Abstract
Background
Ulnar neuropathy at the elbow (UNE) is the second most common entrapment neuropathy after carpal tunnel syndrome. Treatment may be conservative or surgical, but optimal management remains controversial. This is an update of a review first published in 2010 and previously updated in 2012.
Objectives
To determine the effectiveness and safety of conservative and surgical treatment in ulnar neuropathy at the elbow (UNE). We intended to test whether:
‐ surgical treatment is effective in reducing symptoms and signs and in increasing nerve function;
‐ conservative treatment is effective in reducing symptoms and signs and in increasing nerve function;
‐ it is possible to identify the best treatment on the basis of clinical, neurophysiological, or nerve imaging assessment.
Search methods
On 31 May 2016 we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, AMED, CINAHL Plus, and LILACS. We also searched PEDro (14 October 2016), and the papers cited in relevant reviews. On 4 July 2016 we searched trials registries for ongoing or unpublished trials.
Selection criteria
The review included only randomised controlled clinical trials (RCTs) or quasi‐RCTs evaluating people with clinical symptoms suggesting the presence of UNE. We included trials evaluating all forms of surgical and conservative treatments. We considered studies regarding therapy of UNE with or without neurophysiological evidence of entrapment.
Data collection and analysis
Two review authors independently reviewed titles and abstracts of references retrieved from the searches and selected all potentially relevant studies. The review authors independently extracted data from included trials and assessed trial quality. We contacted trial investigators for any missing information.
Main results
We identified nine RCTs (587 participants) for inclusion in the review, of which three studies were found at this update. The sequence generation was inadequate in one study and not described in three studies. We performed two meta‐analyses to evaluate the clinical (3 trials, 261 participants) and neurophysiological (2 trials, 101 participants) outcomes of simple decompression versus decompression with submuscular or subcutaneous transposition; four trials in total examined this comparison.
We found no difference between simple decompression and transposition of the ulnar nerve for both clinical improvement (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.80 to 1.08; moderate‐quality evidence) and neurophysiological improvement (mean difference (in m/s) 1.47, 95% CI ‐0.94 to 3.87). The number of participants to clinically improve was 91 out of 131 in the simple decompression group and 97 out of 130 in the transposition group. Transposition showed a higher number of wound infections (RR 0.32, 95% CI 0.12 to 0.85; moderate‐quality evidence).
In one trial (47 participants), the authors compared medial epicondylectomy with anterior transposition and found no difference in clinical and neurophysiological outcomes.
In one trial (48 participants), the investigators compared subcutaneous transposition with submuscular transposition and found no difference in clinical outcomes.
In one trial (54 participants for 56 nerves treated), the authors found no difference between endoscopic and open decompression in improving clinical function.
One trial (51 participants) assessed conservative treatment in clinically mild or moderate UNE. Based on low‐quality evidence, the trial authors found that information on avoiding prolonged movements or positions was effective in improving subjective discomfort. Night splinting and nerve gliding exercises in addition to information provision did not result in further improvement.
One trial (55 participants) assessed the effectiveness of corticosteroid injection and found no difference versus placebo in improving symptoms at three months' follow‐up.
Authors' conclusions
We found only two studies of treatment of ulnar neuropathy using conservative treatment as the comparator. The available comparative treatment evidence is not sufficient to support a multiple treatment meta‐analysis to identify the best treatment for idiopathic UNE on the basis of clinical, neurophysiological, and imaging characteristics. We do not know when to treat a person with this condition conservatively or surgically. Moderate‐quality evidence indicates that simple decompression and decompression with transposition are equally effective in idiopathic UNE, including when the nerve impairment is severe. Decompression with transposition is associated with more deep and superficial wound infections than simple decompression, also based on moderate‐quality evidence. People undergoing endoscopic surgery were more likely to have a haematoma. Evidence from one small RCT of conservative treatment showed that in mild cases, information on movements or positions to avoid may reduce subjective discomfort.
Plain language summary
Treatment for ulnar neuropathy at the elbow (UNE)
Review question
What are the effects of treatments for ulnar neuropathy at the elbow (UNE)?
Background
Ulnar neuropathy at the elbow is the second most common type of condition in which a nerve becomes trapped or compressed (the most common affects the wrist). The ulnar nerve travels down the side of the elbow. This nerve is important for movement and the sense of touch in the hand at the little finger side. Symptoms of UNE are tingling of the fourth and fifth finger at night, pain at the elbow, and a change in sense of touch if the elbow is bent for a long time. When UNE is severe, some hand muscles can become weak. Diagnosis is by the symptoms and signs of the condition, as well as neurophysiological tests. Treatment of UNE can be surgical or nonsurgical (e.g. splints, physical therapy, and rehabilitation). The best way to treat UNE remains unclear.
Study characteristics
We found two randomised controlled trials (RCTs) of nonsurgical treatment. One RCT compared three groups of people with mild or moderate UNE (51 people in total). All three groups received written instructions to avoid movements or positions that provoked symptoms. The second group had the same information with elbow splints at night for three months. The third group had the same information with nerve gliding exercises. The other nonsurgical study (55 people) compared a corticosteroid injection with a sham injection.
Seven RCTs compared different surgical methods:
• simple decompression or transposition of the nerve (submuscular or subcutaneous transposition) (4 trials, 327 participants);
• medial epicondylectomy or anterior transposition (1 trial, 47 participants);
• anterior subcutaneous transposition or anterior submuscular transposition (1 trial, 48 participants);
• keyhole or open surgery (1 trial, 54 participants with 56 trapped nerves).
Key results and quality of the evidence
Written information alone was as effective in improving work activities and reducing pain at night as when people also used splints or did exercises.
Researchers found no evidence that corticosteroid injection was effective in improving symptoms of UNE.
We were able to combine results from three trials comparing two surgical techniques: simple decompression and transposition of the ulnar nerve (subcutaneous or submuscular). We found no important difference in symptom scores between the techniques at 6 to 12 months. Decompression with transposition may result in more deep and superficial wound infections. Trialists found no clinical differences between surgical techniques in the other surgical trials. People undergoing endoscopic surgery were more likely to have a haematoma (an abnormal collection of blood) after surgery.
Evidence was insufficient for us to choose the best treatment for UNE. However, we did find that in mild cases, information on movements and positions to avoid may reduce discomfort. Moreover, the combined results from three surgical trials provided moderate‐quality evidence that simple decompression surgery and decompression with transposition may be equally effective, but that decompression with transposition may result in more deep and superficial wound infections.
The evidence is up to date to 31 May 2016.
Summary of findings
Summary of findings for the main comparison. Simple decompression versus transposition for ulnar neuropathy at the elbow.
| Simple decompression versus transposition for ulnar neuropathy at the elbow | ||||||
| Patient or population: people with ulnar neuropathy at the elbow Settings: surgery department Intervention: simple decompression Comparison: transposition | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transposition | Simple decompression | |||||
| Proportion of participants with clinical improvement in function compared to baseline Follow‐up: 6 to 12 months | 746 per 10001 | 694 per 1000 (597 to 806) | RR 0.93 (0.8 to 1.08) | 261 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus subcutaneous transposition Follow‐up: 12 months | 730 per 10001 | 672 per 1000 (540 to 832) | RR 0.92 (0.74 to 1.14) | 147 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Subgroup: proportion of participants with clinical improvement in function compared to baseline ‐ simple decompression versus submuscular transposition Follow‐up: 6 months | 768 per 10001 | 730 per 1000 (591 to 899) | RR 0.95 (0.77 to 1.17) | 114 (2 studies) | ⊕⊕⊕⊝ moderate4 | |
| Adverse events: proportion of participants with deep/superficial wound infections Follow‐up: 6 to 12 months | 115 per 10001 | 37 per 1000 (14 to 98) | RR 0.32 (0.12 to 0.85) | 261 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The assumed risk was considered as the median of the risks in the control groups across studies. We did not consider the mean, since the number of studies was low, and the median is the best measure of central tendency in this case. 2Evidence downgraded once for study design (risk of bias). In one study, the method for sequence generation was not adequate and allocation concealment method was unclear. In two studies, allocation concealment method was unclear. In one study, the examiner was not blinded, and in another it was unclear if the examiner was blinded. 3Evidence downgraded once for study design. In the study contributing data for this outcome, the examiner was not blinded and allocation concealment method was unclear. 4Evidence downgraded once for study design. In one study, the method for sequence generation was not adequate and allocation concealment method was unclear, and in the other, the allocation concealment method was unclear. In one study, it was unclear if the examiner was blinded.
Background
Description of the condition
Ulnar neuropathy at the elbow (UNE) is the second most common entrapment neuropathy after carpal tunnel syndrome. Its mean annual crude incidence is 24.7 cases per 100,000 person‐years (Mondelli 2005). One of the sites of entrapment is the cubital tunnel. The tunnel is defined by Osbourne's ligament, the medial collateral ligament of the elbow, the elbow joint capsule, and the olecranon (Palmer 2010). The clinical picture is typically characterised by paraesthesias involving the fourth and fifth finger, pain at the elbow, sensory symptoms with prolonged flexion of the elbow, and in severe cases motor deficit of the ulnar innervated hand muscles (Dellon 1989). Diagnosis is based on signs, symptoms, and electrodiagnostic studies (Robertson 2005). Imaging, particularly ultrasound, in Beekman 2004, and magnetic resonance imaging (MRI), in Bordalo 2004, is gaining more attention as a sensitive diagnostic tool. Provocative clinical tests are not reliable or useful in the diagnosis of UNE (Beekman 2009). Electrodiagnostic examination is necessary to confirm the diagnosis, quantify the severity, and identify the exact site of ulnar nerve compression (AAEM 1999; Padua 2001).
Description of the intervention
Treatment of UNE may be conservative (splint device, physical therapy, rehabilitation) or surgical (Bartels 2005a; Bartels 2005b; Biggs 2006). The goal of conservative treatment is to eliminate or reduce the frequency of external compression on the nerve (Dellon 1993; Robertson 2005). Regarding surgical therapy, many procedures are employed for the treatment of cubital tunnel syndrome, including simple decompression, anterior transposition (subcutaneous, submuscular, and intramuscular), and medial epicondylectomy (Eaton 1980; Kleinman 1989; Kuschner 1996; Tsai 1999; Robertson 2005).
Why it is important to do this review
The basis for choosing a surgical technique must relate to the pathophysiology of the compression of the ulnar nerve at the elbow, an understanding of the aetiology of the ulnar nerve compression in a particular case, and the potential drawbacks of the various operative procedures. Despite the different options for treating UNE, optimal management remains controversial. This review was first published in 2010 and previously updated in 2012.
Objectives
To determine the effectiveness and safety of conservative and surgical treatment in ulnar neuropathy at the elbow (UNE). We intended to test whether:
surgical treatment is effective in reducing symptoms and signs and in increasing nerve function;
conservative treatment is effective in reducing symptoms and signs and in increasing nerve function;
it is possible to identify the best treatment on the basis of clinical, neurophysiological, or nerve imaging assessment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) using truly random or quasi‐random allocation of treatment. We would consider prospective, consecutive series of more than 10 participants where outcomes were collected by an observer other than the operating surgeon in the Discussion. We did not consider single‐case reports.
Types of participants
People with clinical symptoms suggesting the presence of UNE with or without neurophysiological evidence of entrapment.
Types of interventions
All forms of surgical and conservative treatments.
Types of outcome measures
Primary outcomes
We defined the primary outcome measure as clinically relevant improvement in function compared to baseline. We assessed function with whatever scale was used by the trial authors, with a preference for validated scales such as the Disability of the Arm, Shoulder and Hand questionnaire, in Hudak 1996, or the UNE questionnaire (Mondelli 2006). When self administered scales were used, we would have evaluated if statistically significant changes were reported regarding the main scores in the questionnaires. We dichotomised the primary outcome measure into improvement or no improvement, regardless of the differences between the tools used. If a study evaluated more than one functional outcome measure, a better score on at least one of the functional outcome measures was enough to be considered as an improvement.
Secondary outcomes
-
Change in neurological impairment measured by:
the strength of ulnar nerve innervated muscles with the Medical Research Council (MRC) sum score (BMRC 1976);
the presence and extent of sensory deficit measured with whatever instrument was used by the authors, but with a preference for cotton wool or Semmes‐Weinstein filaments (Bell‐Krotoski 1987).
Change from baseline of the motor nerve conduction velocity across the elbow.
Change from baseline in the nerve diameter at the elbow, evaluated by ultrasound or MRI.
Change in quality of life.
Adverse events.
We evaluated primary and secondary outcomes at a short follow‐up (one to six months) and at a long follow‐up (between six months and two years).
Search methods for identification of studies
Electronic searches
On 31 May 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Register of Studies Online (CRSO)), MEDLINE (January 1966 to May 2016), Embase (January 1980 to May 2016), AMED (Allied and Complementary Medicine) (January 1985 to May 2016), CINAHL (Cumulative Index to Nursing and Allied Health Literature) Plus (January 1937 to May 2016), and LILACS (Latin American and Caribbean Health Science Information database) (January 1982 to May 2016). On 14 October 2016, we searched PEDro (Physiotherapy Evidence Database) (January 1980 to October 2016). We applied no limitations as to language.
The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register (Appendix 1), CENTRAL (Appendix 2), MEDLINE (Appendix 3), Embase (Appendix 4), AMED (Appendix 5), LILACS (Appendix 6), CINAHL Plus (Appendix 7), PEDro (Appendix 8), ClinicalTrials.gov (Appendix 9), and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (Appendix 10).
Searching other resources
We searched the references of relevant trials identified by the search strategy and in June 2015 contacted authors of identified papers to determine whether other published or unpublished trials were available.
We also searched the following trials registers.
ClinicalTrials.gov (clinicaltrials.gov) (4 July 2016)
WHO ICTRP (who.int/ictrp/en/) (4 July 2016)
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts of the references retrieved from the searches and selected all potentially relevant studies. We compared the results of our literature search to the review articles found using the previously mentioned databases. Furthermore, when information from one paper was re‐published by the same author in a larger investigation, or written in English, we considered only the most recent article. We obtained copies of these articles, and the same review authors independently reviewed them against the inclusion criteria of the review. The review authors then independently extracted data from included trials and assessed risk of bias with a data extraction form specifically designed for this purpose.
Data extraction and management
We extracted the following data.
Study methods
Design (e.g. randomised or quasi‐randomised, cohort study, case‐control study)
Randomisation method (including list generation)
Method of allocation concealment
Blinding method
Stratification factors
Participants
Inclusion and exclusion criteria
Number (total, per group)
Age distribution
Associated morbidities
Treatments
Pre‐treatment quality of life and functional status, as measured by validated scales
Intervention and control
Type of therapy
Type of control
Details of control treatment including duration of non‐operative treatment
Concomitant treatments
Follow‐up data
Duration of follow‐up
Loss to follow‐up
Outcome data
British Medical Research Council (BMRC) scale
Presence of sensory deficits (evaluated with cotton wool or Semmes‐Weinstein filaments)
Self administered scales including questionnaires assessing regional function and symptoms (Disability of the Arm, Shoulder and Hand questionnaire; UNE questionnaire; visual analogue scale (VAS) (Sriwatanakul 1983), and quality of life measures (such as the 36‐Item Short Form Health Survey (SF‐36)) (Ware 1992)
Neurophysiology
Ultrasound
MRI
We considered the BMRC scale, presence of sensory deficits, and a regional self administered questionnaire the main outcome measures.
Analysis data
Methods of analysis (intention‐to‐treat or per‐protocol analysis)
Comparability of groups at baseline (age, gender, clinical impairment, neurophysiological impairment, associated diseases)
Statistical techniques
Other data
Date
Location
Conflicts of interest
Funding
Single or multicentre
The first review author entered data into the Cochrane statistical software Review Manager 5, and the second review author checked the data entry.
At this update the review included information on trial funding and conflicts of interest.
Assessment of risk of bias in included studies
We evaluated the risk of bias in the trials by scoring the following items and reported our assessments in the 'Risk of bias' tables:
sequence generation;
allocation concealment;
blinding of participants;
blinding of outcome assessors;
incomplete outcome data;
selective outcome reporting;
other risk of bias.
We assessed the included studies under each domain and judged the risk of bias as 'high', 'low', or 'unclear'. We used unclear when the report included insufficient information to make a judgement or when the risk of bias was uncertain (Higgins 2011).
Measures of treatment effect
We used risk ratio (RR) estimations with 95% confidence intervals (CI) for binary outcomes and mean difference (MD) estimations with 95% CI for continuous outcomes. All analyses included all participants in the treatment groups to which they were allocated.
If we had collected data from case‐control studies, we would have considered using odds ratios and 95% CI.
Dealing with missing data
In the first instance, we contacted study authors to supply data missing from included studies. We assessed missing data and dropouts or attrition for each included study, and assessed and discussed the extent to which the missing data could have altered the results or conclusions of the review. If less than 70% of participants allocated to treatments provided data at the end of the trial for a particular outcome, we would have discarded those data as we would have considered them to be too prone to bias.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between trials (age, gender, clinical impairment, neurophysiological impairment, and associated diseases) and trial factors (randomisation concealment, blinding of outcome assessment, losses to follow‐up, treatment type, and co‐interventions). We assessed statistical heterogeneity by examining the I2 statistic, a quantity which approximately describes the proportion of variation in point estimates that is due to heterogeneity rather than sampling error. In addition, we planned to employ a Chi2 test to determine the strength of the evidence that heterogeneity was genuine.
Assessment of reporting biases
In order to detect potential publication bias, we would plot RRs and 95% CIs against standard errors in each study (funnel plots). Asymmetry in such plots could be due to publication bias, but could also be due to a relationship between trial size and effect size. In the event of finding a relationship, we planned to examine the clinical diversity of the studies (Egger 1997).
Data synthesis
Where the interventions were the same, or similar enough, we carried out a meta‐analysis (DerSimonian 1986). We undertook statistical analysis using Review Manager 5 (RevMan 2014). We planned to synthesise results in a meta‐analysis.
'Summary of findings' table
We included a 'Summary of findings' table for comparisons for which information from more than one study was available and assessed the quality of the body of evidence using the GRADE approach for the following outcomes:
proportion of participants with clinically relevant improvement in function compared to baseline at 6 to 12 months (showing subgroups);
change in quality of life;
adverse events.
We downgraded the quality of the evidence for each outcome using the five GRADE considerations: limitations in design or implementation of studies suggesting a high risk of bias; indirectness of evidence; unexplained heterogeneity; imprecision; and high probability of publication bias (Schünemann 2011). RCTs start with a high quality assessment, which may be downgraded to moderate, low, or very low if these considerations are present to a serious degree.
Subgroup analysis and investigation of heterogeneity
If possible, we would have conducted subgroup analysis for the following groups.
Two age groups: (≤ 45 years old, > 45 years old).
-
Two groups with different electrophysiological abnormalities, namely:
participants with pathological motor conduction velocity across the elbow and no other neurophysiological abnormality;
-
participants with concomitant pathological motor conduction velocity across the elbow and one of the following criteria:
denervation signs in the ulnar innervated muscle of the hand; or
reduction of amplitude of sensory response in the fifth digit‐wrist segment.
Sensitivity analysis
If possible, we would have conducted sensitivity analyses to assess the impact of study quality. This would have included separate analyses of RCTs and quasi‐RCTs.
Adverse events
Since randomised studies rarely deal with adverse events adequately because the numbers are small and follow‐up too short, we discussed adverse events (infections, worsening of symptoms) taking into account the non‐randomised literature.
Cost‐benefit analyses
We considered cost‐effectiveness of interventions in the Discussion, where relevant data were available.
Results
Description of studies
Results of the search
The previous version of this review included six studies. In 2016 we identified 253 new papers from database searches as potentially relevant and after we reviewed these, a total of nine RCTs (587 participants) met the inclusion criteria for the review. The following list reports the number of papers identified in each database by the new current strategies and the number newly identified at this update.
MEDLINE: 264 (60 new papers)
Embase: 137 (23 new papers)
CENTRAL: 78 (31 new papers)
Cochrane Neuromuscular Specialised Register: 31 (9 new papers)
AMED: 15 (1 new paper)
LILACS: 3 (1 new paper)
CINAHL Plus: 74 (8 new papers)
PEDro: 5 (2 new papers)
ClinicalTrials.gov: 86
WHO ICTRP: 32
Figure 1 illustrates the study selection process.
1.
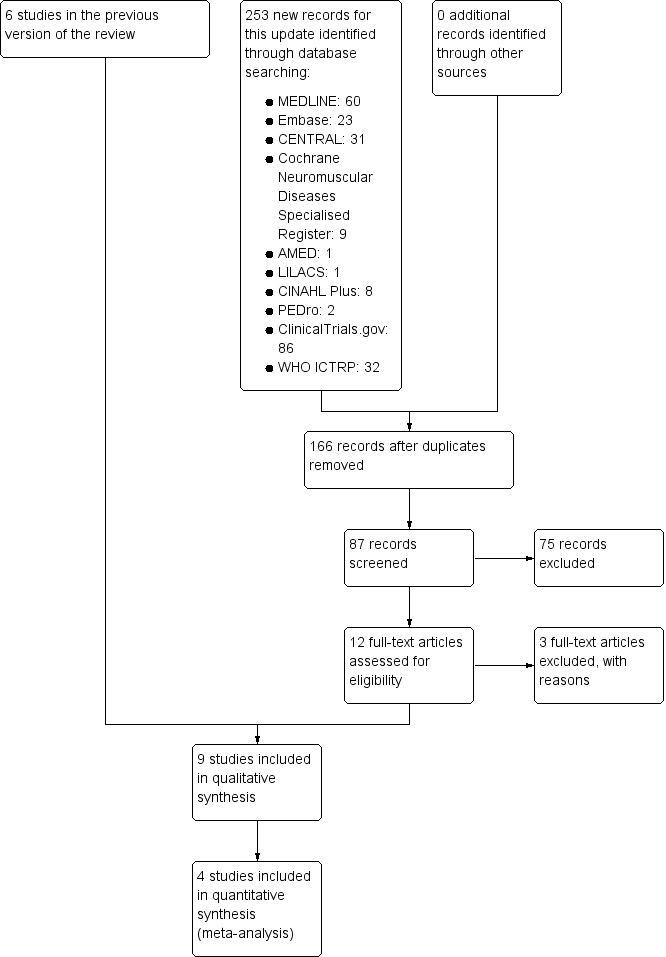
A flow diagram illustrating the study selection process.
Included studies
See Characteristics of included studies.
To evaluate the clinical outcome we included three studies in the meta‐analysis: Bartels 2005 (152 participants; 5 lost to follow‐up), Gervasio 2005 (70 participants), and Biggs 2006 (44 participants). The three studies compared simple decompression with transposition of the ulnar nerve. In two studies the latter was a submuscular transposition (Gervasio 2005; Biggs 2006), and in one study, it was anterior subcutaneous transposition (Bartels 2005). A total of 131 participants were treated by simple decompression, and a total of 130 participants were treated by transposition of the nerve (submuscular or subcutaneous). In all three studies the participants had clinical and electrophysiological evidence of ulnar nerve impairment.
We performed two different evaluations to assess the effectiveness of surgery. In the first, we compared simple decompression with subcutaneous transposition (Bartels 2005), and in the second analysis, we compared simple decompression with submuscular transposition (Gervasio 2005; Biggs 2006).
Bartels 2005 and Biggs 2006 used clinical scores to assess changes after surgical treatment. Gervasio 2005 used a clinical score and a neurophysiological evaluation. The clinical outcome measures in the three studies varied. Bartels 2005 used a clinical scale that included a combination of historical and physical findings (evaluation of sensation and muscular strength); Biggs 2006 used the McGowan score and Louisiana State University Medical Center (LSUMC) score, both of which graded the sensory and muscular deficits; and Gervasio 2005 evaluated the clinical picture preoperatively by Dellon’s classification and postoperatively by the Bishop score. Dellon’s staging system includes the assessment of sensory and motor function and the response to provocative tests. The Bishop score assesses objective and subjective parameters: grip strength, sensory measurement of static two‐point discrimination, severity of residual symptoms, subjective improvement compared with the preoperative period, and preoperative and postoperative work status.
Bartels 2005 performed a clinical follow‐up at one year after surgery; Biggs 2006 at six weeks and six months after surgery; and Gervasio 2005 at six months after surgery (clinical and neurophysiological assessment). At the six‐month follow‐up, Gervasio 2005 performed a neurophysiological assessment in all the participants in both groups. These investigators also performed a second clinical follow‐up (the mean duration of the second follow‐up was 47 months for the simple decompression group and 46.9 months for the anterior submuscular transposition group).
We also performed a meta‐analysis of neurophysiological outcome, including two papers (Gervasio 2005; Nabhan 2005).
Nabhan 2005 (66 participants) compared two surgical procedures: subcutaneous anterior transposition and decompression without transposition. Thirty‐two participants underwent simple nerve decompression, and 34 had subcutaneous transposition. All participants had clinical and electrophysiological evidence of ulnar neuropathy. The main outcome measure was motor conduction velocity across the elbow, although the trialists also assessed muscular strength of ulnar innervated muscles pre‐ and postoperatively. All the participants in both groups underwent neurophysiological assessment. The investigators performed follow‐up examinations at three months and nine months after surgery.
We included five other studies in the review but not in the meta‐analyses (Geutjens 1996; Svernlov 2009; Zarezadeh 2012; Schmidt 2014; vanVeen 2015).
Geutjens 1996 (47 participants) compared medial epicondylectomy with anterior transposition in people with clinical and electrophysiological evidence of ulnar neuropathy. The authors measured the clinical outcome by:
the MRC scale;
evaluation of sensation by light touch and static two‐point discrimination; and
assessment of pain in the hand by a non‐validated five‐item scale.
The neurophysiological outcome was measured by ulnar motor nerve conduction velocity in all the participants in both groups. All the evaluations were performed before treatment and at 12 months. Participant satisfaction was assessed by a non‐validated tool.
Svernlov 2009 (51 participants) compared three groups treated conservatively with:
night splinting for three months and "written information of the anatomy of the ulnar nerve, an explanation of the probable pathomechanics and the regimen regarding the avoidance of movements and positions provoking the symptoms";
nerve gliding exercises and the same written information;
written information only.
All the participants had clinically mild or moderate UNE, classified with the Dellon’s staging system. All participants underwent electrophysiological assessment preoperatively, but only 12 participants had abnormal findings. The clinical outcome measures were: (1) evaluation of fifth‐digit and grip strength, measured by two different dynamometers; and (2) the VAS. Electrophysiological outcomes were ulnar motor and sensory nerve conduction studies and electromyography. Participants assessed their symptoms according to the Canadian Occupational Performance Measure (COPM). The COPM is a 10‐point scale that measures the person’s own opinion of his or her ability to perform occupational activities and satisfaction with performance. The investigators evaluated the participants in the three groups before the treatment and at six months.
Zarezadeh 2012 (48 participants) compared anterior subcutaneous transposition with anterior submuscular transposition in participants with clinical and electrophysiological evidence of ulnar neuropathy. The authors measured the clinical outcome by:
MRC scale;
subjective evaluation of muscle atrophy;
Yale Sensory Scale; and
VAS.
The authors measured the neurophysiological outcome by ulnar motor nerve conduction velocity in all participants but did not report the findings. All the evaluations were performed before treatment and at 12 months. The clinical outcome was assessed by a total score based on the results of the four outcome measures; however, no information was provided on the score generation process.
Schmidt 2014 (54 participants with 56 ulnar entrapments) compared endoscopic surgery with open decompression in people with clinical, neurophysiological, and ultrasonographic findings of ulnar neuropathy. The clinical outcome was measured by a modified Bishop scale, and the neurophysiological outcome by ulnar motor nerve conduction velocity. The trialists performed clinical and neurophysiological evaluations before surgery, during an early follow‐up (a mean of 16 weeks), and a long‐term follow‐up (a mean of 16.8 months).
vanVeen 2015 (63 participants enrolled, 55 participants analysed) compared an ultrasound‐guided injection of a 1 mL injection containing 40 mg methylprednisolone acetate and 10 mg lidocaine hydrochloride with a placebo injection. The trialists measured the clinical outcome by two subjective scales evaluating change in symptoms and severity of symptoms. Moreover, the trial authors evaluated the neurophysiological outcome by ulnar motor nerve conduction velocity and the utrasonographic outcome by measuring the ulnar nerve cross‐section in a segment of 4 cm across the medial epicondyle.
Excluded studies
See Characteristics of excluded studies.
We excluded two studies from the review (Chen 2006; Zhong 2011). Chen 2006 compared two groups of participants. In the first group, participants "were immobilized with the plaster slab for an external fixation for 3 weeks" after operation; in the second group, they "began an immediate range of motion on the 2nd day after operation". It seems that both groups were comprised of participants who underwent ulnar neurolysis or nerve anterior transposition. We excluded the study because the authors did not compare different therapeutic approaches, but rather two management approaches after surgery. From the translation it was difficult to evaluate the quality of the study. Zhong 2011 compared two groups of participants treated surgically. Participants in the first group were treated with subcutaneous transposition, and in the second group with submuscular transposition. The main outcome measures were the cross‐sectional area (CSA) of the ulnar nerve at the elbow and neurophysiological parameters. The authors concluded that submuscular transposition produced greater improvement than subcutaneous transposition in severe cases. The preoperative values of CSA and of neurophysiological parameters were identical for the two compared groups in the report (even the same decimal values); since this is statistically improbable, we had serious concerns about the methodological quality of the work and therefore excluded it.
Risk of bias in included studies
Figure 2 summarises the risk of bias in the included RCTs.
2.
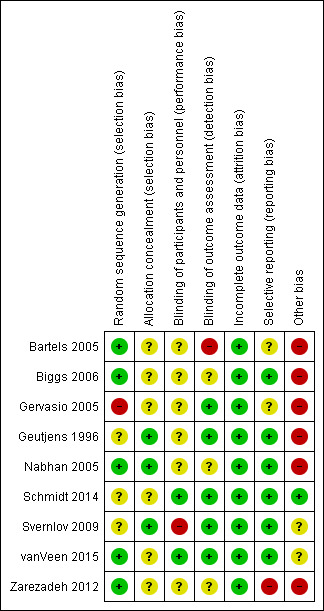
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation was adequate in five studies (Bartels 2005; Nabhan 2005; Biggs 2006; Zarezadeh 2012; vanVeen 2015). Three studies did not clearly describe the method of sequence generation (Geutjens 1996; Svernlov 2009; Schmidt 2014). In one study the method of randomisation was inadequate (Gervasio 2005).
The allocation concealment method was described and adequate in three studies (Geutjens 1996; Nabhan 2005; Svernlov 2009). In six studies the method of allocation concealment was not described (Bartels 2005; Gervasio 2005; Biggs 2006; Zarezadeh 2012; Schmidt 2014; vanVeen 2015); we concluded that no allocation concealment procedure was used in these studies.
Blinding
In six studies the authors did not describe if the participants were blinded (Geutjens 1996; Bartels 2005; Gervasio 2005; Nabhan 2005; Biggs 2006; Zarezadeh 2012); in two studies the participants were blinded (Schmidt 2014; vanVeen 2015); and in one study the participants were not blinded (Svernlov 2009).
In five studies the examiner was blinded (Geutjens 1996; Gervasio 2005; Svernlov 2009; Schmidt 2014; vanVeen 2015). In one study only a subgroup of 30 participants was evaluated by an independent examiner (Bartels 2005). In two studies the authors did not specify whether the examiner was blinded (Nabhan 2005; Biggs 2006). In one study the authors specified that the neurophysiological evaluation was blinded, but no information was reported about the assessment of clinical outcomes (Zarezadeh 2012).
Incomplete outcome data
In three studies no participants were lost to follow‐up (Gervasio 2005; Nabhan 2005; Zarezadeh 2012). In the remaining six studies a low number of participants were lost to follow‐up: five participants (3.3%) in Bartels 2005, three (6.4%) in Biggs 2006, nine (4.7%) in Geutjens 1996, 13 (6.6%) in Svernlov 2009, three (5.5%) in Schmidt 2014, and five (5%) in vanVeen 2015. In the study evaluating conservative treatments (Svernlov 2009), six participants were dropouts because they underwent surgical decompression.
Selective reporting
It was unclear whether two studies were free of selective outcome reporting (Bartels 2005; Gervasio 2005). In Bartels 2005, the methods listed two validated self report questionnaires (McGill Pain Questionnaire‐Dutch language version (MPQ‐DLV), SF‐36), but provided no statistical information on the questionnaires in the results. The authors simply reported that the improvement in the MPQ‐DLV and SF‐36 scores did not differ for participants treated with simple decompression or anterior subcutaneous transposition. In Gervasio 2005, trial authors performed the pre‐ and postoperative evaluations using two different staging systems: preoperatively the Dellon scale, and postoperatively the Bishop rating system. No information on the Dellon scale was available for the follow‐up evaluation. In another study, the authors declared that all participants were neurophysiologically evaluated but reported no data (Zarezadeh 2012).
Six studies were free of selective outcome reporting (Geutjens 1996; Nabhan 2005; Biggs 2006; Svernlov 2009; Schmidt 2014; vanVeen 2015).
Other potential sources of bias
In all nine trials the authors did not specify whether the study was designed to be a non‐inferiority or a superiority trial (Geutjens 1996; Bartels 2005; Gervasio 2005; Nabhan 2005; Biggs 2006; Svernlov 2009; Zarezadeh 2012; Schmidt 2014; vanVeen 2015), and in eight trials the authors did not calculate sample size (Geutjens 1996; Bartels 2005; Gervasio 2005; Nabhan 2005; Biggs 2006; Svernlov 2009; Zarezadeh 2012; Schmidt 2014). In two studies, the clinical outcome measures used may have low sensitivity (Geutjens 1996; Nabhan 2005). One study only included people with severe neuropathy (Gervasio 2005).
Since none of the trials were at an overall high risk of bias, we did not conduct a sensitivity analysis.
Effects of interventions
See: Table 1
Surgery: simple decompression versus transposition
Bartels 2005, Biggs 2006, Gervasio 2005, and Nabhan 2005.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
Reported in Bartels 2005, Biggs 2006, and Gervasio 2005. See Table 1.
We found clinical improvement in 70% of participants treated with simple decompression and 75% of those treated with transposition in the period from 6 to 12 months after surgery. We found no significant difference in postoperative clinical improvement between simple decompression and transposition (subcutaneous or submuscular) of the ulnar nerve (RR 0.93, 95% CI 0.80 to 1.08; n = 261) (Analysis 1.1). Figure 3 shows the forest plot for the studies in the meta‐analysis. We observed no significant difference in clinical outcome between simple decompression and subcutaneous transposition (RR 0.92, 95% CI 0.74 to 1.14; n = 147) or between simple decompression and submuscular transposition (RR 0.95, 95% CI 0.77 to 1.17; n = 114) (Analysis 1.1; Figure 3).
1.1. Analysis.

Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 1 Proportion of participants with clinical improvement in function compared to baseline.
3.
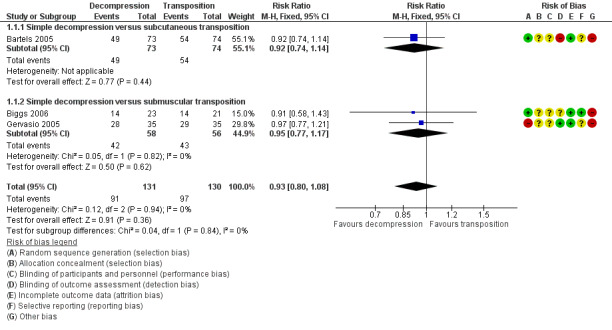
Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.1 Proportion of participants with clinical improvement in function compared to baseline.
Secondary outcomes
Change in neurological impairment
Reported in Nabhan 2005.
Nabhan 2005 found a slight improvement in the mean value of the MRC sum scale (measuring specifically strength in ulnar intrinsic muscles) (BMRC 1976), and in the mean value of a non‐validated sensory scale after simple decompression (pre‐surgery MRC 4.0 ± 1.0, postsurgery MRC 4.5 ± 0.7) and after decompression with anterior subcutaneous transposition (pre‐surgery MRC 3.8 ± 1.0, postsurgery MRC 4.3 ± 0.6). No difference was found between the two procedures.
Change from baseline of the motor nerve conduction velocity across the elbow
Reported in Gervasio 2005 and Nabhan 2005.
We found a statistically significant improvement in motor nerve conduction velocity after simple decompression and after transposition at the six months' follow‐up (Gervasio 2005; Nabhan 2005). We observed no difference in postoperative motor nerve conduction velocity (m/s) between the two procedures (MD 1.47, 95% CI ‐0.94 to 3.87; n = 101) (Analysis 1.2). Figure 4 shows the forest plot for this outcome.
1.2. Analysis.
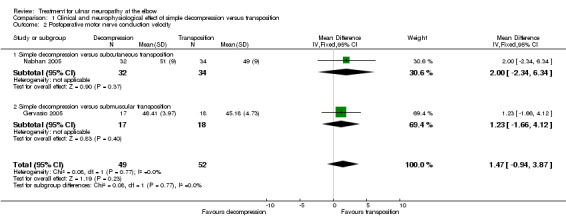
Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 2 Postoperative motor nerve conduction velocity.
4.
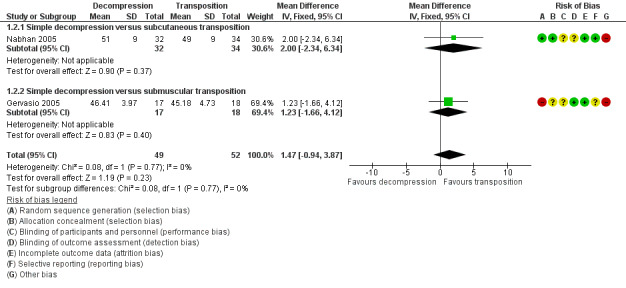
Forest plot of comparison: 1 Clinical and neurophysiological effect of simple decompression versus transposition, outcome: 1.2 Postoperative motor nerve conduction velocity.
For the Gervasio 2005 study, we only included in the meta‐analysis the participants who preoperatively had motor responses at the neurophysiological assessment (17 out of 35 participants in the simple decompression group and 18 out of 35 participants in the transposition group). The motor response, preoperatively absent in 18 participants in the simple decompression group, reappeared postoperatively in eight participants, while in the transposition group, it was absent in 17 participants and reappeared in six. Among the 30 participants with preoperative absence of sensory response in the simple decompression group, the sensory response reappeared in 16 participants. Among the 29 participants with absence of sensory response in the transposition group, the sensory response reappeared in 14 participants.
The available data did not allow a subgroup analysis between participants with pathological motor conduction velocity across the elbow only and participants who also had denervation signs or reduction of amplitude of sensory responses, or both, or between age groups.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
No data were available on change from baseline in the ulnar nerve diameter at the elbow.
Change in quality of life
No data were available for the outcome change in quality of life.
Adverse events
Reported in Bartels 2005, Gervasio 2005, and Biggs 2006.
Decompression with transposition was associated with a higher number of deep and superficial wound infections on meta‐analysis of data from three trials (RR 0.32, 95% CI 0.12 to 0.85; n = 261; Analysis 1.3) (4 deep infections and 11 superficial infections in the transposition group, 0 deep infections and 5 superficial infections in the simple decompression group). Bartels and colleagues also found a higher scar area sensory loss in the transposition group (14 cases) than in the simple decompression group (2 cases). Other adverse events affected small numbers of participants.
1.3. Analysis.
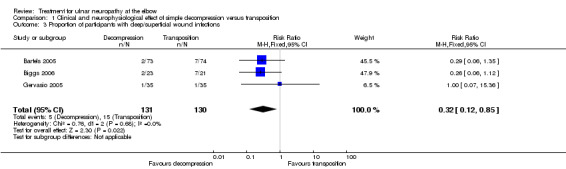
Comparison 1 Clinical and neurophysiological effect of simple decompression versus transposition, Outcome 3 Proportion of participants with deep/superficial wound infections.
Surgery: medial epicondylectomy versus anterior transposition
Studied in Geutjens 1996.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
Clinical improvement in function was not evaluated.
Secondary outcomes
Change in neurological impairment
In Geutjens 1996, the authors found no difference between medial epicondylectomy (n = 25) and anterior transposition (n = 22) in modifying muscular strength and sensory deficits after 12 months. After surgery, the number of participants with muscular deficits at the MRC grading was 13 in the medial epicondylectomy group and 10 in the anterior transposition group (P = 0.119). We were unable to report the grade 2 comparison as the data in the report were unclear. The mean (± standard deviation (SD)) two‐point discrimination scores were 6.8 ± 2.8 in the medial epicondylectomy group and 9.2 ± 3.3 in the anterior transposition groups(P = 0.711).
Change from baseline of the motor nerve conduction velocity across the elbow
Medial epicondylectomy and anterior transposition had similar efficacy on neurophysiological outcomes (postoperative motor nerve conduction velocity was 32.6 ± 7.55 in the medial epicondylectomy group and 34.0 ± 8.01 in the anterior transposition group, P = 0.772)
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
Postoperative pain in the hand occurred with anterior transposition. The mean (0‐to‐5 scale) pain score (± SD) after medial epicondylectomy (n = 25) was 0.0 (± 0) compared to 0.45 ± 0.86 after anterior transposition (n = 22) (P = 0.029).
Surgery: submuscular transposition versus anterior subcutaneous transposition
Studied in Zarezadeh 2012.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
Clinical improvement in function was not evaluated.
Secondary outcomes
Change in neurological impairment
In Zarezadeh 2012, the authors found no difference between submuscular transposition (n = 24) and anterior subcutaneous transposition (n = 24) in improving sensory and strength deficits after 12 months. After surgery, in the submuscular group, 0% of participants had absence of sensation, 50% had decreased or abnormal sensation, and 50% had normal sensation; in the subcutaneous group, 0% of participants had absence of sensation, 54.2% had decreased or abnormal sensation, and 45.8% had normal sensation (P = 1.0). No participant in either group had severe strength deficits; 37.5% of participants in the submuscular group and 29.2% in the subcutaneous group had moderate deficits; and 62.5% in the submuscular group and 70.8% in the subcutaneous group had slight or no deficit. Submuscular transposition was associated with a greater pain reduction (after surgery, in the submuscular group, 0% of participants had severe pain, 12.5% had slight pain, and 87.5% had no pain; in the subcutaneous group, 0% of participants had severe pain, 66.7% had slight pain, and 33.3% had no pain; P = 0.0004).
Change from baseline of the motor nerve conduction velocity across the elbow
The authors declare that neurophysiological studies were performed before and after treatment, but no data on the change from baseline of the motor conduction velocity across the elbow are available.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
No adverse event was reported.
Surgery: endoscopic versus open decompression
Studied in Schmidt 2014.
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
The authors found no difference between the endoscopic group (n = 29 nerves) and the open decompression group (n = 27 nerves) in improving clinical function measured by Bishop score (early (mean 16 weeks) follow‐up, P = 1.00; long‐term (mean 16.8 months) follow‐up, P = 0.47). In the endoscopic group at early follow‐up, the clinical outcome, measured by the modified Bishop score, was poor in 2 arms, fair in 1, good in 11, and excellent in 15 arms. At long‐term follow‐up, the outcome was poor in 4 arms, fair in 1, good in 2, and excellent in 22 arms. In the open decompression group, at early follow‐up the clinical outcome was poor in 3 arms, fair in 1, good in 10, and excellent in 13 arms. At long‐term follow‐up, the outcome was poor in 5 arms, fair in 0, good in 3, and excellent in 19 arms.
Secondary outcomes
Change in neurological impairment
In Schmidt 2014, the trial authors found no difference between the two procedures in improving pain at the sulcus or in the supplemented area of the nerve both in early follow‐up (P = 0.84) and late follow‐up (P = 0.84). In the endoscopic group, the postoperative value of numeric analogue scale (NAS) was 0.97 in the early follow‐up and 0.64 in the long‐term follow‐up. In the open decompression group, the NAS score was 0.85 in the early follow‐up and 0.79 in the long‐term follow‐up. Two‐point discrimination was assessed, but no data comparing intervention groups were available.
Change from baseline of the motor nerve conduction velocity across the elbow
At long‐term follow‐up, after a mean of 13.8 months, the authors found no difference between the procedures in improving electrophysiological findings (in the endoscopic group 21 cases out of 27 improved, and in the open group 22 out of 26 cases improved; P = 0.62).
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
A significantly higher rate of postoperative haematoma occurred in the endoscopic group (7/29 (24.14%) of arms in the endoscopic group and 1/27 (3.7%) of arms in the open group, P = 0.05). No difference was found in the rate of disturbance of wound healing (3/29 (10.34%) of arms in the endoscopic group and 1/27 (3.7%) of arms in the open group, P = 0.61).
Conservative treatment: information provision versus information provision and nerve gliding exercises or versus information provision and night splinting
Assessed in one study (Svernlov 2009).
Primary outcome: proportion of participants with a clinically relevant improvement in function compared to baseline
In clinically mild or moderate UNE, night splinting plus information on the movements and positions provoking the symptoms (n = 26), nerve gliding exercises plus information (n = 23), and just information (n = 21) determined an improvement of occupational activity at six‐month follow‐up (P = 0.0001, P = 0.0003, and P = 0.039, respectively). Night splinting for three months and nerve gliding exercises did not provide further improvement in occupational activities and nocturnal pain at six months when compared with just information. Nerve gliding exercises and information alone improved satisfaction and diurnal pain (P = 0.0001 for both treatments), while night splinting did not.
Secondary outcomes
Change in neurological impairment
Conservative treatments (night splinting plus information to avoid movements or positions provoking the symptoms, nerve gliding exercises plus information, and information alone) did not improve muscular strength (Svernlov 2009).
Change from baseline of the motor nerve conduction velocity across the elbow
Before treatment, 12 out of 51 participants had impaired nerve conduction velocity over the elbow segment. At six months' follow‐up, 58% of these participants had normal conduction velocity.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
Not measured.
Change in quality of life
Not measured.
Adverse events
No adverse event was reported.
Conservative treatment: corticosteroid injection versus placebo
Studied in vanVeen 2015.
Primary outcome: proportion of participants with a clinically relevant improvement in symptoms
The authors found no difference between the corticosteroid group (n = 30 participants) and the placebo group (n = 25 participants) in improving symptoms at three months' follow‐up (P = 0.871). In the corticosteroid group, 9 out of 30 participants (30%) had a favourable outcome, and 21 participants (70%) had an unfavourable outcome. In the placebo group, 7 out of 25 participants (28%) had a favourable outcome, and 18 participants (72%) had an unfavourable outcome.
Secondary outcomes
Change in neurological impairment
In vanVeen 2015, at baseline and at follow‐up the authors found no difference between the two groups regarding the severity of symptoms and the neurological examination findings. The authors reported data only at the baseline; no data are given at follow‐up. At baseline in the corticosteroid group, 30 participants (100%) had sensory symptoms, and 5 participants (17%) had atrophy; the mean MRC score was 19.7 (range 18 to 20). In the placebo group, 25 participants (100%) had sensory symptoms and 5 participants (20%) had atrophy; the mean MRC score was 19.6 (range 15 to 20).
Change from baseline of the motor nerve conduction velocity across the elbow
The trial authors found no difference between the groups in improving electrophysiological findings at follow‐up. The mean motor nerve conduction velocity across the elbow at follow‐up was 48.3 m/s in the corticosteroid group (n = 26) and 50.3 m/s in the placebo group (n = 23) (these velocities were 45.1 m/s and 46.2 m/s at inclusion). The paper does not report SD, preventing calculation of a MD and 95% CI for the change.
Change from baseline in the nerve diameter at the elbow evaluated by ultrasound or MRI
The nerve cross‐sectional area changed significantly (P = 0.043) in the corticosteroid group (n = 26), decreasing from 11.9 mm2 to 10.9 mm2. In the placebo group (n = 23), the cross‐sectional area was unchanged (13.2 mm2 at baseline and at follow‐up). Without measures of variability, we were unable to calculate a MD and 95% CI.
Change in quality of life
Not measured.
Adverse events
In the corticosteroid group, four participants experienced adverse events: one participant reported swelling at the injection site, one had pain at the injection site, one had a swollen hand, and one had depigmentation at the injection site. In the placebo group, one participant reported pain at the injection site.
Discussion
Summary of main results
Participants who underwent surgical procedures in the included studies had, in the majority of cases, improved symptoms and nerve function, but there were no studies comparing surgical treatment to conservative management to support this in a controlled trial environment.
The available evidence suggests that simple decompression and decompression with transposition are equally effective in the treatment of the clinical and neurophysiological impairment of the ulnar nerve. Transposition is associated with a greater possibility of deep wound infections. In 2005, Bartels and colleagues performed a cost analysis in the Netherlands and found that the total median costs per patient were EUR 1124 for simple decompression and EUR 2730 for anterior subcutaneous transposition. This difference was mainly due to the costs related to sick leave, which was shorter for simple decompression (Bartels 2005b). In 2012, Song and colleagues performed a cost analysis and compared simple decompression, anterior subcutaneous transposition, anterior submuscular transposition, and medial epicondylectomy (Song 2012). They found that simple decompression yielded incremental cost‐effectiveness ratios of less than USD 2027 per quality‐adjusted life‐year, and as a result was the most cost‐effective treatment. Endoscopic and open decompression are equally effective in improving clinical function, but a significantly higher rate of postoperative haematoma occurred with the endoscopic approach.
In clinically mild or moderate UNE, instructions to avoid movements or positions provoking the symptoms were sufficient to improve subjective discomfort, but the quality of the evidence was very poor (some bias, small numbers, electrophysiologically unconfirmed UNE in most cases, dropouts). Corticosteroid injection does not improve symptoms of UNE.
Overall completeness and applicability of evidence
The available evidence is insufficient to identify the best treatment based on clinical, neurophysiological, and imaging characteristics. We did not think there was enough evidence to justify a multiple‐treatment meta‐analysis. Only two RCTs were available on the effectiveness of conservative treatments, and in one of these studies only 24% of participants had neurophysiological evidence of UNE. No RCT compared a surgically treated UNE group and an untreated or conservatively treated group. Currently, the most common practice is to treat patients with mild symptoms and without muscular weakness conservatively, while surgery is reserved for cases that do not show benefit after conservative treatments or with severe neurological symptoms and signs (persistent paraesthesia, objective motor weakness, or muscular atrophy). Our meta‐analysis suggests that simple decompression and decompression with transposition are equally effective in people with severe UNE, but simple decompression is associated with a lower rate of complications (wound infections and scar area sensory loss) than decompression with transposition. Not all studies measured adverse events.
No evidence was available on the effects of surgery on quality of life and imaging characteristics of the ulnar nerve at the elbow.
Quality of the evidence
None of the studies identified and included in our meta‐analysis was at an overall high risk of bias. All were small. All the studies were RCTs, which permitted evaluation of a group of participants with clinical and electrophysiological evidence of ulnar nerve impairment who were sufficiently representative of the UNE population. All degrees of severity of nerve impairment were considered, and the number of participants was high (261 participants for the clinical outcomes and 101 participants for the neurophysiological outcome). The follow‐up rate was very high (only eight participants were lost to follow‐up). The method used to generate the allocation sequence was adequate in three of the four RCTs included in the meta‐analyses.
We observed no significant heterogeneity among studies, which allowed good precision of the estimated intervention effect. Some methodological problems must be highlighted. The four most important methodological weaknesses were:
an unblinded observer in one study (in two studies it was unclear whether the examiner was blinded);
inadequate sequence generation and unclear allocation concealment method in one study;
unclear allocation concealment method in two studies; and
no clear definition of the hypothesis being tested in all the studies.
Moreover, in all seven trials the authors neither specified whether the study was designed to be a non‐inferiority or a superiority trial, nor did they calculate sample size.
Potential biases in the review process
We believe the present review has a low likelihood of language or location bias. Indeed, we searched in different databases without any language limitations. We obtained translations of articles written in languages other than English. We cannot exclude publication bias due to a higher rate of publication among studies with statistically significant effects, or time lag bias due to research findings published after our analysis of the literature.
Agreements and disagreements with other studies or reviews
In a previous review on the surgical treatment of UNE (Zlowodzki 2007), the authors identified the same RCTs that we included in our meta‐analysis up to that point. However, the authors used a different statistical approach. Zlowodzki and colleagues analysed the clinical scores as continuous variables and, because different scoring systems were used in each study, they applied a conversion of effect sizes (standardised mean difference). In accordance with our protocol, we dichotomised the primary outcome measure into improvement or no improvement, regardless of differences between the tools used. Despite the difference in statistical evaluation, Zlowodzki and colleagues concluded that simple decompression and decompression with transposition are equally effective. In their review, two different and not entirely comparable surgical techniques (submuscular and subcutaneous transposition) were considered together in the meta‐analysis. We also performed a meta‐analysis comparing submuscular transposition and simple decompression, and found no difference between the procedures. Macadam and colleagues used a similar approach in their review, but the they also introduced non‐randomised trials into the meta‐analysis, with a higher likelihood of selection bias (Macadam 2008).
Authors' conclusions
Implications for practice.
The available evidence is insufficient to identify the best treatment for idiopathic ulnar neuropathy at the elbow (UNE) on the basis of clinical, neurophysiological, and imaging characteristics. We do not know when to treat a person with UNE conservatively or surgically. However, the results of our meta‐analysis provide moderate‐quality evidence that simple decompression and decompression with transposition are equally effective in idiopathic UNE, including when the nerve impairment is severe, but decompression with transposition may result in more deep and superficial wound infections. In mild cases, evidence from one small randomised controlled trial (RCT) showed that providing information on movements or positions to avoid may reduce subjective discomfort.
Implications for research.
Future research in this area should include RCTs evaluating the effectiveness of conservative treatments. These RCTs should use validated disease‐specific clinical outcome measures, validated patient‐oriented tools, neurophysiological measurements, and neuroimaging. Participants with homogeneous inclusion characteristics need to be included to adequately power the study.
An effort should be made to improve the methodology of RCTs examining surgical treatments. The most important aspects to include in future RCTs are:
the presence of a blinded examiner;
a clear description of the allocation concealment method;
validated disease‐specific clinical outcome measures; and
a definition of the hypothesis the study is testing (is the study designed to be a non‐inferiority or a superiority trial?).
Moreover, there is a need for a trial comparing conservative and surgical treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2016 | New citation required and conclusions have changed | Three trials added. |
| 8 May 2016 | New search has been performed | Searches were updated to 31 May 2016. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 10 April 2012 | New citation required but conclusions have not changed | Searches were updated to 20 February 2012. No new trials found, but one excluded study added. |
| 10 July 2011 | New search has been performed | Reporting of results in the abstract revised. Minor edits throughout. |
Acknowledgements
Ombretta Bugiani for her support in developing the literature research strategies (University of Siena).
Angela Meloni for her support in comparing the results of the different databases.
Michael Biggs and his coauthor for providing the raw data of their RCT.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Ulnar Neuropathies Explode All [REFERENCE] [STANDARD] #2 "ulnar neuropath*" or "ulnar nerve" or "compression syndrome*" [REFERENCE] [STANDARD] #3 #1 or #2 [REFERENCE] [STANDARD] #4 elbow [REFERENCE] [STANDARD] #5 #3 and #4 [REFERENCE] [STANDARD] #6 "cubital tunnel syndrome" [REFERENCE] [STANDARD] #7 #5 or #6 [REFERENCE] [STANDARD] #8 (#5 or #6) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL search strategy
#1 elbow #2 ulnar next neuropath* #3 ulnar next nerve* #4 nerve next compression #5 #2 or #3 or #4 #6 #1 and #5 #7 cubital next tunnel #8 #6 or #7
Appendix 3. MEDLINE (OvidSP) search strategy
Ovid MEDLINE(R) 1946 to May Week 3 2016 Database: Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (417272) 2 controlled clinical trial.pt. (90753) 3 randomized.ab. (354957) 4 placebo.ab. (172532) 5 drug therapy.fs. (1860309) 6 randomly.ab. (254384) 7 trial.ab. (367092) 8 groups.ab. (1586892) 9 or/1‐8 (3789565) 10 exp animals/ not humans.sh. (4247320) 11 9 not 10 (3266063) 12 Ulnar Neuropathies/ or ulnar neuropath$.tw. or Ulnar Nerve Compression Syndromes/ or Ulnar Nerve/ or ulnar nerve$.tw. (9958) 13 Elbow/ or elbow$.tw. (27662) 14 12 and 13 (2081) 15 Cubital Tunnel Syndrome/ or (cubital tunnel adj5 syndrome$).tw. (667) 16 14 or 15 (2452) 17 11 and 16 (265) 18 remove duplicates from 17 (264)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1980 to 2016 Week 22> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (47128) 2 double‐blind procedure.sh. (128476) 3 single‐blind procedure.sh. (22118) 4 randomized controlled trial.sh. (401880) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1252828) 6 trial.ti. (199320) 7 or/1‐6 (1402288) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1483388) 9 animal/ or nonanimal/ or animal experiment/ (3570735) 10 9 not 8 (2954815) 11 7 not 10 (1290837) 12 limit 11 to embase (1064995) 13 Cubital Tunnel Syndrome/ or (Cubital Tunnel adj5 Syndrome).tw. (2023) 14 ulnar neuropath$.tw. or ulnar nerve/ or ulnar nerve.tw. or nerve compression/ (21186) 15 elbow.tw. or elbow/ (33336) 16 14 and 15 (2860) 17 13 or 16 (4114) 18 12 and 17 (138) 19 remove duplicates from 18 (137)
Appendix 5. AMED (OvidSP) search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to May 2016> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Randomized controlled trials/ (1780) 2 Random allocation/ (313) 3 Double blind method/ (585) 4 Single‐Blind Method/ (75) 5 exp Clinical Trials/ (3543) 6 (clin$ adj25 trial$).tw. (6397) 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2664) 8 placebos/ (571) 9 placebo$.tw. (2887) 10 random$.tw. (15869) 11 research design/ (1847) 12 Prospective Studies/ (898) 13 meta analysis/ (175) 14 (meta?analys$ or systematic review$).tw. (2852) 15 control$.tw. (32646) 16 (multicenter or multicentre).tw. (916) 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (11738) 18 or/12‐17 (41971) 19 Cubital Tunnel Syndrome/ or (Cubital Tunnel adj5 Syndrome).tw. (11) 20 ulnar neuropath$.mp. or ulnar nerve/ or ulnar nerve.tw. or nerve compression syndromes/ (381) 21 elbow.tw. or elbow/ (1959) 22 20 and 21 (65) 23 19 or 22 (71) 24 18 and 23 (15) 25 remove duplicates from 24 (15)
Appendix 6. LILACS search strategy
(((MH:C10.668.829.500.850$ or "ulnar neuropathy" or "ulnar neuropathies" or "neuropatias cubitale" or "neuropatias ulnares" or "ulnar nerve" or "nervo ulnar") and (elbow or elbows or codo or cotovelo)) or ("cubital tunnel syndrome" or "sindrome del tunel cubital" or "sindrome do tunel ulnar")) and ((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos)))
Appendix 7. EBSCOhost CINAHL search strategy
Monday, June 06, 2016 11:01:44 AM S30 S28 AND S29 8 S29 EM 20141014‐ 577,335 S28 S18 and S27 74 S27 S25 and S26 312 S26 MH elbow or ti elbow or ab elbow 3,585 S25 S19 or S20 or S21 or S22 or S23 or S24 2,330 S24 nerve compression n5 syndrome* 1,487 S23 (MH "Nerve Compression Syndromes") 1,401 S22 cubital tunnel n5 syndrome* 119 S21 ulnar neuropath* 279 S20 ti (ulnar nerve*) or ab (ulnar nerve*) 298 S19 (MH "Ulnar Nerve") 809 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 836,818 S17 ABAB design* 91 S16 TI random* or AB random* 173,912 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 345,985 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 125,186 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 46,385 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 26,841 S11 PT ("clinical trial" or "systematic review") 132,020 S10 (MH "Factorial Design") 972 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 282,840 S8 (MH "Meta Analysis") 24,634 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 49 S6 (MH "Quasi‐Experimental Studies") 7,850 S5 (MH "Placebos") 9,729 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 33,490 S3 (MH "Clinical Trials+") 198,849 S2 (MH "Crossover Design") 13,769 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 72,718
Appendix 8. PEDro search strategy
Simple search: "cubital tunnel syndrome", "ulnar neuropathy"
Advanced search
Abstract and Title: "cubital tunnel syndrome" OR "ulnar neuropathy elbow"
Appendix 9. ClinicalTrials.gov search strategy
Simple search: "ulnar nerve"
Appendix 10. World Health Organization International Clinical Trials Registry Platform search strategy
Simple search: "ulnar nerve"
Data and analyses
Comparison 1. Clinical and neurophysiological effect of simple decompression versus transposition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with clinical improvement in function compared to baseline | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.08] |
| 1.1 Simple decompression versus subcutaneous transposition | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.14] |
| 1.2 Simple decompression versus submuscular transposition | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 2 Postoperative motor nerve conduction velocity | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [‐0.94, 3.87] |
| 2.1 Simple decompression versus subcutaneous transposition | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐2.34, 6.34] |
| 2.2 Simple decompression versus submuscular transposition | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [‐1.66, 4.12] |
| 3 Proportion of participants with deep/superficial wound infections | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bartels 2005.
| Methods | RCT | |
| Participants | 152 people (age range 20 to 77 years; 94 male, 58 female) with signs and symptoms of idiopathic ulnar nerve entrapment, without benefit after conservative treatment and with electrophysiological evidence of the nerve impairment. Detailed description of exclusion criteria is available. | |
| Interventions | Simple decompression (75 participants), anterior subcutaneous transposition (77 participants) | |
| Outcomes | Clinical outcome:
|
|
| Conflicts of interest | No information provided. | |
| Study funding | Supported by a grant from the National Health Insurance Board and the National Society of University Medical Centers. | |
| Notes | Operations performed between March 1999 and July 2002. Clinical follow‐up at one year after surgery. Single‐centre, the Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment method is described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only a subgroup of 30 participants was evaluated by an independent examiner. |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | Lost to follow‐up: 2 participants in the simple decompression group (1 participant revoked informed consent, the other could not be traced), 3 participants in the anterior subcutaneous transposition group (1 was imprisoned, 2 could not be traced) |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The MPQ‐DLV and SF‐36 scores also improved with time. At any follow‐up interval, there was no statistically significant difference in improvement between groups." Comment: No statistical information is available on the changes after surgery of the MPQ‐DLV and SF‐36 scores. |
| Other bias | High risk | The sample size was not calculated. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. |
Biggs 2006.
| Methods | RCT | |
| Participants | 44 people (age range 27 to 83 years; 33 male, 11 female) with signs and symptoms of idiopathic ulnar nerve entrapment, without benefit after conservative treatment and with electrophysiological evidence of the nerve impairment. Exclusion criteria included:
|
|
| Interventions | Simple decompression (23 participants), submuscular transposition (21 participants) | |
| Outcomes | Clinical outcome:
|
|
| Conflicts of interest | No information provided. | |
| Study funding | No information provided. | |
| Notes | Operations were performed between August 1993 and June 1998 by a single surgeon in 2 hospitals in Australia. Follow‐up at 6 weeks and 6 months after surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment method is described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | Quote: "Three patients were lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | Results from all the outcome measures are reported. |
| Other bias | High risk | The sample size was not calculated. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. |
Gervasio 2005.
| Methods | RCT | |
| Participants | 70 people (age range 32 to 75 years; 48 male, 22 female) with severe cubital tunnel syndrome, grade 3 according to Dellon’s staging system. All the participants had electrophysiological evidence of ulnar impairment. Exclusion criteria:
|
|
| Interventions | Simple decompression (35 participants), anterior submuscular transposition (35 participants) | |
| Outcomes | Clinical outcome: Bishop rating system, which assesses subjective and objective parameters Electrophysiological outcome:
|
|
| Conflicts of interest | "The authors ... have no personal or institutional financial interest in drugs, materials, or devices described in this study." | |
| Study funding | "The authors have received no financial support in conjunction with the compilation of this study" | |
| Notes | Operations were performed between February 1998 and June 2003 at a single centre in Italy. There were 2 surgeons, each of whom performed the operation in 1 of the 2 groups. Follow‐up at 6 months after surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were randomised by use of their reservation numbers in the hospital" |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment method is described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The postoperative outcome, both clinical and electroneuromyographic, was evaluated in all the patients by the same blinded evaluator" |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | No participant lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐ and postoperative evaluations were performed using 2 different staging systems: preoperatively the authors used the Dellon scale, postoperatively they used the Bishop rating system. |
| Other bias | High risk | The sample size was not calculated. Only people with severe disease were included in the study. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. |
Geutjens 1996.
| Methods | RCT | |
| Participants | 52 people (age range 36 to 85 years) with clinical and neurophysiological evidence of ulnar nerve impairment at the elbow. Exclusion criteria:
|
|
| Interventions | Medial epicondylectomy (25 cases), anterior transposition (22 cases) | |
| Outcomes | Clinical outcomes:
Electrophysiological outcome (in all the participants in both groups): motor nerve conduction velocity Patient satisfaction assessed by a non‐validated tool. |
|
| Conflicts of interest | Report states: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" | |
| Study funding | No information provided. | |
| Notes | Operations performed between 1985 and 1992. Appears to be a single‐centre study, UK. 2 surgeons both performed each procedure. Follow‐up at 12 months after surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation is not clearly described. |
| Allocation concealment (selection bias) | Low risk | The participants "were randomly allocated to one of the two operations by the use of sealed envelopes which were only opened in the operating theatre just before the procedure". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neurological assessments were blinded as regards the operation and made without exposing the elbow" |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | Quote: "52 patients had operations for ulnar neuropathy and were entered into the study. Nine were lost to follow‐up of whom two had died, records were untraceable in four, and three had moved away and could not be contacted". 43 participants were evaluated at the follow‐up, of whom 4 had bilateral operations. |
| Selective reporting (reporting bias) | Low risk | Results from all the outcome measures are reported. |
| Other bias | High risk | The outcome measures used to evaluate clinical improvement in terms of sensation and motor strength may have a low sensitivity. The sample size was not calculated. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. |
Nabhan 2005.
| Methods | RCT | |
| Participants | 66 people (mean age 52 years, SD 12; 38 males, 28 females) with clinical and electrophysiological evidence of ulnar nerve neuropathy Exclusion criteria: previous traumas to the elbow causing deformity or distortion of the cubital tunnel, as well as recurrent cubital tunnel syndrome after previous surgery |
|
| Interventions | Simple decompression (32 participants), decompression and anterior subcutaneous transposition (34 participants) | |
| Outcomes | Clinical outcomes:
Electrophysiological outcome: motor nerve conduction velocity |
|
| Conflicts of interest | No information provided. | |
| Study funding | No information provided. | |
| Notes | Operations performed between August 2001 and October 2003. Single centre in Germany. Follow‐up examinations at 3 and 9 months after surgery. The sample size was not calculated. Only people with severe disease were included in the study. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out by drawing cards in sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was carried out by drawing cards in sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All patients underwent nerve conduction velocity studies pre‐operatively by two blinded neurophysiologists according to a standard protocol" Comment: It is not specified if the postoperative evaluation was performed by blinded examiners. |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | No participant lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results from all the outcome measures are reported. |
| Other bias | High risk | The outcome measures used to evaluate clinical improvement in terms of sensation and motor strength may have a low sensitivity. The sample size was not calculated. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. |
Schmidt 2014.
| Methods | RCT | |
| Participants | 54 people (mean age 49.3 years, range 20 to 74; 32 males, 22 females) with 56 clinical, electrophysiological, and ultrasonographic confirmed cases of ulnar nerve neuropathy
Exclusion criteria: contraindications for general anaesthesia, post‐traumatic cubital tunnel syndrome with bony deformity of the elbow, previous surgery of the affected ulnar nerve, severe nerve luxation Investigators appear to have randomised arms rather than participants. |
|
| Interventions | Endoscopic decompression (29 arms), open decompression (27 arms) | |
| Outcomes | Clinical outcomes:
Electrophysiological outcome: motor nerve conduction velocity |
|
| Conflicts of interest | Report states: "The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article." | |
| Study funding | No information provided. | |
| Notes | Operations were performed between October 2008 and April 2011. Single centre in Germany. Early follow‐up at mean of 16 weeks and long‐term follow‐up at mean of 16.8 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization process was performed as a simple randomization without restrictions by drawing a trial envelope by the surgeon directly before each surgery started" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization process was performed as a simple randomization without restrictions by drawing a trial envelope by the surgeon directly before each surgery started" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded examiners performed the postoperative evaluation and statistical analysis. |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | 3 participants were free of symptoms at follow‐up and declined further electrophysiological examination. |
| Selective reporting (reporting bias) | Low risk | Results from all the outcome measures are reported. |
| Other bias | Low risk | The sample size was not calculated. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. It is unclear whether any adjustment was made for the 2 bilateral cases (3.7%), but unlikely to have an important effect. |
Svernlov 2009.
| Methods | RCT | |
| Participants | 70 people (age range 17 to 72 years; 31 male, 39 female) with clinical mild or moderate ulnar neuropathy at the elbow classified with the Dellon’s staging system. 12 participants only had abnormal findings at the electrophysiological evaluation. Exclusion criteria: "Patients with past or present symptoms of neck problems, clinical signs of another nerve problem, previous trauma or surgery to the same arm, arthritis or palpable swelling at the elbow or subluxations of the ulnar nerve" | |
| Interventions |
|
|
| Outcomes | Clinical outcome:
Electrophysiological outcome (preoperatively performed in all participants, postoperatively in 45 participants): ulnar motor and sensory nerve conduction studies, electromyography Participants assessed their symptoms according to the Canadian Occupational Performance Measure (COPM). |
|
| Conflicts of interest | No information provided. | |
| Study funding | No information provided. | |
| Notes | Performed between March 1997 and December 2000. Follow‐up at 6 months after surgery. 2 centres, Denmark and Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation is not clearly described. |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were then randomised using sequentially numbered, sealed envelopes into three groups for different treatments" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Another, independent, occupational therapist at each centre evaluated the patients before and 6 months after starting the study" |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | Quote: "Thirteen patients ... in the primarily randomized patients completed the programme but were unavailable for the follow‐up at 6 months. Six did not attend because of practical or personal reasons and seven had developed other symptoms (such as carpal tunnel syndrome, epicondylalgia, impingement syndrome or neck pain). Another six patients, two from each group, underwent surgical decompression because of persistent symptoms" |
| Selective reporting (reporting bias) | Low risk | Results from all the outcome measures are reported. |
| Other bias | Unclear risk | The sample size was not calculated. The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. Only 24% of participants had neurophysiological evidence of UNE. |
vanVeen 2015.
| Methods | RCT | |
| Participants | 63 people were randomised, but 55 people were statistically analysed (in the intervention group mean age 56 years, range 29 to 91; in the placebo group mean age 53 years, range 24 to 76). All the participants had clinical and neurophysiological or ultrasonographic evidence of ulnar nerve neuropathy. Exclusion criteria: recurrence of UNE, age < 18 years, oral prednisolone or anticoagulant drugs, known allergy to prednisolone, history of subluxation of the ulnar nerve at the elbow |
|
| Interventions | Ultrasound‐guided injection of 1 mL containing 40 mg methylprednisolone acetate and 10 mg lidocaine hydrochloride (30 participants), or a placebo injection with 1 mL of sodium chloride 0.9% (25 participants) | |
| Outcomes | Clinical outcome:
Electrophysiological outcome (preoperatively performed in all participants, postoperatively in 48 participants): ulnar motor nerve conduction velocity Ultrasonographic outcome: cross‐sectional area of ulnar nerve in a segment of 4 cm across the medial epicondyle |
|
| Conflicts of interest | No information provided. | |
| Study funding | No information provided. | |
| Notes | Participants enrolled between September 2009 and April 2014 at the neurology outpatient clinic of the Medical Centre Haaglanden, Netherlands. Follow‐up at 3 months after treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment method is described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. "The physician who collected and entered the data was blinded". Treatment allocation was known during the analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was performed by a blinded physician. |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | Among the 34 participants allocated to the corticosteroid group, 1 participant was not treated because of withdrawal of consent before injection. 3 participants were lost at follow‐up and were not analysed for the primary outcome; a total of 7 participants did not undergo ultrasonography, and 7 did not undergo electrophysiological tests. Among the 29 participants allocated to the placebo group, 2 participants did not receive placebo, 1 participant with symptoms recovered before injection, and 1 participant had previously had surgery. 2 participants were lost at follow‐up and not evaluated for the primary outcome; a total of 4 participants did not undergo ultrasonography, and 5 did not undergo electrophysiological tests. |
| Selective reporting (reporting bias) | Low risk | Results from all outcome measures are reported. |
| Other bias | Unclear risk | The authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. |
Zarezadeh 2012.
| Methods | RCT | |
| Participants | 48 participants were enrolled in the study (age range 25 to 60 years; 27 male, 21 female) with clinical and neurophysiological evidence of ulnar nerve impairment at the elbow. The trial authors declare: "according to clinical and paraclinical tests, severe and moderate patients and mild group that did not respond to conservative treatment were operated"; however, they do not indicate how the severity was defined. Exclusion criteria included deformity or distortion of the cubital tunnel due to previous trauma to the elbow and recurrent cubital tunnel syndrome after previous surgery. | |
| Interventions | Anterior subcutaneous transposition (24 participants), anterior submuscular transposition (24 participants) | |
| Outcomes | Clinical outcomes:
|
|
| Conflicts of interest | None declared. | |
| Study funding | None | |
| Notes | Operations performed between October 2008 and March 2009. Single centre, Iran. Electrophysiological evaluation was performed before and 12 months after surgery, but no data are available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "based on a random table numbers, generated by the random allocation software, the principal investigator allocated patients into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "based on a random table numbers, generated by the random allocation software, the principal investigator allocated patients into two groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "To prevent bias, all patients underwent double‐blind nerve conduction velocity studies, conducted by two neurophysiologists according to a standard protocol, before and after surgery". Comment: Despite this quote, the authors do not unequivocally state that the participants were blinded to the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "To prevent bias, all patients underwent double‐blind nerve conduction velocity studies". Comment: It is not specified if the clinical outcomes were assessed by a blinded examiner. |
| Incomplete outcome data (attrition bias) Clinical or neurophysiological outcome | Low risk | No participant was lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | No neurophysiological data are available. |
| Other bias | High risk | The sample size was not calculated. The trial authors do not specify if the study was designed to be a non‐inferiority or a superiority trial. |
MRC: Medical Research Council RCT: randomised controlled trial SD: standard deviation UNE: ulnar neuropathy at the elbow VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2006 | The authors do not compare therapeutic approaches. |
| Zhong 2011 | The preoperative values of cross‐sectional area and of neurophysiological parameters are identical for the 2 compared groups (even the same decimal values). Since this is statistically improbable, we have serious concerns about the methodological quality of the work. |
Differences between protocol and review
We used updated 'Risk of bias' methodology as described in the Cochrane Handbook for Systematic Reviews of Interventions and included 'Summary of findings' tables (Higgins 2011).
We collected the information on concomitant treatments and the additional information listed under 'Other data' in the Methods.
Data did not allow authors to perform subgroup analyses.
Contributions of authors
Pietro Caliandro: protocol development, searching for trials, quality assessment of trials, data extraction, data analyses, development of final review.
Giuseppe La Torre: protocol development (statistical analysis), searching for trials, quality assessment of trials, data extraction, data analyses.
Roberto Padua: protocol development, searching for trials, quality assessment of trials, data extraction, data analyses, development of final review.
Fabio Giannini: protocol development, searching for trials, quality assessment of trials, data extraction, data analyses, development of final review.
Luca Padua: protocol development, searching for trials, quality assessment of trials, data extraction, data input, data analyses, development of final review; corresponding author.
Sources of support
Internal sources
Don Gnocci Foundation, via Maresciallo Caviglia n.30, Rome 00194, Italy.
External sources
No sources of support supplied
Declarations of interest
PC: none known
GLT: none known
RP: none known
FG: none known
LP: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bartels 2005 {published data only}
- Bartels RH, Verhagen WI, Wilt GJ, Meulstee J, Rossum LG, Grotenhuis JA. Prospective randomized controlled study comparing simple decompression versus anterior subcutaneous transposition for idiopathic neuropathy of the ulnar nerve at the elbow: Part 1. Neurosurgery 2005;56(3):522‐30. [PUBMED: 15730578] [DOI] [PubMed] [Google Scholar]
Biggs 2006 {published data only}
- Biggs M, Curtis JA. Randomized, prospective study comparing ulnar neurolysis in situ with submuscular transposition. Neurosurgery 2006;58(2):296‐304. [PUBMED: 16462483] [DOI] [PubMed] [Google Scholar]
Gervasio 2005 {published data only}
- Gervasio O, Gambardella G, Zaccone C, Branca D. Simple decompression versus anterior submuscular transposition of the ulnar nerve in severe cubital tunnel syndrome: a prospective randomized study. [Erratum appears in Neurosurgery. 2005 Feb;56(2):409]. Neurosurgery 2005;56(1):108‐17. [PUBMED: 15617592] [DOI] [PubMed] [Google Scholar]
Geutjens 1996 {published data only}
- Geutjens GG, Langstaff RJ, Smith NJ, Jefferson D, Howell CJ, Barton NJ. Medial epicondylectomy or ulnar‐nerve transposition for ulnar neuropathy at the elbow?. Journal of Bone and Joint Surgery. British Volume 1996;78(5):777‐9. [PUBMED: 8836069] [PubMed] [Google Scholar]
Nabhan 2005 {published data only}
- Nabhan A, Ahlhelm F, Kelm J, Reith W, Schwerdtfeger K, Steudel WI. Simple decompression or subcutaneous anterior transposition of the ulnar nerve for cubital tunnel syndrome. Journal of Hand Surgery. British Volume 2005;30(5):521‐4. [PUBMED: 16061314] [DOI] [PubMed] [Google Scholar]
Schmidt 2014 {published data only}
- Schmidt S, Kleist Welch‐Guerra W, Matthes M, Baldauf J, Schminke U, Schroeder HW. Endoscopic vs open decompression of the ulnar nerve in cubital tunnel syndrome: a prospective randomized double‐blind study. Neurosurgery 2015;77(6):960‐70. [PUBMED: 26595347] [DOI] [PubMed] [Google Scholar]
Svernlov 2009 {published data only}
- Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. Journal of Hand Surgery. European Volume 2009;34(2):201–7. [PUBMED: 19282413] [DOI] [PubMed] [Google Scholar]
vanVeen 2015 {published data only}
- vanVeen KE, Alblas KC, Alons IM, Kerklaan JP, Siegersma MC, Wesstein M, et al. Corticosteroid injection in patients with ulnar neuropathy at the elbow: a randomized, double‐blind, placebo‐controlled trial. Muscle & Nerve 2015;52(3):380‐5. [PUBMED: 25522919] [DOI] [PubMed] [Google Scholar]
Zarezadeh 2012 {published data only}
- Zarezadeh A, Shemshaki H, Nourbakhsh M, Etemadifar MR, Moeini M, Mazoochian F. Comparison of anterior subcutaneous and submuscular transposition of ulnar nerve in treatment of cubital tunnel syndrome: a prospective randomized trial. Journal of Research in Medical Sciences 2012;17(8):745‐9. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2006 {published data only}
- Chen Y, Hong G, Wang F. Therapeutic effect evaluation of ulnar neurolysis and nerve anterior transposition with an immediate range of motion in the aged. Zhongguo Xiu Fu Chong Jian Ke Za Zhi 2006;20(8):809‐11. [PubMed] [Google Scholar]
Zhong 2011 {published data only}
- Zhong W, Zhang W, Zheng X, Li S, Shi J. Comparative study of different surgical transposition methods for ulnar nerve entrapment at the elbow. Journal of International Medical Research 2011;39(5):1766‐72. [DOI] [PubMed] [Google Scholar]
Additional references
AAEM 1999
- Practice parameter for electrodiagnostic studies in ulnar neuropathy at the elbow: summary statement. American Association of Electrodiagnostic Medicine, American Academy of Neurology, American Academy of Physical Medicine and Rehabilitation. Muscle & Nerve 1999;22(3):408‐11. [PUBMED: 10086904] [DOI] [PubMed] [Google Scholar]
Bartels 2005a
- Bartels RH, Verhagen WI, Wilt GJ, Meulstee J, Rossum LG, Grotenhuis JA. Prospective randomized controlled study comparing simple decompression versus anterior subcutaneous transposition for idiopathic neuropathy of the ulnar nerve at the elbow: Part 1. Neurosurgery 2005;56(3):522‐30. [DOI] [PubMed] [Google Scholar]
Bartels 2005b
- Bartels RH, Termeer EH, Wilt GJ, Rossum LG, Meulstee J, Verhagen WI, et al. Simple decompression or anterior subcutaneous transportation for ulnar neuropathy at the elbow: a cost‐minimization analysis: Part 2. Neurosurgery 2005;56(3):531‐6. [DOI] [PubMed] [Google Scholar]
Beekman 2004
- Beekman R, Schoemaker MC, Plas JP, Berg LH, Franssen H, Wokke JH, et al. Diagnostic value of high‐resolution sonography in ulnar neuropathy at the elbow. Neurology 2004;62(5):767‐73. [DOI] [PubMed] [Google Scholar]
Beekman 2009
- Beekman R, Schreuder AH, Rozeman CA, Koehler PJ, Uitdehaag BM. The diagnostic value of provocative clinical tests in ulnar neuropathy at the elbow is marginal. Journal of Neurology, Neurosurgery and Psychiatry 2009;80(12):1369‐74. [DOI] [PubMed] [Google Scholar]
Bell‐Krotoski 1987
- Bell‐Krotoski J, Tomancik E. The repeatability of testing with Semmes‐Weinstein monofilaments. Journal of Hand Surgery. American Volume 1987;12(1):155‐61. [DOI] [PubMed] [Google Scholar]
BMRC 1976
- Medical Research Council (Great Britain). Aids to examination of the peripheral nervous system. Medical Research Council Memorandum No. 45. London: Her Majesty’s Stationery Office, 1976. [Google Scholar]
Bordalo 2004
- Bordalo‐Rodrigues M, Rosenberg ZS. MR imaging of entrapment neuropathies at the elbow. Magnetic Resonance Imaging Clinics of North America 2004;12(2):247‐63, vi. [DOI] [PubMed] [Google Scholar]
Dellon 1989
- Dellon AL. Review of treatment results for ulnar nerve entrapment at the elbow. Journal of Hand Surgery. American Volume 1989;14(4):688‐700. [DOI] [PubMed] [Google Scholar]
Dellon 1993
- Dellon AL, Hament W, Gittelshon A. Nonoperative management of cubital tunnel syndrome: an 8‐year prospective study. Neurology 1993;43(9):1673‐7. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Eaton 1980
- Eaton RG, Crowe JF, Parkes JC 3rd. Anterior transposition of the ulnar nerve using a non‐compressing fasciodermal sling. Journal of Bone and Joint Surgery. American Volume 1980;62(5):820‐5. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hudak 1996
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder, and hand). The Upper Extremity Collaborative Group (UECG). American Journal of Industrial Medicine 1996;29(6):602‐8. [DOI] [PubMed] [Google Scholar]
Kleinman 1989
- Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. Journal of Hand Surgery. American Volume 1989;14(6):972‐9. [DOI] [PubMed] [Google Scholar]
Kuschner 1996
- Kuschner SH. Cubital tunnel syndrome: treatment by medial epicondylectomy. Hand Clinics 1996;12(2):411‐9. [PubMed] [Google Scholar]
Macadam 2008
- Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta‐analysis. Journal of Hand Surgery. American Volume 2008;33(8):1314.e1‐12. [DOI] [PubMed] [Google Scholar]
Mondelli 2005
- Mondelli M, Giannini F, Ballerini M, Gianneschi F, Martorelli E. Incidence of ulnar neuropathy at the elbow in the province of Siena (Italy). Journal of the Neurological Sciences 2005;234(1‐2):5‐10. [DOI] [PubMed] [Google Scholar]
Mondelli 2006
- Mondelli M, Padua L, Giannini F, Bibbò G, Aprile I, Rossi S. A self‐administered questionnaire of ulnar neuropathy at the elbow. Neurological Sciences 2006;27(6):402‐11. [DOI] [PubMed] [Google Scholar]
Padua 2001
- Padua L, Aprile I, Mazza O, Padua R, Pietracci E, Caliandro P, et al. Neurophysiological classification of ulnar entrapment across the elbow. Neurological Sciences 2001;22(1):11‐6. [DOI] [PubMed] [Google Scholar]
Palmer 2010
- Palmer AB, Hughes TB. Cubital tunnel syndrome. Journal of Hand Surgery. American Volume 2010;35(1):153‐63. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2005
- Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. Journal of Manipulative and Physiological Therapeutics 2005;28(5):345. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Song 2012
- Song JW, Chung KC, Prosser LA. Treatment of ulnar neuropathy at the elbow: cost‐utility analysis. Journal of Hand Surgery. American Volume 2012;37(8):1617‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sriwatanakul 1983
- Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clinical Pharmacology and Therapeutics 1983;34(2):234‐9. [DOI] [PubMed] [Google Scholar]
Tsai 1999
- Tsai TM, Chen IC, Majd ME, Lim BH. Cubital tunnel release with endoscopic assistance: results of a new technique. Journal of Hand Surgery. American Volume 1999;24(1):21‐9. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Zlowodzki 2007
- Zlowodzki M, Chan S, Bhandari M, Kalliainen L, Schubert W. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. A meta‐analysis of randomized, controlled trials. Journal of Bone and Joint Surgery. American Volume 2007;89(12):2591‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Caliandro 2011
- Caliandro P, Torre G, Padua R, Giannini F, Padua L. Treatment for ulnar neuropathy at the elbow. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD006839.pub2] [DOI] [PubMed] [Google Scholar]
Caliandro 2012
- Caliandro P, Torre G, Padua R, Giannini F, Padua L. Treatment for ulnar neuropathy at the elbow. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD006839.pub3] [DOI] [PubMed] [Google Scholar]


