Abstract
Introduction
Puumala hantavirus (PUUV) causes a mild type of hemorrhagic fever with renal syndrome characterized by acute kidney injury (AKI), increased capillary leakage, and thrombocytopenia. Albuminuria and hematuria in dipstick urine test at hospital admission are known to predict the severity of upcoming AKI.
Methods
We analyzed dipstick urine glucose in 195 patients with acute PUUV infection at hospital admission, and divided them into 2 categories according to the presence or absence of glucose in the dipstick urine test. Determinants of disease severity were analyzed in glucosuric and nonglucosuric patients.
Results
Altogether, 24 of 195 patients (12%) had glucosuria. The patients with glucosuria had more severe AKI than patients without glucosuria (median maximum creatinine concentration 459 μmol/l, range 78–1041 μmol/l vs. 166 μmol/l, range 51–1499 μmol/l; P < 0.001). The glucosuric patients had more severe thrombocytopenia (median minimum platelet count 41 × 109/l, range 5–102 × 109/l vs. 62 × 109/l, range 3–249 × 109/l; P = 0.006), and more pronounced signs of increased capillary leakage (change in weight, maximum plasma hematocrit, minimum plasma albumin). The glucosuric patients were more often in clinical shock at admission (20.8% vs. 1.2%; P < 0.001) and the length of hospital stay was longer (median 7.5 days, range 4–22 days vs. 6 days, range 2–30 days; P = 0.009).
Conclusion
Glucosuria is relatively rare, but when present it predicts a more severe disease course in patients with acute PUUV infection.
Keywords: acute kidney injury, glucosuria, hantavirus, Puumala virus, thrombocytopenia
See Commentary on Page 1203
PUUV, found in Europe and Western Russia, causes a mild type of hemorrhagic fever with renal syndrome. An average of 1000 to 3000 serologically confirmed diagnoses are made annually in Finland, but up to 5% of blood donors are positive for PUUV antibodies, indicating that most of the cases are mild and remain undiagnosed.1 The symptoms of the infection include fever, headache, nausea, abdominal pain, backache, and visual disturbances. Bleeding diathesis is rare. The renal involvement includes proteinuria, hematuria and AKI. Proteinuria is mainly albuminuria and can reach nephrotic range; however, the renal histology is that of acute tubulointerstitial nephritis.2, 3, 4 Hospitalized patients often have AKI with transient oliguria followed by polyuria. Few patients (6%) need transient dialysis.2 Patients show signs of increased capillary leakage and are thrombocytopenic. Case fatality is low (<0.1%)5 and ultimate prognosis is good.
Glucosuria is a rather infrequent finding in patients with AKI. In general, glucosuria is detected in diabetic patients with high serum glucose concentration and in patients with renal tubular injury such as the Fanconi syndrome. However, 9% of adults and 12% of children who have acute PUUV infection present with glucosuria.2, 6
We have previously shown that albuminuria and hematuria in dipstick urine test predict the severity of upcoming AKI in patients with acute PUUV infection.7, 8 We now report that glucosuria in these patients is an even stronger predictor of disease severity.
Materials and Methods
Subjects
The study cohort consisted of 220 adult patients treated in Tampere University Hospital, Finland, due to serologically confirmed acute PUUV infection during the years 1994 to 2014. A detailed medical history of the patients was obtained and all patients were carefully clinically examined. Clinical features like blood pressure, heart rate, and weight were measured at least daily. The daily urine output was followed during the hospital stay.
One or several previous diagnoses had been made in 7 of 24 patients with glucosuria (29%) and in 50 of 171 patients without glucosuria (30%). The diagnoses were hypertension (n = 13), coronary heart disease (n = 6), rheumatoid arthritis (n = 3), bronchial asthma (n = 6), celiac disease (n = 3), prostate hyperplasia (n = 2), cerebral infarction (n = 1), juvenile rheumatoid arthritis (n = 1), Crohn’s disease (n = 1), epilepsy (n = 1), and spherocytosis (n = 1). One patient had Henoch-Schönlein purpura diagnosed 25 years earlier with no kidney manifestation and 1 patient had a previously treated kidney tuberculosis. Two of the nonglucosuric patients had type 2 diabetes. One patient was pregnant and 1 was breastfeeding. None of the patients in either group had a known kidney insufficiency before the PUUV infection.
A dipstick urine test was available in 196 patients at hospital admission. One of them had a previously undiagnosed type 2 diabetes with blood glucose level of 26.9 mmol/l at admission and was excluded from further analyses. The patients were divided into 2 categories based on the presence or absence of glucose in the dipstick test. Glucosuria was detected in 24 of 195 patients (12.3%), whereas 171 of 195 patients (87.7%) were negative for glucosuria.
A chest radiograph was taken in 150 patients at hospital admission. All radiographs were analyzed by a radiologist.9
All patients provided a written consent and the study was approved by the Ethics Committee of the Tampere University Hospital (study codes 97166, 99256, R04180, and R09206).
Laboratory Determinations
The diagnosis of PUUV infection in 1982 to 1989 was based on duplicate samples with 4-fold or grater rise in IgG titer by the immunofluorescence assay.10 Since 1989, recent PUUV infection was confirmed from a single serum sample by detecting the typical granular staining pattern in immunofluorescence assay11 and/or low avidity of IgG antibodies to PUUV and /or by detecting PUUV IgM antibodies by an “in-house” enzyme-linked immunosorbent assay based on a recombinant antigen.12 The development and use of these and other diagnostic methods have been described by Vaheri et al.13
A dipstick urine test was performed in the emergency room at hospital admission and a follow-up sample was obtained in 11/24 glucosuric and in 76/171 non-glucosuric patients during the hospital care. The dipstick urine test was obtained more than once in 4 of 24 glucosuric and 24 of 171 nonglucosuric patients. Urine dipstick analysis was made by automated tests based on refractometry using Miditron M (Roche, Basel, Switzerland) from 1997 onward, Urisys 2400 or 1900 (Roche) from 2004 onward, and Siemens (Munich, Germany) Clinitek Atlas or Advantus from 2009. The dipstick assay detects albumin, and it does not react with immunoglobulins, immunoglobulin light chains, or tubular proteins. The sensitivity of the assay to urine albumin is 0.15 to 0.3 g/l (U-Alb 1+), ≥ 1 g/l (U-Alb 2+) and 3 g/l (U-Alb 3+). The assay for hematuria detects heme pseudoperoxidase activity and therefore it also detects red cell casts and dysmorphic red cells. The sensitivity of the assay is approximately 10 × 106 cells/l (approximately 3–5 cells by high-power field). The dipstick test for leukocytes detects granulocyte and macrophage esterase activity. The sensitivity is approximately 30 × 106 cells/l (1–2 cells by high-power field). The dipstick test for glucose detects glucosuria from glucose level 3 to 5 mmol/l upward. Glucosuria 3+ corresponds to a glucose level exceeding 30 mmol/l.
Plasma creatinine was analyzed by Vitros (Johnson & Johnson, Rochester, NY) until the 1999 and by Cobas Integra (F. Hoffmann-La Roche Ltd., Basel, Switzerland) from thereafter. Plasma creatinine value was determined daily during the hospital stay and the first value and the highest value (maximum) were taken into the statistical analysis. In addition, several other blood or plasma samples were analyzed on a daily basis including blood cell count, C-reactive protein, and electrolytes. Plasma creatinine max and blood cell count were available from all patients. Creatinine first was available from 24 of 24 glucosuric and 167 of 171 nonglucosuric patients. C-reactive protein was available from 22 of 24 glucosuric and 169 of 171 nonglucosuric patients. Maximum plasma urea concentration was available from 20 of 24 of the glucosuric and 85 of 171 nonglucosuric patients and minimum plasma albumin from 19 of 24 glucosuric and 86 of 171 nonglucosuric patients. Daily urinary volume was available from all glucosuric and from 163 of 171 nonglucosuric patients. Blood cell count was determined by automated hematological cell counters (Bayer Diagnostics, Elkhart, IN), and sodium, potassium, urea, and albumin concentrations using routine automated chemistry analyzers. All laboratory determinations were performed by the Laboratory Centre of the Pirkanmaa Hospital District (later named Fimlab laboratories), Tampere, Finland. Minimum or maximum of the values were taken to the statistical analysis as indicated in Tables 1 and 2.
Table 1.
Clinical findings in 195 patients with PUUV infection according to the presence of glucosuria at admission
| Glucosuria n = 24 |
No glucosuria n = 171 |
P value | |||
|---|---|---|---|---|---|
| Median/number | Range/% | Median/number | Range/% | ||
| Age | 40 | 25–67 | 41 | 21–73 | 0.908 |
| Men | 21 | 87.5 | 111 | 64.5 | 0.034 |
| BMI | 28.1 | 20.4–37.2 | 25.6 | 18.5–41.9 | 0.031 |
| Shock at admissiona | 5 | 20.8 | 2 | 1.2 | <0.001 |
| First systolic BP (mm Hg) | 115 | 70–167 | 124 | 72–210 | 0.025 |
| Max systolic BP (mm Hg) | 144 | 123–204 | 138 | 95–210 | 0.016 |
| Max diastolic BP (mm Hg) | 94 | 78–111 | 87 | 60–120 | 0.017 |
| Min systolic BP (mm Hg) | 109 | 60–150 | 113 | 68–107 | 0.243 |
| Min diastolic BP (mm Hg) | 75 | 36–91 | 70 | 39–100 | 0.542 |
| Hospital stay (d) | 7.5 | 4–22 | 6 | 2–30 | 0.009 |
| Weight change (kg)b | 4.0 | 0.5–11.3 | 2.5 | 0.1–12.0 | 0.025 |
| Urinary output min (ml/d) | 600 | 0–4900 | 1450 | 0–7000 | 0.001 |
| Dialysis | 3 | 12.5 | 7 | 4.1 | 0.110 |
BMI, body mass index; BP, blood pressure; Max, maximum; Min, minimum; PUUV, Puumala hantavirus.
Glucosuria: urine dipstick glucose from 1+ to 3+; No glucosuria: urine dipstick glucose 0.
Shock was defined as systolic blood pressure less than 90 mmHg and symptoms of shock.
Difference between highest and lowest weight during hospital care reflecting both capillary leakage and fluid accumulation during oliguric phase.
Table 2.
Laboratory findings in 195 patients with PUUV infection according to the presence of glucosuria at admission
| Glucosuria n = 24 |
No glucosuria n = 171 |
P value | |||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Plasma and blood findings | |||||
| Creatinine first (μmol/l) | 184 | 72–617 | 96 | 43–1113 | 0.002 |
| Creatinine max (μmol/l) | 459 | 78–1041 | 166 | 51–1499 | <0.001 |
| Urea max (mmol/l) | 30.3 | 3.2–39.3 | 17.4 | 2.1–54.5 | 0.012 |
| Platelets min (× 109/l) | 41 | 5–102 | 62 | 3–249 | 0.006 |
| Hematocrit max | 0.51 | 0.34–0.60 | 0.43 | 0.33–0.59 | <0.001 |
| Albumin min (g/l) | 24 | 11–33 | 28 | 20–39 | <0.001 |
| Leukocytes max (× 109/l) | 16.0 | 5.7–45 | 10.2 | 4.2–35.5 | <0.001 |
| CRP max (mg/l) | 81 | 21–213 | 77 | 16–269 | 0.446 |
| Sodium min (mmol/l) | 128 | 122–140 | 132 | 109–142 | 0.001 |
| Sodium max (mmol/l) | 143 | 132–159 | 141 | 128–150 | 0.034 |
| Potassium min (mmol/l) | 3.6 | 3.7–5.2 | 3.6 | 3.3–5.5 | 0.554 |
| Potassium max (mmol/l) | 4.4 | 2.7–4.0 | 4.3 | 2.9–4.9 | 0.306 |
| Glucose (mmol/l)a | 8.3 | 4.9–17.6 | 5.9 | 3.6–10.9 | 0.001 |
| Urine findings | |||||
| Leukocyte count (× 106/l) | 4 | 0–195 | 3 | 0–86 | 0.442 |
| Erythrocyte count (× 106/l) | 11 | 2–254 | 4 | 0–401 | <0.001 |
CRP, C-reactive protein; Max, maximum; min, minimum; PUUV, Puumala hantavirus.
Glucosuria: urine dipstick glucose from 1+ to 3+; No glucosuria: urine dipstick glucose 0.
Blood glucose concentration at hospital admission.
Statistical Analysis
For the descriptive analyses, the medians with ranges and frequencies (n) with percentages and cross-tabulation were used for the exploratory analyses. For the comparative analyses, Mann-Whitney U test and Student’s t-test for independent samples were performed. The χ2 tests were used to examine differences in proportions. The Spearman correlations (rS) were used to study the relationship between variables. Binary logistic regression was used to adjust upcoming severe AKI with plasma creatinine at admission. All analyses were performed using IBM SPSS Statistics version 24 (IBM, Armonk, NY).
Results
Clinical, Laboratory, and Radiological Findings
Glucosuria was present in 24 of 195 patients (12.3%) with acute PUUV infection. In most cases, glucosuria was mild. Twenty patients presented with urine glucose 1+ in the dipstick test, 3 patients with urine glucose 2+, whereas only 1 patient had urine glucose 3+. Table 1 shows the clinical data of the patients with and without glucosuria. We found glucosuria in 21 of 132 (15.9%) men but in only 3 of 63 (4.8%) women (P = 0.034). None of the glucosuric patients had a diagnosis of diabetes. Patients who were nonglucosuric at hospital admission were nonglucosuric in the follow-up samples. One glucosuric patient was positive for urine glucose at day 1 after hospital admission but turned urine glucose negative in the following samples. The rest of the glucosuric patients were negative for urine glucose in the follow-up samples. The patients arrived at the hospital a median of 4 days after the onset of fever with no difference between the groups.
The use of drugs potentially influencing blood glucose concentration was studied and we found no differences in the use of beta blockers, thiazide diuretics, or corticosteroids (oral or inhaled) or in the use of antibiotics between the groups (data not shown). The glucosuric patients were more often in clinical shock at admission, but presented with higher maximum systolic and diastolic blood pressure during the hospital stay, the length of which was also longer than in nonglucosuric patients, which can be assumed to reflect the overall severity of the disease (Table 1). There was no difference in smoking habits between the groups (data not shown).
The laboratory findings of the patients according to the presence of glucosuria are presented in Table 2. The first plasma creatinine measured at admission was higher in glucosuric patients. Patients with glucosuria had higher maximum plasma creatinine and urea concentrations. When adjusted to creatinine at admission, glucosuria remained a significant predictor of severe AKI, defined as plasma creatinine ≥ 353.6 μmol/l; odds ratio 5.9 (95% confidence interval 1.9–18.0).14 The association of glucosuria with higher maximum plasma creatinine level was observed in both sexes (data not shown). The minimum number of platelets was also lower in glucosuric patients (Table 2).
The presence of glucosuria was related to variables reflecting capillary leakage. The patients with glucosuria had a greater change in weight (Table 1), higher maximum hematocrit, and lower minimum albumin (Table 2) when compared with patients without glucosuria.
Blood glucose sample taken at the hospital admission was available in 14 of 24 patients with glucosuria and in 62 of 171 patients without glucosuria. The concentration of blood glucose was higher in the glucosuric patients (Table 2). Importantly, blood glucose level was below the kidney glucose threshold of 10 mmol/l in 60/62 nonglucosuric and in 12 of 14 glucosuric patients. Blood glucose sample taken later during the hospital care was available in 15 glucosuric and in 84 nonglucosuric patients. The level of blood glucose decreased and there was no difference in the minimum blood glucose concentration between the groups (data not shown).
There was no correlation between body mass index (BMI) and blood glucose concentration at hospital admission either in patients with glucosuria or in patients without glucosuria (rS = 0.396, P = 0.180 and rS = 0.178, P = 0.203, respectively). BMI did not correlate with maximum plasma creatinine value either in glucosuric (rS = 0.062, P = 0.784) or in nonglucosuric patients (rS = 0.040, P = 0.623).
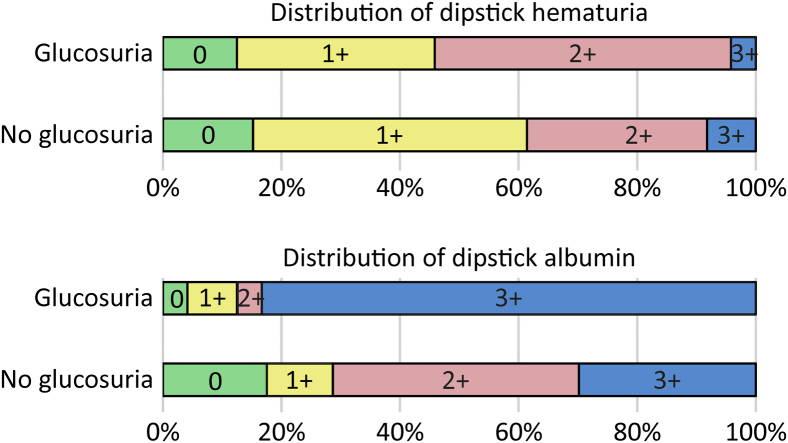
The number of urine erythrocytes was higher in glucosuric patients (Table 2); however, there was no difference in dipstick hematuria between the groups (Figure 1). The prevalence and the amount of albumin in the dipstick urine test at hospital admission was higher in glucosuric patients. Dipstick albumin was positive in 96% of patients and reached 3+ in 83% of patients with glucosuria compared with 83% and 30% of patients without glucosuria, respectively (P < 0.001 for both) (Figure 1).
Figure 1.
Distribution of findings in urine dipstick test for hematuria (U-Eryt) and albuminuria (U-Alb) in patients with acute Puumala hantavirus infection as classified to subgroups according to the presence or absence of glucosuria. Glucosuric patients, n = 24; nonglucosuric patients, n = 171.
The chest radiography was abnormal in 6 of 14 (43%) patients with glucosuria and in 25 of 136 (18%) patients without glucosuria (P = 0.042). There was no difference in the occurrence of pleural effusion (data not shown).
Dipstick Test and the Incidence of AKI
We have previously reported that albuminuria and hematuria in dipstick urine test predict the severity of the upcoming AKI.8 Here we evaluated the impact of dipstick glucosuria, albuminuria, hematuria, and combined albuminuria + hematuria + glucosuria, in predicting the severity of the upcoming AKI.
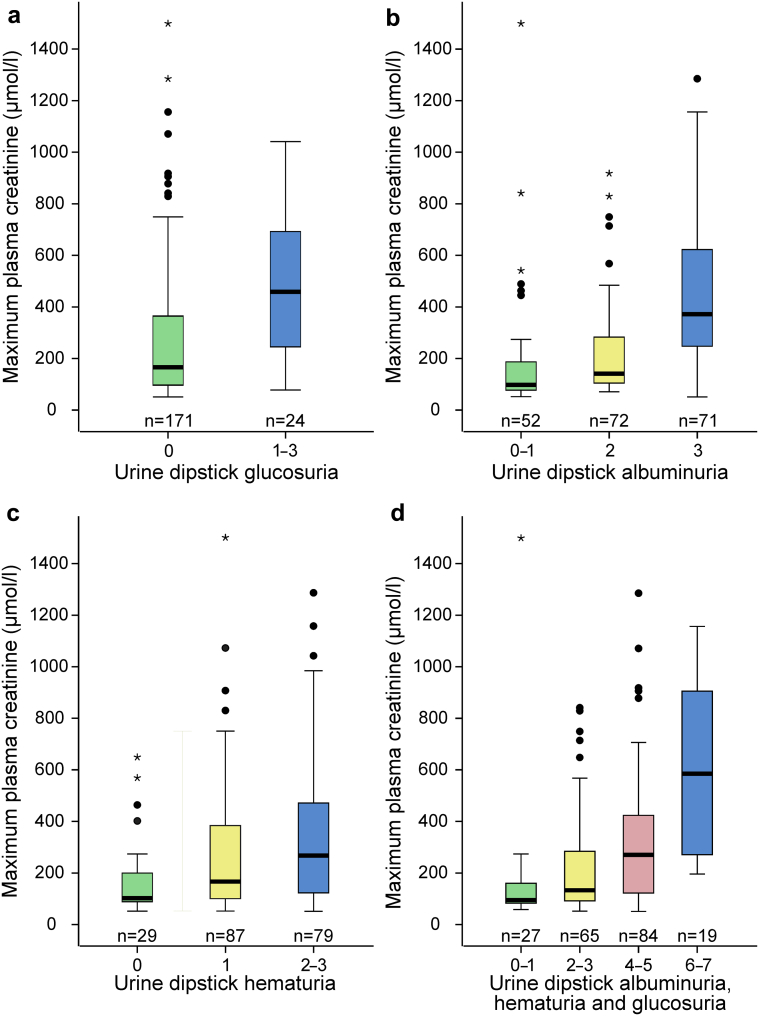
When evaluating the impact of glucosuria, the median maximum creatinine level was higher in patients with glucosuria than in patients without glucosuria (459 μmol/l, range 78–1041 vs. 166 μmol/l, range 51–1499, P < 0.001) (Figure 2a).
Figure 2.
Maximum plasma creatinine value analyzed according to urine dipstick result: glucosuria (a), albuminuria (b), hematuria (c), and combined result of albuminuria + hematuria + glucosuria (d). The number of patients in each group is indicated in the figure. Boxplots are with median, interquartile range, minimum, and maximum within 1.5 interquartile range, and outliers are displayed as circles and extreme values as asterisks.
When assessing the significance of albuminuria, increasing positive result in dipstick urine albumin test predicted higher maximum creatinine level. The median maximum creatinine level was 98 μmol/l (range 52–1499) for albumin 0–1+, 142 μmol/l (range 71–918) for albumin 2+, and 372 μmol/l (range 51–1285) for albumin 3+ (Figure 2b).
When evaluating the impact of hematuria, increasing positive result in the dipstick test also predicted higher maximum creatinine level. The median for maximum plasma creatinine was 102 μmol/l (range 58–648) for 0+; 166 μmol/l (range 52–1499) for 1+; and 267 μmol/l (range 51–1285) for 2 to 3+ (Figure 2c).
Finally, higher positive result in the dipstick test for albuminuria + hematuria + glucosuria predicted higher maximum creatinine. The median maximum creatinine was 95 μmol/l (range 58–1499) for 0 to 1+, 133 μmol/l (range 52–841) for 2 to 3+, 271 μmol/l (range 51–1285) for 4 to 5+, and 585 μmol/l (range 196–1156) for 6 to 7+ (Figure 2d). There were no patients with a sum of positive results exceeding 7+.
Discussion
To our knowledge, this is the first study to show that glucosuria predicts the severity of the upcoming AKI and the overall severity of disease in patients with acute hantavirus infection. Glucosuria correlated with higher systolic and diastolic blood pressure during the hospital stay, which most likely reflects the severity of AKI and fluid retention during the oliguric phase of the infection. Glucosuria correlated with the determinants of capillary leakage: low blood pressure at hospital admission, presence of clinical shock and pathologic chest radiography changes, high hematocrit, low plasma albumin, and greater change in weight during the hospital stay. Glucosuria also correlated with thrombocytopenia. The length of the hospital stay was longer in the glucosuric patients, reflecting the overall severity of the disease. Therefore, glucosuria at admission correlated with all of the main clinical findings of severe PUUV infection: AKI, inflammation, capillary leakage, and thrombocytopenia.
In the current study, 12% of the patients presented with glucosuria at hospital admission. Glucosuria was more common in men than in women, but the same relation between glucosuria and more severe AKI was seen in both sexes. Nonglucosuric patients remained glucose negative in all follow-up samples. One glucosuric patient was glucosuria positive in the first follow-up sample but turned glucosuria negative later during the hospital care. All the rest of the glucosuric patients were glucosuria negative in the follow-up urine samples. It appears that glucosuria is an early and transient sign in PUUV infection.
Although plasma creatinine was higher in glucosuric patients already at the time of the first creatinine measurement, it increased more than in nonglucosuric patients during the hospital care. When the outcome was severe AKI and the result was adjusted to the first plasma creatinine measured, glucosuria still remained a significant predictor of severe AKI. Therefore, glucosuria both associates with and predicts severe AKI. The patients arrived at hospital a median of 4 days after the onset of fever and there was no difference between the groups.
In general, glucosuria most often results from high blood glucose concentration. Glucose is freely filtered into primary urine but it is completely reabsorbed in the tubules. Ninety percent of the reuptake takes place in the proximal tubule by the sodium-glucose cotransporter type 2/glucose transporter 2 (SGLT2/GLUT2) (low affinity–high capacity system). The convoluted part of the proximal tubule also participates in glucose reuptake via the SGLT1/GLUT1 (high affinity-low capacity system). The kidney tubular glucose threshold for blood glucose is approximately 10 to 11 mmol/l at normal glomerular filtration rate, after which the reabsorptive capacity is overwhelmed and glucose appears in the urine.15 When glomerular filtration rate increases, like in the case of pregnancy, the glucose threshold level decreases. Conversely, in chronic renal insufficiency, the level of glucose threshold increases and higher blood glucose levels are tolerated before glucose appears in the final urine. In addition, in diabetes, the maximum reabsorptive capacity of the kidney increases and therefore hyperglycemic diabetic patients may not be glucosuric.15 Little is known about glucosuria in AKI caused by etiologies other than acute tubulointerstitial nephritis (TIN).
We found that blood glucose concentration at hospital admission was higher in the glucosuric than in the nonglucosuric patients. However, none of the glucosuric patients had diabetes. Also, there was no difference in the use of drugs that can influence blood glucose concentration. During the hospital stay, the blood glucose levels decreased and there was no difference in the minimum blood glucose concentration between the groups. Further, at admission, the median blood glucose level in the glucosuric patients was 8.3 mmol /l, which is below the threshold level of a healthy kidney and should not result in glucosuria. It appears that glucosuria detected in the present study was caused by kidney damage and not by hyperglycemia. This is in line with the results obtained in animal studies in which glycerol-induced acute renal failure and proximal tubular damage resulted in glucosuria in normoglycemic rats.16
In the present study, BMI was higher in glucosuric patients but BMI did not correlate with either blood glucose level at hospital admission or maximum plasma creatinine value. Therefore, BMI does not explain the glucosuria or the observed association between glucosuria and severe AKI. It is possible that the higher blood glucose concentration in the glucosuric patients was due to the hypercortisolism caused by the acute PUUV infection,17 and that cortisol raised blood glucose more in the obese patients. However, cortisol metabolism was not systematically analyzed in this study.
Glucosuria can result from proximal tubular damage, such as the Fanconi syndrome. PUUV is known to infect tubular epithelial cells and histologically PUUV infection causes an acute TIN.4 In adult patients, 60% to 80% of TIN cases are drug induced, the second most common cause being autoimmune diseases.18 However, glucosuria is not a common finding in acute TIN in adults. Some causative agents are more likely than others to induce glucosuria in acute TIN. Among these are some non–β-lactam antibiotics, antiviral agents, cytostats, the antiepileptic sodium valproate, and the mood stabilizer lithium.19, 20 In addition, D-serine, a newly acknowledged uremic toxin, can cause damage to the proximal tubule and glucosuria.21 As a comparison, 100% of pediatric patients with TIN and uveitis syndrome had glucosuria.22 The etiology of TIN and uveitis syndrome, remains unknown.
Several infections can cause TIN. In pediatric patients with Yersinia pseudotuberculosis–induced interstitial nephritis, 27% of patients with AKI exhibited glucosuria, whereas none of the patients with normal kidney function had glucosuria.23 In a small case series from the Czech Republic, all 3 pediatric patients with PUUV infection had glucosuria and AKI.24 In our previous reports from patients with acute PUUV infection, 9% of adults and 12% of children had glucosuria.2, 6 However, to our knowledge, this is the first time glucosuria has been used as a maker of disease severity in infection-induced AKI.
The PUUV typically infects kidney tubular epithelial cells. The PUUV RNA is mostly seen in the distal tubuli25 and several adhesion molecules and cytokines are seen in the peritubular area of the distal nephron.26 Subsequent splitting of the tubular basement membrane has been detected in both proximal and distal tubuli.27 The proximal tubule is essential in the reuptake of glucose. In addition, inflammation and cytokine release influence kidney function by decreasing renal tissue perfusion and glomerular filtration rate, decreasing the expression of SGLT2, SGLT3, and GLUT 2, and increasing fractional glucose excretion.28 Several different cytokines are also known to influence the severity of PUUV infection.29, 30, 31 As in a case of glucosuria in experimental animal study,16 glucosuria in our study is most likely a marker of a more severe tubular injury in patients with PUUV infection. Whether glucosuria is a marker of unspecific damage to the proximal tubular cells or a result of a specific pathology needs further investigation.
The prevalence and the amount of albuminuria were higher in patients with glucosuria than in patients without glucosuria (Figure 1). Although urine dipstick hematuria did not differ between the study groups, the number of urine erythrocytes was higher in the glucosuric patients (Table 2). The reason for this discrepancy is unknown.
We previously reported that albuminuria, hematuria, and combined albuminuria + hematuria predicted the severity of the upcoming AKI.7, 8 We now evaluated how different variables or combination of variables in the dipstick urine test predict AKI. Glucosuria is rare, but the presence of even mild glucosuria associates and predicts AKI (Figure 2a). Albuminuria and hematuria are more common and they have a dose-dependent effect on the severity of AKI. Mild albuminuria and mild hematuria indicate benign disease course, whereas abundant albuminuria and hematuria predict severity of AKI (Figure 2b and c); however, the maximum creatinine even with abundant albuminuria or hematuria is still lower than in glucosuric patients. In the combination of albuminuria, hematuria, and glucosuria, there appears to be a threshold level of 4+ (Figure 2d), which separates patients at greater risk of developing severe AKI.
Glucosuria, as well as albuminuria and hematuria, predict the overall severity of the infection. It is of interest, however, that only glucosuria associates with the level of thrombocytopenia.
In conclusion, we show here for the first time that glucosuria in patients with acute hantavirus infection is a good marker for the severity of upcoming AKI and the overall course of the PUUV infection.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported by the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital, Sigrid Jusélius Foundation, Tampere Tuberculosis Foundation, and Magnus Ehrnrooth Foundation. The skillful assistance of Ms. Katriina Ylinikkilä and Ms. Eini Eskola is greatly appreciated.
Footnotes
Strobe Statement.
Supplementary Material
References
- 1.Brummer-Korvenkontio M., Vapalahti O., Henttonen H. Epidemiological study of nephropathia epidemica in Finland 1989–96. Scand J Infect Dis. 1999;31:427–435. doi: 10.1080/00365549950163941. [DOI] [PubMed] [Google Scholar]
- 2.Mustonen J., Brummer-Korvenkontio M., Hedman K. Nephropathia epidemica in Finland: a retrospective study of 126 cases. Scand J Infect Dis. 1994;26:7–13. doi: 10.3109/00365549409008583. [DOI] [PubMed] [Google Scholar]
- 3.Vaheri A., Strandin T., Hepojoki J. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol. 2013;11:539–550. doi: 10.1038/nrmicro3066. [DOI] [PubMed] [Google Scholar]
- 4.Mustonen J., Outinen T., Laine O. Kidney disease in Puumala hantavirus infection. Infect Dis (Lond) 2017;49:321–332. doi: 10.1080/23744235.2016.1274421. [DOI] [PubMed] [Google Scholar]
- 5.Makary P., Kanerva M., Ollgren J. Disease burden of Puumala virus infections, 1995–2008. Epidemiol Infect. 2010;138:1484–1492. doi: 10.1017/S0950268810000087. [DOI] [PubMed] [Google Scholar]
- 6.Mustonen J., Huttunen N., Brummer-Korvenkontio M. Clinical picture of nephropathia epidemica in children. Acta Pediatr. 1994;83:526–529. doi: 10.1111/j.1651-2227.1994.tb13073.x. [DOI] [PubMed] [Google Scholar]
- 7.Outinen T.K., Mantula P., Laine O.K. Haematuria is a marker for the severity of acute kidney injury but does not associate with thrombocytopenia in acute Puumala hantavirus infection. Infect Dis (Lond) 2017;49:840–846. doi: 10.1080/23744235.2017.1358461. [DOI] [PubMed] [Google Scholar]
- 8.Mantula P.S., Outinen T.K., Clement J.P.G. Glomerular proteinuria predicts the severity of acute kidney injury in Puumala hantavirus-induced tubulointerstitial nephritis. Nephron. 2017;136:193–201. doi: 10.1159/000459634. [DOI] [PubMed] [Google Scholar]
- 9.Paakkala A., Mäkelä S., Hurme M. Association of chest radiography findings with host-related genetic factors in patients with nephropathia epidemica. Scand J Infect Dis. 2008;40:254–258. doi: 10.1080/00365540701633012. [DOI] [PubMed] [Google Scholar]
- 10.Brummer-Korvenkontio M., Vaheri A., Hovi T. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980;141:131–134. doi: 10.1093/infdis/141.2.131. [DOI] [PubMed] [Google Scholar]
- 11.Vapalahti O., Kallio-Kokko H., Närvänen A. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early serological response. J Med Virol. 1995;46:293–303. doi: 10.1002/jmv.1890460402. [DOI] [PubMed] [Google Scholar]
- 12.Hedman K., Vaheri A., Brummer-Korvenkontio M. Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet. 1991;338:1353–1356. doi: 10.1016/0140-6736(91)92235-t. [DOI] [PubMed] [Google Scholar]
- 13.Vaheri A., Vapalahti O., Plyusnin A. How to diagnose hantavirus infections and detect them in rodents and insectivores. Rev Med Virol. 2008;18:277–288. doi: 10.1002/rmv.581. [DOI] [PubMed] [Google Scholar]
- 14.Section 2: AKI definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moe O.W., Wright S.H., Palacín M. Renal handling of organic solutes. In: Brenner B.M., Rector F.C., editors. Elsevier Saunders; Philadelphia: 2008. pp. 252–292. (Brenner & Rector's The Kidney. Vol 1). [Google Scholar]
- 16.Westenfelder C., Arevalo G.J., Crawford P.W. Renal tubular function in glycerol-induced acute renal failure. Kidney Int. 1980;18:432–444. doi: 10.1038/ki.1980.156. [DOI] [PubMed] [Google Scholar]
- 17.Mäkelä S., Jaatinen P., Miettinen M. Hormonal deficiencies during and after Puumala hantavirus infection. Eur J Clin Microbiol Infect Dis. 2010;29:705–713. doi: 10.1007/s10096-010-0918-y. [DOI] [PubMed] [Google Scholar]
- 18.Muriithi A.K., Leung N., Valeri A.M. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int. 2014;87:458–464. doi: 10.1038/ki.2014.294. [DOI] [PubMed] [Google Scholar]
- 19.Mehta R.L., Awdishu L., Davenport A. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88:226–234. doi: 10.1038/ki.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knights M., Thekkekkara T., Morris A. Sodium valproate-induced Fanconi type proximal renal tubular acidosis. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213418. bcr2015213418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganote C.E., Peterson D.R., Carone F.A. The nature of D serine induced nephrotoxicity. Am J Pathol. 1974;77:269–282. [PMC free article] [PubMed] [Google Scholar]
- 22.Saarela V., Nuutinen M., Ala-Houhala M. Tubulointerstitial nephritis and uveitis syndrome in children: a prospective multicenter study. Ophthalmology. 2013;120:1476–1481. doi: 10.1016/j.ophtha.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Cheong H.I., Choi E.H., Ha I.S. Acute renal failure associated with Yersinia pseudotuberculosis infection. Nephron. 1995;70:319–323. doi: 10.1159/000188611. [DOI] [PubMed] [Google Scholar]
- 24.Dusek J., Pejcoch M., Kolsky A. Mild course of Puumala nephropathy in children in an area with sporadic occurrence Hantavirus infection. Pediatr Nephrol. 2006;21:1889–1892. doi: 10.1007/s00467-006-0250-z. [DOI] [PubMed] [Google Scholar]
- 25.Sironen T., Klingström J., Vaheri A. Pathology of Puumala hantavirus infection in Macaques. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temonen M., Mustonen J., Helin H. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin Immunol Immunopathol. 1996;78:47–55. doi: 10.1006/clin.1996.0007. [DOI] [PubMed] [Google Scholar]
- 27.Collan Y., Lahdevirta J., Jokinen E.J. Electron microscopy of nephropathia epidemica. Renal tubular basement membrane. Am J Pathol. 1978;92:167–172. [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt C., Höcherl K., Bucher M. Regulation of renal glucose transporters during severe inflammation. Am J Physiol Renal Physiol. 2007;292:F811. doi: 10.1152/ajprenal.00258.2006. [DOI] [PubMed] [Google Scholar]
- 29.Outinen T.K., Mäkelä S.M., Ala-Houhala I.O. The severity of Puumala hantavirus induced nephropathia epidemica can be better evaluated using plasma interleukin-6 than C-reactive protein determinations. BMC Infect Dis. 2010;10:132. doi: 10.1186/1471-2334-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koskela S., Laine O., Mäkelä S. Endothelial nitric oxide synthase G894T polymorphism associates with disease severity in puumala hantavirus infection. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Outinen T.K., Mäkelä S., Huhtala H. High pentraxin-3 plasma levels associate with thrombocytopenia in acute Puumala hantavirus-induced nephropathia epidemica. Eur J Clin Microbiol Infect Dis. 2012;31:957–963. doi: 10.1007/s10096-011-1392-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.