Abstract
Rheumatic fever (RF) is a common cause of acquired heart disease in children worldwide. It is a delayed, nonsuppurative, autoimmune phenomenon following pharyngitis, impetigo, or scarlet fever caused by group A β-hemolytic streptococcal (GAS) infection. RF diagnosis is clinical and based on revised Jones criteria. The first version of the criteria was developed by T. Duckett Jones in 1944, then subsequently revised by the American Heart Association (AHA) in 1992 and 2015. However, RF remains a diagnostic challenge for clinicians because of the lack of specific clinical or laboratory findings. As a result, it has been difficult for some time to maintain a balance between over- and underdiagnosis of RF cases. The Jones criteria were revised in 2015 by the AHA, and the main modifications were as follows: the population was subdivided into moderate- to high-risk and low risk; the concept of subclinical carditis was introduced; and monoarthritis was included as a feature of musculoskeletal inflammation in the moderate- to high-risk population. This review will highlight the major changes in the AHA 2015 revised Jones criteria for pediatricians and general practitioners.
Keywords: American Heart Association, Low/moderate- to high-risk population, Rheumatic fever
Abbreviations
- RF
Rheumatic fever
- RHD
Rheumatic heart disease
- GAS
Group A β-hemolytic streptococcal infection
- AHA
American heart association
- ASO
anti-streptolysin O titer
- ESR
Erythrocyte Sedimentation Rate
- CRP
C-reactive protein
- NSAIDs
non-steroidal anti-inflammatory drugs
- Penicillin V
Phenoxymethylpenicillin
- Penicillin G
Benzylpenicillin
1. Introduction
Rheumatic fever (RF) is a common autoimmune disorder, particularly in developing countries. It is caused by group A β-hemolytic streptococcal (GAS) infection in genetically susceptible individuals [1]. The five cardinal manifestations of RF outlined by Dr. Jones and published in 1944 were carditis, arthritis, chorea, erythema marginatum, and subcutaneous nodules [2]. These features have been memorized by health professionals for several decades with little amendment over time. In Saudi Arabia, a few studies from different regions have demonstrated the mild nature of acute rheumatic fever (ARF) attacks, with frequent carditis ranging from 58% to 65% of patients with ARF [3], [4], [5], [6]. Incidence of RF has declined remarkably in developed countries, but it has not yet been eliminated. In developing countries, it remains a major health challenge and results in lifelong, devastating sequelae.
In view of the high prevalence of the disease, especially in developing countries, it is important to emphasize the revision of the Jones criteria for pediatricians and general practitioners to improve diagnostic sensitivity and achieve earlier disease detection. This will consequently lead to better clinical outcomes for the disease.
2. Methods and discussion
Peer-reviewed articles written in English and indexed in PubMed and EMBASE were screened by four investigators to review ARF diagnosis and management. The following keywords were used: acute rheumatic fever, rheumatic heart disease, Jones criteria, acute rheumatic fever management guidelines, and Saudi Arabia. Article selection was not restricted by year of publication.
3. Epidemiology
RF affects school-age children in the 5- to 14-years age range [7], [8]. Although it occurs all over the world, its epidemiology largely varies. Nowadays, the annual incidence ranges from <0.5/100,000 in developed countries to >100/100,000 in developing countries [9]. The mean incidence rate of RF in the first attack is from five to 51 per 100,000 population [10]. The annual incidence rate is lowest in America and Western European countries (<10/100,000), and there is a relatively higher incidence rate in Eastern Europe, Asia, Australasia, and the Middle East (>10/100,000) [10]. Globally, it has been estimated that approximately 500,000 new RF cases occur annually and that about 230,000 people die each year from the disease [11]. The long-term sequela of acute RF is rheumatic heart disease (RHD), which is the most common cause of heart failure in poor populations [9].
Over the past few years, the global burden of RHD has dramatically reduced in developed countries [12]. However, RHD remains a significant issue in many developing countries, with approximately 1% of all school-age children showing signs of the disease [12]. Arab Gulf countries, Asia, Africa, the Pacific, and the indigenous populations of Australia and New Zealand are most commonly affected by RHD [12], [13]. In Saudi Arabia, published data about the prevalence of RHD are limited. However, it is known that the percentage of children with RHD in Saudi Arabia is still higher than the global rate [14]. In Saudi Arabia, a study was conducted in patients with RF between 1994 and 2003 [15]. The study found that over a 10-year period, 96 children (mean age, 9 years) were diagnosed with RF. The annual incidence was 17 cases in 2 years (1994 and 1995), but only two in 2003, signifying a dramatic decrease in incidence in Saudi Arabia. The average incidence of RF in Saudi Arabia is eight cases annually [15]. Another study conducted on all regions in Saudi Arabia showed a high RF prevalence of 0.3 in 1000 and chronic RHD prevalence of 2.8 in 1000, with an overall rate of 3.1 in 1000 school-age children [4]. Other published studies on different regions in Saudi Arabia reported children older than 5 years as having a high prevalence rate [5], [6]. RF could be the first attack or a relapse. For example, in the study of Abbag et al [6], it was found that 34 out of 40 (85%) patients had initial attacks and 12 (30%) were relapse cases, whereas Al-Eissa et al’s [5] study reported 51 initial attacks out of 67 (76%) children and 22 (32%) relapse cases.
4. Rheumatic fever diagnostic criteria
The diagnosis of RF is based on Dr. Jones’ criteria, which were recently revised (2015). The criteria include major and minor manifestations, and risk stratification has recently been applied to populations, dividing them into low risk and moderate- to high-risk [9]. The major diagnostic criteria are carditis, arthritis, chorea, erythema marginatum, and subcutaneous nodules, whereas the minor criteria are arthralgia, hyperpyrexia, high erythrocyte sedimentation rate (ESR), and/or high C-reactive protein (CRP), and prolonged PR interval [9]. To diagnose a patient with RF as a first episode of the disease, a confirmation of two major criteria or one major and two minor criteria is required, along with evidence of antecedent GAS infection. The diagnosis of subsequent episodes of RF requires either two major criteria, one major and two minor criteria, or three minor criteria [9]. The evidence of GAS infection is confirmed by one of the following: a positive throat culture for GAS; increasing trend anti-streptolysin O titer (ASO) readings rather than a single titer result; or a positive rapid group A streptococcal carbohydrate antigen test in a child who clinically suggests a high pretest probability of streptococcal pharyngitis [9].
4.1. Difference between low-risk and moderate- to high-risk populations
The differences between low-risk and moderate- to high-risk populations in the major criteria are as follows. Arthritis must be polyarthritis in the low risk population, whereas in the moderate- to high-risk population it can be polyarthritis, polyarthralgia, and/or monoarthritis. Meanwhile, the differences in the minor criteria are the following. In arthralgia, the number of affected joints is important in risk stratification. Polyarthralgia is considered a minor criterion in the low-risk population, whereas monoarthralgia is a minor criterion in the moderate- to high-risk population. Moreover, a ≥30 mm/h ESR is considered a minor criterion in the moderate- to high-risk population, but in the low-risk population it must be ≥60 mm/h. Regarding fever, 38.5 °C is considered febrile in the low-risk population, whereas in the moderate- to high-risk population, 38.0 °C is considered as fever [9]. The revised criteria are summarized in Table 1.
Table 1.
Summary of major and minor criteria of RF in low-risk and moderate- to high-risk population.
| Major criteria | |
|---|---|
| Low-risk population | Moderate- to high-risk population |
| - Carditis (clinical or subclinical) | - Carditis (clinical or subclinical) |
| - Arthritis (polyarthritis only) | - Arthritis (polyarthritis, polyarthralgia, and/or monoarthritis) |
| - Chorea | - Chorea |
| - Erythema marginatum | - Erythema marginatum |
| - Subcutaneous nodule | - Subcutaneous nodule |
| Minor criteria | |
| Low-risk population | Moderate- to high-risk population |
| - Polyarthralgia | - Polyarthralgia |
| - Fever (≥38.5 °C) | - Fever (≥38.0 °C) |
| - Elevation of ESR (≥60 mm in the 1st hour) and/or CRP ≥3 mg/dL | - Elevation of ESR (≥30 mm in the 1st hour) and/or CRP ≥3 mg/dL |
| - Prolonged PR interval, corrected for age (only when there is no carditis) | - Prolonged PR interval, corrected for age (only when there is no carditis) |
CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; RF = rheumatic fever.
Universally, the most common presentations of RF major criteria are carditis (50–70%) and arthritis (35–66%) [12], [16], [17], [18], [19], [20]. These are followed by chorea (10–30%), which predominates in females, and then subcutaneous nodules (<10%) and erythema marginatum (<6%), which are rare but highly specific manifestations of RF [9], [18], [19], [20].
In Saudi Arabia, a study published in 2009 reported that the most common presentation of the RF major criteria is arthritis, which was present in 73%, followed by carditis in 17%, and chorea in 10% of cases [15]. None of the patients included in the study presented with erythema marginatum or subcutaneous nodules. For the minor criteria, an elevated ESR was most commonly seen in 94% of patients, followed by high grade fever in 83%, prolonged PR interval in 23%, and arthralgia in the absence of arthritis in 11% [15].
5. Differences between the 1992 Jones criteria and American heart Association criteria 2015
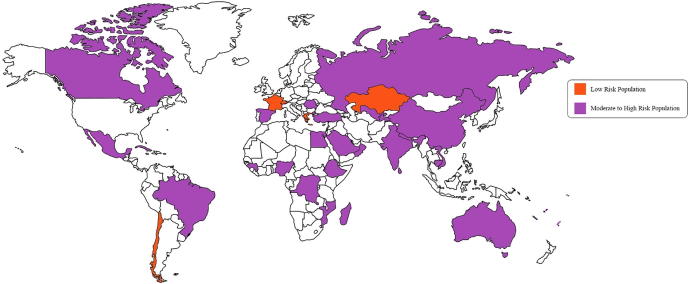
There have been two substantial changes in the recently published 2015 American Heart Association (AHA) criteria compared to the 1992 Jones criteria [21]. One is that susceptible children are divided into two groups on the grounds of epidemiological variation regarding the risk of developing the disease. The reason for this change is that ARF incidence varies significantly from one country to another. Dividing the population into low and moderate- to high-risk would help prevent overdiagnosis in low-risk populations and prevent underdiagnosis in moderate- to high-risk populations [9]. The risk stratification depends on the incidence of RF in the area. Low-risk populations are shown in orange and moderate- to high-risk populations in purple on the world map shown in Fig. 1. Further details of each country are listed in Table 2. For example, children aged 5–14 years living in a community with an incidence of RF of <2/100,000/year, or children of any age where the prevalence of chronic rheumatic carditis is one or more/1000 per year are considered low risk (Class IIa, Level of Evidence C). Meanwhile, children living in areas with an incidence of two or more/100,000/year in children aged 5–14 years or a prevalence of chronic rheumatic carditis more than one/1000/year at any age are considered at a moderate to high risk of developing the disease (Class IIa, Level of Evidence C) [21].
Figure 1.
World map showing low- and moderate- to high-risk populations depending on the reported literature and the new American Heart Association criteria.
Table 2.
Reported data on low and moderate- to high-risk areas.
| Low-risk population | Moderate-to high-risk population | ||
|---|---|---|---|
| Chile [22] | Australia [23], [24] | India [25] | Samoa [26] |
| France [27] | Brazil [28] | Madagascar [29] | Saudi Arabia [30] |
| Greece [31] | Cambodia [12] | Mexico [32] | Spain [33] |
| Kazakhstan [12] | Canada [34] | Mozambique [12] | Sri Lanka [35] |
| Switzerland [36] | China [37] | Nepal [38] | Sub-Saharan Africa [39] |
| Cuba [40] | New Caledonia [41] | Tajikistan [12] | |
| Democratic Republic of Congo [12] | Nigeria [42] | Tonga [43] | |
| Egypt [44] | Oman [45] | Turkey [46] | |
| Ethiopia [47] | Qatar [13] | Uzbekistan [12] | |
| Fiji [48] | Romania [12] | Vietnam [49] | |
| Guinea [12] | Russia [50] | ||
The diagnostic criteria for an initial RF episode in low-risk patients have not changed from the Jones criteria published in 1992. These state that the patient should have two major manifestations or one major plus two minor manifestations [9]. However, in the update published in 2015, polyarthralgia and monoarthritis are considered as major criteria in patients belonging to the moderate- to high-risk group. Moreover, in this risk group, monoarthralgia is considered a minor criterion [9]. The minor criteria stipulate an ESR ≥60 mm in the 1st hour in low-risk individuals but an ESR ≥30 mm/h in moderate- to high-risk patients. There are no other changes in the minor criteria, regardless of the risk stratification group [9].
Most importantly, regardless of the risk stratification, the latest update recommends using echocardiography with Doppler to diagnose carditis and subclinical carditis in all patients [9]. Echocardiography has a high sensitivity and is more reliable in diagnosing valvular involvement in ARF [51]. In addition, it is recommended to repeat echocardiography in case of uncertainty [9]. Carditis has been accepted globally as a major criterion [9]. However, the concept of subclinical carditis, which is defined as positive findings of mitral or aortic valvitis on an echocardiogram without heart murmurs or other clinical signs, has emerged as a major criterion [9]. The echocardiographic features of rheumatic carditis are focused on the aortic and mitral valves. The American College of Cardiology has described, in brief, mitral valve regurgitation detected in two or more views, jet length ≥2 cm, peak velocity >3 m/s, and pansystolic. It also described aortic valve regurgitation if detected in two or more views, jet length ≥1 cm, peak velocity >3 m/s, and pandiastolic. All four above-mentioned criteria must be met to diagnose mitral valve regurgitation and aortic valve regurgitation, respectively [9]. The detailed echocardiographic features of rheumatic carditis are shown in Table 3 [52].
Table 3.
Echocardiographic features of rheumatic carditis.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In all patients diagnosed with rheumatic carditis, the mitral valve is usually involved, and the most common finding in color flow imaging is mitral regurgitation [53]. Mitral regurgitation in rheumatic carditis is associated with restriction of leaflet mobility and/or ventricular dilatation [53]. Rheumatic carditis does not result in congestive heart failure without hemodynamically significant valve lesions [53]. Moreover, it is observed in patients with rheumatic carditis that valve nodules could show echocardiographic equivalents of rheumatic verrucae [53]. Echocardiography is widely available worldwide, and numerous studies have reported echocardiography/Doppler evidence of mitral or aortic valve regurgitation in patients with ARF despite the absence of classic auscultatory findings. However, expert cardiac sonographers are not widely available, particularly in developing countries.
Arthritis in the 1992 Jones criteria is described as migratory polyarthritis in the larger joints, mainly the knees, ankles, wrists, and elbows, which tends to improve significantly with salicylates or nonsteroidal anti-inflammatory drugs (NSAIDs) [54]. However, in the 2015 update, the consideration of monoarthritis and monoarthralgia as major and minor criteria, respectively, make a migratory description impractical. The second change in the 2015 Jones criteria is in the criteria used to diagnose RF relapse. For individuals who have previously had RF, the number of criteria required for diagnosis has been modified. In addition to the typical two major criteria or one major and two minor criteria for diagnosing RF, RF relapse can be diagnosed when three minor criteria are present in the patient [9].
5.1. Impact of AHA 2015 on clinical practice in Saudi Arabia
We believe the study conducted by Kumar et al [55] has provided good insight into how the AHA 2015 update on Jones criteria might impact clinical practice, especially in developing countries. The authors compared the updated AHA criteria to the World Health Organization (WHO) 2004 and Australian guidelines 2012 [56], [52] and found that newer criteria that incorporated subclinical carditis and monoarthritis as major criteria led to a modest increase in the diagnosis of ARF cases. Until local data are available for Saudi Arabia, we think a similar situation is applicable to our population.
6. Treatment of ARF
There are two main goals in treating ARF; the first is to eliminate GAS infection via anti-streptococcal treatment and the second is to treat the clinical manifestations such as arthritis, carditis, and chorea [1], [57].
6.1. Antimicrobial options for eradicating group A β-hemolytic streptococcus infection
-
(1)
Phenoxymethylpenicillin (penicillin V) oral is the anti-streptococcal management of choice for streptococcal pharyngitis. The correct dose of phenoxymethylpenicillin (penicillin V) for patients weighing >27 kg is 500 mg two or three times a day for 10 days. Children weighing ≤27 kg should be given 250 mg two or three times a day for 10 days [1], [57].
-
(2)
Another choice in anti-streptococcal treatment is benzylpenicillin (penicillin G), which is used only in hospital facilities because it is given intramuscularly. It is given as an individual dose of 1.2 MIU for patients with a body weight >27 kg or age >6 years, and 600,000 IU for pediatric patients with a body weight <27 kg or age <6 years [1], [57].
-
(3)
Also, amoxicillin can be given orally for 10 days at a dose of 50 mg per kg with a maximum dose of 1 g every 8 hours. As per AHA guidelines, it is essential to start penicillin treatment because it could prevent initial episodes of RF up to the 9th day of disease onset [57].
6.2. Cases in which the patient is allergic to penicillin
In cases of known allergy to penicillin, a narrow-spectrum oral cephalosporin (cefadroxil or cefalexin) should be administered for 10 days. However, in immediate type hypersensitivity, macrolides such as clindamycin 20 mg/kg/d divided into three times daily (max 1.8 g/d) or clarithromycin 15 mg/kg/d divided into twice daily (max 250 mg, BID) should be administered orally for 10 days, except for azithromycin, which is given for 5 days at a dose of 12 mg/kg once daily (max 500 mg) [57].
One of the causative agents of ARF is GAS infection. A study was conducted by Bhardwaj et al. [58] in 2018 on 296 patients who had GAS infection and who showed antimicrobial resistance. Seventy-nine percent of these patients were resistant to tetracycline, 46% to erythromycin, and 9.5% to ciprofloxacin. Meanwhile, 30.6% showed resistance to both erythromycin and tetracycline [52]. In Saudi Arabia, in the most recent study conducted among 13,750 patients, 7.1% had GAS infection and 1% of them showed antimicrobial resistance to cancomycin [59].
6.3. Managing clinical manifestations of ARF
Arthritis:
-
–
NSAIDs such as acetyl-salicylic acid, ibuprofen (30–40 mg/kg/d), and ketoprofen (1.5 mg/kg/d) are used in cases of arthritis with or without mild carditis [21]. Acetyl-salicylic acid (aspirin) is used for 2–3 weeks at a dose of 100 mg/kg/d, and once the symptoms completely improve, aspirin should be tapered down to 60–70 mg/kg/d. In case of aspirin sensitivity, naproxen at a dose of 10–20 mg/kg/d divided into twice daily can be used instead [60].
Carditis:
-
–
In case of mild cardiac involvement, treatment is aspirin in a similar dose to arthritis mentioned above [60].
-
–
In case of moderate to severe carditis, oral prednisone (2 mg/kg/d, max. 80 mg/d) is used and should be tapered down over 2–4 weeks by decreasing the dose to 2.5–5 mg every 3 days. Aspirin at a dose of 50–75 mg/kg/d should be administered concomitantly and should continue for 12 weeks [60].
Chorea:
-
–
In rare cases in which mild chorea has manifested, a sedative such as diazepam or phenobarbitone is administered. If the patient does not improve, haloperidol (0.25–0.5 mg/kg/d) or valproic acid (15 mg/kg/d) should be administered for 12 weeks or 2–4 weeks after clinical improvement [60]. Conspicuously, extrapyramidal syndrome could occur in high doses of haloperidol, and valproic acid should be given as a substitute [21].
6.4. How to prevent recurrent attacks of ARF
Relapsing episodes of RF could result in RHD or exacerbate the current cardiac condition. The best way to prevent severe RHD is by preventing relapsing episodes of GAS pharyngitis (secondary prevention). Moreover, a patient with a history of RF episodes will be at higher risk of another episode of RF. Benzathine penicillin G is administered intramuscularly as a longstanding prophylaxis every 28 days in this case. (In high-risk patients, it should be administered every 3 weeks.) In children weighing >27 kg, 1.2 million U is administered; however, in children weighing ≤27 kg, 600,000 U is administered. Penicillin V potassium 250 mg orally every 12 hours can be given as an alternative. The duration of the prophylaxis mainly depends on the extent of cardiac involvement; details are summarized in Table 4 [57], [61].
Table 4.
Duration of prophylaxis based on the extent of cardiac involvement.
| Category of patient based on cardiac involvement | Duration of prophylaxis |
|---|---|
| In children with no cardiac involvement | Prophylaxis should continue for 5 yr subsequent to the recent episode or until the age of 21 yr, whichever is longer |
| In children with preceding carditis and mild residual mitral regurgitation or valve lesion which resolved completely | Prophylaxis should continue for 10 yr subsequent to the recent episode or until the age of 21 yr, whichever is longer |
| In children with preceding carditis with moderate to severe valve damage | Prophylaxis should continue for 10 yr subsequent to the recent episode or until the age of 40 yr, whichever is longer |
| In children with relapses or high risk of infection | Prophylaxis must continue forever |
| In children with valve replacement | Prophylaxis must continue forever |
6.5. Challenges in diagnosis and management of ARF in Saudi Arabia
A previous study conducted by Al Qurashi [15] showed that GAS was isolated in only 11% of Saudi children compared to 20–25% in the general literature [62], [63], and this has been attributed to previous antibiotic use. Prevalence of brucellosis-related arthritis and post-streptococcal reactive arthritis make diagnosis of ARF-isolated arthritis very difficult. Al Qurashi [15] also described a high recurrence rate of ARF because of poor compliance with prophylaxis.
7. Conclusion
ARF is still prevalent in developing countries. The updated 2015 AHA criteria provide clinicians with more insight to classify the population into two major groups based on the risk stratification: low risk and moderate to high risk. The concept of subclinical carditis is becoming a widely accepted major criterion in all patients, regardless of their risk group. Primary prevention with eradication of GAS infection remains the most important step in the management of acute RF. However, the high index of suspicion helps in early detection and avoiding the devastating sequela of RF. Extrapolating data from developing countries with a similar ARF risk to Saudi Arabia and the application of newer criteria incorporating subclinical carditis and monoarthritis as major criteria might lead to an increase in the diagnosis of ARF cases. However, this is an area that should invite the interest of local researchers.
Conflicts of interest
The authors declare that they have no conflict of interests.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Szczygielska I., Hernik E., Kołodziejczyk B., Gazda A., Maślińska M., Gietka P. Rheumatic fever–new diagnostic criteria. Reumatologia. 2018;56:37. doi: 10.5114/reum.2018.74748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones T.D. The diagnosis of rheumatic fever. J Am Med Assoc. 1944;126:481–484. [Google Scholar]
- 3.Al-Sekait M.A., Al-Sweliem A.A., Tahir M. Rheumatic heart disease in schoolchildren in Western District, Saudi Arabia. J R Soc Health. 1990;110:15–16. doi: 10.1177/146642409011000107. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sekait M.A., Al-Sweilem A.A., Tahir M. Rheumatic fever and chronic rheumatic heart disease in schoolchildren in Saudi Arabia. Saudi Med J. 1991;12:407–410. [Google Scholar]
- 5.Al-Eissa Y.A., Al-Zamil F.A., Al Fadley F.A., Al Herbish A.S., Al-Mofada S.M., Al-Omair A.O. Acute rheumatic fever in Saudi Arabia: mild pattern of initial attack. Pediatr Cardiol. 1993;14:89–92. doi: 10.1007/BF00796986. [DOI] [PubMed] [Google Scholar]
- 6.Abbag F., Benjamin B., Kardash M.M. Acute rheumatic fever in southern Saudi Arabia. East Afr Med J. 1998;75:279–281. [PubMed] [Google Scholar]
- 7.Carapetis J.R., Kilburn C.J., MacDonald K.T., Walker A.R., Currie B.J. Ten-year follow up of a cohort with rheumatic heart disease (RHD) Aust N Z J Med. 1997;27:691–697. doi: 10.1111/j.1445-5994.1997.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 8.Meira Z.M., Goulart E.M., Colosimo E.A., Mota C.C. Long term follow up of rheumatic fever and predictors of severe rheumatic valvar disease in Brazilian children and adolescents. Heart. 2005;91:1019–1022. doi: 10.1136/hrt.2004.042762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewitz M.H., Baltimore R.S., Tani L.Y., Sable C.A., Shulman S.T., Carapetis J. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806–1818. doi: 10.1161/CIR.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 10.Tibazarwa K.B., Volmink J.A., Mayosi B.M. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart. 2008;94:1534–1540. doi: 10.1136/hrt.2007.141309. [DOI] [PubMed] [Google Scholar]
- 11.Webb R.H., Grant C., Harnden A. Acute rheumatic fever. BMJ. 2015;351 doi: 10.1136/bmj.h3443. [DOI] [PubMed] [Google Scholar]
- 12.Seckeler M.D., Hoke T.R. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eltohami E.A., Hajar H.A., Folger G.M., Eltohami E.A. Acute rheumatic fever in an Arabian Gulf country: effect of climate, advantageous socioeconomic conditions, and access to medical care. Angiology. 1997;48:481–489. doi: 10.1177/000331979704800602. [DOI] [PubMed] [Google Scholar]
- 14.Sims Sanyahumbi A., Colquhoun S., Wyber R., Carapetis J.R. Global disease burden of group A Streptococcus. In: Ferretti J.J., Stevens D.L., Fischetti V.A., editors. Sterptococcus pyogenes: Basic biology to clinical manifestations. University of Oklahoma Health Sciences Center; Oklahoma City: 2016. pp. 1–29. [Google Scholar]
- 15.Al Qurashi M. The pattern of acute rheumatic fever in children: experience at the children’s hospital, Riyadh, Saudi Arabia. J Saudi Heart Assoc. 2009;21:215–220. doi: 10.1016/j.jsha.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cann M.P., Sive A.A., Norton R.E., McBride W.J., Ketheesan N. Clinical presentation of rheumatic fever in an endemic area. Arch Dis Child. 2010;95:455–457. doi: 10.1136/adc.2008.157107. [DOI] [PubMed] [Google Scholar]
- 17.Veasy L.G., Tani L.Y., Hill H.R. Persistence of acute rheumatic fever in the intermountain area of the United States. J Pediatr. 1994;124:9–16. doi: 10.1016/s0022-3476(94)70247-0. [DOI] [PubMed] [Google Scholar]
- 18.Jamal M., Abbas K.A. Clinical profile of acute rheumatic fever in children. J Trop Pediatr. 1989;35:10–13. doi: 10.1093/tropej/35.1.10. [DOI] [PubMed] [Google Scholar]
- 19.Vinker S., Zohar E., Hoffman R., Elhayany A. Incidence and clinical manifestations of rheumatic fever: a 6 year community-based survey. Isr Med Assoc J. 2010;12:78. [PubMed] [Google Scholar]
- 20.Grassi A., Fesslova V., Carnelli V., Boati E., Dell’Era L., Salice P. Clinical characteristics and cardiac outcome of acute rheumatic fever in Italy in the last 15 years. Clin Exp Rheumatol. 2009;27:366–372. [PubMed] [Google Scholar]
- 21.Pereira B.Á., Belo A.R., Silva N.A. Rheumatic fever: update on the Jones criteria according to the American Heart Association review—2015. Rev Bras Reumatol. 2017;57:364–368. doi: 10.1016/j.rbre.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Luque C., Cisternas F.A., Araya M. Cambios del patrón de enfermedad en la postransición epidemiológica en salud en Chile, 1950–2003. Rev Méd Chile. 2006;134:703–712. doi: 10.4067/s0034-98872006000600005. [DOI] [PubMed] [Google Scholar]
- 23.Carapetis J.R., Wolff D.R., Currie B.J. Acute rheumatic fever and rheumatic heart disease in the top end of Australia’s Northern Territory. Med J Aust. 1996;164:146–149. doi: 10.5694/j.1326-5377.1996.tb122012.x. [DOI] [PubMed] [Google Scholar]
- 24.Carapetis J.R., Currie B.J., Mathews J.D. Cumulative incidence of rheumatic fever in an endemic region: a guide to the susceptibility of the population? Epidemiol Infect. 2000;124:239–244. doi: 10.1017/s0950268800003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grover A., Dhawan A., Iyengar S.D., Anand I.S., Wahi P.L., Ganguly N.K. Epidemiology of rheumatic fever and rheumatic heart disease in a rural community in northern India. Bull World Health Organ. 1993;71:59–66. [PMC free article] [PubMed] [Google Scholar]
- 26.Erdem G., Dodd A., Tuua A. Acute rheumatic fever in American Samoa. Pediatr Infect Dis J. 2007;26:1158–1159. doi: 10.1097/INF.0b013e318146236f. [DOI] [PubMed] [Google Scholar]
- 27.Olivier C., Portier H., Cohen R., Schlemmer B., Boucot I., Peyramond D. Rhumatisme articulaire aigu: résultats d’une enquête nationale (1995–1997) BEH. 1999;12:45–47. [Google Scholar]
- 28.Meira Z.M.A., de Castilho S.R.T., Barros M.V.L. Prevalence of rheumatic fever in children from a public high school in Belo Horizonte, Brazil. Arch Bras Cardiol. 1995;65:331–334. [PubMed] [Google Scholar]
- 29.Raobijaona H., Andrianjanaka J.C., Rakotorimanana D.R. Le rhumatisme articulaire aigu (RAA) a antananarivo (Madagascar) Méd d’Afr Noire. 1998;45:686–689. [Google Scholar]
- 30.Abdul-Mohsen M.F., Lardhi A.A. A dramatic decline in university hospital admissions of acute rheumatic fever in the eastern region of Saudi Arabia. J Saudi Heart Assoc. 2011;23:87–91. doi: 10.1016/j.jsha.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kafetzis D.A., Chantzi F.M., Grigoriadou G., Vougiouka O., Liapi G. Incidence and clinical profile of acute rheumatic fever in Greece. Eur J Clin Microbiol Infect Dis. 2005;24:68–70. doi: 10.1007/s10096-004-1274-6. [DOI] [PubMed] [Google Scholar]
- 32.Soto López M.E. Cordera González de Cosío F, Estrada L, Guel L, Abud Mendoza C, Reyes PA. Rheumatic fever in the 5-year period of 1994–1999 at 2 hospitals in San Luis Potosi and Mexico D.F. Arch Cardiol Mex. 2001;71:127–135. [PubMed] [Google Scholar]
- 33.Cortina Greus P., Alfonso Sánchez J.L., Cortés Vizcaíno C., Smeyers Durá P., González Arraez J.I. Epidemiological course of rheumatic fever and rheumatic heart disease in Spain (1951–1986) Rev Sanid Hig Publica (Madr) 1991;65:17–24. [PubMed] [Google Scholar]
- 34.Carceller A., Tapiero B., Rubin E., Miró J. Acute rheumatic fever: 27 year experience from the Montreal’s pediatric tertiary care centers. An Pediatr (Barc) 2007;67:5–10. doi: 10.1157/13108071. [DOI] [PubMed] [Google Scholar]
- 35.Mendis S., Nasser M., Perera K. A study of rheumatic heart disease and rheumatic fever in a defined population in Sri Lanka. Ceylon J Med Sci. 1998;40:31–37. [Google Scholar]
- 36.Bolz D., Tyndall A. Rhumatisme articulaire aigu-encore actuel en Suisse? Forum Med Suisse. 2006;6(27):642–646. EMH Media. [Google Scholar]
- 37.Huang Z.D., Rao X.X., Cen Y.C. An updated epidemiologic survey of acute rheumatic fever among school-age children in China. Chin J Cardiol. 1998;26:94–97. [Google Scholar]
- 38.Rayamajhi A., Sharma D., Shakya U. Clinical, laboratory and echocardiographic profile of acute rheumatic fever in Nepali children. Ann Trop Paediatr. 2007;27:169–177. doi: 10.1179/146532807X220271. [DOI] [PubMed] [Google Scholar]
- 39.Singwe-Ngandeu M., Meli J., Ntsiba H. Rheumatic diseases in patients attending a clinic at a referral hospital in Yaounde, Cameroon. East Afr Med J. 2007;84:404–409. doi: 10.4314/eamj.v84i9.9549. [DOI] [PubMed] [Google Scholar]
- 40.Nordet P., Lopez R., Sarmiento L., Nordet P., Dueñas A. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986–1996–2002) Cardiovasc J Afr. 2008;19:135. [PMC free article] [PubMed] [Google Scholar]
- 41.Le Hello S., Doloy A., Baumann F., Roques N., Coudene P., Rouchon B. Clinical and microbial characteristics of invasive Streptococcus pyogenes disease in New Caledonia, a region in Oceania with a high incidence of acute rheumatic fever. J Clin Microbiol. 2010;48:526–530. doi: 10.1128/JCM.01205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sani M.U., Karaye K.M., Borodo M.M. Prevalence and pattern of rheumatic heart disease in the Nigerian savannah: an echocardiographic study. Cardiovasc J Afr. 2007;18:295–299. [PMC free article] [PubMed] [Google Scholar]
- 43.Carapetis J.R., Hardy M., Fakakovikaetau T. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan schoolchildren. Nat Clin Pract Cardiovasc Med. 2008;5:411–417. doi: 10.1038/ncpcardio1185. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Moula A.M., Sherif A.A., Sallam S.A., Mandil A.M., Kassem A.S., Zaher S.R. Prevalence of rheumatic heart disease among school children in Alexandria, Egypt: a prospective epidemiological study. J Egypt Public Health Assoc. 1998;73:233–254. [PubMed] [Google Scholar]
- 45.Hasab A.A., Jaffer A., Riyami A.M. Rheumatic heart disease among Omani schoolchildren. EMHJ. 1997;3:17–23. [Google Scholar]
- 46.Karademir S., Demirçeken F., Atalay S., Demircin G., Sipahi T., Teziç T. Acute rheumatic fever in children in the Ankara area in 1990–1992 and comparison with a previous study in 1980–1989. Acta Pediatr. 1994;83:862–865. doi: 10.1111/j.1651-2227.1994.tb13158.x. [DOI] [PubMed] [Google Scholar]
- 47.Oli K., Tekle-Haimanot R., Forsgren L., Ekstedt J. Rheumatic heart disease prevalence among schoolchildren of an Ethiopian rural town. Cardiology. 1992;80:152–155. doi: 10.1159/000174993. [DOI] [PubMed] [Google Scholar]
- 48.Steer A.C., Kado J., Jenney A.W. Acute rheumatic fever and rheumatic heart disease in Fiji: prospective surveillance, 2005–2007. Med J Aust. 2009;190:133–135. doi: 10.5694/j.1326-5377.2009.tb02312.x. [DOI] [PubMed] [Google Scholar]
- 49.Minh Hoa T.T., Darmawan J., Chen S.L., Van Hung N., Thi Nhi C., Ngoc An T. Prevalence of the rheumatic diseases in urban Vietnam: a WHO-ILAR COPCORD study. J Rheumatol. 2003;30:2252–2256. [PubMed] [Google Scholar]
- 50.Folomeeva O.M., Benevolenskaia L.I. Rheumatism in the Russian Federation: statistic and reality. Vestn Ross Akad Med Nauk. 1996;11:21–24. [PubMed] [Google Scholar]
- 51.Ramakrishnan S. Echocardiography in acute rheumatic fever. Ann Pediatr Cardiol. 2009;2:61. doi: 10.4103/0974-2069.52812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization . World Health Organization; 2004. Rheumatic fever and rheumatic heart disease: report of a WHO Expert Consultation, Geneva, 29 October–1 November, 2001. [Google Scholar]
- 53.Vasan R.S., Shrivastava S., Vijayakumar M., Narang R., Lister B.C., Narula J. Echocardiographic evaluation of patients with acute rheumatic fever and rheumatic carditis. Circulation. 1996;94:73–82. doi: 10.1161/01.cir.94.1.73. [DOI] [PubMed] [Google Scholar]
- 54.Dajani A.S., Ayoub E., Bierman F.Z. Guidelines for diagnosis of rheumatic fever: Jones criteria. Updated 1992. Circulation. 1993;87:302–307. [Google Scholar]
- 55.Kumar D., Bhutia E., Kumar P., Shankar B., Juneja A., Chandelia S. Evaluation of the American Heart Association 2015 revised Jones criteria versus existing guidelines. Heart Asia. 2016;8:30–35. doi: 10.1136/heartasia-2015-010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carapetis J., Brown A., Maguire G.P., Walsh D., Noonan S.J., Thompson D. An Australian guideline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease. Med J Aust. 2007;186:581–586. doi: 10.5694/j.1326-5377.2007.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 57.Gerber M.A., Baltimore R.S., Eaton C.B., Gewitz M., Rowley A.H., Shulman S.T. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119:1541–1551. doi: 10.1161/CIRCULATIONAHA.109.191959. [DOI] [PubMed] [Google Scholar]
- 58.Bhardwaj N., Mathur P., Behera B., Mathur K., Kapil A., Misra M.C. Antimicrobial resistance in beta-haemolytic streptococci in India: a four-year study. Indian J Med Res. 2018;147:81. doi: 10.4103/ijmr.IJMR_1517_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibl A.M., Memish Z.A., Kambal A.M., Ohaly Y.A., Ishaq A., Senok A.C. National surveillance of antimicrobial resistance among Gram-positive bacteria in Saudi Arabia. J Chemother. 2014;26:13–18. doi: 10.1179/1973947813Y.0000000084. [DOI] [PubMed] [Google Scholar]
- 60.Saxena A., Kumar R.K., Gera R.P., Radhakrishnan S., Mishra S., Ahmed Z. Consensus guidelines on pediatric acute rheumatic fever and rheumatic heart disease. Indian Pediatr. 2008;45:565–573. [PubMed] [Google Scholar]
- 61.Al-Jazairi A., Al-Jaser R., Al-Halees Z., Shahid M., Al-Jufan M., Al-Mayouf S. Guidelines for the secondary prevention of rheumatic heart disease: Endorsed by Saudi Pediatric Infectious Diseases Society (SPIDS) Int J Pediatr Adolesc Med. 2017;4:47–50. doi: 10.1016/j.ijpam.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chagani H.S., Aziz K. Clinical profile of acute rheumatic fever in Pakistan. Cardiol Young. 2003;13:2. doi: 10.1017/s1047951103000064. [DOI] [PubMed] [Google Scholar]
- 63.Dajani A.S., Ayoub E., Bierman F.Z., Bisno A.L., Denny F.W., Durack D.T. special writing group of the committee on rheumatic fever, endocarditis, and Kawasaki disease of the council on cardiovascular disease in the young, American Heart Association. Circulation. 1992;1993:87. [Google Scholar]