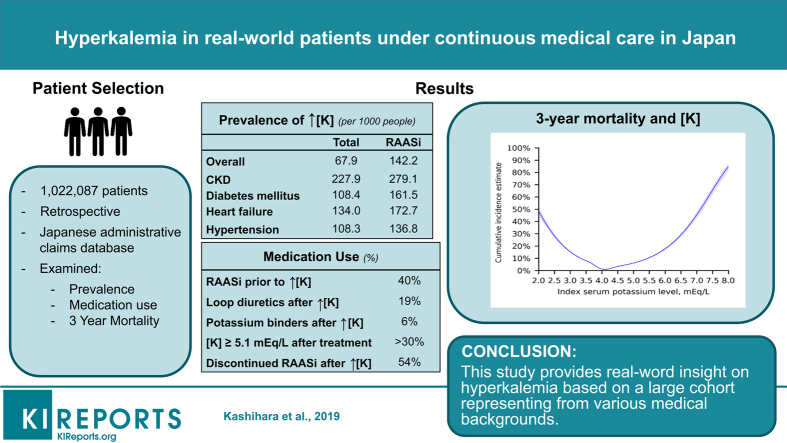
Abstract
Introduction
An abnormal serum potassium (S-K) level is an important electrolyte disturbance. However, its relation to clinical outcomes in real-world patients, particularly hyperkalemia burden, is not extensively studied.
Methods
An observational retrospective cohort study using a Japanese hospital claims database was done (April 2008–September 2017; N = 1,022,087). Associations between index S-K level and 3-year survival were modeled using cubic spline regression. Cox regression model was applied to estimate the time to death according to different S-K levels. Prevalence, patient characteristics, treatment patterns, and management of patients with hyperkalemia from first episode were assessed.
Results
Hyperkalemia prevalence was 67.9 (95% confidence interval [CI]: 67.1–68.8) per 1000 and increased in patients with chronic kidney disease (CKD) (227.9; 95% CI: 224.3–231.5), heart failure (134.0; 95% CI: 131.2–136.8), and renin-angiotensin-aldosterone system inhibitor (RAASi) use (142.2; 95% CI: 139.6–144.7). U-shaped associations between S-K level and 3-year survival were observed with nadir 4.0 mEq/l. The risk of death was increased at S-K 5.1–5.4 mEq with hazard ratio of 7.6 (95% CI: 7.2–8.0). The 3-year mortality rate in patients with CKD stages 3a, 3b, 4, and 5 with normokalemia were 1.51%, 3.93%, 10.86%, and 12.09%, whereas that in patients with CKD stage 3a at S-K 5.1–5.4, 5.5–5.9, and ≥6.0 mEq/l increased to 10.31%, 11.43%, and 22.64%, respectively. Despite treatment with loop diuretics (18.5%) and potassium binders (5.8%), >30% of patients had persistently high S-K (≥5.1 mEq/l).
Conclusion
This study provides real-world insight on hyperkalemia based on a large number of patients with various medical backgrounds.
Keywords: chronic kidney disease, congestive heart failure, hyperkalemia, renin-angiotensin-aldosterone inhibitor, renin angiotensin system
Graphical abstract
Potassium is an ubiquitous electrolyte in the human body and is important in fundamental physiological processes, such as maintenance of cellular membrane potential and transmission of action potentials in synaptic processes.1, 2, 3 Several clinical characteristics are known to alter the S-K levels; including CKD, diabetes mellitus, and heart failure (HF).4, 5 Medical treatment itself, such as renin-angiotensin-aldosterone inhibitors (RAASis) and nonsteroidal anti-inflammatory drugs, can increase the risk of hyperkalemia.4, 5 Overall, the reported incidence of hyperkalemia ranged between 1% and 10% in hospitalized patients.6, 7
There has been an increasing amount of work published on this topic, and recent reports have alluded to increased risk of adverse clinical outcomes in patients with hyperkalemia. In a hospital-based cohort outcome study of 923 Korean patients, dichotomized analysis showed that patients with potassium level ≥6.5 mmol/l had an in-hospital mortality of 31%.8 Similar findings were found in patients with CKD and HF.9, 10
However, the incidence and outcome of potassium abnormality in a broader range of patient population are unclear. Particularly, as hyperkalemia occurs secondary to certain diseases, such as CKD and HF, epidemiological data from large datasets that represent various clinical settings are required to understand its management. Moreover, previous studies have been performed in limited geographical regions, such as the United States and Europe.11 Given the difference in the prevalence of predisposing clinical factors and available treatment strategies, it is important to understand the characteristics and long-term effects of hyperkalemia across various regions.12 Herein, we designed the REVEAL-HK study (an observational research on the prevalence, treatment pattern, and long-term risk of hyperkalemia in real-world clinical practice) to understand the prevalence, incidence, treatment patterns, and management of hyperkalemia in a broad range of Japanese patients in a hospital database with >1 million patient records.
Methods
Data Source
Data were obtained from Medical Data Vision, one of the largest Japanese all-comer hospital claims databases. Medical Data Vision is a database composed of claims data and a diagnostic and procedural coding system, including individual prescription and laboratory data. Data were obtained from >300 hospitals across Japan. Data collection was started in April 2008 and currently covers >20 million inpatients and outpatients. Medical Data Vision uses International Classification of Diseases, 10th Revision coding. This study was reviewed and approved by an independent ethics committee.
Study Design and Patient Selection
This is an observational retrospective cohort study using a Japanese hospital-based database and consisting of 2 parts. The first part demonstrates the overall impact of the index S-K level on long-term clinical outcomes, and the second part describes the clinical characteristics, treatment patterns, and management of hyperkalemia.
Both inpatients and outpatients were included. Hyperkalemia was defined as at least 2 S-K readings ≥5.1 mEq/l, as used in a previous study.11 Hyperkalemia episodes were defined as episodes with a recorded S-K level of ≥5.1 mEq/l. Index date was defined as the date of first hyperkalemia measurement. The follow-up period was measured from the index date until the date of emigration from the database, date of death, or end of the study period, whichever came first. For the overall assessment of index S-K level and prevalence, patients aged ≥18 years with at least 1 S-K measurement were selected from the database within the study period from April 1, 2008, to September 30, 2017. For the second part and the analysis of clinical outcomes, patients aged ≥18 years with at least 2 measurements of S-K ≥5.1 mEq/l and followed up for ≥360 days after the first hyperkalemia episode during the study period were selected. Because each patient had a different index date (different year and month), we defined 1 month as 30 days for a fair comparison among patients. The threshold of follow-up period was set as 12 months after the index hyperkalemia. Patients with a cancer diagnosis during the study period or those undergoing dialysis before the index date were excluded.
Covariates
High-Risk Subgroups
Subgroups of patients with hyperkalemia were defined based on high-risk comorbidities including CKD, diabetes mellitus, HF, and hypertension, which are associated with the occurrence of hyperkalemia.4, 5, 8 CKD severity was based on the estimated glomerular filtration rate (Supplementary Table S1). Other comorbidities of interest were defined using International Classification of Diseases, 10th Revision codes (Supplementary Tables S2 and S3).
Treatment Patterns
Hyperkalemia treatments included thiazide diuretics, loop diuretics, glucose injection, calcium gluconate, sodium bicarbonate, and potassium binders. A low daily dose of a potassium binder was defined as an average daily dose lower than the recommended minimum daily dose in the drug labels. Drugs associated with the occurrence of hyperkalemia, including RAASi (i.e., angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, mineralcorticoid receptor antagonists) and non-RAASi (e.g., nonsteroidal anti-inflammatory drugs, heparin, beta-blockers) treatments, were collected during the 120 days pre-index period, considering the commonly used intervals of drug prescription in Japanese clinical practice. RAASi discontinuation was defined as RAASi treatment not being prescribed ≥30-day gap after the last day of the supply of the previous RAASi prescription during the follow-up period. Dose reduction was the presence of a lower dose of RAASi than the dose of the last RAASi prescription before the index date.
Statistical Analysis
Continuous variables were reported as means, SDs, and medians. Frequencies and percentages were used to document categorical measures of interest. The prevalence of hyperkalemia in 2016 was reported as number of patients per 1000 population. The incidence rate of a first hyperkalemia episode was reported per 100 patient-year at risk. The associations between index S-K and clinical outcomes, including death, hospitalization for HF, rehospitalization, hospitalization for cardiac events, use of calcium gluconate and glucose-insulin therapy, and introduction of renal replacement therapy at 3 years were modeled using cubic spline regression with 5 knots at every one-sixth percentile (0.17, 0.33, 0.50, 0.67, and 0.83), adjusting for covariates of age, sex, Charlson comorbidity index, and high-risk comorbidities. Cox proportional hazard model was applied to estimate the hazard ratio of death in patients with hyperkalemia and hypokalemia compared with patients with normokalemia (3.6–5.0 mEq/l). Univariate and multivariate analyses with adjustment for age, sex, Charlson comorbidity index, and high-risk comorbidities were performed. Although an S-K reading of ≥5.1 mEq/l was defined as a hyperkalemia episode, we further reported the incidence according to the severity of hyperkalemia episode, as S-K ≥5.5 and ≥6.0 mEq/l. Subgroup analyses of prevalence, incidence rates, and cumulative incidences of a first hyperkalemia episode were performed in patients with RAASi treatment and those with high-risk comorbidities, and stratified by different CKD stages and age categories, separately. Cumulative incidences of the first hyperkalemia episode and recurrence of hyperkalemia were estimated using cumulative incidence function, considering death as a competing risk. The Kaplan-Meier method was used to estimate the median time to discontinuation of potassium binder treatment or death. All statistical analyses, except for cubic spline analysis, were performed using SAS version 9.4 (SAS Institute, Cary, NC). Cubic spline regression analysis was performed using R 3.5.1.
Results
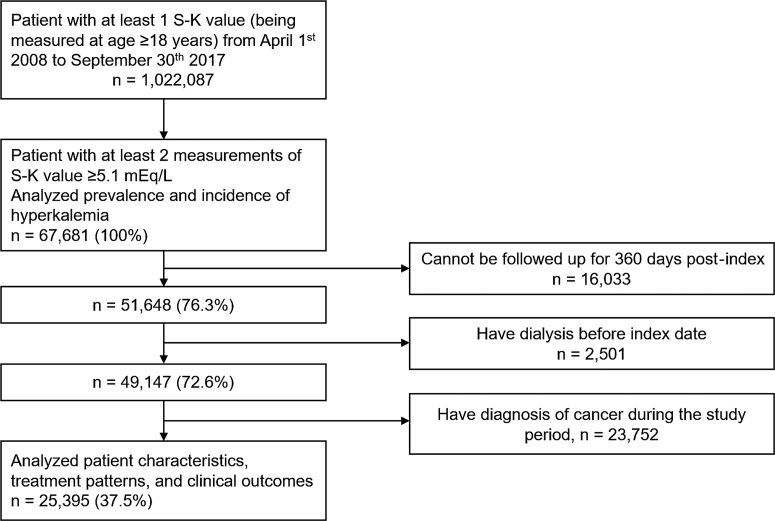
We extracted 1,022,087 patients aged ≥18 years with at least 1 S-K measurement during the study period from the Medical Data Vision database as the overall study population. Of them, we identified 67,681 (6.6%) patients with hyperkalemia. After excluding 16,033 patients who could not be followed up for 360 days except for death after the index hyperkalemia, 2501 patients who had dialysis before index hyperkalemia, and 23,752 patients with a cancer diagnosis, 25,395 (2.5%) patients were identified for the second part of the analysis (Figure 1). The median time interval between the first 2 examinations with S-K level of ≥5.1 mEq/l was 70 days.
Figure 1.
Flow diagram of patient inclusion into the study. S-K, serum potassium.
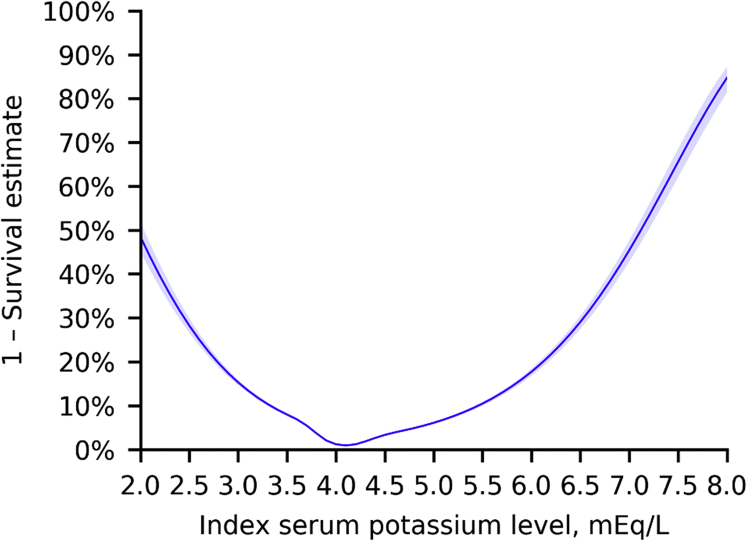
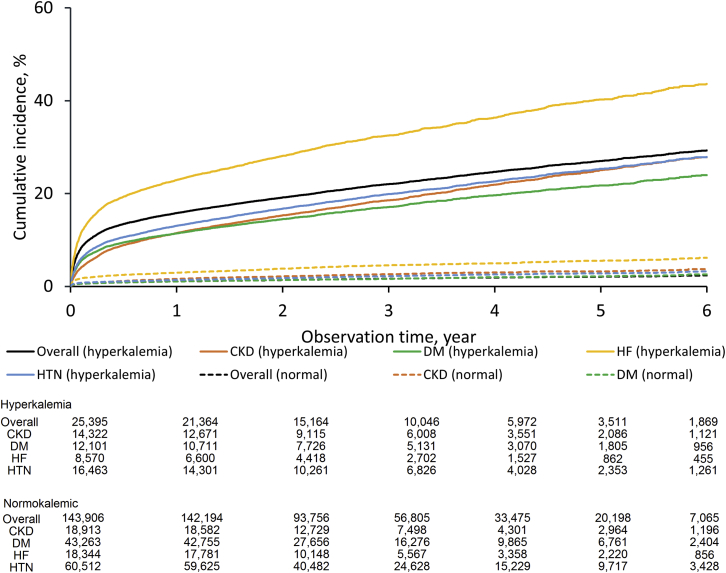
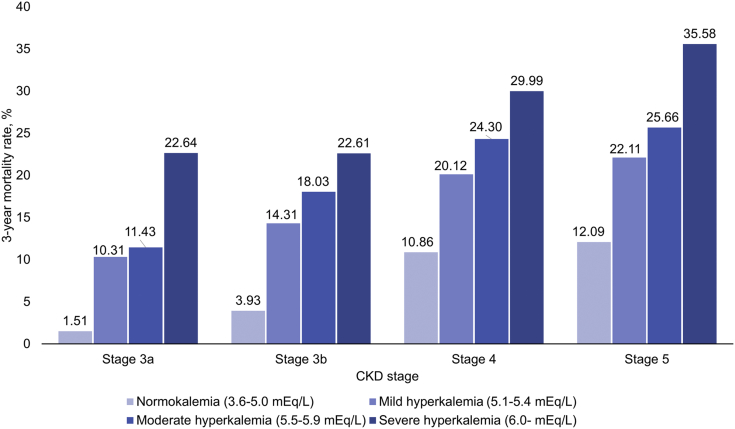
Figure 2 shows the results of cubic spline analysis of the association between the 3-year incidence of death and the S-K level. The incidence was higher according to potassium levels with a nadir of S-K 4.0 mEq/l. An estimated 3-year mortality rate at S-K 6.0 mEq/l was 17.7% (95% CI: 16.9–18.5). Results for other clinical outcomes, including hospitalization for cardiac events, introduction of renal replacement therapy, rehospitalization, hospitalization for HF, and use of calcium gluconate and glucose-insulin therapy, are shown in Supplementary Figure S1. Patients with hyperkalemia had an increased risk of mortality with hazard ratios of 7.6 (95% CI: 7.2–8.0) at S-K 5.1 to 5.4 mEq/l, 10.6 (95% CI: 9.9–11.4) at S-K 5.5 to 5.9 mEq/l, and 17.7 (95% CI: 16.3–19.2) at S-K ≥6.0 mEq/l (Table 1). Death occurred in 22.0% (95% CI: 21.5%–22.6%) of the patients within 3 years after the first hyperkalemia episode and in 1.7% (95% CI: 1.6%–1.7%) of those with normokalemia (Figure 3). The risk difference between 2 groups was 20.4% (95% CI: 19.9%–20.9%). A higher 3-year mortality rate (32.5%; 95% CI: 31.4–33.6%) was observed in the hyperkalemia subgroup with HF. The 3-year mortality rates in patients with CKD stages 3a, 3b, 4, and 5 with normokalemia were 1.51%, 3.93%, 10.86%, and 12.09%, whereas that in patients with CKD stage 3a with mild (5.1–5.4 mEq/l), moderate (5.5–5.9 mEq/l), and severe (>6.0 mEq/l) hyperkalemia increased to 10.31%, 11.43%, and 22.64%, respectively (Figure 4).
Figure 2.
Cubic spline analysis of the 3-year incidence of in-hospital death according to serum potassium levels. Five knots at the 17th, 33th, 50th, 67th, and 83th percentiles were used.
Table 1.
HRs of death at each range of serum potassium level
| S-K category and outcome | Very low n = 5573 | Low n = 56,461 | Normal n = 143,906 | Elevated n = 18,410 | High n = 5006 | Very high n = 1979 |
|---|---|---|---|---|---|---|
| S-K range, mEq/l | ≤3.0 | 3.1≤ S-K ≤3.5 | 3.6≤ S-K ≤5.0 | 5.1≤ S-K ≤5.4 | 5.5≤ S-K ≤5.9 | 6.0≤ |
| Death, n (%) | 1620 (29.1) | 7133 (12.6) | 2310 (1.6) | 3583 (19.5) | 1357 (27.1) | 825 (41.7) |
| Univariate analysis, HR (95% CI) | 22.1 (20.7–23.5) | 8.3 (7.9–8.7) | Reference | 12.6 (11.9–13.2) | 18.7 (17.5–20.0) | 33.8 (31.2–36.6) |
| Multivariate analysis,a HR (95% CI) | 12.7 (11.9–13.6) | 6.4 (6.1–6.8) | Reference | 7.6 (7.2–8.0) | 10.6 (9.9–11.4) | 17.7 (16.3–19.2) |
CI, confidence interval; HR, hazard ratio; S-K, serum potassium.
Adjusted by age, sex, Charlson comorbidity index, and high-risk comorbidities including chronic kidney disease, heart failure, diabetes, and hypertension.
Figure 3.
Cumulative incidence of death in hyperkalemic and normokalemic (serum potassium [S-K] 3.6–5.0 mEq/l) patients. CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension.
Figure 4.
Cumulative incidence of death in hyperkalemic and normokalemic (serum potassium [S-K] 3.6–5.0 mEq/l) patients according to chronic kidney disease (CKD) stages and index S-K levels.
Prevalence and Incidence
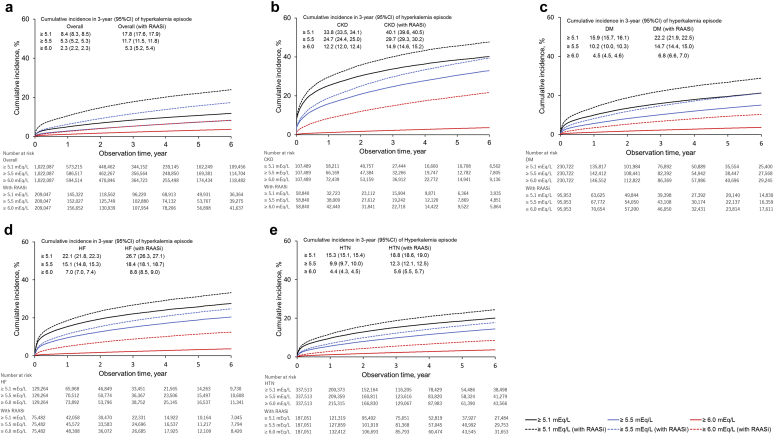
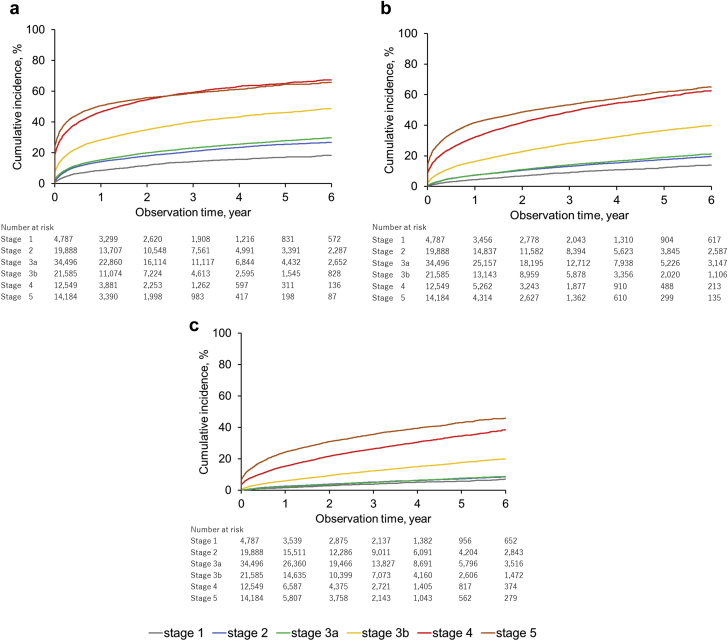
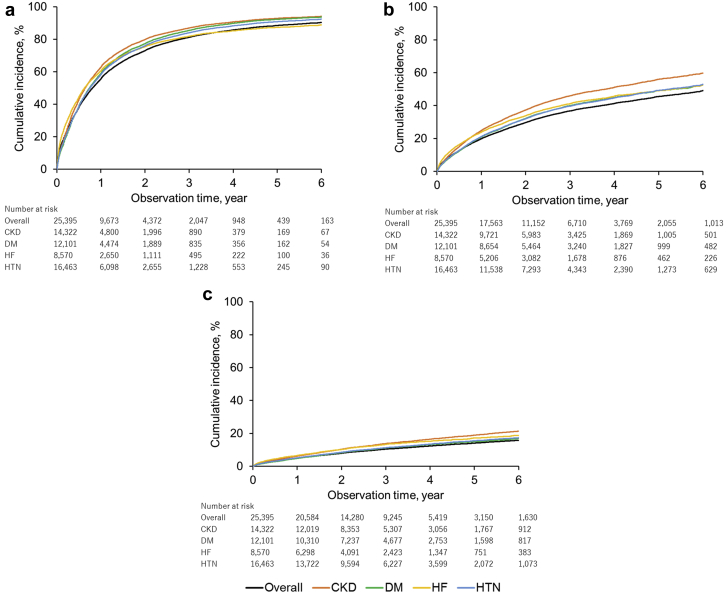
Table 2 shows the prevalence and incidence of hyperkalemia in 2016 in the overall population (n = 332,503) and in the RAASi therapy group (n = 72,822) by high-risk subgroups. The high-risk subgroups had higher prevalence (per 1000) of hyperkalemia, such as CKD (prevalence: 227.9), HF (134.0), diabetes mellitus (108.4), and hypertension (108.3) than the overall study population (67.9). Prevalence increased in accordance with the severity of CKD (stage 1: 72.4, stage 2: 103.3, stage 3a: 132.2, stage 3b: 245.6, stage 4: 436.5, and stage 5: 511.9). The incidence of hyperkalemia in the overall population and in the RAASi therapy subgroup was 2.9 and 5.9 per 100 patient-year-at-risk, respectively. Patients with RAASi therapy had higher prevalence and incidence of hyperkalemia in all subgroups (Figure 5). The highest incidence was seen in patients with stage 5 CKD (66.2) followed by those with stage 4 (51.7) and stage 3b (21.0) (Figure 6). Older patients had higher incidence rates per 100 patient-year-at-risk, as follows: 2.18 in patients aged 18–64 years, 4.75 in patients aged 65–74 years, and 7.73 in patients aged 75+ years (Supplementary Figure S2).
Table 2.
Prevalence of hyperkalemia in 2016 and incidence rate of the first hyperkalemia during follow-up
| Patient category | Prevalence, /1000 population (95% CI) |
Incidence rate, /100 patient-year-at-risk (95% CI) |
||
|---|---|---|---|---|
| Total | With RAASi | Total | With RAASi | |
| Overall | 67.9 (67.1–68.8) | 142.2 (139.6–144.7) | 2.9 (2.9–2.9) | 5.9 (5.8–6.0) |
| CKD | 227.9 (224.3–231.5) | 279.1 (273.5–284.8) | 16.0 (15.8–16.2) | 19.1 (18.9–19.4) |
| Stage 1 | 72.4 (61.4–84.7) | 85.8 (65.8–109.5) | 4.8 (4.4–5.2) | 5.9 (5.3–6.5) |
| Stage 2 | 103.3 (97.4–109.4) | 113.9 (104.6–123.8) | 7.6 (7.4–7.9) | 8.7 (8.4–9.1) |
| Stage 3a | 132.2 (127.1–137.4) | 156.2 (147.9–164.7) | 9.2 (9.0–9.4) | 10.9 (10.6–11.2) |
| Stage 3b | 245.6 (237.8–253.6) | 288.0 (276.5–299.7) | 21.0 (20.5–21.4) | 23.6 (23.0–24.3) |
| Stage 4 | 436.5 (424.1–449.0) | 510.0 (492.9–527.1) | 51.7 (50.5–53.0) | 57.6 (56.0–59.3) |
| Stage 5 | 511.9 (499.1–524.8) | 627.9 (609.0–646.4) | 66.2 (64.7–67.8) | 85.5 (83.1–88.1) |
| DM | 108.4 (106.5–110.3) | 161.5 (157.8–165.2) | 5.8 (5.7–5.9) | 7.7 (7.6–7.8) |
| HF | 134.0 (131.2–136.8) | 172.7 (168.3–177.2) | 9.6 (9.5–9.7) | 11.2 (11.0–11.3) |
| HTN | 108.3 (106.7–110.0) | 136.8 (134.2–139.4) | 5.5 (5.5–5.6) | 6.5 (6.4–6.5) |
CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension.
Figure 5.
Cumulative incidence of first hyperkalemia episode. (a) Overall. (b) Chronic kidney disease (CKD). (c) Diabetes mellitus (DM). (d) Heart failure (HF). (e) Hypertension (HTN). RAASi, renin-angiotensin-aldosterone inhibitor; CI, confidence interval.
Figure 6.
Cumulative incidence of hyperkalemia episode by chronic kidney disease (CKD) stages. (a) Serum potassium (S-K) ≥5.1 mEq/l. (b) S-K ≥5.5 mEq/l. (c) S-K ≥6.0 mEq/l.
Patient Demographics
Table 3 shows the patient characteristics at the time of index hyperkalemia. The mean age was 72.4 ± 13.0 years, and 55% were men. The mean S-K level was 5.4 ± 0.5 mEq/l. In terms of high-risk comorbidities, 64.8% of patients had hypertension, 56.4% had CKD, 47.7% had diabetes mellitus, and 33.7% had HF. Within the CKD group, >70% were in advanced stages (19.6% stage 5, 28.9 % stage 4, and 25.9% stage 3b, respectively). Other comorbidities are listed in Supplementary Table S4.
Table 3.
Patient characteristics
| Patient characteristics | Overall n = 25,395 |
|---|---|
| Age (yr) | |
| Mean ± SD | 72.4 ± 13.0 |
| Age group (yr), n (%) | |
| 18–64 | 6160 (24.3) |
| 65–79 | 10,650 (41.9) |
| 80+ | 8585 (33.8) |
| Sex, male, n (%) | 13,978 (55.0) |
| Length of follow-up period (yr) | |
| Mean ± SD | 2.74 ± 1.89 |
| S-K level at index date (mEq/l) | |
| Mean ± SD | 5.4 ± 0.5 |
| S-K level group at index date, n (%) | |
| ≥ 5.1 mEq/l and < 5.5 mEq/l | 18,410 (72.5) |
| ≥ 5.5 mEq/l and < 6.0 mEq/l | 5006 (19.7) |
| ≥ 6.0 mEq/l | 1979 (7.8) |
| CKD, n (%) | 14,322 (56.4) |
| Stage 1 | 170 (1.2) |
| Stage 2 | 1030 (7.2) |
| Stage 3a | 2464 (17.2) |
| Stage 3b | 3704 (25.9) |
| Stage 4 | 4139 (28.9) |
| Stage 5 | 2803 (19.6) |
| DM, n (%) | 12,101 (47.7) |
| HF, n (%) | 8570 (33.7) |
| HTN, n (%) | 16,463 (64.8) |
| 1 class of antihypertensive | 5716 (22.5) |
| 2 classes of antihypertensive | 5777 (22.7) |
| ≥3 classes of antihypertensive | 610 (2.4) |
CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; S-K, serum potassium.
Treatment Patterns
Table 4 shows the treatment patterns at the time of index hyperkalemia and during the follow-up period; 40.2% of patients were taking RAASis before the index hyperkalemia. More patients were taking mineralcorticoid receptor antagonists in the HF group (28.1%) than in other subgroups. Notably, 53.7% or 13.1% of patients experienced discontinuation or dose reduction of RAASi, respectively, after a first hyperkalemia episode. Loop diuretics were used by 18.5%, followed by potassium binders, which were used by 5.8% of patients. Compared with other subgroups, more patients used loop diuretics (38.2%) in the HF subgroup. The proportion of patients receiving medical treatment including potassium binders was higher among those with advanced CKD stages (Table 5).
Table 4.
Hyperkalemia treatment at the time of index hyperkalemia and during the follow-up period
| Treatment | Overall n = 25,395 | CKD n = 14,322 | DM n = 12,101 | HF n = 8570 | HTN n = 16,463 |
|---|---|---|---|---|---|
| Hyperkalemia treatment on index date, n (%) | |||||
| Thiazide diuretics | 539 (2.1) | 452 (3.2) | 357 (3.0) | 236 (2.8) | 520 (3.2) |
| Loop diuretics | 4698 (18.5) | 3289 (23.0) | 2404 (19.9) | 3271 (38.2) | 3732 (22.7) |
| Glucose injection+insulina | 562 (2.2) | 298 (2.1) | 285 (2.4) | 278 (3.2) | 299 (1.8) |
| Calcium gluconate | 547 (2.2) | 267 (1.9) | 204 (1.7) | 235 (2.7) | 282 (1.7) |
| Sodium bicarbonate | 707 (2.8) | 598 (4.2) | 330 (2.7) | 247 (2.9) | 521 (3.2) |
| Potassium binder | 1468 (5.8) | 1308 (9.1) | 825 (6.8) | 543 (6.3) | 1171 (7.1) |
| SPS | 404 (1.6) | 354 (2.5) | 208 (1.7) | 139 (1.6) | 304 (1.8) |
| CPS | 1264 (5.0) | 1145 (8.0) | 732 (6.0) | 518 (6.0) | 1045 (6.3) |
| Medications associated with hyperkalemia, n (%) | |||||
| RAASi treatment | 10,212 (40.2) | 6785 (47.4) | 5979 (49.4) | 4856 (56.7) | 9453 (57.4) |
| ACEi | 2028 (8.0) | 1334 (9.3) | 1143 (9.4) | 1226 (14.3) | 1844 (11.2) |
| ARB | 7518 (29.6) | 5230 (36.5) | 4687 (38.7) | 3012 (35.1) | 7254 (44.1) |
| MRA | 3161 (12.4) | 2020 (14.1) | 1562 (12.9) | 2412 (28.1) | 2683 (16.3) |
| non-RAASi | 5093 (20.1) | 2193 (15.3) | 2039 (16.8) | 1446 (16.9) | 2735 (16.6) |
| Beta-blockers | 863 (3.4) | 440 (3.1) | 418 (3.5) | 482 (5.6) | 608 (3.7) |
| Cyclosporin | 120 (0.5) | 67 (0.5) | 52 (0.4) | 24 (0.3) | 44 (0.3) |
| Digoxin | 162 (0.6) | 66 (0.5) | 74 (0.6) | 95 (1.1) | 103 (0.6) |
| Heparin | 1896 (7.5) | 741 (5.2) | 690 (5.7) | 593 (6.9) | 915 (5.6) |
| NSAIDs | 1721 (6.8) | 611 (4.3) | 631 (5.2) | 336 (3.9) | 754 (4.6) |
| Potassium supplements | 253 (1.0) | 86 (0.6) | 88 (0.7) | 95 (1.1) | 126 (0.8) |
| Tacrolimus | 57 (0.2) | 21 (0.1) | 25 (0.2) | 11 (0.1) | 19 (0.1) |
| Hyperkalemia treatment during the follow-up period, n (%) | |||||
| Any treatment | 18,595 (73.2) | 12,031 (84.0) | 9641 (79.7) | 7492 (87.4) | 12,839 (78.0) |
| Potassium binder | 6087 (24.0) | 5392 (37.6) | 3303 (27.3) | 2235 (26.1) | 4574 (27.8) |
| Low daily dose | 6084 (100.0)b | 5389 (100.0) | 3301 (99.9) | 2233 (99.9) | 4571 (99.9) |
| Initiation of diuretics | 6491 (25.6) | 4366 (30.5) | 2983 (24.7) | 2338 (27.3) | 4194 (25.5) |
| RAASi treatment before index hyperkalemia | 10,212 (40.2) | 6785 (47.4) | 5979 (49.4) | 4856 (56.7) | 9453 (57.4) |
| Discontinuation of RAASi | 5480 (53.7)c | 3654 (53.9) | 2722 (45.5) | 2628 (54.1) | 4286 (45.3) |
| Dose reduction of RAASi | 1336 (13.1)c | 960 (14.1) | 736 (12.3) | 779 (16.0) | 1210 (12.8) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; CKD, chronic kidney disease; CPS, calcium polystyrene sulfonate; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; MRA, mineral corticoid receptor antagonist; NSAID, nonsteroidal anti-inflammatory drug; RAASi, renin-angiotensin-aldosterone inhibitors; SPS, sodium polystyrene sulfonate.
Glucose injection + insulin was counted only when the glucose injection was used concomitantly with insulin on the same day.
The denominator was the number of patients using potassium binders (n = 6087).
The denominator was the number of patients using RAASis (n = 10,212).
Table 5.
Hyperkalemia treatment at the time of index hyperkalemia according to CKD stages
| Treatment | Hyperkalemia treatment on index date, n (%) |
|||||
|---|---|---|---|---|---|---|
| Stage 1 n = 170 | Stage 2 n = 1030 | Stage 3a n = 2464 | Stage 3b n = 3704 | Stage 4 n = 4139 | Stage 5 n = 2803 | |
| Thiazide diuretics | 0 (0.0) | 16 (1.6) | 65 (2.6) | 135 (3.6) | 152 (3.7) | 83 (3.0) |
| Loop diuretics | 12 (7.1) | 105 (10.2) | 399 (16.2) | 877 (23.7) | 1195 (29.9) | 699 (24.9) |
| Glucose injection+insulina | 1 (0.6) | 10 (1.0) | 14 (0.6) | 51 (1.4) | 104 (2.5) | 117 (4.2) |
| Calcium gluconate | 2 (1.2) | 5 (0.5) | 29 (1.2) | 45 (1.2) | 81 (2.0) | 103 (3.7) |
| Sodium bicarbonate | 0 (0.0) | 9 (0.9) | 15 (0.6) | 49 (1.3) | 136 (3.3) | 389 (13.9) |
| Potassium binder (SPS) | 1 (0.6) | 8 (0.8) | 21 (0.9) | 54 (1.5) | 115 (2.8) | 155 (5.5) |
| Potassium binder (CPS) | 8 (4.7) | 43 (4.2) | 83 (3.4) | 220 (5.9) | 437 (10.6) | 352 (12.6) |
CKD, chronic kidney disease; CPS, calcium polystyrene sulfonate; SPS, sodium polystyrene sulfonate.
Glucose injection + insulin was counted only when the glucose injection was used concomitantly with insulin on the same day.
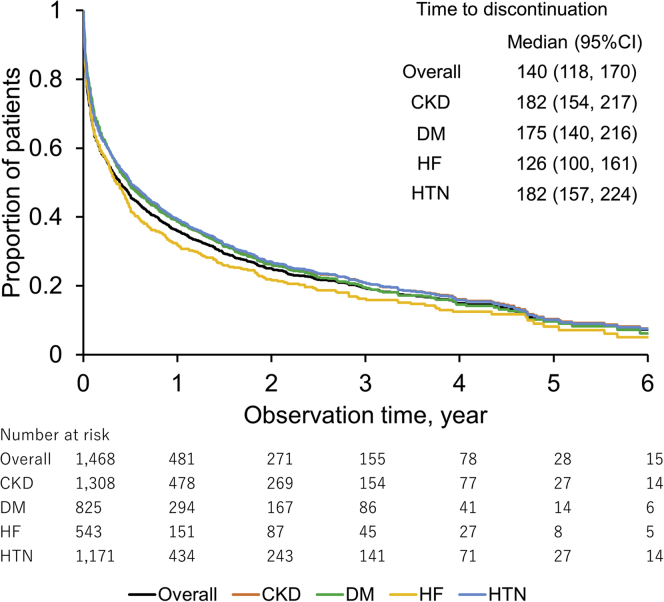
During the follow-up period, 73.2% of patients were taking potassium-lowering agents. Of them, 24.0% were potassium binders, although all patients were taking a low daily dose. Figure 7 shows the survival curve of continuation of potassium binders prescribed at index hyperkalemia. The median time to discontinuation was 140 days (95% CI: 118–170). The mean medication possession ratio in overall patients was 77.2% ± 29.0% (Supplementary Table S5).
Figure 7.
Kaplan-Meier analysis of time to discontinuation of initial potassium binder treatment. Discontinuation of potassium binders was defined as potassium binders not being prescribed ≥30-day gap after the last day of the supply of the previous prescription during the follow-up period. CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension.
Potassium Control After Hyperkalemia
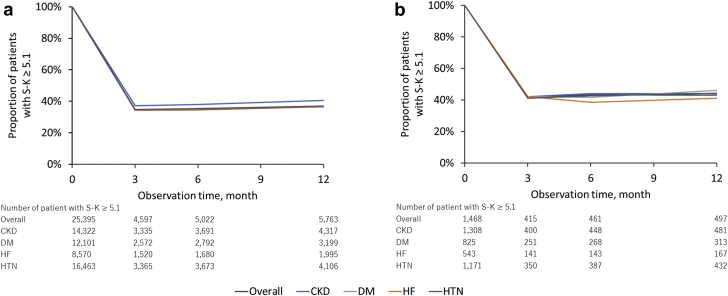
More than 30% of patients had persistently high S-K (≥5.1 mEq/l), with 34.5% at 6 months and 36.6% at 12 months. In patients taking a potassium binder at index hyperkalemia, the proportion of those who retained a high S-K level were 43.2% at 6 months and 43.2% at 12 months (Figure 8). The cumulative incidence of recurrent hyperkalemia at 1 year was 55.6% for S-K ≥5.1 mEq/l, 19.9% for S-K ≥5.5 mEq/l, and 4.9% for S-K ≥6.0 mEq/l (Figure 9).
Figure 8.
Control status of serum potassium level after the index hyperkalemia episode in (a) overall patients and (b) patients taking potassium binders. S-K, serum potassium; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension.
Figure 9.
Cumulative incidence curve of recurrent hyperkalemia episodes for (a) serum potassium (S-K) ≥5.1 mEq/l, (b) S-K ≥5.5 mEq/l, and (c) S-K ≥6.0 mEq/l. CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension.
Discussion
To our knowledge, this is the first report of epidemiological data on hyperkalemia in Japanese clinical practice from a large hospital claims database. The mean duration of patient follow-up was 2.74 years in a cohort of >25,000 patients with hyperkalemia. Hence, the present study provides comprehensive epidemiological data of hyperkalemia with substantial numbers of patients and follow-up time in real clinical settings.
According to the nationwide patient survey in 2014,13 the number of Japanese patients using in-hospital and outpatient care facilities, excluding dentist office, was 7,193,700. If the results of this study are applied, the estimated number of patients with hyperkalemia is approximately 483,000 to 495,000 in Japan. The prevalence of hyperkalemia consistently increased in patients with RAASi treatment in any subgroup. Likewise, the prevalence was greater in patients with advanced CKD stage or HF. The cumulative incidence of first hyperkalemia episodes increased along with the progression of CKD stages, and the increase was more obvious in stage 3a to stage 3b and stage 3b to stage 4. Higher cumulative incidence was also seen in patients using RAASis and in advanced age groups. These results are consistent with previous reports.14, 15, 16 A Danish health registry study with >60,000 patients reported that the incidence increases from moderate to severe CKD, and that hyperkalemia was more prevalent in patients with CKD, HF, or angiotensin-converting enzyme inhibitor use.17 A Swedish observational study reported higher incidences of hyperkalemia in patients with HF, lower estimated glomerular filtration rate, and RAASi use.18 In this study, 6.98% of patients experienced hyperkalemia over 3 years.
In the hyperkalemia cohort, approximately 3 in 4 patients were ≥65 years old. Cardiovascular disease history was prevalent: 22.8% of patients had a history of cerebrovascular disease, 14.9% had a history of peripheral vascular disease, and 4.2% had a history of myocardial infarction (Supplementary Table S4). A Danish registry study reported similar incidences of these conditions except myocardial infarction history.19 Among the high-risk group, patients with HF had several distinctive characteristics compared with others. Comorbidities such as atrial fibrillation/flutter and valvular heart disease were more prevalent in patients with HF. Renal function is a crucial component associated with hyperkalemia development. The results showed that 56.4% of the hyperkalemia cohort had CKD, whereas >70% of patients with CKD had severe or end-stage kidney disease (stage 3b, 4, or 5).
More than 40% of patients received RAASi at the time of index hyperkalemia, and the prevalence of RAASi medication was increased in high-risk groups, such as patients with CKD and HF. For these high-risk conditions, RAASi therapy is an important component of guideline-directed medical therapy,20 which demonstrated cardiorenal-protective effects in numerous studies, by slowing down the progression of kidney disease in the CKD population,21, 22, 23, 24 and improved clinical outcomes in patients with HF.25, 26, 27, 28 However, the results indicated that >50% of patients experienced discontinuation of RAASi after a first hyperkalemia episode even in those with high-risk conditions (53.9% for CKD and 54.1% for HF). These results indicate that a substantial number of patients requiring RAASi therapy for improving cardiorenal outcome are being exposed to nonoptimized RAASi dose because of hyperkalemia. The results also suggest the underestimated incidence and discontinuation rate of RAASi in previous clinical trials in this population. Various factors such as strict enrollment criteria and close monitoring of patients may cause the underestimation of the real frequency of problems caused by hyperkalemia.29 A study using primary care data in the United Kingdom has shown the increased risk of RAASi discontinuation.30 In this study, a J-shaped association between S-K and RAASi discontinuation was observed, and estimated glomerular filtration rate was the most predictive factor for RAASi discontinuation. As patients with cardiac or renal disease prescribed with RAASi are the group that may derive the greatest cardiorenal benefit from RAASi treatment,5, 31 better solutions to manage hyperkalemia while optimizing RAASi treatment are required. A recently published expert consensus statement recommended considering the use of S-K lowering agents, such as non–potassium-sparing diuretics and/or potassium binders, for continuation of RAASi treatment while closely monitoring S-K levels and renal function.32 Patients may experience more adverse cardiorenal outcomes and higher mortality if they discontinue or receive lower doses of RAASi treatment.33 A previous study reported more cardiorenal adverse events and higher mortality in patients who discontinued RAASi or were receiving submaximal doses than in those receiving the maximum dose among the nondialysis CKD population.34 Further studies are warranted on the association between the incidence of clinical outcomes and the discontinuation or dose reduction of RAASis to measure hyperkalemia burden in the context of guideline-directed medical therapy.
Our results indicate that loop diuretics were a mainstay of medical treatments for lowering S-K. Potassium binders were used by relatively few (5.8%) patients at the time of index hyperkalemia; however, a substantial number (24.0%) of patients received potassium binders during follow-up, comparable to the number of patients who initiated diuretics (25.6%). The percentage of patients with hyperkalemia who received a potassium binder was high in the CKD group (37.6%). This result indicates the need for potassium binders in patients with impaired renal function, as the complementary elimination pathway of potassium through the gastrointestinal tract is required. Despite such therapeutic needs, the average daily dose of potassium binders was lower than the recommended daily dose in almost all patients. Moreover, the survival curve of drug continuation indicated that a substantial number of patients experienced discontinuation early after index hyperkalemia. The mean medication possession ratio of potassium binders was less than the commonly used adherence target of 80%.35, 36, 37 Potassium binders, including sodium polystyrene sulfonate and calcium polystyrene sulfonate, were introduced in clinical practice several decades ago and are now widely prescribed; however, patients often discontinue their use for various reasons, such as the occurrence of gastrointestinal adverse effects38 or difficulties in drug administration. Suboptimal dose and treatment persistence can lead to poor control of the S-K level. Newer potassium-binding agents including patiromer and sodium zirconium cyclosilicate are expected to soon be available in clinical practice. With their improved tolerability for the continuous use,29, 39 management of chronic hyperkalemia may be more facilitated.
Our study demonstrated that the S-K level had a U-shaped association with the 3-year survival for Japanese patients. These results are consistent with a previous study of patients with CKD from the United States not undergoing dialysis, which reported that both hyperkalemia and hypokalemia were associated with a higher risk of death, major adverse cardiovascular events, and hospitalization, and there were U-shaped associations between S-K level and adverse clinical outcomes.40, 41 Mortality was increased at higher index S-K levels and in patients with advanced CKD stages. This result suggests that severity of CKD stages and hyperkalemia level might synergistically increase the mortality rate.
This study has several limitations. First, this study used hospital claims data, and such data were not collected for specific research purposes. Although we attempted to adjust for the influence of confounders, some missing values and residual confounding may exist. Several factors, such as nutritional status,42 socioeconomic status,43 and physical activity,44 were reported to be associated with clinical outcomes. Information on such factors could not be collected in the present study; however, they need to be considered in evaluating the effect of S-K on long-term clinical outcomes. Because death records cover only deaths that occurred in hospitals, out-of-hospital deaths could not be captured. However, the patient records were collected systematically and electronically as part of routine clinical practice, which helps avoid recall bias in collecting clinical information. Nearly 100% of prescription information was captured. Although the results of S-K measurement could be affected by hemolysis, it was difficult to assess if the blood sample measurements in this study were affected by hemolysis. To mitigate the inclusion of patients affected by such factor, we required at least 2 S-K measurements ≥5.1 mEq/l to include only patients likely to have persistent hyperkalemia. Second, the data were collected mostly from secondary care hospitals. Therefore, this limitation should be considered when generalizing the present findings to the primary care setting. In fact, some patients had acute conditions at the time of index hyperkalemia. However, the data were collected from 359 hospitals across Japan, which improves their generalizability. The magnitude of risk associated with the S-K level may differ depending on whether the patients had acute or chronic conditions. Further analyses are required to understand the differences in each condition. Finally, because this is an observational study, care needs to be taken when interpreting the results. The association found in this study cannot be directly considered as a causal relationship.
In conclusion, by using a large hospital claims database, we reported the epidemiological data of hyperkalemia in Japanese clinical practice, including its prevalence, incidence, treatment patterns, and management of hyperkalemia. A U-shaped association between S-K level and mortality was observed with the nadir at 4 mEq/l. As for the occurrence of hyperkalemia, there is a notable association with high-risk clinical features (e.g., CKD, HF, and use of RAASi), and a substantial number of patients remain hyperkalemic after their treatment. More than half of the patients discontinued RAASi after the index hyperkalemia episode. Suboptimal doses of potassium binders or poor persistence to treatment can lead to the recurrence of hyperkalemia during long-term clinical care. Continuous monitoring and correction of S-K may optimize patient outcomes over the course of long-term clinical management.
Disclosure
SO and TY are employed by AstraZeneca, K.K. SK reports investigator-initiated grants funding from Bayer and Daiichi Sankyo, and personal fees from Bristol-Meyers Squibb, Pfizer Japan, AstraZeneca, and Bayer, outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
This study and the corresponding analyses were supported and funded by AstraZeneca K.K., Osaka, Japan. The authors thank Piao Yi, Kuan Williams, and all project members of IQVIA Solutions, K.K. for providing technical and editorial support for this study.
Footnotes
Figure S1. Cubic spline analyses of 3-year incidences of other clinical outcomes.
Figure S2. Cumulative incidence of first hyperkalemia episode stratified by age group.
Table S1. Definitions of high-risk subgroups.
Table S2. Definitions of clinical outcomes.
Table S3. List of comorbidities.
Table S4. Comorbidities stratified by high-risk subgroups.
Table S5. Prescription coverage of potassium binder prescribed on index date.
STROBE Statement.
Supplementary Material
References
- 1.Gumz M.L., Rabinowitz L., Wingo C.S. An integrated view of potassium homeostasis. N Engl J Med. 2015;373:60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer B.F. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10:1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaitman M., Dixit D., Bridgeman M.B. Potassium-binding agents for the clinical management of hyperkalemia. P T. 2016;41:43–50. [PMC free article] [PubMed] [Google Scholar]
- 4.Khanagavi J., Gupta T., Aronow W.S. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10:251–257. doi: 10.5114/aoms.2014.42577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer B.F. Managing hyperkalemia caused by inhibitors of the renin–angiotensin–aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 6.Kovesdy C.P. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128:1281–1287. doi: 10.1016/j.amjmed.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh M.F., Wu I.W., Lee C.C. Higher serum potassium level associated with late stage chronic kidney disease. Chang Gung Med J. 2011;34:418–425. [PubMed] [Google Scholar]
- 8.An J.N., Lee J.P., Jeon H.J. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16:R225. doi: 10.1186/cc11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savarese G., Xu H., Trevisan M. Incidence, predictors, and outcome associations of dykalemia in heart failure with preserved, mid-range, and reduced ejection fraction. J Am Coll Cardiol HF. 2019;7:65–76. doi: 10.1016/j.jchf.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Montford J.R., Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28:3155–3165. doi: 10.1681/ASN.2016121344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betts K.A., Woolley J.M., Mu F. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018;34:971–978. doi: 10.1080/03007995.2018.1433141. [DOI] [PubMed] [Google Scholar]
- 12.Sawano M., Kohsaka S., Okamura T. Validation of the european SCORE risk chart in the healthy middle-aged Japanese. Atherosclerosis. 2016;252:116–121. doi: 10.1016/j.atherosclerosis.2016.07.926. [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Health, Labour and Welfare of Japan. Patient Survey 2014. Tokyo, Japan: Ministry of Health, Labour and Welfare of Japan.
- 14.Shemer J., Modan M., Ezra D. Incidence of hyperkalemia in hospitalized patients. Isr J Med Sci. 1983;19:659–661. [PubMed] [Google Scholar]
- 15.Einhorn L.M., Zhan M., Hsu V.D. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarafidis P.A., Blacklock R., Wood E. Prevalence and factor associated with hyperkalemia in predialysis patients in a low-clearlance clinic. Clin J Am Soc Nephrol. 2012;7:1234–1241. doi: 10.2215/CJN.01150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen R.W., Nicolaisen S.K., Adelborg K. Hyperkalaemia in people with diabetes: occurrence, risk factors and outcomes in a Danish population-based cohort study. Diabet Med. 2018;35:1051–1060. doi: 10.1111/dme.13687. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson E., Gasparini A., Ärnlöv J. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277–284. doi: 10.1016/j.ijcard.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen R.W., Nicolaisen S.K., Hasvold P. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes—a Danish population-based cohort study. Nephrol Dial Transplant. 2017;33:1610–1620. doi: 10.1093/ndt/gfx312. [DOI] [PubMed] [Google Scholar]
- 20.Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl. 2016;6:20–28. doi: 10.1016/j.kisu.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann J.F., Gerstein H.C., Pogue J. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ruggenenti P., Perna A., Gherardi G. Renalprotective properties of ACE-inhibition in non-diabetic nephropathies with non-diabetic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 23.Brenner B.M., Cooper M.E., de Zeeuw D. Effects on losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S., Teo K., Anderson C. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intorelant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 25.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 26.Granger C.B., McMurray J.J., Yusuf S. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intorelant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 27.Pitt B., Zannad F., Remme W.J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 28.Zannad F., McMurray J.J., Krum H. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 29.Kovesdy C.P. Management of hyperkalemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 30.Furukand H., MacEwan P., Evans M. Serum potassium as a predictor of adverse clinical outcomes in patients with chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC Nephrol. 2018;19:211. doi: 10.1186/s12882-018-1007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarwar C.M., Papadimitriou L., Pitt B. Hyperkalemia in heart failure. J Am Coll Cardiol. 2016;68:1575–1589. doi: 10.1016/j.jacc.2016.06.060. [DOI] [PubMed] [Google Scholar]
- 32.Rosano G.M.C., Tamargo J., Kjeldsen K.P. Expert consensus document on the management of hyperkalemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the working group on cardiovascular pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2019;4:180–188. doi: 10.1093/ehjcvp/pvy015. [DOI] [PubMed] [Google Scholar]
- 33.Epstein M., Reaven N.L., Funk S.E. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–S220. [PubMed] [Google Scholar]
- 34.Molnar M.Z., Kalantar-Zadeh K., Lott E.H. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sika R., Xia F., Aubert R.E. Estimating medication persistecy using administrative claims data. Am J Manag Care. 2005;11:449–457. [PubMed] [Google Scholar]
- 36.Wade S.W., Curtis J.R., Yu J. Medication adherence and fracture risk among patients on bisphosphonate therapy in a large United States health plan. Bone. 2012;50:870–875. doi: 10.1016/j.bone.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Valenstein M., Copeland L.A., Blow F.C. Pharmacy data identify poor adherent patients with schizophrenia at increased risk for admission. Med Care. 2002;40:630–639. doi: 10.1097/00005650-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Harel Z., Harel S., Shah P.S. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126 doi: 10.1016/j.amjmed.2012.08.016. 264.e9–e24. [DOI] [PubMed] [Google Scholar]
- 39.Rosano G.M.C., Spoletini I., Agewall S. Pharmacology of new treatments for hyperkalemia: patiromer and sodium zirconium cyclosiliate. Eur Heart J Suppl. 2019;21(Supplement A):A28–A33. doi: 10.1093/eurheartj/suy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J., Brunelli S.M., Jensen D.E. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins A.J., Pitt B., Reaven N. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–221. doi: 10.1159/000479802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovesdy C.P., Regidor D.L., Mehrotra R. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(5):999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 43.Saydah S.H., Imperatore G., Beckles G.L. Socioeconomic status and mortality. Contribution of healthcare access and physical distress among U.S. adults with diagnosed with diabetes. Diabetes Care. 2013;36:49–55. doi: 10.2337/dc11-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lear S.A., Hu W., Rangarajan S. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.