Abstract
Hypoxia increases erythropoiesis mediated by hypoxia-inducible transcription factors (HIF), which regulate erythropoietin transcription. Neocytolysis is a physiological mechanism that corrects polycythemia from chronic sustained hypoxemia by transient, preferential destruction of young RBCs after normoxia is restored. We showed that neocytolysis is caused by excessive mitochondrial-derived reactive oxygen species in reticulocytes mediated by downregulation of HIF-controlled BNIP3L regulated mitophagy and a decrease in RBC antioxidant catalase (CAT) in hypoxia-produced erythrocytes. Decreased CAT results from hypoxia-induced miR-21 that downregulates CAT. This correlates with a transient acute decrease of HIF-1 at normoxic return that is associated with normalization of red cell mass.
Keywords: erythropoiesis, hypoxia, neocytolysis, normoxic return from hypoxia, sustained and intermittent hypoxia
obstructive sleep apnea (OSA), characterized by a unique pattern of chronic intermittent hypoxia (CIH), is associated with cardiovascular, endocrine, and neurocognitive comorbidities, and increased cancer risk. The role of the carotid body in OSA-modulated pathophysiologies has been well studied; here we define hematological abnormalities and discuss the possible role of OSA blood changes on health. We found that only ~1% of OSA patients developed polycythemia, which was not attributable to other causes and corrected with continuous positive airway pressure therapy (CPAP) therapy. However, in our pilot OSA studies, we found increased reactive oxygen species (ROS) and mitochondrial mass with decreased catalase (CAT) and BNIP3L in blood cells, along with increased inflammatory markers. After correction of OSA with use of CPAP for >3 mo, some, but not all, of these abnormalities corrected.
The observed blood changes in OSA likely contribute to impaired health; however, their pathophysiological roles need to be carefully defined.
Chronic Sustained Hypoxemia
The number of red blood cells (RBCs) is tightly controlled to maintain oxygen homeostasis. In hypoxia, the red cell mass increases to improve oxygen delivery to tissues by augmented erythropoiesis mediated by hypoxia-inducible factor-2α (HIF-2α; encoded by the EPAS1 gene), the principal regulator of erythropoietin (EPO) gene transcription (32). A rapid change of oxygen tension from hypoxia to normoxia corrects hypoxia-induced RBC expansion by the preferential destruction of young RBCs (neocytes), a process coined neocytolysis (1, 37). Neocytolysis was described in astronauts (1) and people descending from high altitudes (33, 36, 37). Neocytolysis was also reported to be present in pathophysiological conditions, such as anemia in chronic renal diseases, pyruvate kinase deficiency, chronic obstructive pulmonary disease with oxygen treatment, and malaria (6, 15, 28, 35); however, we submit that, in these pathophysiological conditions, the presence of neocytolysis has not been conclusively supported by rigorous evidence. Neocytolysis might be a possible explanation of rapid decrease of hematocrit in neonates after birth (4, 20, 34, 46). Neonates have the highest hematocrit levels at the time of birth, and hematocrit levels rapidly drop to an anemic range within 8 days after birth, whereas it is expected that their hematocrit levels should increase due to loss of body weight (31). End-tidal carbon monoxide (ETCO) measures CO (a product of heme catabolism) in exhaled breath, and the amount of CO in exhaled air correlates with circulating RBC half-life and thus can be used to quantify the hemolytic rate. Our group showed that there is excessive hemolysis after birth by ETCO measurements, indicating that the rapid reduction of hematocrit levels after birth is caused by increased destruction of RBCs (5), which is also supported by hyperbilirubinemia of infancy.
Mouse Model of Neocytolysis
We developed a mouse model of neocytolysis to define its molecular bases and reproduced the hypoxia-mediated expansion of RBC (i.e., polycythemia) (45). In this animal model, we showed overcorrection of polycythemia by rapid but transient hemolysis, with preferential destruction of those young RBCs that were made in hypoxia. The measured changes of hematocrit were not caused by changes of plasma volume. We then searched for the molecular determinants of these changes. We built on evidence that deletion of the HIF-regulated gene BNIP3L, which mediates mitochondrial autophagy (mitophagy), is associated with mitochondrial expansion with resultant increased ROS and hemolysis (39). Our mouse model of neocytolysis also had 1) increased ROS; 2) increased mitochondrial mass in reticulocytes (young RBCs with RNA, ribosome and mitochondria); 3) hemolysis; and 4) decreased Bnip3L transcripts. These observations, however, did not explain the still inexplicable finding of preferential destruction of young RBCs, including reticulocytes. We then searched for defects of ROS scavenging enzymes in erythroid cells and found a reduction of CAT (an antioxidant enzyme that decomposes hydrogen peroxide to oxygen and water), but not of superoxide dismutase and glutathione peroxidase in hypoxia-made RBCs. The observed changes of reduction of both CAT transcripts and its activity were surprising, as HIF-2 (40) and, to a lesser degree, HIF-1 (45) augment CAT gene transcription. We then solved this apparent puzzle by showing that, indeed, acute hypoxia leads to increased CAT mRNA transcripts, but then mRNA and von Hippel-Lindau gene (VHL) enzyme activity rapidly decrease under chronic hypoxia. Searching for the mechanism of this observation, we found several micro-RNAs (miRs) that target CAT mRNA for degradation, one being hypoxia upregulated miR-21, which was found to account for CAT mRNA degradation. As reticulocytes constitute only ~1% of RBCs, changes in reticulocyte half-life would not account for such a rapid decrease of hematocrit after normoxic return. However, administration of an antioxidant agent, N-acetyl-cysteine, or injection of polyethylene glycol conjugated CAT attenuated the hemolytic rate, demonstrating that reticulocyte generated ROS diffuse to plasma, have contact with circulating RBCs, and preferentially destroy those hypoxia-born RBCs with lower CAT activity (i.e., neocytolysis). It is likely that even older RBCs are also affected by plasma ROSs, but likely to a lesser degree than younger RBCs with low CAT. To prove that these findings are modulated by HIF, a mouse model with constitutive upregulation of HIF, having a loss-of-function mutation of VHL, a negative regulator of HIFs [the so-called Chuvash polycythemia mouse (16)] had attenuated neocytolysis. Similarly, augmentation of HIF activity by injection of dimethyloxaloylglycine, an inhibitor of prolyl hydroxylase 2 (the principal negative regulator of HIFs), attenuated neocytolysis, indicating that a rapid decrease of HIF levels is responsible for neocytolysis. The mechanisms of neocytolysis, consisting of increased ROS production and accompanying decreased scavenging ROS ability, are depicted in Fig. 1.
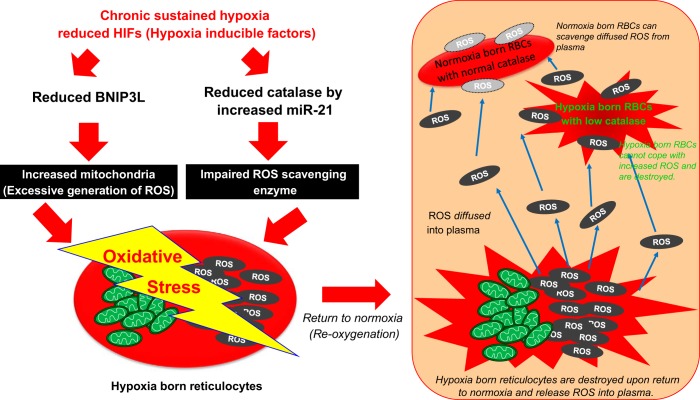
Fig. 1.
Proposed mechanism of neocytolysis. Neocytolysis is caused by increased mitochondrial reactive oxygen species (ROS). ROS are generated by increased mitochondrial mass by downregulated HIF-targeted BNIP3L in reticulocytes. ROS is diffused into plasma from destroyed reticulocytes on return to normoxia. Decreased catalase by hypoxia-induced miR-21 increases the accumulation of ROS. Hypoxia born red blood cells undergo neocytolysis due to impaired ability to cope with increased oxidative stress.
HIF Activity in Acute and Chronic Hypoxia
HIF-1 and HIF-2 share similar structures and undergo the same oxygen-mediated degradation (25). However, at times they target the same genes via a homologous COOH-terminal transactivation domain (TAD), but in other instances they have unique targets utilizing different NH2-terminal TADs (17) [i.e., HIF-1 regulated genes in the glycolytic pathway (18), HIF-2 induced genes promoting tumor growth (12), and the EPO gene (32) in kidney tissue]. In some cases, they have opposite roles in expression of genes such as IL-8 (7). Unexpectedly, we found that acute hypoxia increases HIFs, but continuation of hypoxia [chronic sustained hypoxemia (CSH)] was associated with decreased transcripts of HIF-regulated genes, suggesting that there might be activation of negative regulators of HIFs under CSH. HIF-3α is one of the regulators of decreased HIF-1α (2, 14). HIF-3α has only one TAD at the NH2-terminus, whereas HIF-1α and HIF-2α have two TADs located at the NH2- and COOH-termini (27), and it is thought that transcription factors having this configuration are negative regulators of hypoxia-inducible genes’ expression. HIF-3α negatively regulates hypoxia-induced gene expression by inhibiting HIF-1α and HIF-2α from binding to target DNA elements competitively (14). There are several HIF-3α splicing variants (HIF-3α1–10) with differential DNA binding elements and protein-protein interaction domains (47). While their functional relevance is still largely unknown, one of the splicing variants, HIF-3α4, was shown to suppress HIF-1α activity in renal cell carcinoma (26). However, it remains to be elucidated whether HIF-3α suppresses HIF-1α and/or HIF-2α stabilities under CSH. Another mechanism that may play a role in downregulation of HIF-regulated genes after chronic hypoxic exposure is an E3 ubiquitin ligase named hypoxia-associated factor (HAF), also known as squamous cell carcinoma antigen recognized by T cells (SART1800). HAF was first described in promoting EPO expression by binding to the promoter region of the EPO gene (13). In this study, prolonged hypoxia decreased HIF-1α, but increased HIF-2α by activating HAF independently of oxygen and VHL protein (23). HAF binds to HIF-1α and HIF-2α as an E3 ligase, leading to degradation of HIF-1α but increased HIF-2α transactivation (24). However, it has not yet been shown that HAF downregulates HIF-1α in blood cells in CSH. Still another mechanism that may play a role in downregulation of HIF-regulated genes after chronic hypoxic exposure is hypoxia-induced miRs. In hypoxia, some miRs are upregulated, and some are downregulated. For example, miR-210 and miR-424 stabilize HIF-1, whereas miR-155 negatively regulates HIF-1 (3, 10, 22). In our neocytolysis mouse model, we showed that, while acute hypoxia increases transcription of HIF-2α regulated CAT, CSH then decreases CAT transcript levels by increase of hypoxia-induced miR-21. However, HIF-1α and HIF-2α are differently regulated under acute or prolonged hypoxia in various tissues and by the duration of hypoxia. Thus the detailed mechanism of HIF-1α and HIF-2α activation and degradation under hypoxia should be determined in different tissues and by the duration of hypoxia treatment.
CIH and OSA
In contrast to CSH, CIH consists of repetitious cycles of normoxia and hypoxia. OSA is a prototype of CIH, characterized by recurrent respiratory obstruction due to upper airway collapse, resulting in cyclic oxygen desaturations (30). However, while patients with OSA have significant cycles of severe hypoxia during sleep, most are not polycythemic, as expected (43). Thus we set up the experiments to solve this apparent pathophysiological puzzle. We found that only ~1% of patients develop OSA-related polycythemia, i.e., polycythemia corrected with CPAP therapy and not attributable to other causes (8). Whether polycythemia is beneficial or detrimental is not clear and is at least in part influenced by whole blood volume (42). Under hypoxia at high altitudes, in smokers (42) and in patients with chronic obstructive pulmonary disease or with deficiencies of red cell enzymes, such as cytochrome b5 reductase and 2,3 bisphosphoglycerate mutase, polycythemia is helpful as it delivers more oxygen to tissues (21). However, Chuvash polycythemia caused by a mutation of the VHL gene, a negative regulator of HIF, leads to thrombotic and hemorrhagic vascular complications, resulting in a shorter life span (41); however, these complications are further increased by attempts to correct polycythemia by phlebotomies. In our pilot and yet unpublished studies, we found that OSA patients also have blocks of mitophagy with increased ROS in analogy to CSH in reticulocytes, but, in contrast to blood changes in CSH, also in neutrophils, monocytes, B and T cells, and platelets. We hypothesized that excessive ROS associated with transitions from hypoxia to normoxia (neocytolysis) might explain the virtual absence of polycythemia in OSA. However, when we measured ETCO levels before CPAP treatment, less than one-half of patients had only moderately elevated CO in exhaled breath at >1.0 ppm (normal < 1.0 ppm), indicating a component of mild hemolysis in some OSA subjects that may contribute to the absence of polycythemia. To search for another mechanism of the paucity of polycythemia, we detected increased inflammation (increased IL6 and TNFα transcript) in granulocytes of most OSA patients. It is likely that these inflammatory marker genes are activated by elevated ROS (29). Indeed, inflammation is a well-known suppressor of erythropoiesis, which is, at least in part, mediated by hepcidin (9, 48). Inflammation also increases miR-122, which directly targets EPO mRNA and thus downregulates EPO expression in the kidney, a novel factor in suppressing erythropoiesis (38). In some but not all OSA patients, increased EPO correlates with the severity and duration of hypoxia. To investigate whether these abnormalities are amenable to therapy, we repeated these studies after CPAP use. Importantly, hematocrit levels before and after CPAP were unchanged, whereas EPO levels decreased in at least some patients after CPAP treatment. ROS levels that were increased due to OSA in B cells, T cells, monocytes, granulocytes, and mature RBCs, but not in platelets, corrected after CPAP treatment. CAT transcript levels were lower in OSA and corrected after CPAP treatment, with a coexistent reverse correlation with miR-21. Increased ROS levels in reticulocytes were accompanied by increased mitochondrial mass concomitantly with decreased BNIP3L transcript levels. Additionally, HIF activity, assessed by HIF-regulated gene transcripts in platelets, granulocytes, and reticulocytes, was downregulated in OSA but not always in the same pattern. This indicates that CIH-decreased HIF levels are differently regulated in reticulocytes, platelets, and granulocytes, indicating tissue-specific HIF regulation in CIH (44).
Conclusions
We attempt here to discuss the differences and similarities of blood changes in CSH compared with CIH and the influence of these metabolic and functional modifications on RBC pathophysiology. Rapid oxygen tension change from CSH to normoxia causes neocytolysis via increase of ROS from mitochondrial and impaired CAT activity. Unlike in acute hypoxia, CSH reduces HIF-1 and/or HIF-2 via three possible ways: HIF-3, HAF, and hypoxia regulated miRs. However, the detailed mechanisms of this observation remain to be elucidated. We submit that the oxidative stress from blood cells from CIH in OSA may contribute to impaired health; however, they and their pathophysiological role need to be defined in future studies. Our data suggest that increased ROS and inflammatory markers in blood cells in uncorrected OSA contribute to the pathophysiology of OSA and may be convenient markers of OSA severity, as well as possible targets for therapeutic interventions.
GRANTS
R. Gangaraju and J. T. Prchal were supported by NIH T32DK007115 grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S. performed experiments; J.S., K.S., R.G., and J.T.P. analyzed data; J.S., R.G., and J.T.P. interpreted results of experiments; J.S. prepared figures; J.S. and J.T.P. drafted manuscript; J.S., K.S., R.G., and J.T.P. edited and revised manuscript; K.S. and J.T.P. conceived and designed research; K.S. and J.T.P. approved final version of manuscript.
REFERENCES
- 1.Alfrey CP, Rice L, Udden MM, Driscoll TB. Neocytolysis: physiological down-regulator of red-cell mass. Lancet 349: 1389–1390, 1997. doi: 10.1016/S0140-6736(96)09208-2. [DOI] [PubMed] [Google Scholar]
- 2.Augstein A, Poitz DM, Braun-Dullaeus RC, Strasser RH, Schmeisser A. Cell-specific and hypoxia-dependent regulation of human HIF-3α: inhibition of the expression of HIF target genes in vascular cells. Cell Mol Life Sci 68: 2627–2642, 2011. doi: 10.1007/s00018-010-0575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, Taylor CT. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol 31: 4087–4096, 2011. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen RD, Lambert DK, Henry E, Yaish HM, Prchal JT. End-tidal carbon monoxide as an indicator of the hemolytic rate. Blood Cells Mol Dis 54: 292–296, 2015. doi: 10.1016/j.bcmd.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Christensen RD, Malleske DT, Lambert DK, Baer VL, Prchal JT, Denson LE, Gerday E, Weaver Lewis KA, Shepherd JG. Measuring end-tidal carbon monoxide of jaundiced neonates in the birth hospital to identify those with hemolysis. Neonatology 109: 1–5, 2016. doi: 10.1159/000438482. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Arias C, Arias CF, Rodriguez A. Is malarial anaemia homologous to neocytolysis after altitude acclimatisation? Int J Parasitol 44: 19–22, 2014. doi: 10.1016/j.ijpara.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Florczyk U, Czauderna S, Stachurska A, Tertil M, Nowak W, Kozakowska M, Poellinger L, Jozkowicz A, Loboda A, Dulak J. Opposite effects of HIF-1α and HIF-2α on the regulation of IL-8 expression in endothelial cells. Free Radic Biol Med 51: 1882–1892, 2011. doi: 10.1016/j.freeradbiomed.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangaraju R, Sundar KM, Song J, Prchal JT. Polycythemia is rarely caused by obstructive sleep apnea. Blood 128: 2444, 2016. [Google Scholar]
- 9.Ganz T. Hepcidin and iron regulation, 10 years later. Blood 117: 4425–4433, 2011. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J Clin Invest 120: 4141–4154, 2010. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11: 335–347, 2007. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta M, Mungai PT, Goldwasser E. A new transacting factor that modulates hypoxia-induced expression of the erythropoietin gene. Blood 96: 491–497, 2000. [PubMed] [Google Scholar]
- 14.Hara S, Hamada J, Kobayashi C, Kondo Y, Imura N. Expression and characterization of hypoxia-inducible factor (HIF)-3alpha in human kidney: suppression of HIF-mediated gene expression by HIF-3alpha. Biochem Biophys Res Commun 287: 808–813, 2001. doi: 10.1006/bbrc.2001.5659. [DOI] [PubMed] [Google Scholar]
- 15.Harris BA Jr, Epstein PE. Out of thin air: the evolving enigma of erythropoietin and neocytolysis. Ann Intern Med 134: 710–712, 2001. doi: 10.7326/0003-4819-134-8-200104170-00015. [DOI] [PubMed] [Google Scholar]
- 16.Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. J Clin Invest 117: 3879–3889, 2007. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell 18: 4528–4542, 2007. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23: 9361–9374, 2003. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javier MC, Krauss A, Nesin M. Corrected end-tidal carbon monoxide closely correlates with the corrected reticulocyte count in coombs’ test-positive term neonates. Pediatrics 112: 1333–1337, 2003. doi: 10.1542/peds.112.6.1333. [DOI] [PubMed] [Google Scholar]
- 21.Kaushansky K, Lichtman MA, Prchal J, Levi MM, Press O, Burns L, Caligiuri M. Williams Hematology (9th Ed.). New York: McGraw-Hill Education, 2015, p. 874–877. [Google Scholar]
- 22.Kelly TJ, Souza AL, Clish CB, Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol 31: 2696–2706, 2011. doi: 10.1128/MCB.01242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh MY, Darnay BG, Powis G. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1alpha, leading to its oxygen-independent degradation. Mol Cell Biol 28: 7081–7095, 2008. doi: 10.1128/MCB.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh MY, Lemos R Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res 71: 4015–4027, 2011. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cells 29: 435–442, 2010. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 26.Maynard MA, Evans AJ, Hosomi T, Hara S, Jewett MAS, Ohh M. Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma. FASEB J 19: 1396–1406, 2005. doi: 10.1096/fj.05-3788com. [DOI] [PubMed] [Google Scholar]
- 27.Maynard MA, Ohh M. Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer. Am J Nephrol 24: 1–13, 2004. doi: 10.1159/000075346. [DOI] [PubMed] [Google Scholar]
- 28.Mentzer WC Jr, Baehner RL, Schmidt-Schönbein H, Robinson SH, Nathan DG. Selective reticulocyte destruction in erythrocyte pyruvate kinase deficiency. J Clin Invest 50: 688–699, 1971. doi: 10.1172/JCI106539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20: 1126–1167, 2014. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc 86: 549–554, 2011. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polin RA, Abman SH, Rowitch D, Benitz WE, Uhbgcbi J. Fetal and Neonatal Physiology. New York: Elsevier Health Sciences, 2016. [Google Scholar]
- 32.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117: 1068–1077, 2007. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice L, Alfrey CP. Modulation of red cell mass by neocytolysis in space and on Earth. Pflugers Arch 441, Suppl: R91–R94, 2000. doi: 10.1007/s004240000333. [DOI] [PubMed] [Google Scholar]
- 34.Rice L, Alfrey CP. The negative regulation of red cell mass by neocytolysis: physiologic and pathophysiologic manifestations. Cell Physiol Biochem 15: 245–250, 2005. doi: 10.1159/000087234. [DOI] [PubMed] [Google Scholar]
- 35.Rice L, Alfrey CP, Driscoll T, Whitley CE, Hachey DL, Suki W. Neocytolysis contributes to the anemia of renal disease. Am J Kidney Dis 33: 59–62, 1999. doi: 10.1016/S0272-6386(99)70258-1. [DOI] [PubMed] [Google Scholar]
- 36.Rice L, Ruiz W, Driscoll T, Whitley CE, Tapia R, Hachey DL, Gonzales GF, Alfrey CP. Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass. Ann Intern Med 134: 652–656, 2001. doi: 10.7326/0003-4819-134-8-200104170-00010. [DOI] [PubMed] [Google Scholar]
- 37.Risso A, Turello M, Biffoni F, Antonutto G. Red blood cell senescence and neocytolysis in humans after high altitude acclimatization. Blood Cells Mol Dis 38: 83–92, 2007. doi: 10.1016/j.bcmd.2006.10.161. [DOI] [PubMed] [Google Scholar]
- 38.Rivkin M, Simerzin A, Zorde-Khvalevsky E, Chai C, Yuval JB, Rosenberg N, Harari-Steinfeld R, Schneider R, Amir G, Condiotti R, Heikenwalder M, Weber A, Schramm C, Wege H, Kluwe J, Galun E, Giladi H. Inflammation-induced expression and secretion of microRNA 122 leads to reduced blood levels of kidney-derived erythropoietin and anemia. Gastroenterology 151: 999–1010, 2016. doi: 10.1053/j.gastro.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 39.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 454: 232–235, 2008. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35: 331–340, 2003. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 41.Sergeyeva A, Gordeuk VR, Tokarev YN, Sokol L, Prchal JF, Prchal JT. Congenital polycythemia in Chuvashia. Blood 89: 2148–2154, 1997. [PubMed] [Google Scholar]
- 42.Smith JR, Landaw SA. Smokers’ polycythemia. N Engl J Med 298: 6–10, 1978. doi: 10.1056/NEJM197801052980102. [DOI] [PubMed] [Google Scholar]
- 43.Solmaz S, Duksal F, Ganidağlı S. Is obstructive sleep apnoea syndrome really one of the causes of secondary polycythaemia? Hematology 20: 108–111, 2015. doi: 10.1179/1607845414Y.0000000170. [DOI] [PubMed] [Google Scholar]
- 44.Song J, Sundar KM, Gangaraju R, Sundar S, Thiagarajan P, Prchal JT. Oxidative stress in blood cells associated with obstructive sleep apnea contributes to absence of hypoxia-induced polycythemia. Blood 128: 2442, 2016. [Google Scholar]
- 45.Song J, Yoon D, Christensen RD, Horvathova M, Thiagarajan P, Prchal JT. HIF-mediated increased ROS from reduced mitophagy and decreased catalase causes neocytolysis. J Mol Med (Berl) 93: 857–866, 2015. doi: 10.1007/s00109-015-1294-y. [DOI] [PubMed] [Google Scholar]
- 46.Swenson ER, Bärtsch P. High Altitude: Human Adaptation to Hypoxia. New York: Springer, 2013. [Google Scholar]
- 47.Yang SL, Wu C, Xiong ZF, Fang X. Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function. Mol Med Rep 12: 2411–2416, 2015. doi: 10.3892/mmr.2015.3689. [DOI] [PubMed] [Google Scholar]
- 48.Zhao N, Zhang AS, Enns CA. Iron regulation by hepcidin. J Clin Invest 123: 2337–2343, 2013. doi: 10.1172/JCI67225. [DOI] [PMC free article] [PubMed] [Google Scholar]