Abstract
Background
In patients of various ages undergoing mechanical ventilation (MV), it has been observed that positions other than the standard supine position, such as the prone position, may improve respiratory parameters. The benefits of these positions have not been clearly defined for critically ill newborns receiving MV.
This is an update of a review first published in 2005 and last updated in 2013.
Objectives
Primary objective
To assess the effects of different positioning of newborn infants receiving MV (supine vs prone, lateral decubitus or quarter turn from prone) in improving short‐term respiratory outcomes.
Secondary objective
To assess the effects of different positioning of newborn infants receiving MV on mortality and neuromotor and developmental outcomes over the long term, and on other complications of prematurity.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8), MEDLINE via PubMed (1966 to 22 August 2016), Embase (1980 to 22 August 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 22 August 2016). We also searched clinical trials databases, conference proceedings and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised and quasi‐randomised clinical trials comparing different positions in newborns receiving mechanical ventilation.
Data collection and analysis
Three unblinded review authors independently assessed trials for inclusion in the review and extracted study data. We used standard methodological procedures as expected by The Cochrane Collaboration and assessed the quality of the evidence using the GRADE approach. If the meta‐analysis was not appropriate owing to substantial clinical heterogeneity between trials, we presented review findings in narrative format.
Main results
We included in this review 19 trials involving 516 participants. Seven of the included studies (N = 222) had not been evaluated in the previous review. Investigators compared several positions: prone versus supine, prone alternant versus supine, prone versus lateral right, lateral right versus supine, lateral left versus supine, lateral alternant versus supine, lateral right versus lateral left, quarter turn from prone versus supine, quarter turn from prone versus prone and good lung dependent versus good lung uppermost.
Apart from two studies that compared lateral alternant versus supine, one comparing lateral right versus supine and two comparing prone or prone alternant versus the supine position, all included studies had a cross‐over design. In five studies, infants were ventilated with continuous positive airway pressure (CPAP); in the other studies, infants were treated with conventional ventilation (CV).
Risks of bias did not differ substantially for different comparisons and outcomes. This update detects a moderate to high grade of inconsistency, similar to previous versions. However, for the analysed outcomes, the direction of effect was the same in all studies. Therefore, we consider that this inconsistency had little effect on the conclusions of the meta‐analysis. When comparing prone versus supine position, we observed an increase in arterial oxygen tension (PO2) in the prone position (mean difference (MD) 5.49 mmHg, 95% confidence interval (CI) 2.92 to 8.05 mmHg; three trials; 116 participants; I2= 0). When percent haemoglobin oxygen saturation was measured with pulse oximetry (SpO2), improvement in the prone position was between 1.13% and 3.24% (typical effect based on nine trials with 154 participants; I2= 89%). The subgroup ventilated with CPAP (three trials; 59 participants) showed a trend towards improving SpO2 in the prone position compared with the supine position, although the mean difference (1.91%) was not significant (95% CI ‐1.14 to 4.97) and heterogeneity was extreme (I2= 95%).
Sensitivity analyses restricted to studies with low risk of selection bias showed homogeneous results and verified a small but significant effect (MD 0.64, 95% CI 0.26 to 1.02; four trials; 92 participants; I2= 0).
We also noted a slight improvement in the number of episodes of desaturation; it was not possible to establish whether this effect continued once the intervention was stopped. Investigators studied few adverse effects from the interventions in sufficient detail. Two studies analysed tracheal cultures of neonates after five days on MV, reporting lower bacterial colonisation in the alternating lateral position than in the supine posture. Other effects ‐ positive or negative ‐ cannot be excluded in light of the relatively small numbers of neonates studied.
Authors' conclusions
This update of our last review in 2013 supports previous conclusions. Evidence of low to moderate quality favours the prone position for slightly improved oxygenation in neonates undergoing mechanical ventilation. However, we found no evidence to suggest that particular body positions during mechanical ventilation of the neonate are effective in producing sustained and clinically relevant improvement.
Plain language summary
Best position for newborns who need assisted ventilation
Review question: For newborn infants who need assisted ventilation, can changing the infant's body position improve breathing or other clinical outcomes including survival?
Background: Newborns admitted to an intensive care unit often need help breathing (mechanical ventilation). This support is generally provided by a device placed inside the newborn's nose or mouth (which sometimes reaches the trachea), through which different pressures and concentrations of oxygen are sent. The usual practice is to position the newborn in supine (face‐up) position during ventilation. However, it is not certain whether other positions, for example, “face‐down” (prone position), could be more advantageous for breathing or other pursuits, including survival. This is an update of previously published reviews.
Search date: The evidence is up to date as of August 2016.
Study characteristics: We included in this review 19 trials involving 516 participants. Comparisons included supine position versus prone and different lateral positions (right, left, alternant or quarter for prone). The outcome most often reported in these studies was change in oxygenation.
Key results: We found no clear evidence that particular body positions in newborn babies who need assisted ventilation are effective in producing relevant and sustained improvement. However, putting infants who receive assisted ventilation in the face‐down (prone) position for a short time slightly improves levels of oxygen in the blood (evidence of moderate quality), and these infants undergo fewer episodes of poor oxygenation (evidence of low quality).
Researchers described no adverse effects for any of the positions compared, although studies did not last long enough for investigators to detect all possible effects. What's more, most of the babies participating in the studies were placed in alternate positions. For this reason, medium‐ or long‐term adverse effects cannot be attributed to a given position.
Quality of the evidence: Confidence in review conclusions depends on the characteristics of included studies such as risk of bias (design limitations), consistency (heterogeneity across studies), precision (small confidence interval) and directness (same effect), and requires that all included studies were published independently of their outcomes.
The quality of evidence for these outcomes allows us to have very low to moderate confidence in our conclusions.
Summary of findings
Background
Description of the condition
Mechanical ventilation (MV) is often necessary when ill newborns are treated, particularly when they are preterm. Although it is usually given for a short time, MV occasionally may be necessary for a long period. Mechanical ventilation has been associated with short‐term complications and impaired long‐term respiratory and developmental outcomes (Bancalari 2001; Singer 1997; Walsh 2005), and MV of longer duration is associated with increased risk of complications.
Description of the intervention
Different methods of respiratory support, including high‐frequency ventilation, conventional ventilation (CV) with all its variants and continuous positive airway pressure (CPAP), have been developed with the aim of improving the effectiveness of assisted ventilation and minimising its adverse effects (Brown 2011; Cools 2009; Henderson‐Smart 2009; Wheeler 2010). However, it is uncertain whether the position of the infant while on MV has an impact on clinical outcomes.
How the intervention might work
Positions primarily used in MV are supine, prone, quarter turn from prone and lateral decubitus. Although it is not known which is the best way to position the newborn, the current trend involves keeping ventilated infants in a supine position, mainly for ease of observation and handling of the infant. These patients require frequent monitoring and intervention, and they may need many catheters and tubes (including urinary, umbilical and other vascular catheters and drainage tubes).
Why it is important to do this review
Studies performed on other types of patients suggest that the change from supine to other positions, specifically to prone, can convey some advantages. For example, several studies in adults and children on MV, mainly for acute respiratory failure (ARF) and acute respiratory distress syndrome (ARDS), have reported transitory benefits of the prone position for oxygenation (Chatte 1997; Curley 2000; Douglas 1977; Fridrich 1996; Gattinoni 2001; Gattinoni 2010; Piehl 1976).
Other studies in newborn infants not needing assisted ventilation have noted some benefit of positions other than the traditional supine position, especially the prone position. Observed benefits included reduction of obstructive apnoea (Bhat 2006) and improvement in oxygenation (Kishan 1981; Martin 1979; Schwartz 1975) and in other functional respiratory parameters (Adams 1994; Fox 1993; Itakura 1998; Masterson 1987; Wolfson 1992). It has been suggested that non‐supine positions could provide additional benefit regarding improved feeding, gastric emptying and suctioning (Hewitt 1976; Mizuno 2000; Yu 1975), as well as increased sleep duration (Bhat 2006) and enhancement of some aspects of development during the first months of life (Davis 1998; Dewey 1998; Jantz 1997; Ratliff‐Schaub 2001; Visscher 1998). In addition, postural changes could help to prevent motor abnormalities. These interventions are easy to perform and carry virtually no economic costs. In light of these results, it is of great interest to know whether placing infants in positions other than supine during MV could entail any clinical benefit.
Objectives
Primary objective
To assess the effects of different positioning of newborn infants receiving MV (supine vs prone, lateral decubitus or quarter turn from prone) in improving short‐term respiratory outcomes.
Secondary objective
To assess the effects of different positioning of newborn infants receiving MV on mortality and neuromotor and developmental outcomes over the long term, and on other complications of prematurity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised trials.
Types of participants
Term and preterm neonates requiring any type of positive‐pressure MV including CPAP.
Types of interventions
Placing infants in a supine position compared with placing them in a prone or lateral decubitus position or undertaking a strategy of regular position change. Studies that also evaluated concomitant therapeutic interventions were excluded if the effect of infant positioning could not be assessed independently of the effect of the second intervention.
Types of outcome measures
Primary outcomes
Oxygenation during MV, measured by arterial oxygen tension (PO2), transcutaneous oxygen method (tcPO2), oxy‐haemoglobin saturation measured by a pulse oximeter (SpO2), oxy‐haemoglobin saturation measured by a pulse oximeter/fraction of inspired oxygen ratio (SpO2/FiO2) or arterial oxygen tension/fraction of inspired oxygen ratio (PO2/FiO2)
Decrease in characteristics of ventilator: peak inspiratory pressure or mean airway pressure (cm H2O) and fraction of inspiratory oxygen (FiO2)
Short‐term pulmonary complications: pneumothorax, pulmonary interstitial emphysema (PIE) or ventilator‐associated pneumonia (VAP)
Duration of ventilator support
Bronchopulmonary dysplasia (BPD) or chronic lung disease (CLD) at 28 days after birth or at 36 weeks' postmenstrual age, respectively
Changes in carbon dioxide tension (PCO2)
Changes on pulmonary mechanics: tidal volume (TV) or minute ventilation
Short‐term complications (accidental extubation; dislodgement of central catheter, urinary or drainage tube)
Secondary outcomes
Periventricular or intraventricular haemorrhage
Gastrointestinal or feeding problems: necrotizing enterocolitis (NEC), intolerance of enteral feeding or gastric fluid aspiration (pepsin in tracheal aspirate)
Days of stay in neonatal intensive care unit (NICU)
Days of stay in hospital
Neonatal mortality (death during first 28 days of life)
Infant mortality (death during first year of life)
Long‐term neurodevelopmental outcomes at age two years: rates of cerebral palsy as assessed by physician, developmental delay (i.e. intelligence quotient (IQ) < 2 standard deviations) as determined by validated assessment tools (e.g. Stanford‐Binet Intelligence Scale) or sensory impairment
Cutaneous and joint problems (i.e. dependent oedema, pressure ulcers of the skin, joint contractures or ankylosis)
Bacterial colonisation of the endotracheal tube
Search methods for identification of studies
Electronic searches
For the 2016 update, we identified randomised controlled trials (RCTs), quasi‐randomised controlled trials and systematic reviews by electronically searching the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8), in The Cochrane Library.
MEDLINE via PubMed (from December 2012 to 22 August 2016).
Embase (from December 2012 to 22 August 2016).
Cumulative Index to Nursing and Allied Health Literature (CINAHL; from December 2012 to 22 August 2016).
We followed the search strategy developed by the Cochrane Neonatal Review Group, using free text words and medical subject headings (MeSH) (Furlan 2009), and we applied no language restrictions. Review authors performed the electronic database search independently but did not conduct a search for studies published before December 2012 because the previous Cochrane review included these studies (see descriptions in Appendix 1 and Appendix 2).
Searching other resources
Review authors performed an electronic search of published abstract books from meetings of the Pediatric Academic Societies (PAS), the Perinatal Society of Australia and New Zealand (PSANZ) and the European Society of Pediatric Research (ESPR), held from December 2012 to September 2015.
We sought additional citations using the references in articles retrieved from our searches. We contacted subject experts to ask them to identify unpublished and ongoing studies. We contacted authors of published trials to request clarification or additional information. Review authors independently screened candidate articles to check their eligibility for inclusion in the review. We also searched clinical trials registries (clinicaltrials.gov; controlled‐trials.com; International Clinical Trials Registry Platform (ICTRP) of the World Health Organization (WHO)) up to 22 August 2016 for ongoing and recently completed trials.
Data collection and analysis
We employed the standard methods of the Cochrane Neonatal Review Group in creating this update.
Selection of studies
Three review authors independently assessed retrieved articles for eligibility and resolved discrepancies by discussion and consensus.
Data extraction and management
Three unblinded review authors independently assessed trials for inclusion in the review without prior consideration of study results. We resolved discrepancies by discussion between all review authors. We evaluated only full papers and applied no language restrictions. We contacted authors of individual trials to request missing information and clarification of published data, when necessary. We extracted the following data: study design (RCT), study characteristics (e.g. recruitment modality, source of funding, risk of bias), participant characteristics (e.g. number of participants, age, gender), description of experimental and control interventions, co‐interventions, duration of follow‐up, types of outcomes assessed and results and conclusions of trial authors. All review authors extracted data independently, using prepared data extraction forms. We extracted data from graphs, if necessary, and summarised key findings in a narrative format.
Assessment of risk of bias in included studies
We used the standard methods of the Cochrane Neonatal Review Group and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), in particular, analysis of randomised cross‐over trials. Review authors independently reviewed the methodological quality of each trial.
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions, as detailed below, and resolved disagreements by discussion. We included this information in the Characteristics of included studies table. In addition, for updates in 2006, 2012 and 2015, we evaluated the following issues and included this information in the risk of bias tables (Figure 1 and Figure 2).
1.
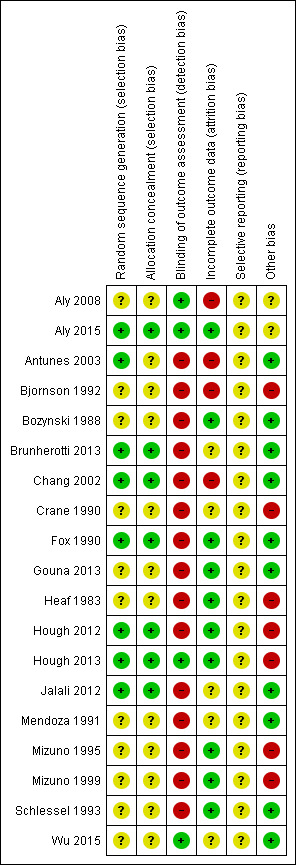
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
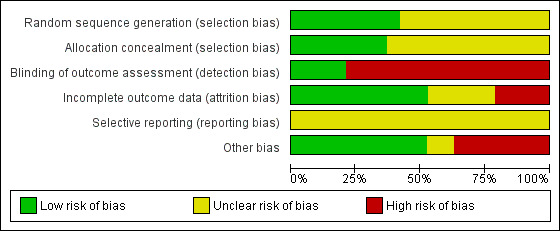
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Random sequence generation (checking for possible selection bias)
For each included study, we assessed whether the method used to generate the allocation sequence was described in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed these methods as: • low risk (any truly random process, e.g. random number table, computer random number generator); • high risk (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or • unclear risk.
Allocation concealment (checking for possible selection bias)
For each included study, we assessed whether the method used to conceal the allocation sequence was described in sufficient detail and determined whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed these methods as: • low risk of bias (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes); • high risk of bias (e.g. open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or • unclear risk of bias.
Blinding of participants, personnel and outcome assessors (checking for possible performance bias and detection bias)
We described for each included study the methods used, if any, to blind study participants and personnel and outcome assessors from knowledge of which intervention each participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes and classes of outcomes.
We assessed these methods as:
• low, high or unclear risk of bias for participants or personnel; and • low, high or unclear risk of bias for outcome assessors.
Incomplete outcome data (checking for possible attrition bias due to the quantity, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, completeness of data, including attrition and exclusion from analysis. We stated whether attrition and exclusions were reported and numbers included in the analysis at each stage (compared with the total number of randomised participants), whether reasons for attrition or exclusion were reported and whether missing data were balanced across groups or were related to outcomes.
We would re‐include missing data in our analyses when sufficient information was reported or could be supplied by trial authors.
We assessed these methods as:
• low risk of bias (e.g. no missing outcome data, missing outcome data balanced across groups); • high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups, ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or • unclear risk of bias.
Selective outcome reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed these methods as:
• low risk of bias (when it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported); • high risk of bias (when not all the study’s prespecified outcomes had been reported, when one or more of the reported primary outcomes were not prespecified, when outcomes of interest were reported incompletely and so could not be used and when the study failed to include results of a key outcome that would have been expected to have been reported); or • unclear risk of bias.
Other sources of bias (checking for bias due to problems not covered by those discussed above)
We described for each included study any important concerns that we had about other possible sources of bias. In the cross‐over design, bias based on the possibility of a ‘carry‐over’ effect of treatment from one period to the next is of particular interest, along with results from both periods.
We assessed whether each study was free of other problems that could put it at risk of bias.
• Low risk of other bias. • High risk of other bias. • Unclear whether risk of other bias was present.
Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the types of bias discussed above, we assessed the likely magnitude and direction of the bias, and whether we considered it likely to have an impact on review findings.
Measures of treatment effect
Continuous data
For continuous outcomes, we expressed treatment effect as mean difference (MD) and its calculated standard deviation (SD).
Dichotomous data
For dichotomous outcomes, we calculated Becker‐Balagtas odds ratios (ORs).
Unit of analysis issues
We analysed cross‐over studies by computing design‐adjusted effect measures according to the methods outlined in Elbourne 2002. Design‐adjusted effect measures take into account the correlation between repeated observations of participants, and thus provide reliable adjusted standard deviations for effect measures. A necessary imputation of the method was the degree of existing correlation between measures taken on the same individual.
For continuous measures, we sought Pearson correlation coefficient values for all included studies, presented directly or indirectly through individual participant data, paired t test values and estimated individual participant data extracted from a graph with the graph digitiser application Getdata (http://www.getdata‐graph‐digitizer.com/). The only studies for which we could estimate a correlation coefficient were Fox 1990 (individual data presented for partial pressure of oxygen), Chang 2002 (paired t test values) and Bjornson 1992 and Crane 1990 (individual data estimated from a graph in both cases). For remaining studies, we applied the correlation coefficient of Fox 1990 because we considered it to be the most reliable estimation.
For the dichotomous variable number of participants with desaturation, estimating the degree of correlation required estimating the number of participants presenting with the outcome of interest under both treatments. In the only cross‐over trials reporting this outcome (Chang 2002), desaturation occurred in as few as five cases in one of the treatment groups. We considered two scenarios: one of the lowest possible correlation between treatments (no participants desaturated under both treatments), and another of the highest correlation assumed (five participants desaturated under both treatments). Results were the same for both scenarios.
Dealing with missing data
In the overall assessment of treatment effect, we performed sensitivity analysis to explore the impact of including studies with high levels of missing data. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include in the analyses all participants randomised to each group). The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We evaluated the presence of heterogeneity within meta‐analyses by using the Cochran Q test and the I² statistic (Higgins 2003). The I² thresholds for heterogeneity include < 25% none, 25% to 49% low, 50% to 74% moderate and 75%+ high. The P value of interest for the Q test (which is quite conservative) is P < 0.1. In the presence of heterogeneity that acted as a trigger in the meta‐analysis (I² > 50% or 75%), we investigated possible sources of heterogeneity through subgroup and sensitivity analyses. Possible sources of heterogeneity examined included differences in types of ventilation received, population (gestational age, birth weight, cointerventions), year in which the trial took place, sample size, control of the 'carry‐over' effect in cross‐over designs and quality of the study.
Assessment of reporting biases
Two review authors independently assessed each included study for possible reporting biases. We were unable to investigate publication bias using funnel plots, as we included fewer than 10 studies in our analyses of outcomes.
We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate this. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias.
Data synthesis
We used Review Manager 5.3 software for statistical analysis. We used the inverse variance method to combine adjusted effect sizes computed for cross‐over trials and usual effect sizes computed for parallel trials. We performed all meta‐analyses using a random‐effects model. If meta‐analysis was not possible, we presented results in a narrative format.
Assessment of the quality of the evidence
We presented the overall quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011; Schünemann 2013). We created the 'Summary of findings' table using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This table provides essential information about the best estimate of the magnitude of effect, in relative terms and absolute differences, for each relevant comparison of alternative management strategies, numbers of participants and studies addressing each important outcome and level of confidence in effect estimates for each outcome. For this update, two review authors independently estimated the quality of evidence for outcomes identified as critical or important in clinical decision making for the comparison 'prone position versus supine or lateral position'.
Mortality: neonatal mortality (death during first 28 days of life) or infant mortality (death during first year of life).
Long‐term complications: long‐term neurodevelopmental outcomes at age two years or BPD or chronic lung disease CLD.
Short‐term complications: pulmonary complications (defined as pneumothorax, PIE or VAP) or general complications (defined as accidental extubation or dislodgement of central catheters, urinary or drainage tube).
Haemorrhage: periventricular or intraventricular haemorrhage.
Oxygenation: measured by PO2 or SpO2.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses on the basis of:
gestational age: 37 or more completed weeks and less than 37 weeks;
birth weight: ≤ 1500 grams and > 1500 grams; and
type of ventilator support: continuous ventilation (VC), synchronised intermittent mandatory ventilation (SIMV), high‐frequency oscillation ventilation (HFOV), high‐frequency jet ventilation (HFJV) and CPAP.
We assessed subgroup differences by performing interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses by quoting the Chi² statistic, the P value and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore effects of adequacy of allocation concealment and other risk of bias components, year in which the trial was carried out, sample size and study quality. Furthermore, we planned sensitivity analyses to explore the quality of evidence through analysis of cross‐over studies when the wash‐out period was not adequately described, but data were insufficient. We tested the robustness of results by repeating different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
Results of the search
See Figure 3 (flow diagram). The updated search identified 28 additional references. Seven studies (Aly 2015; Brunherotti 2013; Gouna 2013; Hough 2012; Hough 2013; Jalali 2012; Wu 2015) verified the inclusion criteria. Another study (Montgomery 2014) contained the mixture of patients included in Hough 2012 and Hough 2013, so it has not been added.
3.
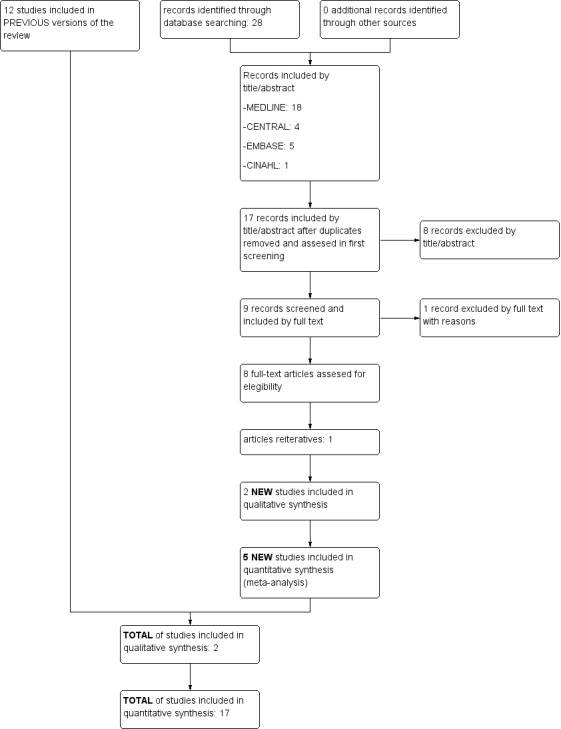
Flow diagram.
We included five of the total number of studies (Brunherotti 2013; Gouna 2013; Hough 2012; Hough 2013; Wu 2015) in the quantitative synthesis (meta‐analysis) and two (Aly 2015; Jalali 2012) in the qualitative synthesis.
Consequently, we included 19 randomised trials in this review. See the Characteristics of included studies table.
Fourteen studies were randomised cross‐over controlled trials, whereas Aly 2008, Aly 2015, Antunes 2003, Jalali 2012 and Wu 2015 were randomised parallel‐group controlled trials.
We listed excluded studies and reasons for their exclusion in the Characteristics of excluded studies table.
A search of online registers of clinical trials (www.clinicaltrials.gov; controlled‐trials.com; ICTRP of the WHO) revealed no studies meeting the inclusion criteria.
Included studies
We included 19 trials (Aly 2008; Aly 2015; Antunes 2003; Bjornson 1992; Bozynski 1988; Brunherotti 2013; Chang 2002; Crane 1990; Fox 1990; Gouna 2013; Heaf 1983; Hough 2012; Hough 2013; Jalali 2012; Mendoza 1991; Mizuno 1995; Mizuno 1999; Schlessel 1993; Wu 2015).
Types of infants
The 19 included studies provided results on 516 term or preterm neonates requiring any type of positive‐pressure MV including CPAP.
Gestational age ranged from 23 to 39 weeks. Most trials (Aly 2008; Aly 2015; Antunes 2003; Chang 2002; Jalali 2012; Mendoza 1991; Mizuno 1995; Mizuno 1999; Schlessel 1993) included term infants. Two studies (Heaf 1983; Wu 2015) did not report gestational age at birth, but when the reported birth weight of study infants was inspected, it was considered that, on average, included participants were born at term. Eight studies (Bjornson 1992; Bozynski 1988; Brunherotti 2013; Crane 1990; Fox 1990; Gouna 2013; Hough 2012; Hough 2013) enrolled preterm infants receiving MV.
We noted important variations in neonate age across studies. In 12 studies (Aly 2008; Aly 2015; Brunherotti 2013; Chang 2002; Crane 1990; Fox 1990; Heaf 1983; Hough 2012; Hough2013; Jalali 2012; Schlessel 1993; Wu 2015), participants were less than one week of age, ranging between 22 hours and seven days. In the other seven trials, neonates were between two and 138 days old.
Participant inclusion and exclusion criteria were not always well defined in the included studies. Investigators applied a variety of exclusion criteria: known congenital defects (Aly 2008; Aly 2015; Antunes 2003; Bjornson 1992; Brunherotti 2013; Chang 2002; Jalali 2012) or severe thoracic deformity (Wu 2015); treatment with sedative or paralysing drugs (Aly 2015; Chang 2002; Fox 1990); asymmetrical lung disease (Bozynski 1988; Schlessel 1993); cardiopulmonary instability or lung collapse (Wu 2015); air leak syndrome; poor skin integrity (Hough 2012; Hough2013); circulatory failure; receipt of vasoactive drugs (Gouna 2013); acute haemorrhage (Wu 2015); periventricular leukomalacia or intraventricular haemorrhage (Aly 2015; Bjornson 1992; Gouna 2013); increased intracranial pressure (Wu 2015); recent surgery (Brunherotti 2013; Hough 2012; Hough2013); diagnosis of BPD (Brunherotti 2013); state of agitation and inability to remain in the decubitus position selected (Brunherotti 2013; Wu 2015); severe anoxia (Aly 2015; Brunherotti 2013); gastrointestinal anomalies such as necrotizing enterocolitis (Aly 2015); use of xanthine derivatives, H2 blockers, prokinetic agents or proton pump inhibitors (Aly 2015); congenital sepsis or pneumonia; inability to be maintained on mechanical ventilation for five (Jalali 2012) or seven full days; and maternal history of substance abuse or documented infection (Bjornson 1992).
The authors of most papers specified (or the review authors deduced) whether patients were fasting. Four studies (Aly 2008; Aly 2015; Mizuno 1995; Mizuno 1999) clearly reported that enteral nutrition was used.
Seven trials (Crane 1990; Fox 1990; Hough 2012; Hough2013; Mizuno 1995; Schlessel 1993; Wu 2015) did not describe simultaneous treatments during the study period. In Bjornson 1992, one infant received sedation, another corticosteroids and two treatment for sepsis (one infant underwent surgical ligation of a patent ductus arteriosus). All infants received surfactant in Gouna 2013, and 18 received surfactant in Chang 2002. Brunherotti 2013 indicated that some participants received surfactant but did not specify the number. In Bozynski 1988, 12 newborns received furosemide, and seven spironolactone plus thiazide diuretics. Three infants were operated on for congenital diaphragmatic hernia in Heaf 1983. In Mendoza 1991, all participants received theophylline, and in Gouna 2013, all received caffeine. In Brunherotti 2013, nine infants received aminophylline or caffeine. In Aly 2008 and Jalali 2012, all participants received an antibiotic until sepsis could be ruled out.
The primary diagnosis of enrolled included infants on MV was respiratory distress syndrome (RDS) in 13 studies (Aly 2015; Bjornson 1992; Brunherotti 2013; Chang 2002; Crane 1990; Fox 1990; Gouna 2013; Hough 2012; Hough2013; Jalali 2012; Mendoza 1991; Schlessel 1993; Wu 2015). In three studies (Bozynski 1988; Mizuno 1995; Mizuno 1999), participants were in stable condition and were ventilated for CLD, and in one trial (Antunes 2003), infants were on intermittent mechanical ventilation (IMV) because of prematurity (presumably due to RDS) and were entered into the study at the beginning of the weaning process. In two trials, there was ventilation due to unilateral lung disease (three infants with congenital diaphragmatic hernia and one infant with hypoplastic right lung in Heaf 1983; and one infant with pneumothorax in Jalali 2012). Aly 2015 included infants with sepsis or pneumonia.
Other trials defined no associated conditions, but Bozynski 1988 described sepsis in two participants, ductus arteriosus in one, intracranial haemorrhage in five and periventricular leukomalacia in three. Bjornson 1992 described two participants with sepsis and one with patent ductus arteriosus. Antunes 2003 described late bronchopneumonia in 11 participants, late sepsis in six and patent ductus arteriosus in 18.
Infants were ventilated with IMV in 14 trials (Aly 2008; Aly 2015; Antunes 2003; Bjornson 1992; Bozynski 1988; Chang 2002; Crane 1990; Hough2013; Jalali 2012; Mendoza 1991; Mizuno 1995; Mizuno 1999; Schlessel 1993; Wu 2015). Two studies (Fox 1990; Heaf 1983) included infants on IMV and others on CPAP. In three trials (Brunherotti 2013; Gouna 2013; Hough 2012), infants received CPAP.
Types of interventions
Interventions included placing infants in a prone versus lateral decubitus or supine position and undertaking a strategy of regular position change. We excluded studies that also evaluated concomitant therapeutic interventions if the effect of infant positioning could not be assessed independently of the effect of the second intervention.
Age and clinical status of infants receiving MV with different positioning were different among the 19 included trials. All trials but two (Antunes 2003; Wu 2015) tested positions for short periods of time, ranging from two minutes in Crane 1990 to three hours in Gouna 2013 and Aly 2015, to eight hours and 16 hours in Wu 2015. In Antunes 2003, the position tested was the same from the start of MV weaning until extubation, except for three hours a day in newborns allocated to the prone position. Aly 2008, Aly 2015 and Wu 2015 maintained the position at all times in the supine group, and changed position from one side to the other every two hours (Aly 2008), four hours (Wu 2015) or six hours (Aly 2015) in the intervention group.
Researchers studied several position changes.
Eleven trials (Antunes 2003; Bjornson 1992; Brunherotti 2013; Chang 2002; Crane 1990; Fox 1990; Gouna 2013; Hough 2012; Mendoza 1991; Mizuno 1995; Mizuno 1999) analysed short‐term respiratory outcomes when infants were placed in a prone versus supine position.
Bjornson 1992, Brunherotti 2013 and Crane 1990 also analysed changes between prone and lateral right positions.
Wu 2015 analysed changes between prone alternant and supine positions and maintained change of position (every four hours) for eight hours and for 16 hours. Review authors carried out all analyses in the review using data for the second period of 16 hours.
Brunherotti 2013 and Gouna 2013 analysed changes between prone and lateral left positions.
Hough 2012 and Hough 2013 analysed prone and supine versus quarter turn from prone with right side uppermost.
Aly 2015,Bjornson 1992,Bozynski 1988,Brunherotti 2013,Crane 1990 and Schlessel 1993 analysed lateral right versus supine position.
Bozynski 1988,Brunherotti 2013,Gouna 2013 and Schlessel 1993 analysed changes between lateral left and supine.
Bozynski 1988,Brunherotti 2013 and Schlessel 1993 analysed lateral right versus lateral left position.
Aly 2008 and Jalali 2012 analysed changes between lateral alternant and supine positions.
Heaf 1983 analysed the impact of position among participants with unilateral lung disease by comparing the good lung dependent versus the good lung uppermost.
Schlessel 1993 randomised participants to the sequences supine‐left‐supine‐right and supine‐right‐supine‐left and presented results for first period supine and second period supine. Review authors carried out all analyses using data for the first period supine.
Outcome measures
Overall, studies showed lack of consistency in types of outcome measures included and in the way data were reported.
Fox 1990, Hough 2013,Schlessel 1993 and Wu 2015 measured oxygenation on MV by PO2; Bozynski 1988 and Heaf 1983 used tcPO2; and Antunes 2003, Bjornson 1992,Brunherotti 2013,Chang 2002,Gouna 2013,Hough 2012,Hough 2013,Mendoza 1991,Mizuno 1995 and Mizuno 1999 measured SpO2. We extracted data from graphs provided by Bjornson 1992 and Bozynski 1988. Wu 2015 measured oxygenation by PO2/FiO2, and Hough 2012 and Hough 2013 by SpO2/FiO2; we extracted data from Gouna 2013.
Bozynski 1988, Crane 1990,Gouna 2013,Heaf 1983,Mizuno 1995 and Mizuno 1999 measured carbon dioxide (CO2) tension using a transcutaneous monitor, and Aly 2008, Hough 2013,Schlessel 1993 and Wu 2015 used arterial samples. Jalali 2012 measured PO2 and CO2 tension, but their results are not valid owing to the significant difference in levels of basal haemoglobin noted in the two groups.
To assess the decrease in ventilator characteristics, Aly 2008, Gouna 2013,Hough 2012,Hough 2013 and Wu 2015 measured FiO2 .
Aly 2008,Aly 2015,Antunes 2003,Chang 2002,Jalali 2012,Mendoza 1991,Mizuno 1995,Mizuno 1999,Schlessel 1993 and Wu 2015 measured hazard ratio (HR) and risk ratio (RR) to assess other ventilator‐associated parameters such as peak inspiratory pressure (PIP) and/or positive end‐expiratory pressure (PEEP).
Some papers measured different physiological pulmonary parameters. Mendoza 1991, Mizuno 1995, Mizuno 1999, Schlessel 1993 and Wu 2015 measured TV (spontaneous breath) in mL/kg and minute ventilation in mL/kg/min.
Investigators defined the outcome of desaturation episodes heterogeneously: Gouna 2013 as a ventilatory pause lasting longer than 10 seconds and/or SpO2 less than 80%; and Chang 2002 as a ventilatory pause lasting longer than 20 seconds and SpO2 less than 90%. Antunes 2003 defined DeSat as the detection of two episodes of SpO2 < 90% that required a temporary increase in FiO2.
Only Wu 2015 studied the duration of ventilator support.
Some studies included other respiratory outcomes not used in this review: Hough 2012 and Hough 2013 analysed regional impedance amplitudes, global inhomogeneity and phase angle using electrical impedance tomography. Gouna 2013 analysed phase angle, rib cage contribution to TV and dynamic elevation of end‐expiratory lung volume using plethysmography. Wu 2015 included dynamic lung compliance; and Mendoza 1991, Mizuno 1999 and Schlessel 1993 total pulmonary resistance, inspiratory pulmonary resistance and expiratory pulmonary resistance.
Other non‐respiratory outcomes reported in this review include gastric fluid aspiration (pepsin in tracheal aspirate) (Aly 2015) and analysed bacterial colonisation of the endotracheal tube (tracheal aspirates cultured on second and fifth days of mechanical ventilation) (Aly 2008;Jalali 2012).
As most of the included trials (14) used a cross‐over design, we could not obtain data to assess the effects of different positioning of newborn infants receiving MV on mortality and neuromotor and developmental outcomes in the long term, as well as other complications of prematurity. Long‐term follow‐up is not relevant for cross‐over trials; by their design, cross‐over trials can address only short‐term effects of treatment. Randomised parallel‐group controlled trials included Aly 2008, Aly 2015, Antunes 2003, Jalali 2012 and Wu 2015.
We have reported additional characteristics of the included trials in the Characteristics of included studies table.
Excluded studies
We identified 19 trials, 10 of which failed to meet the inclusion criteria. We excluded one study (Madlinger‐Lewis 2014) because ventilation therapy is not an inclusion criterion and could have been suspended during the intervention. See the Characteristics of excluded studies table and Appendix 3.
Risk of bias in included studies
We provided details of the methodological quality of each trial in the Characteristics of included studies table and in Figure 1 and Figure 2. Thirteen studies were randomised cross‐over controlled trials, whereas Aly 2008, Antunes 2003, Jalali 2012 and Wu 2015 were randomised parallel‐group controlled trials.
Allocation
Seven studies (Aly 2015; Antunes 2003; Brunherotti 2013; Chang 2002; Fox 1990; Hough 2012; Hough 2013) reported adequate sequence generation. No included studies used quasi‐random methods of participant allocation: None alternately allocated infants to groups. Investigators did not usually state the method of allocation used; only Chang 2002 (randomisation in blocks by a third person to ensure balanced combinations of positions and finally an identification number in a sealed envelope), Fox 1990 (coin toss), Antunes 2003 (drawing of lots in the form of sealed envelopes), Hough 2013 (sequentially numbered set of sealed opaque envelopes), Hough 2012 (sequentially numbered set of sealed opaque envelopes ‐ information obtained by contacting study authors) and Aly 2015 (following consent, opening of an opaque envelope and assignment of participants to allocated groups) provided these descriptions. Only in these six studies could allocation concealment be considered adequate.
Blinding
Owing to the nature of the trials, blinding to the intervention was not possible for participants and personnel in any of the included studies. No studies blinded assessment of outcome measurements. Only Aly 2008, Aly 2015, Hough 2013 and Wu 2015 provided blinding for outcome assessors.
Incomplete outcome data
Ten studies (Aly 2015; Bozynski 1988; Fox 1990; Gouna 2013; Heaf 1983; Hough 2012; Hough2013; Mizuno 1995; Mizuno 1999; Schlessel 1993) provided complete follow‐up of enrolled infants. In Antunes 2003, one participant did not complete the protocol for unknown reasons. In Bjornson 1992, two infants needed changes in ventilatory parameters during the study and could not complete the nine sessions prescribed. In Brunherotti 2013, researchers excluded two randomised infants at the beginning of data collection because they continued to be agitated. In Chang 2002, 10 participants (four in prone, six in supine position) did not complete the two‐hour protocol in the same position because of the need for interventions (airway suctioning, etc), in which case investigators selected a time of equal duration for comparison. In Crane 1990, researchers discarded five infants because of excessive transcutaneous carbon dioxide method (tcPCO2)/partial pressure of carbon dioxide (PaCO2) differences or inaccurate calibration. Mendoza 1991 excluded seven infants from the analysis of some lung mechanics because they did not have good spontaneous breaths in one or both positions. Nineteen participants dropped out of Aly 2008 because of early death (15) or early extubation (four), although it was not clear to which group the dead participants belonged. Jalali 2012 excluded deaths post randomisation without explaining the number of losses, and did not even perform intention‐to‐treat (ITT) analysis. In Wu 2015, five participants (three in supine and two in prone alternant, including one death in each group) did not complete the protocol in the same position for reasons not described.
Selective reporting
No study prespecified primary outcomes, so it was unclear whether studies were free from selective reporting bias.
Other potential sources of bias
No studies used clear prespecified methods, including sample size calculation, and so it was not clear whether studies were likely to be free from other types of bias such as multiple interim analyses or premature stopping. Moreover, lack of blinding may have caused surveillance and ascertainment biases.
We considered that some studies had a particular risk of bias: 10 studies (Aly 2015; Antunes 2003; Crane 1990; Mizuno 1995; Mizuno 1999; Fox 1990; Hough 2012; Hough 2013; Schlessel 1993; Wu 2015 )did not describe co‐interventions provided during the study, and blinding to the intervention was not possible in any of them. This could have been especially detrimental in studies using a cross‐over design. In Jalali 2012, a difference in basal haemoglobin between intervention and control groups invalidated the results. For this reason, we excluded this study from the meta‐analysis.
In studies of cross‐over design, bias due to carry‐over from one period to the next is of particular interest. Although most studies described their wash‐out period, the studies are highly heterogeneous (two minutes to six hours). This could be a source of bias. All studies described randomisation, mentioning that the order of treatments was randomised. Owing to their small size (n < 10), four studies (Bjornson 1992; Heaf 1983; Mizuno 1995; Mizuno 1999) had low power to detect a statistically significant and clinically relevant effect. For this reason, we performed a sensitivity analysis, which did not change review conclusions for the outcome of prone versus supine.
In addition, researchers conducted nine studies before or during the 1990s. Therefore, the applicability of these results to current practice is difficult to assess.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Prone compared with supine for newborn ventilated.
| Prone compared with supine for newborn ventilated | ||||||
| Patient or population: neonates with mechanical ventilation Settings: neonatal intensive care units Intervention: prone Comparison: supine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative risk | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Supine | Prone | |||||
| Death (neonatal or infant) ‐ not reported | No data reported for this outcome | |||||
| Long‐term complications (neurodevelopmental) | No data reported for this outcome | |||||
| Long‐term complications (bronchopulmonary dysplasia, chronic lung disease) | No data reported for this outcome | |||||
| Short‐term complications (respiratory) | No data reported for this outcome | |||||
| Short‐term complications (procedural) | No data reported for this outcome | |||||
| Periventricular or intraventricular haemorrhage | No data reported for this outcome | |||||
| Oxygenation: PO2 (mmHg)a | Mean PO2 ranged across control groups from 51.39 to 73.05 mmHg. | Mean PO2 (mmHg) in intervention groups was 5.49 higher (2.92 to 8.05 higher). | 116 (3 studies)b | ⊕⊕⊕⊝ moderatec,d | Changes in PO2 greater than 2 mmHg are clinically relevant. | |
| Oxygenation: SpO2e | Mean SpO2 in control groups was 89.7% to 97.1%. | Mean SpO2 in intervention groups was 2.18 higher (1.13 to 3.24 higher). | 308 (9 studies)f | ⊕⊝⊝⊝ very lowd,g,h | Changes in SpO2 lower than 2% are not clinically relevant. | |
| SpO2 at ‐ sensitivity low risk; selection bias | Mean SpO2 in control groups was 94.9% to 97.1%. | Mean SpO2 in intervention groups was 0.64 higher (0.26 to 1.02 higher). | 92 (4 studies)i | ⊕⊕⊕⊝ moderatej | Changes in SpO2 lower than 2% are not clinically relevant. | |
| Desaturations | Mean desaturations in control groups ranged from 42.1% to 50%. | Mean desaturations in intervention groups ranged from 4.76% to 26.3%. | Odds ratio 0.11 (0.04 to 0.31) | 178 (3 studies)k | ⊕⊕⊝⊝ lowl,m | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aPO2 (mmHg) = arterial oxygen tension.
bCountries: Australia, Canada and China. Design: two cross‐over, one parallel. Years: 1990; 2013; 2015.
cNo limitations due to methodological concerns. Blinding for participants and personnel is not possible, but for this outcome, this is not considered a source of bias. dDowngrade due to imprecision resulting from a small number of participants.
e SpO2 = haemoglobin oxygen saturation.
fCountries: Australia, Brazil, EEUU, France, Japan and Taiwan. Design: all cross‐over. Years: 1991; 1992; 1995; 1999; 2003; 2012; 2013; 2013; 2013. gDowngraded owing to methodological concerns caused by risk of selection bias in the studies.
hDowngraded owing to inconsistency caused by very high heterogeneity: I² = 89%.
iCountries: Australia, Brazil and Taiwan. Design: all cross‐over. Years: 2002; 2012M; 2013; 2013.
jDowngraded owing to imprecision, because the confidence interval for the estimated effect crosses the threshold of clinical relevance.
kCountries: Brazil, France and Taiwan. Design: two cross‐over, one parallel. Years: 2002; 2003; 2013. lDowngraded owing to inconsistency, because variations in the definition of the outcome across studies limit their comparability.
mDowngraded owing to imprecision, because the confidence interval for the estimated effect crosses the threshold of clinical relevance.
Summary of findings 2. Prone compared with lateral for neonates with mechanical ventilation.
| Prone compared with lateral for neonates with mechanical ventilation | ||||||
| Patient or population: neonates with mechanical ventilation Settings: neonatal intensive care units Intervention: prone Comparison: lateral | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lateral | Prone | |||||
| Death (neonatal or infant) | No data reported for this outcome | |||||
| Long‐term complications (neurodevelopmental) | No data reported for this outcome | |||||
| Long‐term complications (bronchopulmonary dysplasia, chronic lung disease) | No data reported for this outcome | |||||
| Short‐term complications (respiratory) | No data reported for this outcome | |||||
| Short‐term complications (procedural) | No data reported for this outcome | |||||
| Periventricular or intraventricular haemorrhage | No data reported for this outcome | |||||
| Oxygenation SpO2a | Mean SpO2 in control groups was 89.7% to 97.3%. | Mean SpO2 in intervention groups was 0.90 higher (0.25 lower to 2.06 higher). | 206 (5 studies)b | ⊕⊝⊝⊝ very lowc,d,e | Changes in SpO2 lower than 2% are not clinically relevant. | |
| Desaturations | No data reported for this outcome | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSpO2 (%) = haemoglobin oxygen saturation. bNeonatal unit care of Australia, Brazil, EEUU and France. Design: all cross‐over. Years: 1992; 2012; 2013; 2013; 2013. cDowngraded for methodological concerns regarding selection bias. Blinding for participants and personnel is not possible, but for this outcome, this is not considered a source of bias. dDowngraded for inconsistency owing to very high heterogeneity: I² = 91%. eDowngraded for imprecision owing to intervals crossing threshold of clinically relevant effect.
Summary of findings 3. Lateral compared with supine for neonates with mechanical ventilation.
| Lateral compared with supine for neonates with mechanical ventilation | ||||||
| Patient or population: neonates with mechanical ventilation Settings: neonatal intensive care units Intervention: lateral Comparison: supine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Supine | Lateral | |||||
| Death (neonatal or infant) | No data reported for this outcome | |||||
| Long‐term complications (neurodevelopmental) | No data reported for this outcome | |||||
| Long‐term complications (bronchopulmonary dysplasia, chronic lung disease) | No data reported for this outcome | |||||
| Short‐term complications (respiratory) | No data reported for this outcome | |||||
| Short‐term complications (procedural) | No data reported for this outcome | |||||
| Periventricular or intraventricular haemorrhage | No data reported for this outcome | |||||
| Bronchopulmonary dysplasia or chronic lung disease | No data reported for this outcome | |||||
| Oxygenation SpO21 | Mean SpO2 in control groups was 89.7% to 97.1%. | Mean SpO2 in intervention groups was 1.01 higher (0.04 lower to 2.06 higher). | 206 (5 studies)2 | ⊕⊝⊝⊝ very low3,4,5 | Changes in SpO2 lower than 2% are not clinically relevant. | |
| Desaturations | No data reported for this outcome | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSpO2 (%) = haemoglobin oxygen saturation. bNeonatal unit care, Australia, Brazil, EEUU and France. Design: all cross‐over. Years: 1992; 2012; 2013; 2013; 2013. cDowngraded for methodological concerns regarding selection bias. Blinding for participants and personnel is not possible but for this outcome, but this is not considered a source of bias. dDowngraded for inconsistency owing to very high heterogeneity: I² = 90%. eDowngraded for imprecision owing to intervals crossing threshold of clinically relevant effect.
Prone versus supine (comparison 1)
Thirteen trials compared prone versus supine position.
Results for PO2 (mmHg) showed a significant beneficial effect of the prone position (three studies; 116 participants). Two studies in prone (Fox 1990; Hough 2013) and one in prone alternant (Wu 2015) provided data for PO2, showing significant improvement in the prone position (mean difference (MD) 5.49 mmHg, 95% confidence interval (CI) 2.92 to 8.05; I2= 0). Results were similar between prone and prone alternant modalities. Sensitivity analyses by year of publication and by allocation concealment did not modify the results.
Nine studies provided data for SpO2; all showed small improvements in the prone position (1.13 to 3.24) but with extreme heterogeneity (I2= 89%). Results were extremely heterogeneous (I2= 95%), and three studies (59 participants) (Brunherotti 2013; Gouna 2013; Hough 2012) reported no significant effect in subgroup CPAP. We classified six studies (95 participants) (Bjornson 1992; Chang 2002; Hough 2013; Mendoza 1991; Mizuno 1995; Mizuno 1999) into the subgroup of conventional ventilation (CV). In this CV group, extremely heterogeneous results (I2= 84%) suggested a statistically significant increase in SpO2 in the prone position (MD 2.29, 95% CI 1.17 to 3.41). An additional study on newborns during the weaning process (Antunes 2003) presented data on SpO2, revealing no differences in SpO2 between supine and prone, but we could not meta‐analyse these results because a simultaneous reduction in ventilator parameters occurred. In fact, infants in the prone position needed less aggressive ventilator parameters: PIP on the second and third (14.4 ± 1.95 vs 13.0 ± 2.14, P = 0.048) days of weaning. Sensitivity analyses restricted to studies with low risk of selection bias (Analysis 1.11) showed homogeneous results, suggesting a small but significant effect (MD 0.64, 95% CI 0.26 to 1.02; I2= 0; four trials; 92 participants) that nevertheless lacks clinical relevance.
Three studies with 89 participants (Antunes 2003; Chang 2002; Gouna 2013) quantified the number of desaturation episodes (DeSat). All found a lower incidence of DeSat in the prone position. Studies defined and quantified DeSat differently. Antunes 2003 defined DeSat as the detection of two episodes of SpO2 < 90% that required a temporary increase in FiO2. According to this definition, nine of the 21 participants experienced DeSat in the supine position, and one in the prone position (odds ratio (OR) 0.07, 95% CI 0.01 to 0.59). Chang 2002 defined DeSat as SpO2 < 90% that lasted longer than 20 seconds; he found that 14 of 28 infants experienced DeSat in the supine position, and five in the prone position (OR 0.22, 95% CI 0.09 to 0.54). Gouna 2013 studied prone, lateral left and supine positions in the group ventilated with CPAP and defined DeSat as a ventilatory pause longer than 10 seconds and/or SpO2 < 80%. Meta‐analysis of studies showed low heterogeneity (I2= 43%), and analysis with the random‐effects model showed a significant difference in favour of the prone position (OR 0.11, 95% CI 0.04 to 0.31). Antunes 2003 also found that preterm infants who had been weaned in the prone position subsequently had to be reintubated significantly less often (seven out of 21) than those who had been weaned in the supine position (seven out of 21) (P = 0.049). Gouna 2013 found that over three hours, a number of (8 ± 3 SD) desaturations occurred in the supine group compared with 4 ± 2 in the lateral left group and 5 ± 2 in the prone group. Both differences were statistically significant (P < 0.05) compared with the value measured in the supine position.
Six studies with 137 participants (Crane 1990; Gouna 2013; Hough 2013; Mizuno 1995; Mizuno 1999; Wu 2015) provided data for carbon dioxide tension (PCO2; mmHg) with moderate heterogeneity of effect (I2= 62%). The test for subgroup differences was significant (I2 for subgroup differences = 75.1%). In the CPAP subgroup, researchers noted significant reduction in PCO2 in the prone arm (MD ‐6.00, 95% CI ‐9.07 to ‐2.93; one study; 19 participants) but no differences in the CV subgroup in the prone position (MD ‐2.01, 95% CI ‐5.19 to 1.18; I2= 39%; four studies; 51 participants). The single study that assessed prone alternant position with CV reported no differences in PCO2 (MD 1.25, 95% CI ‐2.91 to 5.41; 67 participants). Sensitivity analyses by low risk of selection bias were restricted to Hough 2013, with non‐significant results.
Five studies with 125 participants (Gouna 2013; Mendoza 1991; Mizuno 1995; Mizuno 1999; Wu 2015) assessed pulmonary mechanics by means of TV(mL/kg), and three studies with 39 participants (Mendoza 1991; Mizuno 1995; Mizuno 1999) by minute ventilation (mL/kg/min). Results were heterogeneous for both outcomes (I2= 64% and I2= 68%), and the meta‐analysis found evidence of effect (MD for TV 0.97 mL/kg, 95% CI 0.36 to 1.58) and no evidence of effect (MD for minute ventilation 19.80 mL/kg/min, 95% CI ‐40.54 to 80.14). Investigators noted no differences between subgroups of types of ventilation. We could not conduct sensitivity analyses by selection bias.
The aforementioned study (Antunes 2003) found no differences in the duration of the process of weaning between prone and supine positions. However, once participants had been extubated and placed in the supine position under CPAP, it was found that those who had been weaned in the supine position needed to be reintubated during the first 48 hours more often than those who had been weaned in the prone position (seven vs one; P = 0.049).
Four studies (Gouna 2013; Hough 2012; Hough 2013; Wu 2015) measured the decrease in characteristics of the ventilator by using FiO2 and found no significant differences (MD ‐1.93%, 95% CI ‐4.39 to 0.53; I2= 71%; 134 participants). The test for subgroup differences did not point out different effects by subgroup (I2= 0%). Sensitivity analyses by selection bias restricted to Hough 2012 and Hough 2013 showed non‐significant results with homogeneity (MD ‐0.61, 95% CI ‐3.10 to 1.88; I2= 0; two studies; 48 participants).
Two studies with 48 participants (Hough 2012; Hough 2013) measured oxygenation by SpO2/FiO2. Pooled results showed no significant differences (MD 5.99, 95% CI ‐21.27 to 33.25; I2= 0%) for the CPAP modality study nor for the CV one. Both studies were at low risk of selection bias, thus the sensitivity analysis matched the main analysis.
A single study exploring prone alternant position with 67 participants (Wu 2015) measured the oxygenation index (PaO2/FiO2), showing worse results in the prone alternant position than in the supine position (MD 17.36, 95% CI 7.11 to 27.61).
A single study exploring prone alternant position with 67 participants (Wu 2015) assessed duration of ventilator support. The prone alternant position decreased the duration of ventilator support (3.98 ± 1.63 days) with respect to supine (4.19 ± 1.46 days) (MD ‐0.21, 95% CI ‐0.68 to ‐0.04) without statistical significance (P > 0.05).
Researchers in two studies with 40 participants (Brunherotti 2013; Hough 2012) and moderate heterogeneity of effect (I2= 67%) analysed respiratory rate (RR) only for the CPAP subgroup. Results showed no significant differences (MD ‐4.83, 95% CI ‐10.73 to 1.08). Both studies were at low risk of selection bias, thus the sensitivity analysis matched the main analysis.
1.11. Analysis.
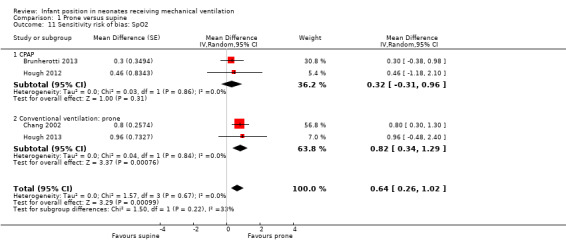
Comparison 1 Prone versus supine, Outcome 11 Sensitivity risk of bias: SpO2.
Prone versus lateral (comparison 2)
Six studies provided data comparing prone and lateral positions.
Lateral right:Bjornson 1992 and Brunherotti 2013 assessed the effect on SpO2, and the meta‐analysis supported a finding of no significant difference compared with prone (MD 1.69, 95% CI ‐0.61 to 3.99; 20 participants). Results were highly heterogeneous (I2= 97%). We restricted sensitivity analysis to a study with low risk of bias (Brunherotti 2013; 16 participants) with non‐significant results. Crane 1990 assessed the effect on PCO2 and found no difference (MD 0.00; 95% CI ‐2.95 to 2.95; 14 participants).
Lateral left: Both studies that measured SpO2(Brunherotti 2013; Gouna 2013) belong to subgroup CPAP. These investigators included 35 participants and found a significant, although discrete, increase in SpO2 in the prone position (MD 0.90%, 95% CI 0.30 to 1.49) with no heterogeneity (I2= 0%). However, sensitivity analysis restricted to a study with low risk of bias (Brunherotti 2013) showed no significant differences (MD 0.80, 95% CI ‐0.03 to 1.63; 16 participants) (Analysis 2.7).
Gouna 2013 found no significant results for PCO2, TV and FiO2. Researchers also measured the number of desaturations that occurred in three hours and reported prone 5 ± 2 versus lateral left 4 ± 2.
Quarter from prone:Hough 2012 and Hough 2013 described SpO2 and FiO2, and Hough 2013 also reported PO2 and PCO2. The meta‐analysis found no difference versus prone for any of the four outcomes (SpO2: MD ‐0.27, 95% CI ‐1.50 to 0.97; 48 participants; I2= 11%). In addition, SpO2/FiO2 showed a non‐significant difference in favour of the quarter turn from prone position (MD ‐1.77%, 95% CI ‐25.59 to 22.05; I2= 0%; 48 participants).
2.7. Analysis.
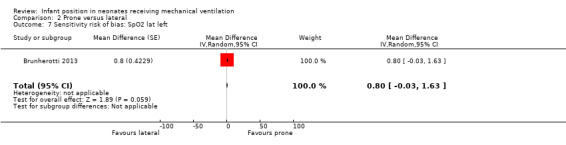
Comparison 2 Prone versus lateral, Outcome 7 Sensitivity risk of bias: SpO2 lat left.
Lateral versus supine (comparison 3)
Ten trials compared the lateral versus supine position.
Aly 2015,Bjornson 1992, Bozynski 1988, Brunherotti 2013 and Crane 1990 focused on the right lateral position.
Bozynski 1988,Brunherotti 2013 and Gouna 2013 focused on the left lateral position. Neither the lateral right position nor the lateral left position showed significant differences compared with the supine position for any outcomes: PO2, SpO2 or PCO2. Only Gouna 2013 with 19 newborns in lateral left who received nasal CPAP found a statistically significant effect for FiO2 and TV (MD for FiO2 ‐4.00%, 95% CI ‐6.21 to ‐1.79; MD for tidal volume 0.70 mL/kg, 95% CI 0.17 to 1.23). However, this study had high risk of selection bias. Investigators also provided the number of desaturations that occurred in three hours at lateral left (4 ± 2) versus supine (8 ± 3) (P < 0.05).
Hough 2012 and Hough 2013 compared quarter turn from prone versus supine. Hough 2013 measured blood gases (PO2 and PCO2) and found no differences. The meta‐analysis (48 participants) found no significant differences for SpO2 (MD 0.94, 95% CI ‐0.23 to 2.10; I2= 0%), for FiO2 (MD ‐0.81%, 95% CI ‐2.58 to 0.95; I2= 0%) and for SpO2/FiO2 (MD 8.16, 95% CI ‐17.87 to 34.19; I2= 7%).
Aly 2008 and Jalali 2012 focused on the lateral alternant. Aly 2008 found no significant difference for PCO2 and FiO2. We did not include outcomes of Jalali 2012 for PO2, PCO2 and SpO2 in our meta‐analysis because we considered that their significant differences in basal haemoglobin invalidated them. Both studies evaluated whether the position is associated with the rate of colonisation, along with the number of reintubations due to accidental extubation. Investigators measured bacterial colonisation of the endotracheal tube using tracheal aspirates cultured on the second and fifth days of mechanical ventilation. Aly 2008 and Jalali 2012 described 50 and 31 newborns with five‐day colonisation count of 87% vs 25% in the supine group, and 30% vs 13% in the lateral position group, respectively. But Jalali 2012 excluded from the study cases of death that occurred post randomisation (with second day positive endotracheal cultures) without mentioning the number of losses or describing researchers who performed ITT analysis. For this reason, we have not meta‐analysed this outcome.
Aly 2015 included 34 preterm infants in CV using a parallel design. Researchers evaluated whether position was associated with gastric fluid aspiration (pepsin in tracheal aspirate) and reported that in the lateral right group, a decreased concentration of pepsin was retrieved from tracheal aspirate (13 ng/mL (interquartile range (IQR) 11.9 to 38.7) to 10 ng/mL (IQR 7 to 12; P < 0.001)), whereas the amount did not change in the control group (P = 0.42).
Lateral right versus lateral left (comparison 4)
Three cross‐over trials (Bozynski 1988; Brunherotti 2013; Schlessel 1993) provided data for this comparison. Neither Bozynski 1988 nor Schlessel 1993 showed significant effects on the outcomes PO2 and PCO2. Meta‐analysis results (with 34 participants) were also non‐significant (MD for PO2 0.05 mmHg, 95% CI ‐6.93 to 6.83; I2= 0; MD for PCO2 0.41 mmHg, 95% CI ‐2.01 to 2.82; I2= 0). Schlessel 1993 analysed TV, and Brunherotti 2013 analysed SpO2; researchers found no significant differences (MD for TV ‐0.80, 95% CI ‐1.87 to 0.27; MD for SpO2 0.30%, 95% CI ‐0.78 to 1.38).
Good lung dependent versus good lung uppermost (comparison 5)
One small cross‐over trial (Heaf 1983) provided data for this comparison and showed no significant differences in oxygen nor in PCO2.
For all position contrasts, we could not assess effects of position on other outcomes defined a priori, including clinically important outcomes, and we could not perform planned subgroup analyses because of lack of data. We were able to carry out such analysis for the subgroup of CPAP but with very scarce data.
Discussion
Summary of main results
Studies included in this review provided no evidence to suggest that any specific body position for infants receiving mechanical ventilation (MV) is more effective than another in producing long‐term clinically relevant benefits. However, neonates on MV who were habitually in the supine position improved their oxygenation when they were changed to the prone position. It cannot be concluded from these studies whether improvement in oxygenation was due to the fact that the prone position is better, or whether it was mainly attributable to a change in position. This improvement in oxygenation lasted only for the short time infants were in the prone position.
We included a total of 19 trials studying different positions: prone versus supine, prone (alternant with supine) versus supine, prone versus lateral right, lateral right versus supine, lateral left versus supine, lateral (alternating sides) versus supine, lateral right versus lateral left, quarter turn from prone ‐ right side uppermost ‐ versus prone and supine and good lung dependent versus good lung uppermost.
The most robust comparison examined the beneficial effect of prone versus supine position on oxygenation outcomes, with a total of 11 studies (246 participants) providing useful data, three of which (59 participants) provided continuous positive airway pressure (CPAP). The three studies that evaluated desaturation episodes concluded that episodes of desaturation were fewer in the prone than in the supine position.
Relatively few small studies, typically with high risk of bias, evaluated the latter comparisons; thus these studies have a high likelihood of type II error stemming from the low power of the study to detect a statistically significant and clinically relevant effect. However, studies with high risk of bias typically overestimate the effect, in contrast to studies with low risk of bias. In small studies that were extremely heterogeneous, the prone position seemed to improve oxy‐haemoglobin saturation measured by a pulse oximeter (SpO2) when compared with the lateral left position, although sensitivity analysis restricted to studies with low risk of bias showed no significant differences. Positions lateral right and quarter from prone do not present significant differences compared with prone, although for this analysis only two small studies, with a total of 20 patients, were included.
Regarding non‐ventilatory outcomes, the parallel study with low risk of bias but a moderate number of participants (Aly 2015) (n = 34) described lateral right as the better position compared with supine in terms of retrieval of a decreased concentration of pepsin from the tracheal aspirate and risk of gastric fluid aspiration. Two studies Aly 2008 and Jalali 2012) compared bacterial colonization of the endotracheal tube in patients in lateral alternant versus supine position, finding less colonization in the lateral position. However, the study by Jalali et al. presented a high risk of bias and therefore the results were excluded from this meta‐analysis.
Strengths and limitations
The primary limitation of this review, which is common to many systematic reviews, is the lack of studies with low risk of bias. Although most of the studies included in this review were published in the two previous decades, methodologically well‐conducted studies remain scarce. A strength of this review was the meta‐analyses, which allowed us to find some significant outcome results. Given that any change in position from the standard supine position may promote a more flexed position (similar to the foetal position) that could positively affect outcomes, most included studies specified that both the standard position and the comparison position promoted flexion through the use of soft cloth rolls, etc.
Nonetheless, the studies included in this review had several limitations. One limitation involved small samples, which added up to only 465 infants analysed for this review. We could not draw firm conclusions because of this limitation and because of variations in the interventions and outcomes assessed, which resulted in multiple subgroups, all including small numbers of participants.
Furthermore, Chang 2002 found that improvement in oxygenation was greater in the subgroup of participants previously treated with surfactant. However, we were unable to conduct a sensitivity analysis due to the lack of data about that co‐intervention.
Use of a cross‐over design in all studies except five (Aly 2008; Aly 2015; Antunes 2003; Jalali 2012; Wu 2015) removed the ability of investigators to measure effects on long‐term outcomes. Because these five studies did not undertake prolonged follow‐up, it was not possible to determine whether positioning might have important long‐term effects.
Overall completeness and applicability of evidence
When compared with the standard supine position, the prone position was shown to be more effective ‐over short periods of time‐ in improving oxygenation for participants in conventional ventilation (CV). This improvement does not appear to occur in the subgroup of ventilated infants with CPAP, although for now, the data are very scarce. Researchers could not reliably establish the magnitude of improvement in oxygenation because the 11 eligible trials (Bjornson 1992; Brunherotti 2013; Chang 2002; Fox 1990; Gouna 2013; Hough 2012; Hough 2013; Mendoza 1991; Mizuno 1995; Mizuno 1999; Wu 2015) analysed a total of only 246 babies and used two different measures of oxygenation.
Oxygenation in the prone position was superior in six out of eight studies that measured oxygenation by pulse oximetry, but not in Brunherotti 2013 (CPAP) and Hough 2013 (CV). Although heterogeneity across studies in the magnitude of effect was evident, results showed an increase in oxy‐haemoglobin saturation measured by a pulse oximeter in the prone position, with a 95% confidence interval (CI) of 1.13% to 3.24% over all studies. Sensitivity analyses restricted to studies with low risk of selection bias showed homogeneous results, suggesting a small but significant effect (95% CI 0.26 to 1.02). In the CPAP subgroup ‐ a sample of 59 participants ‐ these results were not significant (mean difference (MD) 1.79, 95% CI ‐0.06% to 3.65%). Only three studies measured arterial oxygen tension (PO2) (the most precise measure of oxygenation) and showed an increase of 5.49 mmHg (95% CI 2.92 to 8.05) in prone (Fox 1990; Hough 2013) and prone alternant positions (Wu 2015).
Three studies including 58 participants (Bozynski 1988; Hough 2013; Schlessel 1993) provided data on the effect on PO2 of the lateral (right or left) versus lateral, supine or prone position and showed no significant difference in favour of lateral.
It is not possible to conclude whether observed improvements in oxygenation in the prone position would be maintained over the long term. Time of exposure to the prone position for each participant was relatively short, ranging from two minutes to four hours. It also cannot be concluded whether this benefit may be sustained after the intervention has been stopped.
Benefits of the prone position for oxygenation may have been underestimated in that seven out of ten studies (Brunherotti 2013; Chang 2002; Gouna 2013; Hough 2012; Mizuno 1995; Mizuno 1999; Wu 2015) were conducted in relatively stable patients with respiratory insufficiency. Furthermore, three studies (Aly 2015; Bjornson 1992; Gouna 2013) excluded some common conditions such as intraventricular haemorrhage.
One study (Chang 2002) assessed additional secondary outcomes such as motor activity and number, intensity and duration of episodes of desaturation. This study found that, compared with the supine position, the prone position produced fewer episodes of desaturation and lesser levels of activity. Study authors also reported that 74% of the episodes of desaturation were associated with vigorous motor activity and crying. Investigators concluded that the prone position offered indirect benefit by achieving less agitation among infants. Another study examining preterm infants on MV in the weaning phase (Antunes 2003) found that the prone position was better than the supine position as far as desaturation episodes were concerned. Gouna 2013 described the intensity and duration of episodes of desaturation and concluded that these episodes were significantly reduced in both the prone group and the lateral left group compared with the supine group. The meta‐analysis showed that fewer infants experienced desaturation episodes in the prone position. Antunes 2003 also found that preterm infants who had been weaned in the prone position had to be reintubated less frequently than those who had been weaned in the supine position.
The two studies of Mizuno (Mizuno 1995; Mizuno 1999) raised questions about effects that enteral feeding may have on respiratory outcomes. Although both studies offered data suggesting that the prone position may improve oxygenation during and after feeding, we did not include these data in our review because all other included studies were conducted during fasting periods (prefeeding) of at least a few hours.
Six studies including 137 participants (Crane 1990; Gouna 2013; Hough 2013; Mizuno 1995; Mizuno 1999; Wu 2015) provided data on the effect of the prone position on carbon dioxide tension (PCO2) with moderate heterogeneity. Only two studies (Hough 2013; Wu 2015) measured PCO2 in arterial blood. Remaining studies measured transcutaneous partial pressure of CO2 (tcPCO2). Pooled results showed a significant difference in favour of the prone position (MD ‐3.36, 95% CI ‐6.62 to ‐0.10), but sensitivity analyses by low risk of selection bias were restricted to Hough 2013, with non‐significant results.
Several studies measured different pulmonary mechanical parameters. We included these outcomes in our meta‐analysis only when studies using comparable measures were available. We were able to analyses only tidal volume and minute ventilation during spontaneous breathing. We found no significant differences between prone and supine positions.
Other comparisons
Five studies (Bjornson 1992; Brunherotti 2013; Gouna 2013; Hough 2012; Hough 2013) assessed the effect of the prone versus lateral positions (right, left or quarter prone) on oxy‐haemoglobin saturation measured by a pulse oximeter and found no improvement in the prone position (MD 0.90 mmHg, 95% CI ‐0.25 to 2.06); however, results were highly heterogeneous. Only the lateral left subgroup (in which infants received CPAP) showed slight improvement with no heterogeneity (MD 0.90 mmHg, 95% CI 0.30 to 1.49), but sensitivity analysis revealed no significant differences.
One trial (Heaf 1983) compared blood gases among participants with asymmetrical respiratory pathology who were positioned with their good lung dependent versus good lung uppermost. Investigators noted no significant differences between positions in PO2 or PCO2. No reliable conclusions could be made from this study given the very small sample size (only four patients).
Aly 2015 investigated levels of pepsin as a marker of decreased aspiration and described decreased tracheal aspirated pepsin levels with right lateral versus supine position (from 13 ng/mL (interquartile range (IQR) 11.9 to 38.7) to 10 ng/mL (IQR 7 to 12; P < 0.001) (MD 1.69%, 95% CI ‐0.61 to 3.99). In tracheal cultures of neonates after five days of MV, Aly 2008 observed a higher rate of colonisation among those in the supine versus the alternating lateral position (supine 26/30 (87%) vs lateral 9/30 (30%); P < 0.01). Researchers noted no differences when comparing other positions (prone vs lateral right, lateral right vs left), although the possibility of differences cannot be disregarded given the small numbers of included participants. What's more, Jalali 2012 provided data on this outcome, but this study had very high risk of bias with different baselines and selection and attrition bias.
Complications associated with interventions
None of the included trials reported complications or undesirable effects attributable to changes in positioning. Investigators referred to complications in a generic way, and in most trials, they did not explicitly mention complications when stating study objectives. Immediate accidents such as accidental extubation, bleeding and airway loss are practical problems that may be observed in general clinical practice; however, researchers did not observe these events in controlled settings of clinical trials with fairly stable participants.
Quality of the evidence
We assessed none of the 19 trials as having low risk of bias on all seven risk of bias criteria. Given the nature of the intervention, it was not possible to blind participants and personnel, although this fact was not considered a source of bias given the objective nature of the outcomes considered. Moreover, in all studies, we assessed selective reporting of outcomes as having unclear risk of bias because none reported the outcomes as prespecified in the original protocol.
Numbers of participants in the included trials were relatively small (ranging from four to 67), and no studies made a prior estimation of the necessary sample size, which led to concerns about imprecision. We noted that data were missing in some studies: Of 516 participants, data were missing for 51 (corresponding to 9.88% of the total). The missing data were managed by carrying out an analysis of the available data (n=465). The sensitivity analysis performed did not alter the conclusions of the study.
Unfortunately, the included studies did not provide data for relevant outcomes such as neonatal death; long‐term neurodevelopmental, periventricular or intraventricular haemorrhage; bronchopulmonary dysplasia; or chronic lung disease. This reduces the clinical applicability of study results.
We found low to moderate overall quality of evidence of a slight improvement in oxygenation among patients in the prone versus the supine position. Limitations of the evidence are due primarily to the imprecision of estimates, whose confidence intervals crossed the thresholds of clinical relevance.
Evidence from comparisons of prone versus diverse lateral positions and lateral versus supine is of very low quality, precluding any meaningful conclusions.
Potential biases in the review process
We conducted this review by following standard Cochrane methods; however, potential biases in the review process cannot be dismissed entirely because assessment of risk of bias, for example, is subject to individual interpretation.
We sought to identify all relevant trials and to avoid omission of potentially important data by (1) having two review authors independently assess risk of bias and carry out data extraction; (2) contacting study authors if we found study methods or results to be unclear; and (3) consulting a third party if we were unable to resolve dilemmas.
Agreements and disagreements with other studies or reviews
To date, no recent systematic reviews have appraised the best position for infants receiving mechanical ventilation. Results of this updated review are consistent with those of the previous review but have been made more robust by the addition of seven new studies to this update.
Authors' conclusions
Implications for practice.
Evidence from randomised controlled trials indicates that when compared with the supine position, the prone position led to slightly improved oxygenation, including desaturation episodes, when used for short periods, or when the patient's condition had stabilized and they were going through the weaning process. We found no evidence on whether the prone position produces sustained benefit for pulmonary or other parameters.
Although investigators in the studies reviewed did not observe adverse effects from the prone position, we cannot disregard with confidence the possibility of such effects. Researchers have generally tested the effects of position under highly controlled circumstances in patients in stable condition. Use of the prone position should be carefully monitored, particularly to avoid damage to the tracheal tube or to central catheters during manipulation of the neonate.
Implications for research.
Large controlled clinical trials comparing the prone versus the supine position are needed. Controlled trials based on a parallel design could assess not only short‐term outcomes but also long‐term and medium‐term outcomes such as mortality, duration of ventilator support and hospitalisation, cutaneous and joint problems and neurodevelopment. Large studies including different types of patients (including those who are less stable) may help to verify whether the intervention benefits newborns with different diseases or varying disease severity, and whether subgroups of responsive patients may be identified.
It is difficult to design a pragmatic trial that ensures that caregivers, parents and investigators are unaware of the allocated intervention involving position. This lack of blinding may lead to surveillance and ascertainment biases.
Questions on the effects of lateral positioning remain to be answered. Likewise, investigators must assess the lateral decubitus position with the good lung dependent versus good lung uppermost in patients with asymmetrical respiratory pathology.
What's new
| Date | Event | Description |
|---|---|---|
| 1 September 2016 | New citation required but conclusions have not changed | Seven new trials met the inclusion criteria and are included in this update. No change has been made to the conclusions of this review. |
| 1 September 2016 | New search has been performed | This updates the review "Infant position in neonates receiving mechanical ventilation" (Balaguer 2003). The search was updated in August 2016. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 18 February 2009 | Amended | Contact details were updated. |
| 20 October 2008 | Amended | Contact details were updated. |
| 13 May 2008 | Amended | Review was converted to new review format. |
| 16 June 2006 | New citation required but conclusions have not changed | This is an update of the review, "Infant position in neonates receiving mechanical ventilation," published in The Cochrane Library, Issue 2, 2003 (Balaguer 2003). The search was updated in May 2006. One new trial (Antunes 2003) was identified and is included in this review update. The addition of this study did not change the conclusions reported in the previous version of this review. |
Acknowledgements
We thank Dr Albert Balaguer, who led protocol development for the original review.
To Pharmacia Laboratories for help in searching Embase for the first edition of this review. The work of Ms Roqué in the first edition of this review was funded in part by grant 01/A060 from the Instituto de Salud Carlos III, Subdirección General de Investigación Sanitaria.
We acknowledge Dr Judith L. Hough for providing answers to our queries about her study.
We thank Colleen Ovelman, Information Specialist from the Cochrane Neonatal Group, for running new searches for use in this review update.
Appendices
Appendix 1. Search strategies for bibliographic databases
The standard search strategy of the Cochrane Neonatal Review Group, as outlined in The Cochrane Library, was used and combined with the following MeSH search terms (Furlan 2009):
“Posture”, “Prone”, “Position”, “Positioning”, “Lateral decubitus”, “Mechanical ventilation”, “Artificial respiration”, “Positive‐pressure respiration”, “Infant”, “Newborn”, “Neonate”, “Neonatal”, “Premature”, “Very low birth weight”, “Low birth weight”, “VLBW”, “LBW”. The following text word terms were also used:
”infant positioning“ (tw), ”patient positioning“ (tw), ”body position“ (tw), ”prone“ (tw), "prone alternant" (tw), ”lateral decubitus“(tw), "lateral alternant" (tw), "quarter turn from prone" (tw), ”mechanical ventilation“ (tw), ”neonate“ (tw) and ”premature“ (tw).
See Cochrane Neonatal Group methods used in reviews.
Appendix 2. Dates for search of studies published before December 2012
Our search was updated from December 2012 to September 20015. The initial search was carried out from:
• Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 3) (from 2002, Issue 1);
• Ovid MEDLINE, National Library of Medicine (from 1966)
• Embase (from 1980)
• CINAHL (from inception)
• WHO ICTRP (from inception)
• controlled‐trials.com (from inception)
• Oxford Database of Perinatal Trials (from inception to December 2012)
Unpublished studies were identified by handsearching of conference proceedings of the Society for Pediatric Research published from 1990 to January 2002 and Pediatric Academic Societies (PAS) electronically at Abstracts view from 2000 onwards.
Appendix 3. Excluded studies in previous versions of this review
In previous versions of this review, we excluded ten studies; of these, in seven studies (Ancora 2009; Baird 1991; Itakura 1998; McEvoy 1997; Schrod 1993; Wagaman 1979; Zhu 2010), assignment was neither random nor quasi‐random for the position. We excluded Curley 2005, Fineman 2006 and Ibrahim 2007 because of the age of the participants.
Data and analyses
Comparison 1. Prone versus supine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PO2 (mmHg) | 3 | Mean Difference (Random, 95% CI) | 5.49 [2.92, 8.05] | |
| 1.1 Conventional ventilation: prone | 2 | Mean Difference (Random, 95% CI) | 5.56 [2.53, 8.58] | |
| 1.2 Conventional ventilation: prone alternant | 1 | Mean Difference (Random, 95% CI) | 5.3 [0.46, 10.14] | |
| 2 SpO2 | 9 | Mean Difference (Random, 95% CI) | 2.18 [1.13, 3.24] | |
| 2.1 CPAP | 3 | Mean Difference (Random, 95% CI) | 1.91 [‐1.14, 4.97] | |
| 2.2 Conventional ventilation: prone | 6 | Mean Difference (Random, 95% CI) | 2.29 [1.17, 3.41] | |
| 3 Participants desaturating | 3 | OR Becker‐Balagtas (Random, 95% CI) | 0.11 [0.04, 0.31] | |
| 4 PCO2 (mmHg) | 6 | Mean Difference (Random, 95% CI) | ‐2.28 [‐5.20, 0.63] | |
| 4.1 CPAP | 1 | Mean Difference (Random, 95% CI) | ‐6.0 [‐9.07, ‐2.93] | |
| 4.2 Conventional ventilation: prone | 4 | Mean Difference (Random, 95% CI) | ‐2.01 [‐5.19, 1.18] | |
| 4.3 Conventional ventilation: prone alternant | 1 | Mean Difference (Random, 95% CI) | 1.25 [‐2.91, 5.41] | |
| 5 Tidal volume (mL/kg) | 5 | Mean Difference (Random, 95% CI) | 0.79 [0.18, 1.41] | |
| 5.1 Conventional ventilation: prone | 4 | Mean Difference (Random, 95% CI) | 0.75 [‐0.13, 1.62] | |
| 5.2 Conventional ventilation: prone alternant | 1 | Mean Difference (Random, 95% CI) | 0.97 [0.36, 1.58] | |
| 6 Minute ventilation (mL/kg/min) | 3 | Mean difference (Random, 95% CI) | 19.80 [‐40.54, 80.14] | |
| 7 FiO2 (%) | 4 | Mean Difference (Random, 95% CI) | ‐1.93 [‐4.39, 0.53] | |
| 7.1 CPAP | 2 | Mean Difference (Random, 95% CI) | ‐3.10 [‐7.02, 0.81] | |
| 7.2 Conventional ventilation: prone | 1 | Mean Difference (Random, 95% CI) | 1.0 [‐4.66, 6.66] | |
| 7.3 Conventional ventilation: prone alternant | 1 | Mean Difference (Random, 95% CI) | ‐1.0 [‐2.69, 0.69] | |
| 8 SpO2/FiO2 | 2 | Mean Difference (Random, 95% CI) | 5.99 [‐21.27, 33.25] | |
| 8.1 CPAP | 1 | Mean Difference (Random, 95% CI) | 11.82 [‐23.20, 46.84] | |
| 8.2 Conventional ventilation: prone | 1 | Mean Difference (Random, 95% CI) | ‐2.98 [‐46.42, 40.46] | |
| 9 PaO2/FiO2 | 1 | Mean Difference (Random, 95% CI) | 17.36 [7.11, 27.61] | |
| 9.1 Conventional ventilation: prone alternant | 1 | Mean Difference (Random, 95% CI) | 17.36 [7.11, 27.61] | |
| 10 RR (respiratory rate) (CPAP) | 2 | Mean Difference (Random, 95% CI) | ‐4.83 [‐10.73, 1.08] | |
| 11 Sensitivity risk of bias: SpO2 | 4 | Mean Difference (Random, 95% CI) | 0.64 [0.26, 1.02] | |
| 11.1 CPAP | 2 | Mean Difference (Random, 95% CI) | 0.32 [‐0.31, 0.96] | |
| 11.2 Conventional ventilation: prone | 2 | Mean Difference (Random, 95% CI) | 0.82 [0.34, 1.29] |
1.1. Analysis.
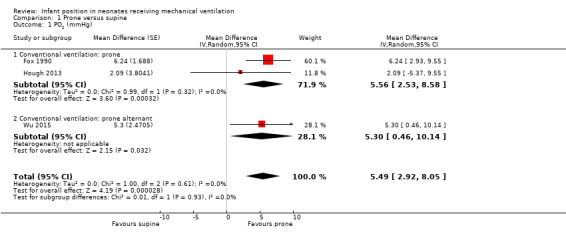
Comparison 1 Prone versus supine, Outcome 1 PO2 (mmHg).
1.2. Analysis.
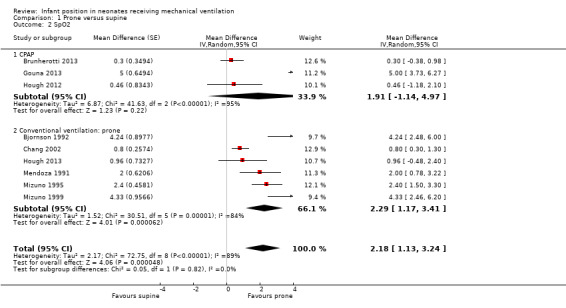
Comparison 1 Prone versus supine, Outcome 2 SpO2.
1.3. Analysis.
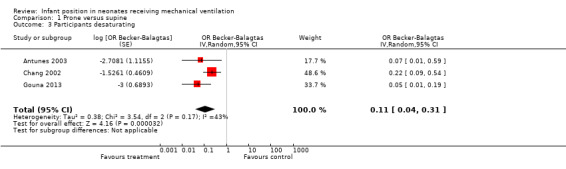
Comparison 1 Prone versus supine, Outcome 3 Participants desaturating.
1.4. Analysis.
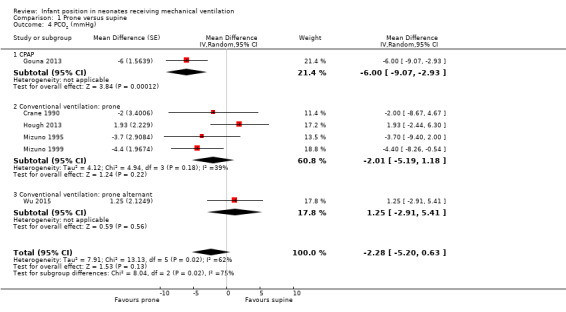
Comparison 1 Prone versus supine, Outcome 4 PCO2 (mmHg).
1.5. Analysis.

Comparison 1 Prone versus supine, Outcome 5 Tidal volume (mL/kg).
1.6. Analysis.
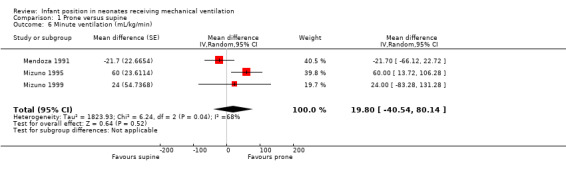
Comparison 1 Prone versus supine, Outcome 6 Minute ventilation (mL/kg/min).
1.7. Analysis.
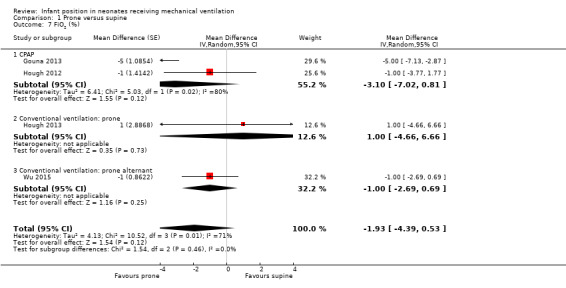
Comparison 1 Prone versus supine, Outcome 7 FiO2 (%).
1.8. Analysis.
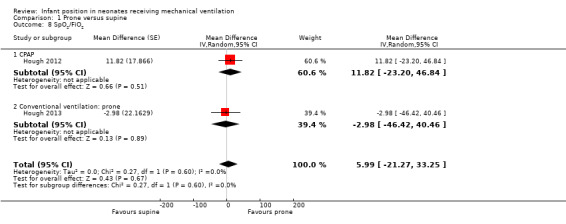
Comparison 1 Prone versus supine, Outcome 8 SpO2/FiO2.
1.9. Analysis.
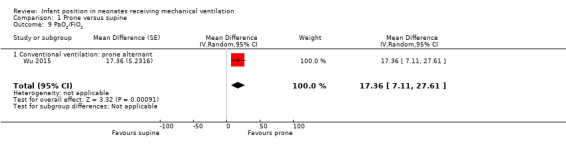
Comparison 1 Prone versus supine, Outcome 9 PaO2/FiO2.
1.10. Analysis.
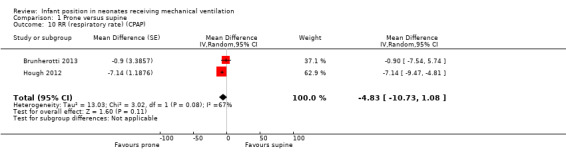
Comparison 1 Prone versus supine, Outcome 10 RR (respiratory rate) (CPAP).
Comparison 2. Prone versus lateral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PO2 (mmHg) | 1 | Mean Difference (Random, 95% CI) | ‐1.9 [‐8.56, 4.76] | |
| 1.1 Quarter prone | 1 | Mean Difference (Random, 95% CI) | ‐1.9 [‐8.56, 4.76] | |
| 2 SpO2 | 5 | Mean Difference (Random, 95% CI) | 0.90 [‐0.25, 2.06] | |
| 2.1 Lateral right | 2 | Mean Difference (Random, 95% CI) | 1.69 [‐0.61, 3.99] | |
| 2.2 Lateral Left (CPAP) | 2 | Mean Difference (Random, 95% CI) | 0.90 [0.30, 1.49] | |
| 2.3 Quarter prone | 2 | Mean Difference (Random, 95% CI) | ‐0.27 [‐1.50, 0.97] | |
| 3 PCO2 (mmHg) | 3 | Mean Difference (Random, 95% CI) | ‐0.59 [‐2.29, 1.11] | |
| 3.1 Lateral Right | 1 | Mean Difference (Random, 95% CI) | 0.0 [‐2.95, 2.95] | |
| 3.2 Lateral Left (CPAP) | 1 | Mean Difference (Random, 95% CI) | ‐1.0 [‐3.37, 1.37] | |
| 3.3 Quarter prone | 1 | Mean Difference (Random, 95% CI) | ‐0.47 [‐4.85, 3.91] | |
| 4 Tidal volume (mL/kg) | 1 | Mean Difference (Random, 95% CI) | 0.2 [‐0.27, 0.67] | |
| 4.1 Lateral left | 1 | Mean Difference (Random, 95% CI) | 0.2 [‐0.27, 0.67] | |
| 5 FiO2 (%) | 3 | Mean Difference (Random, 95% CI) | ‐0.64 [‐1.73, 0.44] | |
| 5.1 Lateral Left (CPAP) | 1 | Mean Difference (Random, 95% CI) | ‐1.0 [‐2.35, 0.35] | |
| 5.2 Quarter prone | 2 | Mean Difference (Random, 95% CI) | 0.0 [‐1.82, 1.82] | |
| 6 SpO2/FiO2 | 2 | Mean Difference (Random, 95% CI) | ‐1.77 [‐25.59, 22.05] | |
| 6.1 Quarter prone | 2 | Mean Difference (Random, 95% CI) | ‐1.77 [‐25.59, 22.05] | |
| 7 Sensitivity risk of bias: SpO2 lat left | 1 | Mean Difference (Random, 95% CI) | 0.8 [‐0.03, 1.63] |
2.1. Analysis.

Comparison 2 Prone versus lateral, Outcome 1 PO2 (mmHg).
2.2. Analysis.
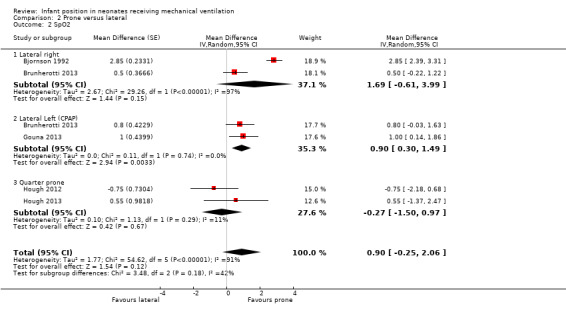
Comparison 2 Prone versus lateral, Outcome 2 SpO2.
2.3. Analysis.

Comparison 2 Prone versus lateral, Outcome 3 PCO2 (mmHg).
2.4. Analysis.
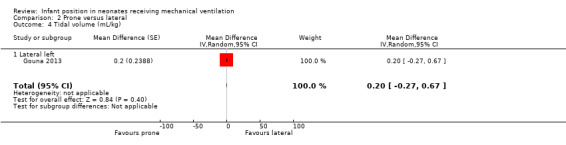
Comparison 2 Prone versus lateral, Outcome 4 Tidal volume (mL/kg).
2.5. Analysis.

Comparison 2 Prone versus lateral, Outcome 5 FiO2 (%).
2.6. Analysis.
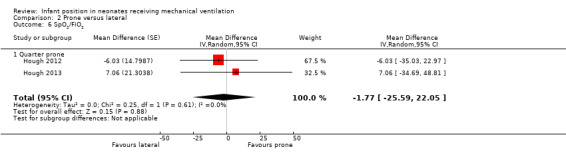
Comparison 2 Prone versus lateral, Outcome 6 SpO2/FiO2.
Comparison 3. Lateral versus supine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PO2 (mmHg) | 1 | Mean Difference (Random, 95% CI) | 1.98 [‐1.69, 5.65] | |
| 1.1 Lateral Right | 1 | Mean Difference (Random, 95% CI) | 1.1 [‐3.71, 5.91] | |
| 1.2 Lateral Left | 1 | Mean Difference (Random, 95% CI) | 3.2 [‐2.48, 8.88] | |
| 2 SpO2 | 5 | Mean Difference (Random, 95% CI) | 1.01 [‐0.04, 2.06] | |
| 2.1 Lateral Right | 2 | Mean Difference (Random, 95% CI) | 0.64 [‐0.91, 2.20] | |
| 2.2 Lateral Left (CPAP) | 2 | Mean Difference (Random, 95% CI) | 1.72 [‐2.69, 6.13] | |
| 2.3 Quarter prone | 2 | Mean Difference (Random, 95% CI) | 0.94 [‐0.23, 2.10] | |
| 3 PCO2 (mmHg) | 5 | Mean Difference (Random, 95% CI) | ‐0.36 [‐3.25, 2.53] | |
| 3.1 Lateral Right | 2 | Mean Difference (Random, 95% CI) | ‐0.69 [‐4.17, 2.79] | |
| 3.2 Lateral Left | 2 | Mean Difference (Random, 95% CI) | ‐3.21 [‐6.93, 0.51] | |
| 3.3 Alternant lateral | 1 | Mean Difference (Random, 95% CI) | 4.7 [‐0.31, 9.71] | |
| 3.4 Quarter prone | 1 | Mean Difference (Random, 95% CI) | 2.4 [‐1.55, 6.35] | |
| 4 FiO2 (%) | 4 | Mean Difference (Random, 95% CI) | ‐1.89 [‐3.98, 0.20] | |
| 4.1 Lateral Left (CPAP) | 1 | Mean Difference (Random, 95% CI) | ‐4.0 [‐6.21, ‐1.79] | |
| 4.2 Quarter prone | 2 | Mean Difference (Random, 95% CI) | ‐0.81 [‐2.58, 0.95] | |
| 4.3 Alternant lateral | 1 | Mean Difference (Random, 95% CI) | 0.0 [‐10.64, 10.64] | |
| 5 SpO2/FiO2 | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 Quarter prone | 2 | Mean Difference (Random, 95% CI) | 8.16 [‐17.87, 34.19] | |
| 6 Tidal volume (mL/kg) | 1 | Mean Difference (Random, 95% CI) | 0.70 [0.17, 1.23] | |
| 6.1 Lateral left | 1 | Mean Difference (Random, 95% CI) | 0.70 [0.17, 1.23] |
3.1. Analysis.
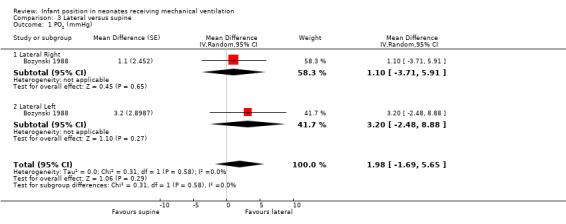
Comparison 3 Lateral versus supine, Outcome 1 PO2 (mmHg).
3.2. Analysis.
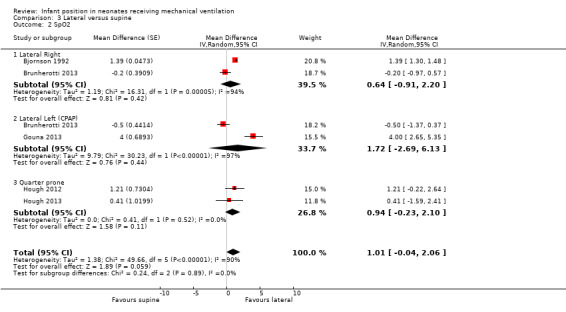
Comparison 3 Lateral versus supine, Outcome 2 SpO2.
3.3. Analysis.

Comparison 3 Lateral versus supine, Outcome 3 PCO2 (mmHg).
3.4. Analysis.
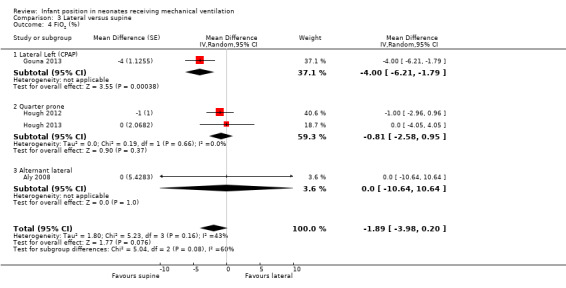
Comparison 3 Lateral versus supine, Outcome 4 FiO2 (%).
3.5. Analysis.

Comparison 3 Lateral versus supine, Outcome 5 SpO2/FiO2.
3.6. Analysis.

Comparison 3 Lateral versus supine, Outcome 6 Tidal volume (mL/kg).
Comparison 4. Lateral right versus lateral left.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PO2 (mmHg) | 2 | Mean difference (Random, 95% CI) | ‐0.05 [‐6.93, 6.83] | |
| 2 PCO2 (mmHg) | 2 | Mean difference (Random, 95% CI) | 0.41 [‐2.01, 2.82] | |
| 3 Tidal volume (mL/Kg) | 1 | Mean difference (Random, 95% CI) | ‐0.8 [‐1.87, 0.27] | |
| 4 SpO2 | 1 | Mean Difference (Random, 95% CI) | 0.3 [‐0.78, 1.38] |
4.1. Analysis.
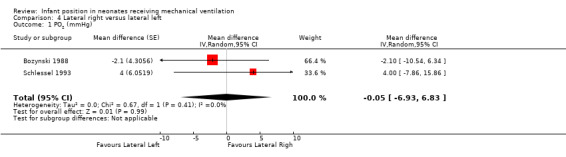
Comparison 4 Lateral right versus lateral left, Outcome 1 PO2 (mmHg).
4.2. Analysis.

Comparison 4 Lateral right versus lateral left, Outcome 2 PCO2 (mmHg).
4.3. Analysis.
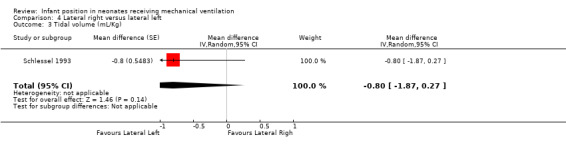
Comparison 4 Lateral right versus lateral left, Outcome 3 Tidal volume (mL/Kg).
4.4. Analysis.
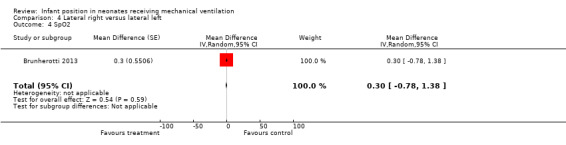
Comparison 4 Lateral right versus lateral left, Outcome 4 SpO2.
Comparison 5. Good lung dependent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PO2 (mmHg) | 1 | Mean difference (Random, 95% CI) | ‐7.75 [‐31.19, 15.69] | |
| 2 PCO2 (mmHg) | 1 | Mean difference (Random, 95% CI) | 0.75 [‐4.16, 5.66] |
5.1. Analysis.

Comparison 5 Good lung dependent, Outcome 1 PO2 (mmHg).
5.2. Analysis.
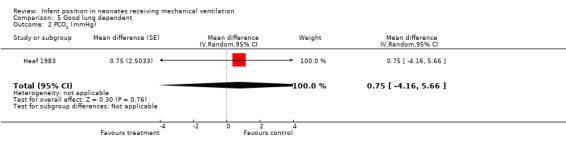
Comparison 5 Good lung dependent, Outcome 2 PCO2 (mmHg).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aly 2008.
| Methods |
DESIGN: randomised parallel controlled trial Method of randomisation: not stated Infant’s position: supine or lateral alternant (left and right every 2 hours) Blinding to intervention: no Complete follow‐up: no; 19 randomised participants dropped out of the study because of early death (15) or early extubation (4); group is unclear Blinding to outcome measurement: simple for tracheal culture |
|
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital of Cairo, Egypt DATE OF SAMPLE COLLECTION: started in 2005 and completed in 13 months PARTICIPANTS
|
|
| Interventions | INTERVENTIONS: lateral (alternating left or right every 2 hours) vs supine CO‐INTERVENTIONS: fewer episodes of self‐extubation and need for reintubation in the lateral group, but none stated | |
| Outcomes |
Outcomes of interest in this review: arterial PCO2
Outcomes not assessed in this review: culture of tracheal aspiration
|
|
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number randomised in each group unclear, no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Unclear risk | Co‐interventions not stated |
Aly 2015.
| Methods |
DESIGN: randomised parallel controlled trial Method of randomisation: opaque envelope technique Infant’s position: for 6 hours all in supine and another 6 hours in the randomly assigned position: supine or lateral right Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: pepsin in tracheal aspirate measured while blinded to group assignment |
|
| Participants |
SETTING: neonatal intensive care unit, Hospital of Cairo, Egypt DATE OF SAMPLE COLLECTION: started in January 2012 and completed in 18 months PARTICIPANTS
|
|
| Interventions | INTERVENTIONS: right lateral vs supine CO‐INTERVENTIONS: none stated | |
| Outcomes | Outcomes of interest in this review: pepsin measurement of tracheal aspiration. The first sample was obtained at the end of 6 hours; the second sample after another 6 hours, while lying in right lateral or supine position. Samples were collected 3 hours after the last feed. Outcomes not assessed in this review: ventilator parameters: PIP, PEEP, IMV; days of ventilation | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: opaque envelope technique |
| Allocation concealment (selection bias) | Low risk | Method of allocation: envelope opened after consent and participant assigned to allocated group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Unclear risk | Co‐interventions not stated |
Antunes 2003.
| Methods |
DESIGN: randomised parallel controlled trial Method of randomisation: drawing of lots in the form of sealed envelopes defining infants Infant’s position: supine or prone Blinding to intervention: no Complete follow‐up: no; 1 randomised participant did not complete the protocol but no reason stated Blinding to outcome measurement: no |
|
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital of Botucatu, Brazil DATE OF SAMPLE COLLECTION: started in 1999 PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine (from start of weaning of CV until his/her extubation, except 3 hours per day in newborns allocated to prone position) CO‐INTERVENTIONS: none stated |
|
| Outcomes |
Outcomes of interest in this review During intervention
Outcomes not assessed in this review During intervention
After intervention
|
|
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: drawing of lots in the form of sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation: not specified whether envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 drop‐out for no explained reason, no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Bjornson 1992.
| Methods |
DESIGN: randomised cross‐over controlled trial Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine, lateral right and prone Blinding to intervention: no Complete follow‐up: no; 2 randomised newborns needed changes in ventilatory parameters during the study and could not complete the 9 sessions prescribed (1 newborn: 6 sessions, another: 7 sessions) Blinding to outcome measurement: no |
|
| Participants |
SETTING: neonatal intensive care unit, Children’s Hospital of Seattle, EEUU DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine, prone vs lateral right, lateral right vs supine CO‐INTERVENTIONS (similar across comparisons): 1 newborn given sedation, 1 corticosteroids, 2 treatment for sepsis (1 surgical ligation for patent ductus arteriosus) |
|
| Outcomes |
Outcomes of interest in this review: oxy‐haemoglobin saturation measured by a pulse oximeter (SpO2) Outcomes not assessed in this review: none stated |
|
| User defined 1 | ||
| Notes | Results extracted from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No complete follow‐up in 2 newborns for reasons explained; no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | High risk | Low power of this study to detect a statistically significant and clinically relevant effect |
Bozynski 1988.
| Methods |
DESIGN: randomised cross‐over controlled trial Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine, lateral right and lateral left Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no |
|
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital of Michigan, EEUU DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: lateral right vs supine, lateral left vs supine, lateral right vs lateral left CO‐INTERVENTIONS (similar across comparisons): furosemide in 12 newborns, spironolactone + thiazide in 7 |
|
| Outcomes |
Outcomes of interest in this review: transcutaneous PO2 (tcPO2) and transcutaneous PCO2 (tcPCO2): only means and ranges Outcomes not assessed in this review: sleep state |
|
| User defined 1 | ||
| Notes | Results extracted from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Brunherotti 2013.
| Methods | DESIGN: randomised cross‐over controlled study Method of randomisation: sequence of positions according to previously drawn lots Infant’s position: 4 positions: A = supine, B = right lateral, C = prone, D = left lateral. Different positions were organised in the following sequences: 1 was A, D, C, B; 2 was C, A, B, D; 3 was B, C, D, A; 4 was D, B, A, C Blinding to intervention: no Complete follow‐up: no; 2 randomised infants were excluded at the beginning of data collection because they continued to be agitated Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, Sao Paulo, Brazil
DATE OF SAMPLE COLLECTION: started in 2009 PARTICIPANTS
|
|
| Interventions | INTERVENTIONS: left lateral vs supine, right lateral vs supine, prone vs supine, right lateral vs left lateral, prone vs left lateral, prone vs right lateral CO‐INTERVENTIONS: none stated | |
| Outcomes | Outcomes of interest in this review: Infants were kept in each position for 60 minutes. During this period, SpO2 was recorded at intervals of 10 minutes. Outcomes not assessed in this review: respiratory rate, heart rate, number of tachypnoea events | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: drawn lots |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 infants excluded for reasons explained, ITT unclear |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Chang 2002.
| Methods |
DESIGN: randomised cross‐over controlled trial Method of randomisation: randomisation in blocks by a third person to ensure balanced combinations of positions; finally by an identification number in a sealed envelope Infant’s position: random assignment to the order of supine and prone positions Blinding to intervention: no Complete follow‐up: 10 participants (4 in prone, 6 in supine) did not complete the 2‐hour protocol in the same position because of the need for interventions (airway suctioning, etc), then equal duration of a selected similarly distributed intervention was used for analysis Blinding to outcome measurement: no |
|
| Participants |
SETTING: neonatal intensive care unit, 2 tertiary care centres in Tainan, Taiwan
DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine CO‐INTERVENTIONS (similar across comparisons): surfactant in 18 newborns |
|
| Outcomes |
Outcomes of interest in this review
Outcomes not assessed in this review: change in motor activity by time and position; ventilator parameters: FiO2 global (mean ± SD, range), PIP, PEEP, mean airway pressure, ventilatory index during study, respiratory rate |
|
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: sealed envelopes |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No complete follow‐up for reason explained, no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Crane 1990.
| Methods | DESIGN: randomised cross‐over controlled study Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine, prone and lateral right Blinding to intervention: no Complete follow‐up: no; only 14 participants included finally in the study; data from 5 discarded by study authors because of excessive tcPCO2/PaCO2 difference or inaccurate calibration; no description as to whether the 5 exclusions were produced post randomisation and, in that case, whether they were analysed by ITT Blinding to outcome measurement: no | |
| Participants |
SETTING: newborn intensive care unit, University of Alabama, EEUU DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine, prone vs lateral right, lateral right vs supine
CO‐INTERVENTIONS: none stated |
|
| Outcomes | Outcomes of interest in this review: transcutaneous PCO2 (tcPCO2) Outcomes not assessed in this review: FiO2 global (mean ± SD, range) | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No complete follow‐up for reason explained, number randomised to each group and ITT unclear |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | High risk | Time of wash‐out only 1 to 4 minutes |
Fox 1990.
| Methods | DESIGN: randomised cross‐over controlled trial Method of randomisation: coin toss Infant’s position: random assignment to the order of positions: supine and prone Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital, Edmonton, Canada DATE OF SAMPLE COLLECTION: September 1986 to May 1988 PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine CO‐INTERVENTIONS: none stated |
|
| Outcomes | Outcomes of interest in the review: arterial PO2 (mmHg) (umbilical catheter attached to a monitor) Outcomes not assessed in this review: none stated | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: coin toss |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Gouna 2013.
| Methods | DESIGN: randomised cross‐over study Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine, prone and lateral left. Body positions were stabilised in a semiflexed posture for infants placed on the left side (tucked position). Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, Lille, France
DATE OF SAMPLE COLLECTION: over a 6‐month period, year not stated PARTICIPANTS
|
|
| Interventions | INTERVENTIONS: left lateral vs supine, prone vs supine, prone vs left lateral position CO‐INTERVENTIONS: All infants received caffeine treatment. | |
| Outcomes | Outcomes of interest in this review: SpO2, FiO2, transcutaneous PCO2, TV in mL/kg (spontaneous breath), apneic episodes; variables recorded 60 to 180 minutes after positioning and during quiet sleep periods only Outcomes not assessed in this review: phase angle between abdominal and thoracic movements, rib cage contribution to TV and dynamic elevation of end‐expiratory lung volume, heart and respiratory rates, arterial blood pressure | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Heaf 1983.
| Methods | DESIGN: randomised cross‐over controlled trial Method of randomisation: not stated; only randomised lateral position (left‐right), not supine position Infant’s position: good lung (uppermost or dependent) Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, Children’s Hospital of London, England DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: good lung dependent vs good lung uppermost CO‐INTERVENTIONS (similar across comparisons): in 3 newborns, surgery for congenital diaphragmatic hernia |
|
| Outcomes | Outcomes of interest in this review: transcutaneous PO2 (tcPO2) and transcutaneous PCO2 (tcPCO2), tidal volume in mL/kg (spontaneous breath) Outcomes not assessed in this review: thoracic gas volume, dynamic lung compliance | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | High risk | Low power of this study to detect a statistically significant and clinically relevant effect (n = 4) |
Hough 2012.
| Methods | DESIGN: randomised cross‐over study design Method of randomisation: sealed opaque envelopes Infant’s position: random assignment to the order of positions: supine, prone and quarter prone (right side uppermost) Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, South Brisbane, Australia DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions | INTERVENTIONS: quarter prone vs supine, prone vs supine, prone vs quarter prone CO‐INTERVENTIONS: none stated | |
| Outcomes | Outcomes of interest in this review: Physiological characteristics of oxy‐haemoglobin saturation measured by a pulse oximeter (SpO2), FiO2 and SpO2/FiO2 were measured 30 minutes after each position change. Outcomes not assessed in this review: respiratory rate; distribution of ventilation was assessed by measurement of regional impedance amplitude, global inhomogeneity index and phase angle analysis, via electrical impedance tomography | |
| User defined 1 | ||
| Notes | We contacted authors of these studies to request clarification of the method of randomisation and the population shared with Hough 2013 and Montgomery 2014, which have the same registration number (Australia New Zealand Clinical Trials Registry: ACTRN12606000210572) and include participants from Hough 2012 and Hough 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: computer‐generated randomisation with 6 different position sequences |
| Allocation concealment (selection bias) | Low risk | Method of allocation: sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | High risk | Shares a clinical trials registry and CPAP group with Montgomery 2014, and a control group with Hough 2013 and Montgomery 2014 |
Hough 2013.
| Methods | DESIGN: randomised cross‐over study design Method of randomisation: sealed opaque envelopes Infant’s position: random assignment to the order of positions: supine, prone and quarter prone (right side uppermost) Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: Assessor was blinded to body position. | |
| Participants |
SETTING: neonatal intensive care unit, South Brisbane, Australia DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions | INTERVENTIONS: quarter prone vs supine, prone vs supine, prone vs quarter prone CO‐INTERVENTIONS: none stated | |
| Outcomes | Outcomes of interest in this review: Physiological characteristics of oxy‐haemoglobin saturation measured by a pulse oximeter (SpO2), arterial PO2 (mmHg), arterial PCO2 (mmHg), FiO2 and SpO2/FiO2 were measured 30 minutes after each position change. Outcomes not assessed in this review: Distribution of ventilation was assessed by measurement of regional impedance amplitude, global inhomogeneity index and phase angle analysis via electrical impedance tomography. | |
| User defined 1 | ||
| Notes | To clarify the method of randomisation and the population shared with Hough 2012 and Montgomery 2014, we contacted study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: computer‐generated randomisation with 6 different position sequences |
| Allocation concealment (selection bias) | Low risk | Method of allocation: sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | High risk | Intervention group was shared with Montgomery 2014, and control group with Hough 2012 and Montgomery 2014. |
Jalali 2012.
| Methods | DESIGN: randomised parallel controlled trial Method of randomisation: random fixed block method Infant’s position: supine or lateral alternant (left and right every 2 hours) Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, Rasht, Iran DATE OF SAMPLE COLLECTION: 2010 to 2011 PARTICIPANTS
|
|
| Interventions | INTERVENTIONS: lateral alternant vs supine CO‐INTERVENTIONS: All received antibiotic treatment. | |
| Outcomes |
Outcomes of interest in this review: FiO2, SpO2, arterial PO2 and PCO2
Outcomes not assessed in this review
|
|
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: fixed block |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Mendoza 1991.
| Methods | DESIGN: randomised cross‐over controlled trial Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine and prone Blinding to intervention: no Complete follow‐up: 7 randomised participants were excluded from analysis of some lung mechanics because they had good spontaneous breaths in neither or only 1 position. Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital, Louisville, EEUU DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine CO‐INTERVENTIONS (similar across comparisons): theophylline |
|
| Outcomes | Outcomes of interest in this review: oxy‐haemoglobin saturation measured by a pulse oximeter (SpO2), TV in mL/kg (spontaneous breath), MV in mL/kg/min Outcomes not assessed in this review: dynamic lung compliance, resistance (expiratory and inspiratory), total work of breathing and heart rate, PIP, PEEP, RR, FiO2 global (mean) | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No complete follow‐up for reason explained, unclear ITT |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Mizuno 1995.
| Methods | DESIGN: randomised cross‐over controlled trial Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine and prone Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital, Tokyo, Japan DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine (before and after feeding) CO‐INTERVENTIONS: none stated |
|
| Outcomes | Outcomes of interest in this review: SpO2, tcPCO2, TV in mL/kg (spontaneous breath), MV in mL/kg/min Outcomes not assessed in this review: the aforementioned measured after feeding; spontaneous respiratory rate and heart rate measured before and after feeding; PIP, PEEP, FiO2 global (range, mean) | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | High risk | Low power of this study to detect a statistically significant and clinically relevant effect |
Mizuno 1999.
| Methods | DESIGN: randomised cross‐over controlled trial Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine and prone Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, Hospital of Chiba, Japan DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone vs supine (before, during and after feeding) CO‐INTERVENTIONS: none stated |
|
| Outcomes | Outcomes of interest in this review: SpO2, tcPCO2, TV in mL/kg (spontaneous breath), MV in mL/kg/min Outcomes not assessed in this review: the aforementioned measured during and after feeding, and the following measured before, during and after feeding: total MV, pulmonary resistance, static compliance, work of breathing for spontaneous breath, spontaneous breaths per minute, PIP, PEEP, FiO2 global (range, mean) | |
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | High risk | Low power of this study to detect a statistically significant and clinically relevant effect |
Schlessel 1993.
| Methods | DESIGN: randomised cross‐over controlled trial Method of randomisation: not stated Infant’s position: random assignment to the order of positions: supine and lateral (right or left) Blinding to intervention: no Complete follow‐up: yes Blinding to outcome measurement: no | |
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital, New York, EEUU DATE OF SAMPLE COLLECTION: not stated PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: lateral left vs lateral right vs supine (participants randomised to a sequence of positions: supine‐left‐supine‐right or supine‐right‐supine‐left) CO‐INTERVENTIONS: none stated |
|
| Outcomes | Outcomes of interest in this review: arterial PO2 (mmHg), arterial PCO2 (mmHg), TV in mL/kg (spontaneous breath) Outcomes not assessed in this review: dynamic lung compliance, total pulmonary resistance, inspiratory pulmonary resistance, expiratory pulmonary resistance, PIP, PEEP (mean ± SD) and FiO2 global (range) | |
| User defined 1 | ||
| Notes | PIP, PEEP and FiO2 global (range, mean) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
Wu 2015.
| Methods |
DESIGN: randomised parallel controlled trial
Method of randomisation: odd numbers into supine position group and even numbers into alternate position group
Infant’s position: supine or prone alternant (supine and prone every 4 hours)
Blinding to intervention: no
Complete follow‐up: 5 randomised participants (3 in supine and 2 in prone alternant, including 1 death in each group) did not complete the 16‐hour protocol in the same position for reasons not described, then equal duration of a selected similarly distributed intervention was used for analysis. Blinding to outcome measurement: Statisticians who analysed these data were not involved in study design and conduct. |
|
| Participants |
SETTING: neonatal intensive care unit, University Children’s Hospital, Xuzhou, China DATE OF SAMPLE COLLECTION: February 2012 to August 2013 PARTICIPANTS
|
|
| Interventions |
INTERVENTIONS: prone alternant position vs supine (participants randomised to a supine group or sequence of positions supine‐prone). All outcomes were measured 8 and 16 hours after each position change. CO‐INTERVENTIONS: none stated |
|
| Outcomes |
Outcomes of interest in this review
Outcomes not assessed in this review: dynamic lung compliance, minute volume, respiratory rate, PIP, PEEP. All dates after 1 hour of ventilator weaning were recorded and compared. |
|
| User defined 1 | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation: sequence by odd and even numbers, but method of generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No complete follow‐up for reasons not explained, no ITT |
| Selective reporting (reporting bias) | Unclear risk | No prespecified primary outcomes, so unclear |
| Other bias | Low risk | |
CLD: chronic lung disease. CPAP: continuous positive airway pressure. FiO2: fraction of inspired oxygen.
HCO3: blood bicarbonate level. IMV: intermittent mechanical ventilation. IPPV: intermittent positive‐pressure ventilation. ITT: intention‐to‐treat. MV: mechanical ventilation. NCPAP: nasal continuous positive airway pressure. PCO2: carbon dioxide tension. PEEP: positive end‐expiratory pressure. PIP: positive inspiratory pressure. PO2: arterial oxygen tension. RDS: respiratory distress syndrome.
RR: respiratory rate. SaO2: arterial oxygen saturation. SD: standard deviation. SpO2: oxy‐haemoglobin saturation measured by a pulse oximeter. tcPCO2: transcutaneous carbon dioxide method. tcPO2: transcutaneous oxygen method. TV: tidal volume.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ancora 2009 | Not randomised |
| Baird 1991 | Not randomised In this study, mechanically ventilated participants were mixed with non‐ventilated participants. |
| Curley 2005 | Age 2.0 years (0.3 to 11.0) (multi‐centre with Fineman 2006) |
| Fineman 2006 | Age 2.1 years (0.3 to 8.6) (multi‐centre with Curley 2005) |
| Ibrahim 2007 | Age 8 months to 9.9 years |
| Itakura 1998 | Not randomised This study did not supply oxygenation data but measured different pulmonary mechanical parameters. |
| Madlinger‐Lewis 2014 | Ventilation therapy is not an inclusion criterion; therefore, it could have been suspended during the intervention. |
| McEvoy 1997 | Not randomised Study of 55 infants, of which only 17 were mechanically ventilated |
| Schrod 1993 | Not randomised |
| Wagaman 1979 | Not randomised |
| Zhu 2010 | Not randomised for position |
Contributions of authors
Review authors made the following contributions to the original and 2013 versions of this review.
Albert Balaguer and May Rivas‐Fernandez independently selected new trials for inclusion on the basis of initial searches. May Rivas‐Fernandez updated ’Risk of bias’ tables for trials already included in the review, and similarly for any new trials identified in the update; these were checked by Albert Balaguer and Marta Roqué. Albert Balaguer and May Rivas‐Fernandez updated the Results section; this work was checked by Marta Roqué and Joaquín Escribano. All review authors participated in restructuring of remaining areas of the review and read and agreed with this updated review.
Review authors made the following contributions to the present revision of this review.
Albert Balaguer, Ana Díez and May Rivas‐Fernandez independently selected new trials for inclusion on the basis of initial searches. Ana Díez and May Rivas‐Fernandez updated ’Risk of bias’ tables for trials already included in the review, and similarly for any new trials identified in the update; these were checked by Albert Balaguer and Marta Roqué. Albert Balaguer, Ana Díez and May Rivas‐Fernandez updated the Results section; this work was checked by Marta Roqué and Joaquín Escribano. All review authors participated in restructuring of remaining areas of the review, and read and agreed with this updated review.
Sources of support
Internal sources
Universitat Internacional de Catalunya, Barcelona, Spain.
Hospital Universitari General de Catalunya, Barcelona, Spain.
External sources
Instituto de Salud Carlos III. Subdirección General de Investigación Sanitaria (01/A060), Spain.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aly 2008 {published data only}
- Aly H, Badawy M, El‐Kholy A, Nabil R, Mohamed A. Randomized, controlled trial on tracheal colonization of ventilated infants: can gravity prevent ventilator‐associated pneumonia?. Pediatrics 2008;122(4):770‐4. [DOI] [PubMed] [Google Scholar]
Aly 2015 {published data only}
- Aly H, Soliman RM, El‐Dib M, Said RN, Abdellatif MA, Sibaii H, et al. Does positioning impact tracheal aspiration of gastric content in ventilated infants?. Journal of Pediatric Gastroenterology and Nutrition 2015;60(3):327‐31. [DOI] [PubMed] [Google Scholar]
Antunes 2003 {published data only}
- Antunes LC, Rugolo LM, Crocci AJ. Effect of preterm infant position on weaning from mechanical ventilation. Jornal de Pediatria 2003;79(3):239‐44. [PubMed] [Google Scholar]
Bjornson 1992 {published data only}
- Bjornson K, Deitz J, Blackburn S, Billingsley F, Garcia I, Hays R. The effect of body position on the oxygen saturation of ventilated preterm infants. Pediatric Physical Therapy 1992;4(3):109‐15. [Google Scholar]
Bozynski 1988 {published data only}
- Bozynski ME, Naglie RA, Nicks JJ, Burpee B, Johnson RV. Lateral positioning of the stable ventilated very‐low‐birth‐weight infant. Effect on transcutaneous oxygen and carbon dioxide. American Journal of Diseases of Children 1988;142(2):200‐2. [DOI] [PubMed] [Google Scholar]
Brunherotti 2013 {published data only}
- Brunherotti MA, Martinez EZ, Martinez FE. Effect of body position on preterm newborns receiving continuous positive airway pressure. Acta Paediatrica 2014;103(3):e101‐5. [DOI] [PubMed] [Google Scholar]
Chang 2002 {published data only}
- Chang YJ, Anderson GC, Dowling D, Lin CH. Decreased activity and oxygen desaturation in prone ventilated preterm infants during the first postnatal week. Heart & Lung 2002;31(1):34‐42. [DOI] [PubMed] [Google Scholar]
Crane 1990 {published data only}
- Crane LD, Snyder JE, Knight P, Philips JB, Cassady G. Effects of position changes on transcutaneous carbon dioxide tension in neonates with respiratory distress. Journal of Perinatology 1990;10(1):35‐7. [PubMed] [Google Scholar]
Fox 1990 {published data only}
- Fox MD, Molesky MG. The effects of prone and supine positioning on arterial oxygen pressure. Neonatal Network 1990;8(4):25‐9. [PubMed] [Google Scholar]
Gouna 2013 {published data only}
- Gouna G, Rakza T, Kuissi E, Pennaforte T, Mur S, Storme L. Positioning effects on lung function and breathing pattern in premature newborns. Journal of Pediatrics 2013;162(6):1133‐7. [DOI: 10.1016/j.jpeds.2012.11.036; PUBMED: 23312684] [DOI] [PubMed] [Google Scholar]
Heaf 1983 {published data only}
- Heaf DP, Helms P, Gordon I, Turner HM. Postural effects on gas exchange in infants. New England Journal of Medicine 1983;308(25):1505‐8. [DOI] [PubMed] [Google Scholar]
Hough 2012 {published data only}
- Hough J, Johnston L, Brauer SG, Woodgate PG, Pham TM, Schibler A. Effect of body position on ventilation distribution in preterm infants on continuous positive airway pressure. Pediatric Critical Care Medicine 2012;13(4):446‐51. [DOI] [PubMed] [Google Scholar]
- Montgomery K, Choy NL, Steele M, Hough J. The effectiveness of quarter turn from prone in maintaining respiratory function in premature infants. Journal of Paediatrics and Child Health 2014;50(12):927‐7. [DOI] [PubMed] [Google Scholar]
Hough 2013 {published data only}
- Hough JL, Johnston L, Brauer S, Woodgate P, Schibler A. Effect of body position on ventilation distribution in ventilated preterm infants. Pediatric Critical Care Medicine 2013;14(2):171‐7. [DOI: 10.1097/PCC.0b013e31826e708a; PUBMED: 23314179] [DOI] [PubMed] [Google Scholar]
Jalali 2012 {published data only}
- Jalali SZ, Mojtabaei SH, Heidarzadeh A, Aghamahdi F, Ahmad‐Soltani M. The influence of lateral and supine position on bacterial colonization of endotracheal tube in neonates admitted to neonatal intensive care unit. Iran Journal of Pediatrics 2012;22(4):499‐504. [PMC free article] [PubMed] [Google Scholar]
Mendoza 1991 {published data only}
- Mendoza JC, Roberts JL, Cook LN. Postural effects on pulmonary function and heart rate of preterm infants with lung disease. Journal of Pediatrics 1991;118(3):445‐8. [DOI] [PubMed] [Google Scholar]
Mizuno 1995 {published data only}
- Mizuno K, Itabashi K, Okuyama K. Effect of body position on the blood gases and ventilation volume of infants with chronic lung disease before and after feeding. American Journal of Perinatology 1995;12(4):275‐7. [DOI] [PubMed] [Google Scholar]
Mizuno 1999 {published data only}
- Mizuno K, Aizawa M. Effects of body position on blood gases and lung mechanics of infants with chronic lung disease during tube feeding. Pediatrics International 1999;41(6):609‐14. [DOI] [PubMed] [Google Scholar]
Schlessel 1993 {published and unpublished data}
- Schlessel JS, Rappa HA, Lesser M, Harper R. Pulmonary mechanics and gas exchange: effect of lateral positioning during recovery from respiratory distress syndrome. Pediatric Pulmonology 1993;15(1):36‐40. [DOI] [PubMed] [Google Scholar]
Wu 2015 {published data only}
- Wu J, Zhai J, Jiang H, Sun Y, Jin B, Zhang Y, et al. Effect of change of mechanical ventilation position on the treatment of neonatal respiratory failure. Cell Biochemistry and Biophysics 2015;72(3):845–9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ancora 2009 {published data only}
- Ancora G, Maranella E, Aceti A, Pierantoni L, et al. Effect of posture on brain hemodynamics in preterm newborns not mechanically ventilated. Neonatology 2010;97(3):212‐7. [DOI] [PubMed] [Google Scholar]
Baird 1991 {published data only}
- Baird TM, Paton JB, Fisher DE. Improved oxygenation with prone positioning in neonates: stability of increased transcutaneous PO2. Journal of Perinatology 1991;11(4):315‐8. [PubMed] [Google Scholar]
Curley 2005 {published data only}
- Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury. JAMA 2005;294(2):229‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fineman 2006 {published data only}
- Fineman LD, LaBrecque MA, Shih MC, Curley MA. Prone positioning can be safely performed in critically ill infants and children. Pediatric Critical Care Medicine 2006;7(5):413‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2007 {published data only}
- Ibrahim T, El‐Mohamady H. Inhaled nitric oxide and prone position: how far they can improve oxygenation in pediatric patients with acute respiratory distress syndrome?. Journal of Medical Science 2007;7(3):390‐5. [Google Scholar]
Itakura 1998 {published data only}
- Itakura Y, Ogawa Y. Effect of body position on tidal volume and minute ventilation in very low birthweight infants. Acta Paediatrica Japonica 1998;40(6):555‐7. [DOI] [PubMed] [Google Scholar]
Madlinger‐Lewis 2014 {published data only}
- Madlinger‐Lewis L, Reynolds L, Zarem C, Crapnell T, Inder T, Pineda R. The effects of alternative positioning on preterm infants in the neonatal intensive care unit: a randomized clinical trial. Research in Developmental Disabilities 2014;35(2):490‐7. Erratum in: Res Dev Disabil 2015 Jun‐Jul; 41‐42:101‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
McEvoy 1997 {published data only}
- McEvoy C, Mendoza ME, Bowling S, Hewlett V, Sardesai S, Durand M. Prone positioning decreases episodes of hypoxemia in extremely low birth weight infants (1000 grams or less) with chronic lung disease. Journal of Pediatrics 1997;130(2):305‐9. [DOI] [PubMed] [Google Scholar]
Schrod 1993 {published data only}
- Schrod L, Frauendienst‐Egger G, Stockhausen HB. Effect of body position and positioning changes on lung function of ventilated premature and newborn infants. Klinische Padiatrie 1993;205(3):145‐9. [DOI] [PubMed] [Google Scholar]
Wagaman 1979 {published data only}
- Wagaman MJ, Shutack JG, Moomjian AS, Schwartz JG, Shaffer TH, Fox WW. Improved oxygenation and lung compliance with prone positioning of neonates. Journal of Pediatrics 1979;94(5):787‐91. [DOI] [PubMed] [Google Scholar]
Zhu 2010 {published data only}
- Zhu L, Xu Z, Ji G, Cai X, et al. Effect of prone or spine position on mechanically ventilated neonates after cardiac surgery with acute lung injury. National Medical Journal of China 2010;90(18):1260‐3. [PubMed] [Google Scholar]
Additional references
Adams 1994
- Adams JA, Zabaleta IA, Sackner MA. Comparison of supine and prone noninvasive measurements of breathing patterns in fullterm newborns. Pediatric Pulmonology 1994;18(1):8‐12. [DOI] [PubMed] [Google Scholar]
Bancalari 2001
- Bancalari E. Changes in the pathogenesis and prevention of chronic lung disease of prematurity. American Journal of Perinatology 2001;18(1):1‐9. [DOI] [PubMed] [Google Scholar]
Bhat 2006
- Bhat RY, Hannam S, Pressler R, Rafferty GF, Peacock JL, Greenough A. Effect of prone and supine position on sleep, apneas, and arousal in preterm infants. Pediatrics 2006;118(1):101‐7. [DOI] [PubMed] [Google Scholar]
Brown 2011
- Brown MK, DiBlasi RM. Mechanical ventilation of the premature neonate. Respiratory Care 2011;56(9):1298‐311. [DOI] [PubMed] [Google Scholar]
Chatte 1997
- Chatte G, Sab JM, Dubois JM, Sirodot M, Gaussorgues P, Robert D. Prone position in mechanically ventilated patients with severe acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 1997;155(2):473‐8. [DOI] [PubMed] [Google Scholar]
Cools 2009
- Cools F, Henderson‐Smart DJ, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD000104.pub3] [DOI] [PubMed] [Google Scholar]
Curley 2000
- Curley MA, Thompson JE, Arnold JH. The effects of early and repeated prone positioning in pediatric patients with acute lung injury. Chest 2000;118(1):156‐63. [DOI] [PubMed] [Google Scholar]
Davis 1998
- Davis BE, Moon RY, Sachs HC, Ottolini MC. Effects of sleep position on infant motor development. Pediatrics 1998;102(5):1135‐40. [DOI] [PubMed] [Google Scholar]
Dewey 1998
- Dewey C, Fleming P, Golding J. Does the supine sleeping position have any adverse effects on the child? II. Development in the first 18 months. ALSPAC Study Team. Pediatrics 1998;101(1):E5. [DOI] [PubMed] [Google Scholar]
Douglas 1977
- Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM. Improved oxygenation in patients with acute respiratory failure: the prone position. American Review of Respiratory Disease 1977;115(4):559‐66. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman D, Higgins J, Curtin F, Worthington H, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fox 1993
- Fox RE, Viscardi RM, Taciak VL, Niknafs H, Cinoman MI. Effect of position on pulmonary mechanics in healthy preterm newborn infants. Journal of Perinatology 1993;13(3):205‐11. [PubMed] [Google Scholar]
Fridrich 1996
- Fridrich P, Krafft P, Hochleuthner H, Mauritz W. The effects of long‐term prone positioning in patients with trauma‐induced adult respiratory distress syndrome. Anesthesia and Analgesia 1996;83(6):1206‐11. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Gattinoni 2001
- Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. New England Journal of Medicine 2001;345(8):568‐73. [DOI] [PubMed] [Google Scholar]
Gattinoni 2010
- Gattinoni L, Carlesso E, Taccone P, Polli F, Guérin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiological review and individual patient meta‐analysis. Minerva Anestesiologica 2010;76(6):448‐54. [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Henderson‐Smart 2009
- Henderson‐Smart DJ, Paoli AG, Clark RH, Bhuta T. High frequency oscillatory ventilation versus conventional ventilation for infants with severe pulmonary dysfunction born at or near term. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002974.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hewitt 1976
- Hewitt VM. Effect of posture on the presence of fat in tracheal aspirate in neonates. Australian Paediatric Journal 1976;12(4):267‐71. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org.
Jantz 1997
- Jantz JW, Blosser CD, Fruechting LA. A motor milestone change noted with a change in sleep position. Archives of Pediatric and Adolescent Medicine 1997;151(6):565‐8. [DOI] [PubMed] [Google Scholar]
Kishan 1981
- Kishan J, Bhargava SK, Rehman F. Effect of posture on arterial oxygen tension in preterm infants. Indian Pediatrics 1981;18(10):701‐4. [PubMed] [Google Scholar]
Martin 1979
- Martin RJ, Herrell N, Rubin D, Fanaroff A. Effect of supine and prone positions on arterial oxygen tension in the preterm infant. Pediatrics 1979;63(4):528‐31. [PubMed] [Google Scholar]
Masterson 1987
- Masterson J, Zucker C, Schulze K. Prone and supine positioning effects on energy expenditure and behavior of low birth weight neonates. Pediatrics 1987;80(5):689‐92. [PubMed] [Google Scholar]
Mizuno 2000
- Mizuno K, Inoue M, Takeuchi T. The effects of body positioning on sucking behaviour in sick neonates. European Journal of Pediatrics 2000;159(11):827‐31. [DOI] [PubMed] [Google Scholar]
Montgomery 2014
- Montgomery K, Choy NL, Steele M, Hough J. The effectiveness of quarter turn from prone in maintaining respiratory function in premature infants. Journal of Paediatrics and Child Health 2014;50(12):972‐7. [PUBMED: 25039401] [DOI] [PubMed] [Google Scholar]
Piehl 1976
- Piehl MA, Brown RS. Use of extreme position change in acute respiratory failure. Critical Care Medicine 1976;4(1):13‐4. [DOI] [PubMed] [Google Scholar]
Ratliff‐Schaub 2001
- Ratliff‐Schaub K, Hunt CE, Crowell D, Golub H, Smok‐Pearsall S, Palmer P, et al. Relationship between infant sleep position and motor development in preterm infants. Journal of Developmental and Behavioral Pediatrics 2001;22(5):293‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Schwartz 1975
- Schwartz FC, Fenner A, Wolfsdorf J. The influence of body position on pulmonary function in low birthweight babies. South African Medical Journal 1975;49(3):79‐81. [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Broek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. www.guidelinedevelopment.org/handbook. Updated October 2013.
Singer 1997
- Singer L, Yamashita T, Lilien L, Collin M, Baley J. A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics 1997;100(6):987‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visscher 1998
- Visscher F, Graaf T, Spaans M, Lingen RA, Fetter WP. Prone position favors motor development of infants. Nederlands Tijdschrift Voor Geneeskunde 1998;142(40):2879‐80. [PubMed] [Google Scholar]
Walsh 2005
- Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Development Neonatal Research Network. Extremely low birthweight neonates with protracted ventilation: mortality and 18‐month neurodevelopmental outcomes. Journal of Pediatrics 2005;146(6):798‐804. [DOI] [PubMed] [Google Scholar]
Wheeler 2010
- Wheeler K, Klingenberg C, McCallion N, Morley CJ, Davis PG. Volume‐targeted versus pressure‐limited ventilation in the neonate. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD003666.pub3] [DOI] [PubMed] [Google Scholar]
Wolfson 1992
- Wolfson MR, Greenspan JS, Deoras KS, Allen JL, Shaffer TH. Effect of position on the mechanical interaction between the rib cage and abdomen in preterm infants. Journal of Applied Physiology 1992;72(3):1032‐8. [DOI] [PubMed] [Google Scholar]
Yu 1975
- Victor YH. Effect of body position on gastric emptying in the neonate. Archives of Disease in Childhood 1975;50(7):500‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Balaguer 2003
- Balaguer A, Escribano J, Roqué i Figuls M. Infant position in neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003668] [DOI] [PubMed] [Google Scholar]
Balaguer 2006
- Balaguer A, Escribano J, Roqué i Figuls M. Infant position in neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003668] [DOI] [PubMed] [Google Scholar]
Balaguer 2013
- Balaguer A, Escribano J, Roqué i Figuls M, Rivas‐Fernández M. Infant position in neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD003668] [DOI] [PubMed] [Google Scholar]


