Abstract
Diseases of the cardiovascular system are the leading cause of morbidity and mortality in men and women in developed countries, and cardiovascular disease (CVD) is becoming more prevalent in developing countries. The prevalence of atherosclerotic CVD in men is greater than in women until menopause, when the prevalence of CVD increases in women until it exceeds that of men. Endothelial function is a barometer of vascular health and a predictor of atherosclerosis that may provide insights into sex differences in CVD as well as how and why the CVD risk drastically changes with menopause. Studies of sex differences in endothelial function are conflicting, with some studies showing earlier decrements in endothelial function in men compared with women, whereas others show similar age-related declines between the sexes. Because the increase in CVD risk coincides with menopause, it is generally thought that female hormones, estrogens in particular, are cardioprotective. Moreover, it is often proposed that androgens are detrimental. In truth, the relationships are more complex. This review first addresses female and male sex hormones and their receptors and how these interact with the cardiovascular system, particularly the endothelium, in healthy young women and men. Second, we address sex differences in sex steroid receptor-independent mechanisms controlling endothelial function, focusing on vascular endothelin and the renin-angiotensin systems, in healthy young women and men. Finally, we discuss sex differences in age-associated endothelial dysfunction, focusing on the role of attenuated circulating sex hormones in these effects.
Keywords: androgens, atherosclerosis, endothelin, endothelium, estradiol, estrogens, menopause, renin-angiotensin system
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among both men and women in developed countries, although CVD is becoming more prevalent in developing countries as well. However, men develop overt CVD at an earlier age compared with women. This sex disparity persists until women reach menopause, at which time the incidence of new onset CVD in women climbs rapidly until it outpaces that of men (180). This rapid increase in the development of CVD coincides with the decline of female sex hormones, characteristic of the menopausal transition, and has long been explained by the loss of cardioprotective effects of estrogens. Furthermore, it is often proposed that androgens are detrimental. However, the roles of sex hormones in cardiovascular health are significantly more complex and are influenced by age, sex differences in the tissues exposed, and route of administration. In this report, we have largely focused on the conducting vessels, as these are the vessels that have been studied most in this field. However, it is important to note that resistance and visceral vessels, while largely unexplored in the context of mechanistic sex differences, may be distinct in their responses and regulation with regard to sex hormones and their receptors.
A healthy endothelium, described as an appropriate balance of endothelial vasoconstrictor and vasodilator factors, is important for vascular homeostasis. Conversely, reductions in endothelial function are detrimental and predict and precede the development of overt CVD. The hallmark of a healthy endothelium is appropriate nitric oxide (NO) synthesis and release by vascular endothelial cells in response to a vasodilatory stimulus. While NO synthesis and downstream NO-mediated dilation are a barometer of healthy endothelial function, there are additional signaling pathways, such as those mediating constrictor tone, that regulate endothelial function and differ between men and women. These include the intracellular angiotensin and endothelin-1 (ET-1) systems. These mechanisms, which contribute to sex differences in endothelial function in young healthy women and men, may explain the cardioprotective phenotype of premenopausal women and the accelerated progression of CVD in men relative to women before menopause.
In this review, we aim to summarize the literature on sex differences in endothelial function. First, we address female and male sex hormones and their receptors and how these interact with the endothelium in healthy young men and women. Second, we discuss sex differences in sex steroid receptor-independent mechanisms controlling endothelial function, focusing on the intracellular vascular ET system and renin-angiotensin system (RAS), in healthy young women and men. Finally, we present sex differences in age-associated endothelial dysfunction, focusing on the role of attenuated circulating sex hormones in these effects in older adults.
FEMALE AND MALE SEX HORMONES AND RECEPTORS
Sex hormones influence endothelial function through their effects on agonists and contribute to key functional differences between women and men related to endothelial function and CVD risk. In women, estrogen receptors (ERs) promote NO release via endothelial NO synthase (eNOS) activation, whereas engagement of the androgen receptor (AR) may result in impaired, agonist-triggered endothelial NO release.
In this section, we provide a brief introduction to sex differences in estradiol, androgen (testosterone), and progesterone signaling in the vasculature. For a more comprehensive examination of this subject, the reader is referred to an excellent review recently provided by Boese et al. (17). It has long been proposed that estrogens play a role in cardioprotection in women (189), whereas androgens may contribute to cardiovascular risk in young women in conditions such as polycystic ovary syndrome (PCOS) (48, 242, 243) or in transgender men receiving testosterone treatment (131, 178, 214). In men, androgens may induce increases in blood pressure via mechanisms including reactive oxygen species (ROS) and decreased NO bioavailability. However, androgens also play a cardioprotective role in men either through direct effects or through conversion to estrogen (157). Thus, the assumption that estrogens are cardioprotective and androgens are detrimental is oversimplified. The effects of these hormones are very much dependent on their environments (124).
Estrogen
Estrogens contribute significantly to the regulation of vasomotor tone, although the receptor- and ligand-specific signaling pathways still need further study. The most well-established ERs are ERα and ERβ, and both of these receptor subtypes are expressed on endothelial cells and vascular smooth muscle cells (VSMCs) (137). While the distinct and specific roles of each subtype continue to be elucidated, both ERα and ERβ contribute to healthy vascular function (141). Receptor expression is dependent on tissue type (72, 141); each subtype is sensitive to circulating plasma estrogen levels (93), and each subtype is affected differently depending on tissue and concentration (141, 165).
The classical view of ERα and ERβ is as ligand-activated nuclear receptors that bind to estrogen response elements in the promoter and regulatory regions of their target genes and so take hours or days to induce their effects. However, ERs and the signaling cascades initiated by ERs can also mediate nongenomic responses (30). These rapid, nongenomic vascular estrogen effects were established in studies showing that estradiol infusion induced vasodilation within 15 min (10, 185). Also, binding sites for ERs were identified on human endothelial cell membranes (24). More recent investigations have demonstrated increases of vascular cAMP content within 30 min of 17β-estradiol (E2) exposure, occurring concomitantly with vasodilation (10, 154), and thus too rapid to be explained by genomic mechanisms. G protein-coupled receptor 30 (GPR30), a G protein-coupled estrogen receptor (GPER), was identified and shown to bind estradiol and also induce rapid signaling events (179), albeit with lower affinity than ERα or ERβ. GPER-estradiol binding leads to rapid and transient activation of a number of intracellular signaling pathways in the reproductive system but also plays a role in the vasculature and in estrogen-mediated endothelial function and thus in estradiol-mediated effects on hypertension and atherosclerosis.
The most well-established vascular effect of estradiol is the production of NO, which occurs via both genomic and nongenomic mechanisms. E2 binding to both ERα and ERβ subtypes triggers signaling cascades that include activation of the kinases c-Src (61, 119), ERK (104, 197), phosphatidylinositol 3-kinase (PI3K) (184), and Akt (PKB) (204). E2 also increases expression of eNOS mRNA and protein expression in endothelial cells (85, 128). In humans, E2 also induces rapid eNOS activation, indicating nongenomic mechanisms, which play an important role in the regulation of vascular function (Fig. 1) (30, 82). Importantly, endothelial cell membranes contain binding sites for E2 leading to the initial activation of eNOS (190). Estrogen binding to activated eNOS promotes the production of NO (103) in the endothelial cell caveola (143) and causes vascular relaxation and endothelial-dependent vasodilation [for the mechanistic details of these pathways, see Orshal and Khalil (166)]. Generally, E2 exposure in women increases vascular relaxation and endothelial-dependent vasodilation, increasing blood flow in numerous vascular beds. Estradiol may also increase sensitivity to vasodilatory factors, such as acetylcholine or prostaglandins, thus reducing the concentrations required to evoke similar vasodilatory responses (142, 230). Recent evidence in human endothelial cells has also demonstrated that GPERs contribute to E2-induced endothelium-dependent vasodilation and NO formation (64).
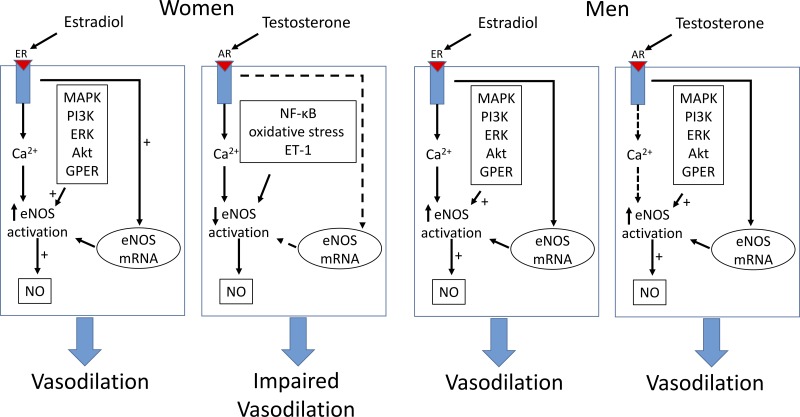
Fig. 1.
Sex differences in the signal transduction pathways of endothelial nitric oxide (NO) synthase (eNOS) activation in endothelial cells by estradiol and testosterone and the subsequent vascular response. Estradiol is generally thought to increase NO availability through genomic and nongenonimc pathways in both men and women, resulting in vasodilation. Testosterone has similar effects in men. Testosterone works through a separate pathway in women, resulting in reduced NO availability and impaired vasodilation. The dashed line indicates effects that are uncertain. ER, estrogen receptor; AR, androgen receptor; PI3K, phosphatidylinositol 3-kinase; GPER, G protein-coupled estrogen receptor 1.
Estrogens in women.
As described above, premenopausal women have a lower risk of CVD than men of similar age, but they lose this advantage with menopause. Chronic E2 supplementation results in an upregulation of eNOS expression and an increase in available NO in isolated vessels (135). In healthy young women, estradiol is associated with increases in flow-mediated dilation (FMD), a measure of conduit artery endothelial function mediated primarily by NO (1, 136, 144). Similarly, short-term ethinyl estradiol administration increases FMD in women with stage 1 hypertension (1), an effect that is mediated by increases in NO (80, 100).
Ethinyl estradiol also attenuates vasoconstrictor responses to norepinephrine infusion in perimenopausal women (215). This effect is likely mediated by reduced α-adrenergic concomitant with greater β-adrenergic receptor contribution to vascular tone (60, 255). However, after menopause, there is a reduction in β-adrenergic receptor contribution to vascular tone, which contributes to increased risk of hypertension in postmenopausal women (77). In ovariectomized (OVX) female rat mesenteric arteries, E2 administration attenuated vasoconstriction during phenylephrine infusion (255) and enhanced vasodilatory responses to isoproterenol infusion (60). Thus, the mechanisms contributing to the greater vascular relaxation by E2 include enhanced NO bioavailability coupled with greater β-adrenergic and lower α-adrenergic sensitivity.
Estrogens in men.
It is not clear whether estrogens provide similar cardioprotection in men as in women. For a discussion of overall estrogen effects in men, we refer the reader to a recent review, Estrogens in male physiology (35). Here, we address the impact of estrogen exposure on the male cardiovascular system. Men express ERα and ERβ (70, 75) and GPER (47) as well as aromatase (70, 75) in their cardiovascular system. GPERs appear to have cardioprotective actions in male rats (47). Consistent with the rapid effects associated with GPERs, E2-mediated vasodilation occurs within minutes in men (140). Estradiol increases vasodilation in men via both prostacyclin and NO mechanisms and so are endothelial dependent and independent (105, 256). An early study (16) demonstrated that acetylcholine induced coronary flow with high levels of equine estrogen infusion, suggesting that estrogens can improve acute endothelial function and avoid ischemia. Testosterone is the major source of E2 in men through its conversion to E2 by aromatase, so circulating E2 depends on concentrations of both testosterone and aromatase. Aromatase is expressed in endothelial cells and VSMCs of male mice (95) and in humans (35, 118). Consistent with effects of E2 on endothelial function, aromatase inhibition decreases FMD in men (118). Estradiol may also have a cardioprotective effect in men by limiting proliferation and migration in VSMCs (40) via both ERα and ERβ mechanisms (86). Conversely, ERβ and E2 exposure increased atherosclerosis in coronary arteries harvested from men, suggesting a role for E2 in early coronary atherosclerosis (123).
Androgens
Androgens are synthesized in testicular Leydig cells and ovarian theca cells and secreted by the testes and adrenal glands. The primary androgen in men is testosterone, which can be converted to dihydrotestosterone (DHT), a more potent form and one not converted to estrogen by aromatase. Like estrogens, androgens are steroid hormones that regulate gene transcription when ligand activated. Androgens bind to ARs in the cytoplasm, undergo conformational changes, and bind to androgen response elements, which move to the nucleus in the target gene to regulate gene expression. As with estrogens, androgens may also induce rapid activation via kinase signaling cascades and mechanisms independent of transcription (9, 17, 83, 177), including activation of intracellular Ca2+ (17), MAPK (83), Akt (9), and PKA/PKC (63) (Fig. 1). ARs are expressed in cells throughout the cardiovascular system, including endothelial cells (226) and VSMCs (125). Transcription-independent mechanisms may also be important for androgen effects on endothelium-dependent vasodilation. Although androgen actions on the endothelium are primarily mediated by ARs, some effects of androgens on the cardiovascular system are mediated indirectly through E2 (157) after testosterone conversion to E2 by aromatase (226). These effects on the endothelium may be ER and NO mediated. Estradiol exposure varies based on both E2 and aromatase levels, and concentrations of these fluctuate in men.
Androgens in men.
Men are at higher risk for developing CVD relative to women of similar age until menopause (89). However, whether this is reflective of negative effects of androgens in men or positive effects of estrogen in women is not entirely clear. While estrogens may play a cardioprotective role in women, normal physiological levels of androgens in men reduce the risk of atherosclerosis (17, 51, 124). Low androgen concentrations are associated with increased risk of coronary heart disease in older men (87, 115), and there is a link between androgen deficiency and atherosclerosis (57, 188). Testosterone administration improves endothelium-mediated vasodilation in some male animal models (34, 36), and knockout of the AR gene increases atherosclerosis in male and female mice (19, 59). Interesting recent data using EAhy926 endothelial cells identified specific androgen-stimulated genes intrinsic to the atheroprotective process (126). In contrast, and adding to the complexity, androgen administration can also blunt endothelium-mediated vasodilation in hypogonadal men by reducing NO availability (12), and testosterone exposure can worsen endothelial function (92) and hypertension in some animal models (155, 208, 251). For example, administration of DHT to male rats induces hypertension, associated with other markers of oxidative stress (155, 208, 251). The variability in these findings is the result of the variations in the concentrations and types of hormones given among the various studies and the numerous cellular [e.g., Ca2+ fluxes, Ca2+-activated K+ channels (97), NF-κB activation, etc.] and hormonal (testosterone metabolism to estrogen by aromatase vs. DHT) mechanisms involved (211).
Androgens in women.
As with men, the impact of androgens on the cardiovascular system in women is also an exciting area of study. Whereas estrogens may play a cardioprotective role, androgens may induce detrimental outcomes on the cardiovascular system in women (51, 124). In general, the engagement of androgen and the AR results in impaired agonist-triggered endothelial NO release in women. This impairment is likely a key causative link between sex differences, infertility, and endothelial dysfunction. Women with high androgen levels, such as women with PCOS, exhibit elevated circulating inflammatory cytokines, mild hypertension, oxidative stress, and NF-κB activation (48), which may reduce NO and lead to endothelial dysfunction (243) and mild hypertension (251). Women with high-androgen PCOS are at risk for CVD (48), and it is likely that androgens drive this risk. In women, serum total testosterone concentration is associated with greater risk of diabetes and related cardiovascular comorbidities (50), all of which are characterized by the presence of endothelial dysfunction (134, 169, 214a). Longitudinal studies of women have supported an association between PCOS and CVD, which is apparent in premenopausal women in their 40s (27), independent of insulin resistance, obesity, or fertility status (27). Androgen excess is associated with endothelial dysfunction in women, including elevated plasma ET-1 levels. In early menopause, when both androgens and estrogens may be fluctuating (116), androgens may be associated with coronary artery calcification (37, 167) and carotid intima media thickness (13), but these findings are not supported by all studies (28, 107).
To examine administration of high doses of testosterone to women, a recent meta-analysis examined androgen (DHT) treatment in female-to-male transgender patients, some of whom also received estrogen and progesterone as well (131). These treatments induced increases in serum triglyceride (TG) and low-density lipoprotein-cholesterol and decreases in high-density lipoprotein-cholesterol at 3, 6, and 24 mo of testosterone treatment (131). Interestingly, cross-sex hormone therapy with testosterone induced blood pressure increases, insulin resistance, and dyslipidemia (131), but cardiovascular morbidity was not yet apparent in these young transgender men (214). None of these studies have yet followed these transgender men into the aging process as CVD risks accelerate, so the data on testosterone administration on cardiovascular morbidity, including changes in the endothelium, are limited.
Methods to study effects of sex hormones and their receptors and between the sexes on the endothelium have proved challenging. Approaches using female mice lacking either ERα or ERβ or male mice lacking the AR may help to define signaling pathways involved in endothelial cell function as well as sex differences in the functioning of these receptors in endothelial cells. For example, both ERα kockout and ERβ knockout mice have been generated and survive into adulthood and have been used to demonstrate the key role that estrogen (via ERα) plays in VEGF regulation in the coronary circulation (96). Furthermore, female mice show increased insulin resistance and obesity compared with male mice (53) after 5α-reductase-1 knockout, indicating a more important role for the AR and glucocorticoids relative to androgens in female as opposed to male mice. Such studies are important not only in elucidating mechanisms but also for demonstrating subtle but important sex differences in the functioning of these receptors. These studies have not been performed specific to endothelial cells but would be of great interest in the future.
Progesterone
Although estrogens have received the majority of attention regarding cardioprotection in women, there is evidence that progesterone also has important cardiovascular effects (147), which are NO (203, 207) and cyclooxygenase (245) mediated. Progesterone administration is associated with increased eNOS in the rat aorta (203) and human endothelial cells (207). The rapid effects of progesterone may be mediated by both nuclear and membrane progesterone receptors (224). Natural progesterone stimulates NO synthesis via transcriptional and nontranscriptional pathways in human endothelial cells (207, 224) and, when added to E2, increases the stimulatory effects of E2 on eNOS (207) that impact vasodilation and vascular tone. Interestingly, synthetic medroxyprogesterone (MPA) does not stimulate eNOS in endothelial cells and may even begin to degrade E2 effects on eNOS expression (207). This suggests that the difference in human endothelial cells is that MPA-bound progesterone receptors do not activate the ERK pathway and so do not subsequently recruit PI3K (207). Thus, the entire signaling pathway for natural versus synthetic progestin is different and may explain the difference in a number of clinical outcomes (207), including mineralocorticoid and inflammatory responses and cancer (192).
Progesterone also impacts VSMCs, inducing rapid Ca2+ influxes, and progesterone receptors have been identified on both endothelial cells and smooth muscle cells. In studies of healthy young women, progesterone enhanced cutaneous adrenergic responsiveness in skin microvessels, which was mediated by prostanoids (245). These findings were similar to earlier studies showing that some progestins can negate vasodilatory effects of E2 (254), and micronized progesterone may attenuate E2 vasodilation during FMD (144). Clearly, much work needs to be done to understand mechanisms by which progesterone may induce vasodilation in some environments but vasoconstriction in others as well as how progesterone and MPA or other progestins interact with E2 on the vasculature in men and women. The few human studies of progesterone effects on the vasculature have been conducted in women, and there have not been studies examining sex differences in the role of progesterone in endothelial function.
SEX DIFFERENCES IN SEX STEROID RECEPTOR-INDEPENDENT MECHANISMS CONTROLLING ENDOTHELIAL FUNCTION
Aside from the marked role(s) of sex steroids and their receptors in the control of endothelial function in men and women, sex differences exist in many additional mechanisms that impact or control endothelial cell biology and function. In particular, sex-specific differences in the RAS and the ET-1 signaling pathway both play large roles in sex differences observed in endothelial function. These sex differences are directly and indirectly mediated by differences in the gonadal hormone milieu, which lead to sex differences in protein expression and/or activity at endothelial cells and VSMCs. In this section, we review sex differences in the role of the RAS and ET-1 signaling in endothelial function in healthy young men and premenopausal women.
ET-1 and Sex Differences in Endothelial Function
The ET-1 system is an important mediator of sex differences in vascular physiology and pathophysiology. ET-1 is a potent vasoconstrictor, and overactivation or dysfunction of the ET system plays a role in the development and progression of CVD in both experimental and clinical studies. ET-1 is produced from its precursor prepro-ET-1 in a two-step enzymatic pathway governed by the constitutively expressed endothelin-converting enzyme (ECE). ET-1 is continuously synthesized and released and acts primarily through autocrine or paracrine signaling at the level of the tissue in which it is synthesized. In the vasculature, the vasoconstrictor effects of ET-1 are mediated by ET-1 binding to the G protein-coupled ET type A receptor (ETAR) and ET type B receptor (ETBR) subtypes on the vascular smooth muscle. ETBRs are also expressed on the vascular endothelium and, upon ET-1 binding, induce the release of endothelium-derived vasodilators such as NO. As such, the net effect of ET-1-mediated vascular tone derives from the balance of vascular smooth muscle ETAR- and ETBR-mediated vasoconstriction and endothelial ETBR-mediated vasodilation. ET-1 also induces the production of growth factors, ROS, and inflammatory mediators, which contribute to vascular pathology associated with overactivity of the ET-1 system. In this section, we focus on sex differences in ET-1-mediated endothelial function. Aside from the vascular effects of ET-1, ET-1 synthesis and signaling are important in a number of other physiological processes, including the renal and immune systems, and sexual dimorphism exists in these systems as well. As these systems are outside the scope of this review, we refer the reader to one of many excellent reviews on these topics (69, 110, 228).
In the vasculature, ET-1 is produced by ECE within the endothelial cell and diffuses out of the cell to act on membrane-bound ETBRs on the endothelium (autocrine) or ETARs and ETBRs on the vascular smooth muscle (paracrine). Circulating ET-1 is cleared by endothelial ETBRs and in the lungs by endocytosis and degradation (133). ECE activity and mRNA expression are augmented in the vasculature of OVX rats (221) and attenuated by treatment with E2 or phytoestrogens (186). Similarly, E2 inhibits ET-1 mRNA expression and ET-1 release in human endothelial cells (15) and VSMCs (220), whereas testosterone increases ET-1 mRNA expression in human aortic endothelial cells (171). Evidence points to a role for circulating female hormones lowering circulating ET-1 in women compared with age-matched men (178). During the menstrual cycle, plasma ET-1 concentrations fluctuate and are lowest during the luteal phase, when both E2 and progesterone are elevated. Creatsas et al. (38) observed a decrease in plasma ET-1 in primary amenorrheic female teenagers after exogenous E2 treatment. Pregnancy, another condition in which endogenous estrogens and progesterone are elevated, is associated with decreases in circulating ET-1 (127). Conversely, women who have preeclampsia, a hypertensive disorder of pregnancy, have attenuated plasma progesterone and elevated plasma ET-1 compared with healthy pregnant women, and progesterone supplementation attenuates ET-1 secretion in human umbilical vascular endothelial cells exposed to serum from preeclamptic women (106). Finally, women with PCOS exhibit elevated circulating androgens and display higher plasma ET-1 than healthy age-matched women (49, 50). These data suggest that ET-1 production in the vascular endothelium is attenuated in the presence of estrogens and progesterone. Complementary to these sex hormone-mediated attenuations in ET-1 synthesis, increases in endothelial ETBR protein expression with estrogen and progesterone may also increase clearance of circulating ET-1 (133). In general, women have a greater ETBR protein expression compared than men, and these differences likely also contribute to sex differences in circulating ET-1 by increasing local ET-1 clearance. Similarly, increases in circulating ET-1 in women with altered estrogen and/or progesterone (i.e., preeclampsia or PCOS) may reflect changes in clearance of ET-1 consequent to changes in ETBR expression. Collectively, sex hormone-mediated differences in both ET-1 synthesis by ECE and clearance through ETBRs contribute to circulating ET-1 concentrations and are reflected in the differences in plasma ET-1 of men compared with women.
Aside from sex differences in endothelial synthesis of ET-1, men have a greater vasoconstrictor response to exogenous ET-1 administration compared than women. Competitive binding studies conducted in human saphenous veins have demonstrated a higher ratio of ET-1 binding to both ETARs and ETBRs in men, resulting in twofold greater constrictor responses in the veins obtained from men compared with those from women (58). Similarly, vascular sensitivity to ET-1 is reduced in cultured cerebral arteries obtained from women compared with those from men (2). Indeed, animal models have demonstrated that physiological concentrations of estrogens attenuate ET-1-mediated vasoconstriction in vivo, whereas testosterone treatment exaggerates these responses (223).
These sex differences in vascular responsiveness to ET-1 are mediated, at least in part, by differences in the expression and location of ET-1 receptor subtypes. In general, women have a greater proportion of endothelial ETBRs relative to vascular smooth muscle ET-1 receptors, favoring more dilation with which to attenuate the overall constrictor tone. Administration of the ETBR antagonist BQ-788 into the cutaneous microvasculature of healthy young human subjects demonstrates that ETBRs mediate tonic constriction in men but contributes to tonic vasodilation in the microvasculature of women (102). Stauffer et al. (213) showed that increases in forearm blood flow in response to intra-arterial infusion of the ETAR antagonist BQ-123 are greater in men, demonstrating that men also have greater tonic ETAR-mediated vasoconstriction. In contrast, saphenous veins and cerebral arteries obtained from women demonstrate greater ETBR expression than tissues obtained from men (2, 58). These human studies are supported by animal models in which OVX increases the vascular vasoconstrictor response to ET-1 and to ETBR antagonism, and this effect is reversed when estrogen is administered (42). Few, if any, studies have examined the role of testosterone in ET-1 receptor subtype location and expression. Interestingly, women with PCOS, who demonstrate hyperandrogenism and consequently elevated circulating testosterone, exhibit attenuated ETBR-mediated dilation compared with normally ovulating control women (242). Furthermore, this effect is abolished during androgen suppression (243). Collectively, this body of literature suggests that healthy premenopausal women benefit from a greater endothelial ETBR-mediated dilation, mitigating ET-1-induced tonic vasoconstrictor tone in the vasculature (Fig. 2). This sex-dependent protective effect is likely governed by circulating ovarian hormones, since OVX animals and women with hyperandrogenism seem to lack this protection, contributing to relative vascular dysfunction compared with their healthy counterparts (Fig. 3).
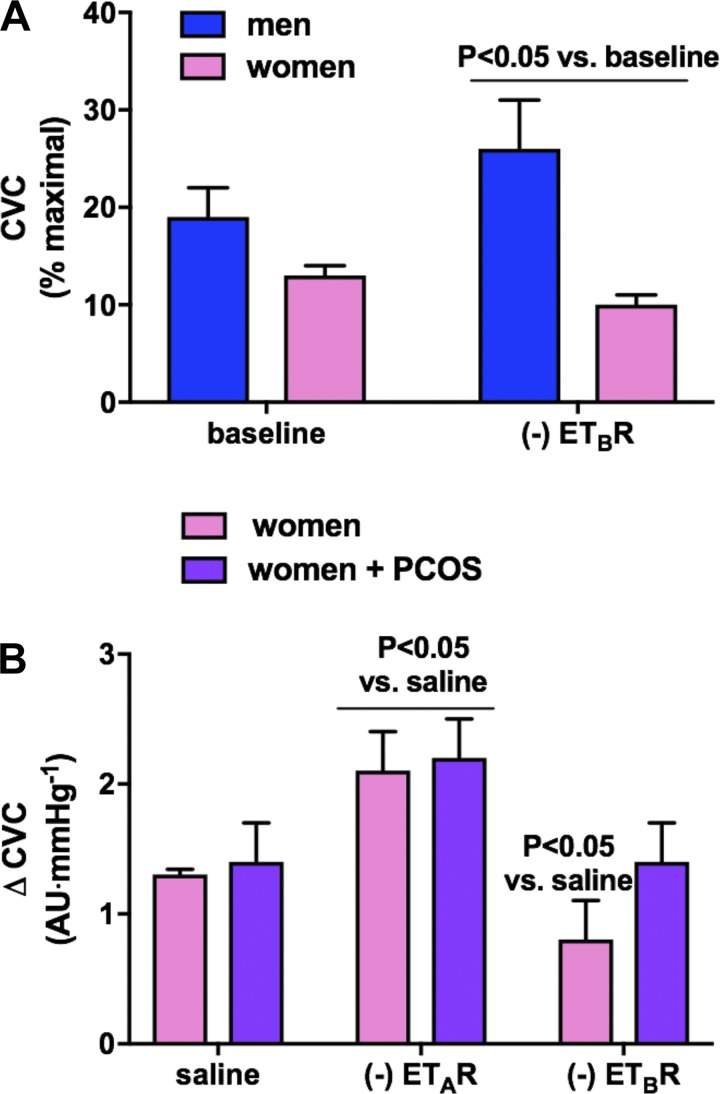
Fig. 2.
A: sex differences in cutaneous microvascular responses to endothelin type B receptor (ETBR) subtype antagonism. Healthy young men demonstrate an increase in cutaneous vascular conductance (CVC) with ETBR blockade, whereas women show a decrease, suggesting that the ETBR mediates tonic constriction in men but contributes to vascular relaxation in women [adapted from Kellogg et al. (102)]. B: however, although there are no differences in tonic constriction mediated by the endothelin type A receptor (ETAR) between healthy women and women with polycystic ovarian syndrome (PCOS), women with PCOS do not demonstrate a decrease in CVC with ETBR antagonism. These data suggest that the ETBR does not contribute to vascular relaxation in women with PCOS, which is characterized by elevated circulating androgens [adapted from Wenner et al. (243)]. AU, arbitrary units.
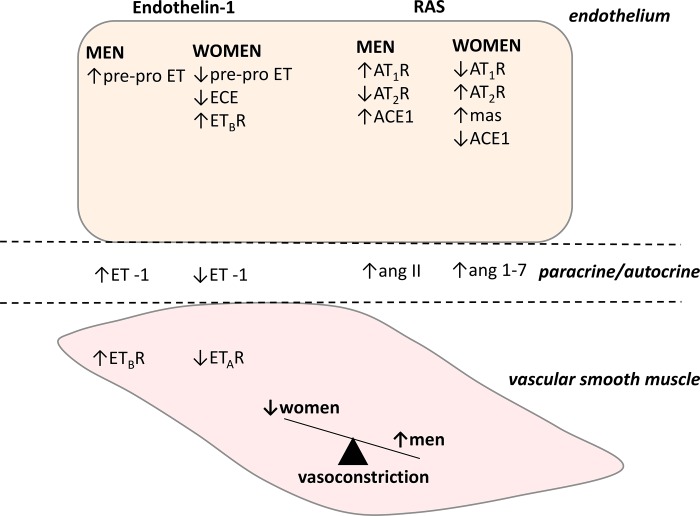
Fig. 3.
Sex differences in the cellular endothelin (ET)-1 system and renin-angiotensin system (RAS). Collectively, men synthesize more ET-1 and angiotensin II (ANG II) and have greater vasoconstrictor responses to both ET-1 and ANG II, mediated by sex differences in receptor subtype expression, whereas women have higher circulating ANG-(1–7) concentrations. These differences contribute to an overall greater tonic vasoconstrictor tone in healthy young men compared with healthy young women. ECE, endothelin-converting enzyme; ETAR, ET type A receptor; ETBR, ET type B receptor; AT1R, ANG II type 1 receptor; AT2R, ANG II type 2 receptor; ACE1, angiotensin-converting enzyme; mas, Mas receptor.
Several clinical trials have demonstrated the efficacy of selective ETAR and combined ETAR/ETBR antagonism in reducing blood pressure in humans with hypertension (112, 156, 240, 253). However, despite this clinical potential, none of these antagonists have been approved for the management of primary hypertension because of their side effects, mainly fluid retention. Clinical trials exploring sex differences in the response to ET receptor antagonism in humans are lacking; however, sex hormones may contribute to the differential responses to ET antagonists in male and female animals. Bosentan, a mixed ETAR/ETBR inhibitor, is more effective at lowering blood pressure in male compared with female DOCA-salt rats (227). This sex-dependent effect is abolished after OVX of female animals and returns with estrogen replacement, along with ETBR expression (41). Conversely, administration of a selective ETAR antagonist more effectively lowers blood pressure in female compared with male rats (101). These findings corroborate the proposed protective role of ETBR-mediated dilation in female subjects and support the idea that differential activation of ET-1 signaling contributes to sexual dimorphism in ET-1 mechanisms contributing to hypertension. Clinical trials in humans focusing on sex differences in efficacy and outcomes of treatment with different ET antagonists will provide further evidence of these sex differences in human vascular physiology and provide clinical insight into sex-specific management of CVD.
The RAS and Sex Differences in Endothelial Function
The RAS is a key system in controlling body fluid balance and blood pressure, and overactivation of the RAS leads to increases in blood pressure and the progression of CVD and renal disease in men and women. Sex hormones affect the components of the RAS by a number of mechanisms, and the net effect of physiological estrogen concentrations in humans seems to be an overall inhibition of angiotensin II (ANG II) synthesis and signaling resulting in a blood pressure reduction. Conversely, circulating testosterone may amplify the RAS, resulting in greater renin activity and circulating ANG II in male rats (32, 54); however, these findings have not yet been explored in humans. In this section, we focus on sex differences in RAS effects on endothelial function. For detailed explanations of the role of sex hormones in upstream regulation of the RAS, including renin activity and ANG II synthesis, we refer the reader to one of many excellent reviews on these topics (62, 216).
In response to systemic graded infusions of ANG II, young healthy men may have exaggerated increases in diastolic blood pressure and mean arterial pressure compared with young healthy women (225). However, these findings contradict earlier studies showing that there are no differences in pressor or forearm vasoconstriction responses to ANG II administration between men and women (20), although Gandhi et al. (67) did find that the duration of the pressor response was significantly longer in men than in women. Despite these equivocal results, tissue responsiveness to exogenous ANG II is attenuated in women and female animals. Cultured cerebral arteries from women have an attenuated vasoconstrictor response to exogenous ANG II compared with those obtained from men, despite a lack of difference in ANG II type 1 receptor (AT1R) protein expression (2). Human studies of renal vasoconstrictor responses to ANG II are lacking; however, animal studies have consistently demonstrated augmented renal constrictor responses to exogenous ANG II in male compared with female animals (163, 200). Furthermore, these effects have been found to be modulated by circulating sex hormones, such that androgens contribute to exaggerated constrictor responses in male animals (164, 212), and estrogens attenuate these responses in female animals (163, 187, 252). Similarly, in humans, the degree of hypertension and renal injury closely parallels increases in RAS activation in men, whereas the same relation is not as strong in women (111).
Recently, ANG II has been demonstrated to enhance ET-1-mediated constriction through increased tissue expression of ET-1 (88), upregulation of ETAR expression (122), and decreasing ETBR function (109), and it is likely that sex differences in the ET-1 system contribute to sex differences in the vascular responses to ANG II. To this end, Kittikulsuth et al. (108) recently demonstrated that ETBR blockade during ANG II infusion and high-salt feeding exacerbated the rise in blood pressure in female rats, such that they demonstrated the same increase in blood pressure as that observed in high-salt/ANG II male rats. However, ETBR blockade had no effect on male rats, suggesting that the ETBR plays a beneficial role in protecting female compared with male rats against ANG II-induced hypertension (108). Intapad et al. (94) recently found that ETAR inhibition reduced ANG II-mediated increases in blood pressure in male, but not female, rats, suggesting that the ETAR contributes to ANG II-mediated increases in blood pressure in male but not female rats. While this mechanistic interplay of the RAS and ET-1 system has not yet been explored in humans, sex differences in the ET-1 system likely contribute to sex differences in the vascular responses to ANG II and vice versa.
Most of the classical effects of the RAS are mediated at the tissue level, primarily by the binding of ANG II to AT1Rs, leading to vasoconstriction, and to some extent by the binding of ANG II to the opposing ANG II type 2 receptor (AT2R). The AT2R is expressed at low levels in the adult vasculature but elicits downstream effects such as NO generation and vasodilation and has anti-inflammatory and antithrombotic actions, which oppose those of the AT1R. Female Sprague-Dawley rats have greater aortic expression of AT2Rs than male Sprague-Dawley rats, and testosterone withdrawal by castration significantly elevates AT2R expression in male rats, suggesting a role for androgens in downregulating AT2Rs and contributing to sex differences in vascular AT2R expression (146). Similarly, female Sprague-Dawley rats have an estrogen-dependent increase in AT2Rs (193, 195). However, because AT2R expression is minimal in the adult human vasculature, and because sex differences in the AT2R have not yet been examined in humans, it remains unclear whether sex differences in the AT2R contribute to differences in the endothelial response to ANG II in men and women. Similar to circulating renin and angiotensin-converting enzyme (ACE), vascular AT1R expression and activity are downregulated in the presence of estrogen and upregulated in the presence of testosterone (33, 45, 159, 160, 168, 187). Downstream of the AT1R, the intracellular RAS operates independently of the circulating RAS and contributes to alterations in vascular tone and arterial structure. All of the components of the circulating RAS are expressed in the vascular wall, and all are modulated by estrogens, mainly at the endothelial level. Tissue ACE1 expression and activity are elevated in overectomized female animals and decreased with estrogen replacement (45, 66, 222). In contrast, oophorectomized male mice have decreased ACE1 protein expression compared with intact male mice, suggesting a role for androgens in the sex-specific increase in tissue ACE1 expression (65). In humans, atrial tissue slices obtained from men demonstrate downregulated ACE1 expression when treated with estradiol (23). These sex hormone-specific effects have been demonstrated to be mediated via tissue ERs and ARs in both human and animal tissues. ERs themselves initiate many downstream vasoprotective signaling mechanisms (see above). Endothelial ER-mediated increases in NO and decreases in ROS likely protect the vasculature from ANG II-mediated vasoconstriction, inflammation, and ROS production, representing a female sex-specific protective mechanism in ANG II signaling biology.
Aside from the classical proconstrictor RAS pathway, the more recently described “nonclassical” RAS pathway has gained attention for its ability to antagonize classical RAS signaling, leading to vasodilation and/or mitigation of ANG II-mediated vasoconstriction and inflammation. In the nonclassical RAS pathway, ACE2 catalyzes the production of ANG-(1–7) directly from ANG II and indirectly from ANG I [via ANG-(1–9)]. Similarly, the peptidase neprilysin directly converts ANG I to ANG-(1–7) and plays a major role in ANG-(1–7) formation in the circulation and vascular endothelium (4). In turn, ANG-(1–7) can bind the AT2R as well as its own receptor, Mas, to oppose the intracellular actions of ANG II-bound AT1Rs. Similarly to the AT2R, sex differences in the ANG-(1–7)/Mas receptor axis may also play a role in divergent responses to RAS stimulation between the sexes. In animal models of hypertension, circulating ANG-(1–7) is higher in female than male animals, and antagonism of the Mas receptor abolishes female sex-specific protection from vascular injury (71, 217, 238). Similarly, Brosnihan et al. (22) demonstrated the ability of E2 to promote ANG-(1–7) production in transgenic hypertensive rats. Human studies of sex differences in ANG-(1–7) are limited, but Sullivan et al. (218) recently reported that healthy premenopausal women have higher circulating plasma ANG-(1–7) concentrations than age-matched healthy men. Women taking oral contraceptives have also been reported to have greater circulating ANG-(1–7) compared with ovulatory women during both the follicular and luteal phases of their ovulatory cycle; however, these increases were accompanied by similarly elevated ANG II and may be compensatory and/or result from a generalized increase in RAS activity (44). Interestingly, women taking oral contraceptives have, on average, ~5-mmHg higher mean arterial pressures compared with ovulatory women not taking hormonal birth control, suggesting that these circulating changes in ANG-(1–7) may not translate to reduced vasoconstrictor tone. Among pregnant women, where circulating E2 and other hormone concentrations increase, urinary ANG-(1–7) increases, supporting a role for female gonadal hormones in mediating an increase in ANG-(1–7) synthesis (231). Downstream of circulating ANG-(1–7), gonadal hormones likely influence Mas receptor expression and/or sensitivity. Female spontaneously hypertensive rats demonstrate greater renal cortical Mas protein expression than male spontaneously hypertensive rats (194, 217), and normotensive female rats demonstrate a reduced renal blood flow response to Mas receptor blockade, which is absent in their male counterparts (191). Along with its vasodilator role in the endothelium, the ACE2/ANG-(1–7)/Mas axis has also been suggested to reduce inflammation and inhibit vascular and cellular growth mechanisms in cerebral, renal, cardiac, and pulmonary vascular tissues (206). While the full scope of this anti-inflammatory mechanism is outside the scope of this review, it is possible that the anti-inflammatory role of ACE2/ANG-(1–7)/Mas contributes to sex differences in the incidence of CVD by inhibiting vascular inflammatory processes in premenopausal women. Collectively, although human studies of ANG-(1–7) synthesis and Mas receptor expression are limited, emerging animal data suggest that the ACE2/ANG-(1–7)/Mas receptor axis likely plays a role in sex differences in endothelial function and contributes to the relatively protected vascular phenotype of premenopausal women (Fig. 3).
While it is commonly hypothesized that the sex differences in vascular RAS mechanisms play a large role in the relative protection of women against CVD before menopause, the pathophysiological significance of the vascular tissue RAS in clinical CVD is unclear. However, patients with hypertension have been shown to have increased vascular responses to exogenous ANG II (205, 229), and patients treated with ANG II receptor blockers (ARB) or ACE inhibitors demonstrate significant regression of vascular remodeling compared with patients treated with atenolol (where blood pressure was lowered independently of RAS activity) (130, 198, 199). Interestingly, women respond more favorably to ARB treatment, whereas ACE inhibitors are more effective in men. In the China STATUS II study, women showed a greater antihypertensive response to treatment with the ARB valsartan combined with amlodipine compared with men on the same treatment (236). Similarly, survival rates for women after congestive heart failure are better for women treated with an ARB compared with those treated with an ACE inhibitor, and yet the opposite was true for men (90). Although few clinical trials have reported efficacy by sex, these few findings are supported by animal studies in which the antihypertensive effects of ACE inhibitors are greater in young male animals compared with young female animals, whereas ARB treatment has a greater depressor effect on blood pressure in young female compared with male animals. While more human data from clinical trials powered to examine sex differences is warranted, these findings suggest that the depressor arm of the RAS is increased in women, and therapeutic interventions leveraging these sex-specific protective mechanisms are more effective in women than they are in men.
Sex Differences in Sympathetic Nerve Activity
While a full discussion of sex differences in sympathetic nerve activity directed to the vasculature is outside the scope of this review, it is important to note that these differences likely play a role in the different relative CVD risk observed between men and women. While there is no relationship between muscle sympathetic nerve activity (MSNA) and blood pressure in healthy young men or women, the mechanisms mediating this are different between the sexes. In young men, there is an inverse relationship between MSNA and cardiac output, such that under conditions of high MSNA, cardiac output is lower to maintain mean arterial pressure (78). However, in young women, sympathetic vasoconstriction is attenuated by β-adrenergic-mediated dilation, abolishing the relationship between MSNA and total peripheral resistance to maintain blood pressure (78). In women, changes in circulating concentrations of sex hormones (across the menstrual cycle, with hormonal contraceptive use, or with pregnancy) have also been shown to influence the relationship between MSNA and mean arterial pressure, highlighting a role for sex hormones in mediating sex differences in these responses. Importantly, changes in vessel stiffness mediated by sex differences in mechanisms contributing to endothelium-dependent dilation and vascular smooth muscle function alter the relationship between sympathetic nerve activity and vascular tone. For further explanation of this topic, we refer the reader to one of many excellent reviews on the topic of sex differences in neurovascular control of blood pressure (7, 21, 79).
SEX DIFFERENCES IN AGE-ASSOCIATED ENDOTHELIAL DYSFUNCTION
The prevalence of CVD increases with age in both men and women. As described above, the prevalence of CVD is generally lower in young women than in age-matched men. However, after menopause, women have a higher prevalence of CVD compared with age-matched men. Declines in endothelial function with aging have been well described. Interestingly, the decline in endothelial function begins around the fourth decade of life in men but is shifted ~10 yr in women, such that declines in endothelial function are most noted during the fifth decade of life, which is at the time of menopause (31). Furthermore, the rate of decline in endothelial function is much steeper in women than in men later in life (31). Indeed, within women, there is a progressive decline in endothelial function across the stages of menopause (151). Drastic changes in sex hormones (particularly E2) have been traditionally thought to mediate these changes in both endothelial dysfunction and CVD with aging; in other words, the loss of the vasoprotective/vasodilatory effects of E2 concomitant with unopposed vasoconstriction due to the loss of E2 and vascular remodeling over time are responsible for the greater decline in endothelial function and the associated rise in CVD in women after menopause.
Given the numerous changes in vascular function with aging, it is challenging to understand what is driven purely by age versus the changing hormonal milieu. Two elegant studies from the laboratory of Dr. Muller-Delp have examined the separate and combined impact(s) of aging and hormonal status in female rats (99, 117). Vascular function was measured in young and old intact female rats, young and old OVX, and young and old OVX rats administered E2. In young female rats, vasodilation was blunted in OVX rats compared with intact rats. Vasodilation was similar between intact rats and OVX rats given E2. As expected, vasodilation was lower in old female intact rats compared with young female intact rats. However, OVX did not cause a further decline in vasodilation, as there were no differences in vessel relaxation between old intact and old OVX female rats. Estrogen administration to old OVX rats improved vasodilation, such that vessel relaxation was similar to that of young intact rats. Importantly, these studies administered E2 immediately after OVX in old female rats and were able to completely restore vasodilation to a similar response to that of young female rats. Although not statistically compared, young OVX rats appeared to have similar vessel relaxation to that of old intact female rats (~30%), suggesting a strong influence of ovarian hormones on vasodilatory function. Taken together, these data show the important influence of both age and hormonal status on endothelial function.
Sex Hormone Changes With Aging
As women approach menopause, E2 and progesterone generally decline and follicle-stimulating hormone increases (76). However, E2 can fluctuate widely before menopause (26, 196). Due to the initial loss of ovarian function during the early perimenopausal period, women may initially experience surges in E2 followed by rapid declines (26, 196). These fluctuations are commonly associated with erratic changes in menstrual cycle length (i.e., shorter, more frequent cycles) and vasomotor symptoms or hot flashes. As women progress through perimenopause, ovarian function continues to decline and E2 levels decrease. During this time (late perimenopause), menstrual cycle frequency declines such that women experience periods of amenorrhea (>60 days). This is followed by a complete loss of ovarian function and permanent cessation of menses (>1 yr), at which time women are considered postmenopausal. As such, levels of E2 and progesterone remain low after menopause, and women remain in a hypoestrogenic state permanently. With regard to androgens, studies have shown that testosterone may either increase (138) or remain stable (25) during menopause. Further evidence suggests a rapid decline in testosterone after menopause but that concentrations begin to increase with advancing age (after 70 yr) (116). Importantly, given the large decline in E2 with menopause, there is an increase in the androgen-to-estrogen ratio in postmenopausal women, which may also have implications for endothelial function and cardiovascular health (174).
As men age, there are declines in circulating androgen concentrations. Although this is generally not as dramatic as the decline in E2 observed in women after menopause, there are instances where the loss of testosterone can be quite significant. As such, the impact of “low testosterone” on cardiovascular function has gained recent attention over the past decade. There appears to be a link between androgen deficiency and developing atherosclerosis (57, 188). Low circulating testosterone in older men is associated with atherosclerosis compared with age-matched contros with testosterone concentrations similar to those in younger men (8, 188). Given that there are many changes that occur to the cardiovascular system with aging, this association does not necessarily prove “cause and effect.” However, data in animal models using castration (3) or AR knockout models (249) suggest a direct association between testosterone and atherosclerosis. To our knowledge, there are no data in humans that demonstrate a cause-and-effect relationship between testosterone and atherosclerosis but it is an important area of investigation.
Mechanisms Contributing to Endothelial Dysfunction With Aging
Due to the interactions of sex hormones on multiple pathways regulating vascular function, the mechanisms contributing to endothelial dysfunction with aging are complex, multifactorial, and not completely understood. As reviewed above, there is a strong and direct influence of E2 on NO production and release from the endothelium via ERα (see Fig. 1). Thus, declines in E2 after menopause likely result in endothelial dysfunction due to reduced eNOS activation and a subsequent reduction in NO production or bioavailability. Moreau et al. (68) demonstrated that expression of ERα and eNOS in endothelial cells harvested from peripheral veins of women are lower in postmenopausal women than in young women (68). Thus, not only does E2 decline with aging but ERα receptor expression also declines. Furthermore, ERα expression and eNOS expression are correlated with flow-mediated vasodilation. These data provide direct evidence in humans that the age-associated decline in endothelial function in postmenopausal women is due in part to a reduction in ERα expression, loss of eNOS, and subsequent reductions in NO production. NO bioavailability can also be impacted by oxidative stress, and it is well known that estrogens have antioxidant capabilities. Therefore, there may be greater generation of ROS, which can scavenge O2 and reduce NO bioavailability in postmenopausal women due to the declines in E2 with menopause. Systemic infusion of the nonspecific antioxidant ascorbic acid increased flow-mediated vasodilation in estrogen-deficient postmenopausal women (152). However, in postmenopausal women using estradiol (either oral or transdermal), there was no further increase in flow-mediated vasodilation with the infusion of ascorbic acid (152). These data demonstrate that oxidative stress is a key mechanism contributing to endothelial dysfunction in estrogen-deficient postmenopausal women and that estrogen treatment improves endothelial function in part via reductions in ROS.
The ET system is also impacted by aging. Most studies have shown that plasma ET concentrations are greater in postmenopausal women (129, 241) and older men (52) compared with their younger counterparts. Furthermore, ET-1 protein expression in harvested endothelial cells is greater in older men than in younger men and associated with the decline in endothelial function (52). As mentioned above, there are known sex differences in plasma ET-1 as well as expression of ETARs and ETBRs. For example, brachial artery infusions of an ETAR antagonist resulted in a greater increase in forearm blood flow in older men compared with women, indicating that ETA-mediated vasoconstriction is greater in older men than in women (213). However, when a combination of ETAR and ETBR antagonists was infused, blood flow increased more in women (213), suggesting that older women have greater ETBR-mediated vasoconstriction. This is in direct contradiction to the primary role of ETBRs in mediating vasodilation in young women, demonstrating the complex interplay between aging and sex hormones in mediating sex differences across the lifespan. These sex differences in heightened ET-1-mediated vasoconstriction in older adults may also contribute to the age-related declines in endothelial function and explain, in part, the sex differences in endothelial function in older adults. Indeed, ETAR-mediated vasoconstriction is greater in older men than in younger men (232). To determine the role of this heightened constriction on the age-related declines in endothelial function in men, Westby et al. (246) infused acetylcholine in the presence and absence of the ETAR antagonist BQ-123 in young and older men. As expected, vasodilatory responses to acetylcholine were blunted in older men. However, coinfusion of BQ-123 improved endothelium-dependent dilation in older men (Fig. 4). These data demonstrate that the reduction in endothelial function in older men is due, in part, to greater ETAR-mediated constriction (246). ET-1 also contributes to the decline in endothelial function in postmenopausal women (241); however, this decline is mediated by a different receptor mechanism, primarily a shift in ETBR responses, as the ETBR antagonist BQ-788 restored vasodilation in postmenopausal women (241). Interestingly, there were no differences in vasodilator capacity during blockade of ETARs between young and postmenopausal women. Thus, the decline in endothelial function in postmenopausal women is due, in part, to a loss of ETBR-mediated dilation (241), whereas ETAR-mediated constriction contributes to the declines in endothelial function in older men (246) (Fig. 4). Whether these changes in ETBR function are related to declines in ERα sensitivity are not known. Taken together, sex differences in the ET-1 system persist throughout aging and contribute to endothelial dysfunction through different receptor mechanisms.
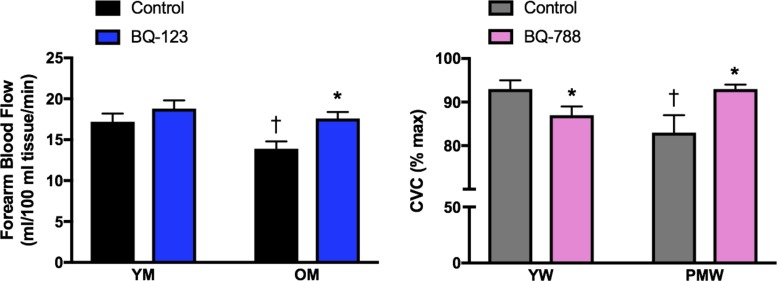
Fig. 4.
Age and sex differences in endothelin (ET)-1 receptor responses on endothelial function. Endothelial function in young men (YM) and older men (OM) (left) was determined by measuring forearm blood flow responses to acetylcholine during control/saline conditions (black bars) or coinfused with the ET type A receptor (ETAR) antagonist BQ-123 (blue bars). Data were adapted from Westby et al. (246) and plotted as blood flow response at the highest dose (16 μg·100 ml tissue−1·min−1) of acetylcholine. The age-associated decline in endothelial function in men was abolished with infusion of ETAR antagonist BQ-123 (246). In contrast, the age-related decline in endothelial function in women is mediated in part by alterations in the ET type B receptor (ETBR; right). Cutaneous microvascular conductance (CVC; expressed as %maximal dilation) was measured in young women (YW) and postmenopausal women (PMW) during local heating with microdialysis perfusions of lactated Ringer solution (control; gray bars) or the ETBR antagonist BQ-788 (pink bars). Data were adapted from Wenner et al. (241). ETBR mediates dilation in YW. However, the age-associated decline in microvascular endothelial function in PMW was abolished with perfusion of the ETBR antagonist BQ-788 (241). *P < 0.05 vs. control; †P < 0.05 vs. young.
Activation of the RAS occurs with aging and likely contributes to the greater prevalence of hypertension in postmenopausal women (120, 121). The balance between ANG II and ANG-(1–7) has gained recent attention as an important pathway for cardiovascular health, and Mas receptor-mediated increases in NO have been proposed to be a new target for improving endothelial function (84, 173). Recent data indicate sex differences in this pathway, showing that the lower pressor response to ANG II in female rats occurs via AT2Rs and is mediated by estrogens (193, 195). However, less is known about aging and menopause on the impact on ANG-(1–7) and Mas receptors. In a surgical model of menopause, constricting responses to ANG II were greater and dilator responses to ANG-(1–7) were blunted in OVX rats (56). Furthermore, ovariectomy resulted in a reduction in Mas receptor expression and ACE2 gene expression in heart tissue of mice (18). Thus, it appears that the abrupt reduction in estrogen exposure with OVX, or a surgical model of menopause, alters the vasodilatory ANG-(1–7)/Mas receptor pathway. However, to our knowledge, there is a dearth of information in humans; whether these findings in animal models translate to humans and specifically to control of endothelial function warrants additional study.
Finally, these age-related declines in endothelial function may also be related to a reduction in ER (or AR) function or sensitivity. Moreau et al. (68) demonstrated that ERα expression is lower in postmenopausal compared with young women. To our knowledge, there have been no parallel studies in men to examine AR expression in relation to changes in endothelial function with age. Given the importance of ERs and ARs on cardiovascular function, we refer the reader to a recent elegant review focusing on these signaling mechanisms (17).
Hormone Therapy Effects on Endothelial Function
Given the potential importance of estradiol on maintaining vascular endothelial health, numerous studies have examined the impact of hormone therapy on endothelial function in postmenopausal women. Menopausal hormone therapy (MHT), but particularly E2 administration, improves endothelial function in postmenopausal women (91, 152, 234). However, it seems as though the magnitude of improvement depends on the timing of when MHT was initiated. In women who were <5 yr postmenopausal, both acute (sublingual dose) and chronic (3 mo, oral) estrogen administration improved FMD more compared with those women who were >5 yr postmenopausal (235). Changes in ER expression or eNOS expression, as highlighted above, may explain, in part, the reason for this discrepancy. Until recently, the timing of MHT initiation had not been a focus of understanding the vascular consequences of menopause and the subsequent importance of MHT. As such, the data reviewed here do not separate postmenopausal women on the basis of time since menopause but demonstrate the influence of estradiol on the mechanisms regulating endothelial function.
Several lines of evidence in cell and animal models demonstrate that ovarian hormones, particularly E2, modulate ET-1. As highlighted above, E2 lowers ET-1 expression (98), attenuates ET-1-mediated vasoconstriction (223), and increases ETBR mRNA expression in coronary vessels (172). In humans, intracoronary infusions of E2 decrease plasma levels of ET-1 in the coronary vasculature of postmenopausal women (239). Furthermore, administration of hormone therapy to postmenopausal women reduces plasma ET-1 concentrations (14, 247). Although recent data indicate that endogenous fluctuations in ovarian hormones modulate ET-1 receptor function in young women (202), additional data are needed to understand the impact of MHT on ET-1 receptor function in postmenopausal women.
Oxidative stress is a key feature of vascular aging in both men and women, contributing to endothelial dysfunction (150, 152, 176). Given the antioxidant capacity of E2, it may not be surprising that improvements in endothelial function after E2 administration are due, in part, to reductions in oxidative stress. Women who underwent OVX had reduced vasodilatory responses to acetylcholine, which was restored after infusion of vitamin C (234). However, 3 mo of estrogen replacement therapy in these women also restored endothelial function; since vitamin C infusion no longer increased vasodilation in women receiving E2, the authors concluded that E2 improves endothelial function by reducing oxidative stress (234). Subsequent studies have also shown that vitamin C infusion increases FMD in estrogen-deficient postmenopausal women (152). Interestingly, aerobic exercise, a common lifestyle modification to improve cardiovascular health, increases FMD in older men but not in postmenopausal women, unless they are receiving E2 therapy (152). One potential mechanism may be related to oxidative stress, as exercise-trained postmenopausal women receiving E2 had no further increase in FMD during vitamin C infusion (152). Taken together, estrogen deficiency elicits oxidative stress and results in endothelial dysfunction, whereas estrogen replacement appears to mitigate oxidative stress to restore endothelial function.
Hormone therapy for postmenopausal women (particularly oral) has been shown to increase angiotensinogen (201) and plasma renin activity (81), whereas other studies have shown a reduction in ACE activity (161). However, systemic changes in the RAS do not necessarily reflect what is happening at the tissue/cellular level. As stated above, E2 treatment increased ANG-(1–7) in human umbilical vein endothelial cells (148), and blockade of the Mas receptor in isolated vessels attenuated the vasodilatory effects of estradiol (209). However, E2 administration to OVX rats attenuated cardiac ANG-(1–7) and Mas receptor expression (237). It is worth noting, however, that these studies were performed in young animals; as mentioned above, it is less clear how aging impacts ANG-(1–7)/Mas receptors and the impact of hormone therapy on this system. Therefore, additional data are needed in both animals and humans to understand the impact of hormone therapy, for both women and men, on an aging vascular system.
Testosterone Therapy
Testosterone falls with advancing age in men, and low circulating testosterone in older men is associated with atherosclerosis compared with age-matched controls with circulating testosterone concentrations similar to those of younger men (8, 188). In low-testosterone aging rat models, androgen administration can induce compensatory downregulation of androgen synthesis and protection against CVD, dependending on dose and timing of administration (181). In humans, testosterone replacement to levels of younger men restores endothelial function (145), although with little impact on markers for atherosclerosis (11). Interestingly, plasma ET-1 levels are higher in hypogonadal men, and testosterone treatment tends to decrease these levels (113). In contrast to these studies, blood pressure was reduced in castrated male rats but increased with testosterone administration (182); these blood pressure responses are mediated by ARs (183). In humans, androgen administration blunts endothelium-mediated vasodilation in hypogonadal men (12), increases peripheral vascular disease and hypertension (233), and impairs vascular reactivity (233). Similarly, testosterone administration does not enhance endothelium-mediated cutaneous microvascular vasodilation during local warming of the skin in older healthy men (210). Given the conflicting findings in the literature, additional studies are warranted to further understand the mechanisms underlying changes in testosterone and endothelial function.
Testosterone therapy has also become more common for use in postmenopausal women (although in lower doses compared with men), particularly to improve libido. As in men, the literature is conflicting regarding the impact of testosterone therapy on cardiovascular health for postmenopausal women. Some studies have demonstrated a positive correlation between testosterone and CVD risk factors in postmenopausal women (73, 114, 132, 170). Additional studies have reported detrimental effects of testosterone on lipid profiles (46, 114, 153) and atherosclerosis, particularly in carotid arteries of postmenopausal women (74, 175, 248). However, others have demonstrated that endogenous testosterone levels were positively correlated with FMD in postmenopausal women, such that those women with the lowest testosterone had the lowest FMD (149). It is important, however, to consider the ratio of testosterone to E2. Testosterone combined with E2 therapy attenuated the development of foam cells and injury to the endothelium as well as reduced inflammation in cell and animal models (39). Similar findings were also shown in humans. In postmenopausal women currently using MHT, the addition of testosterone increased FMD (250). Although there are conflicting reports in the literature with regard to testosterone therapy in postmenopausal women and little is known about the underlying mechanisms, it appears that an appropriate androgen-to-estrogen ratio is an important consideration for vascular health.
SUMMARY AND FUTURE DIRECTIONS
In summary, a complex interplay between circulating sex steroid hormones, their receptors, and sex steroid-independent mechanisms contributes to the overall difference in endothelial function observed between healthy young men and women. The generalized sum of these differences suggests that young men have greater vasoconstrictor tone and attenuated endothelium-dependent dilation compared with premenopausal women, and it is this subclinical reduction in endothelial function that likely contributes to the earlier rise in the incidence of CVD in men. However, aging and the concomitant reduction in circulating sex hormones complicates the delineation of sex differences in older adults, and women may lose cardioprotection compared with age-matched men after menopause. Not all of the mechanistic sex differences that are present in young adults persist into older age, and new differences emerge in this unique period. Collectively, while a great deal of mechanistic work has described the role of sex hormones and other sex differences in endothelial function across the lifespan, further study of these mechanisms is warranted.
One mechanism that has not been fully explored in humans in the context of sex, sex hormones, and relative changes in sex hormones with aging is prostanoid-dependent vascular responses. In particular, the balance between dilating and constricting prostanoids has been demonstrated to shift in numerous CVD states, and the influence of sex on relative protection from or contribution to these shifts has not been examined. OVX rats demonstrate a greater cyclooxygenase-dependent dilation to the endothelial agonist 2-thiazolylethylamine compared with intact or OVX + E2-supplemented rats (29). On the constrictor side, studies in GPER-1 knockout animals have shown no effect of GPER-1 deficiency on NO bioavailability but did show an increased VSMC response to endothelium-derived vasoconstrictor prostanoids, suggesting that E2 signaling via GPER-1 may also contribute to VSMC contraction to cyclooxygenase-derived prostanoid vasoconstrictors (139). Furthermore, E2 administration to OVX rats attenuated prostanoid-dependent vasoconstriction (5, 43). In humans, prostacyclin-mediated dilation is reduced with aging (158) and menopause (162), but the balance between vasodilator and vasoconstrictor prostanoids is not altered with menopause (162). Additional studies are needed to fully characterize the impact of prostanoids on age- and sex-related declines in endothelial function.
Interestingly, there is sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats, such that female animals have greater cyclooxygenase-2 expression and produce more prostaglandin and thromboxane in the renal medulla (219). Moreover, prostaglandin production is sensitive to physiological changes in testosterone but not estrogen in this model (219). However, although selective cyclooxygenase-2 inhibition reduces prostanoid production in female spontaneously hypertensive rats, there is no blood pressure lowing effect of this treatment in either male or female rats, suggesting that cyclooxygenase-2 is not a likely contributor to blood pressure control in either sex (55). Prostanoid production is downstream of many vascular signaling mechanisms that are affected by sex and aging (e.g., ANG II and ET-1), further obscuring distinct effects of sex hormones on prostanoid signaling. While this mechanism has been addressed in animal models, additional research is required in humans to determine the interaction between sex hormones and sex differences and the prostanoid system in endothelial function in humans. Thus, the relative contributions of sex hormone effects on prostanoid-dependent vascular responses, as well as their implications for sex differences in long-term CVD outcomes, is an area that requires future research attention.
In addition to understanding sex differences in the control and maintenance of endothelial function and cardiovascular health, additional study focused on harnessing these differences should aim to target sex-specific mechanisms for the prevention or treatment of CVD. As described above, men and women may respond differently to different cardiovascular medications, and these epidemiological results suggest that targeting or leveraging sex-specific mechanisms may improve patient outcomes across a wide variety of CVDs when the patient’s sex is considered during treatment. Similarly, hormone replacement therapy for aging men and women may mitigate the progression of CVD and/or prevent some of the age-related changes in sex-dependent mechanisms. However, further study, particularly related to timing, formulation, and administration of exogenous hormones to these populations, is necessary. Overall, sex differences in the mechanistic control and maintenance of endothelial function play an important role in supporting cardiovascular homeostasis, and identifying sex-specific recommendations for the treatment or prevention of CVD is essential for the mitigation of CVD morbidity and mortality in men and women across the lifespan.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-138133 (to A. E. Stanhewicz) and P20-GM-113125 (Center of Biomedical Research Excellence) as well as American Heart Association Grant 16SDG30700015 (to M. M. Wenner) and NIH Grant HL-135089 (to N. S. Stachenfeld).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E.S., M.M.W., and N.S.S. prepared figures; A.E.S., M.M.W., and N.S.S. drafted manuscript; A.E.S., M.M.W., and N.S.S. edited and revised manuscript; A.E.S., M.M.W., and N.S.S. approved final version of manuscript.
REFERENCES
- 1.Adler TE, Usselman CW, Takamata A, Stachenfeld NS. Blood pressure predicts endothelial function and the effects of ethinyl estradiol exposure in young women. Am J Physiol Heart Circ Physiol 315: H925–H933, 2018. doi: 10.1152/ajpheart.00188.2018. [DOI] [PubMed] [Google Scholar]
- 2.Ahnstedt H, Cao L, Krause DN, Warfvinge K, Säveland H, Nilsson OG, Edvinsson L. Male-female differences in upregulation of vasoconstrictor responses in human cerebral arteries. PLoS One 8: e62698, 2013. doi: 10.1371/journal.pone.0062698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res 84: 813–819, 1999. doi: 10.1161/01.RES.84.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol 279: F841–F850, 2000. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong SJ, Zhang Y, Stewart KG, Davidge ST. Estrogen replacement reduces PGHS-2-dependent vasoconstriction in the aged rat. Am J Physiol Heart Circ Physiol 283: H893–H898, 2002. doi: 10.1152/ajpheart.00148.2002. [DOI] [PubMed] [Google Scholar]
- 7.Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol 311: R1271–R1275, 2016. doi: 10.1152/ajpregu.00288.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard KD, Kupchak BR, Volk BM, Mah E, Shkreta A, Liptak C, Ptolemy AS, Kellogg MS, Bruno RS, Seip RL, Maresh CM, Kraemer WJ, Volek JS. Acute effects of ingestion of a novel whey-derived extract on vascular endothelial function in overweight, middle-aged men and women. Br J Nutr 109: 882–893, 2013. doi: 10.1017/S0007114512002061. [DOI] [PubMed] [Google Scholar]
- 9.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem 279: 14579–14586, 2004. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 10.Barton M. Not lost in translation: emerging clinical importance of the G protein-coupled estrogen receptor GPER. Steroids 111: 37–45, 2016. doi: 10.1016/j.steroids.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, Pencina KM, Vita J, Dzekov C, Mazer NA, Coviello AD, Knapp PE, Hally K, Pinjic E, Yan M, Storer TW, Bhasin S. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: A randomized clinical trial. JAMA 314: 570–581, 2015. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 12.Bernini G, Versari D, Moretti A, Virdis A, Ghiadoni L, Bardini M, Taurino C, Canale D, Taddei S, Salvetti A. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab 91: 1691–1697, 2006. doi: 10.1210/jc.2005-1398. [DOI] [PubMed] [Google Scholar]
- 13.Bernini GP, Sgro’ M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A. Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab 84: 2008–2012, 1999. doi: 10.1210/jcem.84.6.5824. [DOI] [PubMed] [Google Scholar]
- 14.Best PJ, Berger PB, Miller VM, Lerman A. The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-1 levels in postmenopausal women. Ann Intern Med 128: 285–288, 1998. doi: 10.7326/0003-4819-128-4-199802150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Bilsel AS, Moini H, Tetik E, Aksungar F, Kaynak B, Ozer A. 17Beta-estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc Res 46: 579–584, 2000. doi: 10.1016/S0008-6363(00)00046-8. [DOI] [PubMed] [Google Scholar]
- 16.Blumenthal RS, Heldman AW, Brinker JA, Resar JR, Coombs VJ, Gloth ST, Gerstenblith G, Reis SE. Acute effects of conjugated estrogens on coronary blood flow response to acetylcholine in men. Am J Cardiol 80: 1021–1024, 1997. doi: 10.1016/S0002-9149(97)00596-1. [DOI] [PubMed] [Google Scholar]
- 17.Boese AC, Kim SC, Yin KJ, Lee JP, Hamblin MH. Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am J Physiol Heart Circ Physiol 313: H524–H545, 2017. doi: 10.1152/ajpheart.00217.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borges CC, Penna-de-Carvalho A, Medeiros Junior JL, Aguila MB, Mandarim-de-Lacerda CA. Ovariectomy modify local renin-angiotensin-aldosterone system gene expressions in the heart of ApoE(−/−) mice. Life Sci 191: 1–8, 2017. doi: 10.1016/j.lfs.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Bourghardt J, Wilhelmson ASK, Alexanderson C, De Gendt K, Verhoeven G, Krettek A, Ohlsson C, Tivesten A. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology 151: 5428–5437, 2010. doi: 10.1210/en.2010-0663. [DOI] [PubMed] [Google Scholar]
- 20.Bowyer L, Brown MA, Jones M. Vascular reactivity in men and women of reproductive age. Am J Obstet Gynecol 185: 88–96, 2001. doi: 10.1067/mob.2001.114502. [DOI] [PubMed] [Google Scholar]
- 21.Briant LJ, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol 101: 219–229, 2016. doi: 10.1113/EP085368. [DOI] [PubMed] [Google Scholar]
- 22.Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol Regul Integr Comp Physiol 273: R1908–R1915, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, Bornfleth P, Kutschka I, Gardemann A, Isermann B, Goette A. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med (Maywood) 242: 1412–1423, 2017. doi: 10.1177/1535370217718808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buonassisi V, Venter JC. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci USA 73: 1612–1616, 1976. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab 85: 2832–2838, 2000. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 26.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84: 4025–4030, 1999. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 27.Calderon-Margalit R, Schwartz SM, Wellons MF, Lewis CE, Daviglus ML, Schreiner PJ, Williams OD, Sternfeld B, Carr JJ, O’Leary DH, Sidney S, Friedlander Y, Siscovick DS. Prospective association of serum androgens and sex hormone-binding globulin with subclinical cardiovascular disease in young adult women: the “Coronary Artery Risk Development in Young Adults” women’s study. J Clin Endocrinol Metab 95: 4424–4431, 2010. doi: 10.1210/jc.2009-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderon-Margalit R, Siscovick D, Merkin SS, Wang E, Daviglus ML, Schreiner PJ, Sternfeld B, Williams OD, Lewis CE, Azziz R, Schwartz SM, Wellons MF. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-media thickness: the Coronary Artery Risk Development in Young Adults Women’s study. Arterioscler Thromb Vasc Biol 34: 2688–2694, 2014. doi: 10.1161/ATVBAHA.114.304136. [DOI] [PubMed] [Google Scholar]
- 29.Case J, Davison CA. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J Pharmacol Exp Ther 291: 524–530, 1999. [PubMed] [Google Scholar]
- 30.Caulin-Glaser T, García-Cardeña G, Sarrel P, Sessa WC, Bender JR. 17beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res 81: 885–892, 1997. doi: 10.1161/01.RES.81.5.885. [DOI] [PubMed] [Google Scholar]
- 31.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456–463, 1992. doi: 10.1161/01.HYP.19.5.456. [DOI] [PubMed] [Google Scholar]
- 33.Chinnathambi V, More AS, Hankins GD, Yallampalli C, Sathishkumar K. Gestational exposure to elevated testosterone levels induces hypertension via heightened vascular angiotensin II type 1 receptor signaling in rats. Biol Reprod 91: 6, 2014. doi: 10.1095/biolreprod.114.118968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 94: 2614–2619, 1996. doi: 10.1161/01.CIR.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 35.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev 97: 995–1043, 2017. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costarella CE, Stallone JN, Rutecki GW, Whittier FC. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther 277: 34–39, 1996. [PubMed] [Google Scholar]
- 37.Creatsa M, Armeni E, Stamatelopoulos K, Rizos D, Georgiopoulos G, Kazani M, Alexandrou A, Dendrinos S, Augoulea A, Papamichael C, Lambrinoudaki I. Circulating androgen levels are associated with subclinical atherosclerosis and arterial stiffness in healthy recently menopausal women. Metabolism 61: 193–201, 2012. doi: 10.1016/j.metabol.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Creatsas GC, Malamitsi-Puchner AB, Hassan EA, Economou EB, Aravantinos DI. Endothelin plasma levels in primary amenorrheic adolescents before and after estrogen treatment. J Soc Gynecol Investig 3: 350–353, 1996. [PubMed] [Google Scholar]
- 39.Dai W, Ming W, Li Y, Zheng HY, Wei CD, Rui Z, Yan C. Synergistic effect of a physiological ratio of estradiol and testosterone in the treatment of early-stage atherosclerosis. Arch Med Res 46: 619–629, 2015. doi: 10.1016/j.arcmed.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Dai-Do D, Espinosa E, Liu G, Rabelink TJ, Julmy F, Yang Z, Mahler F, Lüscher TF. 17Beta-estradiol inhibits proliferation and migration of human vascular smooth muscle cells: similar effects in cells from postmenopausal females and in males. Cardiovasc Res 32: 980–985, 1996. [PubMed] [Google Scholar]
- 41.David FL, Carvalho MH, Cobra AL, Nigro D, Fortes ZB, Rebouças NA, Tostes RC. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension 38: 692–696, 2001. doi: 10.1161/01.HYP.38.3.692. [DOI] [PubMed] [Google Scholar]
- 42.David FL, Montezano AC, Rebouças NA, Nigro D, Fortes ZB, Carvalho MH, Tostes RC. Gender differences in vascular expression of endothelin and ETA/ETB receptors, but not in calcium handling mechanisms, in deoxycorticosterone acetate-salt hypertension. Braz J Med Biol Res 35: 1061–1068, 2002. doi: 10.1590/S0100-879X2002000900006. [DOI] [PubMed] [Google Scholar]
- 43.Davidge ST, Zhang Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ Res 83: 388–395, 1998. doi: 10.1161/01.RES.83.4.388. [DOI] [PubMed] [Google Scholar]
- 44.Davis GC, Gibson KJ, Casley D, Brown MA. Angiotensin II/angiotensin (1–7) ratio and 24-h blood pressure throughout the menstrual cycle and in women using oral contraceptives. J Hypertens 35: 1178–1186, 2017. doi: 10.1097/HJH.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 45.Dean SA, Tan J, O’Brien ER, Leenen FH. 17Beta-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol 288: R759–R766, 2005. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- 46.Debing E, Peeters E, Duquet W, Poppe K, Velkeniers B, Van den Brande P. Endogenous sex hormone levels in postmenopausal women undergoing carotid artery endarterectomy. Eur J Endocrinol 156: 687–693, 2007. doi: 10.1530/EJE-06-0702. [DOI] [PubMed] [Google Scholar]
- 47.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 297: H1806–H1813, 2009. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med 10: e3, 2008. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- 49.Diamanti-Kandarakis E, Alexandraki K, Piperi C, Protogerou A, Katsikis I, Paterakis T, Lekakis J, Panidis D. Inflammatory and endothelial markers in women with polycystic ovary syndrome. Eur J Clin Invest 36: 691–697, 2006. doi: 10.1111/j.1365-2362.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 50.Diamanti-Kandarakis E, Spina G, Kouli C, Migdalis I. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab 86: 4666–4673, 2001. doi: 10.1210/jcem.86.10.7904. [DOI] [PubMed] [Google Scholar]
- 51.Dixit KCS, Wu J, Smith LB, Hadoke PWF, Wu FCW. Androgens and coronary artery disease. In: Endotext, edited by De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A. South Dartmouth, MA: MDText.com, 2000. [Google Scholar]
- 52.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dowman JK, Hopkins LJ, Reynolds GM, Armstrong MJ, Nasiri M, Nikolaou N, van Houten EL, Visser JA, Morgan SA, Lavery GG, Oprescu A, Hübscher SG, Newsome PN, Tomlinson JW. Loss of 5α-reductase type 1 accelerates the development of hepatic steatosis but protects against hepatocellular carcinoma in male mice. Endocrinology 154: 4536–4547, 2013. doi: 10.1210/en.2013-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest 83: 1941–1945, 1989. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elmarakby AA, Katary M, Pollock JS, Sullivan JC. Influence of the selective COX-2 inhibitor celecoxib on sex differences in blood pressure and albuminuria in spontaneously hypertensive rats. Prostaglandins Other Lipid Mediat 135: 16–20, 2018. doi: 10.1016/j.prostaglandins.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Endlich PW, Claudio ER, Lima LC, Ribeiro Júnior RF, Peluso AA, Stefanon I, Bissoli NS, Lemos VS, Santos RA, Abreu GR. Exercise modulates the aortic renin-angiotensin system independently of estrogen therapy in ovariectomized hypertensive rats. Peptides 87: 41–49, 2017. doi: 10.1016/j.peptides.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 57.English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J 21: 890–894, 2000. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 58.Ergul A, Shoemaker K, Puett D, Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther 285: 511–517, 1998. [PubMed] [Google Scholar]
- 59.Fagman JB, Wilhelmson AS, Motta BM, Pirazzi C, Alexanderson C, De Gendt K, Verhoeven G, Holmäng A, Anesten F, Jansson J-O, Levin M, Borén J, Ohlsson C, Krettek A, Romeo S, Tivesten Å. The androgen receptor confers protection against diet-induced atherosclerosis, obesity, and dyslipidemia in female mice. FASEB J 29: 1540–1550, 2015. doi: 10.1096/fj.14-259234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res 33: 124–131, 1996. doi: 10.1159/000159140. [DOI] [PubMed] [Google Scholar]
- 61.Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14: 1649–1660, 2000. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 62.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002. doi: 10.1016/S0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 63.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol 29: 169–181, 2008. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fredette NC, Meyer MR, Prossnitz ER. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J Steroid Biochem Mol Biol 176: 65–72, 2018. doi: 10.1016/j.jsbmb.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freshour JR, Chase SE, Vikstrom KL. Gender differences in cardiac ACE expression are normalized in androgen-deprived male mice. Am J Physiol Heart Circ Physiol 283: H1997–H2003, 2002. doi: 10.1152/ajpheart.01054.2001. [DOI] [PubMed] [Google Scholar]
- 66.Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 33: 323–328, 1999. doi: 10.1161/01.HYP.33.1.323. [DOI] [PubMed] [Google Scholar]
- 67.Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension 31: 90–96, 1998. doi: 10.1161/01.HYP.31.1.90. [DOI] [PubMed] [Google Scholar]
- 68.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94: 3513–3520, 2009. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gohar EY, Giachini FR, Pollock DM, Tostes RC. Role of the endothelin system in sexual dimorphism in cardiovascular and renal diseases. Life Sci 159: 20–29, 2016. doi: 10.1016/j.lfs.2016.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grohé C, Kahlert S, Löbbert K, Vetter H. Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J Endocrinol 156: R1–R7, 1998. doi: 10.1677/joe.0.156R001. [DOI] [PubMed] [Google Scholar]
- 71.Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, Karounos M, Cassis LA. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 32: 1392–1399, 2012. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 49: 1358–1363, 2007. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 73.Haffner SM, Newcomb PA, Marcus PM, Klein BE, Klein R. Relation of sex hormones and dehydroepiandrosterone sulfate (DHEA-SO4) to cardiovascular risk factors in postmenopausal women. Am J Epidemiol 142: 925–934, 1995. doi: 10.1093/oxfordjournals.aje.a117740. [DOI] [PubMed] [Google Scholar]
- 74.Hak AE, Westendorp IC, Pols HA, Hofman A, Witteman JC. High-dose testosterone is associated with atherosclerosis in postmenopausal women. Maturitas 56: 153–160, 2007. doi: 10.1016/j.maturitas.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res 84: 1285–1291, 1999. doi: 10.1161/01.RES.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 76.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW + 10 Collaborative Group . Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 97: 1159–1168, 2012. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590: 2069–2079, 2012. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92: 3431–3435, 1995. doi: 10.1161/01.CIR.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 81.Hassager C, Riis BJ, Strøm V, Guyene TT, Christiansen C. The long-term effect of oral and percutaneous estradiol on plasma renin substrate and blood pressure. Circulation 76: 753–758, 1987. doi: 10.1161/01.CIR.76.4.753. [DOI] [PubMed] [Google Scholar]
- 82.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87: 677–682, 2000. doi: 10.1161/01.RES.87.8.677. [DOI] [PubMed] [Google Scholar]
- 83.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol 16: 2181–2187, 2002. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 84.Hilliard LM, Mirabito KM, Denton KM. Unmasking the potential of the angiotensin AT2 receptor as a therapeutic target in hypertension in men and women: what we know and what we still need to find out. Clin Exp Pharmacol Physiol 40: 542–550, 2013. doi: 10.1111/1440-1681.12067. [DOI] [PubMed] [Google Scholar]
- 85.Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett 360: 291–293, 1995. doi: 10.1016/0014-5793(95)00124-R. [DOI] [PubMed] [Google Scholar]
- 86.Hogg ME, Vavra AK, Banerjee MN, Martinez J, Jiang Q, Keefer LK, Chambon P, Kibbe MR. The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res 173: e1–e10, 2012. doi: 10.1016/j.jss.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holmboe SA, Vradi E, Jensen TK, Linneberg A, Husemoen LLN, Scheike T, Skakkebæk NE, Juul A, Andersson A-M. The association of reproductive hormone levels and all-cause, cancer, and cardiovascular disease mortality in men. J Clin Endocrinol Metab 100: 4472–4480, 2015. doi: 10.1210/jc.2015-2460. [DOI] [PubMed] [Google Scholar]
- 88.Hong HJ, Chan P, Liu JC, Juan SH, Huang MT, Lin JG, Cheng TH. Angiotensin II induces endothelin-1 gene expression via extracellular signal-regulated kinase pathway in rat aortic smooth muscle cells. Cardiovasc Res 61: 159–168, 2004. doi: 10.1016/j.cardiores.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 89.Huang CK, Lee SO, Chang E, Pang H, Chang C. Androgen receptor (AR) in cardiovascular diseases. J Endocrinol 229: R1–R16, 2016. doi: 10.1530/JOE-15-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hudson M, Rahme E, Behlouli H, Sheppard R, Pilote L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure—a population study. Eur J Heart Fail 9: 602–609, 2007. doi: 10.1016/j.ejheart.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Hurtado R, Celani M, Geber S. Effect of short-term estrogen therapy on endothelial function: a double-blinded, randomized, controlled trial. Climacteric 19: 448–451, 2016. doi: 10.1080/13697137.2016.1201809. [DOI] [PubMed] [Google Scholar]
- 92.Hutchison SJ, Sudhir K, Chou TM, Sievers RE, Zhu BQ, Sun YP, Deedwania PC, Glantz SA, Parmley WW, Chatterjee K. Testosterone worsens endothelial dysfunction associated with hypercholesterolemia and environmental tobacco smoke exposure in male rabbit aorta. J Am Coll Cardiol 29: 800–807, 1997. doi: 10.1016/S0735-1097(96)00570-0. [DOI] [PubMed] [Google Scholar]
- 93.Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res 91: 814–820, 2002. doi: 10.1161/01.RES.0000038304.62046.4C. [DOI] [PubMed] [Google Scholar]
- 94.Intapad S, Ojeda NB, Varney E, Royals TP, Alexander BT. Sex-specific effect of endothelin in the blood pressure response to acute angiotensin II in growth-restricted rats. Hypertension 66: 1260–1266, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jazbutyte V, Stumpner J, Redel A, Lorenzen JM, Roewer N, Thum T, Kehl F. Aromatase inhibition attenuates desflurane-induced preconditioning against acute myocardial infarction in male mouse heart in vivo. PLoS One 7: e42032, 2012. doi: 10.1371/journal.pone.0042032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jesmin S, Mowa CN, Sultana SN, Shimojo N, Togashi H, Iwashima Y, Kato N, Sato A, Sakuma I, Hiroe M, Hattori Y, Yamaguchi N, Kobayashi H. VEGF signaling is disrupted in the hearts of mice lacking estrogen receptor alpha. Eur J Pharmacol 641: 168–178, 2010. doi: 10.1016/j.ejphar.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 97.Jones RD, Pugh PJ, Jones TH, Channer KS. The vasodilatory action of testosterone: a potassium-channel opening or a calcium antagonistic action? Br J Pharmacol 138: 733–744, 2003. doi: 10.1038/sj.bjp.0705141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Juan SH, Chen JJ, Chen CH, Lin H, Cheng CF, Liu JC, Hsieh MH, Chen YL, Chao HH, Chen TH, Chan P, Cheng TH. 17β-Estradiol inhibits cyclic strain-induced endothelin-1 gene expression within vascular endothelial cells. Am J Physiol Heart Circ Physiol 287: H1254–H1261, 2004. doi: 10.1152/ajpheart.00723.2003. [DOI] [PubMed] [Google Scholar]
- 99.Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, Muller-Delp JM. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol 300: H2105–H2115, 2011. doi: 10.1152/ajpheart.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kauser K, Rubanyi GM. Potential cellular signaling mechanisms mediating upregulation of endothelial nitric oxide production by estrogen. J Vasc Res 34: 229–236, 1997. doi: 10.1159/000159227. [DOI] [PubMed] [Google Scholar]
- 101.Kawanishi H, Hasegawa Y, Nakano D, Ohkita M, Takaoka M, Ohno Y, Matsumura Y. Involvement of the endothelin ETB receptor in gender differences in deoxycorticosterone acetate-salt-induced hypertension. Clin Exp Pharmacol Physiol 34: 280–285, 2007. doi: 10.1111/j.1440-1681.2007.04580.x. [DOI] [PubMed] [Google Scholar]
- 102.Kellogg DL Jr, Liu Y, Pérgola PE. Selected contribution: gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol 91: 2407–2411, 2001. doi: 10.1152/jappl.2001.91.5.2407. [DOI] [PubMed] [Google Scholar]
- 103.Kim KH, Bender JR. Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE 2005: pe28, 2005. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- 104.Kim SC, Boese AC, Moore MH, Cleland RM, Chang L, Delafontaine P, Yin KJ, Lee JP, Hamblin MH. Rapid estrogen receptor-α signaling mediated by ERK activation regulates vascular tone in male and ovary-intact female mice. Am J Physiol Heart Circ Physiol 314: H330–H342, 2018. doi: 10.1152/ajpheart.00841.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kimura M, Sudhir K, Jones M, Simpson E, Jefferis A-M, Chin-Dusting JPF. Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice: regulation of nitric oxide production by endogenous sex hormones in males. Circ Res 93: 1267–1271, 2003. doi: 10.1161/01.RES.0000103172.98986.25. [DOI] [PubMed] [Google Scholar]
- 106.Kiprono LV, Wallace K, Moseley J, Martin J Jr, Lamarca B. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. Am J Obstet Gynecol 209: 44.e1–44.e6, 2013. doi: 10.1016/j.ajog.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kische H, Gross S, Wallaschofski H, Völzke H, Dörr M, Nauck M, Felix SB, Haring R. Serum androgen concentrations and subclinical measures of cardiovascular disease in men and women. Atherosclerosis 247: 193–200, 2016. doi: 10.1016/j.atherosclerosis.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 108.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ETB receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol 40: 362–370, 2013. doi: 10.1111/1440-1681.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kittikulsuth W, Pollock JS, Pollock DM. Loss of renal medullary endothelin B receptor function during salt deprivation is regulated by angiotensin II. Am J Physiol Renal Physiol 303: F659–F666, 2012. doi: 10.1152/ajprenal.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. Br J Pharmacol 168: 318–326, 2013. doi: 10.1111/j.1476-5381.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J Hypertens 28: 1422–1428, 2010. doi: 10.1097/HJH.0b013e3283392673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V; Bosentan Hypertension Investigators . The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med 338: 784–791, 1998. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 113.Kumanov P, Tomova A, Kirilov G. Testosterone replacement therapy in male hypogonadism is not associated with increase of endothelin-1 levels. Int J Androl 30: 41–47, 2007. doi: 10.1111/j.1365-2605.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 114.Lambrinoudaki I, Christodoulakos G, Rizos D, Economou E, Argeitis J, Vlachou S, Creatsa M, Kouskouni E, Botsis D. Endogenous sex hormones and risk factors for atherosclerosis in healthy Greek postmenopausal women. Eur J Endocrinol 154: 907–916, 2006. doi: 10.1530/eje.1.02167. [DOI] [PubMed] [Google Scholar]
- 115.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93: 68–75, 2008. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85: 645–651, 2000. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 117.LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 297: R1713–R1723, 2009. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res 93: 1127–1133, 2003. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- 119.Li L, Hisamoto K, Kim KH, Haynes MP, Bauer PM, Sanjay A, Collinge M, Baron R, Sessa WC, Bender JR. Variant estrogen receptor-c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation. Proc Natl Acad Sci USA 104: 16468–16473, 2007. doi: 10.1073/pnas.0704315104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep 14: 254–260, 2012. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lima R, Yanes LL, Davis DD, Reckelhoff JF. Roles played by 20-HETE, angiotensin II, and endothelin in mediating the hypertension in aging female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 304: R248–R251, 2013. doi: 10.1152/ajpregu.00380.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin YJ, Kwok CF, Juan CC, Hsu YP, Shih KC, Chen CC, Ho LT. Angiotensin II enhances endothelin-1-induced vasoconstriction through upregulating endothelin type A receptor. Biochem Biophys Res Commun 451: 263–269, 2014. doi: 10.1016/j.bbrc.2014.07.119. [DOI] [PubMed] [Google Scholar]
- 123.Liu PY, Christian RC, Ruan M, Miller VM, Fitzpatrick LA. Correlating androgen and estrogen steroid receptor expression with coronary calcification and atherosclerosis in men without known coronary artery disease. J Clin Endocrinol Metab 90: 1041–1046, 2005. doi: 10.1210/jc.2004-1211. [DOI] [PubMed] [Google Scholar]
- 124.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 24: 313–340, 2003. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 125.Lopes RAM, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, Chignalia AZ, Valim YM, Silveira LR, Curti C, Tostes RC. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am J Physiol Heart Circ Physiol 306: H1485–H1494, 2014. doi: 10.1152/ajpheart.00809.2013. [DOI] [PubMed] [Google Scholar]
- 126.Luo C, Pook E, Tang B, Zhang W, Li S, Leineweber K, Cheung SH, Chen Q, Bechem M, Hu JS, Laux V, Wang QK. Androgen inhibits key atherosclerotic processes by directly activating ADTRP transcription. Biochim Biophys Acta Mol Basis Dis 1863: 2319–2332, 2017. doi: 10.1016/j.bbadis.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lygnos MC, Pappa KI, Papadaki HA, Relakis C, Koumantakis E, Anagnou NP, Eliopoulos GD. Changes in maternal plasma levels of VEGF, bFGF, TGF-beta1, ET-1 and sKL during uncomplicated pregnancy, hypertensive pregnancy and gestational diabetes. In Vivo 20: 157–163, 2006. [PubMed] [Google Scholar]
- 128.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res 81: 355–362, 1997. doi: 10.1161/01.RES.81.3.355. [DOI] [PubMed] [Google Scholar]
- 129.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol 95: 336–341, 2003. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 130.Malik RA, Schofield IJ, Izzard A, Austin C, Bermann G, Heagerty AM. Effects of angiotensin type-1 receptor antagonism on small artery function in patients with type 2 diabetes mellitus. Hypertension 45: 264–269, 2005. doi: 10.1161/01.HYP.0000153305.50128.a1. [DOI] [PubMed] [Google Scholar]
- 131.Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, Davidge-Pitts CJ, Nippoldt TB, Prokop LJ, Murad MH. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab 102: 3914–3923, 2017. doi: 10.1210/jc.2017-01643. [DOI] [PubMed] [Google Scholar]
- 132.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism 57: 961–965, 2008. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 133.Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol 84: 147–162, 2012. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCrohon JA, Jessup W, Handelsman DJ, Celermajer DS. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation 99: 2317–2322, 1999. doi: 10.1161/01.CIR.99.17.2317. [DOI] [PubMed] [Google Scholar]
- 135.McNeill AM, Kim N, Duckles SP, Krause DN. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke 30: 2186–2190, 1999. doi: 10.1161/01.STR.30.10.2186. [DOI] [PubMed] [Google Scholar]
- 136.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol 294: H1630–H1637, 2008. doi: 10.1152/ajpheart.01314.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 138.Mesch VR, Siseles NO, Maidana PN, Boero LE, Sayegh F, Prada M, Royer M, Schreier L, Benencia HJ, Berg GA. Androgens in relationship to cardiovascular risk factors in the menopausal transition. Climacteric 11: 509–517, 2008. doi: 10.1080/13697130802416640. [DOI] [PubMed] [Google Scholar]
- 139.Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, Walker MK, Barton M, Prossnitz ER. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension 59: 507–512, 2012. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol 308: 9–16, 2009. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 60: 210–241, 2008. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miller VM, Mulvagh SL. Sex steroids and endothelial function: translating basic science to clinical practice. Trends Pharmacol Sci 28: 263–270, 2007. doi: 10.1016/j.tips.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 143.Mineo C, Shaul PW. Regulation of eNOS in caveolae. In: Caveolins and Caveolae: Roles in Signaling and Disease Mechanisms, edited by Jasmin JF, Frank PG, Lisanti MP. New York: Springer, 2012, p. 51–62. doi: 10.1007/978-1-4614-1222-9_4. [DOI] [PubMed] [Google Scholar]
- 144.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011. doi: 10.1152/ajpheart.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miner M, Canty DJ, Shabsigh R. Testosterone replacement therapy in hypogonadal men: assessing benefits, risks, and best practices. Postgrad Med 120: 130–153, 2008. doi: 10.3810/pgm.2008.09.1914. [DOI] [PubMed] [Google Scholar]
- 146.Mishra JS, Hankins GD, Kumar S. Testosterone downregulates angiotensin II type-2 receptor via androgen receptor-mediated ERK1/2 MAP kinase pathway in rat aorta. J Renin Angiotensin Aldosterone Syst 17: 17, 2016. doi: 10.1177/1470320316674875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Molinari C, Battaglia A, Grossini E, Mary DA, Surico N, Vacca G. Effect of progesterone on peripheral blood flow in prepubertal female anesthetized pigs. J Vasc Res 38: 569–577, 2001. doi: 10.1159/000051093. [DOI] [PubMed] [Google Scholar]
- 148.Mompeón A, Lázaro-Franco M, Bueno-Betí C, Pérez-Cremades D, Vidal-Gómez X, Monsalve E, Gironacci MM, Hermenegildo C, Novella S. Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1–7 production. Mol Cell Endocrinol 422: 1–8, 2016. doi: 10.1016/j.mce.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 149.Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis 18: 9–13, 2007. doi: 10.1097/01.mca.0000236290.79306.d1. [DOI] [PubMed] [Google Scholar]
- 150.Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol 102: 890–895, 2007. doi: 10.1152/japplphysiol.00877.2006. [DOI] [PubMed] [Google Scholar]
- 151.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mudali S, Dobs AS, Ding J, Cauley JA, Szklo M, Golden SH; atherosclerosis risk in communities study . Endogenous postmenopausal hormones and serum lipids: the atherosclerosis risk in communities study. J Clin Endocrinol Metab 90: 1202–1209, 2005. doi: 10.1210/jc.2004-0744. [DOI] [PubMed] [Google Scholar]
- 154.Mügge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 β-oestradiol in vitro. Cardiovasc Res 27: 1939–1942, 1993. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- 155.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol 284: R1055–R1062, 2003. doi: 10.1152/ajpregu.00459.2002. [DOI] [PubMed] [Google Scholar]
- 156.Nakov R, Pfarr E, Eberle S; HEAT Investigators . Darusentan: an effective endothelinA receptor antagonist for treatment of hypertension. Am J Hypertens 15: 583–589, 2002. doi: 10.1016/S0895-7061(02)02933-3. [DOI] [PubMed] [Google Scholar]
- 157.Nettleship JE, Jones TH, Channer KS, Jones RD. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation 116: 2427–2434, 2007. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- 158.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nickenig G, Bäumer AT, Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Böhm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 97: 2197–2201, 1998. doi: 10.1161/01.CIR.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 160.Nickenig G, Strehlow K, Wassmann S, Bäumer AT, Albory K, Sauer H, Böhm M. Differential effects of estrogen and progesterone on AT1 receptor gene expression in vascular smooth muscle cells. Circulation 102: 1828–1833, 2000. doi: 10.1161/01.CIR.102.15.1828. [DOI] [PubMed] [Google Scholar]
- 161.Nogawa N, Sumino H, Ichikawa S, Kumakura H, Takayama Y, Nakamura T, Kanda T, Mizunuma H, Kurabayashi M. Effect of long-term hormone replacement therapy on angiotensin-converting enzyme activity and bradykinin in postmenopausal women with essential hypertension and normotensive postmenopausal women. Menopause 8: 210–215, 2001. doi: 10.1097/00042192-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 162.Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J, Hellsten Y. Early postmenopausal phase is associated with reduced prostacyclin-induced vasodilation that is reversed by exercise training: the Copenhagen Women Study. Hypertension 68: 1011–1020, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07866. [DOI] [PubMed] [Google Scholar]
- 163.Ojeda NB, Royals TP, Alexander BT. Sex differences in the enhanced responsiveness to acute angiotensin II in growth-restricted rats: role of fasudil, a Rho kinase inhibitor. Am J Physiol Renal Physiol 304: F900–F907, 2013. doi: 10.1152/ajprenal.00687.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 298: R1421–R1427, 2010. doi: 10.1152/ajpregu.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Okano H, Jayachandran M, Yoshikawa A, Miller VM. Differential effects of chronic treatment with estrogen receptor ligands on regulation of nitric oxide synthase in porcine aortic endothelial cells. J Cardiovasc Pharmacol 47: 621–628, 2006. doi: 10.1097/01.fjc.0000211749.24196.98. [DOI] [PubMed] [Google Scholar]
- 166.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 167.Ouyang P, Vaidya D, Dobs A, Golden SH, Szklo M, Heckbert SR, Kopp P, Gapstur SM. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 204: 255–261, 2009. doi: 10.1016/j.atherosclerosis.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Owonikoko TK, Fabucci ME, Brown PR, Nisar N, Hilton J, Mathews WB, Ravert HT, Rauseo P, Sandberg K, Dannals RF, Szabo Z. In vivo investigation of estrogen regulation of adrenal and renal angiotensin (AT1) receptor expression by PET. J Nucl Med 45: 94–100, 2004. [PMC free article] [PubMed] [Google Scholar]
- 169.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, Baron AD. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 103: 1410–1415, 2001. doi: 10.1161/01.CIR.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 170.Patel SM, Ratcliffe SJ, Reilly MP, Weinstein R, Bhasin S, Blackman MR, Cauley JA, Sutton-Tyrrell K, Robbins J, Fried LP, Cappola AR. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 94: 4776–4784, 2009. doi: 10.1210/jc.2009-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pearson LJ, Yandle TG, Nicholls MG, Evans JJ. Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides 29: 1057–1061, 2008. doi: 10.1016/j.peptides.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 172.Pedersen SH, Nielsen LB, Mortensen A, Nilas L, Ottesen B. Progestins oppose the effects of estradiol on the endothelin-1 receptor type B in coronary arteries from ovariectomized hyperlipidemic rabbits. Menopause 15: 503–510, 2008. doi: 10.1097/gme.0b013e318156f803. [DOI] [PubMed] [Google Scholar]
- 173.Peiró C, Vallejo S, Gembardt F, Azcutia V, Heringer-Walther S, Rodríguez-Mañas L, Schultheiss HP, Sánchez-Ferrer CF, Walther T. Endothelial dysfunction through genetic deletion or inhibition of the G protein-coupled receptor Mas: a new target to improve endothelial function. J Hypertens 25: 2421–2425, 2007. doi: 10.1097/HJH.0b013e3282f0143c. [DOI] [PubMed] [Google Scholar]
- 174.Phillips GB. Is atherosclerotic cardiovascular disease an endocrinological disorder? The estrogen-androgen paradox. J Clin Endocrinol Metab 90: 2708–2711, 2005. doi: 10.1210/jc.2004-2011. [DOI] [PubMed] [Google Scholar]
- 175.Phillips GB, Pinkernell BH, Jing TY. Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol 17: 695–701, 1997. doi: 10.1161/01.ATV.17.4.695. [DOI] [PubMed] [Google Scholar]
- 176.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10: 1032–1037, 2011. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pihlajamaa P, Sahu B, Jänne OA. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev 36: 357–384, 2015. doi: 10.1210/er.2015-1034. [DOI] [PubMed] [Google Scholar]
- 178.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med 118: 429–432, 1993. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 179.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: a G protein-coupled receptor for estrogen. Mol Cell Endocrinol 265-266: 138–142, 2007. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension 43: 918–923, 2004. doi: 10.1161/01.HYP.0000124670.03674.15. [DOI] [PubMed] [Google Scholar]
- 181.Reckelhoff JF, Roman RJ. Androgens and hypertension: role in both males and females? Hypertension 57: 681–682, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998. doi: 10.1161/01.HYP.31.1.435. [DOI] [PubMed] [Google Scholar]
- 183.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension 34: 920–923, 1999. doi: 10.1161/01.HYP.34.4.920. [DOI] [PubMed] [Google Scholar]
- 184.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 185.Reynolds SR, Foster FI. Peripheral vascular action of estrogen in the human male. J Clin Invest 18: 649–655, 1939. doi: 10.1172/JCI101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rodrigo MC, Martin DS, Eyster KM. Vascular ECE-1 mRNA expression decreases in response to estrogens. Life Sci 73: 2973–2983, 2003. doi: 10.1016/j.lfs.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 187.Roesch DM, Tian Y, Zheng W, Shi M, Verbalis JG, Sandberg K. Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats. Endocrinology 141: 4629–4636, 2000. doi: 10.1210/endo.141.12.7822. [DOI] [PubMed] [Google Scholar]
- 188.Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A, Fini M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res 19: 176–182, 2007. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- 189.Rosenthal T, Oparil S. Hypertension in women. J Hum Hypertens 14: 691–704, 2000. doi: 10.1038/sj.jhh.1001095. [DOI] [PubMed] [Google Scholar]
- 190.Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci USA 97: 5930–5935, 2000. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Safari T, Nematbakhsh M, Hilliard LM, Evans RG, Denton KM. Sex differences in the renal vascular response to angiotensin II involves the Mas receptor. Acta Physiol (Oxf) 206: 150–156, 2012. doi: 10.1111/j.1748-1716.2012.02468.x. [DOI] [PubMed] [Google Scholar]
- 192.Salazar M, Lerma-Ortiz A, Hooks GM, Ashley AK, Ashley RL. Progestin-mediated activation of MAPK and AKT in nuclear progesterone receptor negative breast epithelial cells: The role of membrane progesterone receptors. Gene 591: 6–13, 2016. doi: 10.1016/j.gene.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sampson AK, Hilliard LM, Moritz KM, Thomas MC, Tikellis C, Widdop RE, Denton KM. The arterial depressor response to chronic low-dose angiotensin II infusion in female rats is estrogen dependent. Am J Physiol Regul Integr Comp Physiol 302: R159–R165, 2012. doi: 10.1152/ajpregu.00256.2011. [DOI] [PubMed] [Google Scholar]
- 194.Sampson AK, Moritz KM, Denton KM. Postnatal ontogeny of angiotensin receptors and ACE2 in male and female rats. Gend Med 9: 21–32, 2012. doi: 10.1016/j.genm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 195.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 52: 666–671, 2008. doi: 10.1161/HYPERTENSIONAHA.108.114058. [DOI] [PubMed] [Google Scholar]
- 196.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 81: 1495–1501, 1996. [DOI] [PubMed] [Google Scholar]
- 197.Scaling AL, Prossnitz ER, Hathaway HJ. GPER mediates estrogen-induced signaling and proliferation in human breast epithelial cells and normal and malignant breast. Horm Cancer 5: 146–160, 2014. doi: 10.1007/s12672-014-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation 101: 1653–1659, 2000. doi: 10.1161/01.CIR.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 199.Schiffrin EL, Touyz RM. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart Circ Physiol 287: H435–H446, 2004. doi: 10.1152/ajpheart.00262.2004. [DOI] [PubMed] [Google Scholar]
- 200.Schneider MP, Wach PF, Durley MK, Pollock JS, Pollock DM. Sex differences in acute ANG II-mediated hemodynamic responses in mice. Am J Physiol Regul Integr Comp Physiol 299: R899–R906, 2010. doi: 10.1152/ajpregu.00638.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schunkert H, Danser AH, Hense HW, Derkx FH, Kürzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation 95: 39–45, 1997. doi: 10.1161/01.CIR.95.1.39. [DOI] [PubMed] [Google Scholar]
- 202.Sebzda KN, Kuczmarski AV, Pohlig RT, Lennon SL, Edwards DG, Wenner MM. Ovarian hormones modulate endothelin-1 receptor responses in young women. Microcirculation 25: e12490, 2018. doi: 10.1111/micc.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Selles J, Polini N, Alvarez C, Massheimer V. Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation. Life Sci 69: 815–827, 2001. doi: 10.1016/S0024-3205(01)01174-2. [DOI] [PubMed] [Google Scholar]
- 204.Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β-cells. Endocrinology 152: 3030–3039, 2011. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shoback DM, Williams GH, Moore TJ, Dluhy RG, Podolsky S, Hollenberg NK. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest 72: 2115–2124, 1983. doi: 10.1172/JCI111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1–7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol 169: 477–492, 2013. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Simoncini T, Mannella P, Fornari L, Caruso A, Willis MY, Garibaldi S, Baldacci C, Genazzani AR. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology 145: 5745–5756, 2004. doi: 10.1210/en.2004-0510. [DOI] [PubMed] [Google Scholar]
- 208.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50: 123–129, 2007. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 209.Sobrino A, Vallejo S, Novella S, Lázaro-Franco M, Mompeón A, Bueno-Betí C, Walther T, Sánchez-Ferrer C, Peiró C, Hermenegildo C. Mas receptor is involved in the estrogen-receptor induced nitric oxide-dependent vasorelaxation. Biochem Pharmacol 129: 67–72, 2017. doi: 10.1016/j.bcp.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 210.Sokolnicki LA, Khosla S, Charkoudian N. Effects of testosterone and estradiol on cutaneous vasodilation during local warming in older men. Am J Physiol Endocrinol Metab 293: E1426–E1429, 2007. doi: 10.1152/ajpendo.00535.2007. [DOI] [PubMed] [Google Scholar]
- 211.Somjen D, Kohen F, Gayer B, Sharon O, Baz M, Limor R, Kulik T, Knoll E, Stern N. Role of putative membrane receptors in the effects of estradiol on human vascular cell growth. Am J Hypertens 17: 462–469, 2004. doi: 10.1016/j.amjhyper.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 212.Song J, Kost CK Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res 72: 456–463, 2006. doi: 10.1016/j.cardiores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 213.Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 298: R261–R265, 2010. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Intern Med 167: 256–267, 2017. doi: 10.7326/M17-0577. [DOI] [PubMed] [Google Scholar]
- 214a.Studen KB, Šebeštjen M, Pfeifer M, Preželj J. Influence of spironolactone treatment on endothelial function in non-obese women with polycystic ovary syndrome. Eur J Endocrinol 164: 389–395, 2011. doi: 10.1530/EJE-10-0709. [DOI] [PubMed] [Google Scholar]
- 215.Sudhir K, Ko E, Zellner C, Wong HE, Hutchison SJ, Chou TM, Chatterjee K. Physiological concentrations of estradiol attenuate endothelin 1-induced coronary vasoconstriction in vivo. Circulation 96: 3626–3632, 1997. doi: 10.1161/01.CIR.96.10.3626. [DOI] [PubMed] [Google Scholar]
- 216.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- 217.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sullivan JC, Rodriguez-Miguelez P, Zimmerman MA, Harris RA. Differences in angiotensin (1–7) between men and women. Am J Physiol Heart Circ Physiol 308: H1171–H1176, 2015. doi: 10.1152/ajpheart.00897.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension 45: 406–411, 2005. doi: 10.1161/01.HYP.0000156879.83448.93. [DOI] [PubMed] [Google Scholar]
- 220.Tan Z, Wang TH, Chen Y, Lin GP, Pan JY. [Role of NO signal pathway in the inhibitory of 17beta-estradiol on the production of endothelin-1 in vascular smooth muscle cells]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 23: 347–350, 2007. [PubMed] [Google Scholar]
- 221.Tan Z, Wang TH, Yang D, Fu XD, Pan JY. Mechanisms of 17beta-estradiol on the production of ET-1 in ovariectomized rats. Life Sci 73: 2665–2674, 2003. doi: 10.1016/S0024-3205(03)00605-2. [DOI] [PubMed] [Google Scholar]
- 222.Tanaka M, Nakaya S, Watanabe M, Kumai T, Tateishi T, Kobayashi S. Effects of ovariectomy and estrogen replacement on aorta angiotensin-converting enzyme activity in rats. Jpn J Pharmacol 73: 361–363, 1997. doi: 10.1254/jjp.73.361. [DOI] [PubMed] [Google Scholar]
- 223.Teoh H, Quan A, Leung SW, Man RY. Differential effects of 17beta-estradiol and testosterone on the contractile responses of porcine coronary arteries. Br J Pharmacol 129: 1301–1308, 2000. doi: 10.1038/sj.bjp.0703164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thomas P, Pang Y. Protective actions of progesterone in the cardiovascular system: potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids 78: 583–588, 2013. doi: 10.1016/j.steroids.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 225.Toering TJ, van der Graaf AM, Visser FW, Buikema H, Navis G, Faas MM, Lely AT. Gender differences in response to acute and chronic angiotensin II infusion: a translational approach. Physiol Rep 3: e12434, 2015. doi: 10.14814/phy2.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Torres-Estay V, Carreño DV, San Francisco IF, Sotomayor P, Godoy AS, Smith GJ. Androgen receptor in human endothelial cells. J Endocrinol 224: R131–R137, 2015. doi: 10.1530/JOE-14-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tostes Passaglia RC, David FL, Fortes ZB, Nigro D, Scivoletto R, Catelli De Carvalho MH. Deoxycorticosterone acetate-salt hypertensive rats display gender-related differences in ETB receptor-mediated vascular responses. Br J Pharmacol 130: 1092–1098, 2000. doi: 10.1038/sj.bjp.0703389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, Carvalho MH. Endothelin, sex and hypertension. Clin Sci (Lond) 114: 85–97, 2008. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- 229.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19: 1245–1254, 2001. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 230.Usselman CW, Stachenfeld NS, Bender JR. The molecular actions of oestrogen in the regulation of vascular health. Exp Physiol 101: 356–361, 2016. doi: 10.1113/EP085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Valdés G, Germain AM, Corthorn J, Berrios C, Foradori AC, Ferrario CM, Brosnihan KB. Urinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactation. Endocrine 16: 117–122, 2001. doi: 10.1385/ENDO:16:2:117. [DOI] [PubMed] [Google Scholar]
- 232.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 233.Vigen R, O’Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310: 1829–1836, 2013. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 234.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101: 2258–2263, 2000. doi: 10.1161/01.CIR.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 235.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, Volterrani M, Fini M, Collins P, Rosano GM. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol 28: 348–352, 2008. doi: 10.1161/ATVBAHA.107.158634. [DOI] [PubMed] [Google Scholar]
- 236.Wang H, Chen H. Gender difference in the response to valsartan/amlodipine single-pill combination in essential hypertension (China Status II): An observational study. J Renin Angiotensin Aldosterone Syst 17: 1470320316643903, 2016. doi: 10.1177/1470320316643903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang H, Jessup JA, Zhao Z, Da Silva J, Lin M, MacNamara LM, Ahmad S, Chappell MC, Ferrario CM, Groban L. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats. PLoS One 8: e76992, 2013. doi: 10.1371/journal.pone.0076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang Y, Shoemaker R, Powell D, Su W, Thatcher S, Cassis L. Differential effects of Mas receptor deficiency on cardiac function and blood pressure in obese male and female mice. Am J Physiol Heart Circ Physiol 312: H459–H468, 2017. doi: 10.1152/ajpheart.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Webb CM, Ghatei MA, McNeill JG, Collins P. 17beta-estradiol decreases endothelin-1 levels in the coronary circulation of postmenopausal women with coronary artery disease. Circulation 102: 1617–1622, 2000. doi: 10.1161/01.CIR.102.14.1617. [DOI] [PubMed] [Google Scholar]
- 240.Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet 374: 1423–1431, 2009. doi: 10.1016/S0140-6736(09)61500-2. [DOI] [PubMed] [Google Scholar]
- 241.Wenner MM, Sebzda KN, Kuczmarski AV, Pohlig RT, Edwards DG. ETB receptor contribution to vascular dysfunction in postmenopausal women. Am J Physiol Regul Integr Comp Physiol 313: R51–R57, 2017. doi: 10.1152/ajpregu.00410.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wenner MM, Taylor HS, Stachenfeld NS. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol 589: 4671–4679, 2011. doi: 10.1113/jphysiol.2011.216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 305: E818–E825, 2013. doi: 10.1152/ajpendo.00343.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol 589: 975–986, 2011. doi: 10.1113/jphysiol.2010.194563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Westby CM, Weil BR, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstriction and the age-related decline in endothelium-dependent vasodilatation in men. Clin Sci (Lond) 120: 485–491, 2011. doi: 10.1042/CS20100475. [DOI] [PubMed] [Google Scholar]
- 247.Wilcox JG, Hatch IE, Gentzschein E, Stanczyk FZ, Lobo RA. Endothelin levels decrease after oral and nonoral estrogen in postmenopausal women with increased cardiovascular risk factors. Fertil Steril 67: 273–277, 1997. doi: 10.1016/S0015-0282(97)81910-3. [DOI] [PubMed] [Google Scholar]
- 248.Wild RA, Grubb B, Hartz A, Van Nort JJ, Bachman W, Bartholomew M. Clinical signs of androgen excess as risk factors for coronary artery disease. Fertil Steril 54: 255–259, 1990. doi: 10.1016/S0015-0282(16)53699-1. [DOI] [PubMed] [Google Scholar]
- 249.Wilhelmson AS, Fagman JB, Johansson I, Zou ZV, Andersson AG, Svedlund Eriksson E, Johansson ME, Lindahl P, Fogelstrand P, Tivesten Å. Increased intimal hyperplasia after vascular injury in male androgen receptor-deficient mice. Endocrinology 157: 3915–3923, 2016. doi: 10.1210/en.2016-1100. [DOI] [PubMed] [Google Scholar]
- 250.Worboys S, Kotsopoulos D, Teede H, McGrath B, Davis SR. Evidence that parenteral testosterone therapy may improve endothelium-dependent and -independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab 86: 158–161, 2001. doi: 10.1210/jcem.86.1.7103. [DOI] [PubMed] [Google Scholar]
- 251.Wu CC, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension 57: 788–794, 2011. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xue B, Beltz TG, Guo F, Johnson AK. Sex differences in maternal gestational hypertension-induced sensitization of angiotensin II hypertension in rat offspring: the protective effect of estrogen. Am J Physiol Regul Integr Comp Physiol 314: R274–R281, 2018. doi: 10.1152/ajpregu.00216.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yuan W, Cheng G, Li B, Li Y, Lu S, Liu D, Xiao J, Zhao Z. Endothelin-receptor antagonist can reduce blood pressure in patients with hypertension: a meta-analysis. Blood Press 26: 139–149, 2017. doi: 10.1080/08037051.2016.1208730. [DOI] [PubMed] [Google Scholar]
- 254.Zerr-Fouineau M, Jourdain M, Boesch C, Hecker M, Bronner C, Schini-Kerth VB. Certain progestins prevent the enhancing effect of 17beta-estradiol on NO-mediated inhibition of platelet aggregation by endothelial cells. Arterioscler Thromb Vasc Biol 29: 586–593, 2009. doi: 10.1161/ATVBAHA.108.178004. [DOI] [PubMed] [Google Scholar]
- 255.Zhang Y, Davidge ST. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension 34: 1117–1122, 1999. doi: 10.1161/01.HYP.34.5.1117. [DOI] [PubMed] [Google Scholar]
- 256.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]