Abstract
Background
Heavy menstrual bleeding without an organic lesion is mainly due to an imbalance of the various hormones which have a regulatory effect on the menstrual cycle. Another cause of heavy menstrual bleeding with no pelvic pathology, is the presence of an acquired or inherited bleeding disorder. The haemostatic system has a central role in controlling the amount and the duration of menstrual bleeding, thus abnormally prolonged or profuse bleeding does occur in most women affected by bleeding disorders. Whereas irregular, pre‐menarchal or post‐menopausal uterine bleeding is unusual in inherited or acquired haemorrhagic disorders, severe acute bleeding and heavy menstrual bleeding at menarche and chronic heavy menstrual bleeding during the entire reproductive life are common. This is an update of a previously published Cochrane Review.
Objectives
To determine the efficacy and safety of non‐surgical interventions versus each other, placebo or no treatment for reducing menstrual blood loss in women with bleeding disorders.
Search methods
We searched the Cochrane Cystic Fibrosis Haemoglobinopathies Trials Register (25 August 2016), Embase (May 2013), LILACS (February 2013) and the WHO International Clinical Trial registry (February 2013).
Selection criteria
Randomised controlled studies of non‐surgical interventions for treating heavy menstrual bleeding (menorrhagia) in women of reproductive age suffering from a congenital or acquired bleeding disorder.
Data collection and analysis
Two authors independently assessed studies for inclusion, extracted data and assessed the risk of bias.
Main results
Three cross‐over studies, with 175 women were included in the review. All three studies had an unclear risk of bias with regards to trial design and overall, the quality of evidence generated was judged to be poor.
Two of the studies (n = 59) compared desmopressin (1‐deamino‐8‐D‐arginine vasopressin) with placebo. Menstrual blood loss was the primary outcome for both of these studies. Neither study found clear evidence of a difference between groups. The first of these reported a mean difference in menstrual blood loss in the desmopressin versus placebo group of 21.20 mL (95% confidence interval ‐19.00 to 61.50)
The second study reported that even though there was an improvement of pictorial bleeding assessment chart scores with desmopressin and placebo when compared to pretreatment assessment, there was no clear evidence of difference in these scores when the two were compared to each other (results presented graphically, P = 0.51). The data from these studies could not be combined.
The third study (n = 116) compared desmopressin with tranexamic acid (n = 116). This study found a decrease in pictorial bleeding assessment chart scores after both treatments as compared to baseline. The decrease in these scores was greater for tranexamic acid than for desmopressin, with a mean difference of 41.6 mL (95% confidence interval 19.6 to 63) (P < 0.0002).
In relation to adverse events, across two studies, there was no clear evidence of a difference when placebo was compared to desmopressin, risk ratio 1.17 (95% confidence interval 0.41 to 3.34) . The same was also true when desmopressin was compared to tranexamic acid, risk ratio 1.17 (95% confidence interval 0.41 to 3.34).
Only the study that compared desmopressin to tranexamic acid assessed quality of life. However, we are unable to present any data from this study, since no differences in this outcome between the two intervention groups were reported.
Authors' conclusions
Evidence from randomised controlled studies on the effect of desmopressin when compared to placebo in reducing menstrual blood loss is very limited and inconclusive. Two studies, each with a very limited number of participants, have shown uncertain effects in menstrual blood loss and adverse effects. A non‐randomised comparison in one of the studies points to the value of combining desmopressin and tranexamic acid, which needs to be tested in a formal randomised controlled study comparison.
When tranexamic acid was compared to desmopressin, a single study showed a reduction in menstrual blood loss with tranexamic acid use compared to desmopressin.
There is a need to evaluate non‐surgical methods for treating of menorrhagia in women with bleeding disorders through randomised controlled studies. Such methods would be more acceptable than surgery for women wishing to retain their fertility. Given that women may need to use these treatments throughout their entire reproductive life, long‐term side‐effects should be evaluated.
Keywords: Adult, Female, Humans, Blood Coagulation Disorders, Blood Coagulation Disorders/complications, Blood Coagulation Disorders/drug therapy, Deamino Arginine Vasopressin, Deamino Arginine Vasopressin/adverse effects, Deamino Arginine Vasopressin/therapeutic use, Hemostatics, Hemostatics/adverse effects, Hemostatics/therapeutic use, Menorrhagia, Menorrhagia/drug therapy, Menorrhagia/etiology, Randomized Controlled Trials as Topic, Tranexamic Acid, Tranexamic Acid/adverse effects, Tranexamic Acid/therapeutic use
Plain language summary
Medical therapies for treating heavy menstrual bleeding in women with bleeding disorders
Review question
We reviewed the evidence about the effect and safety of non‐surgical treatments versus each other, placebo or no treatment for reducing menstrual blood loss in women with bleeding disorders. This is an update of a previously published Cochrane Review.
Background
Heavy menstrual bleeding is one of the most common symptoms in women with bleeding disorders. A sizeable population of women with heavy menstrual bleeding are affected by either inherited or acquired bleeding disorders and at the time of presentation these women are considerably younger than the women who suffer from this due to other reasons. Since heavy menstrual bleeding starts at the very onset of menarche and continues throughout reproductive life, the quality of life of these women is severely affected and they are at an increased risk of developing iron‐deficiency anaemia.
Search date
The evidence is current to: 25 August 2016.
Study characteristics
The review included three studies on non‐surgical treatments in 175 women with a bleeding disorder who were experiencing heavy menstrual bleeding. Twostudies compared desmopressin to placebo and one study compared desmopressin to tranexamic acid. The women included in the studies were selected for one treatment or the other randomly. The studies lasted from two to four months.
Key results
Two studies of the three studies (with a total of 59 women) found no clear evidence of a difference in desmopressin (1‐deamino‐8‐D‐arginine vasopressin) in reducing menstrual blood loss when compared to placebo. One of these studies continued with an open non‐randomised comparison of a combination of desmopressin with tranexamic acid versus placebo and found a significant reduction in menstrual blood loss. However, the non‐randomised design of this comparison is an additional potential source of bias.
The third study (116 women), which had more participants than the other two studies combined, found a greater reduction in menstrual blood loss with tranexamic acid use than with desmopressin. We were unable to present any data on quality of life from this study, since no differences in between the two intervention groups were reported. There was no clear evidence of difference in the risk of side effects with desmopressin as compared to tranexamic acid. None of the studies dealt with cost effectiveness.
Quality of the evidence
We were not able to adequately assess the studies in relation to how the women were allocated to the treatment groups and we judged the overall quality of the evidence as poor.
Background
Description of the condition
Heavy menstrual bleeding (menorrhagia)
Heavy menstrual bleeding or menorrhagia is clinically defined as greater than or equal to 80 mL blood loss per menstrual cycle (Cole 1971; Halberg 1966). It is, however, the woman’s perception of her own menstrual loss which is the key determinant in her referral and subsequent treatment. Heavy menstrual bleeding is a public health challenge (James 2006). Insurance data and healthcare services research estimate that at least 5% to 10% of women of reproductive age will seek medical attention for menorrhagia (Oeheler 2003; Vessey 1992). Many women who seek treatment for heavy menstrual bleeding do not actually have greater losses than average (Haynes 1977). Heavy menstrual bleeding without an organic lesion is mainly due to an imbalance of the various hormones which have a regulatory effect on the menstrual cycle. Another cause of heavy menstrual bleeding with no pelvic pathology, is the presence of an acquired or inherited bleeding disorder. Eighty per cent of women treated for heavy menstrual bleeding have no anatomical pathology and over one third of the women undergoing hysterectomies for heavy menstrual bleeding have anatomically normal uteri removed (Clarke 1995; Gath 1982).
Heavy menstrual bleeding and the likelihood of bleeding disorders
Acquired and inherited platelet disorders can present with bleeding symptoms during adolescence. In adolescent females with platelet disorders, both of number and function, heavy menstrual bleeding is a common symptom (Philipp 2010). However, other studies have found an "infrequency of hematological problems" in adolescents presenting with heavy menstrual bleeding (Falcone 1994). Also, the severity of menstrual loss is not predictive of a bleeding disorder, as a significant cause of teenage heavy menstrual bleeding is anovulatory dysfunctional uterine bleeding (Jayasinghe 2005). Others have concluded that menorrhagia has a high predictive value for coagulation and platelet disorders and have suggested that laboratory tests to diagnose these disorders should be included in the investigation of heavy menstrual bleeding, and heavy menstrual bleeding per se can be a guideline in looking for mild bleeding disorders (Edlund 1996).
Besides inherited bleeding disorders, heavy menstrual bleeding may also occur in women with acquired bleeding disorders (van Eijkeren 1990). Antiplatelet drugs are the most common cause of acquired platelet disorders leading to bleeding. Uremia, hepatic cirrhosis, myeloma and related disorders, polycythaemia, essential thrombocythaemia, and cardiopulmonary bypass have long been recognized as clinical situations in which platelet dysfunction may contribute to bleeding (Hassan 2005). Data also support the close association between heavy menstrual bleeding and oral anticoagulant use (van Eijkeren 1990). Acquired haemophilia is a spontaneous autoimmune disorder in which individuals with previously normal haemostasis develop autoantibodies against clotting factors, most frequently FVIII (Von Depka 2002). Acquired von Willebrand disease (AVWD) is a rare, under‐diagnosed hemorrhagic disorder, which is similar to congenital VWD with regard to the clinical and laboratory parameters; however, it is found in individuals with no positive family history and has no genetic basis. The etiology is varied, the commonest being secondary to haemato‐proliferative disorders and cardiovascular disorders. Other disorders associated with AVWD are autoimmune disorders such as systematic lupus erythematosus, hypothyroidism, and neoplasia, or it may also be drug induced. In quite a few cases, the etiology is unknown (Shetty 2011).
Heavy menstrual bleeding in women with bleeding disorders
The haemostatic system has a central role in controlling the amount and the duration of menstrual bleeding, thus abnormally prolonged or profuse bleeding does occur in most women affected by bleeding disorders. Whereas irregular, pre‐menarchal or post‐menopausal uterine bleeding is unusual in inherited or acquired haemorrhagic disorders, severe acute bleeding and heavy menstrual bleeding at menarche and chronic heavy menstrual bleeding during the entire reproductive life are common manifestations (Rodeghiero 2008). Studies have reported that one out of five women who consulted their doctor because of heavy, prolonged bleeding during their periods actually had a bleeding disorder. This means that heavy menstrual bleeding caused by bleeding disorders is much more common than previously thought (Kadira 1998). Subsequently, several studies have reported on the prevalence of heavy menstrual bleeding in women with bleeding disorders. Inherited bleeding disorders are found in a substantial proportion of women with heavy menstrual bleeding and a normal pelvic examination (Kadir 2002). In general the prevalence of bleeding disorders, among adult women with objectively documented heavy menstrual bleeding is consistently reported to be 10% to 20% (El‐Hemaidi 2007) and is even higher in adolescents presenting with the condition (Demers 2006).
While an estimated 13% of women with unexplained heavy menstrual bleeding have VWD, the frequency of other potential bleeding disorders has been uncertain. Laboratory abnormalities of haemostasis, especially platelet function defects, were common among women with unexplained heavy menstrual bleeding within a multi‐racial population in the USA (Miller 2011; Phillip 2003).
In a large majority of women with heavy menstrual bleeding, this is caused by hereditary defects in platelet function; with VWD being the commonest coagulation defect. It has been suggested that screening tests for haemostasis, especially for VWD and inherited platelet function defects, must be performed in women presenting with heavy menstrual bleeding (Saxena 2003). The World Federation of Hemophilia (WFH) reports the prevalence of heavy menstrual bleeding with various bleeding disorders as: VWD 74% to 92%; Bernard Soulier syndrome 51%; Glanzmann thrombasthenia 98%; Factor XI deficiency 59%; carriers of haemophilia 57%; and in other rare factor deficiencies 35% to 70% (Shankar 2004).
Description of the intervention
A range of medical therapies are prescribed in order to reduce excessive menstrual blood loss (MBL) or heavy menstrual bleeding. Women having heavy menstrual bleeding with bleeding disorders may also need the specific deficient factor. Thus the therapy aimed at reducing the blood loss could act at different levels:
tranexamic acid (antifibrinolytic agent);
mefenamic acid;
combined oral contraceptives (OCPs);
progesterone (oral, parenteral, transdermal, progesterone containing IUCD);
danazol;
ethamsylate;
desmopressin (1‐deamino‐8‐D‐arginine vasopressin) (subcutaneous or intranasal);
factor replacement therapy (plasma‐derived and recombinant).
How the intervention might work
Tranexamic acid
Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of haemostatic fibrin by plasmin. In the presence of tranexamic acid, the lysine receptor binding sites of plasmin for fibrin are occupied, preventing binding to fibrin monomers, thus preserving and stabilizing fibrin’s matrix structure.
Common side effects of tranexamic acid include headaches, sinus and nasal symptoms, back pain, abdominal pain, musculoskeletal pain, joint pain, muscle cramps, migraine, anaemia and fatigue.
In relation to drug interactions, given that tranexamic acid is an antifibrinolytic, concomitant use of hormonal contraception may further exacerbate the increased thrombotic risk associated with combination hormonal contraceptives. For the same reason tranexamic acid is not recommended in people taking either factor IX complex concentrates or anti‐inhibitor coagulant concentrates. Individuals (with promyelocytic leukaemia) taking all‐trans retinoic acid (oral trenitoin) when given tranexamic acid have severe thrombotic complications. In people with renal impairment doses of tranexamic acid need to be adjusted as plasma concentrations may be much higher than persons with normal renal function (FDA 2011).
Mefenamic acid
This is a non‐steroidal anti‐inflammatory drug which is a potent prostaglandin inhibitor. Prostagladin inhibitors have been found to decrease menorrhagia by 30% to 50% (van Eijkeren 1992). Mefenamic acid has been used effectively for the control of long‐standing menorrhagia (Fraser 1983). When compared with tranexamic acid and levonorgestrel‐containing intrauterine devices, mefenamic acid significantly decreased MBL (Reid 2005). Side effects of this drug are vomiting, diarrhoea, headache and hematuria.
Combined oral contraceptives (OCPs)
When taken in a cyclical fashion, OCPs induce regular shedding of a thinner endometrium and inhibit ovulation. Using this method, good cycle control can be achieved and, together with the provision of contraception, this makes OCP a most acceptable longer‐term therapy for some women with heavy menstrual bleeding (Farquhar 2009). Besides this, oral pills could also be used continuously to gain the same effect as above and prevent any blood loss for a given period, usually three months. This would be particularly beneficial to women who become anaemic as a result of heavy periods.
Progesterones (oral, parenteral, transdermal, intrauterine)
These induce an arrest of glandular proliferation, pseudo‐secretion, and stromal oedema followed by decidualized stroma with granulocytes and thin sinusoidal blood vessels. Prolonged use results in progressive endometrial atrophy (Deligdisch 2000).
Danazol
This drug acts by means of its anti‐gonadotrophic effects on the pituitary gland; however, a review of the literature reveals that its efficacy in suppressing normal endometrial growth and in causing atrophy of deposits of endometrium cannot be explained solely on this basis. Recent information indicates that, besides acting at the pituitary level, a major mechanism of action may be by a direct inhibitory effect on target tissues. It is suggested that such a mechanism would more readily account for the diverse effects of this drug in the treatment of many disorders, all of which appear to be associated with an imbalanced sensitivity of target organs to steroid hormones (Jenkin 1980). Danazol has androgenic properties (a tendency to cause male characteristics) which may result in acne, seborrhoea (greasy skin) and hirsutism (excessive hair growth). Other side effects include weight gain, irritability, musculoskeletal pains, hot flushes and breast atrophy (loss of breast tissue). Longer‐term treatment with danazol may cause side effects in the liver (including benign hepatic adenomas) in some women.
Ethamsylate
Ethamsylate (2,5‐dihydroxy‐benzene‐sulfonate diethyl ammonium salt) is a synthetic haemostatic drug indicated in cases of capillary bleeding. Ethamsylate acts on the first step of haemostasis by improving platelet adhesiveness and restoring capillary resistance (Lethaby 2000). Recent studies showed that ethamsylate promotes P‐selectin‐dependent, platelet adhesive mechanisms. It is a mild but well‐tolerated drug, particularly useful in dysfunctional uterine bleeding when contraception is not needed (Garay 2006).
Desmopressin (1‐deamino‐8‐D‐arginine vasopressin)
Desmopressin is a derivative of the antidiuretic hormone vasopressin (Mannuci 1997). Desmopressin acts by increasing plasma levels of factor VIII and von Willebrand factor (VWF) (Douglas 1997). The increases in the plasma levels of factor VIII and VWF occur not only in deficient individuals, but also in healthy individuals and in those who already have high levels of these factors. The mechanism by which the plasma levels of FVIII and VWD are increased is by degranulation of endothelial cells. Desmopressin shortens the prolonged activated partial thromboplastin time and the bleeding time (Mannuci 1997). Given this effect, it has been used in the reduction of MBL in women with heavy menstrual bleeding and prolonged bleeding time, both with (Edlund 2002) and without (Kadir 2002) inherited bleeding disorders. Desmopressin is a possible complement for the medical treatment of heavy menstrual bleeding (Edlund 2002). It has also been found to be as effective as mefenamic acid in controlling intrauterine device‐induced heavy menstrual bleeding (Mercorio 2003).
Desmopressin can be used in mild to moderate VWD type 1, mild to moderate haemophilia A and type 2A, and type 2M VWD. However, a test dose needs to be given to monitor whether it results in a sufficient rise in VWF; in type 2B it is contra‐indicated and of no use in type 2N and type 3. Its responsiveness must be assessed in severe type 1 VWD, and effectiveness is unpredictable in people with mild moderate haemophilia A with factor VIII antibodies (Hardman 2001). It is also usually effective in several qualitative platelet function defects.
Desmopressin has a relatively high rate of adverse effects that lead to discontinuation in about 20% of users (Dunn 2000). Reported side effects include water intoxication (with hyponatraemia and occasionally seizures), smooth muscle cramps, vasoconstriction (Hardman 2001) and allergic reactions. Desmopressin should be used with great caution in individuals with vascular diseases, especially coronary atherosclerosis.
Factor replacement therapy
Plasma‐derived clotting factors
factor VII concentrates
factor VIII concentrates
factor VIII / VWF concentrates
cryoprecipitate
plasma fraction with factor VIII inhibitor bypassing activity (FEIBA)
human prothrombin complex concentrates
factor IX concentrates
fibrinogen
factor XI concentrates
factor XIII concentrates
While human blood is a valuable source of many therapeutic proteins, inadequately screened human blood or blood components can transmit a variety of pathogens, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV) and West Nile virus (Fredrick 2008). Since some manufacturing steps (e.g. sterile filtration and freeze‐thaw cycling) remove large pathogens, such as bacteria and parasites, only viruses continue to pose serious threats to the safety of plasma‐derived medicinal proteins (Cai 2005). Recent improvements in protein processing have increased the purity and yields of human plasma‐derived proteins (Cai 2005). No transmission of HBV, HCV or HIV attributable to manufactured plasma derivatives licensed for use in the USA has been reported since the introduction of effective virus inactivation procedures in 1985. Since current screening tests cannot exclude all of the still unknown human pathogens, nor completely anticipate future blood transfusion‐transmitted agents, even adequately screened blood is now deemed to always carry a very low risk for the transmission of pathogens (Klein 2004).
The other concern with the use of blood products is the immunological response of the recipient. While the clinical consequences of immune responses to biopharmaceutical proteins are generally benign, immune responses to some biological proteins can have serious adverse clinical consequences (Chamberlain 2003; Chalmers 2007; Schelkens 2003)
Recombinant factors
recombinant factor VIII (rFVIII)
recombinant factor IX (rFIX)
recombinant factor VIIa (rFVIIa)
recombinant VWF
recombinant factor XIII (FrXIII) (Pipe 2008)
Recombinant factors aim to eliminate all human and animal protein and are therefore theoretically much safer.
Why it is important to do this review
Although several consensus groups have drafted guidelines for the treatment of heavy menstrual bleeding in women with bleeding disorders (NZ Guidelines Group 1999; NICE Guidelines 2007), there is, as yet, no definitive recommendations based on randomised controlled studies. The estimated community prevalence of bleeding disorders is 2%, these disorders are consistently reported to affect 10% to 20% of women with objectively documented heavy menstrual bleeding and are reported to be even higher in adolescents (El‐Hemaidi 2007). Thus, a sizeable population of women with heavy menstrual bleeding are affected by either inherited or acquired bleeding disorders and at the time of presentation these women are considerably younger than the women who have heavy menstrual bleeding due to other reasons. Since heavy menstrual bleeding manifests at the very onset of menarche and continues throughout the reproductive age group, these women are at an increased risk of developing iron‐deficiency anaemia (Chen 2008). Excessive blood loss during every cycle leads to significant impairment in the quality of life (QoL) (Kadir 2010). Given this, non‐surgical interventions are most appropriate. Since this therapy would have to be initiated early in life and continued for a reasonably long time, it is essential that both the efficacy and safety be evidence‐based. This is an update of a previously published Cochrane Review (Ray 2014).
Objectives
To determine the efficacy and safety of non‐surgical interventions versus each other, placebo or no treatment for reducing MBL in women with bleeding disorders.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies.
Types of participants
Participants included were women of reproductive years suffering from a congenital or acquired bleeding disorder with regular heavy periods measured either objectively or subjectively.
The exclusion criteria were women with:
• irregular menses (periods either less than 21 days or more than 35 days apart) and intermenstrual bleeding; • pelvic pathology such as fibroids, endometriosis malignancies, etc; • Iatrogenic causes of heavy menstrual bleeding (IUCD or drug‐induced); • post‐menopausal bleeding (more than one year from the last period).
Types of interventions
We had aimed to compare all the following interventions with each other, with no treatment or with placebo:
desmopressin;
tranexamic acid;
mefenamic acid;
progesterone (oral, transdermal, parental or depot preparations or in IUCD);
OCPs;
ethamsylate;
clotting factor concentrates.
Types of outcome measures
Primary outcomes
-
Menstrual blood loss (MBL)*
objective assessment of blood loss (mL)
-
subjective assessment of blood loss
participant’s satisfaction as regards reduction in blood loss (very satisfied, satisfied, not sure, not satisfied)
participant's perception of blood loss (reduced, same, increased)
-
indirect measures of blood loss
duration of loss (days)
number of sanitary pads
pictorial bleeding assessment charts (PBACs)
Adverse effects
* The preferred technique to estimate the MBL is by determination of the concentration of haemoglobin in menstrual fluid by its conversion to alkaline hematin (Fraser 1985). However, as this method is not normally available except for research purposes, we also included studies with accounts from women on the heaviness of bleeding.
Secondary outcomes
Quality of life (QoL) (using a reproducible and validated format or subjectively by participant questionnaires)
Change from baseline in haemoglobin and hematocrit values
Requirement for additional surgical treatment
Requirement for blood transfusion
Resource cost (for total duration of study)
Search methods for identification of studies
Electronic searches
We searched the Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register for relevant studies using the term: menorrhagia.
The Coagulopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE and the prospective handsearching of one journal ‐ Haemophilia. Unpublished work is identified by searching the abstract books of major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Congress of the World Federation of Hemophilia; the European Association for Haemophilia and Allied Disorders, the American Society of Gene and Cell Therapy and the International Society on Thrombosis and Haemostasis. For full details of all searching activities for the register, please see the relevant section of the Cystic Fibrosis and Genetic Disorders Group Module.
The date of the search of the Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register: 25 August 2016.
We also searched the following:
Embase through OVID (from inception to 3 May 2013) (Appendix 1).
LILACS (from inception to 24 February 2013) (Appendix 2).
WHO International Clinical Trials Registry Platform's search portal (24 February 2013) Appendix 3;
Searching other resources
We searched reference lists of all articles retrieved by the searches. We contacted the individual researchers working in this field, organisations and pharmaceutical companies in order to identify unpublished and ongoing studies. We also searched the congress proceedings of the International Society for Thrombosis and Hemostasis. The date of the search was 18 February 2013.
In our searches, there were no language or year of search restrictions.
Data collection and analysis
Selection of studies
Two authors (AR and SR) independently screened all citations and abstracts identified by the search strategy and included all eligible studies. They assessed the full reports of all studies for inclusion in the review. If eligibility was unclear, they sought more data from the study authors. Authors resolved disagreements through discussion. Authors documented reasons for exclusion of studies. Please see relevant flow chart (Figure 1).
1.
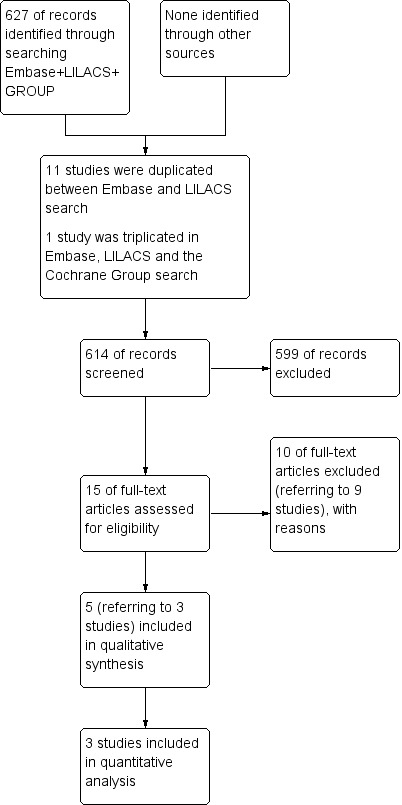
Study flow diagram.
Data extraction and management
Both authors independently extracted the data and information (as listed below) from relevant studies using a pre‐tested data extraction form and resolved disagreements regarding data extraction by discussion.
Study characteristics
Baseline characteristics (randomised controlled study, single or multicentre, etc)
Method of sequence generation
Method of allocation concealment
Blinding (of participants, personnel and outcome assessors)
Number of women randomised, excluded or lost to follow up
Whether an intention‐to‐treat analysis was done
Whether a power calculation was done
Duration, timing and location of the study
Source of funding
Participant characteristics
Age
Type of bleeding disorder
Inclusion criteria
Exclusion criteria
Interventions
Details of interventions, including the dose, route, duration, and combination with other medical interventions
Type of control group (placebo, medical treatment or no treatment)
Outcomes
The authors extracted, where possible, data for the outcomes as listed in the Types of outcome measures section above.
The authors aimed to compare interventions individually and in combination versus placebo or no treatment or other active treatment to reduce MBL in women with bleeding disorders. If in future updates the authors are able to compare other non‐surgical interventions or combinations of such interventions with placebo or no treatment in randomised controlled studies, they will include them for meta‐analysis.
The authors aimed to present data (if available) separately for short‐term MBL (two to seven days) and long‐term MBL (over one week and up to six months). However, the three studies included in the review only presented data on the second and the third day of the menstrual cycle and for two cycles in a cross‐over design (Edlund 2002; Kadir 2000; Kouides 2009). If, for future versions of the review, outcomes are reported at baseline and at short‐ and long‐term follow up, the authors will extract mean change from baseline and the standard deviation (SD) of this mean for each group.
Assessment of risk of bias in included studies
Two review authors (SR an AR) independently assessed the risk of bias of the included studies using the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). They followed guidance given in the handbook when assessing whether adequate steps were taken to reduce the risk of bias across six components:
sequence generation;
allocation concealment;
blinding (of participants, personnel, and outcome assessors);
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
The authors categorised judgements in order to indicate a low, high, or unclear risk of bias. In addition to the 'Risk of bias’ table for each included study, they summarised the results using the 'Risk of bias' graph (Figure 2) and the 'Risk of bias' summary (Figure 3).
2.
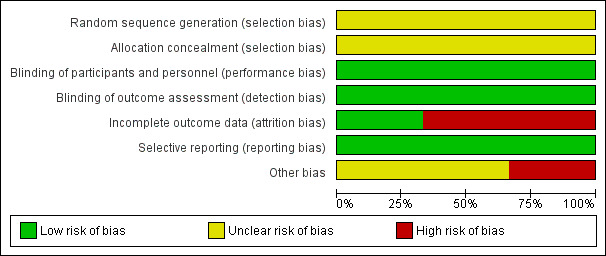
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
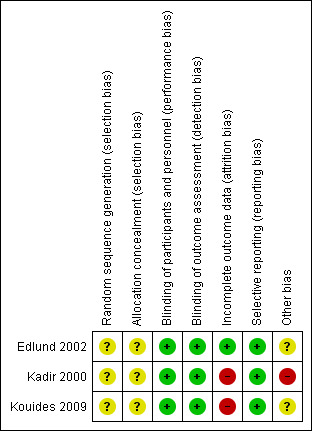
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For dichotomous outcomes, where data were available, the authors used the risk ratio (RR) and with the corresponding 95% confidence intervals (CI). The authors had planned to calculate RR for the first primary outcome of MBL at the end of the first treatment cycle as all three included studies were of a cross‐over design (Elbourne 2002). They were unable to do so as two of the studies had reported MBL as a composite value at the end of both the treatment cycles for desmopressin and placebo (Edlund 2002; Kadir 2000). In addition one of the studies had reported MBL as a median value which they could not synthesize with the other study reporting MBL as a mean value (Kadir 2000). The third study, which did have an actual cross‐over design did not report any comparison which could be pooled with the other two studies (Kouides 2009).
In future updates, if included studies present data for evaluating MBL in different ways (e.g. mean blood loss or the PBAC), the authors plan to use the standardised mean difference (SMD). If this is not possible, they will not pool the data but will analyse these separately. Similarly, the authors will not pool data from studies which measure QoL using different scales.
For some dichotomous outcomes (e.g. the proportion of participants requiring further surgery), a higher proportion represents a negative consequence of that treatment and for other outcomes (e.g. proportion with minimisation of MBL), a higher proportion is considered a benefit of treatment. In future updates the authors will make this discrepancy between the categorising of outcomes clear when they construct the summary graphs for the meta‐analysis for the assessment of the benefits as opposed to the harms of treatment. Thus, for some of the dichotomous outcomes they will display a treatment benefit as RR and CIs to the left of the centre line, while for others they will display a treatment benefit to the right of the centre line. For clarification, the authors will label the forest plots for each outcome. If time‐to‐event outcomes are reported, the authors will extract the estimates of the log hazard ratio and its standard error (SE). If SEs are not available, they will extract alternative statistics.
In future updates, should continuous data be included, the authors plan to calculate the mean difference (MD) with their corresponding 95% CIs. If reports had summarized continuous data using geometric means, the authors plan to combine them on the log scale using the generic inverse variance method and report them on the natural scale. For count data, the authors will extract the total number of events in each group, the total amount of person‐time at risk in each group and the total number of participants in each group. If this information is not available, they will extract alternative summary statistics, such as rate ratios and their CIs. If reports present count data as dichotomous, continuous or time‐to‐event data, authors will analyse these using RR, MD, or log hazard ratios, respectively.
Unit of analysis issues
The authors note the unit of analysis at the level of randomisation was the individual in all three studies and analysed accordingly.
The authors were only able to pool data for adverse events for two studies. They had planned to use the results from the first treatment period only; however, could not do this as the study investigators ignored the cross‐over design and presented the data as though from a parallel study (Edlund 2002; Kadir 2000).
In future updates of this review, if there are more randomised control studies with the individual as the unit of analysis or a group (cluster randomised) as the unit, the authors will analyse accordingly. Also, if in future there are further cross‐over studies, the authors plan to analyse the results from the first treatment period only (Elbourne 2002). They will also consider further methods as described by Elbourne (Elbourne 2002; Higgins 2011b).
Dealing with missing data
The authors were not able to obtain the numerical data in a form which could be synthesized for any of the three studies included in the quantitative analysis.
The published MBL and QoL data for the Kouides study is not in a form that can be entered in the the analysis section of the review, thus the authors report available data narratively and have requested individual patient data in order to included these in a future update.
In future updates of the review, when more studies are included, the authors will attempt to obtain all missing data from study investigators. Where possible they will extract data to allow an intention‐to‐treat (ITT) analysis in which all randomised participants are analysed in the groups to which they were originally assigned. If there is discrepancy in the number analysed and the number randomised, the authors will calculate the percentage lost to follow up in each group and report this loss. If dropouts exceed 10% for any study, they will assign the worst outcome to those lost from that group for dichotomous outcomes and assess the impact for sensitivity analyses with the results of participants who complete the study. For continuous data, if SDs are missing, the authors will calculate these from other available data, such as SEs, or impute them using suggested methods (Higgins 2011a). They will not make assumptions about loss to follow up for continuous data and will analyse results for those who complete the study.
Assessment of heterogeneity
In future updates of this review, when more data are available, the authors will assess heterogeneity between the studies by visual examination of the forest plot to check for overlapping CIs, using the chi2 test for homogeneity and a 10% level of significance, and the I2 statistic. A value for the I2 statistic of less than 25% will denote low heterogeneity, 50% or greater will denote significant heterogeneity, and 75% or greater will denote substantial heterogeneity.
Assessment of reporting biases
The authors do not regard publication bias as likely given the comprehensive nature of our search strategies. Outcome reporting bias was unlikely as all three studies reported the outcomes that were specified in their objectives.
In future versions of this review, the authors will also assess the likelihood of publication bias using funnel plots (provided there are at least 10 studies).
Data synthesis
The authors have synthesized the dichotomous data (adverse effects) from two studies using RR and 95% CI using the RevMan software (Edlund 2002; Kadir 2000) (RevMan 2011). There were no continuous data available for synthesis. The primary outcome of interest to the review was MBL, which was reported by all three studies. The Kadir and the Edlund studies assessed MBL for the same comparison but in different ways (by alkali hematin and by a PBAC). In addition to this, one of the studies reported mean blood loss (Edlund 2002) the other reported median blood loss and we were unable to obtain the mean values from the study authors (Kadir 2000).The Kouides study reported MBL by PBAC for a different comparison and so could not be pooled with the other two studies (Kouides 2009).
Subgroup analysis and investigation of heterogeneity
If more studies are included in future versions of the review, the authors plan the following subgroup analysis as a means of investigating heterogeneous results and also to answer specific questions about the above types of interventions.
Continuous intake of oral pills and cyclical intake
Different doses of progesterone only pills, e.g. high dose or low dose if applicable
Different doses of oestrogen plus progesterones in combined pills
Progestasert® and Mirena®
Transdermal progesterone patches releasing different doses of progestogens
Sensitivity analysis
If more studies are included in future versions of the review, the authors plan to perform the following sensitivity analyses.
Studies with a low risk of bias versus those with a high risk of bias
Studies with or without a power calculation
Studies assessing blood loss objectively versus those assessing subjectively or indirectly as explained above
Studies with dropouts less than 10% versus those with dropouts of more than 10%
Results
Description of studies
Results of the search
A total of 627 studies were identified, of which 11 studies were duplicated and one study was mentioned three times (in the Group's search, in LILACS and in Embase). Therefore, a total of 614 records were screened, out of which 599 of records were excluded. A total of 15 full‐text articles were assessed for eligibility; 11 of these (referring to nine studies) were excluded, with reasons (seeExcluded studies). A total of three studies (five references) are included in the review. No ongoing studies were identified and there are no studies awaiting classification (Figure 1).
Included studies
Two studies investigating desmopressin versus placebo (Edlund 2002; Kadir 2000) and one study investigating desmopressin versus tranexamic acid were eligible for inclusion in the review (Kouides 2009).
Study characteristics
The Edlund study was a single centre randomised, double‐blind, cross‐over study with placebo or desmopressin, in one of the first two treatment cycles (Edlund 2002). The study also had a third treatment cycle in which all women were given a combination of desmopressin and tranexamic acid. A total of 20 women were recruited to the study, four of which were excluded due to events during the study, leaving 16 women included in the analysis. Treatment was preceded by a run‐in phase of one menstrual cycle where in the the blood loss was assessed on a day‐by‐day basis in order to find the part of the period where the most intense bleeding occurred. The study was conducted at the Department of Women and Child Health, and Department of Surgical Sciences/Coagulation Research, Karolinska Hospital, Stockhom, Sweden.
The Kadir study was a single centre randomised double‐blind placebo‐controlled cross‐over study with placebo or desmopressin in one of the two treatment cycles (Kadir 2000). A total of 39 women were recruited to the study, but 10 of these women did not receive any study medication for various reasons. Of the remaining 29, one was known to have taken her first dose of study medication but did not complete a follow up, therefore 28 women were included in the analysis. Again treatment was preceded by a run‐in phase of one menstrual cycle to find the days of most intense bleeding. This study was conducted at the University Department of Obstetrics and Gynecology; the Hemophilia Center and Homeostasis Unit; and at the Department of Primary Care and Population Sciences at the Royal Free Hospital, London, UK. The study was supported by the Ferriing pharmaceutical company.
The Kouides study was a multicentred randomised study with a cross‐over design comparing intranasal desmopressin (IN‐DDAVP) with tranexamic acid therapy for two treatment cycles (Kouides 2009). Each treatment cycle consisted of two menstrual periods. Given the half‐life of the drugs, there was no washout period between the two treatment cycles. After confirmation of menorrhagia (PBAC ≥ 100), identifiable bleeding disorder (laboratory tests) and informed consent, 116 women were found eligible for the treatment phase. Before the treatment phase all eligible women below 46 years of age were offered combined oral contraceptive pills for a period of three cycles as standard care. Only five opted for this treatment and they underwent a washout period of two cycles before receiving either IN‐DDAVP or tranexamic acid. In those undergoing the cross‐over treatment phase, a relatively high dropout rate was observed (43% for the IN‐DDAVP to tranexamic acid sequence and 33% for tranexamic acid to IN‐DDAVP sequence). Only 90 out of 116 women used the medication for at least one cycle (only 28 took medications for all four cycles). The study was conducted between January 2001 at six different USA medical institutes and was approved by the institutional review boards of the Centers for Disease Control and Prevention (CDC) .
Participant characteristics
The Edlund study included non‐pregnant women, 18 years of age or older, with regular menstrual cycles and menorrhagia (defined as MBL greater than 80 mL per cycle) with prolonged bleeding time (more than 570 seconds) not due to known or measured deficiency of coagulation factor II, factor VII, FVIII, factor IX, factor X or of the vWF, and with a normal‐sized uterus (Edlund 2002). Women with signs of lung, heart or endocrine diseases were excluded. Other exclusion criteria were lactation, hormone therapy or curettage within two months prior to the start of the study, drug or alcohol abuse or other conditions possibly jeopardizing the welfare of the individual and abnormal findings at gynaecological examination. Informed consent was taken from all women who participated.
The Kadir study included women aged 18 to 50 years with diagnosed inherited bleeding disorders (Kadir 2000). These included mild to moderate vWD (vWF:Ac ¼ 5 to 50 IU dL), heterozygote FXI‐deficient women (FXI ¼ 15 to 70 IU dL) or carriers of haemophilia (FVIII ¼ 5 to 50 IU dL), and objectively confirmed menorrhagia (PBAC score greater than 100). Women with type 2B VWD, a history of renal and hepatic impairment, endocrine disorders, thromboembolic disease and nasal pathology interfering with absorption of the spray, including rhinitis, nasal polyp or significantly deviated septum were excluded from the study, as were those with a known hypersensitivity to desmopressin or chlorobutanol (or both). Other exclusion criteria included use of hormonal contraception or intrauterine contraceptive devices, medical treatment for menorrhagia, hysteroscopy or dilatation and curettage (or both) in the previous three months.
The Kouides study included women having a laboratory‐detectable bleeding disorder, a MBL amounting to more than 100 mL per bleed, a negative pelvic examination (although women with fibroids with the uterus less than 12 weeks gestational size were included), a negative Papanikolaou (PAP) smear within the last 12 months, having regular periods and not on any medications that might affect coagulation. Participants gave written consent. Women having a blood loss of less than 100 mL per cycle and women having no laboratory‐detectable bleeding disorders were excluded.
A notable difference as regards participants between the studies was that two studies included women with known factor deficiencies (Kadir 2000) or laboratory‐detectable haemostatic disorders (Kouides 2009), whereas one study included women with no known factor deficiencies but prolonged bleeding time (Edlund 2002). However, since the basic pathology of prolonged bleeding resulting in heavy menstrual periods remains the same we have presented all three studies together. More so because other reasons for heavy menstrual bleeding and pelvic pathology have been excluded in all study participants.
Interventions
In the Edlund study there were three study periods (Edlund 2002). For the first two treatment cycles the women were divided into two groups. For one group (n = 8), the first treatment cycle contained desmopressin (Octim®) spray and the second a placebo cycle. For the second group (n = 8), the first treatment cycle contained placebo and the second contained desmopressin (total 16 each for both placebo and desmopressin). Desmopressin nasal spray at a concentration of 300 µg per inhalation was administered twice daily on the two days of maximal blood loss. Saline nasal spray was administered the same way to the placebo group In the third cycle, all women received active treatment with desmopressin combined with tranexamic acid, 1.5 g three‐times daily, during the two treatment days. All participants were thoroughly instructed in the use of nasal inhalation by the nursing staff.
In the Kadir study there were two study periods (Kadir 2000). During each of the study periods, the women were instructed to take one spray in each nostril (i.e. 300 µg of desmopressin (Octim® spray) and the same amount of saline for the placebo)) twice‐daily during the second and third day of the period for two months.
The Kouides study had an optional pre‐treatment phase in which all women below 46 years of age were offered three cycles of combined oral contraceptive therapy (Kouides 2009). The women (n = 5) who opted for this therapy underwent a washout period of two months before commencing the study interventions of IN‐DDAVP or tranexamic acid. Those who completed oral contraceptive therapy and a two‐cycle washout, or who declined to participate in the oral contraceptive arm of the study were offered enrolment in the randomised cross‐over treatment arm comparing the use of IN‐DDAVP with use of tranexamic acid. The IN‐DDAVP group was administered 300 µg of desmopressin (Stimate™) on days two and three of menstrual bleeding (one puff in each nostril each day) and women were instructed to restrict fluid intake. In the other group, tranexamic acid (Cyclokapron™) was administered in tablet form at a dosage of 1 g four times each day for the first five days of menstrual bleeding.
Outcomes
Edlund measured MBL (the primary outcome of both the study and this review) using the alkaline hematin method. The women were instructed to collect all sanitary towels and tampons during the study period. Menstrual blood was extracted from sanitary material with 5% sodium hydroxide, and haemoglobin was thus transformed to alkaline hematin, which was measured spectrophotometrically at 540 nm. Adverse reactions in the form of nausea, vomiting and headache were reported. The other outcomes that were reported, which included coagulation assays were not relevant to this review (Edlund 2002).
Kadir measured MBL (the primary outcome of both the study and this review) using the PBAC method. The women were instructed to maintain a diary for each treatment cycle in which the PBAC was provided and the woman was advised to tick or complete wherever appropriate. The PBACs were then scored by one gynaecologist using the scoring system used by Higham (Higham 1990). The reported adverse events were primarily headache, facial flushing and weight gain. The other outcomes reported, which included the individual's preference and absenteeism from work were not relevant to this review (Kadir 2000).
Kouides also measured MBL (the primary outcome of both the study and this review) using the PBAC method. Side‐effects, most commonly headaches, were reported. At baseline and after the second cycle of both IN‐DDAVP and tranexamic acid, QoL was assessed. Four instruments were used to assess QoL and mean and median scores were calculated for each of the four QoL instruments (Kouides 2009).
Excluded studies
Nine studies were excluded (Ammesse 2005; Chi 2011; Choudry 2009; Halimeh 2012; Kingman 2004; Lukes 2008; Rodeghiero 2008; Rose 2008; Schaedel 2005). Six studies were not randomised controlled studies (Ammesse 2005; Chi 2011; Choudry 2009; Halimeh 2012; Rodeghiero 2008; Rose 2008). A further study was a retrospective case series (Lukes 2008); one was a prospective pilot study in which all women were given the intervention (Kingman 2004); and one was a retrospective case review (Schaedel 2005).
Risk of bias in included studies
Allocation
Sequence generation
All three studies were described as randomised but the way in which the sequence was generated was not specified. Therefore, we have stated that the risk of bias was unclear for all three studies (Edlund 2002; Kadir 2000; Kouides 2009).
Allocation concealment
The method of allocation concealment for all three studies was not specified (Edlund 2002; Kadir 2000; Kouides 2009). Therefore, we have assessed all studies as having an unclear risk of bias for this domain.
Blinding
Two studies have quoted their respective studies to be "double‐blinded", however, it was unclear in either study who exactly was blinded (Edlund 2002; Kadir 2000). The third study did not mention blinding (Kouides 2009). All three studies have assessed the primary outcome of MBL objectively, the Kadir and Kouides studies by PBAC, which is fairly objective even though not as objective as the alkali hematin method used in the Edlund study (Edlund 2002; Kadir 2000; Kouides 2009). Therefore, we have assessed this domain as having a low risk of bias for all.
Incomplete outcome data
In the Kadir study, 39 women were randomised but 10 did not receive any study medication leaving 29 participants (Kadir 2000). One of the women did not complete the PBAC. A total of 28 participants were taken as the ITT population, out of which four did not complete the second treatment phase. We have therefore assessed this study as having a high risk for attrition bias.
In the Edulnd study 20 women were included (Edlund 2002). Four women were excluded from the per protocol analysis due to events during the study (bile stone, kidney stone, goitre and menopause); finally, 16 women remained for the per protocol analysis. The dropout rate was therefore 20%. We have assessed this as having a low risk for attrition bias.
In the Kouides study a relatively high dropout rate was observed (43% for the IN‐DDAVP to tranexamic acid sequence and 33% for tranexamic acid to IN‐DDAVP sequence) (Kouides 2009). Out of 116 women only 90 used medication for at least one cycle (only 28 took medications for all four cycles). Therefore, we have assigned this study as having a high risk for attrition bias.
Selective reporting
Two studies have reported MBL as a composite whole after the whole study period for each of the interventions (placebo and desmopressin) and not at the end of each treatment cycle (Edlund 2002; Kadir 2000). All three studies have reported all outcomes that were stated in the respective studies. We have therefore assessed the studies as having a low risk of bias for this domain (Edlund 2002; Kadir 2000; Kouides 2009).
Other potential sources of bias
The Kadir study was funded by a pharmaceutical company, Ferring Pharma (Kadir 2000). We have therefore assessed this study as having an high risk of bias for this domain (Kadir 2000). The Kouides and the Edlund studies have not mentioned any funding or any other sources of potential bias, and have therefore been assessed as having an unclear risk of bias.
Effects of interventions
DDAVP versus placebo
This comparison was assessed in two trials (n = 59) (Edlund 2002; Kadir 2000).
Primary outcomes
1. Menstrual blood loss
Assessment of MBL was the primary objective of this review and the primary outcome of both included trials.
a. Objective assessment of blood loss (mL)
Both studies (n = 59) used desmopressin as an intervention in a cross‐over design versus placebo (Edlund 2002; Kadir 2000). Both studies reported data only at the end of both treatment cycles, thus ignoring cross‐over design and treating the studies as if they were of parallel design. We have not been able to combine the numerical data and to date we have not received any response to our request for the mean values for the Kadir study (Kadir 2000).
In the Edlund study, for one group, the first of three treatment cycles contained desmopressin and the second placebo. For the second group, the first treatment cycle contained placebo and the second contained desmopressin. In the third (open) cycle, all women received active treatment with desmopressin combined with tranexamic acid. The MBL was measured using the alkali hematin method. As reported in the paper, the MD in MBL in the desmopressin versus placebo group was 21.20 mL (95% CI ‐19.00 to 61.50) and did not reach statistically significant levels.
The study also reports that the MD in MBL in mLwas statistically significant in the desmopressin and tranexamic arm versus placebo (P < 0.05). However, the review authors would like to point out that the non‐randomised design of this comparison is an additional potential source of bias.
The second placebo‐controlled cross‐over study had two treatment cycles (Kadir 2000). The women received either desmopressin first or placebo. The study authors used PBACs to measure MBL reported as a single median value (assumed by the review authors to be the composite median). The authors reported that even though there was a significant improvement of PBAC scores with desmopressin (P = 0.0001) and placebo (P = 0.0001) when compared to pre‐treatment assessment, there was statistically no significant difference (P = 0.51) in the PABC scores when the two were compared to each other. The median values (for MBLin mL) for both desmopressin and placebo have been given in a graphical form in the study and so could not be reported here.
Neither of the studies used any indirect measures of blood loss such as hematocrit values (Edlund 2002; Kadir 2000).
b. Subjective assessment
No subjective assessment of blood loss was done by either of the studies. In the Kadir study, the study authors assessed the preference of the women for the treatment (Kadir 2000). These results implied that 13 women (61.9%) had preferred the placebo and eight (38.1%) had preferred desmopressin. Further analysis, however, revealed that these differences would be expected given the higher proportion of women who preferred the second treatment period. Thus, these results are unlikely to indicate any real preference for the placebo.
c. Indirect measures of blood loss
No other indirect measures of blood loss were assessed in either of the studies (Edlund 2002; Kadir 2000).
2. Adverse effects
Adverse events were reported in both studies (n = 59).
In the placebo‐controlled studies (both cross‐over studies, but reported as parallel studies), the Edlund study had more adverse effects with placebo, although the difference was not significant (primarily headaches, nausea and flushing) (Edlund 2002). The Kadir study (again a cross‐over study reported as a parallel one) had more adverse effects with desmopressin (the reported adverse events were primarily headache, facial flushing and weight gain), although, again, the difference was not significant (Kadir 2000). When combined the meta‐analysis showed no difference between groups, RR 1.04 (95% CI 0.73 to 1.49) (Analysis 1.1; Figure 4)
1.1. Analysis.
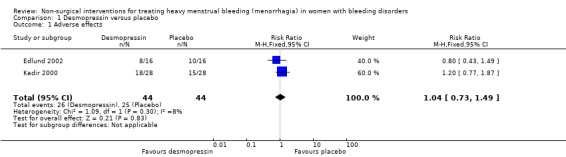
Comparison 1 Desmopressin versus placebo, Outcome 1 Adverse effects.
4.
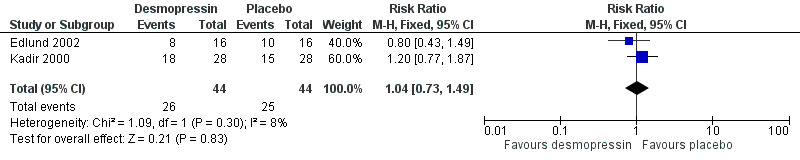
Forest plot of comparison: 1 DDAVP Vs Placebo, outcome: 1.1 Adverse effects.
Secondary outcomes
1. Quality of life
Neither of the included studies reported on this outcome (Edlund 2002; Kadir 2000).
2. Change from baseline in haemoglobin and hematocrit values
Neither of the included studies reported on this outcome (Edlund 2002; Kadir 2000).
3. Requirement for additional surgical treatment
Neither of the included studies reported on this outcome (Edlund 2002; Kadir 2000).
4. Requirement for blood transfusion
Neither of the included studies reported on this outcome (Edlund 2002; Kadir 2000).
5. Resource cost
Neither of the included studies reported on this outcome (Edlund 2002; Kadir 2000).
DDAVP versus tranexamic acid
This comparison was assessed in one trial (n = 116) (Kouides 2009).
Primary outcomes
1. Menstrual blood loss
Assessment of MBL was the primary objective of this review and the primary outcome of the included study (Kouides 2009).
a. Objective assessment of blood loss (mL)
The included study compared tranexamic acid with IN‐DDAVP in a cross‐over design (with no washout period in between) and has reported the primary outcome of MBL at the end of each treatment cycle (Kouides 2009). A carryover effect of treatment was not found. In this study the women received either IN‐DDAVP or tranexamic acid in two menstrual cycles. The study report states that the decrease in the PBAC score was greater for tranexamic acid than for IN‐DDAVP (a difference of 41.6 mL; P = 0.0002, CI = 19.6 to 63.6). The test for treatment‐type effect was significant (P < 0.0001), suggesting a greater reduction in PBAC score with tranexamic acid use than with IN‐DDAVP use. We are unable to enter the available data into the meta‐analysis and have requested further data from the study authors to be incorporated into a future update of the review.
The study did not use any indirect measure of blood loss such as hematocrit values (Kouides 2009).
b. Subjective assessment
No subjective assessment of blood loss was undertaken in the study (Kouides 2009).
c. Indirect measures of blood loss
No other indirect measures of blood loss were assessed in the study (Kouides 2009).
2. Adverse effects
Of the 90 women who completed at least one treatment cycle, 13 reported side effects; seven women reported reactions to IN‐DDAVP and six women reported reactions to tranexamic acid (Kouides 2009). This difference was not significant, RR 1.17 (95% CI 0.41 to 3.34) (Analysis 2.1; Figure 5). The most commonly reported side effect among those taking both IN‐DDAVP and tranexamic acid was headache. Two of the women reported severe side effects. One of them was reported as having hyponatraemia, possibly due to non‐compliance with fluid restriction, and the second took both medications simultaneously which resulted in dizziness, vomiting and double vision.
2.1. Analysis.
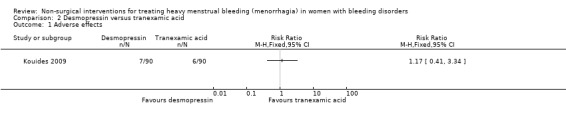
Comparison 2 Desmopressin versus tranexamic acid, Outcome 1 Adverse effects.
5.

Forest plot of comparison: 2 Desmopressin versus Tranaxemic Acid, outcome: 2.1 Adverse effects.
Secondary outcomes
1. Quality of life
The included study assessed QoL at baseline, then after the second cycle of both IN‐DDAVP and that of tranexamic acid (Kouides 2009). Four instruments, reporting mean and median scores, were used to assess QoL: (i) the Health‐Related Quality of Life (HRQOL) instrument, a 14‐item tool assessing the number of physically and mentally unhealthy days in the past 30 days (CDC 1998); (ii) the Short Form‐36 (SF‐36), a 36‐item generic health status survey covering eight health concepts that includes sub scores of a physical component (PCS) and a mental component (MCS) (Ware 1993); (iii) the Center for Epidemiologic Studies Depression (CES‐D) Scale (Radcliff 1997); and (iv) the modified Ruta Menorrhagia Severity Scale, a 13‐item scale measuring the physical, psychological, and social effects of menorrhagia on a woman’s health status (validated for menorrhagia) (Ruta 1995).
We are unable to present any data from this study, since no differences in QoL between the two intervention groups were reported, instead only the differences within each group between baseline and post‐treatment were reported.
2. Change from baseline in haemoglobin and hematocrit values
The included study did not report on this outcome (Kouides 2009).
3. Requirement for additional surgical treatment
The included study did not report on this outcome (Kouides 2009).
4. Requirement for blood transfusion
The included study did not report on this outcome (Kouides 2009).
5. Resource cost
The included study did not report on this outcome (Kouides 2009).
Discussion
Summary of main results
The numerical data for the primary outcome of change in MBL from the three studies could not be pooled for a quantitative analysis. Therefore, the summary of the main results for this review are limited to summarizing the findings of these studies. Two of the studies agree that, even though not significant, there might be a reduction in MBL with the use of desmopressin as compared to placebo (Edlund 2002; Kadir 2000). One of the studies has reported a significant reduction in MBL with a combination of desmopressin and tranexamic acid as compared to placebo, however, the women included in this third treatment cycle were not randomly selected (Edlund 2002). The third study has not compared the interventions with placebo but has reported that tranexamic is significantly more effective in reducing MBL when compared to DDAVP (Kouides 2009). The results of this study could not be pooled with the other two studies.
One study had more adverse effects with placebo though not significant (Edlund 2002). The second study had more adverse effects with desmopressin, again not significant (Kadir 2000). When combined, the meta‐analysis showed a very small increase of side effects with desmopressin, but again this did not reach significant levels. The third study has reported a total of 13 adverse effects, seven with DDAVP and six with tranexamic acid (Kouides 2009). Again these data could not be pooled with the other two studies.
Quality of life was assessed by only one study, which used four different instruments (Kouides 2009). The mean and median scores were calculated for each of the four QoL instruments; however, we were unable to analyse the data as the study does not report differences between the two intervention groups. It may be noted that the within‐group changes from baseline to the first treatment medication tended to exhibit larger improvements in QoL than changes from the first treatment medication to the second treatment medication, irrespective of the sequence in which the medications were administered.
Overall completeness and applicability of evidence
The evidence generated by this review is of limited applicability. The relevance of the evidence to the review question is again very limited. The review was aimed at all non‐surgical interventions. However the included studies have dealt with only two of these, namely desmopressin and tranexamic acid, although there are currently a substantial number of drugs and devices in existence for the effective management of MBL in women with no pelvic pathology. None of the studies have evaluated the cost effectiveness of the studied interventions, which is a highly relevant factor in the applicability of the evidence.
Quality of the evidence
All three studies stated "randomisation" but failed to mention the mode of sequence generation and allocation concealment (unclear risk of bias). Blinding may not have been of consequence as the outcomes were objectively assessed, even though comprehensive details on who was blinded and how were not supplied. Two of the studies had a low number of women (20 in the Edlund study and 39 in the Kadir study) (Edlund 2002; Kadir 2000). Attrition was high in the Kadir study where 10 out of 39 women were withdrawn (Kadir 2000) as well as in the Koudies study which reported a dropout rate of 43% for the IN‐DDAVP to tranexamic acid sequence and 33% for the tranexamic acid to IN‐DDAVP sequence (Kouides 2009). Overall, the equality of evidence generated was poor.
Potential biases in the review process
We undertook a thorough search for studies to include in the review, which were then subject to a rigorous selection process. We extensively searched relevant databases, conference proceedings, trial registries for published and unpublished data, in an iterative search. We regard the potential for missing any relevant or eligible studies to be remote. Although efforts were made, we could not obtain the relevant numerical data from the study authors which led to our inability to pool numerical data and produce a meta‐analysis for MBL (the outcome most relevant to this review).
Agreements and disagreements with other studies or reviews
Although there are several reviews which have generated robust and sound evidence to identify the best non‐surgical intervention for women with heavy MBL, there are none which look at interventions for women with bleeding disorders. Conversely, desmopressin, which has and is still being used with success for women with bleeding disorders, has never been tried as a therapy for heavy MBL in women without bleeding disorders or pelvic pathology.
Authors' conclusions
Implications for practice.
Until stronger evidence is available, evidence of effectiveness of desmopressin for heavy menstrual bleeding in women with bleeding disorders is based on observational evidence, an there is an absence of alternative treatments. The side effects of desmopressin should be explained to women considering this treatment option, especially given that the medication is likely be used for a considerable period of time. Furthermore, the evidence generated by this review indicates uncertainty around the risk of adverse effects with this treatment. A further treatment option might be a combination of desmopressin and tranexamic acid, which one study found to decrease menstrual blood loss. However, the study has not compared this combination with either placebo or no treatment and thus the evidence generated for this combination is also inadequate.
Implications for research.
The evidence from these studies remains inconclusive, partly due to the low number of participants and partly since no study was totally free of bias (method of sequence generation and allocation concealment not specified). Two of the included studies showed a non‐significant reduction in menstrual blood loss when compared to placebo. Further studies of non‐surgical interventions, with a greater number of participants, are needed to evaluate these treatment options for women with MBL and bleeding disorders. These should have a clearly defined method of sequence generation, allocation concealment and also be free of other biases. These studies should ideally be conducted in clinical settings to assess, who is and who is not, responding to treatment. Such studies may be very difficult to organize and recruit women to, because there are numerous surgical options available, in particular the Mirena intrauterine device and endometrial ablation.
Several reviews have generated robust and sound evidence for a gamut of non‐surgical interventions for women with heavy menstrual blood loss. However, there are no studies which look at other interventions for women with bleeding disorders. Danazol, mefenamic acid and progesterone‐containing devices are all used in women with heavy menstrual bleeding without pelvic pathology. Recently, progesterone‐containing devices have been used with good effect in women with menorrhagia, both as a treatment and as a contraceptive option. This may be ideal for women with bleeding disorders suffering from menorrhagia as it offers both a treatment for the increased blood loss and also a contraceptive option, which can be easily reversed when fertility is desired. Therefore, robustly‐designed studies comparing varied interventions independently or in combination are needed to address this issue.
Non‐surgical methods become more important when we consider the fact that these measures preserve fertility, as most women, despite their bleeding problem and the risks associated with pregnancy, would still like to experience motherhood. Another important aspect which should be addressed in future studies, is cost. Whichever intervention is used, it will be necessary for a considerable period of time, from menarche to menopause. Similarly, studies should consider potential side effects, which may not be obvious in the short term. With regular use (every cycle) for a prolonged time, the scenario for adverse effects may be different.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2016 | New citation required but conclusions have not changed | Minor changes have been made throughout, the plain language summary has been reformatted. |
| 8 November 2016 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register identified one reference which has been listed as an additional reference to the already included Kadir study (Kadir 2000). |
History
Protocol first published: Issue 1, 2013 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 13 April 2015 | Amended | Contact details updated. |
Acknowledgements
We gratefully acknowledge the contribution of Ms Gurpreet Rana, Medical Librarian, University of Michigan, USA for designing and conducting the Embase search for this review.
Appendices
Appendix 1. Embase search strategy
Search in Embase 03 May 2013.
#1. 'menorrhagia'/exp
#2. heavy NEAR/2 'menstrual bleeding'
#3. heavy NEAR/2 menstru* AND bleed*:ab,ti
#4. menorrhagia:ab,ti
#5. 'menstrual blood loss' NEAR/2 heavy
#6. 'menorrhagia'/exp OR heavy NEAR/2 'menstrual bleeding' OR (heavy NEAR/2 menstru* AND bleed*:ab,ti) OR menorrhagia:ab,ti OR 'menstrual blood loss' NEAR/2 heavy
#7. 'bleeding disorder'/exp OR 'blood clotting disorder'/exp
#8. 'bleeding disorder':ab,ti
#9. 'blood clotting factor deficiency'/exp
#10. 'blood protein disorder'/exp
#11. 'blood clotting factor'/exp AND deficiency:ab,ti
#12. 'bleeding disorder'/exp OR 'blood clotting disorder'/exp OR 'bleeding disorder':ab,ti OR 'blood clotting factor deficiency'/exp OR 'blood protein disorder'/exp OR ('blood clotting factor'/exp AND deficiency:ab,ti)
#13. 'non surgical':ab,ti
#14. 'tranexamic acid'/exp OR 'mefenamic acid'/exp OR 'oral contraceptive agent'/exp OR 'combined oral contraceptives':ab,ti OR 'progesterone'/exp OR 'danazol'/exp OR 'etamsylate'/exp OR 'desmopressin'/exp OR 'factor replacement therapy':ab,ti
#15. 'non surgical':ab,ti OR 'tranexamic acid'/exp OR 'mefenamic acid'/exp OR 'oral contraceptive agent'/exp OR 'combined oral contraceptives':ab,ti OR 'progesterone'/exp OR 'danazol'/exp OR 'etamsylate'/exp OR 'desmopressin'/exp OR 'factor replacement therapy':ab,ti
#16. 'menorrhagia'/exp OR heavy NEAR/2 'menstrual bleeding' OR (heavy NEAR/2 menstru* AND bleed*:ab,ti) OR menorrhagia:ab,ti OR 'menstrual blood loss' NEAR/2 heavy AND ('bleeding disorder'/exp OR 'blood clotting disorder'/exp OR 'bleeding disorder':ab,ti OR 'blood clotting factor deficiency'/exp OR 'blood protein disorder'/exp OR ('blood clotting factor'/exp ANDdeficiency:ab,ti)) AND ('non surgical':ab,ti OR 'tranexamic acid'/exp OR 'mefenamic acid'/exp OR 'oral contraceptive agent'/exp OR 'combined oral contraceptives':ab,ti OR 'progesterone'/expOR 'danazol'/exp OR 'etamsylate'/exp OR 'desmopressin'/exp OR 'factor replacement therapy':ab,ti)
.....................................................
Appendix 2. LILACS search strategy
Search in LILACS on 24 February 2013 # 1 Menorrhagia ab,ti
# 2 Menorrhagia and bleeding disorders ab,ti
Appendix 3. WHO International Clinical Trials Registry Platform's search portal
Search in WHo International Clinical Trials Registry 24 February 2013
# Menorrhaga ti,AND bleeding disorders
Data and analyses
Comparison 1. Desmopressin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.49] |
Comparison 2. Desmopressin versus tranexamic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Edlund 2002.
| Methods | The study was designed as a randomised, double‐blind, cross‐over study. Treatment was preceded by a run‐in phase of one menstrual cycle in which blood loss was analysed day‐by‐day in order to find the part of the period where the most intense bleeding occurred. The women were then allocated with blinded randomisation. |
|
| Participants | 20 women were included. 4 women were excluded from the per protocol analysis due to events during the study (bile stone, kidney stone, goitre and menopause); finally, 16 women remained for the per protocol analysis. Inclusion criteria: non‐pregnant women, 18 years of age or older, with regular menstrual cycles and menorrhagia (defined as MBL > 80 mL per cycle), prolonged bleeding time (> 570 seconds) not due to known or measured deficiency of coagulation factor II, factor VII, FVIII, factor IX, factor X or of the vWF, and with a normal‐sized uterus, were admitted to the study after receiving information and giving informed consent. Exclusion criteria: women with signs of lung, heart or endocrine diseases were excluded. Lactation, hormone therapy or curettage within 2 months prior to the start of the study, drug or alcohol abuse or other conditions possibly jeopardizing the welfare of the individual were also exclusion criteria. As were abnormal findings at gynaecological examination, including uterine myomas of clinical significance where surgery could be indicated. |
|
| Interventions | For one group (n = 8), the first treatment cycle contained desmopressin and the second placebo. For the other group, (n = 8) the first treatment cycle contained placebo and the second contained desmopressin (total 16 each for both placebo and desmopressin). Desmopressin nasal spray (Octostim®) at a concentration of 300 µg per inhalation was administered twice‐daily on the 2 days of maximal blood loss. Saline nasal spray was administered the same way to the placebo group. In the third (non‐randomised) cycle, all 32 women received active treatment with desmopressin combined with tranexamic acid, 1.5 g 3‐times daily, during the 2 treatment days. |
|
| Outcomes | 1. Measurement of MBL (primary outcome for the study and for this review).
Method: the women were instructed to collect all sanitary towels and tampons during the study period. Menstrual blood was extracted from sanitary material with 5% sodium hydroxide, and haemoglobin was thus transformed to alkaline hematin, which was measured spectrophotometrically at 540 nm. Blood loss could thereafter be calculated from individual blood haemoglobin concentration. 2. Adverse events (the second primary outcome of the study) was also reported. A total of 44 adverse events were reported during the study: 10 during placebo treatment, 8 during desmopressin treatment and 26 during combined treatment. Of the 26 adverse events during combined treatment, several concerned well‐known reactions to tranexamic acid, mainly nausea. The other outcomes reported (but not of interest to the review) were: 1. laboratory tests (B‐hemoglobin, B‐hematocrit, S‐ferritin, B‐platelet count, S‐bilirubin, S‐alanine aminotransferase, S‐aspartate aminotransferase, Palkaline phosphatase, S‐creatinine and differential counts). 2. coagulation assays included INR coagulation factor II, factor VII, factor X. and capillary bleeding time. |
|
| Notes | During whole of the study period concomitant use of drugs known to have an effect on MBL was not permitted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Blinded randomisation " stated but how sequence was generated is not specified. |
| Allocation concealment (selection bias) | Unclear risk | "Blinded randomisation" stated but how allocation was concealed is not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was stated to be double blind (though who was blinded and how the blinding was done remains unstated)and both the medication and the placebo were used as nasal sprays in the same way. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 women were included. 4 women were excluded from the per‐protocol analysis due to events during the study (bile stone, kidney stone, goitre and menopause); finally, 16 women remained for the per protocol analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | No mention of pharmaceutical funding or other potential sources of bias. |
Kadir 2000.
| Methods | This was a randomised, double‐blind, placebo‐controlled, cross‐over single centre study. | |
| Participants | A total of 39 women were randomised to the study. 10 of these women did not receive any study medication for various reasons. Out of the remaining 29, one was known to have taken her first dose of study medication but did not complete a follow up PBAC and therefore was not included in the ITT population (n ¼ 28). Four women did not complete the second period of treatment and thus the per protocol population consisted of 24 women. Inclusion criteria: women aged 18 – 50 years with diagnosed inherited bleeding disorders, including mild to moderate VWD (vWF:Ac ¼ 5 – 50 IU dL)1), heterozygote FXI‐deficient women (FXI ¼ 15 – 70 IU dL)1) or carriers of haemophilia (FVIII ¼ 5 ‐ 50 IU dL)1), and objectively confirmed menorrhagia (PBAC score > 100) were included in the study. Exclusion criteria: women with type 2B VWD, a history of renal and hepatic impairment, endocrine disorders, thromboembolic disease and nasal pathology interfering with absorption of the spray, including rhinitis, nasal polyp or significantly deviated septum were excluded from the study, as were those with a known hypersensitivity to desmopressin or chlorobutanol (or both). Other exclusion criteria included use of hormonal contraception or intrauterine contraceptive devices, medical treatment for menorrhagia and hysteroscopy, and/or dilatation and curettage in the previous 3 months. |
|
| Interventions | During each of the study periods, the women were instructed to take 1 spray in each nostril (i.e. 300 µg of desmopressin ‐ Octim® spray and the same amount of saline for the placebo)) twice daily during the second and third day of the period for 2 months. | |
| Outcomes | 1. Measurement of MBL primary outcome for the study and for this review. Method: the women were instructed to maintain a diary for each treatment cycle in which the PBAC, was provided and the woman was advised to tick or complete wherever appropriate. The PBACs were then scored by one gynaecologist using the scoring system used by Higham (Higham 1990). The study has reported median PBAC scores rather than mean for placebo and desmopressin. 2. Adverse events (the second primary outcome of the review). 3. Participant preference (the review did not have this outcome). 4. Absenteeism from work. |
|
| Notes | The use of diuretics, carbamazepine, non‐steroidal anti‐inflammatory agents, platelet‐impairing medications, clofibrate and chlorpropamide were prohibited during and 10 days before the study. This study was supported by the Ferring pharmaceutical company. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Blinded randomisation" mentioned but method of sequence generation mot mentioned. |
| Allocation concealment (selection bias) | Unclear risk | "Blinded randomisation" mentioned but how allocation was concealed not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Doube blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Out of 39 recruited women outcome data available for 29. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | This study was supported by the Ferring pharmaceutical. |
Kouides 2009.
| Methods | A randomised cross‐over study. | |
| Participants | 116 recruited. Inclusion criteria: women having a lab detectable bleeding disorder, a MBL amounting to more than 100 mL per bleed, a negative pelvic examination (though women with fibroids with the uterus was < 12 weeks gestational size were included), a negative PAP smear within the last 12 months, having regular periods and not on any medications that might affect coagulation. Participants gave written consent. Exclusion criteria: women having a blood loss of < 100 mL per cycle. Women having no laboratory detectable bleeding disorder. |
|
| Interventions | One group received IN‐DDAVP first for 2 menstrual cycles and subsequently received tranexamic acid for 2 menstrual cycles, and the other group received tranexamic acid first followed by IN‐DDAVP. There was no washout period in between washout. Intranasal DDAVP 300 lg, was administered on days 2 and 3 of menstrual bleeding (1 puff in each nostril each day) Trenaxamic acid was administered in tablet form at a dosage of 1 g 4 times each day for the first 5 days of menstrual bleeding. |
|
| Outcomes | 1. Menstraul blood loss PBAC was the method used 2. QoL 3. Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no information on the method used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding not carried out but as the primary outcome is objective, this does not cause bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding not carried out but as the primary outcome is objective, this does not cause bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A high dropout rate (43% for the IN‐DDAVP to tranexamic acid sequence and 33% for the tranexamic acid to IN‐DDAVP sequence). Paper did state "However, comparisons by sequence of the demographic characteristics, race and diagnosis, by attrition, sequence and treatment yielded minimal differences". |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | No mention of pharmaceutical funding or other potential sources of bias. |
IN‐DDAVP: intranasal desmopressin INR:international normalised ratio MBL: menstrual blood loss PAP: Papanikolaou smear PBAC: pictorial blood assessment chart QoL: quality of life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ammesse 2005 | Not a RCT. |
| Chi 2011 | Case‐series. |
| Choudry 2009 | Not a RCT. |
| Halimeh 2012 | Not a RCT. |
| Kingman 2004 | Prospective pilot study in which all women were given the intervention. There was a letter in response to this study as well as a response to this letter among the citations yielded by the electronic searches. Letter: Wahab M, Al Azzawi F. The use of levonorgestrel‐releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG: an International Journal of Obstretrics and Gynaecology 2005;112(10):115‐6 Response to letter: Curtis R. The use of levonorgestrel‐releasing intrauterine system for the treatment of menorrhagia in women with inherited bleeding disorders. BJOG: an International Journal of Obstetrics and Gynaecology 2006;113(2):248‐9. |
| Lukes 2008 | A retrospective case series. |
| Rodeghiero 2008 | Descriptive study. |
| Rose 2008 | Not a RCT. |
| Schaedel 2005 | A retrospective case review. |
RCT: randomised controlled trial
Contributions of authors
Sujoy Ray: conceptualising the review topic; developing and drafting the final version of the protocol; searching for additional references and subsequently developing and drafting the final version of the review and update. Amita Ray: conceptualising the review topic; developing the protocol; providing citations and full text articles for the 'Background' and 'Objectives' sections and drafting the final version of the review and update.
Sources of support
Internal sources
None, Other.
External sources
South Asian Cochrane Network and Centre, BV Moses and ICMR centre for Advanced Research and Training Christian Medical College Vellore, Tamilnadu, India, India.
Declarations of interest
Both authors: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Edlund 2002 {published data only}
- Edlund M, Blombäck M, Fried G. Desmopressin in the treatment of menorrhagia in women with no common coagulation factor deficiency but with prolonged bleeding time. Blood Coagulation and Fibrinolysis 2002;13(3):225‐31. [DOI] [PubMed] [Google Scholar]
Kadir 2000 {published data only}
- Kadir RA, Economides DL, Lee CA. DDAVP nasal spray for treatment of menorrhagia in women with inherited bleeding disorders: a prospective randomized placebo‐controlled cross‐over study [abstract]. Haemophilia 2000;6(4):243. [DOI] [PubMed] [Google Scholar]
- Kadir RA, Lee CA, Economides DL. DDVAP nasal spray for treatment of menorrhagia in women with inherited bleeding disorders: a prospective randomized placebo‐controlled crossover study. XVI FIGO World Congress of O & G. 2000; Vol. Abstract book 2:93. [CENTRAL: 363397; CRS: 5500050000000469]
- Kadir RA, Lee CA, Sabin CA, Pollard D, Economides DL. DDAVP nasal spray for treatment of menorrhagia in women with inherited bleeding disorders: a randomized placebo‐controlled crossover study. Haemophilia 2002;8(6):787‐93. [DOI] [PubMed] [Google Scholar]
Kouides 2009 {published data only}
- Kouides PA, Byams RV, Philipp CS, Stein SF, Heit JA, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. British Journal of Haematology 2009;145(2):212‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ammesse 2005 {published data only}
- Amesse LS, Pfaff‐Amesse T, Leonardi R, Uddin D, French JA 2nd. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD‐associated menorrhagia: a single‐institution study. Journal of Pediatric Hematology/Oncology 2005;27(7):21‐5. [DOI] [PubMed] [Google Scholar]
Chi 2011 {published data only}
- Chi C, Huq FY, Kadir RA. Levonorgestrel‐releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long‐term follow‐up. Contraception 2011;83(3):242‐7. [DOI] [PubMed] [Google Scholar]
Choudry 2009 {published data only}
- Choudry A, Malik A, Choudry H, Bangish N, Hayat N. Effectiveness and safety of Levonorgestrel Releasing Intrauterine System in treatment of menorrhagia secondary to oral anticoagulations and chronic liver disease. Rawal Medical Journal 2009;34(2):187‐90. [Google Scholar]
Halimeh 2012 {published data only}
- Halimeh S. Menorrhagia and bleeding disorders in adolescent females. Hamostaseologie 2012;32(1):45‐50. [DOI] [PubMed] [Google Scholar]
Kingman 2004 {published data only}
- Curtis R. The use of levonorgestrel‐releasing intrauterine system for the treatment of menorrhagia in women with inherited bleeding disorders. BJOG: an International Journal of Obstetrics and Gynaecology 2006;113(2):248‐9. [DOI] [PubMed] [Google Scholar]
- Kingman CE, Kadir RA, Lee CA, Economides DL. The use of levonorgestrel‐releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG: an International Journal of Obstetrics and Gynaecology 2004;111(12):1425‐8. [DOI] [PubMed] [Google Scholar]
Lukes 2008 {published data only}
- Lukes AS, Kouides PA, Moore KA. Tranexamic acid: A novel oral formulation for the treatment of heavy menstrual bleeding. Women's Health 2011;7(2):151‐8. [DOI] [PubMed] [Google Scholar]
Rodeghiero 2008 {published data only}
- Rodeghiero F. Management of menorrhagia in women with inherited bleeding disorders:General principles and use of desmopressin. Haemophilia 2008;14(1):21‐30. [DOI] [PubMed] [Google Scholar]
Rose 2008 {published data only}
- Rose SS, Faiz A, Miller CH, Saidi P, Philipp CS. Laboratory response to intranasal desmopressin in women with menorrhagia and platelet dysfunction. Haemophilia 2008;14(3):571‐8. [DOI] [PubMed] [Google Scholar]
Schaedel 2005 {published data only}
- Schaedel ZE, Dolan G, Powell MC. The use of the levonorgestrel‐releasing intrauterine system in the management of menorrhagia in women with hemostatic disorders. American Journal of Obstetrics and Gynecology 2005;193(4):1361‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Cai 2005
- Cai K, Gierman TM, Hotta J, Stenland CJ, Lee DC, Pifat DY, et al. Ensuring the biologic safety of plasma‐derived therapeutic proteins. Detection, inactivation, and removal of pathogens. Biodrugs 2005;19(2):79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 1998
- Centers for Disease Control and Prevention (CDC). Health related quality of life and activity limitation – eight states. Morbidity and Mortality Weekly Report 1998; Vol. 47:134‐140. [PubMed]
Chalmers 2007
- Chalmers EA, Brown SA, Keeling D, Liesner R, Richards M, Stirling D, et al. Early factor VIII exposure and subsequent inhibitor development in children with severe haemophilia A. Haemophilia 2007;13(2):149–55. [DOI] [PubMed] [Google Scholar]
Chamberlain 2003
- Chamberlain P, Mire‐Sluis AR. An overview of scientific and regulatory issues for the immunogenicity of biological products. Developmental Biology 2003;112:3–11. [PubMed] [Google Scholar]
Chen 2008
- Chen YC, Chao TY, Cheng SN, Hu SH, Liu JY. Prevalence of von Willebrand disease in women with iron deficiency anaemia and menorrhagia in Taiwan. Haemophilia 2008;14(4):768‐74. [DOI] [PubMed] [Google Scholar]
Clarke 1995
- Clarke A, Black N, Rowe P, Mott S, Howie K. Indications for and outcomes of total abdominal hysterectomy for benign disease: a prospective cohort study. British Journal of Obstetrics and Gynaecology 1995;102(6):11–20. [DOI] [PubMed] [Google Scholar]
Cole 1971
- Cole S, Billewicz W, Thomson A. Sources of variation in menstrual blood loss. Journal of Obstetrics and Gynaecology of the British Commonwealth 1971;78(10):939–49. [DOI] [PubMed] [Google Scholar]
Deligdisch 2000
- Deligdisch l. The 1999 long course on pathology of the uterine corpus and cervix. Modern Pathology 2000;13(3):285–94. [DOI] [PubMed] [Google Scholar]
Demers 2006
- Demers C, Derzko C, David M, Douglas J, Society of Obstetricians and Gynaecologists of Canada. Gynaecological and obstetric management of women with inherited bleeding disorders. International Journal of Gynaecology and Obstetrics 2006;95(1):75‐87. [DOI] [PubMed] [Google Scholar]
Douglas 1997
- Douglas JT, Shaw J. High dose desmopressin in bleeding disorders. European Journal of Anaesthesiology 1997;14 Suppl:v‐vi. [DOI] [PubMed] [Google Scholar]
Dunn 2000
- Dunn AL, Powers JR, Ribeiro MJ, Rickles FR, Abshire TC. Adverse events during use of intranasal desmopressin acetate for haemophilia A and von Willebrand disease: a case report and review of 40 patients. Haemophilia 2000;6(1):11‐4. [DOI] [PubMed] [Google Scholar]
Edlund 1996
- Edlund M, Blombäck M, Schoultz B, Andersson O. On the value of menorrhagia as a predictor for coagulation disorders. American Journal of Hematology 1996;53(4):234‐8. [DOI] [PubMed] [Google Scholar]
El‐Hemaidi 2007
- El‐Hemaidi I, Gharaibeh A, Shehata H. Menorrhagia and bleeding disorders. Current Opinion in Obstetrics and Gynecology 2007;19(6):513‐20. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Falcone 1994
- Falcone T, Desjardins C, Bourque J. Dysfunctional uterine bleeding in adolescents. Journal of Reproductive Medicine 1994;39(10):761‐4. [PubMed] [Google Scholar]
Farquhar 2009
- Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000154.pub2] [DOI] [PubMed] [Google Scholar]
FDA 2011
- U.S. Food, Drug Administration (FDA). Highlights of prescribing information: Lysteda™ (tranexamic acid) tablets. Reference ID: 2928912. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022430s002lbl.pdf (accessed 13 March 2014).
Fraser 1983
- Fraser IS, McCarron G, Markham R, Robinson M, Smyth E. Long‐term treatment of menorrhagia with mefenamic acid. Obstetrics and Gynecology 1983;61(1):3‐6. [PubMed] [Google Scholar]
Fraser 1985
- Fraser IS, McCarron G, Markham R, Resta T. Blood and total fluid content of menstrual discharge. Obstetrics and Gynecology 1985;65(2):194–8. [PubMed] [Google Scholar]
Fredrick 2008
- Ofosu FA, Freedman J, Semple JW. Plasma‐derived biological medicines used to promote haemostasis. Thrombosis and Haemostasis 2008;99(5):851–62. [DOI] [PubMed] [Google Scholar]
Garay 2006
- Garay RP, Chiavaroli C, Hannaert P. Therapeutic efficacy and mechanism of action of ethamsylate, a long‐standing haemostatic agent. American Journal of Therapeutics 2006;13(3):236‐47. [DOI] [PubMed] [Google Scholar]
Gath 1982
- Gath D, Cooper P, Day A. Hysterectomy and psychiatric disorder: I. Levels of psychiatric morbidity before and after hysterectomy. International Journal of Psychiatry 1982;140:335–40. [DOI] [PubMed] [Google Scholar]
Halberg 1966
- Hallberg L, Högdahl AM, Nilsson L, Rybo G. Menstrual blood loss‐‐a population study. Variation at different ages and attempts to define normality. Acta Obstetricia et Gynecologica Scandinavica 1966;45(3):320–51. [DOI] [PubMed] [Google Scholar]
Hardman 2001
- Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th Edition. McGraw‐Hill, 2001. [Google Scholar]
Hassan 2005
- Hassan AA, Kroll AH. Acquired Disorders of Platelet Function. Hematology / The Education Program of the American Society of Hematology 2005;1:403‐8. [DOI] [PubMed] [Google Scholar]
Haynes 1977
- Haynes P, Hodgson H, Anderson A, Turnbull A. Measurement of menstrual blood loss in patients complaining of menorrhagia. British Journal of Obstetrics and Gynaecology 1977;84(10):763–8. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higham 1990
- Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. British Journal of Obstetrics and Gynaecology 1990;97(8):734‐9. [DOI] [PubMed] [Google Scholar]
James 2006
- James AH, Ragini MV, Picozzi VJ. Bleeding disorders in premenopausal women: (another) public health crisis for hematology?. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2006;2006(1):474‐85. [DOI] [PubMed] [Google Scholar]
Jayasinghe 2005
- Jayasinghe Y, Moore P, Donath S, Campbell J, Monagle P, Grover S. Bleeding disorders in teenagers presenting with menorrhagia. Australian and New Zealand Journal of Obstetrics and Gynaecology 2005;45(5):439‐43. [DOI] [PubMed] [Google Scholar]
Jenkin 1980
- Jenkin G. Review: The Mechanism of Action of Danazol, a Novel Steroid Derivative. Australian and New Zealand Journal of Obstetrics and Gynaecology 1980;20(2):113–8. [DOI] [PubMed] [Google Scholar]
Kadir 2002
- Kadir RA, Lee CA, Sabin CA, Pollard D, Economides DL. DDAVP nasal spray for treatment of menorrhagia in women with inherited bleeding disorders: a randomised placebo‐controlled crossover study. Haemophilia 2002;8(6):787‐93. [DOI] [PubMed] [Google Scholar]
Kadir 2010
- Kadir RA, EdlundM, Mackensen S. The impact of menstrual disorders on quality of life in women with inherited bleeding disorders. Haemophilia 2010;16(5):832‐9. [DOI] [PubMed] [Google Scholar]
Kadira 1998
- Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet 1998;351(9101):485‐9. [DOI] [PubMed] [Google Scholar]
Klein 2004
- Klein HG. Pathogen inactivation technology:cleansing the blood supply. Journal of Internal Medicine 2004;257(3):224–37. [DOI] [PubMed] [Google Scholar]
Lethaby 2000
- Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000249] [DOI] [PubMed] [Google Scholar]
Mannuci 1997
- Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 Years. Blood 1997;90(7):2515‐21. [PubMed] [Google Scholar]
Mercorio 2003
- Mercorio F, Simone R, Carlo C. Effectiveness and mechanism of action of desmopressin in the treatment of copper intrauterine device‐related menorrhagia: a pilot study. Human Reproduction 2003;18(11):2319‐22. [DOI] [PubMed] [Google Scholar]
Miller 2011
- Miller CH, Philipp CS, Stein SF, Kouides PA, Lukes AS, Heit JA, et al. The spectrum of haemostatic characteristics of women with unexplained menorrhagia. Haemophilia. 2011;17(1):e223‐9. [DOI] [PubMed] [Google Scholar]
NICE Guidelines 2007
- National Institute for Health and Clinical Excellence (NICE). Heavy menstrual bleeding. Clinical Guideline 2007. www.ncbi.nlm.nih.gov/books/NBK56536/pdf/TOC.pdf (accessed 20 October 2011).
NZ Guidelines Group 1999
- New Zealand Guidelines Group. Working Party for Guidelines for the Management of Heavy Menstrual Bleeding. An evidence‐based guideline for the management of heavy menstrual bleeding. New Zealand Medical Journal 1999;112(1088):174‐7. [PubMed] [Google Scholar]
Oeheler 2003
- Oehler MK, Rees MC. Menorrhagia: an update. Acta Obstetrica et Gynecologica Scandinavica 2003;82(5):405‐22. [DOI] [PubMed] [Google Scholar]
Philipp 2010
- Philipp CS. Platelet disorders in adolescents. Journal of Pediatric and Adolescent Gynecology 2010;23(6 Suppl):11‐4. [DOI] [PubMed] [Google Scholar]
Phillip 2003
- Philipp CS, Dilley A, Miller CH, Evatt B, Baranwal A, Schwartz R, et al. Platelet functional defects in women with unexplained menorrhagia. Journal of Thrombosis and Haemostasis 2003;1(3):477‐84. [DOI] [PubMed] [Google Scholar]
Pipe 2008
- Pipe SW. Recombinant clotting factors. Thrombosis and Haemostasis 2008;99(5):840–50. [DOI] [PubMed] [Google Scholar]
Radcliff 1997
- Radloff LS. The CES‐D scale: a self report depression scale for research in the general population. Applied Psychological Measurement 1997; Vol. 1:385‐41.
Reid 2005
- Reid PC, Virtanen‐Kari S. Randomised comparative trial of the levonorgestrel intrauterine system and mefenamic acid for the treatment of idiopathic menorrhagia: a multiple analysis using total menstrual fluid loss, menstrual blood loss and pictorial blood loss assessment charts. British Journal of Obstetrics and Gynecology 2005;112(8):8‐14. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ruta 1995
Saxena 2003
- Saxena R, Gupta M, Gupta PK, Kashyap R, Choudhry VP, Bhargava M. Inherited bleeding disorders in Indian women with menorrhagia. Haemophilia 2003;9(2):193‐6. [DOI] [PubMed] [Google Scholar]
Schelkens 2003
- Schellekens H. Immunogenicity of therapeutic proteins. Nephrology, Dialysis, Transplantation 2003;18(7):1257–9. [DOI] [PubMed] [Google Scholar]
Shankar 2004
- Shankar M, Lee CA, Sabin CA, Economides DL, Kadir RA. von Willebrand disease in women with menorrhagia: a systematic review. BJOG: an International Journal of Obstetrics & Gynaecology 2004;111(7):734‐40. [DOI] [PubMed] [Google Scholar]
Shetty 2011
- Shetty S, Kasatkar P, Ghosh K. Pathophysiology of acquired von Willebrand disease: a concise review. European Journal of Haematology 2011;87(2):99‐106. [DOI] [PubMed] [Google Scholar]
van Eijkeren 1990
- Eijkeren MA, Christiaens GC, Haspels AA, Sixma JJ. Measured menstrual blood loss in women with a bleeding disorder or using oral anticoagulant therapy. American Journal of Obstetrics and Gynecology 1990;162(5):1261‐3. [DOI] [PubMed] [Google Scholar]
van Eijkeren 1992
- Eijkeren MA, Christiaens GC, Geuze HJ, Haspels AA, Sixma JJ. Effects of mefenamic acid on menstrual haemostasis in essential menorrhagia. American Journal of Obstetrics and Gynecology 1992;166(5):1419‐28. [DOI] [PubMed] [Google Scholar]
Vessey 1992
- Vessey MP, Villard‐Mackintosh L, McPherson K, Coulter A, Yeates D. The epidemiology of hysterectomy: findings in a large cohort study. British Journal of Obstetrics & Gynaecology 1992;99(5):402‐7. [DOI] [PubMed] [Google Scholar]
Von Depka 2002
- Depka M. NovoSeven: mode of action and use in acquired haemophilia. Intensive Care Medicine 2002;28(2 Suppl):s222‐7. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow K, Kosinski M, Gandek B. SF‐36 HealthSurvey Manual and Interpretation Guide. The Health Institute, Boston, MA 1993.
References to other published versions of this review
Ray 2014
- Ray S, Ray A. Non‐surgical interventions for treating heavy menstrual bleeding (menorrhagia) in women with bleeding disorders. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010338.pub2] [DOI] [PubMed] [Google Scholar]


