Abstract
Background
Most people with cystic fibrosis (80% to 90%) need pancreatic enzyme replacement therapy to prevent malnutrition. Enzyme preparations need to be taken whenever food is taken, and the dose needs to be adjusted according to the food consumed. A systematic review on the efficacy and safety of pancreatic enzyme replacement therapy is needed to guide clinical practice, as there is variability between centres with respect to assessment of pancreatic function, time of commencing treatment, dose and choice of supplements. This is an updated version of a published review.
Objectives
To evaluate the efficacy and safety of pancreatic enzyme replacement therapy in children and adults with cystic fibrosis and to compare the efficacy and safety of different formulations of this therapy and their appropriateness in different age groups. Also, to compare the effects of pancreatic enzyme replacement therapy in cystic fibrosis according to different diagnostic subgroups (e.g. different ages at introduction of therapy and different categories of pancreatic function).
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. Most recent search: 15 July 2016.
We also searched an ongoing trials website and the websites of the pharmaceutical companies who manufacture pancreatic enzyme replacements for any additional trials. Most recent search: 22 July 2016.
Selection criteria
Randomised and quasi‐randomised controlled trials in people of any age, with cystic fibrosis and receiving pancreatic enzyme replacement therapy, at any dosage and in any formulation, for a period of not less than four weeks, compared to placebo or other pancreatic enzyme replacement therapy preparations.
Data collection and analysis
Two authors independently assessed trials and extracted outcome data. They also assessed the risk of bias of the trials included in the review.
Main results
One parallel trial and 12 cross‐over trials of children and adults with cystic fibrosis were included in the review. The number of participants in each trial varied between 14 and 129 with a total of 512 participants included in the review. All the included trials were for a duration of four weeks. The included trials had mostly an unclear risk of bias from the randomisation process as the details of this were not given; they also mostly had a high risk of attrition bias and reporting bias.
We could not combine data from all the trials as they compared different formulations. Findings from individual studies provided insufficient evidence to determine the size and precision of the effects of different formulations. Ten studies reported information on the review's primary outcome (nutritional status); however, we were only able to combine data from two small cross‐over studies (n = 41). The estimated gain in body weight was imprecise, 0.32 kg (95% confidence interval ‐0.03 to 0.67; P = 0.07). Combined data from the same studies gave statistically significant results favouring enteric‐coated microspheres over enteric‐coated tablets for our secondary outcomes stool frequency, mean difference ‐0.58 (95% confidence interval ‐0.85 to ‐0.30; P < 0.0001); proportion of days with abdominal pain, mean difference ‐7.96% (95% confidence interval ‐12.97 to ‐2.94; P = 0.002); and fecal fat excretion, mean difference ‐11.79 g (95% confidence interval ‐17.42 to ‐6.15; P < 0.0001). Data from another single small cross‐over study also favoured enteric‐coated microspheres over non‐enteric‐coated tablets with adjuvant cimetidine in terms of stool frequency, mean difference ‐0.70 (95% confidence interval ‐0.90 to ‐0.50; P < 0.00001).
Authors' conclusions
There is limited evidence of benefit from enteric‐coated microspheres when compared to non‐enteric coated pancreatic enzyme preparations up to one month. In the only comparison where we could combine any data, the fact that these were cross‐over studies is likely to underestimate the level of inconsistency between the results of the studies due to over‐inflation of confidence intervals from the individual studies.There is no evidence on the long‐term effectiveness and risks associated with pancreatic enzyme replacement therapy. There is also no evidence on the relative dosages of enzymes needed for people with different levels of severity of pancreatic insufficiency, optimum time to start treatment and variations based on differences in meals and meal sizes. There is a need for a properly designed study that can answer these questions.
Plain language summary
Pancreatic enzyme supplements for people with cystic fibrosis
Review question
We reviewed the evidence about how good pancreatic enzyme supplements are in overcoming the enzyme deficiency in people with cystic fibrosis and if these supplements have any side effects.
Background
Between 80% and 90% of people with cystic fibrosis take pancreatic enzyme supplements. In these people, the pancreas is often not able to make the enzymes needed to digest food, which in children can result in a failure to gain weight and to thrive. In adults, it can lead to a loss in body weight and in malnutrition because the body does not absorb vitamins properly. In both children and adults with CF, malnutrition is linked to poorer general health, more severe lung disease and shorter life expectancy. If the pancreas is not making enough enzymes, people with CF can also experience unpleasant symptoms such as painful, frequent, bulky, offensive bowel movements. Pancreatic enzyme supplements are therefore needed to help gain weight, to prevent malnutrition and to avoid some vitamin deficiencies, as well as to control bowel symptoms. This is an updated version of the review.
Search date
We last searched for evidence: 15 July 2016.
Study characteristics
The review included 13 studies with 512 adults and children with cystic fibrosis; in all of them treatment lasted for four weeks. Studies compared different formulations of pancreatic enzyme supplements, so we could not combine many of the results. Also the design of 12 of the studies meant that those taking part received both types of supplement for four weeks each, although the order in which they received them was chosen at random. This also made it difficult to analyse the results. Most of the studies were old; the most recent was from 2015, but the oldest was from 1986.
Key results
We could only combine data from two small studies where individuals took miniature drug capsules (microspheres), which were treated so that the release of the medication is delayed until they have passed from the stomach into the intestine, and normal size tablets which were treated in the same way. The results did not clearly favour one or the other treatment for any of our most important outcomes (weight, height or body mass index). However, those taking the delayed‐release microspheres had less fat in their feces than those taking delayed release tablets (normal size) as well as having less abdominal pain and not needing to go to the toilet as often. In a different study, those people taking the delayed‐release microspheres also had less fat in their feces than those taking supplements that weren't treated so the release of medication was delayed. We didn't find any evidence that one type of these enteric‐coated microspheres was better than another; or that enteric‐coated microspheres were better than enteric‐coated mini‐microspheres (which are smaller).
We didn't find any evidence on different doses of enzymes needed for people who produce different levels of pancreatic enzymes, on the best time for individuals to start treatment and different amounts of supplements based on differences in type of food eaten and meal sizes. A properly designed sudy is needed to answer these questions.
Quality of the evidence
We could not be sure that the people in the included studies had equal chances of being put into the different treatment groups as no details were published about how the decisions were made. Several studies also had large numbers of individuals who dropped out and often reasons for this were not given. In most studies, people took one treatment and then after a while swapped to the alternative treatment; we could only combine results from two studies which were designed in this way, and that design means that the results may seem to be more consistent than they really are when we analyse them. Finally, several studies did not completely report their findings in a way we could analyse in this review. We are not sure how these factors affect our confidence in the results we found.
Background
A glossary of terms and abbreviations can be found in the additional tables (Table 6).
1. Glossary of terms.
| Term/abbreviation | Definition |
| BMI | body mass index |
| CF | cystic fibrosis |
| CFA | coefficient of fat absorption |
| chyme | the semi‐fluid mass of partly digested food expelled by the stomach into the duodenum |
| DIOS | distal intestinal obstruction syndrome |
| ECM | enteric coated microspheres |
| FFE | fecal fat excretion |
| hyperuricemia | an excess of uric acid in the blood |
| hyperuricosuria | the presence of excessive amounts of uric acid in the urine |
| Ileocecum | the combined ileum (end of the small intestine) and cecum (start of the large intestine) |
| NECM | non‐enteric coated microspheres |
| PERT | pancreatic enzyme replacement therapy |
| PI | pancreatic insufficiency |
| porcine | relating to or suggesting swine (pigs) |
| RCT | randomized controlled trial |
| steatorrhea | loss of fat in the stools |
Description of the condition
Cystic fibrosis (CF) is a genetic disorder that affects approximately 80,000 individuals worldwide. The disease can involve many different organs and systems in the body. Between 80% and 90% of people with CF exhibit exocrine pancreatic insufficiency which is caused by decreased production of pancreatic enzymes (Fieker 2011). Pancreatic insufficiency (PI) leads to impaired digestion and absorption from the diet of fat, protein and the fat soluble vitamins A, D, E and K (Dodge 2006); it also predisposes to the development of a distal intestinal obstruction syndrome (DIOS) which is a condition unique to CF and is defined as an acute complete or incomplete fecal obstruction in the ileocecum. This occurs in about 10% to 20% of individuals, mainly in adolescents and adults, and is the result of the absence of CFTR function in the intestine which compromises chloride secretion and increased water absorption. Significant energy (calories) can be lost as fat in the stools (steatorrhea) resulting in a failure to gain weight and a failure to thrive in children and a loss in body weight in adults, with accompanying malnutrition from poor absorption of vitamins. In both children and adults with CF, malnutrition is associated with poorer general health, more severe pulmonary disease and shorter life expectancy (Corey 1988; Stallings 2008). Exocrine PI can result in unpleasant bowel symptoms such as pain and frequent, bulky, offensive stools. Pancreatic enzyme replacement therapy (PERT) is therefore required to promote weight gain, to prevent malnutrition, to avoid deficiency of fat‐soluble vitamins and essential fatty acids, as well as to control abdominal symptoms of steatorrhea and maldigestion (Dodge 2006).
It is known that exocrine pancreatic function declines over the first months of life in infants with CF (Greer 1991; Waters 1990); and that this occurs earlier in those individuals who have "severe mutations" (Class 1 or Class 2, where the CFTR is absent) (Walkowiak 2005). Measurement of pancreatic elastase (a pancreatic enzyme) in the feces is one recognised technique for assessing PI and a fecal pancreatic elastase‐1 concentration below 100 mcg per g of stool is diagnostic of PI. As steatorrhea is one of the most prominent clinical manifestations of PI and stool fat content can be reliably measured, fat‐balance determination is a measure often used to assess this pathology and steatorrhea is assessed by measurement of fat excretion in the stool and by calculation of the co‐efficient of fat absorption (CFA).
Description of the intervention
Pancreatic enzymes mainly of porcine origin (i.e. from pigs), have been used in treating pancreatic insufficiency since the 1930s. Currently all available preparations of PERT are porcine in origin. First preparations were obtained by freeze drying hog pancreas, then extracting and purifying the enzymes which were subsequently administered as lyophilised total pancreatic extracts (TPE). These extracts reduced lipid malabsorption, but most of the enzyme was inactivated in the acidic environment of the stomach; to prevent this, bicarbonate or medication that suppressed acid was co‐administered. Later, enteric‐coated enzymes that were resistant to acid were developed, but these preparations did not completely prevent malabsorption as they did not empty into the duodenum as quickly as the smaller food particles. To overcome this problem, enteric‐coated microspheres (ECM) were developed. They allow for a smaller size of the preparations and stable delivery. The microsphere technology also allows a more uniform mixture of the enzymes with chyme (partly digested food); however, studies of labelled capsules suggest that even with varying sizes of microspheres, the entry of the enzyme into duodenum maybe later than that of food particles. At present the main formulations in use are immediate‐release enteric‐coated microspheres and mini‐microspheres, enteric‐coated microtablets and enteric‐coated microspheres with a bicarbonate buffer (Baker 2008; Fieker 2011). Newer non‐porcine products are in clinical trials (Borowitz 2005).
Though PERT is generally considered to be safe, there are potential significant side effects. The available enzyme products also vary greatly in their potency and properties. Even though PERT has been used for a many years, not all enzymes are equally effective at correcting maldigestion and sustaining normal growth and nutrition on a normal diet. A number of factors contribute to this, including those related to the preparations, such as the delivery of the enzymes in the correct strength and at the correct location; and disease‐related factors such as abnormal bile acid secretion, more acidic intestinal pH.
Some CF centres routinely administer pancreatic enzyme supplements from diagnosis. In countries that have neonatal screening programs, this is commonly in the first few weeks of life. Other centres administer PERT, once growth falters in children or malabsorption and weight loss is evident clinically in older children and adults. Yet other centres conduct formal assessment of pancreatic function such as pancreatic‐stimulation tests or by measuring pancreatic enzyme levels in the stool most commonly fecal pancreatic elastase‐1 or chymotrypsin or by measurement of fecal fat excretion such as the 72‐hour (3‐day) fat balance or the CFA. Other indicators of excess fecal fat excretion include stool microscopy or acid steatocrit (Leus 2000; Schibli 2002).
Pancreatic enzyme preparations currently available are given to CF patients orally 10 to 20 minutes before meals and snacks, either as tablets, enteric‐coated or non‐enteric‐coated capsules (for those individuals able to swallow a capsule) or as granules (for infants and young children). The number of enzyme capsules a person needs to take varies depending on the type of food being eaten, the degree of malabsorption, etc. Currently marketed pancreatic enzyme preparations differ in their composition, enzymatic activities, formulation, stability, and bioavailability.
How the intervention might work
The normal pancreas secretes digestive enzymes and bicarbonate into the duodenum to effect the breakdown of dietary protein, fats and starch. Pancreatic enzyme supplements contain all three main groups of digestive enzymes, namely lipase, amylase and protease, that respectively digest fats, carbohydrates and proteins into their basic components so that they can be absorbed and utilised by the body for growth and development. Thus PERT should facilitate sufficient digestion and absorption of food to support weight gain and growth and improve the bowel symptoms that arise from maldigestion and malabsorption.
Pancreatic enzymes normally act in the alkaline environment of the duodenum. They are denatured by pepsin and gastric acid, so PERT is usually administered as enteric‐coated preparations to prevent inactivation by stomach acid. The coating is designed to dissolve only when the pH exceeds 5.5 within the duodenum. However non‐enteric‐coated enzyme preparations are also available.
Clinical practice may differ between CF centres around the world depending on several factors such as: the level of expertise in certain centres in dealing with CF; the number of individuals with CF and PI as well as the diet and type of food that these people actually have access to; the different brands of ECM enzymes available in different countries, etc.
Why it is important to do this review
The most important reason to optimise PERT is to promote normal growth and to improve the nutritional status in people with CF and PI. This review aims to compare different preparations of PERT for their efficacy and safety in people with CF.
People with CF have a heavy burden of treatment and PERT significantly adds to that burden since enzymes are taken whenever food is eaten and doses need to be constantly adjusted according to what is being eaten, the level of malabsorption and weight gain. It can also be challenging for parents to administer these supplements to babies and young children as liquid preparations are not available. Also, excessive doses of pancreatic enzymes in infants have been associated with side effects such as abdominal pain, perianal irritation, constipation, hyperuricemia and hyperuricosuria (BNF for Children 2014) and very occasionally with serious complications of the gastrointestinal system such as fibrosing colonopathy in both children and adults (CSM 1995).
A systematic review on the efficacy of PERT in people with CF would help to guide clinical practice. Currently the approach to the assessment of pancreatic function, the commencement of pancreatic enzyme supplements, the dose and choice of enzyme supplement for infants and young children with CF is variable between centres. Optimising fat absorption and avoiding malnutrition are important for children with CF to achieve the best possible growth, improve their respiratory disease, their general health and ultimately their life expectancy. It is therefore very important to establish the evidence for benefit and risk with PERT; to compare different formulations; to determine the optimum treatment for different age groups; and to clarify the role of tests of pancreatic function in therapy. This is an update of a previously published review (Somaraju 2014).
Objectives
1. To evaluate the efficacy and safety of PERT in children and adults with CF associated with PI.
2. To compare the efficacy and safety of different formulations of PERT and their appropriateness in different age groups.
3. To compare the effects of PERT in CF according to different diagnostic subgroups (e.g. different ages at introduction of therapy and different categories of pancreatic function).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (using allocation methods such as alternate allocation to treatment and control groups) will be included.
Types of participants
People of any age with CF, either diagnosed clinically and confirmed with sweat test, or by genetic testing or by newborn screening.
Types of interventions
Any dose of PERT and in any formulation, in either home or hospital setting, for a period of not less than four weeks, compared either to placebo or other PERT preparations, commenced either at diagnosis of cystic fibrosis, at the onset of symptoms or at confirmation of abnormal pancreatic function.
We have selected this minimum treatment period for the following reasons. While clinicians and patients would expect to see the effect of treatment in terms of content of fat in stools and bowel motion, etc., within a week after changes have been made, in order to properly assess any impact on weight gain, it is necessary to follow the patients for at least two to four weeks. However, this may also depend on the patient’s age, as newborn babies and infants gain weight faster than older children and adolescents. Furthermore, longer periods may be necessary for the assessment of body mass index (BMI) and the evaluation of quality of life (QoL).
Types of outcome measures
Primary outcomes
-
Changes in nutritional status (absolute or relative change)
weight
height
BMI
Where weight has been adjusted for age or a z score used, we will request data (either individual patient data or aggregate data) from the study authors. If this is not available, then we will report z scores and centiles and include in a meta‐analysis where possible.
Secondary outcomes
-
Bowel symptoms
stool frequency
abdominal pain
flatulence
constipation
distal intestinal obstruction syndrome (DIOS)
Days in hospital (for any reason during the study period)
QoL (as assessed by a validated questionnaire to families )
Number of times vitamin deficiency diagnosed
-
Adverse events attributed to pancreatic enzyme replacement therapy
fibrosing colonopathy
any other adverse events
Fecal fat excretion (FFE) or co‐efficient of fat absorption (CFA)
-
Lung disease
number of exacerbations (as defined by study authors) requiring oral or intravenous antibiotics
-
rate of decline (absolute or relative change) in lung function as measured by:
forced expiratory volume at one second (FEV1) (either in litres or % predicted)
forced vital capacity (FVC) (either in litres or % predicted)
Search methods for identification of studies
Electronic searches
We identified relevant trials from the Group's Cystic Fibrosis Trials Register using the term: pancreatic enzymes.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated with each new issue of theCochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference.
Date of last search: 15 July 2016.
We have searched the ClinicalTrials.gov (clinicaltrials.gov) website and also the websites of the pharmaceutical companies who manufacture pancreatic enzyme replacements for any additional trials. Date searched: 22 July 2016.
Searching other resources
We have contacted the companies for further information on 27 July 2016 and are waiting for reply. If we receive any further information we will include them in future updates of the review.
Data collection and analysis
Selection of studies
Two authors independently selected the trials to be included in the review. We resolved any disagreements through discussion.
Data extraction and management
Two authors independently extracted data from the included studies using standard data acquisition forms to ensure consistency. We resolved any disagreements through discussion. Since all the studies included in the review had a treatment period of four weeks, we were only able to report data in the graphs at the time‐point of 'at one month'. In future updates, if data reported at any other time periods are available, we will report these as well.
Assessment of risk of bias in included studies
We independently assessed the risk of bias for each included study using the established criteria as set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Criteria that we assessed included: how allocation sequence was generated; how the treatment allocation schedule was concealed; whether the study was blinded; whether intention‐to‐treat analyses were possible from the data and if the number of participants who did not complete the study or who were excluded for some reason was recorded; as well as selective reporting and any other potential risk of bias. We resolved any disagreements through discussion.
Measures of treatment effect
The authors did not record any dichotomous data; however, if in future we report such data, we plan to report the odds ratio and calculate the odds of an outcome among treated participants to the corresponding odds in the control group and their 95% confidence intervals (CIs).
For the continuous outcomes, we recorded mean post‐treatment values or the mean change from baseline for each group with corresponding standard deviations (SDs). We entered the data into RevMan to produce a pooled estimate of treatment effect showing the mean difference (MD) between groups and the corresponding 95% CIs (RevMan 2012). Where papers provided the standard errors (SEs) instead of SDs, we converted SEs to SDs so that we were able to enter the data into RevMan.
Unit of analysis issues
We treated cross‐over studies as if they were parallel studies. We are aware that by doing so, we are assuming a correlation of zero and that this may produce conservative results which ignore any within‐patient correlation there may be; and furthermore the two groups will not be independent as each participant will appear in both treatment and control groups (Elbourne 2002).
Where the available data did not allow any analysis to be carried out and so incorporated within the review, we described the results individually.
Dealing with missing data
For an intention‐to‐treat analysis, we tried to obtain data for all participants who were later excluded from either treatment or follow up, for whatever reason, including poor compliance with treatment. We contacted the primary authors for any missing data.
Assessment of heterogeneity
In future updates, if we are able to combine more studies in the review, we will assess the trials for heterogeneity using the Chi2 test and the I2 statistic (Higgins 2003). We will assess heterogeneity such that we will consider values of under 40% as relatively unimportant; values between 40% and 60% as indicating moderate heterogeneity; and values above 60% as indicating substantial heterogeneity.
Assessment of reporting biases
Where possible we compared the original trial protocols, obtained from clinicaltrials.gov, with the final publications to identify any outcomes that were measured but not reported. We also tried to identify any instances of multiple publications of positive results and single publication of negative or neutral results.
We also made note of any language biases and assessed whether papers were published in multiple languages.
Data synthesis
We analysed the extracted data using a fixed‐effect model. In future updates, if we are able to add more studies, where the between‐study variability is not statistically significant, we will use a fixed‐effect model and if the between‐trial variability is statistically significant, we will use a random‐effects model.
Subgroup analysis and investigation of heterogeneity
In future updates, if we are able to add and combine more trials (n = 10) and we identify significant heterogeneity between them, we will perform a subgroup analysis looking at the different formulations and dosages, the presence of symptoms and if possible look at effects at different ages.
Sensitivity analysis
In future updates, if we are able to include sufficient studies in the review (n = 10), we will assess results when including and excluding quasi‐RCTs in addition to RCTs. We will also assess any differences from using a fixed‐effect or a random‐effects model.
In future updates, if we have sufficient data, we will undertake a meta‐analysis including only the first‐arm or last‐arm data and present this as a sensitivity analysis. In the present review, none of the included cross‐over trials presented first‐arm or last‐arm data separately, so we could not undertake this analysis.
Results
Description of studies
Results of the search
The literature search identified 92 studies and, after reviewing the abstracts 20 studies were identified as potentially meeting the inclusion criteria. After obtaining full published reports, 13 studies have been included in the review and six have been listed as 'Awaiting classification'. One eligible study is ongoing with estimated primary completion date in May 2017. We excluded a total of 72 studies from the review.
Included studies
Trial characteristics
All 13 included studies were RCTs. One study was of parallel design (Borowitz 2005) and the remaining 12 studies were of cross‐over design (Assoufi 1994; Elliott 1992; Henker 1987; Lacy 1992; Patchell 1999; Petersen 1984; Stead 1986; Stead 1987; Taylor 2015; Vidailhet 1987; Vyas 1990; Williams 1990). The duration of treatment was 28 days in the parallel study (Borowitz 2005); for the cross‐over studies, each arm of the study was for a period of 28 days (Assoufi 1994; Elliott 1992; Henker 1987; Lacy 1992; Patchell 1999; Petersen 1984; Stead 1986; Stead 1987; Vidailhet 1987; Vyas 1990; Williams 1990 ; Taylor 2015). Three studies were multicentre (Borowitz 2005; Patchell 1999 ; Taylor 2015); Borowitz recruited participants from 26 Cystic Fibrosis Foundation centres in the USA (Borowitz 2005) and Patchell recruited from three hospitals in the UK (Patchell 1999). Taylor recruited participants from 34 sites in seven European countries including Belgium, Bulgaria, Germany, Hungary, Italy, Poland, and the UK (Taylor 2015). For two studies, one based in Denmark (Petersen 1984) and one in the UK (Williams 1990), we could not ascertain whether they were single or multicentre studies from published reports. The remaining eight studies were single‐centre studies; five of these were run in the UK (Assoufi 1994; Lacy 1992; Stead 1986; Stead 1987; Vyas 1990) and one each in New Zealand (Elliott 1992), in the former East Germany (Henker 1987) and in France (Vidailhet 1987). All the studies included in the review were based in a home setting.
Participants
Eight trials included children with CF and the age of children varied from one to 17 years (Elliott 1992; Henker 1987; Lacy 1992; Patchell 1999; Petersen 1984; Vidailhet 1987; Vyas 1990; Williams 1990). The participants in four studies were adults with mean ages varying between 21.4 and 24.8 years (Assoufi 1994; Borowitz 2005; Stead 1986; Stead 1987). One study included participants aged 12 years and older (Taylor 2015).The number of participants in the studies was variable and ranged between 11 (Petersen 1984) and 129 participants (Borowitz 2005). The total number of participants in all included studies was 512.
Interventions
The interventions used were heterogenous between the studies; 10 studies compared enteric‐coated microspheres (ECM) with other preparations of PERT including other ECM.
ECM versus other enteric‐coated preparations
Seven studies compared ECM to other enteric‐coated preparations. Two studies compared ECM (Creon®) with enteric‐coated tablets (ECT) (Pancrex®) (Stead 1986; Vyas 1990). One study compared ECM (Creon 8000®) with enteric‐coated mini‐microspheres (ECMM) (Creon 10000®) (Patchell 1999). Petersen compared ECM (Pancrease®) with enteric‐coated granules (Pancreatin®) (Petersen 1984). Two studies compared ECM with non‐enteric‐coated tablets (Henker 1987; Stead 1987). One of these studies compared ECM (Creon®) with pancreatin (Pankreon Forte®) (Henker 1987); while the second compared ECM (Creon®) with non‐enteric‐coated pancreatin (Pancrex V®) in combination with cimetidine (Stead 1987). Another study compared ECM (Creon®) with lyophilised TPEs (Vidailhet 1987).
ECM versus another ECM
Four studies compared different preparations of ECM; three of these compared two preparations (Creon® versus Pancrease®; Kreon versus Zenpep®) (Elliott 1992; Williams 1990 ; Taylor 2015) and one study compared three preparations of ECM (Nutrizyme GR® versus Nutrizyme MP® versus Creon®) (Lacy 1992).
Note: Kreon is the trade name for Creon® used in German‐speaking regions, for clarity we will use Creon® in this review.
Different doses of PERT
Two studies compared PERT in different doses; one compared high‐dose enzyme replacement therapy (Nutrizyme 22®) with low‐dose therapy (Nutrizyme GR®) (Assoufi 1994) and the remaining study assessed different doses of a novel microbial preparation (Altu‐135) (Borowitz 2005).
Outcomes
None of the studies included measured all of the outcomes of interest to the review, and we looked at both relative and absolute changes in the outcomes.
Eleven studies measured the change in weight from baseline (Assoufi 1994; Elliott 1992; Henker 1987; Lacy 1992; Petersen 1984; Stead 1986; Stead 1987; Taylor 2015; Vidailhet 1987; Vyas 1990; Williams 1990). Four studies gave details on what they reported (absolute change in weight) (Stead 1986; Stead 1987; Taylor 2015; Vyas 1990); the remaining studies provided insufficient details to know exactly which type of change was considered. Stool frequency was also reported in 11 trials (Assoufi 1994; Borowitz 2005; Elliott 1992; Henker 1987; Patchell 1999; Petersen 1984; Stead 1986; Stead 1987; Taylor 2015; Vyas 1990; Williams 1990), while seven studies reported measuring abdominal pain (Elliott 1992; Patchell 1999; Stead 1986; Stead 1987; Taylor 2015; Vyas 1990; Williams 1990). Constipation was reported in one trial (Elliott 1992) and DIOS was reported in one study (Borowitz 2005). Only three studies reported on adverse events (Assoufi 1994; Borowitz 2005; Taylor 2015). All included studies reported FFE or CFA; but one study only measured it at 14 days and hence we did not include those data in our analysis (Borowitz 2005). Only two studies measured QoL (Borowitz 2005; Taylor 2015) and one study measured lung disease (Borowitz 2005).
Excluded studies
We excluded a total of 72 studies identified in the searches. Three studies were excluded as they were neither an RCT or quasi‐RCT (Araujo 2011; Katona 2000; Morrison 1992). Nine studies were excluded as they did not employ a relevant intervention (Breuel 1996; Butt 2001; Colombo 2001; Hubbard 1984; Lubin 1979; Ritz 2004; Stapleton 2001; van der Haak 2016; Vitti 1975) and two studies were excluded as they did not measure any outcomes relevant to this review (Hill 1993; Mack 1991). In the remaining 58 studies, the intervention was given for less than 28 days leading to exclusion.
Studies awaiting classification
Six studies are currently listed under 'Studies awaiting classification' for a number of reasons and we have contacted the investigators for further information (Dalzell 1992; Holsclaw 1980; Knill 1973; Lenoir 2008; Regele 1996; Taylor 1993). One study was deemed eligible, but data were not in a usable form (Dalzell 1992). The methodology with regards to randomisation was unclear in three studies (Holsclaw 1980; Lenoir 2008; Taylor 1993). One study presented data combined for 11 participants with CF and one with pancreatic insufficiency, but data were not available for just those participants with CF (Knill 1973). One cross‐over study presented data for each participant at the end of each treatment period, but did not make clear which treatment group the participant was part of in each period (Regele 1996).
Ongoing studies
One ongoing multicentre study by Anthera pharmaceuticals seems eligible for inclusion. It is a phase 3, randomised, open‐label study for evaluating the safety and efficacy of Lipromatase (non‐porcine PERT) against porcine PERT. Both males and females with CF aged seven years and above are eligible to participate in the study. The estimated enrolment is 126 participants. The outcome measures of the study are CFA (baseline up to eight weeks) and safety as measured by number of participants with adverse events or laboratory abnormalities (baseline up to 18‐20 weeks). The expected date of completion of the study is May 2017. Once results for this study are published we will fully assess it for inclusion in a future update of this review.
Risk of bias in included studies
Allocation
Generation of sequence
All 13 studies included in the review were described as RCTs. However, since the details of randomisation were not given for any of the studies, we have graded them all as having an unclear risk of bias.
Allocation concealment
LIkewise, we graded all 13 studies as having an unclear risk for allocation concealment as again no details were provided.
Blinding
Four of the included studies were open studies with no blinding and we graded them as having a high risk of bias (Henker 1987; Patchell 1999; Stead 1986; Stead 1987). One study was single‐blind and cross‐over in design (Williams 1990). The study medication was issued by pharmacist and the order of treatment was not known to the doctor; but since the participants were not blinded, we also graded this study as having high risk of bias.
For one study the details of blinding were not given; we therefore judged it to have an unclear risk of bias (Vidailhet 1987).
The remaining seven studies were described as double blind and in each of these studies all the participants received equal number of ungraded capsules (Assoufi 1994; Borowitz 2005; Elliott 1992; Lacy 1992; Petersen 1984; Taylor 2015; Vyas 1990). We judged these studies to have a low risk of bias.
Incomplete outcome data
We judged four studies to have a high risk of bias (Assoufi 1994; Lacy 1992; Vyas 1990; Williams 1990). For two studies, the reasons for withdrawals were not described (Assoufi 1994; Lacy 1992). In a further study, there were 20 participants, but only 12 paired stool samples were analysed for fecal fat excretion; the reason for the exclusion of the other participants was not given (Vyas 1990). A fourth study enrolled 39 participants and 12 of these withdrew for various reasons (Williams 1990). Although withdrawals were described clearly, because the proportion of participants withdrawing was 31%, we graded the study as having a high risk of bias.
We judged two studies to have an unclear risk of bias due to incomplete outcome data (Henker 1987; Vidailhet 1987). The first of these did not give any details about whether there were any withdrawals (Henker 1987). The second study appears to have included all the participants in the analysis, but the details were not given (Vidailhet 1987).
We graded seven included studies as having low risk of bias due to incomplete outcome data (Borowitz 2005; Elliott 1992; Patchell 1999; Petersen 1984; Stead 1986; Stead 1987; Taylor 2015). In the Borowitz study, 12 of the 129 enrolled participants withdrew early; Borowitz described the withdrawals and 117 participants were included in a modified intention‐to‐treat analysis (Borowitz 2005). Elliott described three withdrawals out of 30 children; two withdrew consent prior to randomisation and one withdrew from the study due to respiratory exacerbations during the run‐in period (Elliott 1992). In one multicentre study, 54 out of 59 randomised participants completed the study; stool collection data were analysed in one centre on an intention‐to‐treat basis (Patchell 1999). In one study, there were no withdrawals, with all 11 participants completing (Petersen 1984). In the two remaining studies by Stead reasons for withdrawal were described fully; in the earlier study, two out of 23 participants withdrew (Stead 1986) and in the later study one out of 14 participants withdrew (Stead 1987). There were 10 withdrawals from the Taylor study and the reasons were described (Taylor 2015).
Selective reporting
We graded eight studies as having a high risk of bias since some of the outcomes were reported in a way that could not be included in the analysis (Assoufi 1994; Elliott 1992; Henker 1987; Lacy 1992; Patchell 1999; Petersen 1984; Vidailhet 1987; Williams 1990). For four of these, the results were reported in a narrative fashion only (Assoufi 1994; Elliott 1992; Henker 1987; Lacy 1992). Another of these studies measured stool frequency, wind and abdominal pain, but full details were not given (Patchell 1999). A further study presented the results as medians, which could not be included in analysis (Petersen 1984). Another study measured change in body weight but reported this incompletely so it could not be analysed in the review (Vidailhet 1987) and the remaining study measured change in body weight, stool frequency and abdominal pain again without sufficient detail to allow analysis in the review (Williams 1990).
For five studies the outcomes were reported adequately and we graded them as having a low risk of bias (Borowitz 2005; Stead 1986; Stead 1987; Taylor 2015; Vyas 1990).
Other potential sources of bias
We judged seven studies as having a high risk of bias as they were funded or supported by pharmaceutical companies (Borowitz 2005; Elliott 1992; Stead 1986; Stead 1987; Taylor 2015; Vyas 1990; Williams 1990). For three of these the intervention drug (Creon®) was supplied by Duphar laboratories (Stead 1986; Stead 1987; Vyas 1990). One study was sponsored and actively supported by Altus pharmaceuticals (Borowitz 2005) and the primary author of another study was financially supported by Cilag Limited (Williams 1990). For one study, Boehringer Ingelheim (NZ) Ltd Kali Chemie provided funding and materials (Elliott 1992). For the final study, the corresponding author is a consultant to Aptalis and Profile Pharma and the study was funded by Aptalis Pharma (Taylor 2015).
For three studies there was no information and we judged them to have an unclear risk of bias (Assoufi 1994; Henker 1987; Lacy 1992).
For three studies we could not identify any other potential source of bias and judged them to have a low risk of bias (Patchell 1999; Petersen 1984; Vidailhet 1987).
Although with cross‐over studies there is a potential source of bias due to a lack of a washout period, since these enzymes are given orally and act locally within the gastro‐intestinal tract (no systemic absorption), we do not believe a washout period is necessary (Law 2014). Further to these cross‐over studies, none of them presented the first‐arm data and last‐arm data individually, so that they could be included in sensitivity analysis. In the only comparison where we could combine data, the fact that these were cross‐over studies is likely to underestimate the level of inconsistency between the results of the studies due to over‐inflation of confidence intervals from the individual studies. This could potentially be a risk of bias.
Effects of interventions
We have included 13 studies in the review, but were only able to include seven of these in the analysis within the review (Patchell 1999; Stead 1986; Stead 1987; Taylor 2015; Vidailhet 1987; Vyas 1990; Williams 1990). We have presented summary statistics for both significant and non‐significant results below.
We did not have sufficient information to include the remaining six studies in the analysis at this time and have contacted the primary authors for further information. For these studies we have described the results, and we will update the review with information if we receive any at a later date (Assoufi 1994; Borowitz 2005; Elliott 1992; Henker 1987; Lacy 1992; Petersen 1984).
Many of the outcomes of interest to our review were not reported in any of the included studies.
Primary outcomes
1. Changes in nutritional status
a. weight
This outcome was reported in 11 studies with 318 participants (Assoufi 1994; Elliott 1992; Henker 1987; Lacy 1992; Petersen 1984; Stead 1986; Stead 1987; Taylor 2015; Vidailhet 1987; Vyas 1990; Williams 1990); but of these 11 trials, only four (n = 136) provided data for the analysis (Stead 1986; Stead 1987; Taylor 2015; Vyas 1990).
ECM versus non‐enteric‐coated tablets (NECT) with adjuvant cimetidine
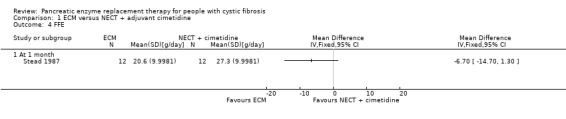
One study with 12 participants compared ECM to non‐enteric‐coated tablets (NECT) with adjuvant cimetidine (Stead 1987). The change in weight in favour of ECM when compared to NECT with adjuvant cimetidine at one month was not statistically significant, MD 0.40 kg (95% CI ‐0.10 to 0.90) (Analysis 1.1).
1.1. Analysis.
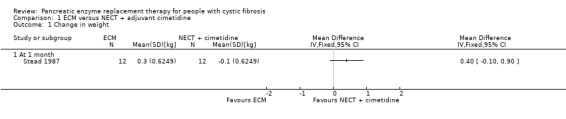
Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 1 Change in weight.
ECM versus ECT
Two studies with data from 41 participants compared ECM to ECT (Stead 1986; Vyas 1990). At one month, there was a higher increase in body weight in participants receiving ECM than those receiving ECT but this was not statistically significant, MD 0.32 kg (95% CI ‐0.03 to 0.67) and heterogeneity was substantial (I2 = 73%) (Analysis 2.1).
2.1. Analysis.
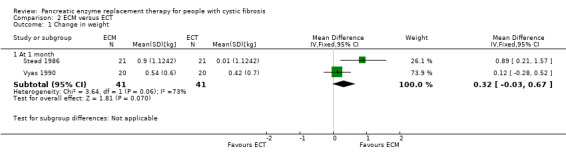
Comparison 2 ECM versus ECT, Outcome 1 Change in weight.
ECM (Creon®) versus another ECM
There were three studies comparing two different forms of ECM (Elliott 1992; Taylor 2015; Williams 1990) and one study comparing three different forms of ECM (Lacy 1992); only one study provided data for analysis (Taylor 2015). There was no difference in treatment effect between Creon® and Zenpep®, MD 0.00 kg (95% CI ‐0.28 to 0.28) (Analysis 4.1). The remaining studies did not report any significant difference in change in body weight (Elliott 1992; Lacy 1992; Williams 1990).
4.1. Analysis.
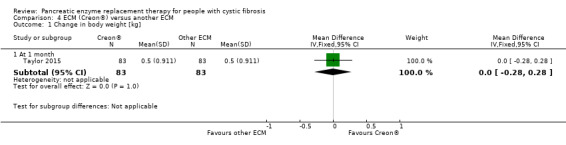
Comparison 4 ECM (Creon®) versus another ECM, Outcome 1 Change in body weight [kg].
ECM versus TPE
One study comparing ECM to TPE did not report any significant difference in change in body weight (no data available for analysis) (Vidailhet 1987)
ECM versus other enteric‐coated preparations
One tudy compared ECM to a conventional pancreatin preparation and did not report any significant difference in change in body weight (Henker 1987). One study compared ECM to enteric‐coated granules and reported that weight gain was significantly better with ECM (Petersen 1984). Neither study provided data for analysis.
Different doses of PERT
One study compared a high dose of enzymes to a low dose, maintaining lipase intake equal, but halving the number of capsules of high‐dose preparation, and reported finding no significant difference in weight gain (Assoufi 1994).
b. height
ECM versus other enteric‐coated preparations
This outcome was measured in only one of the included studies (45 participants) comparing ECM to conventional pancreatin preparation (Henker 1987). The study did not give any details, but the investigators reported no difference between the two preparations.
c. BMI
ECM versus other enteric‐coated preparations
This outcome was not measured in any of the included studies. Furthermore, we could not calculate the BMI as only one study measured the height of participants, but did not report the actual data (Henker 1987).
Secondary outcomes
1. Bowel symptoms
ECM (Creon®) versus another ECM
One study described measuring symptom scores, but did not give any details as to which symptoms were measured or the results (Lacy 1992).
Different doses of PERT
In one study of 117 participants comparing different doses of ALTU‐135, 106 participants reported gastrointestinal adverse events which were mostly mild in intensity and were not significantly different between the three treatment arms; further details were not given (Borowitz 2005). However, four participants did withdraw due to gastrointestinal events.
a. stool frequency
This outcome was reported in 10 studies with 326 participants (Assoufi 1994; Elliott 1992; Henker 1987; Patchell 1999; Petersen 1984; Stead 1986; Stead 1987; Taylor 2015; Vyas 1990; Williams 1990). Of the these, only four studies (n = 136) have provided sufficient data for inclusion in our analysis (Stead 1986; Stead 1987; Taylor 2015; Vyas 1990).
ECM versus NECT with adjuvant cimetidine
The study (n = 12) comparing ECM to NECT with cimetidine reported a significant decrease in stool frequency (number per day) at one month, MD ‐0.70 (95% CI ‐0.90 to ‐0.50) (Stead 1987) (Analysis 1.2).
1.2. Analysis.
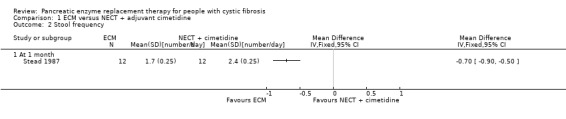
Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 2 Stool frequency.
ECM versus ECT
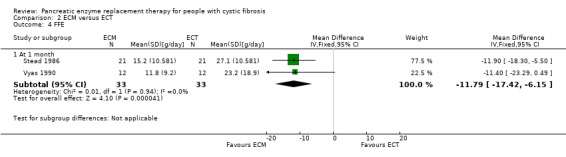
The two studies (n = 41) comparing ECM to ECT also reported a significant decrease in stool frequency (number per day) in favour of ECM, MD ‐0.58 (95% CI ‐0.85 to ‐0.30) (Stead 1986; Vyas 1990) (Analysis 2.2) (Figure 1).
2.2. Analysis.
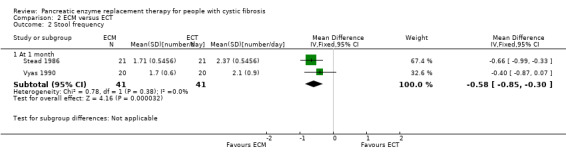
Comparison 2 ECM versus ECT, Outcome 2 Stool frequency.
1.
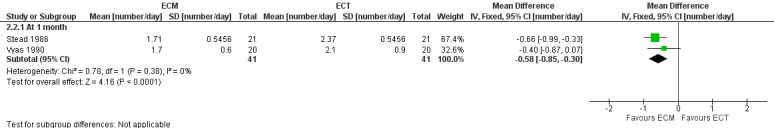
Forest plot of comparison: 2 ECM versus ECT, outcome: 2.2 Stool frequency [number/day].
ECM versus ECMM
One study (59 participants) compared ECM to ECMM and reported no difference between treatment groups with a median stool frequency of two stools per day in both treatment groups (Patchell 1999).
ECM (Creon®) versus another ECM
The three studies comparing different formulations of ECM also reported that there was no significant difference in either treatment period (Elliott 1992; Taylor 2015; Williams 1990) (Analysis 4.2).
4.2. Analysis.
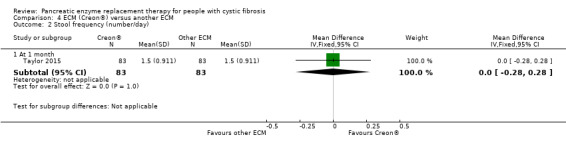
Comparison 4 ECM (Creon®) versus another ECM, Outcome 2 Stool frequency (number/day).
Different doses of PERT
The study comparing a high dose of enzymes to a low dose, while maintaining lipase intake as equal but halving the number of capsules of high dose preparation, found no significant difference in stool frequency (Assoufi 1994).
ECM versus other enteric‐coated preparations
Two studies did not provide sufficient data for inclusion in analysis, the trial comparing ECM to conventional pancreatic preparations (Henker 1987) and the trial comparing ECM to enteric‐coated granules (Petersen 1984) reported finding significantly decreased stool frequency with ECM.
b. abdominal pain
This outcome was reported in seven studies with 253 participants using clinical scores or questionnaires (Elliott 1992; Patchell 1999; Stead 1986; Stead 1987; Taylor 2015; Vyas 1990; Williams 1990). Of the seven studies, only four reported sufficient data (n = 136), such that they could be included in analysis. (Stead 1986; Stead 1987; Taylor 2015; Vyas 1990).
ECM versus NECT with adjuvant cimetidine
The study (n = 12) comparing ECM to NECT with cimetidine reported a non‐significant decrease in abdominal pain when receiving ECM compared to NECT with adjuvant cimetidine, MD ‐10.50 (95% CI ‐21.40 to 0.40) (Stead 1987) (Analysis 1.3).
1.3. Analysis.
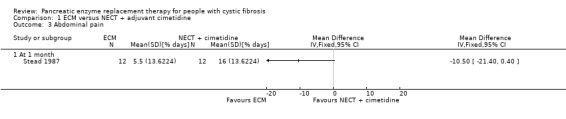
Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 3 Abdominal pain.
ECM versus ECT
Combined data from the two studies (n = 41) comparing ECM to ECT reported a significant decrease in the percentage of days when abdominal pain is present in participants in the ECM group compared to those in the ECT group, MD ‐7.96 (95% CI ‐12.97 to ‐2.94) (Stead 1986; Vyas 1990) (Analysis 2.3) (Figure 2).
2.3. Analysis.
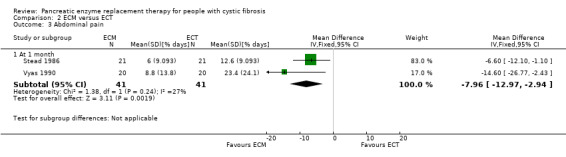
Comparison 2 ECM versus ECT, Outcome 3 Abdominal pain.
2.
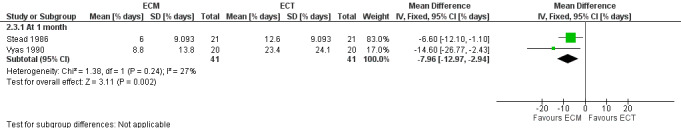
Forest plot of comparison: 2 ECM versus ECT, outcome: 2.3 Abdominal pain [% days].
ECM versus ECMM
The authors of the study comparing ECM and ECMM observed no significant difference between the groups and reported that abdominal pain was mainly absent or mild throughout the study (no data for analysis) (Patchell 1999).
ECM (Creon®) versus another ECM
Likewise, the studies comparing different formulations of ECM found no significant difference between the groups for this outcome (Elliott 1992; Williams 1990; Taylor 2015); only one study provided any data for analysis (Analysis 4.3).
4.3. Analysis.
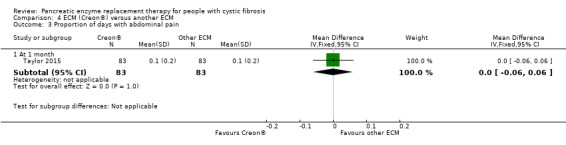
Comparison 4 ECM (Creon®) versus another ECM, Outcome 3 Proportion of days with abdominal pain.
c. flatulence
Two studies reported on this outcome (Patchell 1999; Taylor 2015).
ECM versus ECMM
The authors observed no treatment difference between the groups and flatulence was stated to be absent or mild throughout the study; however no actual data were reported (Patchell 1999).
ECM (Creon®) versus another ECM
In the Taylor study, there was no treatment difference between the two interventions, MD 0.00 (95% CI ‐0.12 to 0.12) (Analysis 4.4) (Taylor 2015).
4.4. Analysis.
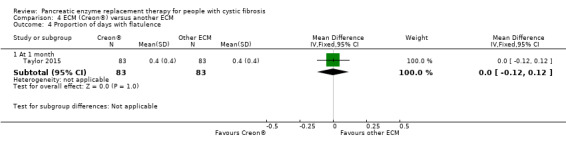
Comparison 4 ECM (Creon®) versus another ECM, Outcome 4 Proportion of days with flatulence.
d. constipation
ECM (Creon®) versus another ECM
One study comparing two forms of ECM measured this outcome and reported narratively that no significant difference was found between the two treatment periods (Elliott 1992).
e. DIOS
Different doses of PERT
Only the study of ALTU‐135 reported this outcome (Borowitz 2005). There was a single episode of DIOS requiring hospitalisation in a participant in the low‐dose group. It resolved without any sequelae.
2. Days in hospital
This outcome was not measured in any of the included studies.
3. QoL
Only two studies reported this outcome (Borowitz 2005; Taylor 2015).
ECM (Creon®) versus another ECM
The study comparing two different forms of ECM used CFQ‐R‐Parent and CFQ‐R‐Teen/Adult to measure this outcome (Taylor 2015). The two forms were found to have similar effects on well being with a nominally significant difference in favour of Zenpep®, but only for the respiratory domain.
Different doses of PERT
The study of ALTU‐135 reported this outcome (Borowitz 2005). The investigators used the Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) (Quittner 2009) and reported finding little change from baseline after treatment, regardless of dose group. They attributed this to the short duration of the study.
4. Number of times vitamin deficiency diagnosed
This outcome was not measured in any of the included studies.
5. Adverse events attributed to pancreatic enzyme replacement therapy
a. fibrosing colonopathy
This outcome was not measured in any of the included studies.
b. any other adverse events
This outcome was reported in only three studies (Assoufi 1994; Borowitz 2005; Taylor 2015).
ECM (Creon®) versus another ECM
Taylor reported mostly mild adverse events, with abdominal pain, diarrhea and flatulence being most common, and found the number of participants reporting adverse events was lower for Zenpep® (19.6%) than Creon® (25.6%) (Taylor 2015).
Different doses of PERT
There were no noted side effects in the trial by Assoufi (Assoufi 1994), while Borowitz did not find any serious adverse events or deaths (Borowitz 2005).
6. Fecal fat excretion (FFE) or co‐efficient of fat absorption (CFA)
This outcome was measured in all 13 included studies (Assoufi 1994; Borowitz 2005; Elliott 1992; Henker 1987; Lacy 1992; Patchell 1999; Petersen 1984; Stead 1986; Stead 1987; Taylor 2015; Vidailhet 1987; Vyas 1990; Williams 1990). Seven studies (n = 111) provided data which could be included in analysis (Patchell 1999; Stead 1986; Stead 1987; Taylor 2015; Vidailhet 1987; Vyas 1990; Williams 1990). The unit of measurement varied across the studies; some authors drew conclusions based on FFE and others drew conclusions based on CFA. Not all the studies provided detailed information as to how the variable was measured. For the purpose of this review, we have assumed either FFE and CFA were equivalent and the two studies whose data were combined, provided both the variables, as CFA is a calculated outcome, for which FFE is necessary.
ECM versus NECT with adjuvant cimetidine
The study (n = 12) comparing ECM to NECT with adjuvant cimetidine showed no significant difference in FFE between treatment groups, MD ‐6.70 (95% CI ‐14.70 to 1.30) (Stead 1987) (Analysis 1.4).
1.4. Analysis.
Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 4 FFE.
ECM versus ECT
In contrast, the two studies (n = 33) comparing ECM and ECT reported a significant decrease in FFE in favour of ECM, MD ‐11.79 (95% CI ‐17.42 to ‐6.15) (Stead 1986; Vyas 1990) (Analysis 2.4) (Figure 3).
2.4. Analysis.
Comparison 2 ECM versus ECT, Outcome 4 FFE.
3.
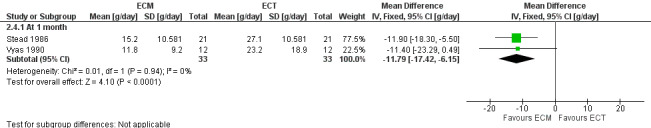
Forest plot of comparison: 2 ECM versus ECT, outcome: 2.4 FFE [g/day].
ECM versus ECMM
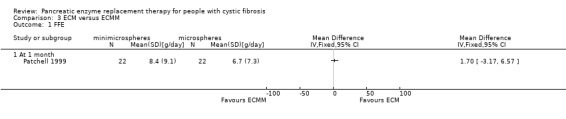
The comparison of ECM and ECMM by Patchell (n = 22) showed no significant difference in FFE between treatments, MD 1.70 (95% CI ‐3.17 to 6.57) (Patchell 1999) (Analysis 3.1; Figure 4).
3.1. Analysis.
Comparison 3 ECM versus ECMM, Outcome 1 FFE.
4.
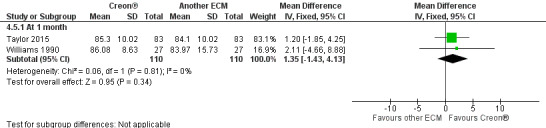
Forest plot of comparison: 4 ECM (Creon®) versus another ECM, outcome: 4.5 Coefficient of fat absorption [%].
ECM (Creon®) versus another ECM
The results from two studies (n = 110) showed no significant difference in CFA when two different formulations of ECM were compared, MD 1.35 (95% CI ‐1.35 to 4.13) (Taylor 2015; Williams 1990) (Analysis 4.5). The study comparing two preparations of ECM (Elliott 1992) and another comparing three preparations of ECM (Lacy 1992) found no significant difference for this outcome (no data available for analysis).
4.5. Analysis.
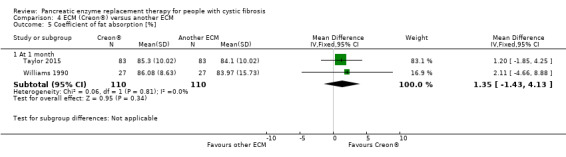
Comparison 4 ECM (Creon®) versus another ECM, Outcome 5 Coefficient of fat absorption [%].
ECM versus TPE
In the study of ECM compared to TPE (n = 17), while FFE was lower in the ECM group, the result was not statistically significant, MD ‐1.60 (95% CI ‐3.31 to 0.11) (Vidailhet 1987) (Analysis 5.1).
5.1. Analysis.
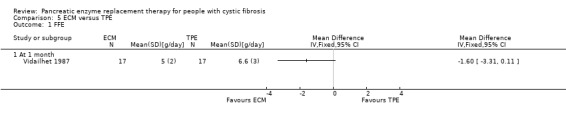
Comparison 5 ECM versus TPE, Outcome 1 FFE.
ECM versus other enteric‐coated preparations
One study comparing ECM to conventional pancreatin reported finding no difference between the two treatment arms (Henker 1987); while another comparing ECM to enteric‐coated granules found improved fat absorption on ECM, but the results were not statistically significant (Petersen 1984).
Different doses of PERT
The study that compared a high dose of enzymes to a low dose (maintaining lipase intake as equal, but halving the number of capsules of the high‐dose preparation) reported an FFE of 15.4 g/day on the high‐dose enzyme and a FFE of 18.7 g/day on the low‐dose enzyme. However, the difference was not statistically significant (Assoufi 1994).
The final study measured this outcome at 14 days on the treatment arm, even though the study period was one month (Borowitz 2005). Since the inclusion criteria of the review require that the treatment period should be at least four weeks before measuring outcome, we have not included the results from that study in our analysis for this outcome.
7. Lung disease
a. number of exacerbations requiring oral or intravenous antibiotics
Different doses of PERT
This outcome was reported in one study comparing different doses of ALTU‐135 (Borowitz 2005). There were a total of 10 pulmonary exacerbations of CF requiring hospitalisation reported, but the distribution of events across groups was not reported.
b. rate of decline in FEV1 and FVC
This outcome was not measured in any of the included studies.
Discussion
Even though pancreatic enzyme replacement therapy (PERT) has been used for many years, not all enzymes are equally effective at correcting maldigestion and sustaining normal growth and nutrition on a normal diet. The available enzyme products also vary greatly in their potency and properties. A number of factors contribute to this, including those related to the preparations, such as the delivery of the enzymes in the correct strength and at the correct location; and disease‐related factors such as abnormal bile acid secretion, more acidic intestinal pH.
This review aimed to compare different preparations of PERT for their efficacy and safety in people with cystic fibrosis (CF).
Summary of main results
In this review we found that people with CF taking enteric‐coated microspheres (ECM) experienced a small increase in body weight; however, this was not statistically significant when compared to enteric‐coated tablets (ECT) or non‐enteric‐coated tablets (NECT) with adjuvant antacids. The people with CF taking ECM also experienced decreases in stool frequency, in abdominal pain and in fecal fat excretion (FFE), all of which were statistically significant when compared to ECT and a statistically significant decrease in stool frequency when compared to NECT with adjuvant antacids. When ECM were compared to total pancreatic extracts (TPE), FFE was found to be lower in participants receiving ECM, but again none of these results were statistically significant. A comparison of different preparations of ECM showed there was no statistically significant difference among the preparations for any of the measured outcomes. Also, a comparison between ECM and enteric‐coated mini‐microspheres (ECMM) identified no statistically significant difference in the efficacy of the two types of preparations.
Overall completeness and applicability of evidence
An important limitation in the review was that we did not find any studies meeting our inclusion criteria which compared the different preparations of PERT to placebo; therefore, we cannot comment on the relative efficacy of PERT in comparison to placebo. Another notable factor identified in our review was the lack of evidence for many outcomes that are likely to be important for people with CF and clinicians in evaluating a response to treatment. There were no comprehensive data on nutritional status (only some data on weight which could not be combined), quality of life (QoL), vitamin deficiencies, or number of days in hospital. The included studies were all of short duration, so the long‐term effects of treatment could not be assessed. Also, there was no information with respect to the severity of disease in participants (either in terms of lung function or degree of pancreatic insufficiency), so we cannot comment on the relative effectiveness of PERT in the different patient groups. This also limits the generalisation of evidence for all patient groups. Finally, with the exception of two studies (Borowitz 2005; Taylor 2015), the included studies were relatively old; probably due to the fact that they were looking at PERT formulations developed in 1970s. There have been more recent studies for recombinant enzymes (Merispase and Altu) which were developed in last 10 years. The company developing Merispase is no longer trading and Altu is the subject of the Borowitz study (Borowitz 2005).
Quality of the evidence
The review included only 13 studies with an unclear risk of bias from randomisation methods; 12 out of the 13 studies were cross‐over in design and mostly also had a high risk of attrition bias and reporting bias. Attrition bias may be due to the duration of the study periods; all studies had a run‐in or dose stabilisation period followed by e.g. eight weeks in each of the two treatment arms. The included studies were of a short duration and this precludes any comments on the long‐term effects of the intervention. Also, there was no evidence on the severity of the pancreatic insufficiency among the participants.
Potential biases in the review process
A potential bias introduced in the review process may be the time frame of four weeks chosen by the authors, which resulted in a number of trials being excluded from the review due to shorter treatment periods. Although clinical changes may be seen within one week of PERT, we felt that a period of four weeks reflects real life clinical practice more accurately.
A further potential bias is that since the included cross‐over studies did not present separate first‐arm data, we had to analyse these studies as if they were parallel studies. We are aware that doing so may produce conservative results which ignore any possible within‐patient correlation and acknowledge that the two treatment groups are not independent as each participant will appear in both treatment and control groups. In the only comparison where we could combine data, the fact that these were cross‐over studies is likely to underestimate the level of inconsistency between the results of the studies due to over‐inflation of confidence intervals from the individual studies.
There was no other additional bias identified in the review. Neither contributing author has any conflict of interest.
Agreements and disagreements with other studies or reviews
In our review, we found that ECM decreased FFE and abdominal symptoms, when compared to TPE or to non‐enteric‐coated preparations. This is similar to the findings of other authors (Beverley 1987; Chazalette 1988; Holsclaw 1979; Mischler 1982). Our finding that the different preparations of ECM showed no significant difference in any of the measured outcomes is in agreement with the findings of other studies (Hilman 1982; Khaw 1977; Santini 2000). That ECMM preparations did not showing any significant difference in FFE, weight gain or clinical symptoms when compared to ECM is corroborated by another study (Duhamel 1998).
Authors' conclusions
Implications for practice.
We found no evidence from any comparison of PERT to placebo. The available evidence suggests that ECM are better at improving clinical symptoms in people with CF compared to non‐enteric‐coated enzyme preparations. This evidence is, however, limited and is from a few small studies which are prone to bias. There is a lack of evidence on the long‐term benefits and risks of treatment and the relative dosages of PERT required for people with different severities of pancreatic insufficiency.
Implications for research.
There is a need for large, multicentre robustly designed parallel randomized controlled trials (RCTs) to study the different forms of PERT, their efficacy, safety, role in improving nutritional status, QoL and their long‐term effects. There is also a necessity to investigate if the same amount of PERT is applicable to all ranges of pancreatic insufficiency in CF. Since the degree of pancreatic insufficiency can decline with age and consequently an adaptation of PERT based on residual function could be necessary for older people, this should be taken into account when planning a trial.
Future studies should be based on "real life clinical scenarios", where participants vary the amount of enzyme everyday with different meals and meal sizes. Different levels of PERT should be compared to placebo whenever possible.
Finally, when planning future studies researchers should take into account the high attrition rates seen in the studies included in this review, possibly due to run‐in or dose stabilisation periods before the actual trial begins.
What's new
| Date | Event | Description |
|---|---|---|
| 22 November 2016 | New citation required but conclusions have not changed | Despite the inclusion of a new study, our conclusions remain the same. |
| 22 November 2016 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's Cystic FIbrosis Trials Register identified eight references which were potentially eligible for inclusion in the review. One new study (two references) has been included (Taylor 2015). Two references were additional references to two already excluded studies (Borowitz 2008; Wooldridge 2009). The remaining four references to two studies have been excluded (Heubi 2016; van der Haak 2016). |
Acknowledgements
Nikki Jahnke and Tracey Remmington have both given considerable advice and support in the drafting of the protocol. Nikki Jahnke also extended considerable support for the development of the review.
The current review team would like to thank the authors who worked on the protocol for this review ‐ Paramita Cifelli, Robyn Huggins and Alan Smyth. They would also like to thank Dhruv Rastogi for his contribution to the early stages of the full review.
Appendices
Appendix 1. Glossary
| Medical term | Lay term |
| acid steatocrit | an estimate of the amount of fat in stool (feces) |
| distal intestinal obstruction syndrome | blockage of the large bowel due to partly digested food |
| duodenum | first part of the small intestine |
| enteric‐coated | protected against damage by acid in the stomach |
| exocrine pancreatic insufficiency | insufficient production of digestive enzymes |
| fibrosing colonopathy | scarring and narrowing of the large intestine, thought to be related to high doses of some enzymes |
| gastrointestinal motility | normal movement of food through the digestive system |
| gastrointestinal tract | digestive system |
| hyperuricemia | high levels of uric acid in the blood |
| hyperuricosuria | high levels of uric acid in the urine |
| perianal redness | sore bottom (in this context) |
Data and analyses
Comparison 1. ECM versus NECT + adjuvant cimetidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Stool frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Abdominal pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FFE | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. ECM versus ECT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.03, 0.67] |
| 2 Stool frequency | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.85, ‐0.30] |
| 3 Abdominal pain | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐7.96 [‐12.97, ‐2.94] |
| 4 FFE | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 1 month | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐11.79 [‐17.42, ‐6.15] |
Comparison 3. ECM versus ECMM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FFE | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. ECM (Creon®) versus another ECM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight [kg] | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 1 month | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 2 Stool frequency (number/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 1 month | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 3 Proportion of days with abdominal pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1 month | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 4 Proportion of days with flatulence | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 1 month | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.12, 0.12] |
| 5 Coefficient of fat absorption [%] | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 1 month | 2 | 220 | Mean Difference (IV, Fixed, 95% CI) | 1.35 [‐1.43, 4.13] |
Comparison 5. ECM versus TPE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FFE | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Assoufi 1994.
| Methods | Randomised, double‐blind cross‐over study. Duration: there was a run‐in period (duration not specified) followed by randomization to 1 of 2 arms. 28 days in each arm. UK based. Home setting. |
|
| Participants | 17 individuals diagnosed with CF. Age: 18 to 42 years. |
|
| Interventions | Group 1: Nutrizyme GR (10000 BP units of lipase). Group 2: Nutrizyme 22 (22000 BP units of lipase). Lipase intake was equivalent to previous intake of participants and was kept constant during the study. |
|
| Outcomes | Weight gain, appetite, stool consistency, stool frequency and FFE. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | When on Nutrizyme 22, participants took an equal number of placebo capsules and high‐dose enzyme capsules to make the total number of capsules the same as when taking Nutrizyme GR. |
| Blinding of participants and personnel (performance bias) Clinicians | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant withdrew after the run‐in period; reason was not given. |
| Selective reporting (reporting bias) | High risk | SDs were not presented for the outcome fecal fat excretion; other outcomes were reported in a way, that could not be included in analysis. |
| Other bias | Unclear risk | Information not given. |
Borowitz 2005.
| Methods | Randomised, double‐blind, 3‐arm parallel, dose‐ranging, multicentre study. Duration: 29 days. Participants recruited from 26 CF Foundation‐accredited centres in the USA. Home setting. |
|
| Participants | 139 participants with previously diagnosed CF and undergoing treatment were screened and 129 enrolled as intention‐to‐treat population. Age: mean (SD) 21.5 (8.5) years. Gender split: 71% were males. |
|
| Interventions | Group 1: Altu‐135 5000 units of lipase. Group 2: Altu‐135 25,000 units of lipase. Group 3: Altu‐135 100,000 units of lipase. Doses were not adjusted on basis of weight or food ingested, but were fixed per meal or snack. Lipase, protease & amylase were in a ratio of 1:1:0.15 |
|
| Outcomes | CFA, CNA, adverse events, QoL using the CFQ‐R. | |
| Notes | The CFA and CNA were measured at baseline and at 14 days after randomization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | All participants received equal number of unlabelled capsules. |
| Blinding of participants and personnel (performance bias) Clinicians | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 129 participants were enrolled as intention‐to‐treat population, of whom 12 withdrew (4 due to gastrointestinal adverse events); 117 participants who received at least 1 dose were included in a modified intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | High risk | Trial sponsored and actively supported by Altus Pharmaceuticals. |
Elliott 1992.
| Methods | Randomised, double‐blind, cross‐over study. Duration: 4 weeks for each treatment arm with a 2‐week run‐in period. Single‐centre study in New Zealand. Home setting. |
|
| Participants | 30 children previously diagnosed with CF using clinical and laboratory data. Age: median 10.1 years. Gender split: 17 girls, 13 boys. |
|
| Interventions | Group 1: Creon® (lipase 8000 BP, amylase 9000 BP, protease 210 BP). Group 2: Pancrease® (lipase 5000 BP, amylase 3000 BP, protease 350 BP). Participants were started on doses of lipase slightly lower or equivalent to pretrial period. Later they were allowed to adjust according to their requirement. |
|
| Outcomes | Mean weight gain, adequate daily intake of energy, fat and nitrogen, stool weight, FFE and nitrogen excretion. | |
| Notes | For the outcomes of interest to the review, the results were given in a descriptive method; means and SDs not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | Both formulations were prepared in identical opaque capsules from commercial stock. |
| Blinding of participants and personnel (performance bias) Clinicians | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew consent and 1 participant was hospitalised due to respiratory exacerbations during the run‐in period. |
| Selective reporting (reporting bias) | High risk | Results were reported in a narrative method and could not be included in analysis |
| Other bias | High risk | Boehringer Ingelheim (NZ) Limited Kali Chemie provided funding and study materials. |
Henker 1987.
| Methods | Randomized, open‐label, cross‐over study. Duration: each arm was for 4 weeks. No run‐in period specified. Single centre in the former East Germany. |
|
| Participants | 45 participants with CF. Age: mean 11.8 years. Gender split: 24 boys and 21 girls. |
|
| Interventions | Group 1: Pancreon forte (conventional). Group 2: Creon® (acid protected microspheres). |
|
| Outcomes | Weight gain, height, stool frequency, FFE. | |
| Notes | Outcomes were given in a descriptive method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) Clinicians | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not given. |
| Selective reporting (reporting bias) | High risk | Narrative results only ‐ could not be included in analysis. |
| Other bias | Unclear risk | Information not given. |
Lacy 1992.
| Methods | Randomized, double‐blind, 3‐arm cross‐over study of 3 different ECMs. Duration: each treatment arm lasted 4 weeks after an initial 2‐week run‐in period. Single‐centre study based in the UK. |
|
| Participants | 22 children with CF. Age: 5 ‐ 16 years. Gender split not given. |
|
| Interventions | Group 1: Nutrizyme GR. Group 2: Nutrizyme MP. Group 3: Creon®. The preparations were compared in a capsule for capsule basis, even though there was a difference in enzyme content. |
|
| Outcomes | Symptom scores, weight gain and CFA. | |
| Notes | Results were given descriptively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Clinicians | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 participants withdrew from study, reasons were not given. |
| Selective reporting (reporting bias) | High risk | Outcomes were reported in a way that could not be included in analysis. |
| Other bias | Unclear risk | Information not given. |
Patchell 1999.
| Methods | Prospective, randomized, open‐label, cross‐over study. Duration: treatment was for a period of 10 weeks; 2‐week run‐in, followed by randomization to 1 of the 2 arms for 4 weeks, and then cross over to alternative treatment for the next 4 weeks. Multicentre study at 3 hospitals in the UK. |
|
| Participants | 59 children with CF, diagnosed by 2 sweat tests or genotype, had proven pancreatic insufficiency. Age : mean (SD) age of 10 (3.5) years. Gender split: not given. |
|
| Interventions | Group 1: ECM (Creon 8000 MS®). Group 2: ECMM (Creon 10000 MMS ®). Dose was lipase for lipase. The median intake of lipase/kg body weight/day was 6689 for Creon 8000® and 8527 for Creon 10000 ®. |
|
| Outcomes | FFE, CFA, stool frequency, abdominal pain, participant preference. | |
| Notes | The stool collection for CFA was done only in 1 centre, with 22 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) Clinicians | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Stool collection data were from 1 hospital only with 22 participants in an intent‐to‐treat analysis. 54 participants completed the trial, 2 dropped out in run‐in period due to abdominal pain and loose stools; a further 2 dropped out during the ECMM phase (1 due to abdominal pain and loose stools and 1 due to meconium ileus equivalent). The 5th participant dropped out during the ECMM phase due to an appendix abscess considered to be unrelated to treatment. |
| Selective reporting (reporting bias) | High risk | Data on stool frequency and abdominal pain reported in a way that could not be included in the analysis. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Petersen 1984.
| Methods | Randomized, double‐blind, cross‐over study. Duration: each treatment arm lasted 4 weeks; no run‐in period. Not clear if a single or multicentre study based in Denmark. |
|
| Participants | 11 children with documented CF. Age: 2 ‐ 11 years of age. Gender split: 2 males and 9 females. |
|
| Interventions | Group 1: pH sensitive ECM Pancrease (1 ‐ 2 capsules containing 330 FIP‐u protease, 6200 FIP‐u lipase, 3600 FIP‐u amylase/capsule). Group 2: conventional ECM Pancreatin (10 ‐ 35 ml containing 525 FIP‐u trypsin, 12000 FIP‐u lipase, 12750 FIP‐u amylase). Participants were allowed to change doses depending on individual requirements |
|
| Outcomes | Symptom scores for stool frequency, consistency, colour, odour and abdominal cramps; weight gain; fat absorption. | |
| Notes | Results reported as medians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | During both periods placebo preparations were given in the form of capsules or granules. |
| Blinding of participants and personnel (performance bias) Clinicians | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals, with all 11 participants completing the study. |
| Selective reporting (reporting bias) | High risk | The results were reported in medians due to which the data could not be included in the analysis. |
| Other bias | Low risk | No other sources of bias were found. |
Stead 1986.
| Methods | Open‐label randomized cross‐over study. Duration: 2 consecutive 28‐day treatment periods. Single centre in the UK (Brompton Hospital Adolescent and Adult Cystic Fibrosis Clinic, London). |
|
| Participants | 23 participants with CF diagnosed by sweat chloride concentration > 70 mmol/L, evidence of pancreatic insufficiency and symptomatic steatorrhea. Age: mean (SD) 24.8 (4.2) years. Gender split: 11 males and 12 females. |
|
| Interventions | Group 1: ECM (Creon® capsules). Group 2: ECT (Pancrex V Forte). Participants received either their usual regimen of ECT or ECM in a ratio of 0.7 capsules for each ECT. Declared lipase of Pancrex V forte to Creon® capsules is 0.7:1, protease is 1.6 : 1. |
|
| Outcomes | Change in weight, frequency of stools, abdominal pain, FFE and CFA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further information given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) Clinicians | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew from study and reasons were given. 1 participant was unable to swallow microsphere capsules and another took more lipase during 1 month than the other. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes are reported. |
| Other bias | Unclear risk | Trial supported by Duphar Laboratories. |
Stead 1987.
| Methods | Open‐label, randomized, cross‐over study. Duration: 2 consecutive 28‐day treatment periods. Single centre in the UK. |
|
| Participants | 14 participants with CF, diagnosed by sweat chloride > 70 mmol/L and typical pulmonary disease. Age: mean 21.4 years. Gender split: 8 males and 6 females. |
|
| Interventions | Group 1: ECM (Creon®) with food. Group 2: NECT (Pancrex V) with food and adjuvant cimetidine 40 min before meals. Both contain 8000 BP units of lipase, number of capsules for each individual was same during both treatment periods. |
|
| Outcomes | Change in weight, stool frequency, abdominal pain, FFE and CFA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) Clinicians | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant withdrew due to inability to control frequency of stools. The treatment arm was not specified. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes are reported. |
| Other bias | Unclear risk | Creon® was supplied by Duphar Laboratories. |
Taylor 2015.
| Methods | Randomized, double‐blind, cross‐over study. Duration: each treatment arm lasted 28 (± 2days); no washout period as investigators considered any residual lipase from the prior treatment period to have a negligible influence on the subsequent CFA‐72 h determination. Multicentre international study ‐ 34 sites in seven European countries including Belgium, Bulgaria, Germany, Hungary, Italy, Poland, and the UK. |
|
| Participants | Participants diagnosed with CF by any of the following criteria: 1 clinical feature of CF and 2 disease causing mutations in genotype or sweat chloride conc >60 mmol/L. Demographics and baseline characteristics were well balanced between groups. Of the 96 participants randomized (48 to each group), 86 completed the study, and CFA‐72 hour data were available from both treatment periods for 83 participants. Age at screening in the 96 participants randomized was mean (SD) 19.2 (7.9) years.Group A mean (range) 20.4 (12 to 42) years, Group B mean (range) 18.0 (12 to 43) years. Gender split: 60.4% in each group were male. |
|
| Interventions | Group A: Zenpep® followed by Kreon® (know as Creon® in English‐speaking regions). Group B: Creon® followed by Zenpep®. Zenpep (APT1008) and Creon® given at a dose as close as possible to participants' established pancreatic enzyme treatment. Daily dose could be rounded up from initial dose to a maximum of 10,000 lipase units/kg of body weight per day or 4000 lipase units/g of fat ingested per day, but not exceeding 10,000 lipase units/kg of body weight per day. Participants began each treatment period at the same starting dose. Dosage adjustment to relieve clinical symptoms allowed during the first 2 weeks of each treatment period. In consultation with a dietician, a mean (SD) daily 100 g (15 g) fat diet was maintained throughout the study and participants were required to abstain from nutritional supplements containing high concentrations of (≥ 30%) medium‐chain triglycerides. |
|
| Outcomes | Primary: CFA over 72 hours calculated from dietary fat intake and stools collected during the last 3 days (72 consecutive hours) of each treatment period. Secondary: CNA, change in body weight, control of signs and symptoms of pancreatic insufficiency (participant diaries), overall well‐being, adverse events, CF symptoms as evaluated by the CFQ‐R (at the beginning of each treatment period and at the end of the study). |
|
| Notes | Stool samples were collected in a hospital or clinic or other controlled environment to ensure adherence to the prescribed diet and quantitative stool collection. Stools were collected for 72 consecutive hours starting on the morning of day 26 (±2 days) of each of the 28‐day (±2 days) treatment periods. Treatment adherence, defined as percentage ≥75% and ≤125% of capsules taken versus capsules prescribed, was determined on the basis of diary entries and study drug reconciliation and was evaluated at each visit. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization process not described. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | Stated that participants were masked. |
| Blinding of participants and personnel (performance bias) Clinicians | Low risk | Stated that caregivers and investigators were masked |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessor masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals from the study were fully described; 13.5% of participants excluded from study. The primary efficacy analysis of CFA‐72 h was based on the completers population, defined as all randomized participants who received at least 1 dose of Zenpep or Creon® and finished both treatment periods with complete CFA‐72 h data. |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported and could be included in study. |
| Other bias | High risk | Corresponding author is a consultant to Aptalis and Profile Pharma. Study was funded by Aptalis Pharma. |
Vidailhet 1987.
| Methods | Randomized, 2‐arm cross‐over study. Duration: after an 8‐day initial washout each treatment given for a period of 30 days. Single‐centre study in France. Home setting. |
|
| Participants | 17 children with documented CF. Age: 1 ‐ 12.5 years. Gender split was not given. |
|
| Interventions | Group 1: ECM (Creon®) (1.2 ‐ 2.4 g/day). Group 2: lyophilised TPE (4 ‐ 8 g/day). |
|
| Outcomes | Body weight, FFE, nutritional indicators (body weight to length index, subscapular skin fold, plasma cholesterol, pre‐albumin, retinol, retinol binding protein, zinc and total essential fatty acids), therapeutic tolerance (drug acceptance, alanine amino transferase, prothrombin time, serum bilirubin and uric acid, urinary uric acid excretion). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Clinicians | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not given. |
| Selective reporting (reporting bias) | High risk | Change in body weight was incompletely reported and cannot be included in the review. |
| Other bias | Unclear risk | Information not given. |
Vyas 1990.
| Methods | Randomized, double‐blind, double‐placebo cross‐over study. Duration: 4 weeks for each treatment arm. Single‐centre study in the UK (London). |
|
| Participants | 20 children with CF diagnosed by sweat test sodium greater than 70 mmol/L Age: mean 9.9 years; range 4.1 ‐ 15.3 years. All had steatorrhea and weight and height above 3rd percentile. The mean weight was 26.0 kg (range 14.2 kg ‐ 50.7 kg) and the mean height was 1.32 metres (range 1.1 metres ‐ 1.63 metres). Gender split not given. |
|
| Interventions | Group 1: active ECM plus placebo ECT. Group 2: placebo ECM plus active ECT. Dosage of ECM was calculated to provide equivalent dosage of lipase to ECT. The day‐to‐day dosage of active drug and placebo varied slightly depending on the participants' diet. |
|
| Outcomes | Change in weight, stool frequency, abdominal pain, FFE. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | Low risk | While taking ECM, participants received a placebo of ECT and while taking ECT, they took a placebo preparation of ECM. |
| Blinding of participants and personnel (performance bias) Clinicians | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 12 paired samples were analysed for FFE. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes are reported. |
| Other bias | High risk | Duphar Ltd, UK supplied pancreatic enzyme supplements and supported the study. |
Williams 1990.
| Methods | Randomized, single blind cross‐over study. Duration: 10 weeks in total, 2‐week run‐in period followed by 4 weeks for each treatment arm. Not clear if multi‐ or single‐centre study based in the UK. Home setting. |
|
| Participants | 39 children with symptoms of CF, at least 2 abnormal sweat chloride results and pancreatic insufficiency. Age: median (range) 9.7 (5 ‐ 17) years. Clinical state, as measured by the Shwachman score (100 = normal) ranged from 37 to 91 with a median value of 79. 12 participants were unsuitable for analysis, the remaining 27 children (15 boys and 12 girls) completed the trial. |
|
| Interventions | Group 1: ECM Creon® (lipase 8000 BP units, amylase 9000 BP units, protease 210 BP units). Group 2: ECM Pancrease® (lipase 5000 BP units, amylase 2900 BP units, protease 330 BP units). Participants took same number of capsules per day during both treatment periods. |
|
| Outcomes | CFA, participant preference, nitrogen excretion, weight change, symptom score for appetite, number, colour and consistency of stools, abdominal pain and general condition. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) Participants | High risk | Blinding not done. |
| Blinding of participants and personnel (performance bias) Clinicians | Low risk | Study medication was issued by pharmacist and order of treatment was not known to the doctor. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 participants (31%) were withdrawn from trial for various reasons and not included in analysis:
|
| Selective reporting (reporting bias) | High risk | Change in weight and symptom scores for abdominal pain, stool frequency were measured but were reported incompletely, so cannot be entered in a meta‐analysis. |
| Other bias | High risk | Corresponding author was financially supported by Cilag Limited (Pancrease). |
CF: cystic fibrosis CFA: co‐efficient of fat absorption CFQ‐R: Cystic Fibrosis Questionnaire‐Revised CNA: co‐efficient of nitrogen absorption ECM: enteric‐coated microspheres ECMM; enteric‐coated mini‐microspheres ECT: enteric‐coated tablets FFE: fecal fat excretion NECT: non enteric‐coated tablets QoL: quality of life SD: standard deviation TPE: total pancreatic extracts
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ansaldi 1988 | Cross‐over study with PERT for 5 days in each arm. |
| Araujo 2011 | Doesn't appear to have a control group, PERT dosage increased according to fecal fat levels. |
| Beker 1994 | Cross‐over study with PERT for 3 days in each arm. |
| Beverley 1987 | Cross‐over study with 3 arms of 15 days each. |
| Borowitz 2008 | The study drugs were given for a period of 6 days only after randomisation. |
| Bouquet 1988 | Cross‐over study with each treatment period only 2 weeks. |
| Bowler 1993 | Crossover study with 2 arms of 2 weeks each. |
| Brady 1991 | Crossover study with 2 arms of 7 days each. |
| Brady 1992 | Cross‐over study of enzymes given before and during meals; 2 periods of 1 week each. |
| Brady 2006 | Cross‐over study with 2 treatment periods of 1 week each. |
| Breuel 1996 | Study assesses pancreatic enzyme activity. |
| Butt 2001 | Study assesses breath tests. |
| Chazalette 1988 | Open label cross‐over study with periods of 1 week each. |
| Chazalette 1993 | Parallel group study for a period of 8 days. |
| Colombo 2001 | Study compares different methods for assessing exocrine pancreatic function. |
| De Boeck 1998 | Single dose intervention. |
| Desager 2006 | Parallel study period of 10 days. |
| Duhamel 1988 | Cross‐over study with 2 periods of 8 days each. |
| Duhamel 1998 | Cross‐over study with 2 periods of 14 days each. Study assessed patient preference, but also assessed clinical symptoms. |
| Durie 1980 | Cross‐over study with 4 preparations given for 1 week each. |
| Dutta 1988 | Crossover study with each intervention given only on a single day. |
| Easley 1998 | Cross‐over study with each intervention given only on a single day. |
| Ellis 1994 | Cross‐over study (assessing coating of PERT) with 2 weeks in each arm. |
| Foucaud 1989 | Placebo‐controlled, parallel study but treatment period for 1 week. |
| Gan 1994 | Cross‐over study with 2 arms of 14 days each. |
| Goodchild 1974 | Cross‐over study with 2 weeks in each arm. |
| Gow 1981 | Cross‐over study of 4 periods of 14 days each. |
| Graff 2010 | Intervention only give for 5 days. |
| Heubi 2007 | Cross‐over placebo‐controlled trial with 2 periods of 1 week each. |
| Heubi 2016 | Cross‐over study with 1 week in each arm. |
| Hill 1993 | Letter reports cross‐over trial to compare patient preference of different formulations; no other outcomes stated. |
| Hilman 1982 | Cross‐over study with a total 2‐week study period. |
| Holsclaw 1979 | Cross‐over study of 6 treatment (Viokase vs Cotazyme vs Pancrelipase with and without bicarbonate) periods of 3 weeks each. |
| Hubbard 1984 | Study assesses use of bentiromide screening test. |
| Kalnins 2005 | Cross‐over study with 2 treatment periods of 2 weeks each. |
| Kalnins 2006 | Cross‐over study with 2 treatment periods of 14 days each. |
| Katona 2000 | Not an RCT or a quasi‐RCT. |
| Khaw 1977 | Cross‐over study with two treatment periods of 12 days each. |
| Konstan 2004 | Intervention given only for 6 days. |
| Konstan 2008 | Cross‐over trial with 2 treatment periods of 5 days each. |
| Konstan 2010 | Treatment only 6 to 7 days in each arm of the 2‐phase cross‐over trial. |
| Kraisinger 1993 | Cross‐over trial with 2 treatment periods of 4 days each. |
| Kuo 2011 | Cross‐over trial with 2 single interventions on separate days. |
| Lancellotti 1996 | Open‐label, cross‐over study with 2 treatment periods of 5 days each. |
| Lazaro 1990 | Cross‐over trial with 2 periods of 6 days each. |
| Leitz 2009 | Placebo‐controlled trial with a treatment period of 1 day. |
| Lubin 1979 | Study assesses use of antacids in conjunction with PERT intervention not relevant. |
| Mack 1991 | Cross‐over study assessing antibiotic absorption with PERT compared to without in a single intervention. |
| Mischler 1980 | Cross‐over study with 2 arms of 5 days each. |
| Mischler 1982 | Cross‐over trial 2 periods of 5 days each. |
| Morrison 1992 | Not an RCT or quasi‐RCT. |
| Munck 2009 | Cross‐over study with 2 treatment periods of 2 weeks each. |
| Munoz 1987 | Cross‐over study with 2 treatment periods of 1 week each. |
| NCT02137382 | Cross‐over study with Creon/Creon N for 5 days in each arm. |
| Neijens 1982 | Cross‐over study with treatment periods of 2 weeks each. |
| Ritz 2004 | Study evaluated a breath test used to assess fat malabsorption and not PERT. |
| Robinson 1989 | Cross‐over study with 2 treatment periods of 2 weeks each. |
| Robinson 1998 | Cross‐over study with 2 periods of 2 weeks each. |
| Santini 2000 | Cross‐over study with 2 treatment periods of 1 week each. |
| Shah 1993 | PERT taken for 2‐week period only. |
| Sinaasappel 1998 | Cross‐over trial with 2 periods of 14 days each. |
| Stapleton 2001 | Study assessing knowledge and education of PERT. |
| Stern 2000 | Parallel RCT but duration only 5 ‐ 7 days. |
| Thomson 1993 | Cross‐over trial with 3 periods of 7 days each. |
| Trapnell 2009 | Cross‐over RCT with 5‐day course of PERT and same for placebo. |
| Van de Vijver 2011 | Treatment period only 5 days. |
| van der Haak 2016 | Intervention not relevant. |
| Vitti 1975 | Study assesses antibiotic absorption when given with PERT. |
| Warwick 1982 | Cross‐over study with 2 treatment periods of 1 week each. |
| Weber 1979 | Cross‐over study with 2 periods of 8 days each. |
| Wooldridge 2009 | Cross‐over study with 2 periods of 7 days each, followed by a non‐randomised extension study. |
| Zentler 1992 | RCT with 3 interventions for 2 weeks each. |
PERT: pancreatic enzyme replacement therapy RCT: randomized controlled trial vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Dalzell 1992.
| Methods | Randomized, double‐blind, cross‐over study. Duration: 12 months. UK‐based study. |
| Participants | 20 participants with CF and chronic distal intestinal obstruction. Mean age: 13.1 years. Gender split: 4 males and 16 females. |
| Interventions | Group 1: low‐dose PERT. Group 2: high‐dose PERT. |
| Outcomes | Weight gain, CFA, episodes of acute distal intestinal obstruction syndrome, abdominal mass, abdominal pain. |
| Notes | Results given in ranges. Await further details from authors. |
Holsclaw 1980.
| Methods | Possibly randomized, not clear. Duration: 14 months for intervention, control not clear. USA‐based study. |
| Participants | 20 participants with CF. |
| Interventions | Pancrease (enteric‐coated) ‐ dose 9 to 11 per day ‐ compared with usual supplement (Viokase or Cotazyme) for a period of 14 months in pancrease arm, duration of other usual supplement not given. |
| Outcomes | Body weight, urine uric acid, serum albumin, abdominal symptoms. |
| Notes | Not stated whether patients were randomized or not. Possible extension of excluded study Holsclaw 1979. |
Knill 1973.
| Methods | Randomized double‐blind cross‐over study with 3 arms. Duration: 3 months in total, each arm lasted 1 month, not clear if washout period was used. |
| Participants | 11 adults with CF and 1 adult with chronic pancreatitis. |
| Interventions | Pancrex V Forte: 3 tablets equivalent to 3 g pancreatine BP. Nutrizym: 2 tablets equivalent to 3.2 g pancreatine BP. Nutrizym plus bromelin: 2 tablets equivalent to 3.2 g pancreatine BP also containing 50g bromelin. Nutrizym tablets looked the same whether containing bromelin or not, but not identical to Pancrex V Forte. Participants not allowed to tell outcome assessors how many tablets they were taking. |
| Outcomes | Self‐reported bowel habits, general health and respiratory symptoms (daily diary), FFE. |
| Notes | Combined data given for all participants (CF data not split out). Full study in French ‐ needs translation. 2 participants who had been on high doses of Pancrex V Forte (9 ‐ 12 per meal) took double the normal preparations in the study. |
Lenoir 2008.
| Methods | Single‐blind cross‐over and parallel design. Single‐centre study. |
| Participants | 24 adults with CF. |
| Interventions | Recombinant acid lipase marketed as MERISPASE®. Session 1: all participants received low‐dose pancreatic extract. Session 2: 3 different doses of lipase (MERISPASE®) compared with 84,000 units of pancreatic extract. Session 3: all participants received low doses of CREON®, 3 groups receiving MERISPASE® continued. |
| Outcomes | Safety, tolerance, CFA. |
| Notes | Meristem Therapeutics went out of business in September 2008; clinical trials blocked in phase II. No one appears to be producing or using this agent. |
Regele 1996.
| Methods | Randomized, double‐blind cross‐over study without placebo. Duration : 28 days in each arm. Not clear if single centre or multicentre, based in Germany. |
| Participants | 16 participants (9 females, 7 males) diagnosed with CF by at least 2 sweat chloride values of ≽ 70 mM and pancreatic insufficient. Age: mean 9.9, range 3 ‐ 27. |
| Interventions | Treatment A: Creon® 25000 (per capsule: 25000 U of lipase, 18000 U of amylase and 1000 U of protease). Treatment B: Panzyrtat (per capsule: 20000 U of lipase, 18000 U of amylase and 1000 U of protease). Both groups received the same number of capsules in each arm. |
| Outcomes | FFE, fecal chymotrypsin, fecal immunoreactive human lipase and serum immunoreactive trypsin. |
| Notes | Mean FFE for both the arms together was given. FFE for individual treatment periods not given. |
Taylor 1993.
| Methods | Open prospective study; not clear if randomized. Cross‐over trial, each arm 3 months. Consecutive so implies no washout. |
| Participants | 23 participants with CF. Age: range 1.3 ‐ 16.8 years. |
| Interventions | Creon 25000 ® compared with conventional microsphere preparations. |
| Outcomes | FFE, BMI, height SD score, lean body mass, clinical symptoms (diary), dietary intake, spirometry, Schwachman and Crispin Norman scores, stool frequency. |
| Notes |
BMI: body mass index CF: cystic fibrosis CFA: co‐efficient of fat absorption ECM: enteric‐coated microspheres FFE: fecal fat excretion PERT: pancreatic enzyme replacement therapy SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
NCT02279498.
| Trial name or title | A Phase 3, Randomized, Open‐Label, Assessor‐Blind, Noninferiority, Active‐Comparator Study Evaluating the Efficacy and Safety of Liprotamase in Subjects With Cystic Fibrosis‐Related Exocrine Pancreatic Insufficiency |
| Methods | Randomized, parallel, open label. Multicentre (54 locations) in USA and Europe. |
| Participants | Estimated enrolment 126 participants aged at least 7 years, males and females. |
| Interventions | Liprotamase (oral, soluble, non‐enterically coated, non‐porcine PERT) versus porcine PERT (oral, enterically‐coated PERT prepared from a porcine source). |
| Outcomes | CFA (change from baseline CFA after up to 8 weeks of stabilized therapy). Safety (number of participants with adverse events or laboratory abnormalities at 18‐20 weeks). |
| Starting date | June 2015 |
| Contact information | Anthera Pharmaceuticals |
| Notes | Expected completion: May 2017. |
CFA: coefficient of fat absorption PERT: pancreatic enzyme replacement therapy
Contributions of authors
Protocol stage: Paramita Cifelli and Robyn Huggins partly drafted the protocol with significant contributions from Alan Smyth.
Review stage: Usha Rani Somaraju and Arturo Solis Moya both selected trials for inclusion in the review, extracted data and assessed the risk of bias. Usha Rani Somaraju drafted the review with comments from Arturo Solis Moya. Usha Rani Somaraju is guarantor of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
None of the authors has any interests to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Assoufi 1994 {published data only}
- Assoufi BK, Doig C, Hodson ME. High dose Nutrizym 22 in adults with cystic fibrosis [abstract]. Pediatric Pulmonology 1994;18 Suppl 10:337. [CFGD Register: GN168] [Google Scholar]
Borowitz 2005 {published data only}
- Borowitz D, Goss C, Limauro S, Murray F, Casey S, Cohen M. A dose‐ranging study of efficacy of TheraCLEC‐Total for treatment of pancreatic insufficiency [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):348. [CFGD Register: GN203a] [Google Scholar]
- Borowitz D, Goss CH, Limauro S, Konstan MW, Blake K, Casey S, et al. Study of a novel pancreatic enzyme replacement therapy in pancreatic insufficient subjects with cystic fibrosis. Journal of Pediatrics 2006;149(5):658‐62. [CFGD Register: GN203f] [DOI] [PubMed] [Google Scholar]
- Borowitz D, Goss CH, Limauro S, Murray F, Casey S, Cohen M, et al. A phase 2 study of TheraCLEC‐Total enzymes in CF patients with pancreatic insufficiency (PI) [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):142. [CFGD Register: GN203b] [Google Scholar]
- Borowitz D, Konstan MW, Goss C, Limauro SE, Murray FT. Enhanced coefficient of fat absorption using a novel pancreatic enzyme preparation, ALTU‐135, with concomitant use of a proton pump inhibitor [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl):S56. [CFGD Register: GN203d] [Google Scholar]
- Borowitz D, Konstan MW, Goss C, Limauro SE, Murray FT, Casey S, et al. Treatment with ALTU‐135 results in a positive inverse relationship between coefficient of fat absorption with stool weight in subjects with cystic fibrosis‐related pancreatic insufficiency [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl):S56. [CFGD Register: GN203c] [Google Scholar]
- Murray FT, Borowitz D, Konstan MW, Limauro SE, Goss C. Changes in coefficients of fat and nitrogen absorption in subjects with cystic fibrosis, pancreatic insufficiency, and cystic fibrosis‐related diabetes mellitus receiving a novel pancreatic enzyme product, ALTU‐135 [abstract]. Pediatric Pulmonology 2006;41(Suppl 29):386. [CFGD Register: GN203e] [Google Scholar]
Elliott 1992 {published data only}
- Elliott RB, Escobar LC, Lees HR, Akroyd RM, Reilly HC. A comparison of two pancreatin microsphere preparations in cystic fibrosis. New Zealand Medical Journal 1992;105(930):107‐8. [CFGD Register: GN152] [PubMed] [Google Scholar]
Henker 1987 {published data only}
- Henker J, Jutta Hein L, Vogt E. Comparison of effectiveness of Pankreon ForteR and KreonR in children with cystic fibrosis [abstract]. 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987:72. [CFGD Register: GN162]
Lacy 1992 {published data only}
- Lacy D, West J, Venkataraman M, Vyas J, MacDonald A, Weller PH, et al. A comparison of Nutrizym GR and Creon in children with CF [abstract]. 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:TP76. [CFGD Register: GN165]
Patchell 1999 {published data only}
- Patchell CJ, Desai M, Weller PH, MacDonald A, Smyth RL, Bush A, et al. Creon® 10000 minimicrospheres™ vs. Creon® 8000 microspheres ‐ an open randomised crossover preference study. Journal of Cystic Fibrosis 2002;1(4):287‐91. [CFGD Register: GN189b] [DOI] [PubMed] [Google Scholar]
- Patchell CJ, Smyth RL, Bush A, Weller PW, Collins SA, Gilbody JS. Creon® 10 000 minimicrospheres™ versus Creon® 8000 MS preference study [abstract]. The Netherlands Journal of Medicine 1999;54(Suppl):S64. [CFGD Register: GN189a] [Google Scholar]
Petersen 1984 {published data only}
- Petersen W, Heilman C, Garne S. A double dummy cross‐over study comparing the effect of pancreatic enzyme as acid‐resistant microsphere (Pancrease R Cilag Chemie) to granules (Pancreatin R Rosco) in cystic fibrosis [abstract]. 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:Poster 8.13. [CFGD Register: GN138a]
- Petersen W, Heilmann C, Garne S. Pancreatic enzyme supplementation as acid‐resistant microspheres versus enteric‐coated granules in cystic fibrosis. A double placebo‐ controlled cross‐over study. Acta Paediatrica Scandinavica 1987;76(1):66‐9. [CFGD Register: GN138b] [DOI] [PubMed] [Google Scholar]
Stead 1986 {published data only}
- Stead RJ, Skypala I, Hodson ME, Batten JC. Enteric coated microspheres of pancreatin in the treatment of cystic fibrosis: comparison with a standard enteric coated preparation. Thorax 1987;42(7):533‐7. [CFGD Register: GN140b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RJ, Skypala I, Hodson ME, Batten JC. Reduction in steatorrhoea in cystic fibrosis using enteric coated microspheres of pancreatin compared with a standard coated preparation [abstract]. 14th Annual Meeting of the European Working Group for Cystic Fibrosis; 1986 Sept 1‐2; Budapest, Hungary. 1986:132. [CFGD Register: GN140a]
Stead 1987 {published data only}
- Stead RJ, Skypala I, Hodson ME. Enteric‐coated microspheres of pancreatin versus non enteric‐coated pancreatin combined with cimetidine in cystic fibrosis [abstract]. 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987:71. [CFGD Register: GN146a]
- Stead RJ, Skypala I, Hodson ME. Treatment of steatorrhoea in cystic fibrosis: a comparison of enteric‐coated microspheres of pancreatin versus non‐enteric‐coated pancreatin and adjuvant cimetidine. Alimentary Pharmacology & Therapeutics 1988;2(6):471‐82. [CFGD Register: GN146b] [DOI] [PubMed] [Google Scholar]
Taylor 2015 {published data only}
- Taylor CJ, Thieroff‐Ekerdt R, Shiff S, Magnus L, Fleming R, Gommoll C. A randomized, double‐blind, multicentre, multinational crossover comparison of the pancreatic enzyme product (PEP) APT‐1008 (ZENPEP®) to KREON® in the treatment of exocrine pancreatic insufficiency (EPI) associated with cystic fibrosis (CF) in patients ≥12 years of age [abstract]. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S120, Abstract no: 240. [CFGD Register: GN251a; CRS: 5500135000001306] [Google Scholar]
- Taylor CJ, Thieroff‐Ekerdt R, Shiff S, Magnus L, Fleming R, Gommoll C. Comparison of two pancreatic enzyme products for exocrine insufficiency in patients with cystic fibrosis. Journal of Cystic Fibrosis 2016;15(5):675‐80. [CENTRAL: 1157598; CFGD Register: GN251b; CRS: 5500135000001564; DOI: 10.1016/j.jcf.2016.02.010; PUBMED: 27013382] [DOI] [PubMed] [Google Scholar]
Vidailhet 1987 {published data only}
- Vidailhet M, Derelle J, Morali A, Gasperi JP. Comparison of effectiveness and tolerance of enteric coated versus unprotected pancreatic extracts in CF patients [abstract]. 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987:69. [CFGD Register: GN164]
Vyas 1990 {published data only}
- Vyas H, Matthew D, Milla P. A double‐blind comparison between enteric coated microspheres and enteric coated tablets of pancreatic enzymes [abstract]. 14th Annual Meeting of the European Working Group for Cystic Fibrosis; 1986 Sept 1‐2; Budapest, Hungary. 1986:52. [CFGD Register: GN148a]
- Vyas H, Matthew DJ, Milla PJ. A comparison of enteric coated microspheres with enteric coated tablet pancreatic enzyme preparations in cystic fibrosis. A controlled study. European Journal of Pediatrics 1990;149(4):241‐3. [CFGD Register: GN148b] [DOI] [PubMed] [Google Scholar]
Williams 1990 {published data only}
- Williams J, MacDonald A, Weller PH, Field J, Pandov H. Enteric‐coated microspheres in cystic fibrosis: Pancrease* vs. Creon [abstract]. Pediatric Pulmonology 1988;5(Suppl 2):143. [CFGD Register: GN149a] [Google Scholar]
- Williams J, MacDonald A, Weller PH, Fields J, Pandov H. Two enteric coated microspheres in cystic fibrosis. Archives of Disease in Childhood 1990;65(6):594‐7. [CFGD Register: GN149b] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ansaldi 1988 {published data only}
- Ansaldi Balocco N, Santini B, Sarchi C. Efficacy of pancreatic enzyme supplementation in children with cystic fibrosis: comparison of two preparations by random crossover study and a retrospective study of the same patients at two different ages [abstract]. Journal of Pediatric Gastroenterology and Nutrition 1988;7(Suppl 1):S40‐5. [CFGD Register: GN141] [PubMed] [Google Scholar]
Araujo 2011 {published data only}
- Araujo M, Goyheneix M, Castanos C. High requirement of pancreatic enzyme dose [abstract]. Journal of Cystic Fibrosis 2011;10 Suppl 1:S75, Abstract no: 294. [CFGD Register: GN223] [Google Scholar]
Beker 1994 {published data only}
- Beker LT, Fink RJ, Shamsa FH, Chaney HR, Kluft J, Evans E, et al. Comparison of weight‐based dosages of enteric‐coated microtablet enzyme preparations in patients with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 1994;19(2):191‐7. [CFGD Register: GN159] [DOI] [PubMed] [Google Scholar]
Beverley 1987 {published data only}
- Beverley DW, Kelleher J, MacDonald A, Littlewood JM, Robinson T. A comparison of four different pancreatic extracts on the absorption of fat in patients with cystic fibrosis [abstract]. 14th Annual Meeting of the European Working Group for Cystic Fibrosis; 1986 Sept 1‐2; Budapest, Hungary. 1986:69. [CFGD Register: GN139a]
- Beverley DW, Kelleher J, MacDonald A, Littlewood JM, Robinson T, Walters MP. Comparison of four pancreatic extracts in cystic fibrosis. Archives of Disease in Childhood 1987;62(6):564‐8. [CFGD Register: GN139b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood M, Kelleher J, Walters MP, Johnson AW. In vivo and in vitro studies of microsphere pancreatic supplements. Journal of Pediatric Gastroenterology and Nutrition 1988;7(Suppl 1):S22‐9. [CFGD Register: GN139c; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borowitz 2008 {published data only}
- Borowitz D, Campion M, Stevens C, Brettman L, Liprotamase 726SG. Reproducibility of coefficient of fat absorption (CFA) in cystic fibrosis patients with pancreatic insufficiency [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S77, Abstract no: 297. [CENTRAL: 848908; CFGD Register: GN211c; CRS: 5500100000010606] [Google Scholar]
- Borowitz D, Falzone RP, Fratazzi C, for the ALTU‐135 Study Group. A phase 3 study of the efficacy and safety of Altu‐135 (Trizytek) for treatment of pancreatic insufficiency in CF [abstract]. Pediatric Pulmonology 2008;43 Suppl 31:423. [CFGD Register: GN211e] [Google Scholar]
- Borowitz D, Stevens C, Brettman LR, Campion M, Chatfield B, Cipolli M, et al. International phase III trial of liprotamase efficacy and safety in pancreatic‐insufficient cystic fibrosis patients. Journal of Cystic Fibrosis 2011;10(6):443‐52. [CENTRAL: 972216; CFGD Register: GN211d; CRS: 5500050000000062; PUBMED: 21831726] [DOI] [PubMed] [Google Scholar]
- Borowitz D, Stevens C, Brettman LR, Campion M, Wilschanski M, Thompson H. Liprotamase long‐term safety and support of nutritional status in pancreatic‐insufficient cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2012;54(2):248‐57. [CENTRAL: 901255; CFGD Register: GN211f; CRS: 5500050000000424; EMBASE: 2012058171] [DOI] [PubMed] [Google Scholar]
- Borowitz D, Stevens C, Campion M, Brettman L. Relationship of baseline & treatment coefficient of fat absorption to growth in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:424, Abstract no: 564. [CENTRAL: 795565; CFGD Register: GN211b; CRS: 5500100000003595] [Google Scholar]
- Borowitz D, Stevens C, Fratazzi C, Cohen D, Campion M. An open‐label international phase 3 study of the long‐term safety of liprotamase for treatment of pancreatic insufficiency in CF [abstract]. Pediatric Pulmonology 2009;44 Suppl 32:397, Abstract no: 520. [CENTRAL: 735842; CFGD Register: GN211a; CRS: 5500100000003414] [Google Scholar]
Bouquet 1988 {published data only}
- Bouquet J, Sinaasappel M, Neijens HJ. Malabsorption in cystic fibrosis: mechanisms and treatment. Journal of Pediatric Gastroenterology and Nutrition 1988;7(Suppl 1):S30‐S35. [CFGD Register: GN142] [DOI] [PubMed] [Google Scholar]
Bowler 1993 {published data only}
- Bowler IM, Wolfe SP, Owens HM, Sheldon TA, Littlewood JM, Walters MP. A double blind lipase for lipase comparison of a high lipase and standard pancreatic enzyme preparation in cystic fibrosis. Archives of Disease in Childhood 1993;68(2):227‐30. [CFGD Register: GN155b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood JM, Bowler IM, Wolfe SP, Owens HM. A double‐blind comparison of a high lipase and standard pancreatic extract [abstract]. Pediatric Pulmonology 1992;14 Suppl S8:267. [CFGD Register: GN155a] [Google Scholar]
Brady 1991 {published data only}
- Brady MS, Rickard K, Yu PL, Eigen H. Effectiveness and safety of small vs. large doses of enteric coated pancreatic enzymes in reducing steatorrhea in children with cystic fibrosis: a prospective randomized study. Pediatric Pulmonology 1991;10(2):79‐85. [CFGD Register: GN150] [DOI] [PubMed] [Google Scholar]
Brady 1992 {published data only}
- Brady MS, Rickard K, Yu PL, Eigen H. Effectiveness of enteric coated pancreatic enzymes given before meals in reducing steatorrhea in children with cystic fibrosis. Comment in: Journal of the American Dietetic Association 1993 Jan;93(1):14. Journal of the American Dietetic Association 1992;92(7):813‐7. [CFGD Register: GN153] [PubMed] [Google Scholar]
Brady 2006 {published data only}
- Brady MS, Garson JL, Krug SK, Kaul A, Rickard KA, Caffrey HH, et al. An enteric‐coated high‐buffered pancrelipase reduces steatorrhea in patients with cystic fibrosis: a prospective, randomized study. Journal of the American Dietetic Association 2006;106(8):1181‐6. [CFGD Register: GN205] [DOI] [PubMed] [Google Scholar]
- Brady MS, Stevens J, Rickard K, Fineberg N. Effectiveness of a new enteric coated (EC) pancreatic enzyme with bicarbonate (pancrecarb®) in reducing steatorrhea in patients with cystic fibrosis (CF): a prospective randomized study [abstract]. Pediatric Pulmonology 1998;26(Suppl 17):354‐5. [CFGD Register: GN187] [Google Scholar]
Breuel 1996 {published data only}
- Breuel K, Wutzke KD, Radke M, Gurk S. Evaluation of pancreatic enzyme activity in cystic fibrosis (CF) by use of a 13C‐Breath test: A comparison of different galemic preparations [abstract]. Israel Journal of Medical Sciences 1996;32(Suppl):S175. [CFGD Register: GN145] [Google Scholar]
Butt 2001 {published data only}
- Butt AM, Kelly E, Pike J, Myilvanasuntharam M, Sequeira K, Morson N, et al. Comparison of the dynamics of lipid assimilation using 13CO2 breath tests with both long‐ and medium‐chain 13C‐labeled stable isotope lipid substrates [abstract]. Pediatric Pulmonology 2001;32(Suppl 22):342. [CFGD Register: GN195] [Google Scholar]
Chazalette 1988 {published data only}
- Chazalette JP, Dain MP, Castaigne JP. Efficacy of pancrease: crossover comparative study versus eurobiol in cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 1988;7(Suppl 1):S46‐8. [CFGD Register: GN183; MEDLINE: ] [PubMed] [Google Scholar]
Chazalette 1993 {published data only}
- Chazalette JP. A double‐blind placebo‐controlled trial of a pancreatic enzyme formulation (Panzytrat (R) 25 000) in the treatment of impaired lipid digestion in patients with cystic fibrosis. Drug Investigation 1993;5(5):274‐80. [CFGD Register: GN184] [Google Scholar]
Colombo 2001 {published data only}
- Colombo C, Giunta A, Moretti E, Corbetta C, Valmarana L, Moretto E, et al. Comparison of two stable isotope breath tests (BT) and faecal elastase to assess exocrine pancreatic status in cystic fibrosis (CF) [abstract]. Pediatric Pulmonology 2001;32 Suppl 22:339. [CFGD Register: GN194; MEDLINE: ] [Google Scholar]
De Boeck 1998 {published data only}
- Boeck K, Delbeke I, Eggermont E, Veereman Wauters G, Ghoos Y. Lipid digestion in cystic fibrosis: comparison of conventional and high‐lipase enzyme therapy using the mixed‐triglyceride breath test. Journal of Pediatric Gastroenterology and Nutrition 1998;26(4):408‐11. [CFGD Register: GN185] [DOI] [PubMed] [Google Scholar]
Desager 2006 {published data only}
- Desager KN, Mulberg AE, Verkade H, Malfroot A, Veereman G, Bodewes F, et al. A phase 2, randomized, investigator‐blinded, parallel‐group, pilot study evaluating the safety, palatability and efficacy of four doses of pancrelipase microtablets in the treatment of infants and toddlers with cystic fibrosis‐related pancreatic insufficiency and fat malabsorption [abstract]. Pediatric Pulmonology 2006;41 Suppl 29:377. [CFGD Register: GN206] [Google Scholar]
Duhamel 1988 {published data only}
- Duhamel JF, Vidailhet M, Luyer B, Douchain F, Jehanne M, Clavel R, et al. Multicenter comparative study of a new formulation of pancreatin in gastro‐resistant microgranules for the treatment of exocrine pancreatic insufficiency in children with mucoviscidosis [Etude multicentrique comparative d'une nouvelle presentation de pancreatine en microgranules gastroresistants dans l'insuffisance pancreatique exocrine de la mucoviscidose chez l'enfant.]. Annales de Pediatrie 1988;35(1):69‐74. [CFGD Register: GN143] [PubMed] [Google Scholar]
Duhamel 1998 {published data only}
- Duhamel JF, Lenoir G, Druon D, Grimfeld A, Vidailhet M, Chazalette JP, et al. Open‐label, multicentre, cross‐over study to investigate the patients' preference for Creon 10 000 MinimicrospheresTM or Creon in patients with pancreatic exocrine insufficiency due to cystic fibrosis [abstract]. Proceedings of 22nd European Cystic Fibrosis Conference; 1998 June 13‐19; Berlin, Germany. 1998:96. [CFGD Register: GN181]
Durie 1980 {published data only}
- Durie PR, Bell L, Linton W, Corey ML, Crozier DN, Forstner GG. The effect of cimetidine and sodium bicarbonate on pancreatic replacement therapy in cystic fibrosis [abstract]. Proceedings of 20th Annual Meeting Cystic Fibrosis Club Abstracts; 1979 May 1; Atlanta, Georgia. 1979:37. [CFGD Register: GN134b]
- Durie PR, Bell L, Linton W, Corey ML, Forstner GG. Effect of cimetidine and sodium bicarbonate on pancreatic replacement therapy in cystic fibrosis. Gut 1980;21(9):778‐86. [CFGD Register: GN134a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dutta 1988 {published data only}
- Dutta SK, Hubbard VS, Appler M. Critical examination of therapeutic efficacy of a pH‐sensitive enteric‐coated pancreatic enzyme preparation in treatment of exocrine pancreatic insufficiency secondary to cystic fibrosis. Digestive Diseases and Sciences 1988;33:1237‐44. [CFGD Register: GN183; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Easley 1998 {published data only}
- Easley D, Krebs N, Jefferson M, Miller L, Erskine J, Accurso F, et al. Effect of pancreatic enzymes on zinc absorption in cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 1998;26(2):136‐9. [CFGD Register: GN180] [DOI] [PubMed] [Google Scholar]
Ellis 1994 {published data only}
- Ellis L, Durie P, Corey M, Davis L, Kalnin D. Changing the coating of pancreatic enzyme microspheres to dissolve at a lower PH does not improve efficacy [abstract]. Pediatric Pulmonology 1994;18 Suppl 10:338. [CFGD Register: GN169] [Google Scholar]
Foucaud 1989 {published data only}
- Foucaud P, Saint‐Marc Girardin MF, Jehanne M, Loeuille G, Gilly R, Lenoir G, et al. Compared efficacity of two pancreatic extracts: eurobiol 25.000 U and eurobiol in children with mucovisidosis [abstract] [Efficacite comparee de deux extraits pancreatiques: eurobiol 25.000 U et eurobiol chez des enfants atteints de mucoviscidose.]. Gastroenterologie Clinique et Biologique 1989;13:A34. [CFGD Register: GN196] [Google Scholar]
Gan 1994 {published data only}
- Gan KH, Heijerman HGM, Geus W, Bakker W, Lamers C. Comparison of a high lipase pancreatic enzyme extract with a regular pancreatin preparation in adult cystic fibrosis patients. Alimentary Pharmacology & Therapeutics 1994;8(6):603‐7. [CFGD Register: GN158b] [DOI] [PubMed] [Google Scholar]
- Gan KH, Heijerman HGM, Lamers CBHW, Bakker W. Comparison of a high lipase pancreatic enzyme extract with a regular pancreatin preparation in pancreatic insufficient CF patients [abstract]. Pediatric Pulmonology 1994;18 Suppl 10:340. [CFGD Register: GN158a] [Google Scholar]
Goodchild 1974 {published data only}
- Goodchild MC, Sagaro E, Brown GA, Cruchley PM, Jukes HR, Anderson CM. Comparative trial of pancrex V forte and nutrizym in treatment of malabsorption in cystic fibrosis. British Medical Journal 1974;3(5933):712‐4. [CFGD Register: GN133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gow 1981 {published data only}
- Gow R, Bradbear R, Francis P, Shepherd R. Comparative study of varying regimens to improve steatorrhoea and creatorrhoea in cystic fibrosis: Effectiveness of an enteric‐coated preparation with and without antacids and cimetidine. Lancet 1981;2(8255):1071‐4. [CFGD Register: GN135a] [DOI] [PubMed] [Google Scholar]
- Shepherd RW, Gow R. Comparative trial of measures to prevent acid‐peptic inactivation of pancreatic enzyme supplements in pancreatic insufficiency due to cystic fibrosis. [abstract]. Australian and New Zealand Journal of Medicine 1982;12:106. [CFGD Register: GN134b] [Google Scholar]
Graff 2010 {published data only}
- Boydd D, Caras SD, Zipfel L, Sander‐Struckmeier S. Evaluation of sparse stool collections compared to 72‐hour collections as an efficacy measure of pancreatic enzyme therapy in patients with exocrine pancreatic insufficiency [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:420, Abstract no: 554. [CENTRAL: 793676; CFGD Register: GN218b; CRS: 5500100000003566] [Google Scholar]
- Caras S, Boyd D, Zipfel L, Sander‐Struckmeier S. Evaluation of stool collections to measure efficacy of PERT in subjects with exocrine pancreatic insufficiency. Journal of Pediatric Gastroenterology and Nutrition 2011;53(6):634‐40. [CENTRAL: 892801; CFGD Register: GN218d; CRS: 5500050000000061; EMBASE: 2011653202] [DOI] [PubMed] [Google Scholar]
- Graff GR, Maguiness K, McNamara J, Morton R, Boyd D, Beckmann K, et al. Efficacy and tolerability of a new formulation of pancrelipase delayed‐release capsules in children aged 7 to 11 years with exocrine pancreatic insufficiency and cystic fibrosis: a multicenter, randomized, double‐blind, placebo‐controlled, two‐period crossover, superiority study. Clinical Therapeutics 2010;32(1):89‐103. [CENTRAL: 743659; CFGD Register: GN218a; CRS: 5500100000003422; PUBMED: 20171415] [DOI] [PubMed] [Google Scholar]
- Maguiness KM, Graff G, Boyd D, Caras D. Efficacy of pancrealipase delayed‐release capsules (CREON®) in correcting nitrogen malabsorption in subjects with CF in randomized, controlled trials [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:417, Abstract no: 547. [CENTRAL: 793539; CFGD Register: GN218c // GN214c; CRS: 5500100000003561] [Google Scholar]
Heubi 2007 {published data only}
- Heubi J, Boas SR, Blake K, Nasr SZ, Woo MS, Graff GR, et al. EUR‐1008, a PEP, is safe and effective in cystic fibrosis (CF) patients with exocrine pancreatic insufficiency (EPI) [abstract]. Pediatric Pulmonology 2007;42 Suppl 30:392. [CFGD Register: GN207b] [Google Scholar]
- Heubi JE. The efficacy of EUR‐1008, a novel pancreatic enzyme product (PEP), in the absence of concurrent agents affecting gastric pH, in patients with exocrine pancreatic insufficiency (EPI) [abstract]. American Journal of Respiratory and Critical Care Medicine 2009;179:A1187. [CFGD Register: GN207d] [Google Scholar]
- Heubi JE, Boas SR, Blake K, Nasr SZ, Woo MS, Graff GR, et al. EUR‐1008 (a new pancreatic enzyme product, PEP) was shown to be safe and effective in cystic fibrosis (CF) patients with exocrine pancreatic insufficiency (EPI) [abstract]. Journal of Cystic Fibrosis 2007;6 Suppl 1:S61. [CFGD Register: GN207a] [Google Scholar]
- Heubi JE, Straforini C, Thieroff‐Ekerdt R. Switching CF patients from previous pancreatic enzyme replacement therapy to ZENPEP™ (pancrelipase delayed‐release capsules) improves symptom control of exocrine pancreatic insufficieny [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S77, Abstract no: 299. [CFGD Register: GN207g] [Google Scholar]
- Wooldridge J, Straforini C, Broussard D, Thieroff‐Ekerdt R. Switching CF patients from previous pancreatic enzyme products to EUR‐1008 (ZENPEP® [pancrelipase] delayed‐release capsules) improves symptom control of EPI in the absence of proton pump inhibitors or H2 receptor agonists [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:422, Abstract no: 559. [CFGD Register: GN207f] [Google Scholar]
- Wooldridge JL, Heubi JE, Amaro‐Galvez R, Boas SR, Blake KV, Nasr SZ, et al. EUR‐1008 pancreatic enzyme replacement is safe and effective in patients with cystic fibrosis and pancreatic insufficiency. Journal of Cystic Fibrosis 2009;8:405‐17. [CFGD Register: GN207e] [DOI] [PubMed] [Google Scholar]
- Wooldridge JL, Heubi JE, Lee C. The efficacy of EUR‐1008 (Zenpep), a novel pancreatic enzyme product, in the absence of concurrent agents affecting gastric pH in patients with exocrine pancreatic insufficiency [abstract]. Pediatric Pulmonology 2009;44 Suppl 32:411, Abstract no: 555. [CFGD Register: GN207c] [Google Scholar]
Heubi 2016 {published data only}
- Heubi JE, Schaeffer D, Ahrens R, Sollo N, Strausbaugh S, Graff G, et al. Safety and efficacy of a novel microbial lipase (NM‐BL) in patients with exocrine pancreatic insufficiency due to cystic fibrosis [abstract]. Pediatric Pulmonology 2015;50 Suppl 41:402, Abstract no: 555. [CENTRAL: 1092206; CFGD Register: GN252a; CRS: 5500135000001395] [Google Scholar]
- Heubi JE, Schaeffer D, Ahrens RC, Sollo N, Strausbaugh S, Graff G, et al. A randomized, controlled clinical trial of a liquid microbial lipase (NM‐BL) in patients with exocrine pancreatic insufficiency due to cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2016;15 Suppl 1:S29, Abstract no: WS17.6. [CENTRAL: 1157595; CFGD Register: GN252b; CRS: 5500135000001561] [DOI] [PubMed] [Google Scholar]
- Heubi JE, Schaeffer D, Ahrens RC, Sollo N, Strausbaugh S, Graff G, et al. Safety and Efficacy of a Novel Microbial Lipase in Patients with Exocrine Pancreatic Insufficiency due to Cystic Fibrosis: A Randomized Controlled Clinical Trial. Journal of Pediatrics 2016 Jun 10 [Epub ahead of print]:pii: S0022‐3476(16)30257‐8. [CENTRAL: 1157596; CFGD Register: GN252c; CRS: 5500135000001562; DOI: 10.1016/j.jpeds.2016.05.049; PUBMED: 27297209] [DOI] [PubMed]
Hill 1993 {published data only}
- Hill CM, Rolles CJ, Keegan P, Chand RA. Pancreatic enzyme supplementation in cystic fibrosis [letter]. Archives of Disease in Childhood 1993;68(1):150. [CFGD Register: GN171; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hilman 1982 {published data only}
- Hilman B, LeBlanc M, Hawley S, Dufton‐Gross N, Gines D, Tolman N, et al. Cross‐over bioequivalence study of two pH sensitive enteric coated pancreatic enzymes in patients with cystic fibrosis [abstract]. 23rd Annual Meeting Cystic Fibrosis Club Abstracts; 1982 May 14; Washington D.C. 1982:122. [CFGD Register: GN174]
Holsclaw 1979 {published data only}
- Holsclaw DS, Fahl JC, Keith HH. Enhancement of enzyme replacement therapy in cystic fibrosis (CF) [abstract]. Proceedings of 20th Annual Meeting Cystic Fibrosis Club Abstracts; 1979 May 1; Atlanta, Georgia. 1979:19. [CFGD Register: GN177]
Hubbard 1984 {published data only}
- Hubbard VS, Wolf RO, Lester LA, Egge AC. Diagnostic and therapeutic applications of bentiromide screening test for exocrine pancreatic insufficiency in patients with cystic fibrosis. Comparison with other tests of exocrine pancreatic disease. Digestive Diseases and Sciences 1984;29(10):881‐9. [CFGD Register: GN198] [DOI] [PubMed] [Google Scholar]
Kalnins 2005 {published data only}
- Kalnins D, Corey M, Ellis L, Durie PR, Pencharz PB. Combining unprotected pancreatic enzymes with pH‐sensitive enteric‐coated microspheres does not improve nutrient digestion in patients with cystic fibrosis. Journal of Pediatrics 2005;146(4):489‐93. [CFGD Register: GN202] [DOI] [PubMed] [Google Scholar]
Kalnins 2006 {published data only}
- Kalnins D, Ellis L, Corey M, Pencharz PB, Stewart C, Tullis E, et al. Enteric‐coated pancreatic enzyme with bicarbonate is equal to standard enteric‐coated enzyme in treating malabsorption in cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2006;42(3):256‐61. [CFGD Register: GN188b] [DOI] [PubMed] [Google Scholar]
- Kalnins D, Stewart C, Ellis L, Corey M, Tullis E, Pencharz PB, et al. Does the addition of bicarbonate to an enzyme microsphere preparation improve efficacy? [abstract]. Pediatric Pulmonology 1998;26(Suppl 17):355. [CFGD Register: GN188a; MEDLINE: ] [Google Scholar]
Katona 2000 {published data only}
- Katona DR, Nakamura CT, Bowman CM, Margetis M, Keens TG, Woo MS. Does pancreacarb improve the treatment of pancreatic insufficiency in cystic fibrosis [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3):A72. [CFGD Register: GN197; MEDLINE: ] [Google Scholar]
Khaw 1977 {published data only}
- Khaw KT, Adeniyi‐Jones S, Gordon D, Palombo J, Coryer R, Schlaman C, et al. Comparative effectiveness of viokase, cotazym and pancrease in children with cystic fibrosis [abstract]. 18th Cystic Fibrosis Club Abstracts; 1977. 1977:57. [CFGD Register: GN160]
Konstan 2004 {published data only}
- Konstan M, Stern R, Trout R, Sherman J, Eigen H, Wagener J, et al. Ultrase MT12® and Ultrase MT20® in the treatment of exocrine pancreatic insufficiency in cystic fibrosis: safety and efficacy [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):339. [CENTRAL: 797027; CFGD Register: GN201a; CRS: 5500100000003612] [DOI] [PubMed] [Google Scholar]
- Konstan MW, Stern RC, Trout JR, Sherman JM, Eigen H, Wagener JS, et al. Ultrase MT12 and Ultrase MT20 in the treatment of exocrine pancreatic insufficiency in cystic fibrosis: safety and efficacy. Alimentary Pharmacology & Therapeutics 2004;20(11‐12):1365‐71. [CENTRAL: 514057; CFGD Register: GN201b; CRS: 5500050000000064; PUBMED: 15606399] [DOI] [PubMed] [Google Scholar]
Konstan 2008 {published data only}
- Konstan MW, Accurso FJ, Nasr SZ, Ahrens RC, Graff GR. Efficacy and safety of a unique enteric‐coated bicarbonate‐buffered pancreatic enzyme replacement therapy in children and adults with cystic fibrosis. Clinical Investigation 2013;3(8):723‐9. [CENTRAL: 918902; CFGD Register: GN209b; CRS: 5500050000000053; EMBASE: 2013513339] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan MW, Strausbaugh SD, Ahrens RC, Accurso FJ, Graff GR, Nasr SZ. A randomized, double‐blind, placebo‐controlled, multicenter, cross‐over study to evaluate the effectiveness and safety of a novel pancrelipase (Pancrecarb® MS‐16) in reducing steatorrhea in children and adults with cystic fibrosis [abstract]. Pediatric Pulmonology 2008;43 Suppl 31:425. [CFGD Register: GN209a] [Google Scholar]
Konstan 2010 {published data only}
- Konstan MW, Liou TG, Strausbaugh SD, Ahrens R, Kanga JF, Graff GR, et al. Efficacy and safety of a new formulation of pancrelipase (Ultrase MT20) in the treatment of malabsorption in exocrine pancreatic insufficiency in cystic fibrosis. Gastroenterology Research and Practice. United States: Hindawi Publishing Corporation (410 Park Avenue, 15th Floor, 287 pmb, New York NY 10022, United States), 2010. [CENTRAL: 889209; CFGD Register: GN239; CRS: 5500050000000060; EMBASE: 2011059347] [DOI] [PMC free article] [PubMed]
Kraisinger 1993 {published data only}
- Kraisinger M, Hendeles L, Stecenko A, Neiberger R. Hyperuricosuria associated with microencapsulated pancreated enzymes [abstract]. Pediatric Pulmonology 1993;16 Suppl 9:269. [CFGD Register: GN166] [Google Scholar]
Kuo 2011 {published data only}
- Kuo P, Stevens JE, Russo A, Maddox A, Wishart JM, Jones KL, et al. Gastric emptying, incretin hormone secretion, and postprandial glycemia in cystic fibrosis‐‐effects of pancreatic enzyme supplementation. Journal of Clinical Endocrinolgy and Metabolism 2011;96(5):E851‐5. [CFGD Register: GN224] [DOI] [PubMed] [Google Scholar]
Lancellotti 1996 {published data only}
- Lancellotti L, Cabrini G, Zanolla L, Mastella G. High‐ versus low‐lipase acid‐resistant enzyme preparations in cystic fibrosis: a crossover randomized clinical trial. Journal of Pediatric Gastroenterology and Nutrition 1996;22(1):73‐8. [CFGD Register: GN172; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lazaro 1990 {published data only}
- Lazaro A, Olivan G, Ros MI, Nebreda JR, Bueno M. Comparative trial between two pancreatine in enteric coated microspheres preparations in children with cystic fibrosis and steatorrhoea [Estudio comparativo cruzado de dos preparados de pancreatina en microesferas en una serie de ni±os con fibrosis quÝstica y esteatorrea.]. BoletÝn de la Sociedad de PediatrÝa de Arag¾n, La Rioja y Soria 1990;20:170‐6. [CFGD Register: GN193] [Google Scholar]
Leitz 2009 {published data only}
- Leitz GJ, Trapnell B, Strausbaugh S, Woo M, Shin‐Yir T, Silber S, et al. A randomised double‐blind (withdrawal) phase 3 study to evaluate the efficacy and tolerability of pancrease‐mt capsules compared with placebo in the treatment of subjects with cystic fibrosis‐dependent exocrine pancreatic insufficiency [abstract]. Pediatric Pulmonology 2009;44 Suppl 32:407, Abstract no: 544. [CFGD Register: GN213a] [Google Scholar]
- Trapnell BC, Strausbaugh SD, Woo MS, Tong S‐Y, Silber SA, Mulberg AE, et al. Efficacy and safety of PANCREAZE for treatment of exocrine pancreatic insufficiency due to cystic fibrosis. Journal of Cystic Fibrosis 2011;10(5):350‐6. [CENTRAL: 887395; CFGD Register: GN213b ; CRS: 5500050000000059; EMBASE: 2011499244] [DOI] [PubMed] [Google Scholar]
Lubin 1979 {published data only}
- Lubin AH, Scott DH, Bonner JL. Failure of antacids to improve the effectiveness and/or utilization of pancreatic extracts in children with cystic fibrosis [abstract]. Proceedings of 20th Annual Meeting Cystic Fibrosis Club Abstracts; 1979 May 1; Atlanta, Georgia. 1979:57. [CFGD Register: GN190]
Mack 1991 {published data only}
- Mack G, Cooper PJ, Buchanan N. Effects of enzyme supplementation on oral absorption of ciprofloxacin in patients with cystic fibrosis. Antimicrobial Agents and Chemotherapy 1991;35:1484‐5. [CFGD Register: PI135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mischler 1980 {published data only}
- Mischler EH, Parrel SJ, Odell GB, Farrell PM. A controlled study of enteric coated pancreatic enzyme microspheres in patients with cystic fibrosis [abstract]. 21st Annual Meeting Cystic Fibrosis Club Abstracts; 1980 April 29; San Antonio, Texas. 1980:80. [CFGD Register: GN161]
Mischler 1982 {published data only}
- Mischler EH, Parrell S, Farrell PM, Odell GB. Comparison of effectiveness of pancreatic enzyme preparations in cystic fibrosis. American Journal of Diseases of Children 1982;136(12):1060‐3. [CFGD Register: GN136] [DOI] [PubMed] [Google Scholar]
Morrison 1992 {published data only}
- Morrison G, Morrison J, Redmond A, Byers C, McCracken K, Dodge JA. Pancreatic enzyme supplements in cystic fibrosis [letter]. Lancet 1991;338:1596‐7. [CFGD Register: GN154b; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morrison G, Morrison JM, Redmond AO, Byers CA, McCracken KJ, Dodge JA, et al. Comparison between a standard pancreatic supplement and a high enzyme preparation in cystic fibrosis. Alimentary Pharmacology & Therapeutics 1992;6(5):549‐55. [CFGD Register: GN154a] [DOI] [PubMed] [Google Scholar]
Munck 2009 {published data only}
- Munck A. Creon® for children is preferred by parents of CF patients to Creon® 10000 MMS [abstract]. Journal of Cystic Fibrosis 2006;5 Suppl 1:S57. [CFGD Register: GN204a] [Google Scholar]
- Munck A, Duhamel JF, Lamireau T, Luyer B, Tallec C, Bellon G, et al. Pancreatic enzyme replacement therapy for young cystic fibrosis patients. Journal of Cystic Fibrosis 2009;8(1):14‐8. [CFGD Register: GN204b] [DOI] [PubMed] [Google Scholar]
Munoz 1987 {published data only}
- Munoz Conde J, Perez Perez M, Dapena Fernandes J. Cross‐over comparison of creonR and pankreon granulateR in cystic fibrosis with steatorrhoea: [abstract]. 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987. [CFGD Register: GN163]
NCT02137382 {unpublished data only}
- NCT02137382. A double‐blind, randomized, multicenter, cross‐over study to compare the effect of Creon N and Creon® on fat digestion in subjects ≥ 12 years of age with pancreatic exocrine insufficiency due to cystic fibrosis. clinicaltrials.gov/ct2/show/NCT02137382 (first received 12 February 2014).
Neijens 1982 {published data only}
- Neijens HJ, Bouquet J, Sinaasappel M, Kerrebijn KF, Grose WFA. Comparison of the effects of pancreatic enzyme as acid‐resistant microspheres to granules and of the addition of N‐acetyl‐cysteine on the malabsorption in CF [abstract]. 11th European Cystic Fibrosis Conference; 1982; Brussels. 1982:197. [CFGD Register: GN179]
Ritz 2004 {published data only}
- Ritz MA, Fraser RJ, Matteo AC, Greville H, Butler R, Cmielewski P, et al. Evaluation of the 13C‐triolein breath test for fat malabsorption in adult patients with cystic fibrosis. Journal of Gastroenterology and Hepatology 2004;19(4):448‐53. [CFGD Register: GN200] [DOI] [PubMed] [Google Scholar]
Robinson 1989 {published data only}
- Robinson PJ, Olinsky A. Evaluation of a new enteric coated pancreatic enzyme [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:G.I.(b)6. [CFGD Register: GN147a] [Google Scholar]
- Robinson PJ, Olinsky A, Smith AL, Chitravanshi SB. High compared with standard dose lipase pancreatic supplement. Archives of Disease in Childhood 1989;64(1):143‐5. [CFGD Register: GN147b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 1998 {published data only}
- Robinson PJ, Natoli G. Comparison of high‐dose and standard‐dose pancreatic enzyme capsules in children with cystic fibrosis. Australian Journal of Hospital Pharmacy 1998;28(3):160‐4. [CFGD Register: GN191] [Google Scholar]
Santini 2000 {published data only}
- Santini B, Antonelli M, Battistini A, Bertasi S, Collura M, Esposito I, et al. Comparison of two enteric coated microsphere preparations in the treatment of pancreatic exocrine insufficiency caused by cystic fibrosis. Digestive Liver Disease 2000;32(5):406‐11. [CFGD Register: GN178b] [DOI] [PubMed] [Google Scholar]
- Santini B, Bertasi S, Collura M, Febbaro L, Ferrari R, Ferrero L, et al. Open label multicenter cross over study on two enteric coated microspheres in the treatment of CF pancreatic insufficiency [abstract]. 21st European Cystic Fibrosis Conference; 1997 June 1‐6; Davos, Switzerland. 1997:170. [CFGD Register: GN178a]
Shah 1993 {published data only}
- Shah A, Dinwiddie R, Madge S, Prescott P, Hudson G. High dose Nutrizym 22 in cystic fibrosis. European Journal of Pediatrics 1993;152(9):763‐4. [CFGD Register: GN156b] [DOI] [PubMed] [Google Scholar]
- Shah A, Hudson G, Madge S, Dinwiddie R. High dose Nutrizym 22 in cystic fibrosis [abstract]. Proceedings of 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:TP78. [CFGD Register: GN156a]
Sinaasappel 1998 {published data only}
- Sinaasappel M, Swart GR, Hoekstra JH, Houwen RH, Laag J, Gijsbers CF, et al. Double‐blind, cross‐over, study to prove the equivalence of creon 10000 minimicrospheres (MMS) versus creon 8000 microspheres (MS) in patients with cystic fibrosis (CF) [abstract]. Pediatric Pulmonology 1998;26 Suppl 17:358. [CFGD Register: GN186] [Google Scholar]
Stapleton 2001 {published data only}
- Stapleton DR, Gurrin LC, Zubrick SR, Silburn SR, Sherriff JL, Sly PD. The effect of 'Go and Grow with CF' on nutrition and pancreatic enzyme knowledge of children with cystic fibrosis. Australian Journal of Nutrition and Dietetics 2001;58(3):164‐8. [CFGD Register: GN199] [Google Scholar]
Stern 2000 {published data only}
- Stern RC, Eisenberg JD, Wagener JS, Ahrens R, Rock M, doPico G, et al. A comparison of the efficacy and tolerance of pancrelipase and placebo in the treatment of steatorrhea in cystic fibrosis patients with clinical exocrine pancreatic insufficiency. American Journal of Gastroenterology 2000;95(8):1932‐8. [CFGD Register: GN192] [DOI] [PubMed] [Google Scholar]
Thomson 1993 {published data only}
- Thomson M, Clague A, Cleghorn GJ, Shepherd RW. Comparative in vitro and in vivo studies of enteric‐coated pancrelipase preparations for pancreatic insufficiency. Journal of Pediatric Gastroenterology and Nutrition 1993;17(4):407‐13. [CFGD Register: GN157] [DOI] [PubMed] [Google Scholar]
Trapnell 2009 {published data only}
- Maguiness K, Graff G, Boyd D, Caras S, Beckmann K. Efficacy of Pacrealipase delayed‐release capsules (CREON®) in correcting nitrogen malabsorption in subjects with CF in randomized controlled trials [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:417, Abstract no: 547. [CFGD Register: GN214c // GN218c] [Google Scholar]
- Trapnell B, Maguiness K, Graff G, Boyd D, Caras S, Beckmann K. Pancrelipase delayed‐release capsules (Creon®) in patients with pancreatic insufficiency due to CF: age and severity analyses [abstract]. Pediatric Pulmonology 2009;44 Suppl 32:402, Abstract no: 531. [CFGD Register: GN214] [Google Scholar]
- Trapnell BC, Maguiness K, Graff G, Boyd D, Beckmann K, Caras S. Efficacy and safety of Creon® 24,000 in subjects with exocrine pancreatic insufficiency due to cystic fibrosis. Journal of Cystic Fibrosis 2009;8(6):370‐7. [CFGD Register: GN214d] [DOI] [PubMed] [Google Scholar]
- Trapnell BC, Maguiness K, Graff GR, Boyd D, Caras SD. Efficacy and safety of a new formulation of CREON® in patients with exocrine pancreatic insufficiency due to CF [abstract]. Journal of Cystic Fibrosis 2009;8 Suppl 2:S80, Abstract no: 323. [CFGD Register: GN214b] [DOI] [PubMed] [Google Scholar]
van der Haak 2016 {published data only}
- Haak N, Boase J, Davidson G, Butler R, Miller M, Kaambwa B, et al. Preliminary report of the 13C‐mixed triglyceride breath test to assess timing of pancreatic enzyme replacement therapy in children with cystic fibrosis. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2016 Apr 19 [Epub ahead of print]:pii: S1569‐1993(16)30017‐0. [CENTRAL: 1157597; CFGD Register: GN258; CRS: 5500135000001563; DOI: 10.1016/j.jcf.2016.03.008; PUBMED: 27102891] [DOI] [PubMed]
Van de Vijver 2011 {published data only}
- Vijver E, Desager K, Mulberg AE, Staelens S, Verkade HJ, Bodewes FA, et al. Treatment of infants and toddlers with cystic fibrosis‐related pancreatic insufficiency and fat malabsorption with pancrelipase MT. Journal of Pediatric Gastroenterology and Nutrition 2011;53(1):61‐4. [CENTRAL: 799167; CFGD Register: GN240; CRS: 5500050000000065; PUBMED: 21694537] [DOI] [PubMed] [Google Scholar]
Vitti 1975 {published data only}
- Vitti TG, Berg TJ, Pagtakhan RD. The effect of pancreatic enzyme supplement on the intestinal absorption of ampicillin and cloxacillin in children with cystic fibrosis [abstract]. Proceedings of 16th Cystic Fibrosis Club Abstracts; 1975. 1975:56. [CFGD Register: PI81]
Warwick 1982 {published data only}
- Warwick WJ, Budd JR. Comparison of cotayme‐S and pancrease [abstract]. 23rd Annual Meeting Cystic Fibrosis Club Abstracts; 1982 May 14; Washington D.C.. 1982:145. [CFGD Register: GN175]
Weber 1979 {published data only}
- Weber AM, Gheldere B, Roy CC, Fontaine A, Dufour OL, Morin CL, et al. Effectiveness of enteric coated pancrease in cystic fibrosis (CF) children under 4 years old [abstract]. 20th Annual Meeting Cystic Fibrosis Club Abstracts; 1979 May 1; Atlanta, Georgia. 1979:18. [CFGD Register: GN176]
Wooldridge 2009 {published data only}
- Wooldridge JL, Heubi JE, Amaro‐Galvez R, Boas SR, Blake KV, Nasr SZ, et al. EUR‐1008 pancreatic enzyme replacement is safe and effective in patients with cystic fibrosis and pancreatic insufficiency. Journal of Cystic Fibrosis 2009;8(6):405‐17. [CFGD Register: GN212b] [DOI] [PubMed] [Google Scholar]
- Wooldridge JL, Heubi JE, Thieroff‐Ekerdt R. Exploratory analysis of the effect of dosing options on the efficacy of EUR‐1008 (Zenpep) in young children with CF and exocrine pancreatic insufficiency [abstract]. Pediatric Pulmonology 2009;44 Suppl 32:411, Abstract no: 554. [CFGD Register: GN212a] [Google Scholar]
- Wooldridge JL, Schaeffer D, Jacobs D, Thieroff‐Ekerdt R. Long‐term safety assessment of Zenpep® (pancrelipase delayed‐release capsules) in infants aged 1‐12 months with exocrine pancreatic insufficiency associated with cystic fibrosis [abstract]. Pediatric Pulmonology 2012;47(S35):418, Abstract no: 531. [CFGD Register: GN225] [Google Scholar]
Zentler 1992 {published data only}
- Assoufi BA, Zentler‐Munro P, Cornell S, Northfield TC, Hodson ME. Efficacy of acid resistant fungal lipase in the treatment of steatorrhoea due to adult cystic fibrosis [abstract]. Pediatric Pulmonology 1988;5(Suppl 2):145. [CFGD Register: GN151a] [DOI] [PubMed] [Google Scholar]
- Zentler Munro PL, Assoufi BA, Balasubramanian K, Cornell S, Benoliel D, Northfield TC, et al. Therapeutic potential and clinical efficacy of acid‐resistant fungal lipase in the treatment of pancreatic steatorrhoea due to cystic fibrosis. Pancreas 1992;7(3):311‐9. [CFGD Register: GN151b] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dalzell 1992 {published data only}
- Dalzell AM, Heaf DP. High dose pancreatic enzymes in distal intestinal obstruction syndrome [abstract]. Proceedings of Paediatric Research Society Meeting. 1992:148. [CFGD Register: GN170; MEDLINE: ]
Holsclaw 1980 {published data only}
- Holsclaw DS, Keith H. Long‐term benefits of pH‐sensitive enteric‐coated enzymes in CF [abstract]. Proceedings of 8th International Cystic Fibrosis Congress; 1980; Toronto, Canada. 1980:19a. [CFGD Register: GN137]
Knill 1973 {published data only}
- Knill Jones RP, Pearce H, Batten PJ, Williams R. Comparative double‐blind experience of a polyenzymatic preparation in chronic pancreatic insufficiency (author's transl) [Essai comparatif en double insu d'une preparation polyenzymatique dans l'insuffisance pancreatique chronique.]. Acta Gastroenterologica Belgica 1973;36(9):489‐504. [CFGD Register: GN132] [PubMed] [Google Scholar]
Lenoir 2008 {published data only}
- Lenoir G, Dubray C, Hubert D, Chiron R, Philippe P, Sarles J. Phase 2 study in young CF adults with the recombinant acid lipase MERISPASE® [abstract]. Journal of Cystic Fibrosis 2008;7 Suppl 2:S29. [CFGD Register: GN208] [Google Scholar]
Regele 1996 {published data only}
- Regele S, Henker J, Munch R, Barbier Y, Stern M. Indirect parameters of pancreatic function in cystic fibrosis (CF) during a controlled double‐blind trial of pancreatic supplementation. Journal of Pediatric Gastroenterology and Nutrition 1996;22(1):68‐72. [CFGD Register: GN173; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taylor 1993 {published data only}
- Taylor CJ, McGaw J, Barraclough M, Ghosal S, Beckles‐Wilson N, Keegan P. Clinical trial of High lipase pancreatic enzyme supplementation in cystic fibrosis (CF) ; Evaluation of Creon 25,000 [abstract]. Pediatric Pulmonology 1993;16 Suppl 9:306. [CFGD Register: GN167] [Google Scholar]
References to ongoing studies
NCT02279498 {unpublished data only}
- NCT02279498. Study of oral liprotamase unit‐matched therapy of non‐porcine origin in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT02279498 (first received 28 October 2014).
Additional references
Baker 2008
- Baker SS. Delayed release pancrelipase for the treatment of pancreatic exocrine insufficiency associated with cystic fibrosis. Therapeutics and Clinical Risk Management 2008;4(5):1079‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF for Children 2014
- BMJ Publishing Group Ltd. BNF for Children. Section 1.9.4: Pancreatin. www.medicinescomplete.com/mc/bnf/current/PHP732‐pancreatin.htm (accessed 25 June 2014).
Corey 1988
- Corey M, McLaughlin FJ, Williams M, Levinson H. A comparison of the survival, growth and pulmonary function in patients with cystic fibrosis. Journal of Clinical Epidemiology 1988;41:583‐91. [DOI] [PubMed] [Google Scholar]
CSM 1995
- Committee on safety of medicines (CSM). Report of the pancreatic enzymes working party. London. Committee on the Safety of Medicines: Medicines Control Agency 1995.
Dodge 2006
- Dodge JA, Turck D. Cystic fibrosis: nutritional consequences and management. Best practice & research. Clinical Gastroenterology 2006;20(3):531‐46. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fieker 2011
- Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clinical and Experimental Gastroenterology 2011;4:55‐73. [DOI: 10.2147/CEG.S17634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greer 1991
- Greer R, Shepherd R, Cleghorn G, Bowling FG, Holt T. Evaluation of growth and changes in body composition following neonatal diagnosis of cystic fibrosis. Journal of Pediatric Gastroenterology & Nutrition 1991;13(1):52‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Law 2014
- Law V, Knox C, Djoumbou Y, Jewison J, Guo AC, Liu Y, et al. Drugbank 4.0: Shedding new light on drug metabolism. Nucleic Acids Research 2014;42(1):1091‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leus 2000
- Leus J, Biervliet S, Robberecht E. Detection and follow up of exocrine pancreatic insufficiency in cystic fibrosis: a review. European Journal of Pediatrics 2000;159(8):563‐8. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schibli 2002
- Schibli S, Durie PR, Tullis ED. Proper usage of pancreatic enzymes. Current Opinion in Pulmonary Medicine 2002;8(6):542‐6. [DOI] [PubMed] [Google Scholar]
Stallings 2008
- Stallings V, Stark L, Robinson K, Feranchak A, Quinton H. Evidence‐based practice recommendations for nutrition‐related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. Journal of the American Dietetic Association 2008;108(5):832‐9. [DOI] [PubMed] [Google Scholar]
Walkowiak 2005
- Walkowiak J, Sands D, Nowakowska A, Piotrowski R, Zybert K, Herzig KH, et al. Early decline of pancreatic function in cystic fibrosis patients with class 1 or 2 CFTR mutations. Journal of Paediatric Gastroenterology & Nutrition 2005;40(2):199‐201. [DOI] [PubMed] [Google Scholar]
Waters 1990
- Waters DL, Dorney SF, Gaskin KJ, Gruca MA, O'Halloran M, Wilcken B. Pancreatic function in infants identified as having cystic fibrosis in a neonatal screening program. New England Journal of Medicine 1990;322(5):303‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Somaraju 2014
- Somaraju UR, Solis‐Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008227.pub2] [DOI] [PubMed] [Google Scholar]


