Highlights
-
•
Phenolic acids are key class of dietary polyphenols, natural antioxidants.
-
•
They exhibit a variety of functions including plant growth, development, and defense.
-
•
They are precursors of other significant bioactive molecules regularly used for therapeutic, cosmetics, and food industries.
-
•
These dietary antioxidants shields against growth and evolution in pathological conditions arise from oxidative stress.
Keywords: Phytochemical, Phenolic acids, Natural medicine, Antioxidant, Biomedical applications
Abstract
Plant phenolics are considered to be a vital human dietary component and exhibit a tremendous antioxidant activity as well as other health benefits. Epidemiology evidence indicates that a diet rich in antioxidant fruits and vegetables significantly reduces the risk of many oxidative stress related diseases viz. cancers, diabetes and cardiovascular. The number and position of hydroxyl group in a particular phenolic compound leads to the variation in their antioxidant potential. Polyphenols are the main source of dietary antioxidants, and are effortlessly absorbed in the intestine. Phenolic acids, a sub class of plant phenolics, possess phenol moiety and resonance stabilized structure which causes the H-atom donation results in antioxidant property through radical scavenging mechanism. Other mode such as radical quenching via electron donation and singlet oxygen quenching are also known for the antioxidant activity of phenolic acids. Furthermore, phenolic acids are found ubiquitously and well documented for other health protective effects like antimicrobial, anticancer, anti-inflammatory, anti-mutagenic etc. The contribution emphasize on the phenolic acids potential in drug discovery. In addition their occurrence, biosynthesis, metabolism and health effects are discussed in detail.
1. Introduction
Human beings are continuously using natural substances for their basic needs such as food, clothes, shelter, medicine and other industrial purposes since the ancient time. It is documented that peoples from all ethnicity used either whole plant or its specific part for medical purpose with an earliest record found for the use of around 1000 plant-derived substances in Mesopotamia in 2600 BC. Similarly, Indian Ayurveda system which is around 5,000 years old also has the documentation for natural drug molecules. Chinese literature is also filled by the use of herbal medicine 2500 years before [1,2]. From past two decades, novel natural product offers prospects for innovation in development and discovery of drug(s) and plays an important role in pharmaceutical and other industries [3,4]. Epidemiological evidence indicates that a diet rich in plant derived foods significantly reduces the risk of many types of cancers and cardiovascular diseases, suggesting that certain dietary antioxidants could be effective agents for the prevention of cancer incidence and mortality [5]. Polyphenols, found in these remedies, are considered to be the chief agents responsible for the biological functions and disease cure [6]. Plant phenolics including simple phenols, phenolic acids, flavonoids, coumarins, stilbenes, hydrolyzable and condensed tannins, lignans, and lignins are the most abundant secondary metabolites, produced mainly through the shikimate pathway from L-phenylalanine and L-tyrosine, and containing one or more hydroxyl groups attached directly to aromatic ring [7,8]. Secondary metabolites originates from primary metabolites (carbohydrates, amino acids, and lipids) principally for protection against UV radiation, competitive warfare against viruses, bacteria, insects and other plants, as well as responsible for smell, color and flavor in plant products [9]. Plant phenolics are similar in many ways to alcohols with aliphatic structure but the presence of aromatic ring, hydrogen atom of phenolic hydroxyl group makes them as weak acids. They are well known to exhibits a variety of functions including plant growth, development, and defense and also have beneficial effects on mankind. They are acknowledged as strong natural antioxidants having key role in wide range of biological and pharmacological properties such as anti-inflammatory, anticancer, antimicrobial, antiallergic, antiviral, antithrombotic, hepatoprotective, food additive, signaling molecules and many more [4,[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]].
The term “phenolic acids” generally describes the phenolic compounds having one carboxylic acid group. Phenolic or phenolcarboxylic acids (a type of phytochemical called a polyphenol) are one of the main classes of plant phenolic compounds. They are found in the variety of plant-based foods viz. seeds, skins of fruits and leaves of vegetables contain them in highest concentrations. Typically, they are present in bound from such as amides, esters, or glycosides and rarely in free form [23]. Phenolic acids are mainly divided in to two sub-groups: hydroxybenzoic and hydroxycinnamic acid [24]. Phenolic acids possess much higher in vitro antioxidant activity than well known antioxidant vitamins [25]. Hydroxycinnamic acids, derived from cinnamic acid, present in foods often as simple esters with quinic acid or glucose. The most abundant soluble bound hydroxycinnamic acid present is chlorogenic acid (a combined from form of caffeic and quinic acids). The four most common hydroxycinnamic acids are ferulic, caffeic, p-coumaric, and sinapic acids. On the other hand, hydroxybenzoic acids possess a common structure of C6-C1 and derived from benzoic acid. They are found in soluble form (conjugated with sugars or organic acids) and bound with cell wall fractions as lignin [26,27]. As compared to hydroxycinnamic acids, hydroxybenzoic acids are generally found in low concentration in red fruits, onions and black radish etc. [28]. The four commonly found hydroxybenzoic acids are p-hydroxybenzoic, protocatechuic, vanillic, and syringic acids. This review summarizes the classifications, extraction methods, mode of metabolism, bioavailability and usages of plant phenolic acids in biomedical, pharmaceutical, food, cosmetic and other industries.
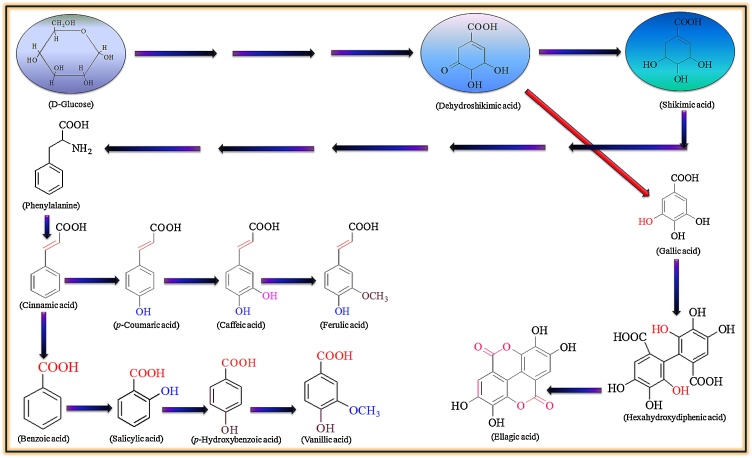
2. Biosynthesis of phenolic acids
The plants produce enormously miscellaneous series of low molecular weight compounds, known as ‘natural products’ or secondary metabolites that confer the metabolic flexibility necessary for biotic and abiotic responses [16] and usually derived from the phenylpropanoid, isopropanoid, alkaloid and fatty acid pathways [29]. Phenolic acids are a diverse class of plant polyphenols and most studied too, and are produced through shikimic acid by phenylpropanoid pathway, during monolignol pathway as by-products, by the breakdown of cell wall polymers such as lignin and some are produced by microbes also [[30], [31], [32]]. The shikimate or shikimic acid pathway is concerned with the production most of the phenolics in bacteria, fungi and plants by converting the simple carbohydrate molecules (resulting from pentose phosphate pathway and glycolysis) into phenylalanine and tryptophan. The pictorial representation of phenolic acids biosynthesis is given as Fig. 1 which is modified from literature [16]. Larger carbohydrate molecules degraded into glucose which converted to dehydroshikimic and shikimic acids in multistep metabolic process. Dehydroshikimic acid transformed into gallic acid, a basic hydroxybenzoic acid whereas shikimic acid into phenylalanine. Through the action of phenylalanine ammonia lyase (PAL) converts the phenylalanine into trans-cinnamic acid and release of ammonia which serves as the key point for the synthesis of phenolic acids as well as in primary and secondary metabolism while some plants and grasses convert 4-hydroxycinnamic with the help of tyrosine ammonia lyase (TAL). The PAL gene expression and its protein activity by metabolite channeling, feedback regulation, and post transcriptional changes greatly affects the production of phenolics [33]. The absence of PAL and chalcone synthase in bacteria results in lack of phenylpropanoids and flavonoids while the some marine bacterium such as Streptomyces maritimes, Streptomyces verticillatus and Sorangium cellulosum are known to possess the PAL [34,35]. In 2007 Moffitt and coworkers discovered a PAL protein in cyanobacteria having similar tertiary and quaternary structure as yeast and plant PALs and associate with the secondary metabolite biosynthetic gene clusters. The prokaryotic PAL has been suggested as an alternate for phenylketonuria patients in enzyme substitution therapy [36]. Recently the information about enzymes used in phenylpropanoid pathway has enhanced which can be utilized in metabolite/ enzyme engineering [37]. Plant cell wall contains the integrated esterified phenolic acids which gets release upon acid/alkali hydrolysis [38]. Cinnamic acid is converted into the p-coumaric acid upon addition of a hydroxyl group at pera position by the action of monooxygenase (use cytochrome P450 as oxygen binding site). The p-coumaric acid undergoes for hydroxylation and oxymethylation and produces caffeic, and ferulic acids, respectively. These phenolic acids contain C6-C3 structure and used as ancestor in the synthesis of lignins and other phenolics. By losing two carbon-atoms cinnamic acid transformed into benzoic acid and its derivatives. For the large scale production of phenolic acids, biotechnological approaches have been used. The cell and tissue cultures models are used to reveal the regulation of different biosynthetic pathways for many secondary metabolites of chemical-pharmaceutical interest, as well as to enhance their production [39]. Plant tissue culture in flask and bioreactors has the advantage over traditional approach (growing the whole plant in natural environment or agricultural farms) as it provides superior control in terms of limitations of natural factors like seasonal variation and geographical locations [16,[40], [41], [42]]. The biotechnological production of phenolic acids has offered a promising way for their mass production [43,44].
Fig. 1.
Schematic representation of phenolic acids biosynthesis through phenylpropanoid pathway.
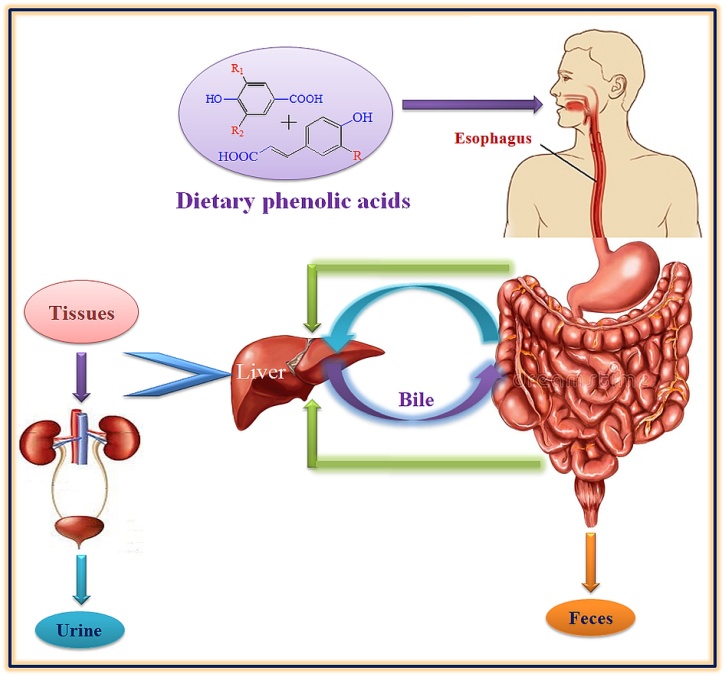
3. Metabolism of phenolic acids
The association of parent phenolic acids and their metabolites has been confirmed by in vitro and in vivo studies of intestinal, hepatic and microbial metabolism. In addition to dietary sources, phenolic acids can also be produced through the metabolism of other kind of polyphenols by microflora in colon [45]. Dietary phenolic acids, occurs ubiquitously in plants, play a major role protective role in oxidative stress conditions. They are (especially caffeic and ferulic acids) metabolized extensively in human after absorption from GI tract followed by methylation, glucuronidation and sulfation derivatives resulting in the change of their structures and exert biological effects [[46], [47], [48], [49]]. Sulfation and glucuronidation occurs at reducing hydroxyl group of phenolic acids so decreases their antioxidant activity. There are too much data available for biological activities of phenolic acids but very less is reported for their metabolites except for the metabolites of caffeic and ferulic acids [[49], [50], [51]]. The metabolic fates of chlorogenic (combination of caffeic and quinic acids), ferulic, caffeic and sinapic acids have been explored well and these are metabolized mainly by colonic microflora [52,53]. The excretion of ferulic acid in free or bound form has been reported as about 11–25% of total ingested amount [54]. The general metabolic routes of dietary phenolic acids have been summarized in Fig. 2. The excretory products from phenolic acids metabolism are removed through the excretory system primarily in feces and urine.
Fig. 2.
Representation of general metabolic pathway for phenolic acids in human beings.
4. Bioavailability of phenolic acids
The intensity of biological activities shown by phenolic acids primarily depends on their bioavailability which accounts for the proportion of their absorption, digestion, and metabolism after entering in the circulation system. There are numerous epidemiological and experimental evidences present describing the protective role of phenolic acids in degenerative diseases such as cardiovascular, cancer, diabetes, inflammation and many more [4,6,[55], [56], [57]]. Their health effects recognized particularly due to their strong antioxidant nature. Though, for the better understanding related to their health effects, we have to advance our database for the bioavailability of phenolic acids as they received less attention than flavonoids. Bioavailability of various phenolic acids has been summarized in Table 1 which is modified from literature [58]. In hydroxycinnamic acids, the majority of experiments have been carried out for caffeic, ferulic, and chlorogenic acids. Ingestion of free from of hydroxycinnamic acids results in their rapid absorption from stomach and small intestine followed by the conjugation through detoxification enzymes [58]. As the dietary concentration of hydroxybenzoic acids is much lower than hydroxycinnamic acids, hence we have limited data for their bioavailability studies [59]. Gallic acid is the most abundantly studied hydroxybenzoic acid for absorption and metabolism and confirm its great absorption ability.
Table 1.
Selected bioavailability of various phenolic acids in their purified administered form.
| Phenolic acid | Dose | Intake (duration) | Metabolites and aglycone |
Reference | ||
|---|---|---|---|---|---|---|
| Tsample (h) {after last dose} | Cdetermined (μM) | 24 h urinary Excretion (dose %) | ||||
| Ferulic acid | 1.94 mg/day | 21 days | 12 | ND | 42.5 | |
| 9.7 mg/day | 21 days | 12 | 1.1 | 51.8 | [60] | |
| 48.55 mg/day | 21 days | 12 | 7.6 | 38.6 | ||
| Caffeic acid | 100 μmol/kg | 1(GI) | 0.33 | 30.39 | -- | [61] |
| 10 mg/kg | 1(GI) | 0.5 | 6.0 | -- | [62] | |
| 45 mg/day | 8 days | 12 | 108.7 | 40.9 | [63] | |
| 110 mg/day | 1(GI) | 0.5 | ≈550 | 13.7 | [64] | |
| 125 mg/kg | 1(GI) | 2.0 | 60 | -- | [53] | |
| 500 mg | ||||||
| p-Coumaric acid | 100 μmol/kg | 1(GI) | 0.16 | 139 | -- | [65] |
| Gallic acid | 100 μmol/kg | 1(GI) | 1 | 3.51 | -- | [65] |
| Chlorogenic acid | 50 mg/kg | 1(GI) | -- | -- | -- | [66] |
| 248 mg/kg | 1(GI) | 0.5-1.0 | ≈8 | -- | [53] | |
| 88.6 mg/day | 8 days | 12 | 113.4 | 58.8 | [63] | |
| Rosmarinic acid | 36.3 mg/kg | 1(GI) | 0.5 | 0.96 | -- | [61] |
| 50 mg/kg | 1(GI) | 0.5 | 4.63 | 5.47 | [67] | |
| 200 mg/kg | 1(GI) | -- | -- | 31.8 | [68] | |
Bold values correspond to Tmax and Cmax; (GI) = gastric intubation.
Red coloured values correspond to concentration of aglycone+conjugated metabolites+microbial metabolites.
5. Extraction and quantification
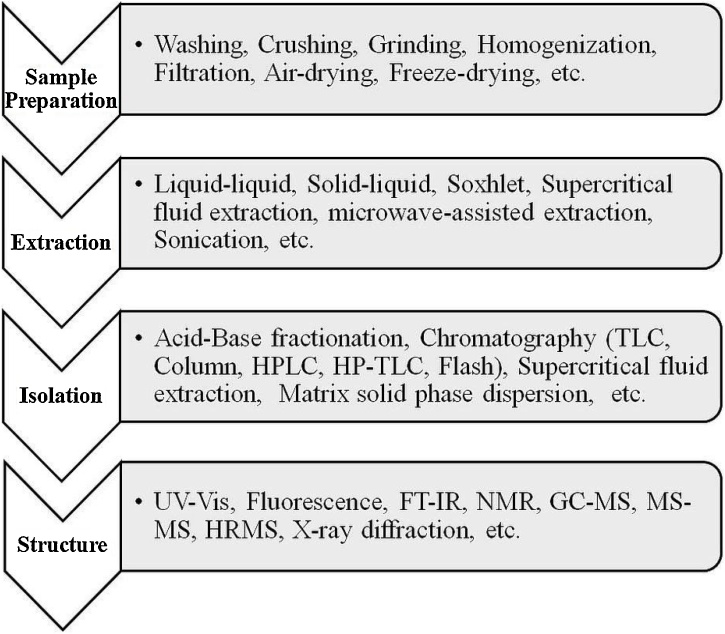
Extraction of phenolic compound(s) from plant materials mainly depends on the nature of sample matrix and also on chemical properties of desired phenolic(s) such as number of aromatic rings and hydroxyl groups in its structure, polarity, and concentration [27]. Therefore, it is tricky to select a single method for phenolic(s) extraction from different plant products. Phenolic compound(s) can be extracted from fresh, frozen as well as dried plant samples. Mostly; the phenolic compounds are found as esters or glycosides than free molecules, so their extraction method should be planned carefully as it affects the selectivity, yield and recovery of desired bioactive compound(s). On the basis of extraction source and type of phenolics, different solvents such as water, acetone, meth/eth/prop-anol, ethyl acetate etc. either alone or in mixture have been used to get high yield of desired phenolic(s) [6,[69], [70], [71], [72], [73], [74]]. Final recovery of extracted phenolic compound in stable form is also affected by pH, temperature, sample-solvent volume ratio, and number and time intervals of each steps in extraction plays a major role in the extraction protocol [75]. The choice of solvent used to extract the phenolic compounds can influence the final composition and bioactivity of extract [76]. After extraction; analytes are separated by different chromatographic techniques (TLC, column, HPLC) for quantification followed by structure elucidation by elemental analysis, FT-IR, NMR, UV–vis., GC—MS/HRMS and X-ray crystallography [6,20]. Even though, there are huge literature but quantification of different phenolic(s) remains a challenge [77]. Therefore, an immense scope is available to develop suitable quantification method for specific phenolic(s) as it offers comprehensible business opportunity since they are widely used in pharmaceuticals, phytochemicals, food ingredients, cosmetics dietary supplements and nutraceuticals [8,78]. Chromatographic techniques viz. HPLC and GC alone or in combination with mass spectrometry provides the best results for phenolic(s) quantification [79]. Quantification of phenolic molecule(s) through spectrophotometry is relatively a simple method. Folin-Ciocalteu and Folin-Denis assay were the commonly used specrophotometric methods for measurement of total phenolic content. These methods were based on the reduction of tungsten and molybdenum, respectively and final products show the blue color at 760 nm in the presence of phenolic compound(s) [80,81]. Both these methods were failing in terms of specificity and also produce colour for other materials such as aromatic amines, vitamin c, and sugars [82]. Quantification of total phenolics, flavonoids, and tannins also carried out through different colorimetric methods [[83], [84], [85]]. Gas chromatography (GC) is another important method to separate, indentify and quantify the phenolics viz. phenolic acids [86], flavonoids [87] and tannins [88]. The key concern during GC analysis is derivatization and volatile property of phenolic molecule(s). Thus, several reagents such as methy/ethyl chloroformate, DMSO, diazomethane, methyl iodate, trifluoroacetymide, trimethylsilyl derivatives, N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide and have been used to amend and creation of volatile derivatives of phenolics [77,89]. For the quantification of phenolic(s) by GC, fused silica capillaries of 30 m lengths, 25–32 μm internal diameters and 0.25 μm particle sizes are the most frequent columns. In combination with flame ionization detector in mass spectrometry, GC gives more selectivity and sensitivity [77]. High Performance Liquid Chromatography (HPLC) is the most favored method used to separate and quantify the phenolic compound(s). Selection of mobile phase, column, column temperature, types of detectors, and sample purification are the major factors affecting the HPLC [79,90,91]. Methanol, acetonitrile and their aqueous forms are leading mobile phase for phenolics quantification through HPLC in gradient elution [92]. Ethanol, 2-propanol and tetrahydrofuran are also used. The maintenance of pH between 2 to 4 is suggested to avoid ionization of phenolic compounds, thus mobile phase contain formic acid/acetic acid/ammonium acetate and citrate buffers/phosphoric acids [[93], [94], [95]]. Choice of HPLC column plays a key role in HPLC. On the basis of polarity of different phenolic compounds, C18 or reverse phase column were used predominantly with 10–30 cm long, 3.9–4.6 mm internal diameter and 3–10 μm particle size. However, development of new column types with 3–25 cm length, 1–4.6 internal diameter and 1.7–10 μm superficial porous particles are used in advanced HPLC methods [27,96]. Identification of phenolics in UV–vis and photodiode array detectors are carried out at 190–380 nm, colorimetric, fluorimetric, photodiode coupled with fluorescence are among the other used detection methods [[97], [98], [99], [100]]. Schematic representation of different extraction and structure determination methods used for plant phenolics are given in Fig. 3.
Fig. 3.
General scheme for the extraction of phenolic compounds from natural sources.
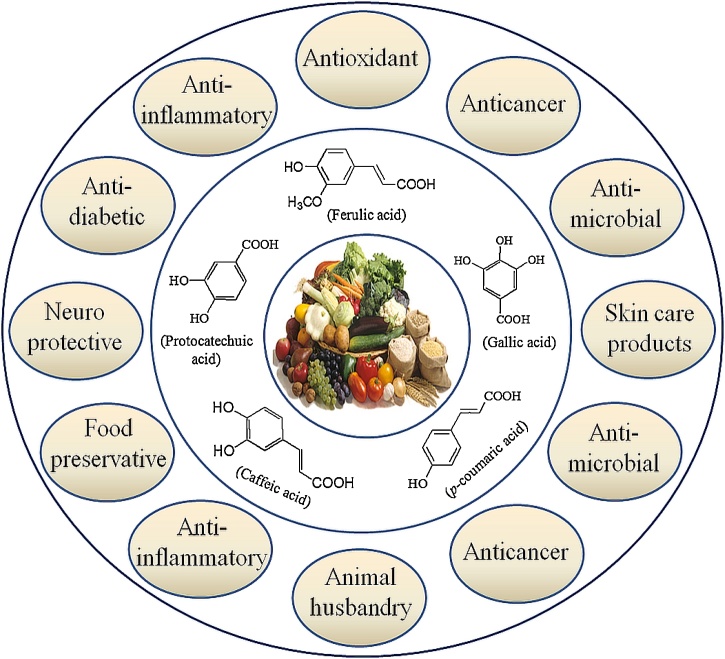
6. Applications of phenolic acids
Even though the complete role of phenolic acids in plants is still unknown but it is confirmed that they have diverse utility including a role in nutrient uptake, structural components, enzyme activity, protein synthesis, photosynthesis, and allelopathy [101]. Phenolic acids, known for diverse biological applications, are the main polyphenols produced by plants and work as ancestor for bioactive molecules regularly used in therapeutics, cosmetics, and food industries [102]. The key advantage of using phenolic acids is their metabolizing ability by natural microbes; therefore provide an essential alternate to man-made chemicals which are harmful to environment also. The pictorial representation of wide applications of phenolic acids has been summarized in Fig. 4 which is inspired from literature [8].
Fig. 4.
Schematic representation of different applications of phenolic acids.
6.1. Phenolic acids as signaling molecules
Plant phenolics particularly phenolic acids work as signaling molecules during the initiation of establishment of arbuscular mycorrhizal and legumerhizobia symbioses, and can also have a role in plant defense. These molecules offer themselves as an alternative source of carbon for some diazotrophs during inadequate environmental conditions and also work as precursors for phenolic lipids [103,104]. Phenolic acids are quickly released from rising roots during the seed germination and growth of seedling in legumes plants [105,106]. In the recent time, some phenolic acids have been found in the nodules of Vigna mungo and stimulate the production of indole acetic acid and also regulate the morphogenesis in Rhizobium sp. [107]. Several phenolic acids reported to stimulate whereas others was noticed to suppress the expression of nod gene Rhizobium trifolii [108]. The possible mechanism of protein profiling behind this work was also discussed which postulated that the effect of phenolic acids depends on their chemical structure, concentration, and also specific to strain [109].
6.2. Phenolic acids in food
According to a German study of 1998, the scientist reported the average daily consumption of phenolic acids is 211 and 11 mg/day for hydroxycinnamic and hydroxybenzoic acids, respectively although the studied population showed the variability from 6 to 987 mg of phenolic acids [110]. There are plenty of literature available which confirmed that a diet rich in vegetables and fruits provide better health as they mainly work as antioxidant and inhibits the oxidative damage induced diseases viz. stroke, coronary heart disease, and cancers [4,6,20,90,111,112]. Exploring the possible role of phenolic acids in plant life is only one aspect in many investigations while their role in food quality is also a huge one area of interest [113]. They have been used for enhancing the organoleptic (flavor, astringency, and hardness), color, sensory qualities, nutritional and antioxidant properties in food items [114,115]. Also the effect of phenolic acids on fruit maturation, prevention of enzymatic browning, and their roles as food preservatives have been investigated [28,116]. Generally, caffeic and ferulic acids (free/esterified) are the most abundant phenolic acids found in most fruits and cereal grains, respectively [117]. Flavonoids alone account roughly two-third of the total dietary phenolics in plant based food, however phenolic acids are also the major part and reports remaining one-third. The interest of scientific community in these simple phenolic acids are increasing continuously due to their antioxidant behavior and promising health benefits [118,119]. As phenolic acids are found ubiquitously, human beings consume them on daily basis through the vegetables, fruits, grains, tea/coffee, and spices etc. which collectively account for about 25 mg per day depending on diet chart [24,120]. Still the exact reason for the antioxidant and health protective effects of all the phenolic acids are not studied well via in vivo but there are many in vitro reports confirmed their beneficial effects [121,122].
6.3. Phenolic acids as antioxidants
Exposure to UV irradiation causes sunburn, DNA damage, and connective tissue degradation and can become the reason for skin cancer and premature skin aging [123]. Epidemiological studies pointed toward the unavailability of sunscreens and sun blockers to completely stop the UV induced skin cancer [124,125]. Therefore, we have to search for new targeted chemopreventive approaches to treat the UV-irradiation induced conditions [126]. Considering the deleterious effects of reactive oxygen species (ROS) such as superoxide (•O2¯), hydroxyl (•OH), and peroxyl (•RO2¯) in the skin, many studies have focused to establish and evaluate the antioxidant compounds for the augmentation of endogenous cutaneous protection system against the oxidative stress-mediated diseases. For this, researchers focused on the natural antioxidants and their derivatives particularly phenolic acids, flavonoids and other phenolics [57,127]. Phenolic compounds are known as direct antioxidants; however they also showed indirect antioxidant activity by inducing endogenous protective enzymes and positive regulatory effects on signalling pathways [128]. Phenolic acids act as antioxidants (due to the reactivity of phenol moiety; hydroxyl substituent on aromatic ring). Even though several mechanisms are known for the antioxidant activity (due to the reactivity of phenol moiety) of phenolic acids but radical scavenging via hydrogen atom donation is believed as the main method. Substituents on the aromatic ring in phenolic acids affect the stabilization of structure and consequently affect the radical-quenching ability. Different phenolic acids therefore have different antioxidant activity, and the antioxidant activities of free, esterified, glycosylated, and nonglycosylated phenolics has been reported and found different [121,129].
6.4. Phenolic acids as antidiabetic agent
Diabetes is identified as an oxidative stress disorder, consequence due to an imbalance between the formation of free radical and individual’s ability to oxidize them. The oxidative stress is vastly associated with the damage of organs through ROS which poorly neutralized by antioxidants leads to the inflammation and variety of metabolic disorders [130]. Antioxidants hinder the free radicals activity by numerous mechanisms and phenolic compounds particularly phenolic acids (possess a high antioxidant and free radical scavenging potential) work against the oxidative stress and its impediments by inhibiting the ROS producing enzymes [131]. The phenolic acids influence the function of glucose and insulin receptors (have a crucial role in diabetes). They augment the expression of glucose transporter GLUT2 in pancreatic β-cells (produces the insulin) and promote the translocation of GLUT4 through PI3K/Akt and AMP activated protein kinase pathways. Chlorogenic and ferulic acids proved the same transporter stimulation mechanism and work as antidiabetic agents [[132], [133], [134], [135], [136], [137], [138], [139], [140], [141]]. Among all the properties shown by phenolic acids, the best is the inhibition of α-glucosidase and α-amylase (two key enzymes) accountable for the conversion of dietary carbohydrates into glucose [142]. More details about the different mechanism followed by phenolic acids to inhibit/reduce the diabetes have been summarized in a recent review [143].
6.5. Phenolic acids as antimicrobial agent
The phenolic acids accounts as the most abundant compounds among all the phenolics present in fecal water [144]. They exhibits antimicrobial activity and also work as food preservatives. The antimicrobial potentials of phenolic acids have been determined through the chemical structure especially by saturated chain length, position and number of substitution in core benzene ring. The phenolic acids display reduction in antimicrobial activity than their methyl and butyl esters [144]. An increase in alkyl chain length greatly enhances the activity such as phenolic acids oligomers have higher activity as compared to their monomers [145,146]. The hydroxybenzoic and hydroxycinnamic acids also show different antimicrobial activity which depends on the number of hydroxyl (−OH) and methoxy (−OCH3) functional groups in a particular compound and their structure–function relationships does not describe for the diversity of these compounds [147]. Like other weak organic acids, hydroxybenzoic acids also followed the diffusion pattern across the membrane by un-dissociated acid for antimicrobial activity through the acidification of cytoplasm which leads to cell death. So the pKa and lipophilicity are the key factors in determining the solubility of phenolic acids in microbial membrane and corresponding antimicrobial ability [148]. The pH also plays an important role as it establish a charge on carboxyl (−COOH) group, substitutions of ring (hydroxyl and methoxy groups) and finally saturation of side chain. Antibacterial activity of phenolic acids is inversely proportional to change in pH [149]. A decrease of double bonds in hydroxycinnamic acids significantly reduces their antibacterial activity.
6.6. Phenolic acids in cancer cure and treatment
Cancer is one of the major health problems around the world, and according to a report of world health organization (WHO) approximately more than twice people died every year from cancer than AIDS, malaria, and tuberculosis together, whereas it is expected that these number will soar 80% by 2030 [150]. As per a report from Indian Council of Medical Research (ICMR), there will be a high increase of 25% in cancer patients in India alone. Epidemiology evidence indicates a diet with high consumption of antioxidant-rich fruits and vegetables significantly reduces the risk of many cancers, suggesting that certain dietary antioxidants could be effective agents for the prevention of cancer incidence and mortality. Phenolic acids such as hydroxybenzoic and hydroxycinnamic acids and their derivatives play a vital role in prevention and treatment of cancer [3,4,13,17,57,151]. Plant phenolics can offer an opportunity in this regard and more than half of all anticancer prescription drugs approved internationally between the 1940s and 2006 were natural products or their derivatives and lots of clinical trials are continuing [152]. They reduces the tumor initiation through several mechanisms viz. prevent the formation of genotoxic molecules and blocking the activity of mutagens-transforming enzymes [153,154]; regulate the heme-containing phase I enzymes [155,156], carcinogen-detoxifying phase II enzymes [157,158], and also stop the DNA adducts formation [159]. Most of the phenolics act at different points to cure or inhibit the different types of cancer [160].
7. Concluding remarks
Consumption of cereals, vegetables and fruits based on their phenolics content reduce the disease risk and enhanced human health. The wide variety of health benefits and industrial applications of phenolic compound(s) draw the attention of scientific community to improve the techniques used in extraction and purification of these natural gifted molecules. Phenolic acids, non-flavonoid polyphenols, are key class of polyphenols and abundantly used in human diet. One should daily intake of phenolic acids around 200 mg/day or more depending on his/her consumption of vegetables, fruits, and whole grains such as apples, mangos, berries, plums, cherries, kiwis, citrus fruits, onions, tea, coffee, and flour made from whole wheat, rice, corn or oats [24,161]. Phenolic acids are also available commercially as dietary supplements viz. grape seed extract or green tea extract. The supplements are typically supplied as antioxidants, but the current research confirmed that vegetables, fruits, and whole grains provide more health benefits rather than antioxidant supplements, so there is a tremendous scope in their extraction business. Phenolic acids, readily absorbed through intestinal tract walls, are beneficial to human health due to their potential antioxidants and avert the damage of cells resulted from free-radical oxidation reactions. On regular eating, phenolic acids also promote the anti-inflammation capacity of human beings. The demand of phenolic acids is very high in industries as they work for precursors of other significant bioactive molecules which are needed on regular basis for therapeutic, cosmetics, and food industries. The increasing demand of phenolic acids is also associated with their environment saving attempts as metabolized by means of natural microorganisms.
Declaration of competing interest
None.
Acknowledgments
Authors are grateful to IIT Indore and BHU for research environment. NK acknowledges University Grant Commission, Government of India for financial support as Postdoctoral Fellowship (Award letter No. 201718-PDFSS-2017-18-UTT-17095).
References
- 1.Alves R.R.N., Rosa I.M.L. Biodiversity, traditional medicine and public health: where do they meet? J. Ethnobiol. Ethnomed. 2007;3:14–23. doi: 10.1186/1746-4269-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2013;4:1–10. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang W.Y., Cai Y.Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for Cancer prevention. Nutr. Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 4.Kumar N., Gupta S., Yadav T.C., Pruthi V., Varadwaj P.K., Goel N. Extrapolation of phenolic compounds as multi-target agents against cancer and inflammation. J. Biomol. Struct. Dyn. 2019;37(9):2355–2369. doi: 10.1080/07391102.2018.1481457. [DOI] [PubMed] [Google Scholar]
- 5.Wahle K.W., Brown I., Rotondo D., Heys S.D. Plant phenolics in the prevention and treatment of cancer. Adv. Exp. Med. Biol. 2010;698:36–51. doi: 10.1007/978-1-4419-7347-4_4. [DOI] [PubMed] [Google Scholar]
- 6.Kumar N., Pruthi V., Goel N. Structural, Thermal and Quantum Chemical Studies of p-coumaric and caffeic acids. J. Mol. Struct. 2015;1085:242–248. [Google Scholar]
- 7.Chirinos R., Betalleluz-Pallardel I., Huamán A., Arbizu C., Pedreschi R., Campos D. HPLC-DAD characterisation of phenolic compounds from Andean oca (Oxalis tuberosa Mol.) tubers and their contribution to the antioxidant capacity. Food Chem. 2009;113:1243–1251. [Google Scholar]
- 8.Kumar N., Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heleno S.A., Martins A., Queiroz M.J., Ferreira I.C. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chem. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Mori H., Kawabata K., Yoshimi N., Tanaka T., Murakami T., Okada T., Murai H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999;19:3775–3778. [PubMed] [Google Scholar]
- 11.Middleton E., Jr, Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 12.Medina I., Gallardo J.M., Gonzalez M.J., Lois S., Hedges N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agric. Food Chem. 2007;55:3889–3895. doi: 10.1021/jf063498i. [DOI] [PubMed] [Google Scholar]
- 13.Badhani B. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5:27540–27557. [Google Scholar]
- 14.Bodini S.F., Manfredini S., Epp M., Valentini S., Santori F. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett. Appl. Microbiol. 2009;49:551–555. doi: 10.1111/j.1472-765X.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya A., Sood P., Citovsky V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010;11:705–719. doi: 10.1111/j.1364-3703.2010.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal S.M., Chakraborty D., Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010;5:359–368. doi: 10.4161/psb.5.4.10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha L.D., Monteiro M.C., Teodoro A.J. Anticancer properties of hydroxycinnamic acids – a review. Cancer Clin. Oncol. 2012;1:109–121. [Google Scholar]
- 18.Karimi E., Oskoueian E., Hendra R., Oskoueian A., Jaafar H.Z. Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules. 2012;17:1203–1218. doi: 10.3390/molecules17021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcan T., Akpinar-Bayizit A., Yilmaz-Ersan L., Delikanli B. Phenolics in human health. IJCEA. 2014;5:393–396. [Google Scholar]
- 20.Kumar N., Pruthi V. Structural elucidation and molecular docking of ferulic acid from Parthenium hysterophorus possessing COX-2 inhibition activity. 3 Biotech. 2015;5:541–551. doi: 10.1007/s13205-014-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Działo M., Mierziak J., Korzun U., Preisner M., Szopa J., Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016;17:160–201. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sytar O., Hemmerich I., Zivcak M., Rauh C., Brestic M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2016:407–443. doi: 10.1016/j.sjbs.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira D.M., Valentão P., Pereira J.A., Andrade P.B. Phenolics: from chemistry to biology. Molecules. 2009;14(6):2202–2211. [Google Scholar]
- 24.Clifford M.N. Chlorogenic acids and other cinnamates - nature, occurrence, and dietary burden. J. Sci. Food Agric. 1999;79:362–372. [Google Scholar]
- 25.Tsao R., Deng Z. Separation procedures for naturally occurring antioxidant hytochemicals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;812:85–99. doi: 10.1016/j.jchromb.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Strack D. Phenolic metabolism. In: Dey P.M., Harborne J.B., editors. Plant Biochemistry. Academic; London: 1997. p. 387. [Google Scholar]
- 27.Khoddami A., Wilkes M.A., Roberts T.H. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahidi F., Nacsk M. Technomic Publishing Co., Inc.; Lancaster, PA: 1995. Food Phenolics: Sources, Chemistry, Effects, and Application. [Google Scholar]
- 29.Dixon R.A. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 30.Moorman T.B., Becerril J.M., Lydon J., Duke S.O. Production of hydroxybenzoic acids by Bradyrhizobium japonicum strains after treatment with glyphosphate. J. Agric. Food Chem. 1992;40:289–293. [Google Scholar]
- 31.Croteau R., Kutchan T.M., Lewis N.G. Natural products (secondary metabolites) In: Buchanan, editor. Biochemistry Andmolecular Biology of Plants. American Society of Plant Physiologists; Rockville MD: 2000. pp. 1250–1318. [Google Scholar]
- 32.Boudet A.M. Evolution and current status of research in phenolic compounds. Phytochem. 2007;68:2722–2735. doi: 10.1016/j.phytochem.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Cheng S.H., Sheen J., Gerrish C., Bolwell G.P. Molecular identification of phenylalanineammonia-lyase as a substrate of aspecific constitutively active Arabidopsis CDPK expressed in maize protoplasts. FEBS Lett. 2001;503:185–188. doi: 10.1016/s0014-5793(01)02732-6. [DOI] [PubMed] [Google Scholar]
- 34.Moore B.S., Hertweck C., Hopke J.N., Izumikawa M., Kalaitzis J.A., Nilsen G., O’Hare T., Piel J., Shipley P.R., Xiang L., Austin M.B., Noel J.P. Plant-like biosynthetic pathways in bacteria: from benzoic acid to chalone. J. Nat. Prod. 2002;65:1956–1962. doi: 10.1021/np020230m. [DOI] [PubMed] [Google Scholar]
- 35.Hill A.M., Thompson B.L., Harris J.P., Segret R. Investigation of the early stages in soraphen A biosynthesis. Chem. Commun. 2003;21:1358–1359. doi: 10.1039/b303542p. [DOI] [PubMed] [Google Scholar]
- 36.Moffitt M.C., Louie G.V., Bowman M.E., Pence J., Noel J.P., Moore B.S. Discovery of two cyanobacterial phenylalanine ammonia lyases: kinetic and structural characterization. Biochem. 2007;46:1004–1012. doi: 10.1021/bi061774g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrer J.L., Austin M.B., Stewart C., Jr, Noel J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008;46:356–370. doi: 10.1016/j.plaphy.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Ascensao A.R., Dubery I.A. Soluble and wall bound phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f. Sp. cubense. Phytochem. 2003;63:679–686. doi: 10.1016/s0031-9422(03)00286-3. [DOI] [PubMed] [Google Scholar]
- 39.Verpoorte R., Contin A., Memelink J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002;1:13–25. [Google Scholar]
- 40.Mirjalili M.H., Moyano E., Bonfill M., Cusido R.M., Palazon J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palacio L., Baeza M.C., Cantero J.J., Cusido R., Goleniowski M.E. In vitro propagation of “jarilla” (Larrea divaricata cav.) and secondary metabolites production. Biol. Pharm. Bull. 2008;31:2321–2325. doi: 10.1248/bpb.31.2321. [DOI] [PubMed] [Google Scholar]
- 42.Palacio L., Cantero J.J., Cusido R., Goleniowski M.E. Effect of inoculum age on kinetic of biomass formation and phenolic accumulations in Larrea divaricata Cav. Cell suspension culture. Mol Med Chem. 2010;21:64–69. [Google Scholar]
- 43.Grzegorczyk I., Krolicka A., Wysokinska H. Establishment of Salvia officinalis L. Hairy root cultures for the production of rosmarinic acid. Z Naturforsch. 2006;61:351–356. doi: 10.1515/znc-2006-5-609. [DOI] [PubMed] [Google Scholar]
- 44.Park S.U., Uddin R., Xu H., Kim Y.K., Lee S.Y. Biotechnological applications for rosmarinic acid production in plant. African. J Biotechnol. 2008;7:4959–4965. [Google Scholar]
- 45.Piazzon A., Vrhovsek U., Masuero D., Mattivi F., Mandoj F., Nardini M. Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012;60:12312–12323. doi: 10.1021/jf304076z. [DOI] [PubMed] [Google Scholar]
- 46.Nardini M., Forte M., Vrhovsek U., Mattivi F., Viola R., Scaccini C. White wine phenolics are absorbed and extensively metabolized in humans. J Agric.Food Chem. 2009;57:2711–2718. doi: 10.1021/jf8034463. [DOI] [PubMed] [Google Scholar]
- 47.Stalmach A., Mullen W., Barron D., Uchida K., Yokota T., Cavin C., Steiling H., Williamson G., Crozier A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009;37:1749–1758. doi: 10.1124/dmd.109.028019. [DOI] [PubMed] [Google Scholar]
- 48.Jimenez J.P., Hubert J., Hooper L., Cassidy A., Manach C., Williamson G., Scalbert A. Urinary metabolites as biomarkers of polyphenol intake in humans: a systemic review. Am. J. Clin. Nutr. 2010;92:801–809. doi: 10.3945/ajcn.2010.29924. [DOI] [PubMed] [Google Scholar]
- 49.Omar M.H., Mullen W., Stalmach A., Auger C., Rouanet J.M., Teissedre P.L., Caldwell S.T., Hartley R.C., Crozier A. Absorption, disposition, metabolism and excretion of [3−14C]caffeic acid in rats. J. Agric. Food Chem. 2012;60:5205–5214. doi: 10.1021/jf3001185. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Z., Egashira Y., Sanada H. Ferulic acid sugars are recovered in rat plasma and urine mainly as the sulfoglucuronide of ferulic acid. J. Nutr. 2003;133:1355–1361. doi: 10.1093/jn/133.5.1355. [DOI] [PubMed] [Google Scholar]
- 51.Stachulski A.V., Harding J.R., Lindon J.C., Maggs J.L., Park B.K., Wilson J.D. Acyl glucuronides: biological activity, chemical reactivity and chemical synthesis. J. Med. Chem. 2006;49:6931–6945. doi: 10.1021/jm060599z. [DOI] [PubMed] [Google Scholar]
- 52.Plumb G.W., Barcia-Conesa M.T., Kroon Paul A., Rhodes M., Ridley S., Williamson G. Metabolism of chlorogenic acid by human plasma, liver, intesting and gut microflora. J. Sci. Food Agric. 1999;79:390–392. [Google Scholar]
- 53.Azuma K., Ippoushi K., Nakayam M., Ito H., Higashio H., Terao J. Adsorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000;48:5496–5500. doi: 10.1021/jf000483q. [DOI] [PubMed] [Google Scholar]
- 54.Bourne L.C., Rice-Evans C. Bioavailability of ferulic acid. Biochem. Biophys. Res. Commun. 1998;253:222–227. doi: 10.1006/bbrc.1998.9681. [DOI] [PubMed] [Google Scholar]
- 55.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 56.Scalbert A., Manach C., Morand C., Remesy C., Jime´nez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 57.Kumar N., Kumar S., Abbat S., Nikhil K., Sondhi S.M., Bharatam P.V., Roy P., Pruthi V. Ferulic acid amide derivatives as anticancer and antioxidant agents: synthesis, thermal, biological and computational studies. Med. Chem. Res. 2016;25:1175–1192. [Google Scholar]
- 58.Lafay S., Gil-Izquierdo A. Bioavailability of phenolic acids. Phytochemy Rev. 2008;7:301–311. [Google Scholar]
- 59.Tomas-Barberan F.A., Clifford M.N. Dietary hydroxybenzoic acid derivatives-nature, occurence and dietary burden. J. Sci. Food Agric. 2000;80:1024–1032. [Google Scholar]
- 60.Adam A., Crespy V., Levrat-Verny M.A., Leenhardt F., Leuillet M., Demigne C., Remesy C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J. Nutr. 2002;132:1962–1968. doi: 10.1093/jn/132.7.1962. [DOI] [PubMed] [Google Scholar]
- 61.Konishi Y., Hitomi Y., Yoshida M., Yoshioka E. Pharmacokinetic study of caffeic and rosmarinic acids in rats after oral administration. J. Agric. Food Chem. 2005;53:4740–4746. doi: 10.1021/jf0478307. [DOI] [PubMed] [Google Scholar]
- 62.Uang Y.S., Kang F.L., Hsu K.Y. Determination of caffeic acid in rabbit plasma by high-performance liquid chromatography. J. Chromatogr. B, Biomed. Appl. 1995;673:43–49. doi: 10.1016/0378-4347(95)00243-c. [DOI] [PubMed] [Google Scholar]
- 63.Gonthier M.P., Verny M.A., Besson C., Remesy C., Scalbert A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003;133:1853–1859. doi: 10.1093/jn/133.6.1853. [DOI] [PubMed] [Google Scholar]
- 64.Camarasa J., Escubedo E., Adzet T. Pharmacokinetics of caffeic acid in rats by a high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 1988;6:503–510. doi: 10.1016/0731-7085(88)80017-7. [DOI] [PubMed] [Google Scholar]
- 65.Konishi Y., Hitomi Y., Yoshioka E. Intestinal absorption of p-coumaric and gallic acids in rats after oral administration. J. Agric. Food Chem. 2004;52:2527–2532. doi: 10.1021/jf035366k. [DOI] [PubMed] [Google Scholar]
- 66.Choudhury R., Srai S.K., Debnam E., Rice-Evans C.A. Urinary excretion of hydroxycinnamates and flavonoids after oral and iv administration. Free Radic. Biol. Med. 1999;27:278–286. doi: 10.1016/s0891-5849(99)00054-4. [DOI] [PubMed] [Google Scholar]
- 67.Baba S., Osakabe N., Natsume M., Terao J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004;75:165–178. doi: 10.1016/j.lfs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 68.Nakazawa T., Ohsawa K. Metabolism of rosmarinic acid in rats. J. Nat. Prod. 1998;61:993–996. doi: 10.1021/np980072s. [DOI] [PubMed] [Google Scholar]
- 69.Verma B., Hucl P., Chibbar R.N. Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem. 2008;85:544–549. [Google Scholar]
- 70.Kalia K., Sharma K., Singh H.P., Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008;56:10129–10134. doi: 10.1021/jf802188b. [DOI] [PubMed] [Google Scholar]
- 71.Casazza A.A., Aliakbarian B., Mantegna S., Cravotto G., Perego P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010;100:50–55. [Google Scholar]
- 72.Garcia-Salas P., Morales-Soto A., Segura-Carretero A., Fernández-Gutiérrez A. Phenolic compound-extraction systems for fruit and vegetable samples. Molecules. 2010;15(12):8813–8826. doi: 10.3390/molecules15128813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taha F.S., Mohamed G.F., Mohamed S.H., Mohamed S.S., Kamil M.M. Optimization of the extraction of total phenolic compounds from sunflower meal and evaluation of the bioactives of chosen extract. Am. J. Food Technol. 2011;6(12):1002–1020. [Google Scholar]
- 74.Biesaga M., Pyrzynska K. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 2013;136:46–54. doi: 10.1016/j.foodchem.2012.07.095. [DOI] [PubMed] [Google Scholar]
- 75.Robards K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A. 2003;1000:657–691. doi: 10.1016/s0021-9673(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 76.Martins N., Barros L., Henriques M., Silva S., Ferreira I.C.F.R. Activity of phenolic compounds from plant origin against Candida species. Indus. Crops Prod. 2015;74:648–670. [Google Scholar]
- 77.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 78.Liu Q., Cai W., Shao X. Determination of seven polyphenols in water by high performance liquid chromatography combined with preconcentration. Talanta. 2008;77:679–683. [Google Scholar]
- 79.Naczk M., Shahidi F. Review: extraction and analysis of phenolics in food. J. Chromatogr. A. 2004;1054:95–111. [PubMed] [Google Scholar]
- 80.Naczk M., Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharmaceut Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Stalikas C.D. Review: extraction, Separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 82.Box J.D. Investigation of the Folin-Ciocalteu phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983;17:511–525. [Google Scholar]
- 83.Hartzfeld P.W., Forkner R., Hunter M.D., Hagerman A.E. Determination of hydrolysable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002;50:1785–1790. doi: 10.1021/jf0111155. [DOI] [PubMed] [Google Scholar]
- 84.Huang W., Xue A., Niu H., Jia Z., Wang J. Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chem. 2009;114:1147–1154. [Google Scholar]
- 85.Jun X., Shouqin Z. Antioxactivity of ethanolic extract of propolis by hydrostatic pressure extraction. Int. J. Food Sci. Tech. 2007;42:1350–1356. [Google Scholar]
- 86.Martin J.G.P., Porto E., Corrêa C.B., De Alencar S.M., Da Gloria E.M., Cabral I.S.R., De Aquino L.M. Antimicrobial potential and chemical composition of agro-industrial wastes. J. Nat. Prod. 2012;5:27–36. [Google Scholar]
- 87.Proestos C., Boziaris I.S., Nychas G.J.E., Komaitis M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: investing ation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006;95:664–671. [Google Scholar]
- 88.Shadkami F., Estevez S., Helleur R. Analysis of catechins and condensed tannins by thermally assisted hydrolysis/methylation-GC/MS and by a novel two step methylation. J. Anal. Appl. Pyrolysis. 2009;85:54–65. [Google Scholar]
- 89.Zadernowski R., Czaplicki S., Naczk M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana) Food Chem. 2009;112:685–689. [Google Scholar]
- 90.Robbins R.J. Phenolic Acids in Foods: an overview of analytical methodology. J. Agric. Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- 91.Stalikas C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 92.Zarena A.S., Udaya Sankar K. Phenolic acids, flavonoid profile and antioxidant activity in mangosteen (Garcina mangostana L.) pericarp. J. Food Biochem. 2011;36:627–633. [Google Scholar]
- 93.Diagone C.A., Colombo R., Lancas F.M., Yariwake J.H. CZE/PAD and HPLC-UV/PAD profile of flavonoids from Maytenus aquifolium and Maytenus ilicifolia “espinheira santa” leaves extracts. Chromatogr. Res. Int. 2012;2012:1–7. [Google Scholar]
- 94.Reichelt K.V., Peter R., Paetz S., Roloff M., Ley J.P., Krammer G.E., Engel K.H. Characterization of flavor modulating effects in complex mixtures via high temperature liquid chromatography. J. Agric. Food Chem. 2010;58:458–464. doi: 10.1021/jf9027552. [DOI] [PubMed] [Google Scholar]
- 95.Takenaka Y., Morimoto N., Hamada N., Tanahashi T. Phenolic compounds from the cultured mycobionts of Graphis proserpens. Phytochem. 2011;72:1431–1435. doi: 10.1016/j.phytochem.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 96.Kalili K.M., de Villiers A. Recent developments in the HPLC separation of phenolic compounds. J. Sep. Sci. 2011;34:854–876. doi: 10.1002/jssc.201000811. [DOI] [PubMed] [Google Scholar]
- 97.Cicchetti E., Chaintreau A. Comparison of extraction techniques and modeling of accelerated solvent extraction for the authentication of natural vanilla flavors. J. Sep. Sci. 2009;32:3043–3052. doi: 10.1002/jssc.200800650. [DOI] [PubMed] [Google Scholar]
- 98.de Villiers A., Kalili K.M., Malan M., Roodman J. Improving HPLC separation of polyphenols. LCGC Eur. 2010;23:466–478. [Google Scholar]
- 99.do Amaral F.P., Napolitano A., Masullo M., dos Santos L.C., Festa M., Vilegas W., Pizza C., Piacente S. HPLC-ESIMSn profiling, isolation, structural elucidation, and evaluation of the antioxidant potential of phenolics from Paepalanthus geniculatus. J. Nat. Prod. 2012;75:547–556. doi: 10.1021/np200604k. [DOI] [PubMed] [Google Scholar]
- 100.Liang Z., Li K., Wang X., Ke Y., Jin Y., Liang X. Combination of off-line two-dimensional hydrophilic interaction liquid chromatography for polar fraction and two-dimensional hydrophilic interaction liquid chromatographyxreversed-phase liquid chromatography for medium-polar fraction in a traditional Chinese medicine. J. Chromatogr. A. 2012;1224:61–69. doi: 10.1016/j.chroma.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 101.Lyu S.W., Blum U., Gerig T.M., O’Brien T.E. Effects of mixtures of phenolic acids on phosphorus uptake by cucumber seedlings. J. Chem. Ecol. 1990;16:2559–2567. doi: 10.1007/BF01017478. [DOI] [PubMed] [Google Scholar]
- 102.Croft K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998;854:435–442. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 103.Chan Y.K. Utilization of simple phenolics for dinitrogen fixation by soil diazotrophic bacteria. Plant Soil. 2006;90:141–150. [Google Scholar]
- 104.Funa N., Ozawa H., Hirata A., Horinouchi S. Phenolic lipid synthesis by type III polyketide synthases is essential for cyst formation in Azotobacter vinelandii. Proc. Nat. Acad. Sci. 2006;103:6356–6361. doi: 10.1073/pnas.0511227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bekkara F., Jay M., Viricel M.R., Rome S. Distribution of phenolic compounds within seed and seedlings of two Vicia faba cvs differing in their seed tannin content, and study of their seed and root phenolic exudations. Plant Soil. 1998;203:27–36. [Google Scholar]
- 106.Staman K., Blum U., Louws F., Robertson D. Can simultaneous inhibition of seedling growth and stimulation of rhizosphere bacterial populations provide evidence for phytotoxin transfer from plant residues in the bulk soil to the rhizosphere of sensitive species? J. Chem. Ecol. 2001;27:807–829. doi: 10.1023/a:1010362221390. [DOI] [PubMed] [Google Scholar]
- 107.Mandal S.M., Mandal M., Das A.K., Pati B.R., Ghosh A.K. Stimulation of indoleacetic acid production in a Rhizobium isolate of Vigna mungo by root nodule phenolic acids. Arch. Microbiol. 2009;191:389–393. doi: 10.1007/s00203-008-0455-6. [DOI] [PubMed] [Google Scholar]
- 108.Djordjevic M., Rolfe B. Recognition process in the Rhizobium trifolii-white clover symbiosis. In: Bothe H., de Bruijn F., Newton F., editors. Nitrogen Fixation: Hundred Years after. Gustav Fischer; Stuttgart, Germany: 1988. pp. 431–436. [Google Scholar]
- 109.Seneviratne G., Jayasinghearachchi H.S. Phenolic acids: possible agents of modifying N2-fixing symbiosis through rhizobial alteration? Plant Soil. 2003;252:385–395. [Google Scholar]
- 110.Radtke J., Linseisen J., Wolfram G. Phenolsa¨urezufuhr erwachsener in einem bayerischen teilkollektiv der Nationalen Verzehrsstudie. Z. Ernahrungswiss. 1998;37:190–197. doi: 10.1007/s003940050016. [DOI] [PubMed] [Google Scholar]
- 111.Ness A.R., Powles J.W. Fruit and vegetables, and cardiovascular disease: a review. Int. J. Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 112.Paganga G., Miller N., Rice-Evans C.A. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radical Res. 1999;30:153–162. doi: 10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- 113.Tomás-Barberán F.A., Espin J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001;81:853–876. [Google Scholar]
- 114.Brenes-Balbuena M., Garcia-Garcia P., Garrido-Fernandez A. Phenolic compounds related to the black color formed during the processing of ripe olives. J. Agric. Food Chem. 1992;40:1192–1196. [Google Scholar]
- 115.Tan S.C. Determinants of eating quality in fruits and vegetables. Proc. Nutr. Soc. Aust. 2000:24. [Google Scholar]
- 116.Fernandez-Zurbano P., Ferreira V., Escudero A., Cacho J. Role of hydroxycinnamic acids and flavanols in the oxidation and browning of white wines. J. Agric. Food Chem. 1998;46:4937–4944. [Google Scholar]
- 117.Shahidi F., Naczk M. CRC Press; London: 2003. Phenolics in Food and Nutraceuticals. [Google Scholar]
- 118.Oomah B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001;81:889–894. [Google Scholar]
- 119.Stacewicz-Sapuntzakis M., Bowen P.E., Hussain E.A., Damayanti-Wood B., Farnsworth N.R. Chemical composition and potential health effects of prunes: a functional food? Crit. Rev. Food Sci. Nutr. 2001;41:251–286. doi: 10.1080/20014091091814. [DOI] [PubMed] [Google Scholar]
- 120.Lodovici M., Guglielmi F., Meoni M., Dolara P. Effect of natural phenolic acids on DNA oxidation in vitro. Food Chem. Toxicol. 2001;39:1205–1210. doi: 10.1016/s0278-6915(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 121.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 122.Morton L.W., Croft K.D., Puddey I.B., Byrne L. Phenolic acids protect low-density lipoproteins from peroxynitrite-mediated modification in vitro. Redox Rep. 2000;5:124–125. doi: 10.1179/135100000101535429. [DOI] [PubMed] [Google Scholar]
- 123.Vicentini F.T., He T., Shao Y., Fonseca M.J., Verri W.A., Jr, Fisher G.J., Xu Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. J. Dermatol. Sci. 2011;61:162–168. doi: 10.1016/j.jdermsci.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 124.Azizi E., Iscovich J., Pavlotsky F., Shafir R., Luria I., Federenko L. Use of sunscreen is linked with elevated naevi counts in Israeli school children and adolescents. Melanoma Res. 2000;10:491–498. doi: 10.1097/00008390-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 125.Bauer J., Buttner P., Wiecker T.S., Luther H., Garbe C. Effect of sunscreen and clothing on the number of melanocytic nevi in 1,812 German children attending day care. Am. J. Epidemiol. 2005;161:620–627. doi: 10.1093/aje/kwi086. [DOI] [PubMed] [Google Scholar]
- 126.Verschooten L., Claerhout S., Van Laethem A., Agostinis P., Garmyn M. New strategies of photoprotection. Photochem. Photobiol. 2006;82:1016–1023. doi: 10.1562/2006-04-27-ir-884.1. [DOI] [PubMed] [Google Scholar]
- 127.Fonseca Y.M., Catini C.D., Vicentini F.T.M.C., Nomizo A., Gerlach R.F., Fonseca M.J.V. Protective effect of Calendula officinalis extract against UV-B-induced oxidative stress in skin: evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010;127:596–601. doi: 10.1016/j.jep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 128.Stevenson D.E., Hurst R.D. Polyphenolic phytochemicals-just antioxidant or much more? Cell. Mol. Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chalas J., Claise C., Edeas M., Messaoudi C., Vergnes L., Abella A., Lindenbaum A. Effect of ethyl esterification of phenolic acids on low-density lipoprotein oxidation. Biomed. Pharmacother. 2001;55:54–60. doi: 10.1016/s0753-3322(00)00011-1. [DOI] [PubMed] [Google Scholar]
- 130.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu Y., Ding Y., Tanaka Y., Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014;11:1185–1200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jung U.J., Lee M.K., Park Y.B., Jeon S.M., Choi M.S. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J. Pharmacol. Exp. Ther. 2006;318:476–483. doi: 10.1124/jpet.106.105163. [DOI] [PubMed] [Google Scholar]
- 133.Jung E.H., Kim S.R., Hwang I.K., Ha T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007;55:9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- 134.Prasad C.N., Anjana T., Banerji A., Gopalakrishnapillai A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010;584:531–536. doi: 10.1016/j.febslet.2009.11.092. [DOI] [PubMed] [Google Scholar]
- 135.Prabhakar P.K., Doble M. Interaction of cinnamic acid derivatives with commercial hypoglycemic drugs on 2-deoxyglucose uptake in 3T3-L1 adipocytes. J. Agric. Food Chem. 2011;59:9835–9844. doi: 10.1021/jf2015717. [DOI] [PubMed] [Google Scholar]
- 136.Prabhakar P.K., Doble M. Interaction of phytochemicals with hypoglycemic drugs on glucose uptake in L6 myotubes. Phytomed. 2011;18:285–291. doi: 10.1016/j.phymed.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 137.Choi R., Kim B.H., Naowaboot J., Lee M.Y., Hyun M.R., Cho E.J., Lee E.S., Lee E.Y., Yang Y.C., Chung C.H. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011;43:676–683. doi: 10.3858/emm.2011.43.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ong K.W., Hsu A., Tan B.K. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ong K.W., Hsu A., Tan B.K. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharmacol. 2013;85:1341–1351. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 140.Cherng Y.G., Tsai C.C., Chung H.H., Lai Y.W., Kuo S.C., Cheng J.T. Antihyperglycemic action of sinapic acid in diabetic rats. J. Agric. Food Chem. 2013;61:12053–12059. doi: 10.1021/jf403092b. [DOI] [PubMed] [Google Scholar]
- 141.Gandhi G.R., Jothi G., Antony P.J., Balakrishna K., Paulraj M.G., Ignacimuthu S., Stalin A., Al-Dhabi N.A. Gallic acid attenuates high-fat diet fed streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014;745:201–216. doi: 10.1016/j.ejphar.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 142.Hanhineva K., Törrönen R., Bondia-Pons I., Pekkinen J., Kolehmainen M., Mykkänen H., Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010;11:1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vinayagam R., Jayachandran M., Xu B. Antidiabetic effects of simple phenolic acids: a comprehensive review. Phytother. Res. 2016;30:184–199. doi: 10.1002/ptr.5528. [DOI] [PubMed] [Google Scholar]
- 144.Cueva C., Moreno-Arribas M.V., Martín-Alvarez P.J., Bills G., Vicente M.F., Basilio A., Rivas C.L., Requena T., Rodríguez J.M., Bartolomé B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010;161:372–382. doi: 10.1016/j.resmic.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 145.Elegir G., Kindl A., Sadocco P., Orlandi M. Development of antimicrobial cellulose packing through laccase-mediated grafting of phenolic compounds. Enzyme Microb. Technol. 2008;43:84–92. [Google Scholar]
- 146.Merkl R., Hrádková I., Filip V., Šmidrkal J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010;28(4):275–279. [Google Scholar]
- 147.Sanchez-Maldonado A.F., Schieber A., Ganzle M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microb. 2011;111:1176–1184. doi: 10.1111/j.1365-2672.2011.05141.x. [DOI] [PubMed] [Google Scholar]
- 148.Campos F.M., Couto J.A., Figuereido A.R., Toth I.V., AOSS Rangel, Hogg T.A. Cell membrane damage induced by phenolic acids on wine lactic acids bacteria. Int. J. Food Microbiol. 2009;135:144–151. doi: 10.1016/j.ijfoodmicro.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 149.Almajano M.P., Carbo R., Delgado M.E., Gordon M.H. Effect of pH on the antimicrobial activity and oxidative stabiliy of oil-in-water emulsions containing caffeic acid. J. Food Sci. 2007;72:258–263. doi: 10.1111/j.1750-3841.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 150.Reddy L., Odhav B., Bhoola K.D. Natural products for cancer prevention: a global perspective. Pharmacol. Ther. 2003;99:1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 151.Kumar N., Goel N., Yadav T.C., Pruthi V. Quantum Chemical, ADMET and Molecular Docking studies of ferulic acid amide derivatives with a novel anti-cancer drug target. Med. Chem. Res. 2017;26:1822–1834. [Google Scholar]
- 152.Efferth T., Li P.C., Konkimalla V.S., Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007;13:353–361. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 153.Frassinetti S., Della Croce C.M., Caltavuturo L., Longo V. Antimutagenic and antioxidant activity of Lisosan G in Saccharomyces cerevisiae. Food Chem. 2012;135:2029–2034. doi: 10.1016/j.foodchem.2012.06.090. [DOI] [PubMed] [Google Scholar]
- 154.Słoczynska K., Powroznik B., Pekala E., Waszkielewicz A.M. Antimutagenic compounds and their possible mechanisms of action. J. Appl. Genet. 2014;55:273–285. doi: 10.1007/s13353-014-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rodeiro I., Donato M.T., Jimenez N., Garrido G., Molina-Torres J., Menendez R., Castell J.V., Gómez-Lechón M.J. Inhibition of human p450 enzymes by natural extracts used in traditional medicine. Phytother. Res. 2009;23:279–282. doi: 10.1002/ptr.2613. [DOI] [PubMed] [Google Scholar]
- 156.Basheer L., Kerem Z. Interactions between cyp3a4 and dietary polyphenols, Oxid. Med. Cell Longev. 2015;2015:1–15. doi: 10.1155/2015/854015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Munday R., Munday C.M. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: Comparison of allyl isothiocyanate with sulforaphane and related compounds. J. Agric. Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 158.Kou X., Kirberger M., Yang Y., Chen N. Natural products for cancer prevention associated with nrf2-ARE pathway. Food Sci. Hum. Wellness. 2013;2:22–28. [Google Scholar]
- 159.Lu F., Zahid M., Wang C., Saeed M., Cavalieri E.L., Rogan E.G. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Choi J., Jiang X., Jeong J.B., Lee S.H. Anticancer activity of protocatechualdehyde in human breast cancer cells. J. Med. Food. 2014;17:842–848. doi: 10.1089/jmf.2013.0159. [DOI] [PubMed] [Google Scholar]
- 161.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]