V.Y., M.L.S.S.-L., and R.S. approved final version of manuscript; M.L.S.S.-L., R.S., and A.D.G.M. performed experiments; R.S. drafted manuscript.
ACKNOWLEDGEMENTS
We thank Xiuyin Liang for technical support.
REFERENCES
- 1.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 2.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell 15: 781–791, 2008. doi: 10.1016/j.devcel.2008.09.007. [Erratum in: Dev Cell 16: 482, 2009.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 4.Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, Olson EN, Perantoni AO, Carroll TJ. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol 15: 1035–1044, 2013. [Erratum in: Nat Cell Biol 15: 1260, 2013.] doi: 10.1038/ncb2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fetting JL, Guay JA, Karolak MJ, Iozzo RV, Adams DC, Maridas DE, Brown AC, Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development 141: 17–27, 2014. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 7.Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez ML. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol 296: H1255–H1262, 2009. doi: 10.1152/ajpheart.01266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grobstein C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp Cell Res 10: 424–440, 1956. doi: 10.1016/0014-4827(56)90016-7. [DOI] [PubMed] [Google Scholar]
- 9.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev 10: 1467–1478, 1996. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 10.Hirose T, Hashimoto M, Totsune K, Metoki H, Asayama K, Kikuya M, Sugimoto K, Katsuya T, Ohkubo T, Hashimoto J, Rakugi H, Takahashi K, Imai Y. Association of (pro)renin receptor gene polymorphism with blood pressure in Japanese men: the Ohasama study. Am J Hypertens 22: 294–299, 2009. doi: 10.1038/ajh.2008.357. [DOI] [PubMed] [Google Scholar]
- 11.Hirose T, Hashimoto M, Totsune K, Metoki H, Hara A, Satoh M, Kikuya M, Ohkubo T, Asayama K, Kondo T, Kamide K, Katsuya T, Ogihara T, Izumi S, Rakugi H, Takahashi K, Imai Y. Association of (pro)renin receptor gene polymorphisms with lacunar infarction and left ventricular hypertrophy in Japanese women: the Ohasama study. Hypertens Res 34: 530–535, 2011. doi: 10.1038/hr.2010.274. [DOI] [PubMed] [Google Scholar]
- 12.Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, Devor-Henneman DE, Saiki Y, Kutsuna H, Tessarollo L, Jenkins NA, Copeland NG. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J 23: 450–459, 2004. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hum S, Rymer C, Schaefer C, Bushnell D, Sims-Lucas S. Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One 9: e88400, 2014. doi: 10.1371/journal.pone.0088400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanda S, Tanigawa S, Ohmori T, Taguchi A, Kudo K, Suzuki Y, Sato Y, Hino S, Sander M, Perantoni AO, Sugano S, Nakao M, Nishinakamura R. Sall1 maintains nephron progenitors and nascent nephrons by acting as both an activator and a repressor. J Am Soc Nephrol 25: 2584–2595, 2014. doi: 10.1681/ASN.2013080896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 107: 30–34, 2010. doi: 10.1161/CIRCRESAHA.110.224667. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi H, Liu Q, Binns TC, Urrutia AA, Davidoff O, Kapitsinou PP, Pfaff AS, Olauson H, Wernerson A, Fogo AB, Fong GH, Gross KW, Haase VH. Distinct subpopulations of FOXD1 stroma-derived cells regulate renal erythropoietin. J Clin Invest 126: 1926–1938, 2016. doi: 10.1172/JCI83551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539, 2005. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Hartwig S, Rosenblum ND. Developmental origins and functions of stromal cells in the normal and diseased mammalian kidney. Dev Dyn 243: 853–863, 2014. doi: 10.1002/dvdy.24134. [DOI] [PubMed] [Google Scholar]
- 21.Lin EE, Sequeira-Lopez ML, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol 306: F249–F258, 2014. doi: 10.1152/ajprenal.00313.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4: a008300, 2012. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schägger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee E, Maringer K, Papke E, Bushnell D, Schaefer C, Kramann R, Ho J, Humphreys BD, Bates C, Sims-Lucas S. Endothelial marker-expressing stromal cells are critical for kidney formation. Am J Physiol Renal Physiol 313: F611–F620, 2017. doi: 10.1152/ajprenal.00136.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohmori T, Tanigawa S, Kaku Y, Fujimura S, Nishinakamura R. Sall1 in renal stromal progenitors non-cell autonomously restricts the excessive expansion of nephron progenitors. Sci Rep 5: 15676, 2015. doi: 10.1038/srep15676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshima Y, Kinouchi K, Ichihara A, Sakoda M, Kurauchi-Mito A, Bokuda K, Narita T, Kurosawa H, Sun-Wada GH, Wada Y, Yamada T, Takemoto M, Saleem MA, Quaggin SE, Itoh H. Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol 22: 2203–2212, 2011. doi: 10.1681/ASN.2011020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramser J, Abidi FE, Burckle CA, Lenski C, Toriello H, Wen G, Lubs HA, Engert S, Stevenson RE, Meindl A, Schwartz CE, Nguyen G. A unique exonic splice enhancer mutation in a family with X-linked mental retardation and epilepsy points to a novel role of the renin receptor. Hum Mol Genet 14: 1019–1027, 2005. doi: 10.1093/hmg/ddi094. [DOI] [PubMed] [Google Scholar]
- 29.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 9: 63–71, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001. doi: 10.1152/ajprenal.2001.281.2.F345. [DOI] [PubMed] [Google Scholar]
- 31.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004. doi: 10.1016/S1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 32.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol 308: R138–R149, 2015. doi: 10.1152/ajpregu.00428.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sihn G, Burckle C, Rousselle A, Reimer T, Bader M. (Pro)renin receptor: subcellular localizations and functions. Front Biosci (Elite Ed) E5: 500–508, 2013. doi: 10.2741/E631. [DOI] [PubMed] [Google Scholar]
- 34.Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E, Gittes G, Bates CM. Endothelial progenitors exist within the kidney and lung mesenchyme. PLoS One 8: e65993, 2013. doi: 10.1371/journal.pone.0065993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song R, Preston G, Ichihara A, Yosypiv IV. Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS One 8: e63835, 2013. doi: 10.1371/journal.pone.0063835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song R, Preston G, Yosypiv IV. Ontogeny of the (pro)renin receptor. Pediatr Res 74: 5–10, 2013. doi: 10.1038/pr.2013.63. [DOI] [PubMed] [Google Scholar]
- 37.Song R, Preston G, Kidd L, Bushnell D, Sims-Lucas S, Bates CM, Yosypiv IV. Prorenin receptor is critical for nephron progenitors. Dev Biol 409: 382–391, 2016. doi: 10.1016/j.ydbio.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song R, Lopez MLSS, Yosypiv IV. Foxd1 is an upstream regulator of the renin-angiotensin system during metanephric kidney development. Pediatr Res 82: 855–862, 2017. doi: 10.1038/pr.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348, 2004. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]