Highlights
-
•
SBRT is associated with better survival compared to TARE or CRT for unresectable ICC.
-
•
Association remained after adjustment using inverse probability weighting.
-
•
These modalities should be compared prospectively.
Keywords: Intrahepatic cholangiocarcinoma, Stereotactic body radiation therapy, Chemoradiation, Transarterial radioembolization
Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) is a highly lethal malignancy. For patients with locally advanced, unresectable disease, numerous liver-directed therapy options exist, including chemoradiation (CRT), stereotactic body radiation therapy (SBRT), and transarterial radioembolization (TARE). There is no randomized data to inform clinicians regarding the optimal treatment modality.
Method
We used the National Cancer Database (NCDB) to study the overall survival (OS) of patients with ICC treated with CRT, SBRT, and TARE. We used Cox proportional hazards modeling and inverse probability of treatment weighting (IPTW) to account for confounding variables.
Results
We identified 170 patients with unresected ICC treated with SBRT (n = 37), CRT (n = 61), or TARE (n = 72). SBRT was associated with higher OS compared to CRT (hazard ratio [HR] = 0.37; 95% confidence interval [CI] 0.20–0.68; p = 0.001) and TARE (HR = 0.40; 95% CI 0.22–0.74; p = 0.003). On multivariable analysis, SBRT remained associated with higher OS compared to CRT (HR = 0.44; 95% CI 0.21–0.91; p = 0.028) and TARE (HR = 0.42; 95% CI 0.21–0.84; p = 0.014). After IPTW (Bonferroni-adjusted significance threshold, α = 0.017), SBRT again had a statistically significant association with higher OS compared to CRT (HR = 0.22; 95% CI 0.11–0.44; p < 0.0001) and was nominally associated TARE (HR = 0.58; 95% CI 0.37–0.91; p = 0.019).
Conclusions
We found SBRT is associated with higher OS when compared to CRT or TARE for the treatment of unresectable ICC. Due to the retrospective nature of the study and potential selection bias, these findings should be evaluated prospectively.
1. Introduction
Cholangiocarcinoma is the second most common primary hepatobiliary malignancy and accounts for over 7000 deaths annually in the United States [1], [2]. Intrahepatic cholangiocarcinoma (ICC) comprises 10% of cholangiocarcinomas.1 Complete surgical resection offers the only possibility of cure for these patients, however only 12% of ICCs have resectable disease at diagnosis, accounting for one-third of patients with localized disease [3]. For the remaining patients with localized disease not amenable to surgical resection, several liver-directed treatment options exist, including conventionally fractionated external beam radiation therapy, chemoradiation (CRT), stereotactic body radiation therapy (SBRT), transarterial chemoembolization (TACE), and transarterial radioembolization (TARE) using yttrium-90 microspheres [4], [5].
In part due to the rarity of the disease, there is no randomized evidence to guide decision making with regard to liver-directed therapies for unresectable ICC. Within radiation oncology, CRT has historically been the preferred treatment in the setting of hepatobiliary malignancies [6], [7], [8], [9], but in more recent years SBRT and TARE have emerged as effective, conformal modalities that deliver high doses to tumor while limiting normal tissue toxicity [10]. The former modality employs hypofractionated external beam radiation and stereotactic immobilization, while the latter is a transcatheter intra-arterial procedure (typically performed by interventional radiology) that entails injection of yttrium-90 microspheres into the hepatic artery, thus capitalizing on the differential dependence of malignancy and normal parenchyma on hepatic arterial and portal venous blood supply, respectively. While there are retrospective studies comparing external beam radiotherapy to non-radiotherapeutic modalities [11], there are no studies comparing SBRT to conventional chemoradiation or TARE for this group of patients. We studied the overall survival of patients treated with SBRT, CRT, and TARE using the National Cancer Database (NCDB), a nationwide hospital-based registry accounting for 70% of newly diagnosed cancers in the United States.
2. Materials and methods
2.1. Study population
Using the NCDB, we identified patients with histologically- or cytologically-confirmed intrahepatic adenocarcinoma of the biliary tract diagnosed between 2004 and 2014. We included patients no more than 85 years old with a Charlson comorbidity score of 0–2. Patients who received surgery, had metastatic or lymph node-positive disease, or had missing T-stage or tumor size data, were excluded. Only patients who received CRT, SBRT, or TARE were included. SBRT was defined as external beam radiation therapy to a total dose of ≥30 Gy delivered in ≤5 fractions [12]. Patients who were treated with <5 Gy per fraction with concurrent chemotherapy, defined as chemotherapy initiated within 14 days of radiation start, were allotted to the CRT group. Receipt of chemotherapy during the first line of treatment was permitted in the SBRT and TARE cohorts.
2.2. Statistical analysis
Patient characteristics were summarized by treatment groups using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Kruskal-Wallis tests were used to compare the differences in continuous variables among the treatment groups, while Chi-square tests or Fisher’s exact tests were used for categorical variables. Kaplan-Meier curves were generated and log-rank testing was used to compare the differences in overall survival among the treatment groups. To further study the association between treatment and overall survival, and to adjust for possible confounders, Cox proportional hazard models were used to estimate the hazard ratios between groups. Variables with statistically significant differences in distributiuon between the treatment groups were selected as covariates for Cox proportional hazards. Missing data was handled as a unique level for multivariable analysis. In order to rule out the potential biases from variables that predict for receipt of treatment, age, year of diagnosis, Charlson comorbidity score, vascular invasion, clinical T stage, tumor focality, and tumor size were used to generate propensity scores that were used to calculate inverse probability of treatment weights, which were accounted for in a weighted Kaplan-Meier analysis. Due to the three pairwise comparisons, the Bonferroni adjusted significance threshold was set at p = 0.05/3 = 0.017 for the Kaplan-Meier comparisons of the inverse probability of treatment-weighted cohorts. All statistical analyses were performed using SAS (version 9.4, SAS Institute Inc, Cary, NC).
3. Results
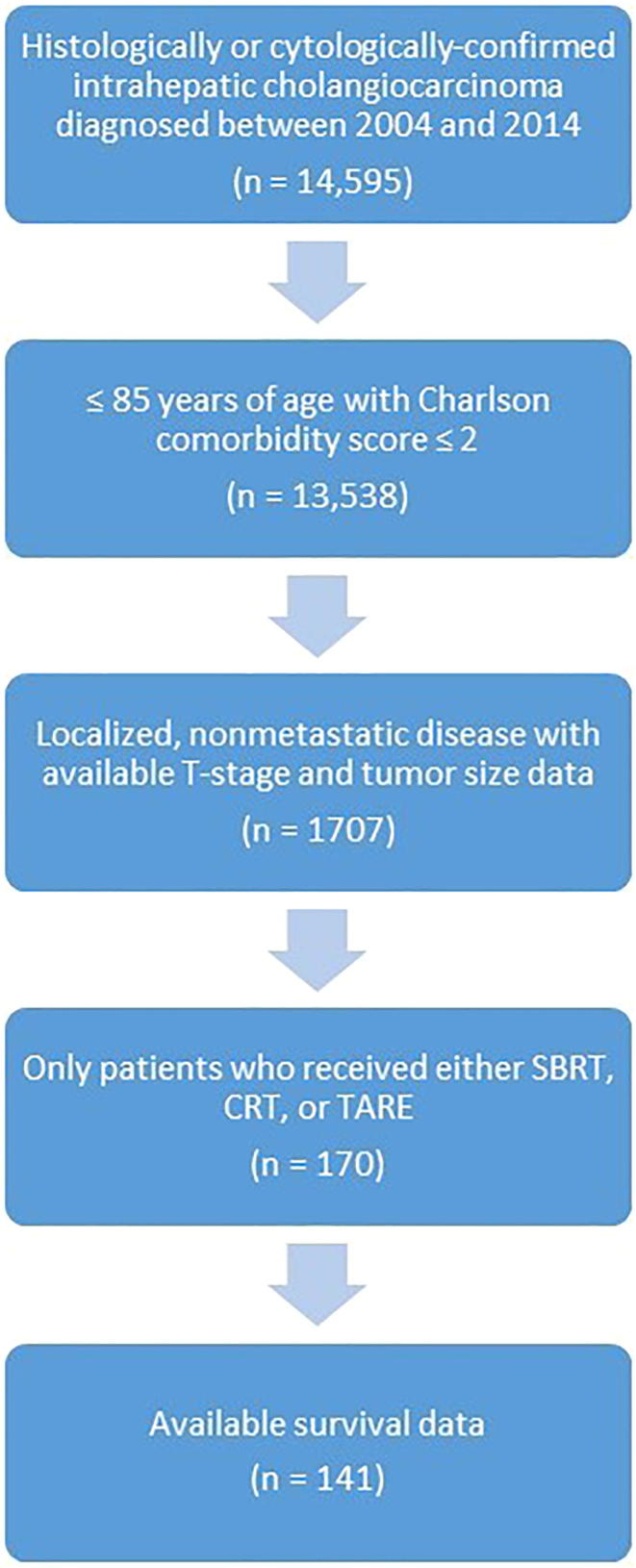
Fig. 1 shows the patient selection schema for the analyzed cohort. There were a total of 141 analyzable patients with unresectable ICC treated with SBRT (n = 27), CRT (n = 54), or TARE (n = 60). Table 1 shows the patient and disease characteristics of the cohort. SBRT, CRT, and TARE cohorts were respectively characterized by increasing proportion of patients with vascular invasion and tumor multifocality. ICC treated with TARE, specifically, was characterized by larger tumor size and higher T-stage, as well as more recent year of diagnosis. Roughly half of patients who received SBRT or TARE also received chemotherapy during the first course of treatment. The median dose and number of fractions for CRT was 50.4 Gy (interquartile range [IQR] 45–54 Gy) and 28 fractions (IQR 25–30), respectively. The median dose and number of fractions for SBRT was 45 Gy (40–50 Gy) and 5 fractions (IQR 3–5) respectively. Median follow-up time for all patients was 17 months. On univariate analysis, SBRT was associated with higher overall survival compared to CRT (hazard ratio [HR] = 0.37; 95% confidence interval [CI] 0.20–0.68; p = 0.001) and TARE (HR = 0.40; 95% CI 0.22–0.74; p = 0.003). There was no statistically significant difference in overall survival between patients treated with TARE and CRT (HR = 0.92; 95% CI 0.60–1.40; p = 0.69) (Fig. 2). The median overall survival for patients treated with SBRT, TARE, and CRT was 48 months (95% CI 20, upper limit not reached), 20 months (95% CI 14–24), and 14 months (95% CI 11–20), respectively.
Fig. 1.
Patient selection schema for the analyzed cohort.
Table 1.
Patient and disease characteristics of the analyzed cohort, stratified by radiotherapeutic modality*.
| Characteristic | SBRT | TARE | CRT | P |
|---|---|---|---|---|
| n (%) | 27 (100%) | 60 (100%) | 54 (100%) | |
| Age (Years) | ||||
| Median (IQR) | 71 (61, 80) | 65 (57.5, 74.5) | 67 (61, 74) | 0.11† |
| Sex | ||||
| Male | 18 (66.7%) | 27 (45.0%) | 25 (46.3%) | 0.14‡ |
| Female | 9 (33.3%) | 33 (55.0%) | 29 (53.7%) | |
| Race | ||||
| White | 25 (92.6%) | 51 (85.0%) | 46 (85.2%) | |
| Black | 1 (3.7%) | 2 (3.3%) | 2 (3.7%) | 0.85§ |
| Other | 1 (3.7%) | 7 (11.7%) | 6 (11.1%) | |
| Charlson Comorbidity Score | ||||
| 0 | 17 (63.0%) | 41 (68.3%) | 39 (72.2%) | 0.35§ |
| 1 | 9 (33.3%) | 13 (21.7%) | 14 (25.9%) | |
| 2 | 1 (3.7%) | 6 (10.0%) | 1 (1.9%) | |
| Year of diagnosis | ||||
| Median (IQR) | 2011 (2008, 2013) | 2013 (2011, 2014) | 2010 (2007, 2012) | <0.0001† |
| T-stage | ||||
| 1 | 16 (59.3%) | 18 (30.0%) | 13 (24.1%) | |
| 2 | 7 (25.9%) | 34 (56.7%) | 15 (27.8%) | <0.0001§ |
| 3 | 3 (11.1%) | 8 (13.3%) | 22 (40.7%) | |
| 4 | 1 (3.7%) | 0 (0.0%) | 4 (7.4%) | |
| Tumor Size (cm) | ||||
| Median (IQR) | 4.5 (3.2, 5.9) | 6.5 (4.5, 9.2) | 4.4 (2.9, 6.5) | <0.0001† |
| Vascular Invasion | ||||
| No | 16 (59.3%) | 23 (38.3%) | 12 (22.2%) | 0.038‡ |
| Yes | 7 (25.9%) | 21 (35.0%) | 22 (40.7%) | |
| Unknown | 4 (14.8%) | 16 (26.7%) | 20 (37.0%) | |
| Tumor Focality | ||||
| Unifocal | 3 (11.1%) | 19 (31.7%) | 3 (5.6%) | 0.032§ |
| Multifocal | 17 (63.0%) | 34 (56.7%) | 24 (44.4%) | |
| Unknown | 7 (25.9%) | 7 (11.6%) | 27 (50.0%) | |
| Chemotherapy | ||||
| No | 16 (59.3%) | 27 (45.0%) | 0 (0.0%) | |
| Yes | 11 (40.7%) | 32 (53.3%) | 54 (100.0%) | |
| Unknown | 0 (%) | 1 (1.7%) | 0 (0.0%) | <0.0001§ |
Abbreviations. SBRT- stereotactic body radiation therapy. TARE- transarterial radioembolization. CRT- chemoradiation. IQR- Interquartile range.
Mann-Whitney U (Wilcoxon rank-sum) test.
Chi-square test.
Fisher’s exact test.
Fig. 2.
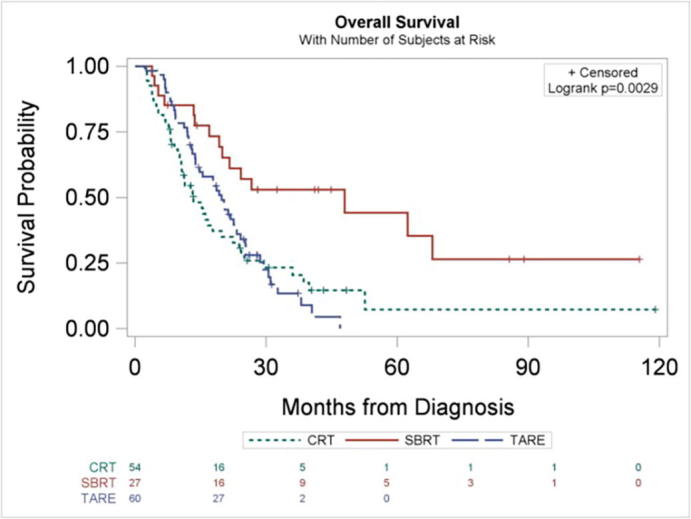
Kaplan-Meier curves for overall survival, stratified by radiotherapeutic modality.
On multivariable analysis (Table 2), SBRT remained associated with higher overall survival when compared to CRT (adjusted HR = 0.44; 95% CI 0.21–0.91; p = 0.028) and TARE (HR = 0.42; 95% CI 0.21–0.84; p = 0.014). There was no statistically significant difference in overall survival with TARE when compared to CRT (HR = 1.04; 95% CI 0.58–1.86; p = 0.89). None of the other covariates tested on multivariable analysis had a statistically significant association with overall survival.
Table 2.
Cox proportional hazards multivariable hazard ratios.*
| Characteristic | HR | Lower CI | Upper CI | P |
|---|---|---|---|---|
| Treatment | ||||
| CRT | 1.0 | 1.0 | 1.0 | – |
| TARE | 1.04 | 0.58 | 1.86 | 0.89 |
| SBRT | 0.44 | 0.21 | 0.91 | 0.028 |
| Year of diagnosis | 1.03 | 0.94 | 1.12 | 0.57 |
| T-stage | ||||
| 1–2 | 1.0 | 1.0 | 1.0 | – |
| 3–4 | 1.05 | 0.56 | 1.96 | 0.89 |
| Tumor size (cm) | 1.00 | 0.99 | 1.00 | 0.31 |
| Vascular invasion | ||||
| No | 1.0 | 1.0 | 1.0 | – |
| Yes | 0.88 | 0.53 | 1.48 | 0.64 |
| Tumor focality | ||||
| Unifocal | 1.0 | 1.0 | 1.0 | – |
| Multifocal | 1.13 | 0.59 | 2.19 | 0.71 |
| Chemotherapy | ||||
| No | 1.0 | 1.0 | 1.0 | – |
| Yes | 0.83 | 0.47 | 1.43 | 0.50 |
Abbreviations. HR- hazard ratio. CI- confidence interval. CRT- chemoradiation. TARE- transarterial radioembolization. SBRT- stereotactic body radiation therapy.
After excluding patients with missing covariates for propensity weighting, there were 19, 25, and 43 patients in the SBRT, CRT, and TARE cohorts, respectively. After propensity weighting adjustment, SBRT maintained a statistically significant higher overall survival compared to CRT (HR = 0.22; 95% CI 0.11–0.44; p < 0.0001) and was nominally associated with higher overall survival compared to TARE (HR = 0.58; 95% CI 0.37–0.91; p = 0.019) without reaching the Bonferroni-adjusted significance threshold (ɑ = 0.017). There was again no statistically significant difference in overall survival between patients treated with TARE and CRT (HR = 1.05; 95% CI 0.67–1.63; p = 0.84) (Fig. 3).
Fig. 3.
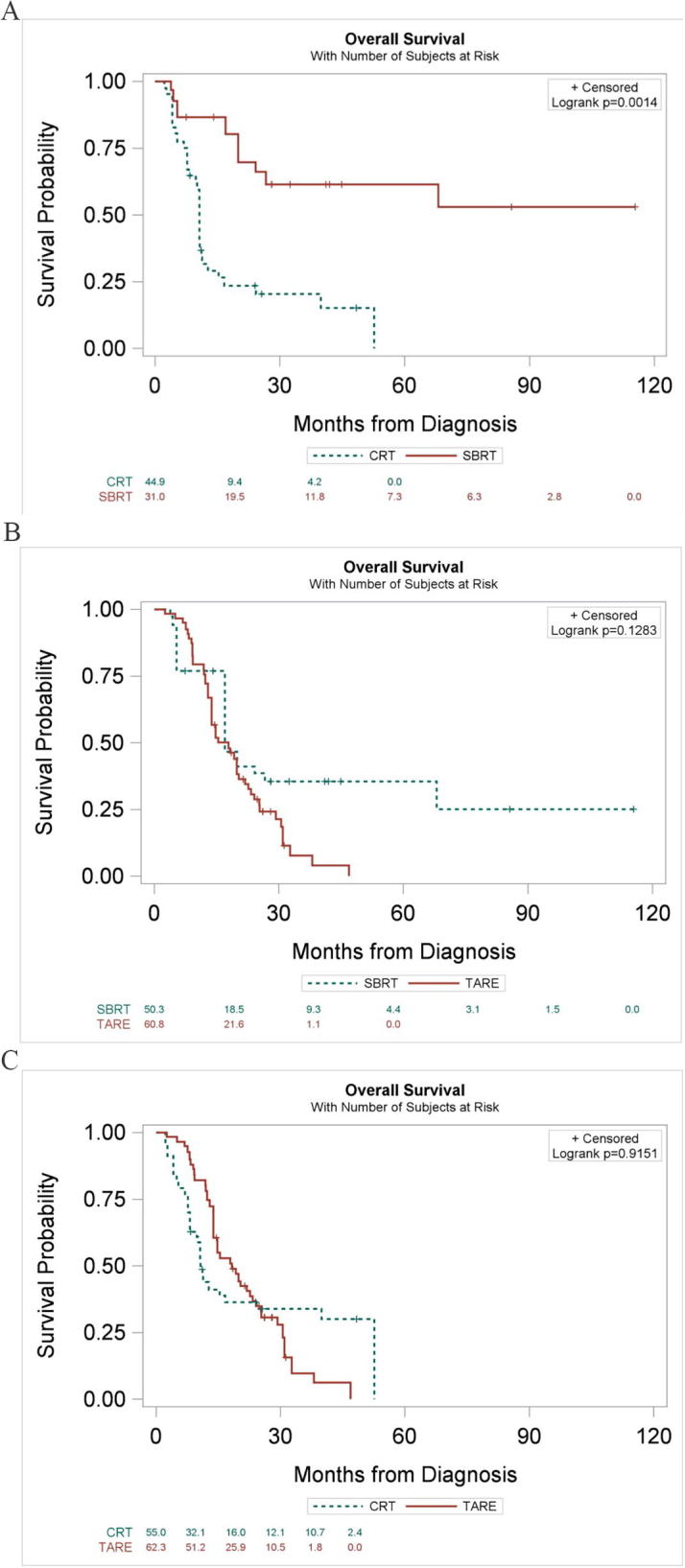
Kaplan-Meier curves for overall survival of inverse probability of treatment-weighted cohorts, comparing (A) SBRT to CRT (B) SBRT to TARE and (C) CRT to TARE. Note that risk tables have been excluded due to variable patient weighting.
4. Discussion
In what is, to our knowledge, the first study comparing outcomes of radiotherapeutic modalities for unresectable ICC, we found SBRT was associated with higher overall survival when compared to CRT or TARE. Importantly, these results were similar after adjusting for relevant prognostic and confounding variables, including T-stage, tumor size, focality, and vascular invasion [13], [14], [15].
The rarity of ICC has complicated efforts to prospectively and comparatively evaluate radiotherapy modalities for nonsurgical management of unresectable disease, although several studies have shown promising results for individual techniques. Studies have shown a modest prognostic and palliative benefit to external beam radiation therapy for unresectable ICC [16], [17], [18]. Additionally, multiple studies suggest favorable survival associated with addition of concurrent chemotherapy to radiation for biliary malignancies [6], [7], [8], [9]. Based on these studies, as well as the survival benefit associated with concurrent chemotherapy seen in other gastrointestinal malignancies, including rectal, anal, and pancreatic cancers, addition of chemotherapy to conventionally fractionated radiotherapy is encouraged [5]. More recently, studies have shown that high ablative doses typified by SBRT lead to improved overall survival and local control, irrespective of chemotherapy administration [12], [19], [20], [21], [22]. In tandem, radioembolization using yttrium-90 microspheres has emerged as an effective treatment option, favored for its ability to deliver high dose of radiation with minimal collateral hepatotoxicity [23], [24], [25].
Given the lack of randomized data, the choice of radiotherapy modality is currently made on the basis of various clinical- and tumor-related factors, such as tumor size and location, particularly given that historical comparisons are limited due to the heterogeneity of these variables between studies. Nevertheless, in the absence of factors clearly precluding a specific modality (such as excessive hepatopulmonary shunting when considering TARE), such a framework does little to clarify which modality is associated with superior outcomes. For a 4 cm tumor, for example, TARE, SBRT, or CRT may all be reasonable approaches for management [4].
Our data suggests that SBRT is the preferred modality in the absence of contraindications, such as a prohibitively large tumor size. While it may be argued that these findings reflect the preferential use of SBRT for more limited and less aggressive disease, our findings persisted after adjusting for discrepancies in stage, size, and focality. Furthermore, the survival benefit associated with SBRT when compared to CRT is congruent with studies suggesting survival advantage with dose-escalation in the setting of external beam radiotherapy. A retrospective study of 79 patients treated at MD Anderson Cancer Center showed that treatment of localized inoperable ICC to a biologically equivalent dose (BED) of >80.5 Gy, when compared to lower doses, was associated with a 3-year overall survival of 73% versus 38% and a 3-year local control of 78% versus 45%. Median overall survival for the entire cohort was 30 months and median overall survival for the high-BED group, specifically, was not reached [12]. Additionally, in a multi-institutional phase II study of high-dose hypofractionated proton beam therapy for ICC and hepatocellular carcinoma, the 37 patients with ICC had encouraging 2-year local control and overall survival of 94.1% and 46.5%, respectively, after treatment with a maximum total dose of 67.5 Gy equivalent in 15 fractions. Median overall survival for the ICC group was 22.5 months [26]. Although we did not explicitly evaluate patients treated with hypofractionated regimens delivered in greater than 5 fractions, in comparison to the aforementioned studies, the SBRT patients in our study had a favorable median overall survival of 48 months. Such comparisons should be interpreted with caution, however, as the SBRT patients in our study have more favorable disease characteristics such as smaller tumor size and no prior therapy.
We also found that SBRT is associated with improved overall survival compared to TARE, a modality that theoretically offers high dose delivery and conformity. Retrospective evidence in the setting of hepatocellular carcinoma suggests that both modalities offer good pathologic response and minimal toxicity as a bridge to transplant [27]. However, although TARE is characterized by a high selectivity for tumor versus normal hepatic parenchyma, and potential to deliver high dose to tumor [28], it is limited by significant variability in absorbed dose [29], [30], [31]. It is unclear if our findings reflect this disadvantage, microenvironment-based resistance that is specific to ICC, superior vascular injury and ablation conferred by SBRT, or unrecognized selection bias. It is also worth noting that novel TARE techniques such as radiation segmentectomy, which permits further dose escalation and conformity compared to lobar infusion, have shown promising results in early-stage hepatocellular carcinoma [32], [33] and may be a comparable alternative to SBRT for limited ICC in the future.
Despite adjustment for key confounders, there are limitations of this study that should be noted. First, the caveats associated with any retrospective study are inherent to this NCDB study, including possible selection bias and inability to account for a variety of potential confounders, such as performance status or departmental/clinical expertise. Along these lines, despite inverse probability of treatment weighting, variability in baseline characteristics such as tumor size and multifocality may reflect potential lead-time bias and/or tumor biology that cannot be accounted for. Second, there is a lack of toxicity data provided for each of the modalities, and baseline liver function may play a role in modality selection and/or radiation dose choice. Furthermore, therapies might be chosen on the basis of anatomic location or radiologic appearance which serve as further confounders in this study. For example, a tumor along the inner edge of the liver might be less likely to receive SBRT due to concern of damaging adjacent bowel, or a tumor with an infiltrative pattern on MRI might be more likely to receive TARE compared to SBRT or CRT since delineating the target may be more difficult or poorly defined. Unfortunately, such detailed data regarding tumor location is not available through NCDB. The lack of dose and microsphere-type data for TARE is also limiting, as biologic effect is known to vary with dose and it is possible that response may vary with microsphere brand [34], [35], [36]. Also, we acknowledge that the statistical power of the study is limited by the relatively low patient numbers in each of the cohorts. Finally, the lack of local recurrence and toxicity data through the NCDB limits complete evaluation of the potential benefit of these modalities. Nevertheless, as the majority of patients with ICC die of tumor-related liver failure, survival is strongly correlated with local control [37].
In summary, we found SBRT was associated with higher overall survival when compared to CRT and TARE in the management of unresectable ICC. These findings suggest that for clinical scenarios amenable to any of these treatments, SBRT may be the preferred option. However, these results should be interpreted with caution given the possibility of selection bias, and these modalities should be compared prospectively to clarify the clinical conditions for which these modalities are best suited.
Funding disclosures
None
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This data was presented in part at the American Society of Radiation Oncology (ASTRO) Annual Meeting 2018 (San Antonio, TX).
References
- 1.Everhart J.E., Ruhl C.E. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Yao K.J., Jabbour S., Parekh N., Lin Y., Moss R.A. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16 doi: 10.1186/s12876-016-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakeeb A., Tran K.Q., Black M.J., Erickson B.A., Ritch P.S., Quebbeman E.J. Improved survival in resected biliary malignancies. Surgery. 2002;132:555–563. doi: 10.1067/msy.2002.127555. discission 563–564. [DOI] [PubMed] [Google Scholar]
- 4.Koay E.J., Odisio B.C., Javle M., Vauthey J.-N., Crane C.H. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobil Surg Nutr. 2017;6:105–116. doi: 10.21037/hbsn.2017.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Hepatobiliary Cancers (Version 1.2019). https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed January 30, 2019. n.d.
- 6.Foo M.L., Gunderson L.L., Bender C.E., Buskirk S.J. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929–935. doi: 10.1016/s0360-3016(97)00299-x. [DOI] [PubMed] [Google Scholar]
- 7.Whittington R., Neuberg D., Tester W.J., Benson A.B., Haller D.G. Protracted intravenous fluorouracil infusion with radiation therapy in the management of localized pancreaticobiliary carcinoma: a phase I Eastern Cooperative Oncology Group Trial. J Clin Oncol. 1995;13:227–232. doi: 10.1200/JCO.1995.13.1.227. [DOI] [PubMed] [Google Scholar]
- 8.Kopelson G., Harisiadis L., Tretter P., Chang C.H. The role of radiation therapy in cancer of the extra-hepatic biliary system: an analysis of thirteen patients and a review of the literature of the effectiveness of surgery, chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 1977;2:883–894. doi: 10.1016/0360-3016(77)90186-9. [DOI] [PubMed] [Google Scholar]
- 9.Crane C.H., Macdonald K.O., Vauthey J.N., Yehuda P., Brown T., Curley S. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys. 2002;53:969–974. doi: 10.1016/s0360-3016(02)02845-6. [DOI] [PubMed] [Google Scholar]
- 10.Tanguturi S.K., Wo J.Y., Zhu A.X., Dawson L.A., Hong T.S. Radiation therapy for liver tumors: ready for inclusion in guidelines? Oncologist. 2014;19:868–879. doi: 10.1634/theoncologist.2014-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolarich A.R., Shah J.L., George T.J., Hughes S.J., Shaw C.M., Geller B.S. Non-surgical management of patients with intrahepatic cholangiocarcinoma in the United States, 2004–2015: an NCDB analysis. J Gastrointest Oncol. 2018;9:536–545. doi: 10.21037/jgo.2018.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao R., Krishnan S., Bhosale P.R., Javle M.M., Aloia T.A., Shroff R.T. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016;34:219–226. doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright G.P., Perkins S., Jones H., Zureikat A.H., Marsh J.W., Holtzman M.P. Surgical resection does not improve survival in multifocal intrahepatic cholangiocarcinoma: a comparison of surgical resection with intra-arterial therapies. Ann Surg Oncol. 2018;25:83–90. doi: 10.1245/s10434-017-6110-1. [DOI] [PubMed] [Google Scholar]
- 14.Blechacz B., Komuta M., Roskams T., Gores G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavros M.N., Economopoulos K.P., Alexiou V.G., Pawlik T.M. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–574. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara E.T., Mitra N., Guo M., Metz J.M. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1495–1501. doi: 10.1016/j.ijrobp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.-X., Zeng Z.-C., Tang Z.-Y., Fan J., Zhou J., Jiang W. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 84 patients. BMC Cancer. 2010;10:492. doi: 10.1186/1471-2407-10-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Z.-C., Tang Z.-Y., Fan J., Zhou J., Qin L.-X., Ye S.-L. Consideration of the role of radiotherapy for unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 75 patients. Cancer J. 2006;12:113–122. [PubMed] [Google Scholar]
- 19.Tse R.V., Hawkins M., Lockwood G., Kim J.J., Cummings B., Knox J. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. JCO. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 20.Kopek N., Holt M.I., Hansen A.T., Høyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94:47–52. doi: 10.1016/j.radonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Gkika E., Hallauer L., Kirste S., Adebahr S., Bartl N., Neeff H.P. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer. 2017;17:781. doi: 10.1186/s12885-017-3788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunner T.B., Blanck O., Lewitzki V., Abbasi-Senger N., Momm F., Riesterer O. Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol. 2019;132:42–47. doi: 10.1016/j.radonc.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Mouli S., Memon K., Baker T., Benson A.B., Mulcahy M.F., Gupta R. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24:1227–1234. doi: 10.1016/j.jvir.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Adra D.P., Gill R.S., Axford S.J., Shi X., Kneteman N., Liau S.-S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120–127. doi: 10.1016/j.ejso.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riaz A., Kulik L.M., Mulcahy M.F., Lewandowski R.J., Salem R. Yttrium-90 radioembolization in the management of liver malignancies. Semin Oncol. 2010;37:94–101. doi: 10.1053/j.seminoncol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Hong T.S., Wo J.Y., Yeap B.Y., Ben-Josef E., McDonnell E.I., Blaszkowsky L.S. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed M., Katz A.W., Tejani M.A., Sharma A.K., Kashyap R., Noel M.S. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Adv Radiat Oncol. 2015;1:35–42. doi: 10.1016/j.adro.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox R.A., Klemp P.F., Egan G., Mina L.L., Burton M.A., Gray B.N. Dose distribution following selective internal radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21:463–467. doi: 10.1016/0360-3016(91)90797-8. [DOI] [PubMed] [Google Scholar]
- 29.Tong A.K.T., Kao Y.H., Too C.W., Chin K.F.W., Ng D.C.E., Chow P.K.H. Yttrium-90 hepatic radioembolization: clinical review and current techniques in interventional radiology and personalized dosimetry. BJR. 2016;89:20150943. doi: 10.1259/bjr.20150943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy A.S., McNeillie P., Dezarn W.A., Nutting C., Sangro B., Wertman D. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494–1500. doi: 10.1016/j.ijrobp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Gulec S.A., Mesoloras G., Dezarn W.A., McNeillie P., Kennedy A.S. Safety and efficacy of Y-90 microsphere treatment in patients with primary and metastatic liver cancer: the tumor selectivity of the treatment as a function of tumor to liver flow ratio. J Transl Med. 2007;5:15. doi: 10.1186/1479-5876-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riaz A., Gates V.L., Atassi B., Lewandowski R.J., Mulcahy M.F., Ryu R.K. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–171. doi: 10.1016/j.ijrobp.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 33.Lewandowski R.J., Gabr A., Abouchaleh N., Ali R., Al Asadi A., Mora R.A. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287:1050–1058. doi: 10.1148/radiol.2018171768. [DOI] [PubMed] [Google Scholar]
- 34.Claudio Traino A., Boni G., Mariani G. Radiodosimetric estimates for radioembolic therapy of liver tumors: challenges and opportunities. J Nucl Med. 2012;53:509–511. doi: 10.2967/jnumed.111.100537. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z., Fardanesh M.R., Machac J., Heiba S., Knesaurek K., Zaretsky V. Comparison of therapeutic response using RECIST criteria: Y-90 SIR-Spheres and TheraSphere treatment of unresectable hepatocellular carcinoma. J Nucl Med. 2013;54:224. [Google Scholar]
- 36.Bhangoo M.S., Karnani D.R., Hein P.N., Giap H., Knowles H., Issa C. Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2015;6:469–478. doi: 10.3978/j.issn.2078-6891.2015.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita S., Koay E.J., Passot G., Shroff R., Raghav K.P., Conrad C. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: a comprehensive analysis of 362 consecutive patients. Cancer. 2017;123:1354–1362. doi: 10.1002/cncr.30488. [DOI] [PMC free article] [PubMed] [Google Scholar]