Abstract
The chronic intrinsic diuretic and natriuretic tone of sodium-glucose cotransporter 2 (SGLT2) inhibitors is incompletely understood because their effect on body fluid volume (BFV) has not been fully evaluated and because they often increase food and fluid intake at the same time. Here we first compared the effect of the SGLT2 inhibitor ipragliflozin (Ipra, 0.01% in diet for 8 wk) and vehicle (Veh) in Spontaneously Diabetic Torii rat, a nonobese type 2 diabetic model, and nondiabetic Sprague-Dawley rats. In nondiabetic rats, Ipra increased urinary excretion of Na+ (UNaV) and fluid (UV) associated with increased food and fluid intake. Diabetes increased these four parameters, but Ipra had no further effect, probably because of its antihyperglycemic effect, such that glucosuria and, as a consequence, food and fluid intake were unchanged. Fluid balance and BFV, determined by bioimpedance spectroscopy, were similar among the four groups. To study the impact of food and fluid intake, nondiabetic rats were treated for 7 days with Veh, Ipra, or Ipra+pair feeding+pair drinking (Pair-Ipra). Pair-Ipra maintained a small increase in UV and UNaV versus Veh despite similar food and fluid intake. Pair-Ipra induced a negative fluid balance and decreased BFV, whereas Ipra or Veh had no significant effect compared with basal values. In conclusion, SGLT2 inhibition induces a sustained diuretic and natriuretic tone. Homeostatic mechanisms are activated to stabilize BFV, including compensatory increases in fluid and food intake.
Keywords: bioimpedance analysis, body fluid volume, diuresis, natriuresis, SGLT2 inhibition
INTRODUCTION
Inhibitors of the sodium-glucose cotransporter (SGLT) 2 are a new class of antihyperglycemic drugs that lower blood glucose by suppressing glucose reabsorption in the kidneys and can have cardiorenal protective effects (15, 47). SGLT2 is located in the brush border membrane of the early proximal tubule (S1 segment) and mediates the majority of glucose reabsorption of the kidney (47). In normoglycemia, SGLT2 is responsible for ~97% of renal glucose reabsorption, whereas SGLT1 reabsorbs the remaining glucose reabsorption in the late proximal tubule (S2/S3 segments) (9, 32, 44).
Because SGLT2 and SGLT1 mediate the transport of glucose together with Na+ (in a 1:1 and 1:2 ratio, respectively) (17), these cotransporters contribute to glucose and Na+ reabsorption in the proximal tubule (44). As a consequence, inhibition of SGLT2 reduces Na+ reabsorption in the proximal tubule, thereby enhancing Na+ excretion, and the osmotic effect of nonreabsorbed Na+ and glucose enhances fluid excretion (20). In accordance, recent studies in humans and animals have shown that SGLT2 inhibitors increase urine volume and Na+ excretion (19, 21, 25, 39, 41). The SGLT2 inhibitor empagliflozin transiently increases the urine volume and Na+ excretion as well as fluid intake in nondiabetic db/m mice and obese type 2 diabetic db/db mice (21). In prediabetic SHR/NDmcr-cp (+/+) rats, the SGLT2 inhibitors luseogliflozin and empagliflozin significantly increased both urine volume and fluid intake for up to 10 wk (19, 30). Sustained diuretic and natriuretic effects of SGLT2 inhibitors, however, are associated with increased fluid and food intake (19, 21, 30), which makes it difficult to discriminate primary and secondary effects.
Furthermore, the effect of SGLT2 inhibitors on body fluid volume has not been fully evaluated. We recently reported that the SGLT2 inhibitor dapagliflozin transiently increased urinary Na+ excretion and decreased body fluid volume in diabetic patients with kidney disease and fluid retention (25). Other studies also showed that SGLT2 inhibitors can decrease body fluid volume and plasma volume in patients with type 2 diabetes (11, 20). Unfortunately, many studies using sustained administration of SGLT2 inhibitors in humans lack detailed information on food and fluid intake (11, 14, 20, 25), and many patients are taking diuretics at the same time (14, 25, 40). Thus, the specific and sustained impact of SGLT2 inhibitors on body fluid volume remains unclear. Furthermore, no animal study has explored in detail the effect of sustained SGLT2 inhibition on body fluid volume.
We therefore first examined the effect of sustained SGLT2 inhibition on body fluid volume in nondiabetic and diabetic rats while closely monitoring food and fluid intake. In a second set of studies, we used pair feeding and pair drinking to potentially unmask a sustained diuretic and natriuretic effect of SGLT2 inhibition that is independent of the increase in fluid and food intake, and we analyzed the impact on body fluid volume.
MATERIALS AND METHODS
Experimental animals.
The study protocol was approved by the Animal Ethics Committee of Jichi Medical University. Male Spontaneously Diabetic Torii (SDT) rats and age-matched male Sprague-Dawley (SD) rats at 9 wk of age were purchased from CLEA Japan, Inc. (Tokyo, Japan) and housed in a 12:12-h light-dark cycle in standard rat cages with free access to food (1.01% K+, 0.35% Na+, 4.92% fat, CLEA Rodent Diet CE-2, CLEA Japan, Inc.) and water. The SDT rat is a nonobese type 2 diabetic model, which spontaneously develops gradual impairment of insulin secretion and hyperglycemia (over 500 mg/dl by 20 wk) in the absence of obesity (35, 36). Blood glucose levels were measured once weekly starting at 15 wk of age, and the onset of diabetes was defined as blood glucose concentration >250 mg/dl, as previously described (24). The measurement of serum aldosterone, serum and urine osmolality, and urinary vasopressin concentration were carried out in the SRL laboratory (Hachioji, Tokyo, Japan). Electrolyte-free water clearance was calculated as described previously (12).
Experiment 1: effect of ipragliflozin treatment for 8 wk with free access to food and water on urine volume and electrolytes and body fluid status.
The main purpose of this experiment was to investigate the long-term effect of the SGLT2 inhibitor ipragliflozin (37, 38) on food and fluid intake and on urinary electrolyte and fluid excretion and balance. At the onset of diabetes, which we defined as 0 week, SDT rats and age-matched SD rats were randomly divided into four groups of matched body weight (BW): SDT rats with vehicle (SDT+Veh) or ipragliflozin (SDT+Ipra), and SD rats with Veh (Cont+Veh) or Ipra (Cont+Ipra). The SDT+Veh and Cont+Veh groups were initiated on the standard powdered food (CE-2), and the SDT+Ipra and Cont+Ipra were given the powdered food (CE-2) containing ipragliflozin [100 mg/kg diet (37, 38); Astellas Pharma Inc., Tokyo, Japan], as described previously (24). This treatment was performed for 8 wk in standard rat cages with free access to food and water. BW and blood glucose were measured every week and food and fluid intake every day. Body fluid analysis by bioimpedance spectroscopy (BIS, see below for details) was performed at the beginning of the 4th and 8th wk of treatment, respectively. One day after completion of BIS in the 8th wk, rats were placed in metabolic cages for 24-h urine collection to determine urine volume and urinary excretion of glucose, protein, and electrolytes. Subsequently, systolic blood pressure was measured over 2 days in the conscious state by a noninvasive tail-cuff system (BP-98A, Softron, Tokyo, Japan), as previously described (16, 24). Finally, rats were anesthetized with isoflurane, and blood was collected by cardiac puncture to measure biochemical and hormonal data at SRL (Tokyo, Japan), and kidneys were harvested.
Experiment 2: effect of ipragliflozin treatment for 7 days with restricted food and fluid intake on urine volume and electrolytes and body fluid status.
The main purpose of this experiment was to investigate the influence of fluid and food intake on the effect of ipragliflozin treatment on urine volume and electrolytes and body fluid status. Three groups of SD rats were used: Veh, Ipra, and Pair-Ipra groups. The Veh group was initiated on the standard powered food (CE-2), and the Ipra group was given the powdered food (CE-2) containing ipragliflozin (100 mg/kg diet), both with free access to food and water. The Pair-Ipra group was given the powdered food (CE-2) containing ipragliflozin (100 mg/kg diet) with pair feeding and pair drinking to match the intake of the Veh group, as previously described (23). The rats were acclimatized to the metabolic cages for 3 days before initiating 24-h urine sample collections and measurement of BW, food, and fluid intake for 7 consecutive days. BIS was performed before (day 0) and on day 7 of pair feeding and pair drinking.
Body fluid analysis by BIS.
Under 2.5% isoflurane (Dainippon Sumitomo Pharma, Osaka, Japan) anesthesia, BIS was performed using the ImpediVet BIS1 system (ImpediMed, San Diego, CA) to analyze total body water, extracellular fluid, and intracellular fluid, as previously described (2, 4, 23). BIS determines body composition based on its electrical characteristics in response to the application of low-amplitude alternating electrical currents (2). Results were expressed as percentage of total BW, absolute value, or percent change of baseline.
Western blot analysis.
In experiment 1, whole kidneys were harvested under terminal isoflurane anesthesia and prepared for Western blot analysis of the membrane fraction as previously described (34, 44). Lysates at 40 μg/lane were separated by SDS-PAGE (4–12% Nu-PAGE Novex Bis Tris Gel) in MOPS buffer (Invitrogen, Carlsbad, CA), and gel proteins were transferred to a polyvinylidene difluoride membrane (Hybond-P, GE Healthcare, Little Chalfont, UK) and immunoblotted with the appropriate primary antibody. The secondary antibody was horseradish peroxidase-conjugated for autoradiographic detection by ECL Plus (Amersham Pharmacia, Piscataway, NJ) and visualized by a luminescent image analyzer (LAS-1000, Fuji Film, Tokyo, Japan). Immunoblotting was performed at 4°C overnight with the primary antibodies as follows: polyclonal rat SGLT2 (dilution 1:1,000) (44), SGLT1 (dilution 1:2,000) (1, 44), glucose transporter 2 (dilution 1:5,000, ab95256, Abcam, Cambridge, MA), sodium-hydrogen exchanger 3 (NHE3; dilution 1:1,000, AB3085, Merck Millipore, Billerica, MA) (51), phosphorylated (S605) NHE3 (dilution 1:1,000, sc-53961, Santa Cruz Biotechnology, Santa Cruz, CA) (31), phosphorylated (S552) NHE3 (dilution 1:1,000, NB110–81529, Novus Biologicals, Littleton, CO) (31), aquaporin 1 (AQP1; dilution 1:1,000, AB2219, Merck Millipore) (26), Na+-K+-Cl− cotransporter-2 (NKCC2; dilution 1:1,000, NKCC21-A, Alpha Diagnostic International, San Antonio, TX) (18), Na+-Cl− cotransporter (NCC; dilution 1:1,000, AB3553, Merck Millipore) (27), α epithelial Na+ channel (α-ENaC; 1:1,000, AB3530P, Merck Millipore) (8), β-ENaC (1:1,000, AB3532P, Merck Millipore) (8), γ-ENaC (1:1,000, AB3534P, Merck Millipore) (8), and monoclonal mouse anti-β-actin (1:2,000, sc-47778, Santa Cruz Biotechnology) (22). Bands were detected by enhanced chemiluminescence (GE Healthcare). The signal band density was quantified using ImageJ Software (version 1.49v, National Institutes of Health, Bethesda, MD) and normalized to β-actin.
Statistical analysis.
Data are presented as means ± SE. Statistical differences were analyzed by two-way ANOVA with post-hoc analysis using the Holm-Sidak method in experiment 1 and by one-way ANOVA with post-hoc analysis as appropriate in experiment 2. Log-transformed values were used for not normally distributed parameters. P < 0.05 was considered to be statistically significant.
RESULTS
SGLT2 inhibitor ipragliflozin increased food and fluid intake, urine volume, and urinary Na+ excretion in nondiabetic SD rats but not in diabetic SDT rats.
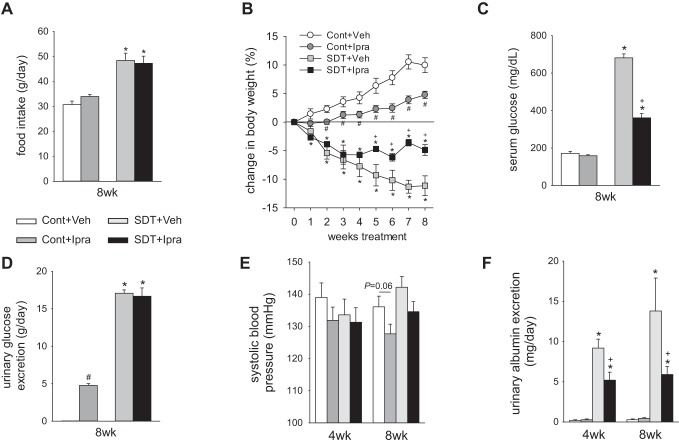
The onset of diabetes in SDT rats varies from 16 to 22 wk of age (average age 18.1 ± 0.5 wk of age). Basal BW before treatment was similar among the SD rats [Cont+Veh 564 ± 22 g vs. Cont+Ipra 569 ± 16 g, not significant (NS)] and the SDT rats [SDT+Veh 517 ± 17 g vs. SDT+Ipra 519 ± 8 g, NS]. Ipragliflozin suppressed the BW gain in SD rats and attenuated the BW loss in SDT rats during the 8-wk treatment (Fig. 1B). Kidney weight in SDT rats was significantly higher than in SD rats, but ipragliflozin did not change kidney weight in SD or SDT rats (Table 1).
Fig. 1.
Ipragliflozin treatment for 8 wk increased urinary glucose excretion and food intake in nondiabetic SD rats but not in diabetic SDT rats. Effect of 8-wk treatment with ipragliflozin on food intake (A), change in body weight from baseline (B), serum glucose (C), and urinary glucose excretion (D). At 4 and 8 wk, ipragliflozin numerically decreased systolic blood pressure both in SD and SDT rats (E). At 4 and 8 wk, ipragliflozin decreased diabetic-induced increase in urinary albumin excretion in SDT rats (F). *P < 0.05 vs. Cont same treatment; #P < 0.05 vs. Cont+Veh; +P < 0.05 vs. SDT+Veh. Values are means ± SE; n = 6–12/group. AQP1, aquaporin 1; Cont, nondiabetic SD rat; Ipra, standard diet containing ipragliflozin (100 mg/kg diet); SD, Sprague-Dawley; SDT, Spontaneously Diabetic Torii; Veh, standard diet (CLEA Rodent Diet CE-2).
Table 1.
Blood and urine parameters after ipragliflozin treatment for 8 wk
| 8 Wk |
||||
|---|---|---|---|---|
| Characteristic | Cont+Veh (n = 5) | Cont+Ipra (n = 12) | SDT+Veh (n = 10) | SDT+Ipra (n = 10) |
| Kidney weight, g | 1.84 ± 0.08 | 2.01 ± 0.07 | 2.44 ± 0.11‡ | 2.29 ± 0.08‡ |
| Urinary protein, mg/day | 9.3 ± 1.4 | 9.7 ± 0.9 | 62.0 ± 14.9‡ | 26.5 ± 4.3† |
| Hematocrit, % | 43.8 ± 0.5 | 43.1 ± 0.8 | 45.9 ± 0.5‡ | 46.3 ± 0.3 |
| Serum creatinine, mg/dl | 0.33 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 |
| Creatinine clearance, L/day | 7.5 ± 0.6 | 7.5 ± 0.4 | 6.9 ± 0.3 | 7.4 ± 0.4 |
| Electrolyte-free water clearance, mL/day | −28.5 ± 9.9 | −15.8 ± 7.9 | 110.7 ± 8.3‡ | 97.2 ± 8.3‡ |
| Serum Na+, mEq/L | 144 ± 1 | 144 ± 1 | 137 ± 1 | 143 ± 1† |
| Serum Cl−, mEq/L | 102 ± 1 | 103 ± 1 | 94 ± 1 | 102 ± 1† |
| Serum K+, mEq/L | 5.7 ± 0.3 | 5.6 ± 0.1 | 5.0 ± 0.2 | 4.8 ± 0.2 |
| Fractional excretion of glucose, % | 0.2 ± 3.1 | 37.2 ± 2.7* | 37.3 ± 2.8‡ | 63.4 ± 2.8†‡ |
| Fractional excretion of Na+, % | 0.29 ± 0.03 | 0.35 ± 0.03 | 0.65 ± 0.05 | 0.56 ± 0.03 |
| Fractional excretion of Cl−, % | 0.46 ± 0.04 | 0.52 ± 0.04 | 0.95 ± 0.07 | 0.80 ± 0.05† |
| Fractional excretion of K+, % | 14.1 ± 1.0 | 17.1 ± 1.3 | 30.8 ± 1.8 | 30.0 ± 1.7 |
| Fractional excretion of fluid, % | 0.5 ± 0.2 | 0.8 ± 0.2* | 3.4 ± 0.2‡ | 2.9 ± 0.2‡ |
| Serum aldosterone, pg/mL | 272 ± 39 | 299 ± 26 | 260 ± 62 | 239 ± 19 |
| Urinary vasopressin, pg/day | 2,940 ± 1,223 | 3,728 ± 476 | 1,3481 ± 1,429 | 13,601 ± 1,728 |
| Serum osmolality, mosmol/kgH2O | 301 ± 2 | 299 ± 1 | 324 ± 2‡ | 316 ± 2†‡ |
| Urine osmolality, mosmol/kgH2O | 1,589 ± 254 | 1,638 ± 57 | 907 ± 23‡ | 957 ± 49‡ |
Values are means ± SE. Cont+Ipra, SD rats with Ipra; Cont+Veh, SD rats with Veh; Ipra, standard diet containing ipragliflozin (100 mg/kg diet); SD, Sprague-Dawley; SDT, Spontaneously Diabetic Torii; SDT+Ipra, SDT rats with Ipra; SDT+Veh, SDT rats with Veh; Veh, standard diet (CLEA Rodent Diet CE-2).
P < 0.05 vs. Cont+Veh;
P < 0.05 vs. SDT+Veh;
P < 0.05 vs. Cont same treatment.
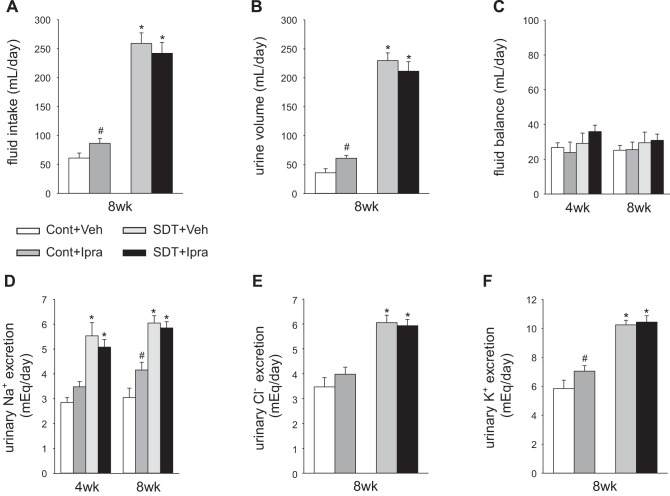
Ipragliflozin did not change serum glucose in anesthetized SD rats (Cont+Veh 172 ± 11 mg/dl vs. Cont+Ipra 158 ± 5 mg/dl, P = 0.584) (Fig. 1C). Ipragliflozin significantly increased urinary glucose excretion and numerically increased food intake in SD rats (Fig. 1, A and D). The results indicate a “partial” compensatory increase in food intake in SD rats because BW of glucosuric SD rats was lower than in their nonglucosuric counterparts (Fig. 1B). Ipragliflozin significantly increased fluid intake and urine volume in nondiabetic SD rats at 8 wk (Fig. 2, A and B). As a result, fluid balance was not significantly different among the two groups (Fig. 2C). Urinary excretions of Na+ and K+ were significantly increased, and Cl− was numerically increased in SD rats treated with ipragliflozin (Fig. 2, D–F). At 8 wk, ipragliflozin tended to decrease systolic blood pressure in SD rats (Fig. 1E), whereas heart rate was not significantly changed (Cont+Veh: 354 ± 12 min−1; Cont+Ipra: 333 ± 11 min−1; NS). Ipragliflozin increased fractional excretion of glucose in SD rats and tended to increase electrolyte-free water clearance (Table 1). Fractional excretion of fluid was increased in SD rats treated with ipragliflozin compared with Veh, whereas the fractional excretion of Na+ and K+ tended to be increased by ipragliflozin (Na+: P = 0.072; K+: P = 0.057) (Table 1).
Fig. 2.
Ipragliflozin treatment for 8 wk increased fluid intake, urine volume, and urinary Na+ excretion in nondiabetic SD rats but not in diabetic SDT rats. Effect of 8-wk treatment with ipragliflozin on fluid intake (A), urine volume (B), fluid balance (C), urinary Na+ excretion (D), urine Cl− excretion (E), and urine K+ excretion (F). #P < 0.05 vs. Cont+Veh; *P < 0.05 vs. Cont same treatment. Values are means ± SE; n = 8–12/group. Cont, nondiabetic SD rat; Ipra, standard diet containing ipragliflozin (100 mg/kg diet); SD, Sprague-Dawley; SDT, Spontaneously Diabetic Torii; Veh, standard diet (CLEA Rodent Diet CE-2).
In SDT rats, ipragliflozin significantly lowered hyperglycemia (Fig. 1C) but did not change food intake and urinary glucose excretion (Fig. 1, A and D). The serum glucose lowering effect of ipragliflozin in SDT rats appeared to match the tubular glucose transport inhibition such that glucosuria and, as a consequence, food intake were not changed compared with Veh-treated SDT rats (Fig. 1, A, C, and D). Ipragliflozin did not change fluid intake and urine volume in SDT rats at 8 wk (Fig. 2, A and B). Thus, fluid balance was not significantly different between SDT rats with and without ipragliflozin (Fig. 2C). Similarly, urinary excretions of Na+, K+, and Cl− were not changed in SDT rats treated with ipragliflozin (Fig. 2, D–F). At 8 wk, ipragliflozin tended to decrease systolic blood pressure in SDT rats (Fig. 1E), whereas heart rate was similar among the 2 groups (SDT+Veh: 336 ± 12 min−1; SDT+Ipra: 347 ± 12 min−1; NS). Ipragliflozin decreased diabetic-induced increase in urinary protein excretion and albumin excretion in SDT rats (Table 1 and Fig. 1F). Ipragliflozin increased serum Na+ and Cl− concentration in SDT rats (Table 1). Fractional excretion of glucose strongly increased, and fractional excretion of Cl− slightly decreased in SDT rats with ipragliflozin (Table 1).
Ipragliflozin treatment with free access to food and water did not change body fluid volume in nondiabetic and diabetic rats.
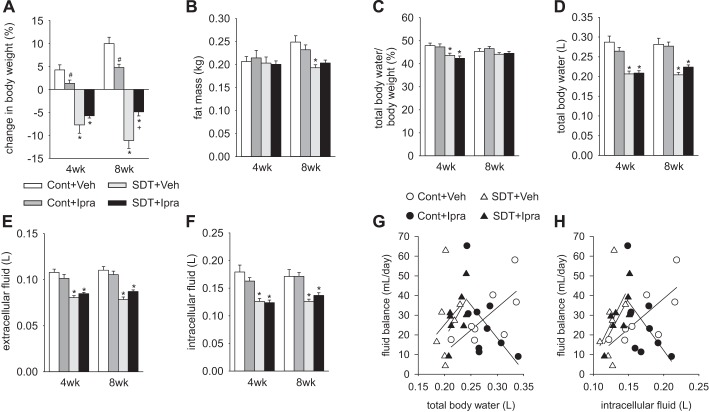
As determined by BIS, fat mass at 4 wk was similar between the four groups, but it was significantly lower in SDT rats than in SD rats at 8 wk (Fig. 3B). Fat mass of each group at 8 wk had a similar trend to the change in BW (Fig. 3, A and B). At 4 and 8 wk of treatment, total body water, extracellular fluid, and intracellular fluid measured by BIS were similar among Veh- and ipragliflozin-treated SD and SDT rats, respectively (Fig. 3, C–F), consistent with the observed similar fluid balance (Fig. 2C). Similarly, hematocrit, serum aldosterone, and urinary vasopressin, which vary depending on plasma volume (10, 49), were not significantly changed by ipragliflozin in SD and SDT rats (Table 1). These data suggest that ipragliflozin did not detectably change body fluid volume in nondiabetic and diabetic rats with free access to food and water.
Fig. 3.
Ipragliflozin treatment for 8 wk did not change total body water, extracellular fluid, and intracellular fluid in nondiabetic SD or diabetic SDT rats. At 8 wk, changes in body weight from baseline (A) had a similar trend to fat mass (B). Total body water/body weight (C), total body water (D), extracellular fluid (E), and intracellular fluid (F) were not changed by ipragliflozin either in nondiabetic or diabetic rats at 4 and 8 wk. At 8 wk, total body water and intracellular fluid were positively associated with fluid balance except for ipragliflozin-treated SD rats (G and H). #P < 0.05 vs. Cont+Veh; *P < 0.05 vs. Cont same treatment; +P < 0.05 vs. SDT+Veh. Values are means ± SE; n = 7–9/group. Cont, nondiabetic SD rat; Ipra, standard diet containing ipragliflozin (100 mg/kg diet); SD, Sprague-Dawley; SDT, Spontaneously Diabetic Torii; Veh, standard diet (CLEA Rodent Diet CE-2).
At 8 wk, trends for positive relationships (r values) between total body water and intracellular fluid versus fluid balance were found in Veh-treated SD rats (total body water: r = 0.707, P = 0.050; intracellular fluid: r = 0.757, P = 0.030), Veh-treated SDT rats (total body water: r = 0.244, P = 0.598; intracellular fluid: r = 0.401, P = 0.373), and ipragliflozin-treated SDT rats (total body water: r = 0.603, P = 0.114; intracellular fluid: r = 0.762, P = 0.028) (Fig. 3, G and H). Only in ipragliflozin-treated SD rats, total body water and intracellular fluid tended to be negatively correlated with fluid balance (r = −0.595, P = 0.091) and intracellular fluid (r = −0.586, P = 0.097) (Fig. 3, G and H). This may indicate that the lower total body or intracellular water in response to ipragliflozin, the more positive the fluid balance in an effort to stabilize the former.
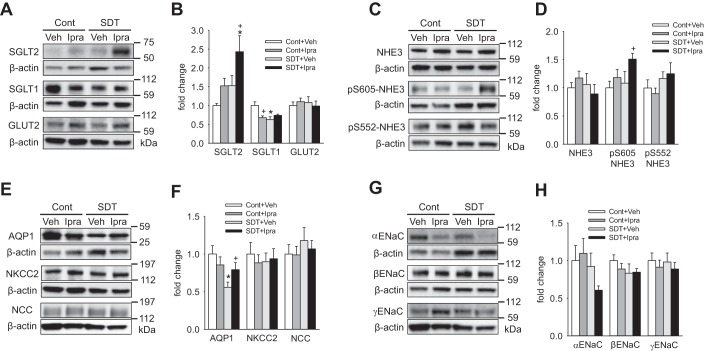
Ipragliflozin treatment with free access to food and water did not increase the renal protein expression of the main sodium transporters in nondiabetic and diabetic rats.
The renal membrane protein expression of SGLT2 was increased and that of SGLT1 was decreased in diabetic SDT rats compared with nondiabetic SD rats (Fig. 4, A and B), similarly to a previous study in diabetic Akita mice (43). Ipragliflozin treatment numerically and significantly increased the expression of SGLT2 in SD and SDT rats, respectively, whereas it significantly decreased the expression of SGLT1 only in SD rat (Fig. 4, A and B). The expression of the basolateral facilitative glucose transporter GLUT2 was similar among the four groups (Fig. 4, A and B). The expression of NHE3, which is another important sodium transporter in the proximal tubule (46), was not changed by hyperglycemia and ipragliflozin, but ipragliflozin increased NHE3 phosphorylated at S605 (pS605), which may indicate reduced activity (see below), in SDT rats (Fig. 4, C and D). The expression of AQP1, which is expressed in proximal tubule and thin descending limbs of Henle’s loop, and mediates water transport (48), was lower in SDT rats compared with SD rats, and ipragliflozin ameliorated the reduction in SDT rats (Fig. 4, E and F). The renal expression of NKCC2 and NCC, which are the main sodium transporters expressed in the thick ascending limb of Henle’s loop and the distal convoluted tubule (3, 33), respectively, was similar among the four groups (Fig. 4, E and F). The expression of the three ENaC subunits, αENaC, βENaC and γENaC, which together mediate most of the Na+ reabsorption in the connecting tubule and collecting duct (28), was not different between the four groups (Fig. 4, G and H).
Fig. 4.
Renal membrane protein expression of sodium and water transporters in nondiabetic SD rats and diabetic SDT rats treated with Veh or ipragliflozin for 8 wk. A and B: ipragliflozin treatment numerically and significantly increased SGLT2 expression in nondiabetic SD rats and diabetic SDT rats, respectively, and significantly decreased SGLT1 in nondiabetic SD rat. C and D: ipragliflozin treatment increased NHE3 phosphorylated at S605 (pS605) in diabetic SDT rats. E and F: AQP1 expression in diabetic SDT rats was lower than that in nondiabetic SD rats, but ipragliflozin treatment ameliorated the reduction. G and H: ipragliflozin treatment did not significantly change the expression of αENaC, βENaC, and γENaC both in nondiabetic SD and diabetic SDT rats. *P < 0.05 vs. Cont same treatment; +P < 0.05 vs. Cont+Veh or SDT+Veh. Values are means ± SE; n = 7–13/group. Cont, nondiabetic SD rat; ENaC, epithelial Na+ channel; GLUT2, glucose transporter 2; Ipra, standard diet containing ipragliflozin (100 mg/kg diet); NCC, Na+-Cl− cotransporter; NHE3, sodium-hydrogen exchanger 3; NKCC2, Na+-K+-Cl− cotransporter-2; SD, Sprague-Dawley; SDT, Spontaneously Diabetic Torii; SGLT, sodium-glucose cotransporter; Veh, standard diet (CLEA Rodent Diet CE-2).
Pair feeding and drinking unmasked the sustained diuretic and natriuretic tone of ipragliflozin.
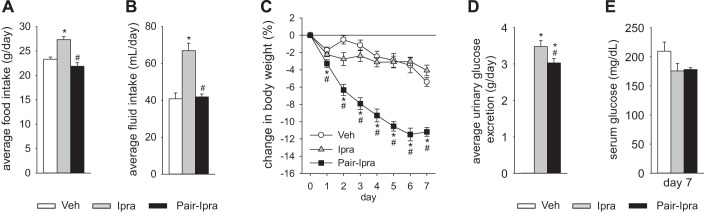
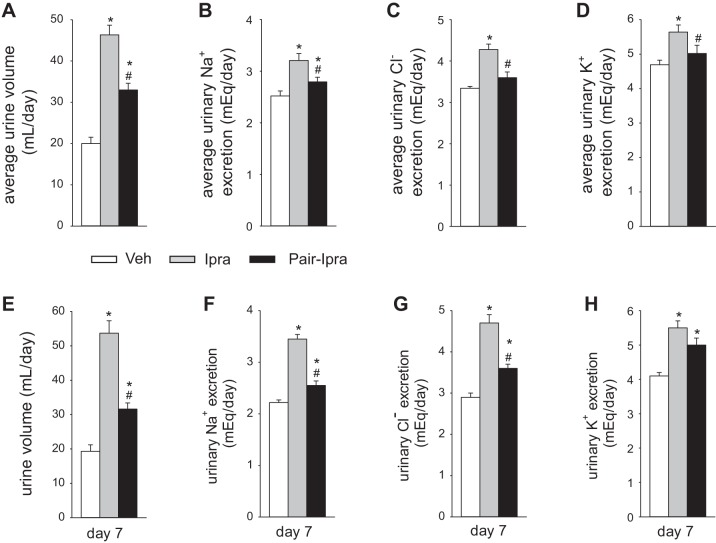
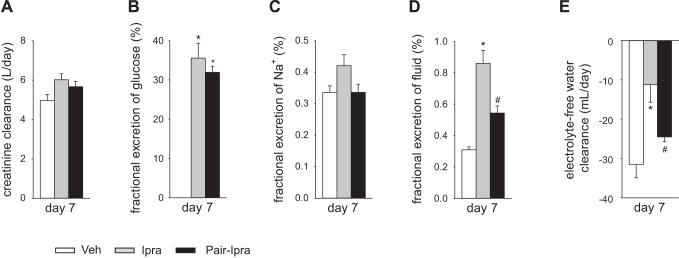
Ipragliflozin treatment in nondiabetic SD rats increased urine volume and urinary Na+ excretion but did not change the body fluid volume, possibly because of the compensatory increase in food and fluid intake. We therefore performed a pair feeding and drinking experiment in SD rats (average 20.5 ± 0.5 wk of age). Average daily food and fluid intake for 7 days was similar between the Veh group (standard diet with free access to food and water) and the Pair-Ipra group [standard diet containing ipragliflozin (100 mg/kg diet) with pair feeding and pair drinking matching the Veh group], whereas these parameters were increased in the Ipra group [standard diet containing ipragliflozin (100 mg/kg diet) with free access to food and water] (Fig. 5, A and B). BW in Pair-Ipra gradually decreased, and the change in BW reached −11% by day 7 (Fig. 5C). Urinary glucose excretion increased in both Ipra and Pair-Ipra, but values were lower in Pair-Ipra, likely reflecting the lower dosage of ipragliflozin because of lower food intake (Fig. 5, A and D). Serum glucose on day 7 was modestly and similarly reduced in Ipra and Pair-Ipra (Fig. 5E). Daily average (7 days) and 24-h urine volume and urinary Na+, Cl−, and K+ excretion on day 7 were increased in Ipra versus the other 2 groups (Fig. 6, A–H). Furthermore, these parameters were modestly increased in Pair-Ipra versus Veh (Fig. 6, A–H). Creatinine clearance was similar among the three groups (Fig. 7A). Fractional excretion of glucose was increased to a similar degree in the Ipra and the Pair-Ipra group (Fig. 7B). Fractional Na+ excretion in Ipra was higher than in Veh and Pair-Ipra (P = 0.086) (Fig. 7C). Fractional excretion of fluid and electrolyte-free water clearance were likewise increased in Ipra, but it was significantly lower in the Pair-Ipra than in the Ipra group (Fig. 7, D and E).
Fig. 5.
Pair feeding and pair drinking experiment with ipragliflozin for 7 days in nondiabetic SD rats. Average food and fluid intake were similar between Veh (standard diet) and Pair-Ipra [standard diet containing ipragliflozin (100 mg/kg diet) with pair feeding and pair drinking matching values in Veh] (A and B). Body weight in the Pair-Ipra gradually decreased over 7 days (C). Average urinary glucose excretion was increased in Ipra [standard diet containing ipragliflozin (100 mg/kg diet)] and Pair-Ipra (D), and serum glucose on day 7 numerically decreased in Ipra and Pair-Ipra (E). *P < 0.05 vs. Veh; #P < 0.05 vs. Ipra. Values are means ± SE; n = 8/group. SD, Sprague-Dawley.
Fig. 6.
Ipragliflozin increased urine volume and urinary excretion of Na+, Cl−, and K+ despite pair feeding and pair drinking. A–D: daily average (days 1–7). E–H: twenty-four-hr urine excretion on day 7. *P < 0.05 vs. Veh; #P < 0.05 vs. Ipra. Values are means ± SE; n = 8/group. Ipra, standard diet containing ipragliflozin (100 mg/kg diet); Pair-Ipra, standard diet containing ipragliflozin (100 mg/kg diet) with pair feeding and pair drinking matching values in Veh; Veh, standard diet (CLEA Rodent Diet CE-2).
Fig. 7.
Fractional excretion of glucose, Na+, and fluid and electrolyte-free water clearance on day 7 in the pair feeding and pair drinking experiment. Creatinine clearance was similar among the three groups (A). Fractional excretion of glucose was similarly increased in Ipra and Pair-Ipra vs. Veh (B). Fractional excretion of Na+ in the Ipra tended to increase (C). Fractional excretion of fluid and electrolyte-free water clearance were increased in Ipra vs. Veh, but significantly reduced in Pair-Ipra (D and E). *P < 0.05 vs. Veh; #P < 0.05 vs. Ipra. Values are means ± SE; n = 5–6/group. Ipra, standard diet containing ipragliflozin (100 mg/kg diet); Pair-Ipra, standard diet containing ipragliflozin (100 mg/kg diet) with pair feeding and pair drinking matching values in Veh; Veh, standard diet (CLEA Rodent Diet CE-2).
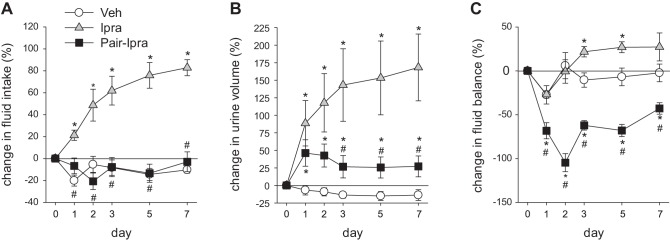
The time course of fluid intake, urine volume, and fluid balance is shown in Fig. 8. Fluid intake in the Pair-Ipra group was similar to the Veh group through the 7-day period, but urine volume was modestly increased in Pair-Ipra with a peak at day 1 (Fig. 8, A and B). As a result, the Pair-Ipra group developed and maintained a negative fluid balance during the 7 days (Fig. 8C).
Fig. 8.
Pair feeding and pair drinking induces a negative fluid balance in SD rats treated with ipragliflozin for 7 days. Change in fluid intake (A), urine volume (B), and fluid balance (C) during 7 days. *P < 0.05 vs. Veh; #P < 0.05 vs. Ipra. Values are means ± SE; n = 8/group. Ipra, standard diet containing ipragliflozin (100 mg/kg diet); Pair-Ipra, standard diet containing ipragliflozin (100 mg/kg diet) with pair feeding and pair drinking matching values in Veh; SD, Sprague-Dawley; Veh, standard diet (CLEA Rodent Diet CE-2).
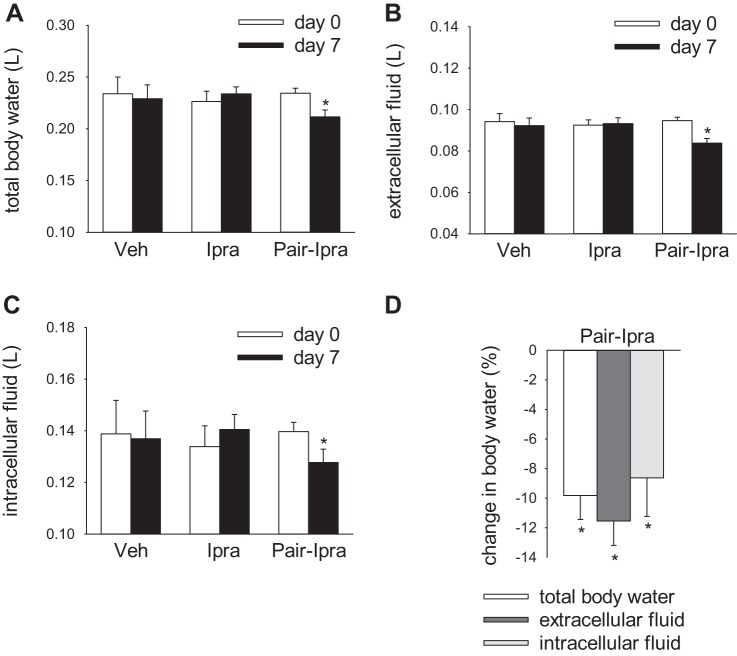
Pair feeding and drinking unmasked the body fluid volume-lowering effect of ipragliflozin.
Total body water, extracellular fluid, and intracellular fluid measured by BIS were similar in the Veh and the Ipra group on 0 and day 7 (Fig. 9, A–C), but these parameters were decreased in the Pair-Ipra group, consistent with negative fluid balance (Fig. 8C and Fig. 9, A–D). These data suggest that ipragliflozin can decrease body fluid volume when food and water intake are not allowed to increase.
Fig. 9.
Pair feeding and pair drinking reduces body fluid volume in SD rats treated with ipragliflozin for 7 days. Pair-Ipra decreased total body water, extracellular fluid, and intracellular fluid after 7 days (A–C). Change in body water during 7 days in the Pair-Ipra group (D). *P < 0.05 vs. day 0. Values are means ± SE; n = 7–9/group. Ipra, standard diet containing ipragliflozin (100 mg/kg diet); Pair-Ipra, standard diet containing ipragliflozin (100 mg/kg diet) with pair feeding and pair drinking matching values in Veh; SD, Sprague-Dawley; Veh, standard diet (CLEA Rodent Diet CE-2).
DISCUSSION
The main finding of this study is that the SGLT2 inhibitor ipragliflozin induces a sustained diuretic and natriuretic effect that is independent of food and fluid intake and that leads to volume depletion when not compensated by an increased fluid and food intake. This interpretation is based on the fact that urine volume and urinary Na+ excretion in nondiabetic rats treated with ipragliflozin remained increased for 7 days, even when food and fluid intake was clamped to the levels of Veh treatment. Moreover, this was associated with a reduction in body fluid volume. Furthermore, restricting food and fluid intake in ipragliflozin-treated rats reduced the fractional excretion of fluid and electrolyte-free water clearance. Thus, in the absence of a compensatory increase in food and fluid intake in response to SGLT2 inhibition, the kidney enhances fluid reabsorption to maintain body fluid volume. To the best of our knowledge, this is the first study to demonstrate a sustained diuretic and natriuretic effect of an SGLT2 inhibitor that is not associated with, and thus potentially the consequence of, an increase in food or fluid intake. Previous studies have shown that SGLT2 inhibitors including ipragliflozin increase urine volume and Na+ excretion in human and animal studies (19, 21, 25, 39–41, 50). However, food and fluid intake were also increased in response to SGLT2 inhibitors, and thus these studies were not designed to show a sustained diuretic and natriuretic effect of SGLT2 inhibition that is independent of intake (19, 21, 30). Similarly, in our study in nondiabetic SD rats with free access to food and water, the SGLT2 inhibitor increased urine volume and Na+ excretion, which was associated with increased food and fluid intake and unchanged body fluid volume. The combined results suggest that the increase in fluid and food intake in response to SGLT2 inhibition serves to compensate for the primary diuretic, glucosuric, and natriuretic effect of the drug to maintain fluid and electrolyte balance.
Consistent with preserved fluid homeostasis during free access to fluid and food, the SGTL2 inhibitor ipragliflozin induced a sustained increase in urinary flow rate in nondiabetic rats but did not change body fluid volume, as shown by BIS data (total body water, extracellular fluid, and intracellular fluid) and suggested by similar hematocrit, serum aldosterone, urine vasopressin, and serum osmolality. Similar to SD rats treated with ipragliflozin, SGLT2 knockout mice did not associate with obvious volume depletion, suggested by similar BW, hematocrit, urine vasopressin, and plasma osmolality compared with wild-type mice (44). Furthermore, food and fluid intake were increased in SGLT2 knockout mice (44), similar to SD rats treated with ipragliflozin. Thus, pharmaceutical and genetic SGLT2 inhibition activate homeostatic mechanisms to maintain body fluid volume that include compensatory increases in food and fluid intake.
In contrast to Veh-treated SD rats, Veh-treated SDT rats, and ipragliflozin-treated SDT rats, total body water and intracellular fluid tended to be negatively correlated with fluid balance only in ipragliflozin-treated SD rats, which may indicate a compensatory mechanism for maintaining body fluid volume when SGLT2 inhibition attempts to decrease body fluid volume. In other words, when the diuretic action of the SGLT2 inhibitor induces body fluid reduction, this may stimulate protective mechanisms against excess fluid loss by increasing fluid balance. The ipragliflozin-treated SD rats were the group in which ipragliflozin maintained a glucosuric effect and an enhanced urinary flow rate.
The renal membrane protein expression of SGLT2 was increased in diabetic SDT rats compared with nondiabetic SD rats, which was similar to previous studies in genetic models of type 1 and type 2 diabetic mice that used Sglt2 knockout mice as negative antibody controls (43, 45). SGLT2 upregulation in diabetes may reflect the hypertrophy of proximal tubule, or it may be caused by the feedback from a glucose sensor downstream of the early proximal tubule (47). Actually, kidney weight in SDT rats was higher than in SD rats, suggesting the involvement of proximal tubule hypertrophy in SGLT2 upregulation. Furthermore, ipragliflozin increased the expression of SGLT2 in SDT rats. Upregulation of SGLT2 expression in diabetic rodents and humans would enhance the blood glucose lowering potential of SGLT2 inhibition (47). In accordance, the strong blood glucose lowering effect (~47%) observed with ipragliflozin in SDT rats may in part be caused by the significant increase in SGLT2 expression (~60%). On the other hand, other studies have reported that treatment with SGLT2 inhibitors decreases or does not change SGLT2 expression in the kidney (6, 7, 30, 39). Possible explanations for this discrepancy remain unclear but may relate to differences in animal models (SHRcp rats, OLETF rats, and BTBR ob/ob mice versus SD and SDT rats) (6, 7, 30, 39).
Renal SGLT1 expression was decreased in SDT versus SD rats, and ipragliflozin reduced SGLT1 expression in the kidney of SD rats. As discussed in previous studies (43, 44), glucose transport through SGLT1 is increased in diabetes or when glucose reabsorption via SGLT2 in the upstream early proximal tubule is inhibited by genetic or pharmacologic means. Thus, the renal membrane SGLT1 expression may be downregulated under these conditions to limit glucose reabsorption and glucotoxicity in the distal segments of the proximal tubule (43, 44).
Does SGLT2 inhibition affect the renal expression of other sodium and water transporting proteins in the kidney? The renal expression of AQP1, a channel that mediates water transport in proximal tubules and thin descending limbs of Henle’s loop (47), was reduced in SDT rats compared with SD rats, and ipragliflozin attenuated this effect. The mechanisms underlying the renal downregulation of AQP1 in diabetic rats and the recovery in response to ipragliflozin are unclear, although an influence of hyperosmotic agents and impermeable osmolytes (like glucose) on AQP1 expression has been well established (42). Nevertheless, the changes in AQP1 are expected to facilitate diuresis in diabetic rats, whereas AQP1 upregulation in response to ipragliflozin in the diabetic setting might induce a compensatory upregulation of water reabsorption in the late proximal tubule and/or thin descending limbs of Henle’s loop. The renal expression of furosemide-sensitive NKCC2 in the thick ascending limb of Henle’s loop, which reabsorbs ~25% of the filtered Na+ (3, 13), was unchanged in SD rats treated with ipragliflozin. Likewise, the renal protein expression of other distal nephron sodium transporters, namely NCC and ENaC (α-ENaC, β-ENaC, and γ-ENaC), was also not significantly altered in SD rats in response to ipragliflozin. NHE3 contributes to renal Na+ reabsorption in the proximal tubule and thick ascending limb (47), and SGLT2 may be functionally linked to NHE3 (5, 29). The protein expression of NHE3 and its phosphorylation at S605 and S552 were not changed by ipragliflozin in nondiabetic SD rats. However, ipragliflozin enhanced the renal expression of NHE3 phosphorylated at S605 in diabetic SDT rats, similar to the previous observation with the SGLT2 inhibitor empagliflozin in diabetic Akita/+ mice, whereas the effect was not observed in nondiabetic control mice (5). Because the phosphorylation of NHE3 at S605 has been associated with reduced NHE3 activity (31), the enhancement of NHE3 phosphorylation at S605 in SDT rats with ipragliflozin may indicate inhibition of NHE3. The underlying mechanisms why NHE3 phosphorylation at S605 is increased in diabetic rats and mice but not in nondiabetic rodents in response to SGLT2 inhibition remains unclear, but the lowering of blood pressure in SDT rats with ipragliflozin could be partially related to the inhibition of NHE3-mediated Na+ reabsorption. Further investigation is required to determine the interaction between SGLT2 inhibition, functional activity of NHE3, and blood pressure in nondiabetic and diabetic settings.
In diabetic SDT rats, ipragliflozin did not change food and fluid intake, urine volume, and urinary glucose and Na+ excretion. Similar to our results, genetic and pharmacological inhibition of SGLT2 in diabetic mice did not increase glucosuria in steady state (43, 45). The reduction in glomerular filtration of glucose by ipragliflozin (~47% reduction of serum glucose but no significant change of creatinine clearance in the present study) appeared large enough to nullify its impact of reducing tubular reabsorption (~42% reduction of fractional glucose reabsorption). Potentially as a consequence of similar glucosuria and urinary calorie loss, ipragliflozin did not stimulate food intake (Na+ intake) in SDT rats, which may contribute to the observed unchanged urinary Na+ excretion. Moreover, similar increases in urinary glucose and Na+ excretion between SDT rats with and without ipragliflozin trigger similar levels of osmotic diuresis and, as a consequence, fluid intake.
Ipragliflozin numerically decreased blood pressure both in nondiabetic and diabetic rats. In addition to the natriuretic and diuretic effect of inhibiting SGLT2, the observed BW loss and fat mass reduction in nondiabetic SD rats may have contributed to the decrease in blood pressure. Recent studies have shown that SGLT2 inhibitors can also turn the circadian rhythm of blood pressure from a nondipper to a dipper pattern, which was associated with increased urinary Na+ excretion (30, 39). Further studies are necessary to better understand the mechanism for reducing blood pressure in nondiabetic and diabetic animals with SGLT2 inhibitors.
In conclusion, chronic application of the SGLT2 inhibitor ipragliflozin induces a sustained diuretic and natriuretic effect that is not secondary to enhanced food and fluid intake. The pair feeding and drinking experiment unmasked this effect and showed that it can cause fluid depletion. Body fluid volume is maintained in response to SGLT2 inhibition, however, because of activation of compensatory homeostatic mechanisms that stabilize body fluid volume, including increases in food and fluid intake.
GRANTS
This study was supported in part by Grant-in-Aid for Young Scientists 15K21321 to T. Masuda; Jichi Medical University Young Investigator Award to T. Masuda; NIH Grants R01DK112042 and R01DK106102, and UAB/UCSD O’Brien Core Center for Acute Kidney Injury Research NIH Grant P30DK079337 to V. Vallon; and Japan Agency for Medical Research and Development (AMED) Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases to D. Nagata.
DISCLOSURES
Astellas Pharma (Tokyo, Japan) provided ipragliflozin for this project. Over the past 36 months, V. Vallon has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Intarcia Therapeutics, Astra-Zeneca, Janssen Pharmaceutical, Eli Lilly, and Merck and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.M. and V.V. conceived and designed research; T.M., Y.W., K.F., and M.W. performed experiments; T.M., K.F., M.W., and V.V. analyzed data; T.M., A.O., and K.O. interpreted results of experiments; T.M. prepared figures; T.M. and V.V. drafted manuscript; T.M., A.O., K.O., T.I., H.K., S.M., V.V., and D.N. edited and revised manuscript; T.M., A.O., K.O., T.I., H.K., S.M., V.V., and D.N. approved final version of manuscript.
REFERENCES
- 1.Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008. doi: 10.1152/ajpcell.00180.2008. [DOI] [PubMed] [Google Scholar]
- 2.Chapman ME, Hu L, Plato CF, Kohan DE. Bioimpedance spectroscopy for the estimation of body fluid volumes in mice. Am J Physiol Renal Physiol 299: F280–F283, 2010. doi: 10.1152/ajprenal.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards A, Castrop H, Laghmani K, Vallon V, Layton AT. Effects of NKCC2 isoform regulation on NaCl transport in thick ascending limb and macula densa: a modeling study. Am J Physiol Renal Physiol 307: F137–F146, 2014. doi: 10.1152/ajprenal.00158.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Y, Gerasimova M, Batz F, Kuczkowski A, Alam Y, Sanders PW, Ronzaud C, Hummler E, Vallon V. PPARγ agonist-induced fluid retention depends on αENaC expression in connecting tubules. Nephron 129: 68–74, 2015. doi: 10.1159/000370254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y, Gerasimova M, Mayoux E, Masuda T, Vallon V. SGLT2 inhibitor empagliflozin increases renal NHE3 phosphorylation in diabetic Akita mice: possible implications for the prevention of glomerular hyperfiltration (Abstract) Diabetes 63, Suppl 1: A132, 2014. [Google Scholar]
- 6.Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, Harvie BM, Kinneally TL, Yeh SM, McCarthy DA, Koepsell H, Vallon V, Pollock C, Panchapakesan U, Forbes JM. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep 6: 26428, 2016. [Erratum in Sci Rep 6: 28124, 2016.] 10.1038/srep26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, Hugo C. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 307: F317–F325, 2014. doi: 10.1152/ajprenal.00145.2014. [DOI] [PubMed] [Google Scholar]
- 8.González AA, Céspedes C, Villanueva S, Michea L, Vio CP. E Prostanoid-1 receptor regulates renal medullary alphaENaC in rats infused with angiotensin II. Biochem Biophys Res Commun 389: 372–377, 2009. doi: 10.1016/j.bbrc.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 9.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196, 2012. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi M, Arima H, Goto M, Banno R, Watanabe M, Sato I, Nagasaki H, Oiso Y. Vasopressin gene transcription increases in response to decreases in plasma volume, but not to increases in plasma osmolality, in chronically dehydrated rats. Am J Physiol Endocrinol Metab 290: E213–E217, 2006. doi: 10.1152/ajpendo.00158.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hirose S, Nakajima S, Iwahashi Y, Seo A, Takahashi T, Tamori Y. Impact of the 8-week administration of tofogliflozin for glycemic control and body composition in Japanese patients with type 2 diabetes mellitus. Intern Med 55: 3239–3245, 2016. doi: 10.2169/internalmedicine.55.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang DY, Pfaff I, Serradeil-Le Gal C, Vallon V. Acute renal response to the non-peptide vasopressin V2-receptor antagonist SR 121463B in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol 362: 201–207, 2000. doi: 10.1007/s002100000282. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B. Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol 310: F958–F971, 2016. doi: 10.1152/ajprenal.00476.2015. [DOI] [PubMed] [Google Scholar]
- 14.Imai T, Akimoto T, Ito C, Masuda T, Nagata D. Management of diabetes associated with nephrotic syndrome: therapeutic potential of dapagliflozin for protracted volume retention. Drug Target Insights 9: 29–31, 2015. doi: 10.4137/DTI.S31710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito M, Tanaka T. The anticipated renoprotective effects of sodium-glucose cotransporter 2 inhibitors. Intern Med 57: 2105–2114, 2018. doi: 10.2169/internalmedicine.9842-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwazu Y, Muto S, Fujisawa G, Nakazawa E, Okada K, Ishibashi S, Kusano E. Spironolactone suppresses peritubular capillary loss and prevents deoxycorticosterone acetate/salt-induced tubulointerstitial fibrosis. Hypertension 51: 749–754, 2008. doi: 10.1161/HYPERTENSIONAHA.107.104901. [DOI] [PubMed] [Google Scholar]
- 17.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93: 397–404, 1994. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemter E, Rathkolb B, Bankir L, Schrewe A, Hans W, Landbrecht C, Klaften M, Ivandic B, Fuchs H, Gailus-Durner V, Hrabé de Angelis M, Wolf E, Wanke R, Aigner B. Mutation of the Na(+)-K(+)-2Cl(-) cotransporter NKCC2 in mice is associated with severe polyuria and a urea-selective concentrating defect without hyperreninemia. Am J Physiol Renal Physiol 298: F1405–F1415, 2010. doi: 10.1152/ajprenal.00522.2009. [DOI] [PubMed] [Google Scholar]
- 19.Kusaka H, Koibuchi N, Hasegawa Y, Ogawa H, Kim-Mitsuyama S. Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc Diabetol 15: 157, 2016. doi: 10.1186/s12933-016-0473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853–862, 2013. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim-Mitsuyama S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol 13: 148, 2014. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol 302: F308–F315, 2012. doi: 10.1152/ajprenal.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuda T, Fu Y, Eguchi A, Czogalla J, Rose MA, Kuczkowski A, Gerasimova M, Feldstein AE, Scadeng M, Vallon V. Dipeptidyl peptidase IV inhibitor lowers PPARγ agonist-induced body weight gain by affecting food intake, fat mass, and beige/brown fat but not fluid retention. Am J Physiol Endocrinol Metab 306: E388–E398, 2014. doi: 10.1152/ajpendo.00124.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda T, Muto S, Fujisawa G, Iwazu Y, Kimura M, Kobayashi T, Nonaka-Sarukawa M, Sasaki N, Watanabe Y, Shinohara M, Murakami T, Shimada K, Kobayashi E, Kusano E. Heart angiotensin II-induced cardiomyocyte hypertrophy suppresses coronary angiogenesis and progresses diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 302: H1871–H1883, 2012. doi: 10.1152/ajpheart.00663.2011. [DOI] [PubMed] [Google Scholar]
- 25.Masuda T, Ohara K, Murakami T, Imai T, Nakagawa S, Okada M, Miki A, Myoga A, Onishi A, Sekiguchi C, Miyazawa Y, Akimoto T, Saito O, Muto S, Nagata D. Sodium-glucose cotransporter 2 inhibition with dapagliflozin ameliorates extracellular volume expansion in diabetic kidney disease patients. POJ Diabetes Obesity Management 1: 1–8, 2017. [Google Scholar]
- 26.Montiel V, Leon Gomez E, Bouzin C, Esfahani H, Romero Perez M, Lobysheva I, Devuyst O, Dessy C, Balligand JL. Genetic deletion of aquaporin-1 results in microcardia and low blood pressure in mouse with intact nitric oxide-dependent relaxation, but enhanced prostanoids-dependent relaxation. Pflugers Arch 466: 237–251, 2014. doi: 10.1007/s00424-013-1325-x. [DOI] [PubMed] [Google Scholar]
- 27.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 17: 573–580, 2011. [Errata in Nat Med 17:1020, 2011; Nat Med 18: 630, 2012.] 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- 28.Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Renal Physiol 313: F135–F140, 2017. doi: 10.1152/ajprenal.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25: 2028–2039, 2014. doi: 10.1681/ASN.2013060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman A, Kittikulsuth W, Fujisawa Y, Sufiun A, Rafiq K, Hitomi H, Nakano D, Sohara E, Uchida S, Nishiyama A. Effects of diuretics on sodium-dependent glucose cotransporter 2 inhibitor-induced changes in blood pressure in obese rats suffering from the metabolic syndrome. J Hypertens 34: 893–906, 2016. doi: 10.1097/HJH.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 31.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–F971, 2012. doi: 10.1152/ajprenal.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, Vallon V. Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC. Am J Pathol 182: 96–106, 2013. doi: 10.1016/j.ajpath.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabolić I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasase T, Ohta T, Masuyama T, Yokoi N, Kakehashi A, Shinohara M. The spontaneously diabetic torii rat: an animal model of nonobese type 2 diabetes with severe diabetic complications. J Diabetes Res 2013: 976209, 2013. doi: 10.1155/2013/976209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinohara M, Masuyama T, Shoda T, Takahashi T, Katsuda Y, Komeda K, Kuroki M, Kakehashi A, Kanazawa Y. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. Int J Exp Diabetes Res 1: 89–100, 2000. doi: 10.1155/EDR.2000.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Qun L, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Antidiabetic effects of SGLT2-selective inhibitor ipragliflozin in streptozotocin-nicotinamide-induced mildly diabetic mice. J Pharmacol Sci 120: 36–44, 2012. doi: 10.1254/jphs.12089FP. [DOI] [PubMed] [Google Scholar]
- 38.Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Qun L, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol 385: 423–436, 2012. doi: 10.1007/s00210-011-0713-z. [DOI] [PubMed] [Google Scholar]
- 39.Takeshige Y, Fujisawa Y, Rahman A, Kittikulsuth W, Nakano D, Mori H, Masaki T, Ohmori K, Kohno M, Ogata H, Nishiyama A. A sodium-glucose co-transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt-treated obese rats. Hypertens Res 39: 415–422, 2016. doi: 10.1038/hr.2016.2. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi T, Dohi K, Omori T, Moriwaki K, Sato Y, Nakamori S, Fujimoto N, Fujii E, Yamada N, Ito M. Diuretic effects of sodium-glucose cotransporter 2 inhibitor in patients with type 2 diabetes mellitus and heart failure. Int J Cardiol 201: 1–3, 2015. [Erratum in Int J Cardiol 206: 173, 2016.] 10.1016/j.ijcard.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H, Takano K, Iijima H, Kubo H, Maruyama N, Hashimoto T, Arakawa K, Togo M, Inagaki N, Kaku K. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther 34: 436–451, 2017. doi: 10.1007/s12325-016-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umenishi F, Schrier RW. Hypertonicity-induced aquaporin-1 (AQP1) expression is mediated by the activation of MAPK pathways and hypertonicity-responsive element in the AQP1 gene. J Biol Chem 278: 15765–15770, 2003. doi: 10.1074/jbc.M209980200. [DOI] [PubMed] [Google Scholar]
- 43.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallon V, Schwark JR, Richter K, Hropot M. Role of Na(+)/H(+) exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol 278: F375–F379, 2000. doi: 10.1152/ajprenal.2000.278.3.F375. [DOI] [PubMed] [Google Scholar]
- 47.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60: 215–225, 2017. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallon V, Verkman AS, Schnermann J. Luminal hypotonicity in proximal tubules of aquaporin-1-knockout mice. Am J Physiol Renal Physiol 278: F1030–F1033, 2000. doi: 10.1152/ajprenal.2000.278.6.F1030. [DOI] [PubMed] [Google Scholar]
- 49.West CA, Sasser JM, Baylis C. The enigma of continual plasma volume expansion in pregnancy: critical role of the renin-angiotensin-aldosterone system. Am J Physiol Renal Physiol 311: F1125–F1134, 2016. doi: 10.1152/ajprenal.00129.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada K, Nakayama H, Yoshinobu S, Kawano S, Tsuruta M, Nohara M, Hasuo R, Akasu S, Tokubuchi I, Wada N, Hirao S, Iwata S, Kaku H, Tajiri Y. Effects of a sodium glucose co-transporter 2 selective inhibitor, ipragliflozin, on the diurnal profile of plasma glucose in patients with type 2 diabetes: A study using continuous glucose monitoring. J Diabetes Investig 6: 699–707, 2015. doi: 10.1111/jdi.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol 287: F896–F906, 2004. doi: 10.1152/ajprenal.00160.2004. [DOI] [PubMed] [Google Scholar]