Abstract
The increasing resistance of infectious microbial organisms to the existing arsenal of antibiotic drugs is on the rise. There is a growing demand for the new antibiotics that are cost effective and easily available to the common people. In search of new antimicrobial entities, this report deals with the in vitro antimicrobial activities of the crude extracts of leaves of Ehretia Serrata. The methanolic extract and its sub-fractions namely n-hexane, chloroform, ethyl acetate, n-butanol and residual water fraction were screened against a range of 30 different bacterial strains and their zones of inhibition (ZI) and minimum inhibitory concentration (MIC) were subsequently evaluated. Methanolic extract has shown activity against all the tested microorganisms such as Azospirillum lipoferum, Escherichia coli, Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Enterococcus sp. with ZOI ranged from 10.3 to 29.0 mm. Moreover, the MIC values of methanolic extract and its sub-fractions against the tested bacteria ranged from 0.8 to 5.1 mg/mL. GC–MS analysis of sub-fractions revealed the presence of mono (2-ethylexyl) 1,2-benzenedicarboxylate, diisooctyl-1,2-benzenedicarboxylate, 3,5-dehydro-6-methoxypivalate-cholest-22-ene-21-ol, and 3,5-bis (1,1-dimethylethyl)-4-hydroxybenzene-propanoic acid. This is the first report on the in vitro antimicrobial activities of leaves of E. Serrata.
Keywords: Antimicrobial activity, Ehretia serrata, Boraginacae, GC–MS analysis, Phytochemical studies
1. Introduction
Antibiotics are crucial in the treatment of several severe infections such as pneumonia, tuberculosis and meningitis. Moreover, they are used in the prevention of surgical site infections and management of immunocompromised individuals (Herbst et al., 2009, Finch, 2007). However, the increasing resistance of infectious microbial organisms to the existing arsenal of antibiotic drugs is considered to be responsible for more than 25,000 deaths in the European Union annually and 23,000 in the United States (Antibiotic Resistance Threats in the United States, 2013, ECDC/EMEA Joint Technical Report, 2009: The bacterial challenge: time to react). Thus, the emergence of the multi drug resistant strains poses a huge challenge for humanity to combat (Pallavali et al., 2017). Therefore, there is an ever growing demand for the new antibiotics that are cost effective and easily available to the common people.
The genus Ehretia of the Boraginacae family has about 50 species, distributed in tropical areas of Africa and Asia. Ehretia serrata Roxb. (syn. E. acuminata var. serrata (Roxb.) is a deciduous tree found in Pakistan and India that is spread in the sub-Himalayan and outer Himalaya from Indus eastward to Sikkim (Johnston, 1951). The plants of genus Ehretia are known for rich folkloric medicinal applications including in the treatment of abdominal and chest pains (Mncwangi et al., 2012), syphilis, toothache, cachexia, cough, diarrhea, stomach diseases and eczema (Torane et al., 2011, Khare, 2007). Moreover, they have been used to treat venereal diseases, pneumonia, epilepsy, dry cough, asthma, tonsils, mental problems, malaria, typhoid, wounds, aphrodisiac (Jeruto et al., 2008), nervous disorder and kidney inflammation (Arenas et al., 2013). A quinonoid xanthene, ehretianone, isolated from the root bark of Ehretia buxifolia has been found to possess anti-snake venom activity (Gomes et al., 2010).
Motivated by the medicinal importance of plants of genus Ehretia, herein we wish to disclose antimicrobial studies of the crude extracts of leaves of E. serrata. Based on the available literature, this plant has been rarely used for the phytochemical and biological screening studies in the past. Earlier, we have reported a comparative study on the antioxidant capacity of different parts of the same plant (Zara, et al., 2012). Our present study is directed to determine the antimicrobial activities of the methanolic extract and its sub-fractions of E. serrata against a range of bacterial strains. In addition, phytochemical screening and GC–MS analysis was also performed to identify the chemical constituents responsible for therapeutic effects.
2. Materials and methods
2.1. Chemicals and instruments
Solvents used for extraction and fractionation were of commercial grade. Dimethyl sulfoxide (DMSO) was purchased from Fisher Scientific and Mueller-Hinton agar from Merck. Standard antibiotic drugs amoxylin, cefixime and levofloxacin were obtained from Pharmagen, Lahore, Pakistan. GC–MS analysis was performed on Agilent Technologies GCMS system GC-7890A/MS-5975C model (Agilent Technologies, Santa Clara, CA, USA) equipped with HP-5MS column ((30 m in length × 250 μm in diameter × 0.25 μm in thickness of film). The ionization mode was electron ionization with high energy electron (70 eV). The injection volume of the sample (prepared by dissolving 0.5 mg/mL of respective solvents) was 1 µL and flow pressure for helium carrier gas (99.999%) was maintained at 1.0 mL/min. The split mode was used having 10:1 ratio. The sample was injected at 200 °C while the initial temperature of oven 35 °C and was gradually increased to 270 °C with an increase rate of 5 °C per minute followed by raising temperature to 320 °C with the rate of 10 °C per minute. The mass spectrometer was set to scan mode ranging from m/z 50–600 with ion source temperature of 250 °C and interface temperature of 150 °C. The analyses were performed in triplicate and a blank run was run after each analysis.
2.2. Microorganisms and growth conditions
The microorganisms used for this study were both gram negative and gram positive bacteria. The gram negative bacteria were: Achromobacter xylosoxidans, Stenotrophomonas maltophilia, Klebsiella pneumonia, Enterobacter cloacae, pseudomonas sp., Escherichia coli, Salmonella typhi, Azospirillum lipoferum, Rhizobium sp., Citrobactor freundii whereas gram positive bacteria include Staphylococcus epidermidis, Staphylococcus aureus, Bacillus sp., Bacillus subtilis, Bacillus megatherium, Enterococcus sp. The bacterial strains were obtained from the Department of Biotechnology, Forman Christian College, University, Lahore, and were subsequently grown at 37 °C and maintained on nutrient agar (Merck, Germany). All the clinical isolates were identified by standard morphological, cultural and biochemical profile (API-20E, bioMerieux, France). Serological identification was performed by using antisera (BD Difco, USA). The isolates were preserved in micro bank tubes containing beads (Pro-Lab Diagnostics, UK) and 16% (v/v) glycerol in brain heart infusion (Oxoid Ltd, UK) and were stored at -70 °C.
2.3. Plant material
The leaves of Ehretia serrata were collected from the campus of Forman Christian College University Lahore, Pakistan. The collected leaves were washed with distilled water and dried under shade for 15 days. The air-dried leaves were pulverized into powdered form by using heavy duty blander.
2.4. Extraction and fractionation
500 g of powdered leaves were soaked in 2000 mL of methanol (100%) at a ratio of 1:4 (powder/solvent) for 7 days. The mixture was agitated from time to time during soaking period in order to ensure homogeneity and to maximize the extraction of the plant constituents. The mixture was first filtered by cheese cloth and then by Whatman No.1 filter paper. The filtrate was concentrated under reduced pressure by using rotary evaporator at 40 °C to complete dryness to yield 42.6 g (8.5%) of crude methanolic extract (Zara et al., 2012). In order to obtain n-hexane, chloroform, ethyl acetate and n-butanol fractions, 32 g of methanolic extract was dissolved in 100 mL of distilled water and then sequentially extracted three times each with 150 mL of n-hexane, chloroform, ethyl acetate and n-butanol. The combined organic extractions were evaporated to dryness under vacuum to obtain the corresponding extracts of n-hexane (3.02 g), chloroform (4.01 g), ethyl acetate (7.03 g) and n-butanol (6.05), respectively. Finally, the aqueous residue (10.9 g) was also obtained by drying the aqueous layer under vacuum at 45 °C for 1 h. The methanolic extract and its sub-fractions were subjected to antimicrobial activity, phytochemical screening and GC–MS analysis.
2.5. Antimicrobial assays
2.5.1. Sample preparation
The stock solutions were prepared by dissolving dried methanolic extract and its fractions in DMSO (50 mg/mL). Stock solutions were then diluted into five different concentrations of 10, 20, 30, 40, and 50 mg/mL in DMSO. Solutions of standard drugs were also prepared in the same manner.
2.5.2. Determination of zones of inhibition (ZOI)
A literature known agar well diffusion assay was used according to the method recommended by Clinical and Laboratory Standards Institute (CLSI) (Njeru et al., 2015, Al Akeel et al., 2014). A well-mixed nutrient agar was prepared by dissolving 32 g of NA (Nutrient Agar) in 1 L of distilled water which was autoclaved at 121 °C for 1 h. For the preparation of bacterial suspension, a lyophilized bacterial culture was diluted before it was streaked onto a slant made of tryptic soya agar and placed in an incubator at 32.5 °C for 24 h. Bacterial growth was observed in the incubated slant, which was then diluted carefully by mixing 3 mL of normal saline in the slant. 2 mL of the diluted culture was then taken and its absorbance was standardized with 0.5% McFarland standard solution at 550 nm. In order to make seed agar, 2 mL of the diluted suspension were added into100 mL of the liquid nutrient agar which was kept at 50 °C. Since, B. subtilis is a spore forming bacterial strain which required extended period of incubation of 7 days for maximum spore formation on the slant. Then 20 mL of the 0.9% of saline solution was added and the resulting mixture was heated at 70 °C for 30 min to ensure complete killing of all vegetative cells of the B. subtilis. In addition, 2 mL of the suspension was taken and standardized with 0.5% McFarland standard solution at 550 nm. The rest of the procedure was same. Then 20 mL of NA was poured into each petri plate of 90 mm diameter and allowed to solidify. After solidification, 4 mL of the seed agar was poured onto the base agar to make a thin layer of seed agar in the petri plate, which was then placed in refrigerator for 1 h for cooling. Holes were made into the petri plates at equal distances with a sterilized agar borer (9 mm). Each of holes was filled with 140 µL of a plant extract prepared in DMSO at a concentration of 40 mg/L. The plates were then kept in an incubator at 37 °C for overnight. The clear ZOI of the samples were measured in millimeters by using digital Vernier’s clippers. The same method was used for all plant samples, DMSO was used as negative standard whereas known antibiotics i.e. amoxicillin, cefixime and levofloxacin were employed as positive standard (Mohapatra et al., 2011). All tests were performed in triplicate.
2.5.3. Determination of minimum inhibitory concentration (MIC)
The MIC of a sample was determined by agar well dilution method (Wiegand, Hilpert, and Hancock, 2008). Mueller-Hinton agar was dissolved in distilled water with concentration 32 g/L, and autoclaved at 121 °C for 1 h. The agar was allowed to cool to 50 °C and poured into Petri plates for sample preparation. Different dilutions of each extract/fraction were prepared in this agar with concentrations 10–50 µg/20 mL. The content of each plate was mixed well and allowed to solidify. Likewise, stock solution of each extract/fraction was prepared in DMSO (5 mg/mL) and dilutions were made. For inoculum, 4–5 colonies of a pure culture were selected and introduced into a test tube containing 5 mL of 5% sterilized NS solution and the mixture was compared with 0.5% McFarland solution to adjust the density of bacterial suspension at 106 CFU/mL. The inoculum for each bacterium was poured into the wells. Then these thirty bacterial strains (3 µL) were transferred on to the MHA medium. The plates were kept in an incubator at 37 °C for overnight. The growth of the bacterial strains was observed according to the template and the MIC values were recorded. All tests were performed in triplicate.
2.5.4. Statistical analysis
In order to keep up the reproducibility of the results statistical analysis was also performed. Mean values of the results were calculated as all of the experiments were done in triplicate. The data was subjected to one-way analysis of variance (ANOVA) and results were expressed (where appropriate) as mean ± standard deviation.
2.5.5. Identification of chemical constituents
The chemical compounds isolated from the extracts of E. serrata were identified by GC–MS analysis. The known compounds were identified by using database library NIST05 comparing them with their known GC–MS profile.
3. Results
3.1. Antimicrobial activity
3.1.1. Zones of inhibition (ZI)
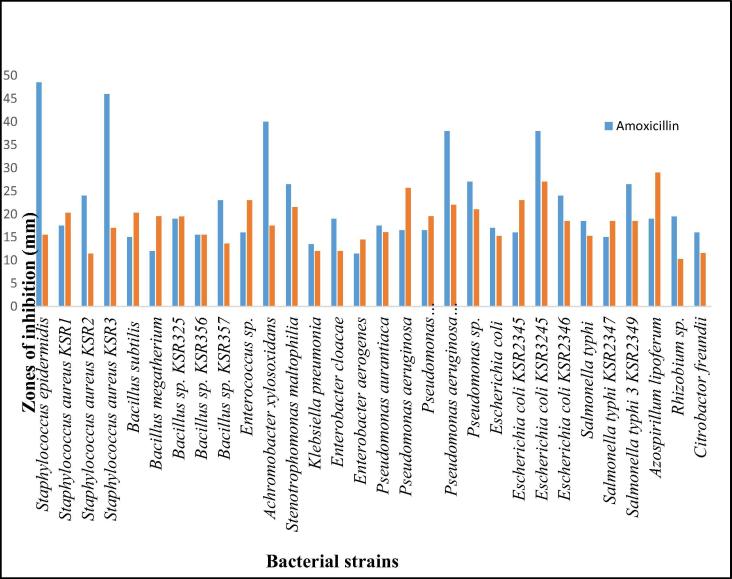
The antimicrobial properties of the methanolic extract were studied against 30 bacterial strains and the results in zones of inhibition (mm) are displayed in Table 1. For comparison, three antibiotics were used in the study namely amoxicillin, cefixime and levofloxacin. The methanolic extract showed a broad range of antimicrobial properties against the tested microorganisms including human pathogens. It showed notable activity at a concentration of 40 mg/mL against Azospirillum lipoferum (29.0 ± 2.64), Pseudomonas aeruginosa (1) (25.7 ± 0.57) and Staphylococcus aureus KSR1 (20.3 ± 0.57). The methanolic extract also showed considerable activity at concentration of 20 mg/mL against Escherichia coli (28.0 ± 0.00), Bacillus megatherium (19.6 ± 2.08), Bacillus subtilis (32.5 ± 2.12) and at concentration of 10 mg/mL against Pseudomonas aeruginosa (1) (30 ± 0.000). In addition, these inhibition zones demonstrated by methanolic extracts are higher than the zones of inhibition of standard antibiotic amoxicillin (Fig. 1). Moreover, amoxicillin turned out to be highly active against Staphylococcus epidermidis, Staphylococcus aureus KSR3, Achromobacter xylosoxidans, Pseudomonas aeruginosa KSR360 and Escherichia coli KSR3245 whereas in other cases the methanolic extract has either equal or higher activity then amoxicillin (Fig. 1).
Table 1.
Comparison of antimicrobial activity* of the methanolic extract of leaves of E. serrata with standard antibiotics against different bacterial pathogens in terms of ZOI (mm).
| Sr. No. | Bacterial strains | Gram +/− | Standard antibiotics (40 mg/mL) |
Methanolic extract (mg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aa | Cb | Lc | 40 | 20 | 10 | 5 | 2.5 | |||
| 1 | Staphylococcus epidermidis | + | 48.5 ± 2.12 | 41.5 ± 0.70 | 48.0 ± 2.82 | 15.5 ± 0.70 | 13.5 ± 0.70 | 12.5 ± 0.70 | 11.0 ± 0.00 | 10.5 ± 0.00 |
| 2 | Staphylococcus aureus KSR1 | + | 17.5 ± 2.12 | 24.5 ± 0.50 | 32.0 ± 1.00 | 20.3 ± 0.57 | 19.5 ± 2.12 | 17.5 ± 0.70 | 15.5 ± 0.70 | 14.5 ± 0.70 |
| 3 | Staphylococcus aureus KSR2 | + | 24.0 ± 1.41 | 32.0 ± 1.41 | 32.0 ± 1.41 | 11.5 ± 0.70 | 32 ± 0.000 | 31 ± 1.212 | 29.0 ± 1.41 | 27.0 ± 1.41 |
| 4 | Staphylococcus aureus KSR3 | + | 46.0 ± 0.00 | 53.0 ± 2.82 | 54.5 ± 0.70 | 17.0 ± 0.00 | 12.0 ± 0.00 | 00.0 ± 0.00 | 00.0 ± 0.00 | 00.0 ± 0.00 |
| 5 | Bacillus subtilis | + | 15.0 ± 1.41 | 24.5 ± 2.12 | 26.0 ± 0.00 | 20.3 ± 0.50 | 32.5 ± 2.12 | 29.0 ± 5.65 | 28.5 ± 0.70 | 26.0 ± 0.00 |
| 6 | Bacillus megatherium | + | 12.0 ± 0.00 | 23.5 ± 0.50 | 31.0 ± 1.00 | 19.6 ± 2.08 | 20.5 ± 7.77 | 19.0 ± 5.65 | 18.5 ± 6.63 | 17.5 ± 6.36 |
| 7 | Bacillus sp. KSR325 | + | 19.0 ± 0.00 | 25.5 ± 1.50 | 30.0 ± 2.50 | 19.5 ± 0.50 | 26.0 ± 1.41 | 24.0 ± 1.41 | 22.0 ± 0.00 | 19.5 ± 3.53 |
| 8 | Bacillus sp. KSR356 | + | 15.5 ± 1.50 | 24.5 ± 1.00 | 27.5 ± 0.50 | 15.5 ± 0.58 | 22.5 ± 2.12 | 18.0 ± 1.41 | 17.0 ± 0.00 | 15.5 ± 1.12 |
| 9 | Bacillus sp. KSR357 | + | 23.0 ± 3.00 | 27.0 ± 1.50 | 43.0 ± 0.70 | 13.6 ± 0.57 | 20.0 ± 1.41 | 18.0 ± 0.00 | 15.0 ± 4.24 | 14.5 ± 2.12 |
| 10 | Enterococcus sp. | + | 16.0 ± 1.00 | 24.5 ± 2.12 | 30.0 ± 0.00 | 23.00 ± 1.0 | 21.0 ± 8.34 | 17.5 ± 3.53 | 11.0 ± 1.41 | 10.5 ± 0.70 |
| 11 | Achromobacter xylosoxidans | − | 40.0 ± 1.41 | 46.5 ± 0.70 | 51.0 ± 1.41 | 17.5 ± 0.70 | 17.0 ± 0.00 | 16.5 ± 0.70 | 15.5 ± 0.70 | 15.0 ± 0.00 |
| 12 | Stenotrophomonas maltophilia | − | 26.5 ± 1.41 | 32.5 ± 0.70 | 47.0 ± 1.41 | 21.5 ± 0.70 | 18.0 ± 0.00 | 15.0 ± 0.00 | 13.0 ± 0.00 | 12.5 ± 0.70 |
| 13 | Klebsiella pneumonia | − | 13.5 ± 2.12 | 21.0 ± 0.00 | 38.5 ± 0.70 | 12.0 ± 0.00 | 11.5 ± 0.00 | 11.0 ± 0.00 | 10.5 ± 0.70 | 00.0 ± 0.00 |
| 14 | Enterobacter cloacae | − | 19.0 ± 1.41 | 39.0 ± 2.82 | 41.0 ± 2.82 | 12.0 ± 1.41 | 12.0 ± 0.00 | 11.0 ± 1.41 | 10.5 ± 0.70 | 00.0 ± 0.00 |
| 15 | Enterobacter aerogenes | − | 11.5 ± 0.70 | 23.0 ± 0.00 | 38.0 ± 0.00 | 14.5 ± 0.70 | 13.5 ± 0.00 | 13.0 ± 0.00 | 12.0 ± 1.41 | 11.0 ± 0.00 |
| 16 | Pseudomonas aurantiaca | − | 17.5 ± 2.12 | 25.0 ± 1.00 | 30.5 ± 2.50 | 16.1 ± 0.76 | 19.5 ± 0.70 | 21.5 ± 0.70 | 19.5 ± 2.12 | 17.5 ± 0.70 |
| 17 | Pseudomonas aeruginosa | − | 16.5 ± 0.70 | 24.5 ± 0.50 | 29.0 ± 0.50 | 25.7 ± 0.57 | 28 ± 0.000 | 30 ± 0.000 | 24.5 ± 0.70 | 23.5 ± 0.70 |
| 18 | Pseudomonas aeruginosaKSR125 | − | 16.5 ± 0.50 | 24.0 ± 0.00 | 35.0 ± 2.00 | 19.6 ± 0.57 | 22 ± 0.000 | 20.0 ± 1.41 | 19 ± 2.828 | 17 ± 0.000 |
| 19 | Pseudomonas aeruginosa KSR360 | − | 38.0 ± 0.00 | 44.0 ± 1.41 | 43.5 ± 0.70 | 22.0 ± 1.41 | 20.0 ± 0.00 | 18.5 ± 0.70 | 18.00 ± 0.0 | 16.0 ± 0.00 |
| 21 | Pseudomonas sp. | − | 27.0 ± 0.00 | 39.5 ± 0.70 | 33.0 ± 1.41 | 21.0 ± 1.41 | 19.5 ± 0.70 | 18.0 ± 0.00 | 17.5 ± 0.70 | 16.0 ± 0.00 |
| 22 | Escherichia coli | − | 17.0 ± 1.41 | 27.0 ± 3.00 | 35.0 ± 2.00 | 15.3 ± 0.57 | 28 ± 0.000 | 26.0 ± 0.00 | 23.5 ± 3.53 | 22.5 ± 0.70 |
| 23 | Escherichia coli KSR2345 | − | 16.0 ± 1.41 | 26.0 ± 0.00 | 30.0 ± 1.00 | 23.00 ± 1.0 | 21 ± 1.414 | 20.0 ± 0.00 | 18.5 ± 0.70 | 17.5 ± 0.70 |
| 24 | Escherichia coli KSR3245 | − | 38.0 ± 0.00 | 43.5 ± 2.12 | 47.0 ± 0.00 | 27.0 ± 1.41 | 23.5 ± 2.12 | 20.5 ± 0.00 | 19.0 ± 0.00 | 18.0 ± 0.00 |
| 25 | Escherichia coli KSR2346 | − | 24.0 ± 1.41 | 30.0 ± 1.41 | 30.0 ± 1.41 | 18.5 ± 0.70 | 22.0 ± 0.00 | 19.0 ± 0.00 | 17.0 ± 0.00 | 16.5 ± 0.70 |
| 26 | Salmonella typhi | − | 18.5 ± 0.70 | 26.5 ± 0.50 | 43.0 ± 3.00 | 15.3 ± 0.57 | 20 ± 1.414 | 19.5 ± 0.70 | 18.5 ± 0.70 | 18.0 ± 4.21 |
| 27 | Salmonella typhi KSR2347 | − | 15.0 ± 1.41 | 24.0 ± 0.00 | 38.5 ± 1.41 | 18.5 ± 0.50 | 20 ± 0.000 | 19.5 ± 0.70 | 18.0 ± 0.00 | 16.0 ± 4.24 |
| 28 | Salmonella typhi 3 KSR2349 | − | 26.5 ± 0.70 | 36.5 ± 2.12 | 36.5 ± 2.12 | 18.5 ± 0.70 | 23.5 ± 2.21 | 21.0 ± 1.41 | 18.0 ± 1.41 | 16.0 ± 2.82 |
| 29 | Azospirillum lipoferum | − | 19.0 ± 1.41 | 25.5 ± 0.70 | 34.5 ± 1.50 | 29.0 ± 2.64 | 21.5 ± 1.41 | 19.0 ± 2.82 | 18.5 ± 2.12 | 17.0 ± 1.41 |
| 30 | Rhizobium sp. | − | 19.5 ± 1.50 | 27.0 ± 0.00 | 35.5 ± 0.50 | 10.33 ± 0.5 | 27.0 ± 0.00 | 24.5 ± 4.94 | 23.5 ± 3.53 | 22.0 ± 0.00 |
| 31 | Citrobactor freundii | − | 16.0 ± 0.00 | 25.0 ± 1.00 | 35.5 ± 1.50 | 11.68 ± 0.5 | 24.0 ± 4.94 | 23.0 ± 0.00 | 13.5 ± 0.70 | 15.0 ± 0.00 |
Negative control = DMSO (dimethyl sulfoxide), showed no activity.
A = Amoxylin.
C = Cefixime.
L = Levofloxacin.
Fig. 1.
Comparison of antimicrobial activity of the methanolic extract of leaves of E. serrata with standard amoxicillin against different bacterial pathogens in terms ZOI (mm).
3.1.2. Antimicrobial activity of fractions of methanolic extracts
Driven by the promising antimicrobial activities of the methanolic extract of leaves of E. serrata, the activity of the sub-fractions of the crude extract against highly sensitive bacterial strains was also explored. The results are exhibited in Tables 2. The n-hexane fraction at a concentration of 40 mg/mL showed considerably high activities against against Stenotrophomonas maltophilia (19.5 ± 0.707), Staphylococcus aureus KSR1 (18.5 ± 0.707) and Salmonella typhi KSR2347 (17.5 ± 0.70 mm). Likewise, the chloroform fraction at a concentration of 40 mg/mL showed good activities against Staphylococcus aureus KSR1 (17.5 ± 0.707), Stenotrophomonas maltophilia (16.0 ± 0.000) and Achromobacter xylosoxidans (17.5 ± 0.707) (Table 2). Similarly, ethyl acetate fraction (40 mg/mL) also exhibited significant activity against Staphylococcus aureus (23.5 ± 0.707), Escherichia coli (1) (28.5 ± 0.707), Azospirillum lipoferum (26.0 ± 0.000) (Table 2). The n-butanol extract (40 mg/mL) displayed significant activities against Staphylococcus epidermidis (20.0 ± 1.414), Bacillus subtillis (20.0 ± 1.414), Escherichia coli KSR2345 (28.5 ± 0.707) and Azospirillum lipoferum (26.0 ± 0.000) (Table 2). In addition, the aqueous fraction at a concentration of 40 mg/mL also showed high value of ZOI against Staphylococcus aureusKSR1 (20.0 ± 1.414) and Bacillus subtilis (22.0 ± 1.414) (Table 2).
Table 2.
Antimicrobial activity of sub-fractions of leaves of E. serrata against different bacterial pathogens in terms of ZOI (mm).
| Sr.No. | Bacterial stains Gram +/− |
n-Hexane sub-fraction | Chloroform sub-fraction | Ethyl acetate sub-fraction | n-Butanol sub-fraction | Aqueous residue | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentrations (mg/mL) |
Concentrations (mg/mL) |
Concentrations (mg/mL) |
Concentrations (mg/mL) |
Concentrations (mg/mL) |
||||||||
| 40 | 20 | 40 | 20 | 40 | 20 | 40 | 20 | 40 | 20 | |||
| ZI (mm) | ZI (mm) | ZI (mm) | ZI (mm) | ZI (mm) | ZI (mm) | ZI (mm) | ZI (mm) | ZI (mm) | ZI (mm) | |||
| 1 | Staphylococcus epidermidis | + | 13.5 ± 0.7 | 12.0 ± 0.00 | 14.0 ± 0.00 | 13.5 ± 0.70 | 10.5 ± 0.70 | 0.00 ± 0.00 | 20.0 ± 1.41 | 18.5 ± 0.70 | 00.0 ± 0.000 | 00.0 ± 0.00 |
| 2 | Staphylococcus aureus KSR1 | + | 18.5 ± 0.70 | 15.0 ± 0.00 | 17.5 ± 0.70 | 16.0 ± 0.00 | 23.5 ± 0.70 | 22.0 ± 0.00 | 18.5 ± 2.12 | 17.5 ± 2.12 | 20.0 ± 1.414 | 19.0 ± 0.00 |
| 3 | Pseudomonas aeruginosaKSR125 | + | 16.0 ± 1.41 | 14.5 ± 0.70 | 17.5 ± 2.12 | 14.5 ± 0.70 | 14.0 ± 1.41 | 13.0 ± 1.41 | 14.5 ± 0.70 | 13.5 ± 0.70 | 14.0 ± 1.414 | 13.5 ± 0.70 |
| 4 | Bacillus sp. | + | 12.5 ± 0.70 | 10.0 ± 0.00 | 14.5 ± 0.70 | 13.5 ± 0.70 | 15.0 ± 1.21 | 12.0 ± 0.00 | 14.0 ± 1.21 | 12.0 ± 0.0 | 14.5 ± 0.707 | 12.0 ± 0.000 |
| 5 | Bacillus subtilis | + | 12.5 ± 0.70 | 10.0 ± 0.00 | 16.0 ± 1.41 | 13.0 ± 0.00 | 19.5 ± 0.70 | 18.5 ± 0.70 | 20.0 ± 1.41 | 18.5 ± 0.70 | 22.0 ± 1.414 | 20.5 ± 0.70 |
| 6 | Enterococcus sp. | + | 14.5 ± 0.70 | 13.0 ± 0.00 | 14.0 ± 0.00 | 12.5 ± 0.70 | 18.0 ± 1.41 | 16.5 ± 2.12 | 18.0 ± 1.41 | 16.5 ± 2.12 | 18.5 ± 2.121 | 17.5 ± 2.12 |
| 7 | Achromobacter xylosoxidans | − | 12.5 ± 0.70 | 10.0 ± 0.00 | 17.5 ± 0.70 | 16.0 ± 1.41 | 14.0 ± 1.41 | 12.5 ± 0.70 | 17.0 ± 1.41 | 15.0 ± 0.00 | 13.5 ± 0.707 | 13.0 ± 0.00 |
| 8 | Stenotrophomonas maltophilia | − | 19.5 ± 0.70 | 17.5 ± 0.70 | 16.0 ± 0.00 | 15.0 ± 0.00 | 13.5 ± 0.70 | 11.0 ± 0.00 | 15.5 ± 0.70 | 14.0 ± 1.41 | 00.0 ± 0.00 | 00.0 ± 0.00 |
| 9 | Klebsiella pneumonia | − | 12.0 ± 0.00 | 10.0 ± 0.00 | 13.0 ± 1.41 | 12.5 ± 0.70 | 15.5 ± 0.70 | 12.5 ± 0.70 | 13.0 ± 0.00 | 12.0 ± 1.41 | 12.0 ± 0.000 | 9.0 ± 0.000 |
| 10 | Salmonella typhi KSR2347 | − | 17.5 ± 0.70 | 14.0 ± 0.00 | 14.0 ± 0.00 | 13.5 ± 0.70 | 16.0 ± 0.00 | 14.5 ± 0.70 | 16.0 ± 0.00 | 14.5 ± 0.70 | 13.0 ± 1.141 | 12.5 ± 0.70 |
| 11 | Escherichia coli KSR2345 | − | 15.5 ± 0.70 | 12.5 ± 0.70 | 15.0 ± 0.00 | 13.5 ± 0.70 | 28.5 ± 0.70 | 27.0 ± 1.41 | 28.5 ± 0.70 | 27.0 ± 1.41 | 00.0 ± 0.000 | 00.0 ± 0.00 |
| 12 | Azospirillum lipoferum | − | 16.0 ± 0.00 | 13.0 ± 0.00 | 14.5 ± 0.00 | 12.5 ± 0.00 | 26.0 ± 0.00 | 19.0 ± 1.41 | 26.0 ± 0.00 | 19.0 ± 1.41 | 12.0 ± 0.000 | 10.0 ± 1.41 |
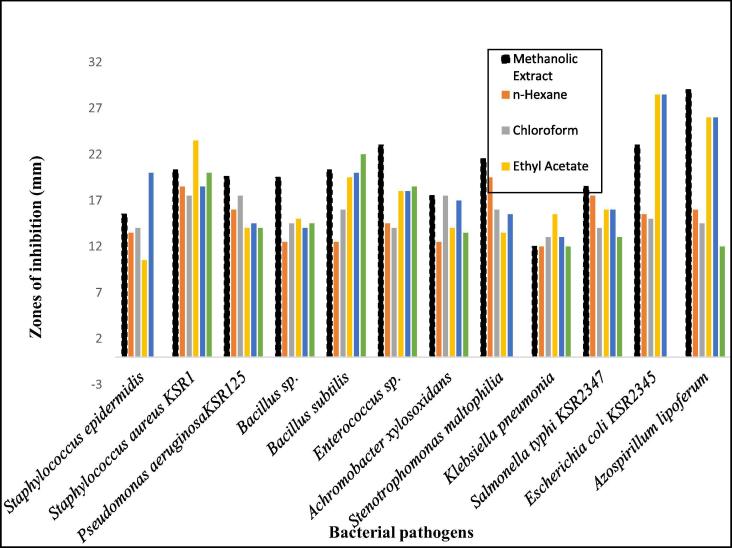
Fig. 2 shows a comparison of the efficacy of the methanolic extract with its sub-fractions at 40 mg/mL which reveals that all the fractions have activities against the given bacterial strains. However, n-butanol extract was found to be more active against Staphylococcus epidermidis whereas ethyl acetate fraction turned out to be more active against Staphylococcus aureusKSR1 compared to other fractions. Moreover, both n-butanol and ethyl acetate fractions were found to be very active against Escherichia coli KSR3245 in comparison to other fractions. Likewise, methanolic extract has shown higher efficacy against all of the remaining tested bacterial strains compared to its sub-fractions (Fig. 2).
Fig. 2.
Comparison of antimicrobial activity of the methanolic extract with its sub-fractions of leaves of E. serrata against different bacterial pathogens in terms of ZOI (mm).
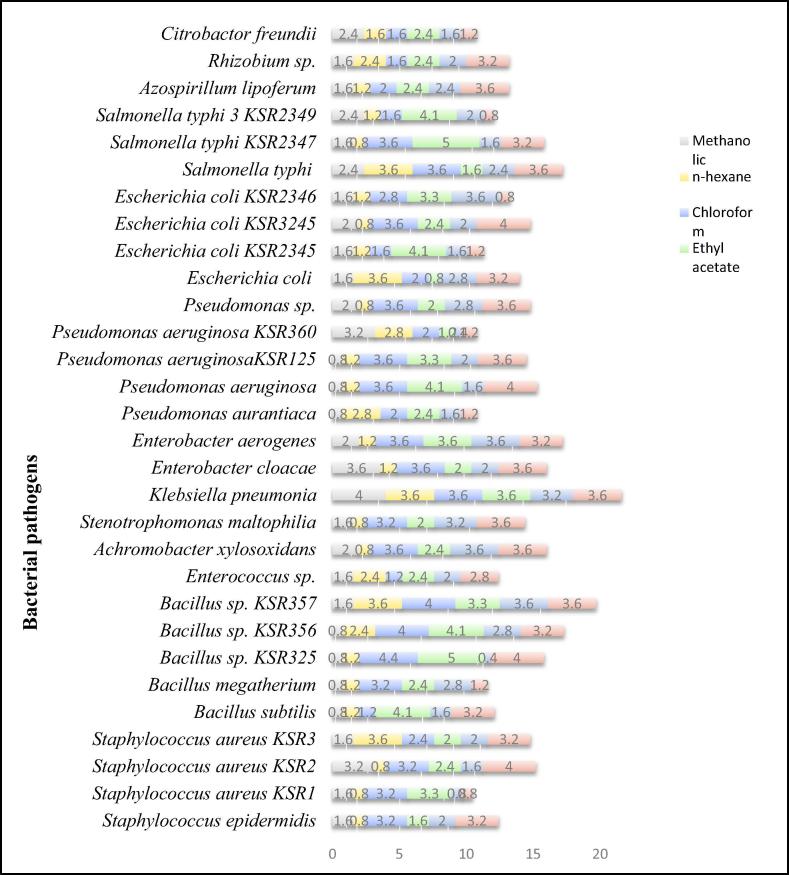
3.1.3. The MIC values
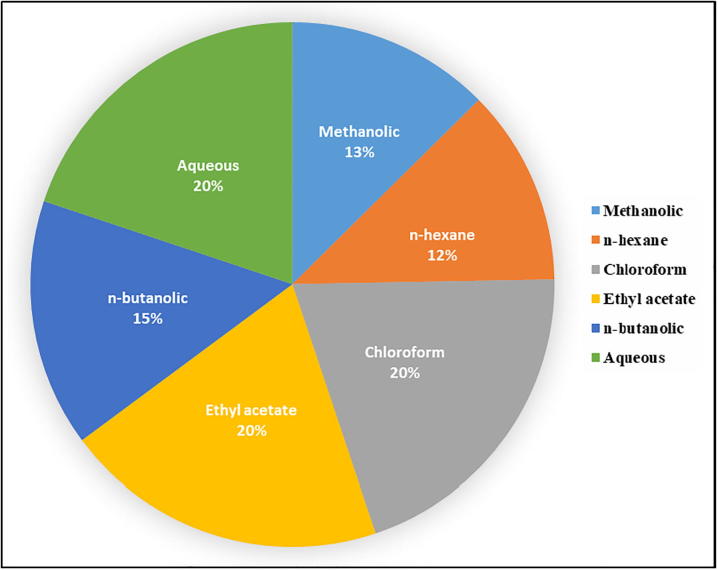
The results of antimicrobial screening by agar well diffusion assay prompted us to calculate the MIC. The methanolic extract and its fractions showed notable results (Table 3). The methanolic extract was found to be the most effective among all the samples. The MIC values were ranged between 0.8 and 4.0 mg/mL against different strains (Table 3 and Fig. 3). The methanolic extract at 0.8 mg/mL concentration inhibited the growth of most of the bacterial strains including Bacillus subtilis, Bacillus megatherium, Bacillus sp. KSR325, Bacillus sp. KSR356, Pseudomonas aurantiaca, Pseudomonas aeruginosa, Pseudomonas aeruginosaKSR125. The n-hexane fraction also displayed significant MIC values ranging from 0.8 to 3.6 mg/mL. Likewise, the MIC values of chloroform fraction were found between 1.2 and 4.4 mg/mL. At a concentration of 3.6 mg/mL, it inhibited the growth of most of the bacterial strains. Moreover, the MIC values of ethyl acetate and n-butanol fractions were ranged between 0.8 and 5.0 and 0.8 to 3.6 mg/mL, respectively. On the other hand, ethyl acetate fraction at a concentration of 2.4 mg/mL inhibited the greater number of bacterial strains whereas in case of n-butanol fraction the 2.0 mg/mL was found to be the most effective concentration for the inhibition of the growth of large number of bacteria (Table 3). Likewise, the aqueous fraction also showed considerable activity against the tested microorganisms, MIC values were ranged between 0.8 and 4.0 mg/mL (Table 3). The pie chart shown in Fig. 4 indicates that fractions were effective in the following descending order n-hexane (12%) > methanolic (13%) > n-butanol (15%) whereas the remaining fraction including chloroform, ethyl acetate and aqueous fractions were equally effective (20%).
Table 3.
MICs of the extracts of E. serrata (mg/mL) against various bacterial strains.
| S. No. | Bacterial strains | Gram +/− | Methanolic | n-hexane | Chloroform | Ethyl acetate | n-butanolic | Aqueous |
|---|---|---|---|---|---|---|---|---|
| 1 | Staphylococcus epidermidis | + | 1.6 | 0.8 | 3.2 | 1.6 | 2.0 | 3.2 |
| 2 | Staphylococcus aureus KSR1 | + | 1.6 | 0.8 | 3.2 | 3.3 | 0.8 | 0.8 |
| 3 | Staphylococcus aureus KSR2 | + | 3.2 | 0.8 | 3.2 | 2.4 | 1.6 | 4.0 |
| 4 | Staphylococcus aureus KSR3 | + | 1.6 | 3.6 | 2.4 | 2.0 | 2.0 | 3.2 |
| 5 | Bacillus subtilis | + | 0.8 | 1.2 | 1.2 | 4.1 | 1.6 | 3.2 |
| 6 | Bacillus megatherium | + | 0.8 | 1.2 | 3.2 | 2.4 | 2.8 | 1.2 |
| 7 | Bacillus sp. KSR325 | + | 0.8 | 1.2 | 4.4 | 5.0 | 0.4 | 4.0 |
| 8 | Bacillus sp. KSR356 | + | 0.8 | 2.4 | 4.0 | 4.1 | 2.8 | 3.2 |
| 9 | Bacillus sp. KSR357 | + | 1.6 | 3.6 | 4.0 | 3.3 | 3.6 | 3.6 |
| 10 | Enterococcus sp. | + | 1.6 | 2.4 | 1.2 | 2.4 | 2.0 | 2.8 |
| 11 | Achromobacter xylosoxidans | − | 2.0 | 0.8 | 3.6 | 2.4 | 3.6 | 3.6 |
| 12 | Stenotrophomonas maltophilia | − | 1.6 | 0.8 | 3.2 | 2.0 | 3.2 | 3.6 |
| 13 | Klebsiella pneumonia | − | 4.0 | 3.6 | 3.6 | 3.6 | 3.2 | 3.6 |
| 14 | Enterobacter cloacae | − | 3.6 | 1.2 | 3.6 | 2.0 | 2.0 | 3.6 |
| 15 | Enterobacter aerogenes | − | 2.0 | 1.2 | 3.6 | 3.6 | 3.6 | 3.2 |
| 16 | Pseudomonas aurantiaca | − | 0.8 | 2.8 | 2.0 | 2.4 | 1.6 | 1.2 |
| 17 | Pseudomonas aeruginosa | − | 0.8 | 1.2 | 3.6 | 4.1 | 1.6 | 4.0 |
| 18 | Pseudomonas aeruginosaKSR125 | − | 0.8 | 1.2 | 3.6 | 3.3 | 2.0 | 3.6 |
| 19 | Pseudomonas aeruginosa KSR360 | − | 3.2 | 2.8 | 2.0 | 1.2 | 0.4 | 1.2 |
| 20 | Pseudomonas sp. | − | 2.0 | 0.8 | 3.6 | 2.0 | 2.8 | 3.6 |
| 21 | Escherichia coli | − | 1.6 | 3.6 | 2.0 | 0.8 | 2.8 | 3.2 |
| 22 | Escherichia coli KSR2345 | − | 1.6 | 1.2 | 1.6 | 4.1 | 1.6 | 1.2 |
| 23 | Escherichia coli KSR3245 | − | 2.0 | 0.8 | 3.6 | 2.4 | 2.0 | 4.0 |
| 24 | Escherichia coli KSR2346 | − | 1.6 | 1.2 | 2.8 | 3.3 | 3.6 | 0.8 |
| 25 | Salmonella typhi | − | 2.4 | 3.6 | 3.6 | 1.6 | 2.4 | 3.6 |
| 26 | Salmonella typhi KSR2347 | − | 1.6 | 0.8 | 3.6 | 5.0 | 1.6 | 3.2 |
| 27 | Salmonella typhi 3 KSR2349 | − | 2.4 | 1.2 | 1.6 | 4.1 | 2.0 | 0.8 |
| 28 | Azospirillum lipoferum | − | 1.6 | 1.2 | 2.0 | 2.4 | 2.4 | 3.6 |
| 29 | Rhizobium sp. | − | 1.6 | 2.4 | 1.6 | 2.4 | 2.0 | 3.2 |
| 30 | Citrobactor freundii | − | 2.4 | 1.6 | 1.6 | 2.4 | 1.6 | 1.2 |
Fig. 3.
MICs values of extracts of E. serrata against various bacterial pathogens (mg/mL).
Fig. 4.
The dose dependent efficiency of extracts of E. serrata against various bacterial pathogens.
3.2. GC–MS analysis
The GC–MS analysis was performed to explore the volatile chemical constitutes in different fractions (Table 4). Compound 2,2′-Methylenebis[6-(1,1-dimethylethyl)-4-methyl phenol was present in hexane (2.126%), ethyl acetate (2.586%) and chloroform (16.026%). Similarly, phytol existed in hexane (4.420%), chloroform (8.439%) and ethyl acetate (2.410%). Likewise, n-hexadecanoic acid was found in chloroform (14.806%), ethyl acetate (10.048%) and n-butanol (17.853%). Compounds such as Diisooctyl ester-1,2-benzenedicarboxylic acid (84.260%) heptacosane (3.148%) and 3-ethyl-5-(2-ethylbutyl)-octadecane (3.320%) were also found in hexane fraction. Moreover, di-n-octyl phthalate (12.950%) and 3,5-dehydro-6-methoxy-, pivalate, cholest-22-ene-21-ol (10.570%) found in chloroform fraction are believed to render activity to kill all the tested microorganisms (Frank et al., 2016). Furthermore, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-benzenepropanoic acid (38.810%) was present in the n-butanol fraction. In short, the presence of variety of these metabolites was responsible for the different activities against the tested bacterial strains.
Table 4.
GC–MS analysis of the different extracts of leaves of E. serrata.
| Sr. No | Retention time (min) | % of total | Compounds | Molecular formula |
|---|---|---|---|---|
| n-hexane fraction | ||||
| 1 | 23.022 | 4.420 | Phytol | C20H40 |
| 2 | 25.749 | 2.126 | 2,2′-Methylenebis(6-tert-butyl-4-methylphenol) | C23H32O2 |
| 3 | 26.700 | 84.260 | Diisooctyl ester-1,2-benzenedicarboxylic acid | C24H38O4 |
| 4 | 29.127 | 3.148 | Heptacosane | C27H56 |
| 5 | 30.672 | 3.320 | 3-Ethyl-5-(2-ethylbutyl)- octadecane | C26H54 |
| 6 | 33.468 | 2.726 | 2-Hexadecanol | C16H34O |
| Chloroform fraction | ||||
| 1 | 21.564 | 14.806 | n-Hexadecanoic acid | C16H32O2 |
| 2 | 23.022 | 8.439 | Phytol | C20H40 |
| 3 | 25.749 | 16.026 | 2,2′-Methylenebis(6-tert-butyl-4-methylphenol) | C23H32O2 |
| 4 | 26.687 | 112.950 | Di-n-octyl phthalate | C24H38O4 |
| 5 | 29.127 | 10.570 | Cholest-22-ene-21-ol, 3,5-dehydro-6-methoxy, pivalate | C33H54O3 |
| 6 | 30.666 | 23.480 | 3-Ethyl-5-(2-ethylbutyl)-octadecane | C26H54 |
| Ethyl acetate fraction | ||||
| 1 | 19.838 | 1.292 | (E)-5-Eicosene | C20H40 |
| 2 | 21.664 | 10.048 | n-Hexadecanoic acid | C16H32O2 |
| 3 | 23.022 | 2.410 | Phytol | C20H40O |
| 4 | 23.309 | 3.072 | 4-Chloro-3-n-hexyltetrahydropyron | C11H21ClO |
| 5 | 25.749 | 2.586 | 2,2′-Methylenebis(6-tert-butyl-4-methylphenol) | C23H32O2 |
| 6 | 26.694 | 181.884 | Di-n-octyl phthalate | C24H38O4 |
| n-butanol fraction | ||||
| 1 | 21.445 | 7.421 | Cyclohexanol, 5-methyl-2-(1-methylethyl) | C10H20O |
| 2 | 21.621 | 17.853 | n-Hexadecanoic acid | C16H32O2 |
| 3 | 23.022 | 12.888 | 1-Cyclohexylnonene | C15H28 |
| 4 | 25.743 | 23.028 | Diisooctyl phthalate | C24H38O4 |
| 5 | 26.687 | 38.810 | 3,5-Bis(1,1-dimethylethyl)-4-hydroxy-benzenepropanoic acid | C35H62O3 |
| Aqueous fraction | ||||
| 1 | 21.564 | 26.358 | n-Hexadecanoic acid | C16H32O2 |
| 2 | 26.700 | 73.642 | Mono(2-ethylexyl) ester-1,2-benzenedicarboxylic acid | C16H22O4 |
4. Discussion
The higher activities of the plant extracts have been attributed to the presence of different types of secondary metabolites (Joanne et al., 2007). Further, the secondary metabolites like flavonoids, polyphenols, coumarins, tannins, triterpenes, saponins, cardiac glycosides and alkaloids have been reported to have considerable antimicrobial activities (Teke et al., 2011). GC–MS analysis of sub-fractions of methanolic extract revealed the presence of n-hexadecanoic acid and mono(2-ethylhexyl) ester of 1,2-benzenedicarboxylic acid that are known to have antimicrobial activity (Agoramoorthy et al., 2007).
The higher activity of n-hexane fraction at a concentration of 40 mg/mL is attributed to the presence of compounds such as phytol and 2,2′-Methylenebis(6-tert-butyl-4-methylphenol) (Hernández-Villegas et al., 2012). Similarly, other fractions including chloroform, ethyl acetate, n-butanol and aqueous fractions were found active against the tested microbes indicating that both polar and non-polar fractions were active against the microbes (Rocha et al., 2011).
The methanolic extract proved to be highly efficient in terms of lowest dose needed to kill the tested microbes. Compared to methanolic extract, n-hexane fraction required higher dose (3.6 mg/mL) to inhibit the growth of the same bacterial strains since at concentration of 0.8 mg/mL it did not inhibit the growth of any of the bacteria killed by methanolic extract (Table 3). This suggested that the composition of the natural products in n-hexane fraction is quite different from that of methanolic fraction (Khan et al., 2016). However, n-hexane fraction showed the best performance in killing the tested microbes since most of the bacterial strains were killed at very low dosages (Fig. 3). This fact was further confirmed by evaluating overall dosage required by all the fractions to kill germs in the MIC experiment. The n-hexane fraction required the lowest dosage to inhibit the growth of 32 bacterial strains. The behavior of the remaining fractions was also appreciable in terms of their MIC values. The antimicrobial properties of methanolic extract and its sub-fractions were attributed to the presence of various metabolites confirmed by GC–MS analysis. These compounds contain different functional groups such as hydroxyl and carboxylic groups, which are required for antimicrobial activity (Omosa et al., 2016).
5. Conclusion
The antimicrobial activity of methanolic extract and its sub-fractions of leaves of Ehretia serrata were evaluated against a wide range of pathogens. Both the methanol and n-hexane extracts have shown considerably promising results against the tested multi drug resistant human pathogens like Escherichia coli, Staphylococcus aureus and Azospirilum lipoferum. In some cases, crude methanolic extract and n-hexane fraction have shown superior activity than the existing antibiotics. This manifests the medicinal importance of E. serrata. This is the first report to explore the potential of this plant against pathogens which warrants further extensive studies on the phytochemical studies, which in turn may result in the discovery of new and more potent antibiotics.
Competing interests
The authors have no competing interests.
Acknowledgement
The authors are highly thankful to Dr. James A. Tebbe, rector FCCU and former rector Dr. Peter H. Armacost for their support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agoramoorthy G., Chandrasekaran M., Venkatesalu V., Hsu M.J. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Brazilian J. Microbiol. 2007;38:739–742. [Google Scholar]
- Al Akeel R., Al-Sheikh Y., Mateen A., Syed R., Janardhan K., Gupta V.C. Evaluation of antibacterial activity of crude protein extracts from seeds of six different medical plants against standard bacterial strains. Saudi J. Biol. Sci. 2014;21:147–151. doi: 10.1016/j.sjbs.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antibiotic Resistance Threats in the United States, 2013. Antibiotic/Antimicrobial Resistance|CDC. Available at: https://www.cdc.gov/drugresistance/threat-report-2013/index.html (accessed October 17, 2017).
- Arenas P.M., Molares S., Aguilar Contreras A., Doumecq B., Gabrielli F. Ethnobotanical, micrographic and pharmacological features of plant-based weight-loss products sold in naturist stores in Mexico City: the need for better quality control. Acta Bot. Brasilica. 2013;27:560–579. [Google Scholar]
- ECDC/EMEA Joint Technical Report, 2009. The Bacterial Challenge-time to React. Available at: https://ecdc.europa.eu/en/publications-data/ecdcemea-joint-technical-report-bacterial-challenge-time-react (accessed October 17, 2017).
- Finch R. Innovation—drugs and diagnostics. J. Antimicrob. Chemother. 2007;60:i79–i82. doi: 10.1093/jac/dkm165. [DOI] [PubMed] [Google Scholar]
- Frank D.J., Zhao Y., Wong S.H., Basudhar D., De Voss J.J., Ortiz de Montellano P.R. Cholesterol analogs with degradation-resistant alkyl side chains are effective mycobacterium tuberculosis growth inhibitors. J. Biol. Chem. 2016;291:7325–7333. doi: 10.1074/jbc.M115.708172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A., Das R., Sarkhel S., Mishra R., Mukherjee S., Bhattacharya S., Gomes A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010;48:865–878. [PubMed] [Google Scholar]
- Herbst C., Naumann F., Kruse E.-B., Monsef I., Bohlius J., Schulz H., Engert A. Prophylactic antibiotics or G-CSF for the prevention of infections and improvement of survival in cancer patients undergoing chemotherapy. Cochrane Database Syst. Rev. 2009:CD007107. doi: 10.1002/14651858.CD007107.pub2. [DOI] [PubMed] [Google Scholar]
- Hernández-Villegas M.M., Borges-Argáez R., Rodríguez-Vivas R.I., Torres-Acosta J.F.J., Méndez-González M., Cáceres-Farfán M. In vivo anthelmintic activity of Phytolacca icosandra against Haemonchus contortus in goats. Vet. Parasitol. 2012;189:284–290. doi: 10.1016/j.vetpar.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Jeruto P., Lukhoba C., Ouma G., Otieno D., Mutai C. An ethnobotanical study of medicinal plants used by the Nandi people in Kenya. J. Ethnopharmacol. 2008;116:370–376. doi: 10.1016/j.jep.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Joanne B., Anderson L.A., Phillipson J.D. third ed. Pharmaceutical Press; London: 2007. Herbal Medicines. [Google Scholar]
- Khan S.U., Khan A., Shah A.-H.A., Shah S.M., Hussain S., Ayaz M., Ayaz S. Heavy metals content, phytochemical composition, antimicrobial and insecticidal evaluation of Elaeagnus angustifolia. Toxicol. Ind. Health. 2016;32:154–161. doi: 10.1177/0748233713498459. [DOI] [PubMed] [Google Scholar]
- Khare C.P. Springer; New York: 2007. Indian Medicinal Plants: An Illustrated Dictionary. [Google Scholar]
- Johnston I.M. Ehretia acuminata var. serrata (Roxb.) J. Arn. Arb. 1951;32:23. [Google Scholar]
- Mncwangi N., Chen W., Vermaak I., Viljoen A.M., Gericke N. Devil’s Claw—a review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012;143:755–771. doi: 10.1016/j.jep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Mohapatra D.P., Thakur V., Brar S.K. Antibacterial efficacy of raw and processed honey. Biotechnol. Res. Int. 2011;2011:1–6. doi: 10.4061/2011/917505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njeru S.N., Obonyo M.A., Nyambati S.O., Ngari S.M. Antimicrobial and cytotoxicity properties of the crude extracts and fractions of Premna resinosa (Hochst.) Schauer (Compositae): Kenyan traditional medicinal plant. BMC Complement. Altern. Med. 2015;15:295. doi: 10.1186/s12906-015-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omosa L.K., Midiwo J.O., Mbaveng A.T., Tankeo S.B., Seukep J.A., Voukeng I.K., Dzotam J.K., Isemeki J., Derese S., Omolle R.A., Efferth T., Kuete V. Antibacterial activities and structure–activity relationships of a panel of 48 compounds from Kenyan plants against multidrug resistant phenotypes. Springerplus. 2016;5:901. doi: 10.1186/s40064-016-2599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavali R.R., Degati V.L., Lomada D., Reddy M.C., Durbaka V.R.P. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS One. 2017;12:0179245. doi: 10.1371/journal.pone.0179245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha O.P., Felício R. De, Rodrigues A.H.B., Ambrósio D.L., Cicarelli R.M.B., Albuquerque S. De, Young M.C., Yokoya N.S., Debonsi H.M. Chemical profile and biological potential of non-polar fractions from Centroceras clavulatum (C. Agardh) Montagne (Ceramiales, Rhodophyta) Molecules. 2011;16:7105–7114. doi: 10.3390/molecules16087105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teke G.N., Lunga P.K., Wabo H.K., Kuiate J.-R., Vilarem G., Giacinti G., Kikuchi H., Oshima Y. Antimicrobial and antioxidant properties of methanol extract, fractions and compounds from the stem bark of Entada abyssinica Stend ex A. Satabie. BMC Complement. Altern. Med. 2011;11:57. doi: 10.1186/1472-6882-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torane R.C., Kamble G.S., Kale A.A., Gadkari T.V., Deshpande N.R. Quantification of dioctyl phthalate from Ehretia laevis Roxb by HPTLC. J. Chem. Pharm. Res. 2011;3:48–51. [Google Scholar]
- Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Zara S., Ahmed D., Baig H., Ikram M. Evaluation of antioxidant activities of various solvent extracts of fruits and leaves of Ehretia serrata. Asian J. Chem. 2012;24:4345–4351. [Google Scholar]