Summary
Background
More than 70 million people worldwide are estimated to have hepatitis C virus (HCV) infection. Emerging evidence indicates an association between HCV and atherosclerotic cardiovascular disease. We aimed to determine the association between HCV and cardiovascular disease, and estimate the national, regional, and global burden of cardiovascular disease attributable to HCV.
Methods
For this systematic review and meta-analysis, we searched MEDLINE, Embase, Ovid Global Health, and Web of Science databases from inception to May 9, 2018, without language restrictions, for longitudinal studies that evaluated the risk ratio (RR) of cardiovascular disease in people with HCV compared with those without HCV. Two investigators independently reviewed and extracted data from published reports. The main outcome was cardiovascular disease, defined as hospital admission with, or mortality from, acute myocardial infarction or stroke. We calculated the pooled RR of cardiovascular disease associated with HCV using a random-effects model. Additionally, we calculated the population attributable fraction and disability-adjusted life-years (DALYs) from HCV-associated cardiovascular disease at the national, regional, and global level. We also used age-stratified and sex-stratified HCV prevalence estimates and cardiovascular DALYs for 100 countries to estimate country-level burden associated with HCV. This study is registered with PROSPERO, number CRD42018091857.
Findings
Our search identified 16 639 records, of which 36 studies were included for analysis, including 341 739 people with HCV. The pooled RR for cardiovascular disease was 1·28 (95% CI 1·18–1·39). Globally, 1·5 million (95% CI 0·9–2·1) DALYs per year were lost due to HCV-associated cardiovascular disease. Low-income and middle-income countries had the highest disease burden with south Asian, eastern European, north African, and Middle Eastern regions accounting for two-thirds of all HCV-associated cardiovascular DALYs.
Interpretation
HCV infection is associated with an increased risk of cardiovascular disease. The global burden of cardiovascular disease associated with HCV infection was responsible for 1·5 million DALYs, with the highest burden in low-income and middle-income countries.
Funding
British Heart Foundation and Wellcome Trust.
Introduction
Globally, more than 70 million people are estimated to have hepatitis C virus (HCV) infection.1 Prevalence of HCV is particularly high in the eastern Mediterranean region and Europe, where approximately 2·3% and 1·5% of the general population have HCV infection, respectively.1 In the USA, the estimated prevalence of past or current HCV infection is 1·4%, affecting 4·6 million people, of whom at least 3·5 million have active HCV infection (1% of the general population).2 The number of new incident cases of HCV infections in the USA has been increasing since 2010.3
After acute HCV infection, most patients develop chronic infection.4 Usually, these patients remain asymptomatic, with less than a third progressing to liver cirrhosis in the subsequent 20–30 years.4 Although mortality due to cirrhosis and hepatocellular carcinoma are well recognised long-term complications of chronic HCV infection,5, 6 patients with chronic infection are also at increased risk of non-liver-related mortality, including cancer and circulatory death.7
Atherosclerotic cardiovascular disease is the most common cause of death worldwide and the burden of disease is projected to rise substantially over the next few decades, particularly in low-income and middle-income countries (LMICs).8 HCV transmission is also projected to rise considerably in LMICs due to unsafe health-care practices and injection drug use.9, 10, 11 Published data12, 13, 14 suggest that the long period of chronic HCV infection might lead to the development of atherosclerotic cardiovascular disease because of derangements in metabolic pathways and chronic inflammation. However, the direction and strength of the association between HCV infection and cardiovascular disease remains uncertain.15, 16, 17, 18, 19
Research in context.
Evidence before this study
We searched PubMed from database inception to Jan 1, 2018, for systematic reviews and meta-analyses evaluating the association between hepatitis C virus (HCV) infection and atherosclerotic cardiovascular disease using the search terms “myocardial infarction”, “stroke”, “cerebrovascular disease”, “cardiovascular disease”, and “hepatitis C”. We found no studies that assessed the risk of cardiovascular disease or calculated the burden from all major atherosclerotic cardiovascular events associated with hepatitis C. Previous meta-analyses have evaluated the association between HCV infection and stroke and surrogate markers of subclinical atherosclerotic disease.
Added value of this study
To our knowledge, our study is the first meta-analysis to investigate the risk of major atherosclerotic cardiovascular disease in people with HCV infection and to estimate the burden of atherosclerotic cardiovascular disease attributed to HCV infection at the global, regional, and national level.
Implication of all the available evidence
Our findings show that people with HCV infection have a higher risk of cardiovascular disease than those without. The global burden of cardiovascular disease attributable to HCV accounted for a substantial number of disability-adjusted life-years in 2015, and the majority of the burden was borne by low-income and middle-income countries. These finding highlights the importance of public health strategies to eradicate HCV infection to reduce the burden of not only hepatic, but extrahepatic complications (such as cardiovascular disease), especially in regions with high HCV prevalence.
Our study aimed to determine the association between HCV infection and the risk of cardiovascular disease to establish the global burden of cardiovascular disease attributable to HCV.
Methods
Search strategy and selection criteria
We searched MEDLINE, EMBASE, Ovid Global Health, and Web of Science from database inception to May 9, 2018, for original peer-reviewed articles using the search terms “myocardial infarction”, “stroke”, “cerebrovascular disease”, “cardiovascular disease”, and “hepatitis C” with no language restrictions. Full search terms are in the appendix (pp 2, 3). Additionally, we manually searched relevant review articles and bibliographic reference lists of studies selected for inclusion in our meta-analysis.
We included all longitudinal studies (case-control studies, cohort studies, and randomised controlled trials) that reported risk ratios (RRs) for hospital admission due to atherosclerotic cardiovascular disease or cardiovascular mortality in people with HCV compared with people without HCV. When there were multiple publications using data from the same cohort, we selected the article that reported the longest follow-up period. Detailed full-text review and data extraction was done independently by at least two investigators (KKL, DS, RB, or MA) and any disagreements were resolved by a third investigator (ASVS). We contacted authors for additional data or clarification if required. This study was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (appendix pp 4, 5).20 The study protocol is available online.
For studies that stratified the study population according to the presence of HCV RNA viraemia, we used the RR estimates pertaining to individuals who were HCV RNA positive. We defined a cardiovascular event as hospital admission with, or mortality from, acute myocardial infarction or stroke. Studies that evaluated a composite of acute cardiovascular events that included myocardial infarction or stroke but were not exclusive to these conditions were also included. For studies that stratified stroke events into haemorrhagic and ischaemic strokes, we included only ischaemic strokes in the analysis because haemorrhagic strokes have distinct pathophysiological mechanisms that are unrelated to atherosclerosis.21
Data analysis
We extracted RR estimates comparing cardiovascular events in people with HCV versus those without HCV from published reports using a standardised data extraction sheet. We estimated pooled RRs with 95% CIs. Since this outcome was relatively uncommon, we pooled studies that reported odds ratio and RR. We also assumed independence between risk estimates for different endpoints reported within studies, consistent with our previous analysis.22 We did a subgroup analysis stratified by outcome, HIV co-infection, publication year, risk of bias, definition of outcome event, and geographical location.
Two independent investigators (KKL and DS) assessed individual studies for risk of bias, using the degree of adjustment for confounders as the primary domain, and any disagreements were adjudicated by a third investigator. Studies that had adjusted for age, sex, and at least one other confounder were classified as being at low risk of bias. Studies that adjusted for fewer confounders than this were classified as moderate or high risk: studies that adjusted for either age or sex without any other confounders were classified as moderate risk of bias and those that did not adjust for both age or sex were classified as high risk of bias. We did the subgroup analysis stratified by risk of bias.
We estimated the burden of cardiovascular disease attributable to HCV at the national, regional, and global level. We obtained 2015 global prevalence estimates of viraemic HCV (HCV RNA positive) for 100 countries from the Polaris Observatory, with estimates stratified by Global Burden of Disease region.23 These 100 countries represent more than 85% of the global population and where more than 89% of all HCV viral infections (HCV RNA positive) are estimated to occur worldwide.23 The national prevalence estimates obtained were age-specific and sex-specific. We obtained age-specific and sex-specific disability-adjusted life-year (DALY) estimates for cardiovascular disease (DALYs due to ischaemic heart disease and stroke) for all adults aged older than 20 years in 2015 from the Institute of Health Metrics and Evaluation.24 The extraction databases from the systematic review and the data from the Polaris Observatory and Institute of Health Metrics and Evaluation used to derive the pooled estimates and the burden estimates alongside the R code script are available online.
We estimated the population attributable risk fraction at the national, regional, and global level using the pooled RR for cardiovascular disease in patients with hepatitis C and the prevalence estimates of HCV. The population attributable fraction (PAF) for cardiovascular disease attributable to HCV was calculated as described previously:25, 26
We then used national, regional, and global level attributable fractions to calculate the burden as previously described (appendix pp 6–10):
We provided estimates of PAF and burden in 5-year age groups and presented data graphically using a linear model to interpolate the intervening years. We further provided burden estimates by income of nation stratified by high-income versus LMICs. National income status was defined according to the 2018 World Bank classification.27
We anticipated heterogeneity in the RRs across studies because of differences in study design, patient population, geographical location, statistical methods, and adjustment for confounders. We pooled RRs using a random effects model to account for within and between study heterogeneity. We assessed heterogeneity in the pooled meta-estimate of the RR using the I2 statistic. We assessed publication bias using visual inspection of funnel plots of the RR estimates and using Egger's regression test for asymmetry.28 We corrected for asymmetry using Duval and Tweedie's trim and fill method.29 Full statistical methods are in the appendix (pp 6–10). All analyses were done using R (version 3.4.1). A two-sided p value of less than 0·05 was considered to indicate statistical significance.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We identified 16 650 articles, of which 2270 were duplicates (figure 1). 343 full-text articles were assessed for eligibility. After full-text review, 36 studies,7, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 which provided 47 estimates, were included in our analyses (table). These studies included 341 739 people with HCV. 31 (86%) of 36 studies were done in North America, Europe, and east Asia. Only two studies30, 31 originated from LMICs. Most studies used the International Classification of Diseases coding or physician diagnosis to define the outcome events.
Figure 1.
Study selection
Table.
Baseline characteristics of studies included in the meta-analysis
| Cohort name | Country or region | Study type | Data source | Participants, n | Events, n | Men, n (%) | Mean age at baseline, years | Study period | Hepatitis C virus status | Outcome | Outcome definition | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heo et al, 201833 | .. | USA | Cohort study | Organ Procurement and Transplant Network | 2728 | 117 | 1996 (73%) | 50·9 | 2004–14 | Seropositive | Cardiovascular disease | Undefined |
| Alvaro-Meca et al, 201734 | .. | Spain | Case-control study | Spanish Minimum Basic Data Set | 4091 | 369 | 3248 (79%) | 45 | 1997–2013 | Seropositive | Stroke* | ICD-9 |
| Butt et al, 201735 | ERCHIVES | USA | Cohort study | Veterans Health Administration | 171 726 | 5949 | 171 726 (100%) | 54 | 2001–15 | Viraemic | Myocardial infarction | ICD-9 |
| Chew et al, 201736 | ERCHIVES | USA | Cohort study | Veterans Health Administration | 168 256 | 11 753 | 168 256 (100%) | 55 | 2001–14 | Seropositive | Cardiovascular disease | ICD-9 |
| Goodkin et al, 201730 | DOPPS | Multiple† | Cohort study | Hospital records | 76 689 | 6790 | 45 016 (59%) | 62·5 | 1996–2015 | Seropositive | Cardiovascular disease, myocardial infarction, stroke | Undefined |
| Kovari et al, 201737 | .. | Switzerland | Cohort study | Swiss HIV Cohort Study | 5006 | 143 | 3624 (72%) | 50 | 1994–2014 | Seropositive | Cardiovascular disease* | Physician diagnosis |
| Piazza et al, 201638 | .. | USA | Cohort study | Hospital records | 143 | 19 | 101 (71%) | 55 | 2005–10 | Undefined | Cardiovascular disease | Undefined |
| Fernandez-Montero et al, 201539 | .. | Spain | Cohort study | Hospital register | 1066 | 29 | 842 (79%) | 42·7 | 2004–15 | Viraemic | Cardiovascular disease† | Physician diagnosis |
| Tsai et al, 201540 | NHIRD–HCV | Taiwan | Cohort study | National Health Insurance Research Database | 69 915 | 848 | 35 936 (51%) | 54·7 | 1998–2008 | Undefined | Myocardial infarction | Undefined |
| Vajdic et al, 201541 | .. | Australia | Cohort study | Pharmaceutical Drugs of Addiction System | 29 571 | 122 | 20 403 (69%) | 26 | 1993–2007 | Seropositive | Cardiovascular disease | ICD-9, ICD-10 |
| Enger et al, 201442 | ORD | USA | Cohort study | Optum Research Database (insurance plans) | 90 931 | 534 | 56 740 (62%) | 49 | 2000–06 | Seropositive | Myocardial infarction, stroke | ICD-9 |
| Gillis et al, 201443 | OCS | Canada | Cohort study | Clinic register | 4152 | 167 | 3483 (84%) | 36 | 1995–2011 | Seropositive | Cardiovascular disease† | Physician diagnosis |
| Hsu et al, 201444 | .. | Taiwan | Cohort study | National Health Insurance Research Database | 7055 | 429 | 4599 (65%) | 54·9 | 2003–11 | Seropositive | Myocardial infarction, stroke | ICD-9 |
| Pothineni et al, 201445 | UAMS | USA | Cohort study | Enterprise Data Warehouse at University of Arkansas for Medical Sciences | 23 050 | 951 | 12 631 (55%) | 50·9 | 2001–13 | Viraemic | Cardiovascular disease | ICD-9 |
| Tripathi et al, 201446 | .. | USA | Cohort study | Medicaid | 13 632 | 1284 | 7661 (56%) | 38 | 1994–2011 | Seropositive | Cardiovascular disease | ICD-9 |
| Womack et al, 201447 | Veterans Aging Cohort Study–virtual cohort | USA | Cohort study | Veterans Health Administration, Medicare, Medicaid, and Quality Enhancement Research Initiative in ischaemic heart disease | 2187 | 86 | 0 | 43·6 | 2003–09 | Seropositive | Cardiovascular disease* | ICD-9 |
| Adinolfi et al, 201348 | .. | Italy | Case-control study | Hospital records | 820 | 123 | 524 (64%) | 76 | 2010–12 | Seropositive | Stroke | Physician diagnosis |
| Hsu et al, 201349 | LHID2000 | Taiwan | Cohort study | Longitudinal Health Insurance Database 2000 | 15 565 | NR | 8078 (52%) | Not reported | 2004–07 | Viraemic | Stroke | ICD-9 |
| Younossi et al, 201350 | NHANES III | USA | Cohort study | National Health and Nutrition Examination Survey | 8985 | NR | 4178 (46%) | Not reported | 1988–2006 | Viraemic | Cardiovascular disease | ICD-10 |
| Campbell et al, 201251 | .. | UK | Cohort study | Hospital records | 4068 | 32 | 4068 (100%) | 36·5 | 2004–09 | Seropositive | Cardiovascular disease* | Physician diagnosis |
| Carrieri et al, 201252 | APROCO-COPILOTE | France | Cohort study | Medical questionnaires | 1154 | 49 | 900 (78%) | 37·7 | 1997–2010 | Seropositive | Cardiovascular disease* | ICD-10 |
| Forde et al, 201253 | THIN | UK | Cohort study | General practice medical records | 76 477 | 264 | 46 727 (61%) | 38·6 | 1996–2008 | Undefined | Myocardial infarction | Read diagnostic code |
| Lee et al, 201254 | REVEAL–HCV | Taiwan | Cohort study | Questionnaires and interviews | 19 636 | 477 | 9523 (48%) | 47·6 | 1991–2008 | Viraemic | Cardiovascular disease | ICD-9 |
| Liao et al, 201255 | NHIRD | Taiwan | Cohort study | National Health Insurance Research Database | 20470 | 1981 | 10235 (50%) | 52 | 2002–08 | Viraemic | Stroke | ICD-9 |
| Freiberg et al, 201156 | Veterans Aging Cohort Study–virtual cohort | USA | Cohort study | Veterans Aging Cohort Study and Large Health Study of Veteran Enrollees | 8579 | 194 | 8579 (100%) | 48·1 | 2000–07 | Seropositive | Cardiovascular disease* | ICD-9 |
| Kristiansen et al, 201157 | .. | Norway | Cohort study | Department of Microbiology, University Hospital of North Norway | 1010 | 5 | 686 (68%) | 40 | 1990–2000 | Seropositive | Cardiovascular disease | ICD-10 |
| Ohsawa et al, 201158 | KAREN | Japan | Cohort study | KAREN cohort | 1077 | 194 | 682 (63%) | 60·4 | 2003–08 | Seropositive | Cardiovascular disease | ICD-10 |
| Bedimo et al, 201059 | HIV Clinical Care Registry | USA | Cohort study | Veterans Registry | 19 424 | 1146 | 18 938 (97%) | 46·2 | 1984–2004 | Viraemic | Myocardial infarction, stroke* | ICD-9 |
| Belloso et al, 201031 | LATINA | Brazil, Mexico | Cohort study | LATINA cohort | 160 | 40 | Not reported | Not reported | 1997–2007 | Seropositive | Cardiovascular disease* | Physician diagnosis |
| DAD Study Group, 201060 | DAD study | Europe, USA, Australia | Cohort study | DAD cohort | 21 815 | 517 | 16 143 (74%) | 38 | 1999–2007 | Seropositive | Myocardial infarction* | WHO MONICA Project |
| Lee et al, 201061 | .. | Taiwan | Cohort study | National Death Certification Registry | 23 665 | 22 | 11 879 (50%) | 47·1 | 1991–92 | Viraemic | Stroke | ICD-9 |
| Tsui et al, 200932 | The Heart and Soul study | USA | Cohort study | Veterans Administration electronic records | 981 | 151 | 803 (82%) | 66·3 | 2000–06 | Seropositive | Cardiovascular disease | Physician diagnosis |
| Guiltinan et al, 200862 | .. | USA | Cohort study | Blood Systems | 20 518 | 88 | 13 254 (65%) | Not reported | 1991–2002 | Seropositive | Cardiovascular disease | ICD-9 CM, ICD-10 CM |
| Kalantar-Zadeh et al, 200763 | .. | USA | Cohort study | DaVita outpatient dialysis database | 13 664 | NR | 7433 (54%) | 60·1 | 2001–04 | Seropositive | Cardiovascular disease | Undefined |
| Arcari et al, 200664 | .. | USA | Case-control study | Clinical registry | 75 834 | 292 | 47 775 (63%) | 40·2 | 1991–2000 | Seropositive | Myocardial infarction | ICD-9 |
| Amin et al, 20067 | .. | Australia | Cohort study | New South Wales Health Department Notifiable Diseases Database | 582 | 450 | 582 (100%) | 34 | 1990–2002 | Seropositive | Cardiovascular disease | ICD-9 and ICD-10 |
ICD=International Classification of Diseases. ERCHIVES=Electronically Retrieved Cohort of Hepatitis C Virus Infected Veterans. DOPPS=Dialysis Outcomes and Practice Patterns Study. NHIRD–HCV=National Health Insurance Research Database–Hepatitis C Virus. ORD=Optum Research Database. OCS=Ontario HIV Treatment Network Cohort Study. UAMS=University of Arkansas for Medical Sciences. LHID2000=Longitudinal Health Insurance Database 2000. NR=not reported. NHANES III=National Health and Nutrition Examination Survey III. APROCO-COPILOTE=Antiprotéases Cohorte. THIN=The Health Improvement Network. REVEAL–HCV=Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer–Hepatitis C Virus. DAD=Data Collection on Adverse Events of Anti-HIV Drugs. MONICA=Multinational Monitoring of Trends and Determinants in Cardiovascular Disease. CM=Clinical Modification.
Risk ratio reported for hepatitis C virus and HIV co-infection versus HIV infection only.
Australia, Belgium, Canada, mainland China, France, Germany, Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates, Italy, Japan, New Zealand, Spain, Russia, Sweden, Turkey, the UK, and the USA.
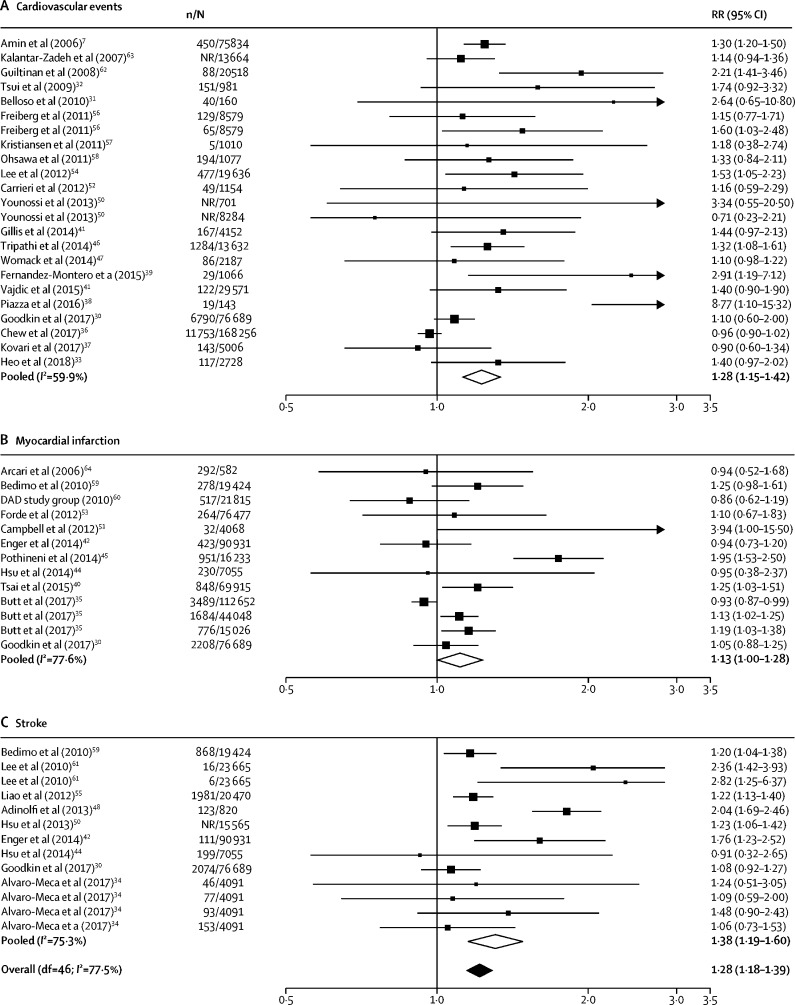
24 studies defined HCV infection as anti-HCV antibody seropositivity, nine studies used detectable HCV RNA levels, and three studies did not explicitly define their approach (table). Overall, the meta-analysis showed that individuals with HCV had a higher risk of cardiovascular disease than individuals without HCV (pooled RR 1·28, 95% CI 1·18–1·39; figure 2). When stratified by outcome the risk ratio was 1·13 (95% CI 1·00–1·28) for myocardial infarction, 1·38 (1·19–1·60) for stroke, and 1·39 (1·24–1·55) for cardiovascular mortality (appendix p 11). Individuals with HCV and HIV co-infection had a higher risk of cardiovascular disease than those with HIV mono-infection (RR 1·20, 1·09–1·32). Post-hoc analyses showed that the RR estimates from studies published before 2014, which was the median publication year, were marginally higher than those from studies published after 2014 (1·39 [1·25–1·54] vs 1·22 [1·11–1·34]). Studies that ascertained outcome with physician diagnosis had higher RRs than did those that used International Classification of Diseases codes (1·68 [1·24–2·29] vs 1·31 [1·20–1·42]). Nine studies in patients with HCV viraemia had marginally higher RRs than the overall pooled RR (1·32, 1·15–1·51). Nearly two-thirds of studies originated from the USA or Taiwan (21 [58%] of 36 studies). In the subgroup analysis, studies from these two countries had a similar pooled RR as those from the rest of the world (1·28 [1·16–1·40] vs 1·29 [1·12–1·48] respectively).
Figure 2.
Forest plots of pooled RRs for cardiovascular disease in people with hepatitis C virus versus people without
Pooled RRs for composite cardiovascular events (A), myocardial infarction (B), and stroke (C). n=number of events. N=number of participants. RR=risk ratio. NR=not reported. df=degrees of freedom. DAD=Data Collection on Adverse Events of Anti-HIV Drugs.
There was significant heterogeneity (I2=77·5%) and publication bias in the overall estimate (Egger's test p=0·003). Using the trim and fill method to correct for funnel plot asymmetry did not change the direction of effect but did attenuate the effect size (appendix p 20). 11 studies were at moderate or high risk of bias (appendix pp 12, 13). Compared with studies with a low risk of bias, those with moderate or high risk of bias had a similar pooled RR (1·30 [95% CI 1·10–1·55] for studies with moderate or high risk of bias vs 1·29 [1·19–1·40] for studies with low risk of bias).
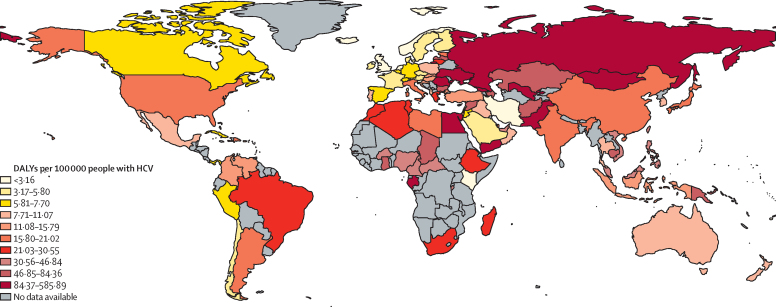
We estimated that in 2015, 1·5 million (95% CI 0·9–2·1) DALYs from cardiovascular disease were attributable to HCV, with marked geographical variation in the estimated burden. LMICs had the highest disease burden, with South Asia, eastern Europe, north Africa, and the Middle East accounting for nearly two-thirds of the global burden of cardiovascular disease attributable to HCV in 2015 (920·7 thousand DALYs; appendix p 14).
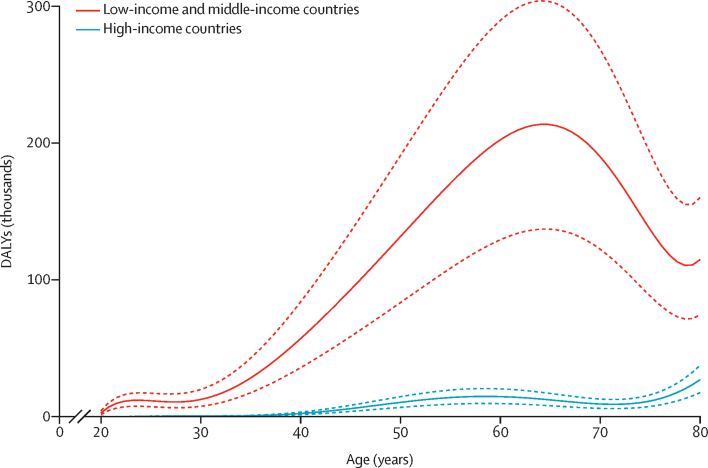
Of the 100 countries with available age-specific and sex-specific viraemic HCV prevalence estimates for 2015, the highest burden (ie, cardiovascular DALYs attributable to HCV) was in Ukraine, Mongolia, Gabon, and Egypt (figure 3; appendix pp 15–17). Worldwide, the PAF of cardiovascular disease attributable to HCV was highest in people aged 55–59 years (appendix p 18). DALYs from cardiovascular disease attributable to HCV was highest in people aged 70–74 years (appendix p 18). The burden of cardiovascular disease attributable to HCV was higher in LMICs than in high-income countries (1·4 million [95% CI 0·88–1·95] DALYs vs 0·1 million [95% CI 0·07–0·12] DALYs; figure 4; appendix p 19).
Figure 3.
DALYs per 100 000 people for cardiovascular disease attributable to HCV
Grey colour denotes regions for which no HCV prevalence data were available to estimate burden. HCV=hepatitis C virus. DALYs=disability-adjusted life-years.
Figure 4.
DALYs for cardiovascular disease attributable to hepatitis C virus
Solid lines show the central estimate and dashed lines show the 95% CI. DALYs=disability-adjusted life-years.
Discussion
In this systematic review and meta-analysis, we assessed the association between HCV and cardiovascular disease and estimated the global, regional, and national burden of cardiovascular disease attributable to HCV. We made several key observations. First, people with HCV have an increased risk of cardiovascular disease compared with those without HCV (RR 1·28). When stratified by type of cardiovascular event, the overall pooled estimate was higher for stroke than for myocardial infarction. Second, our pooled risk estimate was derived from 341 739 people with HCV infection included in 36 studies from 51 countries. Only two studies30, 31 reported findings from populations of LMICs, highlighting the paucity of data from these regions. Third, the most up-to-date annual global burden of cardiovascular disease attributable to HCV was 1·5 million DALYs. Most of this burden was concentrated in the 55–75 year age group, reflecting more premature development of cardiovascular disease in people with HCV. Fourth, considerable geographical variation was identified in the burden of cardiovascular disease attributable to HCV, with the highest burden observed in south Asia, eastern Europe, north Africa, and the Middle East. The majority of the burden was borne by LMICs rather than high-income countries. This observation is likely to reflect both a high prevalence of chronic hepatitis C in these regions and an increasing burden of cardiovascular disease.
Our analysis has several strengths. We included longitudinal studies that evaluated the association between HCV and hospital admissions with, or mortality from, cardiovascular disease. Furthermore, the endpoint of our analysis was major adverse cardiovascular events, which enabled accurate risk estimation and assessment of cardiovascular burden. Previous systematic reviews and meta-analyses,65, 66, 67 which included cross-sectional studies and studies that used surrogate endpoints that might not be fully reflective of a causal relationship, have reported divergent findings. We also analysed burden using age-specific, sex-specific, and country-specific cardiovascular burden and HCV prevalence estimates, allowing us to provide HCV attributable burden estimates for specific age groups, which could be useful for policy makers. Moreover, our estimates for HCV prevalence obtained from the Polaris Observatory23 reflect viraemia rather than just seropositivity alone, and the countries for which we had prevalence estimates accounted for over 89% of all chronic HCV infections globally. Our estimates for the PAF and subsequent HCV associated cardiovascular burden are therefore based on a high-risk population with active HCV infection, in whom both long-term hepatic and extrahepatic complications remain common.
This study has a number of limitations. Most studies included in this meta-analysis originated from high-income countries in North America and western Europe, but estimates were applied to all regions. This approach is commonly used in this type of analysis because of paucity of data from LMICs.68, 69 This highlights an ongoing need for research in these low-resource settings, in which the disease prevalence of HCV and cardiovascular disease is high, to improve the accuracy of the burden estimates in these regions. We also observed significant heterogeneity in our RR estimates. However, the direction of effect was consistent and robust across all subgroup analyses. The observed heterogeneity is likely to reflect the diverse patient population, viraemic status of the study population, differences in health-care systems, access to treatment, and geographical location of the studies pooled in this analysis. Many studies did not fully account for the competing risk of non-cardiovascular mortality, thus some methodological heterogeneity exists. Most people with HCV infection die from non-cardiovascular causes,7, 70 therefore this is an important competing risk that might distort the exposure–outcome association with cardiovascular disease. People with HIV and HCV co-infection have a higher risk for cardiovascular disease than those with HIV mono-infection. This increased risk highlights the importance of risk stratification in this patient population considering that people with HIV are twice as likely to have cardiovascular events than those without HIV.22 Although most studies evaluating the RR of cardiovascular disease adjusted for risk factors for cardiovascular disease, a substantial possibility of residual confounding remains. Furthermore, there was substantial publication bias in the literature, which might have influenced the risk estimates. However, there was little attenuation of the RRs when analysis was restricted to studies without moderate to high risk of bias or after accounting for publication bias using the trim and fill method. Additionally, we pooled RR estimates of myocardial infarction or stroke to estimate the PAF and combined this with the DALYs for ischaemic heart disease and cerebrovascular disease to estimate the burden of cardiovascular disease attributable to HCV. We were unable to estimate the burden of angina or peripheral artery disease attributable to HCV since these conditions are often diagnosed in the outpatient setting and are less likely to be captured by electronic health record systems. Therefore, it is possible that we have underestimated the cardiovascular burden associated with HCV. However, the 2010 Global Burden of Disease study24, 71 showed that angina and peripheral artery disease contributed a relatively small proportion of the overall cardiovascular disease burden. The studies included in this meta-analysis are likely to be exposed to a degree of outcome misclassification bias because most studies used routine diagnostic coding to define cardiovascular events rather than clinical adjudication. All of the included studies were observational studies, and thus we are unable to establish causality.
The underlying pathophysiological mechanism for the association between HCV and cardiovascular disease remains unclear.15 HCV infection has been associated with conditions such as type 2 diabetes, a well known cardiovascular risk factor.72 Evidence has emerged showing direct effects of HCV on the development of atherosclerosis,73 beyond that attributable to metabolic derangements alone. Chronic HCV infection results in a chronic state of immune stimulation and inflammation evidenced by increased circulating levels of proinflammatory cytokines, such as interleukin 6, tumour necrosis factor-α, C-reactive protein, and fibrinogen, all of which are associated with the development of atherosclerotic cardiovascular disease.32, 74, 75 Interferon-based antiviral treatments for HCV reduce markers of inflammation, endothelial dysfunction, and diabetes mellitus.76, 77, 78 Sustained viral response with direct-acting antivirals have also been associated with a lower risk of cardiovascular events.79 Whether eradication of HCV infection reduces future risk of adverse cardiovascular events should be further explored in randomised controlled trials of direct-acting antivirals to investigate this finding.
The link between HCV and cardiovascular disease has important implications for the formulation of health policies and resource allocation, particularly in regions with limited health-care resources, where chronic HCV infection remains prevalent and cardiovascular disease burden is increasing. Globally, prevalence of HCV is projected to increase substantially, particularly in LMICs, as a result of transmission via unsafe health-care related injections and injection drug use.9, 10, 11 Consequently, mortality due to hepatic and extrahepatic complications of HCV is likely to increase considerably if efforts to improve early testing and treatment are not implemented. A disproportionate burden of cardiovascular disease is borne by LMICs, where currently more than 80% of the global burden is concentrated.80 Our estimates suggest that more than 90% of the global cardiovascular burden attributable to HCV occurs in LMICs.
The introduction of direct-acting antiviral therapies with the ability to achieve sustained virological response in more than 90% of treated individuals should be a cause for optimism. These new therapeutic options enable the prevention of both hepatic and extrahepatic complications of HCV infection with a shorter duration of treatment and fewer adverse events than previous generations of antiviral therapies. However, at present, public health programmes and access to health-care services for people with HCV lags behind other comparable infectious diseases, such as HIV or malaria.1 The provision of direct-acting antiviral therapies in patients with HCV infection remains low on the global scale, with only one in 15 patients1 currently being treated, the majority of whom reside in high-income countries.81, 82 To have the greatest impact on HCV morbidity and mortality, the delivery of curative HCV treatment needs to be coupled with efficient health systems to provide chronic care services for patients with both hepatic and extrahepatic complications of HCV. Considering our study findings, investment in greater strategic integration and linkage of viral hepatitis services with other relevant services, including cardiovascular disease prevention, might be a cost-effective method of facilitating the prevention and management of concurrent major health conditions. These innovative approaches to health-care delivery might require further research to evaluate feasibility and efficacy in a real-world clinical setting.
People with HCV have a higher risk of developing cardiovascular disease than those without HCV. HCV accounted for 1·5 million DALYs due to cardiovascular disease worldwide in 2015, with the highest burden in South Asia, eastern Europe, north Africa, and the Middle East. Most of the disease burden is borne by LMICs, where HCV prevalence is projected to rise substantially. Our findings are of public health importance and could inform future research and health-care policies to improve risk stratification and treatment strategies aimed at reducing the combined global burden of HCV and extrahepatic sequelae such as cardiovascular disease.
For the extraction databases, data used to derive pooled estimates, burden estimates, and R code script see https://github.com/kk-lee/hcv
Acknowledgments
Acknowledgments
This study was supported by the British Heart Foundation through a Clinical Research Training Fellowship (FS/18/25/33454), Intermediate Clinical Research Fellowship (FS/19/17/34172), Senior Clinical Research Fellowship (FS/16/14/32023) and a Research Excellence Award (RE/18/5/34216). DAM is funded by a Wellcome Trust Intermediate Clinical Fellowship (201492/Z/16/Z). DEN is funded by a Senior Investigator Award (WT103782AIA). The Polaris Observatory is supported by the John C Martin Foundation.
Contributors
KKL, DS, and ASVS conceived and designed the study. KKL, DS, RB, MA, FS, SoB, and ASVS acquired the data. KKL and ASVS analysed and interpreted the data. KKL and ASVS drafted the initial manuscript. KKL, DS, RB, DEN, JSS, MHC, GSB, CTL, ShB, SK, SaB, HR, PRM, NLM, DAM, and ASVS critically reviewed the manuscript for intellectual content. All authors approved the final version of the report.
Declaration of interests
CTL reports grants from Gilead Sciences, outside the submitted work. SaB and HR report grants from Gilead and AbbVie, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO Global Hepatitis Report. 2017. https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=7364FB0B82B1E840F3FCE010F2D76EC3?sequence=1
- 2.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–1363. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Surveillance for viral hepatitis–United States. 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm
- 4.Grebely J, Page K, Sacks-Davis R. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59:109–120. doi: 10.1002/hep.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspinall EJ, Hutchinson SJ, Janjua NZ. Trends in mortality after diagnosis of hepatitis C virus infection: an international comparison and implications for monitoring the population impact of treatment. J Hepatol. 2015;62:269–277. doi: 10.1016/j.jhep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Grebely J, Dore GJ. What is killing people with hepatitis C virus infection? Semin Liver Dis. 2011;31:331–339. doi: 10.1055/s-0031-1297922. [DOI] [PubMed] [Google Scholar]
- 7.Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet. 2006;368:938–945. doi: 10.1016/S0140-6736(06)69374-4. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logez S, Soyolgerel G, Fields R, Luby S, Hutin Y. Rapid assessment of injection practices in Mongolia. Am J Infect Control. 2004;32:31–37. doi: 10.1016/j.ajic.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Mohsen A, Bernier A, LeFouler L. Hepatitis C virus acquisition among Egyptians: analysis of a 10-year surveillance of acute hepatitis C. Trop Med Int Health. 2015;20:89–97. doi: 10.1111/tmi.12410. [DOI] [PubMed] [Google Scholar]
- 11.Luby SP, Qamruddin K, Shah AA. The relationship between therapeutic injections and high prevalence of hepatitis C infection in Hafizabad, Pakistan. Epidemiol Infect. 1997;119:349–356. doi: 10.1017/s0950268897007899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golia E, Limongelli G, Natale F. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16:435. doi: 10.1007/s11883-014-0435-z. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 14.Welsch C, Efinger M, von Wagner M. Ongoing liver inflammation in patients with chronic hepatitis C and sustained virological response. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babiker A, Jeudy J, Kligerman S, Khambaty M, Shah A, Bagchi S. Risk of cardiovascular disease due to chronic hepatitis C infection: a review. J Clin Transl Hepatol. 2017;5:343–362. doi: 10.14218/JCTH.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46(suppl 5):S165–S173. doi: 10.1016/j.dld.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Goossens N, Negro F. Cardiovascular manifestations of hepatitis C virus. Clin Liver Dis. 2017;21:465–473. doi: 10.1016/j.cld.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Negro F. Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol. 2014;61(suppl 1):S69–S78. doi: 10.1016/j.jhep.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Momiyama Y, Ohmori R, Kato R, Taniguchi H, Nakamura H, Ohsuzu F. Lack of any association between persistent hepatitis B or C virus infection and coronary artery disease. Atherosclerosis. 2005;181:211–213. doi: 10.1016/j.atherosclerosis.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 21.Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. JAMA. 2019;321:1295–1303. doi: 10.1001/jama.2019.2413. [DOI] [PubMed] [Google Scholar]
- 22.Shah ASV, Stelzle D, Lee KK. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation. 2018;138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 24.Institute for Health Metrics and Evaluation GBD results tool. http://ghdx.healthdata.org/gbd-results-tool
- 25.Shah AS, Lee KK, McAllister DA. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350 doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AS, Langrish JP, Nair H. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Bank World Bank country and lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 30.Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol. 2017;12:287–297. doi: 10.2215/CJN.07940716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belloso WH, Orellana LC, Grinsztejn B. Analysis of serious non-AIDS events among HIV-infected adults at Latin American sites. HIV Med. 2010;11:554–564. doi: 10.1111/j.1468-1293.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Cardiol Fail. 2009;15:451–456. doi: 10.1016/j.cardfail.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heo NY, Mannalithara A, Kim D, Udompap P, Tan JC, Kim WR. Long-term patient and graft survival of kidney transplant recipients with hepatitis C virus infection in the United States. Transplantation. 2018;102:454–460. doi: 10.1097/TP.0000000000001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvaro-Meca A, Berenguer J, Diaz A. Stroke in HIV-infected individuals with and without HCV coinfection in Spain in the combination antiretroviral therapy era. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butt AA, Yan P, Chew KW. Risk of acute myocardial infarction among hepatitis C virus (HCV)-positive and HCV-negative men at various lipid levels: results from ERCHIVES. Clin Infect Dis. 2017;65:557–565. doi: 10.1093/cid/cix359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chew KW, Bhattacharya D, Horwich TB. Performance of the pooled cohort atherosclerotic cardiovascular disease risk score in hepatitis C virus-infected persons. J Viral Hepat. 2017;24:814–822. doi: 10.1111/jvh.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovari H, Rauch A, Kouyos R. Hepatitis C infection and the risk of non-liver-related morbidity and mortality in HIV-infected persons in the Swiss HIV Cohort Study. Clin Infect Dis. 2017;64:490–497. doi: 10.1093/cid/ciw809. [DOI] [PubMed] [Google Scholar]
- 38.Piazza NA, Singal AK. Frequency of cardiovascular events and effect on survival in liver transplant recipients for cirrhosis due to alcoholic or nonalcoholic steatohepatitis. Exp Clin Transplant. 2016;14:79–85. doi: 10.6002/ect.2015.0089. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Montero JV, Barreiro P, de Mendoza C, Labarga P, Soriano V. Hepatitis C virus coinfection independently increases the risk of cardiovascular disease in HIV-positive patients. J Viral Hepat. 2016;23:47–52. doi: 10.1111/jvh.12447. [DOI] [PubMed] [Google Scholar]
- 40.Tsai MS, Hsu YC, Yu PC, Lin CL, Kao CH. Long-term risk of acute coronary syndrome in hepatitis C virus infected patients without antiviral treatment: a cohort study from an endemic area. Int J Cardiol. 2015;181:27–29. doi: 10.1016/j.ijcard.2014.11.200. [DOI] [PubMed] [Google Scholar]
- 41.Vajdic CM, Marashi Pour S, Olivier J. The impact of blood-borne viruses on cause-specific mortality among opioid dependent people: an Australian population-based cohort study. Drug Alcohol Depend. 2015;152:264–271. doi: 10.1016/j.drugalcdep.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Enger C, Forssen UM, Bennett D, Theodore D, Shantakumar S, McAfee A. Thromboembolic events among patients with hepatitis C virus infection and cirrhosis: a matched-cohort study. Adv Ther. 2014;31:891–903. doi: 10.1007/s12325-014-0138-4. [DOI] [PubMed] [Google Scholar]
- 43.Gillis J, Smieja M, Cescon A. Risk of cardiovascular disease associated with HCV and HBV coinfection among antiretroviral-treated HIV-infected individuals. Antivir Ther. 2014;19:309–317. doi: 10.3851/IMP2724. [DOI] [PubMed] [Google Scholar]
- 44.Hsu YC, Lin JT, Ho HJ. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 45.Pothineni NV, Delongchamp R, Vallurupalli S. Impact of hepatitis C seropositivity on the risk of coronary heart disease events. Am J Cardiol. 2014;114:1841–1845. doi: 10.1016/j.amjcard.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tripathi A, Liese AD, Winniford MD. Impact of clinical and therapeutic factors on incident cardiovascular and cerebrovascular events in a population-based cohort of HIV-infected and non-HIV-infected adults. Clin Cardiol. 2014;37:517–522. doi: 10.1002/clc.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Womack JA, Chang CC, So-Armah KA. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adinolfi LE, Restivo L, Guerrera B. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231:22–26. doi: 10.1016/j.atherosclerosis.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Hsu CS, Kao JH, Chao YC. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther. 2013;38:415–423. doi: 10.1111/apt.12391. [DOI] [PubMed] [Google Scholar]
- 50.Younossi ZM, Zheng L, Stepanova M, Venkatesan C, Mir HM. Moderate, excessive or heavy alcohol consumption: each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2013;37:703–709. doi: 10.1111/apt.12265. [DOI] [PubMed] [Google Scholar]
- 51.Campbell LJ, Desai M, Hegazi A. Renal impairment is associated with coronary heart disease in HIV-positive men. HIV Clin Trials. 2012;13:343–349. doi: 10.1310/hct1306-343. [DOI] [PubMed] [Google Scholar]
- 52.Carrieri MP, Protopopescu C, Le Moing V. Impact of immunodepression and moderate alcohol consumption on coronary and other arterial disease events in an 11-year cohort of HIV-infected patients on antiretroviral therapy. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forde KA, Haynes K, Troxel AB. Risk of myocardial infarction associated with chronic hepatitis C virus infection: a population-based cohort study. J Viral Hepat. 2012;19:271–277. doi: 10.1111/j.1365-2893.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee MH, Yang HI, Lu SN. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 55.Liao CC, Su TC, Sung FC, Chou WH, Chen TL. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freiberg MS, Chang CC, Skanderson M. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4:425–432. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kristiansen MG, Lochen ML, Gutteberg TJ, Mortensen L, Eriksen BO, Florholmen J. Total and cause-specific mortality rates in a prospective study of community-acquired hepatitis C virus infection in northern Norway. J Viral Hepat. 2011;18:237–244. doi: 10.1111/j.1365-2893.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 58.Ohsawa M, Kato K, Tanno K. Seropositivity for anti-HCV core antigen is independently associated with increased all-cause, cardiovascular, and liver disease-related mortality in hemodialysis patients. J Epidemiol. 2011;21:491–499. doi: 10.2188/jea.JE20100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bedimo RJ, Westfall AO, Mugavero MJ, Drechsler H, Khanna N, Saag MS. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11:462–468. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 60.Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. Weber R, Sabin C. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D Cohort Study. Antivir Ther. 2010;15:1077–1086. doi: 10.3851/IMP1681. [DOI] [PubMed] [Google Scholar]
- 61.Lee MH, Yang HI, Wang CH. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894–2900. doi: 10.1161/STROKEAHA.110.598136. [DOI] [PubMed] [Google Scholar]
- 62.Guiltinan AM, Kaidarova Z, Custer B. Increased all-cause, liver, and cardiac mortality among hepatitis C virus-seropositive blood donors. Am J Epidemiol. 2008;167:743–750. doi: 10.1093/aje/kwm370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584–1593. doi: 10.1681/ASN.2006070736. [DOI] [PubMed] [Google Scholar]
- 64.Arcari CM, Nelson KE, Netski DM, Nieto FJ, Gaydos CA. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis. 2006;43:e53–e56. doi: 10.1086/507031. [DOI] [PubMed] [Google Scholar]
- 65.Petta S, Maida M, Macaluso FS. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational studies. Gastroenterology. 2016;150:145–155. doi: 10.1053/j.gastro.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Ambrosino P, Lupoli R, Di Minno A. The risk of coronary artery disease and cerebrovascular disease in patients with hepatitis C: a systematic review and meta-analysis. Int J Cardiol. 2016;221:746–754. doi: 10.1016/j.ijcard.2016.06.337. [DOI] [PubMed] [Google Scholar]
- 67.Wong RJ, Kanwal F, Younossi ZM, Ahmed A. Hepatitis C virus infection and coronary artery disease risk: a systematic review of the literature. Dig Dis Sci. 2014;59:1586–1593. doi: 10.1007/s10620-014-3222-3. [DOI] [PubMed] [Google Scholar]
- 68.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forouzanfar MH, Liu P, Roth GA. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 70.Mahajan R, Xing J, Liu SJ. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006–2010. Clin Infect Dis. 2014;58:1055–1061. doi: 10.1093/cid/ciu077. [DOI] [PubMed] [Google Scholar]
- 71.Moran AE, Forouzanfar MH, Roth GA. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mostafa A, Mohamed MK, Saeed M. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135–1140. doi: 10.1136/gut.2009.202317. [DOI] [PubMed] [Google Scholar]
- 74.Riordan SM, Skinner NA, Kurtovic J. Toll-like receptor expression in chronic hepatitis C: correlation with pro-inflammatory cytokine levels and liver injury. Inflamm Res. 2006;55:279–285. doi: 10.1007/s00011-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 75.Roed T, Kristoffersen US, Knudsen A. Increased prevalence of coronary artery disease risk markers in patients with chronic hepatitis C—a cross-sectional study. Vasc Health Risk Manag. 2014;10:55–62. doi: 10.2147/VHRM.S53557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chew KW, Hua L, Bhattacharya D. The effect of hepatitis C virologic clearance on cardiovascular disease biomarkers in human immunodeficiency virus/hepatitis C virus coinfection. Open Forum Infect Dis. 2014;1 doi: 10.1093/ofid/ofu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pateria P, Jeffrey GP, MacQuillan G. The association between chronic hepatitis C infection and cardiovascular risk. Intern Med J. 2016;46:63–70. doi: 10.1111/imj.12936. [DOI] [PubMed] [Google Scholar]
- 78.Berenguer J, Rodriguez-Castellano E, Carrero A. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology. 2017;66:344–356. doi: 10.1002/hep.29071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nahon P, Bourcier V, Layese R. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017;152:142–156. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 80.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.WHO Progress report on access to hepatitis C treatment. 2018. https://apps.who.int/iris/bitstream/handle/10665/260445/WHO-CDS-HIV-18.4-eng.pdf?sequence=1
- 82.Vutien P, Jin M, Le MH. Regional differences in treatment rates for patients with chronic hepatitis C infection: systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.