Abstract
Continuous laminar shear stress increases the process of autophagy, activates endothelial nitric oxide (NO) synthase phosphorylation at serine 1177 (p-eNOSS1177), and generates NO in bovine and human arterial endothelial cells (ECs) compared with static controls. However, the translational relevance of these findings has not been explored. In the current study, primary ECs were collected from the radial artery of 7 men using sterile J-wires before (Pre) and after (Post) 60 min of rhythmic handgrip exercise (HG) performed with the same arm. After ECs were identified by positive costaining for vascular endothelial cadherin and 4′,6′-diamidino-2-phenylindole, immunofluorescent antibodies were used to assess indices of autophagy, NO generation, and superoxide anion (O2·−) production. Commercially available primary human arterial ECs were stained and processed in parallel to serve as controls. All end points were evaluated using 75 ECs from each subject. Relative to Pre-HG, HG elevated arterial shear rate (P < 0.05) ~3-fold, whereas heart rate, arterial pressure, and cardiac output were not altered. Compared with values obtained from ECs Pre-HG, Post-HG ECs displayed increased (P < 0.05) expression of p-eNOSS1177, NO generation, O2·− production, BECLIN1, microtubule-associated proteins 1A/1B light chain 3B, autophagy-related gene 3, and lysosomal-associated membrane protein 2A and decreased (P < 0.05) expression (i.e., enhanced degradation) of the adaptor protein p62/sequestosome-1. These novel findings provide evidence that elevated arterial shear rate associated with functional hyperemia initiates autophagy, activates p-eNOSS1177, and increases NO and O2·− generation in primary human ECs.
NEW & NOTEWORTHY Previously, our group reported in bovine arterial and human arterial endothelial cells (ECs) that shear stress initiates trafficking of the autophagosome to the lysosome and increases endothelial nitric oxide (NO) synthase phosphorylation at serine 1177, NO generation, and O2·− production. Here, the translational relevance of these findings is documented. Specifically, functional hyperemia induced by rhythmic handgrip exercise elevates arterial shear rate to an extent that increases indices of autophagy, NO generation, and O2·− production in primary arterial ECs collected from healthy men.
Keywords: blood vessel, exercise, immunofluorescence, shear stress
INTRODUCTION
Voluntary contraction of skeletal muscle during dynamic exercise requires an increase in blood flow to meet elevated oxygen demands. This functional hyperemia increases endothelial cell (EC) shear stress from basal levels. EC shear stress is the product of blood viscosity and the velocity gradient, in the radial direction, and is sensed by mechanoreceptors on the EC surface. We (1, 2, 11) and others have documented that bovine arterial (BAECs) and human arterial (HAECs) ECs exposed to physiological increases in continuous laminar shear stress exhibit increases in the activation of endothelial nitric oxide (NO) synthase phosphorylation at serine 1177 (p-eNOSS1177), NO production, and reactive oxygen species generation.
Of significant importance, in addition to NO- and free radical-related responses, BAECs and HAECs exposed to laminar shear stress also display increased indices of the intracellular degradation process autophagy (1). Autophagy is a highly conserved trafficking process by which damaged intracellular components are targeted and delivered to lysosomes (10). Cargo transported to lysosomes via autophagy is degraded by lysosomal acid hydrolases, producing metabolites that can be recycled for use in new biosynthetic reactions or diverted to metabolic pathways that can generate ATP. Autophagy is critical for maintaining quality control of the cell (16) and integrity of the cardiovascular system (7, 13, 18). However, there is a paucity of data translating the mechanisms responsible for regulating the important process of autophagy in ECs from in vitro studies to the human.
A recent study, focused on the influence of shear stress on p-eNOSS1177 (4), demonstrated that in vitro results can be translated to ECs freshly isolated from the brachial artery of humans in response to functional hyperemia induced by dynamic handgrip (HG) exercise. Specifically, relative to values before HG, forearm contractions increased brachial artery shear rate (blood velocity/arterial diameter) approximately fivefold and elevated p-eNOSS1177 in ECs collected from the brachial artery of the same arm after exercise (4). However, this study did not translate other previously shear-linked responses studied in vitro, such as EC autophagy, NO generation, and superoxide anion (O2·−) production (1, 2). Therefore, utilizing sterile J-wires to collect primary arterial ECs before and after 60 min of rhythmic HG exercise performed with the same arm, we sought 1) to ascertain whether our in vitro results regarding continuous, laminar shear stress and EC autophagy can be translated to primary arterial ECs obtained from humans performing exercise, 2) to confirm the findings of Casey et al. (4) regarding p-eNOSS1177, and 3) to extend these results by measuring estimates of NO generation and O2·− production.
METHODS
Subjects.
Seven male subjects that were sedentary to recreationally active, nonobese, nonsmokers, free of diagnosed cardiovascular or metabolic complications, and not taking any medications participated in the study. Protocol approval and written, informed consent were obtained according to the University of Utah and Salt Lake City Department of Veterans Affairs Medical Center Institutional Review Boards. Subjects reported to the laboratory on two occasions in an overnight-fasted state, having abstained from caffeine, alcohol, and exercise for 24 h.
Flow-mediated vasodilation and maximal handgrip workload test.
Participants made two visits to the laboratory. During visit 1, a flow-mediated vasodilation test was completed using procedures we (9, 23) have previously described. After a 20-min rest period, a maximal handgrip workload test was completed to estimate an intensity that would result in an approximately threefold elevation of arterial shear rate during rhythmic HG. Subjects lay supine with their right arm extended perpendicular to their body while their right hand grasped a handle attached to a pulley connected to a bucket containing 0.9 kg (Fig. 1A). When the subject squeezed and released the handle, once every 2 s, a weight-containing bucket elevated and descended 3.5 cm, respectively. Additional weight (0.9 kg) was placed in the bucket each minute (i.e., every 30 contractions) until the subject could no longer perform the task or began recruiting accessory muscles to complete the task.
Fig. 1.
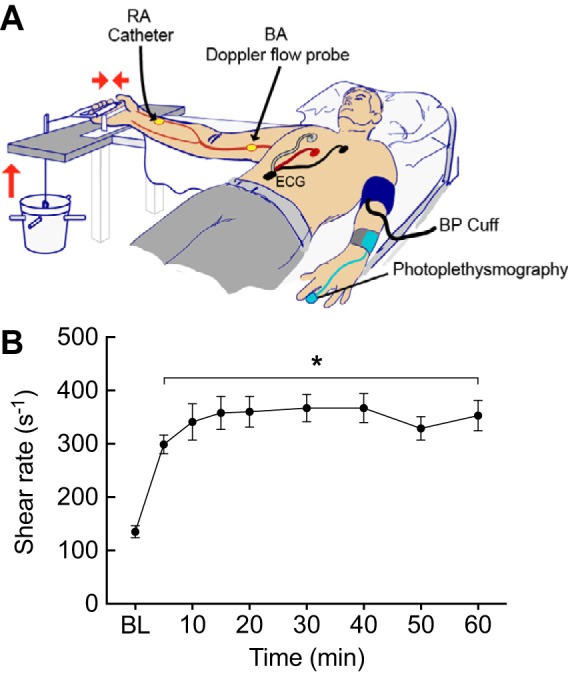
A: subject instrumentation. BA, brachial artery; BP, blood pressure; ECG, electrocardiogram; RA, radial artery. B: rhythmic handgrip exercise (HG) increased arterial shear rate. BL, baseline, i.e., Pre-HG. Values are means ± SE from 7 subjects. *P < 0.01 vs. BL.
EC collection and processing.
Visit 2 occurred within 7 days of visit 1. A 20-gauge, 5-cm catheter (model RA-04020; Arrow, Reading, PA) was inserted into the radial artery of the right arm under aseptic conditions. Immediately after catheter insertion, a blood pressure cuff was placed to occlude the brachial artery and thus radial artery blood flow. A flexible 0.025-in. mesh 3-mm guide wire with a J-shaped tip (i.e., J-wire; GuideRight; St. Jude Medical, Plymouth, MN) was advanced approximately 3–4 cm beyond the termination of the radial artery catheter and retracted (4, 6, 12). The distal portion of the J-wire was placed into dissociation buffer [0.5% BSA, 2 mM EDTA, 18 U/ml heparin in PBS (pH 7.4)]. This represents the Pre-HG collection. Subjects then completed the 60-min HG protocol (see below). Immediately on the cessation of HG exercise, ECs were obtained by advancing the J-wires 4–6 cm beyond the end of the catheter. The J-wire was advanced different distances beyond the end of the arterial catheter at Pre- and Post-HG EC collections so that cells could be collected from different areas.
Within 30 min of collection, the admixture of cell types was dislodged from each J-wire by washing with dissociation buffer. Cells were recovered and treated with an erythrocyte-lysing kit (R&D Systems, Minneapolis, MN). Then, cells were resuspended in EBM-2 (Lonza, Walkersville, MD) and applied evenly to chamber slides pretreated with poly-l-lysine. Gentle centrifugation (450 rpm/~28 g, 10 s with deceleration set to 0; Thermo Fisher Scientific, Waltham, MA), in a microplate swinging-bucket rotor, facilitated adherence of the cells to the slide surface.
Assessment of NO and O2·− generation.
A cohort of cells was stained on the day of collection without fixation to estimate NO and O2·− generation (1, 3, 20, 21, 24). Cells were incubated for 30 min at 37°C with either 5 µM 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM Diacetate; Thermo Fisher Scientific), to estimate NO generation, or 10 µM dihydroethidium (DHE; Thermo Fisher Scientific), to estimate O2·− production. After incubation, all cells were costained with vascular endothelial cadherin (VE-cadherin; R&D Systems) and 4′,6′-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific) to identify ECs and nuclei, respectively. In preliminary studies using HAECs, pyocyanin-induced elevations in O2·− generation were inhibited using N-acetyl-l-cysteine, and insulin-induced stimulation of NO generation was ameliorated using NG-nitro-l-arginine methyl ester (data not shown).
Assessment of protein expression.
A cohort of cells was fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) on the day of collection and frozen at −80°C until staining for protein expression using immunofluorescent antibodies. Commercially available HAECs (P4-6; American Type Culture Collection, Manassas, VA) were treated in an identical manner to serve as intensity controls. On the day of staining, fixed cells were thawed and permeabilized in 0.1% Triton X-100 (Sigma-Aldrich), and nonspecific binding sites were blocked in 0.5% BSA (Thermo Fisher Scientific). Cells were incubated for 1 h at room temperature with primary antibodies to two of the following targets: p-eNOS (1:100 dilution; Thermo Fisher Scientific); eNOS (1:100 dilution; BD Biosciences, San Diego, CA); BECLIN1 (1:100 dilution; Novus Biologicals, Littleton, CO); autophagy-related gene 3 (1:100 dilution; Santa Cruz Biotechnology, Dallas, TX); microtubule-associated proteins 1A/1B light chain 3B (LC3B; 1:100 dilution; Novus Biologicals); p62 (1:50 dilution; Abcam, Cambridge, United Kingdom); and lysosomal-associated membrane protein 2A (1:100 dilution; Abcam). Cells were simultaneously stained with VE-cadherin (1:10 dilution; R&D Systems) to identify endothelial cells. Then, slides were incubated with conjugated secondary antibody [i.e., Alexa Fluor 488, Alexa Fluor 555, and Alexa Fluor 647 (1:500 dilution; Abcam)] for 30 min at room temperature. Finally, slides were mounted with ProLong Diamond Antifade Mountant with DAPI for nuclear identification (Life Technology, Carlsbad, CA; Refs. 6, 12).
All slides were imaged with a confocal microscope (A1R; Nikon) at ×60 magnification. Images were captured at the same exposure time and corrected for background fluorescence. Seventy-five ECs were identified on each slide by positive costaining for VE-cadherin and DAPI. Images were captured and analyzed with NIS-Elements Advanced Research software. Fluorescence intensity was measured using ImageJ (NIH, Bethesda, MD). For protein expression, data are expressed as the ratio of Pre-HG or Post-HG staining intensity divided by HAEC staining intensity × 100 to control for differences between staining sessions (4, 6). For NO and O2·−, data are expressed as arbitrary units.
HG protocol and hemodynamics.
HG was performed during visit 2 to increase shear rate using procedures that we have described (22). Thirty minutes after Pre-HG EC collection, subjects squeezed and released a handle that elevated a bucket containing 5–10% of the weight achieved during their maximal handgrip workload test. The duty cycle was 1:2, and subjects contracted to the sound of a metronome. The weight was designed to provide a workload that required approximately threefold elevations in radial artery shear rate for 60 min. The elevation of radial artery shear rate was estimated throughout the 60-min HG protocol by directly measuring brachial artery (BA) shear rate (see below) over 6 cardiac cycles at 10-min intervals. Rating of perceived exertion was assessed according to the modified 10-point Borg scale, i.e., mild (0–3), moderate (4–7), or difficult (8–10).
With the use of BA diameter and BA blood velocity (Vmean) collected using Doppler ultrasound, BA blood flow [Vmeanπ (vessel diameter/2)2 × 60] and shear rate (8Vmean/BA diameter) were calculated (22). Heart rate was monitored using a standard 3-lead ECG, stroke volume and cardiac output by photoplethysmography, and mean arterial pressure with an automated sphygmomanometer before and during HG (22).
Statistics.
All values are expressed as means ± standard error. Hemodynamic variables before and during HG were averaged over 6 cardiac cycles at 10-min intervals. A one-way repeated-measures ANOVA was used to detect differences over time. Pre-HG vs. Post-HG NO, O2·−, and protein expression were compared using a paired t-test. All statistical analyses were performed using Prism software version 7 (La Jolla, CA). Differences were considered statistically significant when P < 0.05.
RESULTS
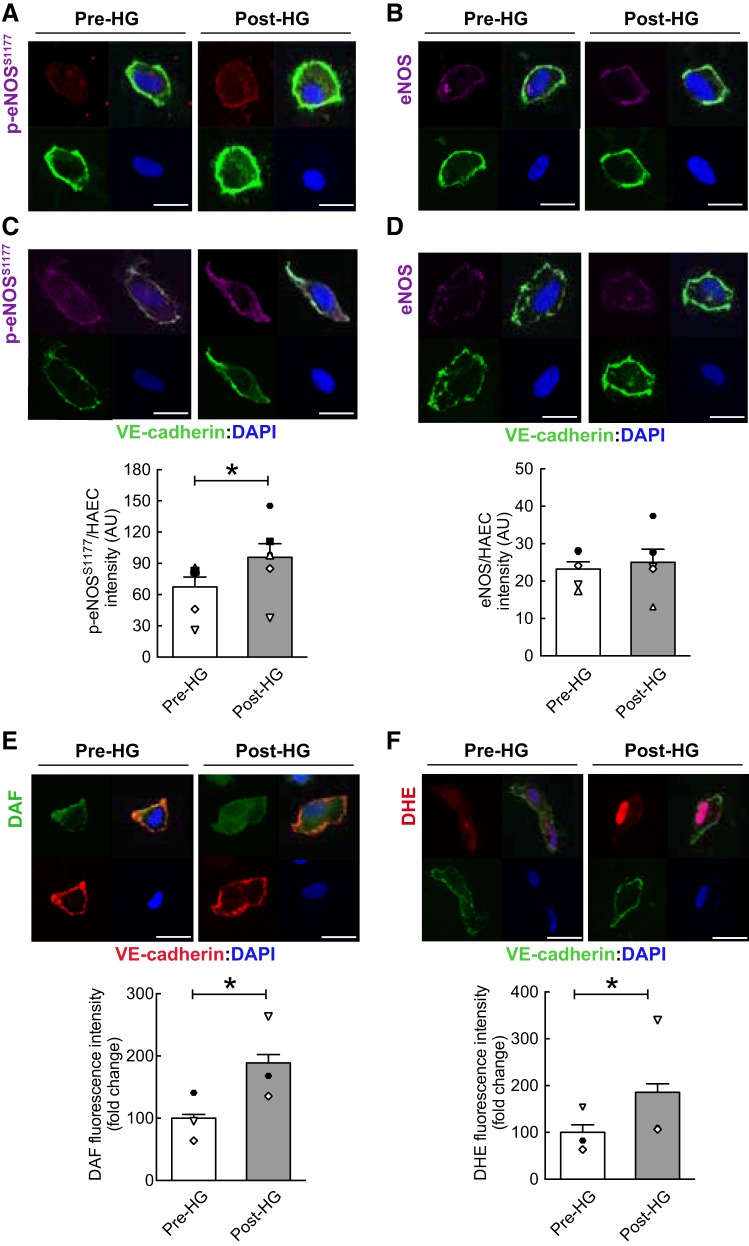
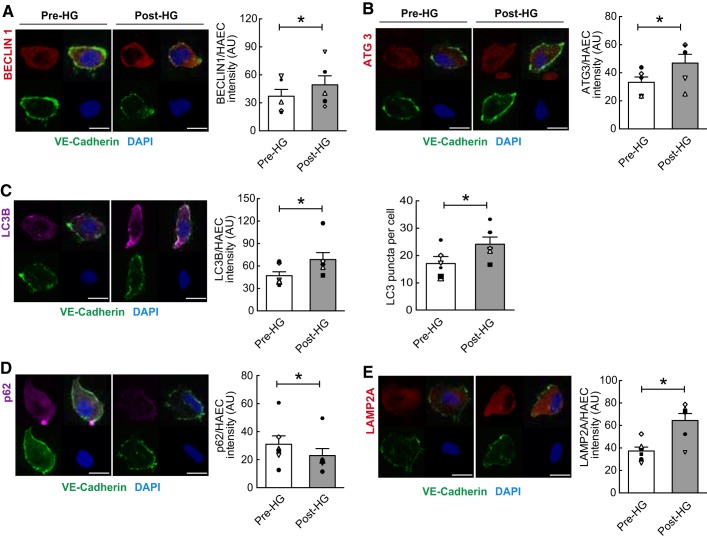
Characteristics of the seven participants are documented in Table 1. Flow-mediated vasodilation exhibited by these subjects was similar to healthy age-matched men reported by others (5). Relative to measures obtained at baseline, HG elevated (P < 0.05) BA blood velocity and arterial shear rate for 60 min, whereas there were no differences in systemic hemodynamics (Table 1; Fig. 1B). Compared with values obtained Pre-HG, ECs collected Post-HG displayed increased (P < 0.05) p-eNOSS1177 but not eNOS protein expression, and this was associated with elevated (P < 0.05) NO generation (Fig. 2, A–E). A cohort of ECs treated with NG-nitro-l-arginine methyl ester Pre- and Post-HG did not display shear-induced NO generation assessed via DAF (data not shown). In addition, ECs exhibited increased (P < 0.05) O2·− production Post- vs. Pre-HG (Fig. 2F). A cohort of ECs treated with N-acetyl-l-cysteine Pre- and Post-HG failed to exhibit shear-induced O2·− production assessed via DHE (data not shown). ECs obtained at 60 min (i.e., Post-HG) displayed increased (P < 0.05) expression of BECLIN1, autophagy-related gene 3, LC3B, and lysosomal-associated membrane protein 2A (Fig. 3, A–C and E) and decreased (P < 0.05) expression (i.e., enhanced degradation) of the adaptor protein p62/sequestosome-1 (Fig. 3D) compared with results obtained from Pre-HG ECs. Likewise, LC3-bound puncta were greater (P < 0.05) Post-HG (i.e., 24 ± 3) vs. Pre-HG (i.e., 17 ± 2). Subjects perceived the intensity of 60-min HG as mild (i.e., 3.0 ± 0.4).
Table 1.
Subject characteristics and hemodynamics
| Hemodynamics during HG |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 10 min | 20 min | 30 min | 40 min | 50 min | 60 min | |||
| Age, yr | 23 ± 1 | BA diameter, mm | 4.64 ± 0.30 | 4.64 ± 0.40 | 4.65 ± 0.40 | 4.64 ± 0.40 | 4.65 ± 0.40 | 4.63 ± 0.40 | 4.63 ± 0.40 |
| Height, cm | 181 ± 3 | BA velocity, cm/s | 7.9 ± 2.0 | 19.8 ± 5.1* | 20.8 ± 3.9* | 21.1 ± 2.6* | 21.2 ± 3.5* | 18.9 ± 2.0* | 20.2 ± 2.9* |
| Weight, kg | 78 ± 2 | Shear rate, fold increase | 0.00 | 2.56 ± 0.60* | 2.74 ± 0.70* | 2.82 ± 0.80* | 2.80 ± 0.70* | 2.54 ± 0.70* | 2.71 ± 0.80* |
| Body mass index, kg/m2 | 23 ± 1 | HR, beats/min | 56 ± 2 | 61 ± 4 | 60 ± 4 | 60 ± 3 | 60 ± 4 | 61 ± 3 | 59 ± 3 |
| FMD, % | 8.4 ± 0.7 | SV, ml | 102 ± 12 | 103 ± 13 | 105 ± 12 | 105 ± 12 | 104 ± 11 | 107 ± 15 | 105 ± 15 |
| Maximal workload, kg | 25 ± 2 | CO, l/min | 5.73 ± 0.70 | 6.29 ± 0.80 | 6.31 ± 0.90 | 6.25 ± 0.90 | 6.20 ± 0.90 | 6.55 ± 1.10 | 6.18 ± 0.90 |
| MAP, mmHg | 90 ± 7 | 93 ± 6 | 95 ± 5 | 94 ± 6 | 93 ± 6 | 96 ± 5 | 97 ± 5 | ||
| RPE | 0.00 | 3.08 ± 0.42 | 3.17 ± 0.38 | 3.25 ± 0.38 | 3.33 ± 0.42 | 3.17 ± 0.31 | 3.25 ± 0.31 | ||
Values are means ± SE. Maximal workload was weight added to bucket during maximal handgrip workload test. BA, brachial artery; CO, cardiac output; FMD, flow-mediated vasodilation; HG, rhythmic handgrip exercise; HR, heart rate; MAP, mean arterial pressure; RPE, rating of perceived exertion; SV, stroke volume.
P < 0.05 vs. baseline.
Fig. 2.
Elevated arterial shear rate increases endothelial nitric oxide (NO) synthase phosphorylation at serine 1177 (p-eNOSS1177), NO generation, and O2·− production. Relative to values obtained before rhythmic handgrip exercise (Pre-HG), Post-HG endothelial cells (ECs) exhibited elevated p-eNOSS1177 (A and C), NO generation (E), and O2·− production (F), whereas eNOS (B and D) protein expression was not altered by HG. Each panel presents a representative EC from each staining protocol: bottom left, vascular endothelial cadherin (VE-cadherin; green); bottom right, 4′,6′-diamidino-2-phenylindole (DAPI; blue); top left, protein of interest; and top right, merge. Mean intensity of the protein of interest (top left) is documented below each representative image. For A–D, n = 7 subjects. Note: two representative images are shown for p-eNOSS1177 and eNOS. For E and F, n = 3 subjects. *P < 0.05 vs. Pre-HG. Scale bar represents 10 µm. Magnification = ×60. Symbols aligned with the histogram indicate individual subject data points. AU, arbitrary units; DAF, diaminofluorescein diacetate; DHE, dihydroethidium; HAEC, human arterial EC.
Fig. 3.
Elevated arterial shear rate increases indices of autophagy in primary arterial endothelial cells (ECs). Relative to values obtained before rhythmic handgrip exercise (Pre-HG), Post-HG ECs exhibited increased expression of BECLIN1 (A), autophagy-related gene 3 (ATG3; B), microtubule-associated proteins 1A/1B light chain 3B [LC3B; C; quantification of LC3 puncta from figures of each group are analyzed by mean LC3 puncta per cell (n = 30 cells per subject)], and lysosomal-associated membrane protein 2A (LAMP2A; E) and decreased expression (i.e., enhanced degradation) of the adaptor protein p62/sequestosome-1 (D). The 4 quadrants of each panel are described in the legend of Fig. 2. Data are reported as the ratio of Pre-HG or Post-HG EC staining intensity divided by human arterial EC (HAEC) staining intensity. Mean intensity of the protein of interest (top left) is documented to the right of each representative image. For A–E, n = 7 subjects. *P < 0.05 vs. Pre-HG. Scale bar represents 10 µm. Magnification = ×60. Symbols aligned with the histogram indicate individual subject data points. AU, arbitrary units; DAPI, 4′,6′-diamidino-2-phenylindole; VE-cadherin, vascular endothelial cadherin.
DISCUSSION
Here, we provide novel evidence that ECs obtained from the radial artery of subjects performing dynamic exercise display elevated autophagy, p-eNOSS1177, NO generation, and O2·− production. These results confirm a recent investigation concerning p-eNOSS1177 (4) and extend those findings to include NO and O2·− generation. Most importantly, we document for the first time that autophagy is initiated in primary arterial ECs obtained from healthy men in response to functional hyperemia.
To determine mechanisms responsible for dysregulated eNOS enzyme function in the context of aging or disease, many laboratories, including our own, employ in vitro reductionist approaches to identify defective nodes in signal transduction pathways. The results from these in vitro cell culture systems are commonly used to explain observations made ex vivo in arteries and/or in vivo in intact animals. However, because cells such as ECs are exposed to a combination of biomechanical stimuli and blood flow patterns ex vivo and in vivo that cannot be precisely recapitulated in vitro, caution should be exercised when extrapolating results from one model system to the next. These are particularly important considerations when evaluating EC autophagy.
First, with regard to biomechanical stimuli, ECs are exposed in vivo to pressure and circumferential tension exerted by the flow of blood in addition to shear stress (17). Second, in terms of flow patterns in vivo, blood flow is pulsatile and the pattern of shear is heterogeneous within the vasculature. For example, shear stress is estimated to range from 10–70 dyn/cm2 in arteries to 1–6 dyn/cm2 in veins (19).
Our (2) first report that continuous laminar shear stress of 20 dyn/cm2 increased trafficking of the autophagosome to the lysosome (i.e., autophagic flux) in BAECs was confirmed by others in human umbilical vein ECs (HUVECs; 12–20 dyn/cm2; Refs. 8, 15) and later by us (1) in BAECs and HAECs. In contrast, autophagic flux is impaired by oscillatory shear stress in HAECs (±3 dyn/cm2; Ref. 14) and HUVECs (±5 dyn/cm2; Ref. 15) and by exposure to continuous low-magnitude flow in HUVECs (4 dyn/cm2; Ref. 15). As shear stress and shear rate are positively associated, we sought to determine whether elevated arterial shear rate concurrent with exercise hyperemia increases autophagy in human primary ECs in vivo. Our results reveal that 60 min of HG exercise 1) elevates BA shear rate approximately threefold, 2) does not alter heart rate, cardiac output, or arterial pressure, and 3) increases EC autophagy activation in healthy male subjects.
In earlier studies, we addressed the contribution of EC autophagy per se to NO generation using a reductionist approach. Genetic and pharmacological inhibition of autophagy prevented shear-induced p-eNOSS1177, negated NO generation, amplified reactive oxygen species production, and unleashed proinflammatory and adhesive responses. These findings strongly imply that intact EC autophagy is critical to maintain EC homeostasis (1, 2). In an effort to ascertain the clinical relevance of these findings, it first was requisite to determine whether elevated arterial shear rate increases autophagy in primary ECs from healthy humans. Here, we provide evidence that elevated arterial shear rate, evoked by functional hyperemia, initiates autophagy, activates p-eNOSS1177, generates NO, and produces O2·− in primary arterial ECs obtained from humans performing dynamic exercise.
To our knowledge, only two investigations have evaluated autophagy in primary human ECs, and both have done so only under basal conditions. LaRocca et al. (12) reported that protein expression of BECLIN1, a key member of the lipid-kinase complex involved in the initiation and regulation of macroautophagy, was lower, whereas p62/sequestosome-1, a marker of undegraded autophagy substrates, was greater, in ECs acquired from the brachial artery of older compared with younger adult subjects. Additionally, in that study, acetylcholine-evoked hyperemia in the brachial artery was lower in the older participants, whereas responses to sodium nitroprusside were similar between groups. These results collectively indicate that impaired EC autophagy is associated with endothelial dysfunction but not vascular smooth muscle dysfunction in the context of aging. Fetterman et al. (6) observed elevated p62 expression, similar BECLIN1 expression, and blunted insulin-mediated p-eNOSS1177 in ECs collected from a forearm vein of subjects with diabetes compared with those without diabetes. In that report, both flow-mediated vasodilation and responses to nitroglycerin were impaired in the brachial artery of the subjects with diabetes compared with controls. These results indicate that a defect in the terminal phase of EC autophagy is associated with endothelium-dependent and -independent dysfunction in individuals with diabetes. Risk factors concurrent with aging and type 2 diabetes mellitus have potential to impair eNOS enzyme function independent of dysregulated EC autophagy. As such, ongoing investigations are designed to determine whether shear-induced EC autophagy and NO generation (present study) are blunted in the context of aging or type 2 diabetes.
This study does have limitations. First, the extent to which our results can be extrapolated to populations other than healthy men is unknown. Second, we did not address the mechanism(s) whereby elevated shear rate initiates autophagy. Both issues are topics of ongoing investigation in our laboratory.
GRANTS
Support was provided for S.-K. Park by American Heart Association (AHA) Grant 17POST33670663; R. S. Richardson by National Heart, Lung, and Blood Institute Grant P01-HL-091830, Veterans Affairs Rehabilitation Research & Development Service (VA RR&D) Merit Grants E6910-R and E1697-R, and VA RR&D Small Projects in Rehabilitation Research (SPiRE) Grant R1433-P; S. Boudina by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants 1-R01-DK-098646-01A1 and R01-DK-099110 and AHA Grant 16GRNT30990018; Y.-T. Shiu by NIDDK Grant R01-DK-100505 and VA BLR&D Merit Review Award I01BX004133; J. D. Trinity by VA RR&D Grant CDA-2 IK2RX001215 and AHA Grant 14SDG1850039; and J. D. Symons by AHA Grant AHA16GRNT31050004, NIH Grants R03-AG-052848 and R01-HL-141540, Seed Grants from the University of Utah (UU) Office of the Vice President for Research, the UU College of Health, the UU Center on Aging, the UU Diabetes and Metabolism Center, and the Diabetes Research Center at Washington University in St. Louis Grant 5-P30-DK-020579.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.-K.P., D.T.L.S., J.C., J.M.C., J.D.T., and J.D.S. conceived and designed research; S.-K.P., D.T.L.S., J.C., J.M.C., A.B., A.N., D.E.M., and J.D.T. performed experiments; S.-K.P., D.T.L.S., J.C., J.M.C., and J.D.T. analyzed data; S.-K.P., D.T.L.S., J.C., J.M.C., J.D.T., and J.D.S. interpreted results of experiments; S.-K.P., D.T.L.S., J.C., and J.M.C. prepared figures; S.-K.P. and J.D.S. drafted manuscript; R.S.R., Y.-T.S., S.B., J.D.T., and J.D.S. edited and revised manuscript; J.D.S. approved final version of manuscript.
REFERENCES
- 1.Bharath LP, Cho JM, Park SK, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, Sargsyan A, Pires K, Anandh Babu PV, Boudina S, Graham TE, Symons JD. Endothelial cell autophagy maintains shear stress-induced nitric oxide generation via glycolysis-dependent purinergic signaling to endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 37: 1646–1656, 2017. doi: 10.1161/ATVBAHA.117.309510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol 92: 605–612, 2014. doi: 10.1139/cjpp-2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PV, Holland WL, Zhang QJ, Abel ED, Symons JD. Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes 64: 3914–3926, 2015. doi: 10.2337/db15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey DP, Ueda K, Wegman-Points L, Pierce GL. Muscle contraction induced arterial shear stress increases endothelial nitric oxide synthase phosphorylation in humans. Am J Physiol Heart Circ Physiol 313: H854–H859, 2017. doi: 10.1152/ajpheart.00282.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 6.Fetterman JL, Holbrook M, Flint N, Feng B, Bretόn-Romero R, Linder EA, Berk BD, Duess MA, Farb MG, Gokce N, Shirihai OS, Hamburg NM, Vita JA. Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis 247: 207–217, 2016. doi: 10.1016/j.atherosclerosis.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res 116: 456–467, 2015. doi: 10.1161/CIRCRESAHA.114.303788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, Wang GX. Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng 42: 1978–1988, 2014. doi: 10.1007/s10439-014-1033-5. [DOI] [PubMed] [Google Scholar]
- 9.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ives SJ, Andtbacka RH, Kwon SH, Shiu YT, Ruan T, Noyes RD, Zhang QJ, Symons JD, Richardson RS. Heat and α1-adrenergic responsiveness in human skeletal muscle feed arteries: the role of nitric oxide. J Appl Physiol (1985) 113: 1690–1698, 2012. doi: 10.1152/japplphysiol.00955.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest 125: 55–64, 2015. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R, Jen N, Wu L, Lee J, Fang K, Quigley K, Lee K, Wang S, Zhou B, Vergnes L, Chen YR, Li Z, Reue K, Ann DK, Hsiai TK. Disturbed flow induces autophagy, but impairs autophagic flux to perturb mitochondrial homeostasis. Antioxid Redox Signal 23: 1207–1219, 2015. doi: 10.1089/ars.2014.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ, Liu GS, Zhang Y, Bu P, Jiang F. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis 6: e1827, 2015. doi: 10.1038/cddis.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol 335: 71–84, 2009. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- 17.Nigro P, Abe J, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal 15: 1405–1414, 2011. doi: 10.1089/ars.2010.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circ Res 116: 480–488, 2015. doi: 10.1161/CIRCRESAHA.116.303805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol 46: 9–15, 2005. [PubMed] [Google Scholar]
- 20.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104: 1085–1094, 2009. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Symons JD, Rutledge JC, Simonsen U, Pattathu RA. Vascular dysfunction produced by hyperhomocysteinemia is more severe in the presence of low folate. Am J Physiol Heart Circ Physiol 290: H181–H191, 2006. doi: 10.1152/ajpheart.00765.2005. [DOI] [PubMed] [Google Scholar]
- 22.Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V, Zhao J, Richardson RS. Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide-mediated mechanism. Am J Physiol Heart Circ Physiol 310: H765–H774, 2016. doi: 10.1152/ajpheart.00817.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witman MA, Garten RS, Gifford JR, Groot HJ, Trinity JD, Stehlik J, Nativi JN, Selzman CH, Drakos SG, Richardson RS. Further peripheral vascular dysfunction in heart failure patients with a continuous-flow left ventricular assist device: the role of pulsatility. JACC Heart Fail 3: 703–711, 2015. doi: 10.1016/j.jchf.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61: 1848–1859, 2012. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]