Abstract
Introduction
This double-blind, randomized controlled trial compared the safety and efficacy of subcutaneous epoetin alfa-epbx, an epoetin alfa biosimilar, with the reference product, epoetin alfa, in hemodialysis patients with end-stage kidney disease (ESKD) and anemia who were receiving epoetin alfa maintenance treatment.
Methods
Eligible patients (n = 320) were randomized (1:1) to subcutaneous epoetin alfa-epbx or epoetin alfa in a titration phase; patients who demonstrated stable subcutaneous dosing (n = 246) were re-randomized to receive subcutaneous epoetin alfa-epbx or epoetin alfa 1 to 3 times per week in a 16-week maintenance phase. Co-primary endpoints were least-squares mean difference between treatments in mean weekly hemoglobin concentration and mean weekly epoetin dose per kilogram body weight (BW) during the last 4 weeks of treatment in the maintenance phase.
Results
The least-squares mean difference (95% confidence interval [CI]) between treatments in weekly hemoglobin was 0.04 g/dl (−0.17 to 0.24 g/dl) and weekly epoetin dose/kg BW was −2.34 U/kg per week (−14.51 to 9.82 U/kg per week). The 95% CIs were contained within the prespecified equivalence margins of ±0.5 g/dl (weekly hemoglobin) and ±45 U/kg per week (weekly epoetin dose/kg BW). In the epoetin alfa-epbx and epoetin alfa groups, respectively, 4.0% and 4.1% of patients required blood transfusions, 69.7% and 70.5% reported adverse events, 18.9% and 27.0% reported serious adverse events, and 3 and 2 deaths were reported. Five patients were confirmed positive for anti-recombinant human erythropoietin antibody, 2 of whom tested positive at baseline. All patients tested negative for neutralizing antibodies.
Conclusions
This comparative clinical trial demonstrated equivalence in efficacy and similar safety of subcutaneously administered epoetin alfa-epbx to epoetin alfa.
Keywords: biosimilar, efficacy, end-stage kidney disease, epoetin alfa, subcutaneous administration, safety
See Commentary on Page 1199
Anemia is a serious and common complication of chronic kidney disease (CKD), often developing early in the course of CKD and worsening with disease progression.1, 2 Anemia in patients with CKD is primarily caused by inadequate production of erythropoietin by damaged kidneys.3 Epoetin alfa (EPOGEN/PROCRIT; Amgen Inc., Thousand Oaks, CA, USA/Janssen Products LP, Horsham, PA, USA) was the first recombinant human erythropoietin (epoetin) approved by the US Food and Drug Administration for treatment of anemia in patients with CKD,4, 5 and is one of the most commonly used erythropoiesis-stimulating agents (ESAs) among hemodialysis patients in the United States.6 Subcutaneous (s.c.) or i.v. administration of epoetin in patients with CKD and anemia results in clinically significant increases in hemoglobin, reduces the need for transfusion, and demonstrates efficacy in both corrective and maintenance treatment.7, 8, 9 However, costs of ESAs are significant. For example, in 2016, costs of ESAs for Medicare-certified dialysis facilities for patients with ESKD exceeded $1.7 billion (estimate provided by Mark Stephens, personal communication, August 2, 2018, based on an analysis of Centers for Medicare & Medicaid Services, Renal Cost Reports, 2018, available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Cost-Reports/RNL-2011-form.html).
Biosimilars are biologic drugs that are highly similar to a licensed (i.e., originator or reference) biologic product.10, 11 Specific guidance for biosimilar approval was first developed by the European Medicines Agency in 2005,10 and multiple approved biosimilar epoetin products have been in use in Europe since 2007.12, 13, 14, 15, 16 In the United States, the Food and Drug Administration developed a regulatory framework for biosimilar approval following passage of the Biologics Price Competition and Innovation Act of 2009, which created an abbreviated licensure pathway for biosimilars.11, 17 This prompted development of epoetin biosimilars that may provide additional treatment options and have the potential to lower the costs of epoetin treatment.18
Epoetin alfa-epbx (RETACRIT; Hospira Inc, a Pfizer company, Lake Forest, IL) is a biosimilar of epoetin alfa.19 Epoetin alfa-epbx and epoetin alfa are identical in amino acid sequence and comparable in carbohydrate composition.20 Epoetin alfa-epbx was approved by the US Food and Drug Administration in May 2018 for all indications of epoetin alfa, and can be administered i.v. or s.c.19 As part of the clinical development program that supported regulatory approval of epoetin alfa-epbx, a comparative clinical study demonstrated equivalence in efficacy and similar safety of epoetin alfa-epbx to epoetin alfa when administered i.v. to hemodialysis patients with anemia.21 As part of the same development program, the current study was conducted to evaluate the equivalence of epoetin alfa-epbx to epoetin alfa when administered s.c. to hemodialysis patients with anemia. This s.c.-administration study was additionally requested by the US Food and Drug Administration because the immunogenicity of a protein can be influenced by the route of administration.22, 23 Subcutaneous administration was selected because it is generally considered to be the route most likely to elicit an immune reaction,23, 24 and was therefore considered a more sensitive assessment for detecting potential differences in immunogenicity between epoetin alfa-epbx and epoetin alfa, should they exist.
Methods
Patient Population
The study included male and nonpregnant female hemodialysis patients, aged 18 to 80 years, with ESKD and anemia who, before randomization, were on stable i.v. or s.c. epoetin alfa 1 to 3 times per week and had stable hemoglobin (mean 9.0–11.0 g/dl) for ≥4 weeks; were on stable, adequate dialysis for ≥12 weeks; and had adequate iron stores (plasma ferritin >100 μg/l, transferrin saturation >20%). Exclusion criteria included active, uncontrolled systemic inflammatory, or malignant conditions; uncontrolled hypertension; recent myocardial infarction, stroke, major thrombotic event, seizure, or decompensated heart failure; required maintenance doses of epoetin alfa >600 U/kg per week; received long-acting ESAs within 12 weeks before randomization; or had recently donated or lost >475 ml of blood.
Study Design
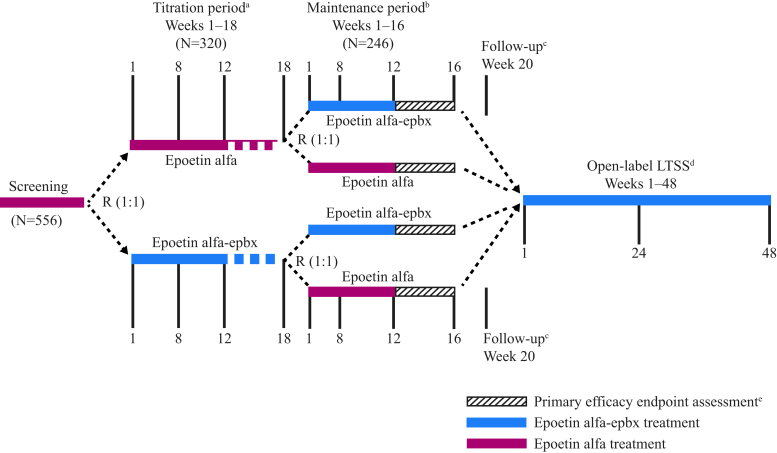
This was a multicenter, randomized, double-blind, active-controlled, parallel-group efficacy and safety trial (ClinicalTrials.gov; identifier NCT01473420) conducted in the United States to evaluate the equivalence of s.c.-administered epoetin alfa-epbx to epoetin alfa in hemodialysis patients with ESKD and anemia who were receiving epoetin alfa maintenance treatment. All patients who satisfied the entry criteria during a 4-week screening period were randomized (1:1) into a titration phase (Figure 1). Patients who were on i.v. epoetin alfa during screening received s.c. epoetin (epoetin alfa-epbx or epoetin alfa) 1 to 3 times per week in the titration phase; the starting dose was 20% to 30% lower than the i.v. epoetin alfa dose received during the last week of screening. Patients were treated for 12 to 18 weeks in the titration phase, based on evidence that safe and effective transition from chronic i.v. to s.c. epoetin alfa requires approximately 12 weeks, or longer, because of variability in patient response.25
Figure 1.
Study design. aPatients who were on subcutaneous treatment at the time of screening and demonstrated optimal stable dosing for 4 consecutive weeks were randomized (R) into the titration phase, received study drug assignment, and then proceeded directly to the maintenance phase if they satisfied all other study criteria for entry into the maintenance phase. Patients who did not demonstrate optimal and stable dosing by week 18 did not continue to the maintenance phase. bPatients who discontinued the study drug early during the maintenance phase received standard-of-care erythropoiesis-stimulating agent treatment until either the follow-up visit or entry into the long-term safety study (LTSS). cPatients who did not enter the LTSS underwent a follow-up visit, 4 weeks after the last schedule of study activities. dPatients had up to 28 days from completion of week 16 of the maintenance phase to enter into the LTSS. ePrimary efficacy endpoints will be assessed during the last 4 weeks of the maintenance phase.
Patients who demonstrated optimal stable dosing on s.c. epoetin by the last 4 weeks of the titration phase were randomized into a 16-week maintenance phase to receive s.c. study drug 1 to 3 times per week at the optimal dose demonstrated during the titration phase. Optimal stable dosing was defined as having met all of the following requirements during the 4 weeks before randomization and before entrance into the maintenance phase: a change in epoetin dosing of no more than 10% from the mean, a mean hemoglobin of 9.0 to 11.0 g/dl, no more than 1 hemoglobin result outside of range from 9.0 to 11.0 g/dl, and no hemoglobin result more than ±1 g/dl from the mean hemoglobin level. Conversely, patients who did not demonstrate optimal stable dosing were excluded from the maintenance phase and discontinued from study. Patients who were on s.c. epoetin alfa during screening received study drug assignment, without undergoing dose titration, and were immediately re-randomized into the maintenance phase to receive s.c. epoetin alfa-epbx or epoetin alfa at the same dose received during screening.
Randomization (1:1) into the maintenance phase was stratified by titration-phase drug assignment and dose level (low, medium, or high). This second randomization into the maintenance phase was intended to evenly distribute potential bias (resulting from possible carryover effect of variable treatment, study drug dose, allocation, and duration in the titration phase) on the primary efficacy variables measured for the parallel treatment groups in the fixed-length maintenance phase. Re-randomization into the maintenance phase also was intended to evenly distribute baseline characteristics. Study drug assignments for each phase were obtained by site personnel, unblinded with respect to study treatment, via an Interactive Voice Response System/Interactive Web-based Response System.
During the titration and maintenance phases, dosing for study drugs was individually adjusted, following the epoetin alfa US prescribing information,26, 27 to maintain hemoglobin within the target range (9.0–11.0 g/dl). Dose-adjustment consideration during the maintenance phase was made at least weekly by the investigator, based on hemoglobin levels over the previous 4 weeks. Dosing frequency was at the investigator’s discretion. Patients who discontinued treatment during the maintenance phase received short-acting, non–study-drug, standard-of-care (SoC) ESAs (epoetin alfa) and completed scheduled study assessments for the remainder of the maintenance phase. All patients who completed the maintenance phase qualified for entry into an open-label extension study under a separate protocol to receive s.c. epoetin alfa-epbx for up to an additional 48 weeks. Patients not entering the extension study continued with SoC treatment and had a follow-up assessment 4 weeks after the end of the maintenance phase (Figure 1).
The study was conducted from January 2012 to February 2014, in compliance with Institutional Review Board regulations, the Declaration of Helsinki, the International Conference on Harmonization guidelines, Good Clinical Practice guidelines, and all applicable regulatory requirements. Patients provided written informed consent before the performance of any study-specific procedures.
Study Drug
Epoetin alfa-epbx (RETACRIT; Hospira Inc) was supplied as an aqueous, phosphate-buffered, isotonic solution, containing polysorbate 20. Epoetin alfa (EPOGEN; Amgen Inc.) was used as the reference epoetin product. All injections were administered at least 30 to 60 minutes before the end of dialysis by site personnel who were blinded with respect to study treatments. To ensure compliance, study drug was prepared and administrations were recorded by site personnel who were unblinded with respect to study treatment.
Assessments
Hemoglobin levels, epoetin dose/kg BW, concomitant medications, transfusion requirements, vital signs, postdialysis BW, tolerability, and adverse events (AEs) were recorded at each weekly visit. Electrocardiograms were performed at screening, week 16, and follow-up. Clinical chemistry, hematology, coagulation parameters, and iron status were assessed at screening, before initial dosing, and monthly throughout the study. Blood was collected before the first (week 1) and last doses of study drug in the titration phase, before dosing at weeks 1 and 16 of the maintenance phase, and at follow-up for assessment of anti-recombinant human erythropoietin (rhEPO)–binding antibodies by radioimmunoprecipitation. If positive, the anti-rhEPO antibody response was quantified in the radioimmunoprecipitation assay by titrating the samples. Antibody titer was reported as the highest dilution that was equal to or above the titration cut point. Anti-rhEPO antibody-positive samples were further tested for anti-rhEPO neutralizing antibodies by cell-based assay, using current validated methods. All AEs were rated by the investigator for severity and relatedness to study drug. All clinical laboratory tests were performed by a central laboratory. The investigator assessed local tolerability and, with the patient, evaluated general tolerability at every visit at dosing using a 5-point scale.
Statistical Analyses
Sample size was determined from results of a similarly designed trial.28 A sample size of 93 patients/treatment group allowed 90% power to demonstrate equivalence between treatments in mean weekly hemoglobin level and mean weekly epoetin dose/kg BW during the last 4 weeks of treatment in the maintenance phase, using 2 one-sided tests with a 2.5% significance level. To account for an estimated 35% dropout rate, which reflects the estimated dropout from the beginning of the titration phase and is similar to that used in a previous study,28 288 patients (144/group) were planned for enrollment into the titration phase.
The intent-to-treat (ITT) population, comprising all patients randomized into the maintenance phase, was used for the primary efficacy analysis and analysis of secondary efficacy variables. Sensitivity analyses for the primary variable were conducted on the per-protocol population: a subset of ITT patients who had ≥4 weeks of study drug treatment during the maintenance phase, no use of non–study-drug ESAs during the last 4 weeks of treatment, and no transfusions during the study.
Co-primary efficacy endpoints, calculated as the least-squares (LS) mean difference between treatments in mean weekly hemoglobin level and mean weekly epoetin dose/kg BW, were derived from hemoglobin level and dose data collected during the last 4 weeks of treatment. Two-sided 95% CIs for the 2 LS mean differences were calculated using analysis of covariance (containing effect for treatment and baseline value) and compared with prespecified equivalence margins. The primary statistical model for the co-primary endpoints used a hierarchical test strategy: if equivalence between epoetin alfa-epbx and epoetin alfa was concluded for mean hemoglobin level (2-sided 95% CI within ±0.5 g/dl equivalence margin), the difference in mean weekly epoetin dose/kg BW was tested; if equivalence was concluded for mean weekly epoetin dose/kg BW (2-sided 95% CI within ±45 U/kg per week equivalence margin), equivalence in efficacy between treatments was concluded. Because the test on the mean weekly epoetin dose/kg BW calculation was performed only if equivalence for hemoglobin level was determined, no adjustment of alpha values was required.
Mean weekly hemoglobin level and mean weekly epoetin dose/kg BW over the 16-week maintenance phase were summarized descriptively and analyzed by t-test and the Mann–Whitney–Wilcoxon test, respectively. The proportion of patients with a weekly mean hemoglobin level within the target range (9.0–11.0 g/dl) at weeks 8 and 16 of the maintenance phase, and the proportion of patients receiving blood transfusions, were summarized with counts and percentages, and analyzed by Fisher’s exact test.
All summaries of safety data were presented for the safety population (all patients who received ≥1 dose of study drug) by the first treatment actually received during the period of interest (titration or maintenance). Safety data and demographic and baseline characteristics were descriptively assessed. AEs were coded using Medical Dictionary of Regulatory Activities version 14.1 terminology.29 Tolerability was summarized for each visit as both continuous and discrete variables separately by treatment phase (titration and maintenance). In addition, the most severe tolerability a patient experienced was summarized as both continuous and discrete variables.
Results
Patient Populations, Disposition, and Characteristics
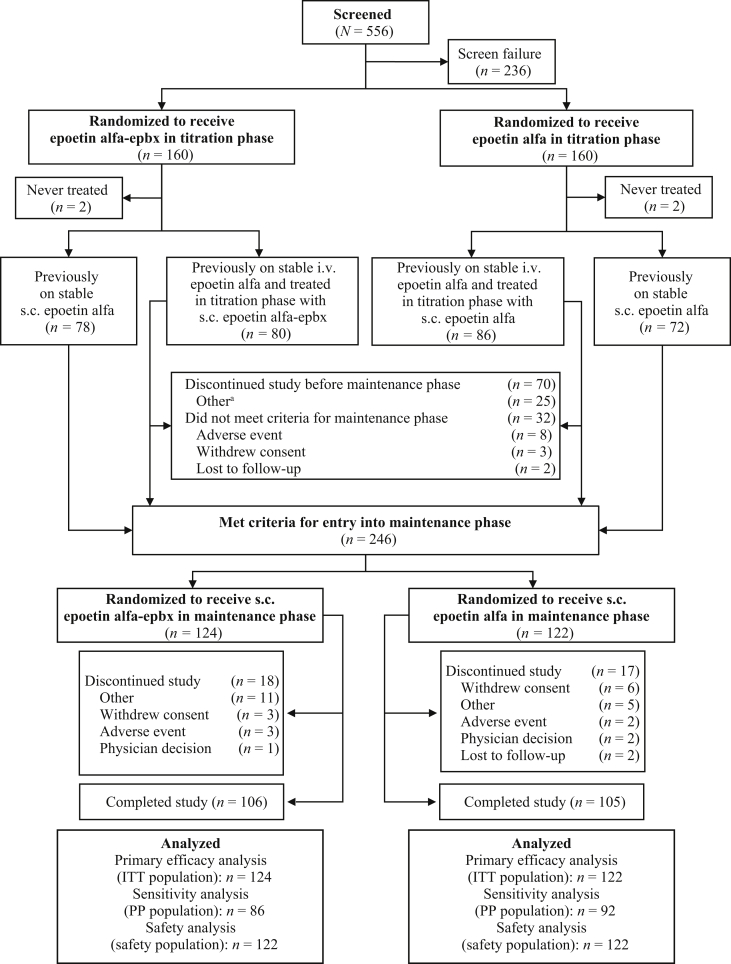
Of the 556 patients screened, 320 across 68 US sites were randomized into the titration phase (Figure 2). Of these, 150 were previously on stable s.c. epoetin alfa and immediately re-randomized into the maintenance phase, 166 were previously on stable i.v. epoetin alfa and entered the titration phase on s.c. study drug (safety population), and 4 were never treated. For the safety population during the titration phase, mean duration of study drug exposure for both treatment groups was approximately 11 to 12 weeks. Seventy patients discontinued study before the maintenance phase, including 15 of 70 (21.4%) and 17 of 70 (24.3%) in the epoetin alfa-epbx and epoetin alfa groups, respectively, who did not meet criteria for entry into the maintenance phase.
Figure 2.
Patient disposition. aOther reasons for study discontinuation before the maintenance phase included temperature excursion (n = 7); standard of care (n = 6); sponsor (n = 5); withdrew from dialysis (n = 2); and did not meet criteria for maintenance phase, elevated hemoglobin, started peritoneal dialysis, patient transferred, and patient went on vacation (n = 1 each). ITT, intent-to-treat; PP, per-protocol; s.c., subcutaneous.
In total, 246 patients (ITT population) qualified for the maintenance phase and were re-randomized to receive epoetin alfa-epbx (n = 124) or epoetin alfa (n = 122) (Figure 2). Of these, 1 of 124 (0.8%) randomized to epoetin alfa-epbx and 1 of 122 (0.8%) randomized to epoetin alfa received no treatment with study drug, 3 of 124 (2.4%) randomized to epoetin alfa-epbx received actual treatment with epoetin alfa, and 2 of 122 (1.6%) randomized to epoetin alfa received actual treatment with epoetin alfa-epbx. Three of 124 (2.4%) patients randomized to epoetin alfa-epbx and 2 of 122 (1.6%) randomized to epoetin alfa withdrew from the maintenance phase because of AEs. The safety population in the maintenance phase comprised 244 patients (epoetin alfa-epbx, n = 122; epoetin alfa, n = 122).
For the safety population during the maintenance phase, 14 of 122 (11.5%) patients in the epoetin alfa-epbx group and 20 of 122 (16.4%) patients in the epoetin alfa group switched to non–study-drug SoC ESA for 1 or more of the following reasons (epoetin alfa-epbx and epoetin alfa, respectively): SoC given in error (n = 2 and n = 3), medical monitor decision (n = 0 and n = 1), randomization delay (n = 2 and n = 0), withdrawal by patient (n = 1 and n = 2), study drug and SoC were both given in the same visit (n = 2 and n = 0), AE/serious AE (SAE) (n = 2 and n = 8), SoC given (n = 2 and n = 5), increased hemoglobin (n = 0 and n = 1), sponsor decision (n = 0 and n = 1), temperature excursion (n = 3 and n = 2), study drug discontinued (n = 0 and n = 1), vacation (n = 1 and n = 0), and other (n = 1 and n = 0). Mean duration of non–study-drug SoC ESA exposure was 6.2 weeks and 4.7 weeks for patients in the epoetin alfa-epbx and epoetin alfa groups, respectively.
Demographic and baseline clinical characteristics were balanced between groups, and all patients had adequate iron stores at maintenance-phase entry (Table 1). In the epoetin alfa-epbx and epoetin alfa groups, respectively, 60.7% and 49.2% of patients received concomitant i.v. iron supplementation at some time during the maintenance phase. Other common concomitant medications are summarized in Supplementary Table S1. One patient each in the epoetin alfa-epbx and epoetin alfa groups tested positive for anti-rhEPO antibodies at baseline, with titers of 1:4 and 1:2, respectively.
Table 1.
Demographic and baseline characteristics (safety population)a
| Characteristic | Epoetin alfa-epbx n = 122 | Epoetin alfa n = 122 |
|---|---|---|
| Male, n (%) | 63 (51.6) | 55 (45.1) |
| Age, yr, mean (SD) | 57.36 (11.93) | 56.50 (13.42) |
| Race, n (%) | ||
| White | 69 (56.6) | 58 (47.5) |
| Black or African American | 48 (39.3) | 60 (49.2) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (0.8) |
| Asian | 4 (3.3) | 2 (1.6) |
| American Indian or Alaska Native | 1 (0.8) | 1 (0.8) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 34 (27.9) | 31 (25.4) |
| Not Hispanic or Latino | 88 (72.1) | 91 (74.6) |
| Weight, kg, mean (SD) | 85.04 (22.88) | 86.59 (25.18) |
| Time from start of dialysis to randomization, mo, mean (SD) | 54.25 (52.44) | 57.94 (41.52) |
| Hemoglobin level, g/dl, mean (SD) | 10.36 (0.78) | 10.28 (0.78) |
| Weekly epoetin dose by BW, U/kg per wk, mean (SD) | 93.53 (112.45)b | 85.91 (82.08) |
| Ferritin level, ng/ml, mean (SD) | 990.9 (413.37) | 927.7 (396.81) |
| TSAT, %, mean (SD) | 36.1 (13.40) | 34.2 (14.50) |
| Anti-rhEPO antibody status, n (%) | ||
| Negative RIP | 108 (88.5) | 104 (85.2) |
| Positive RIP | 1 (0.8) | 1 (0.8) |
| Missingc | 13 (10.7) | 17 (13.9) |
| Primary cause of CKD, n (%)d | ||
| Diabetes | 56 (45.2) | 41 (33.6) |
| Hypertension | 43 (34.7) | 58 (47.5) |
| Nephropathies | 13 (10.5) | 16 (13.1) |
| Congenital renal disease | 5 (4.0) | 3 (2.5) |
| Other | 7 (5.6) | 4 (3.3) |
Anti-rhEPO, anti-recombinant human erythropoietin antibody; BW, body weight; CKD, chronic kidney disease; ITT, intent-to-treat; RIP, radioimmunoprecipitation assay; TSAT, transferrin saturation.
Analyses for all characteristics except primary cause of CKD were performed on the safety population for the maintenance phase. The percentages for ‘race’ may not add up to 100 because patients could select multiple races. Baseline was the last value determined before first dose of study drug in the maintenance phase.
Mean (SD) weekly epoetin dose by BW (U/kg per wk) based on data for 121 patients treated with epoetin alfa-epbx.
Baseline anti-rhEPO antibody samples were missing because of sample not being drawn or sample handling. Missing samples were to be redrawn at the following visit, but were not considered baseline values.
Analyses for primary cause of CKD were performed using the ITT population (epoetin alfa-epbx, n = 124; epoetin alfa, n = 122).
Efficacy
For the primary analysis conducted on the ITT population, the LS mean difference (95% CI) between treatment groups in mean weekly hemoglobin levels during the last 4 weeks of treatment was 0.04 g/dl (−0.17 to 0.24 g/dl) and in mean weekly epoetin dose/kg BW was −2.34 U/kg per week (−14.51 to 9.82 U/kg per week). The 95% CIs were contained within the prespecified equivalence margins of ±0.5 g/dl (weekly hemoglobin) and ±45 U/kg per week (weekly epoetin dose/kg BW) (Table 2). Sensitivity analysis using the per-protocol population demonstrated that the 95% CIs for both co-primary endpoints were also contained within the respective equivalence margins (Table 2).
Table 2.
Primary and secondary efficacy endpoints
| Endpoints | Epoetin alfa-epbx | Epoetin alfa |
|---|---|---|
| Primary efficacy endpoints | ||
| Primary analysis (ITT population) | n = 124 | n = 122 |
| Mean weekly hemoglobin level during last 4 weeks of treatment, g/dl | ||
| LS mean (SE) | 10.16 (0.07) | 10.12 (0.07) |
| Difference (95% CI)a | 0.04 (−0.17 to 0.24) | |
| Mean weekly epoetin dose by BW during last 4 weeks of treatment, U/kg per wk | ||
| LS mean (SE) | 79.57 (4.36) | 81.91 (4.37) |
| Difference (95% CI)b | −2.34 (−14.51 to 9.82) | |
| Sensitivity analysis (per-protocol population) | n = 86 | n = 92 |
| Mean weekly hemoglobin level during last 4 weeks of treatment, g/dl | ||
| LS mean (SE) | 10.19 (0.08) | 10.19 (0.08) |
| Difference (95% CI)a | 0.00 (−0.23 to 0.23) | |
| Mean weekly epoetin dose by BW during last 4 weeks of treatment, U/kg per wk | ||
| LS mean (SE) | 73.85 (5.16) | 72.21 (4.96) |
| Difference (95% CI)b | 1.63 (−12.48 to 15.75) | |
| Secondary efficacy endpoints (ITT population) | ||
| Patients with mean weekly hemoglobin within 9.0–11.0 g/dl at wk 16,cn/N (%) | 83/104 (79.8) | 77/104 (74.0) |
| P value | 0.4108 | |
| Patients who received a red blood cell transfusion at any time during the 16-wk maintenance phase, n/N (%) | 5/124 (4.0) | 5/122 (4.1) |
| P value | >0.9999 | |
BW, body weight; CI, confidence interval; ITT, intent-to-treat; LS, least-squares; PP, per-protocol.
The 95% CI for the LS mean of the difference (epoetin alfa-epbx – epoetin alfa) in mean weekly hemoglobin had to reside within the equivalence margin of ±0.5 g/dl for equivalence to be concluded.
The 95% CI for the LS mean of the difference (epoetin alfa-epbx – epoetin alfa) in mean weekly dose/kg BW had to reside within the equivalence margin of ±45 U/kg per wk for equivalence to be concluded.
Percentages are calculated using the number of observations at week 16 within a treatment group as the denominator.
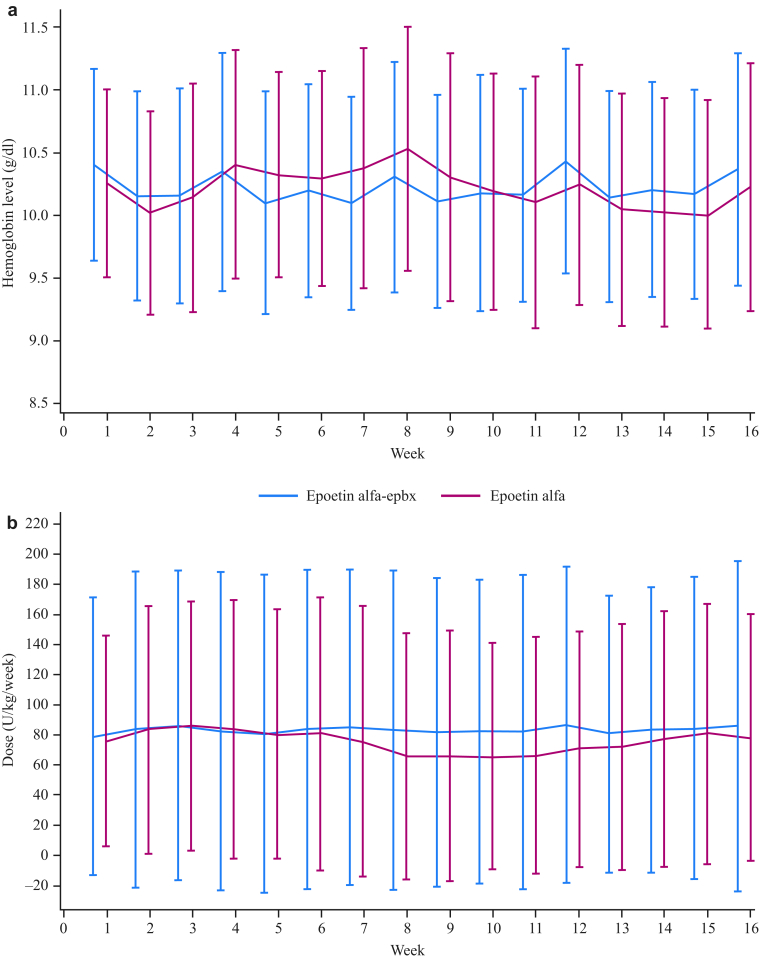
Analysis of secondary efficacy endpoints demonstrated that most epoetin alfa-epbx- and epoetin alfa-treated patients maintained hemoglobin levels within the range of 9.0 to 11.0 g/dl at week 16 of the treatment period; few patients in either group received transfusions during the study (Table 2). Over the 16-week maintenance phase, there was no difference between groups in mean weekly hemoglobin (P = 0.8) or mean weekly epoetin dose/kg BW (P = 0.7) (Figure 3). In addition, the percentages of patients requiring temporary (54.9%, epoetin alfa-epbx and 60.3%, epoetin alfa; P = 0.6) or permanent (29.6%, epoetin alfa-epbx and 20.5%, epoetin alfa; P = 0.3) dose changes of study drug were similar between treatment groups.
Figure 3.
Mean (SD) weekly hemoglobin level (g/dl) (a) and mean (SD) weekly epoetin dose by body weight (BW; U/kg/week) (b) during the maintenance phase. Analyses were performed on the intent-to-treat (ITT) population. Over the 16-week maintenance phase, there was no difference between groups in mean weekly hemoglobin (P = 0.8) or mean weekly epoetin dose/kg BW (P = 0.7).
Safety
For the safety population in the titration phase (epoetin alfa-epbx, n = 80; epoetin alfa, n = 86), 59.6% of patients in both groups reported at least 1 AE; the most common AE was headache, which occurred with similar incidence in both groups (Supplementary Table S2). AEs led to study drug discontinuation in 4 (5.0%, epoetin alfa-epbx) and 6 (7.0%, epoetin alfa) patients. Twelve (15.0%, epoetin alfa-epbx) and 22 (25.6%, epoetin alfa) patients reported SAEs. SAEs reported by 2 or more patients were sepsis (n = 2/group) and chronic obstructive pulmonary disease (epoetin alfa-epbx, n = 0; epoetin alfa, n = 2). In the epoetin alfa-epbx group, 4 (5.0%) patients discontinued study drug due to SAEs of cardiac arrest, asthenia, colon cancer, or dyspnea. In the epoetin alfa group, 6 (7.0%) patients discontinued study drug due to SAEs of acute myocardial infarction, cardiac arrest, coronary artery disease, gastrointestinal angiodysplasia, acute pyelonephritis, or decubitus ulcer. Three (3.8%) patients receiving epoetin alfa-epbx and 1 (1.2%) receiving epoetin alfa died during the titration phase. The SAEs with fatal outcome in the epoetin alfa-epbx (cardiac arrest, multifactorial functional decline, and sepsis [n = 1 each]) and epoetin alfa (acute myocardial infarction) groups were considered not related to study drug.
For the safety population in the maintenance phase (n = 122/group), approximately 70% of patients in each group reported at least 1 AE, the most common being nausea, which occurred with similar incidence in both groups (Table 3). AEs led to study drug discontinuation in 4 (3.3%) patients in each group.
Table 3.
Treatment-emergent adverse events occurring in ≥5% of patients in either treatment group during the maintenance phase
| Preferred term | Epoetin alfa-epbx n = 122 | Epoetin alfa n = 122 |
|---|---|---|
| Any treatment-emergent AE, n (%) | 85 (69.7) | 86 (70.5) |
| Nausea | 10 (8.2) | 8 (6.6) |
| Pyrexia | 8 (6.6) | 4 (3.3) |
| Fall | 8 (6.6) | 3 (2.5) |
| Dizziness | 3 (2.5) | 9 (7.4) |
| Injection-site pain | 3 (2.5) | 8 (6.6) |
AE, adverse event.
Twenty-three (18.9%, epoetin alfa-epbx) and 33 (27.0%, epoetin alfa) patients reported SAEs. The most common SAEs reported by 2 or more patients (epoetin alfa-epbx and epoetin alfa, respectively) were atrial fibrillation (n = 1 and 2), cardiac arrest (n = 2 and 0), cardio-respiratory arrest (n = 0 and 2), impaired gastric emptying (n = 1 and 2), noncardiac chest pain (n = 2 and 1), cellulitis (n = 2 and 0), osteomyelitis (n = 2 and 0), pneumonia (n = 3 and 2), hyperkalemia (n = 2 and 2), hypoglycemia (n = 0 and 4), and mental impairment (n = 1 and 2). In the epoetin alfa-epbx group, 4 (3.3%) patients discontinued study drug due to SAEs of gastrointestinal hemorrhage, multiple fractures, tibia fracture, or respiratory failure. In the epoetin alfa group, 3 (2.5%) patients discontinued study drug due to SAEs of atrial fibrillation, hypoglycemia, or pulmonary edema.
Three (2.5%) patients receiving epoetin alfa-epbx and 2 (1.6%) receiving epoetin alfa died during the maintenance phase. The SAEs with fatal outcome in the epoetin alfa-epbx group (cardiac arrest, gastrointestinal bleed, and azotemia [n = 1 each]) were considered not related to study drug. The 2 deaths in the epoetin alfa group (arrhythmia and aortic stenosis [n = 1 each]) were considered probably not related to study drug.
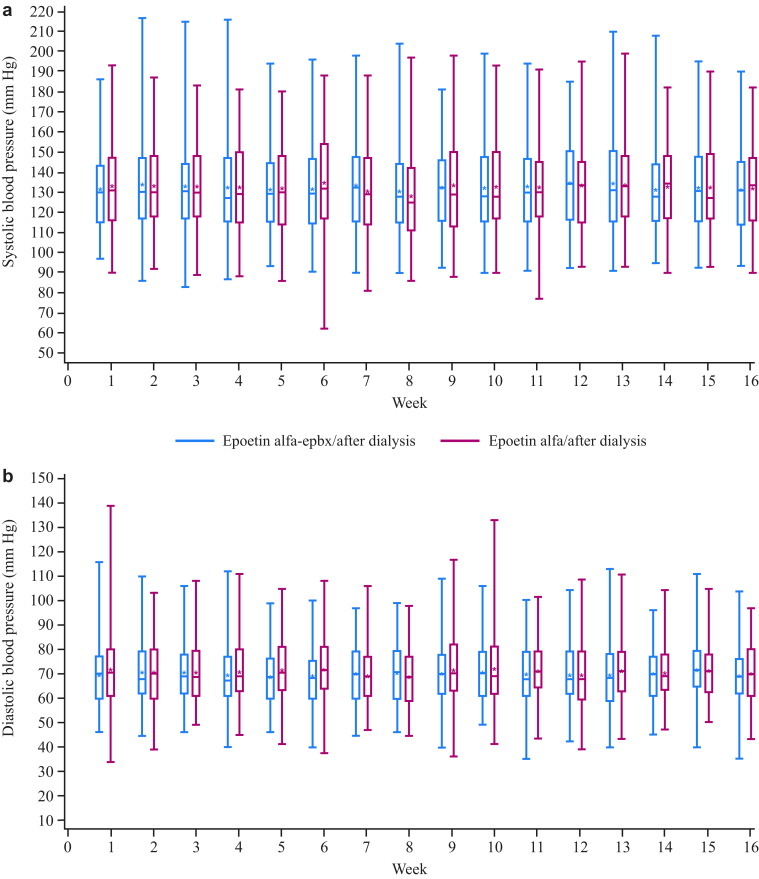
Patients receiving epoetin alfa were more likely to experience a hemoglobin excursion <8.0 or >12.0 g/dl at some point during the maintenance phase than were patients receiving epoetin alfa-epbx; excursions >13.0 or >14.0 g/dl were relatively infrequent (Table 4). Clinical laboratories, vital signs, and postdialysis BW were stable in both groups throughout the duration of the study. During the maintenance phase, blood pressure was similar between the 2 treatment groups over time (Figure 4). Mean changes from baseline to week 16 during the maintenance phase (epoetin alfa-epbx and epoetin alfa, respectively) for plasma ferritin (−11.5 and 42.3 ng/ml) and transferrin saturation (−1.8% and 0.6%) were clinically unremarkable. Throughout the study, iron stores remained adequate in both groups and were able to support erythropoiesis stimulated by epoetin. There were no notable changes in electrocardiogram findings in either group.
Table 4.
Hemoglobin excursions and investigator-rated local tolerability during the maintenance phase
| Variable | Epoetin alfa-epbx n = 122 | Epoetin alfa n = 122 |
|---|---|---|
| Hemoglobin excursions, n (%) | ||
| <8.0 g/dl | 5 (4.1) | 11 (9.0) |
| >12.0 g/dl | 12 (9.8) | 24 (19.7) |
| >13.0 g/dl | 3 (2.5) | 4 (3.3) |
| >14.0 g/dl | 1 (0.8) | 1 (0.8) |
| Most severe local tolerability rating,an (%) | ||
| 1. Excellent tolerability | 82 (67.2) | 66 (54.1) |
| 2. Good tolerability | 30 (24.6) | 37 (30.3) |
| 3. Mild intolerability | 5 (4.1) | 11 (9.0) |
| 4. Moderate intolerability | 2 (1.6) | 4 (3.3) |
| 5. Severe intolerability | 0 | 3 (2.5) |
The most severe response per week is summarized for each patient.
Figure 4.
Box plots of systolic (a) and diastolic (b) blood pressures over time. The box represents the 25th and 75th percentiles and the tails represent the minimum and maximum observed values; the median is indicated by the horizontal line, and the mean by the asterisk.
Using current validated methods, 5 patients (epoetin alfa-epbx, n = 2; epoetin alfa, n = 3) were confirmed anti-rhEPO antibody-positive, 2 of whom (n = 1 each/group) tested positive at baseline before the first dose of study drug in the maintenance phase, and 3 of whom (epoetin alfa-epbx, n = 1; epoetin alfa, n = 2) developed anti-rhEPO antibodies while on study treatment. The patient in the epoetin alfa-epbx group who was confirmed anti-rhEPO antibody-positive at baseline had a titer of 1:4; titers for the remaining 4 patients were between <1:2 and 1:2. All 5 patients tested negative for neutralizing antibodies and were clinically stable.
Both treatments were well-tolerated; the most severe local tolerability rating during the maintenance phase was reported by the investigator as “good” or “excellent” in 112 (91.8%) and 103 (84.4%) patients in the epoetin alfa-epbx and epoetin alfa groups, respectively (Table 4). Three (2.5%, epoetin alfa-epbx) and 8 (6.6%, epoetin alfa) patients experienced injection-site pain, 1 (0.8%, epoetin alfa-epbx) experienced injection-site erythema, and 1 (0.8%, epoetin alfa) experienced injection-site swelling.
Discussion
Regulatory guidance for biosimilar development provides analytical, nonclinical, and clinical testing requirements to assess biosimilarity between a proposed biosimilar and the licensed biologic.10, 11 As part of an overall program to demonstrate biosimilarity of epoetin alfa-epbx to the reference product, epoetin alfa, this study evaluated the equivalence of the 2 s.c. treatments in patients with ESKD and anemia who were on hemodialysis. The study met its prespecified co-primary efficacy endpoints by demonstrating equivalence between epoetin alfa-epbx and epoetin alfa in mean weekly hemoglobin levels and mean weekly epoetin dose/kg BW. Sensitivity and secondary efficacy analyses support the results of the primary analysis.
The co-primary endpoints were selected because both are clinically relevant and sensitive to evaluate change. The equivalence margin (±0.5 g/dl) for evaluating LS mean difference in mean weekly hemoglobin levels was selected because it was used to demonstrate equivalence between biosimilar and reference epoetin products in previous studies,28, 30 and because even well-titrated patients with renal anemia who receive stable doses of epoetin experience intraindividual fluctuations (±1 g/dl) in hemoglobin levels.31, 32 The equivalence margin (±45 U/kg per wk) for evaluating LS mean difference in mean weekly epoetin dose/kg BW was selected based on key principles of a no-effect dose, true-effect dose, guidelines for dose modification in the epoetin alfa US prescribing information,26, 27 and because it was used to demonstrate equivalence in previous studies.28, 30
As noted, s.c. administration was selected for this study because it is generally considered to be the route most likely to elicit an immune reaction,23, 24 and was therefore considered a more sensitive assessment for detecting potential differences in immunogenicity between the biosimilar and the reference product, should they exist. Most patients on hemodialysis receive epoetin i.v.; however, s.c. administration is used in some US hemodialysis units, and in patients on peritoneal dialysis or with nondialysis CKD.33 Patients on hemodialysis with ESKD are the most erythropoietin-deficient population across the licensed indications for epoetin alfa, and therefore the most likely to reveal potential differences in efficacy between products. Patients with ESKD are also less immunocompromised and have higher risk of pure red cell aplasia than other patients for whom epoetin alfa is indicated (e.g., patients with cancer receiving myelosuppressive chemotherapy). Therefore, the current study was designed to evaluate similarity between epoetin alfa-epbx and epoetin alfa using the route of administration and study population deemed most sensitive for identifying a difference in immunogenicity between the 2 products across the conditions of use. Current findings provide important information for the patient populations in which s.c. administration is used, and contributed to the totality of evidence and scientific justification for extrapolation of data that supported a demonstration of biosimilarity and licensure of epoetin alfa-epbx in all indications of the reference product.
Epoetin alfa-epbx and epoetin alfa had similar safety profiles. All AEs (including SAEs) observed in this study were consistent with those expected for an ESKD population receiving epoetin, generally similar between the 2 treatment groups, and concordant with the type and incidence of AEs described for epoetin alfa.26, 27 Patients with CKD have an increased all-cause mortality, which increases as kidney function decreases.34, 35, 36 Cardiovascular disease is the leading cause of death in patients with CKD34, 35; thus, 5 of the 9 deaths in this study resulting from cardiovascular causes are not unexpected. Subcutaneous administration was well-tolerated by patients in both groups; local tolerability ratings were similar and injection-site AEs were infrequent.
Protein therapeutic drugs have the potential to elicit an immune response,37 and neutralizing antibodies to epoetin can result in pure red cell aplasia or severe anemia,26, 27, 38 particularly when administered s.c.39 Three patients (epoetin alfa-epbx, n = 1; epoetin alfa, n = 2) developed anti-rhEPO antibodies while on study treatment; however, no patient in either treatment group had neutralizing antibodies or reported events of pure red cell aplasia or hypersensitivity consistent with immunogenic response to epoetin.
This comparative clinical trial demonstrated equivalence in efficacy and similar safety between s.c.-administered epoetin alfa-epbx and epoetin alfa. One limitation of the study was that investigators were permitted to switch patients from study drug to non–study-drug SoC ESA and retain them in the trial. However, it is unlikely that SoC exposure influenced the efficacy results because the primary analysis of co-primary efficacy endpoints was corroborated by a sensitivity analysis using the per-protocol population, which excluded patients receiving non–study-drug SoC ESA during the last 4 weeks of treatment. The 16-week length of the study also limited the duration of evaluation of epoetin alfa-epbx treatment.
The strengths of this trial include its double-blind nature, use of the ITT population for primary efficacy analyses, individualized study drug dosing per SoC epoetin dosing guidelines, rigor associated with co-primary endpoints required to demonstrate equivalence in efficacy, and robustness added by the sensitivity analysis and secondary endpoints. Additional safety and persistence of efficacy data are under evaluation in the 48-week, open-label extension trial.
The introduction of biosimilars to European markets has been associated with price reductions and increased patient access across product classes.40 A 2017 report estimated a price reduction of 31% and an increase in uptake of 66% across the epoetin drug class (i.e., for reference and biosimilar products) in several European countries following the introduction of epoetin biosimilars.40 In the United States, treatment for patients with ESKD is covered by Medicare, which reimburses dialysis providers for the cost of services, laboratory testing, and medications related to kidney disease, including ESAs (e.g., epoetin), at a bundled rate.41, 42 However, Medicare reimbursement under the bundled payment system may not completely cover the costs of treatment, which could negatively impact dialysis facilities (e.g., limit their operation or services, lead to closure), thereby affecting the quality of patient care or access to treatment.41, 42 Availability of lower-cost epoetin biosimilars could generate savings for dialysis providers and maintain patient access to high-quality care. Results of this comparative efficacy and safety trial demonstrating equivalence of epoetin alfa-epbx to epoetin alfa via s.c. administration support the opportunity to provide patients with additional treatment options that offer the same benefits as epoetin therapy but at a potential lower overall cost.
Disclosure
SF has received consultancy fees from Pfizer Inc; personal fees for advisory boards from Hospira Inc, Keryx Inc, AstraZeneca, and Akebia Inc; and research funding from AstraZeneca, Akebia Inc, and Keryx Inc. BSS is a member of the speakers’ bureau for Pfizer and has received research grant support and/or honoraria from Akebia Therapeutics, FibroGen Inc, Vifor Pharma, and Corvidia Therapeutics. WAW is an employee of and owns stock or options in Hospira Inc, a Pfizer company. NEM was an employee of and held stock or options in Hospira Inc, a Pfizer company, at the time of the study.
Acknowledgments
Trial registration: ClinicalTrials.gov NCT01473420. Medical writing support was provided by Drs. Laurel Mengle-Gaw and Benjamin Schwartz of the Camden Group and was funded by Hospira, which was acquired by Pfizer Inc in September 2015. Medical writing/editorial support was provided by Elyse Smith, PhD, of Engage Scientific Solutions and was funded by Pfizer Inc.
This study was funded by Hospira Inc, which was acquired by Pfizer Inc in September 2015. The study sponsor monitored patient data collected by the investigators for completeness and acceptability throughout the course of the study; generated and validated statistical analyses; provided funding for medical writing and editorial support; and reviewed the final, author-approved version for intellectual property protection.
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union, or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer also will consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Author Contributions
Research idea and study design: SF and NEM; data acquisition: SF, BSS; data analysis/interpretation: all authors; statistical analysis: WAW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Table S1. Common concomitant medications.
Table S2. Treatment-emergent adverse events occurring in ≥5% of patients in either treatment group during the titration phase.
Consort Statement.
Supplementary Material
References
- 1.Kausz A.T., Khan S.S., Abichandani R. Management of patients with chronic renal insufficiency in the Northeastern United States. J Am Soc Nephrol. 2001;12:1501–1507. doi: 10.1681/ASN.V1271501. [DOI] [PubMed] [Google Scholar]
- 2.Kazmi W.H., Kausz A.T., Khan S. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38:803–812. doi: 10.1053/ajkd.2001.27699. [DOI] [PubMed] [Google Scholar]
- 3.Zadrazil J., Horak P. Pathophysiology of anemia in chronic kidney diseases: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:197–202. doi: 10.5507/bp.2013.093. [DOI] [PubMed] [Google Scholar]
- 4.Amgen Inc Epogen (epoetin alfa) US prescribing information. http://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/epogen/epogen_pi_hcp_english.ashx Available at:
- 5.Janssen Products L.P. Procrit (epoetin alfa) US prescribing information. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/PROCRIT-pi.pdf Available at:
- 6.The DOPPS Practice Monitor ESA use, by type (national sample) https://www.dopps.org/dpm/DPMSlideBrowser.aspx?type=Topic&id=1 Available at:
- 7.Eschbach J.W., Egrie J.C., Downing M.R. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 8.Eschbach J.W., Kelly M.R., Haley N.R. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med. 1989;321:158–163. doi: 10.1056/NEJM198907203210305. [DOI] [PubMed] [Google Scholar]
- 9.Macdougall I.C. Treatment of renal anemia with recombinant human erythropoietin. Curr Opin Nephrol Hypertens. 1992;1:210–219. doi: 10.1097/00041552-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Committee for Medicinal Products for Human Use (CHMP), European Medicines Agency. Guideline on similar biological medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf Available at:
- 11.US Food and Drug Administration Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. https://www.fda.gov/media/82647/download Available at:
- 12.European Medicines Agency Retacrit (epoetin zeta): European public assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/retacrit Available at:
- 13.European Medicines Agency Abseamed (epoetin alfa): European public assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/abseamed Available at:
- 14.European Medicines Agency Binocrit (epoetin alfa): European public assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/binocrit Available at:
- 15.European Medicines Agency Epoetin Alfa Hexal (epoetin alfa): European public assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/epoetin-alfa-hexal Available at:
- 16.European Medicines Agency Silapo (epotin zeta): European public assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/silapo Available at:
- 17.The Patient Protection and Affordable Care Act, 42 USC 18001. Pub. L. 111–148 (H.R. 3590). 124 Stat. 119 (2010) https://www.gpo.gov/fdsys/pkg/STATUTE-124/pdf/STATUTE-124-Pg119.pdf Available at:
- 18.Fishbane S., Shah H.H. The emerging role of biosimilar epoetins in nephrology in the United States. Am J Kidney Dis. 2015;65:537–542. doi: 10.1053/j.ajkd.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Hospira Inc Retacrit (epoetin alfa-epbx) US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125545s000lbl.pdf Available at:
- 20.Pfizer Inc Epoetin Hospira, a proposed biosimilar to Epogen/Procrit (epoetin alfa): briefing document for the Oncologic Drugs Advisory Committee. https://www.fda.gov/files/20170525-ODAC-02-Hospira_Backgrounder.pdf Available at:
- 21.Fishbane S., Singh B., Kumbhat S. Intravenous epoetin alfa-epbx versus epoetin alfa for treatment of anemia in end-stage kidney disease. Clin J Am Soc Nephrol. 2018;13:1204–1214. doi: 10.2215/CJN.11631017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamuro L., Kijanka G., Kinderman F. Perspectives on subcutaneous route of administration as an immunogenicity risk factor for therapeutic proteins. J Pharm Sci. 2017;106:2946–2954. doi: 10.1016/j.xphs.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration Immunogenicity Assessment for Therapeutic Protein Products. https://www.fda.gov/downloads/drugs/guidances/ucm338856.pdf Available at:
- 24.Kuriakose A., Chirmule N., Nair P. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. J Immunol Res. 2016;2016:1298473. doi: 10.1155/2016/1298473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paganini E.P., Eschbach J.W., Lazarus J.M. Intravenous versus subcutaneous dosing of epoetin alfa in hemodialysis patients. Am J Kidney Dis. 1995;26:331–340. doi: 10.1016/0272-6386(95)90654-1. [DOI] [PubMed] [Google Scholar]
- 26.Amgen Inc Epogen (epoetin alfa) US prescribing information (June 2011) https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103234Orig1s5166_103234Orig1s5266lbl.pdf Available at:
- 27.Amgen Inc Epogen (epoetin alfa) US prescribing information (December 2013) https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103234s5323lbl.pdf Available at:
- 28.Krivoshiev S., Wizemann V., Czekalski S. Therapeutic equivalence of epoetin zeta and alfa, administered subcutaneously, for maintenance treatment of renal anemia. Adv Ther. 2010;27:105–117. doi: 10.1007/s12325-010-0012-y. [DOI] [PubMed] [Google Scholar]
- 29.Medical Dictionary of Regulatory Activities, version 14.1. 2011. Available at: https://www.meddra.org/. Accessed 24 June 2019.
- 30.Krivoshiev S., Todorov V.V., Manitius J. Comparison of the therapeutic effects of epoetin zeta and epoetin alpha in the correction of renal anaemia. Curr Med Res Opin. 2008;24:1407–1415. doi: 10.1185/030079908x297402. [DOI] [PubMed] [Google Scholar]
- 31.Berns J.S., Elzein H., Lynn R.I. Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int. 2003;64:1514–1521. doi: 10.1046/j.1523-1755.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- 32.Lacson E., Jr., Ofsthun N., Lazarus J.M. Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am J Kidney Dis. 2003;41:111–124. doi: 10.1053/ajkd.2003.50030. [DOI] [PubMed] [Google Scholar]
- 33.Hayat A., Haria D., Salifu M.O. Erythropoietin stimulating agents in the management of anemia of chronic kidney disease. Patient Prefer Adherence. 2008;2:195–200. doi: 10.2147/ppa.s2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perazella M.A., Khan S. Increased mortality in chronic kidney disease: a call to action. Am J Med Sci. 2006;331:150–153. doi: 10.1097/00000441-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Tonelli M., Wiebe N., Culleton B. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 36.US Renal Data System 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 1: chronic kidney disease in the United States. https://www.usrds.org/2016/download/v1_CKD_16.pdf Available at:
- 37.Buttel I., Voller K., Schneider C. Immunogenicity and its impact on benefit/risk considerations in the authorisation of biopharmaceuticals. Curr Drug Saf. 2010;5:287–292. doi: 10.2174/157488610792245993. [DOI] [PubMed] [Google Scholar]
- 38.Macdougall I.C., Roger S.D., de Francisco A. Antibody-mediated pure red cell aplasia in chronic kidney disease patients receiving erythropoiesis-stimulating agents: new insights. Kidney Int. 2012;81:727–732. doi: 10.1038/ki.2011.500. [DOI] [PubMed] [Google Scholar]
- 39.Porter S. Human immune response to recombinant human proteins. J Pharm Sci. 2001;90:1–11. doi: 10.1002/1520-6017(200101)90:1<1::aid-jps1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 40.QuintilesIMS The impact of biosimilar competition in Europe. https://ec.europa.eu/docsroom/documents/23102/attachments/1/translations/en/renditions/pdf Available at:
- 41.Borelli M., Paul D.P., 3rd, Skiba M. Renal dialysis and its financing. Hosp Top. 2016;94:33–38. doi: 10.1080/00185868.2016.1175203. [DOI] [PubMed] [Google Scholar]
- 42.Wish D., Johnson D., Wish J. Rebasing the Medicare payment for dialysis: rationale, challenges, and opportunities. Clin J Am Soc Nephrol. 2014;9:2195–2202. doi: 10.2215/CJN.03830414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.