Abstract
Ventilation and cerebral blood flow (CBF) are both sensitive to hypoxia and hypercapnia. To compare chemosensitivity in these two systems, we made simultaneous measurements of ventilatory and cerebrovascular responses to hypoxia and hypercapnia in 35 normal human subjects before and after acclimatization to hypoxia. Ventilation and CBF were measured during stepwise changes in isocapnic hypoxia and iso-oxic hypercapnia. We used MRI to quantify actual cerebral perfusion. Measurements were repeated after 2 days of acclimatization to hypoxia at 3,800 m altitude (partial pressure of inspired O2 = 90 Torr) to compare plasticity in the chemosensitivity of these two systems. Potential effects of hypoxic and hypercapnic responses on acute mountain sickness (AMS) were assessed also. The pattern of CBF and ventilatory responses to hypercapnia were almost identical. CO2 responses were augmented to a similar degree in both systems by concomitant acute hypoxia or acclimatization to sustained hypoxia. Conversely, the pattern of CBF and ventilatory responses to hypoxia were markedly different. Ventilation showed the well-known increase with acute hypoxia and a progressive decline in absolute value over 25 min of sustained hypoxia. With acclimatization to hypoxia for 2 days, the absolute values of ventilation and O2 sensitivity increased. By contrast, O2 sensitivity of CBF or its absolute value did not change during sustained hypoxia for up to 2 days. The results suggest a common or integrated control mechanism for CBF and ventilation by CO2 but different mechanisms of O2 sensitivity and plasticity between the systems. Ventilatory and cerebrovascular responses were the same for all subjects irrespective of AMS symptoms.
NEW & NOTEWORTHY Ventilatory and cerebrovascular hypercapnic response patterns show similar plasticity in CO2 sensitivity following hypoxic acclimatization, suggesting an integrated control mechanism. Conversely, ventilatory and cerebrovascular hypoxic responses differ. Ventilation initially increases but adapts with prolonged hypoxia (hypoxic ventilatory decline), and ventilatory sensitivity increases following acclimatization. In contrast, cerebral blood flow hypoxic sensitivity remains constant over a range of hypoxic stimuli, with no cerebrovascular acclimatization to sustained hypoxia, suggesting different mechanisms for O2 sensitivity in the two systems.
Keywords: hypoxic ventilatory response, cerebral blood flow, high altitude, hypoxia, magnetic resonance imaging, acute mountain sickness
during adaptation to hypoxia, the partial pressures of arterial carbon dioxide () and oxygen play a major role in cerebral blood flow (CBF) regulation. Hypoxia per se is a potent vasodilator, but the falling , as a result of hypoxic hyperventilation, opposes this dilation (33, 46, 57), leading to vasoconstriction, which may reduce the magnitude of any increases in CBF and has the potential to decrease CBF (26, 62). The overall outcome is generally increased CBF (15, 25, 51); however, the picture may be mixed depending on the balance between hypocapnic and hypoxic influences [reviewed in Dyer et al. (15)]. The role of in regulating CBF has been well described [reviewed in Ainslie and Duffin (1)], and an emerging concept is that cerebrovascular reactivity and ventilatory response to are tightly linked. Cerebrovascular reactivity to CO2 has received much attention (1, 2); however, the influence of hypoxia on the cerebral circulation has received considerably less focus, especially in humans (2, 48). CBF initially rises on ascent to altitude (25, 51, 53). However, it is not clear if there is adaptation for this response following sustained hypoxia exposure. Some reports indicate no changes in CBF hypoxia reactivity (28), whereas others find evidence of CBF adaptation (25, 51).
In the context of the cerebral hemodynamic response to hypoxia, tissue perfusion is typically the true variable of interest rather than indirect measures, such as arterial velocity in proximal arteries (15, 19, 42). Several techniques have been proposed for measuring CBF, and the results vary depending on the degree of hypoxia encountered, as well as the technique used [reviewed by Dyer et al. (15)]. Although commonly referred to as a measure of CBF, the different methods are not measuring exactly the same physiological process. Inert gas techniques (51) and xenon-133 (25) make measurements of global or broadly regional perfusion. Radionucleotide single photon computed emission tomography (37) or PET (7) maps the quantified or relative regional cerebral perfusion. Due to its ease of use and noninvasive nature, previous studies have also promoted Doppler sonography measures of arterial velocity in the proximal intracranial or neck arteries (20, 23, 42, 44, 56, 59), but changes in vessel diameter during hypoxia or hypercapnia can compound estimates of actual CBF from these proximal velocity measurements (19). For these studies, we used MRI to measure CBF, as this provides a quantified measure of true regional cerebral perfusion (i.e., milliliter per minute of blood delivered to the capillary bed per 100 ml cerebral tissue).
The aims of this study were to investigate potential coupling between CBF and ventilation responses for hypoxic and hypercapnic stimuli and to address to what degree these adapt during sustained high-altitude hypoxia. We also investigated what impact this may have on symptoms of acute mountain sickness (AMS). We examined the isocapnic hypoxic ventilatory response (HvR) and isocapnic hypoxic CBF response (HcbfR). Furthermore, we compared the iso-oxic hypercapnic ventilatory response (HcvR) and iso-oxic hypercapnic CBF response (HccbfR). To evaluate the differential impact of acclimatization to sustained hypoxia on these two systems, measurements were repeated following 2 days acclimatization to high altitude.
METHODS
Subjects
Thirty-five healthy, nonsmoking, sea-level residents were recruited: 19 men (age 28 ± 8 yr) and 16 women (age 28 ± 8 yr). Ethical approval for these studies was granted by the Human Research Protection Program of the University of California San Diego. Participants were informed of the experimental procedures and possible risks involved in the study, and written, informed consent was obtained before participation.
Study Design
Measurements of isocapnic HvR used the steady-state paradigm (21, 50, 55), with additional gas challenges to assess the iso-oxic HcvR to hypercapnia and combined hypoxic + hypercapnic stimuli (HHcvR). Resting-state CBF was measured simultaneously using MRI to evaluate the corresponding HcbfR and HccbfR (HHccbfR). These baseline sea-level measurements were repeated after 2 days of acclimatization to sustained hypoxia at altitude. Mean CBF, end-tidal (), arterial oxygen saturation of hemoglobin (), and ventilation were compared for subjects symptomatic for AMS (AMS group) and for those who remained asymptomatic (no-AMS group).
Hypoxic Exposure
For hypoxic exposure, subjects resided at high altitude at the Pace Laboratory at White Mountain Research Station [Bishop, CA; 3800 m, partial pressure of inspired O2 () 90 Torr]. Subjects spent two nights at altitude and then returned to San Diego for MRI. For each subject, the , achieved after 40 h of hypoxic exposure at altitude, was maintained throughout the ~8 h of transportation. This was accomplished via a venturi mask with variable %N2 in the inspiratory port (to maintain consistent despite changing altitude and barometric pressure). Subjects were switched to ambient air to allow ~30 min of normoxia immediately before measurements started. was intermittently monitored while at altitude and continuously monitored during transportation and MRI studies.
Hypoxic and Hypercapnic Responses
Measurements were made while supine within the MRI scanner. Subjects breathed via a close-fitting, low-deadspace, nonrebreathing mask (7900/2600; Hans Rudolph, Shawnee, KS), connected to a partial rebreathing circuit (50). Fresh gas at different partial pressures of O2/N2/CO2 was introduced into the circuit to achieve the desired level of hypoxia or hypercapnia. A large-volume exhaust port ensured that the circuit remained at ambient pressure. A “turbine” flow meter was incorporated into the expiratory limb of the circuit to measure ventilation (Ohmeda 7800 tidal volume monitor, GE healthcare, Chicago, IL). The gas challenge was a step-hypoxia paradigm (Fig. 1) (21, 50). Subjects were first connected to the breathing circuit and allowed to acclimate to the apparatus and reach a steady-state respiration, during which was noted. Subjects breathed a slightly hyperoxic mixture (~200 Torr) to achieve 100% O2 saturation. Inspired CO2 was increased by ~3 Torr to allow for a better dynamic range of control. Subjects acclimated for a further ~10 min to allow steady-state ventilation and CBF before starting measurements. was then held constant (isocapnia) throughout the first six steps of the paradigm and increased by ~5 Torr for the last two steps (Fig. 1).
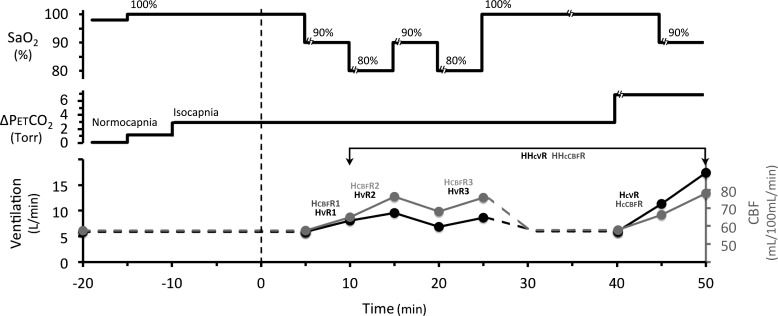
Fig. 1.
Time course of step changes in O2 saturation (; top) and (middle) for hypoxic and hypercapnic responses; bottom: time courses of ventilation and CBF responses. The paradigm is described in detail in Sato et al. (50). The initial 5 min is poikilocapnic ventilation while subjects acclimatize to the breathing apparatus. The rebreathing circuit is then connected, and after a further 5 min of hyperoxia, a small increase in (~3 Torr) is introduced to achieve isocapnic ventilation. The response measurements start once steady-state ventilation (or steady-state CBF) is achieved (indicated as 0 time on the timeline). The changes following step changes define the hypoxic response. Subjects are then again returned to hyperoxia, and after a 10-min period to remove any residual hypoxic drive, the hypercapnic and combined hypoxic hypercapnic responses are measured.
The CBF and ventilatory response to the step-hypoxia paradigm were determined during 5 min periods of steady-state measurement using the following sequence: 1) 100% , 2) 90% , 3) 80% , 4), 90% , and 5) 80% (see Fig. 1). The HvR was defined as –(∆ventilation/∆), and the HcbfR was defined as –(∆CBF/∆) when oxygen was decreased (Fig. 1, and see Table 2). This resulted in three measures of O2 sensitivity for both variables: 1) desaturation from 100 to 90% (HvR1, HcbfR1), 2) 90 to 80% (HvR2, HcbfR2), and 3) another 90 to 80% after 10 min of hypoxia (HvR3, HcbfR3), which allowed us to measure the impact of hypoxic ventilatory decline (or its cerebrovascular equivalent).
Table 2.
Results table
| Baseline (n = 35) | Acclimatized (n = 35) | |||
|---|---|---|---|---|
| Chemosensitivity | ||||
| HvR1 | 100–90% > | 0.26 (0.21) | 0.64 (0.54)ab | l·min−1·%sat−1 |
| HvR2 | 90–80% (1) | 0.12 (0.15)c | 0.26 (0.26)ab | l·min−1·%sat−1 |
| HvR3 | 90–80% (2) | 0.19 (0.20) | 0.29 (0.37) | l·min−1·%sat−1 |
| HcvR | (100%)–(100% + CO2) | 1.08 (0.95) | 1.63 (1.30)ad | l·min−1·Torr−1 |
| HHcvR | (90%)–(90% + CO2) | 1.79 (1.09)e | 2.21 (2.62) | l·min−1·Torr−1 |
| HcbfR1 | 100–90% | 0.88 (0.75) | 0.88 (0.66) | ml·100 ml−1·min−1·%sat−1 |
| HcbfR2 | 90–80% (1) | 1.03 (0.44) | 1.02 (0.52) | ml·100 ml−1·min−1·%sat−1 |
| HcbfR3 | 90–80% (2) | 0.83 (0.57) | 0.99 (0.64) | ml·100 ml−1·min−1·%sat−1 |
| HccbfR | (100%)–(100% + CO2) | 1.62 (1.80) | 2.74 (1.97) | ml·100 ml−1·min−1·Torr−1 |
| HHccbfR | (90%)–(90% + CO2) | 2.51 (1.81)e | 4.74 (7.72) | ml·100 ml−1·min−1·Torr−1 |
| Resting V̇ and CBF | ||||
| V̇100% | HvR1 at 100% | 5.88 (1.68) | 7.28 (2.37)a | l/min |
| V̇100% (CO2 reset) | CO2-corrected HcvR | 18.47 (19.70) | l/min | |
| V̇85%,HvR2 | HvR2 at 85% | 8.82 (2.82) | 13.58 (6.27) | l/min |
| V̇85%,HvR3 | HvR3 at 85% | 7.55 (2.37) | 10.89 (4.81) | l/min |
| ∆V̇85%,HvR2– HvR3 | HvR2– HvR3 at 85% | 1.27 (1.76)f | 2.69 (3.30)ad | l/min |
| CBF100% | HcbfR1 at 100% | 57.50 (13.01) | 60.98 (14.53) | ml·100 ml−1·min−1 |
| CBF100% (CO2 reset) | CO2-corrected HccbfR | 81.08 (32.09) | ml·100 ml−1·min−1 | |
| CBF85%,HcbfR2 | HcbfR2 at 85% | 70.32 (16.21) | 73.65 (16.58) | ml·100 ml−1·min−1 |
| CBF85%,HcbfR3 | HcbfR3 at 85% | 71.10 (16.50) | 73.74 (16.32) | ml·100 ml−1·min−1 |
| ∆CBF85%,HcbfR2–3 | HcbfR2–HcbfR3 85% | −0.78 (3.44) | −0.08 (3.37) | ml·100 ml−1·min−1 |
Sensitivity of ventilation (V̇) and cerebral blood flow (CBF) to hypoxia and hypercapnia and absolute values of V̇ and CBF breathing different gases before (Baseline) and after acclimatization to 2 days of sustained hypoxia. Data are means (1 SD). HvR1, -2, and -3 and HcbfR1, -2, and -3 are the terms that describe the HvRs to O2 desaturation from 100 to 90%, 90 to 80%, and 90 to 80% for a second time (respectively; see Fig. 1). HcvR is the ventilatory sensitivity to an increase in by ~5 Torr during normoxia (50). HHcvR is the ventilatory sensitivity to combined hypoxic and hypercapnic changes [~5 Torr increase in , decreased to 90%]. HcbfR, HccbfR, and HHccbfR are the equivalent sensitivities for CBF. V̇100% and V̇85% are ventilation at 100 and 85% , respectively. V̇100%(CO2 reset) defines the equivalent resting ventilation referred back to the baseline CO2 set point (i.e., “corrected” set point) to assess the full impact on ventilation of both acclimatization and the altered CO2 set point (see Figs. 4 and 5); ΔV̇85% defines the change in ventilation due to hypoxic ventilatory decline assessed at 85% saturation between HvR2 and HvR3. Equivalent measurements were made for CBF responses.
P < 0.05 (relative to baseline).
P = 0.06 (greater change for no-AMS, trend only).
P < 0.05 (relative to HvR1 at baseline).
P < 0.05 (greater change for no-AMS).
P < 0.05 (relative to normoxic hypercapnia at baseline).
P < 0.05.
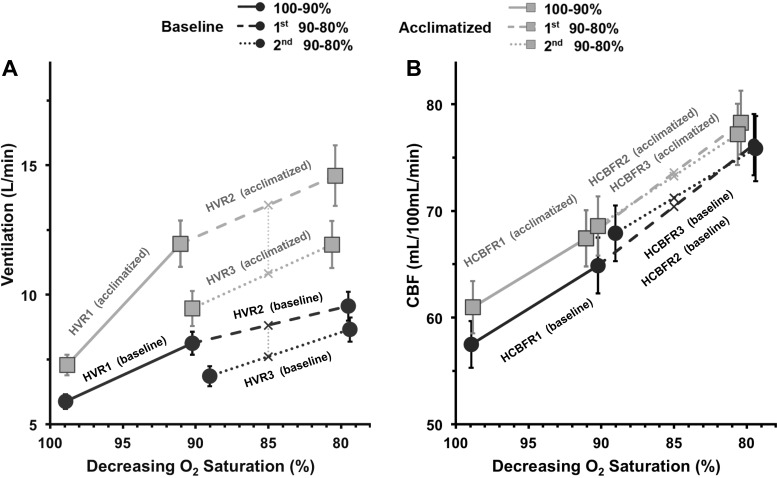
There are two measurements of interest for the hypoxic responses (illustrated in Fig. 2): 1) changes in HvR and HcbfR, which quantify the change in O2 sensitivity as the slope of the lines relating ventilation or CBF to O2 desaturation, and 2) changes in the position of the curves, independent of changes in slope, which provide evidence of hypoxic ventilatory or CBF decline. This can be quantified as the decrease in ventilation or CBF estimated at = 85% between the second and third measurements (4). Subjects returned to 10 min of mild hyperoxia again to remove any residual hypoxic ventilatory drive before measuring hypercapnic responses. Then was increased 5 Torr to quantify CO2 sensitivity as the increase in ventilation or CBF per Torr increase in (HcvR, HccbfR). Finally, we quantified the combined hypoxic and hypercapnic sensitivity with a step change to 90% , with held constant at 5 Torr increase (HHcvR, HHccbfR). In addition to measuring CO2 sensitivity as the slopes of ventilation or CBF vs. , the data show changes in absolute values for baseline ventilation and CBF, as well as resting (see Fig. 4). Adequate time was allowed during step changes to measure 5 min of steady-state ventilation and CBF. The entire measurement paradigm took ~50 min (Fig. 1).
Fig. 2.
Sensitivity to hypoxia for both ventilation (A) and cerebral blood flow (CBF; B). HvR1, -2, and -3 and corresponding HcbfR1, -2, and -3 are the slopes of the lines indicated on the graphs. Absolute values of ventilation and CBF are estimated from these lines at 85% O2 saturation (V̇85% and CBF85%, indicated by “×”). A: following acclimatization, there was a significant increase in resting ventilation during normoxia between baseline and acclimatization to sustained hypoxia (P < 0.0005). There were also significant increases in HvR1 (P < 0.0005) and HvR2 (P < 0.01) and a greater decrease in V̇85% between HvR2 and HvR3 measurements (P < 0.01) following acclimatization. B: by contrast, following acclimatization, the resting CBF in normoxia was not significantly changed. HcbfR1, -2, and -3 did not change with acclimatization, and there was no significant difference in CBF85% between HcbfR measurements 2 and 3, before or after acclimatization. Data are mean values; error bars indicate 1 SE.
Fig. 4.
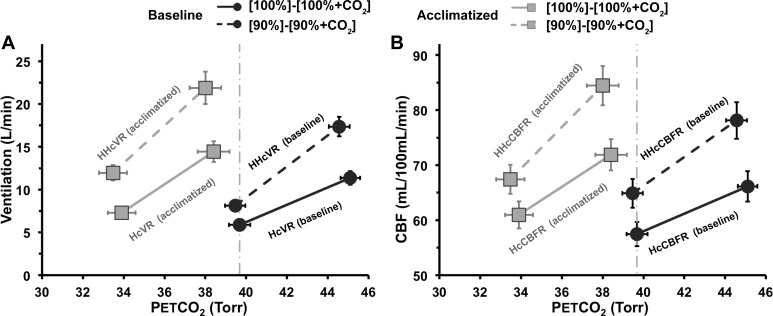
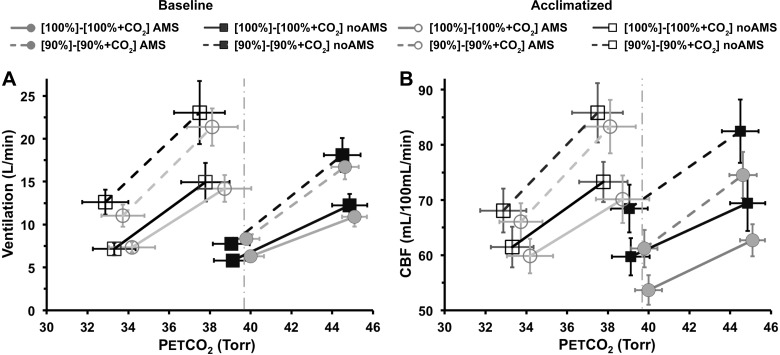
Hypercapnic and combined hypoxic/hypercapnic responses for ventilation (A) and CBF (B). After acclimatization, in normoxia decreases from ~40 Torr (dashed, vertical lines) to ~34 Torr, with a small increase in absolute value of ventilation and CBF. A: hypoxia significantly increased the ventilatory response to hypercapnia at baseline (HHcvR > HcvR, P < 0.0005) but not after acclimatization. The absolute value of ventilation in normoxia increased significantly with hypoxia at baseline and after acclimatization conditions (P < 0.05). B: the same pattern of changes and significant differences are seen for the CBF response to CO2 also. Data are mean values; error bars indicate 1 SE.
AMS Groups
To maximize a potential physiological difference among subjects at altitude, the Lake Louise Score (LLS), an AMS self-report questionnaire (47), was used to divide subjects into two distinct groups: 1) those with no symptoms of AMS (no-AMS group) and 2) those with unambiguous AMS (AMS group) (15). The Lake Louise AMS questionnaire is based on the responses regarding five different symptoms—headache, gastrointestinal symptoms, fatigue, dizziness, and difficulty sleeping—each graded 0–3 in severity. LLSs were determined in each subject on both days 1 and 2 (each immediately following a night at altitude). The mean of these scores was used to characterize subjects into AMS and no-AMS groups. Difficulty sleeping was not included as a criterion for AMS on the first night [to avoid confounds due to the rapid ascent (47)] but was scored on the subsequent day. Subjects with an LLS ≤2 or with no headache were considered AMS nonsufferers (no-AMS group). Those with an LLS ≥5 and a headache plus symptoms of nausea, fatigue, dizziness, or difficulty sleeping were considered unambiguous AMS sufferers (AMS group). Subjects with an intermediate score (3–4) and a headache were grouped into a third “intermediate” group and not included in the analysis when comparing AMS vs. no-AMS but were included in grouped analysis.
Physiological Measurements
Inspired and expired partial pressures of O2 and CO2 were continuously monitored during MRI measurements using a PerkinElmer 1100 medical gas spectrometer (PerkinElmer, Waltham, MA). Hemoglobin concentration was determined from the arterial blood sample using an IL-682 co-oximeter (Instrument Laboratories, Lexington, MA). Ventilation was measured with an Ohmeda 7800 tidal volume monitor (modified for use in the MRI scanner). Hematocrit was determined from direct measurements of packed cell height in a capillary tube following centrifuging. Arterial O2 saturation was measured and logged using a Nonin 3100 wrist pulse oximeter (at altitude and during transportation) and a Nonin 8600FO MRI-compatible pulse oximeter (Nonin Medical, Plymouth, MN; during MRI measurement) that were calibrated in each subject against an arterial blood sample. To ensure that the target hypoxic and cardiorespiratory state remained consistent at each step, throughout measurements, - and -calibrated signals were continuously monitored and recorded using custom LabVIEW (National Instruments, Austin, TX) and Matlab software (MathWorks, Natick, MA).
MRI Measurements—CBF
CBF was measured using a PICORE QUIPPS II arterial spin-labeling technique [echo time = 9.1 ms, repetition time = 2.5 s, inversion time (TI)1 = 700 ms, TI2 = 1,500 ms; 7, 6-mm slices]. The acquisition was ~5 min of steady-state data in each / state. Additional images were collected to determine proton density of cerebrospinal fluid and coil sensitivity profile to allow absolute quantitation of CBF from the MRI signal (16, 41, 61, 64). Measurements focused on gray matter of the superior cerebrum to avoid potential problems with slower transit times in white matter (58). Since 80% of CBF is to the gray matter, this does not have a significant impact on our results (40).
Data Analysis
Cerebral blood flow.
Raw arterial spin-labeled MRI data for each subjects were corrected for T1 relaxation of blood, based on measured (52). The effect of hematocrit on T1 relaxation was small enough to be ignored (~1.4% underestimate of CBF after acclimatization) (31). Images were corrected for cardiorespiratory physiological noise (45) and magnetic field inhomogeneities (36). A CBF time series was determined by averaging CBF at each time point over a cerebral gray-matter mask, generated from a separate high-resolution fast-spoiled gradient-recalled acquisition in the steady state T1-weighted, three-dimensional anatomical MRI (echo time = 4.2 ms, repetition time = 10.1 ms, TI = 450 ms, bandwidth 20.83 kHz, field of view 25 × 25 × 16 cm, matrix 256 × 256 × 128, ~1 × 1 × 1.3 mm resolution, 5.5 min). Cerebral gray matter was automatically segmented using FAST (FMRIB Software Library, Oxford, UK).
Physiological data.
Ventilation, , and were collected as continuous time courses (40 Hz), corresponding to the time series of the CBF MRI data. The mean ventilation, oxygen saturation, , and CBF were determined for each stage by computing the temporal average over these selected 5-min steady-state periods within each challenge.
Statistical analysis.
Data were analyzed with repeated-measures ANOVA of our primary outcome variables (ventilation/CBF slope during decreasing saturation or increasing , ventilation/CBF at normoxia and at 85% saturation set points), with two grouping variables (AMS, no-AMS) and two measurement levels (baseline, acclimatization to 2 days of hypoxia; StatView 5.0.1; SAS Institute, Cary, NC). This repeated-measures (“paired”) design is very powerful, as each subject acts as his/her own control. Data were expressed as means (SD). Changes were considered significant at P < 0.05 two tailed.
RESULTS
All of the 35 subjects analyzed had ventilation and CBF measurements for the two repeated time points, with the exception of four missing data points: 1) baseline ventilation and CBF data missing in one subject (man, no-AMS group), 2) baseline CBF data missing in another subject (woman, no-AMS group), 3) 2 day ventilation and CBF data missing in one subject (woman, intermediate group), and 4) 2 day ventilation and CBF data missing in another subject for gas states six to eight (man, no-AMS group).
Hypoxia
When compared with sea level/baseline, all subjects showed decreased arterial oxygen saturation following 2 days at 3,800 m altitude [ = 90 Torr; 98.2 (0.7)–83.4 (5.0)%, P < 0.0005] and reduced [39.4 (3.3)–31.8 (2.6) Torr, P < 0.0005], consistent with prolonged hypoxia and increased ventilatory drive. Subjects with symptomatic AMS (AMS group) showed a slightly lower mean arterial saturation [81.7 (6.1)%] than the asymptomatic (no-AMS) group [84.5 (2.5)%], but the difference between groups was not significant. Hematocrit was increased in all subjects relative to normoxia [39 (7)–41 (5)%], but this increase did not reach significance (Table 1).
Table 1.
Physiological changes during hypoxia
| Baseline | Acclimatization | |
|---|---|---|
| 38.6 (3.3) | 31.8 (2.4)* | |
| 37.5–39.7 | 31.0–32.6 | |
| 98.2 (0.9) | 83.2 (5.3)* | |
| 97.9–98.5 | 81.5–85.0 | |
| Hct | (n = 29) | (n = 34) |
| 39 (7) | 41 (5) | |
| 37–42 | 39–43 |
Physiological changes during baseline normoxia and after 2 days of acclimatization to hypoxia at 3,800 m altitude ( = 90 Torr). Data are means (1 SD) and 95% confidence limits. Units of end-tidal CO2 tension () are in Torr, arterial saturation () in percent saturation, and hematocrit (Hct) in percent.
P < 0.05.
Acute Mountain Sickness
Of the 35 subjects recruited, 15 developed criteria for AMS (LLS ≥5 and headache; 8 men, 7 women). A further 16 subjects met the criteria for no AMS (no-AMS; LLS ≤2 or no headache; 9 men, 7 women). The remaining four subjects were characterized as intermediate. They were included in the whole-group analysis (Fig. 2, and see Fig. 4) but were not included in AMS comparisons (Fig. 3 and see Fig. 5).
Fig. 3.
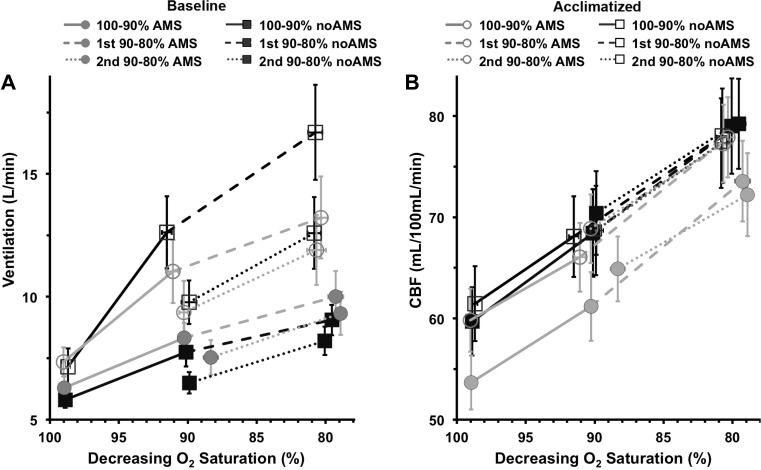
Changes in response to hypoxia, as illustrated in Fig. 2, but split by subjects who were symptomatic (AMS group, circular symbols) or asymptomatic (no-AMS group, square symbols) for AMS at altitude. A: there were no significant differences in the ventilation response with AMS. However, following acclimatization, asymptomatic subjects showed a trend toward a greater increase in HvR1 and HvR2 (i.e., slopes) relative to symptomatic subjects (P = 0.06). Asymptomatic subjects also had greater hypoxic ventilatory decline (decrease in ventilation estimated at 85% saturation between first and second 90–80% O2 saturation tests) after acclimatization (P < 0.05). B: during baseline, subjects symptomatic for AMS showed a lower CBF at all levels of hypoxia relative to asymptomatic or acclimatized subjects, but this was not significant, and there were no changes in O2 sensitivity (slopes). Data represent mean values; error bars indicate 1 SE.
Fig. 5.
Changes in response to hypercapnia, as illustrated in Fig. 4, but split by subjects who were symptomatic (AMS group, circular symbols) or asymptomatic (no-AMS group, square symbols) for AMS at altitude. Subjects symptomatic for AMS show a small but consistent reduced ventilation (A) and CBF (B) at all levels of during normoxia and hypoxia and at baseline and following acclimatization, but this was not significant. Dashed, vertical lines indicate resting before acclimatization. Data are mean values; error bars indicate 1 SE.
Hypoxic Ventilatory Response
At baseline, the sensitivity of ventilation to O2 desaturation decreased with prolonged exposure; the HvR1 slope was significantly greater than the HvR2 slope (P < 0.005). HvR3 showed no significant change in slope from HvR2, but there was a decrease in ventilation at the = 85% midpoint (V̇85%,HVR2 − V̇85%,HVR3, P < 0.0005), consistent with hypoxic ventilatory decline (Fig. 2A).
Following 2 days of acclimatization, there was a significant increase in baseline resting ventilation at normoxia (V̇100%; P < 0.05). The HvR1 slope increased after acclimatization, indicating an increase in hypoxia sensitivity (P < 0.0005 relative to baseline). The postacclimatization HvR2 slope also showed an increase relative to HvR2 at baseline (P < 0.01). The postacclimatization HvR3 slope showed no significant change in hypoxia sensitivity relative to postacclimatization HvR2 or baseline HvR3. Postacclimatization ventilation at = 85% during HvR3 was reduced relative to that at = 85% during HvR2 (V̇85%,HVR2 − V̇85%,HVR3, P < 0.0001). The magnitude of this hypoxic ventilatory decline was also greater postacclimatization (P < 0.05).
Following acclimatization to sustained hypoxia, subjects who were asymptomatic (no-AMS group) showed a trend toward an increase in the HvR1 and HvR2 slopes relative to symptomatic subjects (AMS group; acclimatization state × AMS group interaction, P = 0.06; Fig. 3A). There were no significant differences in the HvR1 or HvR2 slope among AMS groups before acclimatization (Fig. 3A). Ventilation at 85% (V̇85%) also increased more in asymptomatic subjects than in subjects with AMS (V̇85% × AMS group interaction, P < 0.05).
Hypoxic CBF Response
At baseline, initial sensitivity of CBF to O2 desaturation (HcbfR1) did not change significantly during further desaturation (HcbfR2 or HcbfR3). There was no significant change in CBF at the = 85% midpoint between CBF85%,HCBFR2 and CBF85%,HCBFR3 and no cerebrovascular equivalent of hypoxic ventilatory decline (Fig. 2B).
Following 2 days of acclimatization to sustained hypoxia, there was a small increase in resting CBF when compared with baseline, although this did not reach statistical significance [57.50 (13.01)–60.98 (14.53) ml·100 ml−1·min−1, P = not significant]. There was no significant change in HcbfR1, HcbfR2, or HcbfR3 slope with acclimatization. Likewise, there was no significant change in CBF85%,HCBFR2 and CBF85%,HCBFR3 and no postacclimatization CBF equivalent of hypoxic ventilatory decline (Fig. 2B).
At baseline, subjects who were susceptible to AMS (AMS group) showed a lower CBF at all levels of hypoxia (relative to asymptomatic or acclimatized subjects); however, this observation did not reach significance. There was no difference in hypoxia sensitivity (slope) among AMS groups (Fig. 3B).
HcvR and HccbfR
Both CBF and ventilation show an identical pattern of sensitivity to hypercapnia, combined hypoxia/hypercapnia, and acclimatization to altitude (Fig. 4 and Table 2). At baseline, the addition of hypoxia to the hypercapnia significantly increased CO2 sensitivity (HcvRbaseline vs. HHcvRbaseline, P < 0.0005). Acclimatization also significantly increased ventilatory CO2 sensitivity in normoxia (HcvRbaseline vs. HcvRacclimatized,, P < 0.05). Acclimatization also increased ventilation and decreased in normoxia (Fig. 4A). The addition of hypoxia to hypercapnia after acclimatization increased CO2 sensitivity only modestly (HHcvRacclimatized vs. HcvRacclimatized, P = not significant). An identical pattern of significant changes was also seen for the CBF responses to CO2 and acclimatization (Fig. 4B).
In subjects symptomatic for AMS, there was a small but consistent decrease in ventilation and CBF at all and levels compared with subjects with no AMS. This was seen both at baseline and following acclimatization (most prominent for HccbfRbaseline and HHcCBFRbaseline), but none of these reached significance (Fig. 5).
DISCUSSION
The primary findings from this study demonstrate very different effects of hypoxic acclimatization on the pattern of ventilatory and CBF responses to hypoxia (Fig. 2) but very similar effects of acclimatization on the patterns of ventilatory and CBF response to CO2 (Fig. 4).
The HvR has been well described, and our studies confirm similar alterations in ventilation in response to hypoxia and hypercapnia made previously (17). Notably, we observed hypoxic ventilatory decline during 10 min of continued hypoxia, as well as ventilatory acclimatization to hypoxia with increases in the isocapnic HvR after 2 days of sustained hypoxia (38). The resting ventilation in our study is smaller than in other literature studies, since the subjects were lying supine within the MRI scanner (13, 43) (whereas other studies have subjects upright or semirecumbent). Unlike ventilation, CBF shows a constant sensitivity (slope) to desaturation across a wide range of , and there is no cerebrovascular equivalent of hypoxic ventilatory decline in the cerebral circulation.
The apparent increase in resting CBF in response to acute hypoxia following acclimatization in Fig. 4 appears modest and smaller than previous studies reporting 103% increases in CBF sensitivity to acute hypoxia following 2 days of acclimatization to hypoxia (42). To appreciate the actual magnitude of the increase in cerebrovascular sensitivity, we extrapolated the HccbfR and HHccbfR curves back to the original 40 Torr preacclimatization CO2 set point at baseline to mirror the described by Poulin et al. (42). The expected postacclimatization CBF increase can be seen to be ~16 ml·100 ml−1·min−1 compared with the baseline CBF increase of ~8 ml·100 ml−1·min−1. This concurs with the findings of Poulin et al. (42) of an ~100% increase in sensitivity. Similarly, the extrapolation of the HcvR and HHcvR curves allows a better appreciation of the even greater effect of acclimatization on the ventilation sensitivity.
Ventilatory Adaptation
In this study, we focused on the ventilatory and cerebrovascular changes following 2 days of acclimatization. Our findings are consistent with more long-term studies. The HvR is reported to increase continuously at this altitude over 12 days without a plateau (49), but most of the increase occurs in the first 2 days, and there is no significant difference between 2 days and 2 mo (21). The HcvR increases more slowly at this altitude and typically does not increase significantly after 2 days (21, 49), consistent with our results.
Cerebrovascular Adaptation
One question that we set out to address is whether the cerebrovascular system is capable of acclimatization during sustained hypoxia. We applied an established HvR protocol (21, 50, 55) and measured changes in CBF with arterial spin-labeled MRI. The absence of any slope change for the hypoxic cerebrovascular response after 2 days of hypoxic acclimatization provides strong evidence for an absence of any cerebrovascular acclimatization to sustained hypoxia during the first 2 days of acclimatization to hypoxia. This is in stark contrast to the ventilatory hypoxia sensitivity, where there is an increase in the HvR slope after acclimatization to sustained hypoxia and a change in sensitivity to . Previous studies have also observed an absence of hypoxic CBF adaptation for short durations of hypoxic acclimatization (28, 48). The Severinghaus et al. (51) study of CBF acclimatization at 3,800 m altitude showed an initial CBF rise that fell during a prolonged stay at altitude, which has been interpreted as cerebrovascular acclimatization to hypoxia. However, Krasney et al. (28) reviewed these data and concluded that the changes in hematocrit and saturation alone could account for the apparent normalization in CBF and argue that CBF did not adapt. Evidence that CBF sensitivity may adapt for more prolonged periods of acclimatization is mixed; Rupp et al. (48) observed no increases in proximal artery blood velocity after 5 days of acclimatization compared with 1 day. However, Jensen et al. (24) noted some augmentation of the cerebrovascular response after 5 days of acclimatization with a 34% increase in HcbfR but still considerably smaller than the HvR increase of 60% over the same period. It is not clear if this represents an adjustment of the set point or a true change in sensitivity. Jensen et al. (24) argues that during the initial few weeks at moderate altitude, cerebrospinal fluid pH tends to remain alkaline (10), and the rise in hematocrit is negligible. Vascular reactivity to hypoxia might gradually fall, as a part of adaptation to chronic hypoxia may remain unchanged or may rise as does carotid chemosensitivity.
It is well described from clinical studies that dramatic differences exist in focal hypoxia vulnerability for different cerebral regions (14). This study focused on the global cerebrovascular response, so we have not evaluated potential regional differences in cerebrovascular adaptation.
Integration of CBF and Ventilation Control
CBF is exquisitely sensitive to changes in arterial . Hypercapnia causes vasodilation of cerebral arterioles and a subsequent increase in CBF, whereas hypocapnia produces vasoconstriction and a subsequent decrease in CBF (26, 62). The vasodilation or vasoconstriction action of is believed to be at the level of the arterioles and the precapillary sphincters (3, 8). Several mechanisms have been proposed for this action of CO2 [reviewed by Ainslie and Duffin (1)]. CO2 may act via K+ channels in cerebral endothelial cells in response to reduced pH (5, 65). This hyperpolarization can be transmitted to the underlying smooth muscle, where reduced intracellular Ca2+ causes vascular relaxation (22, 27, 35). CO2 may also act via pH-sensitive vasoactive factors, such as nitric oxide and prostaglandins (30, 39, 63).
The remarkable similarity in the pattern of ventilation and CBF responses to CO2 is intriguing and suggests that these may both represent a common physiological mechanism in response to CO2, which is similar whether via a neuronal response that is impacting ventilation at the central chemoreceptor sites, e.g., the retrotrapezoid nucleus within the medulla (18), or affecting CBF at the cerebral arterioles. The similar response also points toward a shared homeostatic role. Previous authors have proposed that CBF and ventilation may be under a common integrated control [reviewed by Ainslie and Duffin (1)]. The dramatic sensitivity of CBF to CO2 is needed to attenuate swings in central partial pressure of CO2 and hence, control central pH (9). is under ventilatory control via the central chemoreceptors. , in turn, has a major role in regulation of CBF (6). Thus CBF-induced changes directly affect the central pH, which impacts pulmonary ventilation, and pulmonary ventilation, in turn, impacts CBF via the resultant changes in (1). An integrated ventilation/CBF response may also be necessary for acclimatization/deacclimatization to sustained hypoxia, based on newer concepts of interdependent central-peripheral chemoreceptor function (12). In the present study, we observed that the pattern of changes for CBF and for pulmonary ventilation is almost identical, and both showed similar plasticity in their sensitivity to CO2 following hypoxic acclimatization. We believe this provides support for a broadly similar control mechanism and potentially, for an integrated ventilation/CBF response to .
In contrast to the very similar ventilation and CBF responses to CO2, our HvR/HcbfR results indicate that ventilation/CBF responses to hypoxia are quite different. CBF reactivity to isocapnic hypoxia has a constant sensitivity (slope) across a wide range (and duration) of hypoxic stimuli, which is unaffected by 2 days of acclimatization to sustained hypoxia at high altitude and does not show the same plasticity seen with the CO2 response. Conspicuously absent in the HcbfR are features that are characteristic of the HvR [reviewed by Pamenter and Powell (38)]. Specifically, we did not see changes in the slope between HvR1 and HvR2 with prolonged isocapnic hypoxia, which may involve changes in carotid body sensitivity and central processing of their afferent input or changes in absolute values of CBF with sustained hypoxia, in contrast to an hypoxic ventilatory decline or other factors determining normoxic ventilatory drive. Thus CBF reactivity to hypoxia does not appear to include characteristics attributed to plasticity in ventilatory chemoreflexes following sustained hypoxic exposure.
The phase of the menstrual cycle is known to have an effect on both resting ventilation (32, 54), as well as cerebrovascular reactivity (11, 29), and this was not specifically controlled in this study. However, the ventilation and cerebrovascular measurements were made simultaneously so that variations in luteal-phase ovarian hormones between imaging sessions (as well as other confounds, such as medications, caffeine, sleep, nutrition, etc.) would impact both ventilation and cerebrovascular dynamics. The common pattern of response for ventilation and CBF to hypercapnia, despite these potential variations across measurements, provides further support for an integrated control mechanism.
Impact of Ventilation and Cerebrovascular Response on AMS
Our study design to separate the subjects into two very disparate groups with unambiguous AMS or with no AMS symptoms (excluding those with intermediate AMS scores) was implemented to emphasize any potential difference in cerebral/respiratory physiology associated with AMS and provides more power than a parametric regression based on AMS scores.
For ventilation, there was no significant difference in the CO2 response for subjects with or without AMS symptoms. Similarly, no association was present between CBF reactivity to CO2 and AMS symptoms. There was a trend toward an augmented HvR slope after acclimatization in AMS asymptomatic subjects. This appears to follow the previously observed association between a brisk HvR response and diminished sensitivity to AMS (34) and emphasizes the potential importance of an increase in the baseline HvR (i.e., ventilatory acclimatization) in reducing AMS.
In looking at the association between the cerebrovascular response to hypoxia or hypercapnia and susceptibility to AMS symptoms, the observation of reduced CBF in AMS-susceptible subjects is intriguing, but the effect size for this observation is small (Cohen’s d = 0.327); thus to achieve a power of 0.8 and P < 0.05 would require 76 subjects to reach significance. Thus this observation appears unlikely to be biologically relevant. Our overall finding that CO2 sensitivity of CBF (slope) was not associated with AMS symptoms agrees with previous reports (60). Our finding of a consistent CBF sensitivity to acute isocapnic hypoxia over a wide range/duration of stimuli and without evidence of acclimatization and unrelated to AMS symptoms is, however, new. Thus altered cerebral hemodynamics per se are not a key determinant of susceptibility to AMS.
Conclusion
With the use of identical interventions in a single group of subjects and an MRI method that quantifies actual cerebral perfusion, the current studies provide experimental evidence for a similar pattern of CBF and ventilatory responses to CO2, as well as in plasticity of these responses during acclimatization to sustained hypoxia. In contrast, the effects of O2 on ventilation and CBF appear quite different. Whereas the HvR is capable of adapting following 2 days of sustained hypoxia, there is no equivalent plasticity in the mechanisms responsible for hypoxic cerebrovascular control. These findings were similar for both subject groups, irrespective of their symptoms at altitude. We hypothesize that similar physiological mechanisms underlie the time-dependent changes in CO2 sensitivity of ventilation and CBF during acclimatization, and differences in time-dependent effects of hypoxia reflect the involvement of arterial chemoreceptors on ventilation but not CBF.
GRANTS
Support for this work was sponsored by grants from the U.S. National Institutes of Health [R01 NS053934 (to D. J. Dubowitz), R21 NS075812 (to D. J. Dubowitz), and R01 HL 081823 (F. L. Powell Jr.)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.D. conceived and designed research; Z.M.S., E.K., R.C.S., E.T.L., M.S., and D.J.D. performed experiments; Z.M.S., E.K., R.C.S., E.T.L., F.L.P., and D.J.D. analyzed data; Z.M.S., F.L.P., and D.J.D. interpreted results of experiments; Z.M.S. and D.J.D. prepared figures; Z.M.S. and D.J.D. drafted manuscript; Z.M.S., M.S., F.L.P., and D.J.D. edited and revised manuscript; Z.M.S., R.C.S., M.S., F.L.P., and D.J.D. approved final version of manuscript.
REFERENCES
- 1.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Exp Physiol 95: 251–262, 2010. doi: 10.1113/expphysiol.2008.045575. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson JL, Anderson RE, Sundt TM Jr. The effect of carbon dioxide on the diameter of brain capillaries. Brain Res 517: 333–340, 1990. doi: 10.1016/0006-8993(90)91046-J. [DOI] [PubMed] [Google Scholar]
- 4.Basaran KE, Villongco M, Ho B, Ellis E, Zarndt R, Antonova J, Hopkins SR, Powell FL. Ibuprofen blunts ventilatory acclimatization to sustained hypoxia in humans. PLoS One 11: e0146087, 2016. doi: 10.1371/journal.pone.0146087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger MG, Vandier C, Bonnet P, Jackson WF, Rusch NJ. Intracellular acidosis differentially regulates KV channels in coronary and pulmonary vascular muscle. Am J Physiol 275: H1351–H1359, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Brian JE Jr, Faraci FM, Heistad DD. Recent insights into the regulation of cerebral circulation. Clin Exp Pharmacol Physiol 23: 449–457, 1996. doi: 10.1111/j.1440-1681.1996.tb02760.x. [DOI] [PubMed] [Google Scholar]
- 7.Buck A, Schirlo C, Jasinksy V, Weber B, Burger C, von Schulthess GK, Koller EA, Pavlicek V. Changes of cerebral blood flow during short-term exposure to normobaric hypoxia. J Cereb Blood Flow Metab 18: 906–910, 1998. doi: 10.1097/00004647-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol 101: 161–211, 1984. doi: 10.1007/BFb0027696. [DOI] [PubMed] [Google Scholar]
- 9.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 10.Crawford RD, Severinghaus JW. CSF pH and ventilatory acclimatization to altitude. J Appl Physiol Respir Environ Exerc Physiol 45: 275–283, 1978. [DOI] [PubMed] [Google Scholar]
- 11.Debert CT, Ide K, Poulin MJ. Effects of estrogen and progesterone on cerebrovascular responses to euoxic hypercapnia in women. Climacteric 15: 621–631, 2012. doi: 10.3109/13697137.2011.631231. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey JA, Powell FL, Bisgard GE, Blain GM, Poulin MJ, Smith CA. Role of chemoreception in cardiorespiratory acclimatization to, and deacclimatization from, hypoxia. J Appl Physiol (1985) 116: 858–866, 2014. doi: 10.1152/japplphysiol.01126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donina ZA, Danilova GA, Aleksandrova NP. Effects of body position on the ventilatory response to hypercapnia. Eur J Med Res 14, Suppl 4: 63–66, 2009. doi: 10.1186/2047-783X-14-S4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubowitz DJ, Bluml S, Arcinue E, Dietrich RB. MR of hypoxic encephalopathy in children after near drowning: correlation with quantitative proton MR spectroscopy and clinical outcome. AJNR Am J Neuroradiol 19: 1617–1627, 1998. [PMC free article] [PubMed] [Google Scholar]
- 15.Dyer EA, Hopkins SR, Perthen JE, Buxton RB, Dubowitz DJ. Regional cerebral blood flow during acute hypoxia in individuals susceptible to acute mountain sickness. Respir Physiol Neurobiol 160: 267–276, 2008. doi: 10.1016/j.resp.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging 18: 649–655, 2003. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- 17.Garcia N, Hopkins SR, Elliott AR, Aaron EA, Weinger MB, Powell FL. Ventilatory response to 2-h sustained hypoxia in humans. Respir Physiol 124: 11–22, 2001. doi: 10.1016/S0034-5687(00)00183-3. [DOI] [PubMed] [Google Scholar]
- 18.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoiland RL, Ainslie PN. CrossTalk proposal: the middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. J Physiol 594: 4073–4075, 2016. doi: 10.1113/JP271981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang SY, Moore LG, McCullough RE, McCullough RG, Micco AJ, Fulco C, Cymerman A, Manco-Johnson M, Weil JV, Reeves JT. Internal carotid and vertebral arterial flow velocity in men at high altitude. J Appl Physiol (1985) 63: 395–400, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Hupperets MD, Hopkins SR, Pronk MG, Tiemessen IJ, Garcia N, Wagner PD, Powell FL. Increased hypoxic ventilatory response during 8 weeks at 3800 m altitude. Respir Physiol Neurobiol 142: 145–152, 2004. doi: 10.1016/j.resp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 12: 113–127, 2005. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen GF, Kagenaar DA, Basnyat B, Odoom JA. Basilar artery blood flow velocity and the ventilatory response to acute hypoxia in mountaineers. Respir Physiol Neurobiol 133: 65–74, 2002. doi: 10.1016/S1569-9048(02)00152-0. [DOI] [PubMed] [Google Scholar]
- 24.Jensen JB, Sperling B, Severinghaus JW, Lassen NA. Augmented hypoxic cerebral vasodilation in men during 5 days at 3,810 m altitude. J Appl Physiol (1985) 80: 1214–1218, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Jensen JB, Wright AD, Lassen NA, Harvey TC, Winterborn MH, Raichle ME, Bradwell AR. Cerebral blood flow in acute mountain sickness. J Appl Physiol (1985) 69: 430–433, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492, 1948. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitazono T, Faraci FM, Taguchi H, Heistad DD. Role of potassium channels in cerebral blood vessels. Stroke 26: 1713–1723, 1995. doi: 10.1161/01.STR.26.9.1713. [DOI] [PubMed] [Google Scholar]
- 28.Krasney JA, Jensen JB, Lassen NA. Cerebral blood flow does not adapt to sustained hypoxia. J Cereb Blood Flow Metab 10: 759–764, 1990. doi: 10.1038/jcbfm.1990.133. [DOI] [PubMed] [Google Scholar]
- 29.Krejza J, Rudzinski W, Arkuszewski M, Onuoha O, Melhem ER. Cerebrovascular reactivity across the menstrual cycle in young healthy women. Neuroradiol J 26: 413–419, 2013. doi: 10.1177/197140091302600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leffler CW, Mirro R, Pharris LJ, Shibata M. Permissive role of prostacyclin in cerebral vasodilation to hypercapnia in newborn pigs. Am J Physiol Heart Circ Physiol 267: H285–H291, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 52: 679–682, 2004. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 32.MacNutt MJ, De Souza MJ, Tomczak SE, Homer JL, Sheel AW. Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J Appl Physiol (1985) 112: 737–747, 2012. doi: 10.1152/japplphysiol.00727.2011. [DOI] [PubMed] [Google Scholar]
- 33.Markwalder TM, Grolimund P, Seiler RW, Roth F, Aaslid R. Dependency of blood flow velocity in the middle cerebral artery on end-tidal carbon dioxide partial pressure—a transcranial ultrasound Doppler study. J Cereb Blood Flow Metab 4: 368–372, 1984. doi: 10.1038/jcbfm.1984.54. [DOI] [PubMed] [Google Scholar]
- 34.Milledge JS. The ventilatory response to hypoxia: how much is good for a mountaineer? Postgrad Med J 63: 169–172, 1987. doi: 10.1136/pgmj.63.737.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Noll DC, Fessler JA, Sutton BP. Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Trans Med Imaging 24: 325–336, 2005. doi: 10.1109/TMI.2004.842452. [DOI] [PubMed] [Google Scholar]
- 37.Pagani M, Ansjön R, Lind F, Uusijärvi J, Sumen G, Jonsson C, Salmaso D, Jacobsson H, Larsson SA. Effects of acute hypobaric hypoxia on regional cerebral blood flow distribution: a single photon emission computed tomography study in humans. Acta Physiol Scand 168: 377–383, 2000. doi: 10.1046/j.1365-201x.2000.00649.x. [DOI] [PubMed] [Google Scholar]
- 38.Pamenter ME, Powell FL. Time domains of the hypoxic ventilatory response and their molecular basis. Compr Physiol 6: 1345–1385, 2016. doi: 10.1002/cphy.c150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parfenova H, Shibata M, Zuckerman S, Leffler CW. CO2 and cerebral circulation in newborn pigs: cyclic nucleotides and prostanoids in vascular regulation. Am J Physiol Heart Circ Physiol 266: H1494–H1501, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Pasztor E, Symon L, Dorsch NW, Branston NM. The hydrogen clearance method in assessment of blood flow in cortex, white matter and deep nuclei of baboons. Stroke 4: 556–567, 1973. doi: 10.1161/01.STR.4.4.556. [DOI] [PubMed] [Google Scholar]
- 41.Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. Neuroimage 40: 237–247, 2008. doi: 10.1016/j.neuroimage.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulin MJ, Fatemian M, Tansley JG, O’Connor DF, Robbins PA. Changes in cerebral blood flow during and after 48 h of both isocapnic and poikilocapnic hypoxia in humans. Exp Physiol 87: 633–642, 2002. doi: 10.1113/eph8702437. [DOI] [PubMed] [Google Scholar]
- 43.Prisk GK, Elliott AR, West JB. Sustained microgravity reduces the human ventilatory response to hypoxia but not to hypercapnia. J Appl Physiol (1985) 88: 1421–1430, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Reeves JT, Moore LG, McCullough RE, McCullough RG, Harrison G, Tranmer BI, Micco AJ, Tucker A, Weil JV. Headache at high altitude is not related to internal carotid arterial blood velocity. J Appl Physiol (1985) 59: 909–915, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Restom K, Behzadi Y, Liu TT. Physiological noise reduction for arterial spin labeling functional MRI. Neuroimage 31: 1104–1115, 2006. doi: 10.1016/j.neuroimage.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 46.Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke 19: 963–969, 1988. doi: 10.1161/01.STR.19.8.963. [DOI] [PubMed] [Google Scholar]
- 47.Roach RC, Bärtsch P, Oelz O, Hackett PH. The Lake Louise acute mountain sickness scoring system. In: Hypoxia and Mountain Medicine, edited by Sutton JR, Houston CS, Coates G. Burlington, VT: Queen City, 1993, p. 272–274. [Google Scholar]
- 48.Rupp T, Esteve F, Bouzat P, Lundby C, Perrey S, Levy P, Robach P, Verges S. Cerebral hemodynamic and ventilatory responses to hypoxia, hypercapnia, and hypocapnia during 5 days at 4,350 m. J Cereb Blood Flow Metab 34: 52–60, 2014. doi: 10.1038/jcbfm.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato M, Severinghaus JW, Bickler P. Time course of augmentation and depression of hypoxic ventilatory responses at altitude. J Appl Physiol (1985) 77: 313–316, 1994. [DOI] [PubMed] [Google Scholar]
- 50.Sato M, Severinghaus JW, Powell FL, Xu FD, Spellman MJ Jr. Augmented hypoxic ventilatory response in men at altitude. J Appl Physiol (1985) 73: 101–107, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Severinghaus JW, Chiodi H, Eger EI II, Brandstater B, Hornbein TF. Cerebral blood flow in man at high altitude. Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res 19: 274–282, 1966. doi: 10.1161/01.RES.19.2.274. [DOI] [PubMed] [Google Scholar]
- 52.Silvennoinen MJ, Kettunen MI, Kauppinen RA. Effects of hematocrit and oxygen saturation level on blood spin-lattice relaxation. Magn Reson Med 49: 568–571, 2003. doi: 10.1002/mrm.10370. [DOI] [PubMed] [Google Scholar]
- 53.Smith ZM, Krizay E, Guo J, Shin DD, Scadeng M, Dubowitz DJ. Sustained high-altitude hypoxia increases cerebral oxygen metabolism. J Appl Physiol (1985) 114: 11–18, 2013. doi: 10.1152/japplphysiol.00703.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takano N. Changes of ventilation and ventilatory response to hypoxia during the menstrual cycle. Pflugers Arch 402: 312–316, 1984. doi: 10.1007/BF00585515. [DOI] [PubMed] [Google Scholar]
- 55.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 56.Ter Minassian A, Beydon L, Ursino M, Gardette B, Gortan C, Richalet JP. Doppler study of middle cerebral artery blood flow velocity and cerebral autoregulation during a simulated ascent of Mount Everest. Wilderness Environ Med 12: 175–183, 2001. doi: 10.1580/1080-6032(2001)012[0175:DSOMCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 57.Tominaga S, Strandgaard S, Uemura K, Ito K, Kutsuzawa T. Cerebrovascular CO2 reactivity in normotensive and hypertensive man. Stroke 7: 507–510, 1976. doi: 10.1161/01.STR.7.5.507. [DOI] [PubMed] [Google Scholar]
- 58.van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med 59: 788–795, 2008. doi: 10.1002/mrm.21515. [DOI] [PubMed] [Google Scholar]
- 59.Van Osta A, Moraine JJ, Mélot C, Mairbäurl H, Maggiorini M, Naeije R. Effects of high altitude exposure on cerebral hemodynamics in normal subjects. Stroke 36: 557–560, 2005. doi: 10.1161/01.STR.0000155735.85888.13. [DOI] [PubMed] [Google Scholar]
- 60.Villien M, Bouzat P, Rupp T, Robach P, Lamalle L, Troprès I, Estève F, Krainik A, Lévy P, Warnking JM, Verges S. Changes in cerebral blood flow and vasoreactivity to CO2 measured by arterial spin labeling after 6 days at 4350 m. Neuroimage 72: 272–279, 2013. doi: 10.1016/j.neuroimage.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Qiu M, Constable RT. In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magn Reson Med 53: 666–674, 2005. doi: 10.1002/mrm.20377. [DOI] [PubMed] [Google Scholar]
- 62.Wasserman AJ, Patterson JL Jr. The cerebral vascular response to reduction in arterial carbon dioxide tension. J Clin Invest 40: 1297–1303, 1961. doi: 10.1172/JCI104359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei EP, Ellis EF, Kontos HA. Role of prostaglandins in pial arteriolar response to CO2 and hypoxia. Am J Physiol Heart Circ Physiol 238: H226–H230, 1980. [DOI] [PubMed] [Google Scholar]
- 64.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med 39: 702–708, 1998. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- 65.Xu H, Cui N, Yang Z, Wu J, Giwa LR, Abdulkadir L, Sharma P, Jiang C. Direct activation of cloned K(atp) channels by intracellular acidosis. J Biol Chem 276: 12898–12902, 2001. doi: 10.1074/jbc.M009631200. [DOI] [PubMed] [Google Scholar]